 Open Access Article
Open Access ArticleWetting effect of branched anionic Gemini surfactant aqueous solution on PMMA surface
Dengxi
Zhang
a,
Zhicheng
Xu
b,
Zhiqiang
Jin
b,
Lei
Zhang
b,
Lu
Zhang
b,
Fenrong
Liu
*a and
Wangjing
Ma
 *b
*b
aSchool of Material Science and Engineering, Inner Mongolia University, Hohhot 010020, P. R. China. E-mail: fenrongl@163.com
bTechnical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: wjma@mail.ipc.ac.cn
First published on 19th May 2023
Abstract
In this paper, the adsorption behaviour and wetting modification ability of the sodium salts of bis-octadecenoyl succinate (GeminiC3, GeminiC6) and monomers on polymethyl methacrylate (PMMA) surfaces were investigated. The difference in spacer length led to slightly different behaviour of surfactant molecules in solution. The large molecular structure and short flexible spacer of GeminiC3 led to a complex self-aggregation behaviour in solution, forming micelles at low concentrations, leading to a rapid decrease in surface tension and subsequent transition to monolayer or multilayer vesicles. In GeminiC6, the longer flexible spacer groups act as spatial structure modifiers that hinder the formation of vesicles. The adsorption behaviour of the gas–liquid interface was analysed in three stages for the peculiar inflection points where surface tension appears. Combining contact angle measurements, adhesion tension and interfacial tension data showed that GeminiC3 and C6 formed a saturated monolayer on the adsorbed PMMA surface at low concentrations and a bilayer structure at high concentrations. Due to the low resistance of molecular space sites, the monomers adsorbed heavily on the PMMA surface, forming semi-colloidal aggregates with the lowest contact angle of monomeric surfactant solutions reaching 38° on the PMMA surface. Also, the monomer and GeminiC3 and C6 surfactants in this paper have a very high hydrophilic modification ability on the PMMA surface compared to other literature.
1. Introduction
The wetting phenomenon on solid surfaces has received considerable attention in a variety of fields such as adhesion, oil extraction, flotation, membrane distillation, washing and lubrication.1–5 Surfactants play a crucial role in regulating the wetting properties of solid surfaces. Researchers have been continuously innovating the structure of surfactants to achieve better wetting performance. Over time, surfactants have evolved from single-chain to various special structures including alkyl-branched double-zwitterionic head Gemini surfactants,6,7 double-chained surfactants with dual hydrophobic chains,8 inserted functionalized monomeric units,4 amphoteric ions,9 and alkyl-branched structures.4 Among these, Gemini and double-chained surfactants have recently garnered much focus. In particular, Gemini surfactants and double-chained surfactants have demonstrated significant progress in the wetting behaviour on the surface of polymethyl methacrylate (PMMA) and are thus worth studying.Polymethyl methacrylate (PMMA) has attracted much attention for its exceptional aging resistance, flexibility, biocompatibility and heat resistance, and has become one of the widely used acrylate plastics.10,11 PMMA is a weakly polar polymer compound with a surface containing various functional groups such as –CH3, –CO, and –OCH3.12 These functional groups lead to a variety of adsorption methods of surfactants on the PMMA surface. Therefore, the wetting behaviour of PMMA surfaces is well worth studying.
In recent studies, it has been established that the use of Gemini surfactants can enhance the surface tension and wettability of polymers, which have the ability to significantly increase the surface wetting of polymers within a confined temperature range, thus improves electrostatic properties on the surface.12 Furthermore, Gemini surfactants exhibit superior chemical stability and antioxidant properties, which render them effective in safeguarding metal surfaces against chemical corrosion and aging.13 When compared to conventional surfactants, Gemini surfactants exhibit higher surface activity and lower CMC. However, there has been limited research on the adsorption behaviour of cationic Gemini surfactants on polymer surfaces,7 although the adsorption behaviour of amphoteric surfactants14 and cationic Gemini surfactants15 on PMMA surfaces has been already reported. Zhang16,17 found that certain surfactants possess unique wetting properties due to the incorporation of polyethylene oxide (EO) units or branched hydrophobic chains into their structures. These surfactants are capable of interacting with the PMMA surface via polar groups at low concentrations, resulting in a slightly hydrophobic PMMA surface. Nonetheless, once the CMC is attained, the adsorption behaviour of these surfactants shifts to hydrophobic interaction, ultimately leading to a decrease in γSL with increasing surfactant concentration.
Lv7et al. investigated the unique adsorption behaviour and wettability changes of alkyl-branched Gemini surfactants (quaternary ammonium salts) were investigated on the surface of PMMA and PTFE (Polytetrafluoroethylene) by steric hindrance effect, as well as the changes in polymer wetting properties caused by the length of the bridge-linking group between C3 and C6. This study found that the difference in the interval length had limited impact on the wetting properties, and the surfactants adhered to the PMMA surface through polar interactions, forming a double-layer saturated adsorption film above the CMC. At the same time, due to the significant steric hindrance, the adsorption amount on the PTFE surface was only one-third of that at the gas–liquid interface. Garcia18 studied the effects of hydrophobic alkyl chain length, bridge-linking group properties and structure, and ionic head polarity on the aggregation behaviour of a series of C12 and C18 alkyl chain quaternary ammonium-based Gemini surfactants with different bridge-linking groups and C12 alkyl chain quaternary ammonium trimeric surfactants in aqueous solution. The results showed that Gemini surfactants were easier to reach the critical micelle concentration (CMC) compared to single-chain surfactants, and rigid and flexible bridge-linking groups were key factors influencing the self-assembly behaviour of Gemini surfactants in water: (1) the trimers of rigid bridge groups are more likely to agglomerate than dimers. (2) The flexible spacer group has a lower CMC than the surfactant of the corresponding rigid spacer group. (3) With the increase of hydrophilicity of flexible spacer groups, the CMC of gemini surfactant also increases, and the elongation to CMC is no longer obvious when the spacer group is rigid.
In addition, double-stranded surfactants are also widely used in the study of the wettability of polymer surfaces. The experimental results have shown that double-chain surfactants can effectively form a dense wetting surface on the surface of polymer, improving surface wettability without affecting surface glossiness.19,20 In addition, double-chain surfactants also have good antibacterial activity and can effectively prevent Gram-positive bacteria.21Nihar Ranjan Biswal19 found that compared to traditional single-chain cationic surfactants (CTAB), double-chain cationic surfactants (DDAB) and double-chain anionic surfactants (AOT) have lower minimum surface tensions and far lower contact angle. The contact angle reduction of the two double-chain surfactants on the surface of PTFE was similar, and their adsorption on the PTFE–water interface was 0.759 times lower than that at the air–water interface.
Based on the above studies, we found that Gemini surfactant and double-chain surfactant have very promising research prospects, so in this paper, we synthesized two configurations of sodium bis-octadecenyl succinate surfactant and tried to explore the adsorption behavior of Gemini and double-chain co-existing surfactants on polymer surfaces and their applications by studying their adsorption mechanisms on polymer surfaces, the variation pattern of adsorption equilibrium, etc. The study is intended to reveal the mechanism of their action on polymer surfaces and to explore the prospects of their application in polymer material modification. This study is of theoretical and practical significance for understanding the adsorption behavior of surfactants on polymer surfaces, expanding the surface modification technology and improving the performance of polymer materials.
In this paper, the wetting properties of Gemini Cn anionic surfactants on the surface of poly (methyl methacrylate) (PMMA) was systematically studied. Three different carbons bridged Gemini Cn anionic surfactants, i.e., the sodium salts of dimeric 18-carbon chains linked by a 3-carbon bridge (GeminiC3) and 6-carbon bridge (GeminiC6), as well as the sodium octadecyl succinimide as a monomer, were synthesized. The gas–liquid–solid contact angles were measured using the drop method, and the surface tension was measured using the plate method. The obtained data were analysed to investigate the wetting behaviour of Gemini Cn anionic surfactants on PMMA surfaces.
2. Experimental
2.1. Materials
A proprietary catalyst from previous studies22 was used in the preparation of octadecenyl succinic anhydride, which contained two alkyl chains of differing lengths. Subsequently, the synthesized compounds were transformed into Gemini surfactants. Surfactants were synthesized according to Dix,23 and their chemical structures are presented in Scheme 1. The purity of the compounds was determined to be in excess of 95%, ascertained through Mass spectrum and nuclear magnetic resonance hydrogen spectroscopy (H-NMR). The experiments were using ultrapure water, characterized by a resistivity of 18.2 MΩ cm. | ||
| Scheme 1 Molecular structure and formula of GeminiC3, C6 and monomer surfactants CX: denotes the Xth carbon position (2 ≤ X ≤ 17). | ||
2.2. Surface tension measurement
The surface tension of the Gemini Cn was measured by Wilhelmy plate technology, and the platinum plate was thoroughly cleaned and flame-dried at 298 ± 0.5 K with a tensiometer (Data Physics AG, DCAT21). In all cases, more than three measurements were made with a standard deviation of no more than 0.2 mN m−1.2.3. Contact angle measurement
The contact angle of the aqueous surfactant solution on PTFE or PMMA plates was determined by OCA20 fixed titration method of Dataphysics Instruments GmbH in Germany at a constant temperature (298 ± 0.5 K). The measurement on both sides of the droplet is performed immediately after the droplet settles to the solid surface. The measurement was repeated several times at different positions on the solid surface with a standard deviation of less than 3°.24 The details of contact angle measurements are described elsewhere.Polymethyl methacrylate (PMMA) plates are cut from large thin plates for contact angle measurement. The plates are ultrasonically cleaned with ethanol, then rinsed with ultrapure water, and then washed in an ultrasonic bath for 20 min. These surfaces are subsequentially heated at 378 K for 2 hours.
3. Results and discussion
3.1. Surface tension of surfactants solution
According to Fig. 1, the surface tension of GeminiC6 exhibits a significant decrease at low concentrations and reaches a critical micelle concentration (CMC) plateau at 28.1 mN m−1, which is in agreement with previous studies, confirming the reliability and reproducibility of the experimental data in this work. Notably, both the GeminiC3 and monomer displayed two critical micelle concentration values, CMC1 and CMC2. CMC1 was observed at low concentrations and accompanied by a substantial drop in surface tension. Phan25et al. attributed this behaviour of monomer to the double-chain structure of the surfactant, which leads to the formation of surface pre-micelles that interact with surfactants already present in the solution as they spread over the gas–liquid interface. The formation of surface pre-micelles leads to the sparse arrangement of surfactant molecules at the gas–liquid interface, the adsorption rate decreases, and therefore the rate of surface tension decrease slows down after reaching CMC1. The concentration of surface micelle formation (CMC1) is much lower than that of the micelles inside the solution. The monolayer adsorbed at the gas–liquid interface acts as a “seed” to reduce the entropy required for aggregation. The relative ease of micelle formation is similar to heterogeneous/homogeneous nucleation. Due to this unique adsorption behavior, CMC1 was also used as the critical micelle concentration for this study system, and the critical micelle concentration of the monomeric surfactant solution itself (CMC2), dividing the study process into three parts for analysis. Unlike the monomer, the mechanism behind the plateau formation of GeminiC3 is more intricate. Based on literatures,18,26 due to large molecular structure and steric hindrance, the two polar carboxylic acid groups produced by carboxylate hydrolysis led to complex self-aggregating behaviour of GeminiC3, and the transition of micelles to vesicle aggregates at high concentrations leads to the formation of two plateaus of surface tensions.Although the bridge groups of GeminiC3 and GeminiC6 differ by only three –CH2–, the surface tensions curves indicates that C3 exhibits two CMC while C6 has only one. The CMC of Gemini surfactants initially increases with the increasing length of the spacer group between the head groups, but it decreases when the chain length exceeds five carbon atoms, Geminis behaves as a double chain surfactant when the interval is short.26 Therefore, the GeminiCn in this paper under- goes different aggregation behaviour in solution, leading to the appearance of different surface tensions. Unlike monomeric surfactants, the vacancy barrier of the bridging group hinders the formation of surface micelles.
The slope of the surface tension of the monomer is calculated by the average of the slopes of the two segments, and the maximum interfacial excess concentration is calculated by combining with the Gibbs adsorption eqn (1):
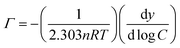 | (1) |
Where γ refers to the surface tension (mN m−1), C refers to molar concentration (mol/1), R refers to gas constant (8.314 J mol−1 k−1), and T refers to absolute temperature. Two ions are ionized in the monomer and three ions can be ionized in GeminiCn, where Gemini surfactant n is taken as 3 and the monomer as 2. The minimum area Amin for each molecule at the air–water interface can be calculated using the following formula:
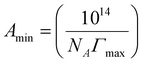 | (2) |
The values for CMC, γCMC, γmax and Amin are listed in Table 1.
| Surfactant | CMC1/(10−6mol l−1) | CMC2/(10−4mol l−1) | γ CMC1(mN m−1) | γ CMC2 (mN m−1) | 10−10Γ1 (mol cm−2) | A min1 (nm2) |
|---|---|---|---|---|---|---|
| GeminiC6 | 1.8 | 28.1 | 1.67 | 1.00 | ||
| GeminiC3 | 5.2 | 5.0 | 40.3 | 28.6 | 1.25 | 1.33 |
| monomer | 1.0 | 4.0 | 43.7 | 31.6 | 6.44 | 0.26 |
The adsorption amounts and adsorption areas in Table 1 are calculated from the slope of the surface tension in the first segment. According to Table 1, the adsorption at the gas–liquid interface during the reduction of surface tension of GeminiC6 is greater than that of GeminiC3, which is attributed to the rapid formation of micelles inside the GeminiC3 solution and then conversion to vesicles, which saturates the adsorption at the gas–liquid interface in advance, thus compensating for the effect of the spatial site resistance of GeminiC6 molecules.
For GeminiC3, C6 and monomer, their lowest surface tension plateau values are 28.1, 28.6 and 31.6mN m−1, respectively. C3 and C6 structures contain four hydrophobic chains, all of which are strongly hydrophobic, and the hydrophilic groups in the molecule are interconnected by strong chemical bonds, and this connection weakens the electrostatic repulsive forces between the hydrophilic groups and their repulsion between the hydrated layers, resulting in a better the adsorption capacity. In the first stage the adsorption of the monomer is greater than that of GeniniCn, but in the range of CMC1–CMC2, the adsorption of the monomer is only 0.43 mol cm−2, which combined with the lowest surface tension plateau value can be concluded that the adsorption of the monomer throughout the gas–liquid interface is lower than that of GeniniCn.
3.2. Contact angles on the PMMA surface
Fig. 2 shows the change in contact angle of the three surfactants solutions. The contact angle of the three surfactants in the concentration range of 5E-7 mol l−1 to 1E-4 mol l−1 is about 76° without significant fluctuation, and the contact angle begins to decrease after the concentration is greater than 1E-4 mol l−1. The contact angles of GeminiC3 and GeminiC6 on the PMMA surface decreased to 58.63° and 60.62°, respectively, which proved that the change of bridge group length did not have much effect on the contact angle change of surfactant on the PMMA surface. On the other hand, it is noteworthy that the contact angle of the monomer significantly decreased to 38.06°. This result was expected, as the smaller molecular size of the monomer facilitated a more tightly packed semi-micellar space on the surface of PMMA, resulting in a greater extent of hydrophilic modification of the PMMA surface.3.3. Adhesion tension on the PMMA surface
Adhesion tension is an important parameter for evaluating the wettability of solid surfaces, obtained by Young's equation and equilibrium contact angle and three interfacial free energies:γLVcos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = γSV − γSL θ = γSV − γSL | (3) |
 | (4) |
Γ
SV, ΓSL and ΓLV indicate the maximum surface adsorption capacity of surfactants at the solid–gas, solid–liquid and gas–liquid interfaces, respectively. Assuming ΓSV = 0, the value of ΓSL/ΓLV can be obtained by the slope of the γLV cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ and γLV curves under CMC.
θ and γLV curves under CMC.
The hydrophilic groups are adsorbed on the solid surface during the hydrophobic interaction of surfactant molecules. In this case, the ratio of adsorption capacity at the solid–liquid interface to that at the gas–liquid interface is negative:
 | (5) |
The alkyl chain is adsorbed on the solid surface through hydrophobic interactions. In this case, the ratio of adsorption capacity at the solid–liquid interface to that at the gas–liquid interface is positive:
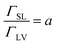 | (6) |
Generally, the adsorption of the hydrophilic head group and PMMA surface of ionic surfactants is weaker than that of the hydrophobic end and PMMA. Therefore, the hydrophobic tail of the surfactant is polarly adsorbed on the surface of PMMA, and the ion head is oriented towards the aqueous phase, resulting in a decrease in energy at the solid–liquid interface with a negative slope, such as anionic surfactants sodium decylsulfate(SDS)27 and bis(2-ethylhexyl) sodium sulfosuccinate (AOT),20 cationic surfactants cetyltrimeth-ylammonium bromide(CBAT), cetylpyridinium bromide (CPyB),28 dodecylethyldimethylammonium bromide C12(EDMAB), and benzyldimethyldodecylammonium bromide (BDDAB).29
Surfactants with a positive slope usually have more than one hydrophilic group. C16PC and C16GPC surfactants have 3 groups of –OH and –N(CH3). C16Pb and C16GPb molecules have –OH, –N(CH3)3+ and –COO– groups. C16(EO)3PC and C16G(EO)3PC molecules have –OH, –N(CH3)3+ and EO groups. C16(EO)3Pb and C16G(EO)3Pb surfactants have –OH, –N(CH3)3+, EO and –COO– groups.30,31
The GeminiCn and monomeric surfactants synthesized in this study contains a –COO– and –CONH– group as the ion head, which can form hydrogen bonds with PMMA and tightly adsorbed on the surface, orienting the hydrophobic end towards the aqueous phase. As shown in Fig. 3, before CMC1, the adhesion tension of GeminiC3 decreased gradually with the decrease of surface tension in a linear relationship. The second segment of surface tension reaches a CMC2 and remains constant, while adhesion tension increases vertically. This is attributed to the fact that with increasing concentration, the surfactant forms a saturated adsorption film at the air–liquid interface, while continuous adsorption occurs at the solid–liquid interface, leading to the vertical increase of adhesion tension data. The surface tension value forms a plateau that leads to a break in the middle of the first and second segments. In the middle of CMC1–CMC2, the monomer still maintains the linear relationship of the first segment because the reduction of surface tension and the reduction of interfacial tension are consistent. In particular, the slopes of GeminiC3, monomer and GeminiC6 surfactants before CMC1 were all consistent at 0.22, indicating that the surfactant adsorption capacity at the gas–liquid interface was 4.5 times higher than that at the PMMA–liquid interface.
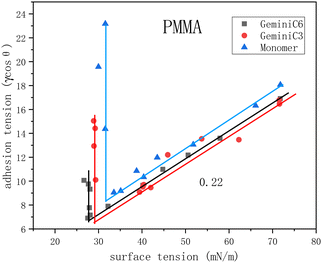 | ||
| Fig. 3 Adhesion tension of C3, C6 and monomer surfactants on the surface of PMMA in relation to surface tension. | ||
3.4. Interfacial tension of the PMMA–solution interfaces
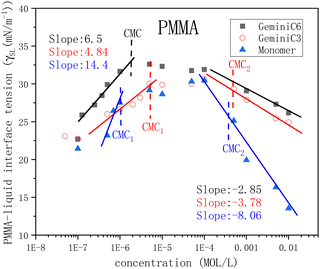
In order to further study the adsorption behaviour of the three surfactants on the surface of PMMA, the solid–liquid interfacial tension of the three surfactants was analysed. The surface free energy of PMMA solid is 39.5 mJ m2, and the interfacial tension γSL of PMMA and liquid can be obtained by eqn (3).The change curve of the interfacial tension γSL with concentration of the three surfactants is plotted in the Fig. 4. It was found that the adsorption behaviour of the three extended surfactants on the PMMA surface had three stages.
 | ||
| Fig. 4 The interfacial tension of C3, C6 and monomer surfactants on the surface of PMMA varies with concentration. | ||
In the low concentration stage, the hydrophobic tails of C3 and C6 are oriented towards the solution, and the hydrophilic part is adsorbed on the surface of PMMA. As a result, the interfacial tension increases and PMMA is modified to a slightly hydrophobic surface. In the concentration range of CMC1 to 1E-4 mol l−1, the interfacial tensions of C3 and C6 achieved the plateau, indicating that the surfactants formed a Colloids in the solution, and the monolayer membrane adsorption reached saturation, resulting in the emergence of the interfacial tension platform shown in Fig. 4. Above the concentration of 1E-4 mol l−1, due to the hydrophobic interaction surfactant molecules adsorbed on the monolayer membrane to form a double-layer film, the hydrophilic part is oriented towards the solution, PMMA becomes a hydrophilic surface, and the interfacial tension gradually decreases. It can be seen from Fig. 4 that the slopes of C3 and C6 at low concentrations are 4.85 and 6.5, respectively. At high concentrations, C3 and C6 are −3.78 and −2.85 which are smaller than the slope before CMC. The results indicate that the large molecular size of Gemini surfactants leads to a relatively loose and difficult-to-adsorb bilayer membrane structure, and limited hydrophobic modification ability on the surface of PMMA.
There are three main adsorption behaviours of surfactants after CMC: (1) monolayer adsorption (hydrophobic tail adsorption or polar adsorption); (2) double-layer adsorption (the second layer of surfactant adsorbed on the original monolayer surfactant); (3) formation of aggregates (semi-micelles formed by adsorption of surfactant molecules on solid surfaces by hydrophobic action).32 From Fig. 4, it can be seen that the slope of monomer in concentration range 5E-7–1E-6 mol l−1 is 14.4, the monomer surface activity and a large number of adsorptions on the surface, the interfacial tension rises rapidly. At the concentration range of 1E-6–1E-4 mol l−1, micelles start to form inside the solution, the molecular monolayer adsorption at the PMMA–liquid interface gradually reaches saturation, the adsorption rate becomes smaller, and the interfacial tension changes slowly. At concentrations greater than 1E-4 mol l−1, surfactant molecules continue to adsorb in PMMA in large numbers to form semi-micellar aggregates, so the slope of the rapid decrease curve of interfacial tension becomes larger.
The minimum value of γSL at high concentrations represents the maximum hydrophilic capacity. For C3, C6 and monomer, γSLmin is 26.1, 24.9 and 13.5, respectively. It shows that the monomer surfactant achieves the maximum modification of the hydrophilicity of the PMMA surface. As shown in Table 2, the theoretical value (AminT) calculated from the Gibbs equation with the slope of the adhesion tension matches exactly with the calculated value of the actual data (Amina), which proves the reliability of the experimental results.
| (<CMC1) | (<CMC1) | A minLV/(ΓSL/ΓLV) | (>1E-4 mol l−1) | (>1E-4 mol l−1) | |
|---|---|---|---|---|---|
| Surfactant | 10−10Γmax (mol cm−2) | A mina (nm2) | A minT (nm2) | 10−10Γmax (mol cm−2) | A minb (nm2) |
| A mina: Calculated value of the minimum adsorption area for the first layer of adsorption.AminT: Theoretical value of minimum adsorption area for first layer adsorption.Aminb: Calculated value of minimum adsorption area for second layer adsorption. | |||||
| GeminiC3 | 0.28 | 6.00 | 6.00 | 0.21 | 7.7 |
| GeminiC6 | 0.37 | 4.45 | 4.50 | 0.16 | 10.0 |
| Monomer | 1.24 | 1.34 | 1.18 | 0.69 | 2.4 |
The maximum value of γSL and the minimum value of γSL can be obtained from the interfacial tension curves, which represent the maximum hydrophobic and hydrophilic modification ability of surfactants on PMMA surfaces, respectively. Some surfactants with special structures were selected for comparison with this study. In order to avoid the effect of different initial values of γSL due to different PMMA sheets in the literature, the difference between the initial and maximum values of γSL (ΔγSL) was used to represent the maximum hydrophobic modification ability of each special structured surfactant molecule, while the maximum hydrophilic modification ability was represented by the minimum value of γSL at high concentration, and the data were listed in Table 3:
In Table 3, GeminiCn and monomer were compared with The stronger hydrophobic modification ability of GeminiCn and monomer compared to other specific structures of Geminis surfactants and betaine is due to the strong adsorption of GeminiCn ionic head to PMMA surface. The monomer exhibited superb hydrophilic modification ability at high concentration, reflecting the advantage of one long and one short double alkyl chain forming semi-micelles on the PMMA surface.
3.5. Work of adhesion on the PMMA surface
The work of adhesion (WA) of a liquid to a solid is defined by the reversible work required to separate a liquid per unit area from a solid surface, as shown in eqn (7).| WA = γSV + γLV − γSL | (7) |
Where WA can also be calculated by the Young formula.
WA = γLV(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ + 1) θ + 1) | (8) |
From eqn (7), the value of the adhesion work is determined by the values of γSL, γSV, and γSL. As shown in Fig. 5, increasingly concentration led to a WA decreasing, which is due to a decrease in both γLV and bonding tension. At high concentrations, the adsorption of surfactants at the gas–liquid interface is saturated, retaining γLV constantly. While the surfactant continues to adsorb on the PMMA–liquid interface, γSL reduced resulting in an increasing trend in WA.
 | ||
| Fig. 5 Effect of GeminiC6, C3 and monomer concentration on the adhesion function WA of PMMA surface. | ||
The higher the adhesion work, the easier the surfactant solution wets on the surface. It is obvious that the monomer after CMC has the largest adhesion work and is the most favourable for wetting on PMMA surface. The adhesion work of GeminiC3 experienced a short plateau and started to continue to decrease with the decrease of γLV until γLV was not changing, the decrease of γSL led to the increase of adhesion work because the PMMA surface became hydrophilic surface, the adhesion work of GeminiC3 was higher than that of GeminiC6. It was verified that the adsorption of GeminiC3 on the hydrophilic modified part of PMMA surface in Table 3 was greater than that of GeminiC6.
3.6. Mechanism responsible for surfactant adsorption behaviour on the PMMA surface
To enhance our comprehension of the adsorption mechanisms of C3, C6, and monomer on the surface of PMMA, the variation of surface tension, solid–liquid interfacial tension, and contact angle as a function of concentration were graphically presented in Fig. 7a–c. Moreover, in order to visually illustrate the adsorption behaviour of the three surfactants on the PMMA surface, mechanism diagrams were also created and depicted in Fig. 6. Based on the information depicted in Fig. 6 and 7, the three surfactants were examined and discussed in three distinct stages.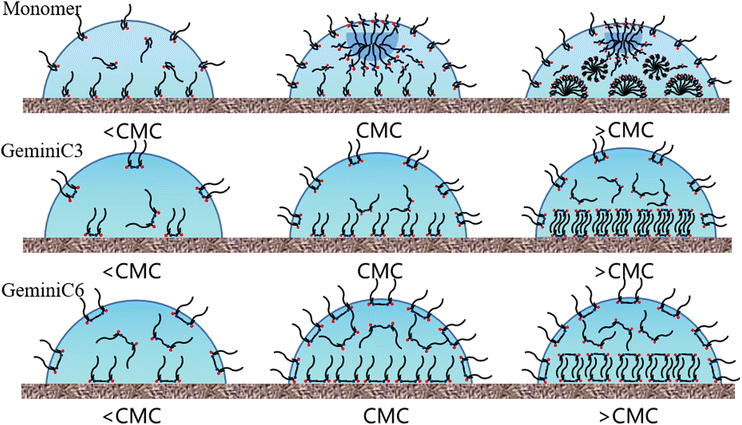 | ||
| Fig. 6 Schematic diagram of the possible molecular arrangement of surfactant molecules on the surface of PMMA. | ||
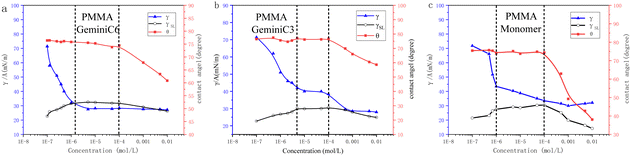 | ||
| Fig. 7 (a–c) Concentration dependence of PMMA surface adhesion data for C6, C3 and monomer surfactants, respectively. | ||
The first stage is defined as the concentration range from 1E-7 mol l−1 to CMC. In this phase, as the concentration increases, the monomer rapidly adsorbs on the gas–liquid interface to form surface micelles, followed by a break in surface tension to reach CMC1. While C6 directly reaches the only CMC, attributed to the longer flexible spacer group of C6 (–CH2–CH2–), which weakens the polarity and spatial hindrance of the two ionic heads. At this stage, all C3, C6 and monomers are continuously adsorbed at the PMMA interface. As shown in Fig. 7, the ionic heads of C3 and C6 are adsorbed on the PMMA surface by hydrogen bonding and the hydrophobic alkyl chains are facing the solution. The increase in solid–liquid interfacial tension compensates for the decrease in surface tension and the contact angle remains constant. The monomer adsorbs heavily on the PMMA surface at this stage due to the smaller spatial site resistance. The second stage is defined as the concentration range from CMC1 for C3 and monomer and CMC for C6 to 1E-4 mol l−1. During this stage, C3 and C6 reach temporary saturation at the gas–liquid interface, and surface tension no longer changes. The first layer of saturated adsorption film is continuously formed on the PMMA surface while retains a constant interfacial tension and contact angle. The surface tension of the monomer continues to decrease, compensated by the increased interfacial tension, and the contact angle remains constant.
The third stage is defined as 1E-4 mol l−1 to 1E-2 mol l−1. C3, C6 continue to adsorb on the first layer of saturated adsorption membrane due to hydrophobic interaction to form a double-layer membrane structure, the ion head group is facing the solution, and the interfacial tension is reduced. However, the surface tension of C6 remains unchanged, the surface tension of C3 decreases, the contact angle rapidly decreases. The decrease in surface tension of C3 can be inferred from the literature18,26 that the shape of the surface micelles changes to vesicles-like aggregates, further reducing the liquid interface activation energy. At this stage, the monomer molecules formed micelles in solution with the increase of concentration, the adsorption area on PMMA decreased significantly, forming the semi-micelles, the adsorption capacity increased significantly.
The ion head of the surfactant orientated to the solution, modifying the PMMA surface to be a hydrophilic surface, the interfacial tension decreases rapidly, the surface tension keeps unchanged, resulting in a rapid decrease in the contact angle.
4. Conclusion
In this paper, the unique adsorption behaviour and wettability modification of baryonic anionic surfactants and monomers with double-chain alkyl C3, C6 spacer groups on PMMA surfaces were investigated using C3, C6, monomeric surfactants synthesized with octadecenyl succinic anhydride with double alkyl chains. Based on the determination of contact angle and surface tension, combined with calculations, it was found that the monomeric surfactants with double-chain structure formed semi-micelles on the PMMA surface with good wetting water modification ability. Baryonic surfactants, on the other hand, are adsorbed on the PMMA surface through bilayers, the same as most baryonic surfactants, but the different lengths of spacer groups and double hydrophobic chains confer different adsorption of GeminiC3 and C6 at the gas–liquid interface. C3 surface tension shows two plateaus at low and high concentrations, respectively, because the more compact molecular and spatial structure of C3 leads to self-polymerization in solution The behaviour is complicated by the occurrence of multiple self-assembly behaviours and the formation of micelles followed by the formation of vesicle structures. In conclusion, the anionic Gemini surfactants in this paper showed completely different adsorption behaviours on PMMA surfaces compared to monomers and had lower surface tension values and CMC. However, during the wettability behaviour, monomers were stronger than C3, C6 baryonic surfactants in wetting modification of PMMA surfaces.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the National Key R&D Program of China (No. 2019YFA0708700), TIPC Director's Fund of Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Key Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences.References
- Y. Chen, J. Meng, Z. Gu, X. Wan, L. Jiang and S. Wang, Adv. Funct. Mater., 2019, 30(5), 1905287 CrossRef.
- S. Deb Barma, B. Banerjee, K. Chatterjee and S. Paria, ACS Sustainable Chem. Eng., 2018, 6(3), 3615–3623 CrossRef CAS.
- M. Gharabaghi and S. Aghazadeh, Curr. Opin. Colloid Interface Sci., 2014, 19(4), 266–282 CrossRef CAS.
- Q. Jiang, Y. Du, L. Zhang, W. Ma, F. Yan, L. Zhang and S. Zhao, Molecules, 2021, 26(4), 863 CrossRef CAS PubMed.
- Y. Jiang and C. H. Choi, Adv. Mater. Interfaces, 2020, 8(2), 2001205 CrossRef.
- C.-C. Lai and K.-M. Chen, Colloids Surf., A, 2008, 320(1–3), 6–10 CrossRef CAS.
- W. F. Lv, Z. H. Zhou, Q. Zhang, W. L. Luo, H. Z. Wang, D. S. Ma, L. Zhang, R. Wang and L. Zhang, Soft Matter, 2019, 15(33), 6725–6731 RSC.
- Y. Shen, Y. Jin, S. Lai, L. Shi, W. Du and R. Zhou, J. Mol. Liq., 2019, 296, 33798 CrossRef.
- I. Svanedal, F. Andersson, E. Hedenstrom, M. Norgren, H. Edlund, S. K. Satija, B. Lindman and A. R. Rennie, Langmuir, 2016, 32(42), 10936–10945 CrossRef CAS PubMed.
- K. Hiramatsu, K. Kameyama, R. Ishiguro, M. Mori and H. Hayase, Bull. Chem. Soc. Jpn., 2003, 76(10), 1903–1910 CrossRef CAS.
- J. Martin-de Leon, V. Bernardo and M. A. Rodriguez-Perez, Materials, 2019, 12(5), 797 CrossRef CAS PubMed.
- P. R. Bhut, N. Pal and A. Mandal, ACS Omega, 2019, 4(23), 20164–20177 CrossRef CAS PubMed.
- B. Brycki, A. Szulc, I. Kowalczyk and J. Brycka, Molecules, 2022, 27(19), 6664 CrossRef CAS PubMed.
- Q. Zhang, Z.-H. Zhou, S.-S. Hu, S.-M. Li, D.-S. Ma, X.-Y. Zhou, L. Han, L. Zhang and L. Zhang, J. Mol. Liq., 2019, 277, 571–576 CrossRef CAS.
- M. Gao, X. G. Wang, W. F. Lv, Z. H. Zhou, Q. Zhang, D. S. Ma, H. Z. Wang, F. Yan, L. Zhang and L. Zhang, Soft Matter, 2020, 16(23), 5450–5457 RSC.
- J. Krawczyk, K. Szymczyk, A. Zdziennicka and B. Jańczuk, Int. J. Adhes. Adhes., 2013, 45, 106–111 CrossRef CAS.
- W. Wu, D.-D. Liu, Z.-C. Xu, Q.-T. Gong, J.-B. Huang, L. Zhang and L. Zhang, Acta Phys.-Chim. Sin., 2016, 32(5), 1214–1220 CAS.
- M. T. Garcia, O. Kaczerewska, I. Ribosa, B. Brycki, P. Materna and M. Drgas, J. Mol. Liq., 2017, 230, 453–460 CrossRef CAS.
- N. R. Biswal and S. Paria, Ind. Eng. Chem. Res., 2012, 51(30), 10172–10178 CrossRef CAS.
- J. Harkot and B. Jańczuk, Appl. Surf. Sci., 2008, 254(9), 2825–2830 CrossRef CAS.
- A. Pinazo, V. Petrizelli, M. Bustelo, R. Pons, M. P. Vinardell, M. Mitjans, A. Manresa and L. Perez, Colloids Surf., B, 2016, 141, 19–27 CrossRef CAS PubMed.
- S Z W J Ma. CN, 2019: 1131728.7.
- L. R. Dix, J. Colloid Interface Sci., 2001, 238(2), 447–448 CrossRef CAS PubMed.
- L. Zhang, Z. L. Wang, Z. Q. Li, L. Zhang, Z. C. Xu, S. Zhao and J. Y. Yu, Langmuir, 2010, 26(24), 18834 CrossRef CAS PubMed.
- C. M. Phan, S. I. Yusa, T. Honda, K. K. Sharker, A. E. Hyde and C. V. Nguyen, ACS Omega, 2018, 3(9), 10907–10911 CrossRef CAS PubMed.
- S. H. H. Hoffmann, Prog. Colloid Polym. Sci., 1996, 101, 131–134 Search PubMed.
- K. Szymczyk and B. Jańczuk, J. Adhes. Sci. Technol., 2012, 25(19), 2641–2657 CrossRef.
- K. Szymczyk, A. Zdziennicka, B. Janczuk and W. Wojcik, J. Colloid Interface Sci., 2006, 293, 172–180 CrossRef CAS PubMed.
- J. Harkot and B. Jańczuk, Appl. Surf. Sci., 2009, 255(6), 3623–3628 CrossRef CAS.
- L. I. U. Dan-Dan, X. U. Zhi-Cheng, Z. Qun, Z. Lei, Z. Lu and Z. Sui, Acta Phys.-Chim. Sin., 2013, 29(03), 569–575 Search PubMed.
- S.-S. Hu, L. Zhang, Z.-C. Xu, Q.-T. Gong, Z.-Q. Jin, L. Luo, L. Zhang and S. Zhao, Appl. Surf. Sci., 2015, 355, 868–877 CrossRef CAS.
- Y. Du, Z. H. Zhou, M. Gao, L. Han, L. Zhang, F. Yan, M. Wang and L. Zhang, Soft Matter, 2021, 17(26), 6426–6434 RSC.
- Y. Fu-Qing, L. I. U. Dan-Dan, G. U. O. Lan-Lei, Z. H. U. Yang-Wen, X. U. Zhi-Cheng, H. Jian-Bin, Z. Lei and Z. Lu, Acta Phys.-Chim. Sin., 2015, 31(4), 715–721 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |


