Recent advances in modified commercial separators for lithium–sulfur batteries
Andrew
Kim
 a,
Seok Hyeon
Oh
b,
Arindam
Adhikari
a,
Seok Hyeon
Oh
b,
Arindam
Adhikari
 cd,
Bhaskar R.
Sathe
cd,
Bhaskar R.
Sathe
 e,
Sandeep
Kumar
f and
Rajkumar
Patel
e,
Sandeep
Kumar
f and
Rajkumar
Patel
 *g
*g
aDepartment of Chemical Engineering, The Cooper Union for the Advancement of Science and Art, New York City, NY 10003, USA
bNano Science and Engineering, Integrated Science and Engineering Division (ISED), Underwood International College, Yonsei University, 85 Songdogwahak-ro, Yeonsugu, Incheon, 21983, South Korea
cAdarsh Innovations, Pune, 411045, Maharashtra, India
dOrmecon Pvt. Ltd, CBD Belapur, Navi Mumbai, 400614, Maharashtra, India
eDepartment of Chemistry, Dr Babasaheb Ambedkar Marathwada University, Aurangabad, India
fPerkinElmer Inc., 501 Rowntree Dairy Rd, Woodbridge, ON L4L dH1, Canada
gEnergy & Environmental Science and Engineering (EESE), Integrated Science and Engineering Division (ISED), Underwood International College, Yonsei University, 85 Songdogwahak-ro, Yeonsugu, Incheon, 21983, South Korea. E-mail: rajkumar@yonsei.ac.kr
First published on 14th March 2023
Abstract
Lithium–sulfur batteries (LSBs) are one of the most promising next-generation batteries because they have higher theoretical capacities, lower cost, and smaller environmental impact than lithium-ion batteries (LIBs). However, one of the main issues preventing widespread LSB adoption is its low cycle stability due to the formation and diffusion of intermediate lithium polysulfides (LiPSs). Some of the most cutting-edge advancements of LSBs address this issue by using functional separators modified from commercial polyolefin separators used in most LIBs. Popular and promising modifiers include (a) many functionalized or conducting polymers, (b) different carbon nanostructures like graphene or carbon nanotubes, (c) covalent–organic or metal–organic frameworks, and (d) various inorganic modifiers like metal oxides and MXenes. This review analyzes the latest insights into designing and fabricating modified polyolefin membranes that minimize polysulfide shuttling in LSBs. Other benefits, including enhanced rate capability, specific capacity, sulfur utilization, electrolyte wettability, Li-ion conductivity, thermal resilience, and structural integrity, are also discussed.
1 Introduction
The increasing popularity of electric vehicles, dependence on portable technology, and recent global crises have brought battery technology innovation to the forefront. Increased production in electric vehicles has required major developments in LIB technology, emphasizing energy density, charge rate, and safety.1,2 The coronavirus disease (COVID-19) pandemic that started in 2020 caused millions of workers and students to become more dependent on phones, laptops, and other portable information technologies powered primarily by LIBs.3,4 Russia's invasion of Ukraine in 2022 led to many European nations facing energy shortages,5,6 emphasizing the need to develop energy storage technologies to facilitate renewable energy adoption, including solar,7–11 thermoelectric,12 microbial,13 and hydrogen14–18 power. LIBs and their derivatives currently dominate the battery market for portable electronics and large-scale energy storage.19–21 However, new energy storage technologies like supercapacitors22–24 with high power densities and next-generation batteries25–28 with large energy densities derived from low-cost and environmentally friendly materials are necessary to meet the exponentially rising energy storage demands.Among the candidates for next-generation batteries, lithium–sulfur batteries (LSBs) are especially promising for their high theoretical capacity, natural abundance, and safety.29,30 LSBs have a theoretical energy density of 2600 W h kg−1 and a specific capacity of 1675 mA h g−1 for a sulfur cathode,31,32 which is around 5 times higher than that of LIBs (150–220 W h kg−1 and 150–200 mA h g−1).33,34 We do not extensively describe the redox mechanism and battery operation of LSBs because such principles have been described in great detail in more general reviews by Li et al.,35 Zhao et al.,36 Yin et al.,37 and Wild et al.38 The components and construction of an LSB have been excellently summarized by Manthiram et al.39 Briefly, as shown in Fig. 1a, an LSB is discharged when Li-ions from the Li anode diffuses through a porous separator to the sulfur cathode where S8 is reduced into Li2S through a series of reactions that produce semi-stable intermediates (Li2Sx; 2 ≤ x ≤ 8) called lithium polysulfides (LiPSs). A typical charge/discharge profile of an LSB is shown in Fig. 1b, and it highlights the two discharge plateaus that correspond to the soluble high-order LiPS (high plateau around 2.3 V) and insoluble low-order LiPS (low plateau around 2.1 V).
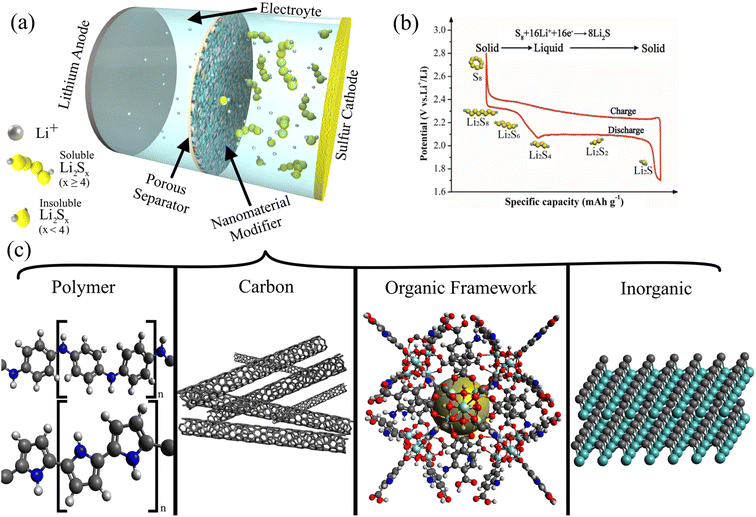 | ||
| Fig. 1 (a) Schematic of a typical LSB configuration with a Li metal anode, porous separator modified with nanomaterials, electrolyte, and sulfur cathode. The shuttle effect is prevented by the nanocomposite separator. (b) A typical charge/discharge profile of LSBs, highlighting the conversion of sulfur (S8) into Li2S via various polysulfide species (Li2Sx; 2 ≤ x ≤ 8).78 (Reproduced with permission from ref. 78 Copyright 2017, the Royal Society of Chemistry). (c) A preview of the common modifications to commercial separators analyzed in this review. | ||
LSBs have several drawbacks preventing commercialization, including poor cyclability, self-discharging, Li dendrite formation, low sulfur loading, and large volume expansion (∼80%).40,41 To address these issues, many recent studies investigated various Li–S cathode materials, cathode modifications, electrolyte combinations, catalyst additives, and other optimizations.41–56 Promising nanomaterials used in other renewable technologies, such as graphene,57–59 quantum dots,60–64 double-layered hydroxides,65,66 and other nanoparticles,67–71 have been used to enhance LSBs. Similarly, advanced polymer techniques enabled the fabrication of various polymer blends and composites72–77 that may be promising to critical LSB components, such as the separator and storage pack.
One of the largest issues with LSBs is the shuttle effect, which severely reduces cycle stability, decreases coulombic efficiency, and increases self-discharging. During the multi-step discharging process (Fig. 1b), the soluble LiPSs (Li2S8, Li2S6, and Li2S4) are solvated by the liquid electrolyte and “shuttled” from the cathode to the anode primarily via diffusion. The shuttle effect harms the cathode by decreasing the amount of active material on the cathode side and limiting the sulfur loading in the cathode, reducing the total capacity of the LSB.79 While some LiPSs may be recovered during the charging process as they shuttle back from the anode to the cathode, most long-chain LiPSs are lost because of parasitic reactions with the Li anode. Specifically, soluble LiPSs bypass the unstable solid–electrolyte interface (SEI), are reduced by the Li metal, and irreversibly precipitate on the Li anode. These parasitic side reactions increase the impedance of the LSB, corrode the Li anode, facilitate LSB self-discharging, and ultimately decrease the total capacity with each battery cycle.80 Recently, Pai et al. made an enormous breakthrough, with their LSB cycling through 4000 cycles without producing soluble polysulfide intermediates. This was due to the formation of a rare and stable γ-monoclinic sulfur on the carbon host material via an altered redox mechanism.81 While cathode improvements can reduce LiPS shuttling by reducing LiPS formation and using catalytic and chemisorptive materials, a significant amount of LiPS still migrates to the anode, resulting in sub-optimal cyclability.82,83 Others have tried to reduce the parasitic LiPS reactions at the anode by improving the stability of the SEI by adding electrolyte additives like LiNO3 or engineering artificial SEI interlayers.84 The electrolyte solvent can also be designed to create strong solvation shells that encapsulate long-chain LiPSs, preventing reactions with the Li metal anode.85 Alternatively, liquid electrolytes may be replaced with solid electrolytes to eliminate the shuttle effect.86 As shown in Fig. 1c, the separator may also be modified to reject LiPSs.
Another important obstacle to overcome is Li dendrite growth on the anode after repeated cycling. The exact mechanism behind Li dendrite formation is still under investigation, but the main culprits are generally considered to be the unstable SEI, uneven current density at the anode, and concentration polarization.87 The Li dendrites reduce coulombic efficiency, consume electrolyte, decrease active material, and can ultimately short-circuit the LSB and become a safety hazard.27
One of the most facile methods of reducing LiPS shuttling and Li dendrite formation is to use modified separators. The separator is a critical component in modern batteries that act as a physical barrier between the cathode and anode, preventing short-circuiting while allowing fast ion diffusion via the permeating electrolyte.88 The most widely available separators are microporous polyolefin-based membranes often used in LIBs, which are fabricated from polypropylene (PP), polyethylene (PE), or a blend of both. These separators are sold commercially under brand names like Celgard. However, polyolefin separators are poor options for LSBs, owing to high polysulfide shuttling, poor thermal stability, and low electrolyte wettability.89 Glass fiber-based separators have recently gained popularity for improving LSB performance but are not as widely used as polyolefin-based separators.90–94 Various polymer nanofiber separators like polyacrylonitrile (PAN) nanofiber95–97 and cellulose nanofiber98 separators have also been developed for LSBs, but these are still in their development stage and are not readily available for commercialization.
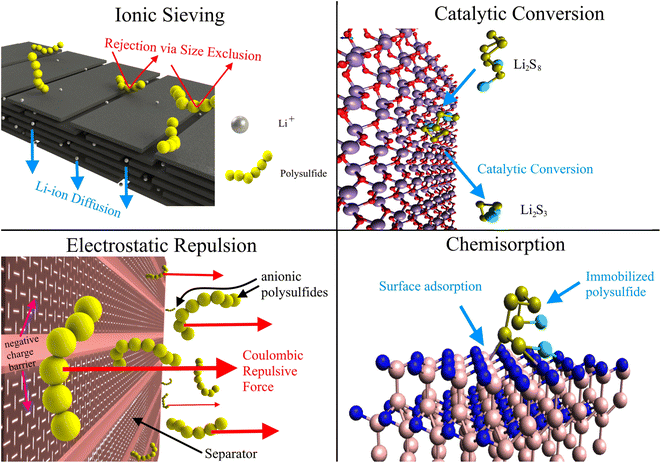
Instead of developing new separators for LSBs, we can modify the ubiquitous polyolefin separators to leverage existing manufacturing methods and supply chains for the ubiquitous polyolefin separators. In doing so, we can reduce production costs and smoothly scale up LSBs to market. Various physical and chemical modifications have been made to commercial membranes, resulting in composite separators with improved polysulfide rejection via enhanced ionic sieving, catalytic conversion, electrostatic repulsion, and surface chemisorption, as shown in Fig. 2. Ionic sieving occurs when long-chain polysulfides are unable to pass through the small nanopores in the separator due to size exclusion. Complex pore systems like hierarchical pore structures can also trap LiPSs.99 Catalytic conversion reduces the shuttle effect by facilitating the conversion of soluble LiPSs into insoluble LiPSs. Modifiers promote LiPS redox kinetics by improving electron mobility, increasing Li2S nucleation, and lowering activation energy.100,101 Anionic polysulfides are also rejected via coulombic repulsion if the surface of the separator is endowed with a net negative charge. The electrostatic shield is often generated with electronegative functional groups on the modified separator surface.102 Lastly, chemisorption plays a large role in immobilizing LiPSs, with many modifiers selected for their high LiPS adsorption affinity. The sulfur atoms are adsorbed by electron-deficient metal centers while electron-rich functional groups bind to the Li component of LiPSs.103,104 These modifications may also decrease Li dendrite formation by homogenizing Li-ion flux and distributing local current densities.105 Functional separators may also provide improved rate capability, sulfur utilization, Li-ion conductivity, and thermal stability, yielding improved specific capacity and battery safety.106
Due to the popularity of battery research, thousands of studies have been published on LSBs, followed by numerous summaries and overviews. However, most reviews focus on advances in LSB cathodes,107–115 electrolytes,116–124 or other important LSB components, like electrocatalysts125 or binders.126 Others present a general overview of improving LSBs, with brief sections on separator modifications.127–132 The few latest reviews focused on separators for LSBs do not organize developments by materials,133 do not specialize in modified polyolefin separators,134 nor focus on specific material composites.135–139 In contrast, this review primarily focuses on cutting-edge developments in modified polyolefin separators for LSBs organized by additive material: polymer-based, carbon-based, organic framework-based, and inorganic-based composites. There is a heavy focus on popular materials like conducting polymers (polyaniline and polypyrrole), functionalized carbon nanomaterials (graphene and carbon nanotubes), metal oxides (TiO2), and metal sulfides (MoS2), amongst many other composites. The advantages of less common yet promising materials like electrically conductive polymers, metal–organic frameworks, and Mxenes are also discussed. We hope this critical review of the latest modified polyolefin separators will guide future work in making LSBs commercially viable.
2 Polymer-based modifications
Polymer-based modifications to commercial membranes are frequently performed via surface modification methods, including grafting,140 surface polymerization,141 and layer-by-layer techniques.142 The main goals of polymer-based modifications are to (a) decrease pore sizes for improved polysulfide sieving, (b) endow a negative charge for the coulombic repulsion of anionic polysulfide, (c) improve compatibility between the polyolefin separator and other modifiers, and (d) to increase separator hydrophilicity for improved electrolyte wetting. To achieve this, common polymers, functionalized polymers, and electrically conductive polymers have been investigated as surface modifiers for commercial polyolefin separators in LSBs. The performance of exemplary polymer-modified separators is summarized in Table 1.| Membrane | Specific charge (mA h g−1) | C rate (C) | Cycles | % Loss per cycle | Highlights | Ref. |
|---|---|---|---|---|---|---|
| a Current density measured in mA cm−2 instead of C rate. | ||||||
| Common polymers | ||||||
| PP/Nafion/KB | 6701 | 0.2 | 150 | 0.06 | Filling interparticle gaps in KB | 143 |
| PP/PDA/PEI | 1250 | 0.2 | 100 | 0.28 | Smoother separator surface | 144 |
| PP/PAA | 562 | 0.5 | 600 | 0.07 | UV grafting decreased pore size | 166 |
| PP/Nafion/Cu-MOF | 680 | 0.5 | 300 | 0.07 | Electrostatic shield | 153 |
| PP/PDA/g-CN | 764 | 0.5 | 500 | 0.05 | Improved g-CN adhesion to separator | 161 |
| PP/Nafion/super P | 807 | 0.5 | 250 | 0.22 | Synergized ionic sieving | 154 |
| PP/silicone/PDA | 982 | 1.0 | 1000 | 0.03 | Blocked side reaction of silicone and Li metal | 162 |
| PP/Co-carbon/PDDA | 872 | 2.0 | 1200 | 0.02 | Small size of PDDA increases LiPS contact | 167 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Functionalized polymers | ||||||
| PP/SPEEK | 1228 | 0.2 | 100 | 0.23 | Electrostatic shield | 168 |
| SPEI/PEI | 1285 | 0.2 | 200 | 0.21 | Improved electrolyte uptake and Li-ion conductivity | 170 |
| PP/PVDF/super P | 1040 | 0.5 | 100 | 0.67 | Functionalized PP separator pre-coating | 173 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Conducting polymers | ||||||
| PP/PSS | 1300 | 0.05 | 30 | 1.00 | Minimum PSS loading required | 190 |
| PP/PANI/V2O5 | 1132 | 0.2 | 100 | 0.11 | Improved electron mobility | 182 |
| PP/PE/PEDOT:PSS | 985 | 0.25 | 1000 | 0.04 | Electrostatic barrier | 179 |
| PP/PEDOT:PSS | 1096 | 0.5 | 500 | 0.03 | PEDOT chelated structures with LiPSs | 178 |
| PP/PANI/CFP | 723 | 1.0 | 100 | 0.74 | PANI used as a binder | 183 |
| PP/PPy/GO | 809 | 1.0 | 1000 | 0.04 | Pyrrolic nitrogen for LiPS chemisorption | 185 |
| PP/RPM | 947 | 5.0 | 700 | 0.06 | Conductive bridge between MoS2 and rGO | 184 |
| PP/PPy-Li-MMT | 775 | 1a | 600 | 0.04 | Improved thermal stability | 189 |
2.1 Common polymers
Popular polymers like Nafion, polyethylene glycol (PEG), polydopamine (PDA), polyacrylic acid (PAA), and polyethylenimine (PEI) are easily appended to PP or PE separators via graft polymerization or filtration methods.140 Upon modification, the composite separators may have reduced pore sizes to promote polysulfide sieving. Unlike PP and PE, polymer modifiers have highly electronegative functional groups, like carboxyls, carbonyls, and sulfonates, which endow a net negative surface charge to repel anionic polysulfides.143 Because various polymers can have strong interactions with polyolefins and polar additives simultaneously, they are useful binders for other modifiers.142 Hydrophilic functional groups in common polymers also improve electrolyte wettability for improved Li-ion conduction.144 Despite all the benefits of using polymer modifiers, common polymer modifications alone are usually insufficient in mitigating the shuttle effect and do not significantly improve the rate capability of LSBs, limiting their charge rate and power density.Nafion is the brand name of a popular and commercially available sulfonated tetrafluoroethylene-based fluoropolymer-copolymer modified to reduce polysulfide shuttling in LSBs via physical and electrostatic mechanisms.145–150 Although Nafion may be used as a separator directly for its high proton conductivity and good thermal stability,151 it is a very expensive polymer, so it is better used as a modifier for polyolefin separators to save material cost. Wang et al.152 modified a standard PP separator with Nafion/MXene composite nanosheets via vacuum filtration. The Nafion contributed to the electrostatic repulsion of polysulfides and decreased the pore size from 200 nm to 40 nm. MXenes were added to create a stacked pore structure that further improved rejection via size exclusion. Recently, Diao et al.153 composited Nafion with a porous Cu-based MOF that mitigated Li dendrite formation by homogenizing Li-ion flux with its negatively charged sulfonate groups. In a Li//Li symmetric cell overpotential test, the Nafion-modified separator had a low overpotential for over 100 h, whereas the pristine PP separator had short-circuited after only 80 h.
While many porous modifiers like porous carbons (Section 3.3) and MOFs (Section 4.1) have excellent adsorptive ability and can trap long-chain LiPSs in their complex pore structure, they have poor adhesion for PP and PE. As a polymer with strong polar groups, Nafion can fill the interparticle gaps and anchor polar additives. As shown in Fig. 3a, Rana et al.143 used Nafion as filler to hold the Ketjen black particles together and reduce the interparticle gaps. The resulting Nafion/Ketjen composite was slurry coated onto a standard PP separator, which showed excellent polysulfide rejection even at high sulfur loadings up to 7.88 mg cm−2. Hao et al.154 similarly synthesized a Nafion/Super P composite and coated it onto a commercial Celgard membrane that enabled polysulfide trapping.
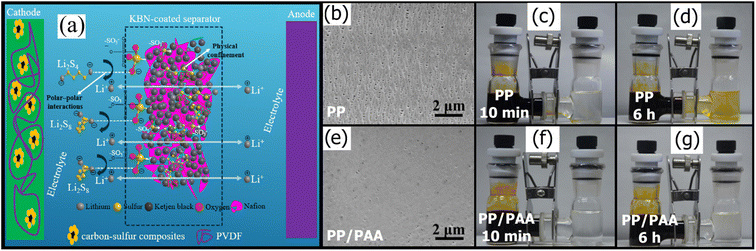 | ||
| Fig. 3 (a) Schematic of the mechanism of physical confinement of the PS in the porous structure of the KB and polar–polar interaction with –SO3−, with Nafion filler143 (Reproduced with permission from ref. 143. Copyright 2020, Elsevier). SEM of (b) PP and (e) PP/PAA. Digital photograph of a polysulfide diffusion test with a PP separator after (c) 10 min and (d) 6 h and PP/PAA after (f) 10 min and (g) 6 h (h)166 (Reproduced with permission from ref. 166. Copyright 2018, Elsevier). | ||
PEG is a low-cost, hydrophilic, and non-toxic polymer frequently used to modify LIB separators due to its excellent electrolyte wettability and ionic conductivity.155–157 For LSBs, PEG modifications can restrict polysulfide shuttling while maintaining high Li-ion conductivity. However, PEG modifications alone are insufficient to reduce polysulfide shuttling and are often composited with porous materials like MWCNTs or porous carbon that can trap polysulfides. For example, Wang et al.158 coated a Celgard 2400 separator with a PEG/MWCNT composite, resulting in a 67% higher initial specific capacity than a pristine Celgard separator. Moreover, after 500 cycles, the LSB with the PEG/MWCNT membrane had a 4-times-higher specific capacity than with the unmodified membrane. Similar improvements with PEG were reported by Chung et al.159 and Luo et al.160
PDA is another promising material for LSB separator modification, owing to its hydroxyl groups that may provide an electrostatic barrier and improved Li-ion diffusion. Recently, Pei et al.144 modified a Celgard membrane with a PDA/PEI composite. The significantly increased hydrophilicity resulted in a 75% increase in electrolyte uptake rate, a 13% increase in ionic conductivity, and a 28% higher initial specific capacity. PDA is a particularly useful binding molecule between standard polyolefin separators and other additives. For example, Tong et al.161 coated a Celgard 2500 separator with PDA to anchor graphitic carbon nitride (g-CN) nanofibers to the membrane substrate. The resulting PP/PDA/g-CN membrane showed good physical and thermal stability, with higher PDA content improving thermal stability. Yang et al.162 used a PDA coating to prevent the reaction between silicone nanofilaments and Li anode. Specifically, a Celgard 2400 membrane was modified with silicone nanofilaments to increase electrolyte wettability and physical polysulfide trapping, but the PP/silicone had poor cycling due to its reaction with Li. The PDA coating prevented the silicone–Li reaction and improved the lifespan of the LSB to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours, whereas the battery with a Celgard separator lasted 100 h of voltage cycling and PP/silicone lasted 460 h. Similarly, Hu et al.163 prevented CoFe/C nanoparticles from aggregating on the Celgard surface by encapsulating the nanoparticles with PDA. Interestingly, PDA may also be a precursor to carbon-based core/shell modifications to polyolefin separators.164,165
000 hours, whereas the battery with a Celgard separator lasted 100 h of voltage cycling and PP/silicone lasted 460 h. Similarly, Hu et al.163 prevented CoFe/C nanoparticles from aggregating on the Celgard surface by encapsulating the nanoparticles with PDA. Interestingly, PDA may also be a precursor to carbon-based core/shell modifications to polyolefin separators.164,165
By grafting a polymer layer on the cathode side of mesoporous membranes with various polymers, the pore sizes can be easily controlled to reject polysulfides while allowing high Li-ion diffusion. For example, Song et al.166 used a facile and inexpensive photografting method to append a PAA layer to a standard PP-based Celgard 2325 membrane. As shown in Fig. 3b and e, the pore size decreased from 100 nm for the pristine membrane by more than 50%, and the total number of pores decreased. Increasing the time under UV irradiation increased the grafting rate, resulting in smaller pores and lower pore density, which improved polysulfide rejection. Fig. 3c and d and Fig. 3f and g show how much the PAA layer improved polysulfide diffusion via a simple diffusion test with a dark yellow LiPS solution on the left compartment and clear solvent on the right compartment separated by either a pristine PP or PAA-modified separator. The PP separator fails almost immediately, with yellow LiPSs diffusing within 10 minutes and completely turning the right compartment yellow in 6 h. In contrast, the PAA-modified PP separator test showed no noticeable color change even after 6 hours of testing. This kind of visual diffusion method qualitatively shows the improved LiPS rejection ability of the PAA-modified separator. However, a UV-vis absorbance test following the diffusion test would be a non-invasive method of providing quantitative results for LiPS diffusion through the separator. While greater PAA grafting improved LiPS rejection via highly selective ionic sieving, the composite membrane exhibited greater hydrophobicity, which decreased electrolyte wettability and, consequently, ionic conductivity. In more recent work, Pei et al.144 similarly found that appending a polydopamine (PDA) layer to a Celgard membrane reduced average pore sizes by 35% (130 nm). However, the PP/PA membrane surface was rough with only PDA. Modifying PDA with PEI yielded a smoother surface finish, which improved Li-ion conductivity. Having multiple layers of PDA/PEI further decreased surface roughness and polysulfide rejection without significantly increasing the LSB's impedance.
Gao et al.167 compared the performance of LSBs with PP/cobalt-doped porous carbon composite separator modified with chenodeoxycholic acid, berberine chloride hydrate, or poly(diallyl dimethyl ammonium chloride) (PDDA). LSBs with the PDDA modification showed one of the highest capacity retentions, with a decay rate of 0.031% per cycle after 1200 cycles at a high current of 4C. The small molecular size of PDDA allowed it to exhibit stronger repulsion against polysulfides and improved ionic sieving than the other organic molecules.
2.2 Functionalized polymers
Polymers may be functionalized prior to their implementation in polyolefin separators to have more hydrophilic and anionic functional groups. Common polymers like PEI (Section 2.1) and other less common polymers like poly(ether ether ketone) (PEEK) can benefit from additional functionalization.168 Newly appended polar groups like sulfonates and carboxyl/hydroxyl groups increase the polymer's ability to immobilize LiPSs with greater chemisorption and repel LiPSs with a more negative surface charge. These groups can also improve Li-ion conductivity and electrolyte wettability for improved rate capability. While the additional functionalization of various polymer modifiers can improve LiPS rejection, it is unsure whether the added material cost and synthesis time are justified, as the cost-benefit analysis is often overlooked.Polymers often undergo sulfonation, where sulfonate groups are appended via reaction in sulfuric acid. For example, Li et al.168 sulfonated PEEK before modifying a PP separator. Similar to Nafion, the SPEEK separator had abundant sulfonate groups that formed an electrostatic barrier against LiPSs, but SPEEK can be fabricated at a lower cost with eco-friendly and facile one-step synthesis. Similarly, Babu et al.169 used SPEEK and Nafion to modify a commercial Celgard membrane. The SPEEK improved the stability of the LSB, while Nafion improved specific capacity. Liu et al.170 sulfonated PEI, which is known for its excellent thermal, chemical, and mechanical stability. In all aforementioned studies, the sulfonation decreased polysulfide shuttling by increasing electrostatic repulsion against polysulfides. Other sulfonated polymers, such as lithiated poly(diphenylether oxadiazole) sulfonate171 and sulfonated PEG,172 have been used in LIBs but have not yet been investigated for LSBs.
Other common functional group modifications to LSB separators include carboxyl and hydroxyl groups. In a recent study, Paniagua-Vásquez et al.173 used plasma treatment to functionalize the PP substrate with hydroxyl and carboxyl groups before coating with polyvinylidene fluoride (PVDF)/super P. The pre-functionalized PP substrate increased compatibility with the PVDF/super P coating, resulting in a smooth coating with no visible cracks and small interparticle gaps. After 100 cycles, the LSB with the modified membrane had a higher specific capacity than the initial capacity of the battery with a pristine membrane. This was due to the PVDF/super P modification enabling improved Li-ion conduction and sulfur utilization. Song et al.166 and Yu et al.174 similarly found that modifying a PP membrane by grafting poly(acrylic acid) (PAA) and hydrolyzed acrylamide, respectively, introduced carboxyl functional groups that provided a highly negative surface charge. The improved hydrophilicity and electrostatic repulsion significantly reduced polysulfide shuttling while improving Li-ion diffusion. Gu et al.175 controlled the ratio of charged amine and carboxylate groups on the surface of a PE/poly(allylamine hydrochloride)/PAA (PE/PAH/PAA) separator by changing the acidity of the electrolyte solution. The microporous PE membrane was modified with alternating layers of PAH and PAA via a layer-by-layer technique. Decreasing the pH from 8.5 to 3 increased specific capacity retention due to the increase in charged carboxylate groups that provided an electrostatic barrier against polysulfides.
2.3 Electrically conductive polymers
Conductive polymers are a special class of polymers that are electrically conductive due to their backbones comprising contiguous sp2 hybridized covalent bonds. The delocalized electrons in these large polymer structures are free to move, resulting in metallic or semiconducting behavior.176 Similar to common polymers (Section 2.1) and functionalized polymers (Section 2.2), conductive polymers can reduce the pore sizes of polyolefin separators for improved LiPS sieving. Separators modified with conductive polymers can also exhibit high ionic conductivity because conductive polymers improve electrolyte wettability. Uniquely, the high electrical conductivity of conductive polymers can improve the conversion kinetics of LiPSs and synergize with other modifiers. Despite these benefits, conductive polymers are limited in use due to their difficult processing and have variable conductivities depending on external factors like the presence of other dopants and coating thickness.177Polystyrene sulfonate (PSS) is often applied together with poly(3,4-ethylenedioxythiophene) (PEDOT) to yield an electrically conductive polymer called PEDOT:PSS. PEDOT also helps contain polysulfides by forming chelated structures with LiPSs.178 Abbas et al.179 spray-coated a layer of PEDOT:PSS onto the cathode side of a Celgard 2500 membrane. Due to the highly negative surface charge of the modified membrane, the separator effectively reduced polysulfide shuffling via coulombic repulsion, decreasing the charge decay rate by 67%. While PEDOT:PSS-modified separators have been shown to potentially reduce the kinetics of the battery due to its thickness,180 the nano-scale thick PEDOT:PSS layer (approx. 800 nm) after spray coating was thin enough to allow high Li-ion conduction.179 Moreover, the hydrophilicity increased with the PEDOT:PSS modification, resulting in improved electrolyte wettability and decreased the charge transfer resistance of the LSB by 14%. Similarly, Hareendrakrishnakumar et al.178 and Zhong et al.181 reported significant polysulfide rejection due to the negatively charged PEDOT:PSS barrier.
Polyaniline (PANI) is another popular conducting polymer for LSBs with its electrostatically repulsive imine (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) groups and excellent conductivity. PANI is particularly useful for providing a conductive coating for other modifiers like metal oxides that have an excellent chemisorption affinity for polysulfides but poor catalytic ability due to their electrically insulating nature. For example, Chen et al.182 coated V2O5 nanowires with PANI to enhance the ionic conductivity of Li-ions, electrical conductivity for Li polysulfide conversion, and mechanical strength. After the PANI/V2O5 composite was vacuum filtered through a standard PP separator, the resulting LSB exhibited a 19% higher initial capacity and 11% higher capacity retention after 200 cycles at 0.5C than the LSB with a PP/V2O5 membrane. This was due to the improved reaction kinetics and sulfur utilization because of the significantly lower charge transfer resistance with PANI. Jo et al.183 found similar improvements to initial capacity and cyclability after coating Co–Fe Prussian Blue analogs (CFPs) with PANI and applying the PANI/CFP to a standard Celgard membrane. The PANI coating on the CFPs allowed more effective binding with the PVDF and NMP binder solution, yielding a more structurally stable surface modification on the PP substrate. Shi et al.184 used PANI as a conductive intermediate between a conductive rGO backbone and highly catalytic and polysulfide-adsorptive MoS2 nanosheets. Fig. 4a shows the synthesis of the rGO-PANI/MoS2 (RPM) composite, and Fig. 4b shows the “trapping-interception-conversion” mechanism for LiPS rejection enabled by the RPM composite. While MoS2 has weak interactions with carbon, PANI interacted with MoS2via strong Mo–N bonds and rGO via strong electrostatic effects. The high electrical conductivity of PANI allowed fast electron transfer from the rGO to MoS2, unlike other polymer connectors. Hence, when cycled at a high rate of 5C, the LSB with an RPM-modified PP separator showed an excellent 99.7% coulombic efficiency and retained 55% of its initial capacity.
N–) groups and excellent conductivity. PANI is particularly useful for providing a conductive coating for other modifiers like metal oxides that have an excellent chemisorption affinity for polysulfides but poor catalytic ability due to their electrically insulating nature. For example, Chen et al.182 coated V2O5 nanowires with PANI to enhance the ionic conductivity of Li-ions, electrical conductivity for Li polysulfide conversion, and mechanical strength. After the PANI/V2O5 composite was vacuum filtered through a standard PP separator, the resulting LSB exhibited a 19% higher initial capacity and 11% higher capacity retention after 200 cycles at 0.5C than the LSB with a PP/V2O5 membrane. This was due to the improved reaction kinetics and sulfur utilization because of the significantly lower charge transfer resistance with PANI. Jo et al.183 found similar improvements to initial capacity and cyclability after coating Co–Fe Prussian Blue analogs (CFPs) with PANI and applying the PANI/CFP to a standard Celgard membrane. The PANI coating on the CFPs allowed more effective binding with the PVDF and NMP binder solution, yielding a more structurally stable surface modification on the PP substrate. Shi et al.184 used PANI as a conductive intermediate between a conductive rGO backbone and highly catalytic and polysulfide-adsorptive MoS2 nanosheets. Fig. 4a shows the synthesis of the rGO-PANI/MoS2 (RPM) composite, and Fig. 4b shows the “trapping-interception-conversion” mechanism for LiPS rejection enabled by the RPM composite. While MoS2 has weak interactions with carbon, PANI interacted with MoS2via strong Mo–N bonds and rGO via strong electrostatic effects. The high electrical conductivity of PANI allowed fast electron transfer from the rGO to MoS2, unlike other polymer connectors. Hence, when cycled at a high rate of 5C, the LSB with an RPM-modified PP separator showed an excellent 99.7% coulombic efficiency and retained 55% of its initial capacity.
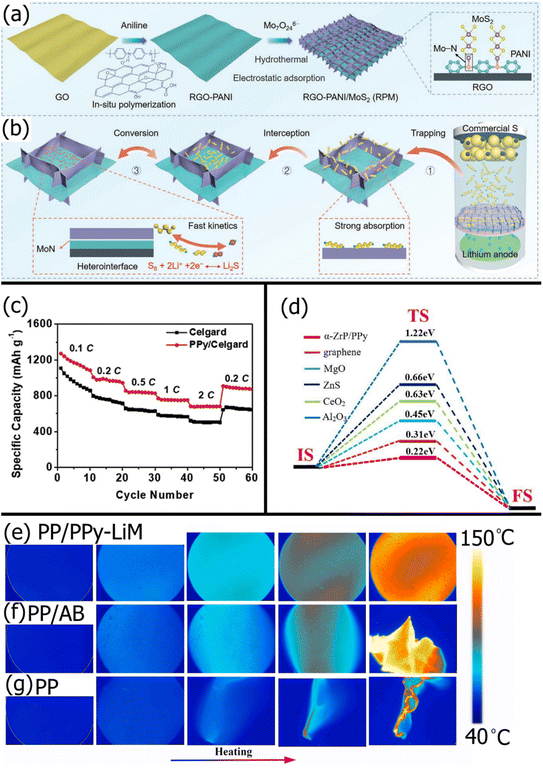 | ||
| Fig. 4 Schematic showing the (a) synthesis process of RPM and (b) the “trapping-interception-conversion” mechanism of the RPM modification on a PP separator substrate184 (Reproduced with permission from ref. 184. Copyright 2022, John Wiley and Sons). (c) Rate performance of an LSB with a standard Celgard separator and a PPy-modified Celgard separator141 (Reproduced with permission from ref. 141. Copyright 2019, Elsevier). (d) Transition state search of lithium ions diffusion in the α-ZrP/PPy interface compared with other materials188 (Reproduced with permission from ref. 188. Copyright 2022, Elsevier). In situ infrared thermography of (e) PP/PPY-LiM, (f) PP/AB, and (g) PP separators189 (Reproduced with permission from ref. 189. Copyright 2021, Elsevier). | ||
Polypyrrole (PPy) is another excellent conducting polymer for LSB separator modifications due to its adsorptive amine groups and facile synthesis. Li et al.141 modified a Celgard separator on both sides with a thin layer of PPy via in situ vapor phase polymerization to reduce Li-dendrite formation on the anode side, with no visible corrosion even after 250 charge/discharge cycles. The improved conductivity and nitrogen functional groups in PPy improved the reversibility of sulfur at the cathode, resulting in a smoother cathode surface. The hydrophilicity of the 15 nm thick PPy coating improved electrolyte wettability, increasing electrolyte uptake from 70% to 108% for higher Li-ion conductivity. Moreover, the rate capability of the LSB was significantly enhanced due to the increased conversion kinetics provided by the conductive PPy (Fig. 4c). While carbon-based modifiers are often modified with a mix of pyridinic, pyrrolic, and graphitic nitrogen atoms, it is possible to modify carbon matrices with mostly pyrrolic nitrogen sites by functionalizing with PPy.185 This is important because pyrrolic nitrogen sites have shown excellent polysulfide adsorption and catalytic conversion compared to the other nitrogen sites.186,187 In a recent study, Zhang et al.188 used DFT to show that a composite comprising PANI chains and α-ZrP nanosheets was highly catalytic to polysulfide conversion. The Li polysulfides adsorbed by the α-ZrP nanosheets acted as an electronic switch that allowed electrons to flow between PPy and α-ZrP, which triggered the multiphase conversion of the adsorbed polysulfide. The PPy was also shown to limit excessive polysulfide oxidation by controlling the output of electrons. As shown in Fig. 4d, the relative energy barrier of Li-ion diffusion through α-Zr/PPy is significantly lower than other common materials. Interestingly, PPy composites can also be used to reduce the effects of thermal shrinkage and thermal runaway. Yang et al.189 compared the thermal stability of a PPy-Li-montmorillonite (PPy-Li-MMT)-modified PP separator, acetylene black-modified PP separator, and pristine PP separator. As shown in Fig. 4e, after heating 160 °C, the PP/PPy-Li-MMT retained its shape and had an evenly spread heating profile. In contrast, PP/acetylene black (Fig. 4f) and PP (Fig. 4g) quickly deformed and disintegrated, owing to the generation of hot spots. While MMT is a well-known flame retardant, the addition of PPy improved the uniformity of MMT on the PP surface while allowing the composite to retain high thermal conductivity, resulting in better thermal stability than a PP modified with MMT without PPy.
3 Carbon-based modifications
Carbon-based modifications are most frequently implemented into polyolefin separators via vacuum filtration,103 layer-by-layer techniques,191 and slurry coating methods.192 Carbon-based modifications are primarily to (a) improve the LiPS conversion kinetics by improving electron mobility during the redox reaction, (b) decrease Li dendrite formation by decreasing local current density and Li surface reactions, and (c) decrease the charge transfer resistance of the LSB as a whole for improved rate capability (d) decrease pore sizes for improved LiPS sieving, and (e) to improve the performance of other additives.193 While there is no direct current flow through the separator, increasing the conductivity of the separator reduces the charge transfer resistance of the whole LSB by improving the electron mobility of sulfur in the cathode and polysulfides.194 Moreover, modifying a separator's anode side with conductive carbon nanostructures can mitigate Li dendrite formation by reducing local current densities at the anode, reducing Li surface reactions, and homogenizing Li-ion flux. A carbon layer can also physically shield the LSB against Li dendrite penetration with its small nanopores.195 Thus, various forms of carbon, including graphene, CNTs, porous carbons, and other graphitic carbons, have been used as surface modifiers for commercial LSB separators. Unlike most modifiers, carbon-based nanomaterials may be easily synthesized from biomass or waste, making carbon materials excellent options for sustainability-focused manufacturing.196 The performance of exemplary LSBs with carbon-modified separators is summarized in Table 2.| Membrane | Specific charge (mA h g−1) | C rate (C) | Cycles | % Loss per Cycle | Highlights | Ref. |
|---|---|---|---|---|---|---|
| Graphene | ||||||
| PP/PNCG | 1192 | 0.1 | 800 | 0.05 | In situ formation of PCN on GO | 101 |
| PP/NG nanoscroll | 950 | 1.0 | 800 | 0.02 | Improved electrolyte wettability | 195 |
| PP/NG/Ni3Sn2 | 1022 | 1.0 | 400 | 0.07 | Nitrogen defects for improved chemisorption | 201 |
| PP/rGO/MoS2 | 1040 | 1.0 | 300 | 0.08 | Increased sulfur utilization | 210 |
| PP/GO/CoPc | 1092 | 1.0 | 400 | 0.08 | Improved thermal stability | 219 |
| PP/tungsten-NG | 1100 | 2.0 | 100 | 0.05 | Adsorptive tungsten metal centers in graphene lattice | 207 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Carbon nanotubes | ||||||
| PE/CNT/Ti4O7 | 888 | 0.5 | 250 | 0.1 | Mitigated Ti4O7 overgrowth | 234 |
| PE/MWCNT–OH | 1058 | 0.5 | 400 | 0.11 | Hydroxyl groups for LiPS immobilization | 240 |
| PP/SWCNT/MnS | 876 | 0.5 | 500 | 0.07 | Thinner modification layer | 238 |
| PP/MWCNT/TiO | 1527 | 0.5 | 1000 | 0.06 | Increased sulfur capacity | 229 |
| PP/SWCNT/TB-BAA | 880 | 1.0 | 500 | 0.06 | Decreased TB-BAA layer thickness | 241 |
| PP/MWCNT/CTF | 1000 | 1.0 | 1000 | 0.05 | Conductive bridging between CTFs | 223 |
| PP/NCNT/MoS2 | 1173 | 1.0 | 1000 | 0.05 | Reduce MoS2 nanosheet aggregation | 236 |
| PP/OCNT/NiFe-LDH | 730 | 2.0 | 600 | 0.09 | Ozone treatment for oxygen functionalization | 239 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Porous carbons | ||||||
| PP/Carbon | 1198 | 0.3 | 100 | 0.42 | Porous carbon from sucrose and egg white | 266 |
| PP/N-doped KB/Co | 1058 | 0.3 | 500 | 0.1 | Nitrogen heteroatom increased LiPS affinity | 252 |
| PP/NC | 1098 | 0.5 | 600 | 0.07 | Chlorella (algae) powder | 265 |
| PP/Carbon | 906 | 1.0 | 500 | 0.10 | Rice paper plant | 264 |
| PP/PC | 912 | 1.0 | 800 | 0.06 | Lowered redox the activation energy | 261 |
| PP/HNPC | 1302 | 1.0 | 900 | 0.06 | P & N heteroatom doping | 260 |
| PP/Co–N-CNT/Carbon | 782 | 2.0 | 500 | 0.04 | One-step pyrolysis synthesis | 262 |
| PP/INC | 1151 | 2.0 | 500 | 0.11 | I-doped carbon from kelp | 263 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Graphitic carbon | ||||||
| PP/CF | 1063 | 0.5 | 500 | 0.07 | Clogged large PP pores | 271 |
| PP/CNP | 699 | 1.0 | 200 | 0.08 | Low dimensionality for interparticle space filling | 269 |
| PP/NSCNP | 650 | 2.0 | 500 | 0.09 | Nitrogen and sulfur heteroatom doping | 270 |
3.1 Graphene
Graphene is a 2D nanosheet of a monolayer, honeycomb-arranged carbon lattice with excellent electrical conductivity, mechanical strength, thermal stability, and high functionalizability, among other properties.197 Graphene derivatives, such as graphene oxide (GO) and reduced graphene oxide (rGO), have many or some oxygen functionalization and are much more common in literature as they are easier to prepare while sharing similar properties with perfect graphene.198 As modifiers for commercially available polyolefin separators, graphene and its derivatives reduce the shuttle effect via ionic sieving with controllable pore sizes and enhanced LiPS reduction kinetics owing to increased electron mobility. Because GO has abundant oxygen groups, they can contribute to LiPS rejection via chemisorption and electrostatic repulsion. Functionalized graphene moieties are also beneficial for improving electrolyte wettability. However, graphene-based modifiers alone are unable to completely mitigate the shuttle effect and must be co-doped with more adsorptive or catalytic modifiers.Graphene dopants can control the pore size of the composite membrane to improve LiPS sieving. Ou et al.199 reduced the pore sizes of a PP/PE/PP membrane by transferring a layer of graphene to the Celgard membrane using a wet transfer method. The average pore size of the graphene-modified separator was 2.45 nm, but the pore size was increased to 3.76 nm via O2 plasma treatment for improved Li-ion conductivity while still being small enough to sieve LiPSs. Improved sieving was indicated by a 15% higher specific capacity after 95 cycles with the graphene-modified separator than with the pristine membrane. Nylon-66 was appended to the composite separator via interfacial polymerization to decrease pore sizes by clogging larger pores in the graphene sheets. The result was a 10% higher capacity retention than the unclogged graphene-modified separator due to improved size exclusion. While the nylon modification reduced polysulfide shuttling, the charge transfer resistance increased slightly by 8.5%, resulting in a 1% lower initial capacity. Similarly, Lee et al.200 reduced the pore sizes of a commercial PE membrane via electrospraying an rGO/PEDOT:PSS mixture. The spray-coated rGO/PEDOT:PSS layer yielded a 2-times-higher specific capacity than the pristine membrane after 100 cycles. Ni3Sn2 doping was used to decrease the pore size of NrGO by 32%, improving polysulfide rejection.201
Because the ion-sieving mechanism is often insufficient, graphene is usually modified via heteroatom doping, yielding nitrogen-doped graphene and sulfur-doped graphene.202,203 By adding nitrogen defects to the graphene lattice, lone pairs from the graphitic, pyridinic, or pyrrolic nitrogen enable LiPS chemisorption. Recently, Qi et al.201 used the adsorption ability of nitrogen-doped graphene (NG) to reduce the shuttle effect. The LSB with the PP/NG separator had 112% higher capacity retention than the battery with a pristine separator. While nitrogen doping improves polysulfide adsorption, NG does not exhibit significant catalytic ability. The adsorption energy of various polysulfides on different parts of the NGO/Mo2N composite was calculated with computational simulations and is shown in Fig. 5d.204 As expected, the strongest binding energies with all polysulfide forms were by pyridinic and pyrrolic nitrogen groups on the NGO. Still, the nitrogen heteroatoms usually have lower binding energies for LiPSs than metal centers like Mo.
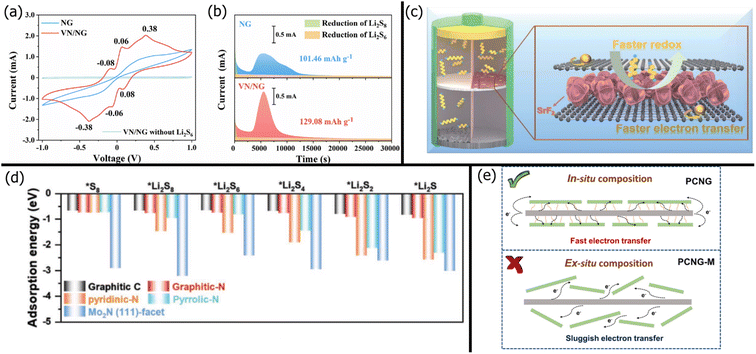 | ||
| Fig. 5 (a) Cyclic voltammetry curves of Li2S6 symmetric cells with an NG/VN separator in a voltage range between −1.0–1.0 V and a scan rate of 1 mV s−1. (b) Potentiostatic discharge profiles at a voltage of a cell with an NG/VN separator at 2.05 V206 (Reproduced with permission from ref. 206. Copyright 2021, Elsevier). (c) Schematic of a battery with a PP/graphene/SrF2 separator in LSBs, showing faster redox of polysulfides and electron transfer through graphene.208 (Reproduced with permission from ref. 208. Copyright 2022, The Royal Society of Chemistry). (d) Comparison of adsorption energies between polysulfides and Mo2N (111) surface204 (Reproduced with permission from ref. 204. Copyright 2022, John Wiley and Sons). (e) Schematic diagram of electron transfer path across PCNG and PCNG-M101 (Reproduced with permission from ref. 101. Copyright 2022, Elsevier). | ||
Thus, graphene derivatives are usually paired with various inorganic modifiers for LiPS adsorption. The properties and benefits of different inorganic modifiers are discussed in detail in Section 5. In this section, we focus on graphene's role in supporting the inorganic modifier. Gai et al.205 slurry coated a Celgard separator with nitrogen-doped rGO (NrGO) and Co nanoparticles. The NrGO was responsible for ion-sieving and polysulfide adsorption, while the Co nanoparticles enhanced the reversible conversion of polysulfides. Similarly, Jing et al.206 used vanadium nitride (VN) nanoparticles to enhance the reduction and oxidation kinetics of the polysulfides. Fig. 5a shows that adding VN yielded clear redox peaks for the conversion of polysulfides in both directions, whereas NG alone did not have any distinct redox peaks. The NG/VN also promoted Li2S nucleation and growth, yielding higher specific capacity than pristine NG (Fig. 5b). Likewise, Wang et al.207 doped a graphene lattice with tungsten metal centers to improve polysulfide adsorption and catalytic conversion. Recently, Jing et al.208 sandwiched SrF2 between graphene nanosheets (Fig. 5c) before vacuum filtration onto a commercial PP membrane. The graphene provided ion-sieving effects as expected but also improved electron transfer for faster polysulfide conversion kinetics. In another study by Jing et al.,209 a standard PP membrane was modified with CaF2-decorated rGO. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio between adsorptive and catalytic CaF2 and conductive rGO was the best compromise between cycle stability from CaF2 and specific capacity from rGO.
3 ratio between adsorptive and catalytic CaF2 and conductive rGO was the best compromise between cycle stability from CaF2 and specific capacity from rGO.
Graphene plays a significant role in sulfur utilization. This was exemplified by Cheng et al.210 who modified a Celgard separator with MoS2-doped rGO nanosheets. The highly conductive rGO enabled a high sulfur utilization as high as 80 wt%. Similar synergies have been reported between graphene derivatives and metal oxides like CeO2,211 Fe3O4,212 MoO2,213 BaTiO3,214 and defect-rich and amorphous Fe3O4−x.215 Studies involving transition metal nanoparticles like cobalt,216 tungsten,207 iron,217 and nickel218 on graphene have also reported similar benefits.
The synergy between graphene derivatives and inorganic nanoadditives may also improve heat dispersion. Shen et al.219 improved the thermal stability of a PP separator via modification with GO/cobalt phthalocyanine (GO/CoPc). Due to the large thermal capacity of GO/CoPc and the thermally conductive GO, the separator was able to dissipate heat more readily and withstand prolonged heating at 130 °C. Improved thermal stability and heat dissipation will help mitigate battery thermal runaway.
When modifying graphene with other nanoparticles, the distribution and adhesion of the nanoparticles play a significant role in the specific capacity of the LSB. For example, Zhang et al.101 compared the performance of a modified Celgard separator prepared with in situ and ex situ methods. In the in situ process, phenyl-modified carbon nitride (PCN) was directly formed on GO (referred to as PCNG) via pyrolysis of a freeze-dried mixture of 2,4-diamino-6-phenyl-1,3,5-triazine (DPT) and GO. In the ex situ process, DPT was calcined into g-C3N4 and mechanically mixed with GO (referred to as PCMG-M). Fig. 5e illustrates the difference in the microstructural bonding between PCN and GO. Because the PCN was uniformly distributed on GO with strong covalent bonding in PCNG, the PCN layer could adsorb polysulfides without deforming and provide direct conductive pathways for improved polysulfide conversion. In contrast, the ex situ synthesized PCN layer was loosely held with random arrangements, resulting in longer conductive pathways. Thus, the in situ synthesis of nanoparticles is recommended for composition with graphene.
Other than modifying graphene with other nanoparticles, graphene morphology has been altered for improved polysulfide rejection. Li et al.220 synthesized hollow graphene spheres (HGS) to block the pores of a Celgard membrane. The hollow spheres exhibited both physical blocking and adsorption mechanisms, resulting in a 55% higher capacity retention than the pristine separator. Interestingly, the HGS seemed to exhibit stronger adsorption effects than regular graphene, indicated by a decent but relatively high decay rate of 0.15% per cycle compared to other graphene composite membranes presented in Table 2. However, the adsorption effects were limited as graphene itself has weak adsorption properties. Changing the morphology of graphene may also add functionality. Zhang et al.195 synthesized nitrogen-doped graphene nanoscrolls (rolled-up sheets of NG nanosheets) that wrapped around Co3O4 nanoparticles. Compared to an LSB with a PP membrane modified with planar NG/Co3O4, the battery with a nanoscroll-modified membrane retained a 10% higher coulombic efficiency (99.5%) after double the number of cycles (800 cycles). The nanoscroll membrane also reduced lithium dendrite formation on the anode. The benefits stemmed from the nanoscroll morphology promoting better electrolyte wetting, homogenous Li-ion diffusion, and lower overpotentials than NG nanosheets.
3.2 Carbon nanotubes
Single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) are 1D tubes with nanoscale diameters and are often thought of as rolled-up tubes of graphene. Thus, they share many of the same properties as graphene (Section 3.1) and consequently have great potential as modifiers in LSBs because of their excellent electrical conductivity, thermal conductivity, functionalizability, and compatibility with other additives.221 This was clearly demonstrated by Raja et al.,106 who found that MWCNTs on the cathode side of a Celgard separator improved polysulfide reaction kinetics. This allowed more efficient charge/discharge at higher charge rates with around a 20% higher initial charge capacity. The effect of MWCNTs on sulfur was only effective on the cathode side. An LSB with MWCNTs on the anode side (Li side) of the separator yielded similar performance to an LSB with a pristine Celgard membrane.As a popular upper current collector material, CNTs may promote electron transfer to hasten LiPS conversion, but CNTs alone do not adsorb LiPSs effectively. Hence, CNT-based modifications employ catalytic compounds that provide chemisorption. Recently, Fan et al.222 used a poly(diallyl di-methyl ammonium) bis(trifluoromethanesulfonyl)imide (PDDA-TSFI) layer on the CNTs to increase polysulfide adsorption and induced interfacial charge distribution, resulting in a 67% higher capacity retention after 300 cycles. Other polysulfide trapping compounds, such as COFs,223 MOFs,224 MXenes,225 metal dichalcogenides,226 metal nitrides,227 and metal phosphides,228 have also been composited with CNTs for similar benefits.
CNTs have been frequently paired with various metal oxides (Section 5.1) that boast excellent polysulfide trapping properties for higher sulfur utilization and redox kinetics. Li et al.229 fabricated a PP/MWCNT/TiO separator that enabled a high sulfur utilization of 62%. While TiO alone limits polysulfide conversion due to its insulating nature, limiting the discharge capacity, adding conductive MWCNTs improved the specific capacity by about 50% more than the pristine membrane. Essentially, adding MWCNTs made TiO a viable modifier for LSB separators, allowing an astoundingly low capacity fade rate of 0.057% per cycle. Similarly, recent studies combined various CNTs with oxygen-rich MnO nanoflakes,230 TiO2,231 Nb2O5,232 MnO,233 and Ti4O7234to enhance specific capacity while also significantly improving polysulfide rejection. Wang et al.235 sandwiched 1D SWCNTs between 2D V2C/V2O5 nanosheet layers to prevent the nanosheets from agglomerating, resulting in a maximum number of active sites for polysulfide adsorption. Moreover, the interstacked SWCNTs created a conductive network that improved LiPS conversion kinetics. Similarly, Gong et al.236 used N-doped CNTs (NCNTs) to provide a porous structure that reduced MoS2 aggregation and consequently greatly increased polysulfide adsorption and sulfur re-utilization. Liu et al.237 synthesized 2D WS2 nanosheets interwoven with 1D SWCNTs that formed a porous 3D structure that provided abundant active sites for polysulfide adsorption and conversion. In another example, Kannan et al.238 slurry coated a PP membrane with SWCNTs functionalized with MnS nanoparticles. The SWCNTs provided conductive pathways for polysulfide redox reactions while also physically blocking polysulfide shuttling.
CNTs are easily functionalizable with various groups that improve adhesion with other modifiers. For example, Liu et al.239 modified MWCNTs with NiFe layered double hydroxides (NiFe-LDH) by first functionalizing the MWCNTs with carboxyl and hydroxyl groups via ozone treatment (OCNTs). The NiFe-LDHs were subsequently grown on the functionalized MWCNTs via an in situ hydrothermal method. The electronegative oxygen functionalization was necessary for the homogeneous distribution of Ni2+ and Fe2+ in solution, yielding a uniform distribution of NiFe-LDHs. The ability of NiFe-LDH/OCNT/PP separator to reject LiPSs was supported by in situ Raman spectroscopy with a custom-designed Raman battery shown in Fig. 6a. The resulting Raman spectra showed no obvious peaks for any LiPS species during charge and discharge of the battery. This was due to the good chemisorption and electrocatalytic conversion afforded by the NiFe-LDHs on the OCNTs.
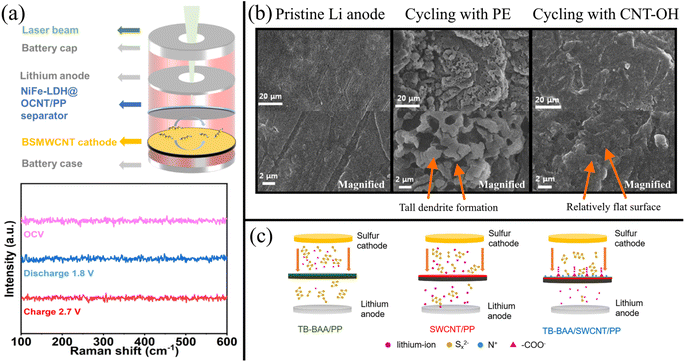 | ||
| Fig. 6 (a) Schematic illustrating a standard cell design for in situ Raman spectroscopy and the corresponding Raman spectra for an LSB with a NiFe-LDH/OCNT-modified PP separator239 (Reproduced with permission from ref. 239. Copyright 2022, John Wiley and Sons). (b) SEM of a Li anode surface before cycling at 1.8–2.6 V at 0.5C for 100 cycles, and after cycling in an LSB with a PE separator versus MWCNT–OH modified separator. The magnified view of the anode surface is shown in the bottom row240 (Reproduced with permission from ref. 240. Copyright 2017, American Chemical Society). (c) Schematic showing the implementation of an SWCNT/TB-BAA gutter layer via modification of a PP separator242 (Reproduced with permission from ref. 242. Copyright 2022, Elsevier). | ||
Moreover, CNTs functionalized with electronegative groups that repel polysulfides and can form porous nanostructures that physically obstruct polysulfide shuttling. Ponraj et al.240 functionalized MWCNTs with abundant hydroxyl functional groups (MWCNT–OH) via a hydrothermal method and used vacuum filtration to coat a standard PE separator with a uniform layer of MWCNT–OH. The MWCNT–OH adsorbed polysulfides, which reduced the shuttling effect. This was observed when stirring MWCNT–OH in a vial of Li2S4 solution turned the yellow solution to a nearly clear solution. In contrast, adding pristine MWCNTs did not show any significant adsorption characteristics. As shown in Fig. 6b, the Li anode with a pristine PE separator suffered from large deposits of inactive sulfur. In contrast, the LSB with a MWCNT-OH–modified separator had a smooth Li anode surface even after 100 cycles at 0.5C. The hydroxyl functionalization of the MWCNTs enabled efficient polysulfide chemisorption, resulting in a nearly perfect coulombic efficiency of 99.5%. The MWCNT–OH also reduced the charge transfer resistance, which resulted in enhanced rate performance and increased re-utilization of sulfur during charge/discharge cycles, indicated by a 52% higher initial charge capacity. Because the MWCNT–OH separator reduced the deposition of inactive sulfur on the Li anode, electrolyte resistance and interfacial resistance decreased. NCNTs have also demonstrated their ability to adsorb polysulfides.236
MWCNTs coatings can also act as a gutter layer that reduces the thickness of highly polysulfide-rejecting layers. A gutter layer prevents the selective material from clogging the support layer's holes and enables a thin, uniform coating of the selective material.241 Sun et al.242 used SWCNTs as a gutter layer between a Celgard 2500 separator support and zwitterionic microporous polymer (TB-BAA) selection layer (Fig. 6c). Without the gutter layer, TB-BAA infiltrated deep into the PP pores (6.5 μm thick layer) while leaving some large microporous holes, leaving pathways for polysulfide shuttling. In contrast, longer SWCNTs were smoothly coated onto the PP substrate and allowed the TB-BBA to be thinly and uniformly coated (400 nm thick layer) on top of the SWCNTs, resulting in a 50% higher specific capacity retention.
3.3 Porous carbons
3D porous carbons are also promising modifiers for commercial LSB separators, owing to their high ionic conductivity, electrical conductivity, and surface area. By controlling their pore size, volume, and density, porous carbon modifications physically block polysulfides with their complex pore structures while allowing high Li-ion diffusion.243 Porous carbons are frequently synthesized starting with a variety of carbon sources via template methods or by using chemical activators followed by pyrolysis at high temperatures.244 The most common implementation of porous carbons to commercial separators is via slurry coating, yielding micrometer-thick, flexible selective layers against polysulfides. Unlike graphene (Section 3.1) and CNTs (Section 3.2) that require more complex processing, porous carbons are readily available as byproducts of industrial processes or as pyrolyzed biowastes, making porous carbon implementations easily scalable and sustainable.Commercially abundant carbon blacks are common modifiers for standard LSB separators. Acetylene black (AB) is produced in large volumes as a byproduct of incomplete coal and oil combustion and is mixed with other porous carbons like graphite to form hybrid 3D structures.245 Similarly, Ketjen black (KB) is a commonly available and popular carbon black frequently used to modify commercial LSB membranes. Its high porosity and excellent electrical conductivity make it an excellent polysulfide sieve and upper current collector that improves sulfur utilization.246 Super P is already one of the most common conductive additives for lithium-based batteries, and super P-modified separators are already commercially available for LSB applications.247,248 Between the many commercially available porous carbons, acetylene black may exhibit the best polysulfide rejection with high ionic conductivity, owing to it having the smallest pore size and pore volume.249 This was shown by Huang et al.,249 who compared LSBs with a PP separator slurry coated with acetylene black, Ketjen black, super P, lamp black, Vulcan black, activated carbon, activated charcoal, and carbon Black Pearls. The main difference between the carbons came down to pore size and pore volume. Non-porous carbons like acetylene black and super P formed closely connected carbon networks that physically blocked polysulfides and limited liquid electrolyte adsorption. However, porous carbons like Ketjen black and activated carbon had large pore volumes that blocked fewer polysulfides and disrupted Li-ion diffusion due to their higher electrolyte uptake. Hence, the separator with non-porous acetylene black, which had the second smallest pore size and second smallest pore volume outperformed the other carbon-modified separators that had mixed rankings of pore size and volume. Still, every carbon-modified separator had at least a 40% capacity retention (acetylene had 64%), which outperformed the pristine separator that had less than 10% retention after 200 cycles at 0.1C.
However, commercial porous carbon modifications alone are not sufficient for polysulfide rejection. Jin et al.247 showed this by comparing the performance of an LSB with a standard PE separator, a commercial super P-modified PE separator, and a Co–N–C powder/super P-modified PE separator. The battery with the PE/super P separator had a 30% higher initial capacity than the pristine separator and lost 73% of its capacity after 200 cycles. Interestingly, the battery with the pristine separator lost only 79% of its initial capacity under the same conditions, suggesting that super P did not significantly stop polysulfide diffusion due to its large pores and minimal electrostatic interactions. However, the addition of Co–N co-doped carbon nanocages to super P increased the initial capacity by 28% and capacity retention by 21%. Hence, carbon blacks are frequently mixed with more catalytic compounds. Because porous carbons have high surface areas and conductivities, they can adsorb a large amount of additives that have high affinities for polysulfides and provide conductive pathways for fast reaction kinetics.
Porous carbons often need to be combined with metal nanoparticles like Ni, Co, Fe, V, Cr, Mo, Nb, and Mn and catalytic metals like Pt and Au to maximize LiPS adsorption and catalytic conversion.247,250,251 For example, Zhen et al.252 found that adding Co nanoparticles to Ketjen black improved capacity retention from 54% to 69% after 300 cycles at 0.3C. Fig. 7a shows the modification of Ketjen black with nitrogen and Co. The nitrogen groups were necessary for the adhesion of Co nanoparticles into the Ketjen pores. The highly adsorptive Co and nitrogen centers embedded in the porous carbon were then able to efficiently adsorb and immobilize LiPSs. Similarly, highly polar metal salts like metal oxides,253–255 sulfides,256,257 fluorides,258 and selenides,259 exhibit good adsorption for polysulfides. While not the most effective against polysulfide rejection, porous carbons have also been composited with doped graphene and CNTs. In such instances, the porous carbons provide high surface areas with large pores to adsorb polysulfides, while the doped graphene or CNTs provide high electron mobility for fast LiPS conversion.
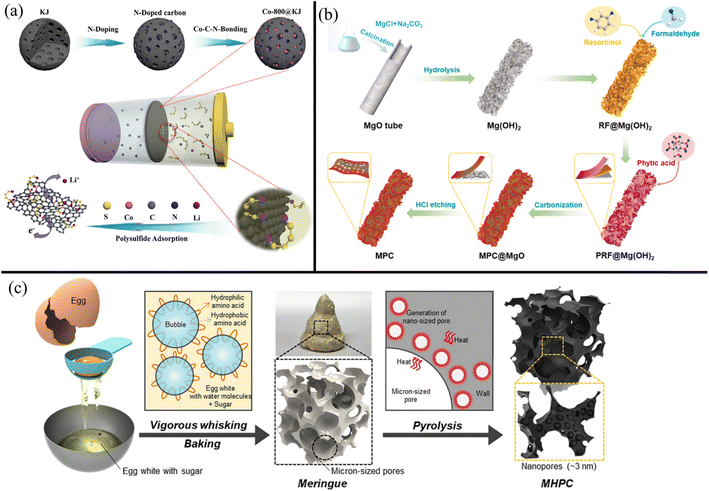 | ||
| Fig. 7 (a) Schematic showing the N and Co modification of Ketjin black to improve LiPS adsorption252 (Reproduced with permission from ref. 252. Copyright 2021, John Wiley and Sons). (b) Schematic showing the formation of P-doped hierarchical porous carbon with flower petal-like protrusions for increased surface area261 (Reproduced with permission from ref. 261. Copyright 2022, Elsevier). (c) Schematic showing the synthesis of porous carbon derived from egg whites and sugar266 (Reproduced with permission from ref. 266. Copyright 2022, Elsevier). | ||
Heteroatom doping with nitrogen, phosphorous, and sulfur improves porous carbon's ability to reject polysulfides by endowing electrostatic and chemisorption effects. The most common doping is with nitrogen atoms, owing to increased electrical conductivity, interlayer spacing, and interaction with polysulfides. Zhang et al.104 synthesized pyridinic N-doped carbon via the calcination of GO with NH4HCO3 at 600 °C. Compared to a pristine Celgard separator or an rGO-modified Celgard separator, the N-doped porous carbon-modified separator reported a higher initial capacity (indicating better sulfur utilization) and lower decay rate per cycle. The improved chemisorption of polysulfides was due to the abundant pyridinic nitrogen groups combined with effects from pyrrolic nitrogen, graphitic nitrogen, and oxygen functional groups in the N-doped porous carbon separator. The reduction of the shuttle effect was determined using time-resolved in situ Raman spectroscopy, wherein significantly lower intensity peaks for soluble LiPSs were detected with the N-doped carbon-modified separator than with the pristine PP separator. Zeng et al.260 synthesized honeycomb-like N, P dual-doped carbon (HNPC) powder by pyrolyzing a mixture of glucose, urea, NH4H2PO4, and SiO2 nanoparticles and applied it to a Celgard membrane via slurry coating. LSBs with an N and P-doped separator exhibited better cycle stability, specific capacity, and rate capability than an only N-doped separator. This was due to the P atoms exhibiting excellent bonding with sulfur atoms in the polysulfides that improved polysulfide adsorption, reaction kinetics, and sulfur loading. Kong et al.261 similarly found that strong P–S and P–Li bonds significantly reduced polysulfide shuttling. Fig. 7b outlines the synthesis of a hierarchically porous P-doped carbon nanosheet structure using MgO as an initial template. HCl etching was used to remove the MgO nanoparticles, leaving uniform pores around 4 nm for effective LiPS sieving. The improved adsorption also lowered the reaction energy barrier for Li2S precipitation, resulting in faster liquid–solid conversion between the polysulfides and Li2S. Metallic dopants like Co were shown by Liu et al.262 to exhibit bi-directional catalytic activity for the redox of polysulfides.
The most promising carbon sources are biomaterials and biowastes, which make porous carbons greener alternatives than other carbon-based modifiers. Yang et al.263 turned supermarket kelp into I and N co-doped carbon powder (INC). While other carbon materials like graphene and CNTs require additional chemicals during synthesis for heteroatom doping, biologically derived porous carbons naturally have abundant atoms like nitrogen, phosphorous, and sulfur in their structure. This makes their synthesis significantly more facile and cost-effective for large-scale production. Recently, Zhu et al.264 carbonized rice paper plant pith commonly used in traditional Chinese pharmacies into a light carbon powder with honeycomb-like pores. Li et al.265 pyrolyzed chlorella (algae) powder to yield N-doped carbon powder (NC). Choi et al.266 used egg whites with sucrose as pore-forming agents, yielding hierarchically porous carbon (Fig. 7c). Wang et al. synthesized heteroatom-doped porous carbon from Ginko leaves.267In situ Raman spectroscopy showed that the Ginko leaf-derived carbon layer mitigated the shuttle effect, with unchanging LiPS peak intensities at the PP separator surface even after 300 min. Recycling such biowaste materials should be encouraged for a more sustainable future. Biologically derived porous carbons are often mixed with small quantities of commercial powders like super P, Ketjen black, and acetylene black as fillers before application to commercial LSB separators.
3.4 Graphitic carbons
While not as popular as graphene, CNTs, and porous carbons modifications, some recent works have been done on modifying commercial separators with other graphitic carbon materials—in particular, carbon nanoparticles (CNPs) and graphitic flakes. As with the other carbon-based materials, graphitic carbon has high conductivity for improved sulfur utilization and the ability to sieve polysulfides.268 Since the primary role of carbon-based modifications in rejecting LiPSs is to improve LiPS conversion kinetics with high electron mobility, lower-dimensional CNPs may be more efficient than 1D CNTs and 2D graphene. Zhang et al.269 compared the performance of LSBs with PP membranes modified with CNPs, CNTs, and graphene nanosheets fabricated via vacuum filtration. Fig. 8 shows SEMs of CNP, CNT, and graphene-coated separators. The 0D CNPs were more densely packed than the intertwined CNTs and flaky graphene, resulting in smaller pore sizes that limited polysulfide shuttling while still having a high pore volume for electrolyte and polysulfide adsorption. Moreover, the charge transfer resistance of the cell with the CNPs was lower than the CNTs and graphene flakes, resulting in better rate capability even at 3C charge/discharge rate. Due to their low dimensionality, CNPs acted as conductive bridges that decreased interparticle gaps between the catalytic modifiers and LiPSs compared to higher dimensional carbon morphologies. The polysulfide rejection was respectable despite the commercial separator having only carbon modifications, with the LSB having a small 0.08% decay per cycle after 200 cycles at 1C. This work shows the potential of CNPs as potential modifiers.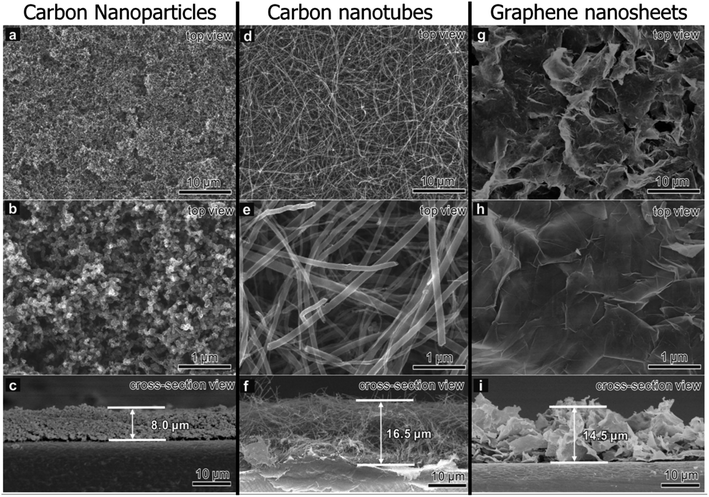 | ||
| Fig. 8 SEM of PP separators coated with a layer of (a–c) CNPs, (d–f) CNTs, and (g–i) graphene nanosheets. An unmagnified and magnified view from the top is shown in the first two rows, respectively. A cross-view of the modified separator is shown in the last row, with the average thickness of the carbon layer labeled269 (Reproduced with permission from ref. 269. Copyright 2021, Elsevier). | ||
CNPs may undergo heteroatom doping with nitrogen or sulfur to improve their interaction with polysulfides. Diez et al.270 co-doped nitrogen and sulfur in CNPs (NSCNPs) by using polypyrrole as a carbon precursor and reaction with sodium sulfate during activation. Having the NSCNP modification resulted in a 23% higher initial capacity of 1020 mA h g−1. Moreover, the cell with the modified separator retained 82% of its initial capacity after 100 cycles at 0.2C (0.089% decay per cycle for 500 cycles at a higher rate of 2C), whereas the cell with the pristine separator retained only 56% after 100 cycles. The superior conductivity and polysulfide adsorption ability of doped CNPs caused the increase in capacity and cyclability.
Graphitic carbon flakes (CFs) are also potential modifiers that exhibit excellent conductivity. Zheng et al.271 synthesized CFs by directly carbonizing sodium citrate and embedded the CFs into a PP separator via vacuum filtration. The CFs clogged the large pores in the PP separator while leaving enough space for Li-ion diffusion. Due to the abundant carboxyl, carbonyl, and hydroxyl groups in the CFs, the CFs had significantly improved electrolyte wettability, indicated by an almost 0° electrolyte contact angle. The rate performance of a battery with PP/CF separators was better than that of a battery with a PP separator, with a 54% higher initial capacity at 0.2C (1207 mA g−1) and 99% coulombic efficiency even after 200 cycles. The capacity fading rate was 0.071% per cycle for 500 cycles at 0.5C.
4 Organic framework-based modifications
Metal–organic frameworks (MOFs) and covalent–organic frameworks (COFs) are highly porous compounds with highly organized crystal structures consisting of metal or organic nodes and organic linkers. Organic frameworks provide polyolefin separators with (a) a highly organized cage structure with controllable pore sizes for highly selective sieving, (b) catalytic metal or organic centers for high LiPS adsorption, and (c) lithiophilic groups that improve Li-ion conductivity. Commercial membranes are often modified with organic frameworks via slurry coating272 but may also be directly crystallized on polyolefin separators.273 The performance of exemplary MOF and COF composite polyolefin separators for LSBs is summarized in Table 3.| Membrane | Specific charge (mA h g−1) | C rate (C) | Cycles | % Loss per cycle | Highlights | Ref. |
|---|---|---|---|---|---|---|
| a Current density measured in A g−1 instead of C rate. | ||||||
| MOFs | ||||||
| PE/UiO-66/Nafion | 1127 | 0.1 | 200 | 0.11 | Nafion helped bind MOFs to PE | 287 |
| PP/ZIF-8/KB | 1235 | 0.1 | 100 | 0.29 | High LiPS chemisorption | 279 |
| PP/aMIL-88 | 1249 | 0.2 | 100 | 0.10 | Amorphous MIL-88 better than crystalline MIL-88 | 102 |
| PP/ZIF-8/MWCNT | 1588 | 0.2 | 100 | 0.45 | 91% sulfur utilization | 278 |
| PP/UiO-66 | 855 | 0.5 | 500 | 0.03 | Improved thermal stability | 274 |
| PE/ZnO/N/KB | 868 | 0.5 | 400 | 0.10 | ZIF-8 carbonization | 283 |
| PP/Ce–UiO-66/super P | 891 | 1.0 | 300 | 0.09 | Super P conductivity required for catalysis | 281 |
| PP/Ce-MOF-808 | 955 | 1.0 | 500 | 0.03 | Homogeneous Li platting/stripping | 273 |
| PP/FJU-90 | 1047 | 1.0 | 500 | 0.04 | Pore-space partitioning increased active sites | 285 |
| PP/ZIF-8/PDA | 750 | 2.0 | 500 | 0.01 | PDA increased MOF density | 286 |
| PP/CSUST-1/CNT | 976 | 2.0 | 1200 | 0.04 | Multi-valent Ce metal centers | 282 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| COFs | ||||||
| PP/COF | 864 | 1.0 | 500 | 0.05 | Lithiophilic properties of the SO3H groups | 290 |
| PP/CTF/MWCNTs | 1156 | 1.0 | 1000 | 0.05 | Decreased overall LSB resistance | 223 |
| PP/CTF/PEDOT:PSS | 1205 | 1.0 | 1000 | 0.05 | Improve adhesion with negatively charged PEDOT:PSS | 293 |
| PP/COF | 666 | 2.5 | 1000 | 0.05 | Electrophilic carboranyl groups | 289 |
| PP/COF | 633 | 4.0 | 400 | 0.04 | Improved Li-ion conductivity | 294 |
| PP/TAPP-ETTB/graphene | 1350 | 0.5a | 400 | 0.08 | Decreased square resistance by 99% | 292 |
4.1 Metal–organic framework (MOF)
MOFs are highly organized, porous, crystalline networks assembled with inorganic metal centers connected with organic ligands. Due to MOFs' easily tunable pore sizes and chemisorptive metal centers, they have been used to reduce polysulfide shuttling via physical and adsorptive mechanisms. While the advantages of MOFs are clear, recent improvements to MOFs involve improving the electrical conductivity of MOFs for higher sulfur utilization and tuning pore sizes for high Li-ion diffusion with minimal polysulfide shuttling. MOFs used to modify commercial LSB separators include274 UiO-66 and ZIF-67.275 More obscure MOFs include Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2 (Ni3(HITP)2)276 and HKUST-1.277One of the most common MOFs is the zeolitic imidazolate framework (ZIF-8), owing to its facile synthesis and easily controllable pore size. Carbon materials (Section 3) are often composited with ZIF-8 to increase the MOF's catalytic ability. Wu et al.278 composited ZIF-8 with MWCNTs. The ZIF-8/MWCNTs slurry-coated PP separator exhibited a high sulfur utilization of 95% due to the combined effects of Lewis acid–base chemisorption effects of the ZIF-8 and reduced charge transfer and internal resistance due to the MWCNTs. More recently, Ma et al.279 coated a PP separator with a slurry mixture of ZIF-8 and Ketjen black. The capacity retention of the PP separator with ZIF-8 and KB was 57%, much higher than the 45% for PP/KB and 36% for pristine PP. While adding ZIF-8 to PP/KB increased the charge-transfer resistance by 8%, the LSB with the PP/ZIF-8/KB had a 9% higher initial capacity. This suggests that the benefits of adding ZIF-8 outweighed the potential loss in conductivity.
Cerium-based MOFs have also recently gained popularity, owing to their excellent hydrophilicity and larger pore sizes compared to ZIF-8 and ZIF-67 MOFs.280 Recently, Dang et al.273 grew cerium-based MOF-808 crystals directly on both sides of a commercial PP separator via a room temperature in situ growth method. The Ce-based MOF-modified separator exhibited a 60% lower charge transfer resistance than a pristine PP separator, owing to the enhanced electrolyte wettability from the hydrophilicity of the Ce-based MOF (Fig. 9c). Moreover, the 1.8 nm pore sizes allowed fast Li-ion diffusion while sieving polysulfides and provided a large surface area for faster redox kinetics, evidenced by the significantly higher, indicated by the small ratio between the steady state current to the initial current shown in Fig. 9d. Ce-based MOFs have also been composited with carbon materials to enhance their electrical conductivity. Recently, Su et al.281 modified a standard Celgard separator with Ce-based UiO-66-MOF and super P, resulting in a composite separator with a higher initial capacity and significantly improved capacity retention compared to the separator modified with only super P. Similarly, Jin et al.282 composited a Ce-based MOF with CNTs before slurry coating the composite onto a commercial PP separator. As shown in Fig. 9a, the mixed-valence Ce-based MOFs were derived from Ce-based UiO-66, referred to as CSUST-1. Compared to the UiO-66-modified, CNT-modified, and un-modified Celgard separators, the CSUST-1/CNT-modified separator exhibited higher overall capacity and superior rate performance shown in Fig. 9b. This was due to the abundant oxygen vacancies stabilized by Ce(IV) metal sites, excellent catalytic properties of the open Ce(III) sites, and the Ce(IV)/Ce(III) redox couple (Fig. 9a).
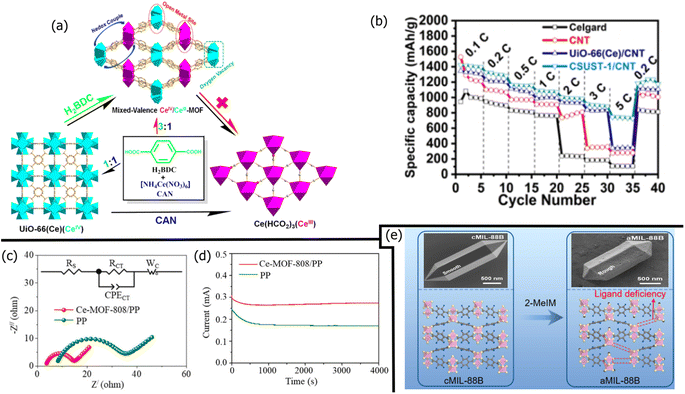 | ||
| Fig. 9 (a) Synthesis and conversion among Ce-based UiO-66, CSUST-1, and Ce(HCO2)3. (b) Comparison of rate performances at various C-rates for a pristine Celgard versus CNT-modified, UiO-66/CNT-modified, and CSUST-1/CNT-modified Celgard separators282 (Reproduced with permission from ref. 282. Copyright 2021, The American Chemical Society). (c) Electrochemical impedance spectrum (EIS) and (d) Current vs. Time plots of a Ce-based MOF-808 modified separator and pristine PP separator273 (Reproduced with permission from ref. 273. Copyright 2022, Elsevier). (e) Schematic diagram of crystalline and amorphous MIL-88. SEMs of crystalline and amorphous MIL-88 are also shown102 (Adapted with permission from ref. 102. Copyright 2021, Elsevier). | ||
MOFs can be used as templates that undergo carbonization to increase electrical conductivity while preserving small pore sizes. In a recent study, Qian et al.283 carbonized a ZIF-8/KB composite to yield a porous ZnO/N/KB composite. Similarly, Zeng et al.181 carbonized a ZIF-8/PAN composite to yield a network of carbon nanofibers. In another example, Qian et al.284 derived Ni-doped porous carbon structures using a Ni-based MOF precursor. Such composites are discussed more fully in our discussion of porous carbons (Section 3.3).
Besides carbon-based improvements to MOF conductivity, amorphous MOFs with abundant defects have exhibited improved conductivity while retaining their catalytic ability. Zhang et al.102 compared the performance of crystalline and amorphous MIL-88 MOF as modifications to a commercial separator. As shown in Fig. 9e, the amorphous MIL-88 MOF (aMIL-88) was rougher and more porous than the crystalline MIL-88 MOF (cMIL-88). The more abundant activation sites with the amorphous MOF improved sulfur utilization and polysulfide rejection, increasing the initial capacity and capacity retention. The amorphous MIL-88 MOF also had a lower charge transfer resistance and interface resistance, owing to the formation of Li-ion transport channels during amorphization.
The ability to control the porosity of MOF-modified commercial separators has been heavily investigated. Jeon et al.99 used rigorous molecular dynamics simulations to show that a nanosheet layer of Cu3(benzene-1,3,5-tricarboxylate)2 MOF was highly selective against polysulfides but favorable for high Li diffusion. Chen et al.285 fabricated pore-space-partitioned FJU-90 MOFs with pore sizes optimized to around 9 Å for a balance between fast Li-ion diffusion, uniform Li-ion electrodeposition, and high polysulfide rejection. Moreover, the pore structure was modified to expose the Co and Ni metal centers for polysulfide adsorption and catalytic conversion. MOFs may also be used to increase the porosity of other separator modifiers.
One of the limitations of MOF modifications to standard PP or PE separators is the low density and adhesion of MOFs to commercial separators. To improve MOF compatibility, intermediate materials like polydopamine (PDA) may be used. For example, Wu et al.286 used PDA as a glue between PP and ZIF-8. The zinc centers in the MOF had strong interactions with the catechol groups in the PDA for improved adhesion to the PP substrate. The PDA interlayer also increased the number of nucleation sites for ZIF-8 growth, resulting in a denser packing of MOFs that closed the larger PP pores more effectively. Similarly, Kim et al.287 could not coat a pristine PE separator completely with UiO-66 MOFs via vacuum filtration due to the weak interactions between the PE and MOF. To overcome this, a Nafion layer was added, which interacted strongly with both the PE separator and the MOF additive.
4.2 Covalent–organic framework (COF)
COFs are highly nanoporous crystalline materials formed by strong covalent links between organic building blocks.288 COF-based modifications have excellent potential in LSBs used in portable technologies like electric vehicles and smart devices, owing to the low density and high thermal stability.289 While they are not yet under intense study due to their difficult synthesis and instability, their tunable pore sizes and excellent functionalizability leave much room for further development.COFs are promising in LSB separators because of their highly tunable pore size and low density. Zhao et al.290 recently modified a PP separator with a 1,3,5-triformylphloroglucinol (Tp) and 2,5-diaminobenzene sulfonic acid (TpPa–SO3H) COF via interfacial polymerization. The PP/COF exhibited regular pore channels and improved electrolyte wettability to its high polarity, resulting in a 157% higher ionic conductivity than pristine PP. The lithiophilic sulfonic groups in the TpPa–SO3H COF helped block polysulfides via coulombic repulsion, resulting in a low 25% decrease in initial capacity after 500 cycles at 1C. Moreover, having the TpPa–SO3H increased lithium conductivity by 158% than PP (Fig. 10a), while the COF with –SO3H groups showed an 11% higher Li-ion transference than the COF with –COOH groups (Fig. 10b). This was due to the lithiophilic interaction from –SO3H and more regular pore structure for the COF with –SO3H groups.
 | ||
| Fig. 10 (a) Ionic conductivity of the TpPa–SO3H@PP, TpPa–COOH@PP, and PP separators; (b) bar graph of ionic conductivity and Li-ion transference number of the TpPa–SO3H, TpPa–COOH, and PP separators290 (Reproduced with permission from ref. 290. Copyright 2022, Elsevier). (c) Schematic showing the rigid structure of the TAPP-ETTB COF with an optimally designed electron transfer system via the porphyrin292 (Reproduced with permission from ref. 292. Copyright 2022, The American Chemical Society). (d) Adsorption energies between the CB-COF molecule and various sulfur species (Li2Sx,1 ≤ x ≤ 8) based on DFT calculation289 (Reproduced with permission from ref. 289. Copyright 2021, The American Chemical Society). | ||
Zhu et al.289 modified a Celgard separator with a carborane-based COF that exhibited a 72% higher initial capacitance and 27% lower % decay per cycle than a pristine Celgard separator. Fig. 10d shows the binding energies between the COF and LiPSs of various chain lengths. The high polysulfide rejection was attributed to the electrophilic boron centers in the COF that easily adsorbed the polysulfides. Work by Sahu et al.291 has also shown the efficacy of boron atoms as adsorptive centers for sulfur. Moreover, the small 1.2 nm pore sizes sieved the large polysulfides while allowing high Li diffusion.
Because COFs suffer from low electrical conductivity, COFs are often combined with conducting materials such as carbon nanomaterials or conductive polymers before modification to commercial battery membranes. For example, Sun et al.292 combined a COF with graphene. 5,10,15,20-tetrakis(4-aminophenyl)porphyrin cores and 4,4′,4′′,4′′′-(ethene-1,1,2,2-tetrayl)tetrabenzaldehyde linkers were reacted to form a COF called TAPP-ETTB (Fig. 10c). The COF was mixed with graphene in a n-methyl-2-pyrrolidone (NMP) solution and combined with a commercial PP via vacuum filtration. Adding graphene to the COF decreased the square resistance from over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Ω square−1 to 33 Ω square−1. Compared to a PP/graphene separator, the PP/COF/graphene separator exhibited about 55% higher specific capacitance at a range of current densities, owing to improved sulfur utilization, decreased polysulfide shuttling, and diffusion during lithiation/delithiation. Similarly, Shi et al.223 modified a COF with MWCNTs before coating a Celgard separator. A 2D covalent triazine framework (CTF) was mixed with MWCNTs in NMP and slurry coated onto a Celgard separator. The CTF/MWCNTs-modified separator exhibited a 42% lower decay per cycle than an MWCNT-modified separator, owing to the abundant pyridinic nitrogen groups in the CTF adsorbing the polysulfides. Interestingly, the PP/CTF/MWCNT separator exhibited a lower total resistance than a PP/MWCNT separator, resulting in the highest rate capability for the CTF/MWCNT separator. CTFs have also been combined with conductive PEDOT:PSS via a layer-by-layer technique onto a Celgard separator.293 The Celgard separator was pretreated with Nafion for stable layer-by-layer assembly. Likewise, the CTF was functionalized with poly(diallyl dimethyl ammonium chloride) (PDDA) to have a positive charge before layering with negatively charged PEDOT:PSS. Ionic conductivity increased by 14% than a pristine separator, and the composite had a low 33 Ω interfacial resistance due to the higher electrolyte wettability and conductivity of the CTF/PEDOT:PSS composite.
000 Ω square−1 to 33 Ω square−1. Compared to a PP/graphene separator, the PP/COF/graphene separator exhibited about 55% higher specific capacitance at a range of current densities, owing to improved sulfur utilization, decreased polysulfide shuttling, and diffusion during lithiation/delithiation. Similarly, Shi et al.223 modified a COF with MWCNTs before coating a Celgard separator. A 2D covalent triazine framework (CTF) was mixed with MWCNTs in NMP and slurry coated onto a Celgard separator. The CTF/MWCNTs-modified separator exhibited a 42% lower decay per cycle than an MWCNT-modified separator, owing to the abundant pyridinic nitrogen groups in the CTF adsorbing the polysulfides. Interestingly, the PP/CTF/MWCNT separator exhibited a lower total resistance than a PP/MWCNT separator, resulting in the highest rate capability for the CTF/MWCNT separator. CTFs have also been combined with conductive PEDOT:PSS via a layer-by-layer technique onto a Celgard separator.293 The Celgard separator was pretreated with Nafion for stable layer-by-layer assembly. Likewise, the CTF was functionalized with poly(diallyl dimethyl ammonium chloride) (PDDA) to have a positive charge before layering with negatively charged PEDOT:PSS. Ionic conductivity increased by 14% than a pristine separator, and the composite had a low 33 Ω interfacial resistance due to the higher electrolyte wettability and conductivity of the CTF/PEDOT:PSS composite.
While not in direct contact, a conductive interlayer between the modified separator and cathode can significantly improve rate capability. Cao et al.294 fabricated a PP/TpPa–SO3Li COF separator that could not sustain capacity at a current density above 2C. However, adding a CNT interlayer enabled a low 0.039% decay per cycle to a respectable 632.7 mA h g−1 even after 400 cycles at 4C. This was because the CNTs lowered the charge transfer resistance of the sulfur-loaded cathode, which decreased the build-up of inert LiS/S.
5 Inorganic modifications
The most common inorganic modifications to LSB separators include transition metal oxides, metal sulfides, and MXenes. Other promising inorganic modifiers include metal nitrides, borides, phosphides, and fluorides. Inorganic modifiers specialize in effective LiPS rejection via (a) immobilization through strong chemisorption, (b) rapid catalytic conversion with their multi-valent metal component (c) sieving or trapping when using 1D or 2D morphologies.295 Inorganic modifiers are often implemented in commercial polyolefin separators via vacuum filtration.296 However, they often require other conductive materials like carbon-based nanomaterials (Section 3) or conductive polymers (Section 2.3) for practical applications due to their poor electrical conductivity. The performance of LSBs with inorganic/polyolefin composite separators is summarized in Table 4.| Membrane | Specific charge (mA h g−1) | C rate (C) | Cycles | % Loss per cycle | Highlights | Ref. |
|---|---|---|---|---|---|---|
| a Current density measured in A g−1 instead of C rate. | ||||||
| Metal oxides | ||||||
| PP/MgAl2O4/MWCNT | 1370 | 0.1 | 50 | 0.61 | Only impacted cathode side | 106 |
| PP/CeO2/graphene | 1039 | 0.5 | 200 | 0.12 | High sulfur loading | 211 |
| PP/TiO2/MWCNT | 1104 | 0.6 | 900 | 0.07 | Increased separator surface area | 231 |
| PP/MnO/NCNTs | 929 | 1.0 | 500 | 0.07 | Upper current collector | 230 |
| Mn3O4/PP | 572 | 2.5 | 2000 | 0.03 | Oxygen vacancies for selective catalyst | 306 |
| PP/V2O5/graphene | 2028 | 1.0a | 100 | 0.62 | Increased Li-ion insertion/extraction capacity | 299 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Metal sulfides | ||||||
| PP/ZnS | 967 | 0.5 | 200 | 0.04 | Quantum dots with abundant active sites | 314 |
| PP/CoS/KB/C3N4 | 810 | 1.0 | 500 | 0.03 | Capacity retention plateaused after 200 cycles | 256 |
| PP/C–SnS2/AB | 919 | 1.0 | 500 | 0.06 | Chemical etching for defects | 257 |
| PP/WS2/SWCNT | 1069 | 1.0 | 1000 | 0.04 | Porous 3D layer | 237 |
| PP/CuS/graphene | 1029 | 1.0a | 200 | 0.19 | Increased electrolyte wettability | 311 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| MXenes | ||||||
| PP/V2C/SWCNT/V2O5 | 1240 | 0.2 | 500 | 0.17 | MWCNTs reduced V2C nanosheets aggregation | 235 |
| PP/Ti3C2 | 849 | 0.5 | 500 | 0.06 | Decreased charge transfer resistance | 316 |
| PP/Ti3C2Tx | 852 | 1.0 | 600 | 0.06 | CNTs prevented MXene restacking | 225 |
| PP/Ti2C/Nafion | 920 | 1.0 | 1000 | 0.03 | Decreased pore size | 152 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Other inorganic modifiers | ||||||
| PP/purified BNNT | 1429 | 0.3 | 200 | 0.12 | Li dendrite suppression | 326 |
| PP/Ni–Co–P/carbon | 961 | 0.5 | 1000 | 0.06 | High rate capability at high sulfur loadings | 338 |
| PP/CaF2/rGO | 1005 | 0.5 | 420 | 0.06 | LiF formation mitigated dendrites | 209 |
| PP/SrF2/graphene | 1140 | 0.5 | 350 | 0.05 | SrF2 lithophilicity suppressed dendrites | 208 |
| PP/TiN–Si3N4 | 1243 | 0.5 | 500 | 0.09 | Molten salt process reduced synthesis cost | 327 |
| PP/Sb2Se3/rGO | 945 | 1.0 | 500 | 0.03 | Defect engineering increased conductivity | 342 |
| PP/ZnSe/carbon | 1026 | 1.0 | 1000 | 0.04 | MOF-derived ZnSe nanoparticles | 341 |
| PP/VN/NG | 1082 | 1.0 | 300 | 0.07 | Dual redox catalysis | 206 |
| PP/f-BN | 1194 | 1.0 | 1000 | 0.01 | Amino and carboxyl functionalization | 323 |
| PP/Ni0.2Mo0.8N/MWCNT | 1421 | 1.0 | 1400 | 0.05 | Bimetallic synergy in metal nitride | 332 |
| PP/Ni3B/rGO | 572 | 2.0 | 500 | 0.06 | Electron deficient adsorbent | 334 |
| PP/Mo–MoB | 671 | 2.0 | 500 | 0.06 | Heterogeneous catalyst for multi-step LiPS reduction | 335 |
| PP/CoN/CNT | 901 | 2.0 | 250 | 0.11 | Nitrogen vacancies | 227 |
5.1 Metal oxides
Transition metal oxides comprise a broad class of nanomaterials that boast abundant active sites, multiple valence states, and low preparation costs. Their use as modifiers for commercial LSB separators is primarily due to their ability to immobilize and catalyze polysulfides via Lewis acid–base interactions and oxygen vacancies.297 Wang et al.298 recently modified a standard PP separator with MnO2via a self-assembly method from KMnO4. The aforementioned properties of transition metal oxides resulted in a low decay rate of 0.058% per cycle after 500 cycles at 0.5C. Cheng et al.299 used V2O5 microspheres on graphene to increase the specific capacity of the LSB. Because V2O5 has a large electrochemical window that can match with the sulfur cathode, it can theoretically act as a secondary cathode for Li-ion insertion/extraction, increasing the cathode-side capacity of the LSB.300 Hence, Cheng et al.299 reported one of the highest initial discharge capacities at 2028 mA h g−1 at 1 A g−1 current. Fig. 11a shows a special LSB cell designed for in situ XRD analysis. The disappearance of α-S8 during discharge and reappearance during the charging process showed the excellent reversibility of the LiPS redox reaction (Fig. 11b). No Li2S was observed during the charge/discharge, suggesting incomplete LiPS reduction. The consistent presence of long-chain LiPS signals also indicated that the soluble LiPSs remained on the cathode side without any significant diffusion to the anode. | ||
| Fig. 11 (a) Schematic showing the configuration of an LSB used for in situ XRD test with a V2O5-modified separator and (b) in situ XRD contour plot of the LSB with a V2O5-modified separator299 (Reproduced with permission from ref. 299. Copyright 2021, Elsevier). Schematic of the LiPS transformation on the surface of (c) N, F, B-doped CoFe2O4−x on MWCNTs and (d) CoFe2O4 on MWCNTs303 (Reproduced with permission from ref. 303. Copyright 2022, John Wiley and Sons). | ||
In another study, a PP separator modified with Eu2O3 and Ketjen Black yielded a low 0.05% decay per cycle for 500 cycles at 1C, primarily due to the adsorption and catalytic conversion by Eu2O3.297 The KB supplemented the poor electrical conductivity of Eu2O3, boosting its rate capability. A similar synergy was reported between Ti4O7 and KB.301 Both Ti4O7 and KB outperformed the pristine separator and KB-modified separator in low and high sulfur loadings, indicated by the higher initial capacity and longer cycle life. Other conductive nanomaterials have been used to improve the charge transfer between insulating polysulfides and metal oxides, including porous carbons,302 CNTs,303 carbon coatings,304 and conductive polymers like PANI.182
While often doped as nanoparticles into conductive hosts, transition metal oxides may have 1D or 2D morphologies that may be directly layered onto standard polyolefin separators. For example, Huang et al.305 synthesized a layered structure of sodium-containing TiO2 nanowires and TiO2 nanosheets via a hydrothermal process. The mixed structure composite was vacuum filtered through PP to yield a composite separator. The polar TiO2 increased electrolyte wettability and decreased the shuttling effect, as expected. Compared to a separator with only TiO2 nanowires, the nanowire/nanosheet mixed-structure composite had an approximately 40% higher initial capacity at 0.2C, with better rate capability for charge densities between 0.2 to 2C. This was partly due to the thinner deposition of the mixed-structure TiO2 than pure TiO2 nanowires, higher Li-ion diffusion rate, and improved polysulfide rejection capability. Other transition metal oxides like V2O5 are commonly used as nanowires.182
Surface engineering has been used to improve the catalytic activity for multiple LiPS species instead of specific LiPSs. Zhu et al.306 created oxygen vacancies in Mn3O4 to reduce the activation energy of converting soluble long-chain LiPS into insoluble short-chain LiPS. Due to the oxygen vacancies, strong Mn–S bonds were formed between the catalyst and long-chain LiPS, whereas short-chain LiPS formed more Li–O bonds. Moreover, the binding energy calculated by DFT was significantly lower for oxygen-vacant Mn3O4−x (−2.7 eV) than pristine Mn3O4 (−2.2 eV). The strong chemisorption of soluble LiPS reduces the shuttle effect, while the favorable Li–O bonds for insoluble LiPS free the sulfur for faster redox kinetics. In situ XRD supported these findings, with fast disappearance of Li2S8 peaks and rapid appearance of Li2S peaks during discharge, with MnS and LiyMnzO4−x intermediate peaks.
Multi-metal oxide heterostructures are promising for LiPS adsorption owing to their abundant defects providing oxygen vacancies. Hu et al.303 decorated MWCNTs with N, F, and B co-doped CoFe2O4−x and coated a Celgard separator (Fig. 11c). The resulting separator exhibited an astoundingly low capacity decay of 0.016% decay per cycle over 1000 cycles at 1C. The excellent rejection was partly due to the heteroatoms increasing polysulfide affinity, resulting in more physiochemical trapping. Moreover, there was more uniform LiPS adsorption with heteroatom doping than without, as illustrated in Fig. 11d. The oxygen vacancies also improved polysulfide conversion kinetics, resulting in decreased shuttling. Liu et al.307 found a low 0.065% decay per cycle over 1000 cycles at a high current density of 2C using NiFe2O4 bimetallic oxides. Multiphase and multi-component NiFe2O4–NiO heterostructures attached to CNTs were directly grown on a PP substrate yielding uniform and crystalline heterostructure interfaces. This minimized electrical impedance and maximized ionic conductivity, resulting in a high capacity of 1350 mA h g−1 at 0.1C.
5.2 Metal sulfides
Transition metal sulfide nanosheets have abundant metal centers for polysulfide adsorption. Fig. 12a shows the adsorption energies of various LiPSs on MoS2 nanosheets,236 respectively. Compared to the nearly non-existent adsorption energy of LiPS on graphene (Fig. 12b), the metal sulfides have a high affinity for LiPSs, indicated by their highly negative adsorption energies. Liu et al.308 used MoS2 nanosheets, which are known to have excellent Li conductivity, to coat a standard PP separator. An LSB with the modified separator exhibited a 51% higher initial capacitance at a low current density of 0.1C and maintained a high 752 mA h g−1 capacity at 2C. The excellent adsorptive properties of the MoS2 nanosheets resulted in a 93% capacity retention after 500 cycles at 1C. The LSB with a MoS2-modified separator had a low 0.083% decay per cycle due to a mix of excellent polysulfide adsorption and physical blocking. Cheng et al.210 found that sulfur-deficient MoS2 nanoflowers exhibited excellent chemisorption and catalytic conversion of polysulfides but required efficient electron transfer from rGO to increase LiPS conversion rates. | ||
| Fig. 12 (a) Adsorption energy of S8 and Li2Sx (2 < x < 8) on a slab of MoS2.236 (Adapted with permission from ref. 236. Copyright 2022, Elsevier). (b) Adsorption energy of Li2S6 on a slab of WS2 without lithilation (left) and with lithilation (center). The adsorption energy of Li2S6 on graphene (right)237 (Adapted with permission from ref. 237. Copyright 2021, American Chemical Society). (c) UV-vis spectra of Li2S6 solutions upon the adsorption by different sorbents (inset: photograph of the corresponding Li2S6 solutions).236 (Adapted with permission from ref. 236. Copyright 2022, Elsevier). Li anode after 100 cycles at 1C of an LSB with a (d) pristine Celgard separator and (e) WS2-modified separator237 (Adapted with permission from ref. 237. Copyright 2021, American Chemical Society). | ||
Other metal sulfide nanosheets like SnS2,309 VS2,310 and WS2237 have reported improved LiPS adsorption and conversion, improving LSB cycle stability and initial capacity. For example, Li et al.311 found that adding CuS nanoflowers greatly increased electrolyte wettability, with an astounding electrolyte contact angle of 0°. Adding WS2 to a Celgard separator decreased the growth of inactive Li, indicated by the smoother and thinner Li anode surface shown in Fig. 12e (WS2) than in Fig. 12d (Celgard).237 As shown in Fig. 12b, the WS2 had much higher affinities for LiPS adsorption, resulting in the reduction of parasitic polysulfide reactions at the anode. Compositing metal sulfide nanosheets with conductive carbon nanomaterials can produce a synergistic effect that improves the catalytic conversion of polysulfides. For example, Gong et al.236 covered nitrogen-doped Co-CNTs with MoS2 nanosheets, resulting in excellent Li-ion diffusion and electrical conductivity shown in the high 610 mA h g−1 capacity at 4C. As shown in Fig. 12c, the vial containing a Li2S6 solution that was dark red initially became nearly transparent after 2 hours with MoS2/Co-CNTs added to the vial. In contrast, with only N-doped CNTs and N-doped Co-CNTs, the vials had much higher absorptions, indicated by their visually brown hues. Ghazi et al.312 found that vacuum filtration of MoS2 through a Celgard membrane yielded a composite separator with a high Li-ion conductivity of 0.2 mS cm−1 (5.4 times higher than with a GO-modified separator).
Metal sulfide nanoparticles have also been used to modify commercial LSB separators. However, the 0D nanoparticles are often used as dopants for carbon-based modifiers (Section 3). Liu et al.256 added CoS nanoparticles to graphitic carbon nitride and KB. The CoS provided Lewis acid–base interactions that immobilized the polysulfide and hastened its conversion. Li et al.313 embedded ZnS nanoparticles on N-doped carbon nanosheets and subsequently carbonized the composite, yielding unsaturated Zn metal centers that formed strong coordinate bonds with polysulfides. ZnS314 quantum dots have shown excellent adsorption and catalytic properties.
5.3 MXenes
MXenes are 2D nanomaterials composed of transition metal carbides, nitrides, or carbonitrides that are electrically conductive with good electrocatalytic properties. In particular, the abundant metal centers may adsorb polysulfides315 (Fig. 13b) and hasten their conversion.315Fig. 13a shows the high binding energies (>2 eV) of various MXenes for all stable LiPSs. Song et al.316 coated a PP separator with Ti3C2 MXenes via vacuum filtration. Due to the high electronic conductivity of MXene nanosheets, the charge transfer resistance decreased from 101.2 Ω for the pristine PP separator to 45.06 Ω. The Ti metal centers in the modified separator exhibited increased LiPS chemisorption, resulting in a low capacity decay rate of 0.062% per cycle at 0.5C. Moreover, the Mxenes increased sulfur utilization and rate capability, resulting in a 72% higher initial capacity at 1C than with a pristine PP membrane. Mxenes have also been used to increase electrolyte wettability with their abundant functional groups and enhance ionic conductivity.152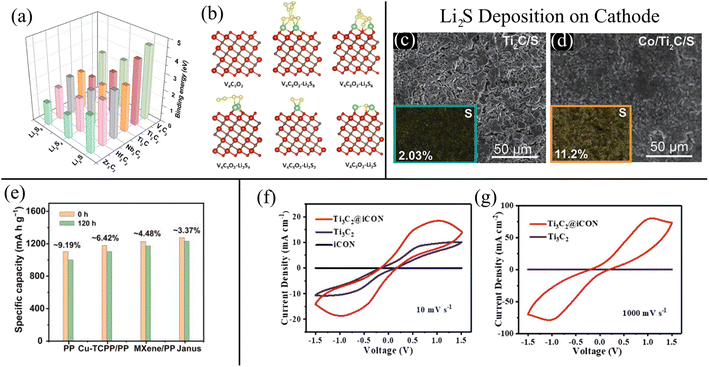 | ||
| Fig. 13 (a) Three-dimensional columnar contrasts of the binding energy of several transition metal carbides for LiPSs. (b) Theoretical structure of V4C3O2 and optimized configurations of Li2Sn (n = 1, 2, 4, 6, 8) adsorption on V4C3O2315 (Reproduced with permission from ref. 315. Copyright 2022, Elsevier). SEM images and the corresponding sulfur distribution map of the (c) Ti2C and (d) Co/Ti2C cathodes after Li2S deposition29 (Reproduced with permission from ref. 29. Copyright 2022, John Wiley and Sons). (e) Self-discharge tests of the LSBs with standard PP, Cu-TCPP-modified, MXene-modified, and Janus (Cu-TPP/MXene) separator configurations after resting for 120 h317 (Reproduced with permission from ref. 317. Copyright 2023, Elsevier). CV curves of symmetric cells at a scan rate of (f) 10 mV s−1 and (g) 1000 mV s−1 for the Ti3C2@iCON and Ti3C2-modified PP separators318 (Reproduced with permission from ref. 318. Copyright 2021, John Wiley and Sons). | ||
MXenes are also highly compatible with other materials that can provide functionality, such as increased electrocatalytic ability and faster Li-ion diffusion via ionic channels. Gu et al.29 added Co nanoparticles to MXenes to improve polysulfide conversion kinetics. As shown in Fig. 13c and d, adding Co nanoparticles increased LiS2 deposition in the cathode, suggesting faster conversion from Li2S8 to Li2S and a smaller loss of sulfur due to a reduced shuttle effect. Liu et al.317 fabricated an asymmetric separator with MXenes facing the cathode side and Cu-TCPP MOFs on the anode side. As shown in Fig. 13e, the pristine separator and MOF/PP separator had a greater self-discharge, owing to the inability to suppress soluble LiPSs on the cathode side. In contrast, the addition of MXenes with almost 50% improved self-discharge resistance than with a standard PP separator. Wang et al.152 used a layer-by-layer to combine MXene with Nafion onto a commercial PP substrate. The composite membrane had a 50% higher initial capacitance than with a pristine PP separator of 1234 mA h g−1, with a low 0.03% decay per cycle for 1000 cycles at 1C. MXenes have also been modified with CNTs to prevent the aggregation of MXene nanosheets while maintaining excellent electrical conductivity.225 Li et al.318 combined a Ti3C2 MXene with guanidinium-based ionic-covalent organic nanosheets (iCON) to suppress the shuttle effect with fast catalytic conversion. While Ti3C2 had decent redox capabilities at low cyclic voltammetry (CV) scan rates (Fig. 13f) but failed at high CV scan rates (Fig. 13g). In contrast, the Ti3C2/iCON composite showed excellent redox capabilities at both CV scan rates, with little change to its shape. The sustainably high catalytic ability of the Ti3C2/iCON composite was due to the high electron mobility afforded by iCON.
5.4 Other inorganic modifiers
Other inorganic modifiers, including nitrides, borides, phosphides, fluorides, and selenides, have been studied for their catalytic abilities as LSB cathodes.319–321 Recently, various catalytic inorganic compounds have also been implemented as modifiers for polyolefin separators. Like the inorganic modifiers discussed previously, these inorganic compounds improve LiPS rejection via strong chemisorption and improve the conversion of soluble LiPSs into insoluble Li2S2 and Li2S. In addition, the materials discussed in this section have unique structures that make them promising for LSB separator modification.Boron nitride (BN), sometimes called inorganic graphite, has recently gained popularity due to its structural similarity with graphene, high thermal conductivity, and chemical stability.322 While the nitrogen groups in BN can bind to the Li in LiPSs, additional functionalization is usually required for improved LiPS chemisorption. Fan et al.323 functionalized BN nanosheets (fBN) with amino and carboxyl groups and vacuum-filtered the modified BN nanosheets through a Celgard separator. The functional groups immobilized LiPSs via chemisorption, resulting in an exceptional 83% capacity retention after 1000 cycles at 3C, whereas an LSB with a Celgard separator retained only 27%. BN coatings on the anode side of the separator can reduce Li dendrite formation. Its high thermal conductivity creates a uniform thermal distribution on the Li anode during charge/discharge, stabilizing the SEI and homogenizing Li plating/stripping.324 Kim et al.325 tested a BN-modified separator through a Li plating/stripping test and found that the BN-modified separators retained a high coulombic efficiency above 85% with no decay even after 100 cycles. SEM analysis showed flat Li granules with a large 1 μm diameter grew on the anode surface with a BN separator, whereas sharp pillars of Li nanowires were formed with a carbon-modified PP separator. BN can also be made into nanotubes (BNNTs) with uniform pore structures for LiPS sieving. Standard synthesis yields impurities such as hexagonal or amorphous boron nitrides, which reduce the BNNT's Li conductivity. In recent work, Kim et al.326 synthesized purified BNNTs of defects before composition with a PP separator via slurry coating. The Li diffusion coefficient was 58% higher for the LSB with purified BNNTs than standard BNNTs and PP. SEM and Li plating/stripping tests showed that purified BNNTs reduced Li dendrite growth and Li inactivation. Purified BNNTs also had strong adsorption for LiPSs, indicated by an 85% decrease in UV-vis absorbance during a simple diffusion test.
Unlike BN, metal nitrides have excellent conductivity and greater adsorption affinity for LiPS due to their abundant metal centers. TiN has shown excellent LiPS adsorption ability and catalytic conversion; however, the high cost, harmful byproducts, and complex synthesis have limited its application.327 To circumvent this, Shen et al.328 devised a one-step solid–state reaction for the growth of TiN on graphene. Zhou et al.327 used a molten salt method (Fig. 14a) to synthesize TiN coated Si3N4 core–shell nanoparticles. Unlike typical high-temperature synthesis, the molten salt process did not require harmful solvents and could be largely recycled, improving the safety and economic viability of TiN. The TiN shell had a dendritic structure that provided a greater surface area for LiPS adsorption.
 | ||
| Fig. 14 (a) Schematic of a molten-salt process for synthesizing TiN/Si3N2 core/shell structures with dendritic TiN327 (Reproduced with permission from ref. 327. Copyright 2023, Elsevier). (b) Schematic illustrating the synthesis of nitrogen-vacant CoN (CoN-Vs) grown on CNT to yield CNT-CoN-Vs. (c) Binding energies of Li2S6, Li2S4, and Li2S on pristine and nitrogen-vacant CoN/CNTs. (d) Diffusion energy barriers of Li2S on pristine and nitrogen-vacant CoN/CNTs.227 (Reproduced with permission from ref. 227. Copyright 2022, Elsevier). (e) Binding energies of Li2S4 on Co2B with boron and cobalt sites, and the binding energy of Li2S4 on Co3O4333(Reproduced with permission from ref. 333. Copyright 2019, American Chemical Society). | ||
Vacancy engineering may also be employed to improve chemisorption and catalytic properties. In recent work, Luo et al.227 synthesized nitrogen-deficient CoN grown on MWCNTs (Fig. 14b) to improve LiPS adsorption and catalytic conversion. As shown in Fig. 14c, DFT calculations confirmed that nitrogen vacancies improved the binding energy, which makes sense as more metal centers are exposed to adsorb the bulky polysulfides. Moreover, the catalytic properties of nitrogen-vacant CoN improved due to its decreased band gap between the d-band in Co and p-band in nitrogen, resulting in improved electron mobility. Fig. 14d shows how nitrogen-vacant CoN/CNTs had smaller energy barriers for Li2S4 diffusion, which would improve LiPS reduction kinetics as LiPS diffusion is critical for the reaction pathway. Yao et al.329 created Te vacancies in a P-doped NiTe2−x electrocatalyst. The bi-metal catalyst was anchored to biologically-derived carbon nanosheets and used to modify a PP separator. With a standard PP separator, in situ Raman spectroscopy showed the formation of soluble LiPSs on the Li anode side during LSB discharge, which remained during recharge. In contrast, no noticeable Raman peaks were detected for the LSB using the electrocatalyst-modified separator. Similarly, selenium vacancies in bimetallic Co9S8−x/FeSe2−y significantly reduced the shuttle effect via excellent chemisorption and electrocatalytic conversion.330 The bimetallic selenide was crystalized onto CNTs before modifying a PP separator. The conversion of soluble LiPSs were detected using in situ XRD, which showed the evolution of α-S8 turning into Li2S during discharge and into β-S8 during recharge. The formation of Se1.1S6.9 was also detected, suggesting the formation of strong chemical bonds between Se from the bimetallic Co9S8−x/FeSe2−y and S from the LiPSs.
Quantum dots are newer but promising additives that boast excellent adsorption properties with high surface area and catalytic abilities. In a recent study by Ma et al.,204 Mo2N quantum dots were also used to improve the Li-ion conduction of nitrogen-doped GO (NGO) by a factor of 3. While the smaller pore sizes of the NGO layer deposited on the PP separator decreased Li-ion conductivity by 56%, the addition of lithiophilic Mo2N supplied attractive forces that improved Li-ion conductivity by 48% more than pristine PP. The excellent polysulfide adsorption resulted in an LSB with one of the highest reported capacity retentions, indicated by a low 0.039% decay per cycle for 800 cycles at a high current of 2C. Similarly, Nitrogen-doped nanodots like NbN331 have been used to increase the rate of LiPS conversion. In situ Raman spectroscopy was performed on a uniquely designed test battery during the charge/discharge process to determine the presence of LiPSs on the Li anode side. During the first charge/discharge, Raman signals for the soluble Li2S8, Li2S6, and Li2S4 were detected for LSBs using a standard PP separator and N-graphene/PP separator. In contrast, no Raman signals were found when employing an NbN/N-graphene/PP nanocomposite separator.
Multi-metal nitrides can also be designed to overcome the limitations of specific metals. For example, Zhang et al.332 synthesized bimetallic Ni0.2Mo0.8N on MWCNTs to overcome the Mo leaching by polysulfides in Mo2N and the low conductivity of Ni3N. Consequently, the LSB with the bimetallic nitride exhibited a 6% and 50% lower capacity decay rate than an LSB with Mo2N and Ni3N-modified separators, respectively.
Metal borides are especially promising for LiPS adsorption because boron atoms have empty orbitals that have a high affinity for polysulfides. Unlike metal oxides, sulfides, nitrides, and MXenes that depend on their metal centers for chemisorption with S atoms, metal borides have both metal and boron components binding to the long sulfur chains.333Fig. 14e shows that the binding energy between the sulfur atom in Li2S4 and boron in Co2B is only slightly lower than the binding energy between Co and S in Co3O4. Moreover, the binding energy is 68% higher between Co and S in Co2B than in Co3O4. In recent work, Shrshr et al.334 synthesized Ni3B nanoparticles uniformly distributed on rGO, which were subsequently slurry coated onto a PP separator. A simple diffusion test showed that Ni3B had less adsorptive ability than Ni3B/rGO, suggesting synergistic properties between the metal boride and conductive carbon host. This was further supported by a self-discharge test wherein Ni3B/rGO lost 2.2% capacity while bare Ni3B lost 4.0% and bare rGO lost 18.8% of its initial capacity. Guo et al.335 improved the catalytic properties of MoB by synthesizing a metallic Mo and MoB heterostructure via a molten salt method. Metallic Mo had a lower Gibbs free energy (ΔG) of 0.31 eV for the reaction of soluble Li2S4 into insoluble Li2S2, whereas MoB had a high ΔG of 1.42 eV. Then, for the subsequent reaction from Li2S2 into Li2S, the reverse happened where metallic Mo had a large ΔG of 0.76 eV, whereas MoB had a lower ΔG of 0.005 eV. The heterogenous composition of the modifier enabled efficient bidirectional catalysis by having the different components favor different steps of LiPS reduction.
While metal fluorides, like other ionic salts, have a good affinity for LiPS adsorption, they have the unique ability to react with Li to form LiF, which may be used to mitigate Li dendrite growth.336 Jing et al.209 found that during cycling, the fluorine in CaF2 and Li in lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) electrolyte formed a layer of LiF. The LiF promoted fast and uniform Li-ion diffusion and deposition. Plating/stripping tests and SEM analysis showed a low and steady overpotential, suggesting minimal dendrite formation. In a more recent study by Jing et al.208 found that SrF2 also reduced Li dendrite formation due to the lithiophilicity of SrF2. The sandwich structure comprising SrF2 and graphene minimized local current densities at the Li anode to further suppress Li dendrites.
Metal phosphides benefit from a similar affinity for LiPSs but have greater electrical conductivities than metal oxides and sulfides.337 The greater electron mobility allows metal phosphides to potentially have great catalytic ability for improved LiPS rejection. Metal phosphides also boast greater thermal and chemical stability for improved battery safety. Wu et al.338 found that Ni and Co-phosphide-modified carbon nanocages promoted faster Li-ion diffusion and improved catalytic conversion of LiPS. Still, the binding energy to LiPSs can be improved. Wang et al.339 recently used defect engineering to increase the electrical conductivity of Mo3P. Mo3P was modified with MoO2 and MoP to create dangling bonds and oxygen vacancies that decreased the formation energy for generating electron holes. Moreover, the defects exposed more metal active sites for LiPS chemisorption.
Like metal sulfides, metal selenides have similar crystal structures, polar characteristics, and adsorption capacity for LiPSs. However, metal selenides boast greater electrical conductivity due to looser valence electrons and greater defect density.340 Zhang et al.341 synthesized ZnSe nanoparticles using ZIF-8 MOF as a template. The highly structured ZnSe nanoparticles showed excellent chemisorption of LiPSs, indicated by nearly transparent UV-vis absorbance. Tian et al.342 used defect engineering to increase the conductivity and catalytic properties of Sb2Se3. Defects were introduced via chemical reduction and thermal shark in an Ar atmosphere. Sb2Se3 with abundant Se vacancies had a 5-times-higher electrical conductivity. When combined with rGO, the Sb2Se3−x maintained stable voltage curves even at a high current rate of 8C. DFT analysis also showed that Se vacancies increased the binding affinity of S–Sb and Li–Se. Consequently, the LSB with Sb2Se3−x-modified separator had a 25% great capacity retention than with a pristine Sb2Se3-modified separator.
6 Future prospects
Various nanocomposite separators have successfully lowered polysulfide shuttling to raise the stability of LSBs to commercially viable standards, withstanding more than 1000 cycles and holding specific capacities above 1300 mA h g−1.343 Composite separators have also improved Li anode stability, decreasing parasitic LiPS reactions and Li dendrite formation. Moreover, the Li-ion diffusion rates, sulfur utilization, thermal stability, and rate capability of LSBs have been greatly enhanced. Still, there is more to be done before LSBs are commercially viable.Firstly, LSBs with modified separators are limited by their polyolefin base. Modifying widely available Li-ion battery separators, such as Celgard membranes, will be important for the initial industrial scale-up and commercial production, but novel separators based on nanofibers may be necessary for competitive performance with LIBs. Nanofiber-based separators have better thermal stability, Li-ion conductivity, electrolyte wettability, flexibility, modifiability, and LiPS adsorption ability than standard polyolefin separators.344 Nonwoven membranes are often made from poly(vinylidene) fluoride (PVDF)345 or polyimide,346 and electrospun membranes can be made from various polymers like polyacrylonitrile (PAN)347 and polyvinylpyrrolidone (PVP).348 However, such membranes tend to have limited LiPS rejection capabilities and high cost of fabrication.349 More work into optimizing fiber roughness,350,351 polymer blend ratios,352 fiber coating,353,354 and nanomaterial doping355–357 may open up solutions to improved fiber-based separators for LSBs.
The complex reaction pathways of LSBs, especially involving the solid–liquid–solid conversion of LiPSs, need to be better understood using improved characterization techniques. Ex situ methods like UV-vis spectroscopy of penetrate experiments and SEM/TEM of electrodes show the macroscopic consequences of using different modifiers. However, the reaction pathways (especially involving catalytic materials) are still unclear and can be improved by designing analysis setups for in situ spectroscopic techniques like Infrared, UV-vis, and Raman spectroscopy and XRD analysis.358 Other in situ analytical techniques used in LIB research, like in situ SEM,359 EDX,360 and mass spectroscopy361 should be employed in LSB investigations to better understand the reactions occurring at the Li anode and modified separator. These in situ tests will require uniquely designed LSBs for testing.
LSBs still require much more development in their overall design before becoming commercially viable. While specific capacities are the primary measure for LSB performance that account for the weight of the electrode, this measure does not account for the weight of the whole battery. The weight contributed by thick modification layers may become significant, especially for smaller devices like drones. For weight-dependent applications like EVs or portable electronics, the energy density of the whole device needs to be accounted for. Similarly, volumetric energy densities are almost never reported but are critical for small form-factor applications. The effect of the modified separator on power densities could also be investigated along side performance measures at varying C rates. The tested cells in laboratory settings also most often use Li metal anodes in coin cell configurations. However, the battery industry has shifted away from Li metal anodes due to safety, cost, and ethical concerns in favor of lithiated carbon-based anodes that have lower capacities.362 Other battery configurations like pouch cells and winding-type cells require LSBs to consider other factors like volume expansion, binder strength, and electrolyte volume, amongst other electrochemical changes. Improvements to the sulfur cathode must also be made for LSBs to sustain higher power densities and greater sulfur loadings.363
The cost and processing complexity of most modifiers are considered before beginning investigations. Hence, many studies design composite separators using cheap, sustainable, or easily processible materials like common polymers, porous carbons, and metal oxides. Studies involving more obscure materials like MOFs and MXenes test the viability of the composite membranes and help answer the question of whether industrial-level production would be worth the cost. Overall, the industrial scale-up of lab-scale composite separator fabrication has still not matured and will be a critical bridge to cross before LSB commercialization. The optimal battery form factor for LSBs has also yet to be tested. Moreover, novel separators for LSBs with highly uniform pores or hierarchically porous structures have also been fabricated via templating methods,364,365 anodic oxidation,366 and advanced polymerization techniques.367 While such fabrication techniques are popular and well-published for membranes used in nanofiltration and wastewater treatment,368 these techniques may be tested for LSB separators.
Lastly, the maintenance and longevity of LSBs still need to be examined. Because the primary focus of LSB research has been on reducing the shuttle effect for higher cycling stability, there is much room for improvement regarding thermal stability, physical stress resistance, and volume expansion resistance, especially at high current rates. As with LIBs, thermal stability is critical if LSBs are to be used in EVs and mobile devices with fast charge/discharge requirements that result in overheating.369 Separators coated with heat-resistant materials like ethylene-vinyl acetate copolymer370 or separators fabricated from thermally stable polymers like polyimide371 should be investigated more to improve LSB safety. With portable and wearable electronics being ubiquitous in society, flexible batteries have become more important due to better resistance against physical stress. Few recent investigations have fabricated highly flexible LSBs,372,373 with many studies using novel separators.374,375 Battery expansion due to gas build-up, volume expansion, and separator swelling is an issue prevalent in Li-ion batteries376 that has not been addressed much for LSBs, despite facing similar problems. Such tests for stability are critical before LSBs may be commercially available.
7 Conclusion
LSBs are one of the most promising next-generation batteries that may displace LIB's dominance in portable technology, electric vehicles, and other energy storage applications. LSBs boast a 5-times-higher theoretical capacity than LIBs and use more affordable, environmentally friendly, safer, and abundant materials than LiBs. However, current LSB materials and designs are not yet commercially viable due to low conductivity, dendrite formation, and the polysulfide shuttle effect. Much development in LSBs in recent years involves reducing LiPS shuttling by modifying polyolefin separators, which are used in most LIBs. Polymer/polyolefin composites have facile preparation methods with easily tunable pore sizes and various functional groups that help repel or immobilize polysulfides. Carbon-modified separators have significantly higher conductivity, allowing fast LiPS conversion, enhanced rate capability, and Li dendrite suppression. The uniform and controllable pore structure of organic framework/polyolefin composites provide selective ion channels for high Li-ion diffusion with limited LiPS shuttling. Inorganic, metal-based additives provide catalytic active sites that promote the adsorption and conversion of soluble LiPS to insoluble Li2S. Modifying polyolefin separators is essential in bringing LSBs to market because it leverages the infrastructure of LIBs' polyolefin separators to lower production costs. However, separator modifications alone are likely insufficient for fully commercializing LSBs, and more work needs to be done in refining the cathode, anode, and electrolyte. Novel separators based on nanofiber technology, graphene sheets, xerogels, or other materials may be required to reach higher efficiencies closer to the theoretical limit of LSBs. With the exponentially growing demand for new energy storage technologies and the potential of LSBs to meet those demands, it is an exciting time to investigate LSBs.Conflicts of interest
There are no conflicts to declare.References
- A. A. Tidblad, K. Edström, G. Hernández, I. de Meatza, I. Landa-Medrano, J. Jacas Biendicho, L. Trilla, M. Buysse, M. Ierides, B. P. Horno, Y. Kotak, H. G. Schweiger, D. Koch and B. S. Kotak, Energies, 2021, 14, 4223 CrossRef CAS.
- M. Khalid, F. Ahmad, B. K. Panigrahi and L. Al-Fagih, J. Energy Storage, 2022, 53, 105084 CrossRef.
- L. Jonnatan, C. L. Seaton, K. L. Rush, E. P. H. Li and K. Hasan, Int. J. Environ. Res. Public Health, 2022, 19, 8231 CrossRef CAS PubMed.
- S. Katsumata, T. Ichikohji, S. Nakano, S. Yamaguchi and F. Ikuine, Comput. Hum. Behav. Rep., 2022, 5, 100168 CrossRef PubMed.
- C. Kuzemko, M. Blondeel, C. Dupont and M. C. Brisbois, Energy Res. Soc. Sci., 2022, 93, 102842 CrossRef.
- C. Sturm, J. Ind. Bus. Econ., 2022, 49, 835–878 CrossRef.
- G. D. Scholes, M. Jones and S. Kumar, J. Phys. Chem. C, 2007, 111, 13777–13785 CrossRef CAS.
- A. Kim, J. K. Dash, P. Kumar and R. Patel, ACS Appl. Electron. Mater., 2022, 4, 27–58 CrossRef CAS.
- S. Günes, K. P. Fritz, H. Neugebauer, N. S. Sariciftci, S. Kumar and G. D. Scholes, Sol. Energy Mater. Sol. Cells, 2007, 91, 420–423 CrossRef.
- S. Kumar and G. D. Scholes, Microchim. Acta, 2007, 160(3), 315–325 CrossRef.
- S. P. Gupta, B. A. Kakade, B. R. Sathe, Q. Qiao, D. J. Late and P. S. Walke, ACS Appl. Energy Mater., 2020, 3, 11398–11409 CrossRef CAS.
- A. Kim, P. Kumar, P. K. Annamalai and R. Patel, Adv. Mater. Interfaces, 2022, 1–27, DOI:10.1002/admi.202201659.
- A. Kim and A. Simson, Int. J. Energy Environ. Eng., 2022 DOI:10.1007/s40095-022-00524-2.
- X. Zou, X. Huang, A. Goswami, R. Silva, B. R. Sathe, E. Mikmeková and T. Asefa, Angew. Chem., 2014, 126, 4461–4465 CrossRef.
- S. S. Narwade, S. M. Mali, P. D. Tanwade, P. P. Chavan, A. V. Munde and B. R. Sathe, New J. Chem., 2022, 46, 14004–14009 RSC.
- S. Kumaravel, E. Kim, B. B. Kale, A. Adhikari, R. Patel and S. Kundu, ChemElectroChem, 2022, 9, e202200724 CrossRef CAS.
- H. Jung, A. Karmakar, A. Adhikari, R. Patel and S. Kundu, Sustainable Energy Fuels, 2022, 6, 640–663 RSC.
- S. S. Narwade, S. M. Mali and B. R. Sathe, New J. Chem., 2021, 45, 3932–3939 RSC.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- M. Winter, B. Barnett and K. Xu, Chem. Rev., 2018, 118, 11433–11456 CrossRef CAS PubMed.
- S. A. Mane, A. A. Kashale, G. P. Kamble, S. S. Kolekar, S. D. Dhas, M. D. Patil, A. V. Moholkar, B. R. Sathe and A. V. Ghule, J. Alloys Compd., 2022, 926, 166722 CrossRef CAS.
- R. Chavan, G. Kamble, A. Kashale, S. Kolekar, B. Sathe and A. Ghule, ChemistrySelect, 2022, 7, e202202166 CAS.
- G. P. Kamble, A. A. Kashale, S. S. Kolekar, I. W. P. Chen, B. R. Sathe and A. V. Ghule, J. Mater. Sci. Mater. Electron., 2021, 325(32), 5859–5869 CrossRef.
- S. H. Chung and A. Manthiram, Adv. Mater., 2019, 31, 1901125 CrossRef PubMed.
- X. Yu and A. Manthiram, Adv. Funct. Mater., 2020, 30, 2004084 CrossRef CAS.
- H. Hao, T. Hutter, B. L. Boyce, J. Watt, P. Liu and D. Mitlin, Chem. Rev., 2021, 122, 8053–8125 CrossRef PubMed.
- H. J. Peng, J. Q. Huang and Q. Zhang, Chem. Soc. Rev., 2017, 46, 5237–5288 RSC.
- Q. Gu, Y. Qi, J. Chen, M. Lu and B. Zhang, Small, 2022, 18, 2204005 CrossRef CAS PubMed.
- Y. Lin, Y. Zhou, S. Huang, M. Xiao, D. Han, J. Qin, S. Wang and Y. Meng, Adv. Energy Mater., 2022, 12, 2201912 CrossRef CAS.
- D. Xiong, S. Huang, D. Fang, D. Yan, G. Li, Y. Yan, S. Chen, Y. Liu, X. Li, Y. Von Lim, Y. Wang, B. Tian, Y. Shi and H. Y. Yang, Small, 2021, 17, 1–10 Search PubMed.
- M. Zhao, X. Chen, X. Y. Li, B. Q. Li and J. Q. Huang, Adv. Mater., 2021, 33, 1–9 Search PubMed.
- A. Eftekhari, ACS Sustainable Chem. Eng., 2019, 7, 3684–3687 CrossRef CAS.
- Q. H. Nguyen, V. T. Luu, S. N. Lim, Y. W. Lee, Y. Cho, Y. S. Jun, M. H. Seo and W. Ahn, ACS Appl. Mater. Interfaces, 2021, 13, 28036–28048 CrossRef CAS PubMed.
- C. Li, Z. Xi, D. Guo, X. Chen and L. Yin, Small, 2018, 14, 1–21 CAS.
- E. Zhao, K. Nie, X. Yu, Y. S. Hu, F. Wang, J. Xiao, H. Li and X. Huang, Adv. Funct. Mater., 2018, 28, 1–21 Search PubMed.
- Y. X. Yin, S. Xin, Y. G. Guo and L. J. Wan, Angew. Chem., Int. Ed., 2013, 52, 13186–13200 CrossRef CAS PubMed.
- M. Wild, L. O'Neill, T. Zhang, R. Purkayastha, G. Minton, M. Marinescu and G. J. Offer, Energy Environ. Sci., 2015, 8, 3477–3494 RSC.
- A. Manthiram, Y. Fu, S. H. Chung, C. Zu and Y. S. Su, Chem. Rev., 2014, 114, 11751–11787 CrossRef CAS PubMed.
- R. Cheng, Y. Guan, Y. Luo, C. Zhang, Y. Xia, S. Wei, M. Zhao, Q. Lin, H. Li, S. Zheng, F. Rosei, L. Sun, F. Xu and H. Pan, J. Mater. Sci. Technol., 2022, 101, 155–164 CrossRef.
- Q. Li, Z. Ma, J. Zhao, K. Shen, T. Shi, Y. Xie, Y. Fan, X. Qin and G. Shao, J. Power Sources, 2022, 521, 230929 CrossRef CAS.
- Y. C. Huang, H. I. Hsiang and S. H. Chung, ACS Sustainable Chem. Eng., 2022, 10, 9254–9264 CrossRef CAS.
- C. Y. Zhang, C. Zhang, J. L. Pan, G. W. Sun, Z. Shi, C. Li, X. Chang, G. Z. Sun, J. Y. Zhou and A. Cabot, eScience, 2022, 2, 405–415 CrossRef.
- X. Liang, Y. Zhang, Y. Ning, D. Huang, L. Lan, S. Li and S. Brutti, Nanomater, 2022, 12, 2614 CrossRef CAS PubMed.
- F. Schmidt, S. Ehrling, K. Schönherr, S. Dörfler, T. Abendroth, H. Althues and S. Kaskel, Energy Technol., 2022, 10, 2100721 CrossRef CAS.
- X. Zhong, D. Wang, J. Sheng, Z. Han, C. Sun, J. Tan, R. Gao, W. Lv, X. Xu, G. Wei, X. Zou and G. Zhou, Nano Lett., 2022, 22, 1207–1216 CrossRef CAS PubMed.
- D. Guo, X. Zhang, M. Liu, Z. Yu, X. Chen, B. Yang, Z. Zhou and S. Wang, Adv. Funct. Mater., 2022, 32, 2204458 CrossRef CAS.
- X. Lang, T. Wang, Z. Wang, L. Li, C. Yao and K. Cai, Electrochim. Acta, 2022, 403, 139723 CrossRef CAS.
- Z. Xiong, J. Li, Y. Sun, Y. Lin, L. Du, Z. Wei, M. Wu, K. Shi and Q. Liu, J. Alloys Compd., 2022, 899, 163245 CrossRef CAS.
- R. Chu, T. T. Nguyen, Y. Bai, N. H. Kim and J. H. Lee, Adv. Energy Mater., 2022, 12, 2102805 CrossRef CAS.
- K. Shi, Y. Lin, Z. Xiong, J. Li, S. Zhang and Q. Liu, Chem. Eng. J., 2022, 430, 132692 CrossRef CAS.
- Y. Wu, D. Li, J. Pan, Y. Sun, W. Huang, M. Wu, B. Zhang, F. Pan, K. Shi and Q. Liu, J. Mater. Chem. A, 2022, 10, 16309–16318 RSC.
- J. Liu, Y. Ding, Z. Shen, H. Zhang, T. Han, Y. Guan, Y. Tian and P. V. Braun, Adv. Sci., 2022, 9, 2103517 CrossRef CAS PubMed.
- T. Tonoya, Y. Matsui, H. Hinago and M. Ishikawa, Electrochem. Commun., 2022, 140, 107333 CrossRef CAS.
- V. Marangon, E. Scaduti, V. F. Vinci and J. Hassoun, ChemElectroChem, 2022, 9, e202200374 CrossRef CAS.
- D. Li, H. Li, S. Zheng, N. Gao, S. Li, J. Liu, L. Hou, J. Liu, B. Miao, J. Bai, Z. Cui, N. Wang, B. Wang and Y. Zhao, J. Colloid Interface Sci., 2022, 607, 655–661 CrossRef CAS PubMed.
- S. S. Narwade, S. M. Mali, A. K. Tapre and B. R. Sathe, New J. Chem., 2021, 45, 20266–20271 RSC.
- A. V. Munde, B. B. Mulik, P. P. Chavan, V. S. Sapner, S. S. Narwade, S. M. Mali and B. R. Sathe, J. Phys. Chem. C, 2021, 125, 2345–2356 CrossRef CAS.
- V. S. Sapner and B. R. Sathe, New J. Chem., 2021, 45, 4666–4674 RSC.
- M. Wang, M. Zhang, J. Li, S. Kumar, G. C. Walker, G. D. Scholes and M. A. Winnik, ACS Appl. Mater. Interfaces, 2010, 2, 3160–3169 CrossRef CAS PubMed.
- M. Wang, S. Kumar, A. Lee, N. Felorzabihi, L. Shen, F. Zhao, P. Froimowicz, G. D. Scholes and M. A. Winnik, J. Am. Chem. Soc., 2008, 130, 9481–9491 CrossRef CAS PubMed.
- L. Vijaya, S. Suresh, R. Patel and E. B. Gowd, ACS Macro Lett., 2022, 11, 1272–1277 CrossRef CAS PubMed.
- L. Shen, A. Pich, D. Fava, M. Wang, S. Kumar, C. Wu, G. D. Scholes and M. A. Winnik, J. Mater. Chem., 2008, 18, 763–770 RSC.
- A. Kim, A. Hosseinmardi, P. K. Annamalai, P. Kumar and R. Patel, ChemistrySelect, 2021, 6, 4948–4967 CrossRef CAS.
- A. Kim, I. Varga, A. Adhikari and R. Patel, Nanomaterials, 2021, 11, 2809 CrossRef CAS PubMed.
- R. Patel, J. T. Park, M. Patel, J. K. Dash, E. B. Gowd, R. Karpoormath, A. Mishra, J. Kwak and J. H. Kim, J. Mater. Chem. A, 2017, 6, 12–29 RSC.
- M. Subhramannia, B. K. Balan, B. R. Sathe, I. S. Mulla and V. K. Pillai, J. Phys. Chem. C, 2007, 111, 16593–16600 CrossRef CAS.
- I. Uddin, S. M. Abzal, K. Kalyan, S. Janga, A. Rath, R. Patel, D. K. Gupta, T. R. Ravindran, H. Ateeq, M. S. Khan and J. K. Dash, ACS Omega, 2022, 46, 42438–42445 CrossRef PubMed.
- B. A. Kakade, S. S. Shintri, B. R. Sathe, S. B. Halligudi and V. K. Pillai, Adv. Mater., 2007, 19, 272–275 CrossRef CAS.
- G. D. Scholes, J. Kim, C. Y. Wong, V. M. Huxter, P. S. Nair, K. P. Fritz and S. Kumar, Nano Lett., 2006, 6, 1765–1771 CrossRef CAS PubMed.
- J. Kim, C. Y. Wong, P. S. Nair, K. P. Fritz, S. Kumar and G. D. Scholes, J. Phys. Chem. B, 2006, 110, 25371–25382 CrossRef CAS PubMed.
- S. Jafarzadeh, E. Thormann, T. Rönnevall, A. Adhikari, P. E. Sundell, J. Pan and P. M. Claesson, ACS Appl. Mater. Interfaces, 2011, 3, 1681–1691 CrossRef CAS PubMed.
- S. Jafarzadeh, A. Adhikari, P. E. Sundall and J. Pan, Prog. Org. Coat., 2011, 70, 108–115 CrossRef CAS.
- S. Radhakrishnan and A. Adhikari, J. Power Sources, 2006, 155, 157–160 CrossRef CAS.
- S. Radhakrishnan, B. T. S. Ramanujam, A. Adhikari and S. Sivaram, J. Power Sources, 2007, 163, 702–707 CrossRef CAS.
- S. Patil, H. Ghadi, N. Ramgir, A. Adhikari and V. R. Rao, Sens. Actuators, B, 2019, 286, 583–590 CrossRef CAS.
- S. H. Oh and R. Patel, Membr. J., 2020, 30, 228–241 CrossRef CAS.
- R. Fang, S. Zhao, Z. Sun, D. W. Wang, H. M. Cheng and F. Li, Adv. Mater., 2017, 29, 1–25 CrossRef PubMed.
- M. Rana, M. Li, X. Huang, B. Luo, I. Gentle and R. Knibbe, J. Mater. Chem. A, 2019, 7, 6596–6615 RSC.
- J. Zheng, M. Gu, H. Chen, P. Meduri, M. H. Engelhard, J. G. Zhang, J. Liu and J. Xiao, J. Mater. Chem. A, 2013, 1, 8464–8470 RSC.
- R. Pai, A. Singh, M. H. Tang and V. Kalra, Commun. Chem., 2022, 5(1), 1–11 CrossRef PubMed.
- M. C. Li, Z. Liu, L. Tan, Q. Y. Zhou, J. J. Zhang, P. P. Hou, X. J. Jin, T. B. Lv, Z. Q. Zhao, Z. Zeng, S. Deng and G. P. Dai, ACS Sustainable Chem. Eng., 2022, 10, 10223–10233 CrossRef CAS.
- X. Sun, D. Tian, X. Song, B. Jiang, C. Zhao, Y. Zhang, L. Yang, L. Fan, X. Yin and N. Zhang, Nano Energy, 2022, 95, 106979 CrossRef CAS.
- N. Akhtar, X. Sun, M. Yasir Akram, F. Zaman, W. Wang, A. Wang, L. Chen, H. Zhang, Y. Guan and Y. Huang, J. Energy Chem., 2020, 52, 310–317 CrossRef.
- L. P. Hou, Z. Li, N. Yao, C. X. Bi, B. Q. Li, X. Chen, X. Q. Zhang and Q. Zhang, Adv. Mater., 2022, 2205284, 1–9 Search PubMed.
- S. Li, W. Zhang, J. Zheng, M. Lv, H. Song and L. Du, Adv. Energy Mater., 2021, 11, 1–24 Search PubMed.
- Y. Luo, L. Guo, M. Xiao, S. Wang, S. Ren, D. Han and Y. Meng, J. Mater. Chem. A, 2020, 8, 4629–4646 RSC.
- Q. Huang, J. Xu, M. Fang, L. Ma, Y. Cao, C. Fan, S. Hu, X. Zhang and D. Niu, ChemistrySelect, 2022, 7, e202201484 CAS.
- A. Liu, S. Li, Z. Jiang, J. Du, Y. Tao, J. Lu, Y. Cheng and H. Wang, J. Power Sources, 2022, 521, 230947 CrossRef CAS.
- J. Zhu, M. Yanilmaz, K. Fu, C. Chen, Y. Lu, Y. Ge, D. Kim and X. Zhang, J. Membr. Sci., 2016, 504, 89–96 CrossRef CAS.
- J. Chen, T. Kang, Y. Cui, J. Xue, H. Xu and J. Nan, J. Power Sources, 2021, 496, 229862 CrossRef CAS.
- S. Ponnada, M. S. Kiai, D. B. Gorle and A. Nowduri, Nanoscale Adv., 2021, 3, 4492–4501 RSC.
- B. Dang, Q. Li, Y. Luo, R. Zhao, J. Li and F. Wu, J. Alloys Compd., 2022, 915, 165375 CrossRef CAS.
- J. Chen, T. Kang, Y. Cui, J. Zhao, J. Xue, H. Xu and J. Nan, Energy Technol., 2022, 10, 2101040 CrossRef CAS.
- S. Jia, K. Huang, J. Long, S. Yang, Y. Liang, N. Yang and J. Xiao, J. Appl. Polym. Sci., 2021, 138, 1–11 Search PubMed.
- N. Yang, Y. Liang and S. Jia, Macromol. Mater. Eng., 2021, 306, 2100300 CrossRef CAS.
- C. Liu, J. Hu, Y. Zhu, Y. Yang, Y. Li and Q.-H. Wu, Materials, 2022, 15, 7527 CrossRef CAS PubMed.
- Z. Zhang, Z. Fang, Y. Xiang, D. Liu, Z. Xie, D. Qu, M. Sun, H. Tang and J. Li, Carbohydr. Polym., 2021, 255, 117469 CrossRef CAS PubMed.
- T. Jeon and S. C. Jung, J. Mater. Chem. A, 2021, 9, 23929–23940 RSC.
- S. Huang, Z. Wang, Y. Von Lim, Y. Wang, Y. Li, D. Zhang and H. Y. Yang, Adv. Energy Mater., 2021, 11, 2003689 CrossRef CAS.
- H. Zhang, Q. Liu, S. Ruan, C. Ma, X. Jia, W. Qiao, L. Ling and J. Wang, Appl. Surf. Sci., 2022, 578, 152022 CrossRef CAS.
- X. Zhang, G. Li, Y. Zhang, D. Luo, A. Yu, X. Wang and Z. Chen, Nano Energy, 2021, 86, 106094 CrossRef CAS.
- G. Wang, J. Li, Z. Du, Z. Ma and G. Shao, Membranes, 2022, 12, 134 CrossRef CAS PubMed.
- J. Zhang, Q. Zhang, X. Qu, G. Xu, B. Fan, Z. Yan, F. Gui and L. Yang, Appl. Surf. Sci., 2022, 574, 151559 CrossRef CAS.
- K. Xie, K. Yuan, K. Zhang, C. Shen, W. Lv, X. Liu, J. G. Wang and B. Wei, ACS Appl. Mater. Interfaces, 2017, 9, 4605–4613 CrossRef CAS PubMed.
- M. Raja, S. Suriyakumar, N. Angulakshmi and A. Manuel Stephan, Inorg. Chem. Front., 2017, 4, 1013–1021 RSC.
- M. Wang, Z. Bai, T. Yang, C. Nie, X. Xu, Y. Wang, J. Yang, S. Dou and N. Wang, Adv. Energy Mater., 2022, 12, 2201585 CrossRef CAS.
- S. F. Ng, M. Y. L. Lau and W. J. Ong, Adv. Mater., 2021, 33, 2008654 CrossRef CAS PubMed.
- K. Liu, H. Zhao, D. Ye and J. Zhang, Chem. Eng. J., 2021, 417, 129309 CrossRef CAS.
- Q. Xiao, J. Yang, X. Wang, Y. Deng, P. Han, N. Yuan, L. Zhang, M. Feng, C. an Wang and R. Liu, Carbon Energy, 2021, 3, 271–302 CrossRef CAS.
- Y. C. Jiang, H. M. U. Arshad, H. J. Li, S. Liu, G. R. Li and X. P. Gao, Small, 2021, 17, 2005332 CrossRef CAS PubMed.
- H. Li, Y. Li and L. Zhang, SusMat, 2022, 2, 34–64 CrossRef CAS.
- X. Liu, J. Q. Huang, Q. Zhang and L. Mai, Adv. Mater., 2017, 29, 1601759 CrossRef PubMed.
- H. J. Peng, J. Q. Huang, X. B. Cheng and Q. Zhang, Adv. Energy Mater., 2017, 7, 1700260 CrossRef.
- J. Zhang, H. Huang, J. Bae, S. H. Chung, W. Zhang, A. Manthiram and G. Yu, Small Methods, 2018, 2, 1700279 CrossRef.
- Y. Liu, Y. Elias, J. Meng, D. Aurbach, R. Zou, D. Xia and Q. Pang, Joule, 2021, 5, 2323–2364 CrossRef CAS.
- J. Guo, H. Pei, Y. Dou, S. Zhao, G. Shao and J. Liu, Adv. Funct. Mater., 2021, 31, 2010499 CrossRef CAS.
- G. Liu, Q. Sun, Q. Li, J. Zhang and J. Ming, Energy Fuels, 2021, 35, 10405–10427 CrossRef CAS.
- X. Q. Zhang, Q. Jin, Y. L. Nan, L. P. Hou, B. Q. Li, X. Chen, Z. H. Jin, X. T. Zhang, J. Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2021, 60, 15503–15509 CrossRef CAS PubMed.
- Q. Pang, X. Liang, C. Y. Kwok and L. F. Nazar, Nat. Energy, 2016, 1(9), 1–11 Search PubMed.
- A. Manthiram, X. Yu and S. Wang, Nat. Rev. Mater., 2017, 24(2), 1–16 Search PubMed.
- H. Shin, M. Baek, A. Gupta, K. Char, A. Manthiram and J. W. Choi, Adv. Energy Mater., 2020, 10, 2001456 CrossRef CAS.
- X. Yang, J. Luo and X. Sun, Chem. Soc. Rev., 2020, 49, 2140–2195 RSC.
- A. Miura, N. C. Rosero-Navarro, A. Sakuda, K. Tadanaga, N. H. H. Phuc, A. Matsuda, N. Machida, A. Hayashi and M. Tatsumisago, Nat. Rev. Chem., 2019, 33(3), 189–198 CrossRef.
- J. He and A. Manthiram, Energy Storage Mater., 2019, 20, 55–70 CrossRef.
- F. Zou and A. Manthiram, Adv. Energy Mater., 2020, 10, 2002508 CrossRef CAS.
- G. Zhou, H. Chen and Y. Cui, Nat. Energy, 2022, 7, 312–319 CrossRef CAS.
- R. Deng, M. Wang, H. Yu, S. Luo, J. Li, F. Chu, B. Liu and F. Wu, Energy Environ. Mater., 2022, 5, 777–799 CrossRef CAS.
- S. H. Chung, C. H. Chang and A. Manthiram, Adv. Funct. Mater., 2018, 28, 1801188 CrossRef.
- J. Zhu, P. Zhu, C. Yan, X. Dong and X. Zhang, Prog. Polym. Sci., 2019, 90, 118–163 CrossRef CAS.
- Z. W. Seh, Y. Sun, Q. Zhang and Y. Cui, Chem. Soc. Rev., 2016, 45, 5605–5634 RSC.
- Y. Huang, L. Lin, C. Zhang, L. Liu, Y. Li, Z. Qiao, J. Lin, Q. Wei, L. Wang, Q. Xie and D. L. Peng, Adv. Sci., 2022, 9, 1–35 Search PubMed.
- B. W. Zhang, B. Sun, P. Fu, F. Liu, C. Zhu, B. M. Xu, Y. Pan and C. Chen, Membranes, 2022, 12, 790 CrossRef CAS PubMed.
- M. Chen, M. Shao, J. Jin, L. Cui, H. Tu and X. Fu, Energy Storage Mater., 2022, 47, 629–648 CrossRef.
- X. Long, S. K. Zhu, Y. Song, M. Zheng, J. J. Shao and B. Shi, New Carbon Mater., 2022, 37, 527–543 CrossRef.
- J. Kang, X. Tian, C. Yan, L. Wei, L. Gao, J. Ju, Y. Zhao, N. Deng, B. Cheng and W. Kang, Small, 2022, 18, 2104469 CrossRef CAS PubMed.
- S. Huang, R. Guan, S. Wang, M. Xiao, D. Han, L. Sun and Y. Meng, Prog. Polym. Sci., 2019, 89, 19–60 CrossRef CAS.
- J. Lopez, D. G. Mackanic, Y. Cui and Z. Bao, Nat. Rev. Mater., 2019, 45(4), 312–330 CrossRef.
- S. Dutta, A. Bhaumik and K. C. W. Wu, Energy Environ. Sci., 2014, 7, 3574–3592 RSC.
- H. Yu, M. Bi, C. Zhang, T. Zhang, X. Zhang, H. Liu, J. Mi, X. Shen and S. Yao, Int. J. Energy Res., 2022, 46, 24565–24577 CrossRef CAS.
- Y. Li, W. Wang, X. Liu, E. Mao, M. Wang, G. Li, L. Fu, Z. Li, A. Y. S. Eng, Z. W. Seh and Y. Sun, Energy Storage Mater., 2019, 23, 261–268 CrossRef.
- L. L. Chiu and S. H. Chung, Polymers, 2021, 13, 535 CrossRef CAS PubMed.
- M. Rana, M. Li, Q. He, B. Luo, L. Wang, I. Gentle and R. Knibbe, J. Energy Chem., 2020, 44, 51–60 CrossRef.
- C. Pei, J. Li, Z. Lv, H. Wang, W. Dong and Y. Yao, Int. J. Energy Res., 2022, 46, 10099–10110 CrossRef CAS.
- M. Li, J. Yang, Y. Shi, Z. Chen, P. Bai, H. Su, P. Xiong, M. Cheng, J. Zhao and Y. Xu, Adv. Mater., 2022, 34, 2107226 CrossRef CAS PubMed.
- Q. Jin, K. Zhao and X. Zhang, J. Power Sources, 2021, 489, 229500 CrossRef CAS.
- I. Bauer, S. Thieme, J. Brückner, H. Althues and S. Kaskel, J. Power Sources, 2014, 251, 417–422 CrossRef CAS.
- A. Mentbayeva, S. Sukhishvili, M. Naizakarayev, N. Batyrgali, Z. Seitzhan and Z. Bakenov, Electrochim. Acta, 2021, 366, 137454 CrossRef CAS.
- Y. Chen, G. Zhou, W. Zong, Y. Ouyang, K. Chen, Y. Lv, Y. E. Miao and T. Liu, Compos. Commun., 2021, 25, 100679 CrossRef.
- Y. Hao, Y. Xing, H. Kong and Y. Jiao, ChemElectroChem, 2021, 8, 2329–2335 CrossRef CAS.
- X. Yu, J. Joseph and A. Manthiram, J. Mater. Chem. A, 2015, 3, 15683–15691 RSC.
- J. Wang, P. Zhai, T. Zhao, M. Li, Z. Yang, H. Zhang and J. Huang, Electrochim. Acta, 2019, 320, 1–11 Search PubMed.
- W. Diao, D. Xie, D. Li, F. Tao, C. Liu, H. Sun, X. Zhang, W. Li, X.-L. Wu and J.-P. Zhang, J. Colloid Interface Sci., 2022, 627, 730–738 CrossRef CAS PubMed.
- Z. Hao, L. Yuan, Z. Li, J. Liu, J. Xiang, C. Wu, R. Zeng and Y. Huang, Electrochim. Acta, 2016, 200, 197–203 CrossRef CAS.
- A. J. Manly and W. E. Tenhaeff, Electrochim. Acta, 2022, 425, 140705 CrossRef CAS.
- F. Z. Chafi, R. X. Qi, L. Ma, Y. J. Cheng and Y. Xia, Energy Fuels, 2021, 35, 18746–18755 CrossRef CAS.
- H. Yang, X. Shi, S. Chu, Z. Shao, Y. Wang, H. Yang, X. Shi, S. Chu, Z. Shao and Y. Wang, Adv. Sci., 2021, 8, 2003096 CrossRef CAS PubMed.
- G. Wang, Y. Lai, Z. Zhang, J. Li and Z. Zhang, J. Mater. Chem. C, 2015, 3, 10715–10722 RSC.
- S. H. Chung and A. Manthiram, Adv. Mater., 2014, 26, 7352–7357 CrossRef CAS PubMed.
- L. Luo, S. H. Chung and A. Manthiram, J. Mater. Chem. A, 2016, 4, 16805–16811 RSC.
- Z. Tong, L. Huang, H. Liu, W. Lei, H. Zhang, S. Zhang and Q. Jia, Adv. Funct. Mater., 2021, 31, 1–10 Search PubMed.
- Y. Yang, W. Wang, L. Li, B. Li and J. Zhang, J. Mater. Chem. A, 2020, 8, 3692–3700 RSC.
- Y. Hu, C. Cheng, T. Yan, G. Liu, C. Yuan, Y. Yan, Z. Gu, P. Zeng, L. Zheng, J. Zhang and L. Zhang, Chem. Eng. J., 2021, 421, 129997 CrossRef CAS.
- J. He, L. Luo, Y. Chen and A. Manthiram, Adv. Mater., 2017, 29, 1–5 Search PubMed.
- Z. Li, J. Zhang, B. Guan, D. Wang, L. M. Liu and X. W. Lou, Nat. Commun., 2016, 7, 1–11 Search PubMed.
- S. Song, L. Shi, S. Lu, Y. Pang, Y. Wang, M. Zhu, D. Ding and S. Ding, J. Membr. Sci., 2018, 563, 277–283 CrossRef CAS.
- H. Gao, S. Ning, J. Lin and X. Kang, Energy Storage Mater., 2021, 40, 312–319 CrossRef.
- Z. Li, Y. Han, J. Wei, W. Wang, T. Cao, S. Xu and Z. Xu, ACS Appl. Mater. Interfaces, 2017, 9, 44776–44781 CrossRef CAS PubMed.
- D. B. Babu, K. Giribabu and K. Ramesha, ACS Appl. Mater. Interfaces, 2018, 10, 19721–19729 CrossRef CAS PubMed.
- J. Liu, Z. Hong, F. Zhu, Q. Li, J. Li, M. Liu, C. Wang, F. Song, L. Bai and F. Zeng, Ionics, 2021, 27, 4749–4759 CrossRef CAS.
- D. Li, H. Wang, L. Luo, J. Zhu, J. Li, P. Liu, Y. Yu and M. Jiang, ACS Appl. Energy Mater., 2021, 4, 879–887 CrossRef CAS.
- L. Ma, P. Nath, Z. Tu, M. Tikekar and L. A. Archer, Chem. Mater., 2016, 28, 5147–5154 CrossRef CAS.
- I. Paniagua-Vásquez, C. C. Zuluaga-Gómez, S. Chacón-Vargas, A. L. Calvo, G. Sáenz-Arce, R. S. Katiyar and J. J. Saavedra-Arias, Energies, 2022, 15, 2183 CrossRef.
- X. Yu, J. Joseph and A. Manthiram, Mater. Horizons, 2016, 3, 314–319 RSC.
- M. Gu, J. Lee, Y. Kim, J. S. Kim, B. Y. Jang, K. T. Lee and B. S. Kim, RSC Adv., 2014, 4, 46940–46946 RSC.
- C. Zhan, G. Yu, Y. Lu, L. Wang, E. Wujcik and S. Wei, J. Mater. Chem. C, 2017, 5, 1569–1585 RSC.
- K. Namsheer and C. S. Rout, RSC Adv., 2021, 11, 5659–5697 RSC.
- H. Hareendrakrishnakumar, R. Chulliyote and M. G. Joseph, Ionics, 2021, 27, 1087–1099 CrossRef CAS.
- S. A. Abbas, M. A. Ibrahem, L. H. Hu, C. N. Lin, J. Fang, K. M. Boopathi, P. C. Wang, L. J. Li and C. W. Chu, J. Mater. Chem. A, 2016, 4, 9661–9669 RSC.
- H. Li, M. Sun, T. Zhang, Y. Fang and G. Wang, J. Mater. Chem. A, 2014, 2, 18345–18352 RSC.
- S. Z. Zeng, Y. Yao, J. Tu, Z. Tian, J. Zou, X. Zeng, H. Zhu, L. B. Kong and P. Han, Ionics, 2021, 27, 3887–3893 CrossRef CAS.
- K. Chen, G. Zhang, L. Xiao, P. Li, W. Li, Q. Xu and J. Xu, Small, 2021, 1–12, DOI:10.1002/smtd.202001056.
- H. Jo, Y. Cho, T. Yoo, Y. Jeon, H. Hong and Y. Piao, ACS Appl. Mater. Interfaces, 2021, 13, 47593–47602 CrossRef CAS PubMed.
- M. Shi, Z. Liu, S. Zhang, S. Liang, Y. Jiang, H. Bai, Z. Jiang, J. Chang, J. Feng, W. Chen, H. Yu, S. Liu, T. Wei and Z. Fan, Adv. Energy Mater., 2022, 12, 1–12 Search PubMed.
- Z. Yu, M. Liu, D. Guo, J. Wang, X. Chen, J. Li, H. Jin, Z. Yang, X. Chen and S. Wang, Angew. Chem., Int. Ed., 2020, 59, 6406–6411 CrossRef CAS PubMed.
- J. Cai, Y. Song, X. Chen, Z. Sun, Y. Yi, J. Sun and Q. Zhang, J. Mater. Chem. A, 2020, 8, 1757–1766 RSC.
- Y. Zhou, H. Gao, S. Ning, J. Lin, J. Wen and X. Kang, ChemElectroChem, 2021, 8, 1798–1806 CrossRef CAS.
- W. Zhang, Y. Liu, A. Wu, L. Ling, Z. Wang, X. Hao and G. Guan, Electrochim. Acta, 2022, 403, 139609 CrossRef CAS.
- M. Yang, J. Nan, W. Chen, A. Hu, H. Sun, Y. Chen and C. Wu, Electrochem. Commun., 2021, 125, 106971 CrossRef CAS.
- J. Conder, A. Forner-Cuenca, E. M. Gubler, L. Gubler, P. Novák and S. Trabesinger, ACS Appl. Mater. Interfaces, 2016, 8, 18822–18831 CrossRef CAS PubMed.
- Y. Li, X. Zhang, Q. Zhang, J. Cui, X. Liang, J. Yan, J. Liu, H. H. Tan, Y. Yu and Y. Wu, ACS Appl. Mater. Interfaces, 2022, 14, 18634–18645 CrossRef CAS PubMed.
- J.-H. Shin, Y.-Y. Park, S.-H. Moon, J.-H. Kim, J.-S. Jang, S.-B. Kim, S.-N. Lee and K.-W. Park, Energies, 2022, 15, 7961 CrossRef CAS.
- X. Wang, L. Yang, Q. Li, Y. Wang, Y. Zhong, Y. Song, Y. Chen, Z. Wu, B. Zhong and X. Guo, Ind. Eng. Chem. Res., 2022, 61, 1761–1772 CrossRef CAS.
- H. Yao, K. Yan, W. Li, G. Zheng, D. Kong, Z. W. Seh, V. K. Narasimhan, Z. Liang and Y. Cui, Energy Environ. Sci., 2014, 7, 3381–3390 RSC.
- Z. Zhang, Y. Dong, Y. Gu, P. Lu, F. Xue, Y. Fan, Z. Zhu, J. Lin, Q. Li and Z. S. Wu, J. Mater. Chem. A, 2022, 10, 9515–9523 RSC.
- A. Benítez, J. Amaro-Gahete, Y. C. Chien, Á. Caballero, J. Morales and D. Brandell, Renewable Sustainable Energy Rev., 2022, 154, 111783 CrossRef.
- X. Liu, Q. He, J. Liu, R. Yu, Y. Zhang, Y. Zhao, X. Xu, L. Mai and L. Zhou, ACS Appl. Mater. Interfaces, 2023, 15, 9439–9446 CAS.
- L. Li, H. Liu, B. Jin, Q. Sheng, Q. Li, M. Cui, Y. Li, X. Lang and Q. Jiang, ACS Appl. Nano Mater., 2023, 6, 1161–1170 CrossRef CAS.
- X. Ou, Y. Yu, R. Wu, A. Tyagi, M. Zhuang, Y. Ding, I. H. Abidi, H. Wu, F. Wang and Z. Luo, ACS Appl. Mater. Interfaces, 2018, 10, 5534–5542 CrossRef CAS PubMed.
- J. H. Lee, J. Kang, S. W. Kim, W. Halim, M. W. Frey and Y. L. Joo, ACS Omega, 2018, 3, 16465–16471 CrossRef CAS PubMed.
- X. Qi, L. Huang, Y. Luo, Q. Chen and Y. Chen, J. Colloid Interface Sci., 2022, 628, 896–910 CrossRef CAS PubMed.
- L. Wang, Z. Yang, H. Nie, C. Gu, W. Hua, X. Xu, X. Chen, Y. Chen and S. Huang, J. Mater. Chem. A, 2016, 4, 15343–15352 RSC.
- H. Li, Y. Zhou, M. Zhao, B. Jin, Z. Wen, H. Xie, S. Dou and Q. Jiang, Adv. Energy Mater., 2020, 10, 1–11 Search PubMed.
- F. Ma, K. Srinivas, X. Zhang, Z. Zhang, Y. Wu, D. Liu, W. Zhang, Q. Wu and Y. Chen, Adv. Funct. Mater., 2022, 1–14, DOI:10.1002/adfm.202206113.
- L. Gai, C. Zhao, Y. Zhang, Z. Hu and Q. Shen, Carbon Energy, 2022, 4, 142–154 CrossRef CAS.
- E. Jing, L. Chen, S. Xu, W. Tian, D. Zhang, N. Wang, Z. Bai, X. Zhou, S. Liu, D. Duan and X. Qiu, J. Energy Chem., 2021, 64, 574–582 CrossRef.
- P. Wang, B. Xi, Z. Zhang, M. Huang, J. Feng and S. Xiong, Angew. Chem., 2021, 133, 15691–15699 CrossRef.
- W. Jing, J. Zu, K. Zou, X. Dai, Y. Song, J. Han, J. Sun, Q. Tan, Y. Chen and Y. Liu, J. Mater. Chem. A, 2022, 10, 4833–4844 RSC.
- W. Jing, K. Zou, X. Dai, M. Shi, J. Sun, D. Zhu, S. Guo, Y. Chen and Y. Liu, J. Colloid Interface Sci., 2021, 601, 305–316 CrossRef CAS PubMed.
- Z. Cheng, Y. Chen, Y. Yang, L. Zhang, H. Pan, X. Fan, S. Xiang and Z. Zhang, Adv. Energy Mater., 2021, 11, 1–9 Search PubMed.
- P. Cheng, P. Guo, K. Sun, Y. Zhao, D. Liu and D. He, J. Membr. Sci., 2021, 619, 118780 CrossRef CAS.
- P. Cheng, P. Guo, D. Liu, Y. Wang, K. Sun, Y. Zhao and D. He, J. Alloys Compd., 2019, 784, 149–156 CrossRef CAS.
- K. Xu, X. Liang, L. L. Wang, Y. Wang, J. F. Yun, Y. Sun and H. F. Xiang, Rare Met., 2021, 40(10), 2810–2818 CrossRef CAS.
- J. Wang, Z. Shi, Y. Luo, D. Wang, H. Wu, Q. Li, S. Fan, J. Li and J. Wang, Nanoscale, 2021, 13, 6863–6870 RSC.
- Y. Zhao, J. Liu, Y. Zhou, X. Huang, Q. Liu, F. Chen, H. Qin, H. Lou, D. Y. W. Yu and X. Hou, ACS Appl. Mater. Interfaces, 2021, 13, 41698–41706 CrossRef CAS PubMed.
- T. Xiao, Q. Chen, W. Zhong, M. Yang, F. Cai, W. Liu, M. Ren and Y. Wang, J. Alloys Compd., 2022, 907, 164486 CrossRef CAS.
- Y. Liu, X. Zhao, S. Li, Q. Zhang, K. Wang and J. Chen, Chem. Res. Chin. Univ., 2022, 38, 147–154 CrossRef CAS.
- C. Chen, Q. Jiang, H. Xu, Y. Zhang, B. Zhang, Z. Zhang, Z. Lin and S. Zhang, Nano Energy, 2020, 76, 105033 CrossRef CAS.
- C. Shen, Y. Li, M. Gong, C. Zhou, Q. An, X. Xu and L. Mai, ACS Appl. Mater. Interfaces, 2021, 13, 60046–60053 CrossRef CAS PubMed.
- P. Li, J. Deng, J. Li, L. Wang and J. Guo, Ceram. Int., 2019, 45, 13219–13224 CrossRef CAS.
- J. He, Z. Gao and X. Li, Jom, 2021, 73, 2516–2524 CrossRef CAS.
- X. Fan, Y. Wang, M. Zeng, H. He, J. Huang, Z. Feng, J. Li, Z. Liang and T. Zhou, J. Alloys Compd., 2022, 894, 162556 CrossRef CAS.
- Q. X. Shi, C. Chang, H. J. Pei, X. Guan, L. L. Yin, X. L. Xie and Y. S. Ye, Electrochim. Acta, 2021, 367, 137418 CrossRef CAS.
- X. Hu, T. Huang, S. Wang, S. Lin, Z. Feng, L. H. Chung and J. He, Electrochim. Acta, 2021, 398, 139317 CrossRef CAS.
- N. Li, W. Cao, Y. Liu, H. Ye and K. Han, Colloids Surf., A, 2019, 573, 128–136 CrossRef CAS.
- Z. T. Shao, L. L. Wu, Y. Yang, X. Z. Ma, L. Li, H. F. Ye and X. T. Zhang, New Carbon Mater., 2021, 36, 219–226 CrossRef CAS.
- M. Luo, Y. Bai, R. Sun, M. Qu, M. Wang, Z. Yang, Z. Wang, W. Sun and K. Sun, J. Energy Chem., 2022, 73, 407–415 CrossRef CAS.
- J. Zhang, Y. Cheng, H. Chen, Y. Wang, Q. Chen, G. Hou, M. Wen and Y. Tang, ACS Appl. Mater. Interfaces, 2022, 14, 16289–16299 CrossRef CAS PubMed.
- Z. Li, L. Tang, X. Liu, T. Song, Q. Xu, H. Liu and Y. Wang, Electrochim. Acta, 2019, 310, 1–12 CrossRef CAS.
- X. Yu, W. Chen, J. Cai, X. Lu and Z. Sun, J. Colloid Interface Sci., 2022, 610, 407–417 CrossRef CAS PubMed.
- Z. Gao, Z. Xue, Y. Miao, B. Chen, J. Xu, H. Shi, T. Tang and X. Zhao, J. Alloys Compd., 2022, 906, 164249 CrossRef CAS.
- L. Zhan, X. Zhou, J. Luo, X. Fan and X. Ning, Int. J. Hydrogen Energy, 2022, 47, 27671–27679 CrossRef CAS.
- X. Qian, F. Li and L. Jin, Microporous Mesoporous Mater., 2022, 329, 111558 CrossRef CAS.
- X. Wu, S. Li, S. Yao, M. Liu, S. Pang, X. Shen, T. Li and S. Qin, Int. J. Energy Res., 2021, 45, 4331–4344 CrossRef CAS.
- T. Wang, Y. Liu, X. Zhang, J. Wang, Y. Zhang, Y. Li, Y. Zhu, G. Li and X. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 56085–56094 CrossRef CAS PubMed.
- Y. Gong, Y. Wang, Z. Fang, S. Zhao, Y. shi He, W. Zhang, J. Mu, L. Zhang and Z. F. Ma, Chem. Eng. J., 2022, 446, 136943 CrossRef CAS.
- J. Liu, K. Li, Q. Zhang, X. Zhang, X. Liang, J. Yan, H. H. Tan, Y. Yu and Y. Wu, ACS Appl. Mater. Interfaces, 2021, 13, 45547–45557 CrossRef CAS PubMed.
- S. K. Kannan, H. Hareendrakrishnakumar, J. Joseph and M. G. Joseph, Energy Fuels, 2022, 36, 8460–8470 CrossRef CAS.
- Q. Liu, X. Han, Q. Dou, P. Xiong, Y. Kang, B. K. Kim and H. S. Park, Int. J. Energy Res., 2022, 46, 9634–9642 CrossRef CAS.
- R. Ponraj, A. G. Kannan, J. H. Ahn, J. H. Lee, J. Kang, B. Han and D. W. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 38445–38454 CrossRef CAS PubMed.
- F. Wang, Z. Yang and C. Y. Tang, ACS ES&T Engg., 2022, 2, 2023–2033 Search PubMed.
- S. Sun, L. Han, J. Hou, Y. Yang, J. Yue, G. Gu, C. Yang, J. Li and Z. Zhang, J. Colloid Interface Sci., 2022, 628, 1012–1022 CrossRef CAS PubMed.
- P. Xie, B. Zhang, Y. Zhou, P. Li and X. Tian, Electrochim. Acta, 2021, 395, 139181 CrossRef CAS.
- Z. Zhao, W. Yin, H. Li, Y. Jiao, D. Lei, Y. Li, J. Guo, W. Bai and M. Xiang, Microporous Mesoporous Mater., 2022, 337, 111946 CrossRef CAS.
- P. Zeng, L. Huang, Y. Han, X. Zhang, R. Zhang and Y. Chen, ChemElectroChem, 2018, 5, 375–382 CrossRef CAS.
- D. Zhao, X. Qian, L. Jin, X. Yang, S. Wang, X. Shen, S. Yao, D. Rao, Y. Zhou and X. Xi, RSC Adv., 2016, 6, 13680–13685 RSC.
- L. Jin, Z. Fu, X. Qian, B. Huang, F. Li, Y. Wang and X. Shen, Microporous Mesoporous Mater., 2021, 316, 110927 CrossRef CAS.
- C. Liao, X. Mu, L. Han, Z. Li, Y. Zhu, J. Lu, H. Wang, L. Song, Y. Kan and Y. Hu, Energy Storage Mater., 2022, 48, 123–132 CrossRef.
- Y. C. Huang, Y. J. Yen, Y. H. Tseng and S. H. Chung, Molecules, 2021, 27, 228 CrossRef PubMed.
- L. Jin, Z. Fu, X. Qian, F. Li, Y. Wang, B. Wang and X. Shen, Electrochim. Acta, 2021, 382, 138282 CrossRef CAS.
- J. He, G. Hartmann, M. Lee, G. S. Hwang, Y. Chen and A. Manthiram, Energy Environ. Sci., 2019, 12, 344–350 RSC.
- S. Zhen, J. Huang, L. Peng, M. Zhang, K. Shi, G. Ma, A. Li and Y. Wang, Energy Technol., 2021, 9, 1–9 Search PubMed.
- H. Han, S. Niu, Y. Zhao, T. Tan and Y. Zhang, Nanoscale Res. Lett., 2019, 14, 1–8 CrossRef CAS PubMed.
- J. Huang, S. Dong and G. Xie, Ionics, 2022, 28(9), 4129–4134 CrossRef CAS.
- W. Jiang, L. Dong, S. Liu, S. Zhao, K. Han, W. Zhang, K. Pan and L. Zhang, Nanomater, 2022, 12, 1347 CrossRef CAS PubMed.
- X. Liu, S. Wang, H. Duan, Y. Deng and G. Chen, J. Colloid Interface Sci., 2022, 608, 470–481 CrossRef CAS PubMed.
- X. Shi, L. Yang, S. Li, Y. Wang, X. Chen, Z. Wu, Y. Zhong, Y. Chen, S. Gao, G. Wang, X. Guo and B. Zhong, Electrochim. Acta, 2021, 390, 138829 CrossRef CAS.
- L. ying Wei, N. ping Deng, J. ge Ju, H. juan Zhao, G. Wang, H. ying Xiang, W. min Kang and B. wen Cheng, Chem. Eng. J., 2021, 424, 130346 CrossRef.
- Y. Luo, H. Bai, B. Li, X. Song, J. Zhao, Y. Xiao, S. Lei and B. Cheng, J. Alloys Compd., 2021, 879, 160368 CrossRef CAS.
- P. Zeng, L. Huang, X. Zhang, R. Zhang, L. Wu and Y. Chen, Chem. Eng. J., 2018, 349, 327–337 CrossRef CAS.
- Z. Kong, Y. Lin, J. Hu, Y. Wang and L. Zhan, Chem. Eng. J., 2022, 436, 132719 CrossRef CAS.
- L. Liu, F. Yang, L. Ge, X. Wang, L. Cui and H. Yang, Electrochim. Acta, 2022, 401, 139380 CrossRef CAS.
- F. Yang and K. Huang, Mater. Res. Express, 2021, 8, 115002 CrossRef CAS.
- L. Zhu, J. Li, H. Xie and X. Shen, Ionics, 2022, 28, 3207–3215 CrossRef CAS.
- Q. Li, Y. Liu, L. Yang, Y. Wang, Y. Liu, Y. Chen, X. Guo, Z. Wu and B. Zhong, J. Colloid Interface Sci., 2021, 585, 43–50 CrossRef CAS PubMed.
- J. R. Choi, E. Kim, B.-I. Park, I. Choi, B.-H. Park, S.-B. Lee, J. H. Lee and S. Yu, J. Ind. Eng. Chem., 2022, 115, 355–364 CrossRef CAS.
- X. Wang, L. Yang, R. Li, Y. Chen, Z. Wu, B. Zhong and X. Guo, Appl. Surf. Sci., 2022, 602, 154342 CrossRef CAS.
- L. Wu, H. Hou, J. Hu, B. Liu, X. Yang, S. Chen, L. Liu, S. Hu, J. Yang, S. Liang, K. Xiao and S. Yuan, Microporous Mesoporous Mater., 2021, 317, 111000 CrossRef CAS.
- X. Zhang, L. Chen, Z. Yang, X. Qiu and Z. Zheng, Mater. Lett., 2021, 290, 129512 CrossRef CAS.
- N. Díez, M. Sevilla and A. B. Fuertes, ACS Appl. Energy Mater., 2020, 3, 3397–3407 CrossRef.
- B. Zheng, L. Yu, Y. Zhao and J. Xi, Electrochim. Acta, 2019, 295, 910–917 CrossRef CAS.
- L. Han, S. Sun, Y. Yang, J. Yue and J. Li, Appl. Surf. Sci., 2023, 610, 155496 CrossRef CAS.
- B. Dang, D. Y. Gao, Y. Luo, Z. Zhang, J. Li and F. Wu, J. Energy Storage, 2022, 52, 104981 CrossRef.
- Y. Fan, Z. Niu, F. Zhang, R. Zhang, Y. Zhao and G. Lu, ACS Omega, 2019, 4, 10328–10335 CrossRef CAS PubMed.
- J. Wang and J. Li, J. Colloid Interface Sci., 2021, 584, 354–359 CrossRef CAS PubMed.
- H. Chen, Y. Xiao, C. Chen, J. Yang, C. Gao, Y. Chen, J. Wu, Y. Shen, W. Zhang, S. Li, F. Huo and B. Zheng, ACS Appl. Mater. Interfaces, 2019, 11, 11459–11465 CrossRef CAS PubMed.
- Y. Guo, M. Sun, H. Liang, W. Ying, X. Zeng, Y. Ying, S. Zhou, C. Liang, Z. Lin and X. Peng, ACS Appl. Mater. Interfaces, 2018, 10, 30451–30459 CrossRef CAS PubMed.
- F. Wu, S. Zhao, L. Chen, Y. Lu, Y. Su, Y. Jia, L. Bao, J. Wang, S. Chen and R. Chen, Energy Storage Mater., 2018, 14, 383–391 CrossRef.
- B. Ma, X. Zhang, X. Deng, S. Huang, M. Xiao, S. Wang, D. Han and Y. Meng, Polymers, 2021, 13, 4210 CrossRef CAS PubMed.
- Z. Hu, Y. Wang and D. Zhao, Chem. Soc. Rev., 2021, 50, 4629–4683 RSC.
- Y. Su, W. Wang, W. Wang, A. Wang, Y. Huang and Y. Guan, J. Electrochem. Soc., 2022, 169, 030528 CrossRef.
- H. G. Jin, M. Wang, J. X. Wen, S. H. Han, X. J. Hong, Y. P. Cai, G. Li, J. Fan and Z. S. Chao, ACS Appl. Mater. Interfaces, 2021, 13, 3899–3910 CrossRef CAS PubMed.
- X. Qian, J. Cheng, L. Jin, Y. Wang, B. Huang and J. Chen, Colloids Surf., A, 2022, 648, 129036 CrossRef CAS.
- X. Qian, Y. Wang, L. Jin, J. Cheng, J. Chen and B. Huang, J. Electroanal. Chem., 2022, 907, 116029 CrossRef CAS.
- Y. Chen, L. Zhang, H. Pan, J. Zhang, S. Xiang, Z. Cheng and Z. Zhang, J. Mater. Chem. A, 2021, 9, 26929–26938 RSC.
- X. Wu, C. Zhou, C. Dong, C. Shen, B. Shuai, C. Li, Y. Li, Q. An, X. Xu and L. Mai, Nano Res., 2022, 15, 8048–8055 CrossRef CAS.
- S. H. Kim, J. S. Yeon, R. Kim, K. M. Choi and H. S. Park, J. Mater. Chem. A, 2018, 6, 24971–24978 RSC.
- S. Y. Ding and W. Wang, Chem. Soc. Rev., 2012, 42, 548–568 RSC.
- Y. Zhu, J. Yang, X. Qiu, M. Li, G. He, Q. Wang, Z. Xie, X. Li and H. Yu, ACS Appl. Mater. Interfaces, 2021, 13, 60373–60383 CrossRef CAS PubMed.
- J. Zhao, G. Yan, X. Zhang, Y. Feng, N. Li, J. Shi and X. Qu, Chem. Eng. J., 2022, 442, 136352 CrossRef CAS.
- T. S. Sahu, V. G. Abhijitha, I. Pal, S. Sau, M. Gautam, B. R. K. Nanda and S. Mitra, Small, 2022, 18, 2203222 CrossRef CAS PubMed.
- K. Sun, C. Wang, Y. Dong, P. Guo, P. Cheng, Y. Fu, D. Liu, D. He, S. Das and Y. Negishi, ACS Appl. Mater. Interfaces, 2022, 14, 4079–4090 CrossRef CAS PubMed.
- Q. X. Shi, C. Y. Yang, H. J. Pei, C. Chang, X. Guan, F. Y. Chen, X. L. Xie and Y. S. Ye, Chem. Eng. J., 2021, 404, 127044 CrossRef CAS.
- Y. Cao, H. Wu, G. Li, C. Liu, L. Cao, Y. Zhang, W. Bao, H. Wang, Y. Yao, S. Liu, F. Pan, Z. Jiang and J. Sun, Nano Lett., 2021, 21, 2997–3006 CrossRef CAS PubMed.
- M. Zhen, K. Jiang, S. Q. Guo, B. Shen and H. Liu, Nano Res., 2021, 15(2), 933–941 CrossRef.
- J. Lin, K. Zhang, Z. Zhu, R. Zhang, N. Li and C. Zhao, ACS Appl. Mater. Interfaces, 2020, 12, 2497–2504 CrossRef CAS PubMed.
- L. Peng, Z. Yu, M. Zhang, S. Zhen, J. Shen, Y. Chang, Y. Wang, Y. Deng and A. Li, Nanoscale, 2021, 13, 16696–16704 RSC.
- X. Wang, L. Yang, Y. Wang, Q. Li, C. Chen, B. Zhong, Y. Chen, X. Guo, Z. Wu, Y. Liu, Y. Liu and Y. Sun, J. Colloid Interface Sci., 2022, 606, 666–676 CrossRef CAS PubMed.
- P. Cheng, D. Cao, K. Sun, Y. Li, Y. Fu, Y. Zhao, D. Liu and D. He, J. Alloys Compd., 2021, 868, 159131 CrossRef CAS.
- S. Wang, P. Zhang, H. Tan, X. Fan and K. Huang, J. Power Sources, 2019, 419, 106–111 CrossRef CAS.
- X. Wu, S. Yao, M. Liu, S. Pang, X. Shen, T. Li and S. Qin, Ionics, 2021, 27, 2397–2408 CrossRef CAS.
- W. Sun, X. Sun, Q. Peng, H. Wang, Y. Ge, N. Akhtar, Y. Huang and K. Wang, Nanoscale Adv., 2019, 1, 1589–1597 RSC.
- S. Hu, M. Yi, H. Wu, T. Wang, X. Ma, X. Liu and J. Zhang, Adv. Funct. Mater., 2022, 32, 1–12 Search PubMed.
- H. Shao, W. Wang, H. Zhang, A. Wang, X. Chen and Y. Huang, J. Power Sources, 2018, 378, 537–545 CrossRef CAS.
- Z. Huang, M. Yang, J. Qi, P. Zhang, L. Lei and Q. Du, Chem. Eng. J., 2020, 387, 1–10 Search PubMed.
- Y. Zhu, Y. Zuo, X. Jiao, R. Manjunatha, E. R. Ezeigwe, W. Yan and J. Zhang, Carbon Energy, 2022, 1–13 Search PubMed.
- Q. Liu, X. Han, Q. Dou, P. Xiong, Y. Kang, S. W. Kang, B. K. Kim and H. S. Park, Batteries Supercaps, 2021, 4, 1843–1849 CrossRef.
- S. Liu, Z. Zhou and D. Liu, Ceram. Int., 2019, 45, 14415–14419 CrossRef CAS.
- B. Moorthy, S. Kwon, J. H. Kim, P. Ragupathy, H. M. Lee and D. K. Kim, Nanoscale Horizons, 2019, 4, 214–222 RSC.
- G. Liu, Q. Zeng, Z. Fan, S. Tian, X. Li, X. Lv, W. Zhang, K. Tao, E. Xie and Z. Zhang, Chem. Eng. J., 2022, 448, 137683 CrossRef CAS.
- H. Li, L. Sun, Y. Zhao, T. Tan and Y. Zhang, Appl. Surf. Sci., 2019, 466, 309–319 CrossRef CAS.
- Z. A. Ghazi, X. He, A. M. Khattak, N. A. Khan, B. Liang, A. Iqbal, J. Wang, H. Sin, L. Li and Z. Tang, Adv. Mater., 2017, 29, 1–6 CrossRef PubMed.
- Z. Li, F. Zhang, L. Tang, Y. Tao, H. Chen, X. Pu, Q. Xu, H. Liu, Y. Wang and Y. Xia, Chem. Eng. J., 2020, 390, 124653 CrossRef CAS.
- Z. Liu, Z. Hu, X. Jiang, Y. Zhang, X. Wang and S. Zhang, Electrochim. Acta, 2022, 422, 140496 CrossRef CAS.
- X. Yu, Y. Yang, L. Si, J. Cai, X. Lu and Z. Sun, J. Colloid Interface Sci., 2022, 627, 992–1002 CrossRef CAS PubMed.
- J. Song, D. Su, X. Xie, X. Guo, W. Bao, G. Shao and G. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 29427–29433 CrossRef CAS PubMed.
- Y. H. Liu, L. X. Li, A. Y. Wen, F. F. Cao and H. Ye, Energy Storage Mater., 2023, 55, 652–659 CrossRef.
- P. Li, H. Lv, Z. Li, X. Meng, Z. Lin, R. Wang and X. Li, Adv. Mater., 2021, 33, 2007803 CrossRef CAS PubMed.
- R. Gao, Z. Wang, S. Liu, G. Shao and X. Gao, Int. J. Miner. Metall. Mater., 2022, 29, 990–1002 CrossRef CAS.
- Z. Wang, X. Xu, Z. Liu, D. Zhang, J. Yuan and J. Liu, Chem. – A Eur. J., 2021, 27, 13494–13512 CrossRef CAS PubMed.
- D. Yang, C. Zhang, J. J. Biendicho, X. Han, Z. Liang, R. Du, M. Li, J. Li, J. Arbiol, J. Llorca, Y. Zhou, J. R. Morante and A. Cabot, ACS Nano, 2020, 14, 15492–15504 CrossRef CAS PubMed.
- W. Luo, Y. Wang, E. Hitz, Y. Lin, B. Yang and L. Hu, Adv. Funct. Mater., 2017, 27, 1–19 Search PubMed.
- Y. Fan, D. Liu, M. M. Rahman, T. Tao, W. Lei, S. Mateti, B. Yu, J. Wang, C. Yang and Y. Chen, ACS Appl. Energy Mater., 2019, 2, 2620–2628 CrossRef CAS.
- W. Luo, L. Zhou, K. Fu, Z. Yang, J. Wan, M. Manno, Y. Yao, H. Zhu, B. Yang and L. Hu, Nano Lett., 2015, 15, 6149–6154 CrossRef CAS PubMed.
- P. J. H. Kim, J. Seo, K. Fu, J. Choi, Z. Liu, J. Kwon, L. Hu and U. Paik, NPG Asia Mater., 2017, 9, e375 CrossRef CAS.
- H. S. Kim, H. J. Kang, H. Lim, H. J. Hwang, J. W. Park, T. G. Lee, S. Y. Cho, S. G. Jang and Y. S. Jun, Nanomaterials, 2021, 11, 471 CrossRef PubMed.
- P. Zhou, D. Yao, H. Liang, Y. Xia and Y. P. Zeng, Ceram. Int., 2022, 49, 1381–1389 CrossRef.
- Q. Shen, L. Huang, G. Chen, X. Zhang and Y. Chen, J. Alloys Compd., 2020, 845, 155543 CrossRef CAS.
- W. Yao, C. Tian, C. Yang, J. Xu, Y. Meng, I. Manke, N. Chen, Z. Wu, L. Zhan, Y. Wang and R. Chen, Adv. Mater., 2022, 34, 1–13 Search PubMed.
- X. Kang, Z. Jin, H. Peng, Z. Cheng, L. Liu, X. Li, L. Xie, J. Zhang and Y. Dong, J. Colloid Interface Sci., 2023, 637, 161–172 CrossRef CAS PubMed.
- F. Ma, X. Zhang, K. Sriniva, D. Liu, Z. Zhang, X. Chen, W. Zhang, Q. Wu and Y. Chen, J. Mater. Chem. A, 2022, 10, 8578–8590 RSC.
- H. Y. Zhang, R. Q. Dai, S. Zhu, L. Z. Zhou, Q. J. Xu and Y. L. Min, Chem. Eng. J., 2022, 429, 132454 CrossRef CAS.
- B. Guan, Y. Zhang, L. Fan, X. Wu, M. Wang, Y. Qiu, N. Zhang and K. Sun, ACS Nano, 2019, 13, 6742–6750 CrossRef CAS PubMed.
- A. E. Shrshr, Y. Dong, M. A. Al-Tahan, X. Kang, H. Guan, X. Zheng and J. Zhang, J. Alloys Compd., 2022, 910, 164917 CrossRef CAS.
- Z. Guo, Y. Zhao, Y. Miao, D. Wang and D. Zhang, ACS Appl. Energy Mater., 2022, 5, 11844–11852 CrossRef CAS.
- Y. Yuan, F. Wu, G. Chen, Y. Bai and C. Wu, J. Energy Chem., 2019, 37, 197–203 CrossRef.
- Y. Chen, W. Zhang, D. Zhou, H. Tian, D. Su, C. Wang, D. Stockdale, F. Kang, B. Li and G. Wang, ACS Nano, 2019, 13, 4731–4741 CrossRef CAS PubMed.
- Z. Wu, S. Chen, L. Wang, Q. Deng, Z. Zeng, J. Wang and S. Deng, Energy Storage Mater., 2021, 38, 381–388 CrossRef.
- X. Wang, N. Deng, Y. Liu, L. Wei, H. Wang, Y. Li, B. Cheng and W. Kang, Chem. Eng. J., 2022, 450, 138191 CrossRef CAS.
- Z. Ren, Z. Zhao, K. Zhang, X. Wang and Y. Wang, ChemElectroChem, 2021, 8, 1531–1536 CrossRef CAS.
- X. Zhang, X. Lv, C. Wei and J. G. Wang, Appl. Surf. Sci., 2021, 568, 150952 CrossRef CAS.
- Y. Tian, G. Li, Y. Zhang, D. Luo, X. Wang, Y. Zhao, H. Liu, P. Ji, X. Du, J. Li and Z. Chen, Adv. Mater., 2020, 32, 1–11 Search PubMed.
- Y. Li, W. Wang, B. Zhang, L. Fu, M. Wan, G. Li, Z. Cai, S. Tu, X. Duan, Z. W. Seh, J. Jiang and Y. Sun, Nano Lett., 2021, 21, 6656–6663 CrossRef CAS PubMed.
- J. Liu, J. Wang, L. Zhu, X. Chen, G. Yi, Q. Ma, S. Sun, N. Wang, X. Cui, Q. Chai, J. Feng and W. Yan, J. Mater. Chem. A, 2022, 10, 14098–14110 RSC.
- S. Luiso, M. J. Petrecca, A. H. Williams, J. Christopher, O. D. Velev, B. Pourdeyhimi and P. S. Fedkiw, ACS Appl. Polym. Mater., 2022, 4, 3676–3686 CrossRef CAS.
- G. Sun, S. Jiang, X. Feng, X. Shi, X. Zhang, T. Li, N. Chen, L. Hou, S. Qi and D. Wu, J. Membr. Sci., 2022, 645, 120208 CrossRef CAS.
- M. Guo, H. Zhu, P. Wan, F. Xu, C. Wang, S. Lu, Y. Zhang, H. Fan and J. Xu, Adv. Fiber Mater., 2022, 2022, 1–14 Search PubMed.
- X. Niu, J. Li, J. Song, Y. Li and T. He, ACS Appl. Energy Mater., 2021, 4, 11080–11089 CrossRef CAS.
- S. Kim, M. S. Kwon, J. H. Han, J. Yuk, J. Y. Lee, K. T. Lee and T. H. Kim, J. Power Sources, 2021, 482, 228907 CrossRef CAS.
- S. Choudhury, M. Azizi, I. Raguzin, M. Göbel, S. Michel, F. Simon, A. Willomitzer, V. Mechtcherine, M. Stamm and L. Ionov, Phys. Chem. Chem. Phys., 2017, 19, 11239–11248 RSC.
- Y. Feng, G. Wang, W. Kang, N. Deng and B. Cheng, Electrochim. Acta, 2021, 365, 137344 CrossRef CAS.
- X. Zhu, Y. Ouyang, J. Chen, X. Zhu, X. Luo, F. Lai, H. Zhang, Y. E. Miao and T. Liu, J. Mater. Chem. A, 2019, 7, 3253–3263 RSC.
- Y. Wang, Z. Zhang, L. Dong and Y. Jin, J. Membr. Sci., 2020, 595, 117581 CrossRef CAS.
- Y. Yang, H. Xu, S. Wang, Y. Deng, X. Qin, X. Qin and G. Chen, Electrochim. Acta, 2019, 297, 641–649 CrossRef CAS.
- M. S. Kiai, O. Eroglu and H. Kizil, J. Appl. Polym. Sci., 2020, 137, 1–9 CrossRef.
- D. Huang, C. Liang, L. Chen, M. Tang, Z. Zheng and Z. Wang, J. Mater. Sci., 2021, 56, 5868–5877 CrossRef CAS.
- L. Y. Yang, J. H. Cao, B. R. Cai, T. Liang and D. Y. Wu, Electrochim. Acta, 2021, 382, 138346 CrossRef CAS.
- L. Zhang, T. Qian, X. Zhu, Z. Hu, M. Wang, L. Zhang, T. Jiang, J. H. Tian and C. Yan, Chem. Soc. Rev., 2019, 48, 5432–5453 RSC.
- S. Ai, M. Xiao, J. Chen, P. Wang, N. Li, J. He, W. L. Song and H. Sen Chen, Energy Technol., 2022, 10, 2200017 CrossRef CAS.
- I. Dienwiebel, M. Winter and M. Borner, J. Phys. Chem. C, 2022, 126, 11016–11025 CrossRef CAS.
- Y. Deng, Y. Pan, Z. Zhang, Y. Fu, L. Gong, C. Liu, J. Yang, H. Zhang and X. Cheng, Adv. Funct. Mater., 2022, 32, 2106176 CrossRef CAS.
- J. Zhao, G. Zhou, K. Yan, J. Xie, Y. Li, L. Liao, Y. Jin, K. Liu, P. C. Hsu, J. Wang, H. M. Cheng and Y. Cui, Nat. Nanotechnol., 2017, 12, 993–999 CrossRef CAS PubMed.
- R. Mori, J. Solid State Electrochem., 2023 DOI:10.1007/s10008-023-05387-z.
- Y. Wang, Z. Zhang, M. Haibara, D. Sun, X. Ma, Y. Jin, H. Munakata and K. Kanamura, Electrochim. Acta, 2017, 255, 109–117 CrossRef CAS.
- C. Choi and D. W. Kim, J. Power Sources, 2020, 448, 1–11 CrossRef.
- J. Cho, Y. K. Ahn, Y. J. Gong, S. Pyo, J. Yoo and Y. S. Kim, Sustainable Energy Fuels, 2020, 4, 3051–3057 RSC.
- J. Wu, S. Liu, J. Huang, Y. Cui, P. Ma, D. Wu and K. Matyjaszewski, Macromolecules, 2021, 54, 2992–2999 CrossRef CAS.
- A. Kim, J. Hak Kim and R. Patel, Bioresour. Technol., 2021, 345, 126501 CrossRef PubMed.
- S.-J. Tan, Y.-F. Tian, Y. Zhao, X.-X. Feng, J. Zhang, C.-H. Zhang, M. Fan, J.-C. Guo, Y.-X. Yin, F. Wang, S. Xin and Y.-G. Guo, J. Am. Chem. Soc., 2022, 144, 18240–18245 CrossRef CAS PubMed.
- Z. Wei, N. Zhang, T. Feng, F. Wu, T. Zhao and R. Chen, Chem. Eng. J., 2022, 430, 132678 CrossRef CAS.
- Y. Li, J. Zhang, C. Zhou, M. Ling, J. Lu, Y. Hou, Q. Zhang, Q. He, X. Zhan and F. Chen, J. Alloys Compd., 2020, 826, 154197 CrossRef CAS.
- B. Yu, Y. Fan, S. Mateti, D. Kim, C. Zhao, S. Lu, X. Liu, Q. Rong, T. Tao, K. K. Tanwar, X. Tan, S. C. Smith and Y. I. Chen, ACS Nano, 2021, 15, 1358–1369 CrossRef CAS PubMed.
- J. Balach, H. K. Singh, S. Gomoll, T. Jaumann, M. Klose, S. Oswald, M. Richter, J. Eckert and L. Giebeler, ACS Appl. Mater. Interfaces, 2016, 8, 14586–14595 CrossRef CAS PubMed.
- X. Liu, J. Zhao, J. Wang, Z. Le, P. Nie, H. Wang, T. Xu, G. Xu, L. Chang and M. Zhu, J. Energy Storage, 2022, 54, 105324 CrossRef.
- V. K. Bharti, A. D. Pathak, C. S. Sharma and M. Khandelwal, Carbohydr. Polym., 2022, 293, 119731 CrossRef CAS PubMed.
- W. D. Hsu, P. W. Yang, H. Y. Chen, P. H. Wu, P. C. Wu, C. W. Hu, L. Saravanan, Y. F. Liao, Y. T. Su, D. Bhalothia, T. Y. Chen and C. C. Chang, Sci. Rep., 2021, 11(1), 1–15 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2023 |