Open Access Article
Open Access ArticleBio-based polycarbonates: progress and prospects
Hao
Wang†
ab,
Fei
Xu†
 ac,
Zhencai
Zhang
ac,
Zhencai
Zhang
 de,
Mi
Feng
de,
Mi
Feng
 a,
Ming
Jiang
a and
Suojiang
Zhang
a,
Ming
Jiang
a and
Suojiang
Zhang
 *ac
*ac
aBeijing Key Laboratory of Ionic Liquids Clean Process, CAS Key Laboratory of Green Process and Engineering, State Key Laboratory of Multiphase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P.R. China. E-mail: sjzhang@ipe.ac.cn
bSchool of Future Technology, University of Chinese Academy of Science, Beijing 100049, P.R. China
cLongzihu New Energy Laboratory, Zhengzhou Institute of Emerging Industrial Technology, Henan University, Zhengzhou, Henan 450000, P. R. China
dHuizhou Institute of Green Energy and Advanced Materials, Huizhou, Guangdong 516081, P. R. China
eSchool of Nano Science and Technology, University of Chinese Academy of Science, Beijing 100049, P. R. China
First published on 14th November 2023
Abstract
Bio-based polycarbonates represent a class of superior-performance polymers prepared from sustainable resources with excellent mechanical properties, thermal stability, and optical transparency, which offer a promising alternative to conventional petroleum-based polycarbonates. This paper provides a comprehensive review of bio-based polycarbonates, covering topics including monomer sources, synthesis methods, structure–property relationships, application areas, and emerging development trends. Two typical types of bio-based polycarbonates represented by poly(isosorbide carbonate)s and poly(limonene carbonate)s are highlighted. Moreover, the effects of catalyst types, reaction conditions, and modification methods on their molecular weights and properties are discussed. Finally, the review addresses the challenges faced by bio-based polycarbonates and provides insights into their prospective directions.
Sustainability spotlightPolymers have become an integral part of modern life due to their versatile range of adjustable properties. Currently, synthetic polymers are mainly made from fossil resources, prompting a quest for more sustainable raw materials to achieve carbon neutrality. Polycarbonate (PC) is one of the most widely used thermoplastic engineering plastics. The global demand for PCs exceeds 4.4 million tons per year and continues to grow. The development of bio-based PCs could fulfill the requirements of environmentally sustainable and performance-advantaged plastics, with a lower carbon footprint and higher bio-safety. The review of bio-based PCs emphasizes the importance of the UN sustainable development goals including: industry, innovation, and infrastructure (SDG9), responsible consumption and production (SDG12), and climate action (SDG14). |
1. Introduction
The utilization of carbon sourced from fossils is a contributing factor to anthropogenic global warming.1,2 It has been projected that by the mid-21st century, the proportion of polymer production in worldwide fossil fuel consumption will increase to one-fifth.3 Nowadays, there is a pressing need for a transition from a fossil fuel-based economy to an ideal circular materials economy, such as through the use of sustainable materials sourced from plants and other non-fossil biogenic feedstocks.4–7 The quest for novel monomers to synthesize polymers without relying on petrochemical feedstock is rapidly growing in scope and scale.8–10Bio-based polymers are derived from renewable feedstock, typically biomass, and offer a sustainable alternative to traditional petroleum-based polymers, with special characteristics such as being green, environmentally friendly, and renewable.11,12 However, there are still many challenges in producing bio-based polymers from biomass. On the one hand, bio-based polymers should exhibit comprehensive performance comparable to petroleum-based polymers to meet application requirements.4 Some reported bio-based monomers have been proven to improve certain properties of bio-based polymers, such as mechanical and optical properties, enabling better use in specific areas. On the other hand, the utilization of multiple bio-based monomers enhances the structural complexity of polymers, thereby promoting synthetic research. Typically, research on the synthesis of bio-based polymers includes two parts: Firstly, novel monomers derived from biomass are investigated to establish new structure–property relationships. Subsequently, this knowledge stimulates the development of innovative catalyst designs and methods.
Polycarbonate (PC) is the only transparent plastic among the engineering plastics, with excellent mechanical, thermal and optical properties that make it ideal for applications in electrical electronics, automotive industry, medical devices, and protective equipment.13,14 Since 2017, the total global PC capacity has exceeded 4 million tons per year and is expected to increase to 9 million tons per year by 2030.15 In 1958, fossil-based PCs were commercialized by Bayer.16 Traditional PC was produced using phosgene and the petroleum-based monomer bisphenol A (BPA).17 However, as an endocrine disruptor, BPA has estrogenic effects and chronic toxicity, limiting the application of PCs in the fields of food, medical apparatus, and baby products.18–21 Consequently, bio-based alternatives to BPA monomers have become a growing field of research.22 In 2012, Mitsubishi Chemical developed a commercial bio-based PC product named “Durabio”, which was produced from corn-derived and non-toxic isosorbide.23 Isosorbide-based PCs not only inherit the desirable properties of BPA-based PCs, but also have a low carbon footprint and high biosafety, in line with the requirements of environmentally sustainable plastics. In addition to isosorbide, synthetic routes of a range of bio-based monomers starting with lignocellulose, terpenoids (typically limonene oxide) and vegetable oils, etc., have been investigated.24–26 The introduction of functional monomers into PCs presents an opportunity to develop novel high-performance materials for emerging applications such as optics, optoelectronic information and biomedicine, owing to their high mechanical strength, transparency, impact resistance, and potential degradability and biocompatibility.27,28 Among bio-based monomers reported for PC synthesis, isosorbide and limonene oxide, which are typical sugar and terpene derived monomers respectively, have been systematically researched and can be represented as prime examples for bio-based PCs.
Recently, many reviews have focused on the sustainable routes of bio-based monomers and the outstanding properties of bio-based polymers.4,8,29–31 Barbaro et al. conducted a review on the sustainable catalytic synthesis of rigid diols derived from biomass, with the potential to act as functional alternatives to BPA.32 Bio-based pre-polymers and polymers including but not limited to epoxy resins, polyethylene, polypropylene and polyethylene terephthalate, have been summarized in reviews.33–35 Zhang et al.36 provided a comprehensive overview of representative bio-based polyesters. Beckham et al.4 summarize some recent advancements in the development of high-performance bio-based polymers with C–O or C–N bonds. In addition, many reviews have summarized the progress of research on aromatic polycarbonates synthesized from BPA and diphenyl carbonate and aliphatic polycarbonates synthesized from epoxide and CO2.37–45 However, there are no reviews that provide a systematic summary of bio-based PCs to our knowledge. So in this review, we give a comprehensive introduction to two representative bio-based PCs, poly(isosorbide carbonate)s and poly(limonene carbonate)s. A brief introduction of the bio-derived monomers is presented in the first section. The majority of this review highlights polymerization and modification strategies. Furthermore, the effects of catalyst types, reaction conditions, and modification methods on their molecular weights and properties are discussed. In conclusion, we outline the opportunities and challenges for advancing the field of bio-based polycarbonates.
2. Bio-based monomers for polycarbonate synthesis
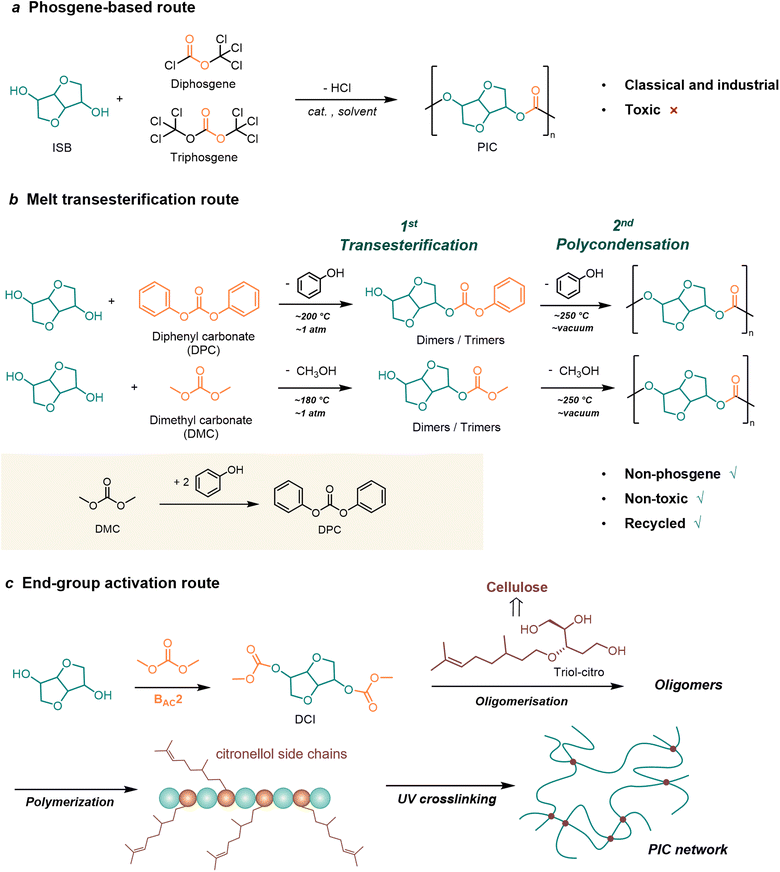
The synthetic methods of bio-based PCs are fundamentally consistent with conventional BPA-PC. Broadly speaking, there are two routes as shown in Fig. 1. The first is the step-growth polycondensation route, referring to bisphenol or aliphatic diols derived from sugars or lignins as bio monomers, followed by transesterification reaction with carbonylation reagents. The second is the ring-opening polymerization (ROP) route, in which epoxides and cyclic carbonates are used as bio-based monomers. Epoxides are mainly derived from terpenoids and vegetable oils, while cyclic carbonates are mainly derived from sugar-based compounds. Based on the two routes described above, typical bio-based monomers used for PC synthesis can be classified into four categories including sugar-derived monomers, lignin-derived monomers, terpene-derived monomers, and vegetable oil-based monomers. These categories are discussed in detail in the following (Fig. 1).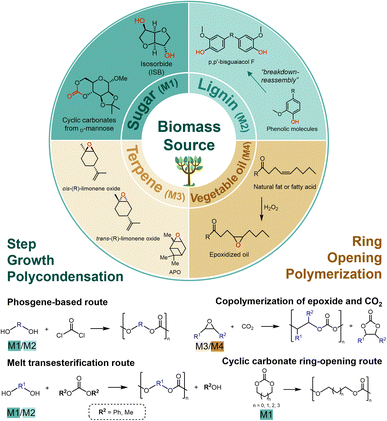 | ||
| Fig. 1 Schematic representation of typical bio-based monomers for synthesizable PCs, and two strategies to transform bio-based monomers to PCs. | ||
Natural sugar is one promising raw material for various applications, which has the advantages of high availability, low toxicity, and structural diversity.46 Specifically, cyclic unit structures derived from sugar can enhance the rigidity of the polymer chain, resulting in a higher glass transition temperature (Tg).47 As the only industrially available bio-based aliphatic bicyclic sugar-based monomer, isosorbide (1,4:3,6-dianhydro-D-glucitol, ISB) has attracted significant research attention.29,47,48 ISB as one of the most promising alternatives to BPA, possesses properties of rigidity, chirality, and non-toxic.29,47 The most common route of ISB by double dehydration of sorbitol under acidic conditions.29,49 The development and maturation of the dual-catalytic system, along with the direct conversion of cellulose to ISB process, have substantially decreased the production cost of ISB.50,51 These developments demonstrate an increasingly obvious cost advantage compared to other biomass monomers. Notably, ISB-based PCs are typical bio-based PCs prepared via step-growth condensation strategies. In addition, saccharides can react with CO2 or compounds containing carbonate linkages to synthesize sugar-based cyclic carbonates, which then produce PCs via cyclic carbonates ring-opening route. Examples are PCs based on glucose,52D-mannose,53 and 2-deoxy-D-ribose,54 among others. The structure of saccharide cyclic carbonate monomer from D-mannose and CO2 was shown in sugar part in Fig. 1. These developments in synthetic methodologies of sugar-based monomers are important steps toward the use of saccharides as effective and innovative feedstocks for PC-based engineering materials.
Lignin, isolated from wood or plant, is the most prevalent biopolymer after cellulose, which can be converted to numerous phenol derivatives.55–57 Lignin is a complex polymer composed of phenylpropane derivatives, which originate from three primary monolignols: p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol.30 Due to the “breakdown-reassembly” of lignin could produce different phenolic molecules, lignin-derived bio-renewable bisphenols, triphenols, and multiphenols have the potential to replace BPA as phenolic monomers in step-growth condensation route.58–61 The reported monomers that can be used to synthesize PCs include vanillyl alcohol,62 eugenol,63–65 and bisguaiacol58,66,67 and their derivatives, of which bisguaiacol derivatives are regarded as the most common bio-based BPA-alternative. The structure of p,p′-bisguaiacol F was shown in lignin part in Fig. 1, which could be made by condensation between vanillyl alcohol and guaiacol. The p,p′-bisguaiacol F could be used to synthesize PCs by interfacial polycondensation with triphosgene.66 In biological assays on lignin-derived phenolic monomers, research has shown that lignin-derived bisphenols extracted from eugenol, carvacrol, and creosol exhibit no estrogenic effects, enabling the production of lower toxicity polycarbonates and potentially reducing long-term health risks.68 However, efficiently catalytic systems for the conversion of lignin into monomers remain to be developed, mainly due to the inherent complexity of lignin materials.
Functional terpenes offer a promising alternative for the synthesis of novel bio-based epoxides. The chemistry of functional terpenes provides an interesting option to obtain new bio-based epoxides.69 Limonene, a commercially available monoterpene that can be easily extracted from citrus fruits, has attracted considerable attention for its potential applications.70 The epoxidation of the double bond on the aliphatic ring gives rise to reactive limonene oxide (LO) or α-pinene oxide (APO), both of which can be used as a platform monomer to obtain polycarbonates.31 The epoxy groups of LO or APO enable ROP reactions. Although there are fewer examples of APO being used as an epoxy monomer,71 the utilization of LO as a renewable terpene feedstock in bio-based PC synthesis has received significantly increased attention. Since (R)-LO is derived from orange peel while (S)-LO is from fir cone oil, (R)-LO is relatively cost-effective compared to (S)-LO,72 so current polymerization studies have focused on the use of (R)-LO.31
The main component of vegetable oil is triglyceride, in which the three fatty acid chains are usually derived from fatty acids containing carbon atoms ranging from 14 to 22. The reactive groups such as double bonds, ester groups, and ester α-carbons in the fatty acid structure can be reactive to introduce functional groups with higher polymerization capacity. Among these, the introduction of epoxy groups into vegetable oil-sourced triglyceride is a common modification method for incorporating functional groups. And the epoxy groups are important groups that can realize copolymerization with CO2. Typically, epoxidized vegetable oils can be employed in the synthesis of PC by ROP reactions in organocatalytic systems under milder conditions.73 The coconut oil-derived glycidyl octanoate,74 linseed oil-based epoxides,75 soybean oil-based terminal epoxides,76,77 castor oil-derived epoxides78,79 and so on were explored to synthesize new biodegradable PCs via the copolymerization with CO2. The presence of long side chains can effectively reduce the Tg of the polymer, addressing the current problems of high Tg and high viscosity of CO2-based PCs to accommodate their applications in coatings among other areas.
In addition to the above sources of bio-based monomers, there are additional bio-based monomers that can be synthesized from natural compounds or are found naturally in limited quantities, such as natural citric acid.80–82 Furthermore, it is noteworthy to mention that the design and synthesis of novel bio-based polymeric monomers have the potential to enhance the structural and performance diversity of PCs. This extension can broaden the range of applications and has also gained significant attention.83–85
Overall, multiple types of bio-based monomers can promote further research in the field of PC synthesis. Generally, research on the synthesis of bio-based polymers includes two parts: structure–property relationships based on novel monomers from biomass and the development of novel catalyst systems for the synthesis of bio-based PCs. Most of the current research on the synthesis of bio-based monomers is still at the stage of synthetic route development. As typical examples, sugar-derived ISB monomers and terpene-derived LO monomers have been commercially available. Thus, in the following, we will further review these two representative bio-based PCs: poly(isosorbide carbonate)s and poly(limonene carbonate)s.
3. Bio-based polycarbonates from isosorbide monomers
As a typical type of sugar-derived product, poly(isosorbide carbonate)s (PICs) have received widespread attention due to their advantageous properties.29,47 Regarding the structure of PIC, the ISB units in PIC have bifuran structures that form 120° dihedral angles in space.47,86 The dihedral angle, which is not easily folded or rotated, imparts superior mechanical and thermal properties to PIC compared to BPA-PC.87 Additionally, the non-toxicity, high transparency, and wear resistance of PIC are superior to traditional BPA-PC.47However, the molecular weight growth of PIC is limited due to the low activity of the hydroxyl groups in the reactant ISB. The solution to this challenge lies in the development of efficient and mild catalysts, which can address the issue of achieving a higher weight-average molecular weight (Mw) of PIC and meet the minimum requirements for industrial processing (Mw > 70 kg mol−1).88 In addition to the study of PIC synthesis, many studies focus on the modification of PIC to meet the industrial demand for high-performance functionalized PC and further expand the application areas of PIC. Hence, research on PICs has focused on two main areas: the synthesis of PICs and the modification of PICs.
3.1. Synthesis of poly(isosorbide carbonate)s
Although the phosgene-based route is the most classical and industrially widespread method for the synthesis of PCs, the raw material phosgene derivatives and solvents are toxic. There is still an urgent need to develop green processes.
Among the alicyclic monomers, ISB is one of the most studied monomer for melt transesterification process.29 Within the studies of transesterification route involving ISB, the reported catalysts can be subdivided into three major types: alkali metal catalysts, organic non-metallic catalysts, and ionic liquid (IL) catalysts (Table 1).
| Catalyst | Carbonyl | Amount cat.a (mol%) | Temp.b (°C) | Reaction timec (h) | Yield (%) | M w (kg mol−1) | PDI | T g (°C) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a The value is based on ISB. b The first value is the reaction temperature of the transesterification stage, and the second value is the reaction temperature of the condensation stage. c The first value is the reaction time of the transesterification stage, and the second value is the reaction time of the condensation stage. d wt% based on DPC. e Conversion of ISB. f The viscosity average molecular weight. g Im: Imidazolate, Lys: lysine, 4-I-Phen: 4- iodophenol, Arg: arginine, Phth: phthalimide, HQ: hydroquinone. | |||||||||
| Alkali salt | |||||||||
| Cs2CO3 | DPC | 2 × 10−5 | 180/240 | 1/1.5 | — | 32.6 | 1.48 | 164 | 93 |
| Ca/SBA-15 | DPC | 0.02d | 180/240 | 1/1 | 95 | 48.8 | 1.64 | 169 | 94 |
| CaCl2 | DPC | 0.16 | 160–220/230–250 | 1.5/1 | — | 17.4f | — | 172 | 95 |
| Zn(OAc)2 | DPC | 0.16 | — | 14.3f | — | 168 | 95 | ||
| LiCl | DPC | 0.16 | 83.5e | 51.0f | — | 168 | 96 | ||
| LiAcac | DMC | 0.10d | 98–180/240 | 7/5 | 93e | 29.7 | 1.99 | 167 | 97 |
| (CH3)3CONa | DMC | 0.80 | 160–180/210 | 2/0.5 | 99e | 55.1 | 1.90 | 167 | 98 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Organic alkali | |||||||||
| TBD | DMC | 0.10 | 180/240 | 1.5/4 | 93 | 53.2 | 1.69 | — | 99 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Ionic liquids | |||||||||
| [N2222][Im] | DPC | 0.0015 | 98/240 | 5/5 | 95 | 25.6 | 1.96 | 162 | 100 |
| [Bmim][CH3CHOHCOO] | DPC | 0.05 | 100/240 | 5/0.5 | 99 | 105.8 | 1.71 | 174 | 101 |
| [Emim][Lys] | DPC | 0.005 | 150/240 | 0.5/0.5 | 99 | 150.0 | 1.63 | 174 | 102 |
| [C2(Min)2][Br]2 | DPC | 0.0045 | 130–160/240 | 3/0.3 | 99 | 98.7 | 1.53 | 170 | 103 |
| [Bmim][4-I-Phen] | DMC | 0.44 | 98/230 | 3.5/4 | — | 50.3 | 1.76 | 160 | 104 |
| [Emim][Br] | DMC | 0.44 | 98/200 | 5/1.5 | 87.4 | 52.1 | 1.82 | 156 | 105 |
| [N1111][Arg] | DMC | 0.10 | 180/250 | 0.5/1 | 72 | 39.9 | 1.65 | 157 | 106 |
| [P4444][Phth] | DMC | 0.50 | 180/250 | 0.5/1 | 99 | 41.7 | 1.66 | 154 | 107 |
| [N2222]2[HQ] | DMC | 0.80 | 180/230 | 1/1 | 99e | 53.6 | 1.69 | — | 108 |
In the past decade, a variety of alkali metal catalysts, represented by cesium carbonate, have been developed.93 Wang et al.94 prepared loaded solid base multiphase catalysts for melt transesterification, and Wu et al.95 explored a series of organometallic catalysts. According to their studies, strong electrophilic reagents represented by alkali metal cations exhibited high reactivity due to their efficient activation of the endo-OH of ISB and the carbonyl group of DPC. Apart from the above-mentioned, metal chlorides have been demonstrated to form intermolecular hydrogen bonds with the hydroxyl groups of ISB.96 This interaction not only improved the endo-OH reaction activity but also increased the ratio of endo–endo structure, resulting in the products with high Tg.
However, since residual metal catalysts can affect product quality, some researchers have turned their attention to non-metallic catalysts. Emerging green media ILs are of great attention to researchers in science and engineering, not only for their designability and low toxicity but also for their excellent performance in catalysis.109–111 Initially, a series of quaternary ammonium-based ILs with different anions were designed.100,112 The relationship between the anions of the ILs and the catalytic activities was investigated. Among them, tetraethylammonium imidazolate ([N2222][Im]) exhibited the highest catalytic activity, resulting in a Mw of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1 and an isosorbide conversion of 92%. The results indicated that the catalytic activity depended on both the basicity of the catalyst and the coordinating strength of the anion in the IL. Based on the above work, a series of imidazolium-based ionic liquid catalysts were further designed and the effect of anion acidity and basicity on the catalytic performance was investigated. The inactivity of strongly acidic anions and the inferior activity of neutral anions also verified the importance of basicity.101 Moreover, the Mw of the PIC synthesized by [Bmim][CH3CHOHCOO] was significantly increased to 105.8 kg mol−1. The anion in catalyst [Bmim][CH3CHOHCOO] exhibits greater electronegativity, where the –OH in lactate anion could facilitate the activation of the carbonyl group through hydrogen bonding, rendering the carbon of the carbonyl group in DPC more susceptible to nucleophilic attack by –OH in ISB. These results indicated that the anions possessing stronger electronegativity and hydrogen bond formation ability exhibited greater advantages in the synthesis of higher molecular weight PICs. Subsequently, based on the aforementioned research, amino acid ionic liquids containing both carboxylic acid anions and hydrogen bond-giving groups were developed, which achieved superior catalytic performance in the synthesis of PIC with the Mw of 150.0 kg mol−1 under the optimal catalyst [Emim][Lys].102 The remarkable catalytic performance was attributed to the fact that [Lys] anion could form a stronger hydrogen bond with the endo-OH of ISB, thereby activating the endo-OH of ISB. Further research has found that [Emim][Lys] can inhibit the formation of cyclic intermediates compared to tetrabutylphosphine ionic liquids. Overall, in the selection of anions, stronger nucleophilic anions containing hydrogen donor groups exhibit activation of carbonyl and hydroxyl groups of the reactants and inhibition of disadvantageous intermediate formation to obtain high molecular weight PICs.
600 g mol−1 and an isosorbide conversion of 92%. The results indicated that the catalytic activity depended on both the basicity of the catalyst and the coordinating strength of the anion in the IL. Based on the above work, a series of imidazolium-based ionic liquid catalysts were further designed and the effect of anion acidity and basicity on the catalytic performance was investigated. The inactivity of strongly acidic anions and the inferior activity of neutral anions also verified the importance of basicity.101 Moreover, the Mw of the PIC synthesized by [Bmim][CH3CHOHCOO] was significantly increased to 105.8 kg mol−1. The anion in catalyst [Bmim][CH3CHOHCOO] exhibits greater electronegativity, where the –OH in lactate anion could facilitate the activation of the carbonyl group through hydrogen bonding, rendering the carbon of the carbonyl group in DPC more susceptible to nucleophilic attack by –OH in ISB. These results indicated that the anions possessing stronger electronegativity and hydrogen bond formation ability exhibited greater advantages in the synthesis of higher molecular weight PICs. Subsequently, based on the aforementioned research, amino acid ionic liquids containing both carboxylic acid anions and hydrogen bond-giving groups were developed, which achieved superior catalytic performance in the synthesis of PIC with the Mw of 150.0 kg mol−1 under the optimal catalyst [Emim][Lys].102 The remarkable catalytic performance was attributed to the fact that [Lys] anion could form a stronger hydrogen bond with the endo-OH of ISB, thereby activating the endo-OH of ISB. Further research has found that [Emim][Lys] can inhibit the formation of cyclic intermediates compared to tetrabutylphosphine ionic liquids. Overall, in the selection of anions, stronger nucleophilic anions containing hydrogen donor groups exhibit activation of carbonyl and hydroxyl groups of the reactants and inhibition of disadvantageous intermediate formation to obtain high molecular weight PICs.
Apart from the above single-active-site ILs, multi-active-site IL catalysts have been exploited with high catalytic activity.103 The superior thermal stability of dicationic ILs compared to conventional monocationic ILs makes them more suitable for high-temperature melt polycondensation to produce PICs. Moreover, the presence of multiple electrophilic sites on the alkyl chain of the cation facilitates the efficient activation of DPC. The halogen anion in the optimal dicationic catalyst [C2(Min)2][Br]2 could break the intramolecular hydrogen bond of ISB and form hydrogen bonds with –OH of ISB. The reactivity of endo-OH and exo-OH is effectively balanced in the synergistic catalysis of [C2(Min)2][Br]2.
More attractive routes to synthesize PICs are prepared by melt polycondensation reactions of ISB with carbon dioxide-derived DMC. CO2-derived DMC is a more environmentally friendly raw material that can effectively reduce carbon dioxide emissions, regarded as the cornerstone of the green chemical industry in the 21st century.108,113
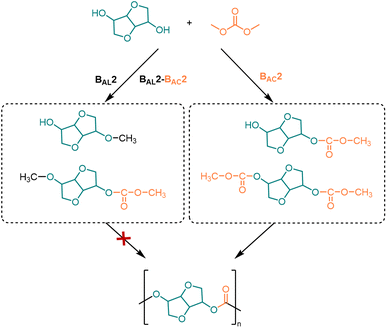
However, using DMC as a carbonyl source faces a challenge: to avoid the DMC-induced methylation (BAL2 mechanism) or methyl-carboxymethylation (BAL2-BAC2 mechanism) side reactions of ISB.114 Due to the fact that the by-products of methylation can inhibit the growth of polymerization chains, it is necessary to inhibit the generation of methylation intermediates. Therefore, efficient catalysts need to be developed to react DMC with ISB according to the BAC2 mechanism to synthesize high molecular weight PICs (Fig. 3).99
The synthesis of PIC through melt polycondensation using ISB and DMC was reported in 2013, utilizing lithium acetylacetonate (LiAcac) as the catalyst.97 The reaction time was 12 h and the Mw of 29.7 kg mol−1 which needed to be improved. In 2020, the metal ion-containing compound catalyst sodium tert-butoxide ((CH3)3CONa) was developed to decrease the reaction time to 2.5 h, meanwhile, the weight-average molecular weight was increased to 55.1 kg mol−1.98 What's worth noting, when the interaction energy of catalyst is high, the side reaction of methylation could be inhibited, but too high interaction energy affects the proton absorption capacity of the anion and thus the catalytic performance. This provides theoretical guidance for the design of subsequent IL catalysts.
The organic alkali catalysts also achieved an improvement in this route, but there are fewer relevant studies. Ochoa-Gómez et al.115,116 used a two-step method with organic alkali catalyst 1,8-diazabicyclo[5.5.0]undec-7-ene (DBU) in the transesterification and polycondensation steps. But the Mw of the PIC is only 1.80 kg mol−1. Furthermore, the organic catalyst 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) was developed via one-pot synthesis.99 TBD has a bifunctional activation mechanism, and its nitrogen bicyclic Lewis base can activate the –OH of ISB as well as enhance the reactivity of DMC.
IL catalysts also showed excellent catalytic effects in the synthetic routes using DMC as the carbon source. Phosphonium-phenolic-based ILs were first found to be effective in catalyzing the transesterification reaction of ISB with DMC to produce methoxy carbonylation intermediates.117 Similar to the reaction mechanism of the DPC route, the reaction is activated by the formation of hydrogen bonds and electrostatic interactions with the IL catalysts. Given this, by modifying the structures of phenolate-based ILs, the nucleophilicity of the anion and the electrophilicity of the cation in ILs could interact with each other to obtain efficient catalysts for the synthesis of high molecular weight PICs.104 A range of phenolic IL catalysts was investigated, revealing that the highest molecular weights could be obtained under the catalyst [Bmim][4-I-Phen], when the hydrogen bonding strength of ILs to the substrate was moderate.104 Subsequently, bromine anion-based,105 amino acid anion-based,106 amide anion-based,107 and dual-site functionalized108 ILs catalysts were developed. Systematic studies have shown that the cation–anion synergistic activation of IL catalysts for the reaction substrates ISB and DMC, where the anions with higher alkalinity, higher negative charge density, and lower steric resistance exhibit better catalytic activity. As an example, the carboxymethylation selectivity of DMC and the conversion of ISB can be improved to 99.6% and 99.0% with phthalimide ILs catalyst ([P4444][Phth]).107 Because of the branching imine group of its amino acid anion, it can form intermolecular hydrogen bonds with DMC through multiple sites. It makes the carbon atom in the carbonyl group more likely to receive attack, thus increasing the selectivity of the carboxymethylation reaction. Aside from the widely studied DPC and DMC, some studies have used other functionalized or green sources of carbonates as carbonyl sources in transesterification reactions. For instance, Kamps et al.118 used bis(methyl salicylic acid) carbonate as a carbonyl source, which has higher reactivity than DPC. It can avoid the discoloration and cross-linking of PICs due to the end groups generated by the elimination reaction of β-H.119 Detrembleur group developed a series of novel CO2-based monomer bis(α-alkylidene carbonate) to synthesize PCs by organocatalytic stepwise polymerization with diols under mild conditions.83 Subsequently, they synthesized a series of regionally regular poly(oxo-carbonate)s by progressive copolymerization of CO2-derived bicyclic carbonate with bio-derived diols such as ISB at 25 °C using DBU as the catalyst.85 This process provides new ideas for the sustainable production of bioplastics under mild conditions.
One good example of end-group activation methods is recent reports on dimethoxycarbonyl isosorbide (DCI) (Fig. 2c). DCI can be obtained in high yield by methoxycarbonylation of isosorbide in the presence of DMC, and it is one of the potential monomers for the synthesis of functional PC.92,120 Recently, Aricò et al.121 used the levoglucosyl triol monomer citrate triol (triol-citro) to achieve polycondensation with DCI to synthesize PC containing both linear and dendritic units. The novel PC is composed entirely of bio-derived groups with citronellol side chains, leading to the cross-linking of the side chains induced by UV light irradiation. The Tg of the cross-linked PC was increased from −60 °C to −6 °C and Td50% from 212 °C to 444 °C, which can be used for biomedical materials requiring low Tg.
Based on the above studies, developing a new synthetic route for green carbonyl source or other functional carbonyl source is the main development direction for the synthesis of PIC in the future, meanwhile enhancing the molecular weight and yield of PIC via designing and developing novel catalyst system and catalytic mechanism remains imperative.
3.2. Modification of poly(isosorbide carbonate)s
| Polymers | Diols | ISB/diola | M n (g mol−1) | T g (°C) | T d-5% (°C) | Ref. |
|---|---|---|---|---|---|---|
| a Determined by 1H NMR spectroscopy. b Diols contain methyl side chains. c The diol comonomer content is virtually indiscernible. d Feed ratio. | ||||||
| PIC | — | — | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
174 | 342 | 101 |
| PEIC | HO–(CH2)2–OH | 1/0.67 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
95 | — | 122 |
| PBIC | HO–(CH2)4–OH | 1/0.95–0.99 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–36 000–36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
68–70 | 310–322 | 97,101,122 |
| PPIC | HO–(CH2)5–OH | 1/1.09 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
48 | 325 | 97 |
| PHIC | HO–(CH2)6–OH | 1/0.98 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
35–52 | 316–330 | 97,101 |
| POIC | HO–(CH2)8–OH | 1/0.97 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 407 407 |
25 | — | 122 |
| PDIC | HO–(CH2)10–OH | 1/0.99 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
20 | — | 122 |
| PGIC-1 | HO–CH2CH2–O–CH2CH2–OH | 1/0.98 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
62 | 331 | 101 |
| PGIC-2 | HO–CH2CH2–O–CH2CH2–OH | 1/0.42 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
105 | 332 | 123 |
| PTIC | HO–(CH2CH2–O)2–CH2CH2OH | 1/0.43 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
83 | 340 | 123 |
| PTTIC | HO–(CH2CH2–O)3–CH2CH2OH | 1/0.40 | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
72 | 334 | 123 |
| PGICb |
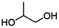
|
1/NAc | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
155 | 324 | 123 |
| PBICb |
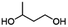
|
1/NAc | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
135 | 297 | 123 |
| PTPICb |
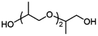
|
1/0.44 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
78 | 299 | 123 |
| P-DHA-IC |
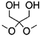
|
1/1d | 4500 | 100 | 194–251 | 127 |
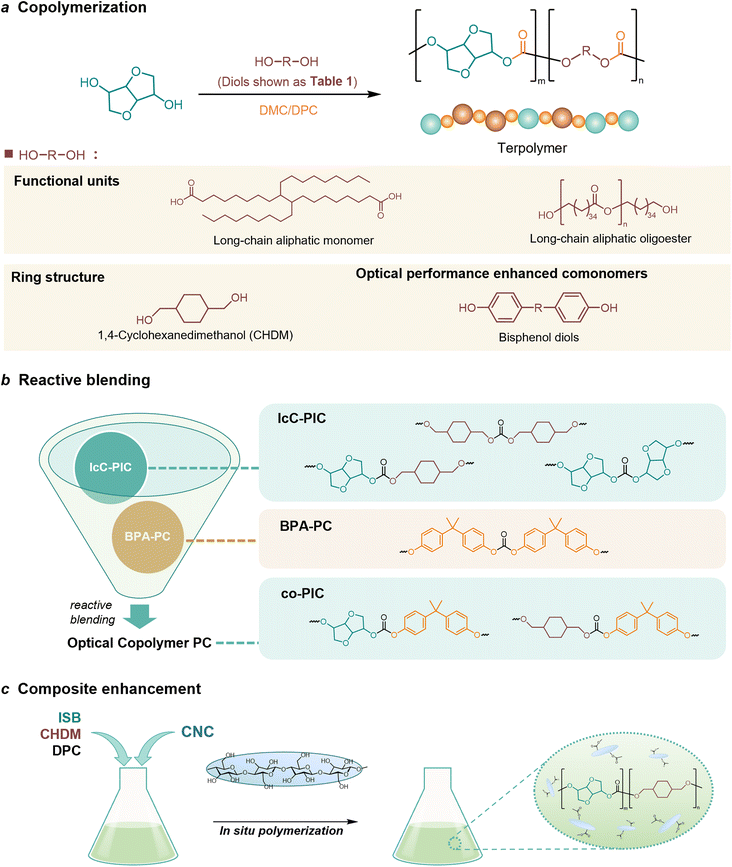
Copolymerization with straight-chain aliphatic diols enhances the flexibility and elongation at the break of PIC, while an increase in flexible structure content leads to a decrease in its mechanical strength.124 The ring structure of cycloaliphatic diols can compensate for the lack of polymer stability. 1,4-Cyclohexanedimethanol (CHDM) is the most widely studied cycloaliphatic diol monomer. Industrially, the copolymerization by CHDM can effectively improve the processing properties and toughness of PIC. Mitsubishi Chemical has developed and commercialized a poly(ISB-co-CHDM carbonate) (IcC-PC), named Durabio-PC, which is synthesized through the polycondensation of ISB and DPC in the presence of flexible CHDM.125 According to the published literature, by changing the ratio of ISB/CHDM, the fragility of solely PIC is overcome and the mechanical properties of the copolycarbonates are adjustable.119 As the content of ISB increased, there was an increase in the Tg and elasticity of copolycarbonates, while the Mw and elongation decreased of copolycarbonates. Optimally formulated IcC-PC exhibits a 1.25-fold increase in Young's modulus, a 1.05-fold increase in ultimate tensile strength, and one grade higher pencil hardness compared to BPA-PC.119 A recent study tested the processability of Durabio-PC in 3D printing.126 Bio-based PC exhibits better tensile strength than other commercial polymers, has good heat and UV resistance, and can reduce harmful emissions from the 3D printing process. In addition, IcC-PC has an excellent performance in the optical field, which is discussed in a later paragraph.
Functional co-polymerization units can specifically improve certain properties of bio-based PCs, resulting in better use in specific areas. For example, Malkoch et al.127 achieved a high Tg amorphous copolymer PIC produced from ISB and sugar metabolic derivative dihydroxyacetone (DHA) (P-DHA-IC in Table 2). The keto-functional DHA has demonstrated the requisite hydrolytic instability to enable co-PIC degradation. The hydrolytic degradation rate of the deprotected polymer microspheres increases with increasing DHA content. This rigid, amorphous, and degradable polycarbonate is expected to be used in bone repair applications. Kamps et al.118 introduced long-chain aliphatic monomers and their oligoesters to copolymerize with ISB. The incorporation of long-chain aliphatic monomers significantly alters the rheological behaviour of the polymer during processing. A certain quantity of soft blocks is present within the separated domain of microphase separation, which can maintain the thermomechanical properties and transparency of the co-PIC, allowing the co-PIC to have good processability with higher Tg performance.
A series of notable examples of the performance enhancement of PICs by copolymerization modification is in the field of optical applications. The PIC has higher transmittance and lower haze compared to those of fossil-based PC.128 However, the refractive index of PIC is around 1.49, while the performance requirements for medium and high-end optical resin lenses (>1.56) are difficult to achieve.129 The incorporation of substituents with higher molar refractions and smaller molar volumes can effectively enhance the refractive index of PIC. For instance, comonomers containing multiple aromatic rings are capable of optimizing the optical properties of PIC. It has been experimentally demonstrated that the refractive index of PCs can be improved by using monomers containing the naphthalene structure and/or cardo structure.130 In addition, bisphenol diol exhibits high polarizability and molar refractive index due to its double benzene ring, heteroatom, and distorted spatial structure, which effectively enhances the polymer's refractive index.131 Xu et al.129 prepared a range of poly(isosorbide carbonate)s via copolymerization modification of bisphenol diols. The co-PICs displayed high molecular weight (144.2–82.8 kg mol−1), a high abbe number (vd = 39.7), low yellowness (YI = 0.93) and a high refractive index (nd = 1.511–1.573).
In addition to copolymer modification, reactive blending has been demonstrated as an effective method for preparing co-PIC with high molecular weight, low discoloration, excellent optical transparency and robust mechanical properties. This is achieved by blending commercially available IcC-PC with BPA-PC under trace amounts of sodium methoxide (Fig. 4b).132–135
In the area of coating development, amorphous bio-derived PC requires further modification compared to standard petroleum-based films due to its low thermal stability, limited ductility, and poor resistance to notched impact.137 Yousfi et al.138 prepared films exhibiting exceptional mechanical and rheological properties using an in situ nanofiber polymer composite concept, in which poly(butyl succinate-co-adipic acid) (PBSA) was homogeneously dispersed in amorphous bio-based IcC-PC in the molten state. Compared with the pure matrix IcC-PC, the elongation of the blends at the point of fracture was enhanced by approximately 670% without sacrificing tensile strength, and the fracture energy of the blends was about ten times that of the pure IcC-PC matrix. There are currently few reports on the composite enhancement with PICs, in-depth research on composite PICs is still worth looking forward to.
At present, the modification studies for PICs are mainly focused on copolymerization strategies to achieve improved mechanical properties of PICs while ensuring their excellent optical and thermal properties. In the future, through specific modification methods, high-performance functional PICs can be designed and synthesized for applications in emerging fields such as optical lenses and biomedical materials.
4. Bio-based polycarbonates from limonene oxide monomers
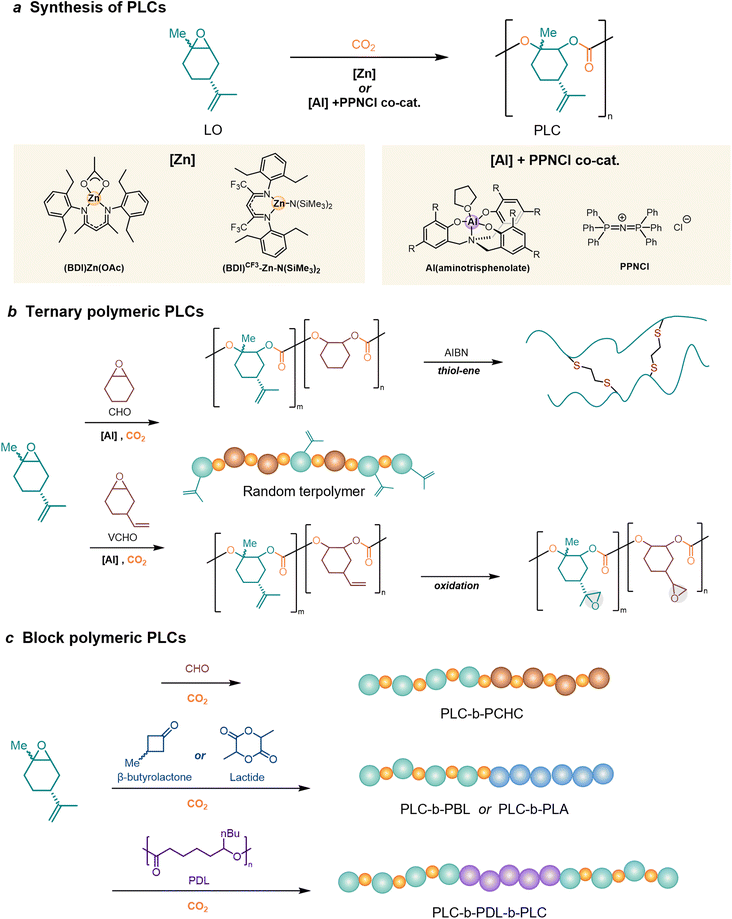
Bio-based epoxides are particularly intriguing due to their potential for creating novel polymer architectures through ring-opening copolymerization (ROCOP) with carbon dioxide (CO2).139 Aliphatic PCs can be produced by the direct addition of epoxy compounds with CO2. Epoxides, such as cyclohexene oxide (CHO), are typically more prone to generating polycarbonates by reacting with CO2.31 As detailed in section 2, the typical bio-based epoxide LO is also attracting significant interest. In the copolymerization process of LO and CO2, it is noteworthy that relatively low reaction pressures (6–20 bar) and temperatures (up to 73 °C) are utilized for the reaction with CO2, resulting in reduced costs and enhanced safety measures during production.140 The resulting poly(limonene)carbonate (PLC) exhibits Tg up to 130 °C, outstanding heat resistance, hardness and transparency, with great potential to replace polystyrene in the near future.141 Sablong et al. have reported fully bio-based PLC could be fully recycled back to LO monomers through the depolymerization initiated by TBD, implying the complete recyclability of the PLC.142 PLC was evaluated as a green platform for functionalized polymers by chemical modification. Recent research found that films of PLC show high gas permeation and excellent selectivity, with permeability to both gases and light. Additionally, these films possess exceptional thermal insulation properties and mechanical strength, thereby presenting a novel opportunity to utilize them as “breathing glass”–transparent materials that allow for gas exchange.1434.1. Synthesis of poly(limonene carbonate)s
To achieve the ROP of LO, an efficient catalyst is necessary to overcome the kinetic barrier for its activation, which is higher than that of terminal epoxides due to the internal trisubstituted nature of LO.144,145 The recent accelerated progress makes developed metal catalysts to enable the synthesis of PLC, as a potential candidate to replace petroleum-based polymers (Fig. 5a).In 2004, Coates et al.146 first used LO and CO2 as feedstocks to synthesize bio-based PLC via the alternating copolymerization method using β-diiminate zinc complexes [(BDI)Zn(OAc)] as a catalyst. The polymerization is stereoselective but with a limited molar mass of the production (25 kDa), incorporating only trans-LO in the polymer. After this initial success, further published research on PLC production was delayed for over a decade. Until 2015, an AlIII-based catalyst, Al(aminotrisphenolate)/PPNCl [PPN = bis(triphenylphosphine)iminium] binary catalysts for stereoregular LO/CO2 copolymerization, was discovered to incorporate both the trans- and cis-isomer of LO.147 It was regarded as an essential step toward higher conversion, as commercial D-limonene, the precursor to its epoxide, is typically found in an isomeric mixture.139 Although aluminum compounds can active both isomers, there is a marked preference for the cis-isomer due to its higher reactivity. When pure cis-monomers are used, higher molecular weights are obtained, as is steric regularity. However, the molar mass remained below 11 kDa and monomer conversion was limited to less than 75%.
A substantial amount of research calls for employing a single isomer to achieve high regioregularity or stereoregularity in polymers.148 A facile synthetic approach has been developed to achieve efficient kinetic resolution of two diastereomers with a purity of up to 98%.149 Building on Coates's original work, Greiner et al.150 optimized the catalytic process and achieved almost complete monomer conversion (>90%) in synthesizing high molecular weight (>100 kDa) at a large scale. The critical factors include the utilization of high trans-isomer content (>85%) LO and the absence of monomer impurities with hydroxyl functionality. Subsequently, a Lewis acid β-diiminato-zinc-complex, bearing two electron-withdrawing groups and an –N(SiMe3)2 initiating group [(BDI)CF3–Zn–N(SiMe3)2], was presented with an expanded range of epoxides, including the sterically demanding monomer LO, for co- and terpolymerization with CO2.151,152 This complex exhibited a TOF of 310 h−1 in the copolymerization of LO and CO2, while (BDI)Zn(OAc) exhibited a TOF of 37 h−1, and Al(aminotrisphenolate)/PPNCl exhibited a TOF of 3 h−1. Besides, this Lewis acidic zinc complex performs exceptional activity towards other epoxides including CHO, propylene oxide or the less studied octene oxide and styrene oxide.
In general, the metal-catalytic systems for the polymerization of LO are well developed and can be divided into two categories: metal Zn with ligand β-diiminate, and aminotriol Al/PPNCl complexes. It is worth mentioning that these catalysts are mutually complementary.140 The Zn catalysts exhibit sensitivity to moisture and demonstrate selective mediation of the copolymerization of LO trans-isomers. Subsequent copolymerization modification, post-modification, and post-functionalization were also based on these two catalytic systems.
Moreover, other novel catalytic systems are currently under exploration and research. For instance, the latest study demonstrated that chromium chloride complexes could catalyze copolymerization reactions with LO to form co-PLC.153 Drawing on the lineage of other aliphatic epoxide catalytic systems, metal-free catalytic systems, i.e., organocatalysts, also present a promising avenue for future PLC synthetic pathways.154–156
4.2. Copolymerization modification of poly(limonene carbonate)s
Despite significant success in synthesis, bio-derived PLCs still show limitations in material properties. Hence, in addition to the traditional alternating copolymerization of LO and CO2, innovative co-polymers have been produced by randomly incorporating multiple epoxide monomers to synthesize terpolymers, or by sequential epoxide addition to synthesize block copolymers.Ternary polymeric PLC can be synthesized by randomly adding LO and multiple epoxide monomers for copolymerization (Fig. 5b).69,157–159 Kleij et al.69 first reported the synthesis of terpolymer PLCs based on CHO, where LO and CO2 are controlled to incorporate both functional LO and non-functional CHO monomers in the polymer backbone. By controlling the number of olefin units, these terpolymers can be post-modified by thiol–ene chemistry, resulting in better thermal properties of the material. The post-modification of terpolymers can introduce new structures while modulating the properties to apply to new applications. According to a recent report,157 when the copolymerization with vinyl cyclohexene oxide (VCHO), the oxidation directly after synthesis resulted in a PC with two different epoxy groups, which was subsequently used for thermoset development.
One drawback of PLC is its limited impact resistance and mechanical properties. Although the incorporation of ethyl oleate additives resulted in improved mechanical properties of PLC,160 using such additives is detrimental due to their tendency to leach out over time. The synthesis of PLC block copolymers by copolymerization with CO2 exploiting living chain ends is a common approach for modifying and fine-tuning the properties (Fig. 5c). Greiner's group161 first reported block copolymers of PLC, poly(limonene carbonate)-block-poly(cyclohexene carbonate) (PLC-b-PCHC), by sequential addition of LO and CHO in the copolymerization with CO2via β-diiminate zinc catalyst. But the properties of PLC-b-PCHC are characterized by Tg of both PCHC and PLC, resulting in a highly brittle plastic. Rieger and colleagues have reported the synthesis of poly(limonene carbonate)-block-poly(β-butyrolactone) (PLC-b-PBL) via a switchable catalytic method, utilizing 50 wt% of PBL to yield a material exhibiting an elongation at break of 18%.162 Recently Agarwal's group163 demonstrated the one-pot living ring-opening copolymerization of lactides, trans-LO, and CO2, to synthesize poly(limonene carbonate)-block-poly(lactide) copolymers (PLC-b-PLA). However, the mechanical and biocompatible properties of the block copolymer need to be supplemented.
Williams's group164 studied ABA block polymers prepared from carbon dioxide, LO, and ε-decalactone (from triglycerides), i.e., poly(limonene carbonate)-block-poly(ε-decalactone)-block-poly(limonene carbonate) (PLC-b-PDL-b-PLC). These polymers include PDL blocks as B-blocks with low Tg and high flexibility, flanked by PLC as A-blocks with high Tg and rigidity. This plastic exhibits a unique combination of tensile strength and high elasticity, with an elongation at break and tensile toughness over 20 times higher than polycarbonate. As such, it effectively overcomes the brittleness and processing limitations associated with PLC.164
In addition, the potential of PLCs for producing more sustainable polymers through blending with petrol- or food-based polymers has been demonstrated by investigating PLCs containing blends of various commercial polymers as minority components.165
Future research could prioritize the synthesis of additional PLC-based random copolymers and block copolymers to modify and fine-tune their properties or better understand how microphase separation can be controlled to modulate these properties.
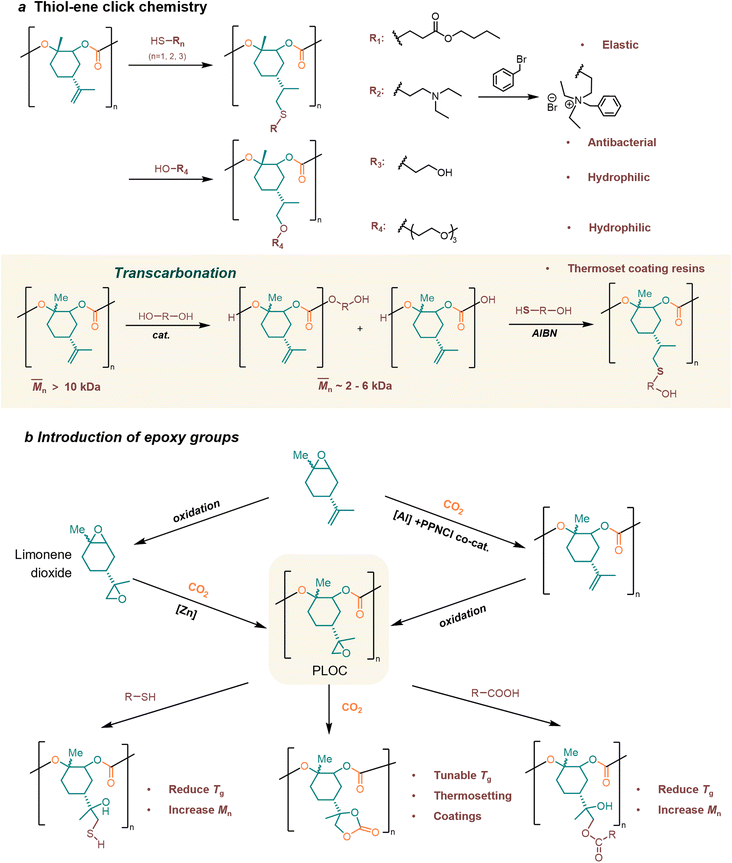
4.3. Post-modification of poly(limonene carbonate)s
Double bonds in LO provide more possibilities for material synthesis and post-modification. So far, two conceptual approaches have been widely employed for the functionalization of PLCs: thiol–ene click chemistry and epoxy group introduction.31Greiner et al. have mentioned that PLC is an ideal green platform polymer, capable of yielding numerous functional materials.166 They demonstrated available examples of facile addition reactions, namely thiol–ene click chemistry, acid-catalyzed electrophilic addition, and metal-catalyzed hydrogenation. As a typical example, in the thiol–ene click chemistry, the thiol addition can induce a transition of the high-Tg thermoplastic to a rubbery state. The modified PLC enhances not only hydrophilicity or pH-dependent solubility but also antimicrobial activity through functionalization with quaternary amines (Fig. 6a).166
Sablong, Koning, and their coworkers' research verifies that cross-linked PLCs can be successfully applied to new bio-based thermoset coating resins.167–170 For coating applications, the polymer resin should possess a minimum of two functional groups to facilitate cross-linking reactions with multifunctional curing agents. A medium molecular weight (2–4 k) is required when the curing reaction is limited to functional end groups. The molecular weight was broken down via transcarbonation reactions with polyols, resulting in the production of α, ω-dihydroxyl PLCs suitable for solvent casting and curing (Fig. 6a).167 Subsequently, the PLCs resulting from the transcarbonation reaction can be subjected to quantitative thiol–ene post-modification, wherein the thermal properties and hydroxyl values can be modulated by controlling the type and amount of incorporated thioether species, which enables their successful application as novel biobased thermoset coating resins.168,169 The study conducted by the group in 2021 demonstrated that biobased coatings, utilizing PLCs as UV-curable powder coating binders, exhibited exceptional properties.170 The poly(thioether-co-carbonate) networks exhibited elevated Tg, reaching up to 125.9 °C, and a broad spectrum of thermomechanical properties, encompassing rubbery moduli ranging from 4.4–27.5 MPa.
Typical post-epoxidation modification products poly(limonene-8,9-oxide carbonate) (PLOC) provide opportunities for the development of “epoxy” chemistry. Limonene dioxide is the diepoxide counterpart of LO, which is obtained by epoxidizing the double bond in LO. Sablong et al.171 prepared a sustainable PLOC by chemoselective copolymerization of limonene dioxide and CO2 (Fig. 6b). The side chain epoxy group in PLOC could react with thiol, carboxylic acid, or CO2, which realized the regulation of Tg without changing the main chain structure.171 Contrary to the use of limonene dioxide by Sablong et al., the Kleji group172 proposed a more cost-effective and easily accessible starting material, cis/trans (+)-LO, in their synthesis of poly(limonene dicarbonate), which was achieved through the functionalization of PLOC using a sequential epoxidation/carboxylation approach (Fig. 6b). The post-functionalization PLC has tunable Tg values reaching up to 180 °C.172
The post-modification of PLOC has a wide range of applications. The bio-based PLOC can be combined with synthetic hardeners to facilitate the formulation of epoxy thermosetting materials.173 The optimal formulation was determined to be a blend of PLOC and Jeffamine, a commercial curing agent, in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio. This formulation is intended for use in coating technology as a partial replacement for diglycidyl ether-based thermosets.173 The post-modification of PLOC can also make it promising for alkyd paint applications.174 A fully renewable limonene-derived polycarbonate-based alkyd resin was developed by copolymerizing limonene dioxide with CO2 to produce PLOC and chemically modifying it with soybean oil fatty acids. This new alkyd resin was evaluated as a coating and performed better than conventional polyester alkyd resin. The alkyd paint, which contains polycarbonates modified with fatty acids, exhibits accelerated chemical drying, elevated König hardness and increased Tg in coating evaluation.174
1 stoichiometric ratio. This formulation is intended for use in coating technology as a partial replacement for diglycidyl ether-based thermosets.173 The post-modification of PLOC can also make it promising for alkyd paint applications.174 A fully renewable limonene-derived polycarbonate-based alkyd resin was developed by copolymerizing limonene dioxide with CO2 to produce PLOC and chemically modifying it with soybean oil fatty acids. This new alkyd resin was evaluated as a coating and performed better than conventional polyester alkyd resin. The alkyd paint, which contains polycarbonates modified with fatty acids, exhibits accelerated chemical drying, elevated König hardness and increased Tg in coating evaluation.174
Overall, the rigidity and functionality of LO and limonene dioxide monomers make them attractive for existing bio-based PCs. PLC and PLOC are expected to replace conventional BPA-based and fossil-sourced propylene oxide-based PCs. However, most of the current research is limited to the design modification and polymerization process of LO monomers, with insufficient research on back-end material applications. In the future, there is still a need for extensive benchmarking of the performance of these terpene-based polymers compared with petroleum-based materials.
5. Perspective and conclusions
Bio-based derivatives as raw materials provide a more environmentally friendly synthesis pathway and possess inherent properties that can adjust the performance of PCs compared to current petroleum-derived materials, thereby expanding the potential application areas of PCs.According to the literature reviewed, bio-based PCs exhibit outstanding sustainability, tunability and functionality. Representative bio-derived ISB monomers and LO monomers have been industrially produced and are commercially available. Moreover, the synthetic processes for bio-based PCs, such as melt transesterification route, have been successfully industrialized. Isosorbide-based PC products, represented by Durabio, have been commercialized. Therefore, bio-based PCs are expected to replace traditional petroleum-based PCs and achieve large-scale industrial production, which will help reduce the demand for fossil fuels.
In terms of properties of bio-based PCs, the structures and properties of bio-based PCs could be modulated by adjusting the ratio of monomers and reaction conditions, and their properties can also be enhanced by compounding or modifying them with other materials. Besides, the inherent properties of bio-based monomers will introduce novel properties for bio-based PCs. Although the synthesis of sustainable monomers and bio-based PC products will increase costs, which can be decreased with continued research and development and increased industrialization scale. On the other hand, by introducing superior material functionality and/or properties into novel bio-based PC products, it is possible to enhance the added value of the products and break through the cost constraints.
Based on the existing research progress, the main directions and challenges for future research related to bio-based PCs are as follows:
(I) Optimize synthetic routes for monomers. Although ISB and LO are already commercially available, their current yields are relatively low by industrial standards, and high-purity, high-quality products are expensive. For example, in the case of isosorbide, the effect of trace impurities on the polymerization process, side reactions, polymer color, and batch stability needs to be studied in depth, thereby improving isosorbide batch yields and product quality. Thus, technological solutions need to be proposed, to increase the yield and production capacity of bio-based monomers. In addition, conventional polymeric monomers, such as BPA, could be accessed through novel routes from renewable resources, which means using renewable resources to create traditional PC plastics.8
(II) Develop novel bio-based monomers for polycarbonate synthesis. The unique structures of biobased monomers can improve the performance of PCs, such as furan structures in sugars, olefinic groups in terpenes, long side chains aliphatic structures in vegetable oils, etc. The structure–property relationship of PCs based on novel bio-based monomers should be further explored in depth. Further, with the development of synthetic routes for monomers, the monomers derived from sustainable feedstocks can be employed as substitutes to produce plastics instead of petrochemicals.
(III) Explore efficient synthetic methods. It is desired to develop more efficient catalytic systems for the synthesis of bio-based PCs under milder reaction conditions without reducing the performance. Take the synthesis of PIC as an example: although high molecular weight PIC has been realized by IL catalysts, in the actual production, there are still problems such as high requirements for the purity of the raw material ISB, the existence of side reactions such as β-H transfer and branching, and the easy discoloration of the PIC product. To achieve industrialization of bio-based PCs, it is imperative to address these challenges by developing highly efficient catalysts and methods that can facilitate the polymerization reaction, as well as enhance PCs' processing properties and application performance.
(IV) Prepare composites and functional materials of bio-based PCs. In studies related to PIC design, more functional copolymerized monomers, such as optical monomers, can be designed and synthesized to directionally tailor the functionality of bio-based PICs. In PLC areas, the presence of olefin groups gives multiple opportunities for chemical modifications, such as addition and epoxidation reactions. Modification methods such as copolymerization, blending, composite enhancement, post-modification, and post-functionalization could be explored, giving them new properties and expanding their application areas and market demand.
(V) Study the degradation mechanism and degradation conditions of bio-based PCs. Although not discussed in detail in this review, researchers have done work on the degradability of bio-based PCs.175–177 Designing easily degradable PCs focuses on controlling their degradation rate and degradation products, improving their environmental adaptability and resource utilization. This is of great significance for the development of sustainable polymer materials.
This review describes polymerization and modification strategies for bio-based PCs, mainly represented by PICs and PLCs. The information gathered in this paper serves as a driving force for future research and development of innovative bio-based PCs in the dynamic field of polymer science. Further research in the area of higher performance biopolymers has the potential to not only advance the field of polymer applications, but also contribute to the development of sustainable economies.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Beijing Natural Science Foundation (Z220024), the Natural Science Foundation of Henan Province (232300421091), the Key Research Program of the Chinese Academy of Sciences, Grant No. ZDRW-CN-2023-6 and No. ZDRW-CN-2023-2–2, the National Natural Science Foundation of China (22078339, 22208342), and the Youth Innovation Promotion Association of Chinese Academy of Sciences (2020050).References
- L. C. Voumik, M. A. Islam, S. Ray, N. Y. M. Yusop and A. R. Ridzuan, Energies, 2023, 16, 1044 CrossRef CAS.
- R. Nandhini, B. Sivaprakash, N. Rajamohan and D. V. N. Vo, Fuel, 2023, 342, 126984 CrossRef CAS.
- World Economic Forum, The New Plastics Economy–Rethinking the Future of Plastics, Ellen MacArthur Foundation & McKinsey World Economic Forum, 2016 Search PubMed.
- R. M. Cywar, N. A. Rorrer, C. B. Hoyt, G. T. Beckham and E. Y. X. Chen, Nat. Rev. Mater., 2021, 7, 83–103 CrossRef.
- R. Mori, RSC Sustainability, 2023, 1, 179–212 RSC.
- F. Versino, F. Ortega, Y. Monroy, S. Rivero, O. V. Lopez and M. A. Garcia, Foods, 2023, 12, 1057 CrossRef CAS.
- M. Krishani, W. Y. Shin, H. Suhaimi and N. S. Sambudi, Gels, 2023, 9, 100 CrossRef CAS.
- G. Hayes, M. Laurel, D. MacKinnon, T. Zhao, H. A. Houck and C. R. Becer, Chem. Rev., 2023, 123, 2609 CrossRef CAS.
- J.-G. Rosenboom, R. Langer and G. Traverso, Nat. Rev. Mater., 2022, 7, 117–137 CrossRef.
- M. Z. Rahman, M. Rahman, T. Mahbub, M. Ashiquzzaman, S. Sagadevan and M. E. Hoque, J. Polym. Res., 2023, 30, 106 CrossRef CAS.
- B. Imre and B. Pukanszky, Eur. Polym. J., 2013, 49, 1215–1233 CrossRef CAS.
- S. T. Gao, G. S. Tang, D. W. Hua, R. H. Xiong, J. Q. Han, S. H. Jiang, Q. L. Zhang and C. B. Huang, J. Mater. Chem. B, 2019, 7, 709–729 RSC.
- D. G. Legrand and J. T. Bendler, Handbook of Polycarbonate Science and Technology, CRC Press, Boca Raton, FL, 1st edn, 1999 Search PubMed.
- S. Paul, Y. Zhu, C. Romain, R. Brooks, P. K. Saini and C. K. Williams, Chem. Commun., 2015, 51, 6459–6479 RSC.
- GlobalData, Global Polycarbonate Market Size and Forecast to 2025–Capacity and Capital Expenditure Forecasts with Details of All Active and Planned Plants, 2022 Search PubMed.
- H. I. Lee and J. S. Lee, Polym. Sci. Technol., 1993, 4, 423 Search PubMed.
- L. R. C. Barclay, M. R. Vinqvist, K. Mukai, H. Goto, Y. Hashimoto, A. Tokunaga and H. Uno, Org. Lett., 2000, 2, 2841–2843 CrossRef CAS.
- E. C. Dodds and W. Lawson, Nature, 1936, 137, 996 CrossRef CAS.
- E. C. Dodds and W. Lawson, Nature, 1938, 141, 247–248 CrossRef CAS.
- H. W. Ng, R. Perkins, W. Tong and H. Hong, Int. J. Environ. Res. Public Health, 2014, 11, 8709–8742 CrossRef CAS.
- G. Ozyildiz, T. Olmez-Hanci and I. Arslan-Alaton, Appl. Catal., B, 2019, 254, 135–144 CrossRef CAS.
- L. Trullemans, S. F. Koelewijn, I. Scodeller, T. Hendrickx, P. Van Puyvelde and B. F. Sels, Polym. Chem., 2021, 12, 5870–5901 RSC.
- T. Setoyama, Catal. Surv. Asia, 2014, 18, 183–192 CrossRef CAS.
- M. Brodin, M. Vallejos, M. T. Opedal, M. Cristina Area and G. Chinga-Carrasco, J. Cleaner Prod., 2017, 162, 646–664 CrossRef CAS.
- C. Zhang, T. F. Garrison, S. A. Madbouly and M. R. Kessler, Prog. Polym. Sci., 2017, 71, 91–143 CrossRef CAS.
- F. Y. Pei, L. J. Liu, H. E. Zhu and H. X. Guo, Polymers, 2023, 15, 829 CrossRef CAS.
- W. Yu, E. Maynard, V. Chiaradia, M. C. Arno and A. P. Dove, Chem. Rev., 2021, 121, 10865–10907 CrossRef CAS.
- W. Zhang, J. Dai, Y.-C. Wu, J.-X. Chen, S.-Y. Shan, Z. Cai and J.-B. Zhu, ACS Macro Lett., 2022, 11, 173–178 CrossRef CAS.
- D. J. Saxon, A. M. Luke, H. Sajjad, W. B. Tolman and T. M. Reineke, Prog. Polym. Sci., 2020, 101, 101196 CrossRef CAS.
- A. Llevot, E. Grau, S. Carlotti, S. Grelier and H. Cramail, Macromol. Rapid Commun., 2016, 37, 9–28 CrossRef CAS.
- F. D. Monica and A. W. Kleij, Polym. Chem., 2020, 11, 5109–5127 RSC.
- F. Liguori, C. Moreno-Marrodan and P. Barbaro, Chem. Soc. Rev., 2020, 49, 6329–6363 RSC.
- E. A. Baroncini, S. K. Yadav, G. R. Palmese and J. F. Stanzione III, J. Appl. Polym. Sci., 2016, 133, 44103 CrossRef.
- V. Siracusa and I. Blanco, Polymers, 2020, 12, 1641 CrossRef CAS.
- G. Z. Papageorgiou, D. G. Papageorgiou, Z. Terzopoulou and D. N. Bikiaris, Eur. Polym. J., 2016, 83, 202–229 CrossRef CAS.
- Q. N. Zhang, M. Z. Song, Y. N. Xu, W. C. Wang, Z. Wang and L. Q. Zhang, Prog. Polym. Sci., 2021, 120, 101430 CrossRef CAS.
- W. B. Kim, U. A. Joshi and J. S. Lee, Ind. Eng. Chem. Res., 2004, 43, 1897–1914 CrossRef CAS.
- X. B. Lu, W. M. Ren and G. P. Wu, Acc. Chem. Res., 2012, 45, 1721–1735 CrossRef CAS.
- Y. S. Qin, X. F. Sheng, S. J. Liu, G. J. Ren, X. H. Wang and F. S. Wang, J. CO2 Util., 2015, 11, 3–9 CrossRef CAS.
- A. H. Liu, J. J. Zhang and X. B. Lv, Chin. J. Catal., 2018, 39, 1320–1328 CrossRef CAS.
- Y. Wang, Y. Zhao, Y. Ye, H. Peng, X. Zhou, X. Xie, X. Wang and F. Wang, Angew. Chem., Int. Ed., 2018, 57, 3593–3597 CrossRef CAS.
- G. W. Yang, Y. Y. Zhang, R. Xie and G. P. Wu, J. Am. Chem. Soc., 2020, 142, 12245–12255 CrossRef CAS.
- W. L. Wang, R. Qu, H. Y. Suo, Y. A. Gu and Y. S. Qin, Front. Chem., 2023, 11, 1202735 CrossRef CAS.
- N. G. Patil, S. K. Boopathi, P. Alagi, N. Hadjichristidis, Y. Gnanou and X. S. Feng, Macromolecules, 2019, 52, 2431–2438 CrossRef CAS.
- C. Chen, Y. Gnanou and X. S. Feng, Macromolecules, 2023, 56, 892–898 CrossRef CAS.
- J. A. Galbis, M. d. G. García-Martín, M. V. de Paz and E. Galbis, Chem. Rev., 2016, 116, 1600–1636 CrossRef CAS.
- F. Fenouillot, A. Rousseau, G. Colomines, R. Saint-Loup and J. P. Pascault, Prog. Polym. Sci., 2010, 35, 578–622 CrossRef CAS.
- T. Sucu and M. P. Shaver, Polym. Chem., 2020, 11, 6397–6412 RSC.
- D. P. Yuan, F. K. Xiao, N. Zhao, C. B. Deng, X. Huang, H. Zhang, Q. M. Yang and Y. G. Qiao, Chem. Eng. J., 2023, 460, 141780 CrossRef CAS.
- M. Y. He, J. X. Guo, X. C. Wang, Y. J. Song, S. S. Liu, H. Wang and C. Q. Li, New J. Chem., 2020, 44, 10292–10299 RSC.
- I. Bonnin, R. Mereau, T. Tassaing and K. D. Vigier, Beilstein J. Org. Chem., 2020, 16, 1713–1721 CrossRef CAS.
- K. Mikami, A. T. Lonnecker, T. P. Gustafson, N. F. Zinnel, P. J. Pai, D. H. Russell and K. L. Wooley, J. Am. Chem. Soc., 2013, 135, 6826–6829 CrossRef CAS.
- G. L. Gregory, L. M. Jenisch, B. Charles, G. Kociok-Köhn and A. Buchard, Macromolecules, 2016, 49, 7165–7169 CrossRef CAS.
- G. L. Gregory, G. Kociok-Kohn and A. Buchard, Polym. Chem., 2017, 8, 2093–2104 RSC.
- Q. Song, F. Wang, J. Cai, Y. Wang, J. Zhang, W. Yu and J. Xu, Energy Environ. Sci., 2013, 6, 994–1007 RSC.
- Q. Song, F. Wang and J. Xu, Chem. Commun., 2012, 48, 7019–7021 RSC.
- W. Deng, Y. Feng, J. Fu, H. Guo, Y. Guo, B. Han, Z. Jiang, L. Kong, C. Li, H. Liu, P. T. T. Nguyen, P. Ren, F. Wang, S. Wang, Y. Wang, Y. Wang, S. S. Wong, K. Yan, N. Yan, X. Yang, Y. Zhang, Z. Zhang, X. Zeng and H. Zhou, Green Energy Environ., 2023, 8, 10–114 CrossRef CAS.
- S. F. Koelewijn, S. Van den Bosch, T. Renders, W. Schutyser, B. Lagrain, M. Smet, J. Thomas, W. Dehaen, P. Van Puyvelde, H. Witters and B. F. Sels, Green Chem., 2017, 19, 2561–2570 RSC.
- H. A. Meylemans, T. J. Groshens and B. G. Harvey, ChemSusChem, 2012, 5, 206–210 CrossRef CAS.
- Q. Chen, W. Huang, P. Chen, C. Peng, H. Xie, Z. K. Zhao, M. Sohail and M. Bao, ChemCatChem, 2015, 7, 1083–1089 CrossRef CAS.
- S. S. Wong, R. Shu, J. Zhang, H. Liu and N. Yan, Chem. Soc. Rev., 2020, 49, 5510–5560 RSC.
- E. D. Hernandez, A. W. Bassett, J. M. Sadler, J. J. La Scala and J. F. Stanzione, ACS Sustain. Chem. Eng., 2016, 4, 4328–4339 CrossRef CAS.
- A. S. Trita, L. C. Over, J. Pollini, S. Baader, S. Riegsinger, M. A. R. Meier and L. J. Goossen, Green Chem., 2017, 19, 3051–3060 RSC.
- M. Q. Huang, D. Bai, Q. Chen, C. B. Zhao, T. H. Ren, C. J. Huang, M. North and H. B. Xie, Polym. Chem., 2020, 11, 5133–5139 RSC.
- Y. Chai, Q. Chen, C. Huang, Q. Zheng, M. North and H. Xie, Green Chem., 2020, 22, 4871–4877 RSC.
- S.-F. Koelewijn, D. Ruijten, L. Trullemans, T. Renders, P. Van Puyvelde, H. Witters and B. F. Sels, Green Chem., 2019, 21, 6622–6633 RSC.
- X. Wu, D. Xu, M. De Bruyn, G. Trimmel and K. Barta, Polym. Chem., 2023, 14, 907–912 RSC.
- M. D. Garrison, P. J. Storch, W. S. Eck, V. H. Adams, P. W. Fedick and B. G. Harvey, Green Chem., 2021, 23, 8016–8029 RSC.
- C. Martin and A. W. Kleij, Macromolecules, 2016, 49, 6285–6295 CrossRef CAS.
- G. Paggiola, S. V. Stempvoort, J. Bustamante, J. M. V. Barbero, A. J. Hunt and J. H. Clark, Biofuels, Bioprod. Biorefin., 2016, 10, 686–698 CrossRef CAS.
- CHN Pat., CN103333329A, 2013 Search PubMed.
- R. Ciriminna, M. Lomeli-Rodriguez, P. Demma Carà, J. A. Lopez-Sanchez and M. Pagliaro, Chem. Commun., 2014, 50, 15288–15296 RSC.
- F. W. Shaarani and J. J. Bou, Sci. Total Environ., 2017, 598, 931–936 CrossRef CAS.
- Y. H. Jiang, Y. S. Qin, L. J. Qiao, X. H. Wang, X. J. Zhao and F. S. Wang, Chin. J. Appl. Chem., 2009, 26, 770–774 CAS.
- M. Alves, B. Grignard, S. Gennen, C. Detrembleur, C. Jerome and T. Tassaing, RSC Adv., 2015, 5, 53629–53636 RSC.
- C. Chang, Y. Qin, X. Luo and Y. Li, Ind. Crops Prod., 2017, 99, 34–40 CrossRef CAS.
- S. Cui, Y. Qin and Y. Li, ACS Sustain. Chem. Eng., 2017, 5, 9014–9022 CrossRef CAS.
- Y. Y. Zhang, X. H. Zhang, R. J. Wei, B. Y. Du, Z. Q. Fan and G. R. Qi, RSC Adv., 2014, 4, 36183–36188 RSC.
- J. Yang, J. C. Dong, Y. P. Wang, X. Zhang, B. Y. Liu, H. F. Shi and L. R. He, Macromolecules, 2021, 54, 8503–8511 CrossRef CAS.
- X. H. Liu, C. C. Pang, J. B. Ma and H. Gao, Macromol. Chem. Phys., 2017, 50, 7949–7958 CAS.
- C. C. Pang, X. S. Jiang, Y. Yu, X. H. Liu, J. Y. Lian, J. B. Ma and H. Gao, ACS Sustain. Chem. Eng., 2018, 6, 17059–17067 CrossRef CAS.
- O. Bonjour, I. Liblikas, T. Pehk, T. Khai-Nghi, K. Rissanen, L. Vares and P. Jannasch, Green Chem., 2020, 22, 3940–3951 RSC.
- S. Gennen, B. Grignard, T. Tassaing, C. Jerome and C. Detrembleur, Angew. Chem., Int. Ed., 2017, 56, 10394–10398 CrossRef CAS.
- F. Ouhib, L. Meabe, A. Mahmoud, N. Eshraghi, B. Grignard, J.-M. Thomassin, A. Aqil, F. Boschini, C. Jérôme, D. Mecerreyes and C. Detrembleur, J. Mater. Chem. A, 2019, 7, 9844–9853 RSC.
- F. Siragusa, E. Van den Broeck, C. Ocando, A. J. Muller, G. De Smet, B. U. W. Maes, J. De Winter, V. Van Speybroeck, B. Grignard and C. Detrembleur, ACS Sustain. Chem. Eng., 2021, 9, 1714–1728 CrossRef CAS.
- R. Montgomery and L. F. Wiggins, J. Chem. Soc., 1946, 390–393, 10.1039/jr9460000390.
- C. H. Lee, H. Takagi, H. Okamoto and M. Kato, Polym. J., 2015, 47, 639–643 CrossRef CAS.
- W. Zhu, X. Huang, C. Li, Y. Xiao, D. Zhang and G. Guan, Polym. Int., 2011, 60, 1060–1067 CrossRef CAS.
- H. Schnell, Chemistry and physics of polycarbonates, Interscience Publishers, New York, 1964 Search PubMed.
- D. Braun and M. Bergmann, Adv. Synth. Catal., 1992, 334, 298–310 CAS.
- S. Chatti, G. Schwarz and H. R. Kricheldorf, Macromolecules, 2006, 39, 9064–9070 CrossRef CAS.
- S. Chatti, H. R. Kricheldorf and G. Schwarz, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 3616–3628 CrossRef CAS.
- Y. S. Eo, H. W. Rhee and S. Shin, J. Ind. Eng. Chem., 2016, 37, 42–46 CrossRef CAS.
- X. L. Shen, Z. Q. Wang, Q. Y. Wang, S. Y. Liu and G. Y. Wang, Chin. J. Polym. Sci., 2018, 36, 1027–1035 CrossRef CAS.
- M. Zhang, W. Q. Lai, L. L. Su and G. Z. Wu, Ind. Eng. Chem. Res., 2018, 57, 4824–4831 CrossRef CAS.
- M. Zhang, W. Q. Lai, L. L. Su, Y. Lin and G. Z. Wu, Polym. Chem., 2019, 10, 3380–3389 RSC.
- Q. Li, W. X. Zhu, C. C. Li, G. H. Guan, D. Zhang, Y. N. Xiao and L. C. Zheng, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1387–1397 CrossRef CAS.
- Z. F. Yang, L. Liu, H. Z. An, C. H. Li, Z. C. Zhang, W. J. Fang, F. Xu and S. J. Zhang, ACS Sustain. Chem. Eng., 2020, 8, 9968–9979 CrossRef CAS.
- W. J. Fang, Z. C. Zhang, Z. F. Yang, Y. Q. Zhang, F. Xu, C. H. Li, H. Z. An, T. Song, Y. J. Luo and S. J. Zhang, Green Chem., 2020, 22, 4550–4560 RSC.
- W. Sun, F. Xu, W. G. Cheng, J. Sun, G. Q. Ning and S. J. Zhang, Chin. J. Catal., 2017, 38, 908–917 CrossRef CAS.
- C. K. Ma, F. Xu, W. G. Cheng, X. Tan, Q. Su and S. J. Zhang, ACS Sustain. Chem. Eng., 2018, 6, 2684–2693 CrossRef CAS.
- Z. C. Zhang, F. Xu, Y. Q. Zhang, C. H. Li, H. Y. He, Z. F. Yang and Z. X. Li, Green Chem., 2020, 22, 2534–2542 RSC.
- W. W. Wang, Y. Q. Zhang, Z. F. Yang, Z. C. Zhang, W. J. Fang, D. H. Niu, H. Y. He and F. Xu, Green Chem., 2021, 23, 973–982 RSC.
- W. Qian, L. Liu, Z. L. Zhang, Q. Su, W. Z. Zhao, W. G. Cheng, L. Dong, Z. F. Yang, R. B. Bai, F. Xu, Y. Q. Zhang and S. J. Zhang, Green Chem., 2020, 22, 2488–2497 RSC.
- W. Qian, X. F. Ma, L. Liu, L. L. Deng, Q. Su, R. B. Bai, Z. L. Zhang, H. B. Gou, L. Dong, W. G. Cheng and F. Xu, Green Chem., 2020, 22, 5357–5368 RSC.
- W. J. Fang, Y. Q. Zhang, Z. F. Yang, Z. C. Zhang, F. Xu, W. W. Wang, H. Y. He, Y. Y. Diao, Y. Q. Zhang and Y. J. Luo, Appl. Catal., A, 2021, 617, 118111 CrossRef CAS.
- W. J. Fang, F. Xu, Y. Q. Zhang, H. Wang, Z. C. Zhang, Z. F. Yang, W. W. Wang, H. Y. He and Y. J. Luo, Catal. Sci. Technol., 2022, 12, 1756–1765 RSC.
- Z. F. Yang, X. Li, F. Xu, W. W. Wang, Y. Q. Shi, Z. C. Zhang, W. J. Fang, L. Liu and S. J. Zhang, Green Chem., 2021, 23, 447–456 RSC.
- J. Sun, J. Q. Wang, L. Wang and S. J. Zhang, Sci. Sin.: Chim., 2014, 44, 1050–1057 Search PubMed.
- K. Dong, X. M. Liu, H. F. Dong, X. P. Zhang and S. J. Zhang, Chem. Rev., 2017, 117, 6636–6695 CrossRef CAS.
- Y. Chen and T. Mu, GreenChE, 2021, 2, 174–186 Search PubMed.
- H. Y. Ju, M. D. Manju, D. W. Park, Y. Choe and S. W. Park, React. Kinet. Catal. Lett., 2007, 90, 3–9 CrossRef CAS.
- M. Selva, A. Perosa and G. Fiorani, Green Chem., 2018, 20, 288–322 RSC.
- F. Arico, U. Toniolo and P. Tundo, Green Chem., 2012, 14, 58–61 RSC.
- J. R. Ochoa-Gomez, S. Gil-Rio, B. Maestro-Madurga, O. Gomez-Jimenez-Aberasturi and F. Rio-Perez, Arabian J. Chem., 2016, 12, 4764–4774 CrossRef.
- J. R. Ochoa-Gomez, L. Lorenzo-Ibarreta, C. Dineiro-Garcia and O. Gomez-Jimenez-Aberasturi, RSC Adv., 2020, 10, 18728–18739 RSC.
- W. Qian, X. Tan, Q. Su, W. G. Cheng, F. Xu, L. Dong and S. J. Zhang, ChemSusChem, 2019, 12, 1169–1178 CrossRef CAS.
- J. H. Kamps, V. Ramakrishnan, T. Hoeks, B. J. P. Jansen, R. P. Sijbesma and J. P. A. Heuts, Macromolecules, 2019, 52, 3187–3198 CrossRef CAS.
- S. A. Park, J. Choi, S. Ju, J. Jegal, K. M. Lee, S. Y. Hwang, D. X. Oh and J. Park, Polymer, 2017, 116, 153–159 CrossRef CAS.
- M. Yokoe, K. Aoi and M. Okada, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 2312–2321 CrossRef CAS.
- S. Fadlallah, A. L. Flourat, L. M. M. Mouterde, M. Annatelli, A. A. M. Peru, A. Gallos, F. Arico and F. Allais, Macromol. Rapid Commun., 2021, 42, 2100284 CrossRef CAS.
- Z. C. Zhang, F. Xu, H. Y. He, W. L. Ding, W. J. Fang, W. Sun, Z. X. Li and S. J. Zhang, Green Chem., 2019, 21, 3891–3901 RSC.
- C. H. Li, Z. C. Zhang, Z. F. Yang, W. J. Fang, H. Z. An, T. Li and F. Xu, React. Funct. Polym., 2020, 155, 104689 CrossRef CAS.
- C. Li, X. Long, Q. Y. Wang, J. G. Li, H. Zhang and G. Y. Wang, J. Polym. Res., 2022, 29, 426 CrossRef CAS.
- JPN Pat., EP2033981A1, 2007 Search PubMed.
- S. J. Park, J. E. Lee, H. B. Lee, J. Park, N. K. Lee, Y. Son and S. H. Park, Addit. Manuf., 2020, 31, 100974 CAS.
- D. Hult, S. Garcia-Gallego, T. Ingverud, O. C. J. Andren and M. Malkoch, Polym. Chem., 2018, 9, 2238–2246 RSC.
- J. H. Park, M. S. Koo, S. H. Cho and M. Y. Lyu, Macromol. Res., 2017, 25, 1135–1144 CrossRef CAS.
- J. Y. Chu, H. Wang, Y. W. Zhang, Z. K. Li, Z. C. Zhang, H. Y. He, Q. Q. Zhang and F. Xu, React. Funct. Polym., 2022, 170, 105145 CrossRef CAS.
- C. Li, X. Long, T. Sun, Q. Wang and G. Wang, ChemistrySelect, 2023, 8, e202204829 CrossRef CAS.
- E. K. Macdonald and A. P. Shaver, Polym. Int., 2015, 64, 6–14 CrossRef CAS.
- W. Q. Lai, L. L. Su, M. Zhang, J. Yan and G. Z. Wu, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 1670–1681 CrossRef CAS.
- S. D. Yan, W. Q. Lai, M. Zhang and G. Z. Wu, Polymer, 2020, 210, 122999 CrossRef CAS.
- S. D. Yan and G. Z. Wu, Polym. Degrad. Stab., 2021, 192, 109703 CrossRef CAS.
- S. D. Yan and G. Z. Wu, Polym. Degrad. Stab., 2022, 202, 110028 CrossRef CAS.
- S. A. Park, Y. Eom, H. Jeon, J. M. Koo, E. S. Lee, J. Jegal, S. Y. Hwang, D. X. Oh and J. Park, Green Chem., 2019, 21, 5212–5221 RSC.
- L. Feng, W. X. Zhu, C. C. Li, G. H. Guan, D. Zhang, Y. N. Xiao and L. C. Zheng, Polym. Chem., 2015, 6, 633–642 RSC.
- M. Yousfi, J. Soulestin, S. Marcille and M. F. Lacrampe, Polymer, 2021, 217, 123445 CrossRef CAS.
- A. Brandolese and A. W. Kleij, Acc. Chem. Res., 2022, 55, 1634–1645 CrossRef CAS.
- F. Parrino, A. Fidalgo, L. Palmisano, L. M. Ilharco, M. Pagliaro and R. Ciriminna, ACS Omega, 2018, 3, 4884–4890 CrossRef CAS.
- D. D. Zhang, E. A. del Rio-Chanona, J. L. Wagner and N. Shah, Sustain. Prod. Consum., 2018, 14, 152–160 CrossRef.
- C. L. Li, R. J. Sablong, R. van Benthem and C. E. Koning, ACS Macro Lett., 2017, 6, 684–688 CrossRef CAS.
- O. Hauenstein, M. M. Rahman, M. Elsayed, R. Krause-Rehberg, S. Agarwal, V. Abetz and A. Greiner, Adv. Mater. Technol., 2017, 2, 1700026 CrossRef.
- F. Castro-Gomez, G. Salassa, A. W. Kleij and C. Bo, Chem.–Euro. J., 2013, 19, 6289–6298 CrossRef CAS.
- M. Palenzuela, D. Sanchez-Roa, J. Damian, V. Sessini and M. E. G. Mosquera, in Advances in Organometallic Chemistry, ed. P. J. Perez, Elsevier Academic Press Inc, San Diego, 2021, vol. 75, pp. 55–93 Search PubMed.
- C. M. Byrne, S. D. Allen, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2004, 126, 11404–11405 CrossRef CAS.
- L. P. Carrodeguas, J. Gonzalez-Fabra, F. Castro-Gomez, C. Bo and A. W. Kleij, Chem.–Euro. J., 2015, 21, 6115–6122 CrossRef.
- S. J. Poland and D. J. Darensbourg, Green Chem., 2017, 19, 4990–5011 RSC.
- D. Steiner, L. Ivison, C. T. Goralski, R. B. Appell, J. R. Gojkovic and B. Singaram, Tetrahedron: Asymmetry, 2002, 13, 2359–2363 CrossRef CAS.
- O. Hauenstein, M. Reiter, S. Agarwal, B. Rieger and A. Greiner, Green Chem., 2016, 18, 760–770 RSC.
- M. Reiter, S. Vagin, A. Kronast, C. Jandl and B. Rieger, Chem. Sci., 2017, 8, 1876–1882 RSC.
- S. Kernbichl and B. Rieger, Polymer, 2020, 205, 122667 CrossRef CAS.
- I. Grimaldi, F. Santulli, M. Lamberti and M. Mazzeo, Int. J. Mol. Sci., 2023, 24, 7642 CrossRef CAS.
- D. Y. Zhang, S. K. Boopathi, N. Hadjichristidis, Y. Gnanou and X. S. Feng, J. Am. Chem. Soc., 2016, 138, 11117–11120 CrossRef CAS.
- M. C. Jia, D. Y. Zhang, G. W. de Kort, C. Wilsens, S. Rastogi, N. Hadjichristidis, Y. Gnanou and X. S. Feng, Macromolecules, 2020, 53, 5297–5307 CrossRef CAS.
- D. D. Zhang, X. S. Feng, Y. Gnanou and K. W. Huang, Macromolecules, 2018, 51, 5600–5607 CrossRef CAS.
- V. B. Moreira, C. Aleman, J. Rintjema, F. Bravo, A. W. Kleij and E. Armelin, ChemSusChem, 2022, 15, e202102624 CrossRef CAS.
- R. Zeinali, L. J. del Valle, L. Franco, I. Yousef, J. Rintjema, C. Aleman, F. Bravo, A. W. Kleij and J. Puiggali, Polymers, 2022, 14, 161 CrossRef CAS.
- T. S. Anderson and C. M. Kozak, Eur. Polym. J., 2019, 120, 109237 CrossRef CAS.
- S. Neumann, L. C. Leitner, H. Schmalz, S. Agarwal and A. Greiner, ACS Sustain. Chem. Eng., 2020, 8, 6442–6448 CrossRef CAS.
- J. Bailer, S. Feth, F. Bretschneider, S. Rosenfeldt, M. Drechsler, V. Abetz, H. Schmalz and A. Greiner, Green Chem., 2019, 21, 2266–2272 RSC.
- S. Kernbichl, M. Reiter, J. Mock and B. Rieger, Macromolecules, 2019, 52, 8476–8483 CrossRef CAS.
- S. Neumann, S. B. Dabritz, S. E. Fritze, L. C. Leitner, A. Anand, A. Greiner and S. Agarwal, Polym. Chem., 2021, 12, 903–910 RSC.
- L. P. Carrodeguas, T. T. D. Chen, G. L. Gregory, G. S. Sulley and C. K. Williams, Green Chem., 2020, 22, 8298–8307 RSC.
- S. Neumann, P. Hu, F. Bretschneider, H. Schmalz and A. Greiner, Macromol. Mater. Eng., 2021, 306, 2100090 CrossRef CAS.
- O. Hauenstein, S. Agarwal and A. Greiner, Nat. Commun., 2016, 7, 11862 CrossRef CAS.
- C. L. Li, R. J. Sablong and C. E. Koning, Eur. Polym. J., 2015, 67, 449–458 CrossRef CAS.
- C. L. Li, S. van Berkel, R. J. Sablong and C. E. Koning, Eur. Polym. J., 2016, 85, 466–477 CrossRef CAS.
- C. L. Li, M. Johansson, R. J. Sablong and C. E. Koning, Eur. Polym. J., 2017, 96, 337–349 CrossRef CAS.
- C. L. Li, M. Johansson, P. Buijsen, G. Dijkstra, R. J. Sablong and C. E. Koning, Prog. Org. Coat., 2021, 151, 106073 CrossRef CAS.
- C. L. Li, R. J. Sablong and C. E. Koning, Angew. Chem., Int. Ed., 2016, 55, 11572–11576 CrossRef CAS.
- N. Kindermann, A. Cristofol and A. W. Kleij, ACS Catal., 2017, 7, 3860–3863 CrossRef CAS.
- V. B. Moreira, J. Rintjema, F. Bravo, A. W. Kleij, L. Franco, J. Puiggali, C. Aleman and E. Armelin, ACS Sustain. Chem. Eng., 2022, 10, 2708–2719 CrossRef.
- C. L. Li, T. Veldhuis, B. Reuvers, R. J. Sablong and C. E. Koning, Polym. Int., 2020, 69, 24–30 CrossRef CAS.
- F. Siragusa, T. Habets, R. Mereau, G. Evano, B. Grignard and C. Detrembleur, ACS Sustain. Chem. Eng., 2022, 10, 8863–8875 CrossRef CAS.
- Y. Yu, L. M. Fang, Y. Liu and X. B. Lu, ACS Catal., 2021, 11, 8349–8357 CrossRef CAS.
- K. Saito, F. Eisenreich, T. Turel and Z. Tomovic, Angew. Chem., Int. Ed., 2022, 61, e202211806 CrossRef CAS.
Footnote |
| † Equal contributions to this manuscript. |
| This journal is © The Royal Society of Chemistry 2023 |