Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Converting benzene into γ-graphyne and its enhanced electrochemical oxygen evolution performance
Qiaodan
Li
,
Chaofan
Yang
,
Lulu
Wu
,
Hui
Wang
and
Xiaoli
Cui
*
Department of Materials Science, Fudan University, Shanghai 200433, China. E-mail: xiaolicui@fudan.edu.cn
First published on 13th September 2023
Abstract
Correction for ‘Converting benzene into γ-graphyne and its enhanced electrochemical oxygen evolution performance’ by Qiaodan Li et al., J. Mater. Chem. A, 2019, 7, 5981–5990, https://doi.org/10.1039/C8TA10317H.
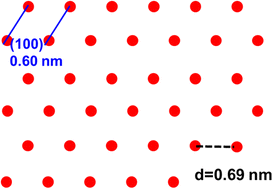
The authors regret errors in the original manuscript in the lattice plane assignment of the lattice fringes in Fig. 2h, the crystal lattices of γ-graphyne in Fig. S3 of the original ESI and in the calculation of bond enthalpies change on page 3 of the original ESI.
The lattice fringes of 0.17 nm (Fig. 2i) and 0.20 nm (Fig. 2h and i) should be assigned to the (220) and (300) lattice planes, respectively, rather than to the (440) and (422) lattice planes stated in the published article. Fig. 2h is revised accordingly.
In the calculation of the Gibbs free energy change in the ESI, the calculation of bond enthalpies change is incorrect. “6N/NA × (332 − 414) + 3N/NA × (−181.5 + 62.8)” should be corrected to “6N/NA × (−332 + 414) + 3N/NA × (−181.5 + 62.8)”. This makes ΔG3 > 0 (ΔG3 = kN × [135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900/R + 260T/R + ln(64/27)T]). A hypothetical two-step reaction follows: (1) mechanochemical dehydrogenation of C–H bonds, and (2) a reaction between generated C6 fragments and CaC2. Though the first step is non-spontaneous, mechanochemistry is a powerful technology for providing enough of a driving force for the reaction. The second step is spontaneous, as calculated below.
900/R + 260T/R + ln(64/27)T]). A hypothetical two-step reaction follows: (1) mechanochemical dehydrogenation of C–H bonds, and (2) a reaction between generated C6 fragments and CaC2. Though the first step is non-spontaneous, mechanochemistry is a powerful technology for providing enough of a driving force for the reaction. The second step is spontaneous, as calculated below.
when T < 9903 K, and ΔG < 0.
The updated Fig. S3 is shown below.
An independent expert has viewed the corrected Fig. 2h, Fig. S3 and the corrections to the calculation of bond enthalpies change, and confirmed that they are consistent with the discussions and conclusions presented.
An ‘Update to the Supplementary information’ with the updated calculation of bond enthalpies and Fig. S3 has been published accordingly as a separate document.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2023 |