Open Access Article
Open Access ArticleAnti-inflammatory role of gold nanoparticles in the prevention and treatment of Alzheimer's disease
Munire
Aili
a,
Kebing
Zhou
a,
Jun
Zhan
b,
Huaping
Zheng
c and
Feng
Luo
 *ad
*ad
aState Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China School of Stomatology, Sichuan University, Chengdu 610041, China. E-mail: luofeng0122@scu.edu.cn; Fax: 86-28-85503671; Tel: +86 28 85503671 Tel: +86-13488904276
bDepartment of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu 610041, China
cDepartment of Dermatology, Rare Diseases Center, Institutes for Systems Genetics, Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University, Chengdu, Sichuan, China
dDepartment of Prosthodontics, West China School of Stomatology, Sichuan University, No. 14, Section 3, Renmin Nanlu, Chengdu 610041, China
First published on 21st August 2023
Abstract
Alzheimer's disease (AD) is a neurodegenerative disease that causes memory and cognitive dysfunction and reduces a person's decision-making and reasoning functions. AD is the leading cause of dementia in the elderly. Patients with AD have increased expression of pro-inflammatory cytokines in the nervous system, and the sustained inflammatory response impairs neuronal function. Meanwhile, long-term use of anti-inflammatory drugs can reduce the incidence of AD to some extent. This confirms that anti-neuroinflammation may be an effective treatment for AD. Gold nanoparticles (AuNPs) are an emerging nanomaterial with promising physicochemical properties, anti-inflammatory and antioxidant. AuNPs reduce neuroinflammation by inducing macrophage polarization toward the M2 phenotype, reducing pro-inflammatory cytokine expression, blocking leukocyte adhesion, and decreasing oxidative stress. Therefore, AuNPs are gradually attracting the interest of scholars and are used for treating inflammatory diseases and drug delivery. Herein, we explored the role and mechanism of AuNPs in treating neuroinflammation in AD. The use of AuNPs for treating AD is a topic worth exploring in the future, not only to help solve a global public health problem but also to provide a reference for treating other neuroinflammatory diseases.
 Munire Aili | Munire Aili is pursuing an undergraduate degree in West China School of Stomatology, Sichuan University. Her current research interests focus on the molecular mechanism of oral diseases. |
1. Introduction
Alzheimer's disease (AD) is the leading cause of dementia in the elderly, and the age-standardized prevalence of dementia in people over 60 is 5–7%. It has become a global health problem, costing up to $604 billion annually.1 In 2018, about 50 million people had dementia worldwide, and age is a major factor contributing to dementia. AD is a neurodegenerative disease that causes memory and cognitive dysfunction and reduces a person's decision-making and reasoning functions.2 People with AD are at higher risk of stroke, chronic obstructive pulmonary disease, and suicide than the general population, placing a heavy burden on patients and society. AD is a multifactorial disease, and the pathogenesis and pathophysiological mechanisms have not been fully elucidated.3 Multiple factors combine to cause AD, with amyloid β-protein (Aβ) deposition, the formation, and proliferation of neurogenic fiber tangles composed of the microtubule-associated protein taurine (Tau), dysregulation of protein degradation pathways, loss of support from neurotrophic factors, and synaptic and neuronal loss being the main pathological features of AD.4Currently, the primary treatment for AD is medication. Cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists can be used to treat AD.5 However, drug therapy can only temporarily relieve symptoms and cannot change the natural history of AD patients.6 As AD patients enter advanced stages, neurodegeneration increases, and they cannot repair damaged neural networks by reducing amyloid, so new drug strategies are urgently needed.7 Recent studies have shown that the inflammatory response is closely related to the onset and progression of AD and that suppressing or eliminating inflammation may help to treat AD.8 Firstly, patients with AD have activated microglia around amyloid plaques, activated T cells in the brain parenchyma, and increased expression of pro-inflammatory cytokines in the peripheral and central nervous systems (CNS). There is an accumulation of Aβ in the nervous system of AD patients. Furthermore, the deposition of Aβ leads to the release of pro-inflammatory cytokines such as interleukin-1α (IL-1α), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), gamma-interferon (IFN-g), reactive oxygen species (ROS), and nitric oxide (NO).9 These factors activate microglia and the complement system, which exacerbate the inflammatory response and induce the expression of amyloid precursor protein (APP).10 The increase of APP will, in turn, upregulate the expression of Aβ, forming a vicious circle, leading to neuronal dysfunction or even death and aggravating AD.11 Generally, inflammation subsides independently, however, persistent inflammatory responses can become chronic inflammation and impair brain function.12 Moreover, long-term use of anti-inflammatory drugs reduces the incidence of AD to some extent.13 This confirms that neuroinflammation may be closely related to the development of AD, so anti-inflammatory medications may be an effective way to treat AD.
Nanomaterials have an important role in disease treatment and diagnosis, novel drug synthesis, and drug delivery, and they have been widely used in biomedical, engineering, and health research fields.14 Among them, nanomedicine is an important branch of nanomaterials that can help medical practitioners to improve the accuracy of diagnosis and have good efficacy.15 Nanomedicine is used in the treatment of cancer, rapid detection of AIDS, and on-demand exogenous insulin release for diabetes, Anti-inflammation, etc.16 Recent studies have shown that nanoparticles have advantages over traditional materials in anti-inflammatory and treating central nervous system diseases.17 AuNPs are an emerging type of nanoparticles. On the one hand, it can penetrate the blood–brain barrier (BBB) and reach the central nervous system to exert therapeutic effects. On the other hand, AuNPs of 40–50 nm in the concentration range of 10 mg kg−1 can effectively reduce the inflammatory response in rats.18 Therefore, given their excellent anti-inflammatory effect, AuNPs may be a promising option for treating Alzheimer's.
Multiple studies have shown that AuNPs reduce the inflammatory response and facilitate tissue repair. AuNPs induce polarization of microglia toward the M2 phenotype, thereby reducing pro-inflammatory cytokines expressed by microglia in the M1 phenotype and increasing anti-inflammatory cytokines expressed by the M2 phenotype, reducing neuroinflammation.19 In addition, AuNPs inhibit the activation of MAPK, NF-κB, JAK/STAT, and IKK-α/β signaling pathways, suppressing their downstream factors.20 AuNPs reduce the expression of ICAM-1 in circulating polymorphonuclear (PMN) leukocytes and endothelial cells and inhibit leukocyte adhesion.21 AuNPs scavenge ROS from mitochondria, inhibit the production of reactive oxygen and nitrogen species, and suppress oxidative stress.22 Injecting 20 nm of AuNPs into the peritoneal cavity of AD model rats, AuNPs were found to reduce Tau phosphorylation caused by okadaic acid significantly. It also increased IL-4 expression levels in the rat cortex and hippocampus, reduced inflammation, and mitochondrial oxidative stress, and prevented cognitive impairment in rats.22 In summary, AuNPs effectively reduce the inflammatory response and may help control AD development.
2. Overview of AuNPs
Nanotechnology is proliferating in the 21st century with its wide range of applications and great potential.23 By observing, manipulating, assembling, and controlling matter from the nanoscale, nanotechnology has prepared new nanomaterials for use in various fields, including chemistry, biology, engineering, and medicine.24 Nanomaterials are classified into inorganic (e.g., metals) and organic nanomaterials (e.g., proteins, lipids) and possess rich structural properties.25 In the biomedical field, nanomaterials show great promise in targeted drug delivery, diagnostic testing, evaluation, and treatment of diseases by engaging in biomolecular interactions through manipulable physical, chemical, and biological properties.26AuNPs, as a kind of noble metal nanostructures, show high activity differentiated from the macroscopic size. The properties of AuNPs are closely related to their shape and size. Currently, many different shapes of AuNPs have been developed, including rod, sphere, cage, shell, etc. (Fig. 1).27 The shapes of AuNPs can confer unique advantages, such as rod-shaped AuNPs with good photothermal properties, while cage-shaped AuNPs are more suitable for loading drugs.28 In addition, the electrical conductivity of AuNPs is affected by the particle size. Au nanocrystals (particle size >2 nm) have conductive properties, while au nanoclusters (particle size <2 nm) have insulating properties.29
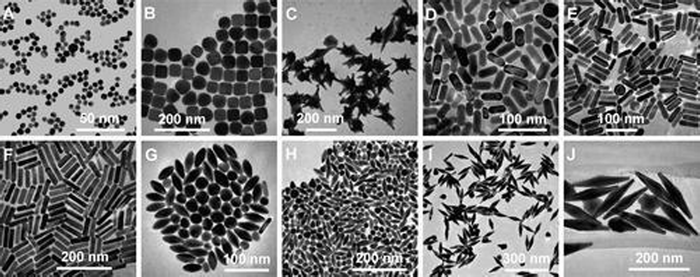 | ||
| Fig. 1 Tem images of AuNPs of different shapes and sizes. (A) Nanospheres. (B) Nanocubes. (C) Nanobranches. (D) Nanorods. (E) Nanorods. (F) Nanorods. (G) Nanobipyramids. (H) Nanobipyramids. (I) Nanobipyramids. (J) Nanobipyramids. Reprinted with permission from ref. 27. Copyright © 2008, American Chemical Society. | ||
In addition to the general properties of nanomaterials, AuNPs have unique properties and thus have received extensive attention from all walks of life. First, AuNPs have a surface plasmon resonance effect (SPR).30 When AuNPs are irradiated by light of a specific wavelength, the incident photons interact with the surface free electrons. When the vibration frequency of the incident light is the same as the resonance frequency of the free electrons on the surface of the metal particles, the resulting resonance is called SPR. The SPR absorption of photons by AuNPs can be detected using UV-vis spectroscopy, and different absorption peaks and peak positions reflect different morphologies, sizes, and structures of AuNPs.31 Meanwhile, the nature of the localized surface plasmon resonance (LSPR) of AuNPs is also affected by factors such as interparticle and surrounding environment.32,33 Since light with wavelengths in the near-infrared (NIR) region has better tissue penetration in water and biological tissues. Therefore, AuNPs with LSPR peaks in the NIR region are more suitable for biomedical applications and have been intensively investigated for diagnostic imaging, drug-targeting delivery, and biosensors.34 Secondly, the optical properties of AuNPs are also developed based on their SPR properties. Different wavelengths of AuNPs absorb different wavelengths of light, thus exhibiting different colors and photothermal effects.35 When a specific wavelength of light irradiates the surface of AuNPs, the light energy is converted into heat energy due to the continuous oscillation of the surface plasma. This property makes AuNPs suitable for cancer treatment.36,37 Thirdly, AuNPs have catalytic properties. The gold catalysts are divided into unloaded and loaded types, and the catalytic of unloaded type is mainly based on the fact that AuNPs have high surface energy and surface-free electrons. In contrast, the principle of loaded AuNPs as catalysts is unclear.38
In biological environments, the low reactivity and biological inertness of AuNPs give them good biocompatibility. Sulfur-containing compounds with different functional groups can also be utilized to react with AuNPs to chemically modify the materials and endow them with rich functionalities.39 The preparation of metallic nanoparticles can be improved using biosynthetic techniques and traditional chemical processes.40 In addition to preparing metal nanoparticles through traditional chemical processes, the use of biosynthesis techniques can better improve the biocompatibility of metal nanoparticles and reduce the toxicity generated in chemical synthesis.41 AuNPs have easy synthesis, superior biocompatibility, catalytic activity, and anti-inflammatory and antioxidant properties. Therefore, the active substance can be given unique surface properties to perform specific functions in living organisms through assembly, recombination, and encapsulation.42 It was shown that in rheumatoid arthritis models, AuNPs produce anti-inflammatory effects by inhibiting the NF-κB signaling pathway and reducing the production of pro-inflammatory cytokines (e.g., TNF-α, IL-1β, COX-2).43 In addition, AuNPs have been shown to penetrate spontaneously through the BBB without external stimulation, enhancing the bioavailability of drugs to treat CNS disorders.44 Therefore, the anti-inflammatory function of AuNPs in preventing and treating Alzheimer's disease has been widely valued.
3. Possible anti-inflammatory mechanism of AuNPs
3.1 Induction of macrophage polarization
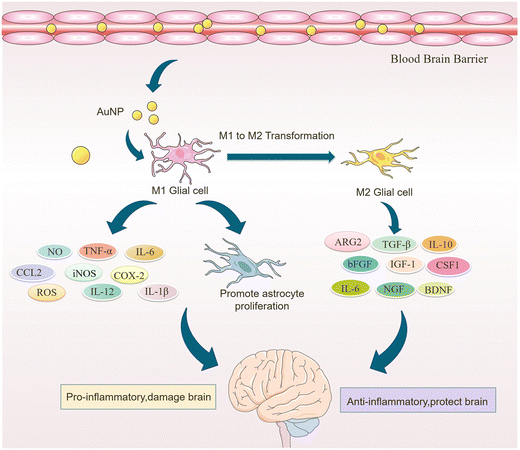
Microglia are a class of macrophages widely distributed in the central nervous system. They are the brain's immune cells, accounting for approximately 10% of all cells in the central nervous system.45 They are involved in the development of the CNS, regulate the learning and memory functions of the brain, recognize CNS injury to initiate immune responses, remove dead cells and cellular debris, misfolded proteins, provide nutrition to the brain, and have an essential role in neuroinflammation and neurodegeneration.46 Microglia generally exist in a resting state and recognize pathogens.47 Upon detection of pathogens or external stimuli, microglia are activated and undergo morphological changes, polarizing into M1-like or M2-like phenotypes.11 Pro-inflammatory cytokines such as endotoxin, peptidoglycan, and interferon gamma (IFNγ) activate the M1 phenotype, while anti-inflammatory cytokines such as interleukin 4 (IL-4) and IL-13 activate the M2 phenotype.48 Microglia in the M1 phenotype produce and release inflammatory mediators such as inducible nitric oxide synthase 2 (iNOS/NOS2), cyclooxygenase-2 (COX-2), chemokines (IL-1β, IL-6, IL-12, TNF-α, and CCL2), NADPH oxidase, integrins, co-stimulatory molecules, protein hydrolases, ROS, and NO, causing neuroinflammation, damaging neural networks, inhibiting neurogenesis, and disrupting the BBB.49 Also, M1-type microglia induce astrocyte proliferation, promote glial scarring, form a barrier around damaged nerves, and inhibit neuronal extension.46 Microglia of the M2 phenotype produce and release anti-inflammatory cytokines and neuroprotective factors such as arginase 2 (ARG2), transforming growth factor-β (TGF-β), IL-10, basic fibroblast growth factor (bFGF), insulin-like growth factor 1 (IGF-1), CSF1, IL-6, neurogenic growth factor (NGF), neuro dystrophin and brain-derived neurotrophin (BDNF), which inhibit inflammatory responses, protect neurons, and promote neurogenesis.50AuNPs can promote microglia polarization toward M2 phenotype and reduce mitosis of astrocytes, which is beneficial for neuronal repair.51 It has been reported that cytokines in the brain of AD patients are mainly produced by microglia and astrocytes, and the inflammatory activity of microglia is upregulated in AD patients.19,52 Promoting microglia polarization toward the M2 phenotype facilitates neuronal repair and regeneration and may help alleviate AD symptoms.53 Recent studies have shown that AuNPs can regulate microglia polarization and promote the repair and regeneration of damaged neurons.54 Xiao et al. produced dihydrolipoic acid-functionalized gold nanoclusters (DHLA-AuNCs). They found that DHLA-AuNCs can promote the mouse microglia-derived cell line BV2 from M1-like phenotype to M2-like phenotype polarization, thereby promoting neurogenesis and axonal extension and inhibiting astrocyte proliferation. In addition, DHLA-AuNCs reduced neuroinflammation and facilitated neuronal regeneration by downregulating the NF-κB signaling pathway, scavenging ROS, inducing autophagy in BV2 cells, and reducing apoptosis.46 Confirm that AuNPs reduce neuroinflammation by inducing microglia polarization toward the M2 phenotype (Fig. 2).
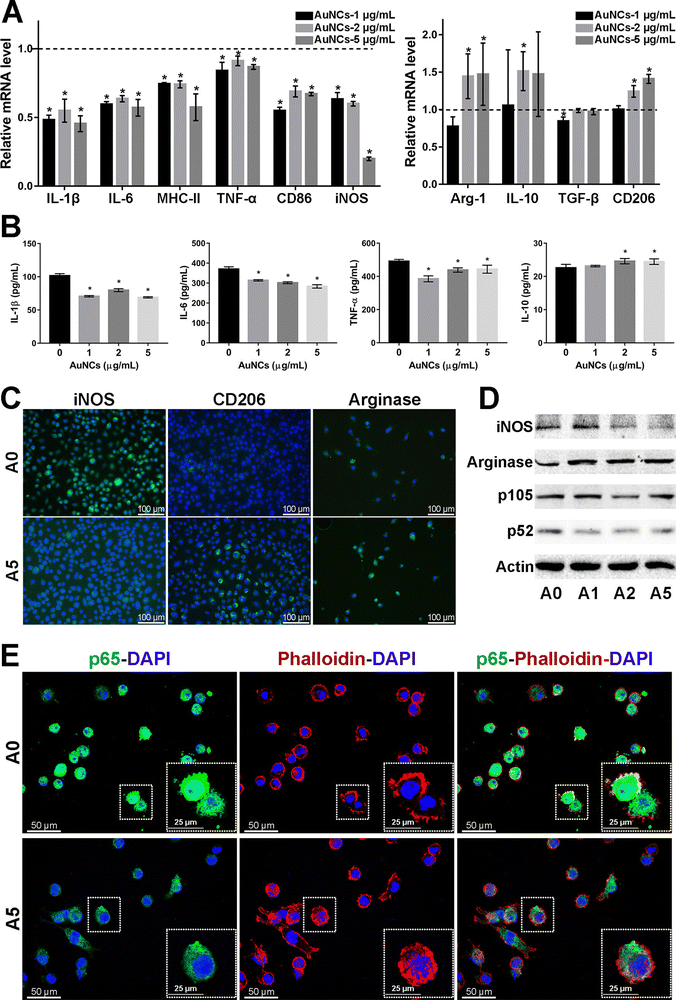 | ||
| Fig. 2 Effect of AuNCs on the pro-inflammatory response of BV2 cells. (A) The represented mrna levels of AuNCs-1/2/5 μg mL−1 groups were normalized to the control group and appeared as fold changes. (B) Quantification of cytokines in BV2 cell supernatant was performed by Elisa. (C) Represented if staining images of iNos, CD206, and arginase (Alexa Fluor488, green) in BV2 cells. Nuclei were counterstained with dapi (blue). (D) Protein levels were examined by western blotting analysis. (E) The cell cytoskeleton was stained with phalloidin (bodipy 558/568, red). Nuclei were stained with dapi (blue). If staining of NF-κB p65 (Alexa Fluor488, green) showed that in comparison with the control group, the cytoskeleton–nuclear translocation of p65 (original magnification, 400×) was reduced in cells treated with AuNCs (5 μg mL−1). Reprinted with permission from ref. 46. Copyright © 2020, American Chemical Society. | ||
There are fewer articles on AuNPs promoting microglia polarization in AD models. However, findings in many other disease models are to be found to suggest that AuNPs promote the polarization of macrophages toward the M2 phenotype, attenuate the inflammatory response, and promote tissue repair. These studies will confirm the function of AuNPs to some extent.55 Can et al. found that 45 nm AuNPs downregulated iNOS expression in periodontal tissue, inhibited macrophage polarization to the M1 phenotype and induced it to the M2 phenotype, decreased TNF-α and IL-6 levels, promoted the release of IL-10 and TGF-β from macrophages, and attenuated inflammatory response of periodontal tissues, improve periodontal inflammation, and inhibit the progression of periodontitis.56 Taratummarat et al. found through in vivo and in vitro experiments that AuNPs reduced M1 phenotype macrophages and increased M2 phenotype macrophages, promoted macrophage polarization toward the M2 phenotype, and upregulated the expression of IL-10, arginase 1 (Arg 1), and PPARγ, reduced the expression of pro-inflammatory mediators such as TNF-α, IL-6, and IL-1β, inducible nitric oxide synthase (iNos) and Nur77, exerting anti-inflammatory effects, thereby reducing bacterial sepsis in mice.57 Wang et al. investigated that hexapeptide-coated AuNPs P12 could inhibit the activation of Toll-like receptor (TLR) signaling in macrophages. They established a lipopolysaccharide-induced mouse ALI model to explain the molecular mechanism of gold nanoparticle anti-inflammation. They recognized that hexapeptide-coated AuNPs P12 exerted its anti-inflammatory effect mainly through regulating macrophages, and P12 could reduce alveolar and interstitial macrophages of M1 phenotype in lung tissue P12 was able to reduce alveolar and interstitial M1 phenotype macrophages and increase alveolar M2 phenotype macrophages in lung tissue. In vivo and in vitro, P12 induced polarization of bone marrow-derived macrophages (BMDMs) toward the M2 phenotype, reduced infiltration of pro-inflammatory mediators and inflammatory cells, upregulated IL-10 expression, reduced lung inflammation, alleviated lung injury, and facilitated the regression of lung inflammation.58 Park et al. found through in vivo and in vitro experiments that trimethoprim AuNPs (Triam AuNPs) promoted the expression of anti-inflammatory mediators by macrophages and fibroblast-like synoviocytes (FLS) and inhibited the expression of pro-inflammatory cytokines, induced a shift from the M1 phenotype to the M2 phenotype of macrophages, increased the number of M2 phenotype macrophages in the joint cavity, affected the inflammatory response in the synovium, and Promoting cartilage regeneration may help to treat arthritis. In contrast, Triam alone only reduces the pro-inflammatory response, does not increase the release of anti-inflammatory cytokines from FLS and macrophages, and induces macrophage polarization to the M2 phenotype.59 These results suggest that AuNPs reduce pro-inflammatory cytokines such as TNF-α and IL-6, IL-1β, iNos, and Nur77 by inducing macrophage polarization.60 Meanwhile, it increases the expression of anti-inflammatory cytokines and neuroprotective factors such as IL-10 and TGF-β, Arg1, and PPARγ, thus exerting anti-inflammatory and neuronal protective effects.61
In skeletal muscle and bone tissues, AuNPs were also found to promote the conversion of macrophages from the M1 phenotype to the M2 phenotype. Theresa et al. modified the surface of AuNPs with polyethylene glycolization and then combined AuNPs with IL-4 to prepare PA4, a complex capable of delivering IL-4. In in vivo experiments, PA4 was injected into the damaged skeletal muscle of mice and was found to induce the conversion of Mφs from M1 to M2 phenotype and improve muscle function. Furthermore, in vitro experiments showed that PA4-polarized Mφs expressed higher levels of CD206 compared to Mφs polarized with soluble IL-4 alone.62 Bai et al. established an LPS-induced inflammatory bone erosion model in mice. They found that AuNPs reduced the proportion of M1 macrophages by 59%, decreased the expression of inflammatory factors in LPS-induced M1 macrophages, and thus reduced bone erosion. At the same time, AuNPs increased the proportion of M2 macrophages by about 3-fold, upregulated type I collagen deposition, increased osteogenic activity, and reduced the number of CD68-positive natural inflammatory cells. In conclusion, AuNPs alleviated bone loss by altering the inflammatory microenvironment at the site of bone erosion, increased bone density by 14%, increased bone mass, and improved bone structure (Table 1).63 These studies suggest that AuNPs promote microglia polarization toward the M2 phenotype, which may help to alleviate or eliminate neuroinflammation and improve AD symptoms (Fig. 3).
| Agent | Modification | Model | Mechanism | Detected inflammatory cytokines | Cognition function | Ref. |
|---|---|---|---|---|---|---|
| DHLA-AuNC | DHLA | Ex vivo brain slice stroke model | Promoting microglia polarization toward the M2 phenotype | NF-κB pathway↓, ROS↓ | Inhibits the inflammatory response of BV2 cells and promotes neurogenesis | 46 |
| AuNPs | — | Periodontitis model | Inhibition of M1 phenotype macrophage polarization and facilitation of phenotype to M2 phenotype conversion | iNOS, TNF-α, IL-6↓; L-10, TGF-β↑ | Reducing periodontal inflammation | 56 |
| AuNPs | — | Cecum ligation and puncture mouse model | Promotion of macrophage polarization toward the M2 phenotype | TNF-α, IL-6, IL-1β, iNos, Nur77↓; IL-10, Arg1, PPARγ↑ | Reduction of bacterial sepsis | 57 |
| Peptide-coated GNPs | Peptide | Acute lung injury model | Induced polarization of BMDMs to the M2 phenotype | TLR↓; IL-10↑ | Promote the reduction of lung inflammation | 58 |
| Triam AuNPs | Triam | Osteoarthritis model | Promoting repolarization of macrophages from M1 to M2 phenotype | — | Reduced pro-inflammatory response and enhanced anti-inflammatory response of fibroblast-like synoviocytes and macrophages, promoting cartilage regeneration | 59 |
| AuNP-IL4 | Polyethylene glycol, IL-4 | Mouse ischemic muscle injury model | Induced conversion of Mφs to the M2 phenotype | CD206↑ | Promotes muscle regeneration and strengthens muscles after ischemia | 62 |
| AuNPs | Mouse inflammatory bone erosion model | Induced polarization of macrophages to the M2 subtype | — | Promotes bone regeneration in areas of bone erosion | 63 |
3.2 Reduction pro-inflammatory cytokine expression
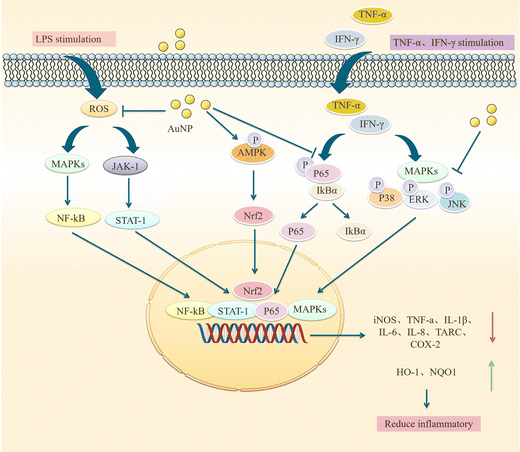
Sustained neuroinflammation becomes chronic and releases pro-inflammatory cytokines excessively, impairing brain function.56 Transcript levels of several pro-inflammatory mediators such as tumor necrosis factor-α, IL-1β, interferon-gamma, IL-18, and IL-6 are increased in the brain of AD patients, which can damage neurons and induce Tau hyperphosphorylation, causing cognitive dysfunction. Pro-inflammatory mediators may be considered biomarkers of AD.64,65 AuNPs may reduce neuroinflammation by decreasing TNF-α and IL-1β expression and inhibiting MAPK and NF-κB pathways.66AuNPs inhibit MAPK, NF-κB, JAK/STAT, and IKK-α/β signaling pathways, suppressing their downstream pro-inflammatory cytokines, and reducing inflammation. Yuan et al. prepared Au25Sv9 with gold atoms and tripeptides and found that Au25Sv9 inhibited the activation of NF-κB and p38MAPK pathways in LPS- and IFN-γ-stimulated microglial cells and the extent of inhibition increased with the concentration of Au25Sv9. Au25Sv9 also decreased the expression levels of TNF-α, NO, and IL-6 in LPS- and IFN-γ-stimulated microglial cells and increased cell survival. Importantly, the same SV peptides did not inhibit the inflammatory cytokines expressed by LPS- and IFN-γ-induced microglia.76 This suggests that the protective effect of Au25Sv9 on microglia is due to the action of gold nanoclusters, indicating that the NF-κB and MAPK pathways are a mechanism by which AuNPs reduce inflammatory cytokines. Alexandre et al. infused streptozotocin (STZ) into the lateral ventricle of rats to establish an AD model and found that 20 nm AuNPs significantly reduced STZ-induced neuroinflammatory responses and ameliorated spatial memory deficits and cognitive impairments in rats. STZ-induced activation of the NF-κB pathway in rats was accompanied by an increase in the expression of IL-1β. AuNPs significantly reduced STZ-induced activation of the NF-κB pathway, and AuNPs may reduce inflammatory cytokines downstream of NF-κB through this effect.77 This experiment was carried out in the animal model of AD, which not only discussed the ways of AuNPs to reduce inflammatory reactions from the molecular level but also tested the cognitive function and spatial memory function of AD animals. It is reasonable to conclude that AuNPs inhibit stimulus-induced activation of the NF-κB pathway in AD models, which may be promising for reducing neuroinflammation to improve AD symptoms (Fig. 4).
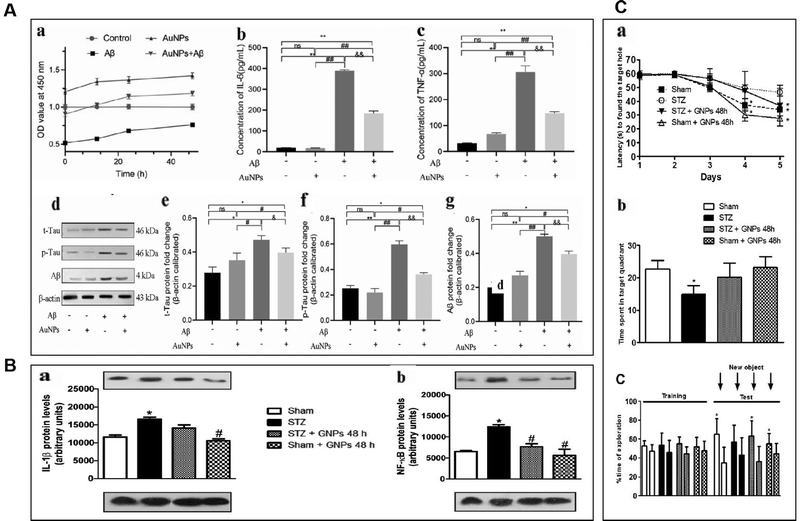 | ||
| Fig. 4 AuNPs reduced neuroinflammation and reduced the toxicity of Aβ. (A) AuNPs promoted cell viability and inhibited inflammatory response in Aβ-treated hNSCs. (a) Aβ inhibited hNSCs viability, and AuNPs significantly enhanced the activity of hNSCs. (b) and (c) The concentrations of IL-6 and TNF-α were measured by elisa kit, and the level of IL-6 and TNF-α remarkably increased after treatment with Aβ. the pro-inflammatory factors IL-6 and TNF-α were significantly decreased in the AuNPs co-treatment group. (d)–(g) The protein expression of t-Tau, p-Tau, and Aβ was measured by western blot, and the results showed that Aβ-induced protein expression was remarkably down-regulated. In contrast, these protein expressions were elevated by AuNPs treatment during cell differentiation. Reprinted with permission from ref. 68. (b) Effects of gnp treatment on the levels of IL-1β (a) and NF-κB (B) at 21 days after induction of dementia by STZ. Adapted with permission from ref. 77. (C) Effects of STZ and AuNPs treatment on cognitive performance. (A) The spatial memory was evaluated using the barnes maze task during acquisition represented by latency to find the escape hole. (B) Retention memory was represented by the time spent within the target quadrant during the retrieval phase of task. (C) Time dedicated to exploration during acquisition memory (equal objects) in object recognition tasks. Reprinted with permission from ref. 77. | ||
AuNPs not only act on NF-κB and MAPK pathways, but some scholars have found that they can also inhibit JAK/STAT and ERK-1/2 pathways. Sun et al. used ephedra as a stabilizer to prepare ES-GNs. They found that in LPS-induced microglia, ES-GNs significantly inhibited the signaling of IKK-α/β, NF-κB, p38MAPK, JAK/STAT, ERK-1/2 and down-regulated the expression of pro-inflammatory mediators and cytokines, such as nitric oxide, prostaglandin E, tumor necrosis factor-α, IL-1β, IL-6, cyclooxygenase-2, and nitric oxide synthase. It also activates AMPK/Nrf2 signaling and upregulates the anti-inflammatory factors quinone oxidoreductase (NQO1) and heme oxygenase-1 (HO-1) to exert anti-neuroinflammatory functions.78 NF-κB, p38MAPK, and JAK/STAT are essential pathways that regulate the immune response in vivo, and AuNPs can act on these pathways to attenuate the inflammatory response of stimulated microglia. However, the mechanism of action of AuNPs should be more than this, and in-depth studies are needed. Scientists have also studied the effects of AuNPs on LPS-induced RAW 264.7 macrophages. Since microglia are also a type of macrophage, there are similarities in their mechanism of action that may provide some clues. Liu et al. synthesized novel EO-AuNPs using extracts of Euphrasia officinalis leaf to investigate their effects on LPS-induced RAW 264.7 macrophages. This study confirmed that EO-AuNPs inhibited LPS-induced NF-κB activation and blocked JAK/STAT pathway activation by inhibiting the phosphorylation and degradation of IκBα and preventing NF-κB p65 from entering the nucleus and reduced LPS-induced NO, iNOS, TNF-α, IL-1β, and IL-6 expression in RAW 264.7 cells, which facilitated suppressing inflammation.79 Another experiment confirmed this result. Ahn et al. synthesized AuNPs in green using Acanthopanacis cortex extract. They showed that in RAW264.7 macrophages, AuNPs inhibited NF-κB translocation and reduced the expression of iNOS, COX-2, NO, and PGE2 by prompting p38MAPK signaling phosphorylation.80 These studies confirm that AuNPs inhibit MAPK, NF-κB, JAK/STAT, and IKK-α/β signaling pathways and activate AMPK/Nrf2 signaling, which facilitates the control of inflammatory responses. Sungeun et al. prepared green synthetic P.g AuNPs using ginseng leaf extract and treated RAW 264.7 macrophages with LPS. They recognized that P.g AuNPs inhibited NF-κB activation in RAW 264.7 macrophages by suppressing LPS-induced activation of the p38 MAPK pathway and inhibiting NF-κB entry into the nucleus, thus reducing mRNA and protein level expression of inflammatory mediators and cytokines, such as IL-6, TNF-α, PEG2, NO, iNOS and COX-2, exerting anti-inflammatory activity and contributing to the treatment of inflammatory diseases.81 In conclusion, AuNPs reduce downstream inflammatory cytokines by attenuating LPS-induced activation of NF-κB, p38MAPK, JAK/STAT, ERK-1/2, and other signaling pathways in microglial cells, attenuating neuroinflammation and promoting neuronal cell survival and differentiation. Exploring the effects of AuNPs on MAPK and NF-κB pathways in AD is an attractive direction for future research, which may be a potential target for treating AD (Fig. 5).
3.3 Restraint of leukocyte adhesion
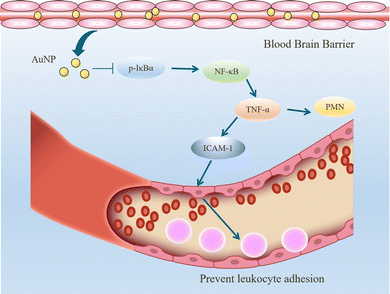
When inflammation occurs, hemodynamics is altered, and vascular permeability increases.82 Leukocytes cross the microvascular wall, swim out of the blood vessels, accumulate at the site of inflammation through chemotaxis of chemokines, and phagocytose and degrade bacteria, necrotic tissue debris, and immune complexes. The most crucial role of the inflammatory response is to deliver leukocytes to the injury site to participate in its repair and regeneration.83 The interaction of leukocytes with endothelial cells is the initial and critical stage of inflammation. In most tissues, leukocyte emergence from the vasculature is a complex and continuous process that includes border set, rolling, adhesion, and emergence.84 Inflammatory mediators (histamine, leukotrienes, and cytokines) stimulate vascular endothelial cells, increase the expression of the adhesion molecule P-selectin glycoprotein ligand 1 (PSGL1), trap motile neutrophils on the endothelial cell surface and roll them along the blood flow.85 Rolling helps neutrophils fully engage with chemokine IL-8-modified endothelial or pro-inflammatory cytokines (TNF-α, IL-1β), activating the G protein-coupled chemokine receptors of neutrophils. Activated neutrophils express constitutively high levels of integrins LFA1 and ICAM1 and show higher affinity for endothelial cell surface molecules such as immunoglobulin-like cell adhesion molecules (CAM).21 It is worth mentioning that the binding between LFA1 and ICAM1 expressed by endothelial cells is essential for the firm adhesion of neutrophils to endothelial surfaces.86 It is worth mentioning that the binding between LFA1 and ICAM1 expressed by endothelial cells is important for the firm adhesion of neutrophils to the endothelial surface.87Inhibition of leukocyte adhesion to endothelial cells during inflammation reduces the secretion of pro-inflammatory factors, prevents heterogeneity of microglia and astrocytes, and maintains neurotransmitter homeostasis. This alleviates the neuroinflammatory response and has implications for treating Alzheimer's disease. It has been shown that AuNPs can reduce circulating TNF concentration and ICAM-1 expression in endothelial cells and reduce leukocyte adhesion to blood vessels.43,57 In the Wistar rat mesenteric microcirculation model constructed by Uchiyama et al., intravenous injection of AuNPs-IgG or 20 nm citrate-fixed alloy nanoparticles (cit-AuNPs) inhibited leukocyte adhesion to the posterior capillary wall.88 Further, Bella et al. applied 20 nm cit-AuNPs to a mouse model of sepsis-associated encephalopathy (SAE). They proposed that 20 nm cit-AuNPs inhibited NF-κB expression or activity by indirectly mediating the reduction of phosphorylated IκBα. Inhibition of NF-κB expression reduces circulating PMN leukocytes and ICAM-1 expression in the cerebral vasculature, inhibits leukocyte adhesion, and thus decreases TNF-α concentrations (Fig. 6).89 In addition, AuNPs were found to reduce the number of leukocytes and exert anti-inflammatory effects in a mouse model of knee osteoarthritis.43,90 In summary, in leukocyte adhesion to endothelial cells, AuNPs inhibit the NF-κB signaling pathway and reduce the expression of inflammatory factors such as TNF-α, which decreases leukocyte activation. On the other hand, AuNPs reduced ICAM1 expression on the surface of circulating leukocytes and endothelial cells, and leukocyte-endothelial cell binding was inhibited, preventing leukocyte adhesion to blood vessels (Fig. 7).
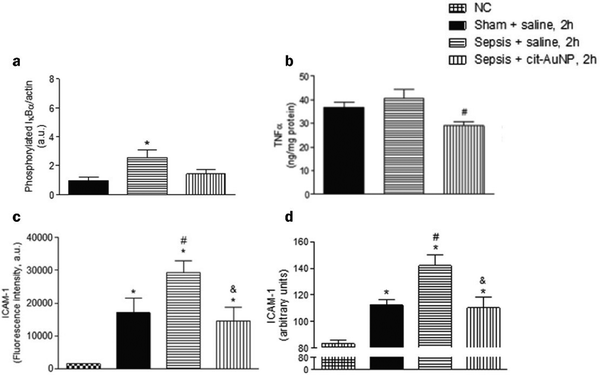 | ||
| Fig. 6 Effect of cit-AuNPs on phosphorylated IκBα (a), TNF-α (b), ICAM-1 in circulating pmn leukocytes (c) and ICAM-1 in cerebral vessels (d) of mice with sepsis. (a) Enhanced phospho-IκBα was demonstrated in the brain of mice 6 h after induction of sepsis, but that increase was not observed in mice previously treated with cit-AuNP. (b) cit-AuNP treatment reduced TNFα concentration. (c) Enhanced ICAM-1 expression was quantified in pmn leukocytes 6 h after induction of sepsis, and cit-AuNP treatment reduced it. (d) Enhanced ICAM-1 expression in cerebral blood vessels was noticed 6 h after induction of sepsis, and cit-AuNP treatment reduced it. Reprinted with permission from ref. 89. | ||
3.4 Reduction of oxidative stress
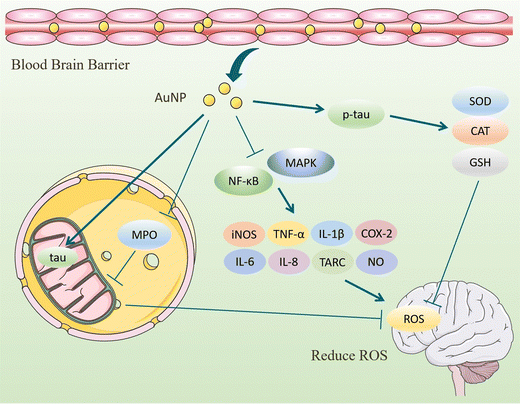
ROS have partially reduced forms of oxygen metabolites (OH−, O2−, H2O2) with strong oxidative properties.91 The NADPH oxidase produces them in phagocytes and as by-products of the electron transport chain (ETC).92 Under normal conditions, small amounts of glutathione in neurons act as antioxidants to eliminate accumulated ROS.93 However, Aβ accumulation and abnormal or increased activity or expression of antioxidant enzymes such as superoxide dismutase (SOD) in the CNS of AD patients.94 Excessive oxidation of lipids, proteins, or nucleic acids accumulates high levels of ROS, creating an oxidative stress environment and increasing the burden on the brain.95 Studies have shown that the mechanisms of oxidative stress formation in Alzheimer's disease patients include mitochondrial dysfunction, metal accumulation, Tau hyperphosphorylation, inflammation, and Aβ accumulation.96,97 Therefore, reducing ROS production and oxidative stress through various pathways is an effective treatment option for AD.AuNPs have good performance in treating AD with their anti-inflammatory and antioxidant properties. On the one hand, AuNPs act as regulators of mitochondria and scavengers of ROS, restoring the mitochondrial membrane potential difference and maintaining the mitochondrial function of neutralizing ROS.98 AuNPs were injected into the ventricles of an Okadaic acid (OA)-induced AD rat model. The results showed that AuNPs controlled Tau phosphorylation in the cortex and hippocampus at normal levels, maintained the antioxidant status (SOD, catalase activity, and GSH levels) of the brain, and prevented OA-induced oxidative stress in brain structures.22,99 Due to the accumulation of Aβ, the activity of critical enzymes in the ETC is reduced, and abnormal mitochondrial dynamics lead to dysfunction, resulting in increased ROS. Xu et al. extracted Hibiscus syriacus L. callus (HCE) to synthesize HCE-AuNPs and evaluated its anti-inflammatory effect in LPS-stimulated macrophages. It was found that using HCE-AuNPs restored the damaged mitochondrial membrane potential and morphology and effectively inhibited ROS production.100 In addition, the combined application of AuNPs and n-acetylcysteine (NAC) reduced the production of myeloperoxidase (MPO) and pro-inflammatory cytokines, improved mitochondrial dysfunction, and reduced brain dysfunction in a rat model of sepsis.101 Thirupathi et al. tried tau-AuNPs as ROS scavengers to eliminate ROS produced by overused muscles and obtained positive results. They hypothesized that AuNPs promote Tau entry into mitochondria and modulate the mitochondrial complex to transfer electrons from the ETC to oxygen molecules, thereby reducing ROS production.102 The above studies suggest that AuNPs maintain the brain's antioxidant levels, repair damaged mitochondria, promote Tau entry into mitochondria, and increase mitochondrial scavenging of ROS, thereby reducing oxidative stress in the brains of AD patients (Fig. 8).
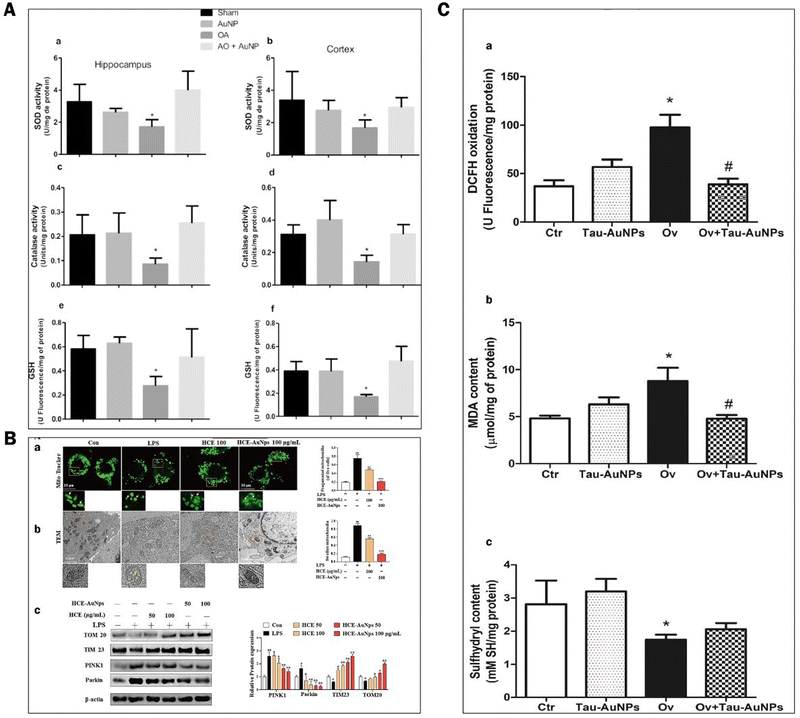 | ||
| Fig. 8 Experimental results of AuNPs to reduce oxidative stress in the brains of ad patients. (A) Effect of AuNPs on the antioxidant status in the brain ventricles of an OA-induced ad rat model. (a) and (b) Superoxide dismutase activity in the hippocampus and cortex was decreased by OA compared with other groups. (c) and (d) Catalase activity in the hippocampus and cortex was decreased by OA compared with the other groups. (e) and (f) glutathione levels in the hippocampus and cortex were decreased by OA compared with other groups. Reprinted with permission from ref. 98. (B) HCE-AuNPs strongly alleviated LPS-induced mitochondrial dysfunction in RAW264.7 cells. Representative images of mitochondrial morphology by mito-tracker (A) and tem images, the white arrows indicated healthy mitochondria, and the yellow arrows demonstrated damaged mitochondria. (B) Immunoblot analysis of tom 20, tim23, pink1, and parkin protein expression and all their bands were analyzed and standardized by β-actin. (C) Adapted with permission from ref. 100. (c) DCFH oxidation (a) and oxidative damage in the lipids (b) and thiol groups (c) of the quadriceps muscles of mice submitted to muscle overuse and Tau-AuNPs administration. Reprinted with permission from ref. 102. | ||
On the other hand, the inflammatory response is also involved in the production of ROS. AuNPs inhibit ROS by inhibiting the activation of NF-κB and MAPK signaling pathways, reducing the secretion of pro-inflammatory cytokines, and decreasing the number of ROS.103 Under the influence of Aβ, microglia, and astrocytes activate to produce cytokines and ROS that lead to neuronal damage. In inflammation models, AuNPs exhibit reduced production of reactive oxygen and nitrogen species, attenuate oxidative stress. and accelerate tissue repair.90,104,105 Peptide CopA3 surface coupled and ginsenoside compound K (CK) loaded AuNPs (GNP-CK-CopA3) interacted with the cysteine (Cys-179) of kinase β (IKKβ). It effectively inhibits the activation of NF-κB and MAPK signaling pathways and reduces pro-inflammatory cytokines, thereby regulating ROS formation at physiological concentrations.23 In summary, AuNPs inhibit oxidative stress in tissues in terms of both ROS production and promotion of ROS clearance, maintaining redox in brain tissues at normal levels and achieving therapeutic effects for AD (Fig. 9).
4. Clinical applications
Currently, there are few drugs with remarkable curative effect for treating AD.106,107 The drugs approved by the FDA2 for AD treatment are memantine, tacrine, galantamine, donepezil, and rivastigmine, which can only relieve the symptoms but cannot eliminate the root cause of the disease and have side effects with long-term use and high drug costs. Therefore, new treatment options need to be explored.108AuNPs can induce good anti-inflammatory and antioxidant functions, and long-term use of anti-inflammatory drugs can reduce the risk of AD, so AuNPs may be a new way to treat AD.109 However, using AuNPs to treat AD also requires consideration of the BBB limitations and biosafety. AuNPs can cross the BBB, and their penetration ability is size-dependent.110,111 15 nm AuNPs have a 500-fold stronger penetration ability into the CNS than 100 nm AuNPs. In addition, among AuNPs from 1.4 nm to 200 nm, AuNPs of 18 nm accumulated the most in the brain, and negatively charged nanoparticles penetrated more than positively charged nanoparticles.112 As for biological safety, there is no conclusive evidence yet. Au is a precious metal that is relatively inert under physiological conditions and has no significant ion release.113 Some scholars believe that AuNPs gold nuclei are not toxic per se, and its capped ligands usually exert toxicity.114
Some scholars have also suggested that AuNPs have potential toxic reactions regarding size, absorption, and metabolism.115 The size of the nanomaterial affects its cellular uptake, distribution in organs and tissues, excretion, and other aspects of biological toxicity. In vitro experiments have found that AuNPs with a 1–2 nm diameter lead to rapid cellular necrosis.116 Khalid et al. investigated the effects of 5 nm, 20 nm, and 50 nm diameter AuNPs on structural and biochemical changes in mouse liver, kidney, and spleen.117 The results showed that 5 nm AuNPs produced significant pathological changes in the liver, followed by a gradual decrease.118 20 nm and 50 nm AuNPs preferentially targeted the spleen, causing significant pathological changes in the spleen structure that persisted. Regardless of the size of the AuNPs, they did not affect the kidney.119 It has also been reported that ultra-small size AuNPs (<2 nm) have a broader biodistribution and longer circulation time in vivo than larger AuNPs.120 In addition, intravenous AuNPs are metabolized primarily by the liver rather than the kidneys because of their larger size. This leads to a higher accumulation of AuNPs in the liver, producing potential hepatotoxicity.119,121 Exploring the appropriate size of AuNPs that can cross the BBB to exert anti-neuroinflammatory effects while avoiding causing cytotoxicity is a worthwhile direction to explore (Table 2).122–124
| Agent | Aize | Model | Cognition function | Ref. |
|---|---|---|---|---|
| PVP, polyvinyl pyrrolidone; ES, ephedra sinica Stapf; EJ, eupatorium japonicum; EO, euphrasia officialize. | ||||
| AuNPs | 9 nm | Type 2 diabetic rat | Anti-inflammatory, relieve diabetes | 20 |
| AuNPs | 40–50 nm | Parkinson's disease rat model | Anti-inflammation, relieves Parkinson's symptoms | 18 |
| ES-GNs | 57.6 ± 3.07 nm | Microglial cells | Anti-neural inflammation | 78 |
| AuNPs | 7.4 ± 1.6 nm | Alcohol-methamphetamine induced liver injury model in rats | Anti-inflammatory, antioxidant | 126 |
| EJ-AuNP | 31–149 nm | Skin inflammation model | Anti-dermatitis | 127 |
| EO-AuNP | 5 nm | RAW 264.7 macrophages | Anti-inflammation | 79 |
| AuNPs | 50 nm | RAW 264.7 macrophages | Anti-inflammation | 80 |
| AuNPs | 20 nm | Chronic subcutaneous injection of AuNPs | Anti-inflammatory and antioxidant effects of gastrocnemius muscle | 104 |
| AuNPs | 10, 20, and 30 nm | HeLa, NIH3T3 and human red blood cells | Weak cytotoxicity of 20 nm AuNPs against NIH3T3 and strong cytotoxicity against HeLa cells | 122 |
| AuNPs | 5, 20, and 50 nm | BALB/c mice | 50 nm AuNPs had the longest circulation time and the widest distribution in liver and spleen tissues, 5 nm AuNPs had increased neutrophils and slightly increased hepatotoxicity | 124 |
| AuNPs | 30, 60, and 100 nm | Eale C57BL/6J mice | AuNPs direct the polarization of M2 macrophages after muscle injury, promoting regeneration and increasing muscle strength. | 62 |
| AuNPs | 85 and 22 nm | RAW-264.7 cells | AuNPs encapsulated with bovine serum albumin are more easily absorbed | 123 |
At present, the therapeutic application of AuNPs in AD is still in the primary research stage, and no clinical trials have been conducted. However, some scholars have coupled AuNPs with other compounds to make new nanomaterials, which will provide a direction for future research. Feng prepared peptide chondroitin sulfate-gold nanoparticles (TAT-CS@Au). In SH-SY5Y cells, TAT-CS@Au reduced the expression of inflammatory cytokines by inhibiting Aβ1-40-induced activation of NF-κB, MAPK pathway, and TAT-CS@Au significantly reduced the Aβ1-40-induced increase in expression levels of TNF-α, IL-1β, IL-6. In addition, TAT-CS@Au reduced malondialdehyde and ROS expression in SH-SY5Y cells, suggesting that TAT-CS@Au can effectively reduce neuroinflammation and oxidative damage.125 Combining AuNPs with other compounds is a potential method for treating AD and is worth exploring.
5. Conclusion and outlook
Neuroinflammation is involved in the pathogenesis of AD. We hypothesize that AuNPs may reduce neuroinflammation and delay AD progression by inducing microglia to polarize toward the M2 phenotype, reducing the expression of pro-inflammatory cytokines such as NO, PGE, TNF-α, IL-1β, IL-6, COX-2, iNOS, and blocking leukocyte adhesion and decreasing oxidative stress. However, this hypothesis requires more in-depth experimental verification. There are fewer studies on the inhibition of NF-KB and MAPK pathways by AuNPs in AD models. However, some scientists have found that in other models, AuNPs have the function of inhibiting NF-κB, MAPK pathways, and downstream inflammatory cytokines. Thaís Gomes et al. established a rat liver injury model with ethanol and METH-injected AuNPs in rats. They found that AuNPs mainly migrated to Kupffer cells in the liver, and 724.96 μg kg−1 AuNPs were able to inhibit the NF-κB pathway and AKT/PI3K pathway in Kuppfer cells, and hepatic stellate cells, MAPK pathway, thereby reducing IL-1β, TNF-α, FGF, SOD-1, and GPx-1 levels, and decreasing Kupffer cell activity in the liver while upregulating IL-10 expression and exerting anti-stress and antioxidant functions.126 Xu et al. prepared EJ-AuNPs from Lycium barbarum to establish a skin inflammation model of HaCaT cells. They reported that EJ-AuNPs might reduce inflammatory mediators such as IL-6, IL-8, TARC, RANTES, and ROS by inhibiting the NF-κB and MAPK pathways, CTACK, ROS production, and release.127 Conclusions in other inflammatory diseases may provide some clues about the role of AuNPs in AD. Further studies are needed on the pathways of AuNPs to inhibit inflammation in AD models. AuNPs can be used not only as a vehicle for drug delivery but also as a drug. However, its biosafety is an issue of concern, and further studies are needed to find the right size as no definite conclusion has been made yet. The use of AuNPs for treating AD is a topic worth exploring in the future, not only to help solve a global public health problem but also to provide a reference for treating other neuroinflammatory diseases. The specific effects of AuNPs on AD and the cellular and molecular mechanisms need to be further explored in the future.Abbreviations
| AD | Alzheimer's disease |
| AuNPs | Gold nanoparticles |
| Aβ | Amyloid β-protein |
| NMDA | N-Methyl-D-aspartate |
| IL-1α | Interleukin-1α |
| IL-1β | Interleukin-1β |
| TNF-α | Tumor necrosis factor-α |
| NO | Nitric oxide |
| APP | Amyloid precursor protein |
| SPR | Surface plasmon resonance |
| IFNγ | Interferon gamma |
| IL-4 | Interleukin 4 |
| iNOS | Inducible nitric oxide synthase 2 |
| COX-2 | Cyclooxygenase-2 |
| ARG2 | Arginase 2 |
| TGF-β | Transforming growth factor-β |
| bFGF | Basic fibroblast growth factor |
| IGF-1 | Insulin-like growth factor 1 |
| NGF | Neurogenic growth factor |
| BDNF | Brain-derived neurotrophin |
| Arg 1 | Arginase 1 |
| iNos | Inducible nitric oxide synthase |
| TLR | Toll-like receptor |
| BMDMs | Bone marrow-derived macrophages |
| FLS | Fibroblast-like synoviocytes |
| HO-1 | Heme oxygenase-1 |
| NQO1 | Quinone oxidoreductase |
| SPR | Surface plasmon resonance |
| CNS | Central nervous systems |
| PSGL1 | P-selectin glycoprotein ligand 1 |
| LFA1 | Lymphocyte Function-associated Antigen 1 |
| ICAM1 | Intercellular cell adhesion molecule-1 |
| CAM | Cell adhesion molecules |
| NF-κB | Nuclear transcription factor-κB |
| PMN | Polymorphonuclear |
| ROS | Reactive oxygen species |
| ETC | Electron transport chain |
| Aβ | β-amyloid |
| SOD | Superoxide dismutase |
| OA | Okadaic acid |
| GSH | Glutathione |
| HCE | Hibiscus syriacus L. callus |
| NAC | N-Acetylcysteine |
| MPO | Myeloperoxidase |
| MAPK | Mitogen-activated protein kinase |
| CK | Compound K |
| BBB | Blood–brain barrier |
Author contributions
Munire Aili: investigation, conceptualization, and writing – original draft; Kebing Zhou: investigation; Jun Zhan: supervision; Huaping Zheng: supervision and writing – review & editing; Feng Luo: conceptualization, supervision, writing – review & editing, and funding acquisition. All authors have read and agreed to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by National Natural Science Foundation of China Youth Science Fund Project (grant no. 82001107) and the Applied Basic Research Project of Sichuan province (grant no. 2022NSFSC1345).References
- J. Graff-Radford, K. X. X. Yong, L. G. Apostolova, F. H. Bouwman, M. Carrillo, B. C. Dickerson, G. D. Rabinovici, J. M. Schott, D. T. Jones and M. E. Murray, Lancet Neurol., 2021, 20, 222–234 CrossRef PubMed.
- E. A. Newcombe, J. Camats-Perna, M. L. Silva, N. Valmas, T. J. Huat and R. Medeiros, J. Neuroinflammation, 2018, 15, 276 CrossRef.
- X. X. Zhang, Y. Tian, Z. T. Wang, Y. H. Ma, L. Tan and J. T. Yu, J. Prev. Alzheimers Dis., 2021, 8, 313–321 Search PubMed.
- M. V. F. Silva, C. M. G. Loures, L. C. V. Alves, L. C. de Souza, K. B. G. Borges and M. D. G. Carvalho, J. Biomed. Sci., 2019, 26, 33 CrossRef PubMed.
- R. Zhou, B. Ji, Y. Kong, L. Qin, W. Ren, Y. Guan and R. Ni, Front. Immunol., 2021, 12, 739130 CrossRef CAS PubMed.
- S. Khan, K. H. Barve and M. S. Kumar, Curr. Neuropharmacol., 2020, 18, 1106–1125 CrossRef CAS PubMed.
- J. Cao, J. Hou, J. Ping and D. Cai, Mol. Neurodegener., 2018, 13, 64 CrossRef CAS PubMed.
- A. Mangalmurti and J. R. Lukens, Curr. Opin. Neurobiol., 2022, 75, 102575 CrossRef CAS PubMed.
- P. S. Sung, P. Y. Lin, C. H. Liu, H. C. Su and K. J. Tsai, Int. J. Mol. Sci., 2020, 21, 701 CrossRef CAS PubMed.
- N. F. Al-Ghraiybah, J. Wang, A. E. Alkhalifa, A. B. Roberts, R. Raj, E. Yang and A. Kaddoumi, Int. J. Mol. Sci., 2022, 23, 10572 CrossRef CAS PubMed.
- T. Ozben and S. Ozben, Clin. Biochem., 2019, 72, 87–89 CrossRef CAS.
- A. Chaney, S. R. Williams and H. Boutin, J. Neurochem., 2019, 149, 438–451 CrossRef CAS.
- P. L. McGeer, J. P. Guo, M. Lee, K. Kennedy and E. G. McGeer, J. Prev. Alzheimers Dis., 2018, 62, 1219–1222 CAS.
- A. B. Cook and P. Decuzzi, ACS Nano, 2021, 15, 2068–2098 CrossRef CAS PubMed.
- R. VanOs, L. L. Lildhar, E. A. Lehoux, P. E. Beaulé and I. Catelas, J. Biomed. Mater. Res., Part B, 2014, 102, 149–159 CrossRef PubMed.
- Y. Wang, C. Wang, K. Li, X. Song, X. Yan, L. Yu and Z. He, J. Controlled Release, 2021, 330, 618–640 CrossRef CAS PubMed.
- H. E. Lee, H. Y. Ahn, J. Mun, Y. Y. Lee, M. Kim, N. H. Cho, K. Chang, W. S. Kim, J. Rho and K. T. Nam, Nature, 2018, 556, 360–365 CrossRef CAS PubMed.
- L. Ling, Y. Jiang, Y. Liu, H. Li, A. Bari, R. Ullah and J. Xue, J. Photochem. Photobiol., B, 2019, 201, 111657 CrossRef CAS.
- M. Fakhoury, Curr. Neuropharmacol., 2018, 16, 508–518 CrossRef CAS PubMed.
- F. Mahmoudi, F. Mahmoudi, K. H. Gollo and M. M. Amini, Biol. Trace Elem. Res., 2022, 200, 2223–2232 CrossRef CAS PubMed.
- M. Singh, M. Thakur, M. Mishra, M. Yadav, R. Vibhuti, A. M. Menon, G. Nagda, V. P. Dwivedi, T. C. Dakal and V. Yadav, Immunol. Lett., 2021, 240, 123–136 CrossRef CAS PubMed.
- N. Dos Santos Tramontin, S. da Silva, R. Arruda, K. S. Ugioni, P. B. Canteiro, G. de Bem Silveira, C. Mendes, P. C. L. Silveira and A. P. Muller, Mol. Neurobiol., 2020, 57, 926–936 CrossRef PubMed.
- R. A. Pinho, D. P. S. Haupenthal, P. E. Fauser, A. Thirupathi and P. C. L. Silveira, J. Inflammation Res., 2022, 15, 3219–3234 CrossRef.
- A. K. Pearce, T. R. Wilks, M. C. Arno and R. K. O’Reilly, Nat. Rev. Chem., 2021, 5, 21–45 CrossRef CAS PubMed.
- C. Snider, D. Grant and S. A. Grant, J. Biomater. Appl., 2022, 36, 1289–1300 CrossRef CAS PubMed.
- B. D. Ratner, Annu. Rev. Biomed. Eng., 2019, 21, 171–191 CrossRef CAS PubMed.
- H. Chen, X. Kou, Z. Yang, W. Ni and J. Wang, Langmuir, 2008, 24, 5233–5237 CrossRef CAS PubMed.
- B. Navyatha and S. Nara, Nanobiomedicine, 2021, 8, 18495435211053945 CrossRef PubMed.
- T. Jennings and G. Strouse, Adv. Exp. Med. Biol., 2007, 620, 34–47 CrossRef.
- V. Amendola, R. Pilot, M. Frasconi, O. M. Maragò and M. A. Iatì, J. Condens. Matter Phys., 2017, 29, 203002 CrossRef.
- P. K. Ngumbi, S. W. Mugo and J. M. Ngaruiya, IOSR J. Appl. Chem., 2018, 11, 25–29 CAS.
- D. M. Kim, J. S. Park, S.-W. Jung, J. Yeom and S. M. Yoo, Sensors, 2021, 21, 3191 CrossRef CAS PubMed.
- A. Agrawal, S. H. Cho, O. Zandi, S. Ghosh, R. W. Johns and D. J. Milliron, Chem. Rev., 2018, 118, 3121–3207 CrossRef CAS.
- A. Timoszyk and R. Grochowalska, Pharmaceutics, 2022, 14, 2599 CrossRef CAS.
- N. Sarfraz and I. Khan, Chem. - Asian J., 2021, 16, 720–742 CrossRef CAS PubMed.
- W. Yang, H. Liang, S. Ma, D. Wang and J. Huang, Sustainable Mater. Technol., 2019, 22, e00109 CrossRef CAS.
- K. Nejati, M. Dadashpour, T. Gharibi, H. Mellatyar and A. Akbarzadeh, J. Cluster Sci., 2021, 1–16 Search PubMed.
- M. J. Ndolomingo, N. Bingwa and R. Meijboom, J. Mater. Sci., 2020, 55, 6195–6241 CrossRef CAS.
- M. Falahati, F. Attar, M. Sharifi, A. A. Saboury, A. Salihi, F. M. Aziz, I. Kostova, C. Burda, P. Priecel and J. A. Lopez-Sanchez, Biochim. Biophys. Acta, Gen. Subj., 2020, 1864, 129435 CrossRef CAS.
- J. H. Cherng, C. J. Lin, C. C. Liu, J. Z. Yeh, G. Y. Fan, H. D. Tsai, C. F. Chung and S. D. Hsu, J. Pers. Med., 2022, 12, 1089 CrossRef.
- S. M. Shahen, M. R. Mohamed, M. R. K. Ali, R. M. Samaka, G. M. Hamdy and R. M. Talaat, Clin. Exp. Pharmacol. Physiol., 2021, 48, 1346–1357 CrossRef CAS PubMed.
- P. Kesharwani, B. Gorain, S. Y. Low, S. A. Tan, E. C. S. Ling, Y. K. Lim, C. M. Chin, P. Y. Lee, C. M. Lee, C. H. Ooi, H. Choudhury and M. Pandey, Diabetes Res. Clin. Pract., 2018, 136, 52–77 CrossRef CAS.
- M. A. Khan and M. J. Khan, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 1149–1158 CrossRef CAS PubMed.
- J. Chen, M. Yuan, C. A. Madison, S. Eitan and Y. Wang, J. Controlled Release, 2022, 345, 557–571 CrossRef CAS.
- H. Yang, S. Lu, S. Wang, L. Liu, B. Zhu, S. Yu, S. Yang and J. Chang, Int. J. Biol. Macromol., 2021, 191, 192–200 CrossRef CAS PubMed.
- L. Xiao, F. Wei, Y. Zhou, G. J. Anderson, D. M. Frazer, Y. C. Lim, T. Liu and Y. Xiao, Nano Lett., 2020, 20, 478–495 CrossRef CAS PubMed.
- S. Zhang, F. Xie, K. Li, H. Zhang, Y. Yin, Y. Yu, G. Lu, S. Zhang, Y. Wei, K. Xu, Y. Wu, H. Jin, L. Xiao, L. Bao, C. Xu, Y. Li, Y. Lu and J. Gao, Acta Pharm. Sin. B, 2022, 12, 3124–3138 CrossRef CAS.
- S. Yang, Y. Zhang, S. Lu, L. Yang, S. Yu and H. Yang, ACS Appl. Bio Mater., 2021, 4, 3214–3223 CrossRef CAS PubMed.
- A. K. Dey, A. Gonon, E. I. Pécheur, M. Pezet, C. Villiers and P. N. Marche, Cells, 2021, 10, 96 CrossRef CAS PubMed.
- A. Serrano-Pozo, S. Das and B. T. Hyman, Lancet Neurol., 2021, 20, 68–80 CrossRef CAS.
- N. Li, Q. Du, Z. Jing, L. Xue, W. He, X. Zhang and Z. Sun, Biomate. Adv., 2022, 138, 212800 CrossRef CAS PubMed.
- B. Xu, Y. He, Y. Zhang, Z. Ma, Y. Zhang and W. Song, ACS Appl. Mater. Interfaces, 2022, 14, 50520–50533 CrossRef CAS PubMed.
- M. Amina, N. M. Al Musayeib, N. A. Alarfaj, M. F. El-Tohamy and G. A. Al-Hamoud, Nanomaterials, 2020, 10, 2453 CrossRef CAS PubMed.
- P. C. L. Silveira, M. S. Rodrigues, D. P. Gelain and J. de Oliveira, Metab. Brain Dis., 2023, 38, 123–135 CrossRef PubMed.
- X. J. Mi, X. Y. Xu, H. S. Choi, H. Kim, I. H. Cho, T. H. Yi and Y. J. Kim, Int. J. Nanomed., 2022, 17, 477–494 CrossRef CAS PubMed.
- C. Ni, J. Zhou, N. Kong, T. Bian, Y. Zhang, X. Huang, Y. Xiao, W. Yang and F. Yan, Biomaterials, 2019, 206, 115–132 CrossRef CAS PubMed.
- S. Taratummarat, N. Sangphech, C. T. B. Vu, T. Palaga, T. Ondee, S. Surawut, A. Sereemaspun, P. Ritprajak and A. Leelahavanichkul, BMC Microbiol., 2018, 18, 85 CrossRef PubMed.
- L. Wang, H. Zhang, L. Sun, W. Gao, Y. Xiong, A. Ma, X. Liu, L. Shen, Q. Li and H. Yang, J. Nanobiotechnol., 2020, 18, 38 CrossRef CAS PubMed.
- J. Y. Park, S. Kwon, S. H. Kim, Y. J. Kang and D. Khang, ACS Appl. Mater. Interfaces, 2020, 12, 38936–38949 CrossRef CAS PubMed.
- M. Sousa de Almeida, P. Taladriz-Blanco, B. Drasler, S. Balog, P. Yajan, A. Petri-Fink and B. Rothen-Rutishauser, Nanomaterials, 2022, 12, 690 CrossRef CAS PubMed.
- H. R. Ali, S. A. Selim and D. Aili, RSC Adv., 2021, 11, 25047–25056 RSC.
- T. M. Raimondo and D. J. Mooney, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 10648–10653 CrossRef CAS PubMed.
- X. Bai, D. Chen, Y. Dai, S. Liang, B. Song, J. Guo, B. Dai, D. Zhang and L. Feng, Nanomedicine, 2021, 38, 102457 CrossRef CAS PubMed.
- M. Hossain, M. Uddin, G. Uddin, D. Sumsuzzman, M. Islam, G. Barreto, B. Mathew and G. Ashraf, Mol. Neurobiol., 2019, 56, 8255–8276 CrossRef CAS PubMed.
- M. C. Chiang, C. J. B. Nicol, C. H. Lin, S. J. Chen, C. Yen and R. N. Huang, Neurochem. Int., 2021, 145, 104992 CrossRef CAS PubMed.
- G. M. Alshammari, M. A. Abdelhalim, M. S. Al-Ayed, L. N. Al-Harbi and M. A. Yahya, Nutrients, 2022, 14, 3327 CrossRef CAS PubMed.
- A. S. M. Aljohani, A. A. H. Abdellatif, Z. Rasheed and W. A. Abdulmonem, J. Biomed. Nanotechnol., 2022, 18, 581–588 CrossRef CAS PubMed.
- G. Wang, X. Shen, X. Song, N. Wang, X. Wo and Y. Gao, Neurotoxicology, 2023, 95, 12–22 CrossRef CAS PubMed.
- R. Akter, L. Ling, E. J. Rupa, J. KyuPark, R. Mathiyalagan, J. Nahar, L. J. Won, K. D. Hyun, M. Murugesan, D. C. Yang, S. C. Kang and G. Y. Kwak, Molecules, 2022, 27, 2795 CrossRef CAS PubMed.
- M. L. Yola and N. Atar, Anal. Bioanal. Chem., 2021, 413, 2481–2492 CrossRef CAS PubMed.
- T. H. M. Tran, A. M. Puja, H. Kim and Y. J. Kim, Biomate. Adv., 2022, 137, 212814 CrossRef CAS PubMed.
- J. Kim, H. J. Lee, S. K. Park, J. H. Park, H. R. Jeong, S. Lee, H. Lee, E. Seol and H. S. Hoe, Int. J. Mol. Sci., 2021, 22, 10637 CrossRef CAS PubMed.
- R. Wang, S. K. Moon, W. J. Kim, S. Dhandapani, H. Kim and Y. J. Kim, ACS Omega, 2022, 7, 35951–35960 CrossRef CAS PubMed.
- R. Dhapola, S. S. Hota, P. Sarma, A. Bhattacharyya, B. Medhi and D. H. Reddy, Inflammopharmacology, 2021, 29, 1669–1681 CrossRef CAS PubMed.
- L. Li, Y. Zhang, M. Wang, J. Zhou, Q. Zhang, W. Yang, Y. Li and F. Yan, Front. Bioeng. Biotechnol., 2021, 9, 631191 CrossRef PubMed.
- Q. Yuan, Y. Yao, X. Zhang, J. Yuan, B. Sun and X. Gao, J. Nanosci. Nanotechnol., 2019, 19, 1986–1995 CrossRef CAS PubMed.
- A. P. Muller, G. K. Ferreira, A. J. Pires, G. de Bem Silveira, D. L. de Souza, J. A. Brandolfi, C. T. de Souza, M. M. S. Paula and P. C. L. Silveira, Mater. Sci. Eng., C, 2017, 77, 476–483 CrossRef CAS PubMed.
- S. Y. Park, E. H. Yi, Y. Kim and G. Park, Int. J. Nanomed., 2019, 14, 2861–2877 CrossRef CAS PubMed.
- Y. Liu, S. Kim, Y. J. Kim, H. Perumalsamy, S. Lee, E. Hwang and T. H. Yi, Int. J. Nanomed., 2019, 14, 2945–2959 CrossRef CAS PubMed.
- S. Ahn, P. Singh, M. Jang, Y. J. Kim, V. Castro-Aceituno, S. Y. Simu, Y. J. Kim and D. C. Yang, Colloids Surf., B, 2018, 162, 398–404 CrossRef CAS PubMed.
- S. Ahn, P. Singh, V. Castro-Aceituno, S. Yesmin Simu, Y. J. Kim, R. Mathiyalagan and D. C. Yang, Artif. Cells, Nanomed., Biotechnol., 2017, 45, 270–276 CrossRef CAS PubMed.
- H. Y. Lorenzo-Anota, D. G. Zarate-Triviño, J. A. Uribe-Echeverría, A. Ávila-Ávila, J. R. Rangel-López, A. C. Martínez-Torres and C. Rodríguez-Padilla, Pharmaceutics, 2021, 13, 942 CrossRef CAS PubMed.
- L. C. Shanley, O. R. Mahon, D. J. Kelly and A. Dunne, Acta Biomater., 2021, 133, 208–221 CrossRef CAS PubMed.
- B. A. David and P. Kubes, Immunol. Rev., 2019, 289, 9–30 CrossRef CAS PubMed.
- P. X. Liew and P. Kubes, Physiol. Rev., 2019, 99, 1223–1248 CrossRef CAS PubMed.
- T. M. Bui, H. L. Wiesolek and R. Sumagin, J. Leukocyte Biol., 2020, 108, 787–799 CrossRef CAS PubMed.
- Z. Y. Soe, E. J. Park and M. Shimaoka, Int. J. Mol. Sci., 2021, 22, 2193 CrossRef CAS PubMed.
- M. K. Uchiyama, D. K. Deda, S. F. Rodrigues, C. C. Drewes, S. M. Bolonheis, P. K. Kiyohara, S. P. Toledo, W. Colli, K. Araki and S. H. Farsky, Toxicol. Sci., 2014, 142, 497–507 CrossRef CAS PubMed.
- D. Di Bella, J. P. S. Ferreira, R. N. O. Silva, C. Echem, A. Milan, E. H. Akamine, M. H. Carvalho and S. F. Rodrigues, J. Nanobiotechnol., 2021, 19, 52 CrossRef CAS PubMed.
- D. P. Dos Santos Haupenthal, C. Mendes, G. de Bem Silveira, R. P. Zaccaron, M. Corrêa, R. T. Nesi, R. A. Pinho, M. M. da Silva Paula and P. C. L. Silveira, J. Biomed. Mater. Res., Part A, 2020, 108, 103–115 CrossRef CAS PubMed.
- M. C. Chiang and C. J. B. Nicol, Free Radical Biol. Med., 2022, 187, 185–201 CrossRef CAS PubMed.
- I. Wahid, P. Rani, S. Kumari, R. Ahmad, S. J. Hussain, S. Alamri, N. Tripathy and M. I. R. Khan, Chemosphere, 2022, 287, 132142 CrossRef CAS PubMed.
- M. Marí, E. de Gregorio, C. de Dios, V. Roca-Agujetas, B. Cucarull, A. Tutusaus, A. Morales and A. Colell, Antioxidants, 2020, 9, 909 CrossRef PubMed.
- G. T. Sen, G. Ozkemahli, R. Shahbazi, P. Erkekoglu, K. Ulubayram and B. Kocer-Gumusel, Int. J. Toxicol., 2020, 39, 328–340 CrossRef CAS PubMed.
- M. S. Uddin and M. T. Kabir, Biological, Diagnostic and Therapeutic Advances in Alzheimer's Disease: Non-Pharmacological Therapies for Alzheimer's Disease, 2019, pp. 91–115 Search PubMed.
- G. Bjørklund, J. Aaseth, M. Dadar and S. Chirumbolo, Mol. Neurobiol., 2019, 56, 7032–7044 CrossRef PubMed.
- M. Rodríguez-Giraldo, R. E. González-Reyes, S. Ramírez-Guerrero, C. E. Bonilla-Trilleras, S. Guardo-Maya and M. O. Nava-Mesa, Int. J. Mol. Sci., 2022, 23, 13630 CrossRef PubMed.
- N. dos Santos Tramontin, S. da Silva, R. Arruda, K. S. Ugioni, P. B. Canteiro, G. de Bem Silveira, C. Mendes, P. C. L. Silveira and A. P. Muller, Mol. Neurobiol., 2020, 57, 926–936 CrossRef PubMed.
- S. Srivastav, B. G. Anand, M. Fatima, K. P. Prajapati, S. S. Yadav, K. Kar and A. C. Mondal, ACS Chem. Neurosci., 2020, 11, 3772–3785 CrossRef CAS PubMed.
- X. Y. Xu, T. H. M. Tran, H. Perumalsamy, D. Sanjeevram and Y. J. Kim, Mater. Sci. Eng., C, 2021, 124, 112035 CrossRef CAS PubMed.
- F. Petronilho, L. Tenfen, A. Della Giustina, L. Joaquim, M. Novochadlo, A. N. de Oliveira Junior, E. Bagio, M. P. S. Goldim, R. J. de Carli, S. Bonfante, K. L. L. Metzker, S. Muttini, T. M. Dos Santos, M. P. de Oliveira, N. A. Engel, G. T. Rezin, L. A. Kanis and T. Barichello, J. Drug Targeting, 2020, 28, 428–436 CrossRef CAS PubMed.
- A. Thirupathi, H. R. Sorato, P. R. L. Silva, A. P. Damiani, V. M. Andrade, P. C. L. Silveira, R. T. Nesi, M. M. S. Paula and R. A. Pinho, An. Acad. Bras. Cienc., 2021, 93, e20191450 CrossRef CAS PubMed.
- V. Ramalingam and R. Rajaram, Process Biochem., 2021, 100, 69–81 CrossRef CAS.
- D. Haupenthal, F. M. Dias, R. P. Zaccaron, G. B. Silveira, M. Corrêa, C. Mendes, L. R. Casagrande, R. A. Pinho, T. A. M. de Andrade, P. E. Feuser, M. Paula and P. C. L. Silveira, Eur. J. Pharm. Sci., 2020, 143, 105120 CrossRef CAS PubMed.
- F. R. da Rocha, D. Haupenthal, R. P. Zaccaron, M. Corrêa, N. D. S. Tramontin, J. P. Fonseca, R. T. Nesi, A. P. Muller, R. A. Pinho, M. Paula and P. C. L. Silveira, J. Drug Targeting, 2020, 28, 307–319 CrossRef PubMed.
- T. G. Götzelmann, D. Strech and H. Kahrass, BMC Med. Ethics, 2021, 22, 32 CrossRef PubMed.
- S. A. Tatulian, Drug Discovery Today, 2022, 27, 1027–1043 CrossRef PubMed.
- S. Tiwari, V. Atluri, A. Kaushik, A. Yndart and M. Nair, Int. J. Nanomed., 2019, 14, 5541–5554 CrossRef CAS.
- J. Li, L. Chen, X. Xu, Y. Fan, X. Xue, M. Shen and X. Shi, Small, 2020, 16, e2005661 CrossRef PubMed.
- M. Khongkow, T. Yata, S. Boonrungsiman, U. R. Ruktanonchai, D. Graham and K. Namdee, Sci. Rep., 2019, 9, 8278 CrossRef PubMed.
- S. Ohta, E. Kikuchi, A. Ishijima, T. Azuma, I. Sakuma and T. Ito, Sci. Rep., 2020, 10, 18220 CrossRef CAS PubMed.
- S. M. D. Rizvi, T. Hussain, A. B. F. Ahmed, T. M. Alshammari, A. Moin, M. Q. Ahmed, G. E. Barreto, M. A. Kamal and G. M. Ashraf, Biomed. Pharmacother., 2018, 107, 7–18 CrossRef CAS PubMed.
- Y. Yu, T. Yang and T. Sun, Nanomedicine, 2020, 15, 1127–1145 CrossRef CAS PubMed.
- B. G. Anand, Q. Wu, G. Karthivashan, K. P. Shejale, S. Amidian, H. Wille and S. Kar, Bioactive Mater., 2021, 6, 4491–4505 CrossRef CAS PubMed.
- M. Magogotya, M. Vetten, M. P. Roux-van der Merwe, J. Badenhorst and M. Gulumian, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2022, 883–884, 503556 CrossRef CAS PubMed.
- A.-L. Bailly, F. Correard, A. Popov, G. Tselikov, F. Chaspoul, R. Appay, A. Al-Kattan, A. V. Kabashin, D. Braguer and M.-A. Esteve, Sci. Rep., 2019, 9, 12890 CrossRef PubMed.
- M. Enea, E. Pereira, J. Costa, M. E. Soares, D. Dias da Silva, M. L. Bastos and H. F. Carmo, Toxicol. In Vitro, 2021, 70, 105046 CrossRef CAS PubMed.
- S. S. I. Abdalla, H. Katas, F. Azmi and M. F. M. Busra, Curr. Drug Delivery, 2020, 17, 88–100 CrossRef PubMed.
- K. E. Ibrahim, M. G. Al-Mutary, A. O. Bakhiet and H. A. Khan, Molecules, 2018, 23, 1848 CrossRef PubMed.
- R. D. Mellor and I. F. Uchegbu, Nanomaterials, 2022, 12, 2476 CrossRef CAS.
- H. P. Borase, A. B. Muley, S. V. Patil and R. S. Singhal, Environ. Toxicol. Pharmacol., 2019, 68, 4–12 CrossRef CAS PubMed.
- L. P. Tartuce, F. Pacheco Brandt, G. Dos Santos Pedroso, H. Rezende Farias, B. Barros Fernandes, B. da Costa Pereira, A. Gonçalves Machado, P. E. Feuser, P. C. Lock Silveira, R. Tiscoski Nesi, M. M. da Silva Paula, M. Andrades and R. A. de Pinho, Colloids Surf., B, 2020, 192, 111012 CrossRef CAS PubMed.
- T. Li, Y. Wang, M. Wang, L. Zheng, W. Dai, C. Jiao, Z. Song, Y. Ma, Y. Ding, Z. Zhang, F. Yang and X. He, Nanomaterials, 2022, 12, 749 CrossRef CAS PubMed.
- Q. Xia, J. Huang, Q. Feng, X. Chen, X. Liu, X. Li, T. Zhang, S. Xiao, H. Li, Z. Zhong and K. Xiao, Int. J. Nanomed., 2019, 14, 6957–6970 CrossRef CAS PubMed.
- Y. Feng, X. Li, D. Ji, J. Tian, Q. Peng, Y. Shen and Y. Xiao, Int. J. Biol. Macromol., 2023, 230, 123125 CrossRef CAS PubMed.
- T. G. de Carvalho, V. B. Garcia, A. A. de Araújo, L. H. da Silva Gasparotto, H. Silva, G. C. B. Guerra, E. de Castro Miguel, R. F. de Carvalho Leitão, D. V. da Silva Costa, L. J. Cruz, A. B. Chan and R. F. de Araújo Júnior, Int. J. Pharm., 2018, 548, 1–14 CrossRef CAS PubMed.
- X. Y. Xu, S. K. Moon, J. K. Kim, W. J. Kim, Y. J. Kim and H. Kim, Front. Pharmacol., 2022, 13, 1055378 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |