Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Valorisation of Sargassum muticum through the extraction of phenolic compounds using eutectic solvents and intensification techniques†
Bárbara C.
Jesus
 a,
Blanca
Sáenz de Miera
b,
Rubén
Santiago
a,
Blanca
Sáenz de Miera
b,
Rubén
Santiago
 b,
Alice
Martins
c,
Rui
Pedrosa
d,
Maria
González-Miquel
b,
Alice
Martins
c,
Rui
Pedrosa
d,
Maria
González-Miquel
 *b and
Isabel M.
Marrucho
*a
*b and
Isabel M.
Marrucho
*a
aCentro de Química Estrutural, Departamento de Engenharia Química, Instituto Superior Técnico, Universidade de Lisboa, 1049-001 Lisboa, Portugal. E-mail: isabel.marrucho@tecnico.ulisboa.pt
bDepartamento de Ingeniería Química Industrial y del Medioambiente, ETS Ingenieros Industriales, Universidad Politécnica de Madrid, 28006 Madrid, Spain
cMARE – Marine and Environmental Sciences Centre, ARNET – Aquatic Research Network, Polytechnic of Leiria, 2520-614 Peniche, Portugal
dMARE/ARNET/ESTM, Polytechnic of Leiria, 2520-614 Peniche, Portugal
First published on 2nd June 2023
Abstract
Seaweeds are naturally abundant and spread all over the globe. They have several biologically active secondary metabolites of great interest. In this work, Sargassum muticum was the algae employed as biomass and the aim was to extract phenolic compounds (PCs) using eutectic solvents (ESs). Several betaine-based, proline-based, and choline-based ESs were tested for the extraction of PCs. All extracts were evaluated according to the total phenolic content (TPC), total flavonoid content (TFC) and antioxidant activity (DPPH). Afterwards, the extracts were characterized using HPLC in terms of 9 target PCs (gallic acid, 3,4-dihydroxybenzoic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, salicylic acid, catechin and quercetin). Proline combined with propylene glycol (Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG) exhibited a higher yield according to HPLC results, followed by proline
PPG) exhibited a higher yield according to HPLC results, followed by proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-butanediol (Pro
1,2-butanediol (Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But) and choline
1,2-But) and choline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid (ChCl
citric acid (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA). Pro
CA). Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG also presented high selectivity towards salicylic acid, while ChCl
PPG also presented high selectivity towards salicylic acid, while ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA towards gallic acid. Optimization studies of water content and temperature were performed for the three best ESs, the optimum conditions being 30% (v/v) water and 60 °C extraction temperature. Ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE) were two intensification methods evaluated to enhance the extraction process, proving their ability to reduce the extraction time when compared with the conventional solid–liquid extraction (SLE) process. In particular, Pro
CA towards gallic acid. Optimization studies of water content and temperature were performed for the three best ESs, the optimum conditions being 30% (v/v) water and 60 °C extraction temperature. Ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE) were two intensification methods evaluated to enhance the extraction process, proving their ability to reduce the extraction time when compared with the conventional solid–liquid extraction (SLE) process. In particular, Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG-based MAE provided a significantly higher extraction yield in comparison with conventional extraction and with the other extraction solvents. In summary, the combination of ESs with intensification techniques was shown to be a valuable valorization strategy of a marine macroalgae waste, in particular Sargassum muticum.
PPG-based MAE provided a significantly higher extraction yield in comparison with conventional extraction and with the other extraction solvents. In summary, the combination of ESs with intensification techniques was shown to be a valuable valorization strategy of a marine macroalgae waste, in particular Sargassum muticum.
Sustainability spotlightThis work contributes to the Sustainable Development Goals established by the United Nations through the valorisation of Sargassum muticum, an invasive brown macroalga on the Portuguese Coast, considered bio-waste, having as objective the extraction of natural phenolic compounds, which have well known biological properties and thus human benefits. |
1. Introduction
The European Commission defines biorefining as the “sustainable processing of biomass into a portfolio of marketable bio-based products, which could include co-production of food and feed, chemicals and materials, and bioenergy (power, heat/cold, fuels)”.1,2 According to the source of biomass, biorefineries can be categorized into the first, second, or third generation. The first and second generation rely mainly on agriculture resources, constituting a part of the green economy, while the third generation exploits ocean resources, in particular seaweeds, leading to the blue economy.3Seaweeds are naturally abundant and do not present competition for food, while producing several biologically active secondary metabolites of great interest.4,5Sargassum muticum (Yendo) Fensholt is a brown macroalga native to Japan which has spread all over the world. It was reported for the first time in Europe in 1973 and currently, it is considered invasive on the European coast.6S. muticum has high colonizing potential and its high dispersion is due to the high growth rates, high fecundity, and self-fertilization.5 This brown seaweed is considered invasive since it may negatively affect some marine environments. Even though several efforts were made to control the invasion of this species, none were successfully and thus, its removal through harvesting is ordered.7 Consequently, this macroalga is considered biowaste, and hence its exploitation brings about not only considerable economical value but also eases its environmental burden. It is well known that seaweeds present a significant number of phenolic compounds (PCs), and species belonging to the Sargassaceae family are recognized to have a particularly high quantity. However, this phenolic content can vary according to the geographical location, season, maturity, environmental factors, and extraction techniques.6,8 Tanniou et al.9 and Montero et al.10 evaluated the geographical impact on the phenolic content in Europe showing that Portuguese and Norwegian S. muticum have the highest phenolic content.
PCs display several human health beneficial properties such as antioxidant, anti-inflammatory, antibacterial, antimicrobial (antifungal and antiviral), anti-cancer, and antidiabetic, which makes them value-added compounds.11,12 Consequently, PCs' market is expected to expand in the near future, due to their use in industries such as functional food and beverages, pharmaceutical, cosmetic, and animal feed, among others.11
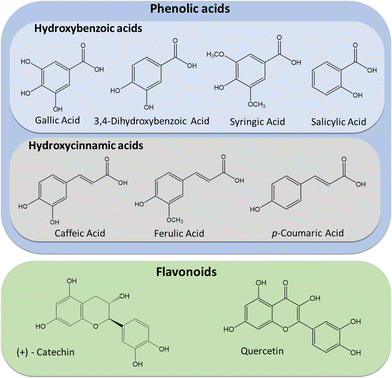
Phenolic acids and flavonoids are two types of phenolic compounds that have been reported to be present in Sargassaceae.12–14 Phenolic acids are small and simple molecules that tend to have high antioxidant activity.15,16 Flavonoids are low molecular weight phenolic compounds and besides the properties mentioned before for PCs, in general, flavonoids can also be good inhibitors of several enzymes.17–20 In this work, phenolic acids and flavonoids were quantified for S. muticum through high-performance liquid chromatographic (HPLC): gallic acid, 3,4-dihydroxybenzoic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, salicylic acid, catechin and quercetin, whose chemical structures can be seen in Fig. 1. There are other phenolic compounds present in S. muticum not easy to quantify by HPLC, such as phlorotannins, that tend to be predominant in brown algae.21 These compounds will have influence on the results of the colorimetric methods used to evaluate the total phenolic content, total flavonoid content and the antioxidant activity.
The solvent used to extract these target compounds is crucial to achieve a high extraction yield and a good separation performance. Parameters as selectivity, solubility, cost, and safety should be considered in the solvent selection. The extraction of PCs from S. muticum is typically carried out with polar organic solvents, due to their high affinity to polyphenols, resulting from their polar behaviour.22,23 There is a need to replace these organic toxic solvents in extraction processes from natural bio-sources with greener solvents, ideally also biocompatible.24 Eutectic solvents (ESs) have emerged as replacing solvents, especially ESs made with natural compounds, the so-called NA(D)ESs. A variety of biomolecules such as PCs, flavonoids, sugars, proteins, and natural pigments, among others from biomass matrices, typically plants, have been successfully extracted using NA(D)ESs, sometimes even showing a higher extraction efficiency with lower extraction times than conventional organic solvents.25
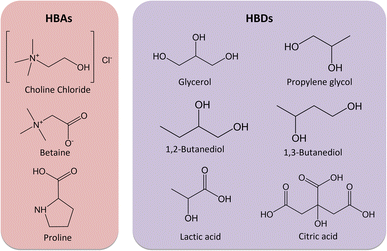
ESs are mixtures of two or more compounds that typically form hydrogen bonds, where usually a hydrogen bond donor (HBD) interacts with a hydrogen bond acceptor (HBA). These interactions cause a shift of the melting temperature to temperatures lower than those of the melting points of the pure compounds.26–29 Intensive research about the extraction of PCs using ESs from different vegetable biomass materials was performed in the literature to identify potential ESs able to replace conventional toxic solvents in the extraction of PCs.30–34 The choice of the different ESs was based on such literature research and previous work from some of us.35 The biocompatibility of the compounds had also to be considered in the choice of the compounds.36–40 There is no information about 1,2-butanediol in FDA, and the European Commission affirms that it is inconclusive if 1,2-butanediol is toxic or not when ingested. However, it can be used in cosmetic applications.41,42 EFSA has restrictions on the ingestion of choline chloride which vary with sex and age.37 In terms of cosmetic applications, among the chosen compounds, only choline chloride is prohibited to be used.43
The HBAs and HBDs used in this work are presented in Fig. 2 and all the mixtures and their compositions in Table 1. It can be seen that the ESs are mostly based on choline chloride (ChCl), proline (Pro), and betaine (Bet). The addition of water was also optimized to reduce their viscosity and promote mass transfer.27–29 A great advantage of ESs is the ease of preparation, just by mixing and heating, with an atom efficiency of 100%.27
| HBA | HBD | HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD ratio HBD ratio |
Abbreviations |
|---|---|---|---|
| Betaine | Glycerol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1 Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
| Propylene glycol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1 PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
|
| 1,2-Butanediol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1 1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1 1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
||
| 1,3-Butanediol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,3-But (1 1,3-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
|
| Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Bet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
||
| Proline | Glycerol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1 Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
| Propylene glycol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1 PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
|
| 1,2-Butanediol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1 1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1 1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
||
| 1,3-Butanediol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,3-But (1 1,3-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
|
| Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
||
| Choline chloride | Glycerol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1 Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
| Propylene glycol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1 PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
|
| 1,2-Butanediol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1 1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
|
| 1,3-Butanediol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,3-But (1 1,3-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
|
| Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
||
| Citric acid | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2 CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (1 CA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (1 CA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
The aim of this work was to replace conventional organic solvents with more benign solvents, in particular NA(D)ESs, that could at least return an equivalent extraction efficiency. With the purpose of enhancing the extraction and making it as greener as possible, intensification techniques such as the ultrasound-assisted technique (UAE) and microwave-assisted technique (MAE) were also evaluated as alternatives to conventional solid–liquid extraction.
2. Materials and methods
2.1. Chemicals and reagents
L-Proline (Pro) (purity ≥ 99% wt), choline chloride (ChCl) (purity ≥ 98% wt), betaine (Bet) (purity ≥ 98% wt), propylene glycol (PPG) (purity 99% wt), citric acid (CA) (purity ≥ 99% wt), 1,2-butanediol (1,2-But) (purity ≥ 98% wt), (±)-1,3-butanediol (1,3-But) (purity ≥ 99.5% wt), and lactic acid (LA) (purity ≥ 85% wt) were all purchased from Sigma-Aldrich. Glycerol (Gly) (purity ≥ 99.5% wt) was purchased from Panreac. The pH of ESs was measured at room temperature using a Consort SP28X pH electrode connected to a Consort multiparameter C3010 analyser. The densities were measured using an Anton Paar DMA 500 densimeter and the viscosities with an Anton Paar (model SVM 3000) automated rotational Stabinger viscometer-densimeter with a temperature uncertainty of ±0.01 K. Standards of gallic acid (purity 97.5–102.5% wt titration), 3,4-dihydroxybenzoic acid (purity ≥ 97% wt), (+)-catechin (purity ≥ 98% wt), caffeic acid (purity ≥ 98% wt), syringic acid (purity ≥ 95% wt), p-coumaric acid (purity ≥ 98% wt), ferulic acid (purity 99% wt), salicylic acid (purity ≥ 99% wt) and quercetin (purity ≥ 95% wt) were purchased from Sigma-Aldrich. The Folin & Ciocalteu phenol reagent was acquired from Sigma Aldrich, sodium carbonate (Na2CO3) (purity 99.5 to 100.5% wt) from Labkem, sodium nitrite (Na2NO3) from Emsure, aluminium chloride (AlCl3) from Sigma-Aldrich (purity ≥98% wt) and sodium hydroxide (NaOH) from J. T. Baker. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) for the antioxidant activity assay was purchased from Sigma-Aldrich and (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) was purchase from Sigma-Aldrich (purity ≥ 97% wt). The UV-Visible spectrophotometer used in the colorimetric methods was a JASCO V-730. The acetic acid used in HPLC was purchased from Labkem (purity ≥ 99.5% wt). The employed solvatochromic probes, 2,6-dichloro-4-(2,4,6-triphenyl-N-pyridino)-phenolate (Reichardt's betaine dye 33), 4-nitroaniline, and N,N-diethyl-4-nitroaniline, were purchased from Fluka (≥97% wt), Sigma Aldrich (≥99% wt) and Frinton Laboratories, respectively. To acquire the UV-vis absorption data for the polarity experiments, a Shimadzu UV-1800 spectrophotometer (UV/Vis) was used, and all spectroscopic measurements were performed in triplicate.2.2. Biomass and pre-treatment methods
The brown seaweed Sargassum muticum was collected at Praia Norte beach, Viana do Castelo, Portugal (41° 41′ 44.2′′ N 8° 51′ 8.1′′ W) in spring/summer 2015 and immediately transported to the laboratory. After being cleaned and washed, firstly with seawater to remove invertebrate organisms, epiphytes, and detritus, and then with distilled water, S. muticum was frozen at −20 °C and freeze-dried (Scanvac Cool Safe, LaboGene, Lynge, Denmark). The dried algal material was ground into a powder in a grinder and stored protected from light, at room temperature.2.3. Preparation of eutectic solvents (ESs)
The ESs were prepared by adding the required masses of HBA (ChCl, Bet and Pro) and HBD (PPG, Gly, LA, CA, 1,2-But, and 1,3-But) at specific molar ratios, all weighed using an analytical balance Sartorius M – POWER AZ1,24 with a repeatability of ±0.0002 g. The mixtures were heated up to 80 °C, except the ESs composed of ChCl and CA which were heated until 100 °C and stirred at 1000 rpm. After acknowledging that a homogeneous liquid solution was attained, the heat was turned off. The ESs composed of ChCl and CA were the only ones that had to be prepared with a small quantity of water (6.4% (w/w)) due to their high viscosity.2.4. Extraction techniques
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid (S
liquid (S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L) ratio of 1
L) ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v) and with a water content of 30% (v/v) using a Vortemp 1550 orbital shaker from Labnet International, Inc. for 100 min at 60 °C and 900 rpm. Subsequently, the sample was subjected to 15 min of centrifugation at 4200 rpm in a Unicen 21 Orto Alresa centrifuge. The three best ESs were selected and water content (10, 20, 30, 40, and 50% (v/v)) and temperature (50, 60 and 70 °C) were optimized.
10 (w/v) and with a water content of 30% (v/v) using a Vortemp 1550 orbital shaker from Labnet International, Inc. for 100 min at 60 °C and 900 rpm. Subsequently, the sample was subjected to 15 min of centrifugation at 4200 rpm in a Unicen 21 Orto Alresa centrifuge. The three best ESs were selected and water content (10, 20, 30, 40, and 50% (v/v)) and temperature (50, 60 and 70 °C) were optimized.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratio was also 1
L ratio was also 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v) and the water content was kept at 30% (v/v). No temperature control was used, and only the power of the probe was controlled. The optimization of the UAE parameters was performed by testing different powers (10 and 20 W) and extraction times (1.5, 3, 6 and 9 minutes).
10 (w/v) and the water content was kept at 30% (v/v). No temperature control was used, and only the power of the probe was controlled. The optimization of the UAE parameters was performed by testing different powers (10 and 20 W) and extraction times (1.5, 3, 6 and 9 minutes).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratio of 1
L ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v) and the water content at 30% (v/v) were kept constant. The optimization of the MAE parameters was performed by testing temperatures of 60 and 100 °C and extraction times of 1.5, 3, 6 and 9 minutes. For ChCl
10 (w/v) and the water content at 30% (v/v) were kept constant. The optimization of the MAE parameters was performed by testing temperatures of 60 and 100 °C and extraction times of 1.5, 3, 6 and 9 minutes. For ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) a time of 12 minutes was also tested.
1) a time of 12 minutes was also tested.
2.5. Total phenolic content
Frequently, the total phenolic content (TPC) is quantified with the Folin–Ciocalteu method. The experimental steps for the Folin method are described in detail by Cañadas et al.35 Initially, 100 μL of the sample were added to a vial followed by 100 μL of the Folin reagent and after 3 min in the dark at room temperature, 2 mL of 2% (v/v) Na2CO3 aqueous solution were added, followed by keeping in the dark for 30 min at room temperature. After that, the absorbance was measured at 765 nm using distillated water as the blank. The Folin–Ciocalteu test used gallic acid aqueous solution as the standard, and therefore the TPC was expressed in mg of gallic acid equivalents per g of dry weight (mg GAE per g). For some samples where the absorbance was too high, a dilution had to be made before performing this assay using distilled water. This assay was also performed for the pure ES used as the solvent in the extraction, in order to be used as a blank. Note that for the ChCl-based ES, the ChCl tends to precipitate while carrying out this method.2.6. Total flavonoid content (TFC)
The Christ–Müllers test allows the quantification of the total flavonoid content (TFC) which was determined by AlCl3 complexation with the flavonoid, that is detected by a colorimetric method at 510 nm in the presence of NaNO2.44,45 The method is described in detail by Cañadas et al.,35 and slight modifications were implemented, using quercetin methanol solution as the standard. Initially, 2 mL of distilled water were added to a vial, followed by the addition of 150 μL of 5% (w/v) Na2NO3 aqueous solution, and then 450 μL of the sample. The vials were then vortexed and kept at room temperature with no need to hide from the light. After 5 min, 150 μL of the 10% (w/v) AlCl3 aqueous solution were added, and after stirring, the vials were kept at room temperature for another 5 min. Finally, 1 mL of 1 M NaOH aqueous solution was added. Then, the solutions were homogenized, and the vials were kept still for 15 min. Afterwards, the absorbance was measured at 510 nm using distillate water as the blank. The TFC was expressed in mg of quercetin equivalents per g of dry weight (mg QE per g). Again, dilutions were performed before this assay for the samples showing high absorbance, and this method was also performed for the pure ES used as solvent in the extraction.2.7. DPPH free radical scavenging
The DPPH radical absorbs at 515 nm and when it undergoes the reduction reaction its purple colour tends to turn yellow, thus causing a decrease in the absorbance at 515 nm. Measuring the absorbance of the extract after the DPPH reduction (Asample) and having the absorbance of the solution of DPPH in MeOH (Ablank), it is possible to calculate the inhibition percentage making use of expression (1).46,47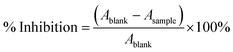 | (1) |
This method is also described in detail by Cañadas et al.35 Initially, a volume of 100 μL of the sample was added to a vial followed by the addition of 2.9 mL of a solution of 24 mg L−1 of DPPH in MeOH, and then the vials were kept in the dark at room temperature for 30 min. Afterwards, the absorbance was measured at 515 nm. This method was also performed for the pure ES in order to evaluate the antioxidant activity of the ES itself. Note that all the proline-based ESs tend to form flakes. Trolox was used as a standard antioxidant and the measurement of the percentage of inhibition was performed for different Trolox concentrations to build a calibration curve. Given this, the Trolox equivalent antioxidant activity (TEAC) will be presented in mg of Trolox equivalents per g of dry weight (mg TEAC per g).
2.8. High-performance liquid chromatographic (HPLC) quantification of phenolic compounds
Reverse high-performance liquid chromatographic (HPLC) analysis was used to quantify the phenolic acids and flavonoids using a JASCO 4000 Series HPLC system with a Fortis C18 column (250 mm × 4.6 mm, 5 μm) and a photodiode array (DAD) detector at 25 °C. The compounds were separated with a gradient elution using a 1.25% (v/v) aqueous acetic acid solution (A) and acetonitrile (B). The gradient method was as follows: the initial composition of the mobile phase was 10% phase B; linear increase to 20% phase B from 0 to 8 min; linear increase to 25% from 8 to 25 min; linear increase to 45% from 25 to 30 min; maintaining the conditions until 33 min; linear increase to 80% phase B until 37 min; maintaining the conditions until 40 min; finally, linear decrease to 10% phase B between 40 and 43 min at a flow rate of 0.5 mL min−1. The re-equilibration of the column was carried out using the starting conditions for 5 min before the next analysis. The total analysis per sample was performed in 48 min. To detect all the different phenolic compounds studied herein, three different wavelengths of the DAD detector were used, one at 271 nm where gallic acid, 3,4-dihydroxybenzoic acid, (+)-catechin, syringic acid, and salicylic acid were detected; the second wavelength at 284 nm, where caffeic acid, p-coumaric acid, and ferulic acid were identified; the third wavelength at 323 nm to detect quercetin. The quantification of each phenolic compound was performed by the integration of the peak area and calculated with the calibration curve prepared with standard solution in a concentration range between 0.5 and 50 mg L−1.2.9. Thermophysical properties: density and viscosity
Density and viscosity are crucial thermophysical properties due to their impact in the mass transport phenomena that will affect the extraction capacity.48 The densities were measured with an Anton Paar DMA 500 densimeter and the viscosities with an Anton Paar (model SVM 3000) automated rotational Stabinger viscometer-densimeter with a temperature uncertainty of ±0.01 K. A quadratic adjustment was used to describe density (ρ in g cm−3) with temperature (T in K), eqn (2) where a, b and c are fitting parameters.| ρ = aT2 + bT + c | (2) |
For the viscosity data the Vogel–Fulcher–Tammann (VFT) model given by expression (3) was used, where Aη, Bη and Cη are fitting parameters, η the viscosity in mPa s and T the temperature in K.48
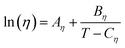 | (3) |
Both density and viscosity results are presented in the ESI.†
2.10. COSMO-RS computational details
COSMO-RS (COnductor-like Screening MOdel for Real Solvents) is a practical computational tool that allows the prediction of the thermodynamic properties of fluids and mixtures. The COSMO-RS method is based on a combination between statistical thermodynamics and computation quantum mechanics, which enables the characterization of the molecular interactions and evaluation of the solution behaviour of a certain solute in a solvent through the calculation of the activity coefficient at infinite dilution (γ∞).49–51 In addition, the COSMO-RS method allows the σ-profile to be obtained, as a probability distribution of a molecule's or mixture's surface-charge density obtained from quantum chemical calculations. The σ-profile can be divided in three regions: hydrogen bond donor (σ < −0.0082 e Å−2), hydrogen bond acceptor (σ > +0.0082 e Å−2) and non-polar (−0.0082 < σ < +0.0082 e Å−2).49–51 The molecular geometries of the compounds were optimized to their minimum energy structure using Turbomole, at the BP86/TZVP computational level. Afterwards, *cosmo files were obtained and implemented in COSMOtherm v.19 software using implicit BP_TZVP_19 parametrization for calculations.2.11. Betaine dye scale and Kamlet–Taft scale
As described by Florindo et al.,52 dichloromethane solutions for all three probes were prepared, with concentrations of 0.1 ppm for 4-nitroaniline and N,N-diethyl-4-nitroaniline and 0.5 ppm for Reichardt's betaine dye 33. Different amounts of each one of the probes, 80 μL of the probe solution for 4-nitroaniline and N,N-diethyl-4-nitroaniline and 400 μL for Reichardt's betaine dye 33 were added to different vials and then the solvent was evaporated under vacuum. Afterwards, 800 μL of each ES was added to each vial, and thoroughly mixed, and the solution was transferred to a 2 mm light path quartz cuvette.48,52There are two scales that can be employed to evaluate the polarity of a certain solvent: betaine dye scale, through the calculation of ET(33) and ETN parameters that measures the overall polarity; and Kamlet–Taft parameters, α, β and π* that measure the ability to be a HBD, HBA and polarizable, respectively. For the betaine dye, ET(33) corresponds to the transition energy for the dissolution of Reichardt's dye 33 expressed in kcal mol−1 and can be determined resorting to expression (4).
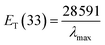 | (4) |
Although Reichardt's dye 30 is the probe typically used, in the case of acidic solvents, such as the case of some of the present ESs, Reichardt's dye 33 should be used instead. The ET(33) values were converted into ET(30) according to the following eqn (5).
| ET(30) = 0.9953(±0.0287) × ET(33) + 8.1132(±1.6546) | (5) |
The normalized polarity, ETN, measures the overall polarity of a solvent and can be determined using the ET(30) parameter through eqn (6).
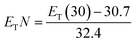 | (6) |
The Kamlet–Taft scale has three parameters: α that is the HBD ability, β which is the HBA ability, and π* that is the polarisability/dipolarity of a certain solvent. The parameter π* gives information about the polarizability and dipolarity of a solvent and it can be calculated with eqn (7), where ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) is the maximum wavenumber in cm−1.
is the maximum wavenumber in cm−1.
π* = 0.314 × (27.52 − ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) N,N-diethyl-4-nitroaniline) N,N-diethyl-4-nitroaniline) | (7) |
The HBD ability, α, measures the capacity of a solvents to donate hydrogen bonds and this can be calculated using expression (8) using ET(33) and π* obtained from eqn (4) and (7), respectively.
| α = 0.0649ET(33) − 2.03 − 0.72π* | (8) |
The HBA ability, β, compares the solvent-induced shifts of 4-nitroaniline and N,N-diethyl-4-nitroaniline, and it is given by expression (9).
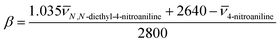 | (9) |
2.12. Statistical test
Every measurement was carried out in triplicate, and it is presented as the mean of the three values and the respective standard deviation is presented in the ESI.† An ANOVA post hoc, called Tukey's test, was made to compare if the means are significantly different or not resorting to IBM SPSS Statistics (p < 0.05).3. Results and discussion
The first step of this work was to identify ESs that could be used as solvents in the extraction of PCs. For that purpose, an experimental screening was carried out at 60 °C with ESs solutions with 30% (v/v) of water for a total extraction time of 100 min with a solid–liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The ESs tested can be organized into three groups: betaine-based, proline-based and choline-based. Extractions with conventional solvents, water and EtOH
10. The ESs tested can be organized into three groups: betaine-based, proline-based and choline-based. Extractions with conventional solvents, water and EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (70
water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v), were also carried out for comparison purposes.
30 v/v), were also carried out for comparison purposes.
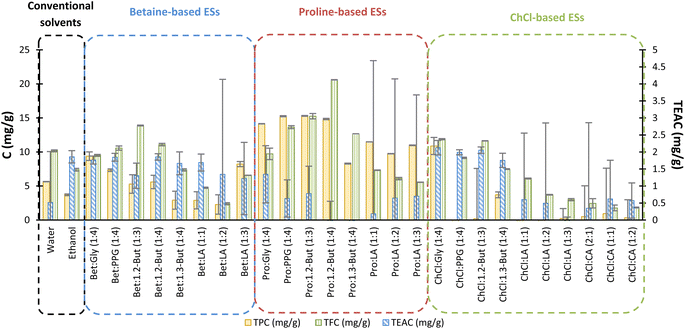
The results of the colorimetric methods used to quantify the TPC, TFC and antioxidant activity of extracts are shown in Fig. 3. It can be observed that the TPC was generally higher for the proline-based ESs, the highest value being 15.30 ± 0.06 mg of GAE per g of dry weight, obtained for Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), followed by Pro
3), followed by Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) with 15.26 ± 0.08 mg of GAE per g of dry weight, and Pro
4) with 15.26 ± 0.08 mg of GAE per g of dry weight, and Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) with 14.84 ± 0.10 mg of GAE per g of dry weight. In comparison with the two conventional solvents, water and EtOH
4) with 14.84 ± 0.10 mg of GAE per g of dry weight. In comparison with the two conventional solvents, water and EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (70
water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v), for which the TPC values were 5.64 ± 0.03 and 3.70 ± 0.13 mg of GAE per g of dry weight, respectively, a notable increase of PCs was obtained when using ESs.
30 v/v), for which the TPC values were 5.64 ± 0.03 and 3.70 ± 0.13 mg of GAE per g of dry weight, respectively, a notable increase of PCs was obtained when using ESs.
Due to the differences in the extraction processes (technique, conditions and solvents) and in the quantification of TPC (e.g., standards used), as well as differences in the biomass depending on the geographical and weather conditions, it is very difficult to directly compare the results obtained herein with other results already published. Silva et al.53 evaluated the TPC of S. muticum using a variety of conventional organic solvents, such as EtOH, and maceration. For pure EtOH and a total extraction time of 24 h at 50 °C, a TPC of 8.31 ± 0.33 mg of GAE per g of dry extract44 was obtained. In the present study, for notably shorter extraction times, 100 min, at 60 °C, an extraction yield 2 times higher was obtained using EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water at a 70
water at a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v) ratio.44 As for the ES that provided the highest TFC, Pro
30 (v/v) ratio.44 As for the ES that provided the highest TFC, Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) yielded 20.61 ± 0.02 mg of quercetin per g, which is twice the value extracted using water and 2.8 times higher than using EtOH
4) yielded 20.61 ± 0.02 mg of quercetin per g, which is twice the value extracted using water and 2.8 times higher than using EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water at a 70
water at a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v) ratio.
30 (v/v) ratio.
In principle, a high TPC should return a high TEAC since the phenolic compounds present antioxidant properties. However, the results in Fig. 3 show a discrepancy between the TPC and TEAC. This can be explained by the fact that in the Folin–Ciocalteu method, other non-phenolic compounds also present in the extract can have impact on the absorbance and consequently influence the final result, causing an overestimation of the TPC. In fact, proline-based ESs that returned the highest TPC tend to return the lowest antioxidant activity. Also, the formation of flakes for proline-based ESs in the antioxidant activity assay and precipitates for ChCl-based ESs in the Folin–Ciocalteu assay were observed. These issues are sources of error, leading to significant error bars, as can be seen in Fig. 3. Given this, it is important to acknowledge that the TPC and TEAC are not very reliable for choline-based and proline-based ESs, respectively. For this reason, HPLC was also used as a more robust method to analyse the solvent extracts, as shown in Fig. 4.
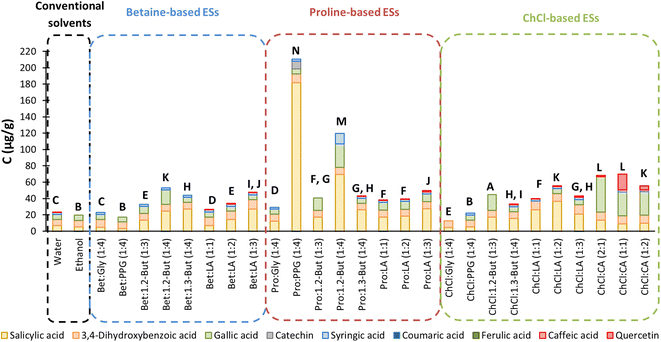
It can be observed that two proline-based ESs, Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and Pro
4) and Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), returned the best extraction yields according to the HPLC results, in agreement with the TPC colorimetric tests. This implies that, although non-phenolic compounds can account for the TPC, the Folin–Ciocalteu method might still indicate the extracts with the highest TPC. However, the discrepancies between the colorimetric method results and those from HPLC are notable, especially for ChCl-based ESs, not only for the TPC but also for the TFC and antioxidant activity.
4), returned the best extraction yields according to the HPLC results, in agreement with the TPC colorimetric tests. This implies that, although non-phenolic compounds can account for the TPC, the Folin–Ciocalteu method might still indicate the extracts with the highest TPC. However, the discrepancies between the colorimetric method results and those from HPLC are notable, especially for ChCl-based ESs, not only for the TPC but also for the TFC and antioxidant activity.
From the HPLC results in Fig. 4, in general ESs greatly improved the extraction yields when compared to conventional solvents. Also, it is worth noting that salicylic acid is the major component of most of the extracts, Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) being the solvent providing the highest yield and the highest selectivity towards salicylic acid. These results are in agreement with those of Caijiao et al.,13 who extracted significant amounts of salicylic acid from Sargassum horneri harvested in China. However, ferulic acid and p-coumaric acid, which were the two most extracted compounds after salicylic acid,13 were not detected in the present extracts, as well as caffeic acid. The studied flavonoids,13 catechin and quercetin, are present in very small quantities in the present extracts as well as syringic acid. Interestingly, 3,4-dihydroxybenzoic acid shows similar extraction yields for all the ESs used.
4) being the solvent providing the highest yield and the highest selectivity towards salicylic acid. These results are in agreement with those of Caijiao et al.,13 who extracted significant amounts of salicylic acid from Sargassum horneri harvested in China. However, ferulic acid and p-coumaric acid, which were the two most extracted compounds after salicylic acid,13 were not detected in the present extracts, as well as caffeic acid. The studied flavonoids,13 catechin and quercetin, are present in very small quantities in the present extracts as well as syringic acid. Interestingly, 3,4-dihydroxybenzoic acid shows similar extraction yields for all the ESs used.
Sabeena Farvin & Jacobsen12 also quantified the different phenolic compounds evaluated in the present work extracted from S. muticum harvested in Denmark using diverse organic solvents and extraction processes. The main difference was that no salicylic acid was extracted. These authors12 observed that when using EtOH, 3,4-dihydroxybenzoic acid was the most extracted phenolic acid, followed by gallic acid, which agrees with the results obtained in this work using EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (70
water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v). All these results clearly show not only the difficulty in comparing results between different authors, but also the superiority of ESs as designable solvents for the valorisation of macroalgae.
30 v/v). All these results clearly show not only the difficulty in comparing results between different authors, but also the superiority of ESs as designable solvents for the valorisation of macroalgae.
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and Pro
4) and Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) were the ESs that in general show the highest extraction capacity. Tukey's test corroborates this observation since it indicates that these two results are significantly different from the rest. Another remarkable fact is the Pro
4) were the ESs that in general show the highest extraction capacity. Tukey's test corroborates this observation since it indicates that these two results are significantly different from the rest. Another remarkable fact is the Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) selectivity towards salicylic acid. The mixture of ChCl with citric acid was shown to be the third best option when it comes to the overall extraction yield. Statistically, according to Tukey's test, the results of ratios (2
4) selectivity towards salicylic acid. The mixture of ChCl with citric acid was shown to be the third best option when it comes to the overall extraction yield. Statistically, according to Tukey's test, the results of ratios (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (1
1) and (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of ChCl
1) of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA are not significantly different, and hence the ratio (2
CA are not significantly different, and hence the ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was selected due to its higher selectivity towards gallic acid. This selectivity could be related to experimental conditions, namely the different pH of the aqueous solutions, as explained in the ESI.†
1) was selected due to its higher selectivity towards gallic acid. This selectivity could be related to experimental conditions, namely the different pH of the aqueous solutions, as explained in the ESI.†
Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) has no restrictions to be used in biocompatible applications such as food, cosmetic and pharmaceutical. In fact, proline is already used orally through nutritional supplements and has been employed in a variety of vaccines already, and may be used as a food additive and it has no restrictions for cosmetic applications.36,54,55 This ES is highly selective toward salicylic acid which is a phenolic compound with current applications in cosmetics as an acne reductor, as a food preservative, and as a starting compound to produce dyes and aspirin. As for Pro
4) has no restrictions to be used in biocompatible applications such as food, cosmetic and pharmaceutical. In fact, proline is already used orally through nutritional supplements and has been employed in a variety of vaccines already, and may be used as a food additive and it has no restrictions for cosmetic applications.36,54,55 This ES is highly selective toward salicylic acid which is a phenolic compound with current applications in cosmetics as an acne reductor, as a food preservative, and as a starting compound to produce dyes and aspirin. As for Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), there is no information about the ingestion of 1,2-butanediol, as previously mentioned and no restrictions for cosmetic applications are imposed. Since proline is also allowed in cosmetic applications, the extract obtained with Pro
4), there is no information about the ingestion of 1,2-butanediol, as previously mentioned and no restrictions for cosmetic applications are imposed. Since proline is also allowed in cosmetic applications, the extract obtained with Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) could follow this purpose. Since choline chloride is forbidden to be used in cosmetics, such application should be discarded for the extracts with ChCl
4) could follow this purpose. Since choline chloride is forbidden to be used in cosmetics, such application should be discarded for the extracts with ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). However, it can be used for food applications, as ChCl and citric acid are allowed by both EFSA and FDA to be used as food additives. According to EFSA reports, gallic acid is naturally contained in some fruits, such as cherries and grapes, as well in some drinks such as whiskey, wine and beer.56
1). However, it can be used for food applications, as ChCl and citric acid are allowed by both EFSA and FDA to be used as food additives. According to EFSA reports, gallic acid is naturally contained in some fruits, such as cherries and grapes, as well in some drinks such as whiskey, wine and beer.56
As mentioned before, COSMO-RS can be of great help in understanding the affinity between solute and solvent. From the σ-profiles obtained with COSMO-RS, Fig. S6,† there is no doubt that ChCl acts as a HBA, since it presents a huge peak in the HBA region (>0.0082 e Å−2), and the CA tends to be a HBD, leading to a highly polar ES. Similarly, the σ-profile of proline indicates its role as a HBA when combined with PPG or 1,2-butanediol, that acts as a HBD (peak located in the most negative part of the histogram). However, the σ-profiles of the latter ESs, especially for the case of proline, are more deviated to the HBD region, indicating a potentially more polar behaviour for ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) than Pro
1) than Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and Pro
4) and Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). This difference in polarities could explain the higher yields towards salicylic acid provided by proline-based ESs, and the higher yield towards gallic acid in the case of ChCl-based ESs, since gallic acid shows a broader sigma profile than salicylic acid. This information is corroborated by the structure of both phenolic acids, where gallic acid presents two more hydroxyl groups than salicylic acid.
4). This difference in polarities could explain the higher yields towards salicylic acid provided by proline-based ESs, and the higher yield towards gallic acid in the case of ChCl-based ESs, since gallic acid shows a broader sigma profile than salicylic acid. This information is corroborated by the structure of both phenolic acids, where gallic acid presents two more hydroxyl groups than salicylic acid.
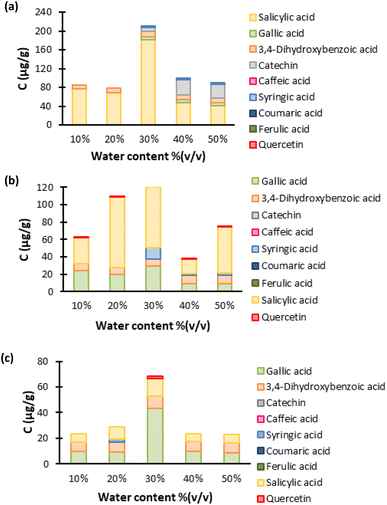
3.1. SLE optimization (water content and temperature)
In order to explain the effect of the amount of water on the extraction, the excess molar volumes were determined, from the experimental density results, and are presented in Fig. S18.† The amount of water from at which it can be considered that the hydrogen bonds began to rupture can be evaluated from the excess molar volumes at different contents of water.
For both Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and Pro
4) and Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), the maximum volume contraction occurs at 30% (v/v). This indicates that at this water concentration the interactions between water and ESs are very favourable, which can be interpreted as a point where the hydrogen bond network of ESs is percolated by water; this can result in more favourable interactions between the ES and water causing a tighter network between the DES and water creating a higher depression on the volume. This change in the ES structure might justify the higher extraction of the studied compounds at 30% (v/v) water. The excess molar volumes for ChCl
4), the maximum volume contraction occurs at 30% (v/v). This indicates that at this water concentration the interactions between water and ESs are very favourable, which can be interpreted as a point where the hydrogen bond network of ESs is percolated by water; this can result in more favourable interactions between the ES and water causing a tighter network between the DES and water creating a higher depression on the volume. This change in the ES structure might justify the higher extraction of the studied compounds at 30% (v/v) water. The excess molar volumes for ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) could not be determined since it was not possible to measure the density of pure ChCl
1) could not be determined since it was not possible to measure the density of pure ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) because of its high viscosity. Even when water is added, the viscosity of ChCl
1) because of its high viscosity. Even when water is added, the viscosity of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is quite high, as can be seen through the viscosity measurements presented in the ESI.†
1) is quite high, as can be seen through the viscosity measurements presented in the ESI.†
Although the σ-profiles in Fig. S6† gave insights into the polarity of the compounds used to formulate the three most performing ESs, they do not quantify the polarity of the ESs. This quantification was conducted through the use of solvatochromic probes for different water contents. The first observation from Fig. S14† is that the amount of water does not significantly change the ETN parameter, and thus cannot explain the differences in the extraction of the model compounds with the water content. However, it can be observed that ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) has a higher overall polarity (1.668 for 30% (v/v) water) than the two proline-based ESs. This result agrees with the conclusions taken from the σ-profiles from COSMO-RS. Pro
1) has a higher overall polarity (1.668 for 30% (v/v) water) than the two proline-based ESs. This result agrees with the conclusions taken from the σ-profiles from COSMO-RS. Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and Pro
4) and Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) have similar ETN values, 0.579 and 0.581, respectively, for 30% (v/v) water content. Florindo et al.52 observed that ETN did not vary much by changing the HBD and maintaining the same HBA. This is corroborated here for the proline-based ESs.
4) have similar ETN values, 0.579 and 0.581, respectively, for 30% (v/v) water content. Florindo et al.52 observed that ETN did not vary much by changing the HBD and maintaining the same HBA. This is corroborated here for the proline-based ESs.
The α values for the two proline-based ESs are much lower than the α values of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), showing a very strong ability to donate hydrogen bonds. These high values of α can be related to the carboxylic groups and the hydroxyl group present in the citric acid. For the proline-based ESs, the HBD ability decreases with the increase of water content. In contrast, for ChCl
1), showing a very strong ability to donate hydrogen bonds. These high values of α can be related to the carboxylic groups and the hydroxyl group present in the citric acid. For the proline-based ESs, the HBD ability decreases with the increase of water content. In contrast, for ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the α tends to slightly increase along with the water content. According to Husanu et al.57 the α parameter is connected with the extraction efficiency of phenolic compounds. Although this might explain the selectivity of Pro
1), the α tends to slightly increase along with the water content. According to Husanu et al.57 the α parameter is connected with the extraction efficiency of phenolic compounds. Although this might explain the selectivity of Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and Pro
4) and Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) towards the salicylic acid, it does not explain the high extraction yields. By contrast to α behavior, the β values for the two proline-based ESs are higher than the β of ChCl
4) towards the salicylic acid, it does not explain the high extraction yields. By contrast to α behavior, the β values for the two proline-based ESs are higher than the β of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which means that the former ESs have higher ability to accept hydrogen bonds. This is corroborated with the σ-profiles from Fig. S6.†
1), which means that the former ESs have higher ability to accept hydrogen bonds. This is corroborated with the σ-profiles from Fig. S6.†
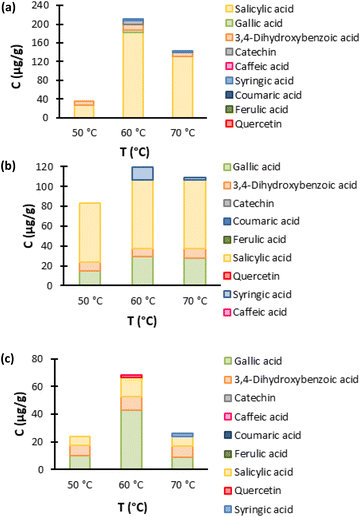
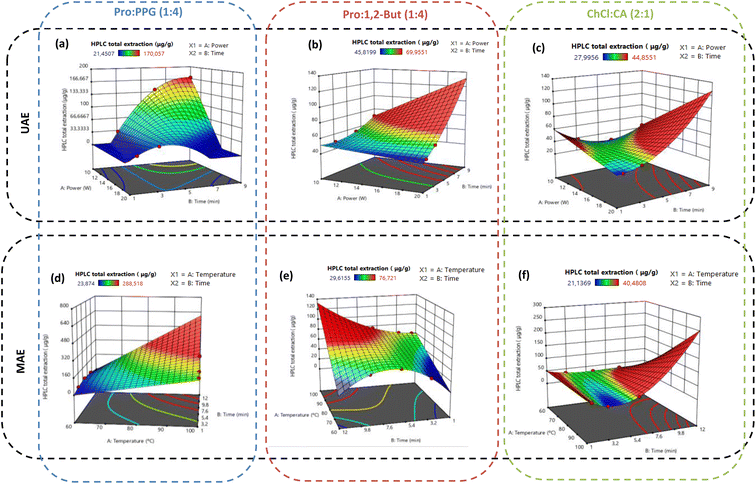
The analysis of the HPLC results of the temperature optimization, Fig. 6, shows that 60 °C is the optimum temperature to perform the extraction for all three ESs. This indicates that the ESs' screening, Fig. 4, was performed under the optimal conditions, not only in terms of temperature but also in terms of water content, as seen above.
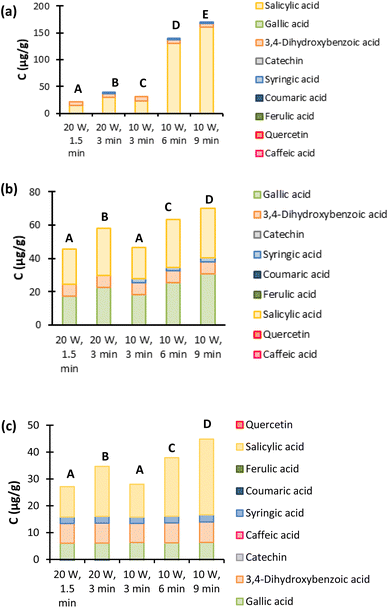
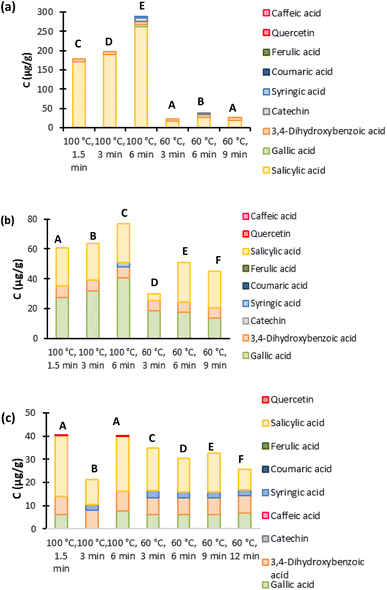
3.2. Intensification techniques
According to the HPLC results for UAE in Fig. 7, lower power and higher extraction times increase the extraction yields for all three ESs. Beyond that, a selectivity switch for ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) can be observed when using this intensification method, from gallic acid to salicylic acid, as can be easily concluded by comparing with the results from Fig. 4. This observation is quite interesting since it allows the extraction selectivity of these ESs to be manipulated. The ultrasound waves could affect the interactions and the structure of the 30% (v/v) aqueous solution of ChCl
1) can be observed when using this intensification method, from gallic acid to salicylic acid, as can be easily concluded by comparing with the results from Fig. 4. This observation is quite interesting since it allows the extraction selectivity of these ESs to be manipulated. The ultrasound waves could affect the interactions and the structure of the 30% (v/v) aqueous solution of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) promoting this selectivity change. The temperatures after the extraction were also measured and are presented in Table S10 in the ESI.† It can be observed that in the extraction using 3 minutes at 10 W, the temperature reaches the lowest value (83.4 °C), compared with the other extractions that all reach temperatures higher than 96 °C. This difference in the temperatures could explain the lower yield obtained using this time and power, as seen in Fig. 7, compared to the remaining extractions. Except when it is imposed a time of 1.5 minutes at 20 W, in these conditions the temperature reaches 98.4 °C and when comparing with 3 minutes at 10 W, they both have similar yields, even though they reach different temperatures. In fact, for both Pro
1) promoting this selectivity change. The temperatures after the extraction were also measured and are presented in Table S10 in the ESI.† It can be observed that in the extraction using 3 minutes at 10 W, the temperature reaches the lowest value (83.4 °C), compared with the other extractions that all reach temperatures higher than 96 °C. This difference in the temperatures could explain the lower yield obtained using this time and power, as seen in Fig. 7, compared to the remaining extractions. Except when it is imposed a time of 1.5 minutes at 20 W, in these conditions the temperature reaches 98.4 °C and when comparing with 3 minutes at 10 W, they both have similar yields, even though they reach different temperatures. In fact, for both Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and ChCl
4) and ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ESs, the Tukey test indicates similar extraction efficiencies.
1) ESs, the Tukey test indicates similar extraction efficiencies.
From Fig. 8, it can be observed that the high temperature (100 °C) improves the extraction, contrary to what was observed in maceration. This is probably due to the low extraction times (up to 6 min) compared with 100 min employed for the conventional extraction, where PC degradation probably occurs. The MAE extractions using ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Fig. 8(c), do not display the same behavior of the other two ESs, Fig. 8(a) and (b). In fact, ChCl
1), Fig. 8(c), do not display the same behavior of the other two ESs, Fig. 8(a) and (b). In fact, ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed some problems, such as difficulties to control the temperature of the equipment in MAE due to its high viscosity. At 60 °C, the behavior of ChCl
1) showed some problems, such as difficulties to control the temperature of the equipment in MAE due to its high viscosity. At 60 °C, the behavior of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Fig. 8(c), is similar to that observed for proline-based ESs but for longer periods of time (i.e., at 6, 9 and 12 min, instead of 3, 6 and 9 min). This can be correlated with the high viscosity of the aqueous solution of ChCl
1), Fig. 8(c), is similar to that observed for proline-based ESs but for longer periods of time (i.e., at 6, 9 and 12 min, instead of 3, 6 and 9 min). This can be correlated with the high viscosity of the aqueous solution of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which probably needs more time to reach the stablished temperature. Viscosity is actually a crucial mass transfer influencer and temperature is an experimental variable that has a significant effect on the viscosity itself. At 100 °C for ChCl
1), which probably needs more time to reach the stablished temperature. Viscosity is actually a crucial mass transfer influencer and temperature is an experimental variable that has a significant effect on the viscosity itself. At 100 °C for ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), an unexpected behavior occurred when the yield decreased at increasing times from 1.5 min to 3 min, Fig. 8(c), without evidence of gallic acid extracted. Additionally, at 100 °C, the yield is similar at 1.5 min or 6 min of extraction, agreeing with the indications provided by Tukey's test. From the results of ChCl
1), an unexpected behavior occurred when the yield decreased at increasing times from 1.5 min to 3 min, Fig. 8(c), without evidence of gallic acid extracted. Additionally, at 100 °C, the yield is similar at 1.5 min or 6 min of extraction, agreeing with the indications provided by Tukey's test. From the results of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), it is clear that the phenomena of the switch of selectivity observed in the UAE can also be seen with MAE. When both UAE and MAE techniques are used, the extraction yields towards gallic acid decrease, while the extraction yields of salicylic acid tend to increase. This selectivity exchange can be seen comparing the conventional extraction results, Fig. 4, with those of the intensification techniques, Fig. 7 and 8 for UAE and MAE, respectively.
1), it is clear that the phenomena of the switch of selectivity observed in the UAE can also be seen with MAE. When both UAE and MAE techniques are used, the extraction yields towards gallic acid decrease, while the extraction yields of salicylic acid tend to increase. This selectivity exchange can be seen comparing the conventional extraction results, Fig. 4, with those of the intensification techniques, Fig. 7 and 8 for UAE and MAE, respectively.
For both UAE and MAE, the response surface models, Fig. 9, were plotted resorting to Design Expert. The response surface methodology uses the central composite design with a quadratic model to fit the UAE results and a cubic model for MAE results. It can be observed that for UAE, for the three ESs, time has more impact on the extraction yield than power. For Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and ChCl
4) and ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with UAE, the surface response models, Fig. 9(b) and (c), indicate that longer time and higher power should increase extraction yields; however, these models do not take into account the heating of the US probe that can cause degradation of PCs. The response surface model of Pro
1) with UAE, the surface response models, Fig. 9(b) and (c), indicate that longer time and higher power should increase extraction yields; however, these models do not take into account the heating of the US probe that can cause degradation of PCs. The response surface model of Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), Fig. 9(a), indicates that longer extraction times at lower power favours the PC extraction. The response surface models for MAE results for the three ESs give the same information as for UAE, i.e., longer time and higher temperature should increase the yields. However, once again, these surface models do not consider the possible degradation of PCs when they are exposed to high temperatures for long periods of time.
4), Fig. 9(a), indicates that longer extraction times at lower power favours the PC extraction. The response surface models for MAE results for the three ESs give the same information as for UAE, i.e., longer time and higher temperature should increase the yields. However, once again, these surface models do not consider the possible degradation of PCs when they are exposed to high temperatures for long periods of time.
An overall comparison of the best extraction results of PCs from S. muticum with the different extraction techniques used in this work is presented in Fig. 10, using 30% (v/v) water solutions of the 3 selectedESs. It is clear that using Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) in combination with MAE improves the extraction in terms of time and yield. Although for Pro
4) in combination with MAE improves the extraction in terms of time and yield. Although for Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-But (1
1,2-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) the intensification methods did not improve the extraction capacity in comparison with the orbital shaker, if the extraction time is taken into account (100 min for maceration, 9 min for UAE and 6 min for MAE), the use of intensification techniques is less energy intensive and thus more sustainable than maceration. A similar situation can be found for ChCl
4) the intensification methods did not improve the extraction capacity in comparison with the orbital shaker, if the extraction time is taken into account (100 min for maceration, 9 min for UAE and 6 min for MAE), the use of intensification techniques is less energy intensive and thus more sustainable than maceration. A similar situation can be found for ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), with the difference that in this case the selectivity changes with the intensification methods as mentioned before.
1), with the difference that in this case the selectivity changes with the intensification methods as mentioned before.
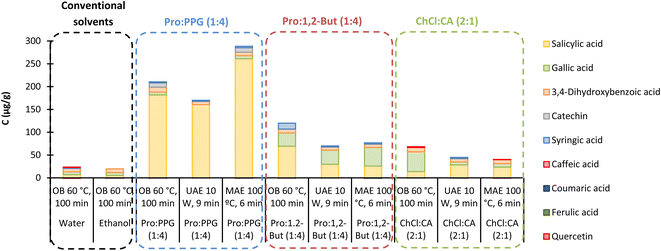 | ||
| Fig. 10 Overall comparison of the conventional solvents and the best conditions for the different techniques used for higher yields of the ESs with 30% (v/v) water. | ||
Considering Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and using the best MAE conditions, extraction with cycles was performed with the objective to enrich the extract. The obtained results are presented in Fig. 11. The extract obtained from one extraction was used as extraction solvent for the next extraction. Solvent losses between extractions were observed, decreasing the amount of solvent available for the subsequent extractions. Therefore, several extractions with small volumes were performed and combined between cycles. After three cycles, a notable increase in the concentration of the extracts was observed. In the optimization of the MAE parameters at 100 °C for 6 minutes, where only a cycle was performed, a final concentration of 26.69 ± 0.63 ppm was determined for salicylic acid. After performing three consecutive cycles, the concentration of the extract was increased up to 156.30 ± 0.39 ppm, which was almost 6 times higher in comparison with that of one cycle.
4) and using the best MAE conditions, extraction with cycles was performed with the objective to enrich the extract. The obtained results are presented in Fig. 11. The extract obtained from one extraction was used as extraction solvent for the next extraction. Solvent losses between extractions were observed, decreasing the amount of solvent available for the subsequent extractions. Therefore, several extractions with small volumes were performed and combined between cycles. After three cycles, a notable increase in the concentration of the extracts was observed. In the optimization of the MAE parameters at 100 °C for 6 minutes, where only a cycle was performed, a final concentration of 26.69 ± 0.63 ppm was determined for salicylic acid. After performing three consecutive cycles, the concentration of the extract was increased up to 156.30 ± 0.39 ppm, which was almost 6 times higher in comparison with that of one cycle.
4. Conclusions
A variety of choline-, proline- and betaine-based ESs were screened for the conventional extraction of phenolic compounds from S. muticum using UV/Vis spectrophotometric methods to determine the total phenolic content (TPC), the total flavonoid content (TFC) and the antioxidant activity (DPPH). For some ESs precipitation and flocculation were observed, sometimes leading to large deviations between replicas. Therefore, high performance liquid chromatography (HPLC) was selected as a more robust quantification method to characterise the composition of the extracts, targeting 9 relevant phenolic compounds (i.e., gallic acid, 3,4-dihydroxybenzoic acid, catechin, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, salicylic acid, and quercetin). Several ESs tested were able to improve the extraction yields of the conventional solvents used, water and ethanol, especially Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), followed by Pro
4), followed by Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-But (1
1,4-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and ChCl
4) and ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). An interesting phenomenon was observed regarding the selectivity, Pro
1). An interesting phenomenon was observed regarding the selectivity, Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) being highly selective towards salicylic acid, while ChCl
4) being highly selective towards salicylic acid, while ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA (2
CA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) presented more affinity towards gallic acid. However, Pro
1) presented more affinity towards gallic acid. However, Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-But (1
1,4-But (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was not very selective despite its good extraction capacity.
4) was not very selective despite its good extraction capacity.
Two experimental variables, water content and temperature, were optimized for the three best performing ESs. It was concluded that 30% (v/v) water content and 60 °C were the best conditions for 100 min of extraction. In order to reduce the extraction time, intensification techniques such as MAE and UAE were evaluated, providing similar extraction yields while significantly reducing the time by one order of magnitude and the energy consumption. In the case of MAE, not only a decrease of the extraction time was observed but also an improvement of the extraction yield for Pro![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPG (1
PPG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). It should be noted that in UAE the optimized parameters were time and power, while in MAE they were time and temperature. A response surface model was plotted for both techniques, leading to the conclusion that longer extraction times, higher power in UAE and higher temperature in MAE should return improved results. However, the surface model does not take into account the degradation of the PCs that tends to occur when they are exposed for a long period of time to high temperatures. This is especially notable in the UAE, where the control of temperature is not possible and the probe tends to reach high temperatures (above 90 °C), and hence this needs attention when setting up the extraction conditions.
4). It should be noted that in UAE the optimized parameters were time and power, while in MAE they were time and temperature. A response surface model was plotted for both techniques, leading to the conclusion that longer extraction times, higher power in UAE and higher temperature in MAE should return improved results. However, the surface model does not take into account the degradation of the PCs that tends to occur when they are exposed for a long period of time to high temperatures. This is especially notable in the UAE, where the control of temperature is not possible and the probe tends to reach high temperatures (above 90 °C), and hence this needs attention when setting up the extraction conditions.
This work showed the advantage of using ESs as extraction solvents in terms of their efficiency in increasing the recovery of natural antioxidants from biomass waste. Moreover, the use of intensification techniques can improve the efficiency of the extraction process by decreasing time and energy requirements.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. Gonzalez-Miquel would like to acknowledge the Comunidad Autónoma de Madrid (Spain) for funding through projects “SUSTEC P2018/EMT-4348” and “Multiannual Agreement with Universidad Politécnica de Madrid in the line Excellence Programme for University Professors, in the context of the V PRICIT (Regional Programme of Research and Technological Innovation)”. This work was financed by FCT/MCTES (Portugal) through the Centro de Química Estrutural (UIDB/00100/2020 and UIDP/00100/2020) and Institute of Molecular Sciences (LA/P/0056/2020). This work was also supported by (FCT/MCTES) through the Strategic Projects granted to MARE—Marine and Environmental Sciences Centre (UIDP/04292/2020 and UIDB/04292/2020), and to Associate Laboratory ARNET (LA/P/0069/2020).Notes and references
- L. Bastiaens, S. van Roy, G. Thomassen and K. Elst, Microalgae-Based Biofuels and Bioproducts: from Feedstock Cultivation to End-Products, 2017, pp. 327–345 Search PubMed.
- R. Platt, A. Bauen, P. Reumerman, C. Geier, R. van Ree, I. V. Gursel, L. Garcia, M. Behrens, P. v. Bothmer, J. Howes, Y. Panchaksharam, K. Vikla, V. Sartorius and B. Annevelink, Directorate-General for Research and Innovation, E4tech, WUR, BTG, FNR and ICONS, EU biorefinery outlook to 2030: studies on support to research and innovation policy in the area of bio-based products and services, European Commission, 2021 Search PubMed.
- P. Choudhary, G. Venkata Subhash, M. Khade, S. Savant, A. Musale, G. Raja Krishna Kumar, M. S. Chelliah and S. Dasgupta, J. Environ. Manage., 2021, 291, 112697 CrossRef PubMed.
- D. Rodrigues, A. C. Freitas, L. Pereira, T. A. P. Rocha-Santos, M. W. Vasconcelos, M. Roriz, L. M. Rodríguez-Alcalá, A. M. P. Gomes and A. C. Duarte, Food Chem., 2015, 183, 197–207 CrossRef CAS PubMed.
- I. N. Caxiano, P. A. Mello, P. H. R. Alijó, L. v. Teixeira, R. F. Cano, J. G. S. S. Maia, J. B. V. Bastos and M. S. G. Pavão, Bioresour. Technol., 2022, 343, 126152 CrossRef CAS PubMed.
- K. le Lann, S. Connan and V. Stiger-Pouvreau, Mar. Environ. Res., 2012, 80, 1–11 CrossRef CAS PubMed.
- M. Barbosa, F. Fernandes, M. J. Carlos, P. Valentão and P. B. Andrade, Algal Res., 2021, 59(102455) Search PubMed.
- P. Pérez-Larrán, M. D. Torres, N. Flórez-Fernández, E. M. Balboa, A. Moure and H. Domínguez, J. Appl. Phycol., 2019, 31, 2481–2495 CrossRef.
- A. Tanniou, L. Vandanjon, M. Incera, E. Serrano Leon, V. Husa, J. le Grand, J. L. Nicolas, N. Poupart, N. Kervarec, A. Engelen, R. Walsh, F. Guerard, N. Bourgougnon and V. Stiger-Pouvreau, J. Appl. Phycol., 2014, 26, 1215–1230 CAS.
- L. Montero, A. P. Sánchez-Camargo, V. García-Cañas, A. Tanniou, V. Stiger-Pouvreau, M. Russo, L. Rastrelli, A. Cifuentes, M. Herrero and E. Ibáñez, J. Chromatogr. A, 2016, 1428, 115–125 CrossRef CAS PubMed.
- Polyphenols Market Size & Share Report, 2022–2030, https://www.grandviewresearch.com/industry-analysis/polyphenols-market-analysis, accessed 13 October 2022 Search PubMed.
- K. H. Sabeena Farvin and C. Jacobsen, Food Chem., 2013, 138, 1670–1681 CrossRef CAS PubMed.
- C. Caijiao, H. Leshan, Y. Mengke, S. Lei, Z. Miansong, S. Yaping, L. Changheng, B. Xinfeng, L. Xue, L. Xin and J. Airong, Russ. J. Mar. Biol., 2021, 47, 380–387 CrossRef.
- K. Chakraborty, A. Maneesh and F. Makkar, J. Aquat. Food Prod. Technol., 2017, 26, 406–419 CrossRef CAS.
- S. C. Foo, F. M. Yusoff, M. Ismail, M. Basri, S. K. Yau, N. M. H. Khong, K. W. Chan and M. Ebrahimi, J. Biotechnol., 2017, 241, 175–183 CrossRef CAS PubMed.
- J. Liu, C. Du, H. T. Beaman and M. B. B. Monroe, Pharmaceutics, 2020, 12(419) Search PubMed.
- A. N. Panche, A. D. Diwan and S. R. Chandra, J. Nutr. Sci., 2016, 5, e47 CrossRef CAS PubMed.
- W. Routray and V. Orsat, Food Bioprocess Technol., 2012, 5, 409–424 CrossRef CAS.
- E. L. Santos, B. H. L. N. S. Maia, A. P. Ferriani and S. D. Teixeira, Flavonoids – From Biosynthesis to Human Health, 2017 Search PubMed.
- S. Kumar and A. K. Pandey, Sci. World J., 2013, 2013(162750) Search PubMed.
- W. Zhao, V. Subbiah, C. Xie, Z. Yang, L. Shi, C. Barrow, F. Dunshea and H. A. R. Suleria, Food Rev. Int., 2022 DOI:10.1080/87559129.2022.2094404.
- B. Pradhan, R. Nayak, P. P. Bhuyan, S. Patra, C. Behera, S. Sahoo, J.-S. Ki, A. Quarta, A. Ragusa and M. Jena, Mar. Drugs, 2022, 20, 403 CrossRef CAS PubMed.
- Q. W. Zhang, L. G. Lin and W. C. Ye, Chin. Med., 2018, 13, 1–26 CrossRef CAS PubMed.
- M. Castro-Puyana, M. L. Marina and M. Plaza, Curr. Opin. Green Sustainable Chem., 2017, 5, 31–36 CrossRef.
- F. Chemat, M. A. Vian, H. K. Ravi, B. Khadhraoui, S. Hilali, S. Perino and A. S. F. Tixier, Molecules, 2019, 24, 3007 CrossRef CAS PubMed.
- C. Florindo, L. C. Branco and I. M. Marrucho, ChemSusChem, 2019, 12, 1549–1559 CrossRef CAS PubMed.
- C. Florindo, F. Lima, B. D. Ribeiro and I. M. Marrucho, Curr. Opin. Green Sustainable Chem., 2019, 18, 31–36 CrossRef.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- J. Afonso, A. Mezzetta, I. M. Marrucho and L. Guazzelli, Green Chem., 2023, 25, 59–105 RSC.
- E. D. Obluchinskaya, A. v. Daurtseva, O. N. Pozharitskaya, E. v. Flisyuk and A. N. Shikov, Pharm. Chem. J., 2019, 53, 243–247 CrossRef CAS.
- J. B. Barbieri, C. Goltz, F. Batistão Cavalheiro, A. Theodoro Toci, L. Igarashi-Mafra and M. R. Mafra, Ind. Crops Prod., 2020, 144, 112049 CrossRef CAS.
- M. Z. Gao, Q. Cui, L. T. Wang, Y. Meng, L. Yu, Y. Y. Li and Y. J. Fu, Microchem. J., 2020, 154, 104598 CrossRef CAS.
- P. Zhou, X. Wang, P. Liu, J. Huang, C. Wang, M. Pan and Z. Kuang, Ind. Crops Prod., 2018, 120, 147–154 CrossRef CAS.
- E. A. Krisanti, K. Saputra, M. M. Arif and K. Mulia, AIP Conf. Proc., 2019, 2175, 020040 CrossRef.
- R. Cañadas, B. Sáenz de Miera, P. Méndez, E. J. González and M. González-Miquel, Molecules, 2023, 28, 1153 CrossRef PubMed.
- Proline, European Medicines Agency, https://www.ema.europa.eu/en/proline, accessed 24 October 2022 Search PubMed.
- C. Chambers, G. Degen, R. Dubakiene, B. Jazwiec-Kanyion, V. Kapoulas, K. Jean, J.-P. M. Carola Lidén, T. Platzek, S. C. Rastogi, R. Jean, R. Vera, T. Sanner, G. Speit, J. Van Engelen and I. R. White, Eur. Comm., 2008, 1–16 Search PubMed.
- European Commission, EUR-Lex – 32011R1130 – EN – EUR-Lex, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32011R1130, accessed 17 September 2022 Search PubMed.
- D. Turck, J. Bresson, B. Burlingame, T. Dean, S. Fairweather-Tait, M. Heinonen, K. I. Hirsch-Ernst, I. Mangelsdorf, H. J. McArdle, A. Naska, M. Neuhäuser-Berthold, G. Nowicka, K. Pentieva, Y. Sanz, A. Siani, A. Sjödin, M. Stern, D. Tomé, M. Vinceti, P. Willatts, K. Engel, R. Marchelli, A. Pöting, M. Poulsen, J. R. Schlatter, E. Turla and H. van Loveren, EFSA J., 2017, 15(11), e05057 Search PubMed.
- A. Anadón, M.-L. Binderup, W. Bursch, L. Castle, R. Crebelli, K.-H. Engel, R. Franz, N. Gontard, T. Haertlé, T. Husøy, K.-D. Jany, C. Leclercq, J.-C. Lhuguenot, W. Mennes, M. Rosaria Milana, K. Pfaff, K. Svensson, F. Toldrá, R. Waring, E. Lampi and D. Wölfle, EFSA J., 2011, 9, 2122 CrossRef.
- RTECS:EK0380000 – 1,2-Butanediol – The Registry of Toxic Effects of Chemical Substances, CDC/NIOSH, https://www.cdc.gov/niosh-rtecs/EK5CC60.html, accessed 17 September 2022 Search PubMed.
- CosIng – Cosmetics – GROWTH, European Commission, https://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.details_v2%26id=54169, accessed 17 September 2022 Search PubMed.
- EUR-Lex – 32009R1223 – EN – EUR-Lex, https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32009R1223, accessed 15 October 2022 Search PubMed.
- A. M. Shraim, T. A. Ahmed, M. M. Rahman and Y. M. Hijji, Antibiotics, 2021, 150, 111932 CAS.
- J. Gudej and M. Tomczyk, Arch. Pharmacal Res., 2004, 27, 1114–1119 CrossRef CAS PubMed.
- R. Cañadas, I. Díaz, M. Rodríguez, E. J. González and M. González-Miquel, J. Cleaner Prod., 2022, 334, 130181 CrossRef.
- M. W. S. Lim, K. M. Tan, L. Y. Chew, K. W. Kong and S. W. Yan, J. Aquat. Food Prod. Technol., 2018, 27, 446–463 CrossRef CAS.
- B. Soares, F. Cunha, I. Silva, C. Florindo, L. C. Branco and I. M. Marrucho, J. Chem. Eng. Data, 2021, 66, 2793–2802 CrossRef CAS.
- A. Klamt, V. Jonas, T. Bürger and J. C. W. Lohrenz, J. Phys. Chem. A, 1998, 102, 5074–5085 CrossRef CAS.
- E. Mullins, Y. A. Liu, A. Ghaderi and S. D. Fast, Ind. Eng. Chem. Res., 2008, 47, 1707–1725 CrossRef CAS.
- E. Chaabani, M. Abert Vian, R. Bott, C. Ginies, C. Defoort, R. Ksouri and F. Chemat, Sep. Sci. Technol., 2019, 55, 716–727 CrossRef.
- C. Florindo, A. J. S. McIntosh, T. Welton, L. C. Branco and I. M. Marrucho, Phys. Chem. Chem. Phys., 2017, 20, 206–213 RSC.
- A. Silva, C. Rodrigues, P. Garcia-Oliveira, C. Lourenço-Lopes, S. A. Silva, P. Garcia-Perez, A. P. Carvalho, V. F. Domingues, M. F. Barroso, C. Delerue-Matos, J. Simal-Gandara and M. A. Prieto, Foods, 2021, 10, 1915 CrossRef CAS PubMed.
- Opinion on Salicylic Acid (CAS 69-72-7), Publications Office of the EU, https://op.europa.eu/en/publication-detail/-/publication/75be19bf-5390-11ea-aece-01aa75ed71a1, accessed 24 October 2022 Search PubMed.
- EUR-Lex – 32009R1223 – EN – EUR-Lex, https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32009R1223, accessed 24 October 2022 Search PubMed.
- Food Contact Materials, Enzymes and Processing Aids, EFSA J., 2010, 8(7), 1405 Search PubMed.
- E. Husanu, A. Mero, J. G. Rivera, A. Mezzetta, J. C. Ruiz, F. D’andrea, C. S. Pomelli and L. Guazzelli, ACS Sustainable Chem. Eng., 2020, 8, 18386–18399 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00051f |
| This journal is © The Royal Society of Chemistry 2023 |