An in situ formed copolymer electrolyte with high ionic conductivity and high lithium-ion transference number for dendrite-free solid-state lithium metal batteries†
Zhiheng
Ren‡
,
Jixiao
Li‡
,
Minghui
Cai
,
Ruonan
Yin
,
Jianneng
Liang
*,
Qianling
Zhang
 ,
Chuanxin
He
,
Chuanxin
He
 ,
Xiantao
Jiang
,
Xiantao
Jiang
 and
Xiangzhong
Ren
and
Xiangzhong
Ren
 *
*
College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, Guangdong 518060, China. E-mail: jlian46@uwo.ca; renxz@szu.edu.cn; Fax: +86-755-26558134; Tel: +86-755-26558134
First published on 13th December 2022
Abstract
Polymer electrolytes (PEs) synthesized from an in situ polymerization strategy are considered as promising candidates to improve interfacial compatibility. However, most in situ formed PEs still suffer from problems such as low lithium-ion transference number (tLi+) and insufficient ionic conductivity at room temperature (RT). Herein, a series of copolymer electrolytes (CPEs) consisting of 1,3-dioxolane (DOL) and 1,3,5-trioxane (TXE) monomers are synthesized. Due to the additive of succinonitrile (SN), and the promoted dissociation of lithium salt, the obtained polymer electrolyte (SN-CPE) exhibits an excellent ionic conductivity (4.06 × 10−4 S cm−1) and a high lithium-ion transference number (tLi+ = 0.881) at RT, as well as a high voltage oxidation window of 5.1 V. Thanks to the in situ formed intimate contacted electrode/electrolyte interface, the symmetric Li/Li cell can achieve long-term cycling stability over 1500 h without a short circuit. A LiF-rich organic–inorganic composite layer is formed on both the solid electrolyte interphase (SEI) and cathode electrolyte interphase (CEI) as demonstrated by XPS, TEM, and SEM analyses. Thereafter, the LiFePO4/Li cell has a high capacity retention of 84.1% after 900 cycles at RT, and it can still work effectively at high temperatures (e.g. 80 °C). Furthermore, SN-CPE exhibits excellent electrochemical performance and safety in the LiCoO2/Li cell and LiFePO4/graphite pouch cell, which again supports the promising application of SN-CPE.
Introduction
Lithium metal is considered as an ideal next-generation anode material because its theoretical capacity is 3860 mA h g−1 and its electrochemical potential is −3.04 V vs. the standard hydrogen electrode, which is the lowest electrochemical potential compared to other anode materials.1,2 Unfortunately, lithium metal batteries (LMBs) experience many challenges in practical applications, including the uncontrolled lithium dendrite growth, interfacial side reactions and huge volume changes during cycling.3–5 To overcome these problems, many novel electrolyte systems have been explored.6–8 Among these electrolyte systems, inflammable solid-state electrolytes (SSEs) are promising candidates for application in solid-state lithium metal batteries (SSLMBs).9,10The promising SSEs for SSLMBs can be generally classified into inorganic solid electrolytes (ISEs) and polymer electrolytes (PEs).11–13 PEs have good flexibility and provide relatively good interfacial contact. However, the performance of PE-based LMBs is still unsatisfactory, which should be attributed to some inherent drawbacks of PEs, including the low ionic conductivity, the narrow electrochemical window and the low lithium-ion transference number.14,15 In addition, the contact between the PE and the electrode only remains at the surface. As a result, no SSE can penetrate the porosities of the thick electrode, and the electrochemical performances of solid-state batteries (SSBs) are bad. Therefore, further investigations on how to build an intimate contact interface between SSEs and electrodes are still critical.16
Recently, in situ polymerization strategies for synthesizing PEs and building SSBs have received plenty of attention. This is because such an approach has great potential for achieving highly ionic conductive PEs, low interfacial resistance and a simple fabrication process for commercial applications.17 The in situ polymerization strategy generally involves pre-injection of a low-viscosity precursor into the cell followed by the initiation of a free-radical polymerization reaction or ring-opening polymerization reaction of the monomer with the help of a thermal or chemical initiator. Therefore, PEs obtained from the in situ process can fill the gaps/voids of the electrode, thus achieving a tight interfacial contact between the PEs and electrodes.18 Up to now, PEs synthesized through an in situ polymerization method have been widely reported, including poly(vinylene carbonate) (PVC),19 poly(methyl methacrylate) (PMMA),20 poly-tetrahydrofuran (PTHF),21 poly(triethylene glycol diacrylate) (PTEGDA),22 poly(1,3-dioxolane) (PDOL)23 and poly(trimethylpropane triacrylate) (ETPTA)24-based PEs. To further improve the interfacial compatibility, mechanical properties and the overall electrochemical performances of in situ polymerized PEs and SSBs, the strategies including integrations of inorganic fillers,24,25 making crosslinking polymers and copolymers,26–28 and introducing functional groups into the polymer chains19,29,30 have also been reported.
1,3-Dioxolane (DOL) is one of the most popular monomers for synthesizing in situ polymerized PEs for SSBs because of the facile initiation of the ring-opening polymerization reaction by some Lewis acid salts such as Al(OTf)3,23 LiFSI,31 LiPF6 (ref. 32) and LiDFOB33 under mild conditions. For example, Archer et al.23 reported the cationic ring-opening polymerization of DOL (PDOL) initiated by a low concentration of Al(OTf)3 salt. Thanks to the good wettability between the liquid precursor and electrodes, the gel polymer electrolyte (GPE) after in situ solidification effectively overcame the high interfacial resistance problem. Guo et al.32 investigated the conversion of a typical DOL/DME-based liquid electrolyte to a quasi-solid-solid GPE through the catalyst of LiPF6. The in situ formed polymer networks changed the solvation structure, disrupting the high voltage limitations of conventional ether-based electrolytes. Though there are many investigations on PDOL-based PEs, they still suffer from the problems of low ionic conductivity, low lithium-ion transference number, poor compatibility towards the lithium metal anode, and poor electrochemical oxidation tolerance properties. As a result, SSLMBs with pristine PDOL PEs show inferior electrochemical performance.
To enhance the overall electrochemical performance of PDOL-based PEs in SSLMBs, herein, we developed a copolymerization strategy to synthesize copolymer electrolytes (CPEs), where two monomers DOL and 1,3,5-trioxane (trioxymethylene (TXE)) were used for the copolymerization reaction. Besides DOL, TXE is also a common industrial chemical and its ring-opening polymerization products (polyformaldehyde (POM)) have been reported for applications in SSLMBs.34 Therefore, in this study, TXE was used to randomly copolymerize with DOL, forming a copolymer P(DOL-TXE), which disrupts the regularity of the PDOL chain and significantly reduces the crystallinity of PDOL-based PEs. Meanwhile, the energy level of the highest occupied molecular orbital (HOMO) of the copolymer is lower compared to other counterparts, which confers a better anti-oxidation capacity. The SN plasticizer is added for increasing the RT ionic conductivity of CPE and labelled as SN-CPE. An ionic conductivity of 4.06 × 10−4 S cm−1 at 25 °C is thus achieved. The strong polar cyano could effectively restrict the movement of anions and loosen the strong coordination effect of Li–O, promoting the transportation of Li+ along the polymer chain. Therefore, SN-CPE has a Li+ transference number of 0.881, which is almost the highest value among the reported PEs. The electrochemical stability window of SN-CPE is 5.1 V. Thereafter, stable lithium plating/stripping cycling over 1500 h is achieved in the Li|SN-CPE|Li symmetric cell. Excellent cycling stability of the LFP|SN-CPE|Li SSB with a capacity retention of 84.1% after 900 cycles at 25 °C is illustrated. Besides, SN-CPE also exhibits good performance in a LiCoO2/Li battery and good stability under high temperature working conditions. This study provides new insights into the design of high-performance PEs for wide working temperature ranges and high energy density SSLMBs.
Experimental part
Electrolyte preparation
The precursors of PEs were prepared in an Ar-filled glove box. H2O and O2 contents in the glove box were controlled below 0.1 ppm. The PDOL PE was obtained by dissolving 1 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI, 99.5%, Aladdin) and 1 M lithium oxalyldifluoroborate (LiDFOB, 99.9%, Adamas) in 1,3-dioxolane (DOL, 99%, Aladdin) solution, following by heating them at 60 °C for 12 h to ensure the completion of the polymerization reaction. For the preparation of CPEs, a mixture of DOL and TXE (99.5%, Macklin) solvents with a weight ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was first prepared. Then, 1 M LiTFSI and 1 M LiDFOB were dissolved in the solvent mixture, followed by heating at 60 °C for 12 h to provide enough time for the polymerization reaction. CPEs with different DOL
1 was first prepared. Then, 1 M LiTFSI and 1 M LiDFOB were dissolved in the solvent mixture, followed by heating at 60 °C for 12 h to provide enough time for the polymerization reaction. CPEs with different DOL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TXE ratios were prepared by the same method. The SN-CPE was also prepared in the same manner as CPE, with the addition of SN. The weight ratio of DOL
TXE ratios were prepared by the same method. The SN-CPE was also prepared in the same manner as CPE, with the addition of SN. The weight ratio of DOL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TXE
TXE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SN (99%, Aladdin) was 4
SN (99%, Aladdin) was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The liquid electrolyte (LE) was prepared by dissolving 2 M LiTFSI in DOL
1. The liquid electrolyte (LE) was prepared by dissolving 2 M LiTFSI in DOL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DME (1
DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution.
1) solution.
Electrode preparation and cell fabrication
The LiFePO4 (LFP) cathode electrode was made by mixing LFP powders (98%, Aladdin), polyvinylidene difluoride (PVDF, Arkema) binder and conductive carbon black powders (Super P, TIMCAL) in N-methyl-2-pyrrolidone (NMP, 99%, Aladdin) solvent. The weight ratio of LFP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVDF
PVDF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SP is 8
SP is 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture then formed a slurry after mixing, and was cast onto Al foil. The electrode was dried at 80 °C for 12 h in a vacuum oven for removing NMP. Then, the dried LFP electrode was punched into disks (12 mm in diameter). The mass loading is 1.5–1.8 mg cm−2. The LiNi0.8Mn0.1Co0.1O2 (NCM811) (Shenzhen Kejing Co., Ltd) and LiCoO2 (LCO, 99.5%, Aladdin) cathode electrodes were prepared by the same method with the active material mass loading of 1.5–1.8 mg cm−2. Coin cells (CR2032) were assembled with Li-metal (∼500 μm thickness) as the anode, LFP, NCM811 or LCO as the cathode, and glass fiber (GF) (≈280 μm thickness) as the separator. The precursor was injected into the separator and the coin cells were then packaged immediately. LFP|SN-CPE|graphite pouch cells were assembled and sealed using the Al-laminated film, and PE separator was used to prevent short circuits. All the cells are assembled in an Ar-filled glove box. All the cells were rested for 2 h before heating for initiating the in situ polymerization process, to ensure that the electrodes were well wetted by the precursor.
1. The mixture then formed a slurry after mixing, and was cast onto Al foil. The electrode was dried at 80 °C for 12 h in a vacuum oven for removing NMP. Then, the dried LFP electrode was punched into disks (12 mm in diameter). The mass loading is 1.5–1.8 mg cm−2. The LiNi0.8Mn0.1Co0.1O2 (NCM811) (Shenzhen Kejing Co., Ltd) and LiCoO2 (LCO, 99.5%, Aladdin) cathode electrodes were prepared by the same method with the active material mass loading of 1.5–1.8 mg cm−2. Coin cells (CR2032) were assembled with Li-metal (∼500 μm thickness) as the anode, LFP, NCM811 or LCO as the cathode, and glass fiber (GF) (≈280 μm thickness) as the separator. The precursor was injected into the separator and the coin cells were then packaged immediately. LFP|SN-CPE|graphite pouch cells were assembled and sealed using the Al-laminated film, and PE separator was used to prevent short circuits. All the cells are assembled in an Ar-filled glove box. All the cells were rested for 2 h before heating for initiating the in situ polymerization process, to ensure that the electrodes were well wetted by the precursor.
Electrochemical characterization
All electrochemical characterization studies were performed at room temperature (25 °C), unless otherwise noted. Galvanostatic charge/discharge tests of the batteries under different current densities were carried out on a LAND battery-testing system (CT2001A).The electrochemical impedance spectroscopy (EIS) study was performed via a CHI760E electrochemical working station, where the frequency ranged from 0.1 M Hz to 0.1 Hz and the voltage amplitude was 5 mV. The ionic conductivities (σ) of PEs were measured in a symmetric stainless steel cell and their values were calculated according to the following eqn (1):
 | (1) |
The activation energy (Ea) of PE was derived from the slope of the Arrhenius plot.
The lithium-ion transference numbers (tLi+) of PEs were calculated according to the chronoamperometry study and EIS measurements of Li|PE|Li symmetric cells. Eqn (2) can be applied for calculating the value of tLi+
 | (2) |
To evaluate the electrochemical stability window, linear sweep voltammetry (LSV) was performed on SS|PE|Li cells in the voltage range of 2.0–6.0 V under the voltage scan rate of 1 mV s−1, where PE was used as the separator. The electrochemical floating test was conducted in an NCM811/Li cell at stepwise voltages from 4.0 V to 4.6 V, with 10 h holding time at each voltage, to examine the oxidative stability of PE. The impedances of LFP/Li and Li/Li cells were evaluated by EIS in the frequency range of 1 M Hz–0.1 Hz, under a voltage amplitude of 10 mV.
Material characterization
A scanning electron microscope (SEM) (Hitachi S-3400N) was used to investigate morphologies. 1H nuclear magnetic resonance (NMR) spectra of samples were recorded via a Bruker AVANCE III 400 MHz NMR spectrometer with dimethyl sulfoxide-d6 as the deuterated solvent. 7Li NMR was performed via a Bruker AVANCE III 600 MHZ spectrometer with dimethyl sulfoxide-d6 as the deuterated solvent. Fourier transform infrared (FTIR) spectra were recorded on an IR affinity-1 FTIR spectrometer (SHIMADZU). Tensile tests were performed using a CMT4304 (LSI) electronic universal tensile machine at a strain rate of 5 mm min−1. X-ray powder diffraction (XRD) was performed using an Empyrean XRD instrument (PANalytical). Thermogravimetric analysis (TGA) tests were performed using a Netzsch STA409PC instrument at a heating rate of 10 °C min−1 under a N2 atmosphere from room temperature to 350 °C to investigate the thermal stability of PEs. A NETZSCH DSC-200F3 was used to perform differential scanning calorimetry (DSC) analysis from −80 to 60 °C at a heating rate of 10 °C min−1 under a N2 atmosphere. The molecular weight of PEs was measured using an Agilent 1260 Infinity gel permeation chromatograph (GPC) in tetrahydrofuran. X-ray photoelectron spectroscopy (XPS) was performed using a K-Alpha+ X-ray photoelectron spectrometer (Thermo Scientific). Transmission electron microscopy (TEM) observations were recorded using a JEOL JEM-2100 instrument. The electrodes used for SEM, XPS and TEM characterization studies were cleaned with DME several times and dried in an Ar-filled glove box.Theoretical calculations
Ab initio calculations were used to calculate the oxidation stabilities of different molecules. The optimized structures and vibrational frequencies of DOL, PDOL, TXE, P(DOL-TXE) and SN molecules were obtained using Gaussian 09 (ref. 35) and the calculations of HOMO and the lowest unoccupied molecular orbital (LUMO) energies were conducted via density functional theory (DFT) with the B3LYP/6-31G(d,p) basis set.36 There are no imaginary frequencies in the optimized structure, which indicated that the structure is the correct minimum point on the potential energy surface. The surface electrostatic potential (ESP) maps were calculated within the background of the Multiwfn program37,38 and plotted using VMD software.39 The binding energies (Eb) between PDOL and free Li+ were calculated using the following eqn (3):| Eb = E(PDOL-Li) − E(Li+) − E(PDOL) | (3) |
The calculating methods of binding energies of CPE-Li+ and SN-Li+ are the same as eqn (3).
Results and discussion
CPE was synthesized through ring-opening polymerization reactions of DOL and TXE monomers catalysed by LiDFOB salt.40 The in situ copolymerization process of SN-CPE is depicted in Fig. 1a, where DOL and TXE monomers first penetrate the pores of the electrode and GF separator, then they in situ transform into a quasi-solid-state PE after the polymerization reaction, resulting in the intimate contact between the electrodes and PE. The copolymerization reaction mechanism is shown in Fig. 1b. Firstly, LiDFOB undergoes a series of decomposition reactions in an alkaline environment to produce BF3 as the Lewis acid initiator, which combines with trace water to produce the BF3–H2O complex. After that, the ionized proton hydrogen attacks the oxygen atoms of monomers to form oxonium ions. Then, other monomers repeatedly attack carbon atoms adjacent to oxonium ions to trigger the growth of copolymer chains and finally form the P(DOL-TXE) copolymer.41 Herein, different PEs obtained by polymerizing DOL, TXE and DOL + TXE copolymer monomer mixtures with different DOL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TXE ratios were synthesized. PE containing a single monomer of DOL (or TXE) and lithium salts is defined as PDOL (or PTXE). For PE consisting of DOL, TXE and lithium salts, it is labelled as CPE. For PE containing DOL, TXE, lithium salts and SN, where SN is introduced to enable CPE an excellent ionic conductivity at RT and a high lithium-ion transference number, it is named SN-CPE. Fig. 1c macroscopically shows photo images of the SN-CPE precursor before and after polymerization. The liquid precursor transforms to a homogeneous quasi-solid state, suggesting the occurrence of polymerization reactions.
TXE ratios were synthesized. PE containing a single monomer of DOL (or TXE) and lithium salts is defined as PDOL (or PTXE). For PE consisting of DOL, TXE and lithium salts, it is labelled as CPE. For PE containing DOL, TXE, lithium salts and SN, where SN is introduced to enable CPE an excellent ionic conductivity at RT and a high lithium-ion transference number, it is named SN-CPE. Fig. 1c macroscopically shows photo images of the SN-CPE precursor before and after polymerization. The liquid precursor transforms to a homogeneous quasi-solid state, suggesting the occurrence of polymerization reactions.
The GF membrane is used as the 3D porous skeleton to sponge the PE precursor, and as the separator for batteries. After filling with PE, the white GF membrane transformed into a translucent membrane, and a uniform interpenetrating network was consequently formed, which ensured continuous and fast Li+ transport (Fig. S1†). It is interesting that the GF membrane with PEs filling exhibited higher elasticity (Fig. S2†), which was beneficial for improving the electrode/electrolyte interface contact and blocking lithium dendrites. To illustrate the characteristics and advantages of in situ polymerization of precursors more clearly inside the cell, a Li (in situ)/SN-CPE/Li (ex situ) cell was constructed, and its interface is studied. As shown in Fig. S3,† the Li (ex situ)/SN-CPE interface prepared by ex situ method is not tight, which will lead to high interfacial impedance and uneven lithium flux. In contrast, the Li (in situ)/SN-CPE interface is intimately contacted. Therefore, the in situ polymerization method can endow the lithium battery with a low interfacial impedance and uniform lithium flux.
Subsequently, 1H NMR was performed to study the chemical structures of samples and prove the ring-open polymerization of monomers,40 and the results are shown in Fig. 1d. First, 1H NMR peaks at 4.78 ppm and 3.78 ppm corresponding to the H (a) and H (b) in DOL molecule were shifted to 4.64 ppm (d) and 3.61 ppm (e), respectively, in PDOL and CPE, SN-CPE samples, representing the formation of –(O–CH2–O–CH2)– and –(O–CH2CH2–O)– units. Secondly, the 5.12 ppm peak comes from H (c) in the O–CH2–O structure of the TXE monomer (Fig. S4†). The NMR peaks from DOL or TXE monomers can be observed in the 1H NMR spectra of PDOL, CPE and SN-CPE samples, which indicates that DOL and TXE monomers are not completely converted into copolymers and the presence of trace amounts of the monomers would be favourable to the increase of ionic conductivity due to the plasticizer effect. In addition, the 4.72 ppm peak should have originated from the H (f) in the –(OCH2)3– structure from the ring-opening polymerization of the TXE monomer. The presence of multiple peaks at the ranges between 3.5 and 3.8 ppm and between 4.6 and 4.9 ppm in CPE and SN-CPE samples indicates the random sequence of DOL and TXE units during the polymerization reaction. The peak at 2.91 ppm comes from the proton of SN. FTIR was used to further demonstrate the structural and molecular interactions of PEs (Fig. 1e). For DOL monomer, a distinct C–H out-of-plane vibrational peak located at 910 cm−1, C–O–C vibrational peak located at 1000–1150 cm−1, and the saturated C–H stretching vibration peak located at 2850–3000 cm−1 were observed. The former belongs to the typical features of the ring-ether structure.42,43 Compared to the DOL monomer, a long-chain –(CH2)n– vibrational mode located at 840 cm−1 appeared in these PEs samples, demonstrating the transition from cyclic monomers to long-chain polymers. In addition, the C–O–C vibrational modes of CPE and SN-CPE were shifted to 1110 cm−1 and 1130 cm−1, respectively, demonstrating interactions among DOL, TXE and SN. Moreover, compared to PDOL and CPE, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration double peaks belong to LiDFOB in SN-CPE shifted from 1753 cm−1 and 1791 cm−1 to 1764 cm−1 and 1801 cm−1, respectively, which implies more dissociation of LiDFOB in SN-CPE, possibly attributed to the strong interaction between LiDFOB and SN. The gel permeation chromatography (GPC) study again supported the polymerization reaction of monomers. As shown in Table S1,† the high average molecular weights (Mw) of PDOL, CPE and SN-CPE are 6975, 5570, and 4479, respectively. It seems that the copolymerization strategy tends to generate smaller molecular weight polymers under the same initiation conditions, which sacrifices some mechanical properties, but facilitates the rapid transport of Li+ ions. In addition, the polydispersity index (Mn/Mw) of SN-CPE is 1.67, which is slightly lower than those of PDOL and CPE, indicating that SN-CPE has better homogeneity.
O stretching vibration double peaks belong to LiDFOB in SN-CPE shifted from 1753 cm−1 and 1791 cm−1 to 1764 cm−1 and 1801 cm−1, respectively, which implies more dissociation of LiDFOB in SN-CPE, possibly attributed to the strong interaction between LiDFOB and SN. The gel permeation chromatography (GPC) study again supported the polymerization reaction of monomers. As shown in Table S1,† the high average molecular weights (Mw) of PDOL, CPE and SN-CPE are 6975, 5570, and 4479, respectively. It seems that the copolymerization strategy tends to generate smaller molecular weight polymers under the same initiation conditions, which sacrifices some mechanical properties, but facilitates the rapid transport of Li+ ions. In addition, the polydispersity index (Mn/Mw) of SN-CPE is 1.67, which is slightly lower than those of PDOL and CPE, indicating that SN-CPE has better homogeneity.
The crystallinity of PE is an important parameter for evaluating the Li+ transport capacity. The more the amorphous polymer phase, the higher the ionic conductivity. The crystallinity of PEs was visually characterized by X-ray powder diffraction (XRD), as shown in Fig. S5.† PDOL showed a clear crystalline peak at 2θ = 22.6°, while no diffraction peak is observed in the other two copolymer systems, indicating that the introduction of TXE would cause the copolymers to be less aligned, thus reducing the crystallinity of PEs.44 The thermal behaviours of PEs were analysed by applying a DSC study. As shown in Fig. S6,† SN-CPE exhibited a lower glass transition temperature (Tg) and a lower melting temperature (Tm), indicating that SN-CPE is almost amorphous at the working temperature.27 Fig. S7† shows the TGA results of these PEs. It was reported that 25–80 °C is the working temperature range of the vast majority of LMBs.45 So, the stability of PEs at this temperature range was studied. TGA results suggest that SN-CPE and CPE had almost no weight loss in this temperature range, indicating their high safety performance.
In general, the ideal electrolyte should have a low HOMO and a high LUMO to obtain a wide voltage stability window thermodynamically.46,47 The HOMO and LUMO of the DOL monomer, PDOL, TXE monomer, P(DOL-TXE) and SN were calculated separately using DFT. The results in Fig. 2a show that TXE and P(DOL-TXE) have a lower HOMO value compared to DOL and PDOL, suggesting that the introduction of the TXE unit will increase the oxidation tolerance of PEs. For SN, the highly polar cyano (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) has a strong electron-absorbing effect, which confers a very low HOMO to SN. Therefore, SN-CPE should have a stronger capacity against oxidation. The LUMO value represents the tendency of the components to be reduced. These results suggest that ether-based DOL and TXE monomers have a higher LUMO compared to PDOL and P(DOL-TXE), respectively, indicating that the polymerization can decrease the capacity against reduction. The relatively lower LUMO for P(DOL-TXE) will not significantly sacrifice its stability toward the Li anode since a LiF and Li3N rich, and stale SEI layer was created at the SN-CPE/Li anode interface, as discussed below. Fig. 2b shows the molecular surface electrostatic potential (ESP) maps of PDOL, P(DOL-TXE), and SN with and without Li+ ion coordination, respectively.37,38 Li+ ions migrate through the coordination with ether oxygen (EO) and hop from one site to another. Fig. 2b shows that the more well-arranged polymer chains can provide a denser negative potential distribution, which can increase the Li+ binding sites.48,49 The binding energies between different polymer chains and Li, which are calculated based on the optimized model, were −1.697 eV for PDOL-Li, and −1.281 eV for P(DOL-TXE)-Li, which means Li+ ions can move faster in the main body of the copolymer (Table S2† displays the calculation details). In addition, the binding energy of SN-Li is −1.786 eV, which is more negative than those between both polymer bodies and Li+, indicating that SN can promote the dissociation of lithium salts. The 7Li NMR results are consistent with DFT calculations. As shown in Fig. S8,† compared to PDOL, CPE had an up-field shift of the Li NMR peak, which confirmed that the copolymer structure formed by DOL and TXE weakened the coordination between Li+ and EO of the polymer chain. With the introduction of SN, a low-field shift of Li peak was exhibited in SN-CPE, which suggested that SN was involved in the coordination with Li+, and the tendency of the coordination was stronger than that between Li+ and EO. Therefore, SN acts synergistically with the copolymer to ensure the solvation of salts and the rapid migration of Li+ ions.50
N) has a strong electron-absorbing effect, which confers a very low HOMO to SN. Therefore, SN-CPE should have a stronger capacity against oxidation. The LUMO value represents the tendency of the components to be reduced. These results suggest that ether-based DOL and TXE monomers have a higher LUMO compared to PDOL and P(DOL-TXE), respectively, indicating that the polymerization can decrease the capacity against reduction. The relatively lower LUMO for P(DOL-TXE) will not significantly sacrifice its stability toward the Li anode since a LiF and Li3N rich, and stale SEI layer was created at the SN-CPE/Li anode interface, as discussed below. Fig. 2b shows the molecular surface electrostatic potential (ESP) maps of PDOL, P(DOL-TXE), and SN with and without Li+ ion coordination, respectively.37,38 Li+ ions migrate through the coordination with ether oxygen (EO) and hop from one site to another. Fig. 2b shows that the more well-arranged polymer chains can provide a denser negative potential distribution, which can increase the Li+ binding sites.48,49 The binding energies between different polymer chains and Li, which are calculated based on the optimized model, were −1.697 eV for PDOL-Li, and −1.281 eV for P(DOL-TXE)-Li, which means Li+ ions can move faster in the main body of the copolymer (Table S2† displays the calculation details). In addition, the binding energy of SN-Li is −1.786 eV, which is more negative than those between both polymer bodies and Li+, indicating that SN can promote the dissociation of lithium salts. The 7Li NMR results are consistent with DFT calculations. As shown in Fig. S8,† compared to PDOL, CPE had an up-field shift of the Li NMR peak, which confirmed that the copolymer structure formed by DOL and TXE weakened the coordination between Li+ and EO of the polymer chain. With the introduction of SN, a low-field shift of Li peak was exhibited in SN-CPE, which suggested that SN was involved in the coordination with Li+, and the tendency of the coordination was stronger than that between Li+ and EO. Therefore, SN acts synergistically with the copolymer to ensure the solvation of salts and the rapid migration of Li+ ions.50
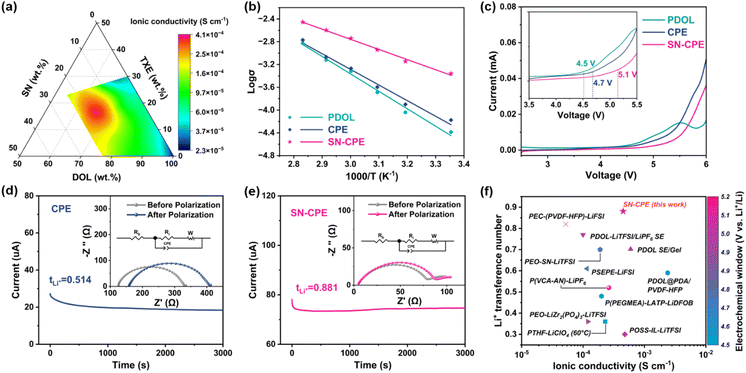
The ionic conductivities of PEs with different mass ratios of DOL, TXE and SN, were evaluated by EIS, and the results are illustrated in Fig. 3a and S9.† It is found that with the mass ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (DOL
2 (DOL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TXE
TXE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SN), SN-CPE can achieve the highest ionic conductivity of 4.06 × 10−4 S cm−1 at 25 °C. The Arrhenius curves of PDOL, CPE and SN-CPE are shown in Fig. 3b, depicting the relationship between the temperature and ionic conductivity at 25–80 °C. All these PEs samples fit the Vogel–Tamman–Fulcher mode well,51 and the calculated activation energies (Ea) of PDOL, CPE, and SN-CPE are 0.61 eV, 0.55 eV and 0.35 eV, respectively.
SN), SN-CPE can achieve the highest ionic conductivity of 4.06 × 10−4 S cm−1 at 25 °C. The Arrhenius curves of PDOL, CPE and SN-CPE are shown in Fig. 3b, depicting the relationship between the temperature and ionic conductivity at 25–80 °C. All these PEs samples fit the Vogel–Tamman–Fulcher mode well,51 and the calculated activation energies (Ea) of PDOL, CPE, and SN-CPE are 0.61 eV, 0.55 eV and 0.35 eV, respectively.
 | ||
| Fig. 3 (a) Ionic conductivity of PEs with various DOL, TXE and SN mass ratios at 25 °C on the ternary contour diagram. (b) Arrhenius plots and (c) linear sweep voltammetry (LSV) of PDOL, CPE and SN-CPE (inset is the enlarged profile at 3.5 to 5.5 V). Chronoamperometry curves with a step voltage of 10 mV (the insets display the EIS plot before and after polarization) of (d) CPE and (e) SN-CPE. (f) The plot of lithium-ion transference number against ionic conductivity for SN-CPE and other reported PEs.18,54–63 The colour scale of the symbol represents the high voltage stability window of the PEs. | ||
The lower activation energy implies a lower energy barrier for Li+ migration, which makes PE easier to achieve a rapid Li+ migration.49 Therefore, the higher ionic conductivity and lower Li+ migration energy barrier of SN-CPE mean it is superior than other PEs.
The electrochemical stability window of PE is crucial when coupling with high-voltage cathodes. It has been well known that the electrochemical stability window of ether-based electrolytes is usually below 4.0 V vs. Li/Li+.52 In this study, we found that the copolymerization strategy can enhance the oxidation tolerance of PE by changing the molecular structure of copolymer chains. The electrochemical oxidation windows of CPE and SN-CPE illustrated a clear increase compared to that of PDOL PE, as determined by LSV studies (Fig. 3c). It presents that PDOL PE exhibited an oxidation voltage of 4.5 V, which increased to 4.7 V and 5.1 V for CPE and SN-CPE, respectively. This result was consistent well with the HOMO result in Fig. 2. Electrochemical float tests were used to further evaluate the oxidation tolerance of PEs in SSBs. As shown in Fig. S10,† the leakage currents of NCM811|SN-CPE|Li cell under high voltage (4.2–4.6 V) are always smaller than those with PDOL and CPE as the electrolytes, and the leakage currents can decay faster, indicating that SN-CPE had better oxidation stability, indicating that it can be matched with high-voltage cathode materials for SSBs.53
The lithium-ion transference number (tLi+) is another important parameter for evaluating the performance of PEs, which reflects how much percentage of the ionic conductivity is contributed by the movement of Li+ ions in the electrolyte. A higher tLi+ can alleviate the concentration polarization and inhibit the formation of lithium dendrites.54 The tLi+ of these PEs was determined using the constant potential polarization method.55 Compared to PDOL PE (tLi+ = 0.478, Fig. S11†) and CPE (tLi+ = 0.514, Fig. 3d), SN-CPE with a tLi+ as high as 0.881 (Fig. 3e) exhibited a closed single ion conductor behaviour. The reasons for such a high tLi+ should be (1) the weak complexation between EO and Li+ ions resulting in fast Li+ transportation; (2) the anions are confined in the copolymer framework, making them less likely to transfer over long distances. In addition, the high dielectric constant of SN (∼55) provides superb lithium salt dissociation, resulting in more free Li+ ions and fast ionic conduction.56 The above computational and experimental results indicate that the as-prepared SN-CPE had a fast Li+ migration ability by suppressing the movement of anions, which is favourable for the electrochemical performance of lithium batteries. Compared to other reported PEs, SN-CPE exhibits excellent properties in terms of tLi+, ionic conductivity, and electrochemical stability window (Fig. 3f).21,57–66
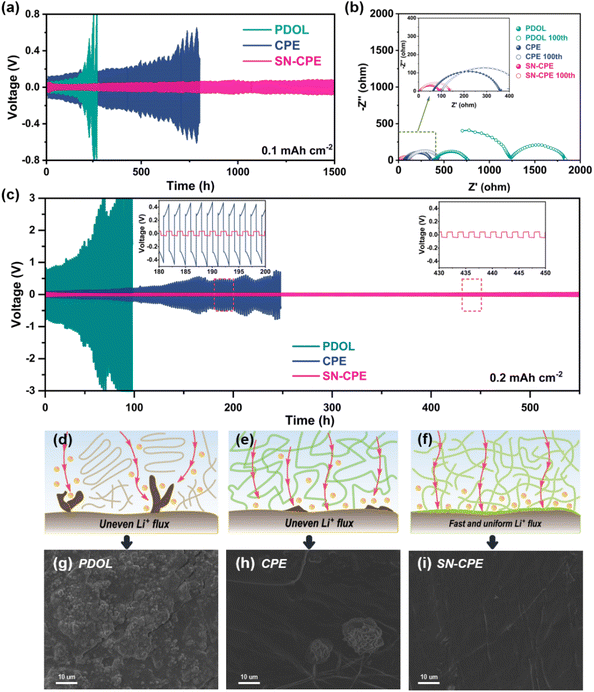
The lithium plating/stripping behaviour of symmetric Li/Li cells with different PEs was studied to evaluate the interface stabilities between the Li metal anode and PEs. As shown in Fig. 4a, at a current density of 0.1 mA cm−2, the symmetric cell using PDOL PE had a relatively high overpotential of up to 120 mV, and an increase in polarization voltage upon cycling. It experienced short circuiting after around 270 h charge/discharge cycling, possibly due to the continuous generation of dead Li and Li dendrites.
The Li/Li symmetric cell with CPE was able to operate stably for 800 h. However, its polarization voltage increased progressively during cycling, meaning the poor interface stability between the CPE and Li metal anode. Encouragingly, the Li/Li symmetric cell using SN-CPE exhibited a small polarization potential (52 mV), and it was stable even after 1500 h cycles, indicating that SN-CPE was stable towards the Li metal anode. On further increasing the current density and the capacity (0.2 mA cm−2, 0.2 mA h cm−2, and 1 mA h cm−2), the Li/Li symmetric cell with SN-CPE can still deliver great cycling stability, indicating the superior electrochemical performance of SN-CPE (Figs. 4c and S12†). However, PDOL and CPE exhibited rapid increases in overpotentials, indicating severe side reactions and poor reaction kinetics at a higher current density.
The interfacial stability between PEs and Li was further evaluated by EIS. As shown in Fig. 4b, the interfacial impedance (Ri) of the Li|SN-CPE|Li cell was pretty low, which was about 80 Ω. It increased to 140 Ω after 100 cycles, which was much lower than those of the Li|PDOL|Li cell (370 Ω) and Li|CPE|Li cell (295 Ω). The low interfacial impedance also suggests good interfacial contact and high compatibility between the SN-CPE and Li metal anode. To verify the effect of SN-CPE in inhibiting Li dendrite growth, the surface morphologies of Li metal anodes after 100 cycles in Li/Li symmetric cells with different PEs were studied by FE-SEM. As shown in Fig. 4g and the schematic illustrated in Fig. 4d, the surface of the Li metal anode from the Li|PDOL|Li cell after cycling showed an uneven and mossy structure. However, the Li metal anode from the Li|CPE|Li cell was flattened but it also presented some irregular protrusions (Fig. 4e and h). Notably, the Li metal anode from the Li|SN-CPE|Li cell had a very smooth and condense surface morphology, indicating uniform Li plating/stripping performance (Fig. 4f and i). The FE-SEM study results again illustrated the stable interface between the SN-CPE and Li metal anode.
The composition of the SEI layer is critical to the stability of the Li metal cathode. An appropriate SEI layer contributes to fast Li+ transport, uniform Li deposition and circumvents unfavourable side reactions between the electrolyte and Li anode.7 To study the chemical components of the SEI layer between the SN-CPE and Li metal anode, and to reveal the mechanism for the stable Li plating/stripping performance, XPS with ion etching for an in-depth analysis was performed.67 As shown in Fig. 5a, in the C 1s XPS spectrum, C–C–O (286.6 eV) and O–C–O (288.0 eV) of the ether-based compounds came from the ring-opening polymerized DOL and TXE. The carbonyl carbon (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 289.5 eV) was originally from the decomposed products of CPE.33 In the F 1s XPS spectrum (Fig. 5b), there were distinct signals at 684.8 eV and 688.4 eV, which could be attributed to C–F and LiF, respectively.68 LiF has been widely regarded as a crucial SEI component for resisting lithium dendrite growth and inhibiting interface side reactions.69 The O 1s spectra in Fig. 5c can be divided into three segments: lithium oxide (ROLi, 530.4 eV), carbonate (Li2CO3/O–C
O, 289.5 eV) was originally from the decomposed products of CPE.33 In the F 1s XPS spectrum (Fig. 5b), there were distinct signals at 684.8 eV and 688.4 eV, which could be attributed to C–F and LiF, respectively.68 LiF has been widely regarded as a crucial SEI component for resisting lithium dendrite growth and inhibiting interface side reactions.69 The O 1s spectra in Fig. 5c can be divided into three segments: lithium oxide (ROLi, 530.4 eV), carbonate (Li2CO3/O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 531.5 eV) and ether oxygen (C–O, 533.0 eV).29 N 1s XPS spectra in Fig. 5d display a peak at 399.5 eV, which can be attributed to TFSI− from LiTFSI, and C
O, 531.5 eV) and ether oxygen (C–O, 533.0 eV).29 N 1s XPS spectra in Fig. 5d display a peak at 399.5 eV, which can be attributed to TFSI− from LiTFSI, and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N from SN.70 Another peak at 398.4 eV belongs to Li3N, which should be the decomposition product from SN.71 Li3N is also considered as a favourable component of SEI because of its high ionic conductivity, which is important in reducing the interfacial resistance and inhibiting Li dendrites.72
N from SN.70 Another peak at 398.4 eV belongs to Li3N, which should be the decomposition product from SN.71 Li3N is also considered as a favourable component of SEI because of its high ionic conductivity, which is important in reducing the interfacial resistance and inhibiting Li dendrites.72
 | ||
| Fig. 5 In-depth XPS analyses of the cycled Li metal after 100 cycles at 0.2 mA cm−2 and 0.2 mA h cm−2 with SN-CPE. (a) C 1s, (b) F 1s, (c) O 1s and (d) N 1s. | ||
As the etching proceeded, chemical information of the inner surface was exposed, and the peak intensities of C–C–O, O–C–O and C–F became weakened and weakened. This means the surfaces of samples were rich with poly-organic materials, while the inside lacks poly-organic materials. On the other hand, the intensities of carbonate, LiF, Li3N and ROLi peaks, which represent inorganic components of the SEI layer, increase as the etching time increase, indicating that the inner layer of the SEI contains more inorganic materials. The organic/inorganic composite SEI layers were beneficial for the stability of the SEI and uniform lithium plating/stripping behaviour.69,73
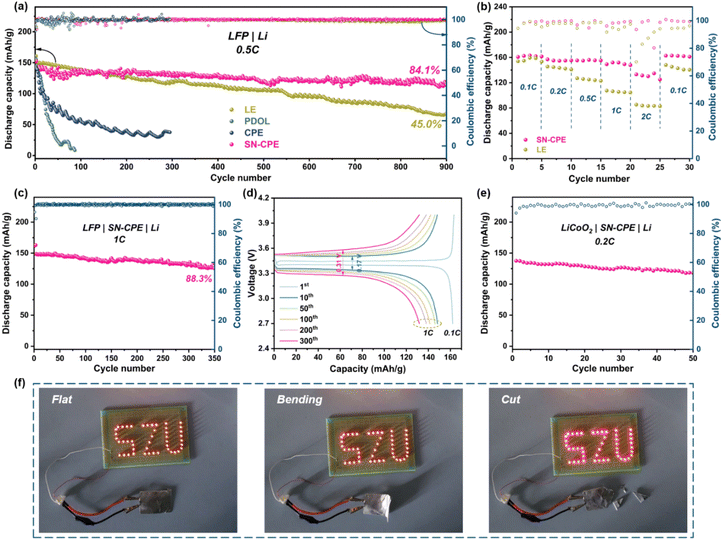
The application of PEs in SSBs was further investigated. Fig. 6a shows the long-term cycling performance of LFP SSBs with different PEs. Compared to LFP SSBs with CPE and PDOL, which had significant capacity decay after tens of cycles due to low ionic conductivity and poor interfacial stability at RT, the LFP|SN-CPE|Li cell can be stably cycled 900 times at a current density of 0.5C, with a coulomb efficiency close to 100% and a capacity retention of 84.1%.
Besides, the capacity retention and the rate performance of LFP battery with SN-CPE also far exceeded those of the LFP battery with ether-based liquid electrolytes (Fig. 6b). At the current density of 1C, the LFP|SN-CPE|Li cell had an initial capacity of 149.8 mA h g−1, which remains 88.3% after 350 cycles (Fig. 6c). Charge/discharge voltage profiles in Fig. 6d also suggest stable and reversible charge/discharge plateaus from 1 to 300 cycles with only a small increase in polarization voltages (0.17 V to 0.31 V), which was attributed to the stable electrode/electrolyte interface and the fast internal and interfacial Li+ transport. As exhibited in Fig. S13,† the impedance of the LFP|SN-CPE|Li cell was relatively small and it slightly increased in subsequent cycles (below 450 Ω), which was beneficial to the electrochemical performance of SSBs. Unfortunately, the LFP|LE|Li cell exhibited a continuous increase in the polarization potential and a fast capacity decay (Fig. S14†).
The performances and safety of batteries are significantly affected by the working temperature during practical operation. Ether-based electrolytes will decompose and generate gases and other side-reaction products at elevated temperatures, resulting in a significant compromise in safety and suitability of batteries. Here, the LFP|SN-CPE|Li cell was found to be able to operate stably at 80 °C, with an initial capacity of ∼165 mA h g−1 at 1C. It experienced almost no decay within 100 cycles (Fig. S15†), which showed excellent high-temperature adaptability.
To further study the compaticity between the PEs and layer-structured cathode, the LiCoO2|SN-CPE|Li cell was studied. As shown in Fig. 6e, this cell had a high initial capacity of 137.6 mA h g−1 at 0.2C and a high capacity retention (∼86.4%) after 50 cycles. Fig. S16† shows corresponding charge/discharge curves of the first 5 cycles, which were smooth and well coincided, suggesting the decent high voltage performance of SN-CPE.
Further study on LFP|SN-CPE|graphite pouch cell was done to evaluate the potential of SN-CPE in industrial applications. As presented in Fig. 6f, the pouch cell can successfully light up LED lamps. And thanks to the flexibility of SN-CPE and the good interfacial contact, the cell can still work after bending and cutting treatment, which suggested excellent safety properties induced by SN-CPE.
SEM, TEM and XPS analyses were further used to investigate the cathode electrolyte interfaces (CEI) in the LFP|LE|Li battery and LFP|SN-CPE|Li cell, respectively, to reveal the reason for the good interface stability with SN-CPE. Fig. 7a and e shows surface SEM images of the cathode after 20 cycles in LFP batteries with LE and SN-CPE, respectively. It shows that the LFP cathode after cycling with SN-CPE prepared by the in situ polymerization method was totally covered by the PE, suggesting the intimate contact interface and the formation of a favourable CEI layer. The morphology of the CEI layer was observed using ex situ TEM, and the results are shown in Fig. 7b and f. It is found that there was a thick and non-uniform CEI layer at the LFP/LE interface, compared to a uniform thin CEI layer (∼4.6 nm) at the LFP/SN-CPE interface. Such a thin and uniform CEI at the LFP/SN-CPE interface indicates less side reaction. Meanwhile, the chemical components of both CEIs were analysed using XPS (Fig. 7c, d, g and h). Compared to LFP/LE, there was more LiF compound at the LFP/SN-CPE interface, which could effectively inhibit the oxidative decomposition of PE at high potentials since LiF has high oxidation resistance.8 Therefore, SSBs with SN-CPE exhibit better electrochemical performance.
Conclusions
In this work, a copolymer electrolyte derived from DOL and TXE monomers and combined with SN was synthesized for SSLMBs. The optimized copolymer electrolyte (SN-CPE) can reach an ionic conductivity of 4.06 × 10−4 S cm−1 at RT, and its lithium-ion transference number was 0.881, which was almost the highest value among the reported data. The Li|SN-CPE|Li symmetric cell exhibited an excellent plating/stripping cycling performance over 1500 h at a current density of 0.1 mA cm−2 and a capacity of 0.1 mA cm−2, as well as a stable performance at a current density of 0.2 mA cm−2 and a capacity of 1 mA cm−2. In addition, the LFP|SN-CPE|Li cell maintained a capacity retention of 84.1% and high coulombic efficiency of around 100% after 900 cycles and this cell can still work effectively at high temperature (80 °C). Moreover, the rate performance and cycling stability performance of SN-CPE were also superior compared to the liquid-based electrolyte, demonstrating its outstanding electrochemical performance and safety. LiCoO2 SSBs with SN-CPE also demonstrated decent cycling performance, suggesting the promising application of SN-CPE in high-voltage SSBs. Besides, the assembled LiFePO4/graphite pouch cell displayed potential application as a high energy density energy storage system. This work applies an in situ copolymerization strategy to synthesize copolymer electrolytes, providing a novel approach for fabricating high-performance PE and disclosing new insights into the development of high-performance and safe SSLMBs.Author contributions
Zhiheng Ren: visualization, validation, formal analysis, writing – original draft. Jixiao Li: validation, formal analysis, investigation, data curation, writing – original draft. Jianneng Liang: conceptualization, formal analysis, project administration, writing – review & editing. Minghui Cai: methodology, resources. Ruonan Yin: methodology, writing – review & editing. Qianling Zhang: resources, writing – review & editing. Chuanxin He: methodology, writing – review & editing. Xiantao Jiang: resources, writing – review & editing. Xiangzhong Ren: supervision, project administration, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Zhiheng Ren and Jixiao Li contributed equally to this work. Extremely thankful for the support from the National Natural Science Foundation of China (21671136), Shenzhen Science and Technology Project Program (JCYJ20210324094204012), Guangdong Basic and Applied Basic Research Foundation (2022A1515011677). The SEM analysis was carried out at the Instrumental Analysis Center of Shenzhen University. Thanks to Chuan Shi and Wei Zhang for their technical guidance in sample characterization. Thanks to Zihao Yang for the assistance with NMR analysis.Notes and references
- D. Lin, Y. Liu and Y. Cui, Nat. Nanotechnol., 2017, 12, 194–206 CrossRef CAS PubMed.
- X. Zhang, A. Wang, X. Liu and J. Luo, Acc. Chem. Res., 2019, 52, 3223–3232 CrossRef CAS.
- B. D. Adams, J. Zheng, X. Ren, W. Xu and J.-G. Zhang, Adv. Energy Mater., 2018, 8, 1702097 CrossRef.
- X.-R. Chen, B.-C. Zhao, C. Yan and Q. Zhang, Adv. Mater., 2021, 33, 2004128 CrossRef CAS.
- R. Xu, X.-B. Cheng, C. Yan, X.-Q. Zhang, Y. Xiao, C.-Z. Zhao, J.-Q. Huang and Q. Zhang, Matter, 2019, 1, 317–344 CrossRef.
- S. Liu, Q. Zhang, X. Wang, M. Xu, W. Li and B. L. Lucht, ACS Appl. Mater. Interfaces, 2020, 12, 33719–33728 CrossRef CAS.
- Q.-K. Zhang, X.-Q. Zhang, L.-P. Hou, S.-Y. Sun, Y.-X. Zhan, J.-L. Liang, F.-S. Zhang, X.-N. Feng, B.-Q. Li and J.-Q. Huang, Adv. Energy Mater., 2022, 12, 2200139 CrossRef CAS.
- P. Bai, X. Ji, J. Zhang, W. Zhang, S. Hou, H. Su, M. Li, T. Deng, L. Cao, S. Liu, X. He, Y. Xu and C. Wang, Angew. Chem., Int. Ed., 2022, 61, e202202731 CAS.
- A. Manthiram, X. Yu and S. Wang, Nat. Rev. Mater., 2017, 2, 16103 CrossRef CAS.
- R. Chen, Q. Li, X. Yu, L. Chen and H. Li, Chem. Rev., 2020, 120, 6820–6877 CrossRef CAS.
- Q. Zhao, S. Stalin, C. Z. Zhao and L. A. Archer, Nat. Rev. Mater., 2020, 5, 229–252 CrossRef CAS.
- J. Liang, J. Luo, Q. Sun, X. Yang, R. Li and X. Sun, Energy Storage Mater., 2019, 21, 308–334 CrossRef.
- J. Li, J. Liang, Z. Ren, C. Shi, Y. Li, L. Zhang, Q. Zhang, C. He and X. Ren, Electrochim. Acta, 2022, 429, 141061 CrossRef CAS.
- P. Ding, Z. Lin, X. Guo, L. Wu, Y. Wang, H. Guo, L. Li and H. Yu, Mater. Today, 2021, 51, 449–474 CrossRef CAS.
- C.-F. Li, K. Zhao, X. Liao, Z.-Y. Hu, L. Zhang, Y. Zhao, S. Mu, Y. Li, Y. Li, G. Van Tendeloo and C. Sun, Energy Storage Mater., 2021, 36, 115–122 CrossRef.
- Y. Liu, D. Lin, Z. Liang, J. Zhao, K. Yan and Y. Cui, Nat. Commun., 2016, 7, 10992 CrossRef CAS.
- C. Ma, W. Cui, X. Liu, Y. Ding and Y. Wang, InfoMat, 2022, 4, e12232 CAS.
- Y.-G. Cho, C. Hwang, D. S. Cheong, Y.-S. Kim and H.-K. Song, Adv. Mater., 2019, 31, 1804909 CrossRef PubMed.
- S.-J. Tan, J. Yue, Y.-F. Tian, Q. Ma, J. Wan, Y. Xiao, J. Zhang, Y.-X. Yin, R. Wen, S. Xin and Y.-G. Guo, Energy Storage Mater., 2021, 39, 186–193 CrossRef.
- A. Hosseinioun and E. Paillard, J. Membr. Sci., 2020, 594, 117456 CrossRef CAS.
- S. Huang, Z. Cui, L. Qiao, G. Xu, J. Zhang, K. Tang, X. Liu, Q. Wang, X. Zhou, B. Zhang and G. Cui, Electrochim. Acta, 2019, 299, 820–827 CrossRef CAS.
- Q. Li, Y. Wang, X. Wang, X. Sun, J.-N. Zhang, X. Yu and H. Li, ACS Appl. Mater. Interfaces, 2020, 12, 2319–2326 CrossRef CAS.
- Q. Zhao, X. Liu, S. Stalin, K. Khan and L. A. Archer, Nat. Energy, 2019, 4, 365–373 CrossRef CAS.
- Z. Ren, J. Li, Y. Gong, C. Shi, J. Liang, Y. Li, C. He, Q. Zhang and X. Ren, Energy Storage Mater., 2022, 51, 130–138 CrossRef.
- Y. Wang, X. Li, Y. Qin, D. Zhang, Z. Song and S. Ding, Nano Energy, 2021, 90, 106490 CrossRef CAS.
- J. Liang, R. Tao, J. Tu, C. Guo, K. Du, R. Guo, W. Zhang, X. Liu, P. Guo, D. Wang, S. Dai and X.-G. Sun, J. Mater. Chem. A, 2022, 10, 12563–12574 RSC.
- F. Liu, T. Li, Y. Yang, J. Yan, N. Li, J. Xue, H. Huo, J. Zhou and L. Li, Macromol. Rapid Commun., 2020, 41, e2000047 CrossRef.
- Q. Liu, J. Tan, Z. Liu, X. Hu, J. Yu, X. Wang, J. Wu, B. Cai, Q. Wang, Y. Fu, H. Liu and B. Li, ACS Appl. Mater. Interfaces, 2022, 14, 26612 CrossRef CAS.
- J. Yu, X. Lin, J. Liu, J. T. T. Yu, M. J. Robson, G. Zhou, H. M. Law, H. Wang, B. Z. Tang and F. Ciucci, Adv. Energy Mater., 2022, 12, 2102932 CrossRef CAS.
- J. Xiang, Y. Zhang, B. Zhang, L. Yuan, X. Liu, Z. Cheng, Y. Yang, X. Zhang, Z. Li, Y. Shen, J. Jiang and Y. Huang, Energy Environ. Sci., 2021, 14, 3510–3521 RSC.
- H. Cheng, J. Zhu, H. Jin, C. Gao, H. Liu, N. Cai, Y. Liu, P. Zhang and M. Wang, Mater. Today Energy, 2021, 20, 100623 CrossRef CAS.
- F. Q. Liu, W. P. Wang, Y. X. Yin, S. F. Zhang, J. L. Shi, L. Wang, X. D. Zhang, Y. Zheng, J. J. Zhou, L. Li and Y. G. Guo, Sci. Adv., 2018, 4, eaat5383 CrossRef CAS.
- Z. Geng, Y. Huang, G. Sun, R. Chen, W. Cao, J. Zheng and H. Li, Nano Energy, 2022, 91, 106679 CrossRef CAS.
- H. Wu, B. Tang, X. Du, J. Zhang, X. Yu, Y. Wang, J. Ma, Q. Zhou, J. Zhao, S. Dong, G. Xu, J. Zhang, H. Xu, G. Cui and L. Chen, Adv. Sci., 2020, 7, 2003370 CrossRef CAS.
- M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci and G. Petersson, Gaussian 09 (Revision D.01), 2009 Search PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- J. Zhang and T. Lu, Phys. Chem. Chem. Phys., 2021, 23, 20323–20328 RSC.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS.
- Q. Liu, B. Cai, S. Li, Q. Yu, F. Lv, F. Kang, Q. Wang and B. Li, J. Mater. Chem. A, 2020, 8, 7197–7204 RSC.
- K. Khan, Z. Tu, Q. Zhao, C. Zhao and L. A. Archer, Chem. Mater., 2019, 31, 8466–8472 CrossRef CAS.
- J. Zhou, T. Qian, J. Liu, M. Wang, L. Zhang and C. Yan, Nano Lett., 2019, 19, 3066–3073 CrossRef CAS.
- Y. Chen, F. Huo, S. Chen, W. Cai and S. Zhang, Adv. Funct. Mater., 2021, 31, 2102347 CrossRef CAS.
- Y. Wang, R. Xu, B. Xiao, D. Lv, Y. Peng, Y. Zheng, Y. Shen, J. Chai, X. Lei, S. Luo, X. Wang, X. Liang, J. Feng and Z. Liu, Mater. Today Phys., 2022, 22, 100620 CrossRef CAS.
- K. Liu, Y. Liu, D. Lin, A. Pei and Y. Cui, Sci. Adv., 2018, 4, eaas9820 CrossRef PubMed.
- Q. Zhou, J. Ma, S. Dong, X. Li and G. Cui, Adv. Mater., 2019, 31, 1902029 CrossRef CAS.
- J. Wan, J. Xie, D. G. Mackanic, W. Burke, Z. Bao and Y. Cui, Mater. Today Nano, 2018, 4, 1–16 CrossRef.
- M. Catti, Chem. Mater., 2007, 19, 3963–3972 CrossRef CAS.
- X. Pei, Y. Li, T. Ou, X. Liang, Y. Yang, E. Jia, Y. Tan and S. Guo, Angew. Chem., Int. Ed., 2022, 61, e202205075 CAS.
- J. Li, F. Huo, Y. Yang, T. Chen, Y. Cui, Y. Cai and H. Zhang, Chem. Eng. J., 2022, 433, 133562 CrossRef CAS.
- J. Vila, P. Ginés, J. M. Pico, C. Franjo, E. Jiménez, L. M. Varela and O. Cabeza, Fluid Phase Equilib., 2006, 242, 141–146 CrossRef CAS.
- S. Yuan, T. Kong, Y. Zhang, P. Dong, Y. Zhang, X. Dong, Y. Wang and Y. Xia, Angew. Chem., Int. Ed., 2021, 60, 25624–25638 CrossRef CAS.
- Y. Wang, S. Chen, Z. Li, C. Peng, Y. Li and W. Feng, Energy Storage Mater., 2022, 45, 474–483 CrossRef.
- C.-Z. Zhao, X.-Q. Zhang, X.-B. Cheng, R. Zhang, R. Xu, P.-Y. Chen, H.-J. Peng, J.-Q. Huang and Q. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 11069–11074 CrossRef CAS PubMed.
- S. Suriyakumar, K. Madasamy, M. Kathiresan, M. H. Alkordi and A. M. Stephan, J. Phys. Chem. C, 2018, 122, 27843–27849 CrossRef CAS.
- P.-J. Alarco, Y. Abu-Lebdeh, A. Abouimrane and M. Armand, Nat. Mater., 2004, 3, 476–481 CrossRef CAS PubMed.
- Z. He, L. Chen, B. Zhang, Y. Liu and L.-Z. Fan, J. Power Sources, 2018, 392, 232–238 CrossRef CAS.
- D. Chen, M. Zhu, P. Kang, T. Zhu, H. Yuan, J. Lan, X. Yang and G. Sui, Adv. Sci., 2022, 9, e2103663 CrossRef PubMed.
- H. Yang, M. Jing, H. Li, W. Yuan, B. Deng, Q. Liu, B. Ju, X. Zhang, S. Hussain, X. Shen and X. Yan, Chem. Eng. J., 2021, 421, 129710 CrossRef CAS.
- S. Xu, Z. Sun, C. Sun, F. Li, K. Chen, Z. Zhang, G. Hou, H. M. Cheng and F. Li, Adv. Funct. Mater., 2020, 30, 2007172 CrossRef CAS.
- B. Deng, M.-x. Jing, R. Li, L.-x. Li, H. Yang, M.-q. Liu, J. Xiang, W.-y. Yuan and X.-q. Shen, J. Colloid Interface Sci., 2022, 620, 199–208 CrossRef CAS.
- L. Jin, G. Jang, H. Lim, W. Zhang, W. Kim and H. Jang, Adv. Mater. Interfaces, 2022, 9, 2101958 CrossRef CAS.
- P. Wang, J. Chai, Z. Zhang, H. Zhang, Y. Ma, G. Xu, H. Du, T. Liu, G. Li and G. Cui, J. Mater. Chem. A, 2019, 7, 5295–5304 RSC.
- Q. Sun, X. Chen, J. Xie, C. Shen, Y. Jin, C. Huang, X. Xu, J. Tu, B. Wang, T. Zhu, X. Zhao and J. Cheng, Mater. Today Energy, 2021, 21, 100841 CrossRef CAS.
- N. Wu, P. H. Chien, Y. Li, A. Dolocan, H. Xu, B. Xu, N. S. Grundish, H. Jin, Y. Y. Hu and J. B. Goodenough, J. Am. Chem. Soc., 2020, 142, 2497–2505 CrossRef CAS PubMed.
- G. Yang, C. Chanthad, H. Oh, I. A. Ayhan and Q. Wang, J. Mater. Chem. A, 2017, 5, 18012–18019 RSC.
- J. Xie, S. Y. Sun, X. Chen, L. P. Hou, B. Q. Li, H. J. Peng, J. Q. Huang, X. Q. Zhang and Q. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202204776 CAS.
- P. N. Didwal, Y. N. Singhbabu, R. Verma, B.-J. Sung, G.-H. Lee, J.-S. Lee, D. R. Chang and C.-J. Park, Energy Storage Mater., 2021, 37, 476–490 CrossRef.
- J. Tan, J. Matz, P. Dong, J. Shen and M. Ye, Adv. Energy Mater., 2021, 11, 2100046 CrossRef CAS.
- N. Angulakshmi, Y. Zhou, S. Suriyakumar, R. B. Dhanalakshmi, M. Satishrajan, S. Alwarappan, M. H. Alkordi and A. M. Stephan, ACS Omega, 2020, 5, 7885–7894 CrossRef CAS PubMed.
- T. Zhao, W. Kou, Y. Zhang, W. Wu, W. Li and J. Wang, J. Power Sources, 2023, 554, 232349 CrossRef CAS.
- Y. Ma, J. Wan, Y. Yang, Y. Ye, X. Xiao, D. T. Boyle, W. Burke, Z. Huang, H. Chen, Y. Cui, Z. Yu, S. T. Oyakhire and Y. Cui, Adv. Energy Mater., 2022, 12, 2103720 CrossRef CAS.
- B. Liu, J.-G. Zhang and W. Xu, Joule, 2018, 2, 833–845 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ta07516d |
| ‡ Zhiheng Ren and Jixiao Li contributed equally to this work and should be considered as co-first authors. |
| This journal is © The Royal Society of Chemistry 2023 |