Enhancing visible-light photocatalytic performance of Au/TiO2 catalysts through light reflection-promoted optical absorption with oriented anatase mesocrystals†
Jingling
Yang
ab,
Shiman
He
b,
Hongwei
Liu
c,
Esa
Jaatinen
d,
Eric
Waclawik
d,
Jiamin
Quan
e,
Sarina
Sarina
 d,
Chun
He
d,
Chun
He
 f,
Senchuan
Huang
b,
Huaiyong
Zhu
f,
Senchuan
Huang
b,
Huaiyong
Zhu
 *d and
Mingmei
Wu
*d and
Mingmei
Wu
 *b
*b
aGuangdong Key Laboratory of Environmental Pollution and Health, School of Environment, Jinan University, Guangzhou 510632, P. R. China
bSchool of Chemical Engineering and Technology, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, P. R. China
cAustralian Centre for Microscopy & Microanalysis, The University of Sydney, Sydney, NSW 2006, Australia
dSchool of Chemistry, Physics and Mechanical Engineering, Queensland University of Technology, Brisbane, QLD4001, Australia
eThe Key Laboratory of Optoelectronic Technology & System, Education Ministry of China, Chongqing University, 400044, China
fSchool of Environmental Science and Engineering, Sun Yat-Sen University, Guangzhou, 510275, P. R. China
First published on 27th January 2023
Abstract
Au nanoparticles (NPs) attached to various TiO2 supports are widely studied as plasmonic catalysts for driving chemical reactions under visible light irradiation. However, plasmonic catalysis still suffers from unsatisfactory efficiencies due to limited light-harvesting abilities. Here we reported a new tactic to enhance the light-harvesting ability of plasmonic Au NPs by utilizing a light reflection-promoted model – a vertically <001> oriented anatase mesocrystal (meso-TiO2) rooted on Ti foil as the support, and thus achieving efficient photocatalytic sulfur-containing volatile organic compound elimination and hydrogen evolution from water. Experimental evidence and theoretical simulations confirm that the Au NPs in this architecture more effectively harvest visible light because of the simultaneous absorption of both the incident and the back-reflected photons from the meso-TiO2/Ti foil surface, where Ti foil serves as the reflective substrate. The enhanced light absorption of Au NPs in this light reflection-promoted model excited strong localized surface plasmon resonance to yield more reactive species that drive the redox reactions. This research could inspire a new paradigm to improve the photocatalytic performance of plasmonic metals by utilizing the light reflection model.
1. Introduction
Plasmonic catalysts of Au nanoparticles (NPs) dispersed on TiO2 supports have been studied for a wide range of applications in chemical synthesis, energy innovation and pollution remediation.1–8 The Au NPs can effectively absorb visible light due to the localized surface plasmon resonance (LSPR) effect, which can promote the migration of hot electrons from the Au NPs to the TiO2 support.9,10 Both the hot electrons transferred to the support and positively charged holes remaining on the Au NPs can induce chemical reactions. Plasmonic catalysts operate in a manner that is fundamentally different from conventional semiconductor catalysts in the nature of their light harvesting, charge generation and migration,11 energy transfer,12 and interaction between the catalyst and reactants.13 Significant effort has been dedicated to improving the performance of the plasmonic catalysts under visible light irradiation.7The quantum yield of light absorption is a primary factor in determining photocatalytic performance.9 Even though the absorption of visible light by a single Au NP a few nanometers in size can be significant, a large fraction of the total incident light is transmitted when light is directed onto a surface that has been coated with a film of the plasmonic metal Au, and a significant fraction is transmitted to the underlying substrate if the effective gold thickness is thin.6,14 For Au NPs with diameters <10 nm the light energy transmitted is considerable. Unlike Au NPs, TiO2 absorbs only a negligible amount of visible light, particularly when in the form of a thin TiO2 film. The crystal properties of TiO2, such as the crystal size, orientation, defects, and inter-crystal voids, all influence its light reflection and scattering and, therefore, the level of photon loss. It follows then that the light absorption by the Au NPs on a TiO2 support will be considerably affected.15–19 If the catalyst is designed to maximize light reflection and their absorption by using Au NPs, the efficiency of the catalyst should be substantially improved.
TiO2 crystals can be essentially transparent to visible light if TiO2 is aligned, which forms an essentially crystalline structure to minimize light scatter. As illustrated in Scheme 1, we envisioned that if the light transmitted through the overlying layer of Au NPs and underlying nanostructured TiO2 could be reflected from the Ti foil back to the metal NPs, the total amount of light being absorbed by these Au NPs would increase,15 which could be expected to improve the yield of photocatalytic reaction for this composite system.
 | ||
| Scheme 1 Illustration of light reflection behavior of oriented anatase TiO2 (a) and non-oriented TiO2 (b) under light irradiation. | ||
To verify the above assumption, in this present study, Au NPs were attached to the <001> oriented TiO2 mesocrystals that were supported by a piece of reflective Ti foil to reflect the light transmitted through the overlying nanostructure (see Scheme 1a). Mesocrystals are highly ordered superstructures with a high degree of crystallinity and oriented subunit alignment.20 For comparison, less transparent films were studied that consisted of aggregated small anatase polycrystals arranged without a preferred orientation (see Scheme 1b). The visible light photocatalytic performance of the three catalysts was evaluated for two model reactions, methanethiol (CH3SH) removal by catalytic oxidation and H2 evolution by reducing water, which are two redox reactions.
CH3SH is a representative sulfur-containing volatile organic compound (S-VOC) with significant toxicity, and photocatalysis is an efficient way for CH3SH elimination,21,22 while hydrogen production from water is a cost-effective approach to promote the development of future hydrogen economies.23 This work using a TiO2 mesocrystal to tune the optical absorption of Au NPs and their performance for catalytic redox reaction highlights the support-effect induced efficient utilization of reflected light, and hence, understanding the effect of light reflection-promoted optical absorption would provide guidelines for designing efficient photocatalysts.
2. Experimental section
2.1 Preparation of a Au/TiO2 film
The fabrication process of the anatase-type TiO2 film on a piece of Ti foil with a size of 1 × 1 cm2 was performed by the acid vapor oxidation (AVO) reaction at 140 °C in an autoclave for 12 or 60 h, respectively.25 The details are shown in the ESI.† The as-synthesized anatase-type TiO2 mesocrystal film with a fabrication time of 12 h was denoted as meso-TiO2, and the TiO2 polycrystal films with a fabrication time of 60 h were denoted as p-TiO2. A Degussa P25 (TiO2) film was prepared by slowly dipping the titanium foil into an ethanol solution containing TiO2 Degussa P25 (10 g L−1) for 5 min and then taking it out; the same procedure was repeated six times, and it was then dried at 100 °C for 12 h. Subsequently, Au NPs were deposited onto these TiO2 films by the UV-assisted photocatalytic reduction method: the TiO2 film was soaked in 5.0 mL of aqueous HAuCl4 (5 mmol L−1), irradiated with UV light for 15 min to reduce the Au3+ adsorbed by TiO2 to Au NPs, then rinsed with distilled water to remove the remaining substances on the film surface and dried in a vacuum oven at room temperature for 12 h.2.2 Characterization
X-Ray diffraction (XRD) patterns were recorded on a Bruker D8 Advance with Cu Kα radiation (40 kV, 40 mA). The microstructural properties were characterized using electron microscopy. Scanning electron microscopy (SEM) images were obtained on an FEI Quanta 400F electron microscope. Transmission electron microscopy (TEM) images, high-resolution TEM (HRTEM) images, and element mapping results were taken on an FEI Tecnai G2 F30 electron microscope operated at 300 kV, and JEOL JEM-2100F electron microscope operated at 200 kV. X-ray photoelectron spectroscopy (XPS) spectra were measured using an X-ray photoelectron spectrometer (ESCALAB 250). A UV-2450 spectrophotometer was used to obtain ultraviolet-visible (UV-vis) diffuse reflectance spectra of these products using BaSO4 as a reference sample. The fluorescence emission enhancement spectra of the samples were collected on an Edinburgh Instruments FLS920 with a 450 W xenon lamp as the excitation source. The sample films for fluorescence enhancement measurement were prepared in the following manner: one piece of the sample film was slowly dipped into a solution of RhB (10−4 mol L−1) for five min and then dried in a vacuum drier at room temperature. The excitation wavelength was 540 nm. The hydroxyl radicals (˙OH) and superoxide radicals (˙O2−) were detected using a Bruker A300-10-12 electron spin resonance spectrometer (ESR, Bruker, Germany) with a 150 W short arc xenon lamp as the irradiation light source and 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin trap agent. The details are shown in the ESI.† The loading amount of Au on the samples was measured using an inductively coupled plasma-mass spectrometer (ICP-MS) (Agilent 7700e). The transient photocurrents were measured on an electrochemical workstation (Metrohm Autolab PGSTAT302N, Herisau, Switzerland), where the samples, Ag/AgCl, and Pt wire served as working, reference, and counter electrodes, respectively. The photocurrent was measured at an applied potential of 0.4 V (vs. Ag/AgCl), and the electrolyte was 0.5 mol L−1 of Na2SO4 aqueous solution. The illumination source was a 300 W Xe lamp (Perfect light PLS-SXE300CUV, Beijing, China) with a visible light filter (λ ≥ 430 nm).2.3 Photocatalytic removal of CH3SH
The photocatalytic removal of CH3SH was conducted by fixing the sample films in the middle of a cylindrical quartz cell equipped with a CH3SH detector (DM-400IS, Detcon) in the range of 0–100 ppm per v (±2%), with a detection limit of 0–100 ppm per v and reproducibility of 2%. Subsequently, the CH3SH flow continuously passed through a catalyst-filled reactor with a constant inflow of 100 mL min−1. Air was employed to dilute CH3SH at an influent concentration of 50 ppm. The inlet and outlet concentrations of CH3SH were analyzed by using a CH3SH detector. A 150 W Xe arc lamp was used as a light source (Beijing Saifan 7ILX150P) with a visible light filter (λ ≥ 430 nm and irradiation power = 10–20 mW cm−2). The light was vertically incident on the samples, and the samples were all immobilized on Ti foil. After adsorption for 1.0 min at room temperature, the light was turned on to reach the adsorption equilibrium. All the tests were carried out under ambient conditions at a temperature of 25 °C.2.4 Photocatalytic hydrogen evolution
The samples were inserted at the bottom of a cylindrical quartz cell filled with 200 mL of a water/methanol mixture (170/30 vol%) at pH = 6.4. The cell was purged with bubbling N2 gas for 20 min and sealed with a rubber septum. A 300 W Xe arc lamp was used as a light source with a visible light filter (20CGA-430, Newport Corporation, visible light λ ≥ 430 nm, and irradiation power = 20 mW cm−2). The light source is vertically incident on the samples, and the samples are all immobilized on Ti foil (Fig. S9†). All the experiments lasted for 8.0 h, and gas samples were withdrawn and analyzed by gas chromatography (GC-7920, Lunan Xinke) to verify H2 evolution.3. Results and discussion
3.1 Synthesis, microstructure observations, and optical property analyses
The film fabrication process, where Au NPs were deposited onto <001> oriented anatase-type TiO2 mesocrystals (meso-TiO2), is schematically illustrated in Fig. 1a. Both <001> orientated meso-TiO2 and non-orientated polycrystalline p-TiO2 rooted at the Ti foil base were fabricated using an acid vapor oxidation (AVO) strategy with Ti foil as tje titanium source under consistent identical reaction conditions but with different duration times. The formation of <001> orientated meso-TiO2 is attributed to a topotactic conversion from the hexagonal TiOF2 intermediate via acid vapor oxidation (AVO) (Fig. S1†), and the prolonged oxidation time results in the formation of a non-orientated TiO2 polycrystal (p-TiO2) as we previously reported.25 Au NPs were deposited on the top surface of the meso-TiO2 and p-TiO2 substrates via a facile photoreduction method.24 Au NPs were also loaded onto the film of the Degussa P25 polycrystal deposited on Ti foil via the same approach.The X-ray diffraction (XRD) peaks of meso-TiO2, p-TiO2, Au/meso-TiO2 and Au/p-TiO2, as shown in Fig. 1b and S2,† can be indexed to tetragonal anatase-type TiO2 (JCPDS No. 21-1272). The XRD patterns of meso-TiO2 and Au/meso-TiO2 show sharp (004) diffraction peaks. The calculated crystallographic preferred orientation (CPO) of meso-TiO2 along the <001> direction reaches nearly 100%, indicating a strong orientation along the <001> direction with large crystal size and high crystallinity.25 In contrast, the p-TiO2 in Au/p-TiO2 has no distinguishable orientation. No obvious peaks from the Au NPs were observed because of the low loading quantity of Au. The scanning electron microscope (SEM) images, transmission electron microscope (TEM) images, and STEM image of Au/meso-TiO2 in Fig. 1c and d confirmed that meso-TiO2 is assembled by highly oriented subunits that grew vertically on the Ti foil, while the corresponding selected area electron diffraction (SAED) pattern shows the single crystalline nature of meso-TiO2 that arranged along the <001> axis (Fig. 1e). The single-crystal-like structure reveals its mesocrystalline nature due to the oriented assembly of nanoflakes where the constituting crystallites are arranged along a shared crystallographic register.25 Moreover, the top-view SEM image (Fig. 1c1) and the element mapping images (Fig. 1f) reveal that Au NPs are attached to the top surface of the meso-TiO2. As a control, dispersed Au NPs are also observed in the p-TiO2 and P25 films (Fig. 1g and S3c–f†). However, the anatase-type p-TiO2 in Au/p-TiO2 and the P25 particles in Au/P25 demonstrate non-oriented polycrystalline properties, with a rough surface structure and voids between the crystals. Au/meso-TiO2, Au/p-TiO2, and Au/P25 are identified to have an identical thickness of 4–5 μm (Fig. 1c2, g2 and S3e†). The XPS spectrum (Fig. S4†) confirms that the Au NPs in Au/meso-TiO2 exist in metallic gold.
The above results collectively confirm the strong orientation, large crystal size and high crystallinity that occurs in the Au/meso-TiO2 sample, and Au/p-TiO2 and Au/P25 samples have a disordered polycrystalline structure. The size distributions of Au NPs in Fig. 1h, i and S3d† show that the size of Au NPs in Au/meso-TiO2 is ca. 9.7 nm, comparable to the Au NPs in Au/p-TiO2 and Au/P25. The specific surface area (SBET) of Au/meso-TiO2, Au/p-TiO2 and Au/P25 is 18.2, 21.8 and 56.3 m2 g−1 (Fig. S5†), respectively. The surface area of Au/meso-TiO2 was slightly lower than that of Au/p-TiO2 because there were fewer inter-crystal voids. The quantity of Au in the as-synthesized samples was revealed; the Au content was found to be 2.0 wt% in Au/meso-TiO2, identical to the 2.0 wt% in the Au/p-TiO2 sample, and similar to the 2.1 wt% in the Au/P25 sample (Fig. S6 and Table S1†). These results collectively confirm the identical size and morphology of Au NPs in these samples. Based on the comparable size distributions and amounts of Au NPs, a comparative study of the influence of the TiO2 support on the light-capturing properties of Au could be made.
Visible light transmission through the Au/TiO2 samples was calculated based on the Beer–Lambert law. The calculation model is shown in Fig. 2a and S7.† The scheme in Fig. 2a shows how the Au NPs absorb the incident light and back reflection from the TiO2-titanium foil. When visible light illuminates the Au NP layer, 80% of the incident light is transmitted through Au NPs and TiO2 to the highly reflective titanium foil. Then, the transmitted light is reflected by the foil back to Au/TiO2. The Au NPs absorb the reflected light with a small fraction of unharvested light (equal to 24% of the incident light). The absorption of the reflected light significantly increases the light-harvesting ability of Au NPs. The calculated specific proportion of light transmission through the substrate depends on the gold thickness and the value of the scatter level of TiO2 (Fig. 2b and c). It is known from Mie scattering theory that for Au NPs less than 20 nm in diameter, the smaller the Au NP diameter, the greater the fraction of incident light that is transmitted to the TiO2 support. For Au NPs which have diameters of 9.7 nm bound to Au/meso-TiO2, Au/p-TiO2 and Au/P25 samples, which are not close-packed, calculations reveal that a minimum of 82% of visible light incident on the Au NP layer can be transmitted into the substrate (Fig. 2b and c). Due to the uniformity of the oriented meso-TiO2 film consisting of vertically aligned anatase crystals, Au/meso-TiO2 can be assumed to exhibit negligible light scatter at visible wavelengths. Hence, it can be expected to permit light reflected off the substrate back to the Au NPs on the top surface. The overall light harvested by the Au NPs is thus increased, as well as the intensity of the fringing EM field around the Au NPs.26–29 In comparison, the p-TiO2 and P25 film samples with a disordered polycrystalline structure have a much higher light scatter than the oriented meso-TiO2 film. Therefore, they cannot reflect light efficiently, thus making it difficult to enhance light harvesting of Au NPs as a consequence. A similar back reflection mechanism has also been shown to be responsible for enhancing surface-enhanced Raman scattering in a sapphire substrate coated with a thin gold film.14
The light-harvesting behavior of each sample was measured by UV-vis adsorption spectra and fluorescence spectroscopy. The Au/TiO2 samples all exhibited intense light absorption in the visible light region with the absorption peak centered at 540 nm corresponding to the intrinsic localized surface plasmon resonance peak. It should be noted that the visible-light harvesting capability of the Au/meso-TiO2 sample is 1.7 times higher than that of the Au/p-TiO2 sample and 1.6 times higher than that of the Au/P25 sample (Fig. 2d), indicating significantly enhanced light harvesting by the Au/meso-TiO2 sample. The weak visible light absorption of the TiO2 samples is partially due to the defects that widen the adsorption to visible light, as reported in our previous work,25 and partially due to the light absorbance of Ti foil (Fig. S8a†). Furthermore, the clearly enhanced fluorescence emission from the RhB on the Au/TiO2 samples excited by the strong LSPR of Au NPs was also demonstrated (Fig. 2e). The strongest fluorescence emission of Au/meso-TiO2 indicates that the excitation and, thus the electromagnetic field (EM) intensity in that sample is the most intense.30 Moreover, the band potentials of meso-TiO2 and p-TiO2 were measured by Mott–Schottky measurement to compare their energy positions. As shown in Fig. S8b and c,† the same flat-band potential (equal to the CB in n-type semiconductor) for meso-TiO2 and p-TiO2 were measured at −0.32 eV vs. Ag/AgCl, while a similar, optical band gap was confirmed from the UV-vis spectra, indicating that the conduction and valence band edge positions of meso-TiO2 and p-TiO2 are similar. It is therefore safe to assume that the enhanced visible light harvesting capability and intense EM field of Au/meso-TiO2 compared to those of Au/p-TiO2 is attributed to the TiO2-effect induced efficient utilization of reflected light.
3.2 Photocatalytic activity evaluation
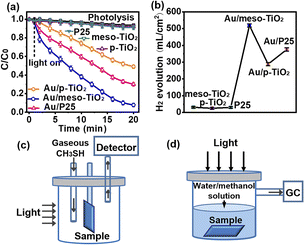
Given that Au/meso-TiO2 displays the greatest visible light absorption and intense fringing EM field, it might be expected to exhibit superior photocatalytic performance to the other two samples. The visible light photocatalytic performance of the catalysts was evaluated for two model reactions: CH3SH removal and hydrogen production from water. The samples immobilized on Ti foil were cut into 1 × 1 cm2 plates, and the light was vertically incident onto the sample (Fig. S9†). As shown in Fig. 3a, meso-TiO2, p-TiO2, and P25 appeared to have no activity when exposed to visible light, while Au/meso-TiO2 exhibited significant photocatalytic activity toward aerobic CH3SH decomposition. An enhanced CH3SH decomposition (92.2%) was demonstrated by Au/meso-TiO2 under visible light irradiation, compared to 51.0% for Au/p-TiO2 (51.0%) and 69.9% for Au/P25. The higher catalytic efficiency of Au/P25 than that of Au/p-TiO2 could be attributed to the mix-phased P25 with high charge carrier transportation and separation properties. Meanwhile, the results of CH3SH adsorption (Fig. S10†) confirm that CH3SH prefers to be adsorbed on Au NPs.Photocatalytic H2 evolution was conducted by inserting the samples at the bottom of a cylindrical quartz cell filled with a water/methanol mixture. It is worth noting that the H2 production of Au/meso-TiO2 (518.46 μL cm−2) is 1.8-fold greater than that of Au/p-TiO2 (287.99 μL cm−2), and 1.4-fold higher than that displayed by Au/P25 (375.40 μL cm−2) (Fig. 3b), while only small amounts of H2 were evolved from meso-TiO2 (30.86 μL cm−2), p-TiO2 (25.12 μL cm−2) and P25 (29.60 μL cm−2) due to their inactivity under visible light. Furthermore, the results of the cyclic experiments with Au/meso-TiO2 demonstrate its good stability toward photocatalytic CH3SH removal and H2 evolution (Fig. S11†). As shown above, the efficiency of both CH3SH removal and H2 production catalyzed by the Au/meso-TiO2 sample is higher than those displayed by Au/p-TiO2 or Au/P25 samples. Given the considerable difference in catalytic performance but the almost identical content, size and morphology of Au NPs in these samples, the superior photocatalytic performance of Au/meso-TiO2 can be attributed to support-effect induced efficient utilization of reflected light.
3.3 Mechanistic insights into enhanced photocatalytic activity
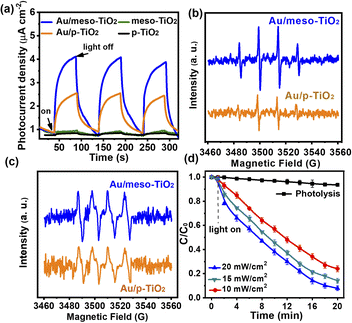
The results of reaction suppression by the chemical scavengers reveal that light-generated electron (e−), hole (h+), ˙O2−, and ˙OH are the main reactive species for photocatalytic redox reactions (Fig. S12b†).34–36 In the photocatalytic oxidation of CH3SH, the reactive oxygen species (ROSs) oxidize CH3SH into SO42− (see the IC results in Table S2†). During photocatalytic H2 evolution, the electrons transferred from Au NPs to TiO2 reduce H2O to H2, while the oxidative ROSs react with methanol (sacrificial agent) in the water/methanol mixed solution.34 As shown in Fig. 4a and S12a,† the photocurrent of Au/meso-TiO2 is 1.9 times and 1.5 times higher than that of Au/p-TiO2 and Au/P25, respectively. The photocurrent is derived from the LSPR-induced electron transfer from plasmonic Au NPs to the conduction band of TiO2.31–33The increased photocurrent of Au/meso-TiO2 reveals that the better light harvesting capability of Au/meso-TiO2 could influence the photocurrent. To identify the generation of ROSs in the samples, we conducted an electron spin resonance (ESR) study. The ESR spectra in Fig. 4b and c show a stronger signal of ˙OH and ˙O2− radicals in the Au/meso-TiO2 sample than that observed with the Au/TiO2-60 sample under identical visible light irradiation, confirming that Au/meso-TiO2 was better able to generate ROSs. As more photoexcited electrons and ROSs are generated by the Au/meso-TiO2 catalyst, deep oxidation of CH3SH and improved photocatalytic H2 evolution were achieved with this photocatalyst. Fig. 4d illustrates the photocatalytic performance of Au/meso-TiO2 for the oxidation of CH3SH at different incident light intensities. When the light intensity is raised, the CH3SH removal rate increases. Clearly, the stronger the light absorption, the higher the reaction performance. We have shown experimentally and through simulations that the utilization of reflected light can enhance the light-harvesting ability of Au NPs for efficient photocatalytic reaction. This light reflection-enhanced architecture with conductive Ti foil as a substrate also has potential for application in other fields of solar energy conversion, such as photoelectrochemistry and solar cells.37–39
4. Conclusions
In summary, this study reported a novel strategy to enhance the visible light-harvesting capability of Au NPs by depositing Au NPs on a film of vertically <001> oriented anatase mesocrystals adhered on a piece of Ti foil for improved photocatalytic performance in both S-VOC elimination and H2 evolution. This architecture enhances the visible light harvesting of Au NPs by increasing the absorption of the back-reflection of transmitted light. The enhanced light absorption increases the intensity of the fringing electromagnetic field near the Au NPs, improving the catalytic activity. Our work demonstrates that light-harvesting of plasmonic catalysts can be enhanced through a light reflection-promoted optical absorption model. This research could inspire a new paradigm to construct efficient visible light photocatalysts that are also expected to be applied to photoelectrochemistry and solar cells.Author contributions
Experiments, J. Y. and S. H.; conceptualization, H. Y. Z. and M. W.; TEM analysis, H. L.; reflection analysis, E. J. and E. R. W,; simulation, J. Q.; result analysis S. S, C. H. and S. H.; investigation, X. Y. W. and Y. C. J.; mechanism, H. Y. Z., H. L., S. S. and E. R. W.; writing, J. Y., M. W., E. R. W. and H. Y. Z.; funding acquisition, M. W. and E. R. W. All authors contributed to revision and approved the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported financially by the National Science Foundation of China (22006051, U1801251, and U1702254), the Government of Guangdong Province for industrial applications (2017B090917001), the Government of Guangzhou City for international joint-project (201704030020), and the Guangdong Basic and Applied Basic Research Foundation (2022A1515010655)We thank Prof. Chung-Yuan Mou for his help in this work.References
- A. Holm, E. D. Goodman, J. H. Stenlid, A. Aitbekova, R. Zelaya, B. T. Diroll, A. C. Johnston-Peck, K.-C. Kao, C. W. Frank, L. G. M. Pettersson and M. Cargnello, J. Am. Chem. Soc., 2020, 142, 14481–14494 CrossRef CAS PubMed.
- Y. Gao, W. Nie, Q. Zhu, X. Wang, S. Wang, F. Fan and C. Li, Angew. Chem., Int. Ed., 2020, 59, 18218 CrossRef CAS PubMed.
- D. Tsukamoto, Y. Shiraishi, Y. Sugano, S. Ichikawa, S. Tanaka and T. Hirai, J. Am. Chem. Soc., 2012, 134, 6309–6315 CrossRef CAS PubMed.
- A. Grirrane, A. Corma and H. García, Science, 2008, 322, 1661–1664 CrossRef CAS PubMed.
- A. Primo, A. Corma and H. García, Phys. Chem. Chem. Phys., 2011, 13, 886–910 RSC.
- S.-i. Naya and H. Tada, J. Catal., 2018, 364, 328–333 CrossRef CAS.
- S. Wang, Y. Gao, S. Miao, T. Liu, L. Mu, R. Li, F. Fan and C. Li, J. Am. Chem. Soc., 2017, 139, 11771–11778 CrossRef CAS PubMed.
- H. Li, S. Wang, M. Wang, Y. Gao, J. Tang, S. Zhao, H. Chi, P. Zhang, J. Qu, F. Fan and C. Li, Angew. Chem., 2022, 134, e202204272 Search PubMed.
- Y. Tian and T. Tatsuma, J. Am. Chem. Soc., 2005, 127, 7632–7637 CrossRef CAS PubMed.
- S. K. Cushing, J. Li, J. Bright, B. T. Yost, P. Zheng, A. D. Bristow and N. Wu, J. Phys. Chem. C, 2015, 119, 16239–16244 CrossRef CAS.
- M. J. Kale, T. Avanesian and P. Christopher, ACS Catal., 2014, 4, 116–128 CrossRef CAS.
- J. Li, S. K. Cushing, F. Meng, T. R. Senty, A. D. Bristow and N. Wu, Nat. Photon., 2015, 9, 601–607 CrossRef CAS.
- E. Peiris, S. Sarina, E. R. Waclawik, G. A. Ayoko, P. Han, J. Jia and H. Y. Zhu, Angew. Chem., Int. Ed., 2019, 58, 12032–12036 CrossRef CAS PubMed.
- M. W. Knight, Y. Wu, J. B. Lassiter, P. Nordlander and N. J. Halas, Nano Lett., 2009, 9, 2188–2192 CrossRef CAS PubMed.
- J. Zhang, X. Jin, P. I. Morales-Guzman, X. Yu, H. Liu, H. Zhang, L. Razzari and J. P. Claverie, ACS Nano, 2016, 10, 4496–4503 CrossRef CAS PubMed.
- Y. Kim, J. G. Smith and P. K. Jain, Nat. Chem., 2018, 10, 763–769 CrossRef CAS PubMed.
- P. Christopher and M. Moskovits, Annu. Rev. Phys. Chem., 2017, 68, 379–398 CrossRef CAS PubMed.
- U. Aslam, V. G. Rao, S. Chavez and S. Linic, Nat. Catal., 2018, 1, 656–665 CrossRef.
- C.-Y. Li, S. Duan, B.-Y. Wen, S.-B. Li, M. Kathiresan, L.-Q. Xie, S. Chen, J. R. Anema, B.-W. Mao, Y. Luo, Z.-Q. Tian and J.-F. Li, Nat. Nanotechnol., 2020, 15, 922–926 CrossRef CAS PubMed.
- Q. Tang, J. Wu, D. Kim, C. Franco, A. Terzopoulou, A. Veciana, J. Puigmartí-Luis, X. Z. Chen, B. J. Nelson and S. Pané, Adv. Funct. Mater., 2022, 32, 2202180 CrossRef CAS.
- D. Xia, W. Xu, Y. Wang, J. Yang, Y. Huang, L. Hu, C. He, D. Shu, D. Y. C. Leung and Z. Pang, Environ. Sci. Technol., 2018, 52, 13399–13409 CrossRef CAS PubMed.
- J. Yang, Q. Zhang, F. Zhang, D. Xia, H. Liu, S. Tian, L. Sun, D. Shu, C. He and S. Runa, J. Hazard. Mater., 2018, 358, 136–144 CrossRef CAS PubMed.
- E. Jin, Z. Lan, Q. Jiang, K. Geng, G. Li, X. Wang and D. Jiang, Chem, 2019, 5, 1632–1647 CAS.
- Y.-H. Lai, S.-W. Chen, M. Hayashi, Y.-J. Shiu, C.-C. Huang, W.-T. Chuang, C.-J. Su, H.-C. Jeng, J.-W. Chang, Y.-C. Lee, A.-C. Su, C.-Y. Mou and U. S. Jeng, Adv. Funct. Mater., 2014, 24, 2544–2552 CrossRef CAS.
- J. Yang, Q. Wu, S. He, J. Yan, J. Shi, J. Chen, M. Wu and X. Yang, Nanoscale, 2015, 7, 13888–13897 RSC.
- Z. Qiu, K. S. Wong, M. Wu, W. Lin and H. Xu, Appl. Phys. Lett., 2004, 84, 2739–2741 CrossRef CAS.
- T. W. van Deelen, C. Hernández Mejía and K. P. de Jong, Nat. Catal., 2019, 2, 955–970 CrossRef CAS.
- X. Liu, Y. Shi, Y. Jin, T. Tana, E. Peiris, X. Zhang, F. Xu, E. R. Waclawik, S. E. Bottle, H. Zhu and S. Sarina, Angew. Chem., 2022, 134, e202203158 Search PubMed.
- S. Atta, A. M. Pennington, F. E. Celik and L. Fabris, Chem, 2018, 4, 2140–2153 CAS.
- S. Lee, B. A. Apgar and L. W. Martin, Adv. Energy Mater., 2013, 3, 1084–1090 CrossRef CAS.
- X.-S. Li, X.-Y. Ma, J.-L. Liu, Z.-G. Sun, B. Zhu and A.-M. Zhu, Catal. Today, 2019, 337, 132–138 CrossRef CAS.
- E. Kazuma, J. Jung, H. Ueba, M. Trenary and Y. Kim, Science, 2018, 360, 521–526 CrossRef CAS PubMed.
- Y. Negrín-Montecelo, M. Testa-Anta, L. Marín-Caba, M. Pérez-Lorenzo, V. Salgueiriño, M. A. Correa-Duarte and M. Comesaña-Hermo, Nanomaterials, 2019, 9, 990 CrossRef PubMed.
- N. L. Reddy, V. N. Rao, M. Vijayakumar, R. Santhosh, S. Anandan, M. Karthik, M. V. Shankar, K. R. Reddy, N. P. Shetti, M. N. Nadagouda and T. M. Aminabhavi, Energy, 2019, 44, 10453–10472 CAS.
- K. Yang, J. Liu, R. Si, X. Chen, W. Dai and X. Fu, J. Catal., 2014, 317, 229–239 CrossRef CAS.
- J. Yang and C.-Y. Mou, Appl. Catal., B, 2018, 231, 283–291 CrossRef CAS.
- Y. Wang, J. Zhang, W. Liang, H. Yang, T. Guan, B. Zhao, Y. Sun, L. Chi and L. Jiang, CCS Chem., 2022, 4, 1153–1168 CrossRef CAS.
- P. S. Tóth, G. Szabó, G. Bencsik, G. F. Samu, K. Rajeshwar and C. Janáky, SusMat, 2022, 2, 749–760 CrossRef.
- R. Shi, L. Shang, C. Zhou, Y. Zhao and T. Zhang, Exploration, 2022, 2, 20210046 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta09982a |
| This journal is © The Royal Society of Chemistry 2023 |