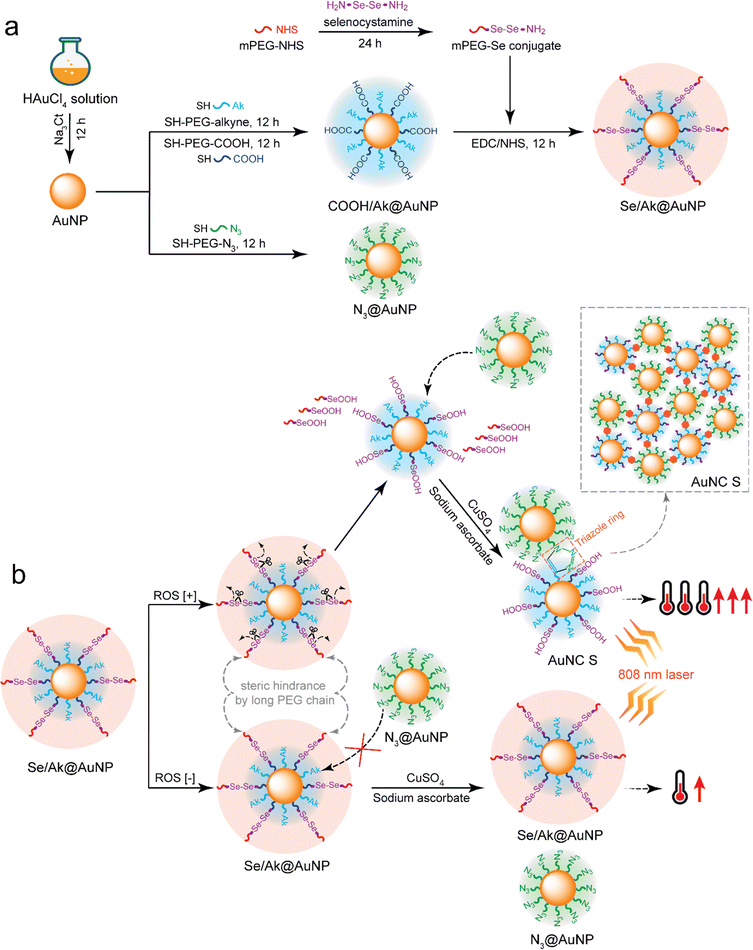
Reactive oxygen species-responsive clicked assembly of gold nanoparticles to enhance photothermal therapy†
Hoai-Thuong Duc
Bui
 a,
Yeonju
Park
b,
Young Mee
Jung
a,
Yeonju
Park
b,
Young Mee
Jung
 bcd,
Sing Yian
Chew
bcd,
Sing Yian
Chew
 efg and
Hyuk Sang
Yoo
efg and
Hyuk Sang
Yoo
 *ab
*ab
aDepartment of Medical Biomaterials Engineering, Kangwon National University, Chuncheon 24341, Republic of Korea. E-mail: hsyoo@kangwon.ac.kr; Web: https://nano-bio.kangwon.ac.kr
bKangwon Radiation Convergence Research Support Center, Kangwon National University, Chuncheon 24341, Republic of Korea
cDepartment of Chemistry, Institute for Molecular Science and Fusion Technology, Kangwon National University, Chuncheon 24341, Republic of Korea
dKangwon Institute of Inclusive Technology, Kangwon National University, Chuncheon 24341, Republic of Korea
eSchool of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, Singapore 637459, Singapore
fLee Kong Chian School of Medicine, Nanyang Technological University, Singapore 308232, Singapore
gSchool of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore
First published on 15th June 2023
Abstract
To enhance the efficacy of photothermal therapy (PTT) at tumor sites, we designed a reactive oxygen species (ROS)-responsive gold nanoparticle (AuNP)-based nanosystem in which azide-decorated AuNPs (N3@AuNPs) and diselenide-coated alkyne-decorated AuNPs (Se/Ak@AuNPs) were separately prepared for selective clicking into nanoclusters when exposed to ROS. Se/Ak@AuNPs were dual-functionalized with alkyne moieties and diselenide linkers embedded in a long chain of polyethylene glycol (PEG) to enable the alkyne moieties of Se/Ak@AuNPs to be inaccessible to the azide moieties of N3@AuNPs owing to steric hindrance. At tumor sites where the ROS level is elevated due to the increased metabolic activity, cellular receptor signaling, mitochondrial dysfunction, and oncogene activity, the diselenide linkers were cleaved, leading to the liberation of the long PEG chains tethered to AuNPs, and the alkyne moieties could be recognized by the surrounding azide moieties to generate a click reaction. The clicked AuNPs formed clustered nanoparticles with increased size. Upon 808 nm laser irradiation, these large clusters of AuNPs significantly enhanced the photothermal conversion efficiency compared with that of isolated AuNPs. In vitro studies revealed that the AuNP clusters exhibited a noticeably higher apoptosis rate than AuNPs. Therefore, ROS-responsive clicked AuNP clusters can be a potential tool for PTT enhancement in cancer treatment.
1. Introduction
Among the emerging cancer treatment modalities, such as gene therapy, immunotherapy, and thermal therapies, photothermal therapy (PTT) has attracted considerable interest as an effective strategy owing to its safety, selectivity in tumor tissues, and minimal invasiveness. PTT employs photothermal agents to achieve hyperthermia from an external laser light, such as near-infrared (NIR), which can penetrate human skin without invading the skin and other tissues.1–3 Studies have shown that proteins undergo denaturation when the cellular temperature exceeds 39 °C, which results in the aggregation of proteins, thereby reducing cell viability. Cellular temperatures above 43 °C may cause long-term cell inactivation. Moreover, the rate of biochemical reactions is significantly increased, which in turn leads to an increase in the intracellular density of reactive oxygen species (ROS), which may cause oxidative damage to proteins, lipids, and nucleic acids.4 In addition, photothermal agents can be chemically functionalized to ensure their accumulation at tumor sites; thus, PTT therapeutics can be localized in cancer cells without causing severe damage to the surrounding normal cells.3,4 Gold nanoparticles (AuNPs) have been recognized as promising photothermal agents owing to their high biocompatibility, stability, photothermal conversion efficiency, and ease of synthesis. In addition, the surfaces of AuNPs are known to exhibit advantageous properties for tumor-targeted chemical functionalization.1,3,5,6 Studies have reported that the optical absorbance of spherical AuNPs can be red-shifted close to the NIR region by modulating their size, which is a noticeable benefit of employing AuNPs to improve photothermal conversion efficiency. Link and El-Sayed7 reported that when the size of spherical AuNPs was increased from 9 to 99 nm, the plasmon absorbance shifted from 517 to 575 nm. Nanoparticles with larger sizes selectively infiltrate the abnormal vasculature at the tumor site and prolong their retention. However, large-sized particles may not fully penetrate the tumor tissue owing to the dense extracellular matrix and elevated interstitial fluid pressure of tumor tissues. Moreover, studies show that the microvasculature of different tumor types exhibits a specific pore-size distribution. For example, the pore size of breast and pancreatic tumors is in the range of 50–60 nm, whereas that of brain tumors may range from 1 to 7 nm.8 Therefore, unchangeable large-sized nanoparticles cannot be flexibly employed for different types of cancers. Larger particles may also increase the attraction and rapid clearance caused by the mononuclear phagocytic system. In contrast, smaller nanoparticles exhibit better cell infiltration, longer blood circulation time, and shorter biological half-life, but they cannot be retained in the tissue and are easily expelled to the blood flow, which causes low therapeutic efficacy. In particular, because of their small size, these particles may penetrate both normal and tumor cells, which may lead to damage to healthy cells and severe systemic side effects in patients.9–11 To overcome these challenges, many researchers have developed stimuli-responsive nanosystems in which small nanoparticles are delivered that form large aggregates selectively under changes in the microenvironment at the tumor site. These systems employ unique characteristics of the tumor microenvironment, such as acidic pH, high levels of ROS, and overexpression of specific enzymes, to control the formation of nanosystems and therapeutic specificity at the tumor site.5,9–12 A 30 nm AuNP-based system was prepared in which AuNP-As were modified with negatively charged peptides, and AuNP-Bs were modified with peptides grafted to 2,3-dimethylmaleic anhydride which were acidity-triggered charge converted. At acidic pH at the tumor site, AuNP-Bs converted to a positively charged state and subsequently aggregated with AuNP-As via electrostatic interaction.5 In another study, 15 nm AuNPs were surface-functionalized with two oppositely charged ligands, 11-mercaptoundecanoic acid and (10-mercaptodecyl)trimethylammonium bromide, to generate a mixed-charge zwitterionic surface. At the tumor site at pH 6.5, AuNP-based aggregates were formed, which increased the localized surface plasmon resonance of AuNPs and further enhanced the effect of PTT as well as photoacoustic imaging signals in the tumor tissue. Although small AuNPs could infiltrate normal cells, aggregates were not formed at pH 7.4. Therefore, these isolated AuNPs can be expelled into the blood flow and then eliminated from the human body, which prevents further damage to healthy tissue.9 ROS, which are elevated in a tumor site due to the imbalance of the redox state, oncogenic activation, increased cellular signaling, metabolic activity, and mitochondrial dysfunction,13,14 have also been utilized for the design of tumor targeted nanosystems. Mao et al.11 employed an H2O2-initiated chemiluminescence-triggered AuNP aggregation strategy in which two types of AuNPs were fabricated by conjugating 2,5-diphenyltetrazole (tAuNPs) and methacrylic acid (mAuNPs) onto the surface of AuNPs separately; luminol was subsequently adsorbed onto the mAuNPs to create self-illuminating mAuNP/Lu nanoparticles that could produce strong chemiluminescence by reacting with H2O2 in the tumor microenvironment. This further led to the aggregation of tAuNPs and mAuNP/Lu, resulting in improved accumulation and retention of AuNPs for the enhancement of photoacoustic imaging and PTT. Although these studies demonstrated optimistic results for enhancing the PTT effect, non-PEGylated AuNPs or short polyethylene glycol (PEG)-chain-decorated AuNPs has been known to exhibit poor colloidal stability for long-term storage and may be easily opsonized by the mononuclear phagocytic system.15ROS-sensitive molecules such as thioketals, boronic esters, peroxalate esters, thioethers, selenoethers, and tellurium have recently been studied for tumor-targeted drug delivery and other biomedical applications. Among these, the diselenide bond has been employed as an effective ROS-responsive linker owing to its easy oxidation in the presence of hydrogen peroxide (H2O2) and cleaving into selenic acid, which is highly biocompatible.16–19 Selenium is well known for its anti-cancer effects as it can regulate the expression of redox-active proteins and modulate the intracellular redox status.20 In a previous study, doxorubicin-encapsulated diselenide-cross-linked micelles were designed. The micelles maintained their structural integrity for at least 6 days under physiological conditions. However, under ROS-rich conditions, the hydrophobic diselenide bond was cleaved into hydrophilic selenic acid derivatives, which caused the micelles to lose their structural integrity and led to the rapid release of encapsulated doxorubicin from the micelles.16
Copper-catalyzed azide–alkyne 1,3-dipolar cycloaddition, popularly known as the click reaction, has become a potential strategy for the facile construction of new structures in the fields of biomaterial science, biochemistry, pharmacology, and medicinal science owing to its high specificity, high yield, biocompatibility, absence of by-products, and ease of modulation.21,22 In our previous study, a clicked AuNP cluster was designed in which azide-functionalized AuNPs were clicked into nanoclusters owing to the presence of an alkyne-grafted matrix metalloproteinase-2 (MMP-2) cleavable peptide as the linker. The size of these nanoclusters could be easily controlled by changing the number of alkyne moieties.22 In another study, AuNPs were dual-functionalized, with azide moieties as the primary PEG shells and MMP-cleavable peptide-conjugated methoxy–PEG as the secondary PEG shells, for selective click reactions with cyclooctyne moieties of quantum dots in an MMP-rich environment. Under the overexpression of MMP at the tumor site, the secondary PEG shell was de-shielded, and the exposed azide moieties were directed towards the quantum dots, which enabled a click reaction between these two particles into nanoclusters.23
In this study, we developed an ROS-sensitive AuNP-based nanosystem in which azide-decorated AuNPs (N3@AuNPs) and diselenide-coated alkyne-decorated AuNPs (Se/Ak@AuNPs) were separately synthesized for ROS-responsive clicking into nanoclusters. In particular, both the azide and alkyne moieties conjugated with PEG were surface-engineered on AuNPs via the Au-S reaction. The decoration of long PEG chains on each AuNP can enhance the colloidal stability and support these nanoparticles to minimize opsonization from the mononuclear phagocyte system, thereby prolonging their circulation in the blood flow.15 To establish the ROS-responsive property of the click reaction, we additionally decorated alkyne-functionalized AuNPs with diselenide linkers embedded in a long chain of PEG to ensure that the alkyne moieties were inaccessible owing to steric hindrance.23–25 In an ROS-rich environment, the diselenide linkers are cleaved, leading to the liberation of the long PEG chains tethered to the AuNPs, and the alkyne moieties can be recognized by the surrounding azide moieties to generate a click reaction.16–18,23 The clicked AuNPs form clustered nanoparticles, and the size of the clusters, which is related to the reaction rate, can be manipulated according to the level of copper.26 Upon appropriate NIR irradiation, these large clusters of AuNPs are anticipated to increase the conversion temperature compared with that of isolated AuNPs owing to the red shift of the surface plasmon resonance absorbance.5,9 We speculate that ROS-responsive clicked AuNP clusters can be a potential strategy for PTT enhancement in cancer treatment.
2. Materials and methods
2.1. Materials
Gold(III) chloride trihydrate (HAuCl4), trisodium citrate dihydrate (Na3Ct), N-hydroxysulfosuccinimide sodium salt (NHS), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), and phosphotungstic acid (PTA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Azide-poly(ethylene glycol)-thiol (N3–PEG–SH, MW 3.4 kDa), alkyne-poly(ethylene glycol)-thiol (Ak–PEG–SH, MW 3.4 kDa), carboxyl-poly(ethylene glycol)-thiol (COOH–PEG–SH, MW 3.5 kDa), and methoxy poly(ethylene glycol) succinimidyl carboxymethyl ester (mPEG-NHS, MW 5 kDa) were purchased from JenKem Technology (TX, USA). Selenocystamine dihydrochloride (Secy) was purchased from BOC Sciences (NY, USA). Copper sulfate (CuSO4) was purchased from ChemCruz™ (TX, USA). H2O2 30% and sodium ascorbate were purchased from Daejung Chemicals and Metals (Gyeonggi, Republic of Korea). Flamma® 648 Azide was purchased from BioActs (Incheon, Republic of Korea). Alexa Fluor 647™ Alkyne was purchased from Thermo Fisher Scientific (Ward Hill, MA, USA). The water-soluble tetrazolium salt (WST-1) reagent was purchased from DoGenBio (Seoul, Republic of Korea). EzWay™ Annexin V-FITC Apoptosis Detection Kit was purchased from Labiskoma (Seoul, Republic of Korea). Dulbecco's modified Eagle's medium (DMEM), Dulbecco's phosphate-buffered saline (PBS), trypsin-EDTA (T/E), and penicillin/streptomycin (P/S) were purchased from Gibco (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from Corning (Woodland, CA, USA). The mouse embryonic fibroblast cell line, NIH3T3, was obtained from the Korean Cell Line Bank (Seoul, Republic of Korea). All other chemicals used were of analytical grade.2.2. Preparation of surface-functionalized AuNPs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N3–PEG–SH = 1
N3–PEG–SH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) to enhance colloidal stability and introduce functional moieties for the click reaction. PEGylation was conducted for 12 h, after which unreacted PEG was removed using centrifugation at 12
000) to enhance colloidal stability and introduce functional moieties for the click reaction. PEGylation was conducted for 12 h, after which unreacted PEG was removed using centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min (three washes with DW). The N3@AuNPs were re-suspended in DW at 1 mg mL−1 and stored at 4 °C until further use.
000 rpm for 10 min (three washes with DW). The N3@AuNPs were re-suspended in DW at 1 mg mL−1 and stored at 4 °C until further use.
The hydrodynamic size and zeta potential of the bare AuNPs and N3@AuNPs were analyzed using dynamic light scattering (DLS, Nano-ZS; Malvern Zetasizer, UK), the morphology was observed under field-emission transmission electron microscopy (FE-TEM, S-4300; Hitachi, Japan), and the concentration (conc.) of Au atoms on the AuNPs was investigated using inductively coupled plasma atomic emission spectroscopy (ICP-OES, 7300DV; Optima, Japan) at the Central Laboratory of Kangwon National University. For FE-TEM, bare and functionalized AuNPs dropped on carbon-supported copper grids (200 mesh; EMS, PA, USA) were stained with a 2% PTA solution in DW (w/v, pH = 7) for 30 min at 25 ± 2 °C in the dark before the investigation. The sizes of the AuNPs obtained from the FE-TEM images were analyzed using ImageJ software (n = 20).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mPEG-NHS = 1.2
mPEG-NHS = 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) into the Secy solution while vigorously stirring. The reaction was maintained for 24 h at 25 ± 2 °C, the remaining Secy was removed via dialysis against DW (MWCO: 3.5 kDa) for 48 h, and the mPEG-Se conjugates were obtained through freeze-drying. To confirm the conjugation, we analyzed the mPEG-Se using proton nuclear magnetic resonance (1H-NMR, JNM-ECZ400S/L1; JEOL, Japan) at the Central Laboratory of Kangwon National University and Fourier-transform infrared spectrometer (FT-IR, iS50; Thermo Fisher Scientific, MA, USA) with a built-in attenuated total reflection (ATR, diamond crystal, 45°) mode and a deuterated triglycine sulphate (DTGS) detector at the Kangwon Radiation Convergence Research Support Center of the Korea Basic Science Institute, Kangwon National University.
1) into the Secy solution while vigorously stirring. The reaction was maintained for 24 h at 25 ± 2 °C, the remaining Secy was removed via dialysis against DW (MWCO: 3.5 kDa) for 48 h, and the mPEG-Se conjugates were obtained through freeze-drying. To confirm the conjugation, we analyzed the mPEG-Se using proton nuclear magnetic resonance (1H-NMR, JNM-ECZ400S/L1; JEOL, Japan) at the Central Laboratory of Kangwon National University and Fourier-transform infrared spectrometer (FT-IR, iS50; Thermo Fisher Scientific, MA, USA) with a built-in attenuated total reflection (ATR, diamond crystal, 45°) mode and a deuterated triglycine sulphate (DTGS) detector at the Kangwon Radiation Convergence Research Support Center of the Korea Basic Science Institute, Kangwon National University.
To introduce alkyne moieties to induce selective click reactions towards azide moieties on N3@AuNPs and amine-reactive moieties for conjugation with mPEG-Se, we co-decorated Ak–PEG–SH and COOH–PEG–SH on bare AuNPs using the same strategy as azide functionalization. Specifically, after the synthesis of bare AuNPs, Ak–PEG–SH and COOH–PEG–SH were added to the AuNP suspension at a molar ratio of surface Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ak–PEG–SH:COOH–PEG–SH = 1
Ak–PEG–SH:COOH–PEG–SH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (COOH/Ak@AuNPs). To confirm the click reaction of the alkyne and azide moieties, we prepared wholly alkyne-functionalized AuNPs (Ak@AuNPs) with a molar ratio of surface Au
000 (COOH/Ak@AuNPs). To confirm the click reaction of the alkyne and azide moieties, we prepared wholly alkyne-functionalized AuNPs (Ak@AuNPs) with a molar ratio of surface Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ak–PEG–SH = 1
Ak–PEG–SH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The PEG decoration was conducted for 6 h, after which unreacted PEG was removed via centrifugation at 12
000. The PEG decoration was conducted for 6 h, after which unreacted PEG was removed via centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min (three washes with DW). COOH/Ak@AuNPs and Ak@AuNPs were re-suspended in DW at 1 mg mL−1 and stored at 4 °C until further use.
000 rpm for 10 min (three washes with DW). COOH/Ak@AuNPs and Ak@AuNPs were re-suspended in DW at 1 mg mL−1 and stored at 4 °C until further use.
mPEG-Se was later decorated on the surface of COOH/Ak@AuNPs via an EDC/NHS-catalyzed amidation reaction (molar ratio of mPEG-Se![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) COOH
COOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDC
EDC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NHS = 1
NHS = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Firstly, the carboxyl moieties of COOH/Ak@AuNPs (0.15 mg mL−1 in DW
10). Firstly, the carboxyl moieties of COOH/Ak@AuNPs (0.15 mg mL−1 in DW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO = 8
DMSO = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) were activated using EDC (0.5 mg mL−1 in DMSO) and NHS (0.5 mg mL−1 in DMSO) for 60 min; subsequently, mPEG-Se (1 mg mL−1 in DW) was added into the mixture under vigorous stirring. The reaction was maintained at 25 ± 2 °C for 12 h, and then the mPEG-Se-decorated COOH/Ak@AuNPs (Se/Ak@AuNPs) were purified via centrifugation at 12
2, v/v) were activated using EDC (0.5 mg mL−1 in DMSO) and NHS (0.5 mg mL−1 in DMSO) for 60 min; subsequently, mPEG-Se (1 mg mL−1 in DW) was added into the mixture under vigorous stirring. The reaction was maintained at 25 ± 2 °C for 12 h, and then the mPEG-Se-decorated COOH/Ak@AuNPs (Se/Ak@AuNPs) were purified via centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min (three washes with DW). The hydrodynamic size, zeta potential, absorbance, FT-IR spectrum, and morphology of the Se/Ak@AuNPs were observed using DLS, ultraviolet-visible (UV-Vis) spectrophotometry, FT-IR spectrometer, and FE-TEM, respectively.
000 rpm for 10 min (three washes with DW). The hydrodynamic size, zeta potential, absorbance, FT-IR spectrum, and morphology of the Se/Ak@AuNPs were observed using DLS, ultraviolet-visible (UV-Vis) spectrophotometry, FT-IR spectrometer, and FE-TEM, respectively.
2.3. Oxidation of diselenide linkers and clustering of oxidized Se/Ak@AuNPs
To investigate the ROS dependence of Se/Ak@AuNPs, we used an H2O2 solution to oxidize the nanoparticles. Briefly, 1% and 0.1% H2O2 solutions in DW were added to Se/Ak@AuNPs (Au conc. of 0.10 mg mL−1) under vigorous stirring. Se/Ak@AuNPs without H2O2 were used as the control group. The hydrodynamic size of oxidized Se/Ak@AuNPs was monitored using DLS. After a 0.5–3 h reaction, the oxidized Se/Ak@AuNPs and controlled Se/Ak@AuNPs were purified via centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and utilized for examination of the exposed alkyne moieties by tagging with an alkyne-reactive fluorescent dye (Flamma® 648 Azide). Briefly, Flamma® 648 Azide was reacted with the aforementioned Se/Ak@AuNPs in the presence of 0.5 mM CuSO4 and 5 mM sodium ascorbate for 3 h (molar ratio of oxidized Se/Ak@AuNP:Flamma® 648 Azide = 1
000 rpm for 10 min and utilized for examination of the exposed alkyne moieties by tagging with an alkyne-reactive fluorescent dye (Flamma® 648 Azide). Briefly, Flamma® 648 Azide was reacted with the aforementioned Se/Ak@AuNPs in the presence of 0.5 mM CuSO4 and 5 mM sodium ascorbate for 3 h (molar ratio of oxidized Se/Ak@AuNP:Flamma® 648 Azide = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1). Subsequently, all unreacted catalysts and fluorescent dye were removed via centrifugation at 12
0.1). Subsequently, all unreacted catalysts and fluorescent dye were removed via centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min with three washes with DW. The fluorescence intensities of the purified dye-tagged Se/Ak@AuNPs were measured using a spectrofluorophotometer (RF-6000, Shimadzu, Japan) at the excitation wavelength of 650 nm and emission wavelength of 665 nm.
000 rpm for 10 min with three washes with DW. The fluorescence intensities of the purified dye-tagged Se/Ak@AuNPs were measured using a spectrofluorophotometer (RF-6000, Shimadzu, Japan) at the excitation wavelength of 650 nm and emission wavelength of 665 nm.
A preliminary assessment of the clustering ability between azide and alkyne moieties on AuNPs was conducted using N3@AuNPs and Ak@AuNPs. N3@AuNPs and Ak@AuNPs were mixed at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the presence of CuSO4 (conc. of 0.250–0.750 mM) and sodium ascorbate (molar ratio of CuSO4
1 in the presence of CuSO4 (conc. of 0.250–0.750 mM) and sodium ascorbate (molar ratio of CuSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sodium ascorbate = 1
sodium ascorbate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10); the final conc. of Au was 0.10 mg mL−1. The hydrodynamic size and absorbance of the AuNP mixtures were monitored using DLS and UV-Vis spectrophotometry, respectively, to confirm cluster formation. Additionally, FT-IR spectra of N3@AuNPs, Ak@AuNPs and AuNC were analyzed, and azide-reactive fluorescent dye (Alexa Fluor™ 647 Alkyne) was reacted with N3@AuNPs and AuNC to observe a reduction in fluorescence intensity of AuNC at an excitation wavelength of 650 nm and an emission wavelength of 665 nm to further evidence the participation of azide moieties in the click reaction. The morphology of the gold nanoclusters (AuNCs) prepared in the presence of 0.375 mM CuSO4 was observed using FE-TEM.
10); the final conc. of Au was 0.10 mg mL−1. The hydrodynamic size and absorbance of the AuNP mixtures were monitored using DLS and UV-Vis spectrophotometry, respectively, to confirm cluster formation. Additionally, FT-IR spectra of N3@AuNPs, Ak@AuNPs and AuNC were analyzed, and azide-reactive fluorescent dye (Alexa Fluor™ 647 Alkyne) was reacted with N3@AuNPs and AuNC to observe a reduction in fluorescence intensity of AuNC at an excitation wavelength of 650 nm and an emission wavelength of 665 nm to further evidence the participation of azide moieties in the click reaction. The morphology of the gold nanoclusters (AuNCs) prepared in the presence of 0.375 mM CuSO4 was observed using FE-TEM.
To perform the click reaction between Se/Ak@AuNPs and N3@AuNPs, we reacted these two AuNPs (Au conc. of 0.10 mg mL−1) in the presence of different concentrations of CuSO4 and with or without 0.1% H2O2. The hydrodynamic size and absorption spectrum of the resultant nanoparticles were monitored for 90 min using DLS and UV-Vis spectrophotometry, respectively.
2.4. Photothermal conversion efficiency of AuNCs
To determine the photothermal conversion efficiency of AuNCs in comparison with bare and functionalized AuNPs, 2 mL of bare AuNPs, N3@AuNPs, Ak@AuNPs, and AuNCs prepared through the reaction of N3@AuNP and Ak@AuNP in the presence of 0.375 mM CuSO4, and AuNC Ss prepared through the reaction of N3@AuNPs and oxidized Se/Ak@AuNPs in the presence of 5 mM CuSO4 for 45 min were added to a polystyrene cuvette and irradiated under an 808 nm laser (MDL-III-808-2W; Uniotech, Republic of Korea) with a power output of 1.00 W cm−2. The distance from the laser tip to the surface of the AuNP suspension was 1 cm. The temperature change was recorded every minute for up to 30 min using a digital thermometer (DTM-305C; TECPEL, Taiwan).To evaluate the influence of the size of AuNCs on the efficiency of PTT, we exposed AuNCs of different sizes by changing the CuSO4 conc. (0.250–0.750 mM) to laser irradiation. The effect of Au conc. of AuNCs on temperature change was examined by irradiating AuNCs with various Au conc. of 0.05–0.40 mg mL−1. The effect of the laser power on the temperature change was determined by irradiating AuNCs at an Au conc. of 0.10 mg mL−1 with different laser powers of 0.25–1.00 W cm−2. All studies were conducted using an 808 nm laser, and the temperature changes were recorded every minute for 30 min.
2.5. Cellular uptake of AuNCs
Before evaluating the in vitro PTT efficacy of the AuNCs, the cellular uptake of the AuNCs was quantified by measuring the amount of NIH3T3 cell-endocytosed AuNCs using ICP-OES. Briefly, NIH3T3 cells seeded on 24-well plates at a density of 5 × 104 cells per mL in DMEM supplemented with 10% FBS and 1% P/S (1 mL per well) were incubated in a 5% CO2 atmosphere at 37 °C for 24 h. Subsequently, the spent medium was replaced with fresh medium containing bare AuNPs, N3@AuNPs, Ak@AuNPs, and AuNCs at Au conc. of 0.05–0.20 mg mL−1. The treated cells were further incubated in a 5% CO2 atmosphere at 37 °C for 6 h; subsequently, all remaining AuNPs were removed, followed by two washes with DMEM (0.5 mL each), and 0.2 mL of aqua regia was added to each well to digest the AuNP-uptaken cells. All samples were diluted 50 times in DW and filtered through a 0.45 μm filter before measuring using ICP-OES at the Central Laboratory of Kangwon National University.2.6. Cell viability and cell apoptosis assay
The anti-proliferative effect of the AuNCs was investigated using a WST-1-based cytotoxicity assay. NIH3T3 cells seeded on 24-well plates at a density of 5 × 104 cells per mL in DMEM supplemented with 10% FBS and 1% P/S (1 mL per well) were incubated in a 5% CO2 atmosphere at 37 °C for 24 h. Subsequently, the spent medium was replaced with fresh medium containing bare AuNPs, N3@AuNPs, Ak@AuNPs, and AuNCs at Au conc. of 0.05–0.20 mg mL−1. The treated cells were further incubated in a 5% CO2 atmosphere at 37 °C for 6 h. Thereafter, all remaining AuNPs were removed, cells were washed once with DMEM (0.5 mL), and 1 mL of fresh medium was added to each well. Laser irradiation at 808 nm was applied to cells with and without AuNPs and AuNC-treated cells for 0, 2, 5, and 10 min with a laser power of 1 W cm−2 and a tip-to-surface distance of 1 cm. Subsequently, all non-irradiated and irradiated cells were further incubated in a 5% CO2 atmosphere at 37 °C for 6 h, followed by the replacement with fresh medium (0.2 mL per well) and the addition of WST-1 reagent (0.02 mL per well) in the dark. The cells were incubated at 37 °C for 2 h before measuring the absorbance at 450 nm using a microplate reader (Multiskan GO; Thermo Fisher Scientific, MA, USA), and the absorbance of untreated cells was normalized to 100%.Apoptotic cell death was investigated in non-irradiated and 10 min NIR-irradiated cells. NIH3T3 cells seeded on 24-well plates increased to a cell density of 2.5 × 105 cells per mL, 1 mL per well. After 808 nm laser irradiation, the treated cells were further incubated at 37 °C for 24 h. The spent medium was removed and the cells were washed twice with PBS (0.2 mL each) and detached with 0.1 mL of T/E. The collected cells were washed twice with PBS before suspending in 0.1 mL of 1× binding buffer. These cells were stained with 2.5 μL of Annexin V-FITC at 25 ± 2 °C for 20 min in the dark, and the reagent was removed via centrifugation at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 rpm for 4 min. The washed cells were re-suspended in 0.5 mL of 1× binding buffer and further stained with 10 μL of propidium iodide (PI) at 25 ± 2 °C for 15 min before analysis using a flow cytometer (FACSymphony A3; Becton Dickinson, USA) at the Central Laboratory of Kangwon National University. Stained cells were detected in the FL-1 (FITC, 518 nm) and FL-2 (PI, 620 nm) channels and analyzed using the software BD FACSDiva™.
200 rpm for 4 min. The washed cells were re-suspended in 0.5 mL of 1× binding buffer and further stained with 10 μL of propidium iodide (PI) at 25 ± 2 °C for 15 min before analysis using a flow cytometer (FACSymphony A3; Becton Dickinson, USA) at the Central Laboratory of Kangwon National University. Stained cells were detected in the FL-1 (FITC, 518 nm) and FL-2 (PI, 620 nm) channels and analyzed using the software BD FACSDiva™.
2.7. Statistical analysis
Statistical analysis was performed using one-way ANOVA with the software SigmaPlot 14.0, and p-values <0.001 were considered statistically significant.3. Results and discussion
N3@AuNPs and Se/Ak@AuNPs were separately synthesized for ROS-responsive clicking into nanoclusters, which were further employed to enhance PTT (Fig. 1). After the synthesis of bare AuNPs, N3@AuNPs were prepared by decorating N3–PEG–SH onto the surface of the AuNPs. Using Ak–PEG–SH, a similar strategy was employed to introduce alkyne moieties to other AuNPs.22,23,27 To establish the ROS-responsive property of the click reaction, the AuNPs were surface-engineered with alkyne groups and diselenide linkers integrated within an elongated PEG chain to ensure steric hindrance and prevent access to the alkyne moieties. However, in an ROS-rich environment like tumor sites, the diselenide linkers undergo cleavage due to ROS oxidation, resulting in the liberation of the long PEG chains from the AuNPs. This ROS-triggered de-shielding of the PEG shells allows recognition of the alkynes by adjacent azide moieties present on other AuNPs, facilitating the click reaction. The click reaction leads to the formation of clustered nanoparticles composed of clicked AuNPs. The size of these clusters can be controlled by adjusting the catalyst level. Upon NIR irradiation, the large AuNP clusters are expected to exhibit an increased conversion temperature due to the red shift of the surface plasmon resonance absorbance,1,28 where the temperature increase can be controlled by manipulating the cluster size.9,27,29To prepare two different types of particles for click reactions, we first surface-engineered AuNPs with either (1) N3–PEG–SH or (2) Ak–PEG–SH and COOH–PEG–SH; the latter particles were further decorated with mPEG-Se such that they could be clicked with each other under ROS-rich conditions. Another type of AuNP fully decorated with alkyne moieties was also prepared to investigate the click reaction between azide and alkyne moieties. After the first PEGylation, the hydrodynamic size increased to 61.45 ± 3.14, 65.68 ± 5.03, and 67.59 ± 4.94 nm for the N3@AuNPs, Ak@AuNPs, and COOH/Ak@AuNPs, respectively, compared with 36.07 ± 4.80 nm for the bare AuNPs (Fig. 2a). The zeta potential also exhibited noticeable changes, where all PEGylated particles became less negatively charged (N3@AuNPs: −8.91 ± 2.63 mV, Ak@AuNPs: −5.84 ± 2.56 mV, and COOH/Ak@AuNPs: −25.23 ± 1.97 mV) compared with the bare AuNPs (−30.60 ± 2.10 mV). After the COOH/Ak@AuNPs were decorated with secondary shells containing mPEG-Se, the Se/Ak@AuNPs exhibited much larger hydrodynamic size (95.17 ± 4.48 nm) and increased zeta potential (−5.71 ± 2.68 mV), suggesting that both the primary and secondary shells were decorated on their surfaces. This was supported by both the microscopic and spectroscopic analyses. The FE-TEM images clearly showed that the AuNPs had relatively spherical shapes. The COOH/Ak@AuNPs exhibited a thin shell around the spherical core, whereas the Se/Ak@AuNPs had a thicker shell due to the double-layered PEG shells. With the addition of the primary and secondary shells, the particle size significantly increased from 23.33 ± 3.03 nm (AuNPs) to 28.58 ± 2.30 nm and 32.03 ± 3.54 nm for the COOH/Ak@AuNPs and Se/Ak@AuNPs, respectively (Fig. 2b and Fig. S1, ESI†). The bare AuNPs had an absorption peak at 526 nm, and after the primary PEGylation, there was a red shift of 3, 5, and 5 nm in the absorption spectrum of the N3@AuNPs, Ak@AuNPs, and COOH/Ak@AuNPs, respectively, compared with the plasmonic absorbance of the bare AuNPs. After coating with the secondary shell, a red shift of 7 nm was observed in the absorption spectrum of the Se/Ak@AuNPs compared with that of the bare AuNPs and 2 nm compared with that of the COOH/Ak@AuNPs (Fig. 2d). Previous studies confirmed that AuNPs have a specific plasmon absorbance at approximately 520 nm; this peak can be red-shifted due to the increase in size by PEGylation, and the degree of PEGylation and the length of the decorated PEG can influence the red shift.23,30 Although the FE-TEM images revealed a smaller particle size due to the collapsed PEG chains, combining the hydrodynamic size and spectroscopic results, it is clear that bare AuNPs were surface-engineered with double layers of PEG shells.23
To confirm the amide coupling reactions between selenocystamine and mPEG-NHS and between mPEG-Se and COOH/Ak@AuNPs, FT-IR spectra were obtained (Fig. 2c). After conjugation, the amide I bands were observed at 1612 and 1646 cm−1 in the spectra of mPEG-Se and Se/Ak@AuNPs, respectively.31 The N–H bending bands of selenocystamine were observed in the range of 1569–1597 cm−1,32 and the NHS band of mPEG-NHS was at 1784 cm−1;18 however, these bands were not observed in the spectrum of mPEG-Se, which could indicate that there was a reaction between the amino group of selenocystamine and the NHS group of mPEG-NHS. We also confirmed the conjugation between selenocystamine and mPEG-NHS using 1H-NMR spectroscopy (Fig. S2, ESI†). The presence of the methylene groups of selenocystamine at 2.95–2.88 ppm and 1.99–1.91 ppm, and the methylene group of mPEG-NHS at 3.60–3.21 ppm on the spectrum of mPEG-Se, proved that this conjugate was formed.33 Based on these results, we may conclude that the AuNPs were successfully decorated with the required functional groups (azide and alkyne moieties) for the click reaction and the mPEG-Se conjugate to induce an ROS response.
To investigate the click reaction between azide moieties and alkyne moieties on AuNPs, we employed fully alkyne-decorated AuNPs (Ak@AuNPs) to prepare AuNCs. Different concentrations of CuSO4 were used to evaluate the influence of copper in catalyzing the click reaction between Ak@AuNPs and N3@AuNPs (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
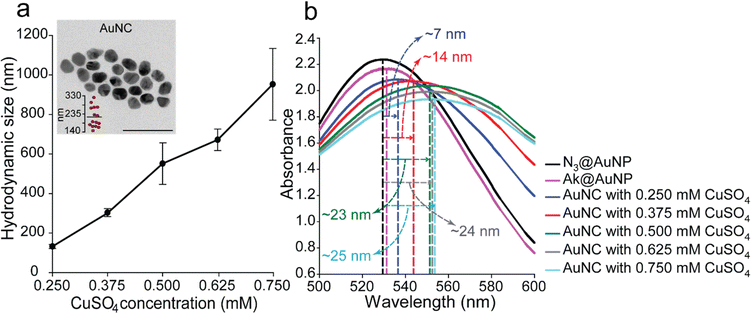
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The DLS results and absorption spectra clearly showed that, when a higher concentration of CuSO4 was used, larger AuNCs were formed because more click reactions could be generated between the azide and alkyne moieties. Specifically, the hydrodynamic size of the AuNCs increased from 132.07 ± 10.10 to 952.53 ± 181.70 nm with the concentration of CuSO4 increasing from 0.250 to 0.750 mM (Fig. 3a and Fig. S1, ESI†). In addition, red shifts were observed in all the spectra of the AuNCs. In detail, red shifts of 7, 14, 23, 24, and 25 nm were observed in the spectra of the AuNCs prepared with 0.250, 0.375, 0.500, 0.625, and 0.750 mM CuSO4, respectively, compared with the N3@AuNPs peak (Fig. 3b). In a previous study, the plasmon absorbance shifted from 517 to 575 nm with an increase in the AuNP size from 9 to 99 nm.7 Rahme et al.30 also reported that when the diameter of the bare AuNPs increased from 30 to 135 nm, the wavelength of maximum absorbance translocated from 525 to 654 nm, which was explained by the effect of surface plasmon modes. In other studies, when isolated AuNPs formed AuNP aggregates, a broad red shift occurred from approximately 520 nm to above 600 nm.9,34,35 In this study, the red shifts of the AuNCs were in accordance with the increase in size with increasing copper concentration, and these results, along with the FE-TEM images, indicated that nanoclusters were formed by the click reaction between azide and alkyne moieties on N3@AuNPs and Ak@AuNPs, and the size of AuNCs could be modulated by changing the concentration of CuSO4. FT-IR spectra were obtained to identify the changes in chemical structure after the click reaction between N3@AuNP and Ak@AuNP. The presence of the azide band at 2112 cm−1 on the N3@AuNP spectrum, which was significantly decreased on the AuNC spectrum, and the newly appeared band of N–H in the range of 3500–2900 cm−1 of the AuNC spectrum indicated that a click reaction successfully occurred (Fig. S3, ESI†). In addition, when the fluorescent dye Alexa Fluor™ 647 Alkyne was reacted with N3@AuNP and AuNC in the presence of 0.1 mM CuSO4 and 1 mM sodium ascorbate for 3 h, the fluorescence intensity of N3@AuNP was nearly double compared to that of AuNC, which demonstrates that most of the azide moieties participated in the click reaction with Ak@AuNP (Fig. S4, ESI†). These data confirmed that the clusters of N3@AuNP and Ak@AuNP were successfully fabricated.
1). The DLS results and absorption spectra clearly showed that, when a higher concentration of CuSO4 was used, larger AuNCs were formed because more click reactions could be generated between the azide and alkyne moieties. Specifically, the hydrodynamic size of the AuNCs increased from 132.07 ± 10.10 to 952.53 ± 181.70 nm with the concentration of CuSO4 increasing from 0.250 to 0.750 mM (Fig. 3a and Fig. S1, ESI†). In addition, red shifts were observed in all the spectra of the AuNCs. In detail, red shifts of 7, 14, 23, 24, and 25 nm were observed in the spectra of the AuNCs prepared with 0.250, 0.375, 0.500, 0.625, and 0.750 mM CuSO4, respectively, compared with the N3@AuNPs peak (Fig. 3b). In a previous study, the plasmon absorbance shifted from 517 to 575 nm with an increase in the AuNP size from 9 to 99 nm.7 Rahme et al.30 also reported that when the diameter of the bare AuNPs increased from 30 to 135 nm, the wavelength of maximum absorbance translocated from 525 to 654 nm, which was explained by the effect of surface plasmon modes. In other studies, when isolated AuNPs formed AuNP aggregates, a broad red shift occurred from approximately 520 nm to above 600 nm.9,34,35 In this study, the red shifts of the AuNCs were in accordance with the increase in size with increasing copper concentration, and these results, along with the FE-TEM images, indicated that nanoclusters were formed by the click reaction between azide and alkyne moieties on N3@AuNPs and Ak@AuNPs, and the size of AuNCs could be modulated by changing the concentration of CuSO4. FT-IR spectra were obtained to identify the changes in chemical structure after the click reaction between N3@AuNP and Ak@AuNP. The presence of the azide band at 2112 cm−1 on the N3@AuNP spectrum, which was significantly decreased on the AuNC spectrum, and the newly appeared band of N–H in the range of 3500–2900 cm−1 of the AuNC spectrum indicated that a click reaction successfully occurred (Fig. S3, ESI†). In addition, when the fluorescent dye Alexa Fluor™ 647 Alkyne was reacted with N3@AuNP and AuNC in the presence of 0.1 mM CuSO4 and 1 mM sodium ascorbate for 3 h, the fluorescence intensity of N3@AuNP was nearly double compared to that of AuNC, which demonstrates that most of the azide moieties participated in the click reaction with Ak@AuNP (Fig. S4, ESI†). These data confirmed that the clusters of N3@AuNP and Ak@AuNP were successfully fabricated.
To analyze the ROS-dependence of the click reaction between Se/Ak@AuNPs and N3@AuNPs due to the presence of a diselenide linker embedded in the long chains of PEG on Se/Ak@AuNPs, we first oxidized the Se/Ak@AuNPs under different concentrations of H2O2.16,36In vitro studies on ROS-mediated drug delivery systems previously reported that, at a pathological concentration of H2O2 (100 μM), 48–80 hours is required to achieve maximum drug release.16,37 Moreover, ROS generation is not solely dependent on H2O2 concentration. Multivalent metal ions, such as copper and iron, have been shown to influence the ROS status according to the cancer stage and/or progression in different types of tumors by generating redox cycles, such as Fenton/Fenton-like reactions, ultimately increasing the activity of ROS.38,39 Therefore, to investigate the cleavage of the diselenide linkers in vitro in a short period, 1.0% and 0.1% H2O2 were studied,36,40 and the hydrodynamic size of the oxidized Se/Ak@AuNPs was monitored for up to 20 h of oxidation (Fig. 4a and Fig. S5, ESI†). After 3 h, the size of 1.0% H2O2-oxidized Se/Ak@AuNPs decreased to 44.89 ± 2.67 nm, and this value was nearly unchanged up to 20 h. In contrast, the size of 0.1% H2O2-oxidized Se/Ak@AuNPs exhibited a small reduction to 84.46 ± 3.79 nm after 3 h, reached 62.33 ± 3.82 nm after 6 h, and 43.15 ± 3.67 after 20 h. Without H2O2, the size of Se/Ak@AuNPs remained unchanged after 20 h (68.77 ± 2.13 nm). The FE-TEM images and absorbance spectra of the oxidized Se/Au@AuNPs were obtained to investigate the de-shielding of the secondary PEG shells. After oxidation with 1.0% H2O2 for 1.5 h, the PEG shells of the oxidized Se/Ak@AuNPs became thinner, and the FE-TEM size was reduced to 27.76 ± 2.49 nm, compared with 32.03 ± 3.54 nm for Se/Ak@AuNPs without oxidation; this was similar to the size of the COOH/Ak@AuNPs (28.59 ± 2.30 nm), which contained a monolayer of PEG (Fig. 2b and Fig. S1, ESI†). Moreover, the plasmonic absorbance of the oxidized Se/Ak@AuNPs exhibited a blue shift of 2 nm, compared with that of the Se/Ak@AuNPs without oxidation (Fig. S6, ESI†); this was close to the peak of the COOH/Ak@AuNPs at 531 nm. The decrease in hydrodynamic and FE-TEM sizes and the blue shift of plasmonic absorbance indicate that, under the oxidation of H2O2, the diselenide linkers were broken, which led to the cleavage of the secondary PEG shells. In our previous study, where AuNPs were surface-engineered with two PEG shells for MMP-responsive click reaction, upon the digestion of MMP, the secondary shells from the double-PEGylated AuNPs were de-shielded, resulting in a size reduction from 43.80 ± 1.96 to 31.50 ± 1.38 nm and from 103.53 ± 11.32 to 84.18 ± 2.36 nm based on FE-TEM and DLS measurements, respectively.23 We further confirmed whether the alkyne moieties were exposed after the de-shielding of the secondary PEG shells using an alkyne-reaction dye tag, Flamma® 648 Azide, which was employed to click with 0.5, 1, 1.5, 2, 2.5, and 3 h-oxidized Se/Ak@AuNP. A higher fluorescence intensity indicated that a higher quantity of Flamma® 648 Azide reacted and more alkyne moieties were exposed (Fig. 4b). The fluorescence intensity increased when the oxidation time was prolonged up to 3 h with 0.1% H2O2, but only up to 1.5 h with 1.0% H2O2, where it decreased dramatically when oxidation was continued for up to 3 h. Previous studies reported that H2O2 can be used to remove thiol on the surface of AuNPs;35 thus, we speculate that during long-time exposure to 1.0% H2O2, the Au–S bond could break and Ak–PEG–SH could be partially cleaved from the bare AuNPs, resulting in fewer alkyne moieties. The lower fluorescence intensities and the reduction in hydrodynamic size of the oxidized Se/Ak@AuNPs (i.e., close to the hydrodynamic size of bare AuNPs when oxidizing Se/Ak@AuNPs with 1.0% H2O2 for more than 1.5 h) could demonstrate our hypothesis. The fluorescence intensities of non-oxidized Se/Ak@AuNPs did not exhibit noticeable changes because of the steric hindrance effect of the secondary long PEG chain, particularly when these chains were longer than those carrying alkyne moieties, which hindered the accessibility of the azide moieties to the alkyne moieties.23,24 Therefore, in the following studies, H2O2 was used to oxidize the diselenide linkers of Se/Ak@AuNPs for selective click reactions with N3@AuNPs. The presence of copper, which was used to catalyze the reaction between the alkyne and azide, can induce faster ROS generation from H2O2 through Fenton-like reactions,41,42 thereby accelerating the oxidation of diselenide linkers. To avoid the cleavage of Ak–PEG–SH from AuNPs, 0.1% H2O2 was used. The ROS-responsive click reaction between Se/Ak@AuNPs and N3@AuNPs was investigated using different concentrations of CuSO4 (Fig. 4d). Although the use of 0.375 mM CuSO4 generated a click reaction between Ak@AuNPs and N3@AuNPs at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, no reaction occurred between Se/Ak@AuNPs and N3@AuNPs with 0.375 mM CuSO4 at both molar ratios of 1
1, no reaction occurred between Se/Ak@AuNPs and N3@AuNPs with 0.375 mM CuSO4 at both molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (data not shown) and 10
1 (data not shown) and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. We assume that, with a low concentration of CuSO4, copper can only act as a part of the Fenton-like reactions. When the CuSO4 concentration increased to 2.5 mM, the click reaction began to occur with the molar ratio of Se/Ak@AuNPs and N3@AuNPs at 10
1. We assume that, with a low concentration of CuSO4, copper can only act as a part of the Fenton-like reactions. When the CuSO4 concentration increased to 2.5 mM, the click reaction began to occur with the molar ratio of Se/Ak@AuNPs and N3@AuNPs at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and an increase in hydrodynamic size to 152.76 ± 20.68 nm was observed. The clicked nanoclusters were obtained at 5 mM CuSO4 (molar ratio of Se/Ak@AuNPs:N3@AuNPs = 10
1, and an increase in hydrodynamic size to 152.76 ± 20.68 nm was observed. The clicked nanoclusters were obtained at 5 mM CuSO4 (molar ratio of Se/Ak@AuNPs:N3@AuNPs = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), with the highest hydrodynamic size at 1032.66 ± 109.04 nm after a 90 min reaction. When the hydrodynamic size of the reaction between Se/Ak@AuNPs and N3@AuNPs was monitored in the presence of 0.1% H2O2 and 5 mM CuSO4 for 90 min, initially, there was a reduction in size to 54.90 ± 8.02 nm after 30 min, which could be the oxidation step where diselenide linkers were broken and the long chains of PEG were released, resulting in the exposure of the alkyne moieties to the azide moieties. Consequently, this led to the click reaction between the oxidized Se/Ak@AuNPs and N3@AuNPs, and the hydrodynamic size increased significantly to 297.67 ± 9.13, 611.03 ± 131.13, 863.12 ± 80.65, and 1032.66 ± 109.04 nm after 45, 60, 75, and 90 min, respectively. With a size larger than 500 nm, AuNCs have poor colloidal stability and tend to drop to the bottom of the container, which may influence the effect of PTT; thus, in this study, we used AuNCs with a size of approximately 300 nm for experiments. The plasmon absorbance of nanoclusters prepared using Ak@AuNPs and N3@AuNPs or oxidized Se/Ak@AuNPs and N3@AuNPs with a similar hydrodynamic size of approximately 300 nm (AuNCs: 301.30 ± 19.98 nm, AuNC Ss: 297.67 ± 9.13 nm) were comparable, with red shifts of 15 and 13 nm from the absorbance peak of bare AuNPs, respectively (Fig. S7, ESI†). Thus, we speculate that these two nanoclusters may have similar PTT effects.
1), with the highest hydrodynamic size at 1032.66 ± 109.04 nm after a 90 min reaction. When the hydrodynamic size of the reaction between Se/Ak@AuNPs and N3@AuNPs was monitored in the presence of 0.1% H2O2 and 5 mM CuSO4 for 90 min, initially, there was a reduction in size to 54.90 ± 8.02 nm after 30 min, which could be the oxidation step where diselenide linkers were broken and the long chains of PEG were released, resulting in the exposure of the alkyne moieties to the azide moieties. Consequently, this led to the click reaction between the oxidized Se/Ak@AuNPs and N3@AuNPs, and the hydrodynamic size increased significantly to 297.67 ± 9.13, 611.03 ± 131.13, 863.12 ± 80.65, and 1032.66 ± 109.04 nm after 45, 60, 75, and 90 min, respectively. With a size larger than 500 nm, AuNCs have poor colloidal stability and tend to drop to the bottom of the container, which may influence the effect of PTT; thus, in this study, we used AuNCs with a size of approximately 300 nm for experiments. The plasmon absorbance of nanoclusters prepared using Ak@AuNPs and N3@AuNPs or oxidized Se/Ak@AuNPs and N3@AuNPs with a similar hydrodynamic size of approximately 300 nm (AuNCs: 301.30 ± 19.98 nm, AuNC Ss: 297.67 ± 9.13 nm) were comparable, with red shifts of 15 and 13 nm from the absorbance peak of bare AuNPs, respectively (Fig. S7, ESI†). Thus, we speculate that these two nanoclusters may have similar PTT effects.
The photothermal conversion efficiencies of different AuNPs and AuNCs were determined using 808 nm laser irradiation with a laser power of 1.00 W cm−2. AuNCs prepared using Ak@AuNPs and N3@AuNPs and AuNC Ss prepared using oxidized Se/Ak@AuNPs and N3@AuNPs with a similar hydrodynamic size of approximately 300 nm were utilized for this study (Fig. 5a and Fig. S8, ESI†). The results indicate that both gold nanoparticles and nanoclusters enhanced the PTT effect with a noticeable increase in temperature compared with that of DW. However, owing to the red shift of the plasmon absorbance of AuNCs closer to the NIR region, it significantly enhanced photothermal conversion, compared with bare AuNPs, N3@AuNPs, and Ak@AuNPs, with an elevated temperature of approximately 13 °C after 30 min of irradiation. Previous studies have also observed a dramatic increase in temperature from 13 to 45 °C when irradiating isolated AuNPs and AuNP aggregates using an 808 nm laser.5,9 As AuNCs and AuNC Ss were observed to have similar photothermal conversion efficiency, AuNCs were used for further experiments. AuNCs of different sizes were prepared by modulating the CuSO4 conc. and then irradiated under an 808 nm laser with an output power of 1.00 W cm−2 for 30 min (Fig. 5b). The temperature increased noticeably with increasing CuSO4 conc., corresponding to the size enlargement of the AuNCs. We further investigated the influence of the Au conc. in the AuNCs on the photothermal conversion efficiency. The AuNCs were prepared through the reaction between Ak@AuNPs and N3@AuNPs (Au conc. at 0.10 mg mL−1) in the presence of 0.375 mM, diluted or concentrated into different Au conc. (0.05–0.40 mg mL−1), and irradiated under an 808 nm laser with an output power of 1.00 W cm−2. A higher Au conc. resulted in a higher temperature (Fig. 5c). Subsequently, we maintained the Au conc. of the AuNCs at 0.10 mg mL−1 and adjusted the laser power from 0.25 to 1.00 W cm−2 to determine its impact on photothermal conversion. We determined that photothermal conversion efficiency was at the highest with the highest laser power of 1.00 W cm−2 (Fig. 5d). The effects of the Au conc. and laser power on the photothermal conversion efficiency were observed to be similar to the results of previous studies, in which Au conc. of 90–360 and 18–140 μg mL−1 and laser powers of 0.5–2.5 and 0.5–2.0 W cm−2 were employed, respectively.5,9 Therefore, the effect of PTT could be modulated by controlling not only the AuNC size but also the Au conc. and laser power.
In vitro studies were conducted to evaluate the cellular uptake, cell viability, and apoptosis effects of AuNCs in comparison with those of bare AuNPs and PEGylated AuNPs (Fig. 6). Different Au conc. (0.05–0.20 mg mL−1) in bare AuNPs, Ak@AuNPs, N3@AuNPs, and AuNCs were incubated with NIH3T3 cells for 6 h, and then the AuNP-taken up cells were lysed and analyzed using ICP-OES (Fig. 6a). At an Au conc. of 0.05 mg mL−1, the AuNCs exhibited a lower cellular uptake compared with that of the other AuNPs, which might have been due to a larger particle size.43 However, when the Au conc. was increased to 0.10 and 0.20 mg mL−1, the cellular uptake of the AuNCs did not exhibit a significant difference compared with that of the other AuNPs. To examine the effect on PTT in vitro, NIH3T3 cells were treated with AuNPs or AuNCs at different Au conc. (0.05–0.20 mg mL−1) (Fig. 6b). Without incubation of the AuNPs or AuNCs, we did not observe any cell cytotoxicity after irradiation with an 808 nm laser for up to 10 min, and the cell viability remained above 90% within all irradiation periods, which means that single NIR irradiation is not effective for inducing cell death. At low Au conc. (0.05 mg mL−1), only the AuNCs were observed to be cytotoxic toward cells after treatment with NIR. With higher Au conc. (0.10 and 0.20 mg mL−1), all AuNP- and AuNC-treated cells exhibited a significant decrease in cell viability compared with that of cells without AuNPs. In particular, the AuNC-treated cells exhibited the most remarkable reduction in cell viability out of all the groups. A previous study has reported that at a very low Au conc. of 18 μg mL−1, cells treated with AuNP aggregates and 808 nm laser irradiation for 10 min exhibited a significant reduction in viability. At Au conc. of 70 and 100 μg mL−1, the cell viability dramatically decreased to 15% and less than 10%, respectively.9 Our results revealed that after 5 and 10 min irradiation, neither an Au conc. of 0.10 nor 0.20 mg mL−1 exhibited a noticeable difference. The cell viability reached its lowest of approximately 6% for AuNC-treated cells with an Au conc. of 0.10 mg mL−1 after 5 and 10 min irradiation and with an Au conc. of 0.20 mg mL−1 after any NIR irradiation period. Therefore, 10 min NIR-exposed AuNC-treated cells with an Au conc. of 0.10 mg mL−1 were employed for the apoptosis assay (Fig. 6c and d). The AuNC-treated cells with 10 min of 808 nm laser irradiation exhibited an exceptional increase in late apoptosis rate (29%) compared with that of all other groups, and AuNC-treated cells without NIR irradiation did not induce a higher cell apoptosis rate compared with that of the control and AuNP-treated groups, which were approximately 6–9% due to natural cell death. The rate of live cells in the AuNC-treated group with 10 min of 808 nm laser irradiation was noticeably reduced to 60.5% compared with that of AuNP-treated groups under the same NIR irradiation conditions or without NIR irradiation (78.9–86.1%). Therefore, we consider that although AuNPs might slightly enhance the PTT effect, it is not sufficient to cause cell apoptosis. Relatively large nanoclusters of AuNPs must be employed to generate a sufficient PTT effect on cells when irradiated with an NIR laser.
4. Conclusions
Se/Ak@AuNPs and N3@AuNPs were ROS-responsively clicked into AuNP clusters with size controllability based on the modulation of copper concentration. The photothermal conversion efficiency was significantly enhanced by increasing the size and concentration of the AuNPs, which was further confirmed using in vitro studies. Cell viability was maintained at a high rate when cells were treated separately with large-sized AuNP clusters at various concentrations or under 808 nm laser irradiation for up to 10 min. However, after dual treatment with AuNCs and NIR irradiation, a significant increase in cell apoptosis was observed. These promising results confirmed that our ROS-responsive nanosystem can be a potential tool for enhancing the PTT effect at tumor sites.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Education and the Kangwon National University (NRF-2019R1I1A2A01040849).References
- W. Yang, B. Xia, L. Wang, S. Ma, H. Liang, D. Wang and J. Huang, Shape effects of gold nanoparticles in photothermal cancer therapy, Mater. Today Sustainability, 2021, 13, 100078, DOI:10.1016/j.mtsust.2021.100078.
- Y. Zhang, X. Zhan, J. Xiong, S. Peng, W. Huang, R. Joshi, Y. Cai, Y. Liu, R. Li, K. Yuan, N. Zhou and W. Min, Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells, Sci. Rep., 2018, 8, 1–9, DOI:10.1038/s41598-018-26978-1.
- Y. Liu, P. Bhattarai, Z. Dai and X. Chen, Photothermal therapy and photoacoustic imaging: Via nanotheranostics in fighting cancer, Chem. Soc. Rev., 2019, 48, 2053–2108, 10.1039/c8cs00618k.
- D. Jaque, L. Martínez Maestro, B. Del Rosal, P. Haro-Gonzalez, A. Benayas, J. L. Plaza, E. Martín Rodríguez and J. García Solé, Nanoparticles for photothermal therapies, Nanoscale, 2014, 6, 9494–9530, 10.1039/c4nr00708e.
- Y. Zhang, J. Chang, F. Huang, L. Yang, C. Ren, L. Ma, W. Zhang, H. Dong, J. Liu and J. Liu, Acid-Triggered in Situ Aggregation of Gold Nanoparticles for Multimodal Tumor Imaging and Photothermal Therapy, ACS Biomater. Sci. Eng., 2019, 5, 1589–1601, DOI:10.1021/acsbiomaterials.8b01623.
- M. Pérez-Hernández, P. Del Pino, S. G. Mitchell, M. Moros, G. Stepien, B. Pelaz, W. J. Parak, E. M. Gálvez, J. Pardo and J. M. De La Fuente, Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms, ACS Nano, 2015, 9, 52–61, DOI:10.1021/nn505468v.
- S. Link and M. A. El-Sayed, Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles, J. Phys. Chem. B, 1999, 103, 4212–4217, DOI:10.1021/jp984796o.
- V. P. Chauhan and R. K. Jain, Strategies for advancing cancer nanomedicine, Nat. Mater., 2013, 12, 958–962, DOI:10.1038/nmat3792.
- R. Zhang, L. Wang, X. Wang, Q. Jia, Z. Chen, Z. Yang, R. Ji, J. Tian and Z. Wang, Acid-Induced In Vivo Assembly of Gold Nanoparticles for Enhanced Photoacoustic Imaging-Guided Photothermal Therapy of Tumors, Adv. Healthcare Mater., 2020, 9, 1–10, DOI:10.1002/adhm.202000394.
- J. Chen, Y. Ma, W. Du, T. Dai, Y. Wang, W. Jiang, Y. Wan, Y. Wang, G. Liang and G. Wang, Furin-Instructed Intracellular Gold Nanoparticle Aggregation for Tumor Photothermal Therapy, Adv. Funct. Mater., 2020, 30, 2001566, DOI:10.1002/adfm.202001566.
- Q. Mao, J. Fang, A. Wang, Y. Zhang, C. Cui, S. Ye, Y. Zhao, Y. Feng, J. Li and H. Shi, Aggregation of Gold Nanoparticles Triggered by Hydrogen Peroxide-Initiated Chemiluminescence for Activated Tumor Theranostics, Angew. Chem., Int. Ed., 2021, 60, 23805–23811, DOI:10.1002/anie.202109863.
- S. Park, W. J. Lee, S. Park, D. Choi, S. Kim and N. Park, Reversibly pH-responsive gold nanoparticles and their applications for photothermal cancer therapy, Sci. Rep., 2019, 9, 1–9, DOI:10.1038/s41598-019-56754-8.
- J. Wang and J. Yi, Cancer cell killing via ROS: To increase or decrease, that is a question, Cancer Biol. Ther., 2008, 7, 1875–1884, DOI:10.4161/cbt.7.12.7067.
- G. Y. Liou and P. Storz, Reactive oxygen species in cancer, Free Radical Res., 2010, 44, 479–496, DOI:10.3109/10715761003667554.
- M. Li, S. Li, H. Zhou, X. Tang, Y. Wu, W. Jiang, Z. Tian, X. Zhou, X. Yang and Y. Wang, Chemotaxis-driven delivery of nano-pathogenoids for complete eradication of tumors post-phototherapy, Nat. Commun., 2020, 11, 1126, DOI:10.1038/s41467-020-14963-0.
- V. G. Deepagan, S. Kwon, D. G. You, V. Q. Nguyen, W. Um, H. Ko, H. Lee, D. G. Jo, Y. M. Kang and J. H. Park, In situ diselenide-crosslinked polymeric micelles for ROS-mediated anticancer drug delivery, Biomaterials, 2016, 103, 56–66, DOI:10.1016/j.biomaterials.2016.06.044.
- P. Han, S. Li, W. Cao, Y. Li, Z. Sun, Z. Wang and H. Xu, Red light responsive diselenide-containing block copolymer micelles, J. Mater. Chem. B, 2013, 1, 740–743, 10.1039/c2tb00186a.
- C. Gong, M. Shan, B. Li and G. Wu, Injectable dual redox responsive diselenide-containing poly(ethylene glycol) hydrogel, J. Biomed. Mater. Res., Part A, 2017, 105, 2451–2460, DOI:10.1002/jbm.a.36103.
- S. Uthaman, S. Pillarisetti, A. P. Mathew, Y. Kim, W. K. Bae, K. M. Huh and I. K. Park, Long circulating photoactivable nanomicelles with tumor localized activation and ROS triggered self-accelerating drug release for enhanced locoregional chemo-photodynamic therapy, Biomaterials, 2020, 232, 119702, DOI:10.1016/j.biomaterials.2019.119702.
- L. Kuršvietienė, A. Mongirdienė, J. Bernatonienė, J. Šulinskienė and I. Stanevičienė, Selenium anticancer properties and impact on cellular redox status, Antioxidants, 2020, 9, 80, DOI:10.3390/antiox9010080.
- V. K. Tiwari, B. B. Mishra, K. B. Mishra, N. Mishra, A. S. Singh and X. Chen, Cu-Catalyzed Click Reaction in Carbohydrate Chemistry, Chem. Rev., 2016, 116, 3086–3240, DOI:10.1021/acs.chemrev.5b00408.
- W. Mao, H. S. Kim, Y. J. Son, S. R. Kim and H. S. Yoo, Doxorubicin encapsulated clicked gold nanoparticle clusters exhibiting tumor-specific disassembly for enhanced tumor localization and computerized tomographic imaging, J. Control. Release, 2018, 269, 52–62, DOI:10.1016/j.jconrel.2017.11.003.
- O. V. Pham-Nguyen, J. U. Shin, Y. Park, S. Jin, S. R. Kim, Y. M. Jung and H. S. Yoo, Fluorescence-Shadowing Nanoparticle Clusters for Real-Time Monitoring of Tumor Progression, Biomacromolecules, 2022, 23, 3130–3141, DOI:10.1021/acs.biomac.2c00169.
- R. De Oliveira, P. Zhao, N. Li, L. C. De Santa Maria, J. Vergnaud, J. Ruiz, D. Astruc and G. Barratt, Synthesis and in vitro studies of gold nanoparticles loaded with docetaxel, Int. J. Pharm., 2013, 454, 703–711, DOI:10.1016/j.ijpharm.2013.05.031.
- P. Kingshott, H. Thissen and H. J. Griesser, Effects of cloud-point grafting, chain length, and density of PEG layers on competitive adsorption of ocular proteins, Biomaterials, 2002, 23, 2043–2056, DOI:10.1016/S0142-9612(01)00334-9.
- C. Uttamapinant, A. Tangpeerachaikul, S. Grecian, S. Clarke, U. Singh, P. Slade, K. R. Gee and A. Y. Ting, Fast, cell-compatible click chemistry with copper-chelating azides for biomolecular labeling, Angew. Chem., Int. Ed., 2012, 51, 5852–5856, DOI:10.1002/anie.201108181.
- E. C. Dreaden, L. A. Austin, M. A. MacKey and M. A. El-Sayed, Size matters: Gold nanoparticles in targeted cancer drug delivery, Ther. Delivery, 2012, 3, 457–478, DOI:10.4155/tde.12.21.
- Z.-Z. J. Lim, J.-E. J. Li, C.-T. Ng, L.-Y. L. Yung and B.-H. Bay, Gold nanoparticles in cancer therapy, Acta Pharmacol. Sin., 2011, 32, 983–990, DOI:10.1038/aps.2011.82.
- J. B. Vines, J. H. Yoon, N. E. Ryu, D. J. Lim and H. Park, Gold nanoparticles for photothermal cancer therapy, Front. Chem., 2019, 7, 1–16, DOI:10.3389/fchem.2019.00167.
- K. Rahme, L. Chen, R. G. Hobbs, M. A. Morris, C. O’Driscoll and J. D. Holmes, PEGylated gold nanoparticles: Polymer quantification as a function of PEG lengths and nanoparticle dimensions, RSC Adv., 2013, 3, 6085–6094, 10.1039/c3ra22739a.
- G. Socrates, Infrared and Raman characteristic group frequencies, 3rd edn, 2001 Search PubMed.
- D. M. Kim, Y. H. Shim, H. Kwon, J. P. Kim, J. I. Park, D. H. Kim, D. H. Kim, J. H. Kim and Y. I. L. Jeong, CD44 Receptor–Specific and Redox-Sensitive Nanophotosensitizers of Hyaluronic Acid–Chlorin e6 Tetramer Having Diselenide Linkages for Photodynamic Treatment of Cancer Cells, J. Pharm. Sci., 2019, 108, 3713–3722, DOI:10.1016/j.xphs.2019.07.024.
- H. H. Jang, S. B. Park, J. S. Hong, H. L. Lee, Y. H. Song, J. Kim, Y. H. Jung, C. Kim, D. M. Kim, S. E. Lee, Y. Il Jeong and D. H. Kang, Piperlongumine-Eluting Gastrointestinal Stent Using Reactive Oxygen Species-Sensitive Nanofiber Mats for Inhibition of Cholangiocarcinoma Cells, Nanoscale Res. Lett., 2019, 14, 58, DOI:10.1186/s11671-019-2887-0.
- C. Hua, W. H. Zhang, S. R. M. De Almeida, S. Ciampi, D. Gloria, G. Liu, J. B. Harper and J. J. Gooding, A novel route to copper(ii) detection using “click” chemistry-induced aggregation of gold nanoparticles, Analyst, 2012, 137, 82–86, 10.1039/c1an15693d.
- J. K. Dae, R. Pitchimani, D. E. Snow and L. J. Hope-Weeks, A simple method for the removal of thiols on gold surfaces using an NH4OH–H2O2–H2O solution, Scanning, 2008, 30, 118–122, DOI:10.1002/sca.20089.
- N. Ma, Y. Li, H. Xu, Z. Wang and X. Zhang, Dual redox responsive assemblies formed from diselenide block copolymers, J. Am. Chem. Soc., 2010, 132, 442–443, DOI:10.1021/ja908124g.
- X. Xu, P. E. Saw, W. Tao, Y. Li, X. Ji, S. Bhasin, Y. Liu, D. Ayyash, J. Rasmussen, M. Huo, J. Shi and O. C. Farokhzad, ROS-Responsive Polyprodrug Nanoparticles for Triggered Drug Delivery and Effective Cancer Therapy, Adv. Mater., 2017, 29, 1–6, DOI:10.1002/adma.201700141.
- A. Gupte and R. J. Mumper, Elevated copper and oxidative stress in cancer cells as a target for cancer treatment, Cancer Treat. Rev., 2009, 35, 32–46, DOI:10.1016/j.ctrv.2008.07.004.
- W. Ma, L. Jia, Q. Xiong, Y. Feng and H. Du, The role of iron homeostasis in adipocyte metabolism, Food Funct., 2021, 12, 4246–4253, 10.1039/d0fo03442h.
- Y. S. Birhan, H. F. Darge, E. Y. Hanurry, A. T. Andrgie, T. W. Mekonnen, H. Y. Chou, J. Y. Lai and H. C. Tsai, Fabrication of core crosslinked polymeric micelles as nanocarriers for doxorubicin delivery: Self-assembly, in situ diselenide metathesis and redox-responsive drug release, Pharmaceutics, 2020, 12, 1–22, DOI:10.3390/pharmaceutics12060580.
- J. W. Moffett and R. G. Zika, Reaction Kinetics of Hydrogen Peroxide with Copper and Iron in Seawater, Environ. Sci. Technol., 1987, 21, 804–810, DOI:10.1021/es00162a012.
- M. Gu, D. C. Bode and J. H. Viles, Copper Redox Cycling Inhibits Aβ Fibre Formation and Promotes Fibre Fragmentation, while Generating a Dityrosine Aβ Dimer, Sci. Rep., 2018, 8, 1–14, DOI:10.1038/s41598-018-33935-5.
- H. S. Kim, S. Yoon, Y. J. Son, Y. Park, Y. M. Jung and H. S. Yoo, High-yield clicking and dissociation of doxorubicin nanoclusters exhibiting differential cellular uptakes and imaging, J. Control. Release., 2015, 217, 64–73, DOI:10.1016/j.jconrel.2015.08.037.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3tb00500c |
| This journal is © The Royal Society of Chemistry 2023 |