 Open Access Article
Open Access ArticleFerroelectricity in oxygen-deficient perovskite-type oxide Sr10Ga6Sc4O25†
Akitoshi
Nakano
 *,
Ichiro
Terasaki
*,
Ichiro
Terasaki
 and
Hiroki
Taniguchi
and
Hiroki
Taniguchi

Department of Physics, Nagoya University, Nagoya 464-8602, Japan. E-mail: nakano.akitoshi@nagoya-u.jp
First published on 5th June 2023
Abstract
We have investigated the physical properties of an oxygen-deficient perovskite-type Sr10Ga6Sc4O25 by means of dielectric permittivity, pyroelectric current, and X-ray diffraction (XRD) measurements using a polycrystalline sample. The real part of the dielectric permittivity shows a clear anomaly around 310 K. Switchable pyroelectric current is also observed around this temperature, indicating a ferroelectric phase transition in the title compound. Synchrotron powder XRD measurements reveal that no discontinuous jump takes place in the temperature dependent cell volume around 310 K, implying that the phase transition is of second order. The candidates of the low and high temperature space groups are discussed based on the group theory.
1. Introduction
Most dielectric materials, which are fundamental to state-of-the-art technologies, belong to simple perovskite-type oxides with the chemical formula of ABO3.1–6 The proper ferroelectric BaTiO3, and P(Zr,Ti)O3 are representative of such compounds.7–10 On the other hand, rich functionalities have also been discovered in the past decade in compounds which have more complex sublattices. For instance, layered perovskite-type oxides show “improper” ferroelectricity with intriguing cross correlation between polarization, strain, and magnetization.11–15 The coexistence of ferroelectricity, anti-ferroelectricity, and ion conductivity in a single thermodynamic phase has also been reported in the derived form of a layered perovskite-type oxide.16–20 A class of aluminate-sodalite-type oxides, which have a cage structure consisting of eco-friendly elements, shows large electromechanical coupling factor and piezoelectric strain coefficient, offering highly efficient pyroelectric power generation and piezoelectric sensing.21,22 Therefore, searching for ferroelectric materials not only in simple perovskite-type oxides but also in compounds with more complex crystal structures will open a frontier of the fields of ferroelectric and other multifunctional materials.In this regard, oxygen-deficient perovskite-type oxides with the composition ratio of A:(B,C):O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 are attractive, because in such compounds oxygen vacancies can be ordered in a periodic manner to form a unique long-range order. The quaternary Sr–Sc–Ga–O system, which forms various crystal structures depending on the ratio of B and C, is the representative of such compounds.23 When Ga
2.5 are attractive, because in such compounds oxygen vacancies can be ordered in a periodic manner to form a unique long-range order. The quaternary Sr–Sc–Ga–O system, which forms various crystal structures depending on the ratio of B and C, is the representative of such compounds.23 When Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sc = 3
Sc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a cubic perovskite-type phase is formed as SrGa0.75Sc0.25O2.5. When Ga
1, a cubic perovskite-type phase is formed as SrGa0.75Sc0.25O2.5. When Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sc = 1
Sc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a brownmillerite-type structure, the framework of which is composed of a two-dimensional ScO6 octahedral network and one-dimensional (1D) GaO4 tetrahedral chains, is formed. The structure, which has a lot of gaps to take in oxygen, has recently attracted keen interest as a playground of oxide ion conductors.24,25 Rom et al. have reported that the brownmillerite-type structure transforms to the simple perovskite-type structure by rare-earth substitution and attains photoluminescent properties.26 Furthermore, Wu et al. have very recently found a dielectric anomaly27 associated with an order-disorder transition of the GaO4 tetrahedral chains.25
1, a brownmillerite-type structure, the framework of which is composed of a two-dimensional ScO6 octahedral network and one-dimensional (1D) GaO4 tetrahedral chains, is formed. The structure, which has a lot of gaps to take in oxygen, has recently attracted keen interest as a playground of oxide ion conductors.24,25 Rom et al. have reported that the brownmillerite-type structure transforms to the simple perovskite-type structure by rare-earth substitution and attains photoluminescent properties.26 Furthermore, Wu et al. have very recently found a dielectric anomaly27 associated with an order-disorder transition of the GaO4 tetrahedral chains.25
Here, we focus on the dielectric properties of an oxygen-deficient perovskite-type oxide with the composition ratio of Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sc = 6
Sc = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, Sr10Ga6Sc4O25. This compound was first synthesized in 2011, and its crystal structure has been analyzed by means of neutron powder diffraction and Monte Carlo simulation.23 The same as Sr2ScGaO5, the ordering of oxygen vacancies creates ScO6 octahedral- and GaO4 tetrahedral-sites, and then they form a new type of long-range order with the space group I41/a. Since the dielectric properties of a compound are strongly related to its crystal structure, such novel crystal structure makes us expect unique dielectric properties. In this paper, we report a second-order ferroelectric phase transition associated with a small spontaneous polarization in Sr10Ga6Sc4O25 revealed by our dielectric, pyroelectric current, and powder X-ray diffraction measurements.
4, Sr10Ga6Sc4O25. This compound was first synthesized in 2011, and its crystal structure has been analyzed by means of neutron powder diffraction and Monte Carlo simulation.23 The same as Sr2ScGaO5, the ordering of oxygen vacancies creates ScO6 octahedral- and GaO4 tetrahedral-sites, and then they form a new type of long-range order with the space group I41/a. Since the dielectric properties of a compound are strongly related to its crystal structure, such novel crystal structure makes us expect unique dielectric properties. In this paper, we report a second-order ferroelectric phase transition associated with a small spontaneous polarization in Sr10Ga6Sc4O25 revealed by our dielectric, pyroelectric current, and powder X-ray diffraction measurements.
2. Experiments
Samples of Sr10Ga6Sc4O25 ceramics were synthesized by a conventional solid-state reaction method. A stoichiometric mixture of SrCO3 (99.9%), Sc2CO3 (99.9%), and Ga2O3 (99.999%) was ground in wet processes with ethanol using an agate mortar and pestle. The powder mixtures were calcined at 1200 °C for 12 h. The resultants were sintered at 1200 °C for 12 h after intermediate regrinding and palletization. The sintered samples were confirmed by powder X-ray diffraction (XRD) measurements at the beamline BL02B2 of SPring-8, Japan.28 The Rietveld refinement was performed by using the Jana2006 program.29 The dielectric measurements were performed by using a Keysight 4284A precision LCR meter with a temperature slope of 10 K min−1. The pyroelectric current was measured by using a Keithley 6430 sub-femto-amp remote source meter. The sample was preliminarily electrically poled on the cooling process and then pyroelectric current was measured on the heating process with a temperature slope of 10 K min−1. The temperatures of the samples were controlled using a LINKAM THMS600 heating/cooling stage for the dielectric and pyroelectric measurements. Dielectric and pyroelectric measurements shown in the main text were performed on the same ceramic pellet, whose diameter and thickness were approximately 10 mm and 200 μm, respectively. The platinum electrodes were sputtered on both surfaces of the samples for the measurements.3. Results and discussion
Fig. 1 shows the crystal structure of Sr10Ga6Sc4O25 visualized by the VESTA code.29 Although this compound has a three-dimensional framework of oxygen-polyhedra, here we treat it as a layered structure that is composed of eight layers. Among them, two layers are symmetrically inequivalent, layer 1 and layer 2 in Fig. 1. In each layer, twelve GaO4 tetrahedra and eight ScO6 octahedra exist, namely, Ga and Sc are ordered with the ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The connection of the oxygen-polyhedra differs between the inequivalent two layers. These two layers are rotated by π/2 and moved up by c/4 by the 41 screw axis by four times, and then the entire structure is formed. The cell volume of Sr10Ga6Sc4O25 is about 100
2. The connection of the oxygen-polyhedra differs between the inequivalent two layers. These two layers are rotated by π/2 and moved up by c/4 by the 41 screw axis by four times, and then the entire structure is formed. The cell volume of Sr10Ga6Sc4O25 is about 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å3, which is roughly 160 times larger than a unit-cell of a simple cubic perovskite-type oxide.
000 Å3, which is roughly 160 times larger than a unit-cell of a simple cubic perovskite-type oxide.
Fig. 2 shows the comparison between the synchrotron powder XRD pattern of Sr10Ga6Sc4O25 and simulation pattern by using the previously reported I41/a crystal structure.23 The measured and simulated patterns show good agreement. The inset shows the expanded pattern around the highest intensity peak. We note that there are substantial amounts of impurity Sr2ScGaO5 phase. The volume of the impurity phase is about 20% against the main phase.
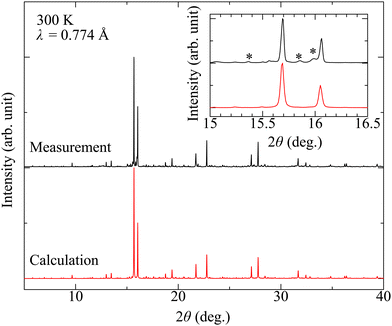 | ||
| Fig. 2 Synchrotron powder X-ray diffraction pattern of polycrystalline Sr10Ga6Sc4O25. The simulated pattern by using the reported structure is also shown. The inset shows close view around the highest peak. There are impurity Sr2ScGaO5 phases, whose volume is 20% against the Sr10Ga6Sc4O25 phase. The structure is visualized by the VESTA code.30 | ||
The temperature dependence of the real (ε′) and imaginary (ε′′) part of the dielectric permittivity for Sr10Ga6Sc4O25 in the temperature range from 250 to 500 K is shown in Fig. 3(a). The real part ε’ is ∼12 at 500 K, which is the same as ordinary oxides, while the imaginary part ε′′ is small enough in the measurement temperature range, indicating good insulating character of Sr10Ga6Sc4O25. Importantly, we observe an anomaly like the Curie–Weiss law in ε′ around 310 K among all measurement frequencies. This is clearly confirmed by the inverse plot of ε′ (the right inset in Fig. 3(a)), which shows linear temperature dependence above 310 K. Although our sample contains impurity Sr2ScGaO5, it shows flat temperature dependence and does not show any dielectric anomalies around 310 K.26 Thus, our observation indicates the occurrence of a phase transition in Sr10Ga6Sc4O25 around 310 K.
Note that the anomaly is not a single peak but associated with a broad tail which shows frequency dependence and spreads to 250 K. We attribute this structure to the response of polar domains formed below the phase transition in Sr10Ga6Sc4O25. Since domain formation depends on defects and/or strain in each polycrystal, the tail structure shows sample-dependence, as shown in Fig. S1 (ESI†). Nevertheless, the 1/T dependence of ε′ above 310 K seems to be almost unchanged between the three different samples, confirming that the anomaly comes from a thermodynamic phase transition in Sr10Ga6Sc4O25. Furthermore, the left inset of Fig. 3(a) shows the expansion of the dielectric anomaly in both heating and cooling processes. The peak temperature is the same in both processes, indicating that the phase transition is of the second order.
To investigate the detail of the dielectric phase transition around 310 K, we conducted pyroelectric current measurements. If the structure changes from a non-polar phase to a polar phase associated with the phase transition, the charges accumulated at the sample surface by spontaneous polarization are released when the temperature of the sample reaches the transition temperature from low temperatures, and pyroelectric current is detected. Fig. 3(a) shows the temperature dependence of the pyroelectric current for Sr10Ga6Sc4O25. We observe a clear peak like signal with its maximum around 315 K under both positive and negative bias fields. This indicates that switchable spontaneous polarization is formed in the low temperature phase of Sr10Ga6Sc4O25, namely the phase transition observed in our dielectric permittivity measurement is a ferroelectric phase transition. Note that this is the first observation of switchable spontaneous polarization in the Sr–Ga–Sc–O quaternary system. In addition, the pyroelectric current emerges diffusively from 340 K to 250 K. Possibly local compositional and/or structural fluctuation originated from the complex crystal structure makes the phase transition diffusive as seen in a disordered perovskite-type ferroelectric material.31 The inset of Fig. 3(b) shows the temperature dependent spontaneous polarization evaluated from the pyroelectric current measurements. The spontaneous polarization at 250 K is about 0.05 μC cm−2, which is 2–3 orders of magnitude smaller than that of ordinary ferroelectric perovskite-oxides.
To further reveal the details of the ferroelectric phase transition, we investigated a structural property. Fig. 4(a) shows the temperature dependent lattice constants from 200 to 520 K. Note that the lattice constants are refined by Rietveld fitting assuming the structural model of I41/a23 among all measured temperatures. Since there are too many refinable structural parameters due to the huge unit cell, we only refined the lattice and profile parameters. The isotropic compression in the a- and c-axes reflects the three-dimensional bonding nature of Sr10Ga6Sc4O25, and differs from anisotropic compression of two dimensional Sr2ScGaO5.25 The cell volume (V) is an essential thermodynamical parameter, which reflects the nature of the phase transition. We do not find a discontinuous jump in temperature dependent V. Rather it seems to show changes in its slope (dV/dT) around 310 K. Since V is the first derivative of the Gibbs free energy, the change in its derivative confirms the second order phase transition.
Now, let us consider the candidate of the space group for the high and low temperature phases. First, the reported space group I41/a is incompatible with our pyroelectric current measurement, since the emergence of pyroelectric current around 310 K proves that the polar space group realizes at 300 K. Nevertheless, the reported crystal structure well reproduces our XRD pattern, namely, the difference between reported and actual crystal structures is expected to be small. Thus, we assume that the space group I41/a is realized in the high temperature phase. Since a second order structural phase transition allows only the transformation to the maximum subgroup of I41/a, we can predict the low temperature space group from the viewpoint of group theory.
According to ref. 32 the maximum subgroups of I41/a are I41, I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) , and C2/a. Fortunately, we can uniquely choose I41 as a candidate for the low temperature space group, since the others are nonpolar space groups, which are incompatible with the result of pyroelectric current measurement. We scrutinized whether the space group I41 is compatible with our XRD data or not. Fig. 4(b) shows a colormap, which is composed of multiple powder X-ray patterns measured at 42 temperature points every 10 K from 100 K to 520 K. The highest intensity reflection 4 −2 8 and the second highest reflection 6 2 0 are always single peaks and do not show splitting or broadening in the measured temperature range, indicating that the low temperature phase maintains tetragonal symmetry.
, and C2/a. Fortunately, we can uniquely choose I41 as a candidate for the low temperature space group, since the others are nonpolar space groups, which are incompatible with the result of pyroelectric current measurement. We scrutinized whether the space group I41 is compatible with our XRD data or not. Fig. 4(b) shows a colormap, which is composed of multiple powder X-ray patterns measured at 42 temperature points every 10 K from 100 K to 520 K. The highest intensity reflection 4 −2 8 and the second highest reflection 6 2 0 are always single peaks and do not show splitting or broadening in the measured temperature range, indicating that the low temperature phase maintains tetragonal symmetry.
Fig. 4(c) and (d) show the comparison of the raw powder XDR data among various temperatures. The dot lines in each picture show the peak position of the 0 0 2 and 0 0 6 reflections, which are prohibited by the 41 screw axis symmetry. Thus, if the low temperature phase is either I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) or C2/a except for I41, additional peaks can emerge at those positions. No additional reflection emerging below 310 K is compatible with the I41 space group. We have also checked the generation rule of a-glide symmetry. However, we cannot find clear violation of it. This indicates that the intensity of additional reflections is several orders of magnitude smaller than the intensity of the primary reflections due to small atomic displacement associated with the phase transition.
or C2/a except for I41, additional peaks can emerge at those positions. No additional reflection emerging below 310 K is compatible with the I41 space group. We have also checked the generation rule of a-glide symmetry. However, we cannot find clear violation of it. This indicates that the intensity of additional reflections is several orders of magnitude smaller than the intensity of the primary reflections due to small atomic displacement associated with the phase transition.
Note that we have not yet succeeded in the refinement of the I41 structure by using the powder XRD data due to too many inequivalent sites (∼100 sites). Here, we guess a possible scenario for the phase transition from the viewpoint of symmetry. At the high temperature phase, there are a- (b-) glide planes at z = 0.25 and 0.75 (z = 0 and 0.5), which give a 1/2 translational operation along the a- (b-) axis and then give mirror operation. Since the mirror symmetry mirrors an upward electric dipole downward, forming macroscopic electric polarization along the c-axis is forbidden. In particular, the layers 3 and 7 (1 and 5) in Fig. 1, which are on the a- (b-) glide planes, cannot form local electric polarization along the c-axis in themselves. In addition, although the layers with even numbers can form local electric polarization in themselves, the glide symmetry cancels out the macroscopic electric polarization. Namely, the disappearance of the a-glide planes can cause local electric polarization in the layers with odd numbers and/or make the cancellation between the local electric polarization imperfect, and then macroscopic nonzero electric polarization emerges. The bending or ordering of the oxygen-polyhedral framework is a candidate of the structural change to break the a-glide planes. It is a future issue to elucidate the crystal structure, phase transition behavior, and microscopic ferroelectric origin by accurate physical property measurements on high-quality single crystals.
In this paper, we have demonstrated that a compound which has a complex long-range order by oxygen vacancies shows ferroelectricity. Although another compound which has the same structure as Sr10Ga6Sc4O25 has not been found yet, we may create this type of material from a large number of perovskite-type oxides by oxygen vacancy engineering. We think that the experimental approach to such complex materials is an important issue to open the frontiers of material science, since the state-of-the-art theoretical calculation cannot precisely predict their physical properties. We hope that our findings will accelerate the development of new functional materials in the future.
4. Summary
The ferroelectric phase transition on the oxygen-deficient perovskite-type Sr10Ga6Sc4GaO25 has been investigated by means of dielectric, pyroelectric current and synchrotron powder XRD measurements in the present study. The dielectric permittivity shows an anomaly which seems to obey the Curie–Weiss law, around 310 K. The pyroelectric current also exhibits a peak-like structure with its maximum around 315 K, and furthermore, it can be switched when opposite bias fields are applied. The estimated spontaneous polarization Ps at 250 K is around 0.05 μC cm−2. The continuous change in the cell volume around 310 K indicates a second-order phase transition. The I41/a and I41 space groups are assigned to high and low temperature phases, respectively, based on the group theory. Further precise structural analyses are needed to clarify the origin of the ferroelectric transition in Sr10Ga6Sc4GaO25.Author contributions
A. N.: conceptualization, formal analysis, funding acquisition, investigation, and writing – original draft. I. T.: resources, supervision, and writing – review & editing. H. T.: resources, supervision, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present work was partially supported by Nippon Sheet Glass Foundation for Materials Science and Engineering. Powder XRD measurements were conducted with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2019B1089, and 2020A1246). This work was partially supported by JSPS Grants-in-Aid for Transformative Research Areas (A) “Hyper-Ordered Structures Sciences” (Grant No. 20H05878, 20H05879).References
- R. E. Cohen, Origin of ferroelectricity in perovskite oxides, Nature, 1992, 358, 136–138 CrossRef CAS.
- M. Posternak, R. Resta and A. Baldereschi, Phys. Rev. B: Condens. Role of covalent bonding in the polarization of perovskite oxides: The case of KNbO3, Matter Mater. Phys., 1994, 50, 8911 CrossRef CAS PubMed.
- M. Kunz and I. D. Brown, Out-of-center Distortions around Octahedrally Coordinated d0 Transition Metals, J. Solid State Chem., 1995, 115, 395–406 CrossRef CAS.
- J. B. Goodenough, Jahn–Teller phenomena in solids, Annu. Rev. Mater. Sci., 1998, 28, 1–27 CrossRef CAS.
- Y. Kuroiwa, S. Aoyagi, A. Sawada, J. Harada, E. Nishibori, M. Takata and M. Sakata, Evidence for Pb–O Covalency in Tetragonal PbTiO3, Phys. Rev. Lett., 2001, 87, 217601 CrossRef CAS PubMed.
- N. A. Benedek and C. J. Fennie, Why Are There So Few Perovskite Ferroelectrics, J. Phys. Chem. C, 2013, 117, 13339 CrossRef CAS.
- M. M. Vijatovic, J. D. Bobic and B. D. Stojanovic, History and Challenges of Barium Titanate: Part I, Sci. Sintering, 2008, 40, 155 CrossRef CAS.
- J. F. Scott and C. A. Paz de Araujo, Ferroelectric memories, Science, 1989, 246, 1400 CrossRef CAS PubMed.
- G. Yi, Z. Wu and M. Sayer, Preparation of Pb(Zr,Ti)O3 thin films by sol gel processing: Electrical, optical, and electro-optic properties, J. Appl. Phys., 1988, 64, 2717 CrossRef CAS.
- C. A. Paz de Araujo, L. D. McMillan, B. M. Melnick, J. D. Cuchiaro and J. F. Scott, Ferroelectrics memories, Ferroelectrics, 1990, 104, 241 CrossRef.
- N. A. Benedek and C. J. Fennie, Hybrid Improper Ferroelectricity: A Mechanism for Contrpllable Polarization- Magnetization Coupling, Phys. Rev. Lett., 2011, 106, 107204 CrossRef PubMed.
- N. A. Benedek, A. T. Mulder and C. J. Fennie, Polar octahedral rotations: A path to new multifunctiona materials, J. Solid State Chem., 2012, 195, 11–20 CrossRef CAS.
- Y. S. Oh, X. Luo, F.-T. Huang, Y. Wang and S.-W. Cheong, Experimental demonstration of hybrid improper ferroelectricity and the presence of abundant charged walls in (Ca,Sr)3Ti2O7 crystals, Nat. Mater., 2015, 14, 407–413 CrossRef CAS PubMed.
- Y. Wang, F. T. Huang, X. Luo, B. Gao and S. W. Cheong, The first Room-Temperature Ferroelectric Sn Insulator and Its Polarization Switching Kinetics, Adv. Mater., 2017, 29, 1601288 CrossRef PubMed.
- S. Yoshida, H. Akamatsu, R. Tsuji, O. Hernandez, H. Padmanabhan, A. Sen Gupta, A. S. Gibbs, K. Mibu, S. Murai, J. M. Rondinelli, V. Gopalan, K. Tanaka and K. Fujita, Hybrid Improper Ferroelectricity in (Sr,Ca)3Sn2O7 and Beyond: Universal Relationship between Ferroelectric Transition Temperature and Tolerance Factor in n = 2 Ruddlesden-Popper Phases, J. Am. Chem. Soc., 2018, 140, 15690 CrossRef CAS PubMed.
- T. Nagai, H. Shirakuni, A. Nakano, H. Sawa, H. Moriwake, I. Terasaki and H. Taniguchi, Weak Ferroelectricity in n = 2 Pseudo Ruddlesden–Popper-Type Niobate Li2SrNb2O7, Chem. Mater., 2019, 31, 6257–6261 CrossRef CAS.
- T. Nagai, Y. Mochizuki, H. Shirakuni, A. Nakano, F. Oba, I. Terasaki and H. Taniguchi, Phase Transition from Weak Ferroelectricity to Incipient Ferroelectricity in Li2Sr(Nb1−xTax)2O7, Chem. Mater., 2020, 32, 744–750 CrossRef CAS.
- Y. Mochizuki, T. Nagai, H. shirakuni, A. Nakano, F. Oba, I. Terasaki and H. Taniguchi, Coexisting Mechanisms for the Ferroelectric Phase Transition in Li2SrNb2O7, Chem. Mater., 2021, 33, 1257–1264 CrossRef CAS.
- A. Nakano, H. Shirakuni, T. Nagai, Y. Mochizuki, F. Oba, H. Yokota, S. Kawaguchi, I. Terasaki and H. Taniguchi, Phase variation of ferroelectric Li2Sr1−xCax(Nb1−xTax)2O7 by selective reinforcement in the (Nb,Ta)-O covalent bonds, Phys. Rev. Mater., 2022, 6, 044412 CrossRef CAS.
- X. Xu, F.-T. Huang, K. Du and S.-W. Cheong, Multifunctionality of Li2SrNb2O7: Memristivity, Tunable Rectification, Ferroelasticity, amd Ferroelectricity, Adv. Mater., 2022, 34, 2206022 CrossRef CAS PubMed.
- Y. Maeda, T. Wakamatsu, A. Konishi, H. Moriwake, C. Moriyoshi, Y. Kuroiwa, K. Tanabe, I. Teradaki and H. Taniguchi, Improper Ferroelectricity in Stuffed Aluminate Sodalites for Pyroelectric Energy Harvesting, Phys. Rev. Appl., 2017, 7, 34012 CrossRef.
- H. Taniguchi, T. Hattori, T. Isobe, A. Nakano, I. Terasaki and M. Hagiwara, A large piezoelectric voltage coefficient in aluminate-sodalite-type improper ferroelectric oxides, J. Mater. Chem. C, 2021, 9, 15649 RSC.
- S. V. Chernov, Y. A. Dobrovolsky, S. Y. Istomin, E. V. Antipov, J. Grins, G. Svensson, N. V. Tarakina, A. M. Abakumov, G. V. Tendeloo, S. G. Eriksson and S. M. H. Rahman, Sr2GaScO5, Sr10Ga6Sc4O25, and SrGa0.75Sc0.25O2.5: a play in the octahedra to tetrahedra ratio in oxygen-deficient perovskites, Inorg. Chem., 2012, 51, 1094 CrossRef CAS PubMed.
- S. Corallini, M. Ceretti, G. Silly, A. Piovano, S. Singh, J. Stern, C. Ritter, J. Ren, H. Eckert, K. Conder, W. Chen, F. Chou, N. Ichikawa, Y. Shimawakawa and W. Paulus, One-Dimensional Oxygen Diffusion Mechanism in Sr2ScGaO5 Electrolyte Explored by Neutron and Synchrotron Diffraction, 17O NMR, and Density Functional Theory Calculations, J. Phys. Chem. C, 2015, 119, 11447 CrossRef CAS.
- C. A. Fuller, Q. Berrod, B. Frick, M. R. Johnson, S. J. Clark, J. S. O. Evans and I. R. Evans, Brownmillerite-Type Sr2ScGaO5 oxide Ion Conductor: Local Structure, Phase Transition, and Dynamics, Chem. Mater., 2019, 31, 7395 CrossRef CAS PubMed.
- T. Rom, S. Laha, S. Gadiyaram, P. K. Maji and A. K. Paul, Rare-earth (Nd and Eu) indued structural transformation and optical properties of brownmillerite-type Sr2ScGaO5 oxide, J. Solid State Chem., 2022, 317, 123696 CrossRef.
- Z. J. Wu, B. H. Zhang, X. Q. Liu and X. M. Chen, Crystal structures, dielectric properties, and phase transitions in Sr2ScGaO5-based brownmillerite ceramics, Ceram. Int., 2023, 49(3), 5298–5304 CrossRef CAS.
- S. Kawaguchi, M. Takemoto, K. Osaka, E. Nishibori, C. Moriyoshi, Y. Kubota, Y. Kuroiwa and K. Sugimoto, High-throughput powder diffraction measurement system consisting of multiple MYTHEN detectors at beamline BL02B2 of SPring-8, Rev. Sci. Instrum., 2017, 88, 085111 CrossRef CAS PubMed.
- V. Petricek, M. Dusek and L. Palatinus, Z. Kristallogr, Crystallographic Computing System JANA2006: General features, Cryst. Mater., 2014, 229, 345–352 CAS.
- K. Monma and F. Izumi, VESTA: a three-dimensional visualization system for electronic and structural analysis, J. Appl. Cryst., 2008, 41, 653 CrossRef.
- N. Setter and L. E. Cross, The role of B-site cation disorder in diffuse phase transition behavior of perovskite ferroelectrics, J. Appl. Phys., 1980, 51, 4356 CrossRef CAS.
- S. Ivantchev, E. Kroumova, G. Madariaga, J. M. Perez-Mato and M. I. Aroyo, SUBGROUPGRAPH: a computer program for analysis of group-subgroup relations between spae group, J. Appl. Cryst., 2000, 33, 1190 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc05118d |
| This journal is © The Royal Society of Chemistry 2023 |



