Open Access Article
Open Access ArticleFluoroalkyl phosphonic acid radical scavengers for proton exchange membrane fuel cells†
Tanya
Agarwal
 ae,
Santosh
Adhikari
ae,
Santosh
Adhikari
 a,
Yu Seung
Kim
a,
Yu Seung
Kim
 *a,
Siddharth
Komini Babu
*a,
Siddharth
Komini Babu
 a,
Ding
Tian
b,
Chulsung
Bae
b,
Nguyet N. T.
Pham
a,
Ding
Tian
b,
Chulsung
Bae
b,
Nguyet N. T.
Pham
 c,
Seung Geol
Lee
c,
Seung Geol
Lee
 d,
Ajay K.
Prasad
e,
Suresh G.
Advani
e,
Allen
Sievert
f,
Wipula Priya
Rasika Liyanage
a,
Timothy E.
Hopkins
d,
Ajay K.
Prasad
e,
Suresh G.
Advani
e,
Allen
Sievert
f,
Wipula Priya
Rasika Liyanage
a,
Timothy E.
Hopkins
 f,
Andrew
Park
f,
Andrew
Park
 f and
Rod
Borup
*a
f and
Rod
Borup
*a
aMAP-11: Materials Synthesis & Integrated Devices, Los Alamos National Laboratory, Los Alamos, NM 87545, USA. E-mail: yskim@lanl.gov; borup@lanl.gov
bDepartment of Chemistry and Chemical Biology, Rensselaer Polytechnic Institute, Troy, NY 12180, USA
cUniversity of Science, Vietnam National University, Ho Chi Minh City, Viet Nam
dSchool of Chemical Engineering, Pusan National University, Busan, 46241, Republic of Korea
eCenter for Fuel Cells and Batteries, Department of Mechanical Engineering, University of Delaware, Newark, DE 19716, USA
fThe Chemours Company FC, LLC, Wilmington, DE 19899, USA
First published on 6th April 2023
Abstract
Radical-induced degradation of proton exchange membranes limits the durability of proton-exchange membrane fuel cells. Cerium is widely used as a radical scavenger, but the migration of cerium ions to the catalyst layer has been an unresolved issue, reducing its effectiveness over time. Here, we report phosphonic acids as a promising class of radical scavengers, showing competent radical scavenging activity compared to cerium without the migration issue. The ex situ Fenton test shows that the fluoride emission rate for Nafion membrane incorporated with fluoroalkyl phosphonic acid ranged from 0.22 to 0.37 μg F cm−2 h−1, lower than that of the cerium-incorporated Nafion™ membrane (0.39 μg F cm−2 h−1). The in situ open circuit voltage hold test confirmed that a phosphonic acid-incorporated Nafion™ membrane has a 58% lower fluoride emission rate compared to the baseline. Density functional theory calculations indicate that the activation energy of the hydroxyl radical scavenging reaction of an alkyl phosphonic acid is only 0.68 eV, suggesting an effective radical scavenging pathway.
Proton exchange membranes (PEMs) transport protons while preventing the diffusion of hydrogen and oxygen between the anode and cathode electrodes. However, the small amounts of gases that cross over through the PEM cause the generation of hydrogen peroxide at the catalyst layers. This peroxide breaks down into radicals that degrade PEMs, leading to the eventual failure of the fuel cell devices.1 The prominent sites for the attack in perfluorosulfonic acid (PFSA) membranes like that discussed in this paper include the terminal carboxylic acid groups, ether groups in the side chains, tertiary carbon atoms, and the sulfonic acid C–S bonds.2,3 Usually, a radical attack causes the polymer chain to fragment, referred to as the unzipping reaction. Unreacted carboxylic groups in PFSA react with hydroxyl radicals forming CO2 and HF. This causes the formation of the terminal CF2 unit. This unzipping proceeds ultimately reaching a side chain which causes loss of the overall side chain.
Attack on the side chain C–S is one of the most important mechanisms of PFSA degradation. Attack of hydroxyl radicals on the terminal RF–CF3–SO3H results in the formation of the RF–CF2 radical. This terminal radical then reacts with the hydroxyl radical forming RF–CF2–OH. RF–CF2–OH reacts with water and results in the subsequent release of HF which causes the formation of –COOH acid groups. Attack on the ether bonds close to the sulfonic acid is perhaps the dominant mechanism of side chain degradation followed by the attack on the tertiary carbon atoms. Attack on the ether bond results in the formation of side group RF–CF2–O radicals which react with water causing HF release and carboxylic acid end group. This further unzips the PFSA chain by the mechanism discussed above.
Cerium is widely used as a radical scavenger because of its ability to switch rapidly between oxidation states. Cerium below 0.6 wt% in the PEM reduces voltage degradation by a factor of 20 while reducing the fluoride emission rate (FER) by orders of magnitude over non-modified Nafion™.4,5 Because of the highly efficient radical scavenging activity, Toyota Motors implemented the cerium technology into their MIRAI fuel cell vehicles to improve fuel cell durability. Although they are effective radical scavengers, cerium ions tend to migrate through the PEM under fuel cell operating conditions,6,7 which reduces the radical scavenging activity over time,8 depriving the PEM of the benefits of cerium incorporation.9 There are several approaches to stabilizing cerium in the membrane. Zirconium doping reduces the dissolution rate of cerium oxide to enhance cerium stability.10,11 Graphene oxides12 and carbon nanotubes13,14 have been used for the durability enhancement of membranes incorporated with cerium. However, the additional material incorporation can cause other issues, such as mechanical property deterioration, conductivity loss, FER reduction, and processing complexity.
Several organic radical scavengers based on phenolic and quinone derivatives have been considered for stabilizing PEMs. α-Tocopherol, hydroquinone, 2,2-bipyridine, and 2,6-dimethoxy-1,4-benzoquinone are investigated as radical scavengers for sulfonated poly(arylene ether sulfone).15 Quercetin was investigated as the radical scavenger for Nafion™ and found to double the durability of the membrane.16 Ferrocyanide,14 cinnamic acid,17 and terephthalic acid18 were tested as radical scavengers for Nafion™. Recently, Alizarin was investigated as a radical scavenger and was found to aid the cerium activity, surpassing the open circuit voltage (OCV) lifetime of 450 h.19 However, the radical scavenging activity of the organic radical scavengers investigated in the literature is low compared to that of cerium.15–17 Moreover, these chemistries also suffer from problems like poor dispersive ability and show limited flexibility of incorporation in the PFSA membrane.
Phosphorus compounds such as phosphoric acids, phosphates, phosphonates, and phosphonic acids are excellent antioxidants in fire extinguishers and biological industries.20 Phosphorus compounds are also known for their exceptional stability under potential conditions of fuel cells over thousands of operating hours.21 However, to the best of our knowledge, phosphorus compounds are not investigated as radical scavengers in fuel cells or other electrochemical devices. Here, we report fluoroalkyl phosphonic acids as effective radical scavengers for PEMs using ex situ Fenton's testing and in situ OCV hold accelerated stress tests (ASTs). We show the impact of phosphonic acid radical scavengers on the properties of the membranes and investigate the migration issue. We then discuss the radical scavenging mechanisms of phosphonic acids that might explain our findings.
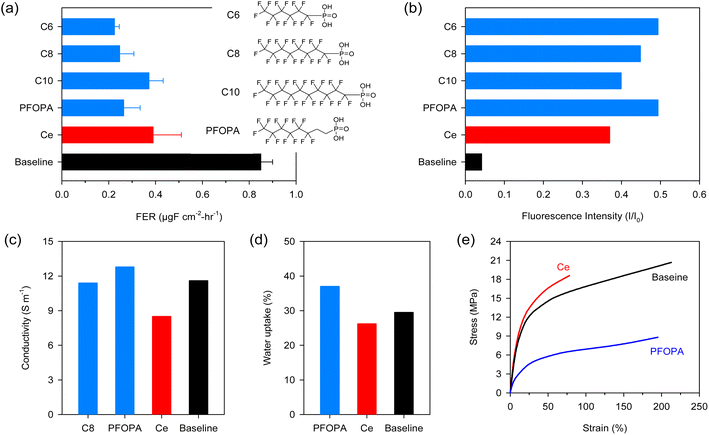
Fig. 1a compares the FER of the non-modified Nafion™ (Baseline), cerium-incorporated Nafion™ (Ce) at a loading of 5 mol%, and fluoroalkyl phosphonic acid-incorporated Nafion™ at a loading of 30 mol% in aqueous Fenton (ex situ) tests. The FER of the Baseline measured with the Fenton test was 0.85 μg F cm−2 h−1, which is significantly higher than those of Ce (0.39 μg F cm−2 h−1) and phosphonic acid-Nafion™ membranes (0.22–0.37 μg F cm−2 h−1). The decay of fluorescence intensity of 6-carboxyfluorescein dye in the presence of the Fenton reagent was monitored with and without radical scavengers to verify the high radical scavenging activity of phosphonic acids observed in the aqueous Fenton test (Fig. S1†). Fig. 1b shows the ratio of fluorescence intensity (I) after the addition of Fenton's reagent with and without scavengers to the fluorescence intensity of the un-degraded dye (I0). The decay of the dye is suppressed significantly in the presence of phosphonic acids, with all phosphonic acids showing higher retention of the intensity of the fluorescent dye (I) compared to cerium. This result confirms the fact that phosphonic acids indeed have higher radical scavenging capability compared to cerium in the presence of transition metal ions.
The radical scavenging activity of the phosphonic acids depends on the fluoroalkyl chain length. The highest radical scavenging activity was obtained with the shortest perfluoro chains (C6) and decreased with increasing the chain length. The lower radical scavenging activity of the longer phosphonic acids may be due to the dilution with non-reactive perfluoro chains. Partially fluorinated phosphonic acid with eight carbon long chain length (PFOPA, pKa = 2.3) has similar radical scavenging activity to C6 and C8 (pKa = 1.85). The insignificant effect of the pKa of the phosphonic acids on radical scavenging activity is in stark contrast with the use of phosphonic acid as an immobilizer for cerium where higher cerium retention was obtained with a phosphonic acid with higher pKa.22
While phosphonic acids show promising radical scavenging activity, it is crucial that the compromise on the other desirable properties of Nafion™, such as conductivity and mechanical strength, should be minimal. PFOPA enhanced the conductivity of Nafion™ by 10%, while comparable conductivity was observed for the C8 incorporated membrane (Fig. 1c). Since phosphonic acids are proton conductors, enhanced conductivity is not surprising. Higher conductivity with the PFOPA-Nafion™ over the C8-Nafion™ may be due to the higher hydrophilicity of PFOPA, facilitating structural diffusion of protons. The cerium incorporated Nafion™ exhibited reduced conductivity because cerium makes a fraction of sulfonic acid sites (based on loading) unavailable for proton conduction leading to a cross-linking effect.11 The PFOPA-incorporated Nafion™ membrane showed higher water uptake (Fig. 1d). This corroborated with the higher conductivity observed for these membranes.
Mechanical properties affect chemical durability and are therefore critical to investigate.22 Incorporating cerium into Nafion™ makes the membrane brittle, i.e., with a higher modulus but lower elongation (Fig. 1e). Incorporating PFOPA decreases the modulus while maintaining elongation. The strength of the PFOPA-incorporated Nafion™ is low, but the elongation at break is slightly lower than the Baseline. As a result, the tensile toughness of the cerium (5 mol%) and PFOPA (30 mol%)-incorporated Nafion™ membranes is comparable (Fig. S2†). The mechanical properties of the PFOPA-incorporated membranes are a function of the content of the phosphonic acid in the composite membrane (Fig. S3†). It appears that phosphonic acids have a plasticizing effect on the membrane with strength and modulus decreasing and elongation increasing with higher loadings of phosphonic acid.
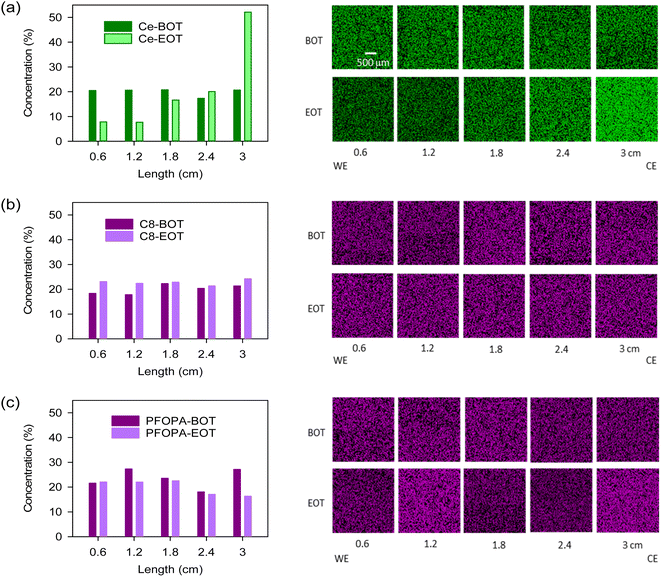
To assess the migration resistance of radical scavengers, we examined the distribution of the cerium and phosphonic acids in Nafion™ by energy-dispersive X-ray spectroscopy (EDX) after the samples were subjected to a 0.4 V potential gradient till a total of 2C charge transfer was achieved. This ex situ test well simulates the in situ migration of cerium during fuel cell operation.23 For the Ce-Nafion™ membrane, the concentration of cerium drastically increased toward the counter electrode (CE) after the migration test (Fig. 2a). The migration of cerium under potential conditions is also evident from the X-ray fluorescence (XRF) line scan (Fig. S4†), where the concentration of cerium towards the counter electrode is approximately 3 times higher than that in the part toward the working electrode (WE) at the end of the test. As opposed to cerium, C8 and PFOPA showed uniform distribution before and after the test (Fig. 2b and c). Slight redistribution occurred likely due to an initial non-uniform distribution of phosphonic acid in the as-cast membrane. This could also be due to the relatively high swelling for these membranes and the non-flat nature of the membrane when performing EDX analysis. This test was designed to study the migration of radical scavengers driven by potential gradients and mobility under high humidities and temperatures. Therefore, the minimal migration of phosphonic acids does not mean that they are resistant to migration under all conditions, such as pressure gradients and water fluxes. Nevertheless, the test shows the increased resistance of these non-water-soluble radical scavengers to migration when compared to cerium.
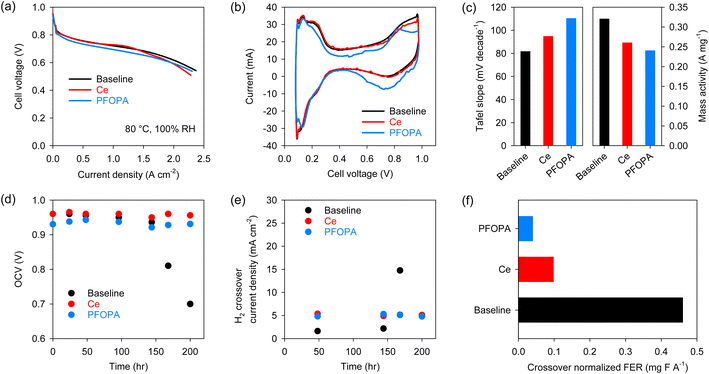
After validating the radical scavenging activity and migration resistance of the phosphonic acids, we examined the in situ performance of the PFOPA-incorporated Nafion™ using the US Department of Energy (DOE) protocols. The H2/air fuel cell performance was measured with 5 cm2 differential cells after conditioning the membranes in humidified conditions over two days. The performance of the cell using the PFOPA-incorporated membrane was lower than that of the cell using the Baseline and Ce-Nafion™ membranes (Fig. 3a). High-frequency resistance (HFR) is similar for all three membranes (∼0.054 Ω cm2) (Fig. S5†). Cyclic voltammogram (CV) data suggests that the active sites on Pt(100) were reduced for the cell using the PFOPA-incorporated membrane (Fig. 3b), causing the lower performance of the PFOPA cell.24 It is also to be noted that the capacitance for the PFOPA cell is lower. The observations from CV data are supported by the high Tafel slope and lower mass activity for the cell using the PFOPA-incorporated Nafion™ (Fig. 3c). This result indicates that phosphonic acid leaching from the membrane can affect catalyst activity, although the impact on overall performance is not substantial. It is to be noted that our conditioning protocol incorporates break-in of the cell under flooded conditions for large periods of time. This situation can aggravate the migration of immobile species. This is not the situation we simulated in the migration test and is unlikely to be experienced in an operating fuel cell.
Next, we validated the radical scavenging activity of phosphonic acids using the US DOE membrane durability AST protocol which consists of holding the fuel cell at 90 °C at 30% RH under OCV conditions.25 The Baseline cell started to fail after 175 hours in the OCV hold test, but the cells using Ce-Nafion™ and PFOPA-Nafion™ were stable for more than 200 hours without signs of failure evident (Fig. 3d). The initial H2 crossover current density of the MEAs using Ce-Nafion™ and PFOPA-Nafion™ was ∼5 mA cm−2, notably higher than that using the baseline MEA (∼1.75 mA cm−2) (Fig. 3e). The higher crossover for the PFOPA membrane could be due to the higher water uptake, unoptimized fabrication process of the MEAs and non-uniformities in the membrane casting process. Fig. 3f shows the H2 crossover normalized FER calculated from the non-normalized total FER emissions during the stable OCV operation of different membranes. The H2 crossover normalized FER of the PFOPA-Nafion™ membrane was 0.04 mg F A−1, which is 58% lower than that of the Ce-Nafion™ membrane for the same duration, confirming the stronger radical scavenging activity of phosphonic acids compared to cerium. Even with a higher crossover, the total FER for the PFOPA membrane is lower than for the Ce membrane (Fig. S6†). The FER trend from Fenton's test and OCV hold test differ considerably, corroborating the long-held belief that Fenton's test may not be an accurate predictor for membrane degradation under fuel cell operating conditions.
The use of phosphonic acids in fuel cell systems for radical scavenging is intriguing, and so is the mechanism of their action. In accordance with the density functional theory (DFT) investigation of reaction pathways for phosphoric acid in the presence of different radicals,26 three pathways are possible (Fig. 4a). Under fuel cell operating conditions, the radical species HO˙, H˙, and HOO˙ have been detected in the MEAs. The hydroxyl radical is the most aggressive intermediate. Primary sources of the hydroxyl radical (HO˙) are slow homolysis of H2O2 (H2O2 → 2HO˙) and the Fenton reaction (Fe2+ + H2O2˙ + H+ → Fe3+ + HO˙ + H2O). The hydroxyl radicals can react with phosphonic acids to produce phosphonate radicals26 (Path 1). The DFT calculations based on the Vienna ab initio simulation package (VASP)27,28 indicate that the activation energy of Path 1 is low (0.65 eV) (Fig. 4b). Hydrogen radicals (H˙) are also generated through the reaction of HO˙ with H2 (HO˙ + H2 → H˙ + H2O). Most of the HO˙ reacts with H2 to form H˙, which then rapidly reacts with O2 to form HOO˙ under fuel cell operating conditions.29 P-centered radicals can react with hydroxyl radicals regenerating phosphonic acid (Path 2–1).30 Alternatively, the P-centered radicals can combine to make phosphonic acid anhydride31 (Path 2–2). The reaction activation energy for Path 2–1 (2.46 eV) is slightly lower than that for Path 2–2 (2.68 eV). However, the activation energy for Path 2 is much higher than that for Path 1, suggesting that the radical scavenging action with phosphonic acids predominantly occurs with Path 1.
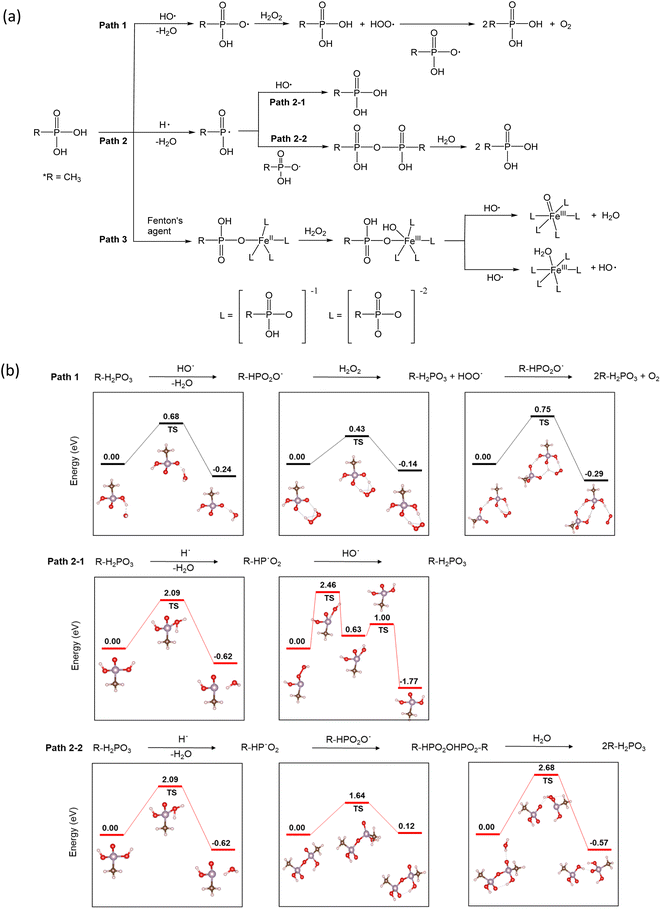 | ||
| Fig. 4 Proposed radical scavenging mechanisms of phosphonic acids: (a) three reaction pathways, (b) reaction activation energy of Path 1 and Path 2. | ||
Under the Fenton test conditions, phosphonic acid can chelate with metal ions such as Fe2+ and affect the catalytic activity of Fe towards radical formation (Path 3). Yoshimura et al.32 observed a decrease in lipid peroxidation in Fenton's solution in the presence of phosphate buffer due to a decrease in ˙OH radical formation. The spin-trapping experiments showed much reduced radical formation in phosphate buffer.33 From the DFT calculations,33 it looks like Path 3 might be a plausible reaction pathway for phosphonic acid in the presence of transition metal ions. The reaction activation energy for Path 3 is relatively low (∼0.6 eV). The highly active radical scavenging activity of alkyl phosphonic acids is consistent with our results that show a lower FER of the phosphonic acid-incorporated Nafion™ under ex situ (with Fe2+) and in situ (without Fe2+) conditions. From the calculations, it appears that when transition metals are present, Path 3 is the dominant pathway while Path 1 is the dominant mechanism for radical scavenging in the presence of hydroxyl radicals only. Path 2 is less likely to contribute to the durability enhancement observed in this study.
Conclusions and outlook
Our results show that phosphonic acids are promising radical scavengers for overcoming the challenges associated with lanthanide and other metal oxide-based radical scavenging systems.34 Chain length, molecular weight, and aromaticity might influence the radical scavenging activity of phosphonic acids. To be effective radical scavengers, phosphonic acids should be non-water soluble. Tethering phosphonic acids into polymeric materials may be desirable for enhancing stability.35 The dependence of phosphonic acid concentration on the radical scavenging efficacy and the impact on membrane properties is currently being investigated. The influence of various metal ions in the system on the radical scavenging properties of phosphonic acids is of interest, particularly for the fuel cell systems using Pt-alloy-based catalysts or Pt group metal-free catalysts as phosphonic acids can chelate with metal ions such as Fe and Co. This can indirectly contribute to the chemical durability enhancement by trapping these peroxide decomposition catalysts. The radical scavenging activity of phosphonic acids under less acidic environments such as sulfonated or quaternized hydrocarbon membranes might be different and needs further study.Author contributions
Tanya Agarwal: conceptualization, methodology, investigation, writing – original draft. Santosh Adhikari: writing – original draft, investigation, formal analysis. Yu Seung Kim: conceptualization, supervision, writing – original draft, writing – review & editing. Siddharth Komini Babu: methodology, investigation, supervision. Ding Tian: investigation. Chulsung Bae: methodology, supervision. Nguyet N. T. Pham: formal analysis, investigation. Seung Geol Lee: methodology, investigation, supervision. Ajay K. Prasad: methodology, supervision, writing – review & editing. Suresh G. Advani: methodology, supervision. Allen Sievert: formal analysis, investigation. Timothy Hopkins: formal analysis, investigation. Andrew Park: methodology, formal analysis, investigation. Rod Borup: writing – review & editing, supervision, funding acquisition.Conflicts of interest
T. A., S. A., S. K. B., Y. S. K., R. B., A. K. P., and S. G. A. filed a US patent application in October 2022, U.S. Serial No. 63/422,732, related to the fluoroalkyl phosphonic acid-incorporated membrane composition in this article. A. S., T. H, and A. P. are employed by The Chemours Company FC, LLC. The remaining authors declare no competing interests.Acknowledgements
The US Department of Energy (US DOE), Office of Energy Efficiency and Renewable Energy (EERE), and Hydrogen and Fuel Cell Technologies office (HFTO) supported this research through the M2FCT (Million Mile Fuel Cell Truck) Consortium.References
- T. H. Yu, Y. Sha, W. G. Liu, B. V. Merinov, P. Shirvanian and W. A. Goddard, J. Am. Chem. Soc., 2011, 133, 19857–19863 CrossRef CAS PubMed.
- M. Zaton, J. Roziere and D. J. Jones, Sustainable Energy Fuels, 2017, 1, 409–438 RSC.
- Z. Rui and J. Liu, Prog. Nat. Sci.: Mater. Int., 2020, 30, 732–742 CrossRef CAS.
- P. Trogadas, J. Parrondo and V. Ramani, Electrochem. Solid-State, 2008, 11, B113–B116 CrossRef CAS.
- F. D. Coms, H. Liu and J. E. Owejan, ECS Trans., 2008, 16, 1735–1747 CrossRef CAS.
- A. M. Baker, S. Komini Babu, R. Mukundan, S. G. Advani, A. K. Prasad, D. Spernjak and R. L. Borup, J. Electrochem. Soc., 2017, 164, F1272–F1278 CrossRef CAS.
- H. Matsui, S. Takao, K. Higashi, T. Kaneko, G. Samjeske, T. Uruga, M. Tada and Y. Iwasawa, ACS Appl. Mater. Interfaces, 2022, 14, 6762–6776 CrossRef CAS PubMed.
- S. M. Stewart, D. Spernjak, R. Borup, A. Datye and F. Garzon, ECS Electrochem. Lett., 2014, 3, F19–F22 CrossRef CAS.
- K. H. Wong and E. Kjeang, J. Electrochem. Soc., 2017, 164, F1179–F1186 CrossRef CAS.
- A. M. Baker, S. T. D. Williams, R. Mukundan, D. Spernjak, S. G. Advani, A. K. Prasad and R. L. Borup, J. Mater. Chem. A, 2017, 5, 15073–15079 RSC.
- J. Choi, J. H. Yeon, S. H. Yook, S. Shin, J. Y. Kim, M. Choi and S. Jang, ACS Appl. Mater. Interfaces, 2021, 13, 806–815 CrossRef CAS PubMed.
- D. C. Seo, I. Jeon, E. S. Jeong and J. Y. Jho, Polymers, 2020, 12, 1375 CrossRef CAS PubMed.
- A. M. Baker, L. Wang, W. B. Johnson, A. K. Prasad and S. G. Advani, J. Phys. Chem. C, 2014, 118, 26796–26802 CrossRef CAS.
- K. R. Yoon, J. M. Kim, K. A. Lee, C. K. Hwang, S. G. Akpe, Y. J. Lee, J. P. Singh, K. H. Chae, S. S. Jang, H. C. Ham and J. Y. Kim, J. Power Sources, 2021, 496, 229798 CrossRef CAS.
- S. H. Shin, A. Kodir, D. Shin, S. H. Park and B. Bae, Electrochim. Acta, 2019, 298, 901–909 CrossRef CAS.
- Z. Y. Rui, J. Y. Wang, J. Li, Y. F. Yao, Y. X. Huo, J. G. Liu and Z. G. Zou, J. Electrochem. Soc., 2019, 166, F3052–F3057 CrossRef CAS.
- Y. Park and D. Kim, J. Membr. Sci., 2018, 566, 1–7 CrossRef CAS.
- Y. Zhu, S. Pei, J. Tang, H. Li, L. Wang, W. Z. Yuan and Y. Zhang, J. Membr. Sci., 2013, 432, 66–72 CrossRef CAS.
- K. Xu, S. Pei, W. Zhang, Z. Han, G. Liu, X. Xu, J. Ma, Y. Zhang, F. Liu and Y. Zhang, J. Membr. Sci., 2022, 655, 120594 CrossRef CAS.
- O. L. Bouamrane, A. Hellal, K. Hachama, L. Touafri, I. Haddadi, H. Layaida, I. Kirouani, A. Hassani, M. Mersellem, A. Madani and C. Bensouici, J. Mol. Struct., 2022, 1250, 131714 CrossRef CAS.
- K. H. Lim, A. S. Lee, V. Atanasov, J. Kerres, S. Adhikari, S. Maurya, E. J. Park, L. D. Manriquez, J. Jung, C. Fujimoto, I. Matanovic, J. Jankovic, Z. Hu, H. Jia and Y. S. Kim, Nat. Energy, 2022, 7, 248–259 CrossRef CAS.
- T. Agarwal, I. Matanovic, S. Adhikari, E. J. Park, S. Komini Babu, Y. S. Kim, D. Tian, C. Bae, O. Morales-Collazo, J. F. Brennecke, A. K. Prasad, S. G. Advani, A. Sievert, T. Hopkins, A. Park and R. Borup, J. Power Sources, 2022, 554, 232320 CrossRef.
- V. M. Ehlinger, A. Kusoglu and A. Z. Weber, J. Electrochem. Soc., 2019, 166, F3255–F3267 CrossRef CAS.
- E. Westsson, S. Picken and G. Koper, Front. Chem., 2020, 8, 163 CrossRef CAS PubMed.
- T. J. Schmidt, ECS Trans., 2006, 1, 19 CrossRef CAS.
- H. C. Li, M. Hua, X. H. Pan, S. C. Li, X. X. Guo, H. Zhang and J. C. Jiang, J. Mol. Model., 2019, 25, 255 CrossRef PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS PubMed.
- M. Danilczuk, F. D. Coms and S. Schlick, J. Phys. Chem. B, 2009, 113, 8031–8042 CrossRef CAS PubMed.
- L. Gubler, S. M. Dockheer and W. H. Koppenol, ECS Trans., 2011, 41(11), 1431–1439 CrossRef CAS.
- C. C. Xia, H. H. Geng, X. B. Li, Y. Y. Zhang, F. Wang, X. W. Tang, R. E. Blake, H. Li, S. J. Chang and C. Yu, RSC Adv., 2019, 9, 31325–31332 RSC.
- Y. Yoshimura, Y. Matsuzaki, T. Watanabe, K. Uchiyama, K. Ohsawa and K. Imaeda, J. Clin. Biochem. Nutr., 1992, 13, 147–154 CrossRef CAS.
- H. Y. Chen, ACS Omega, 2019, 4, 14105–14113 CrossRef CAS PubMed.
- T. Kwon, Y. Lim, J. Cho, R. Lawler, B. J. Min, W. A. Goddard III, S. S. Jang and J. Y. Kim, Mater. Today, 2022, 58, 135–163 CrossRef CAS.
- J. Wang, Y. Dai, R. Y. Wan, W. Wei, S. C. Xu, F. H. Zhai and R. H. He, Chem. Eng. J., 2021, 413, 127541 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta09421e |
| This journal is © The Royal Society of Chemistry 2023 |