Synthesis and optical characterization of lead-free phenylenediammonium bismuth halide perovskites: a long charge carrier lifetime in phenylenediammonium bismuth iodide†
Zumaira
Siddique
abc,
Julia L.
Payne
 *c,
Muhammad Tariq
Sajjad
*c,
Muhammad Tariq
Sajjad
 de,
Natalie
Mica
de,
Natalie
Mica
 d,
David B.
Cordes
d,
David B.
Cordes
 c,
Alexandra M. Z.
Slawin
c,
Alexandra M. Z.
Slawin
 c,
Ifor D. W.
Samuel
d,
Azhar
Iqbal
c,
Ifor D. W.
Samuel
d,
Azhar
Iqbal
 *a and
John T. S.
Irvine
*a and
John T. S.
Irvine
 *c
*c
aDepartment of Chemistry, Quaid-i-Azam University, Islamabad, 45320, Pakistan. E-mail: aiqbal@qau.edu.pk
bDepartment of Chemistry, Government College University, Jhang Road, Faisalabad, 38000, Pakistan
cEaStCHEM School of Chemistry, University of St Andrews, North Haugh, St Andrews, KY16 9ST, UK. E-mail: jlp8@st-andrews.ac.uk; jtsi@st-andrews.ac.uk
dOrganic Semiconductor Centre, SUPA, School of Physics and Astronomy, University of St Andrews, North Haugh, St Andrews, KY16 9SS, UK
eLondon Centre for Energy Engineering, School of Engineering, London South Bank University, 103 Borough Road, London, SE1 0AA, UK
First published on 9th December 2022
Abstract
Toxicity and regulatory concerns over the use of (CH3NH3)PbI3 in photovoltaic devices have resulted in significant interest in lead-free, organic–inorganic metal halides with excellent light absorbing properties and stability. Here we report the synthesis of three new lead-free bismuth halides which accommodate the symmetric conjugated p-phenylenediammonium cation (PPD = (H3NC6H4NH3)2+) in the structures. These are (PPD)BiI5, (PPD)[BiBr4]2·2H2O and (PPD)2BiCl7·H2O. We also synthesized β-(PPD)2Bi2I10. The band gap of the iodide, β-(PPD)2Bi2I10 (1.83 eV) is lower than that of the bromide, (PPD)[BiBr4]2·2H2O (2.64 eV) and chloride, (PPD)2BiCl7·H2O (2.93 eV). Photophysical studies show that β-(PPD)2Bi2I10 has the longest average charge carrier lifetime (>1 μs) of the materials studied here, of the same order of magnitude as that of (CH3NH3)PbI3 and has a low band gap. This suggests that β-(PPD)2Bi2I10 may be a promising candidate for use in lead-free photovoltaic devices.
1. Introduction
Increasing energy consumption and the shortage of fossil fuels are forcing the scientific community to search for new solutions to fulfil energy demands. More than 15 TW energy per year is consumed by the world's population and this is predicted to increase to 30 TW in 2050.1 In one year, the sun can provide 1.7 × 105 TW of energy, which is more than enough to fulfill the energy demands of the whole planet, if a small proportion of this energy could be harnessed.2 For many years, photoelectrochemical and photovoltaic devices have been developed to convert sunlight into electrical energy.3 Following the development of first generation single crystalline silicon solar cells and polycrystalline thin film second generation solar cells, scientists are now focusing on low cost, economical and efficient solar cell materials.4 One notable development was the replacement of organic dyes in dye-sensitized solar cells with hybrid perovskite materials based on (CH3NH3)PbI3, which resulted in significant increases in power conversion efficiencies. The term ‘hybrid’ arises due to the materials containing both an inorganic and organic component. Since 2009, when the use of CH3NH3PbI3 in a photovoltaic device was first reported, the efficiency of these perovskite based devices has increased from 3.8% to 25.5%.5 High absorption coefficients, long charge carrier diffusion lengths, high carrier mobilities, tunable band gaps, weak exciton binding energies and low non-radiative recombination rates make these hybrid perovskites excellent candidates for a variety of photovoltaic applications.6 The diversity of atomic compositions, low temperature device processing methods and device architectures have resulted in the development of cheap and effective photovoltaic devices.7 Despite these benefits, the two main drawbacks of (CH3NH3)PbI3 are the toxicity of lead and the instability of (CH3NH3)PbI3 under ambient conditions, which hinder their commercialization.8Other metal cations such as tin(II), germanium(II) and bismuth(III) have been explored as alternatives to lead in halide perovskites.9–11 Bismuth(III) based hybrid perovskites have been widely used as a result of their lower toxicity, high absorption coefficients, iso-electronicity to Pb2+ (6s2, 6p0) and low oxidation in solution.12 Solution processable hybrid bismuth(III) based halides can form a range of different structure types, where bismuth halide octahedra may exist in the form of isolated zero dimensional octahedra,13,14 one dimensional interconnected chains of octahedra,15 two dimensional layers of octahedra or chains that extend in two directions,16–19 and three dimensional networks of octahedra.20 The connectivity of these octahedra could be through face, edge or corner sharing.21 Due to the greater choice and flexibility in organic cations, unique structural and optoelectronic properties can be linked with 1D, 2D and quasi-2D layered materials.22 So, by choosing versatile fabrication methodologies, exploring new materials and testing different device architectures, these materials should be capable of being fabricated at the industrial scale with the improved stability and performance that are required for commercialization.23
There are several examples of phenylenediammonium metal halides reported in the literature.24–27 In addition, organic cations that are closely related to phenylenediammonium have also been used in the preparation of organic–inorganic metal halides.28 However, many of these studies focus on the crystallography rather than the properties of these materials. The structure of p-phenylenediammonium (PPD) bismuth iodides (and a corresponding hydrate) and a p-phenylenediammonium bismuth chloride have previously been reported.24–27 PPDBi2I8·I2 consists of chains of edge sharing BiI6 octahedra and also contains molecules of I2, which form a pseudo-3-dimensional structure due to the short intermolecular I–I distances between iodine in the I2 molecules and the BiI6 octahedra.27 PPDBi2I8·I2 is stable up to temperatures of 100 °C and has a low band gap of 1.45 eV, although further properties of the compound are currently unexplored.27 PPD2Bi2I10 exists in two polymorphs, α and β, with the α–β phase transition occurring at −26 to −28 °C.24 Both α and β-PPD2Bi2I10 consist of isolated, edge sharing Bi2I10 dimers. In addition, the reported structure of the hydrate, PPD2Bi2I10·4H2O is very similar to the published β-PPD2Bi2I10 and again consists of Bi2I10 dimers.24,25 There is also one report of a p-phenylenediammonium bismuth chloride, PPD2BiCl7·H2O, in the literature which consists of isolated BiCl6 octahedra.26 A peak in differential scanning calorimetry data along with a change in the dielectric constant of PPD2BiCl7·H2O, both at 90 °C, provide evidence for a phase transition.26 It is thought that this phase transition relates to an order–disorder transition, probably driven by the reorganization of water or PPD molecules.26 Despite the improvement in optoelectronic properties by lowering non-radiative recombination in low dimensional materials, and enhanced moisture stability in organic metal halides containing aromatic amines, few studies have explored the use of symmetric conjugated amines, which are more compact and form rigid structural configurations with metal halides.29 Therefore, the aim of this work was to probe the synthesis, structural characterization and optoelectronic properties of p-phenylenediammonium bismuth halide materials. Compounds of this type may also have possible applications in ferroelectrics, lasers, light emitting diodes (LEDs), solar cells or super lattice heterojunction devices.30–32
2. Experimental
2.1. Materials
p-Phenylenediamine (H2NC6H4NH2, 97%, Alfa Aesar), bismuth(III) oxide (Bi2O3, 99.975%, Alfa Aesar), hypophosphorous acid solution (H3PO2, 50 wt% H2O, Sigma Aldrich), hydriodic acid (HI, 57% w/w aqueous solution, stabilized with 1.5% hypophosphorous acid, Alfa Aesar), hydrobromic acid (HBr, 48% w/w aqueous solution, Alfa Aesar), hydrochloric acid (HCl, 37% w/w aqueous solution, Fisher Chemical), absolute ethanol (C2H5OH, VWR BDH Chemicals), and diethyl ether (C4H10O, 99.5%, Riedel-de Haen) were used as received unless otherwise specified.The p-phenylenediamine dihydriodide salt (PPD·2HI) was prepared by the stoichiometric reaction of p-phenylenediamine with hydriodic acid. The p-phenylenediamine was dissolved in in 20 mL ethanol and HI was added dropwise to the solution in a round bottom flask at 0 °C to adjust the pH to around 6–7. The mixture was stirred for two hours, then the solvent was removed using a rotary evaporator at 80 °C. The crude yellowish-white powder was recrystallised three times from ethanol and diethyl ether. Afterwards the white product was filtered and dried at 70 °C in vacuum oven overnight. The equivalent procedure was followed for the synthesis of p-phenylenediamine dihydrobromide (PPD·2HBr) and p-phenylenediamine dihydrochloride salts (PPD·2HCl) by using HBr and HCl precursors instead of HI.
2.2. Synthesis of (PPD)BiI5 and β-(PPD)2Bi2I10
Bismuth(III) oxide was dissolved in a mixture of hydriodic acid (HI, 10 mL) and hypophosphorous acid (HPA, 2 mL) at 180 °C for 10 minutes with constant stirring. A dark red precipitate was immediately formed upon the addition of PPD·2HI (3.64 g, 10 mmol) salt to this hot solution. By changing the ratio of reactants, two different types of bismuth iodide materials were formed. To prepare compound (PPD)BiI5, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio is used (4.66 g, 10 mmol Bi2O3 and 3.64 g, 10 mmol PPD·2HI), whereas to prepare β-(PPD)2Bi2I10 a 1
1 ratio is used (4.66 g, 10 mmol Bi2O3 and 3.64 g, 10 mmol PPD·2HI), whereas to prepare β-(PPD)2Bi2I10 a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio is used (2.33 g, 5 mmol Bi2O3 and 3.64 g, 10 mmol PPD·2HI). This non-stoichiometric ratio of organic cation to metal halide has previously been used in the synthesis of other hybrid perovskites containing diamines, enabling the desired product to be grown from the solution.33
2 ratio is used (2.33 g, 5 mmol Bi2O3 and 3.64 g, 10 mmol PPD·2HI). This non-stoichiometric ratio of organic cation to metal halide has previously been used in the synthesis of other hybrid perovskites containing diamines, enabling the desired product to be grown from the solution.33
To prepare phase pure crystalline β-(PPD)2Bi2I10, stirring was discontinued after 20 minutes and the solution was cooled down to room temperature over 48 hours, which allowed the crystals to grow. After that, the crystals were filtered and dried at 70 °C in a vacuum oven for 24 hours. The atomic% (found/calculated) of Bi (17.76/16.67) and I (82.24/83.33) from EDX analysis of crystals confirmed the Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I ratio in this sample. This compound matches the previously described compound β-PPD2Bi2I10.24
I ratio in this sample. This compound matches the previously described compound β-PPD2Bi2I10.24
2.3. Synthesis of (PPD)[BiBr4]2·2H2O
Bismuth(III) oxide (2.33 g, 5 mmol) was dissolved in a mixture of hydrobromic acid (HBr, 10 mL) and hypophosphorous acid (HPA, 2 mL) at 180 °C for 10 minutes with constant stirring. A light green precipitate was formed upon the subsequent addition of PPD·2HBr salt (2.70 g, 10 mmol) to this hot solution. For the growth of crystals, the stirring was discontinued after 20 minutes and the solution was cooled to room temperature over 48 hours. After that, the crystals were filtered and dried in a vacuum oven at 70 °C for 24 hours. Powder X-ray diffraction showed that (PPD)[BiBr4]2·2H2O was the major phase present, with a small amount of the bromide equivalent of β-PPD2Bi2I10 (i.e. β-PPD2Bi2Br10) present as an impurity phase (see ESI†). The atomic% (found/calculated) of Bi (18.26/16.67) and Br (81.74/83.33) from EDX analysis of crystals confirmed the Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br ratio.
Br ratio.
2.4. Synthesis of (PPD)2BiCl7·H2O
Bismuth(III) oxide (2.33 g, 5 mmol) was dissolved in a mixture of hydrochloric acid (HCl, 10 mL) and hypophosphorous acid (HPA, 2 mL) at 180 °C for 10 minutes with constant stirring. A colourless precipitate was formed upon the addition of PPD·2HCl salt (1.81 g, 10 mmol) to this hot solution. For the growth of crystals, the stirring was discontinued after 20 minutes and the solution was cooled to room temperature over 48 hours. Afterwards the crystals were filtered and dried in a vacuum oven at 70 °C for 24 hours. Powder X-ray diffraction and EDX analysis confirmed the phase purity of (PPD)2BiCl7·2H2O. The atomic% (found/calculated) of Bi (16.57/16.67) and Cl (83.43/83.33) from EDX analysis of crystals confirmed the Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl ratio.
Cl ratio.
Images of the samples of β-(PPD)2Bi2I10, (PPD)[BiBr4]2·2H2O and (PPD)2BiCl7·H2O are shown in Fig. S1 (ESI†).
2.5. Physiochemical techniques
UV-Visible absorption spectra of the powdered samples were measured using a V650 spectrophotometer (Jasco) with BaSO4 as a reference. The room temperature steady-state and time-resolved photoluminescence (PL) of thin films of samples on quartz substrates were performed using an Edinburgh Instruments FLS980 spectrometer. For PL lifetime measurements, samples were excited at 379 nm by a PicoQuant picosecond pulsed laser (PicoQuant, LDH-D-C-375) and PL was detected using a time correlated single photon counting (TCSPC) setup. Powder X-ray diffraction data were collected using a Panalytical Empyrean X-ray diffractometer operating in Bragg-Brentano geometry with CuKα1 radiation and an X'celerator RTMS detector. Data were collected in the range 5–70° 2θ, with a step size of 0.017° and a time per step of 0.94 s. Single crystal X-ray diffraction data were collected at 173 K using a Rigaku FR-X Ultrahigh Brilliance Microfocus RA generator/confocal optics with Rigaku XtaLAB P200 diffractometer [Mo Kα radiation (λ = 0.71075 Å)]. Intensity data were collected using ω steps accumulating area detector images spanning at least a hemisphere of reciprocal space. Data for all compounds were collected using CrystalClear and processed using either CrystalClear34 or CrysAlisPro.35 Structure solution was carried out by dual-space methods using SHELXT36 and structure refinement by full matrix least squares against F2 was carried out with SHELXL (2014/7).37 Non-hydrogen atoms were refined anisotropically and aromatic hydrogen atoms were refined using a riding model. Amine hydrogens were located from the difference Fourier map and refined isotropically subject to a distance restraint. All calculations were performed using the WinGX interface.38 Selected crystallographic data are presented in Table 1 and Table S1 (ESI†). Deposition numbers 2180531–2180533 contains the supplementary crystallographic data for this paper.†| (PPD)BiI5 | (PPD)[BiBr4]2·2H2O | (PPD)2BiCl7·H2O | |
|---|---|---|---|
| CCDC code | 2180531 | 2180532 | 2180533 |
| Formula weight | 953.64 | 601.72 | 695.46 |
| Crystal description | Red chip | Yellow prism | Colourless prism |
| Crystal size (mm3) | 0.05 × 0.03 × 0.02 | 0.09 × 0.06 × 0.03 | 0.15 × 0.13 × 0.03 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | P21 | P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 10.0586(4) | 7.2340(17) | 9.505(2) |
| b (Å) | 8.4358(3) | 12.090(3) | 10.376(2) |
| c (Å) | 10.8613(5) | 12.753(3) | 13.322(3) |
| α (°) | 99.260(4) | ||
| β (°) | 107.582(4) | 98.210(6) | 110.582(4) |
| γ (°) | 105.1820(10) | ||
| Volume (Å3) | 878.55(6) | 1103.9(5) | 1139.6(4) |
| Z | 2 | 4 | 2 |
| ρ (calc, g cm−3) | 3.605 | 3.620 | 2.027 |
| μ (mm−1) | 18.796 | 30.397 | 8.566 |
| F(000) | 816 | 1052 | 664 |
| Reflections collected | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 474 474 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 096 096 |
4063 |
| Independent reflections (Rint) | 3734 (0.0285) | 2018 (0.0770) | 4063 (0.046) |
| Parameters, restraints | 145, 10 | 106, 28 | 269, 82 |
| Goodness-of-fit on F2 | 1.015 | 0.968 | 1.180 |
| R 1 | 0.0235 | 0.0394 | 0.0413 |
| R 1 [I > 2sigma(I)] | 0.0204 | 0.0320 | 0.0402 |
| wR2 | 0.0363 | 0.0757 | 0.1307 |
| wR2 [I > 2sigma(I)] | 0.0357 | 0.0738 | 0.1300 |
| Absolute structure parameter | −0.026(3) | ||
| Largest diff. peak and hole (e Å−3) | 0.672 and −0.883 | 1.680 and −1.815 | 2.968 and −1.945 |
SEM images and EDS (Fig. S7, ESI†) were taken on a Jeol 6700 F microscope. FTIR spectra were obtained using an IR Infinity spectrometer in the range of 400–4000 cm−1.
3. Results and discussion
3.1 Crystal structure analysis
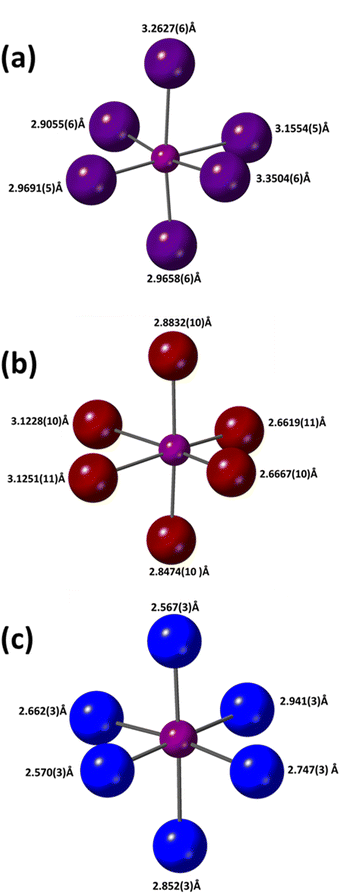
The structure of (PPD)BiI5 was determined from single crystal XRD data and is shown in Fig. 1. It crystallizes in the space group P21 and consists of one-dimensional chains of corner sharing BiI6 octahedra, which run along the b-axis in a zig-zag motif. These chains are separated by PPD cations. Similar chains have been observed in other materials such as N-3-aminopropylimidazolium lead iodide.39 The asymmetric unit comprises of a bismuth atom, five symmetrically inequivalent iodine atoms and a PPD molecule. The Bi–I bond lengths range from 2.9055(6) Å to 3.3504(6) Å (Table 2), with I–Bi–I bond angles ranging from 83.387(8)° to 98.522(17)°. The bismuth atom is off-center within its octahedron. Bismuth atoms are linked through I3; the Bi–I distances in the resulting I–Bi–I chains being long [3.3503(6) Å and 3.2628(6) Å], with a Bi–I–Bi angle of 148.947(19)°. The iodine atoms axial to the plane of the chain show Bi–I distances of 2.9691(5) Å and 3.1554(5) Å, with axial I–Bi–I bond angles ranging from 169.508(18)° to 177.241(19)°, whilst the equatorial I–Bi–I bond angles ranged from 83.387(6)° to 98.522(17)°. The equatorial Bi–I distances range from 2.9055(6) Å to 3.3504(6) Å. Hydrogen bonds form between all six of the ammonium hydrogens and all but one of the iodines, with H⋯I distances of 2.60(3) to 2.91(6) Å, and corresponding N⋯I separations of 3.525(7) to 3.629(7) Å (Fig. S2, ESI†). The hydrogen bonds link the one-dimensional chains into a three-dimensional array. The resulting hydrogen bond arrangement is shown in Fig. S2 (ESI†).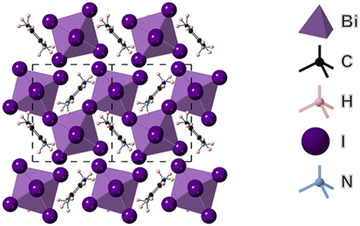 | ||
| Fig. 1 Structure of (PPD)BiI5 obtained from single crystal XRD showing the view in the bc plane, indicating corner-sharing BiI6 octahedra which run along the b-axis. | ||
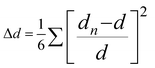 and bond angle variance is given by
and bond angle variance is given by 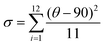
| (PPD)BiI5 | (PPD)[BiBr4]2·2H2O | (PPD)2BiCl7·H2O | β-(PPD)2Bi2I10 | |
|---|---|---|---|---|
| Bi–I (Å) | Bi–Br (Å) | Bi–Cl (Å) | Bi–I (Å) | |
| Bi–X (Å) | 2.9055(6) | 2.6619(11) | 2.567(3) | 2.923(3) |
| Bi–X (Å) | 2.9658(6) | 2.6667(10) | 2.569(3) | 2.951(3) |
| Bi–X (Å) | 2.9691(5) | 2.8474(10) | 2.662(3) | 3.064(2) |
| Bi–X (Å) | 3.1554(5) | 2.8832(10) | 2.747(3) | 3.1013(19) |
| Bi–X (Å) | 3.2627(6) | 3.1228(10) | 2.852(3) | 3.246(3) |
| Bi–X (Å) | 3.3504(6) | 3.1251(11) | 2.941(3) | 3.271(3) |
| Δd (×10−4) | 28.62 | 42.67 | 26.19 | 18.31 |
| σ 2 | 16.72 | 12.21 | 19.52 | 1.65 |
The PPD bismuth iodide system is particularly interesting as a number of different structures have been reported. A summary of structural data from the Cambridge Structural Database40 are included in Table S1 (ESI†).24,25,27 Shestimerova et al. prepared (PPD)(BiI4)2·I2, which contains zig-zag, one-dimensional chains of edge sharing BiI6 octahedra running along the a-axis.27 The shortest inter-chain I–I distance is 3.837(1) Å along the c-axis and 3.862(1) Å along the b-axis. As suggested in the formula (PPD)(BiI4)2·I2, the material contains discrete iodine molecules, with an I–I distance of 2.7286(9) Å, which is typical of that found in molecular iodine (2.722(3) Å) at similar temperatures.27,41 Hrizi et al. have studied α- and β-(PPD)2Bi2I10 and a corresponding hydrate.24,25 These structures both contain isolated dimers of edge sharing bismuth iodide octahedra, forming Bi2I10 units. Our new material, (PPD)BiI5 is therefore the first PPD bismuth iodide to contain corner-sharing BiI6 octahedra. The I–Bi–I angles in (PPD)BiI5 vary further from 90° than other reported PPD bismuth iodides (see Table S1, ESI†). It is also interesting to note that in (PPD)BiI5, the Bi–Bi distances of 6.3718(4) Å are much longer than Bi–Bi distances in the other PPD bismuth iodides, which range from 4.5122(14) Å to 4.770(5) Å.24,25,27 Interestingly, the Bi–Bi distance in PPDBiI5 is comparable to the Pb–Pb distances in (CH3NH3)PbI3, which has a Pb–Pb distance of 6.306 Å in the cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase at 350 K.42
m phase at 350 K.42
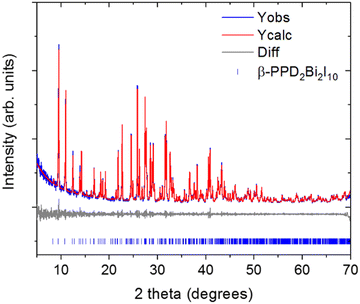
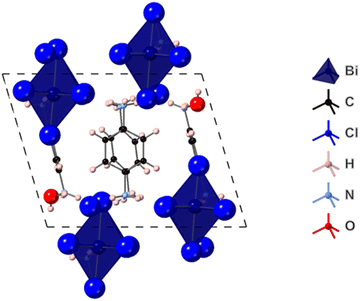
The preliminary PXRD pattern of the sample of (PPD)BiI5 which the single-crystal studied had come from indicated that three phases were present (Fig. S3, ESI†). Optimization of the synthetic procedure resulted in isolation of the second phase present as a pure phase: the PXRD pattern is shown in Fig. 2. The PXRD data can be indexed to a monoclinic cell of a = 11.488(3) Å, b = 12.9039(13) Å, c = 15.026(6) Å, β = 112.520(9)°, space group P21/n. This is in agreement with the published structural model of β-(PPD)2Bi2I10. The resulting Pawley fit to the PXRD data is shown in Fig. 2. The structure of β-(PPD)2Bi2I10 is shown in Fig. S4 (ESI†) and consists of isolated dimers of edge-sharing BiI6 octahedra, forming Bi2I10 units and therefore can be described as a zero dimensional perovskite.24 This is structurally very different to (PPD)BiI5, which consists of chains of corner-sharing octahedra forming one-dimensional chains. Attempts to prepare (PPD)BiI5, in a pure form were unsuccessful, so further studies focused solely on β-(PPD)2Bi2I10.
It was previously shown that the different compounds β-(PPD)2Bi2I10 and its hydrate (PPD)2Bi2I10·4H2O are structurally very similar, showing a small difference in unit cell parameters, which makes it difficult to distinguish between the two forms using powder X-ray diffraction.24,25 In order to investigate the possible incorporation of water into β-(PPD)2Bi2I10 sample, a combined TGA-DTA experiment was carried out (Fig. 3). A mass loss of 0.4% occurred between 50 and 60 °C and is accompanied by an exothermic peak in the DTA. This mass loss is smaller than the 3.6% expected for complete loss of all four water molecules in (PPD)2Bi2I10·4H2O, and is equivalent to the presence of 0.4 H2O per (PPD)2Bi2I10 formula unit. This provides preliminary evidence for the accommodation of a variable water content (i.e. a region of solid solution) in (PPD)2Bi2I10·xH2O, where based on literature and our current work, x can range from 0 to 4.
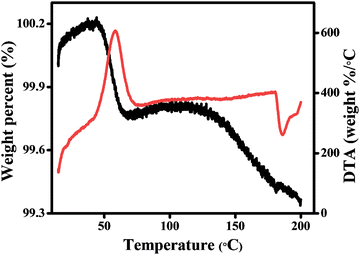 | ||
| Fig. 3 Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) of β-(PPD)2Bi2I10 carried out under an air atmosphere, black line represents TGA and red line represents the DTA. | ||
The structure of (PPD)[BiBr4]2·2H2O was determined from single crystal XRD data and is shown in Fig. 4. It crystallises in the space group P21/c and consists of one-dimensional chains of edge sharing BiBr6 octahedra, which run along the a-axis in a zig-zag motif. The PPD cation is positioned in the spaces between chains of BiBr6 octahedra. The asymmetric unit comprises of one bismuth atom, four crystallographically independent Br atoms, half a PPD cation and a molecule of water. The Bi–Br bond lengths range between 2.6619(11) Å and 3.1251(11) Å (Table 2), with Br–Bi–Br bond angles ranging from 83.86(3)° to 95.82(4)°. Bismuth atoms are linked alternately through Br2 and Br3 atoms; each pair of bringing Br atoms binding asymmetrically, showing one longer and one shorter bond [2.8474(10) and 3.1251(11), and 2.8832(10) and 3.1228(10) for Br2 and Br3, respectively], with Bi–Br–Bi angles of 92.85(3)° and 92.70(3)°, for Br2 and Br3, indicating some distortion of the bismuth octahedra. The inter-chain Br–Br distance is 3.8173(15) Å. Each potential hydrogen bond donor, both ammonium and water, forms a single hydrogen bond, two N–H⋯Br, one N–H⋯O and two O–H⋯Br. These show N–H⋯Br distances of 2.582(17) and 2.69(5) Å [N⋯Br separations of 3.518(8) and 3.564(8) Å], an N–H⋯O distance of 1.73(19) Å [N⋯O separation of 2.713(10) Å], and O–H⋯Br distances of 2.49(2) and 2.61(5) Å [O⋯Br separations of 3.451(8) to 3.508(8) Å]. The hydrogen bonds link the one-dimensional chains into a three-dimensional array (Fig. S5, ESI†).
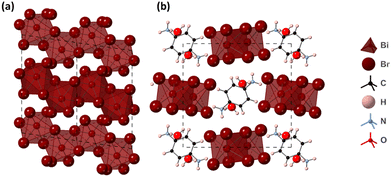 | ||
| Fig. 4 Structure of (PPD)[BiBr4]2·2H2O obtained from single crystal XRD (a) ac plane (showing only chains of BiBr6 octahedra without the PPD cation for clarity) and (b) bc plane. | ||
The powder X-ray diffraction pattern of (PPD)[BiBr4]2·2H2O is shown in Fig. S6 (ESI†) and could be indexed to the same monoclinic cell as the single crystal XRD data. The resulting Pawley fit is shown in Fig. S6 (ESI†). A very small percentage of a secondary phase, attributed to the bromide analogue of β-(PPD)2Bi2I10, was present in the powder X-ray diffraction pattern. This will be discussed in a future publication.
The structure of (PPD)2BiCl7·H2O was determined from single crystal XRD data and is shown in Fig. 5. It crystallizes in the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and contains isolated BiCl6 octahedra. This is in contrast to the other PPD bismuth halides synthesized in this study, which showed either dimers or chains of octahedra. The closest inter-octahedra Cl–Cl distance is 4.836(4) Å. The asymmetric unit comprises one bismuth atom, seven crystallographically independent Cl atoms, (one not coordinated), one whole and two half crystallographically independent PPD cations and a molecule of water. The Bi–Cl bond lengths range from 2.567(3) to 2.941(3) Å (Table 2), with Cl–Bi–Cl bond angles ranging from 84.35(8) to 94.29(9)°. The Bi–Cl distances cluster with three shorter and three longer. Each potential ammonium hydrogen-bond donor, forms at least one hydrogen bond; all bar one to chloride, while the water forms three hydrogen bonds to chlorides. These show N–H⋯Cl distances ranging from 2.20(2) to 2.84(9) Å [N⋯Cl separations of 3.133(10) to 3.317(9) Å], an N–H⋯O distance of 1.84(4) Å [N⋯O separation of 2.786(16) Å], and O–H⋯Cl distances of 2.55(8) to 2.81(10) Å [O⋯Cl separations of 3.278(10) to 3.428(10) Å]. The hydrogen bonds link the BiCl6 octahedra into a three-dimensional array.
and contains isolated BiCl6 octahedra. This is in contrast to the other PPD bismuth halides synthesized in this study, which showed either dimers or chains of octahedra. The closest inter-octahedra Cl–Cl distance is 4.836(4) Å. The asymmetric unit comprises one bismuth atom, seven crystallographically independent Cl atoms, (one not coordinated), one whole and two half crystallographically independent PPD cations and a molecule of water. The Bi–Cl bond lengths range from 2.567(3) to 2.941(3) Å (Table 2), with Cl–Bi–Cl bond angles ranging from 84.35(8) to 94.29(9)°. The Bi–Cl distances cluster with three shorter and three longer. Each potential ammonium hydrogen-bond donor, forms at least one hydrogen bond; all bar one to chloride, while the water forms three hydrogen bonds to chlorides. These show N–H⋯Cl distances ranging from 2.20(2) to 2.84(9) Å [N⋯Cl separations of 3.133(10) to 3.317(9) Å], an N–H⋯O distance of 1.84(4) Å [N⋯O separation of 2.786(16) Å], and O–H⋯Cl distances of 2.55(8) to 2.81(10) Å [O⋯Cl separations of 3.278(10) to 3.428(10) Å]. The hydrogen bonds link the BiCl6 octahedra into a three-dimensional array.
Fig. 6 shows a comparison of the Bi–X octahedra in the three materials and the corresponding bond lengths are summarized in Table 2.
It is interesting to note that a polymorph of (PPD)2BiCl7·H2O has been reported previously by Hrizi et al. although the synthetic conditions are different to those used here and the resulting structure is different to the (PPD)2BiCl7·H2O that we report here, with an approximate doubling of the b-axis.26 Attempts to fit our PXRD data with the model and unit cell parameters previously reported by Hrizi et al.26 were unsuccessful, using both Rietveld and Pawley refinement. However, our PXRD data could be fitted with the unit cell parameters that we obtained from single crystal X-ray diffraction (see Fig. S7, ESI†), indicating the difference in the unit cell parameters and structures of the two materials. PXRD data were collected after 8 months, to judge the long-term stability of the samples and are shown in Fig. S8 (ESI†). The SEM images of the samples synthesized are shown in Fig. S9 (ESI†). A detailed description of crystallographic parameters of the p-phenylenediammonium bismuth halides (PPD)BiI5, (PPD)[BiBr4]2·2H2O and (PPD)2BiCl7·H2O are given in Table 2 and Table S2 (ESI†). The FTIR spectra are shown in Fig. S10 and Table S3 (ESI†) summarizes the characteristic vibrational frequencies of these materials.
3.2 Optoelectronic properties
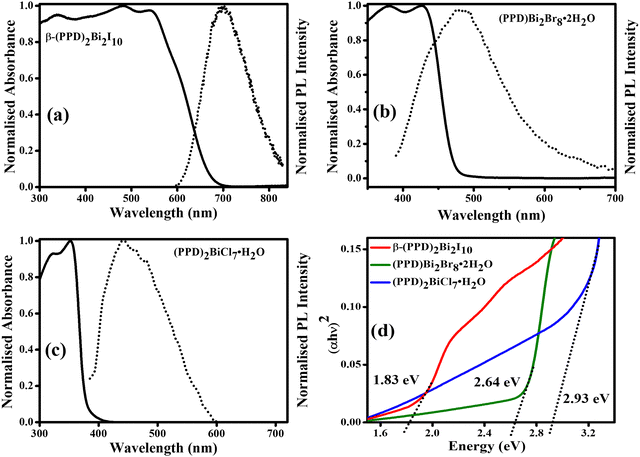
The optical behavior of the powdered samples β-(PPD)2Bi2I10, (PPD)[BiBr4]2·2H2O and (PPD)2BiCl7·H2O were determined by UV-visible absorption and photoluminescence (PL) spectroscopies. Fig. 7a–c shows the absorption spectra of β-(PPD)2Bi2I10, (PPD)[BiBr4]2·2H2O and (PPD)2BiCl7·2H2O with corresponding band gaps assessed by the Tauc plot44 (Fig. 7d). In compound β-(PPD)2Bi2I10, a clear band edge from electronic absorption is observed at 1.83 eV (680 nm). In 3D perovskites, the absorption onset in the low energy region is correlated with the largest Bi–I–Bi bond angles, which favoured the formation of a low energy conduction band and leads to a low band gap.45Table 3 summarizes the band gaps of other bismuth-containing halides. It can be seen that the band gap of 1.83 eV reported here for β-(PPD)2Bi2I10 is low and comparable to those reported for other hybrid bismuth halides. This is desirable because it brings it close to the optimum bandgap of 1.5 eV for a single junction solar cell.46 Therefore, it may be a possible candidate for lead-free light harvesting materials in solar cells. The band gap of (PPD)[BiBr4]2·2H2O is 2.64 eV, and was determined from the Tauc plot with the absorption cutoff wavelength set at 480 nm. Similarly, the data pertaining to (PPD)2BiCl7·H2O showed the absorption cutoff at a wavelength of 395 nm. This compound has a higher octahedral distortion (in terms of bond angle variance, see Table 2) and from the Tauc plot the band gap was determined to be 2.93 eV. A clear red shift was observed from compound (PPD)2BiCl7·H2O to β-(PPD)2Bi2I10. The PPD bismuth halides are able to support different connectivities of BiX6 octahedra, which results in different levels of octahedral distortion for (PPD)BiI5, (PPD)[BiBr4]2·2H2O and (PPD)2BiCl7·H2O and β-(PPD)2Bi2I10 (Table 2). By changing the halide ions, we are able to produce new materials with different band gaps, which may have applications in a range of optoelectronic devices.47,48| Compound | Band gap (eV) | Synthetic technique utilised | Ref. |
|---|---|---|---|
| Cs2AgBiBr6 | 2.02 | Top-seeded solution growth (TSSG) method | 49 |
| Cs2AgBiBr6 | 2.19 | Solid state grinding and solution precipitation | 50 |
| Cs2AgBiCl6 | 2.77 | Solid state grinding and solution precipitation | 50 |
| (CH3NH3)2AgBiBr6 | 2.02 | Hydrothermal method | 51 |
| (CH3NH3)2KBiCl6 | 3.04 | Hydrothermal method | 52 |
| (CH3NH3)2TlBiBr6 | 2.16 | Hydrothermal method | 20 |
| (TMP)[BiI5] | 2.02 | Solvothermal method | 53 |
| (TMP)[BiBr5] | 2.67 | Solvothermal method | 53 |
| (TMP)[BiCl5] | 3.21 | Solvothermal method | 53 |
| (TMP)1.5[Bi2I7Cl2] | 2.10 | Solvothermal method | 53 |
| (C6H13N)2BiI5 | 2.02 | Slow evaporation method | 54 |
| (CH3NH3)3Bi2I9 | 2.06 | Solution grown powder method | 55 |
| (CH3NH3)3Bi2I9 | 2.19 | Thin film in mixed solvents | 14 |
| (CH3NH3)3Bi2I9 | 2.10 | Thin film formation in mixed solvents | 56 |
| Cs3Bi2I9 | 2.20 | Thin film formation in mixed solvents | 56 |
| AgBi2I7 | 1.87 | Solution processed thin film method | 57 |
| [H3N(CH2)6NH3]BiI5 | 1.89 | Slow cooling solution method | 58 |
| Cs2NaBiI6 | 2.43 | First principle study | 59 |
| (CH3NH3)3Bi2Br9 | 2.5 | Collaborative solvent ligand-assisted re-precipitation method | 60 |
| (C3H5N2S)BiI4 | 1.78 | Solution growth using solvent layering approach | 61 |
| KBiI4·H2O | 1.76 | Slow evaporation method | 62 |
| 1,1-(1,n-Alkanediyl)bis(4-methylpyridinium)BiI5/H3C(C5H4N)(CH2)5(C5H4N)CH3 | 1.73 | Slow evaporation from solution | 63 |
| (C17H18N4)BiI5 | 1.59 | Solution growth | 64 |
| (AmV)BiI5, (AmV2+ = 4,4′-amino-bipyridinium) | 1.54 | Slow evaporation | 65 |
| [(Me2DABCO)2(Bi2I10)2], [DABCO = 1,4-diazabicyclo[2.2.2]octane] | 1.44 | Solvothermal with slow cooling | 66 |
The room temperature steady state photoluminescence (PL) spectra of thin films of β-(PPD)2Bi2I10, (PPD)[BiBr4]2·2H2O and (PPD)2BiCl7·2H2O are shown in Fig. 7(a–c). The PL of β-(PPD)2Bi2I10 shows a broad emission peak at 703 nm with a full width half maximum (FWHM) of 119 nm and a separation between the longest wavelength absorption peak and the emission maximum of 164 nm. Compound (PPD)[BiBr4]2·2H2O shows a broad PL emission around 477 nm with FWHM of 146 nm and a shift of 51 nm between absorption and emission. The PL of (PPD)2BiCl7·H2O has a peak around 443 nm with a FWHM of 130 nm and has a peak around 443 nm. The shift between absorption and emission maxima is 91 nm. Table 4 represents the characteristic parameters of β-(PPD)2Bi2I10, (PPD)[BiBr4]2·2H2O and (PPD)2BiCl7·2H2O deduced from UV-vis and SSPL. According to Karunadasa et al. the broad emission is attributed to emission from defect sites which lead to the self-trapping of excitons and the distortion of the crystal structure.67 This distortion in the crystal structure has a strong correlation with the broadband light emission.68,69 There are three aspects of the crystal structure which have been attributed to the broad emission spectra. First, the chloride ions in these materials cause more octahedral distortion due to their small size compared to the other halide anions. Second, the rigid PPD cation also enhances octahedral distortion and contributes to the width of the PL peaks. Third, more hydrogen bonds form between the octahedra and diammonium cations than for monoammonium cations in perovskite structures enables the stronger interaction, which is also responsible for self-trapping of excitons for broad emission in perovskite materials.70
| Compound | Absorption cutoff (nm) | Absorption peak (nm) | Band gap (eV) | PL peak (nm) | FWHM (nm) | Stokes shift (nm) | Stokes shift (eV) |
|---|---|---|---|---|---|---|---|
| β-(PPD)2Bi2I10 | 680 | 539 | 1.83 | 703 | 119 | 164 | 0.54 |
| (PPD)[BiBr4]2·2H2O | 480 | 426 | 2.64 | 477 | 146 | 51 | 0.31 |
| (PPD)2BiCl7·H2O | 395 | 352 | 2.93 | 443 | 130 | 91 | 0.72 |
A long exciton lifetime is desirable for efficient charge transfer and extraction. It is dependent on a number of parameters, but in general single crystals or films with a large grain size exhibit the highest charge carrier lifetimes. Materials which have optimum band gaps and long charge carrier lifetimes can result in photovoltaic devices with higher efficiency. However, the presence of defects or impurities quenches the light emission and is a major non-radiative loss pathway in perovskites. These defects are mostly due to surface halide vacancies.71 It has been shown that oxygen passivation can suppress these trap states efficiently.72,73 We have investigated oxygen passivation by spin-coating the films in the presence and absence of oxygen, i.e. outside and inside a nitrogen-filled glovebox. The time-resolved PL decays of spin-coated films of β-(PPD)2Bi2I10 with and without oxygen passivation are shown in Fig. 8(a). We found that the charge carrier lifetime of the film spin-coated in air is significantly enhanced compared to the film spin-coated inside the glovebox. Oxygen molecules are believed to bind strongly to surface halide vacancies and form superoxide in the presence of photo-excited electrons, which then leads to efficient passivation of these vacancy sites.74
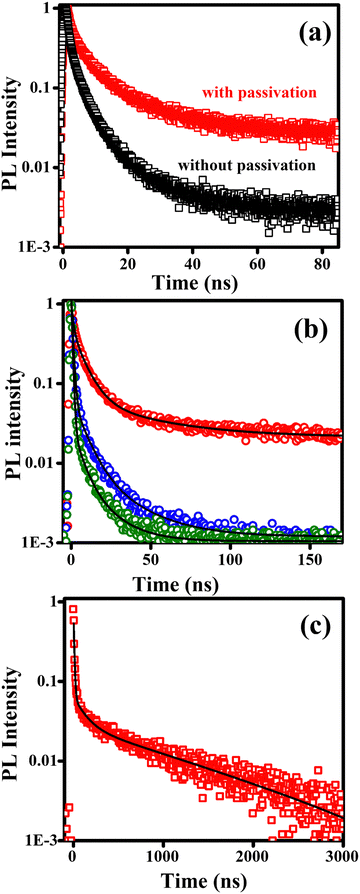 | ||
| Fig. 8 Time-resolved PL decays of bismuth containing perovskite thin films, following excitation at 379 nm by a picosecond pulsed laser. (a) Effect of oxygen passivation on β-(PPD)2Bi2I10. The red curve (“with passivation”) is a decay of a film sample spin-coated in air. The black curve (“without passivation”) is a decay of a film a sample spin-coated in a nitrogen glove box. (b) PL decays of oxygen-passivated films of (PPD)2BiCl7·H2O (blue), (PPD)[BiBr4]2·2H2O (olive) and β-(PPD)2Bi2I10 (red) Black lines are three-exponential fit to the experimental data using eqn (1). (c) PL decay of β-(PPD)2Bi2I10 measured up to 3 μs. Black line is three-exponential fit. | ||
PL decays of passivated films of the other materials are shown in Fig. 8b. For comparison, the PL decay of passivated film of β-(PPD)2Bi2I10 is also plotted in Fig. 8b. The PL of compounds (PPD)[BiBr4]2·2H2O and (PPD)2BiCl7·H2O decayed completely within 200 ns, whereas β-(PPD)2Bi2I10 showed a much slower PL decay after fast initial decay. In order to get information about different processes, the PL decays of compounds (PPD)[BiBr4]2·2H2O and (PPD)2BiCl7·H2O were fitted with eqn (1) and the fitting parameters are given in Table 5.
| R(t) = A1e(−t/τ1) + A2e(−t/τ2) + A3e(−t/τ3) | (1) |
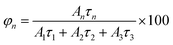 | (2) |
| Compound | τ 1 | τ 2 | τ 3 |
|---|---|---|---|
| β-(PPD)2Bi2I10 | 8.2 ns (11.3%) | 123.6 ns (10.0%) | 1260.4 ns (78.7%) |
| (PPD)[BiBr4]2·2H2O | 0.7 ns (65.7%) | 4.8 ns (21.2%) | 130.3 ns (13.1%) |
| (PPD)2BiCl7·H2O | 0.9 ns (57.1%) | 8.8 ns (32.5%) | 46.5 ns (10.4%) |
To facilitate an approximate comparison with prior work we calculated the average PL lifetime using the intensity weighted average of the fitted exponentials by using eqn (3) and the calculated average lifetimes are given in Table 5.
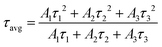 | (3) |
| Compound | τ average | Techniques | Ref. |
|---|---|---|---|
| (CH3NH3)PbI3 | 1500 ns | TR microwave conductance | 78 |
| (CH3NH3)PbI3 | 6.6 ns | TRPL-FTO/TiO2/perovskite | 79 |
| (CH3NH3)PbI3 | 390 ns | Transient photovoltage method | 80 |
| (CH3NH3)Pb(I0.71Br0.29)3 | 560 ns | Transient photovoltage method | 80 |
| (CH3NH3)Pb(I0.46Br0.54)3 | 1070 ns | Transient photovoltage method | 80 |
| (CH3NH3)PbI3 | 208 ns | TRPL-vapor-equilibrated method | 81 |
| (CH3NH3)PbClxI3−x | 113.9 ns | TRPL-vapor-equilibrated method | 81 |
| CsPbBr3 | 5.6 ns | TRPL-thiocyanate treatment | 82 |
| CsPbBr3 | 16.8 ns | TRPL-SiO2 core shell | 83 |
| CsPbBr3 | 3.9 ns | TRPL-pristine | 84 |
| CsPbBr3 | 6.5 ns | TRPL-in situ passivation | 84 |
| (CH5N2)PbBr3 | 180.3 ns | TRPL-rubidium doping | 85 |
| (CH3NH3)PbBr3 | 10.1 ns | TRPL-with benzoquinone | 86 |
| (CH3NH3)PbBr3 | 7.07 ns | TRPL | 87 |
| Cs4PbBr6 | 1.9 ps | Femtosecond TA | 88 |
| (PEA)2PbI4[(CH3NH3)PbI3]n−1 (1, 2, 3) | ∼150 ps | TRPL | 89 |
| Cs2InCl5·H2O | 4.2 μs | TRPL | 90 |
| (BPMA)2PbBr4 | 4.73 ms | Transient fluorescence | 91 |
| (C4N2H14)PbBr4 | 201 ns | Pressure-induced PL | 92 |
| (C4H14N2)2In2Br10 | 3.2 μs | TRPL | 93 |
| (BA)2(CH3NH3)4Pb5I16−10xBr10x | 3.47 ns | TRPL | 94 |
| (p-FC6H4C2H4NH3)2[PbI4] | 63 ns | TRPL | 95 |
| (DMEN)PbBr4 | 0.75 μs | TRPL | 96 |
| β-(PPD)2Bi2I10 | 1245 ns | TRPL | This work |
| (PPD)[BiBr4]2·2H2O | 120 ns | TRPL | This work |
| (PPD)2BiCl7·H2O | 31 ns | TRPL | This work |
4. Conclusions
We have successfully synthesized PPD bismuth halides using a slow cooling solution method. Here, three new perovskite materials were prepared, (PPD)BiI5, (PPD)[BiBr4]2·2H2O and (PPD)2BiCl7·H2O. They differed in their connectivity of bismuth halide octahedra and also in the ratio of organic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) inorganic cations. We also prepared β-(PPD)2Bi2I10. The long-term ambient stability and long charge carrier lifetimes suggests that these materials have potential applications in photovoltaic devices. Specifically, β-(PPD)2Bi2I10 has a small band gap (1.83 eV) and also exhibited long charge carrier lifetime of ∼1.2 μs, which is comparable to (CH3NH3)PbI3 and suggests that has potential for lead-free hybrid perovskite photovoltaic devices.
inorganic cations. We also prepared β-(PPD)2Bi2I10. The long-term ambient stability and long charge carrier lifetimes suggests that these materials have potential applications in photovoltaic devices. Specifically, β-(PPD)2Bi2I10 has a small band gap (1.83 eV) and also exhibited long charge carrier lifetime of ∼1.2 μs, which is comparable to (CH3NH3)PbI3 and suggests that has potential for lead-free hybrid perovskite photovoltaic devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
ZS acknowledges Higher Education Commission (HEC) of Pakistan for indigenous PhD Fellowship Phase-II, Batch II, 2013, PIN 213-66018-2PS2-127 and International Research Support Initiative Programme (IRSIP). She also acknowledges a British council of Pakistan for Pakistan Scottish PhD Research Travel Grants for Women 2017–18. JLP thanks the University of St Andrews for funding and the Carnegie Trust for a Research Incentive Grant (RIG008653). We thank EPSRC for funding (EP/P007821/1).References
- K. G. Reddy, T. G. Deepak, G. S. Anjusree, S. Thomas, S. Vadukumpully, K. R. V. Subramanian, S. V. Nair and A. S. Nair, Phys. Chem. Chem. Phys., 2014, 16, 6838–6858 RSC.
- A. Hagfeldt, G. Boschloo, L. C. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS.
- F. Bella, Electrochim. Acta, 2015, 175, 151–161 CrossRef CAS.
- P. Nagarjuna, K. Narayanaswamy, T. Swetha, G. H. Rao, S. P. Singh and G. D. Sharma, Electrochim. Acta, 2015, 151, 21–26 CrossRef CAS.
- O. Y. Gong, Y. Kim, D. H. Kim, G. S. Han, S. Jeong and H. S. Jung, ACS Energy Lett., 2020, 5, 1032–1034 CrossRef CAS.
- J. L. Yang, Y. Yu, L. Y. Zeng, Y. T. Li, Y. Q. Pang, F. Q. Huang and B. L. Lin, J. Nanomater., 2017, 2017, 1640965 Search PubMed.
- P. P. Boix, S. Agarwala, T. M. Koh, N. Mathews and S. G. Mhaisalkar, J. Phys. Chem. Lett., 2015, 6, 898–907 CrossRef CAS PubMed.
- L. B. Qiu, L. K. Ono and Y. B. Qi, Mater. Today Energy, 2018, 7, 169–189 CrossRef.
- T. Leijtens, R. Prasanna, A. Gold-Parker, M. F. Toney and M. D. McGehee, ACS Energy Lett., 2017, 2, 2159–2165 CrossRef CAS.
- I. Kopacic, B. Friesenbichler, S. F. Hoefler, B. Kunert, H. Plank, T. Rath and G. Trimmel, ACS Appl. Energy Mater., 2018, 1, 343–347 CrossRef CAS.
- H. C. Sansom, G. F. S. Whitehead, M. S. Dyer, M. Zanella, T. D. Manning, M. J. Pitcher, T. J. Whittles, V. R. Dhanak, J. Alaria, J. B. Claridge and M. J. Rosseinsky, Chem. Mater., 2017, 29, 1538–1549 CrossRef CAS.
- Q. Zhang, F. Hao, J. B. Li, Y. Y. Zhou, Y. X. Wei and H. Lin, Sci. Technol. Adv. Mater., 2018, 19, 425–442 CrossRef CAS PubMed.
- S. Oz, J. C. Hebig, E. W. Jung, T. Singh, A. Lepcha, S. Olthof, J. Flohre, Y. J. Gao, R. German, P. H. M. van Loosdrecht, K. Meerholz, T. Kirchartz and S. Mathur, Sol. Energy Mater. Sol. Cells, 2016, 158, 195–201 CrossRef.
- C. S. Ni, G. Hedley, J. Payne, V. Svrcek, C. McDonald, L. K. Jagadamma, P. Edwards, R. Martin, G. Jain, D. Carolan, D. Mariotti, P. Maguire, I. Samuel and J. Irvine, Nat. Commun., 2017, 8 Search PubMed.
- W. X. Chai, L. M. Wu, J. Q. Li and L. Chen, Inorg. Chem., 2007, 46, 1042–1044 CrossRef CAS.
- X. W. Tong, W. Y. Kong, Y. Y. Wang, J. M. Zhu, L. B. Luo and Z. H. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 18977–18985 CrossRef CAS PubMed.
- A. J. Lehner, D. H. Fabini, H. A. Evans, C.-A. Hébert, S. R. Smock, J. Hu, H. Wang, J. W. Zwanziger, M. L. Chabinyc and R. Seshadri, Chem. Mater., 2015, 27, 7137–7148 CrossRef CAS.
- J.-J. Wu, Y. Guo, W.-D. Yao, W.-L. Liu and S.-P. Guo, Dalton Trans., 2022, 51, 4878–4883 RSC.
- X. Li, B. Traoré, M. Kepenekian, L. Li, C. C. Stoumpos, P. Guo, J. Even, C. Katan and M. G. Kanatzidis, Chem. Mater., 2021, 33, 6206–6216 CrossRef CAS.
- Z. Y. Deng, F. X. Wei, S. J. Sun, G. Kieslich, A. K. Cheetham and P. D. Bristowe, J. Mater. Chem. A, 2016, 4, 12025–12029 RSC.
- H. Dammak, A. Yangui, S. Triki, Y. Abid and H. Feki, J. Lumin., 2015, 161, 214–220 CrossRef CAS.
- C. C. Stoumpos, D. H. Cao, D. J. Clark, J. Young, J. M. Rondinelli, J. I. Jang, J. T. Hupp and M. G. Kanatzidis, Chem. Mater., 2016, 28, 2852–2867 CrossRef CAS.
- S. D. Stranks and H. J. Snaith, Nat. Nanotechnol., 2015, 10, 391–402 CrossRef CAS PubMed.
- C. Hrizi, A. Trigui, Y. Abid, N. Chniba-Boudjada, P. Bordet and S. Chaabouni, J. Solid State Chem., 2011, 184, 3336–3344 CrossRef CAS.
- C. Hrizi, A. Samet, Y. Abid, S. Chaabouni, M. Fliyou and A. Koumina, J. Mol. Struct., 2011, 992, 96–101 CrossRef CAS.
- C. Hrizi, C. Chaker and S. Chaabouni, Ionics, 2011, 17, 545–553 CrossRef CAS.
- T. A. Shestimerova, N. A. Golubev, N. A. Yelavik, M. A. Bykov, A. V. Grigorieva, Z. Wei, E. V. Dikarev and A. V. Shevelkov, Cryst. Growth Des., 2018, 18, 2572–2578 CrossRef CAS.
- L. Dobrzycki and K. Wozniak, CrystEngComm, 2008, 10, 577–589 RSC.
- I. C. Smith, E. T. Hoke, D. Solis-Ibarra, M. D. McGehee and H. I. Karunadasa, Angew. Chem., Int. Ed., 2014, 53, 11232–11235 CrossRef CAS.
- Z. K. Tan, R. S. Moghaddam, M. L. Lai, P. Docampo, R. Higler, F. Deschler, M. Price, A. Sadhanala, L. M. Pazos, D. Credgington, F. Hanusch, T. Bein, H. J. Snaith and R. H. Friend, Nat. Nanotechnol., 2014, 9, 687–692 CrossRef CAS PubMed.
- W. Q. Liao, Y. Zhang, C. L. Hu, J. G. Mao, H. Y. Ye, P. F. Li, S. P. D. Huang and R. G. Xiong, Nat. Commun., 2015, 6, 7338 CrossRef PubMed.
- B. R. Sutherland and E. H. Sargent, Nat. Photonics, 2016, 10, 295–302 CrossRef CAS.
- X. T. Li, J. Hoffman, W. J. Ke, M. Chen, H. Tsai, W. Y. Nie, A. D. Mohite, M. Kepenekian, C. Katan, J. Even, M. R. Wasielewski, C. C. Stoumpos and M. G. Kanatzidis, J. Am. Chem. Soc., 2018, 140, 12226–12238 CrossRef CAS PubMed.
- CrystalClear-SM Expert v2.1., Rigaku Americas, The Woodlands, Texas, USA, and Rigaku Corporation, Tokyo, Japan, 2015 Search PubMed.
- CrysAlisPro v1.171.38.46., Rigaku Oxford Diffraction, Rigaku Corporation, Oxford, UK, 2015 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849–854 CrossRef CAS.
- Y. Y. Li, C. K. Lin, G. L. Zheng, Z. Y. Cheng, H. You, W. D. Wang and J. Lin, Chem. Mater., 2006, 18, 3463–3469 CrossRef CAS.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- R. M. Ibberson, O. Moze and C. Petrillo, Mol. Phys., 1992, 76, 395–403 CrossRef CAS.
- P. S. Whitfield, N. Herron, W. E. Guise, K. Page, Y. Q. Cheng, I. Milas and M. K. Crawford, Sci. Rep., 2016, 6, 35685 CrossRef CAS PubMed.
- K. Robinson, G. V. Gibbs and P. H. Ribbe, Science, 1971, 172, 567–570 CrossRef CAS PubMed.
- X. Chen, Y. Myung, A. Thind, Z. N. Gao, B. Yin, M. K. Shen, S. B. Cho, P. F. Cheng, B. Sadtler, R. Mishra and P. Banerjee, J. Mater. Chem. A, 2017, 5, 24728–24739 RSC.
- M. R. Filip, G. E. Eperon, H. J. Snaith and F. Giustino, Nat. Commun., 2014, 5, 5757 CrossRef CAS PubMed.
- W. Shockley and H. J. Queisser, J. Appl. Phys., 1961, 32, 510 CrossRef CAS.
- J. K. Pious, A. Katre, C. Muthu, S. Chakraborty, S. Krishna and V. C. Nair, Chem. Mater., 2019, 31, 1941–1945 CrossRef CAS.
- Y. Bekenstein, J. C. Dahl, J. M. Huang, W. T. Osowiecki, J. K. Swabeck, E. M. Chan, P. D. Yang and A. P. Alivisatos, Nano Lett., 2018, 18, 3502–3508 CrossRef CAS PubMed.
- D. Bartesaghi, A. H. Slavney, M. C. Gelvez-Rueda, B. A. Connor, F. C. Grozema, H. I. Karunadasa and T. J. Savenije, J. Phys. Chem. C, 2018, 122, 4809–4816 CrossRef CAS PubMed.
- E. T. McClure, M. R. Ball, W. Windl and P. M. Woodward, Chem. Mater., 2016, 28, 1348–1354 CrossRef CAS.
- F. X. Wei, Z. Y. Deng, S. J. Sun, F. H. Zhang, D. M. Evans, G. Kieslich, S. Tominaka, M. A. Carpenter, J. Zhang, P. D. Bristowe and A. K. Cheetham, Chem. Mater., 2017, 29, 1089–1094 CrossRef CAS.
- F. X. Wei, Z. Y. Deng, S. J. Sun, F. Xie, G. Kieslich, D. M. Evans, M. A. Carpenter, P. D. Bristowe and A. K. Cheetham, Mater. Horiz., 2016, 3, 328–332 RSC.
- M. Q. Li, Y. Q. Hu, L. Y. Bi, H. L. Zhang, Y. Y. Wang and Y. Z. Zheng, Chem. Mater., 2017, 29, 5463–5467 CrossRef CAS.
- W. C. Zhang, K. W. Tao, C. M. Ji, Z. H. Sun, S. G. Han, J. Zhang, Z. Y. Wu and J. H. Luo, Inorg. Chem., 2018, 57, 4239–4243 CrossRef CAS PubMed.
- M. Abulikemu, S. Ould-Chikh, X. H. Miao, E. Alarousu, B. Murali, G. O. N. Ndjawa, J. Barbe, A. El Labban, A. Amassiana and S. Del Gobbo, J. Mater. Chem. A, 2016, 4, 12504–12515 RSC.
- B. W. Park, B. Philippe, X. L. Zhang, H. Rensmo, G. Boschloo and E. M. J. Johansson, Adv. Mater., 2015, 27, 6806–6813 CrossRef CAS PubMed.
- Y. Kim, Z. Y. Yang, A. Jain, O. Voznyy, G. H. Kim, M. Liu, L. N. Quan, F. P. G. de Arquer, R. Comin, J. Z. Fan and E. H. Sargent, Angew. Chem., Int. Ed., 2016, 55, 9586–9590 CrossRef CAS PubMed.
- H. Y. Zhang, Z. H. Wei, P. F. Li, Y. Y. Tang, W. Q. Liao, H. Y. Ye, H. Cai and R. G. Xiong, Angew. Chem., Int. Ed., 2018, 57, 526–530 CrossRef CAS PubMed.
- S. Zhao, K. Yamamoto, S. S. Iikubo, S. Hayase and T. L. Ma, J. Phys. Chem. Solids, 2018, 117, 117–121 CrossRef CAS.
- M. Y. Leng, Z. W. Chen, Y. Yang, Z. Li, K. Zeng, K. H. Li, G. D. Niu, Y. S. He, Q. C. Zhou and J. Tang, Angew. Chem., Int. Ed., 2016, 55, 15012–15016 CrossRef CAS PubMed.
- T. Li, Q. Wang, G. S. Nichol, C. A. Morrison, H. Han, Y. Hu and N. Robertson, Dalton Trans., 2018, 47, 7050–7058 RSC.
- N. A. Yelovik, A. V. Mironov, M. A. Bykov, A. N. Kuznetsov, A. V. Grigorieva, Z. Wei, E. V. Dikarev and A. V. Shevelkov, Inorg. Chem., 2016, 55, 4132–4140 CrossRef CAS PubMed.
- V. Y. Kotov, A. B. Ilyukhin, A. A. Korlyukov, A. F. Smol’yakovcd and S. A. Kozyukhin, New J. Chem., 2018, 42, 6354–6363 RSC.
- V. Y. Kotov, A. B. Ilyukhin, A. A. Sadovnikov, K. P. Birin, N. P. Simonenko, H. T. Nguyen, A. E. Baranchikov and S. A. Kozyukhin, Mendeleev Commun., 2017, 27, 271–273 CrossRef CAS.
- A. Skorokhod, N. Mercier, M. Allain, M. Manceau, C. Katan and M. Kepenekian, Inorg. Chem., 2021, 60(22), 17123–17131 CrossRef CAS PubMed.
- Z.-P. Zhang, Q.-Y. Feng, Y.-L. Wei, Z.-Y. Gao, Z.-W. Wang and Y.-M. Wang, J. Cluster Sci., 2018, 29, 725–735 CrossRef CAS.
- M. D. Smith, A. Jaffe, E. R. Dohner, A. M. Lindenberg and H. I. Karunadasa, Chem. Sci., 2017, 8, 4497–4504 RSC.
- O. Nazarenko, M. R. Kotyrba, S. Yakunin, M. Aebli, G. Raino, B. M. Benin, M. Worle and M. V. Kovalenko, J. Am. Chem. Soc., 2018, 140, 3850–3853 CrossRef CAS PubMed.
- M. D. Smith and H. I. Karunadasa, Acc. Chem. Res., 2018, 51, 619–627 CrossRef CAS PubMed.
- S. Ahmad, P. Fu, S. W. Yu, Q. Yang, X. Liu, X. C. Wang, X. L. Wang, X. Guo and C. Li, Joule, 2019, 3, 794–806 CrossRef CAS.
- S. D. Stranks, R. L. Z. Hoye, D. W. Di, R. H. Friend and F. Deschler, Adv. Mater., 2019, 31, 1803336 CrossRef CAS PubMed.
- N. Aristidou, C. Eames, I. Sanchez-Molina, X. N. Bu, J. Kosco, M. S. Islam and S. A. Haque, Nat. Commun., 2017, 8, 15218 CrossRef PubMed.
- R. Brenes, C. Eames, V. Bulovic, M. S. Islam and S. D. Stranks, Adv. Mater., 2018, 30, 1706208 CrossRef PubMed.
- T. T. Xuan, S. Q. Lou, J. J. Huang, L. Y. Cao, X. F. Yang, H. L. Li and J. Wang, Nanoscale, 2018, 10, 9840–9844 RSC.
- H. Y. Hsu, L. Ji, M. S. Du, J. Zhao, E. T. Yu and A. J. Bard, J. Phys. Chem. C, 2016, 120, 19890–19895 CrossRef CAS.
- L. F. Chao, T. T. Niu, Y. D. Xia, X. Q. Ran, Y. H. Chen and W. Huang, J. Phys. Chem. Lett., 2019, 10, 1173–1179 CrossRef CAS PubMed.
- A. H. Slavney, T. Hu, A. M. Lindenberg and H. I. Karunadasa, J. Am. Chem. Soc., 2016, 138, 2138–2141 CrossRef CAS PubMed.
- Y. Bi, E. M. Hutter, Y. J. Fang, Q. F. Dong, J. S. Huang and T. J. Savenije, J. Phys. Chem. Lett., 2016, 7, 923–928 CrossRef CAS PubMed.
- A. B. Huang, J. T. Zhu, J. Y. Zheng, Y. Yu, Y. Liu, S. W. Yang, S. H. Bao, L. Lei and P. Jin, Nanotechnology, 2017, 28, 055403 CrossRef PubMed.
- D. Kiermasch, P. Rieder, K. Tvingstedt, A. Baumann and V. Dyakonov, Sci. Rep., 2016, 6, 39333 CrossRef CAS PubMed.
- B. S. Tosun and H. W. Hillhouse, J. Phys. Chem. Lett., 2015, 6, 2503–2508 CrossRef CAS PubMed.
- B. A. Koscher, J. K. Swabeck, N. D. Bronstein and A. P. Alivisatos, J. Am. Chem. Soc., 2017, 139, 6566–6569 CrossRef CAS PubMed.
- Q. X. Zhong, M. H. Cao, H. C. Hu, D. Yang, M. Chen, P. L. Li, L. Z. Wu and Q. Zhang, ACS Nano, 2018, 12, 8579–8587 CrossRef CAS PubMed.
- Y. Wu, C. T. Wei, X. M. Li, Y. L. Li, S. C. Qiu, W. Shen, B. Cai, Z. G. Sun, D. D. Yang, Z. T. Deng and H. B. Zeng, ACS Energy Lett., 2018, 3, 2030–2037 CrossRef CAS.
- Y. F. Shi, J. Xi, T. Lei, F. Yuan, J. F. Dai, C. X. Ran, H. Dong, B. Jiao, X. Hou and Z. X. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 9849–9857 CrossRef CAS PubMed.
- G. H. Ahmed, J. Yin, R. Bose, L. Sinatra, E. Alarousu, E. Yengel, N. M. AlYami, M. I. Saidaminov, Y. Zhang, M. N. Hedhili, O. M. Bakr, J. L. Bredas and O. F. Mohammed, Chem. Mater., 2017, 29, 4393–4400 CrossRef CAS.
- Z. Siddique, J. L. Payne, J. T. S. Irvine, L. K. Jagadamma, Z. Akhter, I. D. W. Samuel and A. Iqbal, J. Mater. Sci.: Mater. Electron., 2020, 31, 19415–19428 CrossRef.
- J. Yin, P. Maity, M. De Bastiani, I. Dursun, O. M. Bakr, J. L. Bredas and O. F. Mohammed, Sci. Adv., 2017, 3, 1701793 CrossRef PubMed.
- W. Peng, J. Yin, K. T. Ho, O. Ouellette, M. De Bastiani, B. Murali, O. El Tall, C. Shen, X. H. Miao, J. Pan, E. Alarousu, J. H. He, B. S. Ooi, O. F. Mohammed, E. Sargent and O. M. Bakr, Nano Lett., 2017, 17, 4759–4767 CrossRef CAS PubMed.
- X. Liu, X. Xu, B. Li, Y. Liang, L. Qi, H. Jiang and D. Xu, CCS Chem., 2020, 2, 216–224 CrossRef CAS.
- H. W. Hu, D. M. Zhao, Y. Gao, X. F. Qiao, T. Salim, B. B. Chen, E. E. M. Chia, A. C. Grimsdale and Y. M. Lam, Chem. Mater., 2019, 31, 2597–2602 CrossRef CAS.
- Y. Q. Wang, S. H. Guo, H. Luo, C. K. Zhou, H. R. Lin, X. D. Ma, Q. Y. Hu, M. H. Du, B. W. Ma, W. G. Yang and X. J. Lu, J. Am. Chem. Soc., 2020, 142, 16001–16006 CrossRef CAS PubMed.
- L. Zhou, J. F. Liao, Z. G. Huang, J. H. Wei, X. D. Wang, H. Y. Chen and D. B. Kuang, Angew. Chem., Int. Ed., 2019, 58, 15435–15440 CrossRef CAS PubMed.
- F. Han, W. Y. Yang, H. Li and L. Zhu, Nanoscale Res. Lett., 2020, 15, 194 CrossRef CAS PubMed.
- Q. Zhou, L. S. Liang, J. J. Hu, B. B. Cao, L. K. Yang, T. J. Wu, X. Li, B. Zhang and P. Gao, Adv. Energy Mater., 2019, 9, 1802593 Search PubMed.
- L. L. Mao, Y. L. Wu, C. C. Stoumpos, M. R. Wasielewski and M. G. Kanatzidis, J. Am. Chem. Soc., 2017, 139, 5210–5215 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2180531–2180533. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2tc02601e |
| This journal is © The Royal Society of Chemistry 2023 |