Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
UN Sustainable Development Goals 7 and 13. How sustainable are the metals in our journey to clean energy storage?
Cristina
Pozo-Gonzalo

Deakin University, Australia
Energy storage systems, to support renewable energy, require a range of metals mostly used as active materials. Due to the exponential growth in consumption in renewables, the metals supply chain is experiencing a great deal of pressure to deliver valuable metals. Some examples of those metals are lithium, cobalt and rare earth elements, which are listed as critical raw materials and are vital to enable the transition to clean energy storage (e.g. batteries, solar cells, wind turbines and electric vehicles). Additionally, common and abundant metals like nickel and copper have been included recently in the list as critical raw materials due to their important role in the manufacturing of Li ion batteries (LIBs). For example, Ni and Cu are extensively available materials in the earth's crust (24th and 27th most abundant elements); however the Ni price has increased by 230% since 2019 due to its demand.1
Mining of metals from primary extraction is not a very sustainable practice as it is high energy-intensive, produces waste streams and requires large volumes of water, which is a precious commodity.
Therefore, secondary sources from spent devices, tailings, mining waste, also known as urban mines, are an economical and environmental alternative to recover valuable and critical metals from waste. In this context traditional technologies such as pyro and hydrometallurgy have been used. However, in the specific case of LIB recycling to recover valuable and critical metals, only 5% of those batteries are recycled as a result of high cost, infrastructure investment and, sometimes, low availability of secondary sources.
Greener methods and usage of chemicals which minimise the generation of waste, pollution and energy consumption have emerged as an ecofriendly alternative to traditional metal recovery processes. This approach is strongly aligned with the global SDG 13 – climate action – by reducing CO2 emissions in comparison with other methods, while maintaining the efficiency of the well-established hydrometallurgy process. Recently the use of organic media has generated the term solvometallurgy with the aim to increase the sustainability of the metal recovery process, minimising harmful waste generation and high energy consumption.
Among those green chemicals, deep eutectic solvents (DESs) are an emerging class of solvents composed of a mixture of hydrogen bond donors (HBD) and hydrogen bond acceptors (HBA) characterized by significant depressions in melting points compared to those of the individual components. It should be highlighted that the most common, and probably cost effective, HBA used in DES is choline chloride (ChCl) as it can be produced in large quantities, it is biodegradable and presents low toxicity.2
Metal-complexation and solvent acidity are the main determining factors to increase leaching efficiency in metal recovery, but not limited to, as discussed below. Based on that, DESs present the ability of coordinating to metals based on their ligands and also have acidic protons to tune the media's acidity, and therefore have potential for greener leaching processes.
But are DES really the solution to sustainable metals recovery? Which other properties and factors do we need to contemplate?
The nature and stability of a metal complex in solution after leaching is governed by the chemistry of the ligand, and this is an obvious strategy to enhance specific species solubility. Following this strategy, it is possible to design ligands for selective metal dissolution. For instance, the formic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl (1
ChCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) DES3 leads to selective dissolution of lithium versus cobalt due to the formation of the insoluble cobalt formate complex, while Li remains in solution.
2 molar ratio) DES3 leads to selective dissolution of lithium versus cobalt due to the formation of the insoluble cobalt formate complex, while Li remains in solution.
The leaching efficiency using the DES ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1
urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) has been reported to be comparable to conventional leaching processes depending on the metal source.4 However, this is not as straightforward as expected, and the leaching ability strongly depends on the chemistry of the DES, the metal source and operational parameters. On the other hand, the DES degradation products generated during the leaching process, and their role in metal dissolution, are not completely understood.
2 molar ratio) has been reported to be comparable to conventional leaching processes depending on the metal source.4 However, this is not as straightforward as expected, and the leaching ability strongly depends on the chemistry of the DES, the metal source and operational parameters. On the other hand, the DES degradation products generated during the leaching process, and their role in metal dissolution, are not completely understood.
Nevertheless, on the bright side, the leaching ability can easily be tuned by careful design of the HBD and HBA chemistry. As an example by tuning the nature of the HBD and shifting from the conventional DES components, i.e. ethylene glycol and urea, to organic acids (e.g. malonic acid or p-toluenesulfonic acid), a significant increase in the dissolution rate was reported.5 Although one could think that the acidity of the HBD is the main parameter that governs metal leaching, it has been observed that the reducing ability of the HBD is more important than its pKa.6 The main reason behind this lies in the higher solubility of metals in a lower oxidation state, requiring an initial reduction process. This is true when comparing L-ascorbic acid versus p-toluenesulfonic acid where the former leads to better leaching efficiency despite being a weaker acid.6 Thus, following this approach, it is possible to decrease harmful waste generation, and also minimise the impact of corrosion from using stronger acids, which are detrimental from both economic and environmental perspectives. Those are important factors to pursue the study of DES as green options for metal recovery.
The ratio of HBD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBA and experimental factors such as solid to liquid ratio and operational parameters can be adjusted to increase the efficiency in the leaching process.
HBA and experimental factors such as solid to liquid ratio and operational parameters can be adjusted to increase the efficiency in the leaching process.
Such exciting news also opens the pathways to new research venues for smart design of solvent compositions to target specific metal dissolution, based on their preferred coordination chemistry.
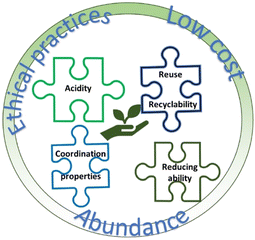
Fig. 1 summarises, from my perspective, some of the key factors that need to be considered in the development of solvents to boost the sustainability of metal recovery from different sources. Thus, in the case of DES and extrapolated to other solvent systems, sustainability can be achieved by using low cost and abundant chemicals, and always under ethical practices. By tuning the chemical composition and physicochemical properties, it would be possible to achieve comparable dissolution efficiency to traditional leaching processes.
Nevertheless, it is important to consider that the DES research area is in its early stages of development, and systematic studies that can add clarity into rational selection and design of the composition of DES are still missing.
Further considerations to promote sustainability of metal leaching processes include:
– Low working temperature: economic benefits and practicability are based on operational conditions using low temperatures, which requires proper design and selection of DES. Initial work on DES for metal leaching was performed at working temperatures mimicking hydrometallurgy practices (>100 °C). However, working at temperature higher than 90 °C leads to the degradation of quaternary ammoniums, such as ChCl, through Hofmann elimination, increasing the pH of the leachate and inhibiting the extraction of metals or causing some metal ions to precipitate. Currently, there is a debate on the actual role of the HBD in the DES as the leaching efficiency is reduced when working at temperatures lower than 90 °C.
– Multiple lifetimes: DES could be recycled and thus further reduce the cost. However due to the complex composition of DES and low volatility, the recycling process is problematic. On the other hand, reuse of the DES has been reported for ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formic acid up to 5 times with similar leaching efficiency showing potential for multiple lifetimes.3
formic acid up to 5 times with similar leaching efficiency showing potential for multiple lifetimes.3
– Metal recovery: after leaching, the metal recovery is usually undertaken as a sequence of precipitation followed by calcination to achieve the metal oxide, with a significant energy consumption. Just for context, the calcination process involved in the manufacturing of Nickel Manganese Cobalt oxide (NMC) from the corresponding hydroxide requires 7 MJ (∼2 kW h) of electricity per kg of material.7 Recovery of metals via electrodeposition from DES can boost the sustainability of the overall process by preventing waste, as the solvent can be reused as a leaching reagent and/or electrodeposition media.
References
- Z. Wang, S. Li, T. Li, T. Hu and X. Ge, Deep Eutectic Solvents (DESs) for Green Recycling of Wasted Lithium-Ion Batteries (LIBs): Progress on Pushing the Overall Efficiency, Min., Metall. Explor., 2022, 39(5), 2149–2165, DOI:10.1007/s42461-022-00660-7.
- K. Binnemans and P. T. Jones, Ionic Liquids and Deep-Eutectic Solvents in Extractive Metallurgy: Mismatch Between Academic Research and Industrial Applicability, J. Sustain. Metall., 2023, 9, 423–438, DOI:10.1007/s40831-023-00681-6.
- L. Chen, Y. Chao, X. Li, G. Zhou, Q. Lu, M. Hua, H. Li, X. Ni, P. Wu and W. Zhu, Engineering a Tandem Leaching System for the Highly Selective Recycling of Valuable Metals from Spent Li-Ion Batteries, Green Chem., 2021, 23(5), 2177–2184, 10.1039/D0GC03820B.
- E. Fan, L. Li, Z. Wang, J. Lin, Y. Huang, Y. Yao, R. Chen and F. Wu, Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects, Chem. Rev., 2020, 120(14), 7020–7063, DOI:10.1021/acs.chemrev.9b00535.
- M. J. Roldán-Ruiz, M. L. Ferrer, M. C. Gutiérrez and F. del Monte, Highly Efficient p-Toluenesulfonic Acid-Based Deep-Eutectic Solvents for Cathode Recycling of Li-Ion Batteries, ACS Sustainable Chem. Eng., 2020, 8(14), 5437–5445, DOI:10.1021/acssuschemeng.0c00892.
- Y. Hua, Y. Sun, F. Yan, S. Wang, Z. Xu, B. Zhao and Z. Zhang, Ionization Potential-Based Design of Deep Eutectic Solvent for Recycling of Spent Lithium Ion Batteries, Chem. Eng. J., 2022, 436, 133200, DOI:10.1016/j.cej.2021.133200.
- J. Porzio and C. D. Scown, Life-Cycle Assessment Considerations for Batteries and Battery Materials, Adv. Energy Mater., 2021, 11(33), 2100771, DOI:10.1002/aenm.202100771.
| This journal is © The Royal Society of Chemistry 2023 |