MXene-decorated flexible Al2O3/TiO2 nanofibrous mats with self-adaptive stress dispersion towards multifunctional desalination†
Yunpeng
Wang
a,
Wanlin
Xu
a,
Xixi
Zou
a,
Wanlin
Fu
 a,
Xiangyu
Meng
a,
Xiangyu
Meng
 a,
Jingyi
Jiang
a,
Yiqun
Zheng
a,
Jingyi
Jiang
a,
Yiqun
Zheng
 b,
Seeram
Ramakrishna
b,
Seeram
Ramakrishna
 c,
Yueming
Sun
a and
Yunqian
Dai
c,
Yueming
Sun
a and
Yunqian
Dai
 *a
*a
aSchool of Chemistry and Chemical Engineering, Southeast University, Nanjing, Jiangsu 211189, P. R. China. E-mail: daiy@seu.edu.cn
bSchool of Chemistry, Chemical Engineering, and Materials, Jining University, Qufu, Shandong 273155, P. R. China
cNUS Centre for Nanotechnology and Sustainability (NUSCNS), National University of Singapore, 117584, Singapore
First published on 31st January 2023
Abstract
Solar-driven interfacial evaporation has shown great promise for desalination due to its high photothermal conversion efficiency. However, for interfacial evaporation, the evaporator must have superior mechanical quality and strong thermal stability. To this end, ceramic nanofibrous mats with good mechanical strength can serve as a stable substrate for desalination. Herein, with the aid of finite element analysis, flexible Al2O3/TiO2 nanofibrous mats with robust mechanical properties are fabricated, where the stress dispersion greatly facilitates the Al2O3/TiO2 nanospheres to be uniformly dispersed into the mat. The flexible nanofibrous mats exhibit a high tensile strength up to 2.18 MPa. Afterward, a two-dimensional (2D) Al2O3/TiO2/MXene mat is constructed by a simple drop-casting method. Interestingly, the evaporation rate of the Al2O3/TiO2/MXene-2 mat reached 1.43 kg m−2 h−1, and the light-to-vapor energy conversion efficiency can reach 102% at a power density of 1.0 kW m−2. To measure the desalination efficiency, five ion concentrations in the collected water were found to be well below the safe salinity levels defined by standards set by the World Health Organization and U.S Environmental Protection Agency. Utilizing basic origami technology, the three-dimensional (3D) photothermal evaporator was integrated with the 2D mat due to its flexibility and ductility. Additionally, the evaporation rate increased to 1.61 kg m−2 h−1. This study is critical in the design of composite ceramic nanofiber materials with high flexibility and photothermal conversion efficiency for multifunctional desalination.
1. Introduction
Since the developments of human civilization have resulted in significant freshwater consumption and pollution, freshwater shortage has emerged as one of the most pressing global concerns currently.1,2 Desalination has become one of the most practical approaches to obtaining fresh water from seawater, which covers 71% of the planet's surface.3,4 It is desirable to create cutting-edge water treatment technologies since water and energy present issues that are interwoven.5 Solar interfacial evaporation, capable of selectively heating the interface, is a long-term answer to the global energy and water crisis.6–8 However, undesired water pollutants, such as organic pollutants, would unavoidably evaporate and concentrate in the evaporated water, which is extremely detrimental to human health.9 In order to directly extract fresh water, it is crucial to combine efficient water purification with water evaporation. TiO2 and Al2O3 are notable examples of semiconductors that can effectively use sunshine to photodegrade organic compounds into non-toxic byproducts. TiO2 has several advantages, including hydrophilicity, and excellent thermal and chemical stability.10,11 Additionally, it has been demonstrated that building evaporators based on nanofibers could be a successful method of preventing salt crystallization.12 The nanopores in the nanofiber-based evaporators allow the salt to be transferred from the evaporation interface to the bulk water.13 An MXene, with its large surface area and high hydrophilicity, has shown great potential for photothermal conversion due to its broad absorption bandwidth of the solar spectrum.14 But in the actual desalination procedure, evaporation may cause salt crystals to accumulate, obstructing water transport channels and causing the evaporator to be damaged permanently.Long-term interfacial evaporation also requires the evaporation to show superior mechanical quality and strong thermal stability. Ceramic nanofiber materials have superior chemical and thermal stability, and nanometer-sized interstitial space.15 Once equipped with good mechanical strength, nanofibrous mats can serve as a stable substrate for desalination.16,17 However, ceramic materials generally suffer from poor mechanical properties.18,19 Therefore, extensive research has been conducted to toughen electrostatically spun ceramic nanofibers.20,21 Unfortunately, TiO2 nanofibers are inherently fragile. Previous research demonstrated that preventing nanocrystal sintering can enhance the mechanical characteristics of TiO2 nanofibers.22 However, the mechanical enhancement of a single nanofiber fails to equate to that of the entire nanofibrous mat.23 Welding nanofibers at their cross points to toughen nanofibrous mats has received much attention.24
Herein, we create a robust, flexible nanofibrous mat with high photocatalytic properties prepared using the stress dispersion method, followed by loading the MXene sheets onto the mat to desalinate and purify seawater. The resulting flexible Al2O3/TiO2 mats exhibited high flexibility with a tensile strength of 2.18 MPa. The results of finite element analysis showed that the pressure at the stress concentration of the flexible nanofibrous mat became only 8.60% that of the traditional mat. This mechanically enhanced nanofibrous mat contributes to sustain MXenes and extend useful life by acting as a skeleton. Through the wicking effect, it also offers direct hydrophilic channels for water transport as well as photocatalytic sites for in situ photodegradation of pollutants when exposed to sunlight. The two-dimensional (2D) photothermal mat and three-dimensional (3D) evaporator showed satisfactory photothermal conversion performance, with the evaporation rates reaching 1.43 and 1.61 kg m−2 h−1 under 1 sun illumination. In addition, the purified water can meet the standards of human drinking water as regulated by the World Health Organization (WHO) and U.S Environmental Protection Agency (EPA).
2. Experimental section
2.1. Materials
Polyvinylpyrrolidone (PVP, Mw ≈ 1.3 × 106), titanium isopropoxide (TTIP), and aluminum acetylacetonate (Al(acac)3) were obtained from Alfa Aesar. Ti3AlC2 was purchased from Macklin. All other chemicals were provided by Sinopharm Chemical Reagent Co., Ltd. All chemicals were used as received. The water used in all experiments was purified through a Millipore filtration system with a resistivity of 18.2 MΩ cm.2.2. Fabrication of the traditional and flexible Al2O3/TiO2 nanofibrous mats
The traditional and flexible Al2O3/TiO2 nanofibrous mats were prepared by the electrospinning–electrospraying method. The electrospinning precursor contained 0.6 g of PVP, 4.5 mL of ethanol, 1.0 g of Al(acac)3, 5.0 mL of acetone, 3.0 mL of acetic acid, and 1.4 mL of TTIP. The electrospraying precursor was prepared by mixing 2.0 mL electrospinning precursor with 6.62 mL of ethanol. The electrospinning process was conducted at a flow rate of 0.6 mL h−1, setting the voltage at 13–15 kV, and the electrospraying process was conducted at a flow rate of 0.1 mL h−1, setting the voltage at 18 kV. Subsequently, the composite nanofibrous mats were calcined at 600 °C for 2 h in air.2.3. Simulations and mechanical performance measurement of stress–strain curves and stress distributions
The mechanical tests were performed on the produced nanofibers using an electronic universal testing machine with a strain rate of 0.1 mm min−1 at room temperature with a humidity of ∼40%. For each test, rectangular samples with a 5 mm width were created. All results of the finite element analysis were simulated by COMSOL Multiphysics 5.4. To guarantee the right direction of force, the load (10 N) was specified on the local coordinate system. The effect of the mesh size was tested and convergence of the results was obtained.2.4. Fabrication of a two-dimensional (2D) photothermal mat and three-dimensional (3D) photothermal evaporator
Delaminated Ti3C2Tx MXenes were fabricated by a milder method. Typically, 1 g of LiF was added to 20 mL of HCl (9 mol L−1) solution and stirred for 20 min. Next, 1 g of Ti3AlC2 powders was gradually added to the etchant, which was stirred for 24 h in a water bath at 35 °C. Subsequently, the product was washed and separated by centrifugation at 3500 rpm until the filtrate pH was 6. The obtained sediment was sonicated for 1 h in 80 mL of deionized water, and then centrifuged at 3000 rpm for 1 h to collect the supernatant. A few layers of Ti3C2Tx MXenes were obtained.Then, the Ti3C2Tx MXene solution was drop-cast on the flexible nanofibrous mats and naturally dried at room temperature. The mass densities of Ti3C2Tx MXenes on the flexible nanofibrous mat was 0.5, 1, and 2 mg cm−2. The photothermal mats were denoted as Al2O3/TiO2/MXene-n, and n was the mass density of MXenes. Additionally, we present a portable 3D structure as a convertible photothermal evaporator that floats on water for effective solar-driven interface desalination based on 2D membranes, inspired by the adaptability of flexible nanofibers.
2.5. Multifunctional performance of the 2D photothermal mat and 3D photothermal evaporator
The 2D photothermal mats were cut to fit the size of 2 × 2 cm2. Subsequently, the nanofibrous mat was wrapped using polystyrene (PS) foam. The photothermal mat was implanted into a hole in the center of the PS foam. The entire PS foam was floated on the simulated seawater stored in a box container. A 3D photothermal evaporator was prepared from a 2D photothermal mat by origami technology. Evaporation was carried out for 1 h under the illumination of a xenon lamp (CEL-HXF300) with light densities of 1, 2, 3, and 4 kW m−2. The mass change was evaluated by using an electronic balance.An infrared thermal imaging instrument simultaneously captured the surface temperature of the photothermal mats. ICP-MS was used to measure the ion concentrations (Na+, K+, B3+, Ca2+, and Mg2+) in the simulated seawater and the condensed water. By placing the evaporation device on an angled glass container, condensed water was collected. Notably, ambient temperature and humidity for all the indoor evaporation studies were set to 25 °C and 50%, respectively.
The light-to-vapor energy conversion efficiency (η) was calculated from the following eqn (1):
 | (1) |
2.6. Characterization studies
Transmission electron microscopy (TEM) images were collected using a transmission electron microscope (Tecnai G2 T20, FEI) operated at 200 kV. Scanning electron microscopy (SEM) images were obtained using an FEI field-emission microscope (Nova Nano SEM 230). The samples were sputter-coated with Au using a high-resolution sputter coater for 120 seconds prior to SEM analysis. The crystal structure information was obtained with X-ray diffraction (Bruker, D8 advance using Cu-Kα radiation, λ = 1.5406 Å). The Brunauer–Emmett–Teller (BET) specific surface area was measured by using an Autosorb-iQ (Quantachrome, U.S.A.). The contact angles of the nanofibrous mats were measured by using a video optical contact angle measuring instrument (OSA 100). An optical power meter was used to measure the power density (S314C, THORLABS). The element contents were determined by using an inductively coupled plasma optical emission spectrometer (ICP-OES) (Optima 7300DV, PerkinElmer Corporation). The tensile experiment of the flexible nanofibrous mat was carried out by using an electronic universal testing machine (STD-500, Yishite Instruments Co., Ltd.).3. Results and discussion
3.1. Mechanical design and synthesis of a flexible nanofibrous mat based on finite element analysis
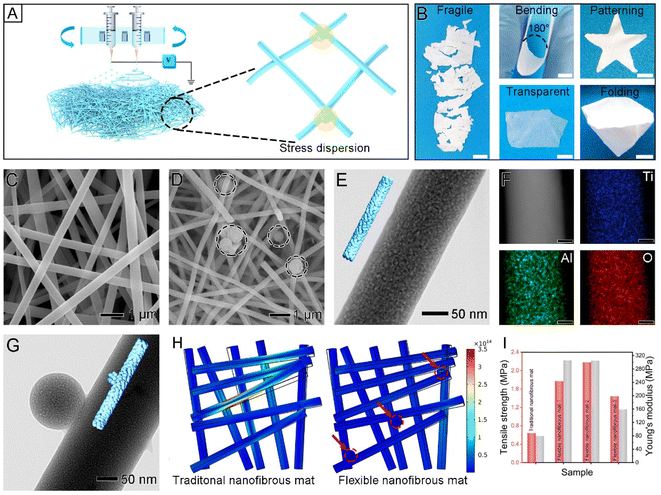
The inherent brittleness of inorganic oxide nanofibers hinders their progress toward practical applications due to stress-induced cracking.25,26 It is generally known that stress concentration further causes this component to fracture, which ultimately leads to the overall fragility. To resist the naturally brittle properties of oxide nanofibers brought about by quick crack development under stress,27 crosslinked nanospheres were designed to be located near the intersection of nanofibers. The traditional nanofibrous mat was simply superimposed, but by using doped nanospheres and the cross-linking of nanofibers, stress can be distributed and the membrane can remarkably become more flexible and robust. Based on this mechanism, a potential strengthening method for a flexible nanofiber mat was presented. When nanofibers are prepared by electrospinning, nanospheres are uniformly electro-sprayed between the nanofibers, allowing them to crosslink and assemble into a whole entity (Fig. 1A). The TiO2 nanofibrous mat is very fragile due to its poor structural integrity. In contrast, the flexible Al2O3/TiO2 nanofibrous mat can sustain its original shape without becoming fragile after being bent, folded, and wound, as schematically illustrated in Fig. 1B. Additionally, the nanofibrous mat was also transparent.The traditional Al2O3/TiO2 nanofibrous mats can be facilely fabricated by electrospinning a precursor containing PVP, ethanol, acetic acid, aluminum(III) acetylacetonate (AP), and titanium isopropoxide (TTIP), followed by calcination at 600 °C (Fig. 1C). It is obvious that the nanofibers were merely piled to form a solid structure with no welding contact. The nanospheres managed to weld the nanofibers and formed a whole mat (Fig. 1D). Moreover, the TEM image showed the porous and uniform structure of the traditional nanofibers (Fig. 1E). The element mappings of Ti, Al, and O confirmed that all these elements have a homogeneous distribution throughout the nanofiber (Fig. 1F). The Ti, Al and O atoms originated from the thermal decomposition of TTIP and AP. Under these conditions, the obtained mat was extremely easy to crack and difficult to handle. Inspiringly, after doping a small number of nanospheres with the same constituents by the electrospraying method, a flexible nanofibrous mat was easily acquired. There was an apparent nanosphere adherence on the surface of the single nanofiber in the presence of nanospheres (Fig. 1G).
Additionally, the crystalline structure of the flexible and traditional nanofibrous mats was examined using XRD. As shown in Fig. S1,† both the flexible and traditional mats showed the same structure and exclusive TiO2 diffraction peaks. No characteristic peak of crystalline Al2O3 was assigned. The diffraction peaks appearing at 25.28°, 37.80°, 48.05°, and 55.06° correspond to the (101), (004), (200), and (211) crystal planes of anatase, respectively. Both the samples exhibit an anatase TiO2 structure with no sign of an impurity phase.28 The average crystal sizes of the traditional and flexible mats can be calculated to be 13.7 nm and 13.4 nm at 600 °C using Scherrer's equation. Therefore, it is clearly shown that adding nanospheres does not affect the composition and grain size of the nanofibrous mats.
Finite element simulations were used for mechanical design at the nanoscale level to generate a flexible Al2O3/TiO2 nanofibrous mat with stable mechanical properties. In the simulation, the applied external stress was set to be 10 N (the diameter of these flexible nanofibers was optimized as 233 nm for traditional nanofibers and 272 nm for flexible nanofibers. The diameter of the nanospheres was 230 nm, the applied stress was equivalent to bearing the weight of 0.1 kg on one nanofiber, the same hereinafter) (Fig. S2 and S3†). When the traditional nanofibrous mat was stretched, the overall length increased, resulting in a reduced cross-sectional area. The rigid nanofibers were easily fragile in this circumstance because of collision and compression. In contrast, nanoparticles were involved in nanofibrous mats to further buffer external stress. The nanospheres can transfer stress effectively and there was almost no stress concentration when stretched. In the presence of nanospheres, the maximum stress on the nanofibrous mat was just 8.60% of that of the traditional nanofibrous mats (Fig. 1H). Therefore, according to the prediction of finite element analysis, the flexible nanofiber mat was more flexible in the presence of nanospheres (Fig. S4†).
The stress–strain curves of traditional and flexible Al2O3/TiO2 nanofibrous mats are exhibited in Fig. S5.† Different flexible nanofibrous mats were obtained by adjusting the electrospraying speed (0.05, 0.1, and 0.2 mL h−1), and named flexible nanofibrous mat-n. Compared with the traditional nanofibrous mat, the tensile strength of flexible nanofibrous mat-2 increased from 0.627 to 2.18 MPa, corresponding to a 248% increase, being competitive with many previously reported nano-adsorbents (Fig. 1I, and Table S1†). In addition, Young's modulus of flexible nanofibrous mat-2 was as high as 305 MPa. Therefore, in the initial stage of stretching, the nanospheres and cross-linking parts can transfer the force acting on the nanofiber to the whole skeleton, realizing the dispersion and reconciliation of mechanics, and thus enhancing the tensile properties. However, with the gradual increase in tensile strength, when the force of a single nanofiber exceeds the limiting stress of the material, microcracks will emerge and rapidly expand and thus the nanofiber will break. In this case, the integrality of the nanofibrous mat decreased, while the force effect was more obvious. When a tension of 2.18 MPa was applied, the mat was fractured instantly. These results indicated that the nanospheres between nanofibers highly reinforced the mechanical properties of nanofibrous mats.
3.2. Fabrication of a 2D photothermal mat and 3D photothermal evaporator
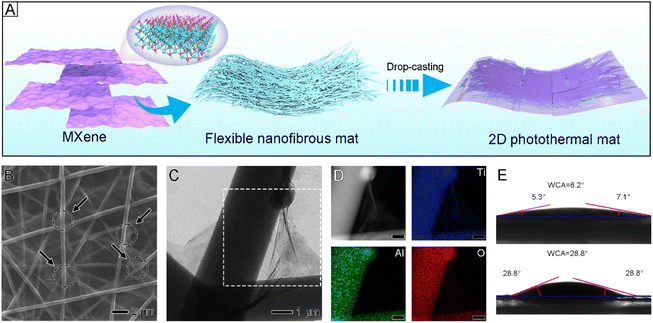
In principle, porous oxides with a larger specific surface area commonly have higher activity and better adsorption capacity, due to the maximized available sites.29,30 The corresponding adsorption–desorption isotherms of flexible Al2O3/TiO2 mats are shown in Fig. S6.† The specific surface area of the flexible nanofibrous mat was determined to be 237.6 m2 g−1, which is remarkable in nanofibrous mats. The average pore width of the flexible Al2O3/TiO2 mats was 3.37 nm, respectively. For flexible mats, the large specific surface areas and the refined mesopores can absorb a large number of water molecules.31By taking advantage of the promoted structure stability of the flexible Al2O3/TiO2 nanofibrous mat, this mat can be loaded on MXene sheets through a simple drop-casting method as a substrate (Fig. 2A and S7†). The MXene sheets, with high specific surface area and abundant oxygen-containing groups, can be bound to nanofibers easily. Therefore, the flexible Al2O3/TiO2 nanofibrous mat was covered equally by the MXene sheets and finally constructed into a double-layer photothermal mat (Fig. 2B). The elemental mappings of Ti, Al, and O also confirmed that the MXene sheets were loaded on nanofibers as shown in Fig. 2C and D. As shown in Fig. S8,† the resulting MXene sheets have ultrathin and transparent layers with a typical two-dimensional structure. Each nanosheet is sub-500 nm in lateral size. These 2D ultrathin nanosheets were produced by ultrasonic processing and corrosion, which produces reactive H˙ and ˙OH radicals. These small nanosheets make it easy to transfer mass between MXene sheets and their surroundings, including water.
Good hydrophilicity and photothermal conversion capabilities are indispensable to the use of photothermal nanofibrous mats as efficient and continuous solar evaporators.32 To measure the hydrophilicity of the flexible nanofibrous mats, the water contact angle was measured. When a water droplet was dropped on the surface of the flexible nanofibrous mats, it spread out quickly, with a contact angle of 6.2° after 0.5 seconds (Fig. 2E). This finding indicated the good hydrophilicity of the flexible nanofibrous mats, which could be attributed to Al2O3 and TiO2. After absorbing water on the surface of Al2O3, it combined with the oxygen element to generate a hydroxyl group, an oxygen-hydroxyl group was formed after dehydration, and then a hydrogen bond was formed.33 Therefore, Al2O3 has extraordinary adsorption capacity for water molecules through strong hydrogen bonds. Surface unstable bridging oxygen of TiO2 reacts with the hole, causing it to separate from the surface and generate an oxygen vacancy. Water molecules in air can absorb oxygen vacancies and break them down into OH− and H+. The holes can also react with water molecules to create active hydroxyl radicals, which are very hydrophilic and can form hydrophilic micro-zones on the surfaces.34,35 In addition, the MXene sheets had a contact angle of 28.8°, which was slightly higher than that of flexible mats. Since the double-layer structures are both hydrophilic, water may be quickly pushed through the microchannels and be used to generate steam efficiently.36
3.3. Multifunctional performance of 2D photothermal mats
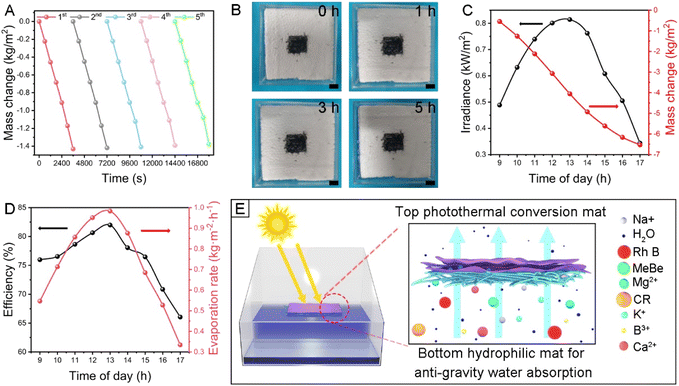
A beaker contained simulated seawater with the prepared photothermal mat floating on the top. The surface temperature of the photothermal mats increased rapidly from room temperature to 63.8 °C within 1 min and 101 °C within 5 min, respectively, under 1 sun illumination (Fig. 3A). In contrast, the temperature of simulated water remained almost unchanged, increasing by only about 33.2 °C in 60 min under 1 sun illumination (Fig. S9†). As shown in Fig. S10,† the light absorption spectra of the Al2O3/TiO2/MXene mat were obtained. The Al2O3/TiO2/MXene photothermal mat hence had an average absorption of over 79.8% in the visible to near-infrared range. The photothermal mat had a special double-layer structure that improves the photothermal performance. The thermal conductivity, measured using a thermal conductivity meter, of the Al2O3/TiO2 nanofiber mat and Al2O3/TiO2/MXene mat was 0.035 and 0.389 W m−1 K−1, respectively. The lower nanofibrous mat had excellent heat-insulating capabilities and the upper MXene layer had a strong photothermal conversion capability, so when the mat was exposed to light and the temperature increased, the heat was rarely transferred downward.The application of the mat could be promising due to this distinguished photothermal conversion capacity.37–39 Because of its exceptional specific surface area and strong hydrophilicity, the photothermal mat was able to quickly absorb water when its periphery was submerged in simulated seawater. Under 1 sun illumination, the evaporation rate of the Al2O3/TiO2/MXene-2 mat was 1.43 kg m−2 h−1 (Fig. 3B), which was significantly more rapid than the natural moisture evaporation rate (0.06 kg m−2 h−1) (Fig. S11†). And 4 sun illumination could be as high as 5.33 kg m−2 h−1. Even for the photothermal Al2O3/TiO2/MXene-0.5 mat, an evaporation rate of 0.984 kg m−2 h−1 was still achieved, and the efficiency can also reach 70.7% (Fig. 3C). As can be seen from Fig. 3D and Table S3,† the photothermal Al2O3/TiO2/MXene-2 mat had great competitiveness among many seawater desalination materials. For the photothermal mat, its exceptional solar evaporation performance promised potential applications in removing soluble contaminants. Meanwhile, sewage with contaminants like rhodamine B (Rh B), Congo red (CR), and methyl blue (MeBe) could also be purified by a similar approach.40 It was discovered that after purification, almost no organic substances could be detected (Fig. 3E). This suggested that the mat can also have a good purification effect and provide fresh water needed by the human body in extremely harsh environments. Practical applications of photothermal mats for desalination in natural light were further investigated. For desalination, salt accumulation on the evaporator surface is detrimental to both sunlight absorption and water transport.41–43 The photothermal mat was floated on the simulated seawater to simulate salt accumulation. When testing simulated seawater, the removal rates of the five main ions (Na+, K+, Ca2+, Mg2+, and B3+ ions) were all higher than 99.1% (Fig. 3F). The salinities of collected freshwater were all significantly decreased and were much lower than the standards of the WHO (1‰) and EPA (0.5‰) (Fig. 3G).
The evaporation rate of the Al2O3/TiO2 mat was 0.150, 0.284, 0.379, and 0.453 kg m−2 h−1, under 1–4 sun illuminations (Fig. S12†). The upper MXene layer is therefore primarily responsible for the mat's ability to convert light energy into heat. This photothermal conversion mechanism of MXenes was attributed to the localized surface plasmon resonance (LSPR) effect similar to that of some noble metal nanoparticles like Au and Ag NPs (Fig. 3H).44 The MXene nanosheets inherit the metallic properties of MAX ceramics and show semi-metallic properties with ultra-high metal conductivity. MXenes combine the properties of ceramics and metals because they binds carbon atoms to a metal lattice.45 MXenes have good photothermal conversion capability because of strong light absorption and the LSPR effect, which can effectively capture solar energy and transform it into thermal energy for utilization.
The salt resistance of the mat must also be taken into account in practical applications, and the mat is further evaluated for 5 h of non-stop evaporation. 99.1% of the light-to-vapor energy conversion efficiency can still be retained after 5 h of continuous testing, as illustrated in Fig. 4A, demonstrating the long-term stability of the mat and proving the great salt resistance. High-concentration ions flowed back into the bulk salt solution through highly porous water transport channels in the water mat, leaving almost no solid NaCl on the evaporated surface after 300 min (Fig. 4B). An evaporation-based desalination system was constructed to collect fresh water from simulated seawater. On a typical sunny day in Nanjing, 9:00 to 17:00 was selected for 8 h of natural illumination of the system, and the changes in ambient air temperature and relative humidity with time were recorded, as shown in Fig. S13.† The solar illumination fluctuated during the day with an average of 0.650 kW m−2 and the amount of freshwater produced through evaporation increased continuously (Fig. 4C). The corresponding hourly evaporation rate varied with the trend of solar illumination with an average energy efficiency of 82.8% (Fig. 4D). After 8 h of evaporation, the calculated amount of fresh water obtained from a photothermal mat with a surface area of 1 m2 is about 6.70 kg, which can meet the daily drinking water needs of 4–5 individuals.
A heat confinement effect was created at the interface of the upper and lower mats as a result of the lower nanofibrous mat's efficient insulation, which effectively focused the heat quantity to promote water evaporation. Because of constant water pumping from the lower mat to the upper mat, salt ions from saltwater such as Na+, Ca2+, Mg2+, K+, B3+, and Cl− will dissolve fast rather than crystallizing on the MXene mat, thereby preventing the obstruction of vapor channels and accumulating on the surface of the photothermal mat46 (Fig. 4E). The current solar evaporation technology using a 2D photothermal mat has unmatched advantages and potential for seawater desalination and wastewater treatment because it is effective, inexpensive, energy-saving, and convenient to maintain compared to other water treatment technologies like nanofiltration, reverse osmosis, and electrodialysis.47,48
Additionally, hydrogen bonds formed on the surface of Al2O3 and TiO2 might make free water less tightly bound together, requiring less energy to evaporate.49 To increase the photothermal conversion efficiency of the current 2D photothermal mat, a random array of MXene sheets outside Al2O3/TiO2 nanofibers may act as light/heat sites at the 1D level by trapping light and heat through repeated reflections. Additionally, by precisely localizing the heat on the MXene sheets that serve as the mass transfer channels inside each Al2O3/TiO2 nanofiber, it was possible to speed up the entire mass-transfer process in the 2D photothermal mat.
3.4. Multifunctional performance of the 3D photothermal evaporator
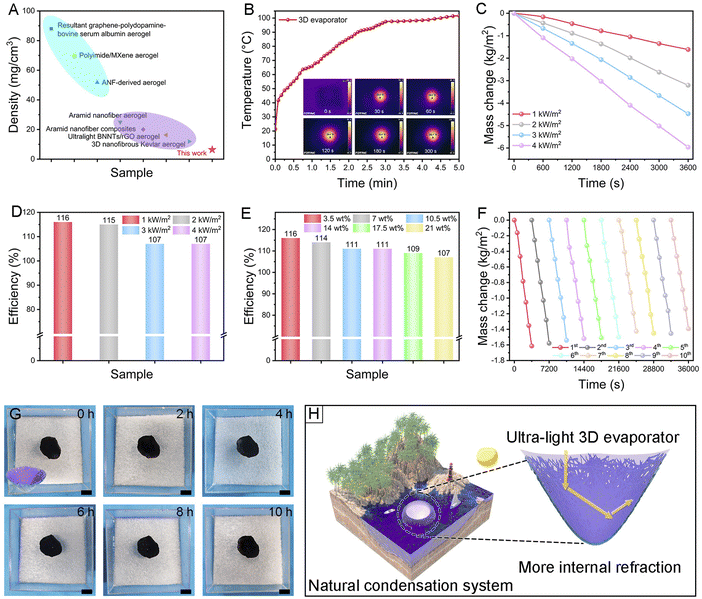
In our design, the MXene sheets absorb most of the light which illuminates the 3D evaporator. Internal reflections of the additional dispersed light onto the MXene sheets will enable it to finally absorb practically all of it. Additional scattered light would continually strike the MXene sheets, eventually being almost entirely absorbed. Utilizing basic origami technology, the 3D Al2O3/TiO2/MXene evaporator with ultralow density (6.41 mg cm−3) was simply fabricated by using the 2D Al2O3/TiO2/MXene-2 photothermal mat, which is lighter than most aerogels (Fig. 5A and Table S4†). Using an infrared thermal imaging instrument, the temperature variation of the 3D evaporator under illumination was observed. Under 1 sun illumination, the surface temperature of the 3D evaporator climbed rapidly from 21.4 °C to 65.9 °C in 1 min (Fig. 5B). Nanopore structures protected the thermal energy within Al2O3/TiO2 nanofibers from the cold seawater and offered a direct conduit for quick water wicking from the overall structure. The 3D evaporator eventually obtained a high light absorbance with a maximum value of 116% under 1 sun illumination (Fig. 5C and D). This demonstrated that the 3D evaporator effectively increased light reflection. Moreover, with the increase in salt concentration, the evaporation rate of the 3D evaporator in 7 wt%, 10.5 wt%, 14 wt%, 17.5 wt%, and 21 wt% simulated seawater remains nearly constant. At a concentration of 21 wt% salt, the evaporation rate can still reach 101% (Fig. 5E).Furthermore, the stability of solar evaporation was very important for the practical large-scale application of evaporators. Therefore, we evaluated the solar evaporation performance of the 3D evaporator during long-term use. The evaporation rates of the 3D evaporator were stable in the range of 1.61–1.39 kg m−2 h−1 for 10 h (Fig. 5F). No salt crystals were observed on the surface of the 3D evaporator even under 1 sun illumination and 10 h illumination demonstrating the superior salt-resistance (Fig. 5G). The efficient, stable evaporation and integrality of the 3D evaporator under harsh conditions could be ascribed to the chemical stability, the flexible mechanical properties, and the strong connection among nanofibers and MXene sheets. In short, benefitting from the porous structure, high-concentration brine could be transported to a low salt concentration between the MXene sheets and nanofibers. Thus, the 3D evaporator could still retain superior salt resistance even if the upper part of the evaporator is not touching the liquid level. Besides the material design, as illustrated in Fig. 5H, the evaporator can be directly used as a natural condensation system to extract freshwater from the sea. The as-mentioned results demonstrated the great potential of our nanofibrous mats for practical water desalination and purification.
4. Conclusions
In this study, a flexible Al2O3/TiO2 nanofibrous mat has been prepared by stress dispersion technology as a new skeleton for 3D evaporators with multifunctional desalination. After electrospraying nanospheres, the ceramic nanofibrous mat becomes more flexible and can be bent, folded, and wound without any trail. After mechanical tests, it was found that the tensile properties of the flexible nanofibrous mat were improved by 248% compared with the traditional nanofibrous mat. The 2D photothermal mat not only exhibited high specific surface areas and remarkable long-term chemical stability but also had good photothermal conversion ability, salt resistance, and wastewater purification ability after loading of MXenes. For the photothermal Al2O3/TiO2/MXene-2 mat, the light-to-vapor energy conversion efficiency can reach up to 102% and the evaporation rate was 1.43 kg m−2 h−1 under 1 sun illumination. After using simple origami technology, the 3D photothermal evaporator shows better evaporation efficiency. Utilizing origami technology, a 3D evaporator with an evaporation efficiency of up to 1.61 kg m−2 h−1 was created. The 3D evaporator also possessed exceptional salt resistance, allowing it to be exposed to light for more than 10 h without salt accumulation. These observations substantiated that electrospraying nanospheres provided a flexible nanofibrous mat for the production of membranes with excellent mechanical properties necessary for photothermal conversion and desalination.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2020YFC1511902, 2022YFA1505700), the National Natural Science Foundation of China (21975042), the Qinglan Project in Jiangsu, the Innovation Platform Project Supported by Jiangsu Province (6907041203), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22_0261), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Open Project of the State Key Laboratory of Physical Chemistry of Solid Surfaces in Xiamen University.References
- R. Abell and I. Harrison, Science, 2020, 370, 38–39 CrossRef CAS PubMed.
- A. Uliana, N. Bui, J. Kamcev, M. Taylor, J. Urban and J. Long, Science, 2021, 372, 296–299 CrossRef CAS PubMed.
- L. Wang, P. Zhang, K. Chen, J. Dong, F. Luo, Q. Huang, Z. Sun, X. Zou and G. Zhu, Sci. China Mater., 2022, 65, 1920–1928 CrossRef CAS.
- M. Zou, Y. Zhang, Z. Cai, C. Li, Z. Sun, C. Yu, Z. Dong, L. Wu and Y. Song, Adv. Mater., 2021, 33, 2102443 CrossRef CAS PubMed.
- J. Li, X. Wang, Z. Lin, N. Xu, X. Li, J. Liang, W. Zhao, R. Lin, B. Zhu, G. Liu, L. Zhou, S. Zhu and J. Zhu, Joule, 2020, 4, 928–937 CrossRef CAS.
- X. Liu, F. Chen, Y. Li, H. Jiang, D. Mishra, F. Yu, Z. Chen, C. Hu, Y. Chen, L. Qu and W. Zheng, Adv. Mater., 2022, 34, 2203137 CrossRef CAS PubMed.
- F. Wang, N. Xu, W. Zhao, L. Zhou, P. Zhu, X. Wang, B. Zhu and J. Zhu, Joule, 2021, 5, 1602–1612 CrossRef CAS.
- L. Chen, J. Ren, J. Gong, J. Qu and R. Niu, Chem. Eng. J., 2023, 454, 140383 CrossRef CAS.
- R. Das, V. Sypu, H. Paumo, M. Bhaumik, V. Maharaj and A. Maity, Appl. Catal., B, 2019, 244, 546–558 CrossRef CAS.
- C. Cheng, W. Fang, R. Long and O. Prezhdo, JACS Au, 2021, 1, 550–559 CrossRef CAS PubMed.
- F. Dalanta, T. Kusworo and N. Aryanti, Appl. Catal., B, 2022, 316, 121576 CrossRef CAS.
- W. Xu, X. Hu, S. Zhuang, Y. Wang, X. Li, L. Zhou, S. Zhu and J. Zhu, Adv. Energy Mater., 2018, 8, 1702884 CrossRef.
- X. Dong, L. Cao, Y. Si, B. Ding and H. Deng, Adv. Mater., 2020, 32, 1908269 CrossRef CAS PubMed.
- P. Ying, B. Ai, W. Hu, Y. Geng, L. Li, K. Sun, S. Tan, W. Zhang and M. Li, Nano Energy, 2021, 89, 106443 CrossRef CAS.
- X. Mao, J. Hong, Y. Wu, Q. Zhang, J. Liu, L. Zhao, H. Li, Y. Wang and K. Zhang, Nano Lett., 2021, 21, 9419–9425 CrossRef CAS PubMed.
- X. Li, Y. Zhang, L. Zhang, S. Xia, Y. Zhao, J. Yan, J. Yu and B. Ding, Small, 2022, 18, 2106500 CrossRef CAS PubMed.
- J. Song, X. Wu, M. Zhang, C. Liu, J. Yu, G. Sun, Y. Si and B. Ding, Chem. Eng. J., 2020, 379, 122269 CrossRef CAS.
- Y. Meng, Y. Zhu, L. Zhou, X. Meng, Y. Yang, R. Zhao, J. Xia, B. Yang, Y. Lu, H. Wu, L. Mao and S. Yu, Adv. Mater., 2022, 34, 2108267 CrossRef CAS.
- L. Li, C. Jia, Y. Liu, B. Fang, W. Zhu, X. Li, L. A. Schaefer, Z. Li, F. Zhang, X. Feng, N. Hussain, X. Xi, D. Wang, Y. Lin, X. Wei and H. Wu, Mater. Today, 2022, 54, 72–82 CrossRef CAS.
- Y. Lei, Q. Wang, S. Peng, S. Ramakrishna, D. Zhang and K. Zhou, Adv. Energy Mater., 2020, 10, 1902115 CrossRef CAS.
- M. Fu, J. Zhang, Y. Jin, Y. Zhao, S. Huang and C. Guo, Adv. Sci., 2020, 7, 2000258 CrossRef CAS PubMed.
- W. Xu, W. Fu, X. Meng, M. Tang, C. Huang, Y. Sun and Y. Dai, Nanoscale, 2021, 13, 20564–20575 RSC.
- J. Cai, C. Griesbach and R. Thevamaran, ACS Nano, 2021, 15, 19945–19955 CrossRef CAS PubMed.
- T. Wu, H. Li, J. Xue, X. Mo and Y. Xia, Angew. Chem., Int. Ed., 2019, 58, 16416–16421 CrossRef CAS PubMed.
- L. Wang, Y. Qiu, H. Lv, Y. Si, L. Liu, Q. Zhang, J. Cao, J. Yu, X. Li and B. Ding, Adv. Funct. Mater., 2019, 29, 1901407 CrossRef.
- F. Wang, L. Dou, J. Dai, Y. Li, L. Huang, Y. Si, J. Yu and B. Ding, Angew. Chem., Int. Ed., 2020, 59, 8285–8292 CrossRef CAS.
- J. Xue, T. Wu, Y. Dai and Y. Xia, Chem. Rev., 2019, 119, 5298–5415 CrossRef CAS PubMed.
- Y. Liang, S. Cao, Q. Wei, R. Zeng, J. Zhao, H. Li, W. Yu and B. Zou, Nano-Micro Lett., 2021, 13, 196 CrossRef CAS PubMed.
- H. Zhou, T. Yang, Z. Kou, L. Shen, Y. Zhao, Z. Wang, X. Wang, Z. Yang, J. Du, J. Xu, M. Chen, L. Tian, W. Guo, Q. Wang, H. Lv, W. Chen, X. Hong, J. Luo, D. He and Y. Wu, Angew. Chem., Int. Ed., 2020, 59, 20465–20469 CrossRef CAS PubMed.
- M. Zhu, C. Zhao, X. Liu, X. Wang, F. Zhou, J. Wang, Y. Hu, Y. Zhao, T. Yao, L. Yang and Y. Wu, ACS Catal., 2021, 11, 3923–3929 CrossRef CAS.
- Y. Fang, D. Luan, S. Gao and X. Lou, Angew. Chem., Int. Ed., 2021, 60, 20102–20118 CrossRef CAS PubMed.
- Y. Kuang, C. Chen, S. He, E. Hitz, Y. Wang, W. Gan, R. Mi and L. Hu, Adv. Mater., 2019, 31, 1900498 CrossRef PubMed.
- Y. Lin, C. Loh, L. Shi, Y. Fan and R. Wang, J. Membr. Sci., 2017, 539, 65–75 CrossRef CAS.
- J. Lin, J. Hu, W. Wang, K. Liu, C. Zhou, Z. Liu, S. Kong, S. Lin, Y. Deng and Z. Guo, Chem. Eng. J., 2021, 407, 125783 CrossRef CAS.
- Z. Xin, X. Zhao, H. Ji, T. Ma, H. Li, S. Zhong and Z. Shen, Chin. Chem. Lett., 2021, 32, 2151–2154 CrossRef CAS.
- R. Niu, Y. Ding, L. Hao, J. Ren, J. Gong and J. Qu, ACS Appl. Mater. Interfaces, 2022, 14, 45533–45544 CrossRef CAS PubMed.
- G. Chen, Z. Jiang, A. Li, X. Chen, Z. Ma and H. Song, J. Mater. Chem. A, 2021, 9, 16805–16813 RSC.
- Z. Fan, J. Ren, H. Bai, P. He, L. Hao, N. Liu, B. Chen, R. Niu and J. Gong, Chem. Eng. J., 2023, 451, 138534 CrossRef CAS.
- F. Meng, Z. Ding, Z. Chen, K. Wang, X. Liu, J. Li, T. Lu, X. Xu and L. Pan, J. Mater. Chem. A, 2022, 10, 9575–9581 RSC.
- P. He, H. Bai, Z. Fan, L. Hao, N. Liu, B. Chen, R. Niu and J. Gong, J. Mater. Chem. A, 2022, 10, 13378–13392 RSC.
- X. Meng, X. Peng, Y. Wei, S. Ramakrishna, Y. Sun and Y. Dai, Chem. Eng. J., 2022, 437, 135444 CrossRef CAS.
- R. Zhu, D. Wang, J. Xie, Y. Liu, M. Liu and S. Fu, Chem. Eng. J., 2022, 427, 131618 CrossRef CAS.
- Z. Li, X. Xu, X. Sheng, P. Lin, J. Tang, L. Pan, Y. Kaneti, T. Yang and Y. Yamauchi, ACS Nano, 2021, 15, 12535–12566 CrossRef CAS PubMed.
- X. Fan, L. Liu, X. Jin, W. Wang, S. Zhang and B. Tang, J. Mater. Chem. A, 2019, 7, 14319–14327 RSC.
- Z. Lei, X. Sun, S. Zhu, K. Dong, X. Liu, L. Wang, X. Zhang, L. Qu and X. Zhang, Nano-Micro Lett., 2021, 14, 10 CrossRef PubMed.
- Q. Zhang, G. Yi, Z. Fu, H. Yu, S. Chen and X. Quan, ACS Nano, 2019, 13, 13196–13207 CrossRef CAS PubMed.
- H. Luo, K. Chang, K. Bahati and G. M. Geise, J. Membr. Sci., 2019, 590, 117295 CrossRef.
- Y. Hou, Q. Wang, S. Wang, M. Wang, X. Chen and X. Hou, Chin. Chem. Lett., 2022, 33, 2155–2158 CrossRef CAS.
- D. Wang, Y. Tian and L. Jiang, Small, 2021, 17, 2100788 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta09488f |
| This journal is © The Royal Society of Chemistry 2023 |