Unravelling the electrochemical activation and the reaction mechanism of maricite-NaFePO4 using multimodal operando techniques†
Abstract
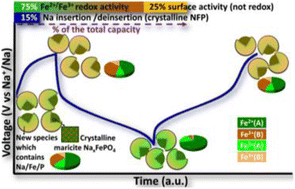
The electrochemical activity of maricite NaFePO4 can be triggered by ball milling with carbon. The origin of such activation is still unclear, as this material does not exhibit open channels for Na+ diffusion. Herein, a characterisation approach including several complementary techniques is applied to investigate the effect of ball milling on the structure of maricite NaFePO4 prepared by hydrothermal synthesis, and how these modifications influence its electrochemical mechanism. Our findings confirm the partial nano-sizing and amorphisation of maricite, going along with the oxidation of the iron centres during ball milling, and allow the elucidation of different mechanisms contributing to the total capacity delivered during (de)sodiation. Although only 15% of the capacity is explained by Na+ insertion/extraction in/from bulk crystalline NaFePO4, 75% of the total capacity is attributed to the simultaneous Fe3+/Fe2+ redox activity. The remaining 25% extra-capacity does not seem to be related to Fe3+/Fe2+ activity, but rather to surface activity, associated with the new species formed during ball milling.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...