Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A cross-linkable, organic down-converting material for white light emission from hybrid LEDs†
Hao
Yang
a,
Jochen
Bruckbauer
 b,
Lyudmyla
Kanibolotska
a,
Alexander L.
Kanibolotsky
ac,
Joseph
Cameron
b,
Lyudmyla
Kanibolotska
a,
Alexander L.
Kanibolotsky
ac,
Joseph
Cameron
 a,
David J.
Wallis
de,
Robert W.
Martin
*b and
Peter J.
Skabara
a,
David J.
Wallis
de,
Robert W.
Martin
*b and
Peter J.
Skabara
 *a
*a
aWestCHEM, School of Chemistry, University of Glasgow, Glasgow, G12 8QQ, UK. E-mail: peter.skabara@glasgow.ac.uk
bDepartment of Physics, SUPA, University of Strathclyde, Glasgow, G4 0NG, UK. E-mail: r.w.martin@strath.ac.uk
cInstitute of Physical-Organic Chemistry and Coal Chemistry, 02160 Kyiv, Ukraine
dDepartment of Materials and Metallurgy, University of Cambridge, Cambridge, CB3 0FS, UK
eCentre for High Frequency Engineering, University of Cardiff, Cardiff, CF24 3AA, UK
First published on 7th July 2023
Abstract
The use of organic materials and the replacement of rare-earth elements in the making of light-emitting devices has been increasingly popular over the last decades. Herein, the synthesis and characterisation of a novel organic green-emitting material (GreenCin), based on a fluorene-benzothiadiazole-fluorene (Flu-BT-Flu) core structure, and its performance as a down-converting layer in tandem with commercial blue light-emitting diodes (LEDs) for white light emission are reported. This material has been functionalised with cinnamate-groups to enable the emissive material to react with the cross-linker tetra(cinnamoyloxymethyl)methane (TCM), to produce stable films with high performance in hybrid LEDs. The hybrid devices can generate white light with a good colour rendering index (CRI) of 69. The hybrid devices also have ×2.6 increased luminous efficacy (107 lm W−1) and ×2.4 increased radiant flux (24 mW) when compared with hybrid devices using non-cross-linked analogues of GreenCin. Additionally, the hybrid devices containing GreenCin have a high blue-to-white efficacy value (defined by dividing the luminous flux of a hybrid device by the radiant flux of the underlying blue LED), of 213 lm W−1, for which inorganic phosphors have values in the range of 200–300 lm W−1.
1. Introduction
With the booming industrial needs for high performance displays and lighting devices, it is crucial to achieve higher white light output efficiencies.1 Commonly, white light-emitting diodes (WLEDs) can be produced through a combination of inorganic LEDs that emit at different colour ranges or by coating inorganic LEDs that emit at blue, violet, or near-UV range with down-converting inorganic phosphors.2,3 White light-emitting devices that consist of a combination of inorganic red, green, and blue (RGB) LEDs offer higher values for the colour rendering index (CRI) but come with high manufacturing and maintenance costs.2 These inorganic LEDs can produce white light with CRI values of over 80.4,5 However, their performance is commonly hindered by the need to balance the output from three separate LEDs and the lack of efficient green output. This is a problem due to how well human eyes respond to green light.6,7To solve these problems, inorganic phosphors can be used as down-converting materials to coat inorganic LEDs and to achieve white light emission.8–15 Commercially, the most popular approach is to combine blue LEDs with inorganic phosphors that absorb a portion of the blue light and emit in the yellow region so that the combination of transmitted blue and emitted yellow appears as white light.16,17 The colour rendering can also be further improved by adding red phosphors to increase the emission coverage. However, despite good device performance through the use of inorganic phosphors, several drawbacks still exist. The synthesis of inorganic phosphors usually involves the use of rare-earth elements such as yttrium (Y), cerium (Ce), gadolinium (Gd), and ytterbium (Yb), which increases the manufacturing costs.18,19 In addition, WLEDs with inorganic phosphors tend to have low colour rendering capability due to the weak green and red emission.20 Finally, the device performance is further hindered due to self-absorption which causes photoluminescence quenching in inorganic phosphors.21
By comparison, organic materials used as the emissive layers in organic light-emitting diodes (OLEDs) possess distinct characteristics such as low manufacturing costs, ease of synthesis and colour tuning of emission, together with mechanical flexibility.22–24 However, they also exhibit some distinct problems which hinder the complete substitution of inorganic components. Organic materials tend to aggregate when in the solid state, which can quench the emission. Additionally, organic materials suffer from inferior stability under device operation conditions that can cause OLEDs to have relatively short device lifetimes when compared to their inorganic counterparts.24
To solve this issue, the design of hybrid inorganic/organic LED devices has been proposed to potentially combine the advantages of both inorganic LEDs and organic materials. Most commonly, this hybrid architecture is achieved by having an organic material coating as a frequency down-converter for an inorganic LED.25–29 One of the early works on white light-emitting devices that combines an inorganic LED with an organic material was conducted by Hide et al.30 In this work, InGaN LEDs that emit in the blue region were coated with two organic polymers as a bilayer that emit within the green (BuEH-PPV) and red (MEH-PPV) regions of the visible spectrum. In general, the colour of the overall emission can be tuned by varying the thickness of each organic layer to achieve an ‘ideal’ value. The commission internationale de l’éclairage (CIE) coordinates of the ‘whitest’ device is (0.34, 0.29), which is close to the most ideal white point of equal-energy that is located at (0.33, 0.33).
Previously, we reported a hybrid inorganic/organic LED device that utilises a yellow-emitting material as an energy down-converter for a blue LED.26 The organic down-converter material was dissolved and cured in a 1,4-cyclohexanedimethanol divinyl ether (CHDV) matrix to give a rigid membrane that forms a dome on the blue LED and serves to provide some stability from air for the organic emitter. Embedding organic down-converters in additional solid matrices can disaggregate the organic dyes and limit the π–π stacking.31,32 However, in order to form a CHDV matrix, a photoacid generator, 4-octyloxy diphenyliodonium hexafluoroantimonate (PAG), is needed to initiate the cross-linking process. PAG is sensitive to short UV wavelength (250 nm) and decomposes during illumination to release protons. The vinyl ether units polymerise due to the presence of protons which eventually leads to the cross-linked matrix network. Although the CHDV matrix can embed the organic material well to prevent photo-oxidation, the degree of cross-linking is rather difficult to control. In addition, we observed that the colour of the membranes has the tendency to change to deep yellow over time with higher PAG concentration, which consequently lowers the down-converting efficiency. CHDV encapsulation was also used for organic light emitting materials based on 2,1,3-benzothiadiazole-fluorene (BT-Flu) core structures. Taylor-Shaw et al. reported novel organic down-converting materials, (TPA-Flu)2BT and (TPA-Flu)2BTBT, that emit in the yellow region and were integrated with a blue LED.33 The materials served well as down-converting materials for blue LEDs with a highest luminous efficacy value of 41 lm W−1 and CIE coordinates of (0.34, 0.31).
Herein, we report a novel organic green-emitting material (GreenCin) which has the advantage that it can be used as an energy down-converter for blue LEDs with high efficiency and also can be applied to blue LEDs as a homogeneous layer without the need of additives in the host matrix materials for encapsulation due to the photoreactive properties of cinnamate groups (Fig. 1). Apart from the photoreactive ability, this material also bears several distinct characteristics—it has an absorbance band that aligns closely with the emission wavelength of blue LEDs and it has a high photoluminescence quantum yield (PLQY) that can lead to higher luminous efficacy values. The material is based on a well-studied green-emitter structure that consists of an electron-deficient BT core flanked by two fluorene units as weak electron donors. The BT-Flu backbone structure is known for its effectiveness as a green-emitter.34 The UV-curability comes from the cinnamate functionality at the end of each fluorene unit. Cinnamate groups are known to undergo a [2+2] cycloaddition reaction under UV irradiation to form a cyclobutane ring structure due to the vinyl groups.35 However, due to the linear structure of GreenCin, the polymeric form is also linear. In order to cross-link GreenCin to form a robust matrix, tetra(cinnamoyloxymethyl)methane (TCM) was added as a cross-linking agent (Fig. 1). The resulting cross-linked GreenCin film is insoluble in most organic solvents, which makes it a physically stable film without the need of additional encapsulation, as well as being a promising candidate as an energy down-converting coating for use with blue LEDs. The resulting devices demonstrate very reasonable colour rendering quality (52 < CRI < 69) and a high luminous efficacy value of 107 lm W−1, as well as an excellent blue-to-white efficacy value of 213 lm W−1 (defined by dividing the luminous flux of a hybrid device by the radiant flux of the underlying blue LED). The blue-to-white efficacy value can be used to determine the amount of white light output from the devices that is generated by colour conversion of the absorber/emitter. By comparison, the blue-to-white efficacy values of commonly used commercial phosphors range between 200–300 lm W−1.3
2. Synthesis
The syntheses of TCM,36 tetrakis(triphenylphosphine)palladium,37 9,9-dihexyl-7-trimethylsilylfluoren-2-yl boronic acid (2), 4,7-bis(7-trimethylsilyl-9,9-dihexylfluoren-2-yl)-2,1,3-benzothiadiazole (3) and 4,7-bis(7-bromo-9,9-dihexylfluoren-2-yl)-2,1,3-benzothiadiazole (4)33 are described in the literature. GreenCin was synthesised in 5 steps according to the following procedure (Scheme 1). 2,7-Dibromo-9,9-dihexylfluorene (1) was functionalised with a trimethylsilane group and a boronic acid group at the brominated positions in two sequential steps to give 9,9-dihexyl-7-trimethylsilylfluoren-2-yl boronic acid 2. The dibromo-BT unit was then coupled with the modified fluorene unit 2via a Suzuki-coupling reaction to yield 4,7-bis(7-trimethylsilyl-9,9-dihexylfluoren-2-yl)-2,1,3-benzothiadiazole 3. Compound 3 was then brominated in high yield to give 4,7-bis(7-bromo-9,9-dihexylfluoren-2-yl)-2,1,3-benzothiadiazole 4, which was further modified via another Suzuki-coupling reaction with 4-(hydroxymethyl)phenylboronic acid pinacol ester to give product 5. Finally, compound 5 was reacted with cinnamoyl chloride to afford the cinnamate-functionalised green emitter material 4,7-bis(7-(4-cinnamoyloxymethylphenyl)-9,9-dihexylfluoren-2-yl)-2,1,3-benzothiadiazole (GreenCin, 6) in a high yield of 96% for the two final steps of the conversion of 4 to 6.Synthesis of 4,7-bis(7-(4-oxymethylphenyl)-9,9-dihexylfluoren-2-yl)-2,1,3-benzothiadiazole (5)
Deionised water (1.66 ml) was added to a mixture of the 4,7-bis(7-bromo-9,9-dihexylfluoren-2-yl)-2,1,3-benzothiadiazole (4) (0.3 g, 0.31 mmol), tetrakis(triphenyl phosphine)palladium (0) (0.07 g, 0.06 mmol), 4-(hydroxymethyl)phenyl boronic acid pinacol ester (0.22 g, 0.94 mmol), Ba(OH)2·8H2O (0.79 g, 2.5 mmol) in 30 ml tetrahydrofuran (THF), and the reaction mixture was degassed and stirred at 80 °C for 20 h. After aqueous work-up with CH2Cl2 and deionised water, the crude product was subjected to column chromatography on silica gel, eluting with toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (10
ethyl acetate (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The main fraction was precipitated from CH2Cl2–methanol, which afforded the product as a yellow-orange solid (97% yield). M.p. 101–104 °C.
1). The main fraction was precipitated from CH2Cl2–methanol, which afforded the product as a yellow-orange solid (97% yield). M.p. 101–104 °C.
MALDI TOF MS (dithranol as a matrix): m/z 1012.22 (M+), 995.22 ([M − OH]+).
Anal. Calcd for C70H80N2O2S: C, 82.96; H, 7.96; N, 2.76; found: C, 82.95; H, 7.65; N, 2.72.
1H NMR (CDCl3, δH, 400 MHz), 8.05 (2H, dd, 3J = 7.9 Hz, 4J = 1.6 Hz), 7.99 (2H, d, 4J = 1.2 Hz), 7.91 (2H, s), 7.90 (2H, d, 3J = 8.0 Hz), 7.84 (2H, d, 3J = 7.8 Hz), 7.70 (4H, d, 3J = 8.2 Hz), 7.62 (2H, dd, 3J = 7.8 Hz, 4J = 1.6 Hz), 7.60 (2H, s), 7.50 (4H, d, 3J = 8.3 Hz), 4.79 (4H, d, 3J = 5.9 Hz), 2.22–1.96 (8H, m), 1.67 (2H, t, 3J = 6.0 Hz), 1.26–0.99 (24H, m), 0.95–0.73 (8H, m), 0.77 (12H, t, 3J = 6.9 Hz).
13C NMR (CDCl3, δC, 100 MHz), 154.5, 152.2, 151.6, 141.3, 141.1, 140.2, 140.1, 140.0, 136.4, 133.8, 128.4, 129.1, 127.7, 127.6, 126.2, 124.1, 121.7, 120.4, 120.0, 65.4, 55.5, 40.5, 31.6, 29.9, 24.1, 22.7, 14.2.
Synthesis of 4,7-bis(7-(4-cinnamoyloxymethylphenyl)-9,9-dihexylfluoren-2-yl)-2,1,3-benzothiadiazole (GreenCin, 6)
Cinnamoyl chloride (22.6 mg, 0.135 mmol) was added to a mixture of 4,7-bis(7-(4-oxymethylphenyl)-9,9-dihexylfluoren-2-yl)-2,1,3-benzothiadiazole (5) (45.9 mg, 0.045 mmol) and 4-dimethylaminopyridine (16.6 mg, 0.136 mmol) in 20 ml of dry toluene. The reaction mixture appeared as a milky suspension with white flakes formed and this was degassed and stirred at 100 °C for 20 h. After filtration and removal of the solvent, the crude product was purified by column chromatography on silica gel, eluting with CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether 40–60 °C (1
petroleum ether 40–60 °C (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Precipitation of the main fraction from CH2Cl2
1). Precipitation of the main fraction from CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol afforded a yellow-orange solid in 99% yield. M.p. 106–107 °C.
methanol afforded a yellow-orange solid in 99% yield. M.p. 106–107 °C.
MALDI-TOF MS (retinoic acid as a matrix): m/z – 1272.38 (M+).
Anal. calcd for C88H92N2O4S: C, 82.98; H, 7.28; N, 2.20; found: C, 82.99; H, 7.24; N, 2.26.
1H NMR (CDCl3, δH, 400 MHz) 8.05 (2H, dd, 3J = 7.9 Hz, 4J = 1.6 Hz), 7.99 (2H, d, 4J = 1.2 Hz), 7.91 (2H, s), 7.90 (2H, d, 3J = 7.6 Hz), 7.84 (2H, d, 3J = 7.8 Hz), 7.77 (2H, d, 3J = 16.0 Hz), 7.72 (4H, d, 3J = 8.3 Hz), 7.62 (2H, dd, 3J = 7.8 Hz, 4J = 1.6 Hz), 7.60 (2H, s), 7.58–7.52 (8H, m), 7.45–7.35 (6H, m), 6.52 (2H, d, 3J = 16 Hz), 5.33 (4H,s), 2.2–2.0 (8H, m), 1.2–1.0 (24H, m), 0.9–0.7 (8H, m), 0.77 (12H, t, 3J = 6.9 Hz).
13C NMR (CDCl3, δC, 101 MHz) 167.0, 154.5, 152.2, 151.6, 145.4, 141.9, 141.1, 140.3, 139.9, 136.4, 135.1, 134.5, 133.7, 130.5, 129.1, 128.4, 128.3, 128.1, 127.6, 126.3, 124.1, 121.8, 120.4, 120.0, 118.1, 66.3, 55.5, 40.5, 31.6, 29.9, 24.1, 22.7, 14.2.
3. Results and discussion
3.1 Optical characterisation of GreenCin
It is essential to fully understand the optical properties of organic emissive materials to evaluate their performance as down-converters. UV-Vis absorption spectroscopy measurements of samples in solid-state as well as their photoluminescence properties were studied. The solid-state measurements were performed on films that were prepared by spin-coating toluene solutions of 2.5 mg ml−1 concentration of GreenCin on quartz substrates at 2000 rpm spin-speed.The UV-Vis spectrum of GreenCin shows three major absorption peaks as seen in Fig. 2 for solution and solid-state measurements. The peak around 280 nm can be assigned to the cinnamoyl groups. The narrow peak around 335 nm corresponds to the π–π* transition of the fluorene arms whereas the broad peak around 430 nm corresponds to the intramolecular charge transfer (ICT) band. The absorption spectrum of GreenCin in the solid film is very similar to that in solution, but with a bathochromic shift (red shift) of 14 nm observed for the ICT band. This shift is likely to be due to dipole–dipole interactions of the molecules in the condensed phase. A similar bathochromic shift has been observed for a different linear quaterfluorene-DPP system.38 The emission spectra in the film are blue-shifted by 11 nm compared to the emission spectra in CH2Cl2, due to a lower degree of excited state structural relaxation before emission in the condensed phase. The results of solution and solid state measurements are summarised in Table 1. The optical properties of GreenCin are suitable for an energy down-converter for blue light as the main absorption band at 436 nm in the solid-state is close to the wavelength of blue light emitted by most commercially used blue LEDs, which typically emit near 450 nm. Additionally, the PLQY value for GreenCin in the solid state is 84% which makes it an excellent light-emitting organic dye and can consequently increase the down-converting performance.
| λ absmax in CH2Cl2a [nm] | λ emmax in CH2Cl2b [nm] | PLQY in CH2Cl2cd [%] | λ absmax film [nm] | λ emmax filme [nm] | PLQY filmc [%] |
|---|---|---|---|---|---|
| a Recorded in CH2Cl2 at 10−5 M. b Recorded in CH2Cl2 at 10−6 M with excitation at 421 nm. c Absolute values. d Recorded in CH2Cl2 at 10−6 M. e Excitation at 421 nm. | |||||
| 280, 334, 422 | 560 | 94 | 284, 339, 436 | 549 | 84 |
3.2 Photoreactive properties
The photoreactive properties of GreenCin and the cross-linking process between GreenCin and TCM were examined by treating the samples with UV irradiation. The samples were prepared by spin-coating the target solutions on quartz substrates at 2000 rpm. The process was monitored by UV-Vis absorption spectroscopy measurements.Initially, the photoreactive properties of the monomer TCM as a single component were studied. Fig. 3(a) shows the changes in the UV-vis absorption spectra of TCM film after exposure to 254 nm UV light over a time span of 90 minutes. The peak at 294 nm corresponds to the absorption of the cinnamate moieties. The peak decreases upon further irradiation and disappears after 90 min, which indicates that the cross-linking process between cinnamate groups by a cycloaddition is complete. In order to test the stability of films when in contact with solvents, the film was rinsed with CH2Cl2, followed by rinsing with chloroform, and finally immersion in hot chloroform for 5 minutes. The UV-Vis spectra were measured after each washing stage (Fig. 3(b)). The results showed no change in the absorption spectra which indicates the insolubility of the cross-linked TCM matrix.
For comparison, the photocycloaddition processes of the GreenCin monomer were studied by the same method. The resulting polymer structure, p(GC), is shown in Fig. 4(a). Fig. 4(b) shows a similar change in the cinnamate absorption band where the intensity of the peak at 291 nm diminished, indicating the completion of polymerisation between GreenCin molecules. However, the films of p(GC) were completely washed-off upon rinsing with CH2Cl2, which proved that the linear polymer of GreenCin created upon UV-irradiation (with a molecular weight of 12.0 kDa), has good solubility.
To study the cross-linking ability of TCM, stock solutions of the same concentration of TCM and GreenCin in toluene were prepared (2 mM) separately. The ratio between GreenCin and TCM was kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in order to have a high degree of cross-linking between these two materials without sacrificing the down-converting efficiency due to the solution being too diluted. Fig. 5 shows the idealised structure of the cross-linked copolymer between GreenCin and TCM.
10 in order to have a high degree of cross-linking between these two materials without sacrificing the down-converting efficiency due to the solution being too diluted. Fig. 5 shows the idealised structure of the cross-linked copolymer between GreenCin and TCM.
The cross-linking process was monitored by performing UV-vis spectroscopy measurements of films at intervals of 20 min. Fig. 6(a) shows the UV-vis absorption spectra of the cross-linking process. The intensity for the absorption of the cinnamate groups (291 nm) was significantly higher than that for the previous samples as additional cinnamate groups were introduced when mixing TCM with GreenCin. After no further reduction in the cinnamate peak could be observed from the UV-Vis spectra, the samples were rinsed with CH2Cl2, which washed off any unreacted materials and by-products such as linear GreenCin polymer and co-polymers of TCM-GreenCin with low molecular weight. This explains the decrease in absorption across the entire spectra after the first wash. The remaining film was then proven to be insoluble after washing with chloroform and then immersing in hot chloroform for 5 min with no change in absorption intensity (Fig. 6(b)). These data confirm the successful cross-linking between GreenCin and TCM.
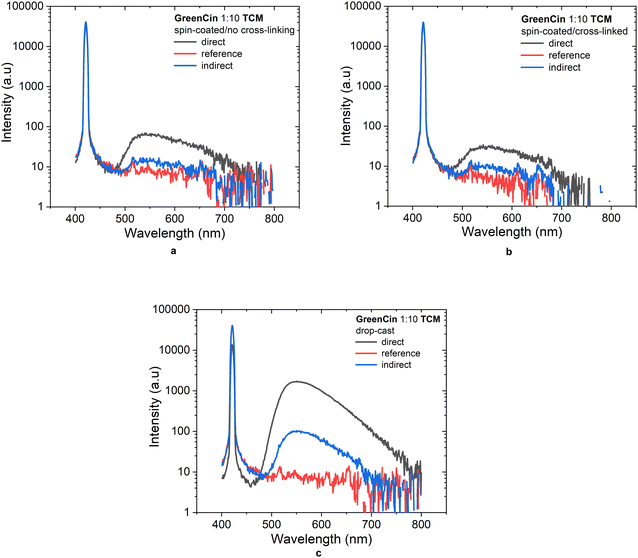
The PLQY values of films obtained from GreenCin and TCM composite material were also studied in thick (drop-cast) and thin (spin-coated) films. The films were prepared by three different ways: spin-coated and not cross-linked; spin-coated, cross-linked and washed with CH2Cl2; drop-casted, cross-linked and washed with CH2Cl2. The summary of PLQY values and PLQY spectrum are shown in Table 2 and Fig. 7. It can be seen that the PLQY values of films that were prepared by spin-coating were significantly lower than the films that were prepared by drop-casting. The reason for this was mainly due to the small quantity of emissive material remaining on the resulting films following the spin-coating process. However, since the deposition method used to fabricate hybrid devices was by drop-casting (details of device fabrication is discussed in the next section), the amounts of emissive materials remaining on the blue LEDs were higher. Thus, the PLQY value obtained from this method shows more relevance and was also consistent with the PLQY value determined for the neat GreenCin films.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 TCM films
10 TCM films
| Deposition method/treatments | PLQY (%) |
|---|---|
| Spin-coated, no cross-linking or washing | 19 |
| Spin-coated, cross-linked and washed with CH2Cl2 | 11 |
| Drop-cast, cross-linked and washed with CH2Cl2 | 87 |
3.3 Down-converting device properties
The blue-emitting InGaN/GaN LEDs used for this work were manufactured by Plessey Semiconductors Ltd. The LEDs emit near 450 nm, which has good overlap with the absorption peak of GreenCin at 436 nm in the solid state. Thus, the emitted blue light from the inorganic LEDs can be absorbed and down-converted by the organic layer. GreenCin and TCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio) were dissolved in tetrahydrofuran at a concentration of 16 mg ml−1 for the emitter material. The solution was drop-cast onto a blue LED and cross-linked under 254 nm UV irradiation to form a green-emitting film on the top. However, due to the presence of two cinnamate-functionalised materials, self-polymerisation of GreenCin and short-chain copolymers of TCM and GreenCin can also be created. Therefore, the down-converting layer was washed with CH2Cl2 to remove any materials before a second layer of cross-linked TCM film was applied on the top of the down-converting film to protect it from the ambient environment which contains oxygen and water. The deposition process and an image of the hybrid LED under operation inside the integrating sphere are shown in Fig. S1 in the ESI.†
10 molar ratio) were dissolved in tetrahydrofuran at a concentration of 16 mg ml−1 for the emitter material. The solution was drop-cast onto a blue LED and cross-linked under 254 nm UV irradiation to form a green-emitting film on the top. However, due to the presence of two cinnamate-functionalised materials, self-polymerisation of GreenCin and short-chain copolymers of TCM and GreenCin can also be created. Therefore, the down-converting layer was washed with CH2Cl2 to remove any materials before a second layer of cross-linked TCM film was applied on the top of the down-converting film to protect it from the ambient environment which contains oxygen and water. The deposition process and an image of the hybrid LED under operation inside the integrating sphere are shown in Fig. S1 in the ESI.†
Fig. 8 shows the electroluminescence (EL) spectra of hybrid devices that were measured under a constant current of 25 mA inside an integrating sphere. Device 1 to 8 follow the trend of increasing amount of GreenCin–TCM solution drop-cast onto the blue LEDs that were later cross-linked under UV to yield films with increasing emission intensities. Fig. 8(a) shows the absolute EL spectra whereas the green-emission peaks are normalised against the blue-emission peaks in Fig. 8(b) so that the difference of intensities can be seen more easily. Two distinct emission peaks were observed in both spectra. The emission peak at 450 nm can be assigned to the blue LED whereas the emission peak at 540 nm can be assigned to the green-emission from the cross-linked copolymer films. With the increase of the amount of down-converting material, the intensity of green emission increases. As a result, the ratio between green emission and blue emission increases. Thus, a higher yield of blue light being absorbed and converted by the organic material can be achieved.
White light emission was confirmed by CIE 1931 chromaticity coordinates, which were calculated from the EL spectra. Several hybrid devices with different green-emission intensity were made and their chromaticity coordinates are shown in the CIE 1931 plot (Fig. 9). Chromaticity coordinates of a bare blue LED and GreenCin were also included as a reference. It can be seen that the data points form a line that cuts through the Planckian locus from the near blue region to near green region, with some lying near the region of ideal white light emission.
In addition to the chromaticity coordinates, correlated colour temperature (CCT) can also be used to quantify the colour of the emission. The CCT value can be calculated by determining the shortest distance between the chromaticity point and the Planckian locus.39 It is also often used to categorise a white light as ‘cool white’ or ‘warm white’. The CCT values for the emissions of hybrid devices that lie in the cool-white region and warm-white region are 7490 K and 4750 K respectively, which covers the colour region between cool and warm white light. Therefore, colour tuning of white light emission from the hybrid devices can be achieved by varying the amount of organic materials coated on blue LEDs. The fidelity and the quality of the white light emission can be described by the colour rendering index (CRI), which ranges between 0 to 100 with the maximum value representing an “ideal” colour rendering source such as an incandescent light bulb.7 The CRI values for the hybrid devices range between 52 and 69, which is reasonable for hybrid devices with only one organic down-converting material, and are similar to the colour rendering ability achieved with similar organic down-converting materials that consist of BT-fluorene core structures.33 Overall, the CRI value reduces as the green emission intensity increases. This indicates that high colour rendering quality is achieved with cool white light. The biggest contribution to the good colour-rendering ability is the low self-absorption of the organic material.
Apart from the characterisation of light produced by the hybrid devices, additional parameters are used to quantify the down-converting efficiencies of the organic materials in the hybrid devices. Radiant flux (W) can be used to determine the optical power of the device whilst luminous efficacy (lm W−1) can be used to determine the ability of a device to convert electrical power to light. It is worth mentioning that luminous efficacy is defined by the ratio of luminous flux and the input electric power, whilst taking into account how the human eye responds to visible light, whereas radiant flux only considers the optical performance of devices without considering how the human eye perceives the light.25,39Table 3 shows the luminous efficacy values for the hybrid devices with different green emission intensities (different amounts of organic materials coated on blue LEDs). The luminous efficacy value increases with the increase of green emission intensity. The highest luminous efficacy value achieved with this type of hybrid device is 107 lm W−1. By comparison, this is more than 2 times higher than the last generation of organic down-converting materials that utilise similar BT-fluorene core structures.33 The high luminous efficacy can be explained by the high PLQY value of 84% in the solid-state. The radiant flux values for hybrid devices decrease with increased green emission intensity (from cool to warm white light), with the highest value being 24 mW. In addition, blue-to-white efficacy can be used as a supplementary parameter to further describe the down-converting efficiency. The term is defined by the ratio of the luminous flux (lm) of the hybrid devices with the radiant flux of the bare blue LED (W).3 This can further illustrate how much of the emitted blue light from the LED is converted to white light by down-converting materials.39 The blue-to-white efficacy values follow a similar trend to the values of luminous efficacy, where the value increases with the increase of green emission intensity. The highest blue-to-white efficacy value is 213 lm W−1 (device 7).
| Hybrid device | Luminous efficacy (lm W−1) | Radiant flux (mW) | Blue-to-white efficacy (lm W−1) |
|---|---|---|---|
| Device 1 | 71.3 | 24.0 | 138 |
| Device 2 | 81.9 | 23.8 | 162 |
| Device 3 | 94.6 | 22.0 | 197 |
| Device 4 | 99.0 | 16.5 | 193 |
| Device 5 | 110.6 | 21.2 | 185 |
| Device 6 | 103.4 | 20.3 | 205 |
| Device 7 | 104.1 | 17.4 | 213 |
| Device 8 | 107.1 | 16.5 | 212 |
It is worth mentioning that this new generation of organic down-converting material based on the BT-fluorene core structure shows some improvements when compared to the similar green-emitter material, (TPA-Flu)2BT.33Table 4 shows some of the key values that were used to compare the down-converting ability of this organic material. In general, GreenCin has a higher PLQY value, higher luminous efficacy and higher radiant flux values, and a similar CRI range when compared to (TPA-Flu)2BT. However, the only drawback of this new material based on the measurements is the lower blue-to-white efficacy value with comparison to the (TPA-Flu)2BT. This might be caused by the area coverage differences between dome-shaped CHDV membranes that contain (TPA-Flu)2BT and the thin films of cross-linked TCM-GreenCin copolymer. Due to the small volume of GreenCin solution that was deposited on the blue LEDs, the material tends to accumulate around the LED edge or wire bonds, which is a common problem for direct deposition of organic dyes on LEDs.40 Thus, fewer blue photons were absorbed and down-converted by GreenCin molecules. The blue-to-white efficacy value can be improved either by tuning the optical properties of the GreenCin material to shift its absorption band to more closely match the wavelength of commercially used blue LEDs (450 nm) or by physically increasing the area coverage of organic down-converting materials on the LEDs. Of strategic importance, an increase in the number of incoming blue photons absorbed will lead to a higher blue-to-white efficacy.
| Material | PLQY | Luminous efficacy (lm W−1) | Radiant flux (mW) | Blue-to-white efficacy (lm W−1) | CRI |
|---|---|---|---|---|---|
| GreenCin | 84% | 107 | 24 | 213 | 52–69 |
| (TPA-Flu)2BT | 61% | 41 | 10.2 | 368 | 51–66 |
3.4 Stability study of GreenCin
Measuring the high performance of organic materials as down-converting layers for inorganic LEDs is important. However, organic materials can suffer from poor thermal or photo-stability and this limits the lifetime of hybrid devices. Ideally, cross-linked polymers are more stable than their monomers. Thermodynamically, the total energy of the monomers combined is higher than the total energy of the polymers.24 Mechanically, due to the entanglements of polymer chains, cross-linked polymers tend to have restricted chain movements which lead to lower degree of freedom and hence, higher stability.41 Therefore, GreenCin in its matrix form should be relatively more stable than other non-cross-linkable organic down-converting monomers.The degradation of the hybrid devices was studied by recording the devices’ EL spectra regularly over several months to check if there was any significant decrease of the green-emission peak as well as a change in chromaticity coordinates. Initial measurements of the early batches of hybrid devices showed a decrease in the green-emission peak (data not shown) and was suspected to be caused by photo-oxidation due to the organic layers being in contact with the atmosphere. Protection of the organic layer was attempted by adding an additional layer such as a poly(CHDV) membrane or a TCM film as encapsulant. However, the green emission continued to decrease over several weeks of time whilst the chromaticity coordinates shifted towards the blue region.
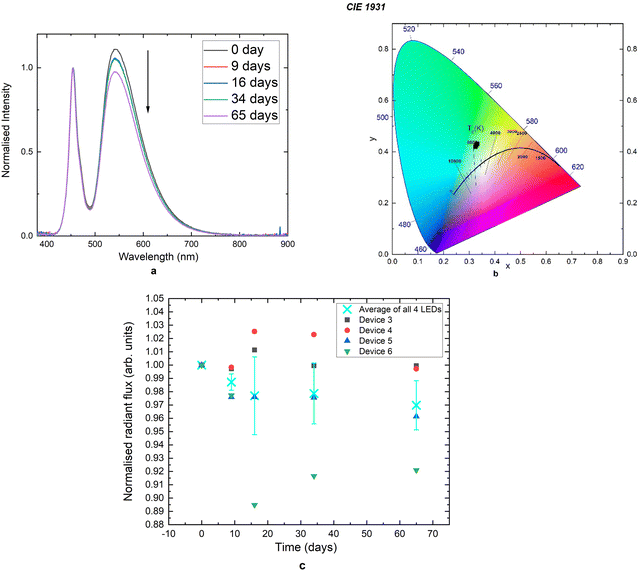
It was later realised that the stability issue could potentially be caused by another polymer material in the organic layer. As mentioned above, the cross-linked organic down-converting layer was prepared by reacting GreenCin and TCM monomers to form a matrix system. However, GreenCin being a cinnamate-functionalised material also has the tendency to self-polymerise to form a linear polymer system (p(GC)) and short-chained copolymer of TCM and GreenCin. These unwanted polymer materials are soluble in most organic solvents whereas the cross-linked polymer is not. After the down-converting material was treated with UV irradiation, the organic layer was washed with CH2Cl2 to wash off the residual unreacted monomers and short-chain polymers. The EL spectra of one of the best performing hybrid devices is promising (device 6). The hybrid LED was studied by driving the LED for a short period of time under a constant current of 25 mA at different time intervals over a time-span of 65 days. The green-emission peaks were normalised against the blue emission peak (Fig. 10(a)). Initially, there was a drop in the green emission intensity during the second measurement that was conducted 9 days after the initial measurement. The degradation could be caused by the residue of small molecules/polymers in the system that were not successfully removed. The organic down-converting material stabilised after the first week and no further change was observed during subsequent measurements that were conducted after 34 days. However, the green emission decreased after the 5th measurement that was performed 65 days after the device was made, although the scale of the degradation is rather minimal. Table 5 lists all the key parameters across the 5 measurements. It can be seen that throughout the measurements, radiant flux, luminous flux, CRI and luminous efficacy values remained basically the same, with small changes in CCT and blue-to-white efficacy ratio. The small degree of change can be further illustrated by the CIE 1931 chromaticity diagram where all the data points lie near the same region with little change in coordinates (Fig. 10(b)). It is worth mentioning that the films that were deposited on LEDs showed relatively good stability when compared to other down-converting materials that were embedded in polymer matrix systems such as poly(CHDV) and poly(urethane) membranes.25,26
| Measurements | Time (days) | Radiant flux (mW) | Luminous flux (lm) | CCT (K) | CRI | Luminous efficacy (lm W−1) | Blue-to-white ratio (lm W−1) |
|---|---|---|---|---|---|---|---|
| 1st | 0 | 17.4 | 7.0 | 5536 | 55.0 | 104.1 | 213.4 |
| 2nd | 9 | 17.4 | 6.9 | 5628 | 55.2 | 103.7 | 210.2 |
| 3rd | 16 | 17.9 | 7.1 | 5612 | 55.0 | 107.3 | 218.2 |
| 4th | 34 | 17.8 | 7.1 | 5633 | 55.1 | 107.1 | 217.5 |
| 5th | 65 | 17.4 | 6.9 | 5759 | 55.5 | 103.2 | 209.7 |
A similar degradation pattern can also be seen from the other hybrid devices. Fig. 10(c) shows the plot of radiant flux against time for 4 hybrid devices with each device's radiant flux value normalised to its initial value. The average values for all 4 hybrid devices are also included.
4. Conclusions
In this work we have reported the synthesis, characterisation and fabrication of hybrid devices for white light emission with a novel organic down-converting material, GreenCin. This green-emitting material can produce good quality white light when incorporated with inorganic InGaN/GaN blue LEDs due to its favourable optical properties such as high PLQY (94% in CH2Cl2 and 84% in the solid state) compared to the last generation of down-converting organic materials. The functionalisation of the material with cinnamate groups allows the cross-linking reaction between GreenCin with TCM to form a physically stable film. Thus, no additional host matrix material such as poly(CHDV) or poly(urethane) is needed for the encapsulation of organic materials. Using this new approach, photoacid generators can be avoided in the cross-linking process, which can degrade the emissive organic emitter in the hybrid device. It has been shown that the resulting hybrid device can produce white light more efficiently than the last generation of green-emission organic materials based on similar chemical structures with 2.6 × luminous efficacy (107 lm W−1), 2.4 × radiant flux (24 mW) and high blue-to-white efficacy (213 lm W−1). Colour tuning can also be achieved with the hybrid device by varying the amount of GreenCin that was deposited on blue LEDs that leads to ‘cool’ to ‘warm’ white light emission. The quality of the white light produced by hybrid devices also proved to be reasonable with a CRI value range between 52–69 with values above 80 being the target for viable white sources. Although some degradation of the down-converting layer was observed during the lifetime measurements, the decrease in emission in the green region was minimal, even when compared to some other down-converting materials that were encapsulated in additional host matrix materials. Despite the green emission decreasing gradually throughout the measurements, both radiant flux and luminous flux remain relatively stable, whilst the luminous efficacy and blue-to-white ratio did not fluctuate drastically. Therefore, we have reported a new approach to creating white light emitting hybrid LEDs, with a new class of photopolymerisable materials used for LEDs with high performance and good stability.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank EPSRC for funding through grants EP/P02744X/2 and EP/R03480X/1. DJW acknowledges support from EPSRC fellowship (EP/N01202X/2).References
- K. T. Kamtekar, A. P. Monkman and M. R. Bryce, Adv. Mater., 2010, 22, 572–582 CrossRef CAS PubMed.
- J. Bruckbauer, C. Brasser, N. J. Findlay, P. R. Edwards, D. J. Wallis, P. J. Skabara and R. W. Martin, J. Phys. D: Appl. Phys., 2016, 49, 405103 CrossRef.
- J. Bruckbauer and N. J. Findlay, in Optoelectronic Organic-Inorganic Semiconductor Heterojunctions, ed. Y. Zhou, CRC Press, Boca Raton, 1st edn, 2021, ch. 10, pp.231–266 Search PubMed.
- V. Anand, R. Mishra and Y. Barot, Dyes Pigm., 2021, 191, 109390 CrossRef CAS.
- Y. Fu, P. Xiong, X. Liu, X. Wang, S. Wu, Q. Liu, M. Peng and Y. Chen, J. Mater. Chem. C, 2021, 9, 303–312 RSC.
- K. P. O’Donnell, M. Auf der Maur, A. Di Carlo, K. Lorenz and T. S. Consortium, Phys. Status Solidi RRL, 2012, 6, 49–52 CrossRef.
- C. J. Humphreys, MRS Bull., 2011, 33, 459–470 CrossRef.
- C. R. Ronda, T. Jüstel and H. Nikol, J. Alloys Compd., 1998, 275–277, 669–676 CrossRef CAS.
- B. Lei, B. Li, H. Zhang and W. Li, Opt. Mater., 2007, 29, 1491–1494 CrossRef CAS.
- G. Li, Z. Wang, Z. Quan, C. Li and J. Lin, Cryst. Growth Des., 2007, 7, 1797–1802 CrossRef CAS.
- T.-C. Liu, B.-M. Cheng, S.-F. Hu and R.-S. Liu, Chem. Mater., 2011, 23, 3698–3705 CrossRef CAS.
- R. K. Tamrakar, D. P. Bisen, I. P. Sahu and N. Brahme, J. Radiat. Res. Appl. Sci., 2014, 7, 417–429 Search PubMed.
- M. Yu, J. Lin, Z. Wang, J. Fu, S. Wang, H. J. Zhang and Y. C. Han, Chem. Mater., 2002, 14, 2224–2231 CrossRef CAS.
- P. Schlotter, J. Baur, C. D. Hielscher, M. Kunzer, H. Obloh, R. Schmidt and J. Schneider, Mater. Sci. Eng., B, 1999, 59, 390–394 CrossRef.
- K. Bando, K. Sakano, Y. Noguchi and Y. Shimizu, J. Light Visual Environ., 1998, 22, 2–5 CrossRef PubMed.
- H. Ju, W. Deng, B. Wang, J. Liu, X. Tao and S. Xu, J. Alloys Compd., 2012, 516, 153–156 CrossRef CAS.
- H. Zhu, C. C. Lin, W. Luo, S. Shu, Z. Liu, Y. Liu, J. Kong, E. Ma, Y. Cao, R.-S. Liu and X. Chen, Nat. Commun., 2014, 5, 4312 CrossRef CAS PubMed.
- Z. Yue, Y. F. Cheung, H. W. Choi, Z. Zhao, B. Z. Tang and K. S. Wong, Opt. Mater. Express, 2013, 3, 1906–1911 CrossRef.
- S. Massari and M. Ruberti, Resour. Policy, 2013, 38, 36–43 CrossRef.
- X. A. Cao, S. Li, X. M. Li and L. Y. Liu, IEEE Trans. Electron Devices, 2018, 65, 4891–4896 CAS.
- J. McKittrick, L. E. Shea-Rohwer and D. J. Green, J. Am. Ceram. Soc., 2014, 97, 1327–1352 CrossRef CAS.
- N. Muhamad Sarih, P. Myers, A. Slater, B. Slater, Z. Abdullah, H. A. Tajuddin and S. Maher, Sci. Rep., 2019, 9, 1–8 CrossRef CAS PubMed.
- Z. He, W. Zhao, J. W. Y. Lam, Q. Peng, H. Ma, G. Liang, Z. Shuai and B. Z. Tang, Nat. Commun., 2017, 8, 1–7 CrossRef CAS PubMed.
- M. Mosca, R. Macaluso and I. Crupi, in Polymers for Light-Emitting Devices and Displays, ed. I. Inamuddin, R. Boddula, M. I. Ahmed and A. M. Asiri, Wiley-Scrivener, Hoboken-Beverly, 2020, ch. 8, pp. 197–262 Search PubMed.
- A. A. Wiles, J. Bruckbauer, N. Mohammed, M. Cariello, J. Cameron, N. J. Findlay, E. Taylor-Shaw, D. J. Wallis, R. W. Martin, P. J. Skabara and G. Cooke, Mater. Chem. Front., 2020, 4, 1006–1012 RSC.
- N. J. Findlay, J. Bruckbauer, A. R. Inigo, B. Breig, S. Arumugam, D. J. Wallis, R. W. Martin and P. J. Skabara, Adv. Mater., 2014, 26, 7290–7294 CrossRef CAS PubMed.
- R. Smith, B. Liu, J. Bai and T. Wang, Nano Lett., 2013, 13, 3042–3047 CrossRef CAS PubMed.
- G. Heliotis, P. N. Stavrinou, D. D. C. Bradley, E. Gu, C. Griffin, C. W. Jeon and M. D. Dawson, Appl. Phys. Lett., 2005, 87, 103505 CrossRef.
- H. Chun, P. Manousiadis, S. Rajbhandari, D. A. Vithanage, G. Faulkner, D. Tsonev, J. J. D. McKendry, S. Videv, E. Xie, E. Gu, M. D. Dawson, H. Haas, G. A. Turnbull, I. D. W. Samuel and D. C. O’Brien, IEEE Photonics Technol. Lett., 2014, 26, 2035–2038 CAS.
- F. Hide, P. Kozodoy, S. P. DenBaars and A. J. Heeger, Appl. Phys. Lett., 1997, 70, 2664–2666 CrossRef CAS.
- E. Aksoy, N. Dem
![[i with combining dot above]](https://www.rsc.org/images/entities/char_0069_0307.gif) r and C. Varlikli, Can. J. Phys., 2018, 96, 734–739 CrossRef CAS.
r and C. Varlikli, Can. J. Phys., 2018, 96, 734–739 CrossRef CAS. - C. Karapire, C. Timur and S. İçli, Dyes Pigm., 2003, 56, 135–143 CrossRef CAS.
- E. Taylor-Shaw, E. Angioni, N. J. Findlay, B. Breig, A. R. Inigo, J. Bruckbauer, D. J. Wallis, P. J. Skabara and R. W. Martin, J. Mater. Chem. C, 2016, 4, 11499–11507 RSC.
- C. R. Belton, A. L. Kanibolotsky, J. Kirkpatrick, C. Orofino, S. E. T. Elmasly, P. N. Stavrinou, P. J. Skabara and D. D. C. Bradley, Adv. Funct. Mater., 2013, 23, 2792–2804 CrossRef CAS.
- A. V. Rami Reddy, K. Subramanian and A. V. Sesha Sainath, J. Appl. Polym. Sci., 1998, 70, 2111–2120 CrossRef.
- S. Y. Cho, J. G. Kim and C. M. Chung, J. Nanosci. Nanotechnol., 2010, 10, 6972–6976 CrossRef CAS PubMed.
- D. R. Coulson, L. C. Satek and S. O. Grim, Inorg. Synth., 1972, pp.121–124 Search PubMed.
- A. L. Kanibolotsky, F. Vilela, J. C. Forgie, S. E. Elmasly, P. J. Skabara, K. Zhang, B. Tieke, J. McGurk, C. R. Belton, P. N. Stavrinou and D. D. Bradley, Adv. Mater., 2011, 23, 2093–2097 CrossRef CAS PubMed.
- E. Taylor, P. R. Edwards and R. W. Martin, Phys. Status Solidi A, 2012, 209, 461–464 CrossRef CAS.
- N. J. Findlay, C. Orofino-Peña, J. Bruckbauer, S. E. T. Elmasly, S. Arumugam, A. R. Inigo, A. L. Kanibolotsky, R. W. Martin and P. J. Skabara, J. Mater. Chem. C, 2013, 1, 2249–2256 RSC.
- S. Koltzenburg, M. Maskos and O. Nuyken, Polymer Chemistry, Springer-Verlag, Berlin Heidelberg, 2017 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc05139g |
| This journal is © The Royal Society of Chemistry 2023 |