A switchable system between magnetic and natural circularly polarised luminescence via J-aggregation using photosynthetic antenna model compounds†
Abstract
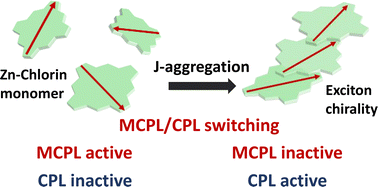
A switchable system between magnetic and natural circularly polarized luminescence via J-aggregation using photosynthetic antenna model compounds is demonstrated for the first time. The J-aggregation provides characteristic dispersion-type circularly polarised luminescence signals based on the degenerate J-band (gCPL = 0.03).
- This article is part of the themed collection: Circularly Polarised Luminescence


 Please wait while we load your content...
Please wait while we load your content...