Controlling the density of storable charge of surface faradaic layers on Fe2O3 photoanodes for solar rechargeable devices†
Dongjian
Jiang
a,
Xiao
Sun
a,
Mengfan
Xue
b,
Pin
Wang
b,
Yingfang
Yao
 a,
Wenjun
Luo
a,
Wenjun
Luo
 *a and
Zhigang
Zou
ab
*a and
Zhigang
Zou
ab
aNational Laboratory of Solid State Microstructures, College of Engineering and Applied Sciences, Nanjing University, Nanjing 210093, China. E-mail: wjluo@nju.edu.cn
bEco-materials and Renewable Energy Research Center (ERERC), Jiangsu Key Laboratory for Nano Technology, National Laboratory of Solid State Microstructures and Department of Physics, Nanjing University, Nanjing 210093, China
First published on 24th January 2023
Abstract
Interface charge transfer plays a key role in a photoelectrochemical cell. Recently, a faradaic junction transfer model was proposed that describes the interface charge transfer process. Electrochemical potential is introduced as a thermodynamic descriptor for the structure and composition of the surface faradaic layer of a semiconductor. However, the kinetic process in the faradaic junction model remains unclear. Herein, we introduce a descriptor, the density of storable charge (DOSC), to describe the number of charges that can be stored in a surface faradaic layer at different applied potentials. Moreover, the DOSC of the faradaic layer on the surface of Fe2O3 was modified by Ti doping, and the results suggest that a larger DOSC leads to higher transient photocurrent in a solar rechargeable device, which is helpful for designing other high-performance devices for solar conversion and storage.
Introduction
Photoelectrochemistry has been widely studied over the past few decades for the conversion and storage of solar energy.1–3 The semiconductor/electrolyte interface plays a key role in the performance of a photoelectrochemical cell.4–6 Three models have been proposed to understand charge transfer at the semiconductor/electrolyte interface: the direct model, the surface states model, and the faradaic junction model.6–8 Different from the direct transfer model, the surface states and the faradaic junction models consider photo-generated carriers that are initially trapped by the surface of a semiconductor and then transferred into the electrolyte.9,10 In the surface states model, no new substance is produced in the surface layer and there is no change in the chemical composition, while a new substance is produced and the chemical composition of the surface layer changes with applied potentials in the faradaic junction model. The composition of a surface faradaic layer depends on potential sensitivity, which can be described by electrochemical potential.9 However, an electrochemical potential is only a thermodynamic parameter, and the kinetic process in the faradaic junction model remains unclear.The density of surface states (DOSS), the number of different electronic states allowed to occupy a particular surface energy level, is introduced to quantitatively describe the kinetics process of interface charge transfer in the surface states model. The values are usually measured by electrochemical impedance spectroscopy (EIS),5,11–13 cyclic voltammetry,14,15 and chronopotentiometry.16,17 However, in the faradaic junction model, the carriers are coupled electrons and ions. The rate of ion transfer is much lower than that of electron transfer, which is the rate-determining step during the interface charge transfer. Therefore, the DOSS in the surface states model is not suitable for describing the number of charges (electrons and ions) that can be stored in the surface faradaic layer. To understand the kinetic process of interface charge transfer in a faradaic junction, it is necessary to introduce a parameter to describe the number of charges that can be stored in a surface faradaic layer at different applied potentials.
Herein, we used Fe2O3 as a model semiconductor and experimentally determined that the same faradaic reactions occur on the surface of Fe2O3 in the dark and under illumination. According to Faraday's law, the product amount of the surface faradaic reaction is proportional to the number of charges through an external circuit. Accordingly, we introduce a descriptor, the density of storable charge (DOSC), to quantitatively describe the number of charges (electrons and ions) that can be stored in the surface faradaic layer at different applied potentials. Moreover, we also modified the DOSC of a Fe2O3 semiconductor by Ti doping to investigate the effect of DOSC on the performance of a solar rechargeable device based on a Fe2O3/NiCoOxHy faradaic junction. The results suggest that a larger DOSC for Fe2O3 enables higher transient photocurrent in the device, which can offer guidance for designing other high-performance solar conversion and storage devices.
Results and discussion
Adjusting the surface composition of a Fe2O3 semiconductor by Ti doping
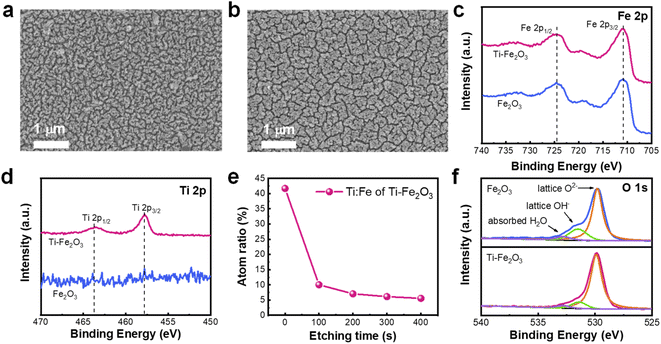
Pristine Fe2O3 and Ti-doped hematite (Ti–Fe2O3) were prepared on fluorine-doped tin oxide (FTO) by a hydrothermal method at 100 °C for 1 h and annealed at 800 °C for 5 min, following a method from a previous study.18Fig. 1a and b shows the surface SEM images of Fe2O3 and Ti–Fe2O3, respectively. The morphology of Fe2O3 and Ti–Fe2O3 is that of a rod-like nanostructure, with an average length of 100 nm. XRD and Raman spectra results suggest that the effect of Ti doping on the bulk structure of Fe2O3 (Fig. S1†) is negligible. Moreover, the UV-vis spectra of the samples did not obviously change after Ti doping (Fig. S2†). According to previous studies,19,20 Ti doping can be used to remarkably adjust the surface composition of Fe2O3. Therefore, the surface compositions of Fe2O3 and Ti–Fe2O3 were investigated by XPS, and the results are shown in Fig. 1c–f. Only Fe3+ was observed on the surface of Fe2O3, while Ti4+ and Fe3+ were present on the surface of Ti–Fe2O3. Moreover, XPS depth analysis (Fig. 1e) indicated that the atomic ratio of Ti to Fe was nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 on the surface of Ti–Fe2O3 and sharply decreased in the bulk, suggesting that Ti4+ ions are mainly located on the surface of Ti–Fe2O3.
2 on the surface of Ti–Fe2O3 and sharply decreased in the bulk, suggesting that Ti4+ ions are mainly located on the surface of Ti–Fe2O3.
These results are in satisfactory agreement with those of previous studies.21,22 Moreover, in previous studies, the concentration of surface OH− played a key role in the charge transfer at the semiconductor/liquid interface.6,23,24 Therefore, we also used XPS to investigate the change in the surface OH− of Fe2O3 after Ti doping (Fig. 1f). Three characteristic peaks at 529.8 eV, 531.4 eV, and 532.8 eV were assigned to lattice O2−, lattice OH−, and adsorbed H2O molecules, respectively.6,25 By calculating the area of characteristic peaks, the ratio of lattice OH− to lattice O2− significantly decreased from 0.30 to 0.13 for the surface of Ti–Fe2O3 (Fig. S3†). These results suggest that the doping of Ti4+ decreases the concentration of lattice OH− on the surface of Fe2O3, which occurs due to the higher activation energy barriers for hydroxylation as well as the reduced adsorption energy of OH− on the superficial Ti4+ sites.26,27 Therefore, the surface of Fe2O3 is covered with hydroxylated FeOx(OH)3−2x, while Ti doping decreases the ratio of lattice OH−/lattice O2− on the surface of FeOx(OH)3−2x of Fe2O3.
The same faradaic reaction on the surfaces of Fe2O3 and Ti–Fe2O3 in the dark and under illumination
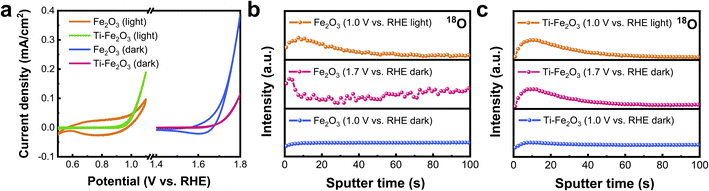
To investigate the effect of the ratio of lattice OH−/lattice O2− on the (photo)electrochemical properties of Fe2O3, CV curves for Fe2O3 and Ti–Fe2O3 were measured in the dark and under illumination, and the results are shown in Fig. 2a. In the dark, an obvious hysteresis is observed in the CV curve for Fe2O3, which is related to the redox reaction of FeOx(OH)3−2x on the surface of electrodes.28,29 The area of hysteresis remarkably decreased in the CV curve for Ti–Fe2O3, which is consistent with previous work.30 Moreover, the electrochemically active surface area of Fe2O3 did not change after Ti doping (Fig. S4†). Therefore, the smaller hysteresis comes from the lower amount of surface FeOx(OH)3−2x of Ti–Fe2O3. Under illumination, larger hysteresis and smaller hysteresis were also observed in the CV curves for Fe2O3 and Ti–Fe2O3, respectively. These results suggest that a similar redox reaction occurs on the surface of semiconductors in the dark and under illumination. Although in situ FT-IR and Mössbauer spectra have been used to observe high valent state Fe4+ at the potentials with hysteresis in previous studies,31,32 the details of the redox reaction remain unclear.Time-of-flight secondary-ion mass spectrometry (TOF-SIMS) was then used to investigate the interface ion transfer process by 18O isotopic labeling in the electrolyte.9,33Fig. 2b and c indicates the 18O depth profiles for Fe2O3 and Ti–Fe2O3 after I–t measurement at different potentials in the dark and under illumination, respectively. Negligible current was observed in Fe2O3 at 1.0 V vs. RHE in the dark (Fig. S5a†). The intensity of 18O remained unchanged at different depths, and no obvious 18O distribution was observed on the surface of the sample (Fig. 2b). In contrast, 18O was distributed on the surface of Fe2O3 after I–t measurement at the potential of 1.7 V vs. RHE in the dark or at the potential of 1.0 V vs. RHE under illumination.
The results suggest that the 18O in the electrolyte diffuse into the surface FeOx(OH)3−2x on Fe2O3 at 1.7 V in the dark or 1.0 V vs. RHE under illumination by a faradaic reaction: Fe3+Ox(OH)3−2x + h+ + OH− ↔ Fe4+Ox(OH)4−2x. The holes come from FTO in the dark and Fe2O3 under illumination, respectively. The same faradaic reaction also occurs on the surface of Ti–Fe2O3 after I–t measurement at the potential of 1.7 V vs. RHE in the dark or at the potential of 1.0 V vs. RHE under illumination (Fig. 2c). Therefore, the faradaic reactions on the surfaces of Fe2O3 and Ti–Fe2O3 are the same in the dark and under illumination, and the smaller hysteresis loop in the CV curve of Ti–Fe2O3 occurs due to the lower amount of surface FeOx(OH)3−2x after Ti doping.
The measurement and calculation of DOSC of an intrinsic faradaic layer on a semiconductor surface in the dark
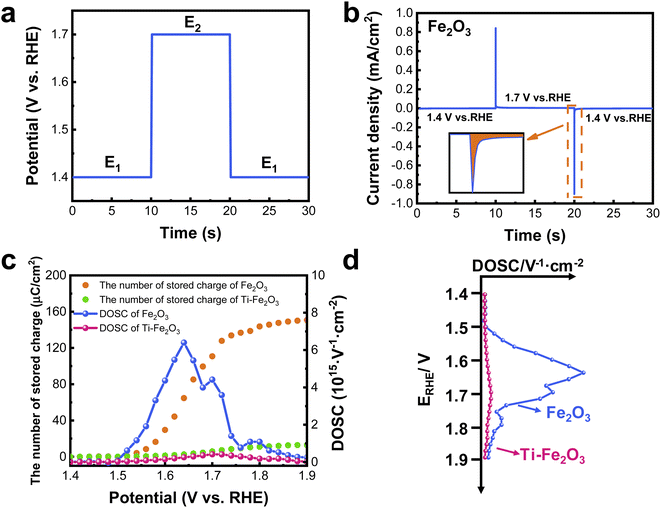
An electrochemical method for double potential step chronoamperometry in the dark was performed according to a previous study,16 and the results are shown in Fig. 3a and b. From Fig. S6,† the onset potential for the faradaic reaction of the surface faradaic layer is approximately 1.4 V vs. RHE. Accordingly, the initial potential of E1 was set at 1.4 V vs. RHE for 10 s (Fig. 3a), and negligible oxidation current was obtained (Fig. 3b). Therefore, there was no faradaic reaction at this potential in the dark. Subsequently, a higher potential of E2 (1.7 V vs. RHE) was applied for an additional 10 s, and an anodic transient current was observed (Fig. 3b) that originated from the oxidation of the surface faradaic layer in the dark.During the oxidation process, the potential of the surface faradaic layer positively shifted. When the potential was the same as the applied potential of E2, the anodic transient current disappeared. When the applied potential returned to the initial lower potential of E1 (Fig. 3a), the oxidized surface faradaic layer was reduced, and the cathodic transient current appeared. After all of the oxidized faradaic layer was reduced, the cathodic current was negligible. Therefore, the amount of oxidized surface faradaic layer (the number of charges stored in the surface faradaic layer) at E2 (1.7 V vs. RHE) can be calculated by integrating the anodic transient current or cathodic transient current (inset in Fig. 3b).
The number of stored charges in the surface faradaic layer on Fe2O3 is 468 μC cm−2 calculated from the anodic transient current, and 117 μC cm−2 calculated from the cathodic transient current (Fig. S7†). Here, the geometric area of the electrode was used to calculate the number of charges that can be stored in the surface faradaic layer because it is difficult to measure the actual area of the nanostructured electrode. The higher value calculated from the anodic transient current is due to the additional current of water oxidation in the anodic current.15,34 Therefore, the number of stored charges in the surface faradaic layer can be more accurately measured by integrating the cathodic transient current.
Using the same method, the number of stored charges in the surface faradaic layer of Fe2O3 and Ti–Fe2O3 was measured at different potentials from 1.4 V vs. RHE to 1.9 V vs. RHE in the dark (Fig. S8†), and the results are shown in Fig. 3c. The number of stored charges in the surface faradaic layer increased at 1.4 V vs. RHE and was then saturated at approximately 1.9 V vs. RHE. After considering the contribution of double layer charging to the stored charge (Fig. S9†), the maximal number of stored charge for Fe2O3 and Ti–Fe2O3 was 152 μC cm−2 and 12 μC cm−2, respectively. The number of stored charges in the surface faradaic layer of two samples under illumination indicate a value similar to that in the dark (Fig. S10†), which further confirms that the same faradaic reaction occurred under illumination and in the dark. Therefore, the number of stored charges in the surface faradaic layer of Fe2O3 decreased to 8% after Ti doping.
Once the numbers of stored charges in the surface faradaic layer of two samples at different potentials are obtained, the DOSC can be calculated by differentiating the number of stored charges with respect to the electrochemical potentials. The DOSC values in the surface faradaic layer of Fe2O3 and Ti–Fe2O3 are shown in Fig. 3. When the potential is higher than 1.5 V vs. RHE, the DOSC of Fe2O3 sharply increases, and the maximal value of 6.4 × 1015 V−1 cm−2 was obtained at approximately 1.65 V vs. RHE and then decreased to zero at the potential of 1.9 V vs. RHE. Similar to the amount of stored charge, there was a much smaller DOSC for Ti–Fe2O3 as compared to Fe2O3. The maximal DOSC for Ti–Fe2O3 was 4.1 × 1014 V−1 cm−2 at approximately 1.7 V vs. RHE. Like the DOSS in the energy band diagram of surface states of a semiconductor, a graph of DOSC vs. applied potential is thus plotted in Fig. 3d.
The effects of DOSC of the surface faradaic layer on the transient photocurrent under illumination
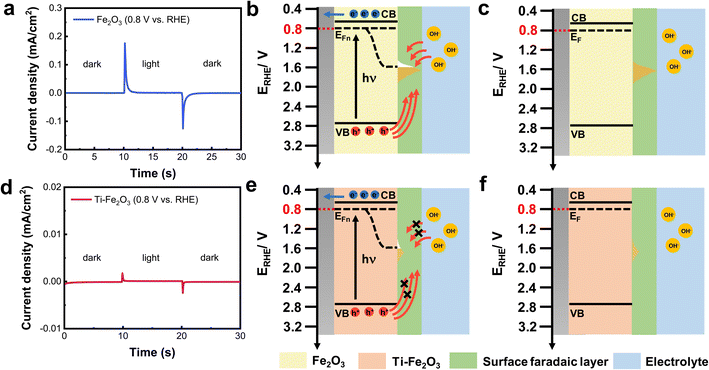
To investigate the effect of DOSC for interface charge transfer kinetics under illumination, I–t curves of Fe2O3 and Ti–Fe2O3 were measured at different applied potentials under chopped light, and the results are shown in Fig. 4 and S11,† respectively. Using 0.8 V vs. RHE as an example, in the I–t curve for the initial 10 seconds, the current of Fe2O3 was negligible in the dark (Fig. 4a). When the light was on, transient photocurrent of 0.18 mA cm−2 was observed in Fe2O3, which decayed to zero in 3 seconds. When the light was off, a reverse transient dark current of 0.13 mA cm−2 appeared, which also decayed to zero in 3 seconds. The I–t curve under chopped light is similar to the I–t curve with the double potential step method in the dark (Fig. 3b).The results suggest that the transient photocurrent comes from the faradaic reaction: Fe3+Ox(OH)3−2x + h+ + OH− ↔ Fe4+Ox(OH)4−2x on the surface of Fe2O3. To explain the origin of the transient current under illumination and in the dark, the band positions and DOSC of the surface of Fe2O3 are illustrated in Fig. 4b and c. In our previous study, when the light was on, reduction and oxidation faradaic layers of TiO2 were observed on the surface of TiO2 in photocatalysis.9 However, in photoelectrocatalysis, only the oxidation of the faradaic layer plays a key role during interface charge transfer when an applied potential is more positive than the potential window of a reduction faradaic layer.
In this study, the applied potential was at 0.8 V vs. RHE, which is more positive than the potential window of the reduction faradaic layer of Fe2O3 (0.25–0.6 V vs. RHE).9 Therefore, only the oxidation faradaic layer of Fe2O3 was plotted in Fig. 4. At the potential of 0.8 V vs. RHE, the Fermi level of Fe2O3 was adjusted at this applied potential. When Fe2O3 was illuminated, 800 mV of photovoltage was measured (Fig. S12†), which led to the quasi-Fermi level of holes at approximately 1.6 V vs. RHE.35 Therefore, the surface faradaic layer is photocharged by Fe3+Ox(OH)3−2x + h+ + OH− ↔ Fe4+Ox(OH)4−2x until the potential of the faradaic layer is equal to the quasi-Fermi level of the holes (Fig. 4b). When the light is off, the partially oxidized faradaic layer is filled with electrons from the substrate and ions from the electrolyte, which leads to reverse transient dark current (Fig. 4c).
The number of charges during dark discharge is the same as that during the photo charge process, which suggests that there is satisfactory reversibility to the faradaic reaction. In contrast, much lower transient photocurrent and dark current were observed in Ti–Fe2O3 (Fig. 4d), which resulted from the much smaller DOSC of the faradaic layer (Fig. 4e–f). Therefore, a larger DOSC of the surface faradaic layer leads to higher transient photocurrent and dark current.
Enhanced performance of solar rechargeable devices by controlling the DOSC of a semiconductor surface
In previous studies,36–38 a higher transient charging photocurrent was used to improve the performance of a solar rechargeable device. Accordingly, Fe2O3 and Ti–Fe2O3 were used as light absorbers and were coated with NiCoOxHy energy storage material and connected with carbon counter electrodes to construct a solar rechargeable device (Fig. 5a). Fig. 5b indicates I–t curves of the two devices at zero bias in the dark and under illumination. The Fe2O3/NiCoOxHy/KOH(aq)/carbon device indicates a transient photocurrent of 0.95 mA cm−2 when the light is on, which decays to a negligible value within dozens of seconds.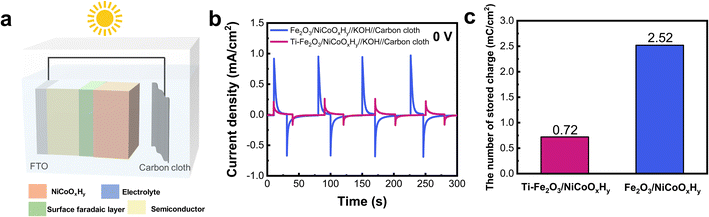 | ||
| Fig. 5 (a) Configuration of a two-electrode solar rechargeable device. (b) Current–time curves of two devices at zero bias. (c) Charge density of two devices during the photo-charging process. | ||
Under illumination, the photo-generated holes, which are initially stored in the surface faradaic layer of Fe2O3, subsequently transfer to the NiCoOxHy and participate in the faradaic reaction of NiCoOxHy39 (Fig. S13†), leading to a higher photocurrent and longer charge time. When the light is off, transient dark current of 0.69 mA cm−2 was obtained. In contrast, the Ti–Fe2O3/NiCoOxHy/KOH(aq)/carbon device showed a transient photocurrent of 0.26 mA cm−2 and transient dark current of 0.17 mA cm−2, which are both much lower than those for the Fe2O3/NiCoOxHy/KOH(aq)/carbon device. The numbers of stored charges in the two devices were calculated and are indicated in Fig. 5c. The number of stored charges in Fe2O3/NiCoOxHy/KOH(aq)/carbon was 2.52 mC cm−2, which was much higher than the value of 0.72 mC cm−2 for Ti–Fe2O3/NiCoOxHy/KOH(aq)/carbon. Moreover, the Fe2O3/NiCoOxHy/KOH(aq)/carbon also exhibited higher photovoltage and satisfactory cycle stability (Fig. S14†).
To exclude the effect of electrodeposited NiCoOxHy on the performance of the devices, we characterized the NiCoOxHy on Fe2O3 and Ti–Fe2O3 by inductively coupled plasma mass spectroscopy (ICP), XPS, and Raman methods (Table S1† and Fig. S15†). The results suggest that the loading amount, chemical composition, and crystal structure of NiCoOxHy are the same on the two samples. Therefore, the higher performance of the Fe2O3/NiCoOxHy/KOH(aq)/carbon solar rechargeable device is derived from the larger DOSC of the surface faradaic layers on Fe2O3.
Conclusions
Electrochemical and TOF-SIMS methods revealed that the same faradaic reactions were observed on the surface of Fe2O3 and Ti–Fe2O3 semiconductors in the dark and under illumination. To describe the number of charges (electrons and ions) that can be stored in the surface faradaic layer of a semiconductor, the DOSC was introduced and was measured by a double potential step chronoamperometry method. Moreover, the DOSC of the faradaic layer on the Fe2O3 surface was modified by Ti doping, which leads to inferior photocharge current for a solar rechargeable device based on a Ti–Fe2O3/NiCoOxHy faradaic junction. The results suggest that the DOSC is an important parameter for understanding the charge transfer kinetic process in the faradaic junction interface.Author contributions
W. L. supervised the project, proposed the concept, and designed the experiments. D. J. carried out sample preparation, characterization, and electrochemistry measurements. W. L. and D. J. analyzed the data and wrote the paper. All authors discussed the results and provided comments on the manuscript.Conflicts of interest
None declared.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22279052).References
- Y. He, T. Hamann and D. Wang, Chem. Soc. Rev., 2019, 48, 2182–2215 RSC.
- J. Zhang, J. Cui and S. Eslava, Adv. Energy Mater., 2021, 11, 2003111 CrossRef CAS.
- Q. Zeng, Y. Lai, L. Jiang, F. Liu, X. Hao, L. Wang and M. A. Green, Adv. Energy Mater., 2020, 10, 1903930 CrossRef CAS.
- S. Corby, R. R. Rao, L. Steier and J. R. Durrant, Nat. Rev. Mater., 2021, 6, 1136–1155 CrossRef CAS.
- P. Y. Tang, L. J. Han, F. S. Hegner, P. Paciok, M. Biset-Peiró, H. C. Du, X. K. Wei, L. Jin, H. B. Xie, Q. Shi, T. Andreu, M. Lira-Cantú, M. Heggen, R. E. Dunin-Borkowski, N. López, J. R. Galán-Mascarós, J. R. Morante and J. Arbiol, Adv. Energy Mater., 2019, 9, 1901836 CrossRef.
- Z. Yin, X. Chen, C. Wang, Z. Guo, X. Wu, Z. Zhao, Y. Yao, W. Luo and Z. Zou, Chem. Sci., 2020, 11, 6297–6304 RSC.
- Q. Wang and K. Domen, Chem. Rev., 2020, 120, 919–985 CrossRef CAS PubMed.
- J. Li, W. Wan, C. A. Triana, H. Chen, Y. Zhao, C. K. Mavrokefalos and G. R. Patzke, Nat. Commun., 2021, 12, 255 CrossRef CAS PubMed.
- M. Xue, Z. Chu, D. Jiang, H. Dong, P. Wang, G. Sun, Y. Yao, W. Luo and Z. Zou, Natl. Sci. Rev., 2022 DOI:10.1093/nsr/nwac249.
- K. George, T. Khachatrjan, M. van Berkel, V. Sinha and A. Bieberle-Hütter, ACS Catal., 2020, 10, 14649–14660 CrossRef CAS.
- B. Klahr and T. Hamann, J. Phys. Chem. C, 2014, 118, 10393–10399 CrossRef CAS.
- B. Klahr, S. Gimenez, F. Fabregat-Santiago, T. Hamann and J. Bisquert, J. Am. Chem. Soc., 2012, 134, 4294–4302 CrossRef CAS PubMed.
- Q. Shi, S. Murcia-López, P. Tang, C. Flox, J. R. Morante, Z. Bian, H. Wang and T. Andreu, ACS Catal., 2018, 8, 3331–3342 CrossRef CAS.
- J. M. Jimenez, G. R. Bourret, T. Berger and K. P. McKenna, J. Am. Chem. Soc., 2016, 138, 15956–15964 CrossRef CAS PubMed.
- B. Klahr, S. Gimenez, F. Fabregat-Santiago, J. Bisquert and T. W. Hamann, Energy Environ. Sci., 2012, 5, 7626–7636 RSC.
- H. N. Nong, L. J. Falling, A. Bergmann, M. Klingenhof, H. P. Tran, C. Spori, R. Mom, J. Timoshenko, G. Zichittella, A. Knop-Gericke, S. Piccinin, J. Perez-Ramirez, B. R. Cuenya, R. Schlogl, P. Strasser, D. Teschner and T. E. Jones, Nature, 2020, 587, 408–413 CrossRef CAS PubMed.
- A. L. Roest, J. J. Kelly, D. Vanmaekelbergh and E. A. Meulenkamp, Phys. Rev. Lett., 2002, 89, 036801 CrossRef CAS PubMed.
- J. W. Jang, C. Du, Y. Ye, Y. Lin, X. Yao, J. Thorne, E. Liu, G. McMahon, J. Zhu, A. Javey, J. Guo and D. Wang, Nat. Commun., 2015, 6, 7447 CrossRef PubMed.
- D. Monllor-Satoca, M. Bärtsch, C. Fàbrega, A. Genç, S. Reinhard, T. Andreu, J. Arbiol, M. Niederberger and J. R. Morante, Energy Environ. Sci., 2015, 8, 3242–3254 RSC.
- J. Zhang, Q. Lin, Z. Wang, H. Liu, X. Li and Y. Zhang, Small Methods, 2021, 5, 2100976 CrossRef CAS PubMed.
- D. Cao, W. Luo, J. Feng, X. Zhao, Z. Li and Z. Zou, Energy Environ. Sci., 2014, 7, 752–759 RSC.
- I. S. Cho, H. S. Han, M. Logar, J. Park and X. Zheng, Adv. Energy Mater., 2016, 6, 1501840 CrossRef.
- C. Tang, B. Sun, M. Li, J. Zhang, X. Fan, F. Gao, Y. Tong, L. Dong and Y. Li, J. Mater. Chem. A, 2019, 7, 8050–8054 RSC.
- B. J. Trześniewski, I. A. Digdaya, T. Nagaki, S. Ravishankar, I. Herraiz-Cardona, D. A. Vermaas, A. Longo, S. Gimenez and W. A. Smith, Energy Environ. Sci., 2017, 10, 1517–1529 RSC.
- W. Luo, C. Jiang, Y. Li, S. A. Shevlin, X. Han, K. Qiu, Y. Cheng, Z. Guo, W. Huang and J. Tang, J. Mater. Chem. A, 2017, 5, 2021–2028 RSC.
- Y. Zhu, Q. Qian, G. Fan, Z. Zhu, X. Wang, Z. Li and Z. Zou, Chin. Chem. Lett., 2018, 29, 791–794 CrossRef CAS.
- P. M. Deleuze, H. Magnan, A. Barbier, M. Silly, B. Domenichini and C. Dupont, J. Phys. Chem. Lett., 2021, 12, 11520–11527 CrossRef CAS PubMed.
- C. Y. Cummings, F. Marken, L. M. Peter, K. G. Wijayantha and A. A. Tahir, J. Am. Chem. Soc., 2012, 134, 1228–1234 CrossRef CAS PubMed.
- P. Wang, D. Li, H. Chi, Y. Zhao, J. Wang, D. Li, S. Pang, P. Fu, J. Shi and C. Li, Angew. Chem., Int. Ed., 2021, 60, 6691–6698 CrossRef CAS PubMed.
- O. Zandi, B. M. Klahr and T. W. Hamann, Energy Environ. Sci., 2013, 6, 634–642 RSC.
- O. Zandi and T. W. Hamann, Nat. Chem., 2016, 8, 778–783 CrossRef CAS PubMed.
- Z. Kuang, S. Liu, X. Li, M. Wang, X. Ren, J. Ding, R. Ge, W. Zhou, A. I. Rykov, M. T. Sougrati, P.-E. Lippens, Y. Huang and J. Wang, J. Energy Chem., 2021, 57, 212–218 CrossRef CAS.
- M. Chen, H. Dong, M. Xue, C. Yang, P. Wang, Y. Yang, H. Zhu, C. Wu, Y. Yao, W. Luo and Z. Zou, Nat. Commun., 2021, 12, 6363 CrossRef CAS PubMed.
- F. Le Formal, K. Sivula and M. Grätzel, J. Phys. Chem. C, 2012, 116, 26707–26720 CrossRef CAS.
- X. Chen, K. Zhu, P. Wang, G. Sun, Y. Yao, W. Luo and Z. Zou, iScience, 2020, 23, 100949 CrossRef CAS PubMed.
- P. Wang, X. Chen, G. Sun, C. Wang, J. Luo, L. Yang, J. Lv, Y. Yao, W. Luo and Z. Zou, Angew. Chem., Int. Ed., 2021, 60, 1390–1395 CrossRef CAS PubMed.
- J. Zhang, D. Jiang, P. Wang, J. Zhong, G. Sun, Y. Yao, W. Luo and Z. Zou, J. Mater. Chem. A, 2022, 10, 1802–1807 RSC.
- P. Wang, M. Xue, D. Jiang, Y. Yang, J. Zhang, H. Dong, G. Sun, Y. Yao, W. Luo and Z. Zou, Nat. Commun., 2022, 13, 2544 CrossRef CAS PubMed.
- X. Deng, X. Kang, M. Li, K. Xiang, C. Wang, Z. Guo, J. Zhang, X.-Z. Fu and J.-L. Luo, J. Mater. Chem. A, 2020, 8, 1138–1146 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta09340e |
| This journal is © The Royal Society of Chemistry 2023 |