A metal–organic framework based propylene nano-trap with dual functionalities for highly efficient propylene/propane separation†
Hui-Min
Wen
 a,
Miaoyu
Liu
a,
Yujia
Ling
a,
Xiao-Wen
Gu
b,
Di
Liu
b,
Chenyi
Yu
a,
Yulan
Liang
a,
Bo
Xie
a,
Miaoyu
Liu
a,
Yujia
Ling
a,
Xiao-Wen
Gu
b,
Di
Liu
b,
Chenyi
Yu
a,
Yulan
Liang
a,
Bo
Xie
 a,
Bin
Li
a,
Bin
Li
 *b and
Jun
Hu
*b and
Jun
Hu
 *a
*a
aCollege of Chemical Engineering, Zhejiang University of Technology, Hangzhou, Zhejiang 310014, China. E-mail: hjzjut@zjut.edu.cn
bState Key Laboratory of Silicon and Advanced Semiconductor Materials, School of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: bin.li@zju.edu.cn
First published on 31st July 2023
Abstract
Propylene/propane (C3H6/C3H8) separation represents one of the most challenging and energy-intensive processes in the petrochemical industry due to their very similar sizes and physical properties. Most of the reported physisorbents still face the challenge of achieving simultaneously high C3H6 uptake and selectivity with moderate adsorption enthalpy. Herein, we realize an efficient propylene nano-trap in a microporous MOF (ZJUT-2, Ni(pyz-SH)2SiF6) for highly efficient C3H6/C3H8 separation. This MOF-based propylene nano-trap features a suitable pore cavity decorated with dual functionalities (–SH and SiF62−) to optimally interact with the C3H6 molecule, affording both large C3H6 capture capacity (123.5 cm3 cm−3 at 296 K and 0.5 bar) and high C3H6/C3H8 selectivity of 17.2 achieved with moderate C3H6 adsorption enthalpy (45 kJ mol−1). Theoretical calculations revealed that the appropriate pore cavity and dual functionalities synergistically construct an efficient nano-trap to match better with the C3H6 molecule and thus provide stronger multipoint interactions with C3H6 over C3H8. Actual breakthrough experiments demonstrated that this material can efficiently capture C3H6 from C3H6/C3H8 mixtures (50/50 and 10/90, v/v) under ambient conditions, affording both top-tier C3H6 capture amount (2.6 mmol g−1) and dynamic selectivity of 10.
Introduction
Propylene (C3H6) is one of the most critical chemical intermediates and an essential raw material used in the production of polypropylene, acrylonitrile, isopropanol, and propylene oxide.1 In 2018, the global production of polypropylene, as the second most important synthetic plastic (second to polyethylene), was estimated at 56 Mt and will continuously increase to 88 Mt by 2026.2 For most end uses, the propylene must have a purity of at least 99.5% (polymer-grade).3 In industry, propylene is typically obtained by steam cracking of naphtha or during fluid catalytic cracking of gas oils in refineries, which involves propane (C3H8) as a coproduct.4 The production of polymer-grade C3H6 involves the separation of C3H6 from a C3H6/C3H8 mixture. A conventional method for this separation mainly relies on cryogenic distillation, executed at about 243 K and 0.3 MPa in a column containing over 100 trays.5 Evidently, such a heat-driven separation process is highly energy-intensive. It is highly demanded to develop alternative and energy-efficient separation technologies to potentially supersede traditional methods.6Non-thermally driven processes, such as adsorptive separation by porous materials, have been considered to dramatically reduce the cost and energy required to purify olefins. In this regard, microporous metal–organic frameworks (MOFs) have been demonstrated to be promising adsorbents for gas separation and purification owing to their tunable pore size/shape and surface functionality.7–11 Amongst various gas separations, C3H6/C3H8 separation represents one of the highest separation difficulties due to the subtle molecular size difference between the two components (<0.4 Å). A number of MOFs have been developed in recent years to show high C3H6/C3H8 separation performance based on equilibrium-based, kinetic-based, molecular sieving or gating-opening mechanisms.12–30 Nevertheless, there commonly exists a trade-off challenge between uptake capacity and separation selectivity for most of the materials. For instance, several size-selective adsorbents with well-matched pore sizes (e.g., KAUST-7 and Co-gallate) enable complete size-exclusion of C3H8 from C3H6 to show record C3H6/C3H8 selectivities but are commonly impaired by their relatively low gas uptakes.16,18 In contrast, those large-pore MOFs (e.g., HKUST-1 and FeMIL-100) show high C3H6 uptakes over 120 cm3 g−1; however, large pores cannot efficiently discriminate the two similar molecules, resulting in low selectivity below 5.29 To overcome this trade-off dilemma, some specific MOFs with gating-opening or thermodynamic–kinetic effects have been realized for benchmark separation properties, while such kinds of materials are difficult to universally design in most cases.12–19 Another more popular strategy is to immobilize strong open metal sites (OMSs) into MOFs for boosting preferential binding of C3H6 over C3H8.25–29 For example, the incorporation of high-density OMSs in MOF-74 or Ag(I) centers in MIL-101-SO3H can improve C3H6/C3H8 selectivity up to ca. 40 while maintaining a high C3H6 adsorption amount.25–28 However, such high selectivities arise from ultra-strong metal−olefin interactions that are commonly greater than 60 kJ mol−1, leading to high regeneration energy. Evidently, there is a high demand to immobilize suitable functional sites with moderate binding affinity to boost C3H6/C3H8 selectivity while maintaining high adsorption amounts.
Recent studies have shown that SIFSIX materials (SIFSIX = hexafluorosilicate (SiF62−)) are very promising adsorbents for hydrocarbon separations because their pore size can be finely tuned and SiF62− anions have moderately strong interactions with hydrocarbon molecules.23,31 For example, two SIFSIX materials (GeFSIX-2-Cu-i and SIFSIX-2-Cu-i) with pore sizes of 4.5–4.7 Å exhibit the selective separation of C3H6 over C3H8, while the relatively large pore sizes lead to the insufficient selectivity of below 5.23b Optimizing the pore size to 3.5 Å in NbOFFIVE-1-Ni can afford full molecular sieving toward C3H6/C3H8 separation; however, the extremely small pore spaces severely delimit its C3H6 uptake.16 Therefore, simple control of pore sizes with single functionality in SIFSIX materials cannot fully address the trade-off dilemma to target both high C3H6 adsorption and selectivity. Herein, we realized the immobilization of dual functionalities in a SIFSIX material (ZJUT-2, Ni(pyz-SH)2SiF6, pyz-SH = 2-mercaptopyrazine),34 to construct an efficient C3H6 nano-trap for highly efficient C3H6/C3H8 separation. This MOF-based nano-trap features not only a small pore cavity with a suitable size of 3.9 × 3.9 × 7.5 Å3 that matches better with a kinetic diameter of C3H6 (4.0 Å) than C3H8 (4.3 Å),12,18 but also is decorated with dual functionalities (–SH and SiF62−) to optimally interact with the C3H6 molecule. This material thus exhibits both top-tier C3H6 capture capacity (123.5 cm3 cm−3 at 0.5 bar and 296 K) and C3H6/C3H8 selectivity of 17.2 under ambient conditions, achieved by a moderate C3H6 heat of adsorption (45 kJ mol−1). The C3H6 uptake and selectivity of ZJUT-2a are obviously higher than those of the pristine SIFSIX-3-Ni (76.6 cm3 cm−3 and 6.6) and most of the top-performing materials reported. Highly efficient separation of C3H6 from both 50/50 and 10/90 C3H6/C3H8 mixtures was confirmed by experimental breakthrough tests, providing both large C3H6 uptake (2.6 mmol g−1) and high dynamic selectivity (10). Both values outperform or are comparable to those of some promising MOFs, such as Y-abtc (1.26 mmol g−1 and 8.3),17 KAUST-7 (1.16 mmol g−1 and 12),16 and Ni-NP (2.3 mmol g−1 and 9.6).22a
Results and discussion
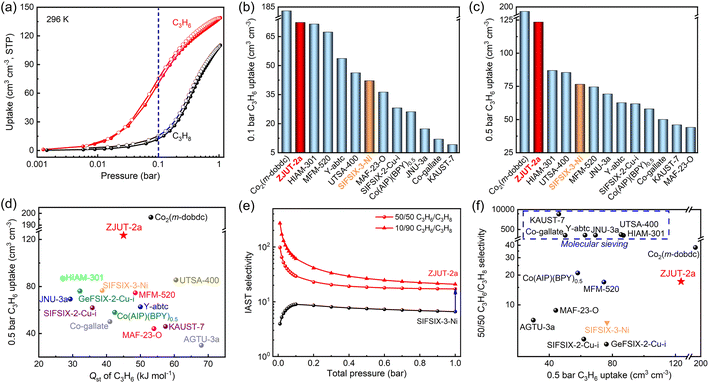
The powder sample of ZJUT-2 was prepared by the reaction of NiSiF6 and pyz-SH in methanol solution at 85 °C according to the previously reported literature.34 The phase purity and crystallinity of bulk ZJUT-2 were confirmed by powder X-ray diffraction (PXRD), which matched well with that of the simulated patterns (Fig. S1, ESI†). As shown in Fig. 1a, detailed structure analysis revealed that this material consists of two-dimensional (2D) nets based on pyz-SH linkers and metal nodes, which are further pillared by SiF62− anions to form the resulting 3D network. Each pore channel is separated by four SiF62− anions to form cylindrical nanocages with a size of 3.9 × 3.9 × 7.5 Å3. From a kinetics point of view, the nanocage aperture of 3.9 Å matches better with the kinetic diameter of C3H6 (4.0 Å) than C3H8 (4.3 Å), making it an ideal single-molecule trap for the capture of single C3H6 molecules (Fig. 1b). Most importantly, the incorporated dual functionalities of –SH and SiF62− groups are located around the pore channels, which can create a multi-binding nano-trap to optimize the adsorption and recognition of C3H6 molecules. Thus, the optimized nano-trap with a suitable size and dual functionalities may provide a single-molecule trap for highly selective capture of C3H6 over C3H8.The permanent porosity of ZJUT-2a was first confirmed using the CO2 adsorption isotherms at 196 K (Fig. S2, ESI†), affording a Brunauer–Emmett–Teller (BET) surface area of 387.8 m2 g−1. Single component gas adsorption isotherms of C3H6 and C3H8 for ZJUT-2a were collected at 296 K and 273 K up to 1 bar (Fig. 2a, S3 and S4, ESI†) and compared with those of pristine SIFSIX-3-Ni (Fig. S6, ESI†). As illustrated in Fig. 2a, ZJUT-2a exhibits a steep and high C3H6 uptake at 296 K, which is larger than that of C3H8 in the whole pressure region. Even at a low pressure of 0.1 bar, ZJUT-2a shows a very high C3H6 uptake of 72.3 cm3 cm−3, which is the highest among the reported MOFs relevant for C3H6/C3H8 separation except for the MOF-74 series (Fig. 2b).25–27 In comparison, the C3H8 uptake at 0.1 bar (11.9 cm3 cm−3) is very low, affording a notably high C3H6/C3H8 uptake ratio of 6.1 at 0.1 bar. Such high low-pressure C3H6 uptake indicates that this MOF-based nano-trap can provide a stronger binding affinity with C3H6 over C3H8, probably attributed to the suitable cage size and dual functionalities that match better with the size and shape of the C3H6 molecule. When the pressure increases to 0.5 bar, the C3H6 uptake amount can be improved to 123.5 cm3 cm−3, which is higher than that of most relevant MOFs except for the MOF-74 series.25–27 It is worth noting that this uptake is superior to that of SIFSIX-3-Ni (76.6 cm3 cm−3) and most of the current best-performing materials reported (Fig. 2c), such as HIAM-301 (87.0 cm3 cm−3),13 UTSA-400 (85.5 cm3 cm−3),14 MFM-520 (74.6 cm3 cm−3),20 JNU-3a (69.3 cm3 cm−3),12 and KAUST-7 (46.0 cm3 cm−3).16 At 1 bar and 296 K, the C3H6 uptake of ZJUT-2a can further increase to 138.9 cm3 cm−3. These adsorption behaviors can be supported by the experimental isosteric heat of adsorption (Qst), wherein the initial Qst value of C3H6 for ZJU-2a (45 kJ mol−1) is higher than that of C3H8 (Fig. S9, ESI†). Further, as shown in Fig. S11 (ESI†), the initial Qst value of ZJUT-2 for C3H6 is much higher than that of SIFSIX-3-Ni (38.7 kJ mol−1), indicating that the immobilization of –SH groups can strengthen the C3H6 binding affinity. As shown in Fig. 2d, due to the lack of strong OMSs, the Qst value of ZJUT-2a is much less than that of the MOF-74 series with high-density OMSs (55–70 kJ mol−1).25 Therefore, ZJUT-2a exhibits a remarkably top-tier C3H6 uptake achieved by a moderate Qst value, compared with all the indicated MOFs as evidenced in Fig. 2d and S12 (ESI†).
The adsorption selectivity of ZJUT-2a for 50/50 and 10/90 C3H6/C3H8 mixtures was calculated by the ideal adsorbed solution theory (IAST) method. As indicated in Fig. 2e, ZJUT-2a shows a high selectivity of up to 17.2 and 20.9 for 50/50 and 10/90 C3H6/C3H8 mixtures at 1 bar and 296 K, respectively, which are much higher than that of the pristine SIFSIX-3-Ni (6.6) and other SIFSIX materials such as SIFSIX-2-Cu-i (4.5),23b GeFSIX-2-Cu-i (4),23b and ZU-36-Ni (10.8).23a These values also outperform those of some promising MOFs including Ni–Np (10.5),22b MAF-23-O (8.8)19 and MFM-520 (17),20 but lower than those of molecular-sieving materials. It should be pointed out that this selectivity value is only for the qualitative comparison purpose. Besides gas selectivity, C3H6 uptake capacity at partial pressure is also an important criterion to determine the final separation performance. As shown in Fig. 3f, we comprehensively compared C3H6 uptake and selectivity of ZJUT-2a with some promising materials. Although those molecular-sieving MOFs exhibit the record-high selectivity due to the small pore sizes, their C3H6 uptakes are relatively low. If we set the C3H6 uptake and selectivity as concurrent objectives, most of the reported materials suffer from either unsatisfactory selectivity or inadequate uptake capacity. Evidently, ZJUT-2a, Co2(m-dobdc),25 UTSA-400 (ref. 14) and HIAM-301 (ref. 13) exhibit more balance between adsorption uptake and gas selectivity for C3H6/C3H8 separation. Thus, both top-tier C3H6 uptake capacity (123.5 cm3 cm−3 at 0.5 bar) and selectivity (17.2) along with moderate adsorption heat make this material among the best-performing materials reported for this separation.
To gain better insight into both high C3H6 uptake and selectivity of ZJUT-2, grand canonical Monte Carlo (GCMC) simulations were performed to study the sorbate–sorbent interactions between the framework and gas molecules. The optimal adsorption sites for C3H6 and C3H8 in the pores of ZJUT-2a are approximated as shown in Fig. 3. Since the cavity size in ZJUT-2a matches well with the C3H6 molecule, each nano-trap can only capture one C3H6 molecule through multiple hydrogen bonding and van der Waals (vdW) interactions between dual functionalities (–SH and SiF62−) and the C3H6 molecule. As shown in Fig. 3a and b, each C3H6 molecule interacts with four SiF62− anions through eleven C–H⋯F hydrogen bonds with the distances of 2.33–3.09 Å and also binds with four –SH groups through six C–H⋯S interactions (2.73–3.27 Å). Evidently, the immobilized SiF62− and –SH groups synergistically contribute to enforcing the interactions with the C3H6 molecule. Further, the dense distribution of these nano-traps within the framework enables the C3H6 molecules to be in close proximity to each other with a contact distance of 4.16 Å along the b axis (Fig. 3c). Such observed contact distances in ZJUT-2a are notably shorter than those found in JNU-3a (4.53 Å), HIAM-301 (6.03 Å) and also comparable to the C3H6⋯C3H6 average distance in the crystalline C3H6 (4.47 Å) collected at 65 K.15,32 This reveals that ZJUT-2a shows the dense packing of C3H6 molecules within the pores, thus resulting in its high C3H6 uptake capacity. In comparison, the overall H-bonding interactions between the pore surface and C3H8 molecule are much less than that of C3H6 (Fig. S14 and S15, ESI†). In addition, the cavity sizes of this nano-trap were found to be smaller than the kinetic diameter of C3H8. The weaker binding affinity and poor size match with the nano-trap may lead to partially populating the binding sites for C3H8. The above reasons thus afford both high C3H6 uptake capacity and selectivity.
To evaluate the actual separation performance of ZJUT-2a, dynamic breakthrough experiments on binary C3H6/C3H8 gas mixtures were carried out under ambient conditions. As presented in Fig. 4a, ZJUT-2a exhibited a clear separation for the 50/50 C3H6/C3H8 mixture, wherein pure C3H8 first eluted through the adsorption bed at 33 min, while C3H6 was retained for a longer time of 65 min. During this breakthrough interval, the C3H6 dynamic uptake was calculated to be 2.6 mmol g−1, which is 79% of the saturated uptake (3.3 mmol g−1) obtained from single-component adsorption isotherms at 296 K and 1 bar. This value is not only much higher than that of other reported SIFSIX materials (Fig. 4b), including SIFSIX-3-Ni (1.25 mmol g−1),23a GeFSIX-2-Cu-i (2.2 mmol g−1),23b and SIFSIX-2-Cu-i (2.0 mmol g−1),23b but also outperforms some top-performing MOFs such as JNU-3a (2.45 mmol g−1),12 HIAM-301 (2.07 mmol g−1)13 and KAUST-7 (1.16 mmol g−1).16 Further, the dynamic C3H6/C3H8 selectivity was estimated to be up to 10, which exceeds that of SIFSIX-3-Ni (2.3)23a and most top-performing materials, such as Y-abtc (8.3),17 Co-MOF-74 (6.5)25 and Ni-NP (9.6).22a As shown in Fig. 4c, ZJUT-2a thus shows a rare combination of simultaneously high C3H6 dynamic uptake and selectivity, placing it among the best-performing materials reported so far for C3H6/C3H8 separation. It should be noted that the feed gases in some production processes might contain only a small amount of C3H6, which requires adsorbents to efficiently capture C3H6 at low partial pressures. Therefore, we performed the breakthrough experiments for 10/90 C3H6/C3H8 mixtures (Fig. 4d). More difference in the breakthrough times of C3H6 and C3H8 was observed, with a high dynamic C3H6 uptake of 1.5 mmol g−1, indicating the superior separation ability of ZJUT-2a for some gas mixtures with low C3H6 content. Three continuous cycles on 50/50 and 10/90 mixtures showed the full retention of the separation performance and easy recyclability of ZJUT-2a (Fig. 4e and S20, ESI†). Given that water vapor is a ubiquitous component in industrial gas mixtures,33 we conducted the breakthrough experiments for a wet C3H6/C3H8 mixture at 50% relative humidity. As shown in Fig. 4f, the almost unchanged breakthrough times of both C3H6 and C3H8 demonstrated the excellent moisture tolerance properties of ZJUT-2a, which can avoid the deleterious effect of water vapor on C3H6/C3H8 separation performance. As inferred from the PXRD performed on associated samples (Fig. S22, ESI†), the framework of ZJUT-2a remains stable after multiple breakthrough experiments.
Conclusions
In summary, we have realized the immobilization of dual functionalities into a suitable MOF to construct a single-molecule nano-trap for highly efficient C3H6/C3H8 separation. The incorporated bifunctional groups (–SH and SiF62−) combined with the appropriate cavity size/shape in ZJUT-2a can synergistically create multiple binding environments to densely and selectively trap the C3H6 molecule, as revealed by theoretical calculations. This MOF-based nano-trap thus exhibited both top-tier C3H6 capture capacity (123.5 cm3 cm−3 at 0.5 bar) and C3H6/C3H8 selectivity of 17.2 under ambient conditions, achieved by a moderate C3H6 adsorption enthalpy (45 kJ mol−1). Breakthrough experiment data revealed both remarkably large C3H6 dynamic uptake of 2.6 mmol g−1 and high dynamic selectivity of 10 for the separation of actual C3H6/C3H8 mixtures, surpassing most of the top-performing materials reported to date. This work provides some guidance to design porous materials with multiple soft functionalities to highly boost C3H6/C3H8 separation performance through moderate gas adsorption enthalpy.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (92163110, U1909214 and 92061126) and the Key Research and Development Program of Zhejiang Province (2021C01182).Notes and references
- (a) I. Amghizar, L. A. Vandewalle, K. M. Van Geem and G. B. Marin, Engineering, 2017, 3, 171–178 CrossRef CAS; (b) C. C. E. Christopher, A. Dutta, S. Farooq and I. A. Karimi, Ind. Eng. Chem. Res., 2017, 56, 14557–14564 CrossRef CAS.
- C. Tsioptsias, K. Leontiadis, E. Tzimpilis and I. Tsivintzelis, J. Plast. Film Sheeting, 2021, 37, 283–311 CrossRef CAS.
- V. F. D. Martins, A. M. Ribeiro, M. G. Plaza, J. C. Santos, J. M. Loureiro, A. F. P. Ferreira and A. E. Rodrigues, J. Chromatogr. A, 2015, 1423, 136–148 CrossRef CAS PubMed.
- T. Ren, M. Patel and K. Blok, Energy, 2006, 31, 425–451 CrossRef CAS.
- S. U. Rege and R. T. Yang, Chem. Eng. Sci., 2002, 57, 1139–1149 CrossRef CAS.
- (a) A. Marinas, P. Bruijnincx, J. Ftouni, F. J. Urbano and C. Pinel, Catal. Today, 2015, 239, 31–37 CrossRef CAS; (b) D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed.
- (a) J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC; (b) M. C. Das, Q. Guo, Y. He, J. Kim, C.-G. Zhao, K. Hong, S. Xiang, Z. Zhang, K. M. Thomas, R. Krishna and B. Chen, J. Am. Chem. Soc., 2012, 134, 8703–8710 CrossRef CAS PubMed; (c) R.-B. Lin, Z. Zhang and B. Chen, Acc. Chem. Res., 2021, 54, 3362–3376 CrossRef CAS PubMed; (d) S. Furukawa, J. Reboul, S. Diring, K. Sumida and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5700–5734 RSC; (e) K. Adil, Y. Belmabkhout, R. S. Pillai, A. Cadiau, P. M. Bhatt, A. H. Assen, G. Maurin and M. Eddaoudi, Chem. Soc. Rev., 2017, 46, 3402–3430 RSC; (f) H. Wang, Y. Liu and J. Li, Adv. Mater., 2020, 32, 2002603 CrossRef CAS PubMed; (g) Z. Chen, K. O. Kirlikovali, P. Li and O. K. Farha, Acc. Chem. Res., 2022, 55, 579–591 CrossRef CAS PubMed.
- (a) L. Li, R.-B. Lin, R. Krishna, H. Li, S. Xiang, H. Wu, J. Li, W. Zhou and B. Chen, Science, 2018, 362, 443–446 CrossRef CAS PubMed; (b) W. Fan, S. Yuan, W. Wang, L. Feng, X. Liu, X. Zhang, X. Wang, Z. Kang, F. Dai, D. Yuan, D. Sun and H.-C. Zhou, J. Am. Chem. Soc., 2020, 142, 8728–8737 CrossRef PubMed; (c) W. Fan, S. B. Peh, Z. Zhang, H. Yuan, Z. Yang, Y. Wang, K. Chai, D. Sun and D. Zhao, Angew. Chem., Int. Ed., 2021, 60, 17338–17343 CrossRef CAS PubMed; (d) Y.-Y. Xue, X.-Y. Bai, J. Zhang, Y. Wang, S.-N. Li, Y.-C. Jiang, M.-C. Hu and Q.-G. Zhai, Angew. Chem., Int. Ed., 2021, 60, 10122–10128 CrossRef CAS PubMed; (e) H. Li, C. Liu, C. Chen, Z. Di, D. Yuan, J. Pang, W. Wei, M. Wu and M. Hong, Angew. Chem., Int. Ed., 2021, 60, 7547–7552 CrossRef CAS PubMed; (f) Y. Yang, L. Li, R.-B. Lin, Y. Ye, Z. Yao, L. Yang, F. Xiang, S. Chen, Z. Zhang, S. Xiang and B. Chen, Nat. Chem., 2021, 13, 933–939 CrossRef CAS PubMed.
- (a) Z. Di, C. Liu, J. Pang, C. Chen, F. Hu, D. Yuan, M. Wu and M. Hong, Angew. Chem., Int. Ed., 2021, 60, 10828–10832 CrossRef CAS PubMed; (b) Y. Ye, S. Xian, H. Cui, K. Tan, L. Gong, B. Liang, T. Pham, H. Pandey, R. Krishna, P. C. Lan, K. A. Forrest, B. Space, T. Thonhauser, J. Li and S. Ma, J. Am. Chem. Soc., 2022, 144, 1681–1689 CrossRef CAS PubMed; (c) L. Li, L. Guo, D. H. Olson, S. Xian, Z. Zhang, Q. Yang, K. Wu, Y. Yang, Z. Bao, Q. Ren and J. Li, Science, 2022, 377, 335–339 CrossRef CAS PubMed; (d) T. He, X.-J. Kong, Z.-X. Bian, Y.-Z. Zhang, G.-R. Si, L.-H. Xie, X.-Q. Wu, H. Huang, Z. Chang, X.-H. Bu, M. J. Zaworotko, Z.-R. Nie and J.-R. Li, Nat. Mater., 2022, 21, 689–695 CrossRef CAS PubMed; (e) A. N. Hong, E. Kusumoputro, Y. Wang, H. Yang, Y. Chen, X. Bu and P. Feng, Angew. Chem., Int. Ed., 2022, 61, e202116064 CAS; (f) G.-D. Wang, R. Krishna, Y.-Z. Li, W.-J. Shi, L. Hou, Y.-Y. Wang and Z. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202213015 CAS.
- (a) H. Zeng, X.-J. Xie, M. Xie, Y.-L. Huang, D. Luo, T. Wang, Y. Zhao, W. Lu and D. Li, J. Am. Chem. Soc., 2019, 141, 20390–20396 CrossRef CAS PubMed; (b) H.-G. Hao, Y.-F. Zhao, D.-M. Chen, J.-M. Yu, K. Tan, S. Ma, Y. Chabal, Z.-M. Zhang, J.-M. Dou, Z.-H. Xiao, G. Day, H.-C. Zhou and T.-B. Lu, Angew. Chem., Int. Ed., 2018, 57, 16067–16071 CrossRef CAS PubMed; (c) H. Yang, Y. Wang, R. Krishna, X. Jia, Y. Wang, A. N. Hong, C. Dang, H. E. Castillo, X. Bu and P. Feng, J. Am. Chem. Soc., 2020, 142, 2222–2227 CrossRef CAS PubMed; (d) Y. Chai, X. Han, W. Li, S. Liu, S. Yao, C. Wang, W. Shi, I. da-Silva, P. Manuel, Y. Cheng, L. D. Daemen, A. J. Ramirez-Cuesta, C. C. Tang, L. Jiang, S. Yang, N. Guan and L. Li, Science, 2020, 368, 1002–1006 CrossRef CAS PubMed; (e) Y. Wang, T. Li, L. Li, R.-B. Lin, X. Jia, Z. Chang, H.-M. Wen, X.-M. Chen and J. Li, Adv. Mater., 2023, 35, 2207955 CrossRef CAS PubMed; (f) J.-X. Wang, C.-C. Liang, X.-W. Gu, H. Wen, C. Jiang, B. Li, G. Qian and B. Chen, J. Mater. Chem. A, 2022, 10, 17878–17916 RSC.
- (a) S. Geng, E. Lin, X. Li, W. Liu, T. Wang, Z. Wang, D. Sensharma, S. Darwish, Y. H. Andaloussi, T. Pham, P. Cheng, M. J. Zaworotko, Y. Chen and Z. Zhang, J. Am. Chem. Soc., 2021, 143, 8654–8660 CrossRef CAS PubMed; (b) K.-J. Chen, D. G. Madden, S. Mukherjee, T. Pham, K. A. Forrest, A. Kumar, B. Space, J. Kong, Q.-Y. Zhang and M. J. Zaworotko, Science, 2019, 366, 241–246 CrossRef CAS PubMed; (c) O. T. Qazvini, R. Babarao, Z.-L. Shi, Y.-B. Zhang and S. G. Telfer, J. Am. Chem. Soc., 2019, 141, 5014–5020 CrossRef CAS PubMed; (d) K. Su, W. Wang, S. Du, C. Ji and D. Yuan, Nat. Commun., 2021, 12, 3703 CrossRef CAS PubMed; (e) K. Shao, H.-M. Wen, C.-C. Liang, X. Xiao, X.-W. Gu, B. Chen, G. Qian and B. Li, Angew. Chem., Int. Ed., 2022, 61, e202211523 CAS; (f) J. Pei, X.-W. Gu, C.-C. Liang, B. Chen, B. Li and G. Qian, J. Am. Chem. Soc., 2022, 144, 3200–3209 CrossRef CAS PubMed; (g) S. C. Pal, R. Ahmed, A. K. Manna and M. C. Das, Inorg. Chem., 2022, 61, 18293–18302 CrossRef CAS PubMed.
- H. Zeng, M. Xie, T. Wang, R.-J. Wei, X.-J. Xie, Y. Zhao, W. Lu and D. Li, Nature, 2021, 595, 542–548 CrossRef CAS PubMed.
- L. Yu, X. Han, H. Wang, S. Ullah, Q. Xia, W. Li, J. Li, I. da Silva, P. Manuel, S. Rudić, Y. Cheng, S. Yang, T. Thonhauser and J. Li, J. Am. Chem. Soc., 2021, 143, 19300–19305 CrossRef CAS PubMed.
- Y. Xie, Y. Shi, E. M. C. Morales, A. El Karch, B. Wang, H. Arman, K. Tan and B. Chen, J. Am. Chem. Soc., 2023, 145, 2386–2394 CrossRef CAS PubMed.
- D. Liu, J. Pei, X. Zhang, X.-W. Gu, H.-M. Wen, B. Chen, G. Qian and B. Li, Angew. Chem., Int. Ed., 2023, 62, e202218590 CrossRef CAS PubMed.
- A. Cadiau, K. Adil, P. M. Bhatt, Y. Belmabkhout and M. Eddaoudi, Science, 2016, 353, 137–140 CrossRef CAS PubMed.
- H. Wang, X. Dong, V. Colombo, Q. Wang, Y. Liu, W. Liu, X.-L. Wang, X.-Y. Huang, D. M. Proserpio, A. Sironi, Y. Han and J. Li, Adv. Mater., 2018, 30, 1805088 CrossRef PubMed.
- (a) P. Krokidas, M. Castier, S. Moncho, E. Brothers and I. G. Economou, J. Phys. Chem. C, 2015, 119, 27028–27037 CrossRef CAS; (b) B. Liang, X. Zhang, Y. Xie, R.-B. Lin, R. Krishna, H. Cui, Z. Li, Y. Shi, H. Wu, W. Zhou and B. Chen, J. Am. Chem. Soc., 2020, 142, 17795–17801 CrossRef CAS PubMed; (c) Y. Chen, Y. Yang, Y. Wang, Q. Xiong, J. Yang, S. Xiang, L. Li, J. Li, Z. Zhang and B. Chen, J. Am. Chem. Soc., 2022, 144, 17033–17040 CrossRef CAS PubMed.
- Y. Wang, N.-Y. Huang, X.-W. Zhang, H. He, R.-K. Huang, Z.-M. Ye, Y. Li, D.-D. Zhou, P.-Q. Liao, X.-M. Chen and J.-P. Zhang, Angew. Chem., Int. Ed., 2019, 58, 7692–7696 CrossRef CAS PubMed.
- J. Li, X. Han, X. Kang, Y. Chen, S. Xu, G. L. Smith, E. Tillotson, Y. Cheng, L. J. M. Mcpherson, S. J. Teat, S. Rudić, A. J. Ramirez-Cuesta, S. J. Haigh, M. Schröder and S. Yang, Angew. Chem., Int. Ed., 2021, 60, 15541–15547 CrossRef CAS PubMed.
- X.-W. Zhang, D.-D. Zhou and J.-P. Zhang, Chem, 2021, 7, 1006–1019 CAS.
- (a) Y. Xie, Y. Shi, H. Cui, R.-B. Lin and B. Chen, Small Struct., 2022, 3, 2100125 CrossRef CAS; (b) Z. Chang, R.-B. Lin, Y. Ye, C. Duan and B. Chen, J. Mater. Chem. A, 2019, 7, 25567–25572 RSC.
- (a) Z. Zhang, Q. Ding, X. Cui, X.-M. Jiang and H. Xing, ACS Appl. Mater. Interfaces, 2020, 12, 40229–40235 CrossRef CAS PubMed; (b) X. Wang, P. Zhang, Z. Zhang, L. Yang, Q. Ding, X. Cui, J. Wang and H. Xing, Ind. Eng. Chem. Res., 2020, 59, 3531–3537 CrossRef CAS.
- H. Wu, Y. Yuan, Y. Chen, F. Xu, D. Lv, Y. Wu, Z. Li and Q. Xia, AIChE J., 2020, 66, e16858 CAS.
- J. E. Bachman, M. T. Kapelewski, D. A. Reed, M. I. Gonzalez and J. R. Long, J. Am. Chem. Soc., 2017, 139, 15363–15370 CrossRef CAS PubMed.
- Y.-S. Bae, C. Y. Lee, K. C. Kim, O. K. Farha, P. Nickias, J. T. Hupp, S. T. Nguyen and R. Q. Snurr, Angew. Chem., Int. Ed., 2012, 51, 1857–1860 CrossRef CAS PubMed.
- E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Science, 2012, 335, 1606–1610 CrossRef CAS PubMed.
- G. Chang, M. Huang, Y. Su, H. Xing, B. Su, Z. Zhang, Q. Yang, Y. Yang, Q. Ren, Z. Bao and B. Chen, Chem. Commun., 2015, 51, 2859–2862 RSC.
- (a) N. Lamia, M. Jorge, M. A. Granato, F. A. A. Paz, H. Chevreau and A. E. Rodrigues, Chem. Eng. Sci., 2009, 64, 3246–3259 CrossRef CAS; (b) S.-Y. Kim, T.-U. Yoon, J. H. Kang, A.-R. Kim, T.-H. Kim, S.-I. Kim, W. Park, K. C. Kim and Y.-S. Bae, ACS Appl. Mater. Interfaces, 2018, 10, 27521–27530 CrossRef CAS PubMed; (c) J. W. Yoon, A.-R. Kim, M. J. Kim, T.-U. Yoon, J.-H. Kim and Y.-S. Bae, Microporous Mesoporous Mater., 2019, 279, 271–277 CrossRef CAS.
- M. H. Mohamed, Y. Yang, L. Li, S. Zhang, J. P. Ruffley, A. G. Jarvi, S. Saxena, G. Veser, J. K. Johnson and N. L. Rosi, J. Am. Chem. Soc., 2019, 141, 13003–13007 CrossRef CAS PubMed.
- (a) P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80–84 CrossRef CAS PubMed; (b) O. Shekhah, Y. Belmabkhout, Z. Chen, V. Guillerm, A. Cairns, K. Adil and M. Eddaoudi, Nat. Commun., 2014, 5, 4228 CrossRef CAS PubMed; (c) X. Cui, K. Chen, H. Xing, Q. Yang, R. Krishna, Z. Bao, H. Wu, W. Zhou, X. Dong, Y. Han, B. Li, Q. Ren, M. J. Zaworotko and B. Chen, Science, 2016, 353, 141–144 CrossRef CAS PubMed; (d) B. Li, X. Cui, D. O'Nolan, H.-M. Wen, M. Jiang, R. Krishna, H. Wu, R.-B. Lin, Y.-S. Chen, D. Yuan, H. Xing, W. Zhou, Q. Ren, G. Qian, M. J. Zaworotko and B. Chen, Adv. Mater., 2017, 29, 1704210 CrossRef PubMed; (e) P. Zhang, Y. Zhong, Y. Zhang, Z. Zhu, Y. Liu, Y. Su, J. Chen, S. Chen, Z. Zeng, H. Xing, S. Deng and J. Wang, Sci. Adv., 2022, 8, eabn9231 CrossRef CAS PubMed; (f) Y. Jiang, J. Hu, L. Wang, W. Sun, N. Xu, R. Krishna, S. Duttwyler, X. Cui, H. Xing and Y. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202200947 CAS.
- R. L. Hudson, P. A. Gerakines, Y. Y. Yarnall and R. T. Coones, Icarus, 2021, 354, 114033 CrossRef CAS.
- S. M. Sadrameli, Fuel, 2016, 173, 285–297 CrossRef CAS.
- H.-M. Wen, C. Liao, L. Li, L. Yang, J. Wang, L. Huang, B. Li, B. Chen and J. Hu, Chem. Commun., 2019, 55, 11354–11357 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Stability tests, PXRD patterns, adsorption isotherms and breakthrough curves. See DOI: https://doi.org/10.1039/d3ta02737f |
| This journal is © The Royal Society of Chemistry 2023 |