Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nitroimino as an energetic group in designing energetic materials for practical use, a tautomerism from nitroamino
Yaxi
Wang
 a,
Lu
Hu
a,
Lu
Hu
 *a,
Siping
Pang
*a,
Siping
Pang
 *a and
Jean'ne M.
Shreeve
*a and
Jean'ne M.
Shreeve
 *b
*b
aSchool of Materials Science & Engineering, Beijing Institute of Technology, Beijing 10081, China. E-mail: lhu@bit.edu.cn; pangsp@bit.edu.cn
bDepartment of Chemistry, University of Idaho, Moscow, Idaho 83844-2343, USA. E-mail: jshreeve@uidaho.edu
First published on 15th June 2023
Abstract
Energetic materials are widely used as energy components in military and civilian applications, which require high energy, and good safety, and are inexpensive and environmentally friendly. As an easily synthesized high-energy group, nitroamino can provide excellent energetic properties to a system. Compared with other energetic groups, nitroamino itself has the ability to form intermolecular hydrogen bonds (HB). The nitroamino undergoes a hydrogen transfer process to generate nitroimino, which forms intramolecular HBs unexpectedly, resulting in a larger conjugated system. The generation of secondary bonds promotes the stability and planarity of the system, thus favoring a series of important parameters such as density, thermal stability, and sensitivity, providing a theoretical basis for the design of nitroamino-substituted explosives with balanced energy and safety. This facilitates the development of high energy density materials (HEDMs) for aerospace, military and civilian applications. The hydrogen transfer process of the nitroamino system can be understood as one in which the nitroamino moiety becomes stable by changing the structure of the backbone or other substituent groups. However, the construction strategies and properties of nitroimino energetics have attracted little attention and are far from being fully exploited. The summarization of views and possible construction ideas will provide new opportunities. In this review, some important nitroimino-substituted explosives have been included to confirm the guiding role of hydrogen transfer strategy by capturing their structural features. Through comprehensive analysis of the skeleton structure, synthesis method, and performance characteristics, we strive to provide a macroscopic view of nitroimino explosives including the mechanism of the self-stabilizing hydrogen transfer, the improved performance, and the blueprint for the future design of HEDMs based on these characteristics.
Introduction
Energetic compounds are important materials for weapon and civilian systems, which given such excellent properties can significantly improve the performance of modern advanced weapons and equipment. The development of new generation high energy density materials (HEDMs) has always been a top goal in the pursuit of superior military and civilian capabilities.1–3 The structure of an energetic material can be divided into two parts, the backbone and the energetic groups.4 Diverse backbones, such as aromatic, aliphatic, caged carbon and heterocyclic frameworks, have been widely reported in the development of energetic materials. With energetic groups (e.g., –NO2, –ONO2, –N3, N-oxide, –NHNO2, –C(NO2)3, among others), energetic materials exhibit high detonation performance.5,6 When the energetic group is covalently or ionically linked to the skeleton to form an explosive, the C and H atoms can be oxidized by the O atoms to generate CO/CO2 and H2O releasing the chemical energy stored in the energetic material.7 However, the energy and safety levels are often contradictory for an explosive, and with the introduction of more energetic groups, the overall stability is diminished.8 Therefore, the question is – is it possible to introduce energetic groups that can balance energy and stability simultaneously?The formation of hydrogen bonds (HBs) and van der Waals forces can significantly influence the overall stability of covalent compounds, which is one of the main routes to enhance the stability of energetic materials.9 Compared to the weaker van der Waals forces, the effects of modulating HB systems are more pronounced. It is important to find an energetic group with the potential to form abundant HBs to provide both energy and stabilization for the entire system. The nitroamino moiety which contains a high-energy N–N bond and nitro group, and promotes density, heat of formation (HOF) and energy of the whole system, is undoubtedly the most compatible with the above requirements among available energetic groups.10,11 Its own self-contained electron donor and accepter provide the basis of intermolecular HB formation and p–π conjugation.12 Additionally, it can also undergo a hydrogen atom transfer process with the backbone under appropriate circumstances, thus forming extra intramolecular HBs to support the stability, which will also further promote intermolecular interactions and enhance van der Waals forces.13
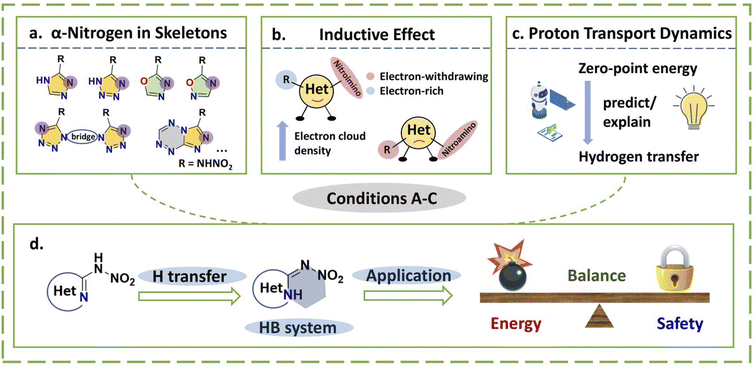
The nitroamino undergoes electron pair transfer, which migrates to α-nitrogen to form the nitroimino. In order for hydrogen transfer to occur, a suitable structure must meet the following conditions (Fig. 1): (A) for the selected skeleton, each substitution position connected with nitroamino must be accompanied by an α-nitrogen, a proton acceptor; (B) for the inductive effect, when the backbone has a low electron cloud density, proton transfer has less chance to occur; (C) even if conditions (A) and (B) are fully satisfied, success is still related to the zero-point energy (ZPE), which is needed for the hydrogen transfer to favor an energy decrease in the system. For a nitroamino explosive, taking structures substitute with nitroamino groups as the energy zero point, a decrease in the ZPE of the nitroimino structure favors hydrogen transfer; an increase is detrimental, which has been promised as proton transport dynamics.14 As shown in Fig. 1d, if nitroimino occurs, the extra intramolecular HB systems will further promote close packing and stability, providing extra advantageous support by improving the energy and safety to realize the application value. Therefore, nitroamino explosives are theoretically structurally advantageous HEDMs. In recent years, many nitroamino-substituted explosives based on different monocyclic, bridged and fused-ring skeletons have been widely reported, demonstrating the strong dynamics and the excellent prospects of the field.15,16 Among them, triazole, tetrazole, and oxadiazole skeletons have been shown to have a hydrogen transfer basis, and a series of bridged or fused-ring skeletons based on them have been reported extensively.5,8,17–21
This review covers 36 kinds of nitroimino-substituted explosives, which are divided into four parts based on the skeleton structures. Five reference classical compounds (TNT, TATB, RDX, HMX, CL-20) are included for comparison. As shown in Table 1, the melting point, decomposition temperature, density, heat of formation and detonation performance are provided.5 Compounds RDX, HMX and CL-20 are three classical nitroamino explosives.22–24HMX and CL-20 are considered to be high-powered explosives and benchmarks for current new HEDMs, showing great promise for the application of nitroamino-based explosives. ICM-101 is the most representative example for nitroimino explosives. Thanks to the planar structure resulting from nitroimino, ICM-101 has a high density of 1.99 g cm−3. Together with excellent detonation performance (9481 m s−1) and good thermal stability (210 °C), the extensive application prospects for developing nitroimino HEDMs is exemplified.20
| Compd | T m (°C) | T d (°C) | d (g cm−3) | ΔHfd (kJ mol−1)/(kJ g−1) | v (m s−1) | P (GPa) | ISg (J) | FSh (N) | Ref.i |
|---|---|---|---|---|---|---|---|---|---|
| a Melting point. b Decomposition temperature (onset). c Density. d Heat of formation (calculated). e Detonation velocity (calculated). f Detonation pressure (calculated). g Impact sensitivity. h Friction sensitivity. i Reference. j Trinitrotoluene. k 2,4,6-Triamino-1,3,5-trinitrobenzene. l Hexahydro-1,3,5-trinitro-1,3,5-triazine. m 1,3,5,7-Tetranitro-1,3,5,7-tetrazocane. n Hexanitrohexaazaisowurtzitane. o [2,2′-Bi(1,3,4-oxadiazole)]-5,5′-dinitramide. | |||||||||
| TNT | 81 | 295 | 1.65 | −59.3/−0.26 | 7303 | 21.3 | 15 | 363 | 5 |
| TATB | 350 | ∼360 | 1.94 | −154.2/−0.60 | 8544 | 32.1 | 50 | 360 | 5 |
| RDX | 205 | 210 | 1.80 | 70.7/0.32 | 8795 | 34.9 | 7.5 | 120 | 5 |
| HMX | — | 280 | 1.91 | 74.8/0.25 | 9144 | 39.2 | 7 | 120 | 5 |
| CL-20 | — | 210 | 2.04 | 397.8/0.91 | 9706 | 45.2 | 4 | 94 | 5 |
| ICM-101 | — | 210 | 1.99 | 166.8/0.65 | 9481 | 41.9 | 5 | 60 | 20 |
The present review covers most nitroimino-based explosives with good comprehensive properties. Since hydrogen transfer can occur mainly in triazole, tetrazole, oxadiazole and some fused skeletons, the review is divided into four parts, which summarize the fundamental methodology of building nitroimino-based explosives and the properties of the combination of nitroimino with the skeletons and other energetic groups. By highlighting the influence of nitroimino on properties such as stability and detonation performance, the extensive application prospects for developing HEDMs are seen. This review will provide readers with a macroscopic perspective in the field of nitroimino-based HEDMs.
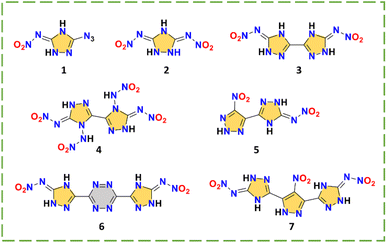
Triazole and triazole bridged nitroimino explosive
Triazole is widely used in the field of energetic materials because it contains three nitrogen atoms in a heterocycle and two carbon substituent sites, which possess high HOF and facilitates the introduction of more energetic groups or bridged structures. The reported mono-, di- and tricyclic nitroamino explosives, demonstrate a range of elegant backbone synthesis methods. There are seven kinds of triazole and triazole bridged nitroimino explosives 1–7 in Scheme 1, and their properties are shown in Table 2.| No. | T m (°C) | T d (°C) | d (g cm−3) | ΔHf (kJ mol−1)/(kJ g−1) | v (m s−1) | P (GPa) | IS (J) | FS (N) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | — | 143 | 1.79 | — | 8972 | 35.7 | 2.5 | — | 25 |
| 2 | — | 125 | 1.83 | 194.0/1.14 | 8880 | 34.3 | <4 | — | 26 |
| 3 | — | 194 | 1.80 | 405.0/2.14 | 8355 | 30.0 | 3 | 108 | 27 |
| 4 | — | 137 | 1.91 | 707.1/1.88 | 9421 | 40.3 | 5 | 60 | 28 |
| 5 | 156 | 168 | 1.88 | 593.3/2.46 | 9067 | 36.2 | 16 | 180 | 29 |
| 6 | — | 162 | 1.88 | 1336.0/3.95 | 9100 | 31.7 | 20 | 270 | 30 |
| 7 | — | 134 | 1.92 | 791.8/2.16 | 9008 | 35.9 | 20 | 270 | 31 |
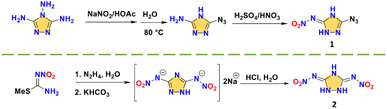
Both 3-azido-5-nitrimino-1,2,4-triazole (1) and 3,5-dinitrimino-1,2,4-triazole (2) are monocyclic nitroimino compounds, indicating the possibility of hydrogen transfer for nitroamino-based triazole.25,26 Their synthesis routes are shown in Scheme 2. Compound 1 links an azide and a nitroimino and compound 2 links two nitroimino groups, providing the basis for a detonation performance superior to RDX. With the nitroimino group, compound 1 forms a larger conjugated system, where all atoms are nearly coplanar (Fig. 2a). The two nitroimino groups indicate that triazole is a good carrier for nitroimino. However, as strong electron-withdrawing group, two nitroimino groups can significantly lead to instability of the skeleton, a monocyclic skeleton obviously cannot support the stability of the system, resulting in the high sensitivity (IS < 4 J) of compounds 1 and 2.
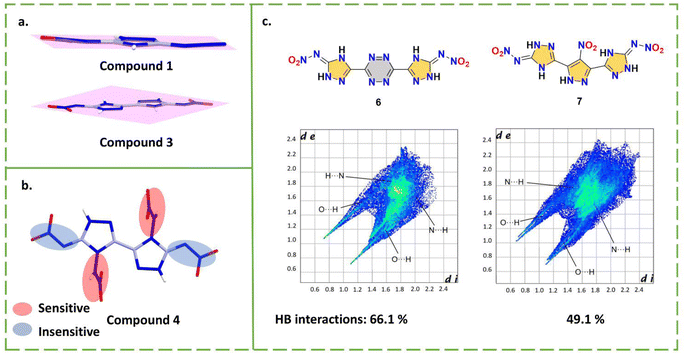 | ||
| Fig. 2 (a) The planarity of compound 1; (b) the different stabilities of N-nitroamino and N-nitroimino groups of compound 4; (c) the fingerprint plots of compounds 6 and 7. | ||
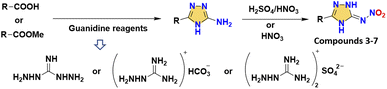
The use of a bridged skeleton is an effective way to disperse the energetic groups. As shown in Scheme 3, compounds 3–7 were all synthesized by an elegant cyclization methodology to construct amino-triazole structures, and then nitroamino was introduced by nitration. For 3,3′-dinitrimino-5,5′-bis(1H-1,2,4-triazole) (3), the decomposition temperature (194 °C) demonstrates the enhanced thermal stability with bridged skeletons and nitroimino.27 Compound 3 also has good planarity. However, it possesses a relatively lower density (1.80 g cm−3) and detonation performance (30.0 GPa; 8355 m s−1). After modifying the two N positions, N,N′-(5,5′-bis(nitroimino)-1,1′,5,5′-tetrahydro-4H,4′H-[3,3′-bi(1,2,4-triazole)]-4,4′-diyl)dinitramide (4)28 has two more N-nitroamino groups than 3, making a great breakthrough in the detonation performance (40.3 GPa; 9421 m s−1) over HMX. Since the hydrogen on N is replaced by nitroamino, the density (1.91 g cm−3) is significantly increased compared to compounds 1–3. However, the N-nitroamino groups without hydrogen transfer capability are obviously sensitive compared to the nitroimino groups, leading to the low thermal stability (137 °C) of compound 4 (Fig. 2b). To better balance the safety and energy for triazole nitroimino compounds, choosing other matching energetic groups is a useful strategy. 4-Nitro-5-(5-nitroimino-1,2,4-triazol-3-yl)-2H-1,2,3-triazolate (5)29 has an acceptable thermal stability (168 °C) and low sensitivity (IS = 16 J; FS = 180 N) while also having higher detonation performance (36.2 GPa; 9067 m s−1) than 1–3, suggesting that the combination with the common energetic group nitro may be a breakthrough in the construction of nitroimino compounds with excellent overall performance.
The strategy for N,N′-((1,2,4,5-tetrazine-3,6-diyl)bis(1,2-dihydro-3H-1,2,4-triazole-5-yl-3-ylidene))dinitramide (6) and N,N′-((4-nitro-1H-pyrazole-3,5-diyl)bis(1H-1,2,4-triazole-5,3-diyl))dinitramide (7) was to introduce tricyclic skeletons to provide stability.30,31 By reacting carbonyl functional groups in the starting materials with guanidine reagents, symmetric bridged skeletons can be efficiently constructed. The nitroimino compounds can be obtained after nitration (Scheme 2). Compounds 6 and 7 show significantly improved HOFs (3.95 and 2.16 kJ g−1), detonation velocities (9100 and 9008 m s−1), high densities (1.88 and 1.92 g cm−3) and low sensitivities (both IS and FS are 20 J and 270 N), achieving the balanced energy and safety. As shown in the fingerprint plots of compounds 6 and 7 (Fig. 2c), the HB interactions (N–H⋯N, N–H⋯O and O–H⋯N) of these two compounds are very extensive, accounting for 66.1% and 49.1% of the total contact interactions, respectively. The symmetric tricyclic structure is favorable to molecular stability, when paired with nitroimino, indicating the promising application of nitroamino-substituted multiple skeletons.
The two carbon substituent sites on triazoles provide the basis for the formation of nitroimino, and also allow the introduction of bridged structures, which greatly increase the diversity of the triazole nitroimino systems. The high density (1.91 g cm−3) and detonation velocity (9421 m s−1) of compound 4 are good proof of the advantage of nitroamino as an energetic group in the design of HEDMs. However, the N–NHNO2 groups also cause compound 4 to have low stability. To further improve the stability, a more stable energetic group like nitro or amino can be used to pair with nitroimino or a stable bridged structure can be introduced to improve overall performance of triazole compounds.
Tetrazole and tetrazole bridged nitroimino explosives
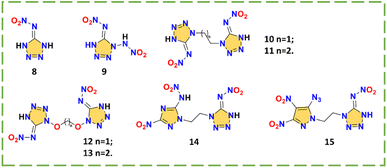
Due to the high nitrogen content, tetrazole is one of the most common structures when designing HEDMs. The nitroamino group connected at the only carbon position has the structural basis for hydrogen transfer. With different N–N′ bridges, a large number of symmetric and asymmetric bridged nitroamino explosives have been reported (representatives 8–15 are selected).4,17,19,32–38 There are 8 kinds of selected tetrazole or tetrazole bridged nitroimino explosives 8–15 in Scheme 4. Their properties are shown in Table 3.| No. | T m (°C) | T d (°C) | d (g cm−3) | ΔHf (kJ mol−1)/(kJ g−1) | v (m s−1) | P (GPa) | IS (J) | FS (N) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 8 | — | 122 | 1.87 | 264.0/2.03 | 9173 | 36.3 | 1.5 | 8 | 39 |
| 9 | — | 110 | 1.93 | 486.3/2.56 | 9967 | 43.4 | 1 | <5 | 40 |
| 10 | — | 194 | 1.86 | 1038.3/3.63 | 9329 | 38.2 | 10 | — | 4 |
| 11 | — | 173 | 1.66 | 1032.0/3.44 | 8374 | 28.1 | 10 | — | 4 |
| 12 | — | 157 | 1.90 | 1088.5/3.58 | 9867 | 46.7 | 1 | — | 17 |
| 13·3H2O | — | 134 | 1.81 | 1103.6/3.47 | 9200 | 38.4 | 1.5 | — | 17 |
| 14 | — | 96 | 1.80 | 511.5/1.55 | 8690 | 32.2 | 12 | 240 | 37 |
| 15 | 81 | 131 | 1.81 | 812.5/2.29 | 8748 | 32.7 | 8 | 80 | 33 |
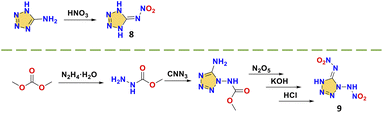
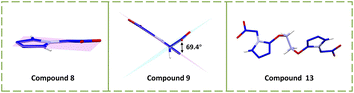
Compounds 5-nitroiminotetrazole (8) and 1-(2-azidoethyl)-5-nitroiminotetrazole (9) are synthesized via 5-aminotetrazole and 1-methoxycarbonyl-1,5-diaminotetrazole, respectively (Scheme 5).39,40 Due to the planarity of the whole molecule and the high nitrogen content, compound 8 exhibits a high density (1.87 g cm−3) and detonation velocity (9173 m s−1), confirming the feasibility of designing nitroimino explosives based on tetrazole or tetrazole bridged skeletons. However, its sensitivity (1.5 J, 8 N) is limited by the low stability of tetrazole. When an additional nitroamino group was introduced into the tetrazole, the density (1.93 g cm−3) and detonation velocity (higher than CL-20, 9967 m s−1) of compound 1,5-di(nitroamino)tetrazole (9) were further enhanced while the thermal and impact and friction stabilities were significantly decreased (Td = 110 °C; IS = 1 J; FS < 5 N), indicating that the nitroamino itself favors the density and energy of the system, but the stability must be improved. As shown in Fig. 3a and b, compared to the coplanar compound 8, the N-nitroamino group leads to low planarity of compound 9, with a twist angle of 69.4°. From this perspective, C-nitroamino is more valuable than N-nitroamino because it can undergo hydrogen transfer.
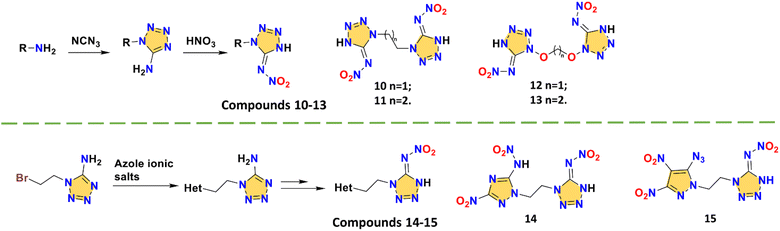
A series of symmetrical/asymmetrical bridged skeletons were designed to solve the stability problem of tetrazole nitroimino explosives. The tetrazole rings of compounds 10–13 are all synthesized by using cyanogen azide. By the reactions of 1-(2-bromoethyl)-1H-tetrazol-5-amine with various energetic azole salts, compounds 14 and 15 can be obtained conveniently (Scheme 6). Compounds 10–13 are alkyl- or ether-bridged symmetrical tetrazole nitroimino compounds. These are 1,1′-ethylenebis(5-nitroiminotetrazole) (10),4 1,1′-propylenebis(5-nitroiminotetrazole) (11),4 1,1′-methylenebis(oxy)bis(5-nitroimino-tetrazole) (12),17 and 1,1′-ethylenebis(oxy)bis(5-nitroimino-tetrazole) (13),17 respectively. Due to the presence of the two nitroimino groups, both systems have high energetic properties, especially compound 12 with a detonation velocity of 9867 m s−1. Obviously, the ether-bridged compounds 12–13 with higher oxygen content have better densities (1.90 and 1.81 g cm−3) than alkyl-bridged compounds 10–11 (1.86 and 1.66 g cm−3). However, the ether bridge also results in a non-coplanar structure (Fig. 3c) and high impact sensitivity (<1.5 J). Compared with compounds 8 and 9, alkyl bridges lead to 10–11 possessing better safety, proving that the overall performance can be tuned by changing the bridge structures. By fixing the ethyl bridge and changing the connected azole ring, asymmetrical compounds 14 and 15 maintain the nitroimino tetrazole on one side to explore more possibilities. The triazole and pyrazole rings in compounds N-(1-(2-(3-nitro-5-(nitroamino)-1H-1,2,4-triazol-1-yl)ethyl)-1,4-dihydro-5H-tetrazol-5-ylidene)nitramide (14) and (E)-N-(1-(2-(5-azido-3,4-dinitro-1H-pyrazol-1-yl)ethyl)-1H-tetrazol-5(4H)-ylidene)nitramide (15) can provide extra carbon positions for other energetic groups. For compound 14, the nitroamino group bonded to the triazole has no hydrogen transfer, which is related to the inductive effect, resulting in the poor thermal stability (96 °C).37 For compound 15, the three extra substituent sites increase the diversity of tetrazole nitroimino bridged compounds.33
Tetrazole has a high nitrogen content and high HOF. The impressive detonation velocity (9967 m s−1) of compound 9 demonstrates the potential of dinitroamino tetrazole structures, which can enhance stability by introducing N–N′ bridges, setting the basis for a series of symmetric and asymmetric tetrazole nitroimino structures. Combining the properties of 10–15, 12 yields the best overall performance with only one carbon number in the bridge structure. The symmetric structure is better than the asymmetric structure. Compound 10 has a detonation performance comparable to HMX and compound 12 exceeds that of CL-20, so that a new generation of HEDMs can be constructed by adjusting the bridge structure to design symmetric dinitroimino tetrazole bridged compounds.
Oxadiazole and oxadiazole bridged nitroimino explosives
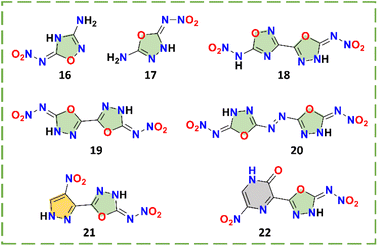
The oxadiazole family is an important branch of explosives because of the high oxygen balance. There are four isomers of oxadiazole, in which the 1,2,3-oxadiazole is now rarely used due to its instability.16 Of the remaining three isomers, 1,2,4-oxadiazole and 1,3,4-oxadiazole are more suitable for combination with nitroamino than furazan. The two isomers greatly increase the diversity of the oxadiazole series. By studying and comparing the comprehensive properties of these isomers, a design of the oxadiazole series of nitroamino explosives can be made more scientific. There are 7 kinds of oxadiazole and oxadiazole bridged nitroimino explosives 16–22 (Scheme 7), and their physicochemical properties are given in Table 4.| No. | T m (°C) | T d (°C) | d (g cm−3) | ΔHf (kJ mol−1)/(kJ g−1) | v (m s−1) | P (GPa) | IS (J) | FS (N) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 16·H2O | 114 | 168 | 1.70 | −196.3/−1.20 | 8033 | 25.6 | 40 | 360 | 18 |
| 17 | — | 153 | 1.88 | 899.1/6.20 | 8668 | 31.2 | 3 | 56 | 41 |
| 18 | — | 176 | 1.86 | 267.7/1.04 | 8888 | 34.1 | 10 | 108 | 42 |
| 19 | — | 210 | 1.99 | 166.8/0.65 | 9481 | 41.9 | 5 | 60 | 20 |
| 20 | — | 185 | 1.97 | 405.7/1.42 | 9358 | 39.1 | 6 | 160 | 44 |
| 21 | — | 205 | 1.90 | 168/0.69 | 8494 | 30.5 | 35 | >360 | 45 |
| 22 | — | 270 | 1.77 | 511.1/1.90 | 8516 | 31.85 | 32 | >360 | 46 |
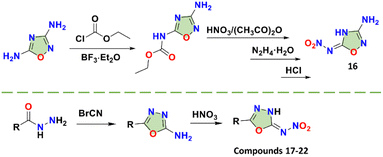
Except for compound 16, which was synthesized by amino protection, the oxadiazoles in all other compounds were obtained using the reaction of cyanogen bromide and hydrazide, such as compounds 17–22 (Scheme 8). For compound 19, the construction of the 1,3,4-oxadiazole ring occurred in yields up to 90.5%. There are two isomers, compounds 3-amino-5-nitroamino-1,2,4-oxadiazole (16)18 and 2-amino-5-nitroamino-1,3,4-oxadiazole (17),41N-(5-(5-(nitroamino)-1,3,4-oxadiazol-2-yl)-1,2,4-oxadiazol-3-yl)nitramide (18)42 and [2,2′-bi(1,3,4-oxadiazole)]-5,5′-dinitramide (19, ICM-101).20 When the properties of these two pairs of isomers are compared, the explosives with the 1,3,4-oxadiazole backbones are significantly better than those with 1,2,4-oxadiazole backbones. As is the case for most nitroimino substituted compounds, compounds 16 and 17 show idealized planar structures, but they have significantly different shortest intermolecular HB lengths (2.133 Å vs. 1.956 Å) and interlayer spacing (3.170 Å vs. 2.927 Å) (Fig. 4).41 Based on these characteristics, compound 17 is more likely to form stronger π–π interactions and HBs than 16, directly resulting in better density (1.88 vs. 1.70 g cm−3) and superior detonation performance (31.2 vs. 25.6 GPa and 8668 vs. 8022 m s−1). Comparing compounds 16 and 18, the nitroamino group on 1,2,4-oxadiazole in compound 16 was transferred to nitroimino, which may be because of the electron-donating group (amino) connected in the β-position of compound 16, which demonstrates that the inductive effect of the other substituents ultimately affects the occurrence of nitroimino.
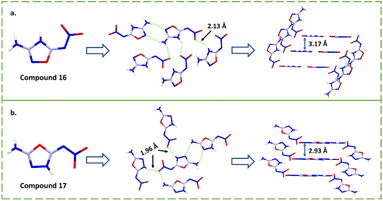 | ||
| Fig. 4 (a and b) Crystal structures, intermolecular shortest HBs and intermolecular stacking of compounds 16 and 17, respectively. | ||
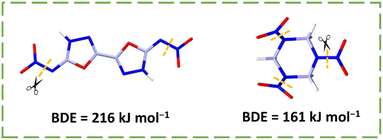
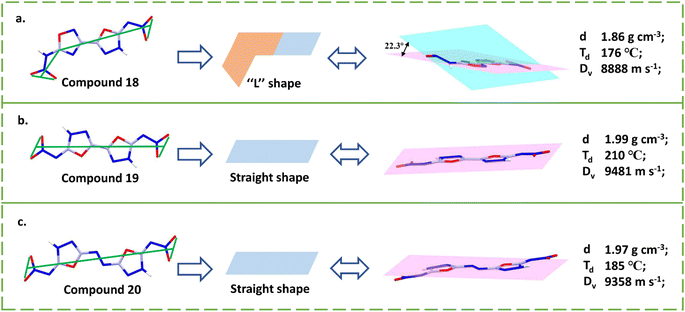
Compared to compound 19, the nitroamino group causes compound 18 to have a significant difference in density (1.88 vs. 1.99 g cm−3) and thermal stability (176 vs. 210 °C), indicating the advantage of nitroimino. Compound 19 is one of the best representatives of nitroamino explosives, with excellent properties such as excellent density (1.99 g cm−3), superb detonation velocity (9481 m s−1), and a lower sensitivity (6 N, 60 J) than CL-20, resulting from the planar molecular structure, high packing coefficient, and remarkably abundant HBs related to the nitroimino. The total intermolecular hydrogen bond energy (EHB) is higher than that in TATB, a classical NH2/NO2 explosive.43 Its good decomposition temperature (210 °C) can be explained by the bond dissociation enthalpy (BDE) of N-nitro bonds (the trigger bond in nitroamino compounds). As shown in Fig. 5, the value was estimated to be 216 kJ mol−1, higher than that of RDX (161 kJ mol−1).20 The rational use of cyanogen bromide is the key to construct 5,5′-dinitramino-3,3′-azo-1,3,4-oxadiazole (20), which obtained by adding an azo bond to compound 18.44
Due to good planarity of the entire large conjugated structure, its density and energetic properties (1.97 g cm−3, 9358 m s−1) are similar to 19 (1.99 g cm−3, 9481 m s−1). While having the same energetic properties, compound 20 has a better sensitivity (6 J; 160 N), confirming the advantage of introducing the azo bridge. There are strong HB systems in compound 20, resulting in the formation of covalent organic framework-like structures and the enhancement of mechanical insensitivity. As shown in Fig. 6a–c, compound 18 exhibits an L-shape due to the presence of the nitroamino group and leads to a dihedral angle of 22.3°, which is significantly different from the linear shape of compounds 19–20. The density, thermal stability, and detonation velocity of compound 18 are also the lowest among the three, further demonstrating the necessity of nitroimino for maintaining good overall performance. Compounds (E)-N-(5-(4-nitro-1H-pyrazol-3-yl)-1,3,4-oxadiazol-2(3H)-ylidene)nitramide (21) and 2-oxo-5-nitro-3-(5-nitroamino-1,3,4-oxadiazole-2-yl)-pyrazine (22) differ from compound 19 in the replacement of the 1,3,4-oxadiazole with pyrazole and pyrazine rings, respectively.45,46 Both compounds 21 and 22 have satisfactory stabilities as demonstrated by decomposition temperatures (205 and 270 °C) and sensitivities (35 J, >360 N; 32 J, >360 N). However, the detonation performance of both compounds is greatly affected because not all substitution sites are fully utilized by energetic groups.
Because of the high oxygen content and the absence of excess hydrogen atoms, oxadiazole facilitates the formation of the high-density property, which in turn promotes good detonation performance, so when combined with the self-stabilization, the oxadiazole nitroimino series exhibits comprehensive excellent performance. Combining the performance of different isomers, the 1,3,4-oxadiazole nitroimino bridge series has the best performance and is the main trend for future development, which can be used to build symmetrical/asymmetrical compounds with direct bridges based on nitroimino-1,3,4-oxadiazole. Whether in terms of its structure, synthetic method and properties, compound 19 (ICM-101) provides a reference, showing that it is possible to construct oxadiazole nitroimino HEDMs which perform better than HMX and CL-20.
Fused-ring nitroimino explosives
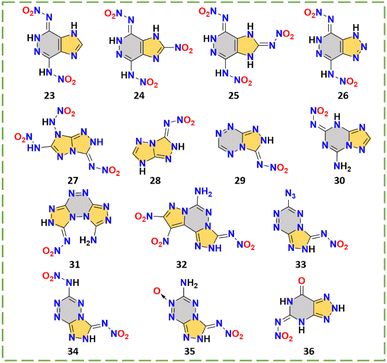
Fused rings exhibit high HOFs due to the abundant ring-strain energy stored in the molecules, showing good energetic performance, enhanced thermal stability and low sensitivities when combined with suitable energetic groups (e.g., –NO2, –ONO2, N-oxide, –NHNO2, etc.). The fused-ring nitroamino explosives have been widely reported in recent years and are leading an important trend in the design of a new generation of HEDMs. The combination of different rings to form fused-ring skeletons increases the diversity for this series and provides many different elegant synthesis methods. There are 14 kinds of fused-ring nitroimino explosives 23–36 (Scheme 9), and their properties are shown in Table 5.| No. | T m (°C) | T d (°C) | d (g cm−3) | ΔHf (kJ mol−1)/(kJ g−1) | v (m s−1) | P (GPa) | IS (J) | FS (N) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 23 | — | 166 | 1.82 | 355.2/1.48 | 8360 | 29.0 | 32 | 120 | 47 |
| 24 | — | 117 | 1.80 | 407.8/1.43 | 8568 | 31.5 | 7 | 160 | 13 |
| 25 | — | 116 | 1.86 | 349.6/1.16 | 8729 | 33.2 | 8 | 20 | 14 |
| 26·H2O | — | 162 | 1.87 | 303.0/1.48 | 8875 | 34.5 | 18 | 120 | 48 |
| 27 | — | 92 | 1.85 | — | — | — | — | — | 49 |
| 28·H2O | — | 169 | 1.73 | 174.9/0.93 | 8271 | 26.6 | 6 | 120 | 50 |
| 29 | — | 132 | 1.81 | 647.8/3.56 | 8880 | 33.2 | 31 | >240 | 51 |
| 30 | — | 225 | 1.77 | 454.3/2.32 | 8308 | 26.6 | >40 | >360 | 52 |
| 31·H2O | — | 166 | 1.88 | 614.7/2.41 | 9073 | 34.0 | >40 | >360 | 53 |
| 32 | — | 172 | 1.91 | 869.0/2.67 | 9202 | 35.5 | 30 | 300 | 54 |
| 33 | — | 150 | 1.85 | 951.8/4.27 | 9236 | 36.3 | 1 | >40 | 51 |
| 34 | — | 138 | 1.91 | 740.9/3.06 | 9301 | 38.3 | 3 | >5 | 55 |
| 35 | — | 182 | 1.88 | 486.0/2.28 | 9047 | 35.1 | 10 | >160 | 56 |
| 36 | — | 242 | 1.94 | 223.0/1.13 | 8845 | 33.0 | 36 | 348 | 58 |
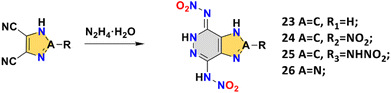
This series of compounds 23–26 with novel dicyclic backbones has been reported by Shreeve's group in recent years, which can be obtained by a very concise synthesis route.13,14,47,48 The construction of these fused-ring skeletons was by reacting hydrazine hydrate with dinitrile (Scheme 10), which also resulted in the introduction of two amino groups. Then the final compound was obtained by a nitration reaction. The synthesis of compound 26 is cost effective (with a total yield of 60%.) and can be obtained in gram scale quantities (2 g), which suggests that it may be suitable for the manufacture and application as an explosive. It should be mentioned that the hydrogen transfer is selective, i.e., only one nitroamino on pyrazine undergoes hydrogen transfer. The phenomenon has been confirmed by proton transport dynamics, a sufficient way to predict the possibility and selectivity of hydrogen transfer. This model greatly improves the efficiency of designing new nitroamino HEDMs with self-stabilizing capabilities.14 As shown in Fig. 7a, the prediction consists of three steps: first, all possible conformations are assumed, then a ZPE calculation is performed for each conformation, and the optimal conformation is determined to be the one with the lowest ZPE value. The optimal conformation is often the real conformation (the crystal structure) of the calculated nitroamino compound.
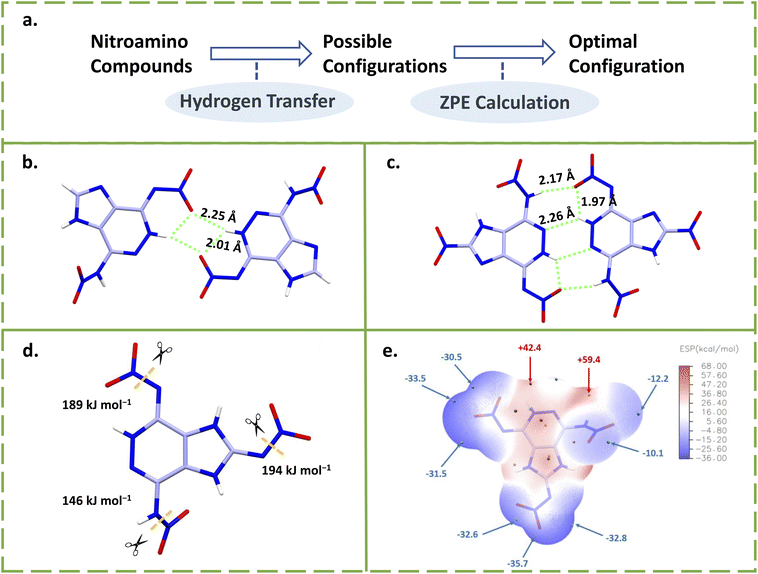 | ||
| Fig. 7 (a) The process of proton transport dynamics; (b and c) the intermolecular HBs of compounds 23 and 24; (d) the BDEs of compound 25; (e) the ESP of compound 25. | ||
As seen in Fig. 7b and c, both 4-nitramino-7-nitrimino-imidazo[4,5-d]pyridazine (23)47 and 2-nitro-4-nitramino-7-nitroimino-1,5-dihydro-4H-imidazolo[4,5-d]pyridazine (24)13 have head-to-head HB systems between nitroamino/nitroimino groups and pyridazine rings, which show short inter- or intra-HB lengths. This demonstrates the existence of strong HB systems for this series of compounds, which were produced by nitroimino. Compared to compounds 23 and 24, the fully nitroamino-substituted compound 2,4-nitramino-7-nitroimino-1,5-dihydro-4H-imidzolo[4,5-d]pyridazine (25)14 has a higher density (1.86 g cm−3) and detonation performance (8729 m s−1) but lower decomposition temperature (116 °C). As shown in Fig. 7d, the BDE (145.5 kJ mol−1) of nitroamino (NH–NO2) in compound 25 can explain the low thermal stability. The BDEs of the other two nitroimino groups (N–NO2) were significantly elevated (197.1 and 196.8 kJ mol−1), which suggests that the effect of nitroimino on BDEs can further enhance thermal stability. The electrostatic potential surface (ESP) analysis of compound 25 can also explain the difference between nitroamino and nitroimino groups. As shown in Fig. 7e, the minimum potential parts of nitro of the nitroimino moiety are obviously lower than the nitro on the nitroamino moiety. The proton on the nitroamino group (+59.4 kcal mol−1) is higher than the proton on the fused ring (+42.40 kcal mol−1), showing the dynamics for hydrogen transfer. The lattice of compound 4-nitroamino-7-nitroiminotriazolo[4,5-d]pyridazine (26)48 contains a cocrystallized water molecule that has so far been impossible to remove. Perhaps containing stronger HBs, compound 26 still maintains a high density of 1.87 g cm−3 and detonation velocity of 8878 m s−1.
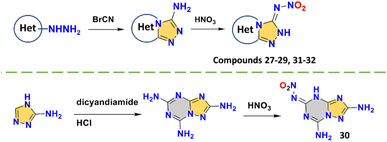
The triazole based fused-ring skeletons of compounds 27–29 and 31–32 are all synthesized by the cyclization of cyanogen bromide and hydrazine groups, which then easily give the nitroimino group by nitration. Compound 30 is synthesized by using dicyandiamide (Scheme 11).49–54 These two straightforward ways to construct amino-triazoles have been widely used in the synthesis of bi- or tricyclic fused-ring skeletons. Triazole is an excellent carrier for the formation of nitroimino. Due to the strong electron-withdrawing effect caused by three nitroamino groups, 3,6,7-trinitro-7H-[1,2,4]triazolo[4,3-b][1,2,4]triazole (27)49 is too acidic to be stable, so its many properties have not been reported, also indicating the instability of the N-nitroamino group (similar to compound 9). 7-Nitroimino-7H-[1,2,4]triazolo[4,3-b][1,2,4]triazole (28),50N-([1,2,4]triazolo[4,3-b][1,2,4,5]tetrazin-3-yl)nitramide (29),51 and N-(7-amino-[1,2,4]triazolo[1,5-a][1,3,5]triazine-5-yl)nitramide (30)52 all sacrifice a substituent site to provide the stability of the backbone, but to some extent lower the detonation performance (8270 m s−1 to 8880 m s−1). Therefore, to ensure balanced performance, it is important to make full use of substituent sites to introduce energetic groups. The [1,3,5]triazine ring is more stable compared to the pyridazine or azole ring, but nitroimino also occurs in compound 30, which is relatively rare. The stability of compound 30 is outstanding among these compounds, with a good thermal stability (225 °C) and sensitivity (IS > 40 J; FS > 360 N). This also provides a new idea for the future design of energetic materials with superior comprehensive performance, the full energetic group substituted nitroimino based [1,3,5]triazine fused compounds.
(Z)-N-(8-Aminobis([1,2,4]triazolo)[4,3-b:3′,4′-f][1,2,4,5]tetrazin-1(2H)-ylidene)nitramide hydrate (31)53 and 9,10-dinitropyrazolo[1,5-d][1,2,4]triazolo[3,4-f][1,2,4]triazine-3-nitroamine-6-amine (32)54 have full substituents and a higher detonation performance than compounds 28–30. Interestingly, only one side of the amino group in compounds 31–32 was successfully nitrated, which can be explained by the different aromaticity or electron cloud density of the precursors. As shown in Fig. 8a, the two amino groups are different, which has been explained by the shielding map, a figure for showing aromaticity. Thus, the precursor compound 31-1 is not a strictly symmetric structure, leading to the selectivity in subsequent nitration. Similarly, compound 32 also has a selectivity for nitration, which has been explained by the natural bond orbital (NBO) analysis of its precursor, showing the different electronic cloud density of the nitrogen atoms in two amino groups. Thus, this all suggests that calculations can assist in predicting whether the designed precursors will undergo nitration as expected. Moreover, the energy and safety performance of compounds 31 and 32 are obviously superior to those of bicyclic fused-ring nitroimino compounds such as 23–26, demonstrating the superiority of stable polycyclic fused-ring skeletons. In the case of a similar skeleton structure, compound 32 outperforms 31, which indicates that the stable energetic groups such as the nitro group may contribute to the overall performance of nitroimino based HEDMs. Compound 32 has surpassed HMX in terms of density (1.91 g cm−3), detonation velocity (9202 m s−1) and sensitivity (30 J; 300 N), indicating that the correct design of suitable multicyclic skeletons is a future trend in the field of fused-ring nitroimino HEDMs.
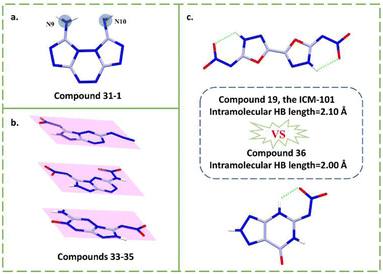 | ||
| Fig. 8 (a) The difference of the two amino groups of compounds 31-1; (b) the planarity structure of compounds 33–35; (c) the comparison of intramolecular HB length of compounds 19 and 36. | ||
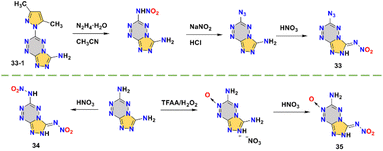
This series of tetrazine–triazole nitroimino explosives 33–35 has been reported by Shreeve's group,51,55,56 which are N-(6-azido-[1,2,4]triazolo[4,3-b][1,2,4,5]tetrazin-3-yl)nitramide (33),51 3,6-dinitramino-1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine (34)55 and 3-nitramino-6-amino-1,2,4-triazolo[4,3-b][1,2,4,5]tetrazine-7-N-oxide (35),56 respectively. The fused-ring skeleton with tetrazine has received much attention in recent years due to its high nitrogen content and good stability. The synthesis routes to compounds 33–35 are shown in Scheme 12. Compound 33-1 is a good tetrazine–triazole precursor from which a series of nitroimino explosives have been derived.57 With different substituents on tetrazine, the properties of this class of compounds 33–35 can be compared and this helps to intuitively understand the effects of nitroimino combined with different energetic groups on energy and safety performance. Compounds 33–35 all have high densities (1.85–1.91 g cm−3) and detonation performances (35.1–38.3 GPa; 9047–9301 m s−1). As shown in Fig. 8b, these three compounds all have good planarity. For compound 33, the azido group interacts with the other two or three molecules, resulting in the irregular distribution in three-dimensional crystal stacking. Compound 34 with an nitroamino group in the tetrazine ring is also coplanar, which may relate to the weak interactions between molecules. This likely contributes to compound 34 possessing the highest density and detonation velocity (1.91 g cm−3; 9301 m s−1) among these three compounds. However, the presence of an nitroamino group also leads to the low thermal stability (138 °C) and high sensitivity (3 J; >5 N). This suggests that although the nitroamino moiety remains planar with the backbone, it can still decrease the stability of the system. Compound 35 has an N-oxide group which increases the oxygen content, giving it a high density (1.88 g cm−3) even in the presence of an amino group. Compared with compounds 33 and 34, which are linked to an azide or a nitroamino group, compound 35 undoubtedly has better sensitivity (10 J; >160 N). Thus, compound 35 has the best overall performance among these three compounds, indicating the stable N-oxide and amino groups may be a good choice for pairing with a nitroimino group.
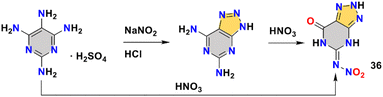
N-(7-Oxo-6,7-dihydro-2H-[1,2,3]triazolo[4,5-d]pyrimidin-5-yl)nitramide (36) was reported by the Cheng group.58 From the same starting material, tetraaminopyrimidine sulfate, compound 36 was synthesized by two different routes, one was obtained by diazotization to a fused ring, then followed by nitration; the other route was by undergoing a rearrangement process under nitric acid conditions (Scheme 13). Compound 36 has balanced comprehensive performance, such as good detonation velocity (8845 m s−1), high density (1.94 g cm−3), and low sensitivity (36 J, 348 N). Especially notable is the high decomposition temperature (241 °C). Compound 36 surpasses the RDX in all aspects. The packing coefficient is up to 0.79, indicating high density. Abundant HBs systems are also observed by the crystal structure. As shown in Fig. 8c, the bond length of the intramolecular N–H⋯O interaction (2.00 Å) of compound 36 is stronger than that of compound 19, the ICM-101 (2.10 Å), illustrating the strong HBs, thereby improving the density, detonation performance and stability.
The fused ring is regarded as a good carrier for nitroamino because of its high HOF, rich variety and good stability. Among them, the triazole-containing fused ring is a strong contender for the backbone of the fused-ring nitroimino explosives due to its simple synthesis method. Since selective hydrogen transfer tends to occur under full nitroamino substitution (compounds 23–26 and 34), as confirmed by proton transfer dynamics. Nitroamino groups obviously have an adverse effect on stability. To avoid this situation, fused-ring nitroimino explosives with other stable substituent groups often show a better overall performance, such as compounds 32 and 35. Therefore, with a good backbone, nitro, amino, and N-oxide groups with higher stability can be introduced into the system, and then combined with nitroimino to synthesize a new generation of competitive HEDMs.
Conclusions
Nitroimino explosives have been developing rapidly in recent years and have attracted much attention for hydrogen transfer. The extra intramolecular HBs which are formed cause the molecules to be more closely aligned, which promotes various intermolecular interactions. These secondary bonding interactions significantly increase the density and enhance the stability, allowing compounds to display high energetic properties while also obtaining stability through this self-stabilization process. There is no doubt that this elegant stability strategy will markedly enhance potential for advancing the next generation of HEDMs beyond RDX, HMX or CL-20.This review summarizes 36 kinds of reported monocyclic, bridged and fused-ring nitroimino explosives. The exploratory works on monocyclic nitroimino explosives (compounds 2, 8, 17), prove that triazole, tetrazole and oxadiazole are good carriers for nitroimino providing the basis for the subsequent design of bridged/fused-ring nitroimino explosives, whose stability is further enhanced due to the more stable multi-ring skeletons. Among them, a number of comprehensive explosives with balanced properties have emerged, such as triazole-bridged nitroimino compound 6, tetrazole-bridged nitroimino compound 12, oxadiazole-bridged nitroimino compounds 19 (ICM-101) and 20 and fused-ring nitroimino compound 32. The analysis of the causes, characteristics and performance enhancement of hydrogen transfer facilitates the understanding of the changes brought by microscopic secondary bonding interactions to crystal stacking, molecular conjugation, aromaticity, etc., and then to macroscopic property changes. The authors believe that the combination of nitroimino with the nitro group and substitution with a fused ring with a high nitrogen content will be the most potential way to design energetic materials for practical use. With high content HBs of nitroimino compounds, the research of host–guest and co-crystals will be another good way to obtain the next generation HEDMs. As has already shown great promise in the last decade, nitroimino-substituted explosives will surely burst out with great vigor in the development of the new generation of HEDMs for aerospace, military and civilian applications.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China under grant no. 22205020 (L. H.) and no. 22235003 (S. P.). JMS is grateful to the Fluorine 19 fund.References
- A. A. Dippold, D. Izsák, T. M. Klapötke and C. Pflüger, Chemistry, 2016, 22, 1768–1778 CrossRef CAS PubMed.
- D. Fischer, J. L. Gottfried, T. M. Klapötke, K. Karaghiosoff, J. Stierstorfer and T. G. Witkowski, Angew. Chem., Int. Ed., 2016, 55, 16132–16135 CrossRef CAS PubMed.
- Y. Tang, C. He, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2016, 4, 3879–3885 RSC.
- Y. H. Joo and J. M. Shreeve, Angew. Chem., Int. Ed., 2009, 121, 572–575 CrossRef.
- H. Gao, Q. Zhang and J. M. Shreeve, J. Mater. Chem. A, 2020, 8, 4193–4216 RSC.
- P. Yin, Q. Zhang and J. M. Shreeve, Acc. Chem. Res., 2015, 49, 4–16 CrossRef PubMed.
- Q. Zhang and J. M. Shreeve, Chem. Rev., 2014, 114, 10527–10574 CrossRef CAS PubMed.
- Y. Tang, L. A. Mitchell, G. H. Imler, D. A. Parrish and J. M. Shreeve, Angew. Chem., Int. Ed., 2017, 56, 5894–5898 CrossRef CAS PubMed.
- J. Zhang, S. Dharavath, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, J. Am. Chem. Soc., 2016, 138, 7500–7503 CrossRef CAS PubMed.
- T. M. Klapötke, C. Petermayer, D. G. Piercey and J. Stierstorfer, J. Am. Chem. Soc., 2012, 134, 20827–20836 CrossRef PubMed.
- P. Yin and J. M. Shreeve, Angew. Chem., Int. Ed., 2015, 54, 14513–14517 CrossRef CAS.
- Q. Sun, N. Ding, C. Zhao, Q. Zhang, S. Zhang, S. Li and S. Pang, Sci. Adv., 2022, 8, eabn3176 CrossRef CAS PubMed.
- L. Hu, C. He, S. Pang and J. M. Shreeve, Org. Lett., 2021, 23, 7860–7864 CrossRef CAS PubMed.
- Y. Wang, L. Hu, R. J. Staples, S. Pang and J. M. Shreeve, ACS Appl. Mater. Interfaces, 2022, 14, 52971–52978 CrossRef CAS PubMed.
- J. Ma, G. Cheng, X. Ju, Z. Yi, S. Zhu, Z. Zhang and H. Yang, Dalton Trans., 2018, 47, 14483–14490 RSC.
- H. Wei, C. He, J. Zhang and J. M. Shreeve, Angew. Chem., Int. Ed., 2015, 54, 9367–9371 CrossRef CAS PubMed.
- Y. H. Joo and J. M. Shreeve, Angew. Chem., Int. Ed., 2010, 49, 7320–7323 CrossRef CAS PubMed.
- Y. Tang, H. Gao, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, Angew. Chem., Int. Ed., 2016, 55, 1147–1150 CrossRef CAS PubMed.
- D. Kumar, C. He, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2016, 4, 9220–9228 RSC.
- W. Zhang, J. Zhang, M. Deng, X. Qi, F. Nie and Q. Zhang, Nat. Commun., 2017, 8, 181–187 CrossRef PubMed.
- C. Lei, H. Yang, Q. Zhang and G. Cheng, ACS Appl. Mater. Interfaces, 2022, 14, 39091–39097 CrossRef CAS PubMed.
- A. Strachan, A. C. van Duin, D. Chakraborty, S. Dasgupta and W. A. Goddard III, Phys. Rev. Lett., 2003, 91, 098301 CrossRef PubMed.
- M. Geetha, U. R. Nair, D. B. Sarwade, G. M. Gore, S. N. Asthana and H. Singh, J. Therm. Anal. Calorim., 2003, 73, 913–922 CrossRef CAS.
- O. Bolton, L. R. Simke, P. F. Pagoria and A. J. Matzger, Cryst. Growth Des., 2012, 12, 4311–4314 CrossRef CAS.
- K. Wang, D. A. Parrish and J. M. Shreeve, Chemistry, 2011, 17, 14485–14492 CrossRef CAS PubMed.
- A. M. Astakhov, D. V. Antishin, V. A. Revenko, A. D. Vasiliev and E. S. Buka, Chem. Heterocycl. Compd., 2017, 53, 722–727 CrossRef CAS.
- A. A. Dippold and T. M. Klapötke, Chemistry, 2012, 18, 16742–16753 CrossRef CAS PubMed.
- Q. Lang, Q. Sun, Q. Wang, Q. Lin and M. Lu, J. Mater. Chem. A, 2020, 8, 11752–11760 RSC.
- Z. Xu, G. Cheng, S. Zhu, Q. Lin and H. Yang, J. Mater. Chem. A, 2018, 6, 2239–2248 RSC.
- S. Zhang, G. Cheng and H. Yang, Dalton Trans., 2020, 49, 5590–5596 RSC.
- M. Xu, G. Cheng, H. Xiong, B. Wang, X. Ju and H. Yang, New J. Chem., 2019, 43, 11157–11163 RSC.
- Y.-H. Joo and J. M. Shreeve, J. Am. Chem. Soc., 2010, 132, 15081–15090 CrossRef CAS PubMed.
- D. Kumar, G. H. Imler, D. A. Parrish and J. M. Shreeve, New J. Chem., 2017, 41, 4040–4047 RSC.
- D. Kumar, G. H. Imler, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2017, 5, 16767–16775 RSC.
- G. Zhao, D. Kumar, C. He, J. P. Hooper, G. H. Imler, D. A. Parrish and J. M. Shreeve, Chemistry, 2017, 23, 16753–16757 CrossRef CAS.
- S. Manzoor, Q. Tariq, X. Yin and J. Zhang, Def. Technol., 2021, 17, 1995–2010 CrossRef.
- D. Kumar, L. A. Mitchell, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2016, 4, 9931–9940 RSC.
- Y.-H. Joo and J. M. Shreeve, J. Am. Chem. Soc., 2010, 132, 15081–15090 CrossRef CAS PubMed.
- J. Stierstorfer and T. M. Klapötke, Helv. Chim. Acta, 2007, 90, 2132–2150 CrossRef.
- D. Fischer, T. M. Klapötke and J. Stierstorfer, Angew. Chem., Int. Ed., 2015, 54, 10299–10302 CrossRef CAS PubMed.
- Q. Sun, N. Ding, C. Zhao, J. Ji, S. Li and S. Pang, Chem. Eng. J., 2022, 427, 130912 CrossRef CAS.
- S. Liao, T. Liu, Z. Zhou, K. Wang, S. Song and Q. Zhang, Dalton Trans., 2021, 50, 13286–13293 RSC.
- Y. Ma, A. Zhang, C. Zhang, D. Jiang, Y. Zhu and C. Zhang, Cryst. Growth Des., 2014, 14, 4703–4713 CrossRef CAS.
- S. Banik, P. Kumar, V. D. Ghule, S. Khanna, D. Allimuthu and S. Dharavath, J. Mater. Chem. A, 2022, 10, 22803–22811 RSC.
- A. K. Yadav, V. D. Ghule and S. Dharavath, ACS Appl. Mater. Interfaces, 2022, 14, 49898–49908 CrossRef CAS PubMed.
- T. Li, F. Yang, L. Ding, Q. Lang and M. Lu, Energ. Mater. Front., 2022, 3, 32–37 CrossRef CAS.
- L. Hu, R. J. Staples and J. M. Shreeve, Chem. Commun., 2021, 57, 603–606 RSC.
- L. Hu, R. J. Staples and J. M. Shreeve, Chem. Eng. J., 2021, 420, 129839 CrossRef CAS.
- C. Bian, W. Feng, Q. Lei, H. Huang, X. Li, J. Wang, C. Li and Z. Xiao, Dalton Trans., 2020, 49, 368–374 RSC.
- Y. Tang, C. He, G. H. Imler, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2017, 5, 6100–6105 RSC.
- Y. Liu, G. Zhao, Y. Tang, J. Zhang, L. Hu, G. H. Imler, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2019, 7, 7875–7884 RSC.
- P. Yang, H. Yang, W. Yang, J. Tang, G. Zhang, W. Hu and G. Cheng, Cryst. Growth Des., 2022, 22, 4221–4227 CrossRef CAS.
- Q. Yu, J. Singh, R. J. Staples and J. M. Shreeve, Chem. Eng. J., 2021, 431, 133235 CrossRef.
- W. Hu, G. Zhang, P. Yang, H. Yang and G. Cheng, Chem. Eng. J., 2023, 451, 138640 CrossRef CAS.
- L. Hu, P. Yin, G. Zhao, C. He, G. H. Imler, D. A. Parrish, H. Gao and J. M. Shreeve, J. Am. Chem. Soc., 2018, 140, 15001–15007 CrossRef CAS PubMed.
- L. Hu, C. He, G. Zhao, G. H. Imler, D. A. Parrish and J. M. Shreeve, ACS Appl. Energy Mater., 2020, 3, 5510–5516 CrossRef CAS.
- D. E. Chavez and M. A. Hiskey, J. Heterocycl. Chem., 1998, 35, 1329–1332 CrossRef CAS.
- G. Zhang, H. Xiong, P. Yang, C. Lei, W. Hu, G. Cheng and H. Yang, Chem. Eng. J., 2021, 404, 126514 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |