Intense 1400–1600 nm circularly polarised luminescence from homo- and heteroleptic chiral erbium complexes†
Abstract
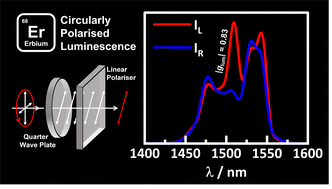
Efficient near-infrared circularly polarised luminescence (CPL) between 1400 and 1600 nm of four enantiomer pairs of homo- and heteroleptic complexes has been successfully measured. Utilising inexpensive optics and a commercial fluorimeter, discrimination of the left and right circularly polarised components was achieved, yielding remarkably high dissymmetry values in emission (glum). Most notably, homoleptic complexes CsEr(hfbc)4 (hfbc = 3-heptafluorobutylyrylcamphorate) and [TMG−H+]3Er(BINOLate)3 (TMG = 1,1,3,3-tetramethylguanidine; BINOLate = 1,1′-bi-2-naphtholate) show maximal |glum| values of 0.83 (at 1510 nm) and 0.29 (at 1545 nm) respectively, with the former complex possessing the strongest CPL within the NIR to date.
- This article is part of the themed collection: Circularly Polarised Luminescence


 Please wait while we load your content...
Please wait while we load your content...