Self-reduction of Mn4+ to Mn2+: NaY9Si6O26:Mn2+ red phosphors with excellent thermal stability for NUV LEDs
Abstract
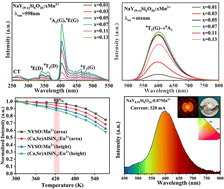
Luminous properties play an essential role in phosphor-converted white light-emitting diodes for high-quality illumination, where the self-reducing behavior of doped activators and their excellent thermal stability have received significant attention. Here, we prepared NaY9Si6O26:Mn2+ red phosphors by a high-temperature solid-state reaction method, which achieved self-reduction of Mn4+ → Mn2+ and excellent thermal stability (luminous intensity at 420 K is 98% of that at room temperature) under ambient conditions. First-principles calculations and X-ray photoelectron spectroscopy elucidated a self-reduction mechanism from Mn4+ to Mn2+. The defect types were calculated in conjunction with thermoluminescence, verifying that the low thermal quenching properties originated from the transfer of electrons from the defect energy level to the Mn2+ excited state center induced by the cationic vacancy defect, which suppressed the luminescence loss from thermal quenching. In this work, the effect of defects on luminescence properties is highlighted and the further exploration of defect control is stimulated, opening up a new avenue for new types of highly thermally stable materials.


 Please wait while we load your content...
Please wait while we load your content...