Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
UN Sustainable Development Goal 2 – Zero hunger
David
Cole-Hamilton
EastChem, School of Chemistry, University of St Andrews, St Andrews, KY16 9LU, Scotland, UK. E-mail: jc@st-and.ac.uk
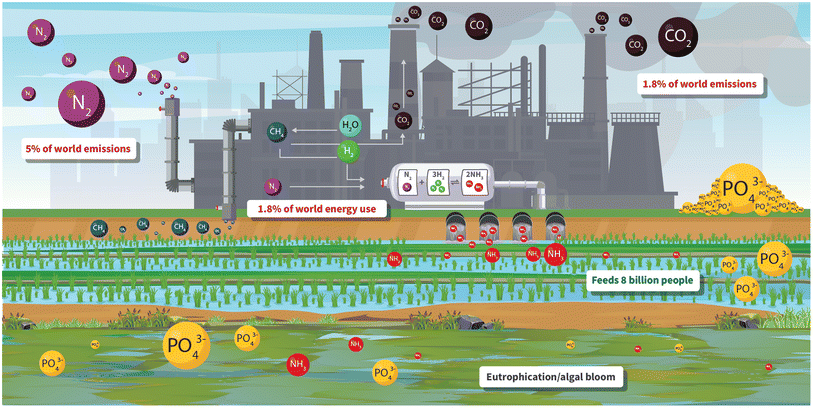
For allowing billions of people to be fed on this planet, the Haber Bosch process must be considered one of the greatest achievements of mankind ever. However, it comes at a price. 1.8% of all the world's energy2 (equivalent to the total used by 145 M people) is consumed in the process and it consumes 5% of all the world's annual methane. This is because almost all ammonia plants make the hydrogen for the reduction of nitrogen by steam reforming of methane to give hydrogen and carbon monoxide, which is then subjected to the water–gas shift reaction to give more hydrogen and carbon dioxide, which is vented to the atmosphere accounting for 1.8% of all CO2 emissions and contributing to global warming. Here is a chemical problem crying out to be solved in the pursuit of sustainability.
One possibility would be to capture the carbon dioxide using carbon capture and storage (CCS) technology but a much better one would be to replace methane as the source of hydrogen by hugely abundant water using electrolysis with electricity generated from renewables when demand is low and supply is high. However, this again presents problems because, although modern electrolysers can operate at up to 80% current efficiency, they use the scarce and expensive elements, platinum and iridium, for the electrodes. There are great chemical opportunities in clever design and operation of electrodes containing earth abundant elements for water electrolysis. Several companies and consortia are beginning to explore electrolysis for the generation of hydrogen for new ammonia plants but there is an urgent need to work on retrofitting the very large plants that are currently in operation.
Another problem with the Haber Bosch process concerns the thermodynamics and kinetics of the reaction. The negative entropy change during the reaction means it should be run at as low a temperature as possible to have high conversion. However, there is a large kinetic barrier to the reaction, because the N–N triple bond must be broken, so a catalyst needs to be used, but to achieve commercially important rates, the reaction must still be run at high temperature with multiple passes because of the decreased conversion per pass at the higher temperature. Alwyn Mittasch, who developed the catalyst, investigated thousands of different combinations of elements and oxides, coming up with the current promoted iron based catalyst composition. Modern catalysts have seldom proven superior or are very expensive but further research in this area could prove fruitful. Another possible approach might involve selective heating of the catalyst with the gas phase being kept cold. This may allow for high rates at the catalyst bed leading to higher conversion. Because of the very high volumes of ammonia produced, even a small increase in conversion per pass could have a very significant positive effect on the current negative impact of the process.
Production is not the only issue with ammonia. Its application in agriculture can cause even greater problems, which are shared by the use of phosphorus. Currently, whole fields are sprayed with fertiliser containing N and P, also potassium. This certainly fertilises the plants but, in many cases, massively over does it. Much of the fertiliser is not taken up by the plants so runs off the fields into waterways and eventually the oceans. Here it continues to do its job but this time on algae causing proliferation to algal blooms and eutrophication. The spread of algae over the water surface reduces light and oxygen supply to the water and adversely influences water life, badly affecting fish survival.
Working with farmers and agriculturalists there are huge opportunities to find ways of delivering fertilisers to the plants that need it in the amount that they need, whilst not delivering them to healthy plants. There are also big opportunities for collection of run-off and recovering the fertilisers from it as well as from human waste.
So, although tackling zero hunger at the moment is a political problem (of food distribution), there are enormous opportunities for chemistry to improve what we are doing at the moment to alleviate hunger by cleaning up the processes we use and tackling the overuse of fertilisers with its very negative consequences.
Nitrogen and phosphorus along with carbon are the elements that currently exceed the planetary boundaries in terms of biogeochemical flows.3 That is, their use already causes problems to the world that may be irreversible. It is extremely urgent that their use should be reduced and that they should be targeted more effectively. Chemists will have to take a major part in making sure this happens.
The advantages and disadvantages of ammonia and phosphate are summarised in Fig. 1.
RSC Sustainability would welcome your papers tacking any of these challenges.
SDGs: although this editorial largely addresses SDG2 Zero hunger, it also impacts on many of the other ones. These include SDG7 Affordable and clean energy since any developments in hydrogen electrolysers will contribute to clean energy storage and the use of hydrogen as a clean fuel, SDG 10 Reduced inequalities by distributing the total world food supply more equitably, SDG 12 Responsible consumption and production by making ammonia production and consumption more sustainable, SDG13 Climate action by eliminating the massive current release of CO2 associated with ammonia production, SDG14 Life under water by reducing fertiliser run-off and eutrophication, SDG15 Life on land by reducing the overuse of fertilisers on the land and SDG 17 Partnerships for the goals especially in tackling the over use of fertilisers.
References
- Extrapolated from: J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, Nat. Geosci., 2008, 1, 636–639 CrossRef CAS.
- Much of the data in this editorial comes from a Royal Society briefing paper: Ammonia: zero-carbon fertiliser, fuel and energy store, https://royalsociety.org/-/media/policy/projects/green-ammonia/green-ammonia-policy-briefing.pdf Search PubMed.
- W. Steffen, K. Richardson, J. Rockstrom, S. E. Cornell, I. Fetzer, E. M. Bennett, R. Biggs, S. R. Carpenter, W. de Vries, C. A. de Wit, C. Folke, D. Gerten, J. Heinke, G. M. Mace, L. M. Persson, V. Ramanathan, B. Reyers and S. Sorlin, Science, 2015, 347, 1259855, DOI:10.1126/science.1259855.
| This journal is © The Royal Society of Chemistry 2023 |