Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Raman spectra and defect chemical characteristics of Sr(Ti,Fe)O3−y solid solution of bulk pellets vs. thin films†
Eva
Sediva
 ab and
Jennifer L. M.
Rupp
ab and
Jennifer L. M.
Rupp
 *abc
*abc
aElectrochemical Materials, Department of Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Av., MA 02139, USA. E-mail: jrupp@mit.edu
bElectrochemical Materials, Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, 77 Massachusetts Av., Cambridge, MA 02139, USA
cDepartment of Chemistry, Technical University of Munich, 85748 Garching, Germany
First published on 28th November 2023
Abstract
Sr(Ti,Fe)O3−y perovskite solid solutions are relevant functional materials for energy conversion and electronic devices such as solid oxide fuel and photoelectrochemical cells, electrolyzers, oxygen sensors, resistive random access memories or synaptic transistors. The Raman spectra and vibrational characteristics of the Sr(Ti,Fe)O3−y materials class are suitable for describing their defect chemistry and the iron valence state, which governs a multitude of its mixed ionic–electronic transport and other characteristics. We synthesize a standard range of compositions containing 1–75 mol% of iron including the end members in the form of macrocrystalline bulk pellets, nanocrystalline poly- and single crystalline thin films. Through the change in both iron substitution level and microstructure, we directly see the effect of defect chemistry such as its phase, transition metal ion valence and oxygen nonstoichiometry on the Raman spectra. These are discussed in terms of in and ex situ experiments under oxidizing/reducing atmosphere. In contrast to long range structural X-ray diffraction measurements, Raman spectroscopy provides valuable insights into oxygen vacancy ordering and oxygen nonstoichiometry for the Sr(Ti,Fe)O3−y material class.
1 Introduction
Solid solutions of the perovskite family Sr(Ti,Fe)O3−y are important due to their ability to vary their physical and chemical properties with composition, iron valence state, and oxygen nonstoichiometry. These can determine the material's transport,1 electronic,2 magnetic3,4 and optical5,6 properties, crucial for applications in electrochemical devices,7 such as electrodes for solid oxide fuel8–10 and photoelectrochemical cells,11,12 oxygen sensors,13,14 synaptic transistors15,16 or memristive devices,17–19Fig. 1 and review in ref. 20. Such devices are often fabricated in thin film form exhibiting conductivities,21,22 optical transmittance23,24 or point defect chemistry25 dependent on the processing and the nanostructure characteristics, which differ from their bulk pellet, tape or single crystalline counterparts. Easy, non-destructive and in situ thin film characterization techniques are essential for controlling the device fabrication and engineering their performance. Raman is a materials characterization tool measuring normal vibrational modes through inelastic scattering of an excitation laser. The strength of the technique is its sensitivity to small and local atomic displacements, that inevitably occur with defect chemical changes. In this work we aim to showcase the abilities of Raman to describe oxygen defect chemistry on the model of Sr(Ti,Fe)O3−y solid solutions. We interpret the Raman spectra of Sr(Ti,Fe)O3−y solid solutions from their defect-chemical perspective in order to highlight Raman as a fast, nondestructive but yet precise materials characterization technique for the broader community of ceramists and electrochemists working with this material class.1.1 Physical and chemical properties of Sr(Ti,Fe)O3−y solid solutions and its end members SrTiO3 and SrFeO3−y
It is illustrative to start the discussion of the materials class with the end members of the solid solution, SrTiO3 and SrFeO3−y, in order to later understand the properties of the intermediate compositions as an alloy of their end members.26,27Strontium titanate, SrTiO3, is a wide band gap oxide (3.2 eV) and is among the most studied electroceramics.28 SrTiO3 has been investigated in terms of its oxide charge transport properties in the bulk and grain boundaries,29–31 defect chemistry32–34 or oxygen exchange.35 The cubic perovskite unit cell, Fig. 2a of SrTiO3 is stable in both reducing and oxidizing atmospheres and over a wide range of temperatures from 105 K36 up to its melting point of 2353 K.37 In contrast, strontium iron oxide, SrFeO3−y, has a rich phase diagram due to structural accommodation of oxygen vacancies as a result of the mixed valence state of the iron ion. This has been recently exploited to control its topotactic phase transitions through electrochemical gating38 resulting in applications in neuromorphic,15,16 electrochromic39,40 or magneto-ionic4 devices. In Fig. 2b the crystal unit cells of the SrFeO3−y phases are depicted in the order of decreasing oxidation. At high oxidation levels SrFeO3 grows in the cubic perovskite structure analogous to SrTiO3.41 The symmetry lowers from cubic to tetragonal, orthorhombic and finally to the brownmillerite phase through the progressive reduction of Fe4+ by Fe3+ in the crystal lattice.42–45 The phase diagram of SrFeO3−y and its relation to the Sr(Ti,Fe)O3−y solid solutions is presented in Fig. 2c, as reported by Mizusaki et al., Fig. 2d.46
 | ||
| Fig. 2 (a) The perovskite cubic lattice unit cell of SrTiO3. (b) Unit cells of the phases of SrFeO3−y in the order of decreasing oxygen content. (c) Phase diagram of the Sr(Ti,Fe)O3−y solid solution as a function of temperature, composition and nonstoichiometry. The iron oxide side of the phase diagram with the different phases is highlighted for clarity,46 (d). | ||
Next we describe the structural properties of the intermediate compositions of the Sr(Fe,Ti)O3−y solid solutions, which can be described as a mixture of strontium titanate, SrTiO3, and strontium ferrite, SrFeO2.5.2,26,27,47 The solid solution is formed without phase separation or oxygen vacancy ordering, Fig. 2c, which has been confirmed by X-ray diffraction (XRD)48–51 and neutron scattering experiments.52 The cubic phase is formed in compositions reaching up to 90 mol% of iron.50,53 However, transmission electron microscopy (TEM) and X-ray absorption spectroscopy (XAS) revealed local unit cell superstructures54 and Fe3+ in a tetrahedral environment55 in compositions above 50 mol% Fe. Generally, the cubic perovskite phase is stabilized with increasing titanium contents2,56 and temperature.57
The description of the Sr(Fe,Ti)O3−y solid solutions from a defect chemical and electronic perspective strongly depends on the content of the mixed-valent iron cation. Compositions with less than 1 mol% iron can be considered dilute solid solutions, and can be described as an acceptor doped wide band gap electroceramic with predominantly electronic conductivity.27,58 As the concentration of iron increases above the dilute solution limit (ca. 1 mol%) the nature of the iron electronic states becomes extended. The Fe3+/Fe4+ states lie on top of the valence band, and form covalent admixtures with the O 2p states. The width of this iron band increases with iron content as the iron states overlap with each other and with O 2p states.2 Consequently, the valence band shifts closer to the conduction band with increasing iron content lowering the Sr(Fe,Ti)O3−y band gap up to ca. 1.9 eV in SrFeO2.5. Concurrently, the ionic and electronic conductivity increase turning the material into a mixed ionic–electronic conductor.2,9,58,59 The oxygen nonstoichiometry generally depends on temperature, oxygen pressure and iron concentration.35 This means that the transport and electronic properties of Sr(Ti,Fe)O3−y solid solutions can be controlled by their composition, environment or voltage, which makes them attractive for many applications.
Lately the thin film form of the Sr(Ti,Fe)O3−y solid solutions is increasingly requested for integration in various electrochemical devices8,18,60,61 and for model electrochemical experiments, which take advantage of the well defined surfaces and geometries of thin films.13,62 In general, thin films have different properties than bulk materials.22,63,64 In polycrystalline films this is a result of an increased grain boundary vs. grain volume due to a reduced average grain size and in single crystalline films it is an absence of grain boundaries. Other effects such as cation nonstoichiometry or dislocation density can create additional differences between thin films and bulk materials. For example, a difference in the electronic properties between bulk and thin film structures was found in films doped up to 5 mol% Fe, which have shown evidence of containing the Fe2+/Fe3+ pair of oxidation states rather than the Fe3+/Fe4+ combination known from bulk pellets.25,65 Also, conductivities of thin films vary from their bulk pellet counterparts. Namely, in 0.37 mol% Fe-doped SrTiO3 thin films the conductivity was three orders of magnitude smaller than in the bulk pellet66 and in Sr(Ti,Fe)O3−y thin films with 35 mol% Fe the difference was one order of magnitude.14 The differences in defect chemistry between bulk and thin films can arise from the non-equilibrium states attained through film deposition techniques, substrate induced strains or space charge effects occurring for nanocrystalline grain sizes, which are close to the Debye length of the material. The different defect states induced by the thin film growth can have an effect on the bond lengths and structural distortions. Traditionally structural properties of Sr(Ti,Fe)O3−y thin films are investigated by electron microscopy and X-ray techniques. These include TEM and XAS, which however require large-scale equipment or facilities. Raman spectroscopy is a lab-scale, thin film-suitable technique that also provides information on short range atomic ordering. We present here a characterization and spectral interpretation of Sr(Ti,Fe)O3−y thin films and bulk pellets to serve as a reference for the broader community of ceramists or electrochemists working with these materials.
1.2 Raman spectroscopic characteristics of the Sr(Ti,Fe)O3−y solid solutions and their end members
In general, the investigations of structure, phase, and defect chemistry of Sr(Ti,Fe)O3−y solid solutions are essential for their integration into any electrochemical devices. Usually, the material's phase is confirmed by XRD, which is an averaging technique providing no information about local atomic arrangements. Raman spectroscopy on the other hand can give useful information on local symmetry breaks caused for example by oxygen vacancy ordering or iron valence state and can therefore serve as a strong complementary technique for structural and defect chemical investigations.Generally in the perovskite lattice Raman can probe the oxygen octahedra tilts, rotations or deformations, that can exclusively activate certain phonon modes.67,68 Sr(Ti,Fe)O3−y solid solutions were investigated with Raman spectroscopy in the form of ceramic powders69 and nanoparticles,70 single crystals71,72 and polycrystalline thin films.62,73 Importantly, we have previously shown that the oxygen nonstoichiometry in Sr(Ti,Fe)O3−y thin films with can be quantified by the frequency of its oxygen vibration.73 However, Sr(Ti,Fe)O3−y spectra of the bulk pellets and thin films including epitaxially grown thin films have not been systematically compared. Since the defect chemistry between the bulk and thin films can substantially vary14,65,66 such a comparative study can serve as a basis for thin film characterization and in situ electrochemical device measurements.
In this paper we synthesize and investigate Sr(Ti,Fe)O3−y solid solution system to describe its Raman characteristics over the whole composition range and for various microstructures ranging from nanocrystalline thin films to macrocrystalline bulk pellets, also including single crystalline thin films for a grain boundary free model material case. First, we present and interpret the Raman spectra of the SrTiO3 and SrFeO3−y end members. Second, we discuss the Raman spectra of the full Sr(Ti,Fe)O3−y solid solutions in the range of 1–75 mol% Fe comparing effects on Raman vibrational characteristics of random and ordered oxygen vacancies, and iron valence state complemented also by in situ electrochemical reduction experiments. The concepts developed here ultimately contribute to the understanding of the influence of defect chemistry on the vibrational modes in the Sr(Ti,Fe)O3−y solid solution, which enables characterization of materials physical and chemical properties based on defect chemistry described crucial for the future design of functional electrode and electrolyte materials in energy and information devices.
2 Experimental
SrTi1−xFexO3−y bulk pellet and thin film samples with compositions of x = 0, 0.01, 0.25, 0.3, 0.5, 0.75, 1 were considered in this study. The bulk pellets were prepared from Sr(Ti,Fe)O3−y powders by uniaxial and isostatic pressing and their phase was confirmed by X-ray diffraction. The relevant powders were produced by a conventional solid state synthesis by ball milling the appropriate amounts of SrCO3, TiO2 and Fe2O3 and sintering powders at 1350 °C for 5 hours. Thin film samples were deposited by pulsed laser deposition (Surface, Germany) with a KrF 248 nm excimer laser. The deposition was performed at 650 °C under 0.027 mbar oxygen pressure for 2000 shots with a laser fluence of 1.9 J cm−2. The distance of the oxide target and the substrate was 8 cm. The film thickness was about 100 nm. The substrates for the oxide thin films were randomly oriented sapphire substrates (Stettler Sapphire, CH) with 80 nm thick bottom layer of e-beam evaporated platinum (Plassys MEB 550, France). The platinum bottom layer serves to absorb the Raman signal and prevent the sapphire substrate Raman peaks to appear in the final spectrum.Additionally, oriented thin films (SrTi0.99Fe0.01O3−δ and SrTi0.7Fe0.3O3−y) were grown on (100) LaAlO3 substrates (CrysTec, Germany) with pulsed laser deposition system. The substrate temperature was 650 °C, 0.027 mbar, the laser pulse frequency 2 Hz, the substrate–target distance was 8 cm, laser fluence 1.9 J cm−2 and the number of shots was 2000.
The XRD of the pellets was measured on the PANalytical X'Pert Pro MPD with the Cu Kα wavelength and the polycrystalline thin films on the PANalytical X'Pert3 MRD with the Cu Kα wavelength in grazing incidence with 0.4° angle. Both scans were measured in the scan range of 2θ = 20–140°. The XRD patterns of the oriented thin films grown on LaAlO3 were measured on the Bruker D8 High-Resolution XRD with the Cu Kα wavelength in the scan range 2θ = 20–60°. The indexing and refinement were performed with the PANalytical X'Pert HighScore Plus software.
Raman spectra were taken with a confocal WITec alpha300 R Raman microscope (WITec, Germany) with three different excitation wavelengths of 457 nm (2.71 eV), 532 nm (2.33 eV) and 633 nm (1.96 eV) and a grating of 1800 grooves per mm. For laser focusing a 100× objective with a numerical aperture (NA) of 0.9 Zeiss microscope was used, which gives an approximate laser spot size of 0.8 μm for the 633 nm laser. The laser energy was adjusted according to the sample between 20 μW and 5 mW. Several different integration times were used according to the specific samples. To minimize the resonant contributions to the spectra, only spectra of the 633 nm excitation wavelength are presented if not stated otherwise.
The in situ oxidation experiments were performed in the HFS600 Linkam (Resultec, Germany) stage using pure oxygen or argon (50 sccm). Here a high working distance (50×, NA = 0.6) objective (Zeiss, Germany) was used to focus on the sample to accommodate for the stage height.
3 Results and discussion
3.1 End members of the solid solution: SrTiO3 and SrFeO3−y
Strontium titanate, SrTiO3, has a cubic crystal structure that has no allowed first-order Raman modes since all atoms sit on a center of inversion. From group theoretical analysis it follows that SrTiO3 has at the Γ point of the Brillouin zone four T1u and one silent T2u mode. Three of the T1u modes are infrared active and one is acoustic.74 In Fig. 3a the T1u first-order lattice vibrational modes in the z direction of the cell are depicted. The first mode, 1T1u, in Fig. 3a corresponds to the Ti–O–Ti bending and is the soft mode connected to the ferroelectric instability.75,76 The second mode corresponds to the translation of Sr against the TiO6 octahedra and the last to Ti–O stretching.In Fig. 3b the comparison of Raman spectra of the SrTiO3 thin film and the bulk pellet at room temperature is shown. Both spectra clearly show pronounced broad Raman features from 200 to 450 cm−1 and from 600 to 800 cm−1. These we assign to second-order Raman scattering in SrTiO3.75,77 Importantly, the material can reveal also first-order modes when processed as a thin film, which was confirmed in single crystalline78,79 as well as polycrystalline80 thin films. This is possible due to the breaking of translational and/or inversion symmetry from intrinsic defects or oxygen nonstoichiometry,80 impurities81 or strain78,79 often present in thin films. Turning to the thin film spectrum, Fig. 3b, modes at 180, 486 and 539 cm−1 are visibly strengthened with respect to the bulk pellet spectrum. We assign these peaks to the infrared active T1u symmetry modes and accordingly to their transverse and longitudinal components: second transverse optical (TO2) and first longitudinal optical mode (LO1), second longitudinal (LO2) and fourth transverse optical mode (TO4).82,83 The large frequency splitting of the Raman vibrational modes into the transverse and longitudinal components is a characteristic of perovskites such as SrTiO3, BaTiO3 (ref. 84) or PbTiO3 (ref. 85) see ref. 86 for details.
We now turn to the strontium iron oxide, SrFeO3−y, being the second end member of the solid solution series. Looking first to the thin film XRD pattern, Fig. 4a, we find major diffraction peaks at 2θ = 32.6, 40.3, 46.9, 58.3 and 126.9°, which correspond to the ref. 43 of cubic SrFeO3 (grey in Fig. 4a). (The strong diffraction peak at 66° marked with (*) belongs to the single crystalline sapphire substrate.) The bulk pellet pattern, Fig. 4a, has the same reflections as the thin film pattern, however, noticeable are also peak splittings and intermediate reflections. A peak splitting example is shown in the inset of Fig. 4a, where reflections corresponding both to the tetragonal and orthorhombic phases of SrFeO3−y are present. Therefore, we conclude, that the phase of the SrFeO3−y bulk pellet is a mixture of the orthorhombic and tetragonal phases, which means that the oxygen nonstoichiometry y in SrFeO3−y is somewhere between 0.15 and 0.35 according to the phase diagram in Fig. 2c.46
In Fig. 4b, the Raman spectrum of the SrFeO3−y as-deposited thin film is compared to the bulk pellet. The spectra are qualitatively similar confirming the transfer of the same phase during the pulsed laser deposition from pellet to thin film. This contrasts the XRD results, which predicted a cubic phase of the thin film. XRD in this case lacks the sensitivity to resolve the superstructure reflections in the thin film. This result highlights the sensitivity of Raman to local atomic arrangements, that can stay unresolved with XRD and shows the suitability of this method for the characterization of the SrFeO3−y reduction extent, critical for its respective electrochemical devices.
The spectra of the SrFeO3−y as-deposited thin film, Fig. 4b, has Raman modes at 135, 181, 220, 332, 428, 476, 617 and 703 cm−1. Apart from the weaker 476 and 703 cm−1 Raman modes, the spectrum is in agreement with experimental spectra of the orthorhombic–tetragonal phase mixture of SrFeO3−y measured by Radheep et al.87 and of spectra measured with y = 0.31 by Adler et al.88 and with y = 0.39 by Barkalov et al.89 The assignment of the weak modes at 476 cm−1 and 703 cm−1 is unclear, however, they could belong to the orthorhombic SrFeO3−y phase, since similar weak modes were reported by Damljanovic.42 Table 1 in the ESI† summarizes the symmetry allowed Raman modes for the tetragonal (I4/mmm), orthorhombic (Cmmm)42 and the brownmillerite (Imma)90,91 phases. The tetragonal phase has 31 allowed Raman active modes, the orthorhombic phase 21 and the brownmillerite phase 51. It is clear that not all modes appear in the spectrum, and we cannot unambiguously assign the phase based solely on the symmetry analysis.
We further reduce the as deposited SrFeO3−y thin film at 500 °C in argon to probe the effect of the different redox states of iron on the phase of SrFeO3−y. After reduction the sample is quenched (150 °C min−1) to room temperature where the Raman spectrum is measured, Fig. 4c. The spectrum shows peaks at 115, 182, 296, 452, 596 and 668 cm−1. The SrFeO2.5 brownmillerite phase Raman spectrum has a distinctive enhanced mode around 660 cm−1,89,91 which has been attributed to the symmetrical stretching of oxygen around the Fe3+ transition metal ion.91 Additional Raman modes of the SrFeO2.5 brownmillerite phase were found around 290, 440 and 600 cm−1.89,91 For the complete assignment of their symmetry, polarized Raman on single crystalline samples would be necessary. In conclusion, the SrFeO3−y thin films reveal for the as-deposited state a mixture of tetragonal and orthorhombic phases, which changes to the brownmillerite phase upon reduction at 500 °C in argon.
3.2 Phase and Raman spectra of the Sr(Ti,Fe)O3−y solid solutions
The XRD patterns of the Sr(Ti,Fe)O3−y solid solutions, Fig. S1,† show the reflections of the cubic perovskite phase for all the considered compositions. The lattice constants were determined through a standard refinement process of the perovskite cubic phase. The empirical Vegard's law describes the change in lattice parameter of a solid solution as the weighted mean two fully intermixable phases (the two end members of the solid solution SrTiO3 and SrFeO3−y). The iron ion changes its size depending on its valence and their respective amounts then determine the average lattice parameter. We plot a reference Vegard's law in Fig. 4 with the assumption that all iron has the 4+ valence. In Fig. 5 we compare the measured lattice constants to the Vegard's law of oxidized Sr(Ti,Fe)O3 (only Fe4+ present) as well as to the lattice constants measured by Vračar et al. of powders annealed under an oxygen pressure of 600 bar.69 The lattice constants of both thin films and pellets decrease with increasing iron content, are however much larger than Vegard's law predicts for complete oxidation (red arrow in Fig. 5). This points to the substitution of Ti by both Fe4+ with a smaller and Fe3+ with a larger ionic radius than Ti4+.58,59,69 The slightly larger lattice constants of Vračar et al.69 as compared to the oxidized prediction from Vegard's law show that full oxidation is not achieved even when annealing under 600 bar O2.92 In summary, the refinement of the XRD patterns show that all the samples contain a mixture of Fe4+ and Fe3+ valence states, however, it is not possible to quantify their respective content. | ||
| Fig. 5 X-Ray diffraction. Refined lattice constants from the XRD patterns of Sr(Ti,Fe)O3−y thin films and pellets as a function of the iron content. For comparison, we show Vegard's law prediction based on oxidized Sr(Ti,Fe)O3 solid solutions and the results of powders oxidized under 600 bar O2 from Vračar et al.43,46,69 | ||
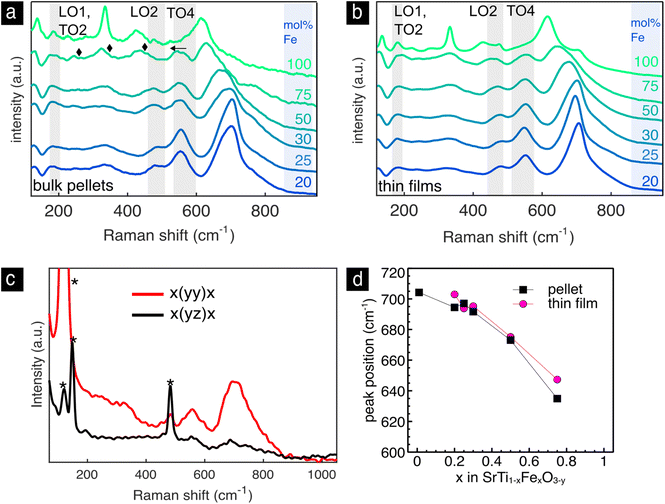
The Raman spectra of the bulk pellets and thin films of the Sr(Ti,Fe)O3−y solid solutions are shown with respect to the iron concentration in Fig. 6a and b. In both pellets and thin films, Fig. 6a and b, we find the T1u modes (LO1, TO2, LO2, TO4) of the cubic lattice analogous to SrTiO3, Fig. 3b, and an additional highly enhanced mode around 700 cm−1. The T1u modes are broadened and strengthened with respect to pure SrTiO3, which can be attributed to the B-site disorder breaking the translational and inversion symmetry. In the bulk pellet with 75% iron we see three extra modes appear at 245, 324 and 435 cm−1. Additionally, the LO2 mode disappears and the TO4 mode red-shifts with respect to the other compositions. The changes mimic the SrFeO3−y spectrum in the tetragonal–orthorhomic phase mixture, and we therefore assign their presence to an oxygen vacancy ordering.54 This is in accordance to previous literature results, which predict a higher degree of structural distortions with iron concentrations above 50 mol% Fe.54,55 Interestingly, these spectral changes are not present in the thin film of the same iron concentration, which preserves the cubic phase by randomly distributing the oxygen vacancies. Raman spectroscopy measures normal vibration modes, whose number and position change upon symmetry breaks or changes in the material such as their redox state. This ability is highlighted in the non-destructive identification of oxygen vacancy ordering in the SrFeO3−y pellet and is accessible via Raman spectroscopy. On the other hand, XRD is an averaging technique that measure mean atomic positions. For these reasons and in this case, it is impossible via XRD to clearly show the evident oxygen vacancy ordering in the pellet sample of SrFeO3−y.
Next, we will discuss the intense mode around 700 cm−1, which is visible in all spectra in Fig. 6. It is connected to an oxygen vibration around Fe4+,73 which has been assigned in prior work through in situ electrochemical titration, see ref. 73 for further details. Similar enhanced modes are commonly measured and characterized in other B site substituted perovskite solid solutions.93–98 They have been assigned to a charge transfer activated oxygen breathing,93,96,99 ordering of the transition metal cations on the B site,98,100 and a Jahn–Teller distortion.69 We do not expect the band to be related to B site ordering as no evidence of this has been found so far in current or previous studies of Sr(Ti,Fe)O3−y.2 A charge transfer process would be possible between two neighboring Fe atoms but unlikely between Fe and Ti. And finally, a local Jahn–Teller distortion around Fe4+, previously suggested to activate the enhanced mode,69,101 diminishes as the iron band broadens merging the d-electron energy levels.102 We conclude therefore, that the mode is clearly connected to the oxidized iron state but the physical origin of it remains unclear.
To assign its symmetry, we have investigated oriented SrTi0.7Fe0.3O3−y films grown on (100) LaAlO3 substrates, Fig. 6d. The XRD patterns in Fig. S2† show the epitaxial nature of the film. The Raman spectra were measured in the backscattering configuration in the quasicubic y–z plane of the LaAlO3 substrate with both parallel and cross polarization configurations. The mode disappears in the cross polarization configuration signifying a symmetrical stretching vibration. This is in accordance to experimental93,103 and theoretical101,104 results of structurally related B site perovskite solid solutions assigning the mode to a local Ag-like oxygen stretching mode.
3.3 Role of the iron valence on the Raman spectra of Sr(Ti,Fe)O3−y
The enhanced oxygen stretching mode red-shifts as the material expands during reduction and Fe4+ is replaced with Fe3+.73 The spectra in Fig. 6 clearly show a red-shift with increasing iron content. This apparent expansion contrasts the average lattice contraction as measured from XRD, Fig. 5. The maximum Raman frequency of the stretching mode occurs in completely oxidized samples (only Fe4+) around 700 cm−1 for all compositions.69,73 With the introduction of Fe3+ the mode down shifts in frequency. In both thin films and pellets this down shift is greater with increasing iron concentration. This has to do with the expansion of the local environment around Fe4+ as more Fe3+ neighbors are introduced. It is also evident from the difference in the measured lattice constant from the theoretical lattice constant at complete oxidation (red arrow in Fig. 5), which increases with iron content. In prior work, the oxygen nonstoichiometry has been calibrated to the position of the oxygen stretching mode for the compositions of 30 and 50 mol% Fe.73 In order to calculate the oxygen nonstoichiometry in the present samples we use the previously measured Raman frequency of completely reduced Sr(Ti,Fe)O3−y (685 and 664 cm−1 for 30 and 50 mol% Fe), and the relative change in wavenumber (ν) with respect to the oxygen nonstoichiometry (y) (123 and 180 cm−1 for 30 and 50 mol% Fe). The resulting nonstoichiometry for the bulk pellets is y = 0.02, 0.12, and for the as-deposited thin films y = 0.074, 0.16, corresponding to 30 and 50 mol% Fe respectively. The thin films are therefore slightly more reduced than the pellets, which is manifested also in their higher oxygen vibrational frequencies, Fig. 6c. We want to stress that for an absolute and non-comparative quantification of the oxygen nonstoichiometry, a reference sample with a known composition is required. In summary, the frequency of the oxygen stretching mode gives a convenient measure of the sample's oxidation extent, and it can be used to determine the oxygen stoichiometry also quantitatively,73 unlike classical XRD.
(123 and 180 cm−1 for 30 and 50 mol% Fe). The resulting nonstoichiometry for the bulk pellets is y = 0.02, 0.12, and for the as-deposited thin films y = 0.074, 0.16, corresponding to 30 and 50 mol% Fe respectively. The thin films are therefore slightly more reduced than the pellets, which is manifested also in their higher oxygen vibrational frequencies, Fig. 6c. We want to stress that for an absolute and non-comparative quantification of the oxygen nonstoichiometry, a reference sample with a known composition is required. In summary, the frequency of the oxygen stretching mode gives a convenient measure of the sample's oxidation extent, and it can be used to determine the oxygen stoichiometry also quantitatively,73 unlike classical XRD.
To further clarify the role of iron valence on the Sr(Ti,Fe)O3−y Raman spectra we measure the thin films under varying atmosphere in ex and in situ experiments, Fig. 7. After reduction at 500 °C under 10−6 mbar vacuum the enhanced oxygen stretching mode either disappears or its intensity significantly decreases consistent with previous reports,69Fig. 7a. This phenomenon was explained by Blokhin et al., who showed with ab initio calculations that the phonon density of states in the region from 620 to 760 cm−1 is zero when  complexes are present in the material.104 The absence of additional Raman modes beyond the T1u cubic modes signifies that the thin films remain in the cubic phase even upon strong reduction.
complexes are present in the material.104 The absence of additional Raman modes beyond the T1u cubic modes signifies that the thin films remain in the cubic phase even upon strong reduction.
To understand the potential of Raman to measure oxygen incorporation kinetics we perform an in situ experiment with the SrTi0.7Fe0.3O3−y thin film, Fig. 7b. First, we reduce the sample under argon at 500 °C until the oxygen stretching mode disappears. Next, we measure spectra at 450 °C under an oxygen atmosphere, and monitor the growth of the oxygen stretching mode, signifying the ongoing oxidation of Fe3+ to Fe4+. With enhanced temperatures, Raman spectra are noisier, the intensity lowers and the peaks broaden due to increased thermal vibrations. This in turn increases the necessary acquisition time per spectrum lowering the time resolution of the in situ experiment. Time resolution of in situ Raman experiments in general will be given by the strength of the Raman bands (sample nature), experimental temperature and oxygen exchange kinetics. Additionally, Raman mode intensity depends on the sample, laser energy, laser focus and instrumentation. Therefore quantification of the laser intensity changes over time are less precise than peak positions. Here, we do not attempt to fully quantify oxygen exchange kinetics. The growing oxygen stretching mode is shifted to lower wavenumbers (ca. 675 cm−1), Fig. 7b. This is a consequence of a combination of chemical expansion during the reduction of the material and thermal expansion at higher temperatures. Since these expansions are strongly coupled,105 it is difficult to separate their contributions to the peak shift. In summary the in situ experiments confirm the connection of the oxygen stretching mode to the Fe4+ cation and also highlight the potential to measure oxidation kinetics with Raman, relevant for operando measurements of electrochemical devices.
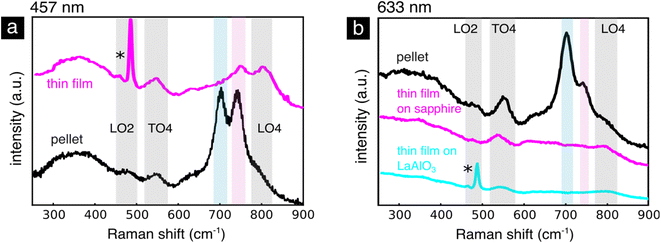
When comparing the bulk pellet with the thin film, Fig. 8a, we notice that the oxygen stretching mode at 704 cm−1 disappears in both oriented (grown on LaAlO3) and polycrystalline (grown on sapphire) thin films, Fig. 8b. Using a combination of X-ray absorption and photoelectron spectroscopy Koehl et al. reported that SrTiO3 thin films doped with iron up to 5 mol% contain the Fe3+ and Fe2+ oxidation states,25 rather than the Fe4+/Fe3+ pair present in the bulk. They argue that the non-equilibrium deposition of the thin film introduces a higher concentration of point defects or that dislocations and anti-phase boundaries introduce a higher number of oxygen vacancies. The absence of Fe4+ in lightly Fe doped SrTiO3 thin films justifies the absence of the enhanced mode at 704 cm−1 associated to Fe4+, Fig. 8a and b. When using the 457 nm excitation wavelength, Fig. 8a, however, the resonantly enhanced mode around 740 nm connected to an electronic transition from Fe4+ does not completely disappear in the thin film sample, Fig. 8. This could be caused by a low concentration of Fe4+, which have been shown to form under the irradiation of wavelengths between 390 and 485 nm.6,109
4 Conclusion
Understanding the Raman mode characteristics of the materials class Sr(Ti,Fe)O3−y and its wide range of compositions is essential to understand and design its defect chemistry by means of oxygen nonstoichiometry, oxygen vacancy ordering and the iron valence state. A multitude of mixed ionic–electronic transport characteristics or even magnetic properties can be described through the assessment of the Raman vibrational characteristics of each solid solution composition. Despite the wide use of this materials class in energy and information devices in both bulk and thin film form, making Sr(Ti,Fe)O3−y almost a model material case, experimental description of its Raman vibration characteristics remains still scarce.In this work we synthesize a wide set of model Sr(Ti,Fe)O3−y solid solution compositions and study their Raman signatures in bulk pellet (macroscrystalline), polycrystalline thin film (nanocrystalline) and epitaxial films (without grain boundaries) to effectively modulate the role of grain boundaries and grain size. In this model solid solution, the presence of Fe3+/Fe4+ redox states is balanced with changes in the oxygen nonstiochiometry to keep overall electroneutrality in the defect chemistry. Clearly, the Raman spectra recorded confirm that all here exemplified compositions of the solid solution exhibit an enhanced oxygen stretching mode around 700 cm−1 connected to the Fe4+ valence state, which may serve as a material's fingerprint. Specifically, through polarized measurements on epitaxial films we assign it to the symmetric stretching of oxygen around the Fe4+ cation. An exception is the lightly iron doped (1 mol%) polycrystalline and epitaxial thin films, where the Fe4+ valence is missing. But also oxygen vacancy ordering has been verified in the pellet with high iron content (75 mol%) established through the occurrence of Raman modes that can be attributed to the tetragonal and orthorhombic phases of SrFeO3−y. Regarding the SrTiO3 and SrFeO3−y end members, we identify the usual cubic phase in SrTiO3 bulk pellets and thin films. In SrFeO3−y we trigger the topotactic phase transition by high temperature reduction, which we attribute to a transition from the tetragonal–orthorhombic phase mixture to the brownmillerite phase by a combination of Raman and XRD.
Collectively, we present a comprehensive study of the Raman characteristics for the model Sr(Ti,Fe)O3−y solid solutions and reveal the varying impact of the iron valence state on oxygen vacancies. These findings can serve as a standard for the determination of defect chemical characteristics in Sr(Ti,Fe)O3−y systems and aid the materials design of energy and information devices integrating this materials class. Typically defect chemical characteristics are determined via electrochemical studies through the application of electrodes. We advocate based on the findings presented that important insights on both the transition metal ion redox state, oxygen nonstoichiometry and ordering relevant for mixed ionic–electronic transport, and other properties can be fully accessed even for thin film samples via Raman spectroscopy.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Swiss National Science Foundation Starting Grant, grant number BSSGI0_155986/1. The authors thank Dr Thomas Weber from the X-ray Service Platform at ETH Zurich.References
- W. Jung and H. L. Tuller, Solid State Ionics, 2009, 180, 843 CrossRef CAS.
- A. Rothschild, W. Menesklou, H. L. Tuller and E. Ivers-Tiffe, Chem. Mater., 2006, 18, 3651 CrossRef CAS.
- H.-S. Kim, L. Bi, G. F. Dionne and C. A. Ross, Appl. Phys. Lett., 2008, 93, 092506 CrossRef.
- S. Ning, Q. Zhang, C. Occhialini, R. Comin, X. Zhong and C. A. Ross, ACS Nano, 2020, 14, 8949 CrossRef CAS PubMed.
- R. Waser, T. Bieger and J. Maier, Solid State Commun., 1990, 76, 1077 CrossRef CAS.
- M. Siebenhofer, A. Viernstein, M. Morgenbesser, J. Fleig and M. Kubicek, Mater. Adv., 2021, 2, 7583 RSC.
- S. J. Skinner and J. A. Kilner, Mater. Today, 2003, 6, 30 CrossRef CAS.
- W. Jung and H. L. Tuller, Energy Environ. Sci., 2012, 5, 5370 RSC.
- W. Jung and H. L. Tuller, ECS Trans., 2009, 25, 2775 CrossRef CAS.
- A. Nenning, L. Volgger, E. Miller, L. V. Mogni, S. Barnett and J. Fleig, J. Electrochem. Soc., 2017, 164, F364 CrossRef CAS.
- J. Fleig, G. Walch, G. C. Brunauer, B. Rotter, E. Esmaeli, J. Summhammer, A. K. Opitz and K. Ponweiser, ECS Trans., 2016, 72, 23 CrossRef CAS.
- G. Walch, B. Rotter, G. C. Brunauer, E. Esmaeili, A. K. Opitz, M. Kubicek, J. Summhammer, K. Ponweiser and J. Fleig, J. Mater. Chem. A, 2017, 5, 1637 RSC.
- W. Menesklou, H.-J. Schreiner, K. H. Härdtl and E. Ivers-Tiffée, Sens. Actuators, B, 1999, 59, 184 CrossRef CAS.
- S. J. Litzelman, A. Rothschild and H. L. Tuller, Sens. Actuators, B, 2005, 108, 231 CrossRef CAS.
- P. Shi, D. Wang, T. Yu, R. Xing, Z. Wu, S. Yan, L. Wei, Y. Chen, H. Ren, C. Yu and F. Li, Mater. Des., 2021, 210, 110022 CrossRef CAS.
- C. Ge, C. Liu, Q. Zhou, Q. Zhang, J. Du, J. Li, C. Wang, L. Gu, G. Yang and K. Jin, Adv. Mater., 2019, 31, 1900379 CrossRef PubMed.
- R. Muenstermann, T. Menke, R. Dittmann and R. Waser, Adv. Mater., 2010, 22, 4819 CrossRef CAS PubMed.
- F. Messerschmitt, M. Kubicek, S. Schweiger and J. L. M. Rupp, Adv. Funct. Mater., 2014, 24, 7448 CrossRef CAS.
- K. Berggren, Q. Xia, K. K. Likharev, D. B. Strukov, H. Jiang, T. Mikolajick, D. Querlioz, M. Salinga, J. R. Erickson, S. Pi, F. Xiong, P. Lin, C. Li, Y. Chen, S. Xiong, B. D. Hoskins, M. W. Daniels, A. Madhavan, J. A. Liddle, J. J. McClelland, Y. Yang, J. Rupp, S. S. Nonnenmann, K.-T. Cheng, N. Gong, M. A. Lastras-Montaño, A. A. Talin, A. Salleo, B. J. Shastri, T. F. de Lima, P. Prucnal, A. N. Tait, Y. Shen, H. Meng, C. Roques-Carmes, Z. Cheng, H. Bhaskaran, D. Jariwala, H. Wang, J. M. Shainline, K. Segall, J. J. Yang, K. Roy, S. Datta and A. Raychowdhury, Nanotechnology, 2020, 32, 012002 CrossRef PubMed.
- J. Irvine, J. L. M. Rupp, G. Liu, X. Xu, S. Haile, X. Qian, A. Snyder, R. Freer, D. Ekren, S. Skinner, O. Celikbilek, S. Chen, S. Tao, T. H. Shin, R. O'Hayre, J. Huang, C. Duan, M. Papac, S. Li, V. Celorrio, A. Russell, B. Hayden, H. Nolan, X. Huang, G. Wang, I. Metcalfe, D. Neagu and S. G. Martín, J. Phys.: Energy, 2021, 3, 031502 CAS.
- M. Kubicek, S. Taibl, E. Navickas, H. Hutter, G. Fafilek and J. Fleig, J. Electroceram., 2017, 39, 197 CrossRef CAS PubMed.
- R. A. De Souza, F. Gunkel, S. Hoffmann-Eifert and R. Dittmann, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 241401 CrossRef.
- J. J. Kim, S. R. Bishop, N. Thompson, Y. Kuru and H. L. Tuller, Solid State Ionics, 2012, 225, 198 CrossRef CAS.
- N. H. Perry, J. J. Kim and H. L. Tuller, Sci. Technol. Adv. Mater., 2018, 19, 130 CrossRef CAS PubMed.
- A. Koehl, D. Kajewski, J. Kubacki, C. Lenser, R. Dittmann, P. Meuffels, K. Szot, R. Waser and J. Szade, Phys. Chem. Chem. Phys., 2013, 15, 8311 RSC.
- B. Ouyang, T. Mueller, N. H. Perry, N. R. Aluru and E. Ertekin, 2018, preprint, arXiv:1801.00456, DOI:10.48550/arXiv.1801.00456.
- N. Kim, N. H. Perry and E. Ertekin, Chem. Mater., 2019, 31, 233 CrossRef CAS.
- R. A. De Souza, J. Fleig, R. Merkle and J. Maier, Z. Metallkd., 2003, 94, 218 CrossRef CAS.
- I. Denk, F. Noll and J. Maier, J. Am. Ceram. Soc., 1997, 80, 279 CrossRef CAS.
- S. Rodewald, J. Fleig and J. Maier, J. Am. Ceram. Soc., 2001, 84, 521 CrossRef CAS.
- M. Vollmann and R. Waser, J. Electroceram., 1997, 1, 51 CrossRef CAS.
- I. Denk, W. Münch and J. Maier, J. Am. Ceram. Soc., 1995, 78, 3265 CrossRef CAS.
- N.-H. Chan, R. K. Sharma and D. M. Smyth, J. Electrochem. Soc., 1981, 128, 1762 CrossRef CAS.
- R. Moos and K. H. Hardtl, J. Am. Ceram. Soc., 1997, 80, 2549 CrossRef CAS.
- R. Merkle and J. Maier, Angew. Chem., Int. Ed., 2008, 47, 3874 CrossRef CAS PubMed.
- K. A. Müller and H. Burkard, Phys. Rev. B: Condens. Matter Mater. Phys., 1979, 19, 3593 CrossRef.
- H. Ohta, Mater. Today, 2007, 10, 44 CrossRef CAS.
- B. M. Lefler, W. M. Postiglione, C. Leighton and S. J. May, Adv. Funct. Mater., 2022, 32, 2208434 CrossRef CAS.
- J. S. Lim, J. Lee, B. J. Lee, Y.-J. Kim, H.-S. Park, J. Suh, H.-H. Nahm, S.-W. Kim, B.-G. Cho, T. Y. Koo, E. Choi, Y.-H. Kim and C.-H. Yang, Sci. Adv., 2020, 6, eabb8553 CrossRef CAS PubMed.
- H. T. D. Nguyen, D. Zhang, T. M. Nguyen, H. Zhang and J. Seidel, Adv. Opt. Mater., 2021, 9, 2100955 CrossRef CAS.
- M. Okube, Y. Furukawa, A. Yoshiasa, T. Hashimoto, M. Sugahara and A. Nakatsuka, J. Phys.: Conf. Ser., 2008, 121, 092004 CrossRef.
- V. Damljanovic, Raman Scattering, Magnetization and Magnetotransport Study of SrFeO3, Sr3Fe2O7−x and CaFeO3, Max-Planck-Institut für Festkörperforschung, 2008 Search PubMed.
- J. P. Hodges, S. Short, J. D. Jorgensen, X. Xiong, B. Dabrowski, S. M. Mini and C. W. Kimball, J. Solid State Chem., 2000, 151, 190 CrossRef CAS.
- Y. Takeda, K. Kanno, T. Takada, O. Yamamoto, M. Takano, N. Nakayama and Y. Bando, J. Solid State Chem., 1986, 63, 237 CrossRef CAS.
- M. Takano, T. Okita, N. Nakayama, Y. Bando, Y. Takeda, O. Yamamoto and J. B. Goodenough, J. Solid State Chem., 1988, 73, 140 CrossRef CAS.
- J. Mizusaki, M. Okayasu, S. Yamauchi and K. Fueki, J. Solid State Chem., 1992, 99, 166 CrossRef CAS.
- N. H. Perry, D. Pergolesi, S. R. Bishop and H. L. Tuller, Solid State Ionics, 2015, 273, 18 CrossRef CAS.
- L. H. Brixner, Mater. Res. Bull., 1968, 3, 299 CrossRef CAS.
- C. Greaves and R. A. Buker, Mater. Res. Bull., 1986, 21, 823 CrossRef CAS.
- H. D. Zhou and J. B. Goodenough, J. Solid State Chem., 2004, 177, 1952 CrossRef CAS.
- M. Ghaffari, T. Liu, H. Huang, O. K. Tan and M. Shannon, Mater. Chem. Phys., 2012, 136, 347 CrossRef CAS.
- T. C. Gibb, P. D. Battle, S. K. Bollen and R. J. Whitehead, J. Mater. Chem., 1992, 2, 111 RSC.
- P. I. Cowin, R. Lan, C. T. G. Petit and S. Tao, Solid State Sci., 2015, 46, 62 CrossRef CAS.
- S. Steinsvik, R. Bugge, J. Gjonnes, J. Taft and T. Norby, J. Phys. Chem. Solids, 1997, 58, 969 CrossRef CAS.
- E. O. Filatova, Y. V. Egorova, K. A. Galdina, T. Scherb, G. Schumacher, H. J. M. Bouwmeester and S. Baumann, Solid State Ionics, 2017, 308, 27 CrossRef CAS.
- M. V. Patrakeev, I. A. Leonidov, V. L. Kozhevnikov and V. V. Kharton, Mater. Sci. Forum, 2006, 514–516, 382 CAS.
- J. B. MacChesney, R. C. Sherwood and J. F. Potter, J. Chem. Phys., 1965, 43, 1907 CrossRef CAS.
- L. F. da Silva, J.-C. M'Peko, J. Andrés, A. Beltrán, L. Gracia, M. I. B. Bernardi, A. Mesquita, E. Antonelli, M. L. Moreira and V. R. Mastelaro, J. Phys. Chem. C, 2014, 118, 4930 CrossRef CAS.
- T. R. Clevenger, J. Am. Ceram. Soc., 1963, 46, 207 CrossRef CAS.
- A. Evans, A. Bieberle-Hütter, J. L. M. Rupp and L. J. Gauckler, J. Power Sources, 2009, 194, 119 CrossRef CAS.
- G. Korotcenkov, Sens. Actuators, B, 2005, 107, 209 CrossRef CAS.
- B. E. Hayden and F. K. Rogers, J. Electroanal. Chem., 2018, 819, 275 CrossRef CAS.
- C. Ohly, S. Hoffmann-Eifert, X. Guo, J. Schubert and R. Waser, J. Am. Ceram. Soc., 2006, 89, 2845 CrossRef CAS.
- S. Schweiger, M. Kubicek, F. Messerschmitt, C. Murer and J. L. M. Rupp, ACS Nano, 2014, 8, 5032 CrossRef CAS PubMed.
- J. Szade, K. Szot, M. Kulpa, J. Kubacki, C. Lenser, R. Dittmann and R. Waser, Phase Transitions, 2011, 84, 489 CrossRef CAS.
- S. Taibl, G. Fafilek and J. Fleig, Nanoscale, 2016, 8, 13954 RSC.
- M. C. Weber, M. Guennou, H. J. Zhao, J. Íñiguez, R. Vilarinho, A. Almeida, J. A. Moreira and J. Kreisel, Phys. Rev. B, 2016, 94, 214103 CrossRef.
- M. N. Iliev, M. V. Abrashev, J. Laverdière, S. Jandl, M. M. Gospodinov, Y.-Q. Wang and Y.-Y. Sun, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 064302 CrossRef.
- M. Vračar, A. Kuzmin, R. Merkle, J. Purans, E. A. Kotomin, J. Maier and O. Mathon, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 174107 CrossRef.
- N. Van Minh and D. T. T. Phuong, J. Sol-Gel Sci. Technol., 2010, 55, 255 CrossRef.
- C. Lenser, A. Kalinko, A. Kuzmin, D. Berzins, J. Purans, K. Szot, R. Waser and R. Dittmann, Phys. Chem. Chem. Phys., 2011, 13, 20779 RSC.
- O. Kurt, D. Ascienzo, S. Greenbaum, T. J. M. Bayer, C. A. Randall, N. Madamopoulos and Y. H. Ren, Mater. Chem. Phys., 2017, 198, 131 CrossRef CAS.
- E. Sediva, T. Defferriere, N. H. Perry, H. L. Tuller and J. L. M. Rupp, Adv. Mater., 2019, 31, 1902493 CrossRef PubMed.
- R. A. Evarestov, E. Blokhin, D. Gryaznov, E. A. Kotomin and J. Maier, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 134108 CrossRef.
- T. Ostapchuk, J. Petzelt, V. Železný, A. Pashkin, J. Pokorný, I. Drbohlav, R. Kužel, D. Rafaja, B. P. Gorshunov and M. Dressel, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 235406 CrossRef.
- A. A. Sirenko, C. Bernhard, A. Golnik, A. M. Clark, J. Hao, W. Si and X. X. Xi, Nature, 2000, 404, 373 CrossRef PubMed.
- D. A. Tenne, I. E. Gonenli, A. Soukiassian, D. G. Schlom, S. M. Nakhmanson, K. M. Rabe and X. X. Xi, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 024303 CrossRef.
- A. A. Sirenko, I. A. Akimov, J. R. Fox, A. M. Clark, H.-C. Li, W. Si and X. X. Xi, Phys. Rev. Lett., 1999, 82, 4500 CrossRef CAS.
- V. I. Merkulov, J. R. Fox, H.-C. Li, W. Si, A. A. Sirenko and X. X. Xi, Appl. Phys. Lett., 1998, 72, 3291 CrossRef CAS.
- S. Gupta and R. S. Katiyar, J. Raman Spectrosc., 2001, 32, 885 CrossRef CAS.
- H. Uwe, H. Yamaguchi and T. Sakudo, Ferroelectrics, 1989, 96, 123 CrossRef CAS.
- J. D. Axe, Phys. Rev., 1967, 157, 429 CrossRef CAS.
- J. Hlinka, J. Petzelt, S. Kamba, D. Noujni and T. Ostapchuk, Phase Transitions, 2006, 79, 41 CrossRef CAS.
- M. DiDomenico, S. H. Wemple, S. P. S. Porto and R. P. Bauman, Phys. Rev., 1968, 174, 522 CrossRef CAS.
- G. Burns, J. A. Sanjurjo and E. López-Cruz, Phys. Rev. B: Condens. Matter Mater. Phys., 1984, 30, 7170 CrossRef CAS.
- W. Zhong, R. D. King-Smith and D. Vanderbilt, Phys. Rev. Lett., 1994, 72, 3618 CrossRef CAS PubMed.
- D. M. Radheep, K. Shanmugapriya, B. Palanivel and R. Murugan, Appl. Phys. A: Mater. Sci. Process., 2016, 122, 778 CrossRef.
- P. Adler, A. Lebon, V. Damljanović, C. Ulrich, C. Bernhard, A. V. Boris, A. Maljuk, C. T. Lin and B. Keimer, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 094451 CrossRef.
- O. I. Barkalov, S. V. Zaitsev and V. D. Sedykh, Solid State Commun., 2022, 354, 114912 CrossRef CAS.
- A. Maity, R. Dutta, B. Penkala, M. Ceretti, A. Letrouit-Lebranchu, D. Chernyshov, A. Perichon, A. Piovano, A. Bossak and M. Meven, et al. , J. Phys. D Appl. Phys., 2015, 48, 504004 CrossRef.
- A. Piovano, M. Ceretti, M. R. Johnson, G. Agostini, W. Paulus and C. Lamberti, J. Phys.: Condens. Matter, 2015, 27, 225403 CrossRef PubMed.
- P. Adler and S. Eriksson, Z. Anorg. Allg. Chem., 2000, 626, 118 CrossRef CAS.
- J. Andreasson, J. Holmlund, C. S. Knee, M. Käll, L. Börjesson, S. Naler, J. Bäckström, M. Rübhausen, A. K. Azad and S.-G. Eriksson, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 104302 CrossRef.
- A. G. S. Filho, J. L. B. Faria, I. Guedes, J. M. Sasaki, P. T. C. Freire, V. N. Freire, J. Mendes Filho, M. M. Xavier, F. A. O. Cabral, J. H. de Araújo and J. A. P. da Costa, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 67, 052405 CrossRef.
- Y. Fujioka, J. Frantti and M. Kakihana, J. Phys. Chem. B, 2004, 108, 17012 CrossRef CAS.
- G. Kotnana and S. N. Jammalamadaka, J. Appl. Phys., 2015, 118, 124101 CrossRef.
- I. G. Siny, R. S. Katiyar and A. S. Bhalla, J. Raman Spectrosc., 1998, 29, 385 CrossRef CAS.
- C. L. Bull and P. F. McMillan, J. Solid State Chem., 2004, 177, 2323 CrossRef CAS.
- J. Andreasson, J. Holmlund, R. Rauer, M. Käll, L. Börjesson, C. S. Knee, A. K. Eriksson, S.-G. Eriksson, M. Rübhausen and R. P. Chaudhury, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 235103 CrossRef.
- M. N. Iliev, M. V. Abrashev, A. P. Litvinchuk, V. G. Hadjiev, H. Guo and A. Gupta, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 104118 CrossRef.
- R. Evarestov, E. Blokhin, D. Gryaznov, E. A. Kotomin, R. Merkle and J. Maier, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 174303 CrossRef.
- V. E. Alexandrov, J. Maier and R. A. Evarestov, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 075111 CrossRef.
- A. Dubroka, J. Humlíček, M. V. Abrashev, Z. V. Popović, F. Sapiña and A. Cantarero, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 224401 CrossRef.
- E. Blokhin, E. A. Kotomin and J. Maier, J. Phys.: Condens. Matter, 2012, 24, 104024 CrossRef CAS PubMed.
- N. H. Perry, J. J. Kim, S. R. Bishop and H. L. Tuller, J. Mater. Chem. A, 2015, 3, 3602 RSC.
- T. Bieger, J. Maier and R. Waser, Ber. Bunsenges. Phys. Chem., 1993, 97, 1098 CrossRef CAS.
- J. N. Baker, P. C. Bowes, D. M. Long, A. Moballegh, J. S. Harris, E. C. Dickey and D. L. Irving, Appl. Phys. Lett., 2017, 110, 122903 CrossRef.
- J. Dashdorj, M. Zvanut and L. J. Stanley, J. Appl. Phys., 2010, 107, 083513 CrossRef.
- K. W. Blazey, Phys. Status Solidi A, 1976, 38, K97 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ta04818g |
| This journal is © The Royal Society of Chemistry 2023 |