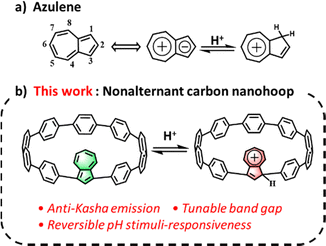
A nonalternant azulene-embedded carbon nanohoop featuring anti-Kasha emission and tunable properties upon pH stimuli-responsiveness†
Xiaonan
Li
a,
Luyang
Jia
a,
Wenguang
Wang
 a,
Ying
Wang
a,
Ying
Wang
 a,
Di
Sun
a,
Di
Sun
 b and
Hua
Jiang
b and
Hua
Jiang
 *a
*a
aCollege of Chemistry, Beijing Normal University, Beijing 100875, P. R. China
bSchool of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, P. R. China
First published on 16th December 2022
Abstract
The insertion of a nonalternant π-system into the skeletons of [n]cycloparaphenylenes ([n]CPPs) can significantly alter their optoelectronic properties. We herein present a nonalternant azulene-embedded carbon nanohoop 1,3-Az[9]CPP. The investigations revealed that 1,3-Az[9]CPP demonstrates unique properties including anti-Kasha emission, reversible pH stimuli-responsiveness and tunable band gaps upon protonation and deprotonation. Importantly, 1,3-Az[9]CPP exhibits a highly selective binding for C60 over C70 (KC60/KC70 = 30) in acidic media. Moreover, the molecular structures of 1,3-Az[9]CPP and its fullerene complex C60@1,3-Az[9]CPP were confirmed by single-crystal X-ray diffraction. Notably, 1,3-Az[9]CPP is the first pH-responsive nonalternant all-carbon nanohoop, which may hold significant promising applications in tunable optoelectronic materials.
Introduction
[n]Cycloparaphenylenes ([n]CPPs), consisting of a cyclic arrangement of para-linked phenylenes, are the shortest conjugated fragment of armchair carbon nanotubes (CNTs).1 Since the initial synthesis by Jasti et al. in 2008,2 numerous CPP derivatives have been developed with various functionalities and thus boosted tremendous advances in the fields of host–guest chemistry and materials science,3 owing to their in-plane radially oriented π-orbitals and size-dependent optoelectronic properties. So far, a majority of CPP derivatives have been synthesized by embedding alternant aromatic units such as naphthalene,4 anthracene,5 pyrene,6 fluorene,7,8 pyridine,9 anthraquinone,10 and graphenoids11 into the skeletons of CPPs, thus leading to fascinating applications in organic light emitting diodes (OLEDs),8 molecular machines,9 and so on.12,13 In contrast, incorporating nonalternant π-systems into nanohoops can not only reduce the aromaticity of nanohoops, but also lower their band gap, which significantly alters their electronic properties.14,16 However, there are limited examples of nonalternant nanohoops from rubicene,14 fluoranthene,15 and dibenzo[a,e]pentenyl16 and a methylene-bridged non-alternant aromatic belt.17 Therefore, the developments of nonalternant aromatic carbon nanohoops would be complementary to the abovementioned alternant aromatic counterparts and thus hold great significance for their applications in materials science.Azulene, a nonalternant isomer of naphthalene, consists of an electron-deficient seven-membered ring and an electron-rich five-membered ring, featuring a large dipole moment of 1.08D and a non-mirror symmetric frontier molecular orbital, which eventually lead to anti-Kasha's rule emission from S2 to S0.18 It is noteworthy that the odd positions of azulene are more electron-rich than the even ones, so protonation can readily occur at the C-1 position to form a stable 6π-azulenylium ion under acidic conditions (Fig. 1a).19 These unique properties endow azulene with great promise for developing advanced materials in organic semiconductors,20 photoswitches,21 nonlinear optical materials,22 chemical sensing, bioimaging,23 and nonplanar polycyclic aromatic hydrocarbons.24 However, investigations associated with nonalternant aromatic all-carbon nanohoops with pH reversible stimuli-responsiveness remained largely unexplored to date because of the lack of suitable stimuli-responsive all-carbon building blocks. Most pH active materials normally require heteroatoms such as nitrogen, oxygen, and sulfur as proton acceptors. Therefore, the synthesis of pH-responsive nonalternant all-carbon nanohoops is still challenging.
The reversible changes in color and physicochemical properties upon protonation/deprotonation make azulene an excellent building block for developing pH stimulus-responsive all-carbon nanohoops. We anticipated that incorporating an azulene unit into the CPP backbone would allow us to obtain a nonalternant aromatic all-carbon nanohoop with appealing properties, such as anti-Kasha's rule fluorescence and a tunable band gap upon protonation/deprotonation. Herein, we report the synthesis and structure of novel nonalternant aromatic azulene-embedded [n]CPP (1,3-Az[9]CPP) (Fig. 1b) and its properties in the absence and presence of acid. Remarkably, the nonalternant aromatic 1,3-Az[9]CPP displays not only reversible stimuli-responsiveness but also high selectivity for binding C60 over C70 in acidic media.
Results and discussion
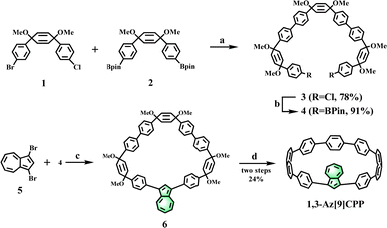
The synthetic procedure for 1,3-Az[9]CPP is shown in Scheme 1. Our synthetic strategy was to couple two cyclohexadiene fragments 1 and 2 by Suzuki–Miyaura cross-coupling to access dichloride intermediate 3, followed by Miyaura borylation to give cyclization precursor 4. In the presence of Pd(PPh3)4 and K2CO3, 1,3-dibromoazulene and C-shaped fragment 4 were subjected to Suzuki–Miyaura cross-coupling in a diluted reaction solution (3.5 mM) to provide the macrocyclic precursor 6. The final reductive aromatization of the macrocyclic precursor 6 was carried out in anhydrous tetrahydrofuran with freshly prepared H2SnCl4 at room temperature, and the target macrocycle 1,3-Az[9]CPP was obtained in 24% yield over two steps. The structure of 1,3-Az[9]CPP was confirmed by 1H NMR, 13C NMR, 1H-1H COSY, 1H-13C HSQC, 1H-13C HMBC, MALDI-TOF MS and single-crystal X-ray analysis (Fig. 2, Fig. S1–S7 and S9 and Table S1, ESI†).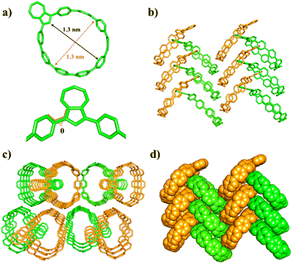 | ||
| Fig. 2 (a) X-ray crystal structures, (b) herringbone packing model, (c) long range channel alignment and (d) densely stacked 1,3-Az[9]CPP. | ||
The structure of 1,3-Az[9]CPP was confirmed by single-crystal X-ray analysis (Fig. 2). Suitable crystals were obtained by slow diffusion of acetonitrile into 1,1,2,2-tetrachloroethane solution at room temperature. Crystallographic analysis reveals that the carbon nanohoop exhibits a nearly circular cavity with an estimated diameter of about 1.30 nm (Fig. 2a), which is similar to that of [10]CPP (1.4 nm) and thus enables 1,3-Az[9]CPP to be a suitable host for fullerenes as [10]CPP.25 As shown in Fig. 2a, the five-membered ring of azulene points towards the interior of CPP due to the strain caused by the 1,3-Az[9]CPP nanohoop, thus leading to the formation of dihedral angles of about 45.0° between the azulene unit and the adjacent benzene molecules. 1,3-Az[9]CPP adopts a herringbone packing in the solid phase when viewed from the side (Fig. 2b and d) and thus forms channels when viewed from the top (Fig. 2c). Furthermore, the unit cell of 1,3-Az[9]CPP belongs to the P21 space group.
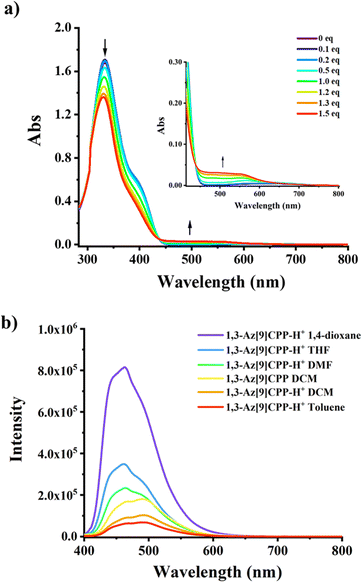
The 1H NMR spectrum of 1,3-Az[9]CPP features a set of signals for the azulene protons with δ = 8.70, 7.68, 7.28, and 7.22 ppm for H4/H8, H6, H2, and H5/H7, respectively, and signals for the CPP segment protons with δ = 7.68–7.55 ppm in CD2Cl2 (Fig. 3 and Fig. S4, ESI†). Titrations of 0.1–1.5 equiv. HBArF·(Et2O)2 (BArF−: B[3,5-(CF3)2C6H3]4−, very hygroscopic and sensitive to oxygen) into the solution of 1,3-Az[9]CPP caused significant changes in the chemical shifts (Fig. 3 and Fig. S11, ESI†). No further changes were observed for the titrations of HBArF·(Et2O)2 beyond 1.0 equiv. The remarkable downfield shifts of protons assigned to the azulene unit were observed, with the largest downfield shift being assigned to the H5 or H7, up to 1.78 ppm. These significant downfield shifts indicate strong deshielding effects due to the formation of an azulenylium cation. Moreover, the proton signals of H4/H8 appear as a doublet peak at 8.70 ppm due to the symmetric structure of the nanohoop, but split into two separate sets of peaks, a doublet peak at 9.34 ppm and a multiplet peak at 9.08 ppm in the protonated asymmetric nanohoop 1,3-Az[9]CPP-H+ (Fig. 3 and S8, ESI†). Similar splits in the triplet peak assigned to H5 and H7 in 1,3-Az[9]CPP were also observed. Furthermore, a new peak at 5.54 ppm can be assigned to the C–H proton of the protonated five-membered ring. Interestingly, the color of the solution also changed significantly from initially green to yellow and finally deep red with the titrations of HBArF·(Et2O)2 (Fig. 3 and Fig. S11, ESI†). All these observations demonstrate that the monoprotonation of 1,3-Az[9]CPP occurs at its C-1 or C-3 position, thus inducing an asymmetric structure of the protonated carbon nanohoop, consistent with the previously reported monoprotonation of azulene derivatives.26
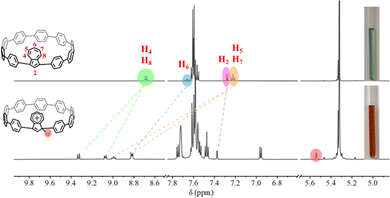 | ||
| Fig. 3 1H-NMR spectra and color change of the nanohoop 1,3-Az[9]CPP before (top) and after protonation (bottom) with 1.0 eq. HBArF·(Et2O)2 in CD2Cl2 (600 MHz, 298 K). | ||
Next, we set about investigating the reversible pH stimuli-responsiveness of 1,3-Az[9]CPP. 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) was selected as a base. As shown in Fig. S12 (ESI†), addition of 1.0 equiv. of HBArF·(Et2O)2 to the 1,3-Az[9]CPP solution resulted in the disappearance of the proton signals in the azulene unit accompanied by the appearance of the proton signals of azulenylium cations, while the color of the solution turned from green to red. When 1.0 equiv. of DBU was added, the chemical shifts of 1,3-Az[9]CPP recovered, concomitantly accompanied with the color recovery from red to green (Fig. S12, ESI†). Notably, the process of protonation and deprotonation can be repeated multiple times, indicating the intriguing reversibility of pH stimuli-responsiveness for 1,3-Az[9]CPP.
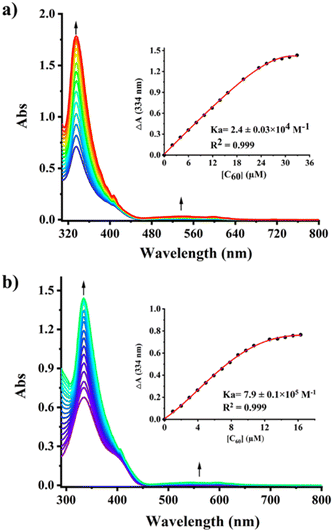
The optical properties of 1,3-Az[9]CPP in the absence and presence of HBArF·(Et2O)2 were subsequently investigated by UV-vis absorption spectroscopy in a dichloromethane (DCM) solution (Fig. 4a). Furthermore, to gain insight into the relationship between the electronic structure and photophysical properties, time-dependent density functional theory (TD-DFT) calculations were performed at the ωB97XD/6-31g(d,p) level (Fig. S20–S22 and Table S3–S6, ESI†). 1,3-Az[9]CPP exhibited an absorption maximum (λmax) at 334 nm, which was attributed to the combined transitions of S0 → S4 and S0 → S5 according to the TD-DFT calculations. Meanwhile, a shoulder peak appeared at about 385 nm is assigned to the S0 → S2 transition. Moreover, there is a weak broad band at 500–670 nm assigned to the S0 → S1 (HOMO → LUMO) transition, which is consistent with that of the parent azulene.27 In contrast, significant changes in the UV-vis spectra of 1,3-Az[9]CPP were observed upon titrations with HBArF·(Et2O)2 (Fig. 4a). Upon the titrations with acid, the intensity of the peak at λmax decreased remarkably accompanied by a blue-shift of 5 nm from 334 nm to 329 nm. Meanwhile, the intensity of the shoulder peak at 385 nm diminished while a new band at about 497 nm appeared, which is attributed to the generation of azulenium cations. Furthermore, an isosbestic point was clearly observed at 439 nm, implying the existence of two interconverted optically different species in the system. Based on the onset of the longest wavelength absorption (λonset), the optical gaps of 1,3-Az[9]CPP and its protonated species were estimated to be 2.74 eV, and 1.82 eV, respectively (Table 1). This tunable optical gap upon protonation/deprotonation was supported by theoretical calculations. In addition, the fluorescence spectrum reveals that 1,3-Az[9]CPP shows an emission band at around 493 nm. Surprisingly, the emission peak of its protonated species remains almost constant (Fig. 4b). No obvious redshift in emission wavelength can be accounted for by that the cyclic conjugation system of 1,3-Az[9]CPP in the presence of acid. The excited-state calculation results suggest that the S1 → S0 (f = 0.0043) transition of 1,3-Az[9]CPP is forbidden; hence the emission band at around 445 nm should come from S2 → S0 (f = 1.18960) transition of 1,3-Az[9]CPP, implying the anti-Kasha emission feature. However, in the presence of HBArF·(Et2O)2, the S1 → S0 (f = 0.3737) transition of the protonated 1,3-Az[9]CPP becomes allowed (Fig. S22 and Table S6, ESI†).
| λ abs ,b (nm) | λ em ,c (nm) | Φ F (%) | E g(opt) (eV) | E HOMO (eV) | |
|---|---|---|---|---|---|
| a UV-vis absorption and fluorescence spectra were measured in CH2Cl2 (1 × 10−5 M) at room temperature. b Wavelength of the maximum absorption. c Emission maximum (λex = highest intensity of absorption plus 10 nm). d Absolute fluorescence quantum yield. e Estimated from absorption onset, Eg(opt) = 1240/λonset. f E HOMO = −(4.74 + Eonsetox) eV (calibration by ferrocene). | |||||
| 1,3-Az[9]CPP | 334 | 493 | 1.4 | 2.74 | −4.97 |
| 1,3-Az[9]CPP-H+ | 329 | 493 | 1.0 | 1.82 | −4.74 |
Furthermore, we have also measured UV-vis and fluorescence spectra of 1,3-Az[9]CPP in the absence and presence of trifluoroacetic acid (TFA) in different solvents including DCM, toluene, N,N-dimethylformamide (DMF), 1,4-dioxane and tetrahydrofuran (THF) (Fig. 4b and Fig. S14, S15, ESI†). Upon titration with TFA, the variations in UV-vis spectra of 1,3-Az[9]CPP in different solvents were similar to those in DCM but to a different extent (Fig. 4a and Fig. S14, ESI†). Interestingly, a significant increase in fluorescence emission intensity was observed in DMF, 1,4-dioxane and THF solutions (Fig. 4b and Fig. S15, ESI†). The emission intensity in 1,4-dioxane was enhanced to be about 10 times higher than that in DCM at the same emission wavelength (λem). However, the fluorescence spectrum in toluene is almost the same as that in DCM. These phenomena suggest that polar solvents are more favorable for the fluorescence emission of the azulenylium cation.
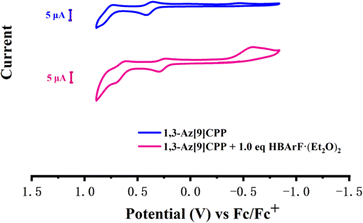
The electrochemical properties of 1,3-Az[9]CPP were further evaluated by CV. In C2H4Cl2-Bu4NPF6, 1,3-Az[9]CPP exhibited two oxidation processes. A quasi-reversible peak at 0.28V (peak anode current/peak cathode current, ipa/ipc = 0.3) and an irreversible peak at 0.79V were observed (Fig. 5). However, in the presence of HBArF·(Et2O)2, a new reduction peak at −0.58 V was detected, which further confirms the protonation of 1,3-Az[9]CPP. The CV data in combination with the results of NMR and UV-vis studies confirm that 1,3-Az[9]CPP features pH reversible stimuli-responsiveness. To the best of our knowledge, it is the first pH-responsive nonalternant all-carbon nanohoop.
The host–guest interaction behaviours between 1,3-Az[9]CPP and fullerenes C60/C70 were finally investigated because 1,3-Az[9]CPP has a cavity similar to that of [10]CPP.12 Single crystals of the C60@1,3-Az[9]CPP complex suitable for X-ray analysis were prepared by slow diffusion of acetonitrile into a mixed solution of chlorobenzene and CS2 (Fig. 6). The unit cell of C60@1,3-Az[9]CPP belongs to the space group C2/c. The crystal structure demonstrates that fullerene C60 resides inside the cavity of 1,3-Az[9]CPP, clearly showing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model. The crystal packing shows columnar stacks along the vertical axis (Fig. 6d).
1 binding model. The crystal packing shows columnar stacks along the vertical axis (Fig. 6d).
The binding behaviours of 1,3-Az[9]CPP with C60 and C70 were further characterized by UV-vis titration experiments in toluene at room temperature. By fitting the UV-vis adsorption changes at 334 nm at different fullerene concentrations, the association constants (Ka) of C60 and C70 with 1,3-Az[9]CPP were estimated to be 2.4 × 104 M−1 for C60 and 1.6 × 104 M−1 for C70 in toluene (Fig. 7a and Fig. S16a and S17, ESI†), which are smaller than those of 10[CPP] (2.7 × 106 M−1 for C60 and 8.4 × 104 M−1 for C70).28 The phenomenon is attributed to the tilting of azulene units in the carbon nanohoop. Subsequently, the Ka values of 1,3-Az[9]CPP with C60 and C70 in the presence of HBArF·(Et2O)2 were also extracted by UV-vis titration experiments to be 7.9 × 105 M−1 and 2.5 × 104 M−1 for C60 and C70, respectively (Fig. 7b and S16b and S17, ESI†). Notably, Ka of 1,3-Az[9]CPP with C60 in the presence of acid is about 30 times higher than that in the absence of acid, suggesting that the binding of 1,3-Az[9]CPP to C60 was significantly enhanced in the presence of acid. This enhanced binding can be accounted for the cation–π interaction between the aromatic tropylium ion and the fullerene rich in π-electrons. This interpretation was verified by NMR titration (Fig. S18 and S19, ESI†). As for C60@1,3-Az[9]CPP-H+ and C70@1,3-Az[9]CPP-H+, due to the enhanced electronic shielding by cation–π-electron interaction, the proton signals of H4'/H8' and H6' attributed to the protonated azulene are observed to have moved to higher fields. Moreover, the perfect size match between 1,3-Az[9]CPP-H+ and symmetrical spherical structure C60 may also make an important contribution for a favorable binding interaction. This acid-tunable selectivity of 1,3-Az[9]CPP for C60 over C70 may have potential application in enriching C60 from fullerene C60/C70 mixtures.29
Conclusions
In summary, we have successfully synthesized the first conjugated nonalternant all-carbon nanohoop by incorporating an azulene unit. The data demonstrated that the 1,3-azulene-embedded carbon nanohoop exhibits quite unique optical and physical properties such as anti-Kasha's rule emission and an adjustable band gap. Notably, 1,3-Az[9]CPP also shows good pH reversible stimuli-responsiveness and this process of protonation and deprotonation can be repeated multiple times. Remarkably, in an acidic environment, 1,3-Az[9]CPP shows a high selectivity (30/1) in binding of C60 over C70. This work can motivate further investigations of conjugated nanohoops containing nonalternant π-systems and develop their potential application in the field of host–guest chemistry as well as functional materials. Currently, incorporations of 2,6- and 4,7- connected azulene units into the CPP skeleton are ongoing projects in our laboratory.Author contributions
X. L. performed the experiments, carried out the characterization, and collected and analyzed the data. L. J. and Y. W. performed the theoretical calculations. W. W. analyzed the data of the electrochemical section. D. S. processed the single crystal data. H. J. and X. L. wrote the manuscript. All authors participated in the discussion and revised the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (21971020) and the Beijing Natural Science Foundation (2212008).Notes and references
-
(a) R. Jasti and C. R. Bertozzi, Chem. Phys. Lett., 2010, 494, 1–7 CrossRef PubMed
; (b) U. H. Bunz, S. Menning and N. Martin, Angew. Chem., Int. Ed., 2012, 51, 7094–7101 CrossRef PubMed
; (c) H. Omachi, T. Nakayama, E. Takahashi, Y. Segawa and K. Itami, Nat. Chem., 2013, 5, 572–576 CrossRef PubMed
; (d) H. Chen and Q. Miao, J. Phys. Org. Chem., 2020, 33, e4145 Search PubMed
.
- R. Jasti, J. Bhattacharjee, J. B. Neaton and C. R. Bertozzi, J. Am. Chem. Soc., 2008, 130, 17646–17647 CrossRef CAS PubMed
.
-
(a) S. E. Lewis, Chem. Soc. Rev., 2015, 44, 2221–2304 RSC
; (b) E. R. Darzi and R. Jasti, Chem. Soc. Rev., 2015, 44, 6401–6410 RSC
; (c) Y. Segawa, A. Yagi, K. Matsui and K. Itami, Angew. Chem., Int. Ed., 2016, 55, 5136–5158 CrossRef CAS
; (d) E. J. Leonhardt and R. Jasti, Nat. Rev. Chem., 2019, 3, 672–686 CrossRef CAS
; (e) I. Roy, A. H. G. David, P. J. Das, D. J. Pe and J. F. Stoddart, Chem. Soc. Rev., 2022, 51, 5557–5605 RSC
.
-
(a) H. Omachi, Y. Segawa and K. Itami, Org. Lett., 2011, 13, 2480–2483 CrossRef CAS PubMed
; (b) Z. Sun, T. Suenaga, P. Sarkar, S. Sato, M. Kotani and H. Isobe, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 8109–8114 CrossRef CAS
; (c) K. Okada, A. Yagi, Y. Segawa and K. Itami, Chem. Sci., 2017, 8, 661–667 RSC
; (d) H. Jia, Y. Gao, Q. Huang, S. Cui and P. Du, Chem. Commun., 2018, 54, 988–991 RSC
.
-
(a) P. Li, B. M. Wong, L. N. Zakharov and R. Jasti, Org. Lett., 2016, 18, 1574–1577 CrossRef CAS PubMed
; (b) P. Della Sala, A. Capobianco, T. Caruso, C. Talotta, M. De Rosa, P. Neri, A. Peluso and C. Gaeta, J. Org. Chem., 2018, 83, 220–227 CrossRef CAS PubMed
.
- T. Iwamoto, E. Kayahara, N. Yasuda, T. Suzuki and S. Yamago, Angew. Chem., Int. Ed., 2014, 53, 6430–6434 CrossRef CAS
.
-
(a) E. Kayahara, R. Qu, M. Kojima, T. Iwamoto, T. Suzuki and S. Yamago, Chem. – Eur. J., 2015, 21, 18939–18943 CrossRef CAS
; (b) L. Sicard, O. Jeannin, J. Rault-Berthelot, C. Quinton and C. Poriel, ChemPlusChem, 2018, 83, 874–880 CrossRef CAS
; (c) L. Sicard, F. Lucas, O. Jeannin, P. A. Bouit, J. Rault-Berthelot, C. Quinton and C. Poriel, Angew. Chem., Int. Ed., 2020, 59, 11066–11072 CrossRef CAS
.
- Y. Y. Liu, J. Y. Lin, Y. F. Bo, L. H. Xie, M. D. Yi, X. W. Zhang, H. M. Zhang, T. P. Loh and W. Huang, Org. Lett., 2016, 18, 172–175 CrossRef CAS
.
-
(a) J. M. Van Raden, B. M. White, L. N. Zakharov and R. Jasti, Angew. Chem., Int. Ed., 2019, 58, 7341–7345 CrossRef CAS
; (b) J. M. Van Raden, N. N. Jarenwattananon, L. N. Zakharov and R. Jasti, Chem. – Eur. J., 2020, 26, 10205–10209 CrossRef CAS
.
- T. Kuwabara, J. Orii, Y. Segawa and K. Itami, Angew. Chem., Int. Ed., 2015, 54, 9646–9649 CrossRef CAS
.
-
(a) D. Lu, H. Wu, Y. Dai, H. Shi, X. Shao, S. Yang, J. Yang and P. Du, Chem. Commun., 2016, 52, 7164–7167 RSC
; (b) Q. Huang, G. Zhuang, H. Jia, M. Qian, S. Cui, S. Yang and P. Du, Angew. Chem., Int. Ed., 2019, 58, 6244–6249 CrossRef CAS PubMed
.
- Y. Xu and M. von Delius, Angew. Chem., Int. Ed., 2020, 59, 559–573 CrossRef PubMed
.
- J. He, M. Yu, M. Pang, Y. Fan, Z. Lian, Y. Wang, W. Wang, Y. Liu and H. Jiang, Chem. – Eur. J., 2022, 28, e202103832 Search PubMed
.
- S. Hitosugi, S. Sato, T. Matsuno, T. Koretsune, R. Arita and H. Isobe, Angew. Chem., Int. Ed., 2017, 56, 9106–9110 CrossRef PubMed
.
- P. Du, Q. Huang, Y. Wu, Y. Zhou, H. Liu, J. Wang and S. Wang, Synthesis, 2020, 2535–2540 CrossRef
.
-
(a) D. Wassy, M. Pfeifer and B. Esser, J. Org. Chem., 2020, 85, 34–43 CrossRef
; (b) J. S. Wossner, D. Wassy, A. Weber, M. Bovenkerk, M. Hermann, M. Schmidt and B. Esser, J. Am. Chem. Soc., 2021, 143, 12244–12252 CrossRef
.
- Y. Li, Y. Segawa, A. Yagi and K. Itami, J. Am. Chem. Soc., 2020, 142, 12850–12856 CrossRef CAS PubMed
.
-
(a) A. Pfau and P. A. Plattner, Helv. Chim. Acta, 1939, 22, 202–208 CrossRef CAS
; (b) M. Beer and H. C. Longuet-Higgins, J. Chem. Phys., 1955, 23, 1390–1391 CrossRef CAS
; (c) J. Michl and E. W. Thulstrup, Tetrahedron, 1976, 32, 205–209 CrossRef CAS
; (d) R. S. H. Liu, J. Chem. Educ., 2002, 79, 183–185 CrossRef CAS
; (e) J. C. Del Valle and J. Catalan, Phys. Chem. Chem. Phys., 2019, 21, 10061–10069 RSC
; (f) H. Wang, J. Wang, T. Zhang, Z. Xie, X. Zhang, H. Sun, Y. Xiao, T. Yu and W. Huang, J. Mater. Chem. C, 2021, 9, 10154–10172 RSC
; (g) K. Veys and D. Escudero, Acc. Chem. Res., 2022, 55, 2698–2707 CrossRef CAS
.
- S. S. Danyluk and W. G. Schneider, Can. J. Chem., 1962, 40, 1777–1785 CrossRef CAS
.
-
(a) Y. Yamaguchi, K. Ogawa, K. Nakayama, Y. Ohba and H. Katagiri, J. Am. Chem. Soc., 2013, 135, 19095–19098 CrossRef CAS PubMed
; (b) Y. Yamaguchi, M. Takubo, K. Ogawa, K. Nakayama, T. Koganezawa and H. Katagiri, J. Am. Chem. Soc., 2016, 138, 11335–11343 CrossRef CAS
; (c) H. Ran, X. Duan, R. Zheng, F. Xie, L. Chen, Z. Zhao, R. Han, Z. Lei and J.-Y. Hu, ACS Appl. Mater. Interfaces, 2020, 12, 23225–23235 CrossRef CAS
; (d) H. Xin, B. Hou and X. Gao, Acc. Chem. Res., 2021, 54, 1737–1753 CrossRef CAS
; (e) H. Ran, F. Li, R. Zheng, H. Zhang, F. Xie, P. Jin, Z. Lei, X.-T. Wang and J.-Y. Hu, Dyes Pigm., 2022, 203, 110311 CrossRef CAS
.
- A. G. Lvov and A. Bredihhin, Org. Biomol. Chem., 2021, 19, 4460–4468 RSC
.
-
(a) Y.-Y. He, J. Chen, X.-L. Zheng, X. Xu, W.-Q. Li, L. Yang and W. Q. Tian, ACS Appl. Nano Mater., 2019, 2, 1648–1654 CrossRef CAS
; (b) C.-C. Yang, J.-Y. Ma, X. Su, X.-L. Zheng, J. Chen, Y.-Y. He, W. Quan Tian, W.-Q. Li and L. Yang, FlatChem, 2022, 33, 100362 CrossRef CAS
.
-
(a) C. M. Lopez-Alled, A. Sanchez-Fernandez, K. J. Edler, A. C. Sedgwick, S. D. Bull, C. L. McMullin, G. Kociok-Kohn, T. D. James, J. Wenk and S. E. Lewis, Chem. Commun., 2017, 53, 12580–12583 RSC
; (b) L. C. Murfin and S. E. Lewis, Molecules, 2021, 26, 353 CrossRef CAS
.
-
(a) N. Ogawa, Y. Yamaoka, H. Takikawa, K. I. Yamada and K. Takasu, J. Am. Chem. Soc., 2020, 142, 13322–13327 CrossRef CAS
; (b) S. Mishra, T. G. Lohr, C. A. Pignedoli, J. Liu, R. Berger, J. I. Urgel, K. Mullen, X. Feng, P. Ruffieux and R. Fasel, ACS Nano, 2018, 12, 11917–11927 CrossRef CAS PubMed
; (c) I. C. Hou, Q. Sun, K. Eimre, M. Di Giovannantonio, J. I. Urgel, P. Ruffieux, A. Narita, R. Fasel and K. Mullen, J. Am. Chem. Soc., 2020, 142, 10291–10296 CrossRef CAS PubMed
; (d) C. Duan, J. Zhang, J. Xiang, X. Yang and X. Gao, Angew. Chem., Int. Ed., 2022, 61, e202201494 CAS
; (e) S. Wang, M. Tang, L. Wu, L. Bian, L. Jiang, J. Liu, Z. B. Tang, Y. Liang and Z. Liu, Angew. Chem., Int. Ed., 2022, e202205658 CAS
.
- Y. Segawa, H. Omachi and K. Itami, Org. Lett., 2010, 12, 2262–2265 CrossRef CAS PubMed
.
-
(a) J. Schulze and F. A. Long, J. Am. Chem. Soc., 1963, 86, 322–326 CrossRef
; (b) F. Wang, T. T. Lin, C. He, H. Chi, T. Tang and Y.-H. Lai, J. Mater. Chem., 2012, 22, 10448 RSC
.
- M. Koch, O. Blacque and K. Venkatesan, J. Mater. Chem. C, 2013, 1, 7400 RSC
.
-
(a) T. Iwamoto, Y. Watanabe, T. Sadahiro, T. Haino and S. Yamago, Angew. Chem., Int. Ed., 2011, 50, 8342–8344 CrossRef CAS PubMed
; (b) T. Iwamoto, Y. Watanabe, H. Takaya, T. Haino, N. Yasuda and S. Yamago, Chem. – Eur. J., 2013, 19, 14061–14068 CrossRef CAS
.
- W. Sun, Y. Wang, L. Ma, L. Zheng, W. Fang, X. Chen and H. Jiang, J. Org. Chem., 2018, 83, 14667–14675 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2177150 and 2177151. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2tc04321a |
| This journal is © The Royal Society of Chemistry 2023 |