Hierarchical Ni2P/Zn–Ni–P nanosheet array for efficient energy-saving hydrogen evolution and hydrazine oxidation†
Abstract
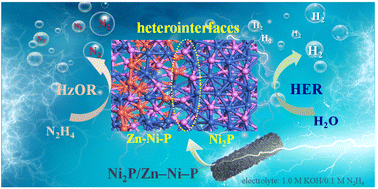
Electrochemical overall water splitting (OWS) includes two half reactions, the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER). Due to the limitation of the slow kinetic process of the OER, hydrazine oxidation reaction (HzOR) instead of OER has attracted extensive attention. Here, we obtained a series of hybrid catalysts-Ni2P/Zn–Ni–P with nanosheet array structures and rich atomic-level heterointerfaces using a simple synthesis approach. Among them, Ni2P/Zn–Ni–P-0.1 only requires low overpotentials of 63 and 27 mV to achieve current density of 10 mA cm−2 in the HER and HzOR, respectively. In the overall hydrazine splitting (OHzS), potentials of 0.165, 0.358 and 0.532 V are needed to achieve current densities of 10, 100 and 400 mA cm−2, which are much lower than that in OWS. Density Functional Theory calculation results show that Ni2P/Zn–Ni–P heterointerfaces not only optimize the hydrogen adsorption free energy, but also promote the dehydrogenation kinetics of hydrazine. Moreover, there are different active sites for the HER and HzOR in Ni2P/Zn–Ni–P. This study provides a new strategy for the development of novel dual-function electrocatalysts and promotes the development of practical energy-saving hydrogen production technology.


 Please wait while we load your content...
Please wait while we load your content...