Open Access Article
Open Access ArticleCleaning steel by devulcanizing rubber from used automotive tires†
Yang
Chen
a,
Saleh
Ibrahim
a,
Sijia
Zheng
a,
Liam
Wittenberg
 a,
Spencer
Chapple
a,
Griffin
LaChapelle
a,
Cheok Hang
Iao
a,
Adam
Bourke
b and
Michael A.
Brook
a,
Spencer
Chapple
a,
Griffin
LaChapelle
a,
Cheok Hang
Iao
a,
Adam
Bourke
b and
Michael A.
Brook
 *a
*a
aChemistry and Chemical Biology, McMaster University, 1280 Main Street West, Hamilton, ON L8S 4M1, Canada. E-mail: mabrook@mcmaster.ca
beTracks, Suite 401, 2275 Upper Middle Road East, Oakville, Ontario L6H 0C3, Canada
First published on 23rd September 2023
Abstract
In many cases, only a fraction of the steel recovered from end-of-life automobile tires can be recycled. About 10–20% of the steel isolated after grinding/shredding the tires is too contaminated with rubber to be accepted by metal recyclers. We report that complete devulcanization to ‘dissolve’ the rubber from the steel is possible, but not necessary. Much less chemical intervention is required, resulting in lower overall efficiency, and lower environmental impact, if the samples are first: magnetically separated from rubber; swollen with toluene and, after physical agitation, again magnetically separated (to remove up to 90% of rubber present on steel) and finally subjected to chemical treatment to chemically strip the rubber from steel by partial devulcanization (using about 1/10 the catalyst required for complete devulcanization); combining these two means that only about 1% of the B(C6F5)3 catalyst is needed to clean steel compared to complete devulcanization of an as received sample. This process is more cost competitive, the toluene solvent remains internal to the process, and the steel produced is sufficiently clean to be sent to recyclers rather than to landfill.
Sustainability spotlightLandfilling industrial products that could otherwise be reused contravenes several of the UN SDGs, particularly, responsible production and consumption (12) and industry, innovation and infrastructure (9). Depending on jurisdiction at end of life, tires are: landfilled; burned as fuel; pyrolyzed to obtain lower quality materials; or shredded for secondary use as fillers. For the latter outcome, and in the best case scenario, much of the steel found in steel-belted automobile tires is recycled by metal recyclers but 10–20% has to be landfilled because of excessive contamination by rubber. This paper takes on these 2 SDGs by reporting a method that allows the remaining portion of steel to be recycled. |
Introduction
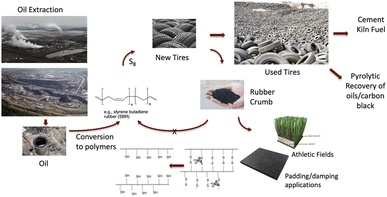
Steel is the most recycled material on the planet,1 and ever increasing fractions of recycling lead to improving circularity. This trend is particularly beneficial for the planet, as the energy needed to recycle steel is only a small fraction of that needed for the initial production of steel from iron ore. Some contaminants, however, compromise the ability of steel to be recycled. One of these is the rubber that contaminates steel recovered from used automobile tires.It is inarguable that the quality of life throughout the world hinges on efficient transportation, most of which depends on rubber tires. Combinations of natural and synthetic rubber are used to create tires that may be categorized in three main areas: enormous tires used for heavy industry, e.g., mining; ‘big rig’ truck tires used to deliver goods across continents; and, automobile tires. The numbers are enormous, with estimates of 2.2 billion automobile tires manufactured in 2021 alone;2 these are the focus of this paper.
Petroleum is converted into resilient and robust elastomer products by converting oil derivatives into polymers – pre-elastomers. A complex cocktail of internal fillers including carbon black and silica, catalysts, accelerators, and other ingredients such as antioxidants are required to first facilitate rubber formation and then stabilize it after production. For the purposes of this paper and a consideration of circularity, the most important ingredients to consider are fiber (cord, sidewall) and steel (tread) reinforcing agents and the sulfur that crosslinks/vulcanizes available alkenes in the pre-elastomer by forming oligosulfide bridges (Fig. 1).3,4 Note that different parts of automobile tires are comprised of different elastomers5 and the constitutions of tires will also depend on the manufacturer. Over the decades, the longevity of automobile tires has dramatically improved and tires guaranteed for 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km in normal use are now common.6 But what happens at the end of life of the automobile tire?
000 km in normal use are now common.6 But what happens at the end of life of the automobile tire?
One can view automobile tires as a problematic example of ‘single use polymers’. Once the tread is too thin, after only a small fraction of the mass of a tire is lost, most of the value of the tire is also lost; while truck tires may be retreaded, this is not common with automobile tires.7 The next stage in a tire ‘life cycle’ is highly dependent on jurisdiction (Fig. 1).
Stockpiling
In some locales tires are collected and just left in large piles – a type of landfilling. Storing tires this way is problematic. Leachate has been shown to be toxic to fish (salmon) found in adjacent waterways.8 Stockpiles of tires also serve as fire hazards. Tire fires are hard to control. They burn very hot and are environmentally problematic because they create a lot of soot and undesirable pollutants like sulfur oxides from sulfur combustion. A tire fire in Hagersville, Ontario, Canada, where 14 million tires were stored, burned for 17 days in 1990 until it could finally be controlled.9 Even with care, such fires are difficult to avoid. There was a fire at a tire recycling facility in Minto, New Brunswick, Canada in 2019.10Controlled combustion
There are markets for used tires as a relatively cheap fuel, for example, in pulp and paper plants or cement kilns.11,12 Pyrolytic processes – thermally induced partial degradation – convert used rubber into oils and alkane gases that can be used as fuel, and permit recovery of carbon black filler in some cases;13,14 these processes can be challenged by the presence of residual sulfur.15 Partial devulcanization (decrosslinking) allows some reuse of rubber in a variety of applications, including new tires, but is limited to about 10 wt% (note: Michelin recently claimed as much as 25% partly devulcanized rubber from used tires can be put in new tires16).Low value applications
Sidewalls may be cut from tires to provide convenient weights, for example, on silage in farmers' fields or temporary highway markers. Much of the rubber from used automobile tires, however, is shredded to give tire rubber crumb (TCR), which is typically converted into low value materials – garden mulch, fillers in artificial turf for athletic fields, as a filler in asphalt, foam pads to walk on, etc.17 However, after these single re-uses, most of these rubber products will end up in landfills.Partial or complete recovery and reuse of tires is legally required in many jurisdictions. In Ontario, Canada (population ∼14 million), government regulations require that 85% of used tires must be diverted from landfills, that is, recycled at least once.18 One extra use before disposal to the environment is better than nothing, but not much. Full circularity would require the tire rubber be infinitely reused; that objective is far away for automotive rubber but need not be for other constituents in tires.
In addition to TCR, the automobile tire shredding process generates polyester and/or nylon fiber which, in the absence of viable applications in Ontario, at least, is mostly landfilled. The other key product is steel fiber/wire from tire tread,19 which constitutes about 13% of the total mass of the tire.20 An ‘average tire’ is considered to weigh 12.5 kg for light truck and on-road passenger tires, which correlates to about 1.6 kg of steel/tire.21 In Ontario, about 80–90% of the steel can be sold directly to metal recycling firms, but the remaining 10–20% is landfilled because it contains too much residual rubber. During recycling of rubber-contaminated steel, the rubber – like in a tire fire – will release SO2 → SO3 and recyclers either don't have the necessary scrubbing facilities to remove large quantities of these acidic gases, or their use is not justified by the value of the product. This paper describes an alternative strategy to better recover steel from used tires that is contaminated with rubber.
We previously described a chemical route – Piers–Rubinsztajn (PR) reduction of oligosulfides using silanes (HSi-containing small molecules and polymers) – to reduce S–S bonds in organic disulfides/polysulfides; only low quantities of B(C6F5)3 catalyst are required.22 The same PR process can be used to completely devulcanize used rubber tires by degrading the sulfur crosslinks.23 The process is mild, efficient and permits recovery of fillers, clean steel, and polymer fluids that can be crosslinked into new rubber (Fig. 2). Essentially all S–S bonds are cleaved in this process. While the PR process is efficient, it is not cost effective. The complex mixture of materials in the rubber partly poison the catalyst and, as a consequence, large ratios of the expensive B(C6F5)3 catalyst to rubber are needed, up to ∼10 wt%.
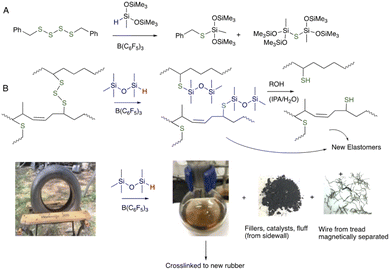 | ||
| Fig. 2 (A) Piers–Rubinsztajn reduction of disulfides.22 (B) Application of the PR process to tire rubber to create devulcanized polymers separated magnetically from metal wire and from catalyst/filler/additives/cord by filtration.23 | ||
We reasoned that only partial devulcanization of the rubber should be necessary to clean contaminated steel just enough that it can be sold to metal recyclers (a); complete rubber degradation, i.e., reduction of all S–S crosslinks in the rubber, is not required. We report a simple multistep process that permits rubber to be stripped from contaminated steel. Much less catalyst is required than for complete rubber devulcanization, and the steel can be diverted from landfill to useful products.
Results
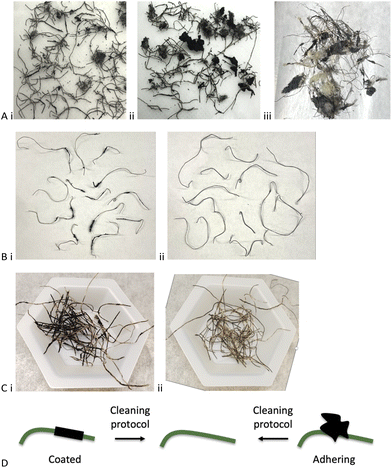
While an appropriate technical term for the rubber contaminated steel wire might be a ‘complex co-adhered mixture’, the pragmatist would call it an inhomogeneous ‘mess’. Different sample lots, even from the same production facility, were dramatically different with respect to homogeneity, the mass fraction of rubber, nominal size of adhering rubber particles and aspect ratio of the steel wires (Fig. 3Ai–iii). The constitution of one sample (Batch B, Table 1) included steel wire with lengths that ranged from about 0.2–5.0 cm, most of which were embedded in chunks of rubber and/or which had rubber coating along the fiber (Batch A, Table 1). The automotive rubber not adhered to the wire – rough, complex shapes – ranged in size from 2–30 mm diameter. Most of the rubber bound to wire carried 1–4 wires per crumb but there were exceptions where a single crumb was bound to a single wire. These crumbs too had a size distribution of about 0.5–3.5 cm.| Mass of magnetically separated rubber (Step 1)b | Mass of toluene-collected rubber (Step 2) | Mass of rubber after mortar (Step 3) | Wire mass after chemical reaction (Step 4) | Residual rubber on wirec | Wire mass in original sample | Total rubber in original sample | Rubber removed by physical means (%) | Rubber removed by physical + chemical protocol | Cleaning efficiencyd (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| a Batch A (low rubber content) and Batch B (high rubber content) are shown in Fig. 3Ai and Aii; we arbitrarily set the values low/high as below or above 5 wt% rubber on steel. b All masses are in g. Steps refer to Fig. 4. c Amount removed by combustion. d Residual rubber/total rubber × 100 (total rubber = 1 g Batch A or 5 g Batch B – wire mass after reaction). | ||||||||||
| Batch A 1 g scale | ||||||||||
| A1 | 0.01 | 0.007 | 0.030 | 0.954 | 0.022 | 0.932 | 0.068 | 69.1 | 0.046 | 67.4 |
| A2 | 0.182 | 0.01 | 0.056 | 0.666 | 0.000 | 0.666 | 0.334 | 74.3 | 0.334 | 100.0 |
| A3 | 0.092 | 0.021 | 0.059 | 0.817 | 0.036 | 0.781 | 0.219 | 78.5 | 0.183 | 83.4 |
| A4 | 0.021 | 0.065 | 0.022 | 0.867 | 0.014 | 0.853 | 0.147 | 73.5 | 0.133 | 90.4 |
| Average | 0.192 | 85.3 | ||||||||
| mg rubber cleaned per mg B(C 6 F 5 ) 3 | 139 | |||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||
| Batch B 5 g scale | ||||||||||
| B1 | 0.0718 | 0.8655 | 1.4222 | 2.4999 | 0.0423 | 2.4576 | 2.5424 | 92.8 | 2.500 | 98.3 |
| B2 | 0.1811 | 0.0104 | 1.1398 | 3.4501 | 0.2360 | 3.2141 | 1.7859 | 74.5 | 1.550 | 86.8 |
| B3 | 0.1388 | 0.0257 | 1.9436 | 2.6917 | 0.1744 | 2.5173 | 2.4827 | 84.9 | 2.308 | 93.4 |
| B4 | 0.2787 | 0.057 | 2.0687 | 2.0666 | 0.0036 | 2.063 | 2.9370 | 81.9 | 2.933 | 99.9 |
| Average | 2.4370 | 94.6 | ||||||||
| mg rubber cleaned per mg B(C 6 F 5 ) 3 | 372 | |||||||||
Initial plans to quantitate the ‘average’ mass fraction of rubber using thermogravimetric analysis (TGA) were abandoned because it was not possible to select an average rubber-on-wire sample; the variability of selected samples was extremely high (Fig. 3A and S1, ESI†). This high variability occurs between suppliers, batches from one supplier and within a given sample (note: various types of shredding processes are practiced commercially.24 In addition to differences arising from individual shredding processes used are the feedstocks for shredders that will vary from batch to batch and may include automobile and truck tires; the samples described here did not contain mining tires). One strategy used to measure rubber content involved physical removal of adhering particles with tweezers; the amount of rubber coating the wires ranged from 2–20 wt% over three sets of 5 randomly selected wires from the same batch (Table S1, ESI†). Alternatively, heating wires to red hot in a flame for 5–10 seconds and recording the differences in mass after burning led to comparable wide ranges of wt% rubber between samples (note: small amounts of char remained on some wires and, of course, even clean steel wires can gain mass as surface iron is converted to iron oxide; see experimental section for an analysis of the error this introduced Fig. 3B). To address the high degree of inhomogeneity in a given sample and between samples, all the experiments below were repeated with multiple sets of samples and changes in mass were followed after both physical and chemical treatments.
Control experiments – complete dissolution of the waste rubber
A positive control for the chemical cleaning process discussed below involved complete dissolution of rubber using the PR process (Fig. 2B), 10 wt% B(C6F5)3vs. rubber, and MHMH (HMe2SiOSiMe2H) as reducing agent in toluene at 100 °C for 1 hour.23 The result was shiny, clean steel wire that was readily removed by magnetic separation from the reaction medium by magnet (Fig. 3C). Note that a very small quantity (<1 wt%) of black material coated small areas of the wires, which could be removed by combustion, suggesting it is mostly carbon black.The catalyst used in this reduction process is expensive (note: over the last 2 years, prices for 100 g of this catalyst have ranged from US$5000–6500 from various suppliers; complete de-crosslinking of rubber can, as noted, require up to 10 wt% B(C6F5)3 catalyst). A series of physical strategies was explored with the hope to develop methods that would remove first at least a portion of the rubber from the steel. Any rubber removed physically would not require chemical reduction, which is desirable for both cost and green chemistry reasons;25 doing less chemistry to achieve a given objective will fundamentally be ‘greener’.
Even simple E-factor calculations make clear that the lower the need for solvents/chemistry per kg of recovered steel the better.26 In addition, it was hoped that only partial devulcanization would be needed to clean the steel, again lowering the degree of chemistry needed. One recognizes, however, that each additional physical/chemical process adds to cost. The studies below were developed as batch processes, but designed to be converted to sequential, continuous processing to enhance the practicality of the steel cleaning process.
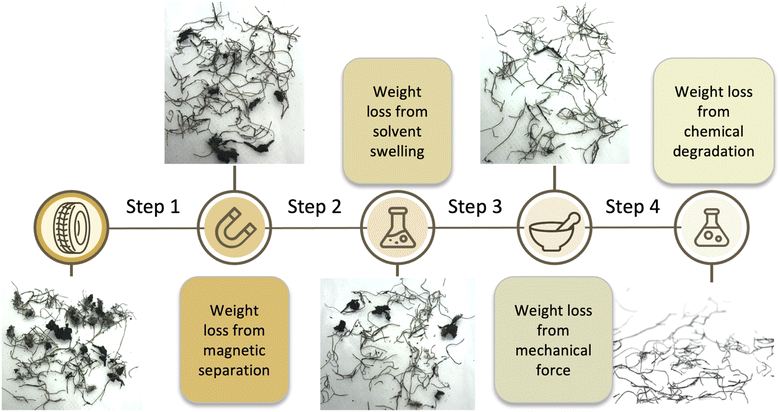
The optimized cleaning process
Extensive experimentation was required to identify and optimize physical processes to remove adhered rubber from wires in addition to the chemical devulcanization protocol. The optimized process shown schematically in Fig. 4 was designed around practicality: as few steps as practically possible were employed, and energetically favorable reactions were used that involve low consumption of chemicals.Mechanical removal of untethered or weakly bound rubber
The ferromagnetic properties of the steel wire facilitated physical separation of wire-containing materials from rubber particles at several stages of the process. Placing a fixed magnet on the outside of a flask, and then agitating, led to a population of rubber/wire associated with the magnet (Fig. S2, ESI†). The separate population of wire-free rubber was readily separated by gravity simply by inverting the flask while holding the magnet against the outside of the flask (Step 1, Fig. 4, Table 1). This separation was surprisingly efficient, given the relatively low magnetic field generated with a fixed magnet. A strong electromagnet, combined with mechanical agitation should allow the continuous separation of wire-free rubber from the raw sample.Swelling of rubber
We reasoned that the stress associated with a large change in volume upon swelling of rubber would both facilitate its dehesion from the surface of the wire and, potentially, break up rubber aggregates. Recovered rubber from automobile tires is comprised of a complex mix of rubbers with different constitutions, crosslink densities and originating from many suppliers; this feedstock stream will vary from batch to batch from a given producer and between producers. It was important to find one solvent that would both facilitate swelling to clean the rubber crumb and permit chemical modification.Rubber from tires swells efficiently in many solvents. Unfortunately, the best solvents to use for this process, based on a green chemistry assessment, e.g., water, alcohols, do not solvate rubber effectively as they are too polar.27 Toluene efficiently swelled automobile tire rubber (e.g., SBR (styrene butadiene rubber), natural rubber and other rubber materials) in the samples examined. It is not an ideal non-polar solvent with respect to green chemistry, although better than many other alternatives.28 A secondary benefit is its convenient boiling point (110–111 °C): not too low, such the off-gassing of the flammable solvent is difficult to manage, recovery is straightforward from a distillation tower; and not too high, as with chlorobenzene, xylenes. Finally, should it be released inadvertently to the environment, it undergoes facile oxidative conversion to relatively benign benzoic acid.
Samples of (wire free) TCR were observed to undergo enormous dimensional change, ∼160 wt%, upon swelling in toluene overnight (in separate experiments, tread we cut from a tire was found to swell 180 wt% and sidewall 140 wt%, thus a ∼160% average swelling is expected). Solvent removal from the crumb by drying under vacuum; sitting in a fume hood; or heating in the oven led to particles that were essentially their original size. In some cases, the toluene solution had turned yellow, or even brown but after solvent evaporation this extract constituted less than 3–4 wt% of the available rubber in the sample. The desired outcome was thus achieved. There was a measurable loss of rubber from the rubber-adhered wires: after swelling; magnetic separation; and evaporation of solvent (Step 2, Fig. 4, Table 1). As with the previous magnetic separation, this process can readily be adapted to be continuous.
Performing an additional mechanical agitation step prior to chemical etching, following by drying, led to a more effective removal of rubber from the wire (Step 3, Fig. 4, Table 1). This was performed simply by using a mortar/pestle or impacting the rubber/wire with a hammer. While it was anticipated that ball milling would effectively dislodge rubber from wire at the various stages of the overall process, it proved less efficient than simple mechanical agitation with induced shear.
Chemical etching
Systematic studies were undertaken to find reaction conditions that would: lead to loss of rubber most rapidly; at the lowest practical temperature; and with the least amount of B(C6F5)3 catalyst possible. It was found that the most practical conditions involved reacting a toluene solution containing 0.5 wt% B(C6F5)3 at 100 °C for 1 hour (Step 4, Fig. 4, Table 1). In addition, the efficiency of the process could be increased by filtering off at intervals dislodged rubber, a process that is facilitated by magnetic removal of wires. Doing so better targets the chemistry to the rubber adhering to the steel rather than all available rubber. As a consequence, the reaction medium could be used 3–5 times with comparable efficiency before it became necessary to add more catalyst and additional silane reducing agent.Table 1 demonstrates that the constitution of the sample can really affect the efficiency of the process. When much of the rubber is found as a coating on the surface of the wire, the process is less efficient, as with Batch A in which 1 mg of B(C6F5)3 cleaned 139 mg of rubber from the wire. By contrast, when the rubber presenting in the sample is mostly adhering as a large pieces to the wire, the process is more efficiently: 1 mg of catalyst cleaned 372 mg of rubber.
While the rubber removal steps were performed in batch mode, it should be possible to modify the process to make it continuous. An advantage of the magnetic character of the wires is ready removal from the reaction solution that facilitates continuous filtration of dislodged rubber that then is removed from chemical processing. It is worth noting that the partially degraded rubber formed elastomeric films upon drying, suggesting the rubber byproduct could be targeted for application as an adhesive, rather than be sent to a waste stream.
Discussion
One of the challenges in recycling automobile tire rubber is the complexity of the product. The rubber itself is a diverse mixture of petroleum-based polymers, sulfur for crosslinking and a soup of fillers, catalysts, accelerators, etc. TCR is obtained by shredding tires at normal temperature or under cryogenic conditions.29 In addition to the desired rubber crumb are several waste streams, including polyester and/or polyamide cord that is used to reinforce sidewalls and the steel belts used to reinforce tread. The ‘fluff’ arising from the polymer cord is, in Ontario, landfilled along with 10–20% of the steel because no viable products have been identified with their use, in part because of rubber contamination. The objective of this research was to develop methods to permit the rubber-contaminated steel that is currently landfilled to instead be sold to metal recyclers, better closing the loop on this tire constituent.Efficient reactions that degrade/devulcanize the rubber that contaminates steel from shredded tires are rare.23 It is possible to completely dissolve rubber adhering to steel using B(C6F5)3-catalyzed reduction of S–S crosslinks. There are two disadvantages to the process: the need for high catalyst concentrations (up to 10 wt%) because the constituents in the tire mixture can poison the catalyst; and, chemical redundancy – while only one S–S bond needs to be broken to cleave each crosslink essentially all the S–S bonds are cleaved in the reducing reaction. In fact, to achieve the objective of cleaning the wires, only the rubber adhering to the steel needs to be partly degraded/devulcanized. To mitigate these deficiencies, the devulcanization process was adapted.
Readily induced mechanical stress, including the use of elastomer swelling, and magnetic separation physically removes a significant portion of the rubber (up to 90%, Table 1).
Toluene is much more benign than its cousin benzene – because it can undergo environmental oxidation30 – and exhibits much lower toxicity, particularly mutagenicity. However, even excellent recovery processes will be imperfect and some toluene can be expected to escape the process from the gas phase, or remain as a residue within the rubber. It is not considered a green solvent (category yellow28), but is much less problematic than many other solvents that are able to swell complex mixtures of elastomers (chlorinated solvents, high boilers).
The complex mixture of rubbers in TCR swell very effectively – ∼160 wt% – in toluene. As shown from Table 1, the generated stress facilitates dehesion of available rubber from the wire per se, and also facilitates the loss of more rubber with subsequent mechanical shear (Step 3, Fig. 4). Any rubber lost in these steps no longer needs to be chemically degraded, saving both costs of reagents and energy.
The chemical process to completely dissolve automobile rubber has already been reported.23 Wire coated with rubber, similar to blocks of rubber or TCR, also required about 5–10 wt% catalyst for complete devulcanization. However, there is no need for complete devulcanization. Once enough rubber degradation has occurred to loosen the particle from the wire, it can be physically/magnetically separated. This was achieved in Batch A (Table 1) using only 0.7 wt% B(C6F5)3 catalyst and 0.27 wt% for Batch B; these are a small fraction of the catalyst needed for complete devulcanization. The differences between Batches A and B – 20 wt% vs. 50 wt% rubber – are consistent with two different populations of rubber. In the first, the rubber is coated along the shaft of the wire and essentially complete degradation is necessary to clean the wire. The other population consists of crumb particles adhering to the wire (coated vs. adhering, Fig. 3Ci vs. Aii and 3D). Note that the liquor recovered after cleaning the steel can be recovered and reused for several batches until the B(C6F5)3 loses potency. It can be seen that the constitution of wire/rubber samples even from a given batch are wildly different, but the process maintains good to excellent cleaning capacity over 4 sequential cycles (different wires, same cleaning solution).
While developed here as a batch process, each of the steps described: magnetic separation of steel from unbound rubber; swelling and mechanical abrasion – also with magnetic separation; and then a chemical cleaning that separates metal from partly digested rubber by filtration – are amenable to continuous, sequential processes. As shown in Fig. 4, Table 1, the process leads to removal of 65–100% of the rubber initially present. To date, we have focused on recovering clean steel. It will be recognized that the partially devulcanized rubber should have attractive properties, a hypothesis we are exploring.
The simple processes described here to recover clean steel and partly degraded rubber are imperfect, yet permit one to divert rubber-contaminated steel from landfills. The utilization of swelling and mechanical separation can remove up to 90% of the rubber before doing any chemistry, which dramatically reduces the magnitude of the problem per se. The need for only partial devulcanization of the low levels of residual rubber means that less than one tenth of the B(C6F5)3 catalyst is needed to achieve clean steel, compared to compete de-crosslinking, a demonstration of enhanced atom economy (Green Principle 2). Thus, the net requirement for B(C6F5)3 catalyst is only about 1% of that needed to complete devulcanize the crude rubber-on-steel samples.
Steel is a valuable resource and possibly the most recycled material on the planet;1 the benefits of recycling rather than sending it to landfill are obvious. While there are, of course, costs to permit that diversion, including the use/recovery of the toluene solvent that dramatically improves the efficiency of the cleaning process, and the catalyst, the value of the recyclable steel should offset the costs of cleaning the metal and avoiding landfill fees. From a sustainability perspective the process described uses (much less) simple chemistry to turn waste into value, improving the circularity of steel in automobile tires.
Experimental section
Materials
Several batches from different suppliers of rubber contaminated steel (Fig. 3) were provided courtesy of eTracks. MHM (97%, HMe2SiOSiMe3, pentamethyldisiloxane), Bis-H (97%, HMeSi(OSiMe3)2, bistrimethylsiloxymethylsilane), and MHMH (99%, HMe2SiOSiMe2H, tetramethyldisiloxane) were purchased from Gelest and stored over 4 Å molecular sieves. A stock solution of B(C6F5)3 (the process catalyst) obtained from Alfa-Aesar was prepared: 40 mg were introduced into dry toluene (10 mL) in a pre-dried flask, sealed with a septum and stored under nitrogen. Toluene (reagent grade, Caledon) was dried using an activated alumina column before use. A horseshoe magnet (∼12 cm × 5.7 cm × 1.2 cm deep, ESI†) was obtained from a local hardware store. A Retsch MM500 Nano Ball mill was used at 35 Hz (∼2100 rpm) for 2 min using 10 mm steel balls.Rubber quantitation
The quantity of rubber adhering to steel wire at different stages of the cleaning process was confirmed by a combination of mechanical removal of large rubber pieces and combustion of the adhering rubber. 5 wires, randomly selected from a given batch of rubber-on-steel wires, held at one end, were heated red hot in the flame of a Bunsen burner for ∼5–10 s. The process was repeated while holding the wires from the other end. After cooling, the change in mass was measured; rubber content on the contaminated wires ranged from about 2–20 wt% (Table S1†). In some cases, even after combustion, some residual char remained on the wire (e.g., Fig. 3Bii, Table 1). A second error in measurement arises from by oxidation of iron: steel burns. Either too hot a flame or exposure in the flame for too long led to partial or, sometimes, complete combustion to give iron oxide powder. The magnitude of the error in the measurements associated with iron oxidation (and its minimization by choice of burn times) was determined by mechanically cleaning wires and following changes in mass due only to iron oxidation in the flame.Controlled complete silylation
Wires from Batch A that were coated with rubber but did not have adhering rubber particles (1.0 g) were placed in a round-bottomed flask containing anhydrous toluene (20 mL). MHMH (1.5 mL, 1.14 g, 8.5 mmol, 17.0 mmol SiH) and B(C6F5)3 (3.3 mL × 1.5 mg mL−1 in toluene, 4.95 mg, 9.7 mmol) solution was added and the reaction heated at 100 °C for 1 h (comparable results were observed after 4 h at 70 °C). The clean steel samples were removed by filtration and dried in air, and showed a loss of 0.048 g of rubber (burning selected wires after cleaning showed less than 0.1 wt% residual rubber).Separation sequence
The external magnet, wrapped with a Kimwipe, was moved to the neck and then the rubber-contaminated wires were transferred out of the flask into a mortar – the Kimwipe was pulled away from the magnet so the wires were never in direct contact with the magnet (Step 3). The material was crushed within the mortar using a pestle. In a reverse operation of the previous process with a Kimwipe, the wires were weighed, and then transferred using the magnet back to the flask. The rubber left in the mortar was put in a 95 °C oven for at least 3 h and, after cooling to room temperature, weighed (Table 1).
Mechanical stress through grinding/shear induced by a mortar/pestle is acceptable for a very small-scale process. Alternatives, including agitation using a rotary evaporator (500 mL flask containing wires ∼0.5 g and glass balls, diameter = 3 mm, 16 g) or a more aggressive treatment with a ball mill (35 Hz, ∼2100 rpm, for 2 min using 10 mm steel balls) were actually less efficient. Mechanical stress created using a high shear Waring blender, normally designed for cooking, was also less efficient and accompanied by abrasion of the cutting blade. Thus, at large scale a mechanical tumbler is recommended to mimic the observations with the mortar and pestle.
Conclusions
Closing the life cycle of any product involves ensuring that materials, at end of life, are in a form that nature can readily process and/or can be completely directed to a new product: one product's waste is another's feedstock. The use of a combination of mechanical, physical (solvent swelling) and, finally partial cleavage of rubber that contaminates steel from shredded used automobile tires efficiently removes rubber from steel. The process is much more sustainable than simple chemical reaction both in net financial cost and, more importantly, the fact that much less chemistry needs to be done to achieve the goal. The consequence is that the steel is of sufficient quality to be recycled by metal processors rather than landfilled.Author contributions
Conceptualization, M. A. B. and A. B.; methodology and investigation Y. C., S. Z., S. I., L. W., G. L., C. H. I.; writing M. A. B.; supervision M. A. B.; project administration M. A. B.; funding acquisition, M. A. B. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support of the Natural Sciences and Engineering Research Council of Canada. It would not have been possible to undertake this work without the generous provision of rubber, and rubber/steel samples from suppliers Emterra (Brampton, Canada), CRM, Ideal Rubber, Evolve Recycling Inc. (all from Brantford Canada). We also thank Mr Mukesh Singh and Dr Rakesh Sahu for ball mill experiments and Dr Ravi Selvaganapathy, all of McMaster Mechanical Engineering, for helpful discussions.Notes and references
- Determination of Steel Recycling Rates in the United States: Technical Report, https://www.steel.org/wp-content/uploads/2021/08/AISI-and-SMA-Steel-Recycling-Rates-Report-Final-07-27-2021.pdf, accessed Aug. 31, 2023 Search PubMed.
- Global Tire Market Report 2022: Industry Trends, Share, Size, Growth, Opportunity and Forecast 2017-2021 & 2022-2027, https://www.businesswire.com/news/home/20220518005677/en/Global-Tire-Market-Report-2022-Industry-Trends-Share-Size-Growth-Opportunity-and-Forecast-2017-2021-2022-2027---ResearchAndMarkets.com, accessed Sept. 2, 2023 Search PubMed.
- A. Y. Coran, in The Science and Technology of Rubber, ed. J. E. Mark, B. Erman and C. M. Roland, Academic Press, Boston, 4th edn, 2013, pp. 337–381, DOI:10.1016/B978-0-12-394584-6.00007-8.
- C. Goodyear, US Pat., 3633, 1844 Search PubMed.
- E. T. McDonel, K. C. Baranwal and J. C. Andries, in Polymer Blends, ed. D. R. Paul and S. Newman, Academic Press, 1978, pp. 263–292, DOI:10.1016/B978-0-12-546802-2.50015-4.
- C. Reports, How to Chose Long Lasting Tread Tires, https://consumerreports.org/cro/news/2014/03/how-to-choose-long-lasting-tires/index.htm, accessed July 13, 2022 Search PubMed.
- S. Dabic-Miletic, V. Simic and S. Karagoz, Environ. Sci. Pollut. Res., 2021, 28, 68053–68070 CrossRef PubMed.
- Z. Tian, H. Zhao, T. Peter Katherine, M. Gonzalez, J. Wetzel, C. Wu, X. Hu, J. Prat, E. Mudrock, R. Hettinger, E. Cortina Allan, G. Biswas Rajshree, C. Kock Flávio Vinicius, R. Soong, A. Jenne, B. Du, F. Hou, H. He, R. Lundeen, A. Gilbreath, R. Sutton, L. Scholz Nathaniel, W. Davis Jay, C. Dodd Michael, A. Simpson, K. McIntyre Jenifer and P. Kolodziej Edward, Science, 2021, 371, 185–189 CrossRef CAS PubMed.
- Archives, CBC, May 16, 2019, https://cbc.ca/archives/1990-was-the-year-of-the-tire-fire-in-canada-1991.5126271 Search PubMed.
- K. Donkin, CBC, Feb 05, 2021, https://cbc.ca/news/canada/new-brunswick/minto-tire-fire-records-1.5900816.
- G. Sai Kishan, Y. Himath kumar, M. Sakthivel, R. Vijayakumar and N. Lingeshwaran, Mater. Today: Proc., 2021, 47, 5483–5488 CrossRef CAS.
- M. A. Barlaz, W. E. Eleazer and D. J. Whittle, Waste Manage. Res., 1993, 11, 463–480 CrossRef CAS.
- J. D. Martínez, N. Puy, R. Murillo, T. García, M. V. Navarro and A. M. Mastral, Renewable Sustainable Energy Rev., 2013, 23, 179–213 CrossRef.
- P. T. Williams, Waste Manage., 2013, 33, 1714–1728 CrossRef CAS PubMed.
- X. Zhang, J. Tang and J. Chen, J. Energy Inst., 2022, 102, 302–314 CrossRef CAS.
- J.-M. Douarre and M. Dorato, Presented in part at the International Symposium on Green Chemistry, La Rochelle, France, 2022 Search PubMed.
- J. Karger-Kocsis, L. Mészáros and T. Bárány, J. Mater. Sci., 2013, 48, 1–38 CrossRef CAS.
- G. Ontario, Resource Recovery and Circular Economy Act, 2016, S.O. 2016, c. 12, Sched. 1, Regulation of Tires 225/18, https://www.ontario.ca/laws/regulation/180225, accessed Sept. 2, 2023 Search PubMed.
- M. Polyakova and A. Stolyarov, Encyclopedia, 2021, vol. 1 Search PubMed.
- T. Amari, N. J. Themelis and I. K. Wernick, Resour. Policy, 1999, 25, 179–188 CrossRef.
- Registry Procedure, Weight Conversion Factors (Tires), https://prod-environmental-registry.s3.amazonaws.com/2018-04/Registry%20Procedure%20-%20Weight%20Conversion%20Factors%20(Tires).pdf, accessed Sept. 2, 2023 Search PubMed.
- M. Liao, S. Zheng and M. A. Brook, Eur. J. Org. Chem., 2021, 2021, 2694–2700 CrossRef CAS.
- S. Zheng, M. Liao, Y. Chen and M. A. Brook, Green Chem., 2020, 22, 94–102 RSC.
- A Beginner's Guide to Tire Shredding and Recycling, https://ecogreenequipment.com/a-beginners-guide-to-tire-shredding-and-recycling/ Search PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry Theory and Practice, OUP, 2000 Search PubMed.
- K. M. D. Reyes, K. Bruce and S. Shetranjiwalla, J. Chem. Educ., 2023, 100(1), 209–220 CrossRef CAS.
- ACS, https://www.acs.org/content/acs/en/greenchemistry/research-innovation/tools-for-green-chemistry/solvent-selection-tool.html, 2018, accessed April 7, 2023.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef.
- D. A.-O. Yerezhep, A. Tychengulova, D. A.-O. Sokolov and A. A.-O. Aldiyarov, Polymers, 2021, 13, 2494 CrossRef CAS PubMed.
- P. Dong, Z. Chen, X. Qin and Y. Gong, Environ. Sci. Technol., 2021, 55, 16316–16325 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional photos showing rubber/wire sample complexity and magnetic separation. See DOI: https://doi.org/10.1039/d3su00218g |
| This journal is © The Royal Society of Chemistry 2023 |