Open Access Article
Open Access ArticlePlant-based, aqueous, water-repellent sprays for coating textiles†
Sara K.
Fleetwood
 a,
Sydney
Bell
a,
Sydney
Bell
 b,
Reinhard
Jetter
b,
Reinhard
Jetter
 bc and
E. Johan
Foster
bc and
E. Johan
Foster
 *a
*a
aDepartment of Chemical and Biological Engineering 421, University of British Columbia, 2360 East Mall, Vancouver, BC V6T 1Z3, Canada. E-mail: johan.foster@ubc.ca
bDepartment of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, BC V6T 1Z1, Canada
cDepartment of Botany, University of British Columbia, 6270 University Boulevard, Vancouver, BC V6T 1Z4, Canada
First published on 7th September 2023
Abstract
Novel superhydrophobic coatings, that are both biodegradable and biosourced, have the potential to revolutionize the water-repellent coating industry. Here, water-repellent coatings were prepared from commercially unavailable plant waxes, isolated using solvent extraction and characterized using DSC, GC-MS and DLS. In the first stage, a plant survey was conducted to identify an ideal plant source for the final spray, in which Whatman filter paper was submerged in a wax-solvent solution with recrystallization occurring upon air-drying. In the second stage, aqueous, PFC-free wax dispersions were prepared, coated onto textiles (cotton and polyester), and heat-treated with a home drying machine to allow for the spreading and recrystallization of the waxes. In both stages, SEM visualization verified the coating's morphology, and contact angle measurements showed them to be superhydrophobic. It was concluded that, using less coating material than commercial coatings, high-performing petroleum-free coatings could be made and applied onto textiles of various polarities.
1 Introduction
Superhydrophobic coatings (contact angle >150°) have gained significant attention in recent years due to their ability to make a wide variety of materials self-cleaning, anti-fogging, and/or anti-biofouling. Therefore, superhydrophobic coatings are useful in a plethora of applications, including rainwear, solar panels, skis, produce, cars, etc. To stay dry and allow thermoregulation of the body, outdoor users currently apply commercial water-repellent sprays, or durable water-repellent coatings (DWR), to their apparel and gear (ex. rainjacket, shirt, softshell, tent) at home by spraying and then heat treating in their drying machine. This prevents the fabric from becoming saturated with water, which prevents discomfort or hypothermia by reducing the heat transfer away from the body caused by evaporative cooling. However, to minimize environmental impact, these coatings need to also be durable, non-toxic, and biodegradable.Water-repellent coatings typically contain perfluorinated compounds (PFCs), or more specifically perfluoroalkyl substances (PFASs), due to their low surface energy. However, PFASs with long chain lengths of seven or more carbons accumulate within the body and do not break down in the environment.1 Therefore, there has been a shift to using shorter perfluoroalkyls with C4 to C6 chains. While shorter fluorinated chains are not considered to bioaccumulate, they persist in the environment, their key performance characteristics are not as good, and there is a lack of long-term data on their impact on the environment and human health.2 For these reasons, concerns of using long-chain PFASs began in the 1960s, and a growing number of restrictions have since been put on the use of these chemicals, with the outdoor apparel industry receiving some of the most scrutiny as a route of exposure to PFASs.3 This has led to large companies such as Arc’teryx and Patagonia posting PFC-free and PFAS-free statements on their websites4,5:
“PFC-free DWR is a serious focus for our R&D efforts …. To date, all of the non-PFC DWR treatments we have tested have fallen short.”4
Environmental and human health concerns for water-repellent coatings using PFAS chemistry have led to the exploration of PFC-free chemistries,3 one solution being waxes. However, the processes used are often not industrially scalable, use complex chemical compositions (negating the benefits of being plant-based), and/or cannot be applied by users at home. Common techniques for dispersing waxes in surfactants include homogenization, either by rotor–stator or high-pressure and ultrasonication, or dispersing in organic alcohols through self-emulsification.6 A more environmentally friendly alternative would be to use an aqueous wax suspension. However, past literature has stated that to stabilize this would require 10% or more emulsifier.7 However, adding more emulsifier can increase the surface energy of the particles, thus reducing waterproof properties.6,8
A few papers have reported aqueous wax dispersions without emulsifiers, all using non-scalable techniques to create the suspension or apply it to the surface. These waxes were either petroleum based, made of a combination of beeswax and carnauba wax, or incorporated nanoparticles into their coating. For example, Bayer et al. spray casted surfactant-free wax-in-alcohol (carnauba wax in isopropyl alcohol or ethanol) emulsions containing suspended ∼150 nm PTFE nanoparticles (prepared using sonication) onto glass slides, followed by thermal annealing on a hot plate at 110 °C for 15 min to achieve superhydrophobic surfaces with 150 μm film thicknesses and PTFE/wax mass fractions >0.6.7 Lozhechnikova et al. took this a step further and prepared an aqueous self-emulsifying carnauba wax layer-by-layer (LBL) coating using probe sonication.6 Forsman et al. used LBL deposition of cationic poly-L-lysine and anionic carnauba wax particles (wax dispersion prepared via probe sonication) to coat linen and cotton, followed by thermal annealing in an oven at 103 °C for 1 h to achieve superhydrophobicity for the two bilayers (2BL) sample on light cotton.9 Wang et al. spray coated wax-in-acetone emulsions of carnauba wax and beeswax (prepared using ultrasonication) onto glass slides and polystyrene cups for easy removal of food container residues to reduce food waste, achieving superhydrophobicity with surface densities of carnauba wax ≥0.55 mg cm−2.10
Within industry, mountainFLOW Eco Wax LLC has developed plant-based waxes for applying to skis and bikes, including an aqueous dispersion that can be sprayed onto ski skins. However, all of their patents pertain to melting solid blocks of plant wax onto skis as the intended use, rather than applying to outdoor apparel and gear that is intended to keep the user dry. Additionally, their chemical composition uses commercially available plant waxes, with the primary contents being either candelilla, carnauba, rice bran, or castor wax.11,12
In all scientifically reported and industrially used systems, the waxes were petroleum-based (ex. paraffin wax) or obtained from commercially available biological sources. Among the biologically sourced waxes, beeswax, carnauba, candelilla, and sugarcane are economically the most important, while ouricury, esparto, bamboo, rice bran, and Japan wax are of local importance.13 Carnauba wax is a natural ester wax found on the leaves of the carnauba palm, Copernicia prunifera, which is only grown in northeastern Brazil. It is a common leaf wax used in commercial applications, as it is one of the hardest natural waxes and has a high melting point of 82.0–85.5 °C. Carnauba wax consists primarily of wax alkyl esters (40%) of acids with average chain length C26 and alcohols with average chain length C32.13 Often, carnauba wax is mixed with beeswax, which has a lower melting point of 62–65 °C,13 to reduce brittleness.
Carnauba palms are evergreens, meaning their leaves must be harvested for wax extraction. To keep the tree healthy, only 20–30 leaves can be harvested per tree each year.14 Instead, it would be better to use waxes from plant waste streams, such as leaf-litter from deciduous plants or conifer needles from slash piles that remain in the forest after logging. Many plants coat their leaves or needles with waxes composed of large amounts of nonacosan-10-ol and nonacosanediols.15 These two compound types are known to spontaneously co-crystallize into nanotubules, a structure that allows the hydrophilic hydroxyl groups to be buried between layers and the hydrophobic methyl groups to form the external surface of the tubules.16 Because of the nano-roughness they add to the surface of the plant, nanotubules can achieve much higher contact angles than smooth wax films (e.g., in the Lotus effect). Moreover, nanotubules give similar contact angles to wax platelets but do so more efficiently, due to higher surface-to-volume ratios and require fewer co-crystallizing compounds for crystal formation to occur. Currently, tubular waxes are not commercially available, as their plant sources have in the past been considered to produce minute quantities of wax in comparison to other sources.14
Herein, we explored using commercially unavailable, tubule-forming plant waxes to produce novel water-repellent coatings for applying to textiles. Through the preparation of these aqueous wax suspensions, we aimed to improve the scalability of the process by using a homogenizer in place of probe sonication. Additionally, we aimed to produce a stable coating using no emulsifiers that could be applied as a single-layer spray-on coating and, with the addition of heat from a home drying machine, would recrystallize into tubules. In doing so, a simpler, two-step coating process would be developed, allowing for coatings to be applied at home. It was anticipated that, by using wax sources containing the secondary alcohol nonacosan-10-ol, the hydrophobicity of this coating would be comparable or greater than that of commercial products, thus producing a petroleum- and PFC-free alternative.
2 Experimental methods
2.1 Materials
Leaves were collected from maple (Acer rubrum), horsetail (Equisetum arvense, sterile shoots), ginkgo (Ginkgo biloba), katsura (Cercidiphyllum japonicum), cedar (Cedrus atlantica), and smoketree (Cotinus coggygria) plants growing on the University of British Columbia campus (Vancouver, Canada) (Fig. 6). 100% pure carnauba wax of T1 Grade was purchased from H&B Oils Center Co. Chloroform (ACS reagent), N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) (GC derivatization, LiChropur™, ≥99.0%), and hexanes (ACS Reagent) were purchased from Sigma-Aldrich (St. Louis, United States of America). n-Tetracosane (≥99%) was purchased from Alfa Aesar (Ward Hill, United States of America). Pyridine (ACS Reagent) was purchased from Caledon (Georgetown, Canada). Helium (≥99%), hydrogen (≥99.95%) and nitrogen (≥99.998%) were purchased from Linde (Mississauga, Canada).Commercial spray-on coatings were purchased directly from the manufacturer's website or through online retailers. Grangers Performance Repel Plus (OWP) is produced in the UK, marketed for use on waterproof clothing, and is PFC-free. Nikwax SoftShell Proof™ Spray-On is also produced in the UK, marketed for use on softshells, and is water-based, PFC-free, and non-petroleum based. Arc’teryx Nμ is a re-branded product of Gear Aid (Bellingham, United States of America), marketed for use on technical outerwear fabrics (including GORE-TEX, softshells and windshells), and contains PFCs, and is petroleum-based. Fjäll Räven Waterproofing Impregnation is produced in Sweden, marketed for use on waterproof/breathable apparel, PFC-free, and contains petroleum based products such as polyurethane. Throughout the remainder of the paper these will be randomly assigned and referred to by the names “Coating 1–4”, respectively.
Hydrophilic and hydrophobic material substrates were used for applying the coatings. Grade 40 Whatman filter paper (100% cotton, nominal pore size 8 μm) was purchased from Fisher Scientific (Waltham, United States of America) and will be referred to as “filter paper” throughout the remainder of the paper. 100% cotton twill (0900801 Cotton Twill, 006 greige) and 100% polyester knit (039400 Scuba, 008 off white) were purchased from Fabricland Online (Toronto, Canada). A t-shirt made of 100% organic cotton jersey (birch white) was purchased from Patagonia, Inc. (Ventura, United States of America). A t-shirt made of 100% recycled polyester spun jersey (classic navy) was purchased from Patagonia, Inc. (Ventura, United States of America). All fabric was first washed with Seventh Generation Laundry Detergent (Fresh Lavender Scent) and then dried.
2.2. Overview of plant wax extraction methods
Plant waxes were extracted using three different methods: (1) small lab-scale (Section 2.3.1), (2) large-scale (Section 2.4.1), and (3) analytical-scale (Section 2.5.6), which are described in further detail in their corresponding sections. A detailed graphic of process steps for the plant survey (Section 2.3) and the scale-up experiments (Section 2.4 and 2.5) are shown in Fig. 1.2.3 Plant wax survey
2.4 Scaling-up isolation and coating processes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, using an IKA (Staufen, Germany) T25 Ultra-Turrax Homogenizer, mounted with a S25 N-18G Dispersing Tool. Subsequently, the emulsion was quenched in an ice bath and filtered through a filter funnel, containing a 40–90 μm nominal maximal pore size fritted disc. The wax particles then solidified and the emulsion became a suspension.
000 rpm, using an IKA (Staufen, Germany) T25 Ultra-Turrax Homogenizer, mounted with a S25 N-18G Dispersing Tool. Subsequently, the emulsion was quenched in an ice bath and filtered through a filter funnel, containing a 40–90 μm nominal maximal pore size fritted disc. The wax particles then solidified and the emulsion became a suspension.
For comparison, commercial coatings were also used to coat the substrates, following the instructions on their bottles. 4 × 4 cm2 squares of cotton and polyester fabric were dipped in RO water to dampen, sprayed 20× from 15 cm away, and cleaned of excess coating by wiping after 2 min. Coating 2 and Coating 4 were dried with a GE 4 cu. ft. Electric Compact Dryer (model PCVH480EK0WW) on medium heat (∼57.2 °C, timed dry) for 30 min, Coating 1 was dried on medium heat for 60 min, and Coating 3 was dried on low heat for 30 min.
Similar to the fabric squares, the suspension was poured into a Dynalon Flip & Spray Bottle, but was instead sprayed onto cotton and polyester t-shirts from a distance of 6 cm away. Samples were initially dried in the drying machine on medium heat for 30 min. However, this was not enough time for the t-shirts to completely dry, so t-shirts were dried for an additional 30 min on medium heat.
2.5 Characterization techniques
The amount of coating applied to each substrate was determined by weighing the substrate before and after coating, allowing the coating to fully dry, and calculating the mass of wax per mass of substrate (wt%).
Gas chromatography-mass spectrometry (GC-MS) was carried out following a procedure similar to Buschhaus and Jetter.21 The wax was dissolved in 20 μL of pyridine, and then 20 μL of BSTFA was added. The solution was reacted at 70 °C for 45 min. Then, a stream of nitrogen gas at 60 °C was used to evaporate any excess derivatization reagent, and the residue was dissolved in 20 μL of chloroform. The derivatized wax samples were separated using GC (6890N, Agilent), equipped with an on-column injector and a HP-1 capillary column (Agilent; 30 m length, 320 μm i.d., 1 μm film thickness). An aliquot of the sample was injected on-column into a 2 mL min−1 constant flow of hydrogren. The oven was held for 2 min at 50 °C, ramped to 200 °C at 40 °C min−1, held at 200 °C for 2 min, ramped to 320 °C at 3 °C min−1, and held at 320 °C for 30 min. A flame ionization detector (FID, Agilent) was used to quantitatively detect analytes, with flame settings of 20 mL per min nitrogen, 30 mL per min hydrogen and 200 mL per min air at 250 °C. The samples were analyzed qualitatively with the same GC and column setup, but a 1.4 mL min−1 column flow of helium and a MS (5793N, Agilent, EI 70 eV, m/z 50–800, 1 scan s−1) were used instead.
To perform a GC-MS analysis of the commercial waterproofing sprays, a liquid–liquid extraction was performed for each spray by combining an aliquot of the spray (1 mL) with deionized water (3 mL) and hexanes (3 × 4 mL). The organic layers were combined and dried under a stream of nitrogen gas to yield the isolated waxy components. The isolated waxy components were derivatized and analyzed following the same procedure as the plant waxes.
3 Results and discussion
3.1 Plant wax survey: source wax properties
Initially, plant species were screened by visual inspection of water repelling properties, and trees with leaves appearing superhydrophobic were selected. This included leaves that were dry during rainstorms, appeared glaucous, caused water to bead on their surfaces, or appeared metallic when submerged in water (Fig. 2). The glaucous appearance is particularly of note, as this has been correlated to surface morphologies of high surface-to-volume ratios and high surface energies.22To understand the impact of cuticular wax composition on contact angle and, thus, coating performance, waxes were extracted with chloroform from fresh leaves (maple, ginkgo, katsura, and smoketree), needles (cedar), or branches (horsetail) of the plant species with chloroform. The extracts were spiked with an internal standard (tetracosane), TMS-derivatized, and analyzed with GC-MS to identify individual wax components (Fig. S1–S6, ESI†). Common contaminants, such as GC column degradation products or environmental pollutants, were not included in the compound class analyses and wax quantifications.
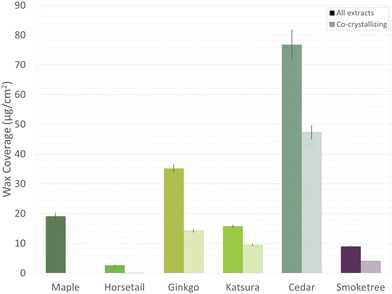
The total wax coverages ranged from 2.6 μg cm−2 in horsetail to 8.9 μg cm−2 in smoketree, 15.7 μg cm−2 in katsura, 19.1 μg cm−2 in maple, 35.2 μg cm−2 in ginkgo, and 76.8 μg cm−2 in cedar (Fig. 3). Because surface areas were calculated using top-down projections assuming flat surfaces, wax coverages must be considered as approximations. In particular, cedar needles are fairly thick and may therefore have slightly larger surface areas and lower wax loads then estimated here.23
While the analytical extraction method used three washes, each with a duration of thirty seconds, the bulk extraction method used one wash with a duration of five minutes to reduce the relative amount of chloroform needed. It has been shown that, when submerged in chloroform, the majority of cuticular waxes are removed within ten seconds.24 Using ginkgo as an example, it was determined that the bulk extraction method was able to achieve an extraction yield of about 43 μg cm−2. When adjusted for the difference in average leaf surface area between fresh leaves (66 cm2) as used in the analytical method and litter leaves as used in the bulk method (45 cm2), it was determined that the bulk method extracted about 80% of the wax that the analytical method achieved.
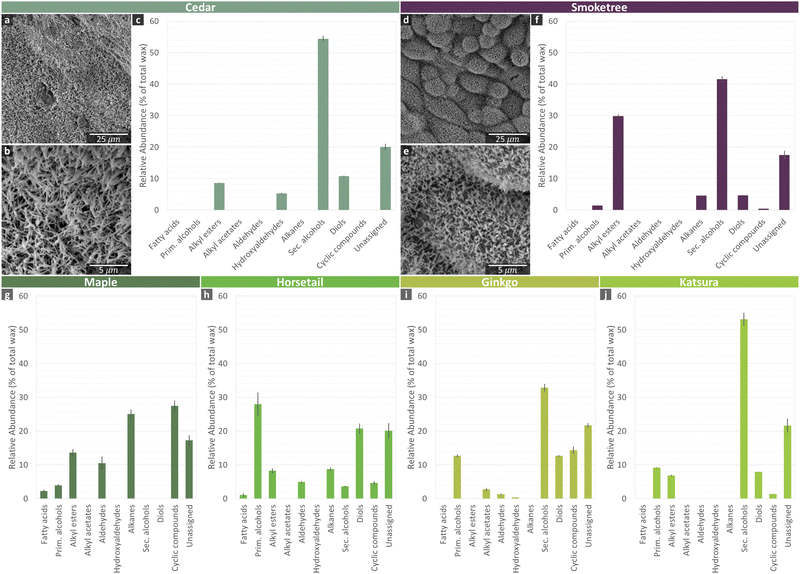
The wax analysis revealed a diversity of compound classes across the species. The most ubiquitous compound classes were the primary alcohols, secondary alcohols, and diols (Fig. 4). Only cedar wax had no detectable primary alcohols, and only maple wax had no detectable secondary alcohols or diols. Also frequently present were alkyl esters (found in cedar, smoketree, maple and horsetail waxes), aldehydes (maple, horsetail, and ginkgo), alkanes (smoketree, maple, and horsetail), and fatty acids (maple and horsetail). The more specialized compounds, alkyl acetates and hydroxyaldehydes, were only found in ginkgo wax. For brevity, the diverse cyclic compounds found in these species have been combined into one class (Table S1, ESI†).
The horsetail wax composition was dominated by primary alcohols (28%), and the maple wax was composed largely of alkanes (25%) and triterpenoids (24%). Secondary alcohols were the major component in cedar (54%), smoketree (42%), ginkgo (33%), and katsura (53%) waxes. In each of these four species, the most abundant compound was nonacosan-10-ol. These four species also all had diols present in their wax mixtures. In cedar, smoketree, and katsura, all the detected diols were 29 carbons long, but the most dominant hydroxyl group position varied from C-10/C-13 in cedar, to C-5/C-10 in smoketree, and C-4/C-10 in katsura. Interestingly, both a mid-chain-functionalized nonacosane-5,10-diol and terminally functionalized 1,3-diols (largely octacosane-1,3-diol) were found in ginkgo wax.
Maple, horsetail, smoketree, and ginkgo waxes have been described previously, and the findings of the present study generally agree with the literature. Further discussion on compounds found in comparison to prior literature can be found in the ESI.†
Nonacosan-10-ol and its diol derivatives are known to co-crystallize into wax tubules.15 Thus, the combined total coverage of these compounds was also determined (Fig. 3). None of the co-crystallizing compounds were detected in maple and horsetail wax, while smoketree wax had 4.05 μg cm−2 (45% of total wax), katsura wax had 9.43 μg cm−2 (60% of total wax), ginkgo wax had 14.16 μg cm−2 (40% of total wax), and cedar wax had 47.30 μg cm−2 (62% of total wax).
The commercial waterproofing sprays were also screened with GC-MS to determine if any were made of common plant leaf waxes. Coating 2 consisted primarily of glyceryl palmitate, while the compositions of Coatings 1 and 4 were primarily erucamide. Glyceryl palmitate and erucamide can be found as natural products in plants, but neither are common leaf wax components. Coating 3 did not contain any compounds that are known to be plant leaf waxes.
The micro-morphologies of two of the more hydrophobic plant surfaces, cedar needles and smoketree leaves, were also investigated with SEM (Fig. 4), which showed tubular structures. These results match with previous reports on a closely related cedar species as well as smoketree.23,25 Other plant waxes documented within the literature to have a tubular structure include ginkgo26 and katsura.27 Horsetail also has nano-scale surface roughness, but from silicon dioxide crystals.28 In contrast, maple contains wax platelets29 and the morphology of carnauba is not documented within the literature.
To determine the optimal temperature for melting of plant waxes and subsequently recrystallization, DSC measurements were taken (Fig. 5). Complete melting of carnauba, smoketree, and ginkgo waxes occurred at approximately 84, 70, and 62 °C respectively. Carnauba and smoketree waxes showed additional heat absorption peaks at roughly 77 and 60 °C, respectively. The additional heat absorption peaks of both waxes were likely due to polymorphic phase transitions, by which the wax mixtures existed in a semi solid–liquid form.10,18 For the smoketree wax, considered to be non-pure, this is due to the melting of different components at different temperatures. Based on these DSC measurements, 60 °C was concluded to be a suitable temperature at which to anneal the fabric samples.
3.2 Plant wax survey: solvent-based wax coating performance
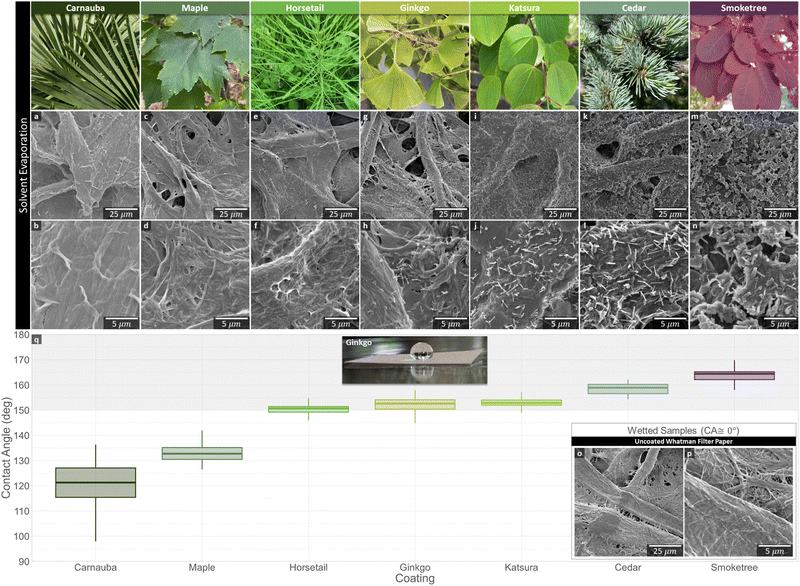
WCAs were measured to observe changes in hydrophobicity of the filter paper before and after coating. Control experiments showed that untreated filter paper immediately absorbed water, confirming its hydrophilic character. In contrast, all plant wax coated filter paper had WCAs greater than 120° (Fig. 6q), which is consistent with previous reports on wax-covered plant surfaces, where smooth wax layers had WCAs close to 90° and micro-structured waxes had WCAs in the range of 120–160°.30 All plant waxes, except carnauba, held these WCAs for the full 5 min of testing, demonstrating their stability as hydrophobic coatings.However, carnauba and maple waxes had considerably lower WCAs than the remainder of the plant waxes. Neither species waxes are known to contain secondary alcohols–carnauba waxes are reported to be mainly composed of alkyl esters,13 and the present study found maple wax to be primarily alkanes and triterpenoids. Moreover, in reports on other species of maple, WCAs on the leaf fall significantly short of superhydrophobic. A tubule structure has not been reported for either wax, and red maple waxes are known to instead crystallize into platelets.13
The results for carnauba wax, exhibiting the lowest WCA and no surface nano-roughness, were particularly of interest, given that carnauba wax has been the focus of prior research on a plant-based alternative to PFC coatings due to its commercial availability and having a high melting point among plant waxes. Within 5 min, carnauba exhibited time-dependent wetting under the Cassie impregnating wetting regime.31 Wetting may have been partially related to the relatively low solubility of carnauba wax, in comparison to other waxes, requiring the addition of heat during the coating procedure and possibly resulting in less uniform wax coverage.
The horsetail wax, which contained only traces of nonacosan-10-ol, but a significant amount of hentriacontane-6,8-diol, was capable of achieving near-superhydrophobicity on the filter paper. However, no wax crystals were visible using SEM.
The remaining waxes, ginkgo, katsura, cedar, and smoketree, all contained significant amounts of the co-crystallizing nonacosan-10-ol and nonacosanediols. Indeed, tubule nanocrystals were visible via SEM after recrystallization on filter paper. The nano-roughness afforded by the tubules led to each of these waxes being able to achieve superhydrophobicity. On the natural leaf surfaces, ginkgo and katsura waxes have both exhibited near-superhydrophobicity.27,32
With maple wax achieving one of the lowest contact angles in the study (Fig. 6), and cedar wax achieving one of the highest, a very general link can be drawn between the relative abundance of co-crystallizing compounds and the contact angle of the re-crystallized waxes. However, this is not the only contributing factor, as smoketree wax has a slightly higher contact angle than cedar wax but a much lower relative abundance of co-crystallizing compounds.
SEM images revealed a difference in surface morphology, specifically nano-roughness, as a potential explanation for the difference in hydrophobicity between plant waxes (Fig. 6a–n). Carnauba, maple, and horsetail coated samples appeared smooth. However, ginkgo, katsura, cedar, and smoketree plant waxes recrystallized into tubules upon air drying and without heat treating (Fig. 6g–n), consistent with Jetter et al.33
In summary, the primary difference in hydrophobicity was that hydrophobic (90° < WCA < 150°) plant wax coatings were smooth and did not show nano-scale surface roughness, whereas superhydrophobic (WCA > 150°) samples contained wax tubules. Due to their high WCAs, we chose to focus further experiments on water-repellent coatings of waxes forming nanotubules based on nonacosan-10-ol. In this context, it was important to develop a scalable wax extraction process, so instead of using freshly picking leaves/needles off of trees, we used leaf litter from deciduous plant sources.
Among plant sources, we chose to focus on ginkgo because of its hydrophobicity, both observed on the leaf (Fig. 2) and filter paper samples (Fig. 6), and dense coverage of tubular microcrystalline wax aggregates (primarily made up of (S)-nonacosan-10-ol [(+)-ginnol]34–36) on both abaxial and adaxial surfaces.26 The dense wax coverage was also observed in our results (Fig. 3). While cedar needles had greater hydrophobicity and wax coverage than ginkgo leaves, cedars are evergreens and therefore do not naturally lose their needles each year, in comparison to ginkgo leaves. Additionally, ginkgo leaves are already harvested for other commercial applications, specifically ginkgo is one of the most sold medicinal plants with a production of 8000 tons per year of dried leaves.37 Therefore, we chose ginkgo wax for scale-up experiments testing water-repellent spray applications, in comparison with carnauba wax and commercial hydrophobic sprays.
3.3 Water-repellent spray: dispersion stability over time
In the following experiments, plant wax suspensions were compared against commercial coatings, prepared as instructed on their labels. The aqueous plant wax particle suspensions, containing either carnauba or ginkgo wax, were prepared with no additional emulsifiers and stabilizers. Bottles of commercial samples were shaken, as instructed. To maintain consistency, plant wax suspensions were also shaken, despite there being visually very little sedimentation over the course of three months. After shaking, no sedimentation was visible with all samples looking similar (Fig. 11).DLS was used to measure the stability of these suspensions with respect to surface charge. Within a day of preparation, ζ-potentials were −57.0 ± 1.5 mV for carnauba and −52.3 ± 0.5 mV for ginkgo at a concentration of 0.01 g mL−1 (Table 1). Due to this net negative surface charge of wax particles, electrostatic double-layer repulsion was present and able to provide a high degree of stability for the suspension with no tendency to coagulate or flocculate.38 Similar electrostatic charges were described by Lozhechnikova et al. as being caused by the amphiphilic nature of wax,6 consisting of a hydrophobic tail attached to hydrophilic functional groups at the head (e.g. –OH, –COOH, –CHO).39 Upon melting and emulsifying in water, these amphiphilic molecules rearrange into micelles, resulting in a hydrophobic core and a hydrophilic particle surface interacting with the polar water phase.
| Material | Z-avg (nm) | PDI | ζ-potential (mV) | Time | Wax concentration (g mL−1) |
|---|---|---|---|---|---|
| Time: time after suspension preparation, PDI: polydispersity index | |||||
| Coating 4 | 169 ± 3 | 0.082 ± 0.019 | 57.3 ± 0.4 | — | — |
| Carnauba | 168 ± 1 | 0.103 ± 0.018 | −57.0 ± 1.5 | 1 day | 0.01 |
| Carnauba | 180 ± 1 | 0.129 ± 0.012 | −65.0 ± 1.6 | 1 month | 0.01 |
| Carnauba | 201 ± 1 | 0.173 ± 0.017 | −68.7 ± 1.1 | 1 day | 0.015 |
| Ginkgo | 184 ± 0 | 0.134 ± 0.016 | −52.3 ± 0.5 | 1 day | 0.01 |
| Ginkgo | 188 ± 1 | 0.119 ± 0.007 | −57.2 ± 1.2 | 1 month | 0.01 |
| Ginkgo | 264 ± 1 | 0.283 ± 0.011 | −57.0 ± 1.4 | 1 day | 0.015 |
| Coating 1 | 214 ± 1 | 0.266 ± 0.014 | −52.0 ± 1.9 | — | — |
| Coating 2 | 184 ± 2 | 0.214 ± 0.014 | 61.6 ± 1.0 | — | — |
| Coating 3 | 666 ± 10 | 0.352 ± 0.035 | 66.9 ± 0.8 | — | — |
The stability of the plant wax suspensions (ginkgo and carnauba) over storage time was also verified using DLS (Table 1). Although a slight increase in particle size was observed, all suspensions had good or excellent stability and showed reasonable particle size homogeneity with polydispersity indexes (PDI) close to 0.1. After a month, the Z-average increased from 168.3 ± 1.4 nm (PDI = 0.103) to 180.0 ± 0.7 nm (PDI = 0.129) for carnauba wax, and from 184.3 ± 0.2 nm (PDI = 0.134) to 188.4 ± 0.5 nm (PDI = 0.119) for ginkgo wax. Within this time, the intensity distributions decreased and broadened slightly, and the ginkgo and carnauba distributions became indistinguishable from one another, suggesting a stable state. Thus, the present work concludes that plant wax suspensions without emulsifiers can be stable, contrasting the findings of prior literature.7,40
Also observed was that particle size can be controlled by wax concentration (Table 1). With an increase in concentration from 0.10 to 0.15 g mL−1, the average partice size increased by 32.7 nm for carnauba wax and 79.4 nm for ginkgo wax. A similar finding was reported by Lozhechnikova et al.6
DLS measurements were also performed on commercial samples to compare against with plant wax suspensions (Fig. 7 and Table 1). All commercial products had a similar particle size to the plant wax suspensions except Coating 3, which had a bimodal distribution with particle size peaks at approximately 150 nm and 920 nm. As the Z-average increased, so did the PDI. All commercial products had a positive charge, except Coating 1, and a ζ-potential with either good or excellent stability. Overall, all plant wax suspensions and commercial coatings, except Coating 3, were relatively similar and showed good or excellent stability with time.
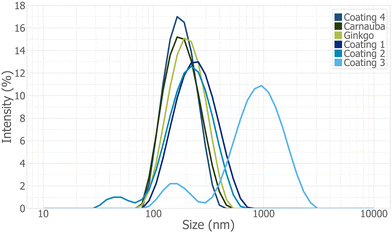 | ||
| Fig. 7 Dynamic light scattering (DLS) of 0.01 g mL−1 plant wax suspensions (carnauba and ginkgo) and commercial products (Coating 1–4), ordered from highest to lowest peak intensity. Plant wax suspensions were filtered beforehand using a 0.45 μm diameter filter. Particle size using DLS, similar to.6 | ||
3.4 The effect of thermal treatment on textile hydrophobicity
The effect of thermal treatment on coated fiber surface morphology and wettability was tested using SEM and WCA measurements. Because users would be heat treating their clothing in a home drying machine, our DSC data had to be compared with typical settings on these machines. The outlet thermostat set point on low heat is 51.7 °C (125 F) and on both medium and high heat is 57.2 °C (135 F). Additionally, the drum itself will reach a temperature of approximately 62.8 °C (145 F) during operation.41 This was in line with the temperature of textiles measured inside the drum with an IR gun (Milwaukee 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Infrared Temp-Gun™) after running the GE dryer on medium heat in 5 min intervals for 30 min. Overall, the dryer set to medium heat thus reached temperatures near the ideal temperature of annealing wax coatings determined by DSC (see above), and in-line with Forsman et al. using a temperature of 70 °C.42 Accordingly, our dryer was used at medium heat to prepare coatings for testing their performance on fabrics.
1 Infrared Temp-Gun™) after running the GE dryer on medium heat in 5 min intervals for 30 min. Overall, the dryer set to medium heat thus reached temperatures near the ideal temperature of annealing wax coatings determined by DSC (see above), and in-line with Forsman et al. using a temperature of 70 °C.42 Accordingly, our dryer was used at medium heat to prepare coatings for testing their performance on fabrics.
To monitor morphological changes of wax coatings during heat treatment, they were investigated using SEM (Fig. 8a–p, Table 2, and Fig. S7, ESI†). Ginkgo wax coatings appeared as small spheres (diameter 852 ± 0.402 nm) when air dried, began to change morphology in the Tenney, and changed to tubules upon heating in the GE dryer. Coatings of carnauba wax remained as small spheres (diameter 820 ± 0.291 nm) in all cases, but the number of spheres appeared to decrease and their diameter slightly increased when heated with the GE dryer. Therefore, some wax may have been partially melting and spreading on the surface, improving coverage and allowing molecular mobility. Both plant wax coatings appeared relatively rough in comparison to commercial coatings.
| Wax source | Drying method | Diameter/length (μm) |
|---|---|---|
| Ginkgo | Air dry | 0.852 ± 0.402 |
| Ginkgo | Tenney | Unmeasurable |
| Ginkgo | GE | 1.283 ± 0.336 by 0.157 ± 0.042 |
| Carnauba | Air dry | 0.820 ± 0.291 |
| Carnauba | Tenney | 0.924 ± 0.451 |
| Carnauba | GE | 1.019 ± 0.301 |
The performance of hydrophobic coatings from commercial and plant wax sources on textiles was assessed using WCA measurements, with coatings, substrates, and drying methods compared. On the polyester substrate, ginkgo and carnauba samples heat-treated using the GE dryer had the highest WCAs and were superhydrophobic (Fig. 8). Additionally, three of the four commercial coatings were superhydrophobic. The fourth commercial coating fell between hydrophobic and superhydrophobic. Carnauba and ginkgo samples heat-treated using the Tenney had hydrophobic WCAs. Samples that wetted within 5 min included polyester substrates that were either uncoated or coated with (air-dried) ginkgo or carnauba waxes. These samples are not shown on the WCA graph and are instead shown as a series of video frames to illustrate the behavior of droplets over time (Fig. 8x). For comparison, ginkgo heat-treated with a GE dryer is also shown in the video frames, which remained superhydrophobic for the full 5 min.
On the cotton substrate, ginkgo wax coatings heat-treated both using the Tenney and GE dryer had the highest WCA and were superhydrophobic (Fig. 9). For the commercial coatings applied to cotton, the order of hydrophobicity was the reverse of that on the polyester substrate. Coatings 1–3 were superhydrophobic, with Coating 1 (GE) having the highest WCA and Coating 3 the lowest. Coating 4 wetted within the 5 min, along with uncoated cotton, ginkgo wax (air dried), and carnauba wax (air dried). Both carnauba wax samples fell between hydrophobic and superhydrophobic.
WCAs can be further explained by local surface polarities as well as micro-reliefs. In making the dispersions, waxes become mobilized by heat and maximize their surface polarities upon cooling in the aqueous environment. It is therefore surmised that, upon melting of the wax, polar OH-groups become exposed as the tubules lose their structure, resulting in decreased hydrophobicity. The new structures are likely meta-stable at room temperature also after spraying/drying, and therefore may persist in “air-dried” controls. Finally, annealing again mobilizes the waxes, but this time they are exposed to air instead of water, leading to different structures now burying polar OH-groups and exposing non-polar hydrocarbons.6,43 A similar, but chemically different, explanation has been given for carnauba wax by Lozhechnikova et al. and Forsman et al.6,42
In terms of micro-relief, various treatments, such as recrystallization from the melt or solution, tend to yield more or less smooth surfaces, due to their lower surface-to-volume ratios and, therefore, lower surface energies. Cheng et al.,44 showed that melting of tubules does not affect the chemical composition or quantity of wax, suggesting that our wax treatments before annealing only resulted in loss of the nano-scale structure, formation of a smooth surface, and decrease in contact angle. After annealing, nonacosan-10-ol and nonacosanediols are thought to co-crystallize in tubular form.43
3.5 The effect of surface roughness on hydrophobicity of coated samples
In order for a surface to be superhydrophobic, low surface energy and high surface roughness must be present.45 This allows for water to be suspended on both the substrate and pockets of air, reducing contact at the solid–liquid interface, and is referred to as the Cassie–Baxter state.10 Among the substrates used here, surface roughness qualitatively increased from filter paper over polyester to cotton (Fig. 10). Ginkgo wax coatings, either recrystallized through solvent evaporation or annealing using the GE dryer, had WCAs increasing with substrate surface roughness (Fig. 6, 8, and 9).3.6 Scaling to t-shirt application
After confirming the ginkgo spray performed well compared to commercial coatings on small-scale fabric samples, we wanted to apply it to real-life applications by testing it on t-shirts. Polyester and cotton outdoor shirts were coated using the ginkgo wax spray, and water was applied to the surface to test the hydrophobicity (Fig. 11). Initially, the shirts were dried on medium heat for 30 min in a GE dryer, as had previously been done with fabric squares. However, shirts were not as hydrophobic as the textile samples discussed in Section 3.4. This was evident even before water fully absorbed into the textile because, as water drops began being absorbed by the textile, the base where the water drop made contact with the textile reflected the textile color, rather than appearing white (similar to the metallic appearance in Fig. 2). This change in color occurs when the air layer between the wax and water is no longer present. This appeared to be related to the increase in water content of the shirt vs. textile samples, resulting in a slower drying time. To test this, we dried the shirts for an additional 30 min (increasing the overall dry time to 60 min), which increased their hydrophobicity to the point that it was difficult to get photos of the water beaded on the person's shoulder rather than have it roll off. Thus, the Lotus effect was exhibited, rather than the Petal effect.Qualitatively, the tactile feel and drape of the shirts were not affected by the water-repellent plant-based sprays. However, it was noticed that, if too much spray was added to a small section of the shirt, slight discoloration to a yellowish tint was present on the white shirt. Discoloration was not noticeable on the blue shirt. This was not a surprise given that the wax suspension had a yellowish tint. In future experiments we will investigate bleaching techniques and determine the optimal number of sprays to balance hydrophobicity and reduce discoloration.
The impact of wash-cycles on coating durability was not tested. However, this was investigated in previous work by Forsman et al., in which their layer-by-layer carnauba wax coated textiles did not hold up to standard washing with detergents.42 Instead, the coating would have to be reapplied after washing or used on clothing that is less frequently washed, such as outdoor clothing.42 Therefore, it would be of interest to test the wash cycle durability of the ginkgo wax coated textiles in future work.
4 Conclusions
Aqueous plant wax suspensions were prepared by homogenization, without the addition of stabilizers or surfactants, to create PFC-free, petroleum-free hydrophobic coatings. This was achieved using wax from plant waste, with a focus on natural annual leaf-litter. These suspensions were then sprayed onto t-shirts and heat treated in home drying machines, using a similar procedure to that of commercial coatings that can be applied by the user at home. On both cotton and polyester textiles, the ginkgo wax produced a coating that was more hydrophobic than commercial products. In conclusion, a water-repellent spray was developed that could be applied by users at home, which was both more environmentally friendly than commercially available products and had greater water repellency.Author contributions
We strongly encourage authors to include author contributions and recommend using https://casrai.org/credit/CRediT for standardised contribution descriptions. Please refer to our general https://www.rsc.org/journals-books-databases/journal-authors-reviewers/author-responsibilities/author guidelines for more information about authorship.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support provided by the NSERC Canfor Industrial Research Chair in Advanced Bioproducts, (#553449–19), NSERC Discovery Grant (RGPIN-2021-03172), the Canada Foundation for Innovation (Project number 022176), the Pacific Economic Development Canada (PacifiCan), and Natural Sciences and Engineering Research Council (Canada) Discovery Grants Program (grant #262461). The authors would also like to thank the UBC Bioimaging facility (RRID: SCR021304) for their technical assistance and use of their facilities.References
- L. Ahrens, J. Environ. Monit., 2011, 13, 20–31 RSC.
- M. P. Krafft and J. G. Riess, Chemosphere, 2015, 129, 4–19 CrossRef CAS PubMed.
- P. J. Hill, M. Taylor, P. Goswami and R. S. Blackburn, Chemosphere, 2017, 181, 500–507 CrossRef CAS PubMed.
- Arc’teryx, ARC’TERYX AND PFCS, https://blog.arcteryx.com/arcteryx-and-pfcs/.
- Patagonia, Fluorinated DWR, https://www.patagonia.ca/our-footprint/dwr-durable-water-repellent.html.
- A. Lozhechnikova, H. Bellanger, B. Michen, I. Burgert and M. Österberg, Appl. Surf. Sci., 2017, 396, 1273–1281 CrossRef CAS.
- I. S. Bayer, D. Fragouli, P. J. Martorana, L. Martiradonna, R. Cingolani and A. Athanassiou, Soft Matter, 2011, 7, 7939–7943 RSC.
- Y. J. Liu, J. N. Shao and P. L. Liu, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 323, 012013 Search PubMed.
- N. Forsman, A. Lozhechnikova, A. Khakalo, L.-S. Johansson, J. Vartiainen and M. Österberg, Carbohydr. Polym., 2017, 173, 392–402 CrossRef CAS PubMed.
- W. Wang, K. Lockwood, L. M. Boyd, M. D. Davidson, S. Movafaghi, H. Vahabi, S. R. Khetani and A. K. Kota, ACS Appl. Mater. Interfaces, 2016, 8, 18664–18668 CrossRef CAS PubMed.
- P. Arlein, Snow equipment wax formulation, U.S. Pat., 11059975B1, 2020, https://patents.google.com/patent/US11059975B1/en?assignee=mountainflow Search PubMed.
- P. Arlein, Snow equipment wax formulation, U.S. Pat., 11021633B1, 2020, https://patents.google.com/patent/US11021633B1/en?assignee=mountainflow Search PubMed.
- E. Krendlinger, U. Wolfmeier, H. Schmidt, F.-L. Heinrichs, G. Michalczyk, W. Payer, W. Dietsche, K. Boehlke, G. Hohner and J. Wildgruber, Waxes, 2015, 1–63 Search PubMed.
- A. H. Warth, The chemistry and technology of waxes, 1947, https://go.exlibris.link/jhNPm06k Search PubMed.
- R. Jetter, L. Kunst and A. L. Samuels, Composition of Plant Cuticular Waxes, Wiley Online Books, 2006 DOI:10.1002/9780470988718.ch4.
- C. Neinhuis and W. Barthlott, Ann. Bot., 1997, 79, 667–677 CrossRef.
- Y. Gaillard, A. Mija, A. Burr, E. Darque-Ceretti, E. Felder and N. Sbirrazzuoli, Thermochim. Acta, 2011, 521, 90–97 CrossRef CAS.
- W. Zhang, P. Lu, L. Qian and H. Xiao, Chem. Eng. J., 2014, 250, 431–436 CrossRef CAS.
- M. Wen, C. Buschhaus and R. Jetter, Phytochemistry, 2006, 67, 1808–1817 CrossRef CAS PubMed.
- M. Abramoff, P. Magalhães and S. J. Ram, Biophotonics Int., 2003, 11, 36–42 Search PubMed.
- C. Buschhaus and R. Jetter, Plant Physiol., 2012, 160, 1120–1129 CrossRef CAS PubMed.
- R. Jetter and M. Riederer, Bot. Acta, 1995, 108, 111–120 CrossRef CAS.
- H. Wang, H. Shi, Y. Li, Y. Yu and J. Zhang, Front. Environ. Sci. Eng., 2013, 7, 579–588 CrossRef.
- L. Schreiber and J. Schönherr, Pestic. Sci., 1993, 38, 353–361 CrossRef CAS.
- G. M. Hunt and E. A. Baker, Chem. Phys. Lipids, 1979, 23, 213–221 CrossRef CAS.
- P.-G. Gülz, E. Müller, K. Schmitz, F.-J. Marner and S. Güth, Z. Naturforsch., C: J. Biosci., 1992, 47, 516–526 CrossRef.
- H. Kang, P. M. Graybill, S. Fleetwood, J. B. Boreyko and S. Jung, PLoS One, 2018, 13, e0202900–e0202900 CrossRef PubMed.
- K. Koch and W. Barthlott, Philos. Trans.: Math., Phys. Eng. Sci., 2009, 367, 1487–1509 CAS.
- T. W. Brakke, W. P. Wergin, E. F. Erbe and J. M. Harnden, Remote Sens. Environ., 1993, 43, 115–130 CrossRef.
- P. J. Holloway, J. Sci. Food Agric., 1969, 20, 124–128 CrossRef CAS.
- E. Bormashenko, R. Pogreb, T. Stein, G. Whyman, M. Erlich, A. Musin, V. Machavariani and D. Aurbach, Phys. Chem. Chem. Phys., 2008, 10, 4056–4061 RSC.
- C. Neinhuis and W. Barthlott, New Phytol., 1998, 138, 91–98 CrossRef.
- R. Jetter and M. Riederer, Planta, 1994, 195, 257–270 CrossRef CAS.
- A. Dommisse, J. Wirtz, K. Koch, W. Barthlott and T. Kolter, Eur. J. Org. Chem., 2007, 3508–3511 CrossRef CAS.
- H. L. Casal and P. Moyna, Phytochemistry, 1979, 18, 1738–1739 CrossRef.
- P. J. Holloway, C. E. Jeffree and E. A. Baker, Phytochemistry, 1976, 15, 1768–1770 CrossRef CAS.
- B. Singh, P. Kaur Gopichand, R. D. Singh and P. S. Ahuja, Fitoterapia, 2008, 79, 401–418 CrossRef CAS PubMed.
- R. J. Hunter, Zeta potential in colloid science: principles and applications, 1981.
- P. Wagner, R. Fürstner, W. Barthlott and C. Neinhuis, J. Exp. Bot., 2003, 54, 1295–1303 CrossRef CAS PubMed.
- Y. Wang, B. He and L. Zhao, BioResources, 2017, 12, 7774–7783 CAS.
- Haier, Dryer - Explanation of Dryer Temperatures, 2022, https://products.geappliances.com/appliance/gea-support-search-content?contentId=20985.
- N. Forsman, L.-S. Johansson, H. Koivula, M. Tuure, P. Kääriäinen and M. Österberg, Carbohydr. Polym., 2020, 227, 115363 CrossRef CAS PubMed.
- H. J. Ensikat, P. Ditsche-Kuru, C. Neinhuis and W. Barthlott, Beilstein J. Nanotechnol., 2011, 2, 152–161 CrossRef CAS PubMed.
- Y. T. Cheng, D. E. Rodak, C. A. Wong and C. A. Hayden, Nanotechnology, 2006, 17, 1359–1362 CrossRef.
- S. Gorb, Functional Surfaces in Biology: little structures with big Effects, Springer, 2009, vol. 1 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sm00720k |
| This journal is © The Royal Society of Chemistry 2023 |