Amplification of dissymmetry factors by dihedral angle engineering in donor–acceptor type circularly polarized luminescence materials†
Abstract
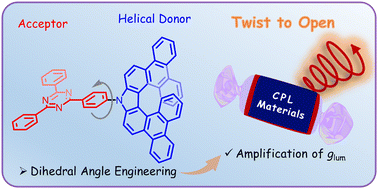
By employing aza[7]helicene as the chiral donor and triazine as the acceptor, we have developed a new type of circularly polarized luminescence material. The influence of the dihedral angle between the donor and acceptor moieties on the dissymmetry factors has been revealed for the first time, providing a novel strategy to amplify dissymmetry factors by engineering the dihedral angles in donor–acceptor type chiral materials.
- This article is part of the themed collection: Circularly Polarised Luminescence


 Please wait while we load your content...
Please wait while we load your content...