Fully recyclable high-performance polyacylsemicarbazide/carbon fiber composites†
Abstract
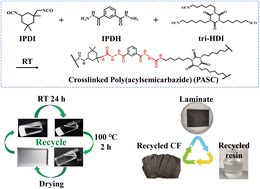
Realizing the complete and non-destructive recycling of carbon fiber reinforced composite materials is of great significance for the pursuit of sustainability and a circular economy. Although the application of resins containing dynamic bonds in carbon fiber composite materials has made great progress in recent years, the development of a completely recyclable resin matrix with high strength, high modulus, and high Tg is still a huge challenge due to the mutual exclusion of the dynamic properties and mechanical properties of the material. Here we report a novel dynamic polyacylsemicarbazide (PASC) material with ultra-high strength and modulus containing supramolecular multiple hydrogen bonding between the polymer chains. The influence of different chemical structures on the intermolecular hydrogen bond structure of the material is studied. The optimized PASC material with a Young's modulus of 4.1 GPa, a stress at break of 97.6 MPa, and a glass transition temperature (Tg) of 174 °C exhibits great reprocessing properties, excellent solvent recycling ability, and excellent water resistance. Furthermore, using this newly developed PASC material as the matrix resin, the carbon fiber reinforced polymer composite was successfully prepared through solution impregnation and thermal pressing. The composite material shows an optimized inter-layer shear strength of 36.7 MPa and a healing efficiency of 70.6%. The great dynamic and reversible characteristics of the material enable nearly 100% completely non-destructive solvent recycling of the carbon fiber and matrix resin, and the regenerated composite material still retains 82.3% of its initial inter-layer shear strength.
- This article is part of the themed collection: Sustainable Composites


 Please wait while we load your content...
Please wait while we load your content...