Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical composition and bioactivity of hemp, reed canary grass and common reed grown on boreal marginal lands†
Marina
Fidelis
 *af,
Jenni
Tienaho
*af,
Jenni
Tienaho
 a,
Hanna
Brännström
b,
Risto
Korpinen
a,
Hanna
Brännström
b,
Risto
Korpinen
 b,
Juha-Matti
Pihlava
b,
Jarkko
Hellström
a,
Paula
Jylhä
c,
Jaana
Liimatainen
b,
Juha-Matti
Pihlava
b,
Jarkko
Hellström
a,
Paula
Jylhä
c,
Jaana
Liimatainen
 b,
Veikko
Möttönen
d,
Jyri
Maunuksela
e and
Petri
Kilpeläinen
b,
Veikko
Möttönen
d,
Jyri
Maunuksela
e and
Petri
Kilpeläinen
 b
b
aFood and Bioproducts, Natural Resources Institute Finland (Luke), Latokartanonkaari 9, Helsinki, FI-00790, Finland
bBiomass Fractionation Technologies, Natural Resources Institute Finland (Luke), Viikinkaari 9, Helsinki, FI-00790, Finland
cForest Technology and Wood Material Solutions, Natural Resources Institute Finland (Luke), Teknologiakatu 7, Kokkola, FI-67100, Finland
dForest Technology and Wood Material Solutions, Natural Resources Institute Finland (Luke), Yliopistokatu 6 B, Joensuu, FI-80100, Finland
eNeova Group, FI-01300, Vantaa, Finland
fFood Sciences Unit, Department of Life Technologies, University of Turku, Turku FI-20014, Finland. E-mail: marina.m.fidelis@utu.fi
First published on 6th October 2023
Abstract
Underutilised agricultural land and former peat production areas in northern Europe are potentially suitable for growing lignocellulosic biomass that could be used in various non-food applications. In this study, the biorefining process of Phalaris arundinacea (reed canary grass), Phragmites australis (common reed), and Cannabis sativa (oil and fibre hemp cultivars) was assessed based on their chemical composition and biological activity using various analytical techniques. Two-stage accelerated solvent extraction was used first with hexane, followed by EtOH/H2O (95/5, v/v) to extract the lipophilic and hydrophilic fractions of the samples collected during and after the growing season. Later, pressurised hot water extraction (PHWE) and two-stage extraction were performed to examine the biorefinery potential of aqueous extracts focusing on extraction efficiency, quality, and chemical composition of the plant materials. Combining two-stage and elevated extraction temperatures with PHWE resulted in high levels of total dissolved solids (TDS), carbohydrates, phenolics, and bioactivities. Data showed that TDS yielded over 400 mg g−1 for summer oil hemp and approximately 300 mg g−1 for reed canary grass and common reed. Summer-harvested plants had carbohydrate yields of 110–155 mg g−1, while autumn yields were 40–60 mg g−1 for hemp and 120–170 mg g−1 for reed canary grass and common reed, respectively. The findings suggest that aboveground biomass from marginal lands holds potential as a valuable source of bioactive compounds for biorefinery feedstocks, thereby presenting new opportunities for sustainable biomass-based valorisation and future optimisation of two-stage extraction methods targeting hemicellulose-rich fractions.
Sustainability spotlightMarginal lands and peatlands contribute to carbon storage, however, when drained for cultivation or peat production, they release greenhouse gas emissions. We explore the sustainable utilisation of plant biomass from these areas for biorefining, promoting sustainable land use and supporting land restoration, biodiversity conservation, and ecosystem protection (SDG 15). We examine eco-friendly green extraction processes, such as two-stage pressurised hot water, exemplifying their efficiency as a sustainable and innovative biorefinery process for recovering and separating valuable antioxidative and antibacterial components from reed canary grass, common reed, and hemp (SDG 9, SDG 12). By investigating plant-derived bioactive compounds as alternatives to synthetic additives, our findings promote responsible consumption and production practices, also contributing to overall well-being (SDG 12, SDG 3). |
Introduction
Abandoned or underutilised agricultural land constitutes approximately 15% of agricultural land worldwide.1 These types of so-called marginal land have potential to significantly contribute to biomass provision for the bioeconomy in the future.2 In Finland in 2020, the area of abandoned agricultural land was estimated to be 85 kha, of which peatlands constitute 22 kha.3 Also, former peat production areas could be harnessed to produce raw materials for various bio-based products. After Russia, Finland has Europe's second largest peatland area (8.3 Mha),4 of which 120 kha have been converted to peat production so far.5 The peat production area was estimated to be 52 kha in 2019,4 but the area is rapidly diminishing due to the aim of reducing the use of fuel peat in energy generation by at least half by 2030.6 Peatlands are wetland ecosystems that play a crucial role in carbon storage. Peat is formed when dead plants have not been fully decomposed in an anaerobic environment.7 When drained for cultivation or peat production, peat starts decomposing and increasingly emitting carbon dioxide and nitrous oxide into the atmosphere.8 Globally, ca. 12% of the existing peatlands are drained or degraded, contributing 4% of greenhouse gas emissions (GHG) annually.4 In Finland, agricultural peatlands and peat production areas are marked GHG emissions sources. Peatlands comprise approximately 10% (260 kha) of Finland's arable land but are estimated to produce as much as 60% of the total climate load in agriculture.9 In 2018, peat production areas emitted 1.8 Mt CO2-eq.10 Consequently, carbon-wise management of these soils is of great importance.Plant species, such as reed canary grass (Phalaris arundinacea L.) and common reed (Phragmites australis (Cav.) Trin. ex Steud) are large grass species that thrive in wet peatlands, whereas hemp species (Cannabis sativa L.) can grow on mineral soils.11 Depending on the environmental conditions and cultivation practices, these plants possess varying carbon sequestration capabilities. For instance, one hectare of P. arundinacea was estimated to sequester 2.7–6.5 t CO2-eq. in a former peat production area in Finland,12 while the estimates of the annual carbon capture potential of C. sativa ranged from 9 to 15 t CO2-eq., reaching up to 22 t CO2-eq.13 While progress has been made, further advancements are needed to accurately model biomass yield and its stability on marginal lands, such as organic soils.14
In fact, common reed (CR), reed canary grass (RCG), fibre and oil hemp species (FH and OH) are among the most promising industrial crops for marginal lands.15 Due to the effects of climate change and rising temperatures, biomass plants like RCG are better adapted to Europe's northern regions, which shows promise for cultivation in Finland's former peat production areas.16,17 Besides providing renewable energy, the cultivation of RCG could mitigate climate change through enhanced carbon sequestration.17 CO2 emissions from peatlands could be further reduced by shifting to paludiculture, where the land is rewetted and cultivated with wet-tolerant plants, such as CR.18 Thus far, CR and RCG has been cultivated predominantly for paper production and phytoremediation of soil and waste. Yet, their potential for biorefinery production from marginal lands remains underexplored.19–21 FH is gaining interest due to its fast growth and utilization in commercial products (e.g., textile, paper, medicine, food, animal feed, paint, biofuel, biodegradable plastic, and construction materials) and its versatile cultivation conditions.22 In turn, OH is a minor crop in Finland. Even though only a part of the plant (seeds) biomass is currently being utilized, hemp oil is used as food and feed for particular purposes due to its health benefits.23
Plant biomass from marginal lands can be valuable sources of compounds for biochemical and bioplastic applications, providing alternatives to petroleum-based products.24 Fresh or dried biomass displays beneficial, value-added compounds such as cellulose, hemicellulose, carbohydrates, polyphenols, proteins, and lipids, with applications transiting various industries, including chemicals, pharmaceuticals, cosmetics, fibre products, fuels, food packaging, and feed.25–28 For instance, cellulose-derived sugars have emerged as critical feedstocks, facilitating a wide range of chemical reactions and enabling the substitution of petrochemicals with diverse chemicals beyond the scope of biofuels.29 Notably, secondary metabolites found in such biomass are known for their multifaceted properties, encompassing antioxidant, antibacterial, antiviral, antihyperglycemic, antihypertensive and antiproliferative properties.30–32 Given the extant evidence on the health and technological benefits of ingredients and natural preservatives, biorefinery potential and alternative valorisation techniques have been explored to replace synthetic antioxidants with plant-derived bioactive compounds. Evidence has shown that hemp fractions can provide natural antioxidants and anti-inflammatory nutritional support. Bioactive compounds were capable of retarding the oxidation of vegetable oils33 and synergising the beneficial omega-6/omega-3 ratio of seed triglycerides.34 Moreover, potentially sustainable and eco-friendly insecticide has been revealed due to hemp waste biomass efficacy in killing malaria vectors.35
A promising approach to effectively recovering phytochemicals includes the multi-step fractionation processes using green technologies.36,37 Fractionation, extraction and chemical processes using solvents are commonly employed due to their efficiency in isolating particular chemicals, ease of use and broad applicability.38 Due to their favourable properties, the CHEM21 solvent selection guide recommends water and ethanol for industry use.39 Ethanol is one of the most used solvents by researchers, mainly due to its miscibility in water and different organic solvents, its ability to dissolve polar and non-polar substances, and its low toxicity.40 Meanwhile, pressurised hot water extraction (PHWE), in turn, is an extraction process in which the temperature stands above 100 °C but below the critical temperature of water (374 °C) while operating either in static (batch) or dynamic (flow-through) mode. The liquid state of water is retained by maintaining the system under sufficiently high pressure. In addition to the secondary metabolites, biomass separation into the three main compound classes (hemicelluloses, cellulose, and lignin) has received considerable attention.41 Methods like PHW extraction of hemicelluloses42 provide advantages over conventional extraction methods43 as it is typically faster, as well as a greener approach. Thus far, PHWE has been successfully utilised and upscaled for investigating the recovery of hemicelluloses and polyphenols.44,45 Even though plant-oriented hemicelluloses are not currently reaching their full potential in industry use; studies have shown their valuable resource for different applications, such as in food emulsions as a delivery system of essential fatty acids26 or different medical and pharmaceutical applications.46
Extant research in the area of marginal land biomasses has mainly focused on the production of fibres from grasses, but the high cost and logistical challenges associated with nonwood fibres have limited their use in pulp and cellulose-based products.47–50 Consequently, this study aimed to (1) assess the biorefinery potential of P. arundinacea (reed canary grass), P. australis (common reed), and two cultivars of C. sativa (oil and fibre hemp) grown in Finland by examining their chemical composition and biological activities and (2) evaluate the biorefining process by extracting the most promising plant fraction through different temperatures, especially, two-stage hot water extractions targeting the selective isolation of extractives and hemicelluloses. Furthermore, this study investigates the biorefinery suitability of selected plant species which can be grown on marginal land and former peat production areas. This research contributes to the sustainable utilisation of plant-based resources by providing an understanding of the chemical composition and biological activities of the plants chosen and implementing green extraction processes for efficient use in further industrial processes.
Experimental
Chemicals
HPLC grade standards of catechin, epicatechin, gallocatechin, epigallocatechin, 2,2′-azobis (2-methylpropionamidine) dihydrochloride, fluorescein, 1,1,1,3,3,3-hexamethyl disilazane and trolox ((±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) were purchased from Sigma-Aldrich (Steinheim, Germany). Phosphate buffer pH 7.5, pyridine, and trimethylsilyl chloride were purchased from Merck (Darmstadt, Germany). N,O-Bis(trimethylsilyl) trifluoroacetamide was purchased from Supelco Analytical (Bellefonte, PA, USA), thiolysed procyanidin B2 was purchased from Extrasynthese (Lyon, France), and Bio-Rad Protein microassay kit was purchased from Bio-Rad Laboratories (Inc., USA).Biomass sampling
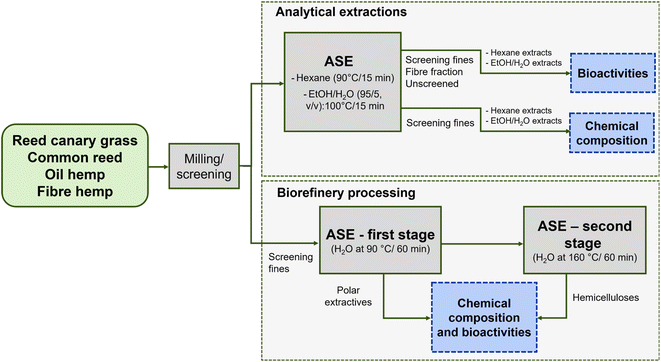
This study examined four herbaceous plants grown in Finland: reed canary grass (Phalaris arundinacea var. Pedja), common reed (Phragmites australis), fibre hemp (Cannabis sativa var. Uso 31), and oil hemp (Cannabis sativa var. FINOLA). The aboveground plant biomass samples were collected during and after the growing season to assess extractive differences. Subsequently, the raw materials were stored in the freezer before processing. Table 1 shows the origin of the plant samples, the soil type and properties at the growing site.51 Mean daily temperatures and monthly rainfalls are shown in Fig. S1.† Oil hemp seeds (25 kg ha−1) were sown on 16.5.2021 and soil was ploughed at 20 cm depth. Fibre hemp seeds (31.8 kg ha−1) were sown on 7.6.2021, while soil was harrowed at 20 cm depth in autumn 2021 and at 5 cm on 6.6.2021. The hemp and RCG samples were collected from plantations managed by private farmers. The RCG plantation was sown in 2019. Since CR is not cultivated in Finland, the samples were collected from a natural population composing of 1.2–1.3 m tall shoots grown on sandy seashore. The collected raw materials were freeze-dried and ground using a mill (Fritsch Pulverisette mill, Idar-Oberstein, Germany) with a 1 mm sieve. Further fractionation of the plant materials was performed using a wire screen with 1 mm openings. The unscreened samples (i.e., a mixture of different aboveground biomass fractions), their screening fines, and the fibre fractions were extracted with accelerated solvent extraction (ASE) and their bioactivities were determined. Next, the screening fines were chosen for further chemical characterisation since this fraction represents the less fibrous part of the material that is unsuitable for pulping and papermaking52 but useable for other value-added purposes.| Species | Abbreviation | Location | Soil type | Soil pH55 | Soil nutrient content – P/K/Ca (mg L−1)56 | Fertilisation – N/P/K (kg ha−1)57 | Sampling week |
|---|---|---|---|---|---|---|---|
| Common reed (Phragmites australis) | CR | Siikajoki (64.8° N, 24.8° E) | Sandy sea shore | NA | NA | NA | 30/2021 (summer) |
| 42/2021 (autumn) | |||||||
| Reed canary grass (Phalaris arundinacea var. Pedja) | RCG | Siikajoki (64.6° N, 25.1° E) | Agricultural peatland (Sphagnum peat) | 3.9 | 2/55/470 | NA | 30/2021 (summer) |
| 42/2021 (autumn) | |||||||
| Fibre hemp (Cannabis sativa var. Uso 31) | FH | Siikajoki (64.6° N, 25.4° E) | Fine-sandy moraine (organic content 6–11.9%) | 6.2 | 18/125/1645 | 94/12/46 | 42/2021 (autumn) |
| Oil hemp (Cannabis sativa var. FINOLA) | OH | Hausjärvi (60.7° N, 25.0° E) | Fine silt (organic content 3–5.9%) | 6.4 | 14/139/1573 | 170/24/47 | 26/2021 (summer) |
| 38/2021 (autumn) |
Analytical extractions were performed for both comparison and as a benchmark for biorefinery processing with hot water. There is comprehensive information about cellulose, hemicellulose and lignin content as well as further pulping experiments in the literature.49,52–54 Our aim was to show that there are bioactive compounds in both lipophilic (obtained with hexane) and hydrophilic fraction (ethanol/water extraction) and further present chemical composition of extracts. Biorefinery processing with water shows how to obtain extractives at low temperature and underutilised hemicelluloses at high temperature. Fig. 1 illustrates the analytical scheme, and Table 2 notes the distribution of the screening fines and fibre fractions.
| Plant biomass | Sampling | Screening fines (%, w/w) | Fibre fraction (%, w/w) |
|---|---|---|---|
| Common reed | Summer | 24 | 76 |
| Autumn | 17 | 83 | |
| Reed canary grass | Summer | 26 | 74 |
| Autumn | 32 | 68 | |
| Oil hemp (seed crop) | Summer | 80 | 20 |
| Autumn | 86 | 14 | |
| Fibre hemp (fibre crop) | Autumn | 51 | 49 |
Chemical composition of the plant biomass
For GC analyses, an aliquot of the extracts, which yielded approximately 0.4 mg of dry solids, was dried under nitrogen gas. Dry samples were derivatised with 150 μL silylation solution containing 25 μL pyridine (Merck KGaA, Darmstadt, Germany), 100 μL N,O-bis(trimethylsilyl) trifluoroacetamide and 25 μL trimethylsilyl chloride. The derivatisation was carried out in an oven at 70 °C for 45 min. Heneicosanoic acid (C21: 0, 0.02 mg mL−1), betulinol (0.02 mg mL−1), cholesteryl heptadecanoate (Ch17, 0.02 mg mL−1) and 1,3-dipalmitoyl-2-oleyl-glycerol (TGstd, 0.02 mg mL−1) were used as internal standards. The silylated samples were analysed using GC-MS (HP6890-5973 GC-MSD instrument, Hewlett Packard, Palo Alto, CA, USA), using an HP-5 GC column (Agilent Technologies, Inc., Santa Clara, CA, USA; 30 m × 0.25 mm i.d., film thickness 0.25 μm). Helium was used as the carrier gas, and the injection was made in splitless mode. The temperature profile was as follows: 150 °C → 230 °C, 7 °C min−1, 230 °C → 310 °C, 4 °C min−1, hold time 10 min. The injector temperature was 260 °C, and the detector 290 °C.
The mass spectrum was obtained in the electron ionisation mode (70 eV), and the fragmentation pattern was compared to standards in commercial (NIST14 and Wiley11) libraries, as well as the MS libraries available at our laboratory. Furthermore, the silylated samples were analysed using the GC-FID for group composition (Shimadzu GC-2010, Kyoto, Japan) with an HP-1 column (Agilent Technologies, Inc., Santa Clara, CA, USA; 15 m × 0.53 mm i.d., film thickness 0.15 μm). The temperature profile was as follows: 100 °C, hold time 1.5 min, 100 °C → 325 °C, 12 °C min−1, hold time 6 min. The temperature profile of the injector was 50 °C, hold time 0.5 min, 50 °C → 340 °C, 200 °C min−1, hold time 18 min. The temperature of the detector was 325 °C.
Bioactivities: antibacterial effects and antioxidant activity
The DPPH free-radical scavenging assay was carried out following the description by Brand-Williams et al.63 This method monitors signal intensity loss over time as the antioxidant scavenges the DPPH radical. An aliquot of 40 μL of a diluted sample and 260 μL of a stock methanolic solution of DPPH (0.10 mM) were pipetted onto a 96-well plate. The obtained mixture was left in the dark at 25 °C for 30 min. A blank sample was prepared by replacing the sample aliquot with water. After the reaction time, the decrease in DPPH absorbance was measured at 517 nm. The procedure was performed in triplicate, and the results were expressed as mg of ascorbic acid equivalent per g of extract (mg AAE per g dw).
The cupric ion-reducing antioxidant capacity (CUPRAC) was estimated using the copper(II)–neocuproine (Cu(II)–Nc) reagent as the chromogenic oxidant.64 The experimental procedure involved adding 100 μL of the diluted sample, or blank (water), mixed with 1 mL of each of the following solutions into a test tube: CuCl2 (1.0 × 10−2 M), neocuproin (7.5 × 10−3 M) solution, NH4Ac (1 M, pH 7.0 buffer), and water to make the final volume reach 4.1 mL. The technical triplicates were transferred to a 96-well format, and the absorbance of the Cu(I)–chelate, formed due to the redox reaction by reducing polyphenols and vitamins, was recorded at 450 nm against a control sample after 30 min of incubation. The results were expressed as mg of ascorbic acid equivalent per g of extract (mg AAE per g dw).
The Fe(II) chelating capacity assay was then assessed by the reaction between a phenolic compound and iron(II). In a slightly acidic medium (pH 6), the remaining Fe2+ reacts with ferrozine, forming a blue-coloured complex that can be monitored spectrophotometrically.65 Initially, a 50 μL aliquot of the sample previously diluted in ultrapure water (or the EDTA-Na2 solution used for the calibration curve), 160 μL of ultrapure water, and 20 μL of FeSO4 (0.30 mM) solution were added to a 96-well plate. In addition, a negative control was prepared to correct the varying colours of the sample solutions: 50 μL of water, 20 μL of FeSO4 (0.30 mM) solution, and 30 μL of water, which replaced the ferrozine solution, were added. After 5 min of incubation, the reaction was initiated by adding 30 μL of ferrozine solution (0.80 mM), and the final mixture was incubated again for 15 min. The colour reduction, which represents an estimation of the binding ability of the extract absorbance of the Fe2+-ferrozine complex, was measured at 562 nm. The results were expressed as mg EDTA-Na2 per g dw.
The oxygen radical absorbance capacity (ORAC) test measures a potential antioxidant's ability to prevent peroxyl radicals from harming the fluorescent fluorescein molecule. The method was modified from those described by Huang et al.66 and Pior et al.67 In brief, two technical replicates of 50 μL were measured in the 96-well format as in Tienaho et al.68 All the samples were measured with a series of five dilutions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320) and additional dilutions were made when necessary to adjust the sample concentrations to the 0.153 mM Trolox ((±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) standard curve. The reaction mixture contained the sample dilution in 75 mM phosphate buffer pH 7.5, 150 μL of 8.16 × 10−5 mM fluorescein and 25 μL of 2,2′-azobis (2-methylpropionamidine) dihydrochloride. The results were expressed as Trolox equivalents (μM TE per g dw).
320) and additional dilutions were made when necessary to adjust the sample concentrations to the 0.153 mM Trolox ((±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) standard curve. The reaction mixture contained the sample dilution in 75 mM phosphate buffer pH 7.5, 150 μL of 8.16 × 10−5 mM fluorescein and 25 μL of 2,2′-azobis (2-methylpropionamidine) dihydrochloride. The results were expressed as Trolox equivalents (μM TE per g dw).
For the PHWE extracts, the procedure was the same as described above, but extracts were not pre-dried and pipetted into the microplate in 25, 12.5, 6.25, and 3.13%. The inhibition (% ± CV%) results are shown for 1 mg mL−1 content for each sample.
Data analysis
The experimental data were expressed as means and standard deviation. The Shapiro–Wilk test was used to assess the data's normality and the Brown–Forsythe test to assess the homogeneity of the data variance. One-way analysis of variances (ANOVA) and the Tukey post hoc test were used to compare the mean values. Differences that reached a confidence level of 95% (p < 0.05) were considered statistically significant. An unpaired Student's t-test was used to compare EtOH/H2O and hexane solvents regarding the TPC and antioxidant activity. A bivariate correlation analysis assessed the Pearson correlation coefficients between different compounds and bioactivity properties. Furthermore, multivariate factor analysis was used to identify the interrelationships among compounds. The statistical analysis was performed using the softwares TIBCO Statistica v.13.3 (2018) and IBM® SPSS® Statistics Version 26 (2019).Results and discussion
Chemical composition and biological activities of the lipophilic and hydrophilic extracts
As noted earlier, this study compares the chemical composition and bioactivities of the biomass from reed canary grass, common reed, oil and fibre hemp to examine the main extractable compounds for biorefinery applications. For this purpose, consecutive hexane and EtOH/H2O extractions were performed to assess the lipophilic and hydrophilic fractions of the biomass collected in the summer and autumn.Compound groups and individual compounds
Through ASE, various compound groups, such as fatty acids, sitosterol, steryl esters, triglycerides, and condensed tannins were extracted (Table 3). Fatty acids can be categorised into four groups according to their length of the hydrocarbon chain: short-chain (C6), medium-chain (C6–C12), long-chain (C13–C21), and very-long-chain fatty acids (C22).72 In this study, compounds containing short-chain fatty acids (C6) were designated as fatty acids group 1, while medium to long chains were called fatty acids group 2. Overall, the lipophilic OH autumn extract mainly contained triglycerides, followed by a group containing mainly sterols, steryl esters and fatty acids 2 (Table 3). A total of nine fatty acids were identified in hexane-rich fractions: acid 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (palmitic acid), acid 18
0 (palmitic acid), acid 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (oleic acid), acid 18
1 (oleic acid), acid 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (linoleic acid), acid 18
2 (linoleic acid), acid 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (stearic acid), acid 20
0 (stearic acid), acid 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (arachidic acid), acid 22
0 (arachidic acid), acid 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (behenic acid), acid 24
0 (behenic acid), acid 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (lignoceric acid), acid 26
0 (lignoceric acid), acid 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (hexacosanoic acid), and acid 28
0 (hexacosanoic acid), and acid 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (octacosanoic acid), the most abundant being oleic and palmitic acids (Table 4). However, only two fatty acids were found in EtOH/H2O
0 (octacosanoic acid), the most abundant being oleic and palmitic acids (Table 4). However, only two fatty acids were found in EtOH/H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acid 16
acid 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (palmitic acid) and acid 18
0 (palmitic acid) and acid 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (stearic acid). In addition, the factor analysis showed high factor loadings between triglycerides, fatty acids 2 and steryl esters (factor 1), and fatty acids 1 and sterols etc. (factor 2) (ESI Fig. 2 and Table 1†). The compounds with high factor loadings in terms of factors 1 or 2 indicate the probability of their co-occurrence in the studied biomass. Notably, the grouping of the compounds was done by following the same screening method commonly used to analyse wood extractives. However, further analysis is critical to determine sterols etc. composition, considering that compounds (e.g., cannabinoids and sterols) might be eluted at the same retention time. Meanwhile, summer oil hemp was the only raw material with detectable amounts of tannin (CT) (Table 3). Hemp tannins were essentially (epi)catechin polymers, i.e., procyanidins agreeing with the study of Mattila et al.73
0 (stearic acid). In addition, the factor analysis showed high factor loadings between triglycerides, fatty acids 2 and steryl esters (factor 1), and fatty acids 1 and sterols etc. (factor 2) (ESI Fig. 2 and Table 1†). The compounds with high factor loadings in terms of factors 1 or 2 indicate the probability of their co-occurrence in the studied biomass. Notably, the grouping of the compounds was done by following the same screening method commonly used to analyse wood extractives. However, further analysis is critical to determine sterols etc. composition, considering that compounds (e.g., cannabinoids and sterols) might be eluted at the same retention time. Meanwhile, summer oil hemp was the only raw material with detectable amounts of tannin (CT) (Table 3). Hemp tannins were essentially (epi)catechin polymers, i.e., procyanidins agreeing with the study of Mattila et al.73
| Compound group (mg per g dw) | RCG summer | CR summer | OH summer | RCG autumn | CR autumn | OH autumn | FH autumn | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexane | EtOH/H2O | Hexane | EtOH/H2O | Hexane | EtOH/H2O | Hexane | EtOH/H2O | Hexane | EtOH/H2O | Hexane | EtOH/H2O | Hexane | EtOH/H2O | |
| a Note: compound groups were analysed using GC, while condensed tannins were analysed using UHPLC after the thiolytic degradation of the tannins. The content is expressed as mg per g dw of the biomass (mean ± SD). Fatty acids group 1 contains short-chain fatty acids (C6) and fatty acids 2 from medium to long-chain fatty acids (C6–C21). RCG = reed canary grass, CR = common reed, OH = oil hemp, FH = fibre hemp, dw = dry weight, ND = not detected, DP = average degree of polymerisation. | ||||||||||||||
| Fatty acids 1 | 1.6 ± 0.3 | 3.8 ± 0.4 | 0.8 ± 0.0 | 4.0 ± 0.5 | 1.4 ± 0.3 | 8.8 ± 0.2 | 0.8 ± 0.0 | 0.9 ± 0.2 | 0.7 ± 0.1 | 0.9 ± 0.4 | 4.3 ± 0.2 | 1.5 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.0 |
| Fatty acids 2 | 0.7 ± 0.1 | 1.2 ± 0.3 | 0.4 ± 0.1 | 1.5 ± 0.3 | 2.4 ± 0.0 | 4.7 ± 0.6 | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.8 ± 0.1 | 0.5 ± 0.2 | 11.9 ± 1.3 | 1.2 ± 0.1 | 0.3 ± 0.0 | 0.2 ± 0.1 |
| Sterols etc. | 2.5 ± 0.2 | 26.4 ± 1.7 | 4.7 ± 0.2 | 31.3 ± 2.7 | 6.2 ± 0.2 | 39.2 ± 12.2 | 3.0 ± 0.6 | 1.7 ± 0.4 | 2.0 ± 0.4 | 8.2 ± 1.2 | 18.2 ± 1.2 | 23.6 ± 2.0 | 1.6 ± 0.3 | 3.3 ± 0.0 |
| Steryl esters | 1.7 ± 0.1 | 1.8 ± 0.2 | 1.9 ± 0.0 | 4.3 ± 0.4 | 2.5 ± 0.5 | 2.7 ± 0.7 | 1.4 ± 0.5 | 0.6 ± 0.0 | 0.7 ± 0.1 | 0.8 ± 0.2 | 7.7 ± 0.3 | 2.5 ± 0.5 | 0.9 ± 0.1 | 0.3 ± 0.0 |
| Triglycerides | 0.7 ± 0.1 | 0.7 ± 0.3 | 0.8 ± 0.1 | 0.3 ± 0.1 | 1.0 ± 0.4 | 0.3 ± 0.3 | 0.4 ± 0.0 | 0.2 ± 0.0 | 1.6 ± 0.0 | 0.6 ± 0.1 | 83.1 ± 6.4 | 3.2 ± 0.4 | 0.6 ± 0.1 | ND |
| Condensed tannins | ND | ND | ND | ND | 0.3 ± 0.0 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Degree of polymerisation (DP) | ND | ND | ND | ND | 4.7 ± 0.7 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Solvent | Compounds | RCG, summer mg per g dw | RCG, autumn mg per g dw | CR, summer mg per g dw | CR, autumn mg per g dw | OH, summer mg per g dw | OH, autumn mg per g dw | FH, autumn mg per g dw |
|---|---|---|---|---|---|---|---|---|
| a Note: ND = not detected, RCG = reed canary grass, CR = common reed, OH = oil hemp, FH = fibre hemp. | ||||||||
| Hexane | Sugars | |||||||
| Sucrose | ND | ND | 0.03 | ND | 0.03 | ND | ND | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Organic acids | ||||||||
Acid 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (palmitic acid) 0 (palmitic acid) |
0.17 | 0.19 | 0.12 | 0.38 | 0.18 | 0.17 | 0.04 | |
Acid 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (oleic acid) 1 (oleic acid) |
0.58 | 0.26 | 0.12 | 0.12 | 0.37 | 0.55 | 0.04 | |
Acid 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (linoleic acid) 2 (linoleic acid) |
0.33 | 0.15 | 0.07 | 0.07 | 0.08 | 0.09 | 0.04 | |
Acid 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (stearic acid) 0 (stearic acid) |
0.04 | 0.05 | 0.04 | 0.08 | 0.07 | 0.43 | 0.03 | |
Acid 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (arachidic acid) 0 (arachidic acid) |
ND | ND | ND | 0.03 | ND | ND | 0.01 | |
Acid 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (behenic acid) 0 (behenic acid) |
ND | 0.01 | ND | 0.04 | ND | ND | 0.01 | |
Acid 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (lignoceric acid) 0 (lignoceric acid) |
ND | ND | ND | 0.04 | ND | ND | ND | |
Acid 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (hexacosanoic acid) 0 (hexacosanoic acid) |
ND | 0.04 | 0.05 | 0.01 | ND | ND | 0.07 | |
Acid 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (octacosanoic acid) 0 (octacosanoic acid) |
ND | 0.09 | 0.12 | 0.03 | ND | ND | 0.07 | |
| Distearyl acid phosphate (phosphoric acid) | 0.03 | 0.03 | ND | 0.03 | 0.03 | ND | ND | |
| Ursolic acid | ND | ND | ND | ND | ND | ND | 0.14 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Fatty alcohols | ||||||||
Alcohol 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (1-tetracosanol) 0 (1-tetracosanol) |
ND | ND | ND | 0.02 | ND | ND | 0.04 | |
Alcohol 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (1-hexacosanol) 0 (1-hexacosanol) |
0.89 | 1.21 | 0.10 | 0.08 | 0.05 | ND | 0.08 | |
Alcohol 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (1-octacosanol) 0 (1-octacosanol) |
ND | 0.07 | 0.67 | 0.21 | ND | ND | 0.15 | |
Alcohol 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (1-triacontanol) 0 (1-triacontanol) |
ND | ND | 0.75 | 0.16 | 0.06 | ND | 0.14 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Sterols | ||||||||
| Campesterol | 0.11 | 0.13 | 0.05 | 0.22 | 0.08 | ND | 0.01 | |
| Stigmastreol | 0.05 | 0.12 | 0.20 | 0.22 | 0.05 | ND | 0.03 | |
| β-Sitosterol | 0.26 | 0.25 | 0.46 | 1.24 | 0.63 | 0.26 | 0.17 | |
| Ergosterol | ND | ND | ND | ND | ND | ND | 0.02 | |
| Cycloartenol | ND | 0.03 | ND | ND | ND | ND | ND | |
| Tocopherol | ||||||||
| α-Tocopherol | ND | ND | ND | ND | 0.15 | ND | ND | |
| β-Tocopherol | ND | ND | ND | ND | 0.03 | ND | ND | |
| Cannabinoids | ||||||||
| Cannabidiol | ND | ND | ND | ND | ND | 1.06 | 0.02 | |
| Cannabivarinic acid | ND | ND | ND | ND | 0.05 | 0.44 | 0.01 | |
| Cannabidiolic acid | ND | ND | ND | ND | 2.49 | 12.84 | 0.23 | |
| Tetrahydrocannabinolic acid | ND | ND | ND | ND | 0.42 | ND | ND | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| EtOH/H 2 O | Sugars | |||||||
| Fructose | 13.06 | 6.78 | 15.44 | 1.09 | 32.29 | 4.21 | 2.06 | |
| Sorbose | 0.59 | 0.25 | 0.30 | 0.05 | 1.04 | 0.13 | 0.08 | |
| Psicose | 1.92 | 0.00 | 1.53 | ND | 4.07 | 0.44 | ND | |
| α-Glucose | 9.32 | 2.38 | 7.26 | 0.60 | 16.07 | 1.64 | 0.96 | |
| Mannose | 0.65 | 0.99 | 0.00 | 0.19 | 0.48 | ND | 0.26 | |
| β-Glucose | 9.97 | 2.61 | 8.10 | 0.67 | 15.30 | 1.76 | 1.06 | |
| Galactose | 0.31 | 0.22 | ND | 0.09 | 0.56 | ND | ND | |
| Sucrose | 38.00 | 0.18 | 38.81 | 7.13 | 30.83 | 19.28 | 1.71 | |
| Raffinose | 0.57 | ND | ND | ND | ND | 2.47 | 0.00 | |
| Trehalose | 0.32 | 0.09 | ND | 1.92 | ND | 0.13 | 0.74 | |
| Xylose | ND | 0.03 | ND | ND | ND | ND | 0.01 | |
| Raffinose | ND | ND | ND | 0.11 | ND | ND | ND | |
| Arabinose | ND | 0.10 | ND | ND | 0.67 | ND | 0.02 | |
| Maltose | ND | ND | ND | ND | 0.35 | ND | ND | |
| Maltose isomer | ND | ND | ND | ND | 0.38 | ND | ND | |
| Cellobiose | ND | ND | ND | ND | 0.30 | ND | ND | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Organic acids | ||||||||
Acid 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (palmitic acid) 0 (palmitic acid) |
ND | 0.06 | ND | 0.08 | 0.19 | ND | 0.15 | |
Acid 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (stearic acid) 0 (stearic acid) |
ND | 0.05 | ND | 0.07 | ND | 0.34 | 0.06 | |
| Quinic acid | ND | ND | 0.67 | ND | ND | ND | ND | |
| Glucuronic acid | ND | 0.06 | ND | ND | ND | ND | ND | |
| p-Coumaric acid | ND | ND | ND | 0.07 | ND | 0.31 | 0.24 | |
| Aconitic acid | 0.17 | ND | 0.30 | ND | ND | ND | ND | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Alcohols | ||||||||
| Arabitol | 0.37 | ND | 0.39 | ND | ND | ND | ND | |
| Mannitol | 0.27 | 2.56 | 0.68 | ND | 0.46 | 0.89 | ND | |
| Myo-inositol | 0.71 | 0.11 | 0.31 | 0.15 | 4.55 | 0.67 | 0.10 | |
| Scyllo-inositol | ND | ND | ND | ND | 1.00 | 0.20 | ND | |
| Isomaltitol | ND | 0.10 | ND | ND | ND | ND | ND | |
| Glucitol | ND | 0.06 | ND | ND | ND | ND | ND | |
| Sorbitol | ND | ND | ND | 3.69 | ND | ND | 2.05 | |
| Pinitol | ND | 0.11 | ND | 0.28 | 9.50 | 7.84 | 0.21 | |
| Xylitol | ND | 1.14 | ND | 2.63 | 0.70 | 0.76 | 0.71 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Sterols | ||||||||
| β-Sitosterol | ND | ND | ND | 0.07 | ND | ND | 0.03 | |
| Cannabinoids | ||||||||
| Cannabidiol | ND | ND | ND | ND | ND | 0.15 | ND | |
| Cannabidiolic acid | ND | ND | ND | ND | ND | 0.87 | ND | |
| Lactone | ||||||||
| Lactone | ND | ND | ND | ND | 0.75 | ND | ND | |
Table 4 shows the individual compounds found in the biomass extracts. Sucrose, fructose, α-glucose, and β-glucose were the primary sugars in EtOH/water samples. Of the nine sugar alcohols identified in the hydrophilic extracts, the major ones were pinitol, myo-inositol, xylitol, and sorbitol, whose biological properties have been studied often. Several studies have been reporting their biological properties. Pinitol has drawn attention due to its properties, such as insulin regulation.74 Sorbitol is a natural sugar alcohol with numerous applications ranging from the food industry (as a sweetener), pharmaceutical applications (as a drug carrier),75 to the cosmetics industry (as an emulsion stabiliser).76 Evidence shows that the administration of myo-inositol decreases the multiplicity and size of surface tumours and the size of adenocarcinoma, showing the potential to be utilised for the chemoprevention of early pulmonary lesions. This component and its derivatives may be an appropriate adjunct therapy in mental afflictions and cognitive diseases.77 Moreover, lignocellulosic biomasses are renewable and cost-effective sources of polysaccharides that can be used for xylitol production, which has applications in food (e.g., chewing gums and sweeteners) and pharmaceutical (e.g., syrups and vitamins) sectors.
Phytocannabinoids were found in both lipophilic and hydrophilic hemp extracts (Table 4). The most abundant one, cannabidiolic acid (CBDA), was identified in lipophilic OH and FH hemp extracts. Cannabidiol (CBD) was found in both OH and FH autumn but mainly in OH lipophilic extract. In contrast, tetrahydrocannabinolic acid (THCA) was identified only in OH summer lipophilic fraction. Comparatively, Pavlovic et al. studied hemp inflorescence (cv. FINOLA vs. Futura 75) extracted with ASE and found CBDA as the primary cannabinoid (23.5 mg g−1 in FINOLA vs. 27.6 mg g−1 in Futura 75). Nevertheless, a similar content was identified for CBD (2.6 mg g−1 in FINOLA vs. 0.6 mg g−1 in Futura 75), THCA (0.38 mg g−1 in FINOLA vs. 0.36 mg g−1 in Futura 75), and CBDVA (2.9 mg g−1 in FINOLA vs. 1.2 mg g−1 in Futura 75) when comparing with the present study.78
Typically, RCG is produced for energy generation, and it is harvested in early spring to reduce plant components with harmful constituents (alkali and chlorine) and to reduce moisture content.79 In Finland, FH is normally harvested during the spring when the soil is frozen. With this timing, unwanted plant components can be reduced, fiber processing or combustion properties can be improved,80 and soil compacting and rutting can be avoided. In the case of OH, generally, only seeds are harvested.81 The present study explored the potential for increasing the yields of valuable compounds by bringing the harvest time forward. However, increasing biomass recovery would increase the need for fertilisation.80 From a biorefining perspective, the harvest time can affect the extractable substances. Since the compounds (e.g. sugars, sterols, fatty acids) with higher concentrations in summer can be of significant value for potential commercial applications, it may be worth exploring the option of harvesting immature plants to capitalise on their higher concentrations. This is the first time that the detailed extractives content and composition of the extracts obtained from different biomasses from marginal land has been investigated, and thus it can form the basis for future research.
Total phenolic content, flavonoids, total dissolved solids, and protein content
In this study, oil and fibre hemp (OH and FH) had the highest TPC for both hydrophilic and lipophilic extracts compared to other grasses (Table 5) revealed by the fine screening and fibre. Specifically, the highest TPC was found in the hemp fine screening fraction (69 ± 1 mg GAE per g dw in FH-ASF-EtOH/H2O and 54 ± 2 mg GAE per g dw in OH-SSF-hexane). André et al.82 analysed the same fibre-type FINOLA cultivar in different harvest periods (15.46 ± 0.22 mg GAE per g dw in July/full flowering vs. 5.38 ± 0.23 mg GAE per g dw August/end of flowering). They investigated the chemical composition of eight fibre-type Cannabis sativa L. inflorescences with different sowing densities from July to September 2019. They found that the plant's harvest period and phenological stage mainly influenced the content of individual flavonoids and terpenes. However, the content of polyphenols and flavonoids decreased during flower development for all cultivars studied, whereas the terpene content increased with maturation. Male and female inflorescences were also found to influence the phenolic composition of the extracts. Similarly, Drinić et al. assessed the effect of extraction solvent on the TPC and antioxidant activity of industrial hemp and reported that the former values ranged from 5.85 to 17.05 mg GAE per g dw depending on the ethanol concentrations in water (30, 50, 70, and 90%).83 Overall, a 50% ethanol/H2O was found to be the best solvent system for extracting phenolic compounds from aerial parts (variety Helena). However, another study observed lower TPC values compared to our findings (26.90 ± 0.53 mg GAE per g dw) in fibre-type hemp (cultivar ‘Beniko’) threshing residues remaining after the harvesting and cleaning of industrial seeds (i.e., a mixture of leaves, floral bracts, flower fragments and immature seeds) extracted by pressurised liquid extraction (EtOH/H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v).84 Even though they applied the same extraction temperature (100 °C) as in the current study, the differences may be due to other factors such as plant cultivar, plant growth environment and circumstances, extraction parameters, technique, and analytical methods. On the one hand, Gunjević et al.85 carried out a complete valorisation of hemp components (aqueous fraction of hemp inflorescences under microwave-assisted extraction), and their TPC content was similar to our findings (53 mg GAE/g). On the other, an investigation of hemp extraction using green technology found up to 109.5 ± 9.3 mg GAE per g of aqueous extract.86 The difference may be attributed to several factors, such as the cultivar (Futura 75), plant fraction, solvent, extraction technique, and analytical method. Furthermore, Padda and Picha have indicated that the TPC estimated using the Folin–Ciocalteu method might produce overestimated values due to the ascorbic acid and carbohydrate presence and interference.87
1 v/v).84 Even though they applied the same extraction temperature (100 °C) as in the current study, the differences may be due to other factors such as plant cultivar, plant growth environment and circumstances, extraction parameters, technique, and analytical methods. On the one hand, Gunjević et al.85 carried out a complete valorisation of hemp components (aqueous fraction of hemp inflorescences under microwave-assisted extraction), and their TPC content was similar to our findings (53 mg GAE/g). On the other, an investigation of hemp extraction using green technology found up to 109.5 ± 9.3 mg GAE per g of aqueous extract.86 The difference may be attributed to several factors, such as the cultivar (Futura 75), plant fraction, solvent, extraction technique, and analytical method. Furthermore, Padda and Picha have indicated that the TPC estimated using the Folin–Ciocalteu method might produce overestimated values due to the ascorbic acid and carbohydrate presence and interference.87
| Biomass | Harvest season/plant fraction | Abbreviation | TPC (mg GAE per g) | Flavonoids (mg quercetin per g) | TDS (mg g−1) | Protein (mg g−1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hexane | EtOH/H2O | Hexane | EtOH/H2O | Hexane | EtOH/H2O | Hexane | EtOH/H2O | |||
| a Note: values are expressed as means followed by the standard deviation (n = 3) and expressed as mg per g dw of extract. Different lowercase letters within the same column of the individual extracts indicate significant differences (one-way ANOVA and Tukey's test, p < 0.05). Different uppercase letters in different columns represent statistically different (p < 0.05) results comparing the EtOH/H2O (95/5, v/v) and hexane extracts. TPC: total phenolic content, GAE: gallic acid equivalent, NA: not analysed, ND: not detected. | ||||||||||
| Reed canary grass | Summer unscreened | RCG-SU | 25 ± 0e | 26 ± 1ij | NA | NA | 16.6 ± 0fgB | 81.3 ± 0dA | 4.4 ± 0.0iB | 8.1 ± 0.3gA |
| Summer screening fines | RCG-SSF | 32 ± 1dA | 29 ± 0gB | ND | 30 | 17.4 ± 0fB | 81.2 ± 1dA | 7.7 ± 0.2gA | 7.3 ± 0.3ghB | |
| Summer fibre fraction | RCG-SFF | 41 ± 2cA | 21 ± 0jB | NA | NA | 12.2 ± 0hiB | 78.3 ± 2deA | 6.6 ± 0.5gh | 7.5 ± 0.5gh | |
| Autumn screening fines | RCG-ASF | 13 ± 1gB | 54 ± 1bA | ND | 10 | 12.8 ± 0ghiB | 29.0 ± 0hA | 20.1 ± 0.9cd | 22.3 ± 0.6b | |
| Autumn fibre fraction | RCG-AFF | 16 ± 1fgB | 51 ± 1cA | NA | NA | 8.5 ± 0ijB | 25.7 ± 2hA | 21.8 ± 0.5bcA | 18.8 ± 0.4cB | |
| Common reed | Summer unscreened | CR-SU | 23 ± 1eB | 29 ± 1gA | NA | NA | 16.8 ± 0fgB | 84.4 ± 3dA | 6.8 ± 0.2gB | 13.1 ± 0.6eA |
| Summer screening fines | CR-SSF | 30 ± 1dA | 27 ± 0hiB | ND | 19 | 15.2 ± 0.0fghB | 101.4 ± 3cA | 5.0 ± 0.5hiB | 6.9 ± 0.1ghA | |
| Summer fibre fraction | CR-SFF | 42 ± 1cA | 28 ± 1ghB | NA | NA | 12.7 ± 0.2ghiB | 80.4 ± 5dA | 5.0 ± 0.5hiB | 13.0 ± 0.2eA | |
| Autumn screening fines | CR-ASF | 18 ± 0fB | 36 ± 1dA | ND | 7 | 15.3 ± 0.0fghB | 41.9 ± 0gA | 18.7 ± 0.8deB | 25.2 ± 0.1aA | |
| Autumn fibre fraction | CR-AFF | 13 ± 0g | 34 ± 1e | NA | NA | 11.0 ± 0.6hijB | 43.9 ± 4gA | 17.7 ± 0.4eA | 17.2 ± 0.6dB | |
| Oil hemp (cv. FINOLA) | Summer unscreened | OH-SU | 47 ± 0bA | 11 ± 0kB | NA | NA | 30.8 ± 0.3dB | 124.9 ± 0bA | 9.5 ± 0.2fA | 6.5 ± 0.5hiB |
| Summer screening fines | OH-SSF | 54 ± 2aA | 5 ± 0lB | ND | 6 | 26.0 ± 0.3eB | 140.8 ± 1aA | 28.5 ± 0.7aA | 2.7 ± 0.1jB | |
| Summer fibre fraction | OH-SFF | 46 ± 2bA | 3 ± 0mB | NA | NA | 23.9 ± 0.2eB | 107.5 ± 2cA | 17.8 ± 1.3eA | 1.7 ± 0.1jB | |
| Autumn unscreened | OH-AU | 47 ± 3bA | 10 ± 0kB | NA | NA | 176.6 ± 3.4bB | 63.2 ± 2fA | 6.5 ± 0.2ghB | 12.6 ± 0.8eA | |
| Autumn screening fines | OH-ASF | 47 ± 1bA | 3 ± 0mB | ND | 1 | 205.4 ± 1.0aB | 70.7 ± 3efA | 2.2 ± 0.0jB | 3.1 ± 0.3jA | |
| Autumn fibre fraction | OH-AFF | 25 ± 0eA | 5 ± 0lB | NA | NA | 158.9 ± 2.7cA | 50.5 ± 0gB | 2.2 ± 0.1jB | 5.1 ± 0.4iA | |
| Fibre hemp (var. Uso 31) | Autumn screening fines | FH-ASF | 30 ± 1dB | 69 ± 1aA | ND | ND | 6.8 ± 0.4jkA | 24.4 ± 1hB | 23.3 ± 0.8bB | 25.6 ± 0.5aA |
| Autumn fibre fraction | FH-AFF | 32 ± 1d | 32 ± 1f | NA | NA | 3.9 ± 0.0kA | 30.8 ± 1hB | 21.8 ± 0.4bcA | 10.6 ± 0.9fB | |
The flavonoid content was evaluated exclusively for screening fines. Results varied from 1 to 30 mg g−1 in ethanol–water extracts, indicating that summer-collected plants provided a higher level of flavonoids. In this regard, a previous study reported the presence of kaempferol, luteolin, and apigenin as the main flavonoids in the hemp-threshing residues.84
The TDS varied from 24.4 mg g−1 to 140.8 mg g−1 for the hydrophilic extracts and from 3.9 mg g−1 to 205.4 mg g−1 for the lipophilic samples. Hydrophilic extraction yielded lower for most biomasses than the consecutive EtOH/H2O extraction, except for autumn oil hemp.
The protein content in all the extracts ranged from 2.2 mg g−1 to 28.5 mg g−1 (hexane) and from 1.7 mg g−1 to 25.6 mg g−1 (EtOH/H2O). In reed canary grass, the protein content ranged from 7.3 mg g−1 (SSF) to 22.3 mg g−1 (ASF) with ethanol–water extraction and from 4.4 mg g−1 (SU) to 21.8 mg g−1 (AFF) with hexane extraction. These values align with the findings of previous studies, wherein depending on the light conditions, soil nitrogen levels, and moisture levels, the protein content of green-house-grown ground reed canary grass leaf tissue varied from 7 to 26 mg g−1.88 In common reed, the protein content varied from 6.9 to 25.2 mg g−1 for ethanol–water extracts, corresponding to 45–113 μg mL−1. Hendricks et al. found that in common reed leaves, combined methanol and ethyl acetate extracts contained protein levels of 43.2 μg mL−1, which makes our findings somewhat higher.89 The solvent choice, plant fraction, growing conditions, and environment variances might have caused the difference. The protein content was lower for the common reed hexane extracts, 5–18.7 mg g−1 corresponding to 5–14.1 μg mL−1. The protein content in oil hemp varied from 1.7 to 12.6 mg g−1 and 2.2–28.5 mg g−1 for the ethanol and hexane extracts, respectively. However, the results were superior for the fibre hemp, varying from 10.6–25.6 mg g−1 for ethanol–water extracts vs. and 21.8–23.3 mg g−1 for hexane extracts. In a study conducted in the USA, the crude protein content of dried industrial hemp biomass was found to range from 53 to 245 mg g−1, depending on the plant part used.90 While drought stress can activate the production of certain low-molecular-weight proteins, prolonged water deficit accumulates the production of reactive oxygen species and leads to protein degradation.91 This means that protein content can vary heavily depending on the growing conditions and is one potential cause of differences in the protein contents found in the literature.
Antioxidant activity (AOX)
In this study, three antioxidant mechanisms were tested: single electron transfer by DPPH and CUPRAC, transition metal ion chelation using Fe2+, and hydrogen atom transfer by ORAC. The DPPH radical scavenging activity values showed different outcomes for the polar and non-polar fractions. The hemp samples harvested in autumn exhibited the highest antioxidant capacity for both hydrophilic and lipophilic extractions (337 ± 9 mg AAE per g in OH-AU-hexane and 56 ± 3 mg AAE per g in FH-ASF EtOH/H2O fraction). These results may be attributed to the increased solubility and affinity of the oil hemp cultivar components of hexane, contributing to the increased AOX (Table 6). Similar results were found (367 mg TE eq. per g) in FINOLA hempseed oil, which is associated with significant amounts of polyphenols, especially flavonoids such as flavanones, flavanols, flavonols and isoflavones.92 Kitrytė et al.84 reported lower values for hemp threshing residue extracted using pressurised liquid (PLE) with acetone (48 ± 3 mg TE per g dw) as a non-polar fraction and PLE-EtOH/H2O (59 ± 2 mg TE per g dw). Palmieri et al.93 analysed hemp inflorescence (cv. Futura 75), an exploitable threshing residue from seed harvest, and found comparatively lower antiradical activity (45 ± 1 mg TE per g dw) in their ethanolic samples extracted using the ultrasound assisted extraction technique. Another study revealed that an aqueous extract obtained using optimised microwave-assisted extraction conditions could be a source of bioactive phenolic constituents, particularly glycosidic flavones. Thus, the abundance of these compounds can be related to the DPPH values (92 ± 5.5 mg TE per g).93| Biomass | Harvest season/plant fraction | Abbreviation | DPPH (mg AAE per g) | CUPRAC (mg AAE per g) | Fe(II) chelating ability (mg EDTA-Na2 per g) | ORAC (μM TE per g) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hexane | EtOH/H2O | Hexane | EtOH/H2O | Hexane | EtOH/H2O | Hexane | EtOH/H2O | |||
| a Note: values are expressed as means followed by the standard deviation (n = 3) and expressed as mg per g dw of extract. Different superscript letters within the same column of individual extracts indicate significant differences (one-way ANOVA and Tukey's test, p < 0.05). Different capital letters in different columns represent statistically different (t-test, p < 0.05) results comparing EtOH/H2O (95/5, v/v) vs. hexane extracts. GAE: gallic acid equivalent, AAE: ascorbic acid equivalent, TE: Trolox equivalent, ND: not detected. | ||||||||||
| Reed canary grass | Summer unscreened | RCG-SU | ND | 24 ± 0de | 102 ± 3dB | 167 ± 0bA | 40 ± 1bcA | 8 ± 1bcB | 194 ± 15jB | 3035 ± 8cdeA |
| Summer screening fines | RCG-SSF | ND | 36 ± 1c | 46 ± 4fB | 104 ± 6eA | 23 ± 6fgA | 13 ± 0bB | 376 ± 22fgB | 4333 ± 78aA | |
| Summer fibre fraction | RCG-SFF | ND | 22 ± 1de | 102 ± 2dA | 44 ± 3hiB | 17 ± 0hiA | 7 ± 1bcB | 294 ± 3hiB | 2974 ± 91deA | |
| Autumn screening fines | RCG-ASF | ND | 7 ± 1ij | ND | 48 ± 4h | 40 ± 3cdA | 25 ± 7aB | 310 ± 7fgB | 2277 ± 127gA | |
| Autumn fibre fraction | RCG-AFF | ND | 43 ± 4b | ND | 43 ± 7hi | 31 ± 2deA | 11 ± 2bcB | 405 ± 22fgB | 2594 ± 22fA | |
| Common reed | Summer unscreened | CR-SU | 21 ± 5gh | 27 ± 1d | 86 ± 5e | 82 ± 0f | 19 ± 0ghA | 7 ± 0bcB | 266 ± 9ijB | 3523 ± 260bA |
| Summer screening fines | CR-SSF | 26 ± 8g | 26 ± 0d | 39 ± 3fB | 98 ± 3eA | 28 ± 2efA | 7 ± 0bcB | 223 ± 20ijB | 3056 ± 57cdA | |
| Summer fibre fraction | CR-SFF | 244 ± 10bA | 20 ± 2eB | 100 ± 4dB | 152 ± 2cA | 18 ± 4hi | 9 ± 0bc | 213 ± 9ijB | 3221 ± 47cA | |
| Autumn screening fines | CR-ASF | 152 ± 7dA | 46 ± 1bB | 25 ± 4gA | 14 ± 0jB | 57 ± 2aA | 9 ± 1bcB | 677 ± 15dB | 3708 ± 29bA | |
| Autumn fibre fraction | CR-AFF | 139 ± 6dA | 13 ± 2gB | ND | 121 ± 6d | 20 ± 7ghA | 4 ± 1cdB | 304 ± 30ghB | 4490 ± 18aA | |
| Oil hemp (cv. FINOLA) | Summer unscreened | OH-SU | 10 ± 1ghB | 13 ± 0fA | 265 ± 2aA | 41 ± 1hiB | 15 ± 0hiA | 2 ± 0deB | 1232 ± 58bB | 1505 ± 55hA |
| Summer screening fines | OH-SSF | 24 ± 4ghA | 5 ± 0ijB | 249 ± 1bA | 18 ± 1jB | 10 ± 0ijA | 2 ± 0deB | 1536 ± 88aA | 597 ± 33jB | |
| Summer fibre fraction | OH-SFF | 7 ± 0hA | 4 ± 0jB | 218 ± 5cA | 11 ± 0jB | 31 ± 2deA | 1 ± 0fB | 1157 ± 6bA | 275 ± 21kB | |
| Autumn unscreened | OH-AU | 337 ± 9aA | 10 ± 0hiB | 211 ± 2cA | 66 ± 1gB | 10 ± 0ijA | 3 ± 0deB | 819 ± 12cB | 1602 ± 43hA | |
| Autumn screening fines | OH-ASF | 57 ± 1fA | 2 ± 0jB | 266 ± 0aA | 12 ± 1jB | 18 ± 2hiA | 1 ± 0fB | 774 ± 63cdA | 406 ± 5jkB | |
| Autumn fibre fraction | OH-AFF | 99 ± 1eA | 4 ± 0jB | 246 ± 2bA | 33 ± 2iB | 2 ± 0jA | 1 ± 0fB | 518 ± 34eA | 636 ± 17jB | |
| Fibre hemp (var. Uso 31) | Autumn screening fines | FH-ASF | 195 ± 9cA | 56 ± 3aB | 95 ± 10deB | 321 ± 7aA | 34 ± 3cd | 30 ± 7a | 412 ± 23fB | 2798 ± 54efA |
| Autumn fibre fraction | FH-AFF | 18 ± 5ghB | 36 ± 4cA | ND | 70 ± 7fg | 49 ± 2abA | 5 ± 2cdB | 372 ± 17fgB | 1004 ± 43iA | |
The CUPRAC values varied from 11 to 321 mg AAE per g for the EtOH/H2O fractions and 25 to 265 mg AAE per g for the hexane fractions. Overall, our data showed that hemp provided the most promising results. Hydrophilic FH-ASF extract (321 ± 7 mg AAE per g) had the highest values, while OH-ASF-hexane (266 mg AAE per g) and OH-SU-hexane extracts (265 ± 2 mg AAE per g) had AOX within lipophilic samples. For example, a previous study reported 94.3 mg AAE per g for PHWE Norway spruce bark extracts.94 It is known that the solvent in which the reaction occurs greatly impacts the results, as the polarity can affect the mechanism of the reaction. According to Moscariello et al., hemp biomass is a valuable resource for the sustainable implementation of second-generation biorefineries, adding value to the conventional production of hemp fibres and seeds. Oil, composite materials, biopolymers, and platform chemicals are a few examples of innovative bioproducts obtained from hemp.95
Meanwhile, the Fe(II) chelating capacity resulted in higher values for lipophilic samples of FH (49 ± 2 mg AAE per g FH-AFF and 34 ± 3 mg AAE per g FH-ASF), indicating contrasting outcomes compared to other tested AOX methods. Sudan et al. found that different solvents affect the chelation extent of the ferrous ions of Arisaema jacquemontii tubers, leaves, and fruit extracts. They also found that methanol was the most promising extractant compared to acetone, ethyl acetate, chloroform, and hexane. The study also compared methanol, water and chloroform and found negligible activity in water, as examined in the present study.96
The ORAC values varied from 275 to 4490 μM TE per g (EtOH/H2O) and 194 to 1536 μM TE per g (hexane). The highest values for each biomass were as follows: CR-AFF (4490 ± 18 μM TE per g, EtOH/H2O) > RCG-SSF (4333 ± 78, EtOH/H2O) > FH-ASF (2798 ± 54 μM TE per g, EtOH/H2O) > OH-AU (1602 ± 43 μM TE per g, EtOH/H2O). Differences in the AOX may be related to the distinctive availability of extractable components resulting from the varied chemical composition of plants.93 El-Borady et al. reported an eco-friendly fabrication of gold nanoparticles (AuNPs) using the aqueous extract of common reed (P. australis) leaf and demonstrated antioxidant, anticancer, and enhanced photocatalytic potential. Based on the favourable results, the study provided a potential option for environmentally managing common reed biomass by biosynthesising AuNPs, contributing to the emerging green medical nano-based technologies.97 Furthermore, studies have revealed the RCG potential as remediation source to recover microelements from municipal sewage sludge thereby decreasing the dependency on mineral fertilizers and promoting the preservation of natural resources.20,21 Thus, the data obtained in this study and the literature suggest that RCG and CR biomass may also be utilised for other biorefinery purposes besides the current energy use.
Notably, all the variations in the AOX determinations may be explained by the fact that the extraction of secondary metabolites highly depends on the processing/analytical methods, extraction solvents, chemical properties, and other external factors, such as environmental conditions and soil type. For instance, the extracting solvent and temperature can reduce the solvent viscosity, facilitating cell membrane permeability and further increasing the diffusion of the phenolic compounds.98
Antibacterial activity
The bacterial inhibition of the extracts was evaluated using whole-cell bacterial biosensor strains: two Gram-negative E. coli strains (constitutively luminescent E. coli K12 + pcGLS11 and genotoxic stress-reactive E. coli DPD2794) and one Gram-positive S. aureus strain (constitutively luminescent S. aureus RN4220+pAT19) (Table 7). Overall, the observed activities were quite low with the non-specific toxicity responsive E. coli K12 + pcGLS11 strain, and the maximum inhibition was detected from the autumn fibre hemp (hexane): 19.1% fibre fraction and 11.8% screening fines. No activities were detected in the reed canary grass ethanol–water extract, whereas the only summer screening fines indicated relatively low activity (2.6%). In the common reed, only summer fibre faction and autumn screening fines indicated low activities (0.5–1.3%) with ethanol–water extraction, and all but the summer unscreened and autumn fibre fraction showed low inhibition with hexane extraction (3.0–5.4%). In oil hemp, only the unscreened summer fraction for both extracts showed deficient activity (0.2% for EtOH/water and 0.5% for hexane extract). With the S. aureus strain, three hexane extracts could inhibit the bacterial luminescent light production completely: reed canary grass fibre fraction from autumn and both autumn fibre hemp samples (screening fines and fibre fraction), indicating high toxicity to the strain. In all but OH fractions, more activity against the S. aureus strain was detected from the hexane-extracted fractions collected in autumn. In all cases, the hexane extracts showed at least slightly higher activities in the constitutively luminescent light-producing strains of E. coli and S. aureus than the ethanol–water extracts. This indicates that the initially conducted hexane extraction can extract more antibacterial compounds from the reed and hemp biomasses, and lesser content of the effective compounds against the E. coli strain is detected from the consecutively obtained ethanol extraction product. In fact, the antioxidant was also found to be superior for hexane extracts in general.| Biomass | Harvest season/plant fraction | Abbreviations | E. coli K12 + pcGLS11 (inhibition %) | E. coli DPD2794 (inhibition %) | S. aureus RN4220 + pAT19 (inhibition %) | |||
|---|---|---|---|---|---|---|---|---|
| Hexane | EtOH/H2O | Hexane | EtOH/H2O | Hexane | EtOH/H2O | |||
| a Note: values are expressed as means followed by the standard deviation (n = 3). Different lowercase letters within the same column of the individual extracts indicate significant differences (one-way ANOVA and Tukey's test, p < 0.05). Different capital letters in different columns represent statistically different (t-test, p < 0.05) results comparing the EtOH/H2O (95/5, v/v) vs. hexane extracts. ND means that activity was not detected. | ||||||||
| Reed canary grass | Summer unscreened | RCG-SU | ND | ND | ND | 1.8 ± 0.9c | 19.8 ± 2.0cdA | 3.9 ± 0.3dB |
| Summer screening fines | RCG-SSF | 2.6 ± 1.3b | ND | ND | ND | 31.2 ± 0.8bcA | 4.6 ± 0.7dB | |
| Summer fibre fraction | RCG-SFF | ND | ND | ND | 1.6 ± 0.5c | 38.6 ± 2.4bA | 4.8 ± 0.8cdB | |
| Autumn screening fines | RCG-ASF | ND | ND | 40.3 ± 10.2a | 26.4 ± 2.1a | 41.9 ± 11.1b | ND | |
| Autumn fibre fraction | RCG-AFF | ND | ND | 17.1 ± 9.1bcd | 16.7 ± 7.9b | 100.0 ± 14.7aA | 13.2 ± 8.0bcB | |
| Common reed | Summer unscreened | CR-SU | ND | ND | ND | 1.2 ± 1.2c | 19.6 ± 2.0cdA | 3.7 ± 0.8dB |
| Summer screening fines | CR-SSF | 3.0 ± 4.1b | ND | ND | ND | 33.5 ± 3.7bcA | 5.8 ± 1.8cdB | |
| Summer fibre fraction | CR-SFF | 5.4 ± 1.7ab | 0.5 ± 0.0b | ND | 1.6 ± 1.8c | 36.0 ± 2.7bcA | 4.3 ± 0.5dB | |
| Autumn screening fines | CR-ASF | 5.2 ± 0.0ab | 1.3 ± 0.8b | ND | 2.1 ± 2.1c | 45.9 ± 2.7bA | 16.0 ± 2.8abB | |
| Autumn fibre fraction | CR-AFF | ND | ND | 25.0 ± 4.6abcA | 7.6 ± 1.6cB | 47.2 ± 5.4bA | 5.4 ± 0.4cdB | |
| Oil hemp (cv. FINOLA) | Summer unscreened | OH-SU | 0.5 ± 0.3b | 0.2 ± 0.0b | ND | 1.3 ± 0.4c | 9.3 ± 2.1dA | 1.7 ± 0.2dB |
| Summer screening fines | OH-SSF | ND | ND | ND | ND | 39.0 ± 2.8bA | 0.7 ± 0.2dB | |
| Summer fibre fraction | OH-SFF | ND | ND | 8.4 ± 4.9cd | 0.8 ± 0.3c | 32.6 ± 3.9bcA | 0.3 ± 0.2dB | |
| Autumn unscreened | OH-AU | ND | ND | ND | 0.4 ± 0.2c | 4.1 ± 0.0d | ND | |
| Autumn screening fines | OH-ASF | ND | ND | ND | 0.3 ± 0.1c | 6.0 ± 0.7d | ND | |
| Autumn fibre fraction | OH-AFF | ND | ND | 0.4 ± 0.2d | 0.3 ± 0.1c | 5.5 ± 0.0d | ND | |
| Fibre hemp (var. Uso 31) | Autumn screening fines | FH-ASF | 11.8 ± 11.2ab | 5.7 ± 4.1a | 24.5 ± 1.7abcA | 7.3 ± 4.6cB | 100.0 ± 3.5aA | 21.8 ± 5.8aB |
| Autumn fibre fraction | FH-AFF | 19.1 ± 8.9a | 2.6 ± 1.3ab | 28.6 ± 11.6ab | 3.7 ± 1.0c | 100.0 ± 11.0aA | 15.2 ± 2.1abB | |
For the stress-responsive strain of E. coli (DPD2794), ethanol–water extracts showed activity in some of the fractions wherein hexane extracts did not, but in all cases where activity was detected in both hexane and ethanol extracts, the one extracted using hexane indicated higher luminescence induction. This phenomenon is likely due to the hexane extract's toxicity becoming too high to bear for the E. coli strain to bear and the light induction is suppressed.
Biorefinery processing
This study also investigated the potential use of plant biomass components as feedstock for biorefinery processing. A green, simple, and flexible biorefinery concept was suggested. As discussed earlier, the aim was to examine the extraction efficiency, quality, and chemical composition of the plant material. The screening fine fractions were chosen as they were considered less fibrous fractions and showed the most promising biomass sources in bioactivities.The samples extracted through PHWE were assessed for their TDS, extractives, carbohydrates, TPC, antioxidant activity (i.e., DPPH, CUPRAC, Fe(II) chelating ability, and ORAC), and antibacterial properties.
Total dissolved solids
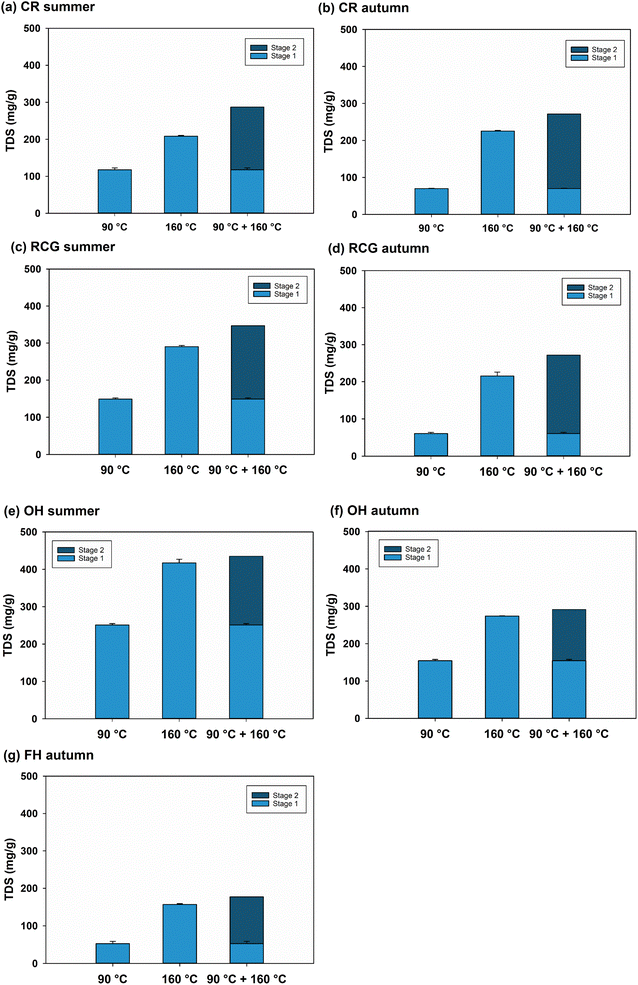
Fig. 2A–G shows the TDS yield obtained through PHWE. As expected, the TDS increased with the temperature, showing higher results from the two-step extraction than in the samples with a single step (90 °C and 160 °C alone). The second stage (160 °C/60 min) constituted more than 50% of the TDS in most samples, except for OH autumn (47% of the TDS). CR and RCG collected in late autumn, when the plants are typically harvested, represented the highest content of extractives in the second stage compared to summer samples (74% in CR autumn vs. 59% in CR summer and 78% in RCG autumn vs. 57% in RCG summer). In contrast, the first phase (90 °C/60 min) resulted in higher extractives (58%) in summer-collected OH. This sample yielded the highest amount of TDS across all tested temperatures and conditions than other biomasses.Carbohydrate composition
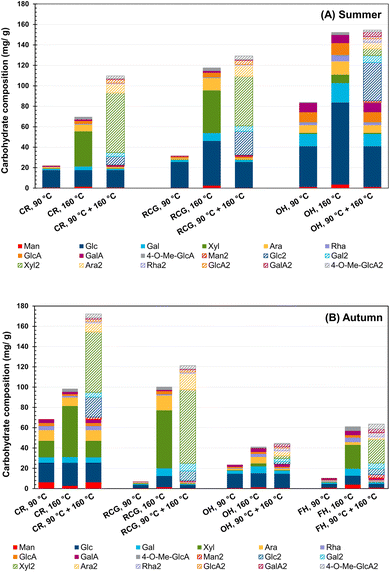
Fig. 3 shows that a higher extraction temperature typically increased the total amount of extracted hemicelluloses (i.e., sum of carbohydrates). The results demonstrate that the two-stage treatment through PHWE was a practical approach to recovering hemicelluloses from the majority of the studied materials. Notably, in the case of hemp extracts (OH and FH), similar efficiency between 160 °C and the two-stage method was observed, indicating that a temperature exceeding 90 °C results in more efficient extraction. Moreover, when comparing the first and second extraction stages, CR and RCG had higher percentages of sugar content extracted in the second stage in both seasons (80% in CR summer vs. 60% in CR autumn and 75% in RCG summer vs. 94% in RCG autumn). The increased temperature helped recover higher xylose content. Summer and autumn OH behave similarly, with some differences in the monosaccharide proportions. Instead, FH seems to benefit from the high-temperature extraction (in the sense of carbohydrate content), but no benefit is evident when comparing 160 °C and two-stage extraction. This observation aligns with the analogous trend found in TDS yield. Overall, over 60% of total carbohydrate content was obtained from the second stage (160 °C/60 min) in most of the extracts, except for OH hemp (approximately 47% in both summer and autumn samples).Comparatively, Väisänen et al. investigated the effect of steam treatment on the chemical composition of industrial hemp at different temperatures (i.e., 100 °C, 120 °C, and 160 °C). They found that the higher the temperature, the greater the extraction of hemicelluloses, while lower temperatures showed the prevalence of glucose and pectin.99 Leppänen et al. found that higher temperatures can positively contribute to obtaining a greater yield of high molar mass hemicelluloses without extensive degradation of the extracted polysaccharides.42 Another study confirmed the critical role of extraction temperature, time and flow rate on the hemicellulose yield.44
Since hemicelluloses consist of various sugar units, the composition and arrangement of these units can vary depending on the plant species and tissue type. Regarding the carbohydrate composition, in both harvest seasons, the CR and RCG extracts mainly presented xylose, glucose, arabinose, and galactose, whereas glucose, arabinose, and galactose were dominant in OH. Since OH had approximately 80% of the screening fines (Table 2), the higher amount of glucose found in these extracts can be attributed to the sample material containing leaves. As for OH, the non-polar compounds should be extracted before the pressurised hot water process, as the yield of autumn carbohydrates can be low. In this context, a study found that hemp leaves contain more glucose than xylose due to the composition of their cell walls.99 Likely, the higher glucose concentration in hemp leaves results from their higher glucan content than the straw's xylose-containing hemicellulose.
The data also demonstrated that the harvesting time could influence the extract composition. Specifically, when collected in late autumn (typical harvesting time), CR showed higher overall contents than those samples collected in summer, when samples were immature. On the other hand, RCG and OH had higher recovery in summer. A previous study identified glucose, xylose and arabinose as the primary carbohydrate components in RCG and confirmed that the soil type, growing locations, and weather conditions could influence the carbohydrate composition and lignin content. The data showed that, unlike sand-rich soil, high soil organic and clay content can result in lower glucose and xylose amounts but higher content of lignin.47
Two-stage extraction (90 °C + 160 °C) was suitable for obtaining hemicellulose-rich fractions. However, further upscaling of the extraction to the pilot scale should be considered as it can provide comprehensive information on the process performance and channelling of water, which could not be detected in laboratory-scale processes.44 The literature has also shown successful conversion of hemicelluloses into higher added value end-use. For instance, studies have recognised that wood hemicelluloses could efficiently stabilise emulsions against lipid oxidation in yoghurt, act as delivery systems for fatty acids, and enhance the bioavailability of bioactive compounds.26,100–102 The marginal lands have shown to be good sources of valuable compounds such as biorefinery feedstocks that can be valorised in diverse fields (e.g., chemical, pharmaceutical, and cosmetics industries).
Total phenolic content, protein content, antioxidant activity, and antibacterial properties
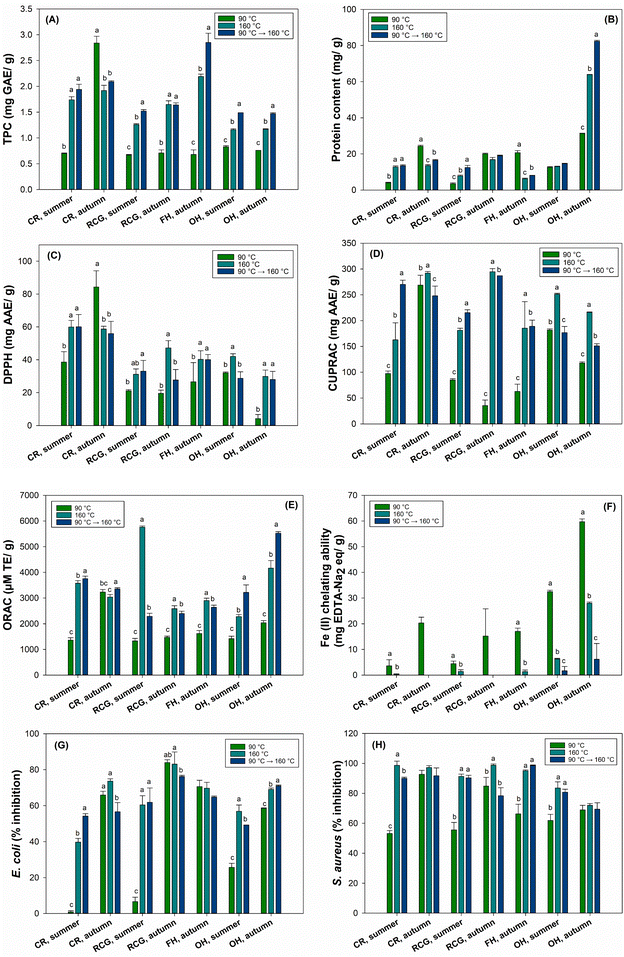
Fig. 4 shows the TPC, protein content, and antioxidant and antibacterial properties of PHW extracts. Overall, the TPC increased for all the samples with increasing extraction temperature and indicated higher values when the two-stage extraction was employed, except for CR autumn (Fig. 4A). The CR and FH collected in autumn had a higher TPC than the other extracts. In general, the two-stage extraction (90 °C + 160 °C) revealed similar or higher values compared to 90 °C or 160 °C separately.Moreover, the protein content of OH autumn (82.4 mg g−1) extracted through a two-stage approach was considerably elevated compared to all other biomasses, even when extracted at 90 °C (Fig. 4B). Väisänen et al. reported a higher protein content (247 mg g−1) in hemp leaves than other hemp fractions, such as stalk and decorticated hemp with hurd.99 These findings corroborate our results as OH screening fines were composed partially of leaves that contain more proteins.
In the present study, we employed a standardised extraction time of 60 min in the PWHE and three cycles of 5 min each stage in the ASE extraction with hexane and ethanol/water. The aim here was to find the best temperature condition and the most effective extracts in terms of AOX. Among the tested plant extracts, CR was the most effective in reducing the DPPH radical (Fig. 4C), showing similar results between summer and autumn. Additionally, the data demonstrated that AOX markedly increased with elevated extraction temperatures (either 160 °C or two-stage extraction) than at 90 °C, possibly due to the extraction of phenol and polyphenols. Since the DPPH assay is suitable for polar to medium polar compounds, the extract's AOX might depend on the plant composition and polarity. A previous study on birch bark supports these findings as it found that the highest AOX determined by a DPPH assay was also found in the water extract of finely ground bark and increased with elevated extraction temperatures (90–180 °C).103 Hosny et al. investigated the efficiency of common reed aqueous extracts to fabricate gold nanoparticles (AuNPs), suggesting an alternative solution to handle the accumulated undesirable biomass of aquatic macrophytes in aquatic ecosystems.104 They found an AOX higher than 10% (DPPH assay) and cytotoxic effects by inhibiting the growth and proliferation of human lung cancer cells (A549 cell line). Therefore, besides serving as an eco-friendly and sustainable approach, the extract also shows promising applicability for synthesising AuNPs that could be used in different biomedical applications.
For the CUPRAC and ORAC methods, the AOX significantly increased with higher temperatures, depicting a similar trend as the DPPH (Fig. 4D and E). The RCG and CR autumn reached a higher AOX by CUPRAC within the investigated extracts, while the two-stage extraction showed higher AOX by ORAC in OH and CR extracts, respectively. Thus, even though the two-stage process was performed to obtain a hemicellulose-rich fraction with few extractives, this procedure was beneficial for the AOX, which is generally associated with the phenolic composition.
Furthermore, unlike other tested methods, the first extraction temperature (90 °C) indicated higher AOX in all the extracts, especially OH autumn, when tested by the Fe(II) chelating ability (Fig. 4F). The overall low outcome may suggest that the hemicellulose-rich extracts have less capacity to chelate ferrous ions. Indeed, a previous study evaluated the AOX of hemicelluloses from Norway spruce galactoglucomannan and found no activity using a similar metal chelating assay, Cu2+ chelating ability.105 Such findings support our outcomes.
The temperature also influenced the antibacterial activity in the constitutively luminescent light-producing strains of E. coli (K12 + pcGLS11) and S. aureus (RN4220 + pAT19). When employing a higher extraction temperature, the plant extracts showed more than 50% inhibition (e.g., RCG at 160 °C) against E. coli, while inhibition of S. aureus (e.g., RCG at 160 °C and FH two-stage extraction) was higher than 70% for some of the extracts (Fig. 4G and H). Overall, the results for PHWE extracts were relatively high, especially autumn fractions yielded higher results against E. coli, while the differences between autumn and summer fractions were not as significant against S. aureus. Based on the results of this study, extracts could also harbour the potential for different antibacterial applications in various fields.
Next, the Pearson correlation analysis showed that, for the summer samples, TPC significantly correlated with DPPH (r = 0.740; p = 0.023), CUPRAC (r = 0.872; p = 0.002), ORAC (r = 0.734; p = 0.024), E. coli (r = 0.831; p = 0.006), and S. aureus (r = 0.948; p < 0.001). For the autumn outcomes, the TPC positively correlated with DPPH (r = 0.724; p = 0.008), CUPRAC (r = 0.651; p = 0.022), and S. aureus (r = 0.696; p = 0.012). When it comes to carbohydrates, we found that (ESI Table 2) that xylose (summer) correlated significantly (p ≤ 0.05) with TPC (r = 0.889, p < 0.01), DPPH (r = 0.662, p < 0.01), CUPRAC (r = 0.846, p < 0.01), E. coli (r = 0.794, p < 0.01), and S. aureus (p = 0.908, p < 0.01), but had a slightly negative correlation with the Fe(II) chelating ability (r = −0.529, p < 0.05). Similar behaviour was also found in arabinose (summer), which significantly correlated with TPC (r = 0.658, p < 0.01), CUPRAC (r = 0.620, p < 0.01), E. coli (r = 0.726, p < 0.01), and S. aureus (r = 0.756, p < 0.01). The sugar-acid 4-O-Me-GlcA (summer) revealed a positive correlation with TPC (r = 0.871, p < 0.01), CUPRAC (r = 0.807, p < 0.01), E. coli (r = 0.872, p < 0.01), and S. aureus (p = 0.922, p < 0.01). Finally, GalA (summer) had a positive correlation with the Fe(II) chelating ability (r = 0.671, p < 0.01), indicating that the chelating effect may also reveal the antioxidant potential of polysaccharides, as previous studies have reported. In most in vitro antioxidant systems, polysaccharides can effectively act as free radical scavengers, reducing agents, and ferrous chelators. Here, synergistic effects might occur when other antioxidants are possibly conjugated or mixed with polysaccharides, such as proteins, peptides, and polyphenols. However, different chemical characteristics influence the antioxidant potential of polysaccharides, including the molecular weight, glycosidic branching, compositions of monosaccharides, and intermolecular associations of polysaccharides.106,107
Comparing solvent extraction by ASE (using hexane and EtOH/H2O) with PHWE, we found that PHW extracts, especially those obtained at 160 °C or two-stage extraction using two temperatures exhibited higher bioactivity tested by CUPRAC, ORAC, and antibacterial properties. However, hexane had higher TPC, DPPH, and Fe(II) chelating abilities (except for OH), while EtOH/H2O had the highest protein content. As water is heated at high temperatures (from 100 to 374 °C) and pressure in the PHWE technique, it behaves as an organic solvent and consequently becomes less polar with a dielectric constant corresponding to that of organic solvents.108,109 Due to the increased solute desorption in matrix sites, higher-temperature extractions typically result in improved extraction efficiency (e.g., faster mass transfer rates and superior extraction yields). Since the solvent's polarity directly affects the solubility of the phenolic compounds, the decrease in the polarity and weakening of hydrogen bonds can also contribute to the dissolution of semi-polar compounds.110,111 Water is a source of hydronium (H3O+) at higher temperatures, allowing the hydrolysis of polysaccharides and proteins into smaller molecules, such as oligosaccharides, monosaccharides, peptides and amino acids.112
The current study's findings highlight the influence of the treatment parameters (e.g., solvent, time and temperature) and harvesting time on the extraction of bioactive compounds and bioactivities. Developing multi-step fractionation processes represents a promising approach for effectively valorising side streams for further applications. Thus, it is crucial to carefully optimise the PHWE process to take full advantage of the enhanced solubility and improved mass transfer while minimising the degradation effects.
Conclusions
This study demonstrates the efficacy of ASE and PHWE in extracting antioxidative and antibacterial compounds from reed canary grass, common reed, oil and fibre hemp. These extraction methods ensure sustainable recovery and separation of valuable compounds in biorefinery processes. In summary, oil hemp and reed canary grass were the most promising biomasses when exploring the lipophilic and hydrophilic fractions. In the PHWE process, oil hemp and reed canary grass showed overall potential regarding chemical composition; common reed and reed canary grass had higher antioxidant activity, while all the biomasses showed promising antibacterial properties. Furthermore, implementing a two-stage PHWE enables the isolation of extractives followed by the sequential extraction of hemicelluloses, yielding over 400 mg g−1 of summer oil hemp and approximately 300 mg g−1 of reed canary grass and common reed. In contrast, fibre hemp yielded under 200 mg g−1. Summer-harvested plants had carbohydrate yields of 110–155 mg g−1, while autumn yields were 40–60 mg g−1 for hemp and 120–170 mg g−1 for reed canary grass and common reed, respectively. This approach not only offers an alternative to utilising biomass from marginal lands but also provides comprehensive information on the chemical composition and bioactivity, addressing the existing gap in characterising these plant fractions. According to the results, the studied plants are attractive feedstocks of extractives, antioxidant and antibacterial compounds, serving as a source for further applications. For example, antioxidants may have applications in the food industry, cosmetics, or pharmaceuticals, while antibacterial compounds may be relevant for healthcare, agriculture, or personal care products.From the biorefining potential viewpoint, the harvest time could affect the extractable substances. Hence, the feasibility of utilising the identified plants as feedstocks for commercial production should consider factors such as availability, cultivation requirements, overall biomass yield and long-term yield stability, scalability, and sustainability. The outcomes show the potential for future two-stage extraction optimisation for hemicellulose or polyphenol extraction, providing a basis for future studies concentrated on isolating specific components for further exploration.
Author contributions
Fidelis, Marina: investigation, writing – original draft, writing – original draft, writing – review & editing, methodology, formal analysis, data curation. Tienaho, Jenni: investigation, methodology, formal analysis, writing – review & editing. Brännström, Hanna: conceptualisation, reviewing the original draft, supervision, project administration, funding acquisition. Korpinen, Risto: investigation, methodology, formal analysis, writing – review & editing. Pihlava, Juha-Matti: investigation, methodology, formal analysis, writing – review & editing. Jylhä, Paula: investigation, methodology, writing – review & editing, project administration, funding acquisition. Liimatainen, Jaana: investigation, methodology, writing – review & editing. Hellström, Jarkko: investigation, methodology, formal analysis, writing – review & editing. Möttönen, Veikko: methodology, statistical analysis, writing – review & editing. Maunuksela, Jyri: investigation, writing – review & editing. Kilpeläinen, Petri: conceptualisation, supervision, methodology, formal analysis, writing – review and editing.Conflicts of interest
The authors have no competing interests to declare.Acknowledgements
The authors thank Natural Resources Institute Finland (Luke) for funding this work through the thematic project BioMargin (41007-00214200). They also thank Ulla Jauhiainen, Riitta Henriksson, Satu Örling, Piia Grandell, Pauli Karppinen, and Kalle Kaipanen for their skilful technical assistance.Notes and references
- H. Ahmadzai, S. Tutundjian and I. Elouafi, Sustainability, 2021, 13, 8692 CrossRef.
- P. Schröder, B. Beckers, S. Daniels, F. Gnädinger, E. Maestri, N. Marmiroli, M. Mench, R. Millan, M. M. Obermeier, N. Oustriere, T. Persson, C. Poschenrieder, F. Rineau, B. Rutkowska, T. Schmid, W. Szulc, N. Witters and A. Sæbø, Sci. Total Environ., 2018, 616–617, 1101–1123 CrossRef PubMed.
- M. Isoniemi, Potentiaalisten metsityskohteiden kartoitus suonpohjilla ja peltoheitoilla. [Mapping of afforestation potential in cutaway peatlands and abandoned agricultural land], Suomen metsäkeskus, 2020, media release 15.10.2020, in Finnish Search PubMed.
- J. Patronen, Selvitys Turpeen Energiakäytön Kehityksestä Suomessa – Raportti Työ-Ja Elinkeinoministeriölle, AFRY Management Consulting, 2020 Search PubMed.
- H. Salo, Turvetuotannosta Poistuneet Suonpohjat Ovat Jo Hiilinieluja – Metsitys Tärkein Jälkikäyttömuoto [Cutaway Peatlands Released from Peat Production Are Already Carbon Sinks – Afforestation Is the Mot Important Form of After-Use], Bioenergia ry, 2019, media release 8.3.2019, in Finnish Search PubMed.
- Finnish Government, Inclusive and Competent Finland – a Socially, Economically and Ecologically Sustainable Society, Programme of Prime Minister Sanna Marin's Government, 2019 Search PubMed.
- H. Joosten and D. Clarke, Wise Use of Mires and Peatlands: Background and Principles Including a Framework for Decision-Making, International Peat Society, 2002 Search PubMed.
- H. Kekkonen, H. Ojanen, M. Haakana, A. Latukka and K. Regina, Carbon Manage., 2019, 10, 115–126 CrossRef CAS.
- H. Lehtonen, S. Saarnio, S. Luostarinen, L. Maanavilja, J. Heikkinen, K. Soini, J. Aakkula, M. Jallinoja, S. Rasi and J. Niemi, Maatalouden Ilmastotiekartta – Tiekartta Kasvihuonekaasupäästöjen Vähentämiseen Suomen Maataloudessa. [Agriculture Climate Roadmap – Roadmap for Reducing Greenhouse Gas Emissions in Finnish Agriculture], MTK ry, 2020, in Finnish Search PubMed.
- Statistics Finland, Suomen Kasvihuonekaasupäästöt 1990–2019. [Finland's Greenhouse Gas Emissions 1990–2019], 2020 Search PubMed.
- M. Von Cossel, I. Lewandowski, B. Elbersen, I. Staritsky, M. Van Eupen, Y. Iqbal, S. Mantel, D. Scordia, G. Testa, S. L. Cosentino, O. Maliarenko, I. Eleftheriadis, F. Zanetti, A. Monti, D. Lazdina, S. Neimane, I. Lamy, L. Ciadamidaro, M. Sanz, J. Esteban Carrasco, P. Ciria, I. McCallum, L. M. Trindade, E. N. Van Loo, W. Elbersen, A. L. Fernando, E. G. Papazoglou and E. Alexopoulou, Energies, 2019, 12, 3123 CrossRef CAS.
- N. J. Shurpali, H. Strandman, A. Kilpeläinen, J. Huttunen, N. Hyvönen, C. Biasi, S. Kellomäki and P. J. Martikainen, GCB Bioenergy, 2010, 2, 130–138 CAS.
- I. Adesina, A. Bhowmik, H. Sharma and A. Shahbazi, Agriculture, 2020, 10, 129 CrossRef CAS.
- J. Haberzettl, P. Hilgert and M. Von Cossel, Agronomy, 2021, 11, 2397 CrossRef CAS.
- E. Alexopoulou, D1.3: List with the Selected Most Promising Industrial Crops for Marginal Lands, Center for Renewable Energy Sources and Saving, 2018 Search PubMed.
- S. Ferdini, M. Von Cossel, V. Wulfmeyer and K. Warrach-Sagi, GCB Bioenergy, 2023, 15, 424–443 CrossRef.
- J. Järveoja, M. Peichl, M. Maddison, A. Teemusk and Ü. Mander, GCB Bioenergy, 2016, 8, 952–968 CrossRef.
- L. Lahtinen, T. Mattila, T. Myllyviita, J. Seppälä and H. Vasander, Ecol. Eng., 2022, 175, 106502 CrossRef.
- H. Brix, S. Ye, E. A. Laws, D. Sun, G. Li, X. Ding, H. Yuan, G. Zhao, J. Wang and S. Pei, Ecol. Eng., 2014, 73, 760–769 CrossRef.
- J. Antonkiewicz, B. Kołodziej, E. J. Bielińska and A. Popławska, Soil Sci. Annu., 2019, 70, 21–33 CAS.
- B. Kołodziej, J. Antonkiewicz, E. J. Bielińska, R. Witkowicz and B. Dubis, Int. J. Phytorem., 2023, 25, 441–454 CrossRef PubMed.
- M. Rehman, S. Fahad, G. Du, X. Cheng, Y. Yang, K. Tang, L. Liu, F.-H. Liu and G. Deng, Environ. Sci. Pollut. Res., 2021, 28, 52832–52843 CrossRef PubMed.
- M. Saastamoinen, M. Eurola and V. Hietaniemi, J. Food Chem. Nanotechnol., 2016, 2(1), 73–76 Search PubMed.
- B. Kumar and P. Verma, Fuel, 2021, 288, 119622 CrossRef CAS.
- P. Walsh and E. de Jong, Bio-Based Chemicals: Value Added Products From Biorefineries, IEA Bioenergy, 2012 Search PubMed.
- F. Valoppi, N. Maina, M. Allen, R. Miglioli, P. O. Kilpeläinen and K. S. Mikkonen, Eur. Food Res. Technol., 2019, 245, 1387–1398 CrossRef CAS.
- J. Chen, Z. Zhu, J. Cai, M. Cao, F. Sha, S. Tao, H. Yang, H. Gan, H. Wu, F. Cao, L. Zhao and P. Ouyang, Green Chem., 2022, 24, 2000–2009 RSC.
- J. Voogt, N.-P. Humblet-Hua, P. Geerdink, B. Beelen, W. Mulder and C. Safi, Food Bioprod. Process., 2023, 139, 1–10 CrossRef CAS.
- S. P.-M. Ung and C.-J. Li, RSC Sustainability, 2023, 1, 11–37 RSC.
- J. Tienaho, D. Reshamwala, T. Sarjala, P. Kilpeläinen, J. Liimatainen, J. Dou, A. Viherä-Aarnio, R. Linnakoski, V. Marjomäki and T. Jyske, Front. Bioeng. Biotechnol., 2021, 9, 797939 CrossRef.
- M. Fidelis, M. A. V. do Carmo, T. M. da Cruz, L. Azevedo, T. Myoda, M. Miranda Furtado, M. Boscacci Marques, A. S. Sant'Ana, M. Inês Genovese, W. Young Oh, M. Wen, F. Shahidi, L. Zhang, M. Franchin, S. M. de Alencar, P. Luiz Rosalen and D. Granato, Food Chem., 2020, 310, 125909 CrossRef CAS PubMed.
- D. Del Rio, A. Rodriguez-Mateos, J. P. E. Spencer, M. Tognolini, G. Borges and A. Crozier, Antioxid. Redox Signaling, 2013, 18, 1818–1892 CrossRef CAS PubMed.
- C. Cantele, M. Bertolino, F. Bakro, M. Giordano, M. Jędryczka and V. Cardenia, Antioxidants, 2020, 9, 1131 CrossRef CAS PubMed.
- O. Werz, J. Seegers, A. M. Schaible, C. Weinigel, D. Barz, A. Koeberle, G. Allegrone, F. Pollastro, L. Zampieri, G. Grassi and G. Appendino, PharmaNutrition, 2014, 2, 53–60 CrossRef CAS.
- P. Rossi, A. Cappelli, O. Marinelli, M. Valzano, L. Pavoni, G. Bonacucina, R. Petrelli, P. Pompei, E. Mazzara, I. Ricci, F. Maggi and M. Nabissi, Molecules, 2020, 25, 3451 CrossRef CAS PubMed.
- H.-J. Huang, S. Ramaswamy, U. W. Tschirner and B. V. Ramarao, Sep. Purif. Technol., 2008, 62, 1–21 CrossRef CAS.
- M. Arshadi, T. M. Attard, R. M. Lukasik, M. Brncic, A. M. da Costa Lopes, M. Finell, P. Geladi, L. N. Gerschenson, F. Gogus, M. Herrero, A. J. Hunt, E. Ibáñez, B. Kamm, I. Mateos-Aparicio, A. Matias, N. E. Mavroudis, E. Montoneri, A. R. C. Morais, C. Nilsson, E. H. Papaioannou, A. Richel, P. Rupérez, B. Škrbić, M. Bodroža Solarov, J. Švarc-Gajić, K. W. Waldron and F. J. Yuste-Córdoba, Green Chem., 2016, 18, 6160–6204 RSC.
- G. Reyes, C. M. Pacheco, E. Isaza-Ferro, A. González, E. Pasquier, S. Alejandro-Martín, L. E. Arteaga-Peréz, R. R. Carrillo, I. Carrillo-Varela, R. T. Mendonça, C. Flanigan and O. J. Rojas, Green Chem., 2022, 24, 3794–3804 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- F. M. Kerton and R. Marriot, Alternative Solvents for Green Chemistry, RSC Publishing, Cambridge, UK, 2nd edn, 2013 Search PubMed.
- P. O. Kilpeläinen, S. S. Hautala, O. O. Byman, L. J. Tanner, R. I. Korpinen, M. K.-J. Lillandt, A. V. Pranovich, V. H. Kitunen, S. M. Willför and H. S. Ilvesniemi, Green Chem., 2014, 16, 3186–3194 RSC.
- K. Leppänen, P. Spetz, A. Pranovich, K. Hartonen, V. Kitunen and H. Ilvesniemi, Wood Sci. Technol., 2011, 45, 223–236 CrossRef.
- A. Sluiter, B. Hames, C. Scarlata, J. Sluiter, D. Templeton and D. L. A. P. Crocker, Determination of structural carbohydrates and lignin in biomass, Laboratory analytical procedure, NREL, 2008, vol. 1617, pp. 1–16 Search PubMed.
- P. O. Kilpelainen, S. S. Hautala, O. O. Byman, L. J. Tanner, R. I. Korpinen, M. K.-J. Lillandt, A. V. Pranovich, V. H. Kitunen, S. M. Willfor and H. S. Ilvesniemi, Green Chem., 2014, 16, 3186–3194 RSC.
- M. Ravber, Ž. Knez and M. Škerget, Cellulose, 2015, 22, 3359–3375 CrossRef CAS.
- X. Liu, Q. Lin, Y. Yan, F. Peng, R. Sun and J. Ren, Curr. Med. Chem., 2019, 26, 2430–2455 CrossRef CAS PubMed.
- M. Finell, M. Arshadi and R. Gref, Biomass Bioenergy, 2011, 35, 1097–1102 CrossRef CAS.
- S. Hellqvist, M. Finell and S. Landström, Agric. Food Sci., 2003, 12, 49–56 CrossRef.
- K. Saijonkari-Pahkala, Dissertation, University of Helsinki, 2001 Search PubMed.
- Handbook of Pulp, ed. H. Sixta, Wiley, Weinheim, Germany, 1st edn, 2006 Search PubMed.
- R. Lemola, R. Uusitalo, J. Hyväluoma, S. Minna and E. Turtola, Luonnonvarakeskus, 2018, 211 Search PubMed.
- L. Paavilainen, J. Tulppala, A. Varhimo, M. Rannua and J. Pere, Production and Use of Agrofibre in Finland. Final Report, Part IV. Reed Canary Grass Sulphate Pulp as Raw Material for Fine Paper, Maatalouden Tutkimuskeskus, Jokioinen, 1997 Search PubMed.
- R. Salo, Production and Use of Agrofibre in Finland Final Report of Biomass as Raw Material for Fibre and Energy. Final Report, Part I. Breeding and Cultivattion of Reed Canary Grass, Maatalouden tutkimuskeskus, Jokioinen, 2000 Search PubMed.
- M. Hemming, T. Maunu, A. Suokannas, M. Järvenpää and A. Pehkonen, Production and Use of Agrofibre in Finland. Final Report, Part II. Harvesting, Storage, Mechanical Pretreatment, Production Costs and Availability of Reed Canary Grass, Maatalouden tutkimuskeskus, Jokioinen, 1996 Search PubMed.
- V. T. Aaltonen, B. Aarnio, E. Hyyppä, P. Kaitera, L. Keso, E. Kivinen, P. Kokkonen, M. J. Kotilainen, M. Sauramo, P. Tuorila and J. Vuorinen, Agric. Food Sci., 1949, 21, 37–66 CrossRef.
- ISO, Soil Quality – Determination of pH, 2005, ISO 10390:2005 Search PubMed.
- A. L. Vuorinen, N. Markkinen, M. Kalpio, K. M. Linderborg, B. Yang and H. P. Kallio, Food Res. Int., 2015, 77, 608–619 CrossRef CAS.
- A. Sundheq, K. Sundherg, C. Lillandt and B. Holmhom, Nord. Pulp Pap. Res. J., 1996, 11, 216–219 CrossRef.
- J.-M. Pihlava, E. Nordlund, R.-L. Heiniö, V. Hietaniemi, P. Lehtinen and K. Poutanen, J. Food Compos. Anal., 2015, 38, 89–97 CrossRef CAS.
- P. Korkalo, R. Korpinen, E. Beuker, T. Sarjala, J. Hellström, J. Kaseva, U. Lassi and T. Jyske, Molecules, 2020, 25, 4403 CrossRef CAS PubMed.
- M. L. Price and L. G. Butler, J. Agric. Food Chem., 1977, 25, 1268–1273 CrossRef CAS.
- T. Margraf, A. R. Karnopp, N. D. Rosso and D. Granato, J. Food Sci., 2015, 80, C2397–C2403 CrossRef CAS PubMed.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, LWT–Food Sci. Technol., 1995, 28, 25–30 CrossRef CAS.
- R. Apak, K. Güçlü, M. Özyürek and S. E. Çelik, Microchim. Acta, 2008, 160, 413–419 CrossRef CAS.
- J. S. Santos, V. R. Alvarenga Brizola and D. Granato, Food Chem., 2017, 214, 515–522 CrossRef CAS PubMed.
- D. Huang, B. Ou, M. Hampsch-Woodill, J. A. Flanagan and R. L. Prior, J. Agric. Food Chem., 2002, 50, 4437–4444 CrossRef CAS PubMed.
- R. L. Prior, H. Hoang, L. Gu, X. Wu, M. Bacchiocca, L. Howard, M. Hampsch-Woodill, D. Huang, B. Ou and R. Jacob, J. Agric. Food Chem., 2003, 51, 3273–3279 CrossRef CAS PubMed.
- J. Tienaho, M. Karonen, R. Muilu-Mäkelä, J. Kaseva, N. de Pedro, F. Vicente, O. Genilloud, U. Aapola, H. Uusitalo, K. Vuolteenaho, R. Franzén, K. Wähälä, M. Karp, V. Santala and T. Sarjala, Planta Med., 2020, 86, 1009–1024 CrossRef CAS PubMed.
- S. Vesterlund, J. Paltta, A. Lauková, M. Karp and A. C. Ouwehand, J. Microbiol. Methods, 2004, 57, 23–31 CrossRef CAS PubMed.
- A. C. Vollmer, S. Belkin, D. R. Smulski, T. K. Van Dyk and R. A. LaRossa, Appl. Environ. Microbiol., 1997, 63, 2566–2571 CrossRef CAS PubMed.
- J. Tienaho, T. Sarjala, R. Franzén and M. Karp, J. Microbiol. Methods, 2015, 118, 78–80 CrossRef CAS PubMed.
- H.-H. Chiu and C.-H. Kuo, J. Food Drug Anal., 2020, 28, 60–73 CrossRef CAS PubMed.
- P. H. Mattila, J.-M. Pihlava, J. Hellström, M. Nurmi, M. Eurola, S. Mäkinen, T. Jalava and A. Pihlanto, Food Qual. Saf., 2018, 2, 213–219 CAS.
- Y. Gao, M. Zhang, T. Wu, M. Xu, H. Cai and Z. Zhang, J. Agric. Food Chem., 2015, 63, 6019–6026 CrossRef CAS PubMed.
- S. Maniganda, V. Sankar, J. B. Nair, K. G. Raghu and K. K. Maiti, Org. Biomol. Chem., 2014, 12, 6564–6569 RSC.
- W. Chanasattru, E. A. Decker and D. J. McClements, Food Hydrocolloids, 2009, 23, 253–261 CrossRef CAS.
- D. R. Chhetri, Front. Pharmacol., 2019, 10, 1172 CrossRef CAS PubMed.
- R. Pavlovic, S. Panseri, L. Giupponi, V. Leoni, C. Citti, C. Cattaneo, M. Cavaletto and A. Giorgi, Front. Plant Sci., 2019, 10, 1265 CrossRef PubMed.
- J. Burvall, Biomass Bioenergy, 1997, 12, 149–154 CrossRef CAS.
- T. Prade, M. Finell, S.-E. Svensson and J. E. Mattsson, Fuel, 2012, 102, 592–604 CrossRef CAS.
- N. Norokytö, Öljyhamppu – Opas Viljelyyn Ja Käsittelyyn. (Oil Hemp – Guide for Cultivation and Handling), Turun ammattikorkeakoulun puheenvuoroja, 2013 Search PubMed.
- A. André, M. Leupin, M. Kneubühl, V. Pedan and I. Chetschik, Plants, 2020, 9, 1740 CrossRef PubMed.
- Z. Drinić, S. Vidović, J. Vladić, A. Koren, B. Kiprovski and V. Sikora, Lek. Sirovine, 2018, 17–21 CrossRef.
- V. Kitrytė, D. Bagdonaitė and P. Rimantas Venskutonis, Food Chem., 2018, 267, 420–429 CrossRef PubMed.
- V. Gunjević, G. Grillo, D. Carnaroglio, A. Binello, A. Barge and G. Cravotto, Ind. Crops Prod., 2021, 162, 113247 CrossRef.
- E. Mazzara, R. Carletti, R. Petrelli, A. M. Mustafa, G. Caprioli, D. Fiorini, S. Scortichini, S. Dall'Acqua, S. Sut, S. Nuñez, V. López, V. D. Zheljazkov, G. Bonacucina, F. Maggi and M. Cespi, J. Sci. Food Agric., 2022, 11971 Search PubMed.
- M. S. Padda and D. H. Picha, J. Food Sci., 2007, 72, C412–C416 CrossRef CAS PubMed.
- J. P. Martina and C. N. von Ende, Environ. Exp. Bot., 2012, 82, 43–53 CrossRef.
- L. G. Hendricks, H. E. Mossop and C. E. Kicklighter, J. Chem. Ecol., 2011, 37, 838–845 CrossRef CAS PubMed.
- M. D. Kleinhenz, G. Magnin, S. M. Ensley, J. J. Griffin, J. Goeser, E. Lynch and J. F. Coetzee, Appl. Anim. Sci., 2020, 36, 489–494 CrossRef.
- M. Farooq, A. Wahid, N. Kobayashi, D. Fujita and S. M. A. Basra, Agron. Sustainable Dev., 2009, 29, 185–212 CrossRef.
- A. Smeriglio, E. M. Galati, M. T. Monforte, F. Lanuzza, V. D'Angelo and C. Circosta, Phytother. Res., 2016, 30, 1298–1307 CrossRef CAS PubMed.
- S. Palmieri, M. Pellegrini, A. Ricci, D. Compagnone and C. Lo Sterzo, Foods, 2020, 9, 1221 CrossRef CAS PubMed.
- N. Pap, D. Reshamwala, R. Korpinen, P. Kilpeläinen, M. Fidelis, M. M. Furtado, A. S. Sant'Ana, M. Wen, L. Zhang, J. Hellström, P. Marnilla, P. Mattila, T. Sarjala, B. Yang, A. dos S. Lima, L. Azevedo, V. Marjomäki and D. Granato, Food Chem. Toxicol., 2021, 153, 112284 CrossRef CAS PubMed.
- C. Moscariello, S. Matassa, G. Esposito and S. Papirio, Resour., Conserv. Recycl., 2021, 175, 105864 CrossRef CAS.
- R. Sudan, M. Bhagat, S. Gupta, J. Singh and A. Koul, BioMed Res. Int., 2014, 2014, 179865 Search PubMed.
- O. M. El-Borady, M. Fawzy and M. Hosny, Appl. Nanosci., 2021, 13, 3149–3160 CrossRef.
- P. C. Setford, D. W. Jeffery, P. R. Grbin and R. A. Muhlack, Trends Food Sci. Technol., 2017, 69, 106–117 CrossRef CAS.
- T. Väisänen, P. Kilpeläinen, V. Kitunen, R. Lappalainen and L. Tomppo, Ind. Crops Prod., 2019, 131, 224–233 CrossRef.
- M. Lehtonen, S. Teräslahti, C. Xu, M. P. Yadav, A.-M. Lampi and K. S. Mikkonen, Food Hydrocolloids, 2016, 58, 255–266 CrossRef CAS.
- K. S. Mikkonen, D. Merger, P. Kilpeläinen, L. Murtomäki, U. S. Schmidt and M. Wilhelm, Soft Matter, 2016, 12, 8690–8700 RSC.
- H. Zhao, K. S. Mikkonen, P. O. Kilpeläinen and M. I. Lehtonen, Foods, 2020, 9, 672 CrossRef CAS PubMed.
- M. Co, P. Koskela, P. Eklund-Åkergren, K. Srinivas, J. W. King, P. J. R. Sjöberg and C. Turner, Green Chem., 2009, 11, 668 RSC.
- M. Hosny, M. Fawzy, O. M. El-Borady and A. E. D. Mahmoud, Adv. Powder Technol., 2021, 32, 2268–2279 CrossRef CAS.
- D. Granato, D. Reshamwala, R. Korpinen, L. Azevedo, M. A. Vieira do Carmo, T. M. Cruz, M. B. Marques, M. Wen, L. Zhang, V. Marjomäki and P. Kilpeläinen, Food Chem., 2022, 381, 132284 CrossRef CAS PubMed.
- T. C.-T. Lo, C. A. Chang, K.-H. Chiu, P.-K. Tsay and J.-F. Jen, Carbohydr. Polym., 2011, 86, 320–327 CrossRef CAS.
- J. Wang, S. Hu, S. Nie, Q. Yu and M. Xie, Oxid. Med. Cell. Longevity, 2016, 2016, 1–13 Search PubMed.
- R. M. Smith, J. Chromatogr. A, 2002, 975, 31–46 CrossRef CAS PubMed.
- P. G. Matshediso, E. Cukrowska and L. Chimuka, Food Chem., 2015, 172, 423–427 CrossRef CAS PubMed.
- M. Co, C. Zettersten, L. Nyholm, P. J. R. Sjöberg and C. Turner, Anal. Chim. Acta, 2012, 716, 40–48 CrossRef CAS PubMed.
- M. Fidelis, J. S. Santos, G. B. Escher, M. Vieira do Carmo, L. Azevedo, M. Cristina da Silva, P. Putnik and D. Granato, Food Chem. Toxicol., 2018, 120, 479–490 CrossRef CAS PubMed.
- M. Plaza and C. Turner, TrAC, Trends Anal. Chem., 2015, 71, 39–54 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00255a |
| This journal is © The Royal Society of Chemistry 2023 |