Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metallic to half-metallic transition driven by pressure and anion composition in niobium oxyfluoride†
Eliza K.
Dempsey
 and
James
Cumby
and
James
Cumby
 *
*
School of Chemistry, University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ, UK. E-mail: james.cumby@ed.ac.uk
First published on 9th January 2023
Abstract
Half-metallic materials play a key role in the development of spintronics, but few suitable materials are known. Continuously varying the ratio of two anions in a single material offers one route to enhance half-metallic behaviour. Here we report the effect of varying the oxide-fluoride ratio in ReO3-type niobium oxyfluoride NbO2−xF1+x using ab initio calculations. Increasing the fluorine content from NbO2F to NbF3 leads to a transition from semiconducting, through metallic, to half-metallic behaviour as x increases. The effect of pressure on this behaviour is also investigated, finding a transition from cubic to rhombohedral symmetry for all compositions below a maximum transition pressure of 5 GPa for x = 0.35. Additionally, we reveal that compositions close to NbF3 are expected to retain rhombohedral symmetry under ambient pressure. The structural phase transition and electronic behaviour are independent for most compositions except those close to NbO1.65F1.35. Here, we discover that the application of moderate pressure can drive the material from metallic to half-metallic, offering a new possibility for controlling spin currents.
I. Introduction
The next generation of computational devices will require novel materials to meet the technological demands of data processing and storage. Spintronics is concerned with utilising the electron spin degree of freedom, in addition to electron charge, for the development of smaller components and increased data-processing speed whilst also decreasing overall power consumption.1 The phenomenon of half-metallicity arises in some magnetic materials where the material is conducting for one electron spin direction, either up or down, whilst semiconducting or insulating in the other. This concept was first introduced in 1983 and the spin control properties of these materials play a crucial role in the field of spintronics.2 In spite of this long history, most existing half-metals do not meet the needs of functional spintronics devices, therefore better half-metallic candidates need to be developed.Atomic doping is one strategy known to induce half-metallicity,3–5 improve thermal stability,6 increase conductivity7 and increase magnetoresistance.8 Solid solutions — where dopant composition can be continuously varied between two parent compounds – provide even greater flexibility in tuning physical properties. One flexible approach towards solid solutions are mixed-anion materials, containing multiple anions in a single phase. This approach offers a route towards tuning composition while maintaining the cationic interactions important for many properties, however, the number of such materials is currently limited.9 Niobium oxyfluorides are one particular class of mixed-anionic materials of interest for functional applications: the variable oxidation state of niobium; similar ionic radii of oxygen and fluorine; and higher covalency of Nb–O bonds cf. Nb–F bonds leads to direct control of physical properties through variable oxygen:fluorine ratios. These materials are built around corner-sharing Nb(O,F)6 octahedra, with a number of shear-related phases also known.10–12 The simplest structure observed in the family is that of cubic ReO3 (Fig. 1a) and this structure has been reported across the entire NbO2−xF1+x solid solution, ranging from x = 0 (NbVO2F) to x = 2 (NbIIIF3).13–15 Such ReO3-type (oxy)fluorides are of wide interest, with a range of properties including lithium incorporation for battery applications16,17 or negative thermal expansion as in ScF3.18 The latter property is linked to a cubic to rhombohedral transition apparent in many ReO3-type trifluorides,19–22 caused by octahedral rotation around the [111] crystallographic direction (a−a−a− in Glazer's tilt notation).23 Such a transition has also been observed in NbO2F under a relatively low hydrostatic pressure of 0.47 GPa (Fig. 1b).24
 | ||
Fig. 1 Structures reported for NbO2−xF1+x compositions showing (a) cubic (Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) and (b) rhombohedral (R m) and (b) rhombohedral (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c) polymorphs. Structures are aligned to show the relationship between unit cells (shown as black lines). Nb shown as red spheres/polyhedra, O/F as blue spheres (figures created using VESTA v3.5.7).25 c) polymorphs. Structures are aligned to show the relationship between unit cells (shown as black lines). Nb shown as red spheres/polyhedra, O/F as blue spheres (figures created using VESTA v3.5.7).25 | ||
Electronically, NbF3 has been predicted to be half-metallic,26 with a large minority spin band gap (8.9 eV) that would minimise spin flipping within a real spintronic device.27 In contrast, NbO2F is known to be a wide band gap semiconductor with a band gap of 3.1 eV.28 However, there is no information on the electronic structure of the intermediate solid solutions. It is therefore unclear how half-metallicity arises in NbF3 and also how the band structure is modified to reach semiconducting NbO2F. Here, we report electronic structure calculations across the NbO2−xF1+x solid solution to understand the relationship between electronic structure, half-metallicity and anionic composition. In addition, we describe the effect of anionic composition on the cubic to rhombohedral phase transition under applied pressure.
II. Methods
All first-principles calculations were performed using a DFT+U approach implemented in CASTEP (v18.1) by the rotationally invariant approach proposed by Dudarev et al.29,30 The generalised gradient approximation (GGA) Perdew–Burke–Ernzehof (PBE) exchange–correlation functional was used for all calculations. A Hubbard U parameter of 3.5 eV was chosen to match, as closely as possible, the previously predicted NbF3 minority spin band gap (Fig. S1, ESI†). The U correction was applied to the niobium d orbitals. The electronic ground state was found by the density-mixing algorithm with a mixing amplitude of 0.6 and 1.5 for the charge and spin density respectively. The default CASTEP (v18.1) ultrasoft pseudopotentials were used for oxygen and fluorine whilst the Materials Studio pseudopotential was used for niobium. The electron wave functions were expanded as plane waves up to a cut-off energy of 850 eV. For the sampling over the Brillouin zone, a Monkhorst–Pack31k-point grid spacing of 0.03 Å−1 was used. The cut-off energy and k-point grid spacing were chosen so that the final energy converged to ±4 meV (Fig. S2, ESI†). Geometry optimisation and density of states calculations were performed using single primitive cubic (ICSD #25596) and rhombohedral (ICSD #280539) cells.24,32–34 To examine antiferromagnetic ordering, single point energy calculations were performed for a cubic 2 × 2 × 2 supercell. Mixed oxygen/fluorine calculations were performed by applying the virtual crystal approximation (VCA) so that anion sites are treated as a stoichiometric mixture of oxygen and fluorine potentials. External pressure values were specified as a tensor within the CASTEP geometry optimisations, with atomic positions and cell geometry optimised with the constraint of either cubic or rhombohedral symmetry. Density of states processing and optical band gap calculations were performed using OptaDOS (v1.2).35 Analysis of octahedral distortion was performed by fitting of a minimum bounding ellipsoid using the PIEFACE (v1.1.0) software package.36III. Results and discussion
A. Magnetic ordering
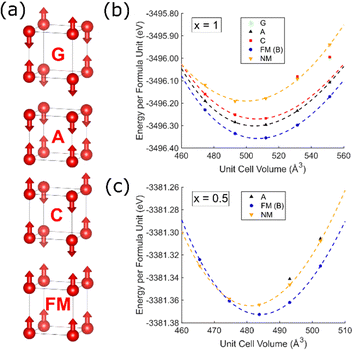
Self-consistent energy calculations at different unit cell volumes for NbOF2 and NbO1.5F1.5 with different magnetic configurations (Fig. 2) were fitted to a third order Birch–Murnaghan equation of state.37 These results indicate that a ferromagnetic (FM) ordering of spins is significantly more stable for both compositions by approximately 0.06 eV and 0.01 eV per formula unit, respectively. This contradicts the previous assertion that FM ordering will be lost on the addition of oxygen to NbF3,27 although it is unclear whether these previous results relate to surface calculations. Many of the calculations show difficult convergence to the desired magnetic state on account of a shallow potential energy landscape and low magnetic moments; only the FM calculation could be reliably converged at all volumes for both compositions. In particular, antiferromagnetic (AFM) calculations for NbO1.5F1.5 (Fig. 2c) often resulted in non-magnetic occupations of Nb t2g orbitals (Fig. S3, ESI†). Despite this, all AFM or non-magnetic states obtained are significantly higher in energy than the FM state except when extremely compressed.B. Electronic band structure
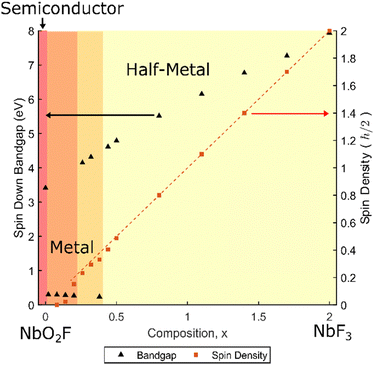
Representative band structures and partial density of states (PDOS) results for NbO2−xF1+x are shown in Fig. 3. For each composition x, the cubic ReO3 lattice parameter was optimised enforcing FM ordering. In each case, the minority spin band gap and overall spin density have been extracted and are summarised in Fig. 4. NbO2F is correctly predicted to be a semiconductor with a band gap of 3.4 eV, in close agreement to the experimental value of 3.1 eV.28 The PDOS for NbO2F (Fig. 3a) shows the valence band is dominated by the O 2p states whilst Nb 3d states dominate the conduction band. Comparing this result with the PDOS for NbO1.8F1.2 (Fig. 3b), the effect of increasing the fluorine content is to reduce Nb(V) towards Nb(IV), donating electrons into the conduction band. This leads to a transition from semiconductor to metal for small x.As well as inducing a metallic phase, increasing the fluorine content x increases the number of unpaired electrons per niobium, resulting in an increase in overall spin density (Fig. 4). This leads to an increase in the energy difference between the Nb 3d majority and minority spin bands as the majority band occupation is increasingly stabilised, until a transition to a half-metallic phase occurs between x = 0.20 and x = 0.44. There is some uncertainty in this transition with several borderline half-metal/metal results, indicated by the overlapping shaded area in Fig. 4. This uncertainty reflects the proximity in energy of metallic and half-metallic states in this region, and the corresponding difficulties in achieving a robust self-consistent minimum energy. The splitting between the minority and majority Nb 3d spin states can be seen in Fig. 3c showing the half-metallic state for x = 1.1. Furthermore, Fig. 4 shows an increase in the minority spin band gap as x increases corresponding to this splitting. The dispersive nature of the conduction bands (Fig. 3a) indicate this half-metallic phase should have good conductivity, desirable for spintronics applications.
The dashed line in Fig. 4 corresponds to the number of unpaired electrons per unit cell and therefore the maximum spin density. From this, it is clear the spin density is equal to the number of unpaired Nb electrons for the half-metallic phase. This is due to the presence of only majority spin states close to the Fermi level which leads to a theoretical 100% spin polarisation. However, as the splitting between the spin states decreases and the phase becomes metallic there are also minority spin states at the Fermi level (Fig. 3b). This results in a deviation from the ideal 100% spin polarisation, eventually resulting in a loss of magnetic ordering around x = 0.14.
The electron density distribution for a cross-section of the NbF3 and NbO2F unit cells is shown in Fig. 5. As expected, the charge density is more delocalised in NbO2F due to the more covalent character of the Nb–O bonds. The highly electronegative fluoride, however, results in a more localised charge density for NbF3.38 The increasing covalency as x decreases corresponds to an increasing anionic contribution to the states near the Fermi level, driving the reduction in minority spin band gap.
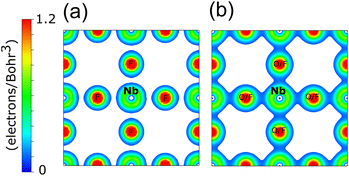 | ||
| Fig. 5 The DFT calculated electron density for (a) cubic NbF3 and (b) cubic NbO2F, both for the (100) plane with a minimum isosurface of 0.1 electrons/Bohr3. | ||
C. Crystal structure under external pressure
Given the relatively low-pressure transition to a rhombohedral structure for NbO2F,39 DFT+U calculations were also performed at elevated external pressures using a rhombohedral unit cell (Fig. 1b) in order to allow additional degrees of freedom for octahedral tilting and distortion during the geometry optimisations. The quality of the rhombohedral geometry optimisations was evaluated by comparison with experimental results for NbO2F (Fig. S4, ESI†).24,39 For the cubic structure at ambient pressure there is good agreement between the experimental and computational results. However, at elevated pressure, the lattice parameter is slightly underestimated, and the cell angle overestimated on the order of 2°.Comparison of the final enthalpy of these optimisations indicate all compositions are predicted to undergo a cubic to rhombohedral phase transition with pressure, while NbO0.3F2.7 and NbF3 are both rhombohedral at ambient pressure. Transition to the rhombohedral structure occurs by a tilting mechanism of the rigid octahedra which can be quantified by an octahedral rotation,  , where y is the anion fractional coordinate.40 The results of the geometry optimisations in terms of this octahedral rotation are shown for each composition and pressure in Fig. 6a. For higher fluorine compositions, the octahedra also experience a trigonal distortion with equal length Nb–(O/F) bonds no longer oriented at 90° to each other. This distortion can be quantified by fitting a minimum bounding ellipsoid to the octahedra and calculating the standard deviation of its principal radii, σ(R), the results of which are shown in Fig. 6b.36 An attempt to determine the transition pressure using a common tangent approach (Fig. S5 and S6, ESI†) produced large errors at higher x compositions due to the low curvature of the rhombohedral energy against volume, therefore ω was used to identify the transition pressures shown in Fig. 6.
, where y is the anion fractional coordinate.40 The results of the geometry optimisations in terms of this octahedral rotation are shown for each composition and pressure in Fig. 6a. For higher fluorine compositions, the octahedra also experience a trigonal distortion with equal length Nb–(O/F) bonds no longer oriented at 90° to each other. This distortion can be quantified by fitting a minimum bounding ellipsoid to the octahedra and calculating the standard deviation of its principal radii, σ(R), the results of which are shown in Fig. 6b.36 An attempt to determine the transition pressure using a common tangent approach (Fig. S5 and S6, ESI†) produced large errors at higher x compositions due to the low curvature of the rhombohedral energy against volume, therefore ω was used to identify the transition pressures shown in Fig. 6.
Previous experimental work reported NbF3 as adopting a cubic ReO3-type structure,14 however the present results suggest a rhombohedral phase is more stable. One explanation may be that the synthesised NbF3 actually contained some quantity of oxygen. The method used for stoichiometric analysis of the final products is unspecified although a titration approach was likely used and could be inaccurate.14 It has also been suggested that NbF3 cannot be synthesised without some quantity of oxygen being present.41
From these results the transition pressure obtains a maximum at approximately x = 0.35, close to one additional electron per three niobium(V) ions. Similar cubic to rhombohedral transitions in other ReO3-type structures are typically governed by cationic repulsion; the reduction in Nb–Nb distance observed in the rhombohedral structure is unfavourable for highly charged cations.42–44 As such, the transition pressure is expected to decrease with decreasing cation charge, as is observed for x > 0.35. For x < 0.35 a different energetic contribution must therefore drive the increase in transition pressure with x. We attribute this to the greater covalency of Nb–O bonds cf. Nb–F bonds. The increased covalency drives hybridisation between O 2p and 2s orbitals, which in turn causes the Nb–O–Nb bond angle to shift away from 180° and oxygen lone pairs to occupy the vacant space within the ReO3 framework. The lone pairs can be seen from electron localisation function (ELF) calculations (Fig. S7, ESI†). This effect is strong enough to reduce the transition pressure for small x, in spite of the significant electrostatic repulsion between adjacent Nb(V) ions.
D. Electronic structures under pressure
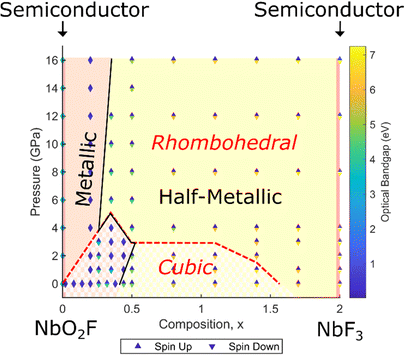
In order to determine the effect of the structural transition and increasing pressure on the electronic structure, a full composition-pressure phase diagram for both cubic and rhombohedral geometries is presented in Fig. 7 (individual cubic and rhombohedral results are shown in Fig. S8, ESI†). The transition from semiconducting NbO2F to a metallic phase on doping with fluorine is maintained at all pressures, as is the metallic to half-metallic transition with x. NbF3 is predicted to be semiconducting for the most stable rhombohedral phase (in contrast to previous cubic predictions).For NbO2F, the structural transition to a rhombohedral phase with pressure has no significant impact on the electronic properties. The phase remains semiconducting with a large band gap between the oxygen 2p and niobium 3d states (Fig. S9, ESI†), although the band gap does show a slight increase (less than 1 eV) with pressure. Additionally, the octahedral splitting in the Nb 3d band is more pronounced for the rhombohedral structure. These effects are due to narrowing of the 3d bands due to the octahedral tilting, caused by the reduced overlap efficiency between niobium 3d t2g and anion 2p states.45–47 This is supported by an observed increase in the octahedral splitting with octahedral rotation.
The most significant observation from the phase diagram (Fig. 7) is the co-occurrence of the transition between metallic and half-metallic behaviour with the cubic to rhombohedral phase transition, observed for compositions around x = 0.35 and pressures of 3–5 GPa. This can be understood by comparing the density of states for each symmetry for x = 0.35 (Fig. 8a and b, corresponding band structures in Fig. S10 and S11, ESI†). Increased narrowing of the minority spin 3d bandwidth in the rhombohedral phase causes it to become unoccupied, resulting in a half-metallic state. In contrast, the broader cubic 3d band (driven by more effective overlap between Nb and O orbitals) causes it to be partially occupied, maintaining a metallic state.
Examination of the PDOS for rhombohedral NbO0.3F2.7 and NbF3 (Fig. 8c and d and Fig. S12–S14, ESI†) shows an additional splitting in the niobium 3d states absent at higher oxygen compositions. This is caused by the trigonal distortion of the octahedra discussed previously (Fig. 6b) with the effect that the cubic 3d t2g states are split into a combination of occupied eg and unoccupied a1g states. The resulting electronic gap (0.9 eV) allows NbF3 to maintain spin polarisation whilst being semiconducting, and contrasts with previous predictions of half-metallic behaviour for x = 2.14,27
E. Effects of anionic ordering
In order to evaluate the effects of anionic ordering on the electronic structure, we performed calculations based on a 2 × 2 × 2 supercell with different arrangements of oxygen and fluorine across the anion sites. These ordered models do not match the true anion arrangement (which exhibits local O/F ordering without 3-dimensional periodicity) but allow deviations from the VCA model due to anion ordering to be studied.Calculations for NbO2F with different anion arrangements reveal that a semiconducting state is retained with anion ordering, but the O2p and F2p sub-bands split according to the differing electronegativities of these species (Fig. S15, ESI†). The resulting bandgaps (1–2 eV) are smaller than the experimental value, but will be highly dependent on the Hubbard on-site repulsion term. Similar supercell models for cubic NbO1.5F1.5 and NbOF2 (Fig. 9) show the same splitting of O and F sub-bands, but the half-metallicity observed from the VCA model is retained. Different anion ordering patterns (Fig. S16 and S17, ESI†) result in changes to the width of the anion p-bands, but the half-metallicity occurs as a result of the splitting of niobium 3d states which are independent of anion order. In real materials, we anticipate that the anion disorder will result in a DOS intermediate between the VCA and supercell model results presented here.
IV. Conclusions
Our first-principles calculations have predicted half-metallicity over a wide range of compositions in the NbO2−xF1+x solid solution. Half-metallic behaviour is expected to occur in both cubic and rhombohedral structures for 0.44 ≤ x < 2, and could allow the electronic band structure to be continuously tuned by varying anionic composition.The impact of external pressure on the ReO3-type structure and the electronic properties of NbO2-xF1+x has also been explored. Anionic control over the cubic to rhombohedral phase transition is predicted with a maximum transition pressure of 5 GPa for x = 0.35. The trigonal octahedral distortion expected in NbF3 is found to produce a spin-polarised semiconducting phase rather than the previously predicted half-metallic cubic phase.27 The cubic to rhombohedral phase transition is also found to be the driving force behind half-metallicity in compositions around x = 0.35. This is caused by a narrowing of the Nb 3d t2g band as a result of octahedral tilting. This structural control over half-metallicity could potentially be exploited to give materials that could be switched between metallic and half-metallic states under moderate applied pressure. Such behaviour would present a new approach in controlling spin currents for spintronic devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work used the Cirrus UK National Tier-2 HPC Service at EPCC (http://www.cirrus.ac.uk) funded by the University of Edinburgh and EPSRC (EP/P020267/1). EKD wishes to thank the University of Edinburgh for funding. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising from this submission.References
- S. A. Wolf, D. D. Awschalom, R. A. Buhrman, J. M. Daughton, S. von Molnár, M. L. Roukes, A. Y. Chtchelkanova and D. M. Treger, Science, 2001, 294, 1488–1495 CrossRef CAS PubMed.
- R. A. de Groot, F. M. Mueller, P. G. van Engen and K. H. J. Buschow, Phys. Rev. Lett., 1983, 50, 2024–2027 CrossRef CAS.
- Y. H. A. Wang, A. Gupta, M. Chshiev and W. H. Butler, Appl. Phys. Lett., 2008, 92, 062507 CrossRef.
- Y. H. A. Wang, A. Gupta, M. Chshiev and W. H. Butler, Appl. Phys. Lett., 2009, 94, 062515 CrossRef.
- W. E. Pickett and D. J. Singh, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 53, 1146–1160 CrossRef CAS PubMed.
- Y. Ding, C. Yuan, Z. Wang, S. Liu, J. Shi, R. Xiong, D. Yin and Z. Lu, Appl. Phys. Lett., 2014, 105, 092401 CrossRef.
- K. G. West, M. Osofsky, I. I. Mazin, N. N. H. Dao, S. A. Wolf and J. Lu, Appl. Phys. Lett., 2015, 107, 012402 CrossRef.
- N. Tezuka, N. Ikeda, A. Miyazaki, S. Sugimoto, M. Kikuchi and K. Inomata, Appl. Phys. Lett., 2006, 89, 112514 CrossRef.
- H. Kageyama, K. Hayashi, K. Maeda, J. P. Attfield, Z. Hiroi, J. M. Rondinelli and K. R. Poeppelmeier, Nat. Commun., 2018, 9, 772 CrossRef PubMed.
- S. Cordier, T. Roisnel and M. Poulain, J. Solid State Chem., 2004, 177, 3119–3126 CrossRef CAS.
- S. Andersson, Acta Chem. Scand., 1964, 18, 2339–2344 CrossRef CAS.
- S. Andersson and A. Åström, Acta Chem. Scand., 1964, 18, 2233–2236 CrossRef CAS.
- F. J. Brink, L. Norén and R. L. Withers, J. Solid State Chem., 2004, 177, 2177–2182 CrossRef CAS.
- M. Pouchard, M. R. Torki, G. Demazeau and P. Hagenmuller, C. R. Seances Acad. Sci., Ser. C, 1971, 273, 1093–1096 CAS.
- F. P. Gortsema and R. Didchenko, Inorg. Chem., 1965, 4, 182–186 CrossRef CAS.
- M. V. Reddy, S. Madhavi, G. V. Subba Rao and B. V. R. Chowdari, J. Power Sources, 2006, 162, 1312–1321 CrossRef CAS.
- N. H. Bashian, M. B. Preefer, J. Milam-Guerrero, J. J. Zak, C. Sendi, S. A. Ahsan, R. C. Vincent, R. Haiges, K. A. See, R. Seshadri and B. C. Melot, J. Mater. Chem. A, 2020, 8, 12623–12632 RSC.
- B. K. Greve, K. L. Martin, P. L. Lee, P. J. Chupas, K. W. Chapman and A. P. Wilkinson, J. Am. Chem. Soc., 2010, 132, 15496–15498 CrossRef CAS PubMed.
- K. S. Aleksandrov, V. N. Voronov, A. N. Vtyurin, A. S. Krylov, M. S. Molokeev, M. S. Pavlovskiĭ, S. V. Goryaĭnov, A. Y. Likhacheva and A. I. Ancharov, Phys. Solid State, 2009, 51, 810–816 CrossRef CAS.
- C. R. Morelock, L. C. Gallington and A. P. Wilkinson, J. Solid State Chem., 2015, 222, 96–102 CrossRef CAS.
- S. U. Handunkanda, E. B. Curry, V. Voronov, A. H. Said, G. G. Guzmán-Verri, R. T. Brierley, P. B. Littlewood and J. N. Hancock, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 134101 CrossRef.
- H. A. Evans, Y. Wu, R. Seshadri and A. K. Cheetham, Nat. Rev. Mater., 2020, 5, 196–213 CrossRef CAS.
- A. Glazer, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1972, 28, 3384–3392 CrossRef CAS.
- S. Carlson, A.-K. Larsson and F. E. Rohrer, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2000, 56, 189–196 CrossRef PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- J. Wang, S. Qi, X. Song, Y. Qu, W. Li and M. Zhao, Appl. Surf. Sci., 2019, 495, 143623 CrossRef CAS.
- B. Yang, J. Wang, X. Liu and M. Zhao, Phys. Chem. Chem. Phys., 2018, 20, 4781–4786 RSC.
- H. Mizoguchi, M. Orita, M. Hirano, S. Fujitsu, T. Takeuchi and H. Hosono, Appl. Phys. Lett., 2002, 80, 4732–4734 CrossRef CAS.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr., 2005, 220, 567–570 CAS.
- S. L. Dudarev, G. A. Botton, S. Y. Savrasov, C. J. Humphreys and A. P. Sutton, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 1505–1509 CrossRef CAS.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- P. Ehrlich, F. Plöger and G. Pietzka, Z. Anorg. Allg. Chem., 1955, 282, 19–23 CrossRef CAS.
- G. Bergerhoff, R. Hundt, R. Sievers and I. D. Brown, J. Chem. Inf. Comput. Sci., 1983, 23, 66–69 CrossRef CAS.
- A. Belsky, M. Hellenbrandt, V. L. Karen and P. Luksch, Acta Crystallogr., Sect. B, 2002, 58, 364–369 CrossRef PubMed.
- A. J. Morris, R. J. Nicholls, C. J. Pickard and J. R. Yates, Comput. Phys. Commun., 2014, 185, 1477–1485 CrossRef CAS.
- J. Cumby and J. P. Attfield, Nat. Commun., 2017, 8, 14235 CrossRef CAS PubMed.
- F. Birch, Phys. Rev., 1947, 71, 809–824 CrossRef CAS.
- A. Agulyansky, in The Chemistry of Tantalum and Niobium Fluoride Compounds, ed. A. Agulyansky, Elsevier Science, Amsterdam, 2004, ch. 1, pp. 1–10 Search PubMed.
- S. Carlson, J. Appl. Crystallogr., 2000, 33, 1175–1176 CrossRef CAS.
- C. Michel, J.-M. Moreau and W. J. James, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1971, 27, 501–503 CrossRef CAS.
- H. Schäfer, H. G. Schnering, K. J. Niehues and H. G. Nieder-Vahrenholz, J. Less-Common Met., 1965, 9, 95–104 CrossRef.
- P. Daniel, A. Bulou, M. Rousseau, J. Nouet and M. Leblanc, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 42, 10545–10552 CrossRef CAS PubMed.
- A. Mogus-Milankovic, J. Ravez, J. P. Chaminade and P. Hagenmuller, Mater. Res. Bull., 1985, 20, 9–17 CrossRef CAS.
- P. Woodward, Acta Crystallogr., Sect. B: Struct. Sci., 1997, 53, 32–43 CrossRef.
- P. G. Radaelli, G. Iannone, M. Marezio, H. Y. Hwang, S. W. Cheong, J. D. Jorgensen and D. N. Argyriou, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 8265–8276 CrossRef CAS.
- M. Medarde, J. Mesot, P. Lacorre, S. Rosenkranz, P. Fischer and K. Gobrecht, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 52, 9248–9258 CrossRef CAS PubMed.
- T. Zhao, L. M. Daniels, B. Slater, M. J. Rosseinsky and F. Corà, J. Phys. Chem. C, 2020, 124, 13045–13052 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc01563c |
| This journal is © The Royal Society of Chemistry 2023 |