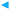Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of scission on alignment of nonionic surfactant micelles under shear flow
Yusuke
Koide
 * and
Susumu
Goto
* and
Susumu
Goto

Graduate School of Engineering Science, Osaka University, 1-3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan. E-mail: y_koide@fm.me.es.osaka-u.ac.jp
First published on 26th April 2023
Abstract
We investigate the alignment of wormlike micelles under shear flow with dissipative particle dynamics simulations of nonionic surfactant solutions. To reveal the effect of micellar scission on alignment, we evaluate the shear-rate dependence of the mean orientation angle and the average lifetime of micelles for fixed aggregation numbers. Our numerical results demonstrate the presence of two distinct shear-rate regimes of micellar alignment. In the low shear-rate regime, where flow-induced scission does not occur, wormlike micelles align more in the flow direction with the shear rate. In contrast, flow-induced scission suppresses micellar alignment in the high shear-rate regime. In addition, comparing the alignment of wormlike micelles with that of polymers without scission, we find that the mean orientation angle of wormlike micelles is larger than that of polymers when flow-induced scission occurs. This comparison confirms that flow-induced scission yields the unique behavior of micellar alignment. Furthermore, we demonstrate that flow-induced scission suppresses micellar alignment for fixed aggregation numbers by reducing the effective longest relaxation time of micelles.
1. Introduction
One of the significant differences between polymers and wormlike micelles is the underlying mechanism of their structure formation. Polymers are based on covalent bonds, whereas surfactant micelles are on weak non-bonded interactions. Thus, unlike polymers, micelles constantly exhibit scission and recombination. It is important to understand how this difference affects their properties. A crucial example is turbulent drag reduction by adding polymers and surfactants.1,2 Since the flow statistics are similar for both additives,3 polymers and surfactant micelles have similar roles in modulating turbulence. However, there is an indispensable difference originating from their formation mechanisms. Since polymers are formed through covalent bonds, their structures never recover once they are broken by strong shear flows.4 Due to this irreversibility, the drag reduction efficiency by polymer additives declines with time.5–7 In contrast, since the driving force for micellization is a non-bonded interaction, surfactants can form micelles again after scission.8 Thus surfactant solutions are less affected by mechanical degradation than polymers.9 This property makes surfactants more suitable for closed-loop systems such as air conditioning systems.10,11 Therefore, developing technologies using polymer and surfactant solutions requires a fundamental knowledge of their different properties arising from their formation mechanisms.Here, we explore whether micellar scission kinetics yields a difference in alignment behavior under shear flow. In general, the alignment of wormlike micelles and polymers is closely related to the rheological properties of their solutions. Since surfactants and polymers are often added to solutions to control rheology,12–14 it is indispensable to understand their alignment properties. Many studies have been conducted on polymer alignment using theoretical, experimental, and numerical approaches.15–18 It is well known that polymers under shear flow align more in the flow direction as the shear rate increases. Similarly to polymers, numerous studies have reported that rodlike and wormlike micelles also align in the flow direction.19–26 However, little is known about the effect of scission on micellar alignment, although recent experimental results suggest that the scission effect on the micellar alignment emerges for high shear rates. Arenas-Gómez et al.25 conducted simultaneous measurements of the viscosity and alignment of wormlike micelle solutions. They revealed that the orientation parameter exhibits nonmonotonic dependence on the shear rate. They attributed the decrease in the orientation parameter to the decreased contour length due to increased scission frequency. Recently, King et al.26 investigated the alignment and viscosity of concentrated wormlike micelle solutions. They demonstrated that the saturation of alignment occurs for high shear rates, indicating the scission effect. However, it is difficult to experimentally measure the scission frequency and observe the structures and dynamics of individual micelles in flowing solutions. Thus the effect of scission on micellar alignment remains unclear.
Molecular simulations allow us to overcome these difficulties and reveal the relationship between alignment and scission. Although some studies investigated the alignment of rodlike and wormlike micelles using molecular simulations,27–30 little has been done to reveal the effect of scission.31 Huang et al.28 used a mesoscopic model incorporating a kinetic model for scission and recombination into linear chains and examined alignment and scission under shear flow. Although they reported the saturation of alignment for high shear rates, they attributed this saturation to finite-size effects under strong shear flows. Sambasivam et al.30 investigated alignment and scission of a single rodlike micelle under shear flow with the coarse-grained molecular dynamics (CGMD) method. They found that when the Weissenberg number Wi = τ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) defined as the product of the shear rate
defined as the product of the shear rate ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) and the rotational relaxation time τ is larger than 1, a rodlike micelle aligns in the flow direction exhibiting tumbling dynamics. They also showed that elongation of micelles by shear flow leads to micellar scission for large Wi. However, there was no comparison between alignment and scission for given Wi. Although it is worth noting that Carl et al.31 found that the saturation of the mean alignment angle can occur due to a decrease in the average chain length, there is still room for studying micellar alignment under shear flow in terms of scission.
and the rotational relaxation time τ is larger than 1, a rodlike micelle aligns in the flow direction exhibiting tumbling dynamics. They also showed that elongation of micelles by shear flow leads to micellar scission for large Wi. However, there was no comparison between alignment and scission for given Wi. Although it is worth noting that Carl et al.31 found that the saturation of the mean alignment angle can occur due to a decrease in the average chain length, there is still room for studying micellar alignment under shear flow in terms of scission.
In the present study, we investigate the effect of scission on micellar alignment through detailed analysis of both alignment and scission using molecular simulations. There are two significant issues for solving this problem. One is the evaluation of statistical properties of scission. Observing many scission events requires a high computational cost. To overcome this difficulty, we employ the dissipative particle dynamics (DPD) method. Thanks to coarse-graining and soft repulsive interactions, DPD allows us to simulate large systems for long time scales with a low computational cost. In fact, our previous study32 demonstrated that DPD is suitable for evaluating the statistical properties of micellar lifetimes. In addition, surfactant and water molecules are explicitly considered in DPD simulations. Thus we do not rely on a kinetic model for the scission and recombination of micelles.33–35 The other issue is the polydispersity of the aggregation number of micelles. When a concentration is high enough for wormlike micelles to exist, micelles of different sizes coexist in the solution. In addition, the distribution changes for high shear rates due to increased scission frequency.32 Thus statistics over all the micelles in the system, often used in previous studies,28,29,33,36 could demonstrate only the combined effect of the changes in the micellar properties and distributions. Here, we employ conditional statistics based on the aggregation number used in our previous study.32 This analysis method separates the effect of individual micellar properties and distributions of the aggregation number. In addition, this method allows us to directly compare the alignment of individual micelles with that of polymers to reveal the effect of micellar scission on alignment. In the following, we will quantitatively show that flow-induced scission suppresses micellar alignment even for fixed aggregation numbers.
2. Method
2.1 DPD governing equations
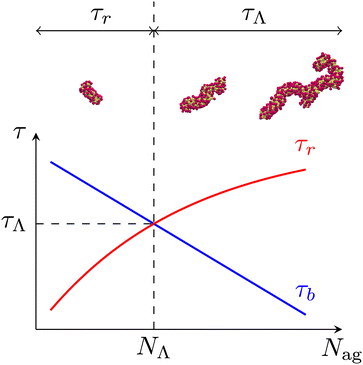
In the present study, we use the DPD method to conduct molecular simulations of nonionic surfactant solutions. In DPD simulations, a single DPD particle represents a group of atoms and molecules. The motion of a DPD particle obeys | (1) |
 | (2) |
| FDij = −γwD(rij)(eij·vij)eij (vij = vi − vj) | (3) |
| FRij = σwR(rij)θijeij, | (4) |
| θij(t) = θji(t) | (5) |
| 〈θij(t)〉 = 0 | (6) |
| 〈θij(t)θkl(t′)〉 = (δikδjl + δilδjk)δ(t − t′) | (7) |
| wD(rij) = [wR(rij)]2 | (8) |
| σ2= 2γkBT, | (9) |
 | (10) |
2.2 Surfactant model
The model of a nonionic surfactant molecule contains a hydrophilic head particle and two hydrophobic tail particles. Three surfactant particles are connected by two harmonic springs expressed by| FBij = −ks(rij − req)eij, | (11) |
| N | ρ | σ | ϕ | k s | r eq | a hh | a ht | a hw | a tt | a tw | a ww |
|---|---|---|---|---|---|---|---|---|---|---|---|
648![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3 | 3 | 0.05 | 50 | 0.8 | 25 | 60 | 20 | 25 | 60 | 25 |
2.3 Polymer model
In the present study, we conduct DPD simulations of polymer solutions for comparison. We employ the fully flexible polymer model used in the previous study.40 Linear polymer chains consist of Nm beads, and adjacent beads in a polymer are connected by finitely extensible nonlinear elastic (FENE) springs expressed by | (12) |
| N | N m | ρ | σ | ϕ | k F | r eq | r max | a pp | a pw | a ww |
|---|---|---|---|---|---|---|---|---|---|---|
375![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
50 | 3 | 3 | 0.05 | 40 | 0.7 | 2 | 25 | 25 | 25 |
2.4 Simulation details
To integrate the equations of motion, we use the modified velocity Verlet method38 with the time step Δt = 0.04 and the parameter λ = 0.65 introduced in this algorithm. We have confirmed that these parameters realize sufficiently accurate temperature control. To generate a uniform shear flow, we use the Lees–Edwards boundary condition41 and the SLLOD equations.42 We impose a random initial configuration and conduct equilibrium simulations for 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 time units for surfactant solutions and 8000 time units for polymer solutions, achieving statistically steady values of the potential energy. After that, we conduct non-equilibrium simulations by imposing shear flow. Conditional statistics for micelles introduced in Section 2.5 require DPD simulations for a long time to obtain sufficiently accurate results. Thus we conduct non-equilibrium simulations of surfactant solutions for 40
000 time units for surfactant solutions and 8000 time units for polymer solutions, achieving statistically steady values of the potential energy. After that, we conduct non-equilibrium simulations by imposing shear flow. Conditional statistics for micelles introduced in Section 2.5 require DPD simulations for a long time to obtain sufficiently accurate results. Thus we conduct non-equilibrium simulations of surfactant solutions for 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–160
000–160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 time units depending on the shear rate.
000 time units depending on the shear rate.
We perform all the DPD simulations using our in-house code.
2.5 Analysis details
To evaluate individual micellar properties, we need to identify micelles in the system. We define micelles adopting the method used in previous studies.43,44 In this method, two surfactant molecules belong to a common cluster if a hydrophobic particle of one surfactant molecule is within rc (=1) of a hydrophobic particle of the other. If a cluster has an aggregation number Nag larger than a threshold value nmic (=10), then we regard the cluster as a micelle. Since we focus mainly on micelles with Nag ≳ 50, our results are practically insensitive to the choice of nmic.To investigate Nag dependence of micellar properties, we use conditional statistics based on Nag instead of statistics over all the micelles in the system. Specifically, to evaluate the micellar properties for given Nag, we use the data for micelles having aggregation numbers that lie in [Nag − ΔNag,Nag + ΔNag], where we set ΔNag = 0.05Nag. In the following, the micellar orientation angle is conditioned by instantaneous Nag, whereas the micellar lifetime is conditioned by the value of Nag before scission.
3. Results
One of the most intriguing observations in the present study is that flow-induced scission, which occurs for high shear rates, suppresses micellar alignment in the flow direction even for fixed aggregation numbers Nag. To quantitatively demonstrate the effect of scission on micellar alignment, we evaluate the shear-rate dependence of the micellar lifetime and alignment.3.1 Alignment
In this subsection, we examine the micellar alignment under shear flow. First, we define micellar orientation angles. In the present study, we define the micellar direction as the eigenvector e(1) of the gyration tensor Gij corresponding to the largest eigenvalue. Here, Gij is defined as | (13) |
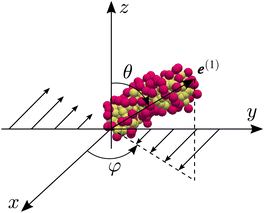 | ||
| Fig. 2 Definition of angles θ and φ of micelles. The micellar direction is defined as the eigenvector e(1) of the gyration tensor Gij corresponding to the largest eigenvalue. | ||
A key point of our analysis is that we rely on conditional statistics based on Nag instead of statistics over all the micelles in the system. The inset of Fig. 3 shows the probability density function (PDF) P(Nag) of Nag for the shear rate ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 0.005. Note that the temporally averaged values are shown. We find that micelles of various sizes coexist in the system for the considered volume fraction ϕ. Fig. 3 shows the PDF P(φ) of φ for various Nag, which demonstrates that the statistical properties of micellar alignment significantly depend on Nag for given
= 0.005. Note that the temporally averaged values are shown. We find that micelles of various sizes coexist in the system for the considered volume fraction ϕ. Fig. 3 shows the PDF P(φ) of φ for various Nag, which demonstrates that the statistical properties of micellar alignment significantly depend on Nag for given ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . Therefore, the analysis using the information on all the micelles in the system, as conducted in previous studies,28,29 reflects not only individual micellar dynamics but also the distribution of Nag. In particular, since P(Nag) for high shear rates greatly differ from that at equilibrium due to flow-induced scission (see Fig. 10 of ref. 32), this change in P(Nag) complicates the interpretation of the results. We overcome this difficulty using conditional statistics based on Nag defined in Section 2.5, which enables us to investigate the alignment properties of individual micelles.
. Therefore, the analysis using the information on all the micelles in the system, as conducted in previous studies,28,29 reflects not only individual micellar dynamics but also the distribution of Nag. In particular, since P(Nag) for high shear rates greatly differ from that at equilibrium due to flow-induced scission (see Fig. 10 of ref. 32), this change in P(Nag) complicates the interpretation of the results. We overcome this difficulty using conditional statistics based on Nag defined in Section 2.5, which enables us to investigate the alignment properties of individual micelles.
 | ||
Fig. 3 PDF P(φ) of the orientation angle φ for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 0.005. Different curves correspond to different values of the aggregation number: Nag = 70, 100, 200, and 400 from thinner (and darker) to thicker (and lighter) lines. The inset shows PDF P(Nag) of Nag for = 0.005. Different curves correspond to different values of the aggregation number: Nag = 70, 100, 200, and 400 from thinner (and darker) to thicker (and lighter) lines. The inset shows PDF P(Nag) of Nag for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 0.005. The vertical black dashed line indicates the mean orientation angle = 0.005. The vertical black dashed line indicates the mean orientation angle ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) defined by eqn (14), and the vertical dotted red line indicates the conventional mean orientation angle χG defined by eqn (15) for Nag = 400. defined by eqn (14), and the vertical dotted red line indicates the conventional mean orientation angle χG defined by eqn (15) for Nag = 400. | ||
In the following, we characterize ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) dependence of micellar alignment with a mean orientation angle. Note that given a set of n angles {φ1, φ2, …, φn}, the arithmetic mean
dependence of micellar alignment with a mean orientation angle. Note that given a set of n angles {φ1, φ2, …, φn}, the arithmetic mean 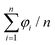 is inappropriate as a mean orientation angle due to the periodicity of φi. For instance, the arithmetic mean of φ1 = π/2 − ε and φ2 = −π/2 + ε is zero even when ε ≪ 1, which is misleading because φ1 ≃ φ2 ≃ π/2 modulo π. Here, we define a mean orientation angle
is inappropriate as a mean orientation angle due to the periodicity of φi. For instance, the arithmetic mean of φ1 = π/2 − ε and φ2 = −π/2 + ε is zero even when ε ≪ 1, which is misleading because φ1 ≃ φ2 ≃ π/2 modulo π. Here, we define a mean orientation angle ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) as
as
 | (14) |
![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) by dividing the mean direction
by dividing the mean direction  of 2φi by 2. We show
of 2φi by 2. We show ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) of micelles with Nag = 400 for
of micelles with Nag = 400 for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 0.005 by a vertical black dashed line in Fig. 3 as an example. It verifies that
= 0.005 by a vertical black dashed line in Fig. 3 as an example. It verifies that ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) is a characteristic angle of wormlike micelles. For comparison, we also show the conventional mean orientation angle χG17,46 defined by
is a characteristic angle of wormlike micelles. For comparison, we also show the conventional mean orientation angle χG17,46 defined by | (15) |
![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) and χG show similar values. Thus the definition of the mean orientation angle has little effect on the following results.
and χG show similar values. Thus the definition of the mean orientation angle has little effect on the following results.
Fig. 4 shows ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) dependence of
dependence of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) for various Nag. Two important features are evident in this result. First, Nag dependence of
for various Nag. Two important features are evident in this result. First, Nag dependence of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) for fixed
for fixed ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) indicates that there exists a threshold aggregation number NΛ above which
indicates that there exists a threshold aggregation number NΛ above which ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) remains constant. For Nag ≥300,
remains constant. For Nag ≥300, ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) collapses on a single function of
collapses on a single function of ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) irrespective of Nag. In contrast,
irrespective of Nag. In contrast, ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) of micelles with Nag ≤ 130 decreases as Nag increases for fixed
of micelles with Nag ≤ 130 decreases as Nag increases for fixed ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . These observations indicate that the longest relaxation time of micelles increases with Nag for Nag ≲ NΛ and remains constant for Nag ≳ NΛ. We will explain how to evaluate the values of the longest relaxation time and NΛ in Section 4. Second,
. These observations indicate that the longest relaxation time of micelles increases with Nag for Nag ≲ NΛ and remains constant for Nag ≳ NΛ. We will explain how to evaluate the values of the longest relaxation time and NΛ in Section 4. Second, ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) dependence of
dependence of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) for fixed Nag exhibits a nonmonotonic behavior. Here, we explain this nonmonotonic dependence focusing on the results for Nag ≥ 300. For
for fixed Nag exhibits a nonmonotonic behavior. Here, we explain this nonmonotonic dependence focusing on the results for Nag ≥ 300. For ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ≤ 0.005,
≤ 0.005, ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) decreases as
decreases as ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) increases, indicating that micelles align more in the flow direction for lager
increases, indicating that micelles align more in the flow direction for lager ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . In contrast, for
. In contrast, for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) > 0.005,
> 0.005, ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) decreases more slowly and slightly increases for
decreases more slowly and slightly increases for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ≥ 0.02. This is an intriguing phenomenon because
≥ 0.02. This is an intriguing phenomenon because ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) of polymers monotonically decreases as
of polymers monotonically decreases as ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) increases, as described below (see Fig. 8). Interestingly, the saturation of alignment was also reported for amphiphilic Janus colloids,47 which suggests the general alignment behavior of self-assembled clusters. In the next subsection, we will explore the origin of the alignment behavior by evaluating the micellar lifetime under shear flow.
increases, as described below (see Fig. 8). Interestingly, the saturation of alignment was also reported for amphiphilic Janus colloids,47 which suggests the general alignment behavior of self-assembled clusters. In the next subsection, we will explore the origin of the alignment behavior by evaluating the micellar lifetime under shear flow.
Before closing this subsection, we refer to the results without using conditional statistics. We define ![[capital Phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c1.gif) as
as ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) obtained with eqn (14) using all the data for micelles with Nag ≥ 50. In Fig. 4, we show
obtained with eqn (14) using all the data for micelles with Nag ≥ 50. In Fig. 4, we show ![[capital Phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c1.gif) as a function of
as a function of ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . In the case of the examined parameters, since a sufficient number of micelles with Nag ≳ NΛ exist in the system,
. In the case of the examined parameters, since a sufficient number of micelles with Nag ≳ NΛ exist in the system, ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) for Nag ≳ NΛ and
for Nag ≳ NΛ and ![[capital Phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c1.gif) exhibit similar behavior. Specifically,
exhibit similar behavior. Specifically, ![[capital Phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c1.gif) does not decrease toward zero when increasing
does not decrease toward zero when increasing ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) , which was also observed in a previous study31 modeling linear micelles as breakable bead-spring chains. However, due to the contributions from micelles with Nag ≲ NΛ,
, which was also observed in a previous study31 modeling linear micelles as breakable bead-spring chains. However, due to the contributions from micelles with Nag ≲ NΛ, ![[capital Phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c1.gif) is larger than
is larger than ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) for Nag ≳ NΛ. In summary, since
for Nag ≳ NΛ. In summary, since ![[capital Phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c1.gif) depends on P(Nag), interpretation of
depends on P(Nag), interpretation of ![[capital Phi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c1.gif) may not be straightforward, especially when comparing results between different parameters.
may not be straightforward, especially when comparing results between different parameters.
3.2 Effect of scission
In the previous subsection, we have seen that![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) of wormlike micelles exhibits a nontrivial dependence on
of wormlike micelles exhibits a nontrivial dependence on ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . To reveal its origin, we investigate the effect of scission on micellar alignment. To quantitatively evaluate micellar scission, we focus on the micellar lifetime tb defined as the time between micellar birth and scission. We estimate a survival function S(tb), which gives the probability that micelles survive beyond a certain time tb, using the Kaplan–Meier method.48 Then, we fit S(tb) with C0
. To reveal its origin, we investigate the effect of scission on micellar alignment. To quantitatively evaluate micellar scission, we focus on the micellar lifetime tb defined as the time between micellar birth and scission. We estimate a survival function S(tb), which gives the probability that micelles survive beyond a certain time tb, using the Kaplan–Meier method.48 Then, we fit S(tb) with C0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−tb/τb) to obtain the average lifetime τb. The definition of micellar scission and the method to evaluate τb are the same as in our previous study.32 In this subsection, we will show the results of micelles with Nag larger than NΛ because more than 65% of surfactants in the system belong to micelles with Nag ≳ NΛ at equilibrium. In addition, micelles with Nag ≳ NΛ have the slowest timescale in the system as described in Section 4. In other words, they align most in the flow direction. We will discuss the alignment of micelles with Nag ≲ NΛ in Section 4.
exp(−tb/τb) to obtain the average lifetime τb. The definition of micellar scission and the method to evaluate τb are the same as in our previous study.32 In this subsection, we will show the results of micelles with Nag larger than NΛ because more than 65% of surfactants in the system belong to micelles with Nag ≳ NΛ at equilibrium. In addition, micelles with Nag ≳ NΛ have the slowest timescale in the system as described in Section 4. In other words, they align most in the flow direction. We will discuss the alignment of micelles with Nag ≲ NΛ in Section 4.
To explore the relationship between alignment and scission, we show ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) dependence of
dependence of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) and τb for various Nag (>NΛ) in Fig. 5. To focus on the flow effect, we normalize τb by the value τ0b at equilibrium for given Nag. We find that τb/τ0b also follows a single function of
and τb for various Nag (>NΛ) in Fig. 5. To focus on the flow effect, we normalize τb by the value τ0b at equilibrium for given Nag. We find that τb/τ0b also follows a single function of ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) irrespective of Nag (>NΛ) as well as
irrespective of Nag (>NΛ) as well as ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) . The bottom panel of Fig. 5 demonstrates that τb/τ0b ≃ 1 for
. The bottom panel of Fig. 5 demonstrates that τb/τ0b ≃ 1 for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ≲ 0.005, indicating that flow-induced scission does not occur, whereas τb/τ0b ≲ 1 for
≲ 0.005, indicating that flow-induced scission does not occur, whereas τb/τ0b ≲ 1 for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ≳ 0.005 due to flow-induced scission. We show this threshold shear rate
≳ 0.005 due to flow-induced scission. We show this threshold shear rate ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c (=0.005) for flow-induced scission in Fig. 5 to emphasize the effect of flow-induced scission. The top panel of Fig. 5 demonstrates that the alignment behavior changes around
c (=0.005) for flow-induced scission in Fig. 5 to emphasize the effect of flow-induced scission. The top panel of Fig. 5 demonstrates that the alignment behavior changes around ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c. This indicates that flow-induced scission, which occurs for
c. This indicates that flow-induced scission, which occurs for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ≳
≳ ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c, suppresses micellar alignment. It is worth emphasizing that Fig. 5 shows
c, suppresses micellar alignment. It is worth emphasizing that Fig. 5 shows ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) of micelles with a given Nag obtained with conditional statistics instead of the average values over all the micelles. Thus, the increase in the number of small micelles due to flow-induced scission cannot explain the suppression of alignment. Although the present study focuses on the relationship between alignment and scission of micelles under shear flow, their effect on the rheological properties is an important aspect. In our previous study,32 we have shown that the considered solutions have viscoelasticity and exhibit shear thinning. In this system, both alignment and flow-induced scission contribute to the shear thinning. Constitutive models based on the microscopic dynamics and kinetics of micelles (e.g.ref. 49) will allow a systematic study on the effect of alignment and flow-induced scission on the rheological properties, which is an important near-future study.
of micelles with a given Nag obtained with conditional statistics instead of the average values over all the micelles. Thus, the increase in the number of small micelles due to flow-induced scission cannot explain the suppression of alignment. Although the present study focuses on the relationship between alignment and scission of micelles under shear flow, their effect on the rheological properties is an important aspect. In our previous study,32 we have shown that the considered solutions have viscoelasticity and exhibit shear thinning. In this system, both alignment and flow-induced scission contribute to the shear thinning. Constitutive models based on the microscopic dynamics and kinetics of micelles (e.g.ref. 49) will allow a systematic study on the effect of alignment and flow-induced scission on the rheological properties, which is an important near-future study.
To confirm whether finite system sizes affect micellar alignment, we show the results of the system composed of N = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 particles in Fig. 5. Since these results agree with those of N = 648
000 particles in Fig. 5. Since these results agree with those of N = 648![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, finite system sizes have little effect on the micellar alignment. In the next section, we demonstrate that the nontrivial
000, finite system sizes have little effect on the micellar alignment. In the next section, we demonstrate that the nontrivial ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) dependence of
dependence of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) (Fig. 5) is specific to wormlike micelles by conducting a similar analysis for polymer models without scission.
(Fig. 5) is specific to wormlike micelles by conducting a similar analysis for polymer models without scission.
Before closing this subsection, we refer to the orientational dynamics of micelles before scission. To observe the typical dynamics of micelles before scission, we show φ of micelles that survive for O(τb) as a function of time t − t* in Fig. 6. Here, ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 0.01, and t* is the time when scission occurs. Since τb is smaller than the rotational relaxation time τr for Nag = 300 as described in Section 4.1, φ does not exhibit significant variations. This is evident by observing that φ of micelles with Nag = 130, for which τb ≳ τr, fluctuates between −π/2 and π/2 before scission. Consequently, micellar recombination plays an essential role in the distribution of φ when τb ≲ τr. Thus it is an important near-future study to investigate micellar alignment in terms of recombination.34,50,51
= 0.01, and t* is the time when scission occurs. Since τb is smaller than the rotational relaxation time τr for Nag = 300 as described in Section 4.1, φ does not exhibit significant variations. This is evident by observing that φ of micelles with Nag = 130, for which τb ≳ τr, fluctuates between −π/2 and π/2 before scission. Consequently, micellar recombination plays an essential role in the distribution of φ when τb ≲ τr. Thus it is an important near-future study to investigate micellar alignment in terms of recombination.34,50,51
4. Discussion
4.1 Comparison with polymers
Comparing the mean orientation angle![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) with the average lifetime τb has demonstrated that flow-induced scission suppresses micellar alignment (Fig. 5). Here, we compare the alignment of micelles with that of polymers to demonstrate the unique properties of micellar alignment.
with the average lifetime τb has demonstrated that flow-induced scission suppresses micellar alignment (Fig. 5). Here, we compare the alignment of micelles with that of polymers to demonstrate the unique properties of micellar alignment.
Since wormlike micelles and polymers have different timescales, we introduce the Weissenberg number Wi = τ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) defined as the product of the longest relaxation time τ and the shear rate
defined as the product of the longest relaxation time τ and the shear rate ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . We estimate τ of polymers by fitting the autocorrelation function C(t) of the end-to-end vector with an exponential function C0
. We estimate τ of polymers by fitting the autocorrelation function C(t) of the end-to-end vector with an exponential function C0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp (−t/τ).40 We obtain τ = 148 in the case of the examined parameters.
exp (−t/τ).40 We obtain τ = 148 in the case of the examined parameters.
We evaluate the longest relaxation time τ(Nag) of unentangled micelles from the rotational relaxation time τr(Nag) and the average lifetime τb(Nag). Specifically, we use the formula
 | (16) |
![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) and τb/τ0b are independent of Nag (>NΛ) for fixed
and τb/τ0b are independent of Nag (>NΛ) for fixed ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) (Fig. 5). The methods to evaluate τr and τb are the same as in our previous study.32 We obtain τ(70) = 72, τ(90) = 203, τ(130) = 586, τ(300) = τ(400) = τ(500) = τΛ = 796 and NΛ = 169.
(Fig. 5). The methods to evaluate τr and τb are the same as in our previous study.32 We obtain τ(70) = 72, τ(90) = 203, τ(130) = 586, τ(300) = τ(400) = τ(500) = τΛ = 796 and NΛ = 169.
Fig. 8 shows Wi dependence of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) for polymers and micelles. We confirm that
for polymers and micelles. We confirm that ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) of polymers obeys
of polymers obeys ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) ∝ Wi−0.45 for Wi ≳ 1. A similar power law was reported in previous studies,16,46,52,53 with a slightly different definition of a mean orientation angle. It is interesting to focus on the threshold Weissenberg number Wic = τΛ
∝ Wi−0.45 for Wi ≳ 1. A similar power law was reported in previous studies,16,46,52,53 with a slightly different definition of a mean orientation angle. It is interesting to focus on the threshold Weissenberg number Wic = τΛ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c for flow-induced scission of micelles with Nag ≳ NΛ. The alignment behavior of micelles with Nag ≳ NΛ deviates from that of polymers above Wic. Two important features are evident in this result. First, we confirm that
c for flow-induced scission of micelles with Nag ≳ NΛ. The alignment behavior of micelles with Nag ≳ NΛ deviates from that of polymers above Wic. Two important features are evident in this result. First, we confirm that ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) of wormlike micelles and polymers collapse on a single function of Wi for Wi ≲ Wic, indicating the similar alignment behavior of wormlike micelles and polymers when flow-induced scission does not occur. In addition, this collapse verifies that eqn (16) proposed in our previous study32 yields a quantitatively valid value of τ(Nag). Second, wormlike micelles have larger values of
of wormlike micelles and polymers collapse on a single function of Wi for Wi ≲ Wic, indicating the similar alignment behavior of wormlike micelles and polymers when flow-induced scission does not occur. In addition, this collapse verifies that eqn (16) proposed in our previous study32 yields a quantitatively valid value of τ(Nag). Second, wormlike micelles have larger values of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) than polymers for Wi ≳ Wic, indicating that flow-induced scission suppresses micellar alignment compared with polymers. So far, we have mainly focused on micelles with Nag ≳ NΛ. Here, we investigate the alignment of micelles with Nag ≲ NΛ in a similar way. Fig. 8 demonstrates that
than polymers for Wi ≳ Wic, indicating that flow-induced scission suppresses micellar alignment compared with polymers. So far, we have mainly focused on micelles with Nag ≳ NΛ. Here, we investigate the alignment of micelles with Nag ≲ NΛ in a similar way. Fig. 8 demonstrates that ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) of micelles with Nag ≲ NΛ collapse on the same function for Wi ≲ Wic. This collapse shows that we can consider Nag dependence of
of micelles with Nag ≲ NΛ collapse on the same function for Wi ≲ Wic. This collapse shows that we can consider Nag dependence of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) shown in Fig. 4 by using eqn (16). Similarly to micelles with Nag ≳ NΛ,
shown in Fig. 4 by using eqn (16). Similarly to micelles with Nag ≳ NΛ, ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) of micelles with Nag ≲ NΛ is larger than that of polymers for Wi ≳ Wic. Thus, the alignment of micelles with Nag ≲ NΛ is also suppressed for high shear rates. However, since
of micelles with Nag ≲ NΛ is larger than that of polymers for Wi ≳ Wic. Thus, the alignment of micelles with Nag ≲ NΛ is also suppressed for high shear rates. However, since ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) has different values depending on Nag for fixed Wi (≳ Wic), more detailed investigations are required to explain the degree of the alignment suppression. In the following, we again focus on the alignment of wormlike micelles with Nag ≳ NΛ, which are characterized by a common longest relaxation time τΛ.
has different values depending on Nag for fixed Wi (≳ Wic), more detailed investigations are required to explain the degree of the alignment suppression. In the following, we again focus on the alignment of wormlike micelles with Nag ≳ NΛ, which are characterized by a common longest relaxation time τΛ.
We also confirm the suppression of micellar alignment through visualization of wormlike micelles and polymers for given Wi. For surfactant solutions, we define the Weissenberg number of the system as Wi = τΛ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . We first focus on the alignment of wormlike micelles and polymers for Wi ≲ Wic, where flow-induced scission of micelles does not occur. Fig. 9(a) shows micelles with 200 ≤ Nag ≤ 600 for Wi = 2.4, and Fig. 9(c) shows some polymers which are randomly chosen for Wi = 3.0. They exhibit a qualitatively similar alignment behavior for Wi ≲ Wic. Incidentally, since surfactants consist of three DPD particles, micelles are slightly thicker than polymers. Indeed, the mean end-to-end distance of surfactants gives a rough estimate of the micellar radius R ≃ 1.43. Using longer surfactant models will reveal the effect of micellar thickness on alignment.
. We first focus on the alignment of wormlike micelles and polymers for Wi ≲ Wic, where flow-induced scission of micelles does not occur. Fig. 9(a) shows micelles with 200 ≤ Nag ≤ 600 for Wi = 2.4, and Fig. 9(c) shows some polymers which are randomly chosen for Wi = 3.0. They exhibit a qualitatively similar alignment behavior for Wi ≲ Wic. Incidentally, since surfactants consist of three DPD particles, micelles are slightly thicker than polymers. Indeed, the mean end-to-end distance of surfactants gives a rough estimate of the micellar radius R ≃ 1.43. Using longer surfactant models will reveal the effect of micellar thickness on alignment.
Next, we investigate their alignment for Wi ≳ Wic, where flow-induced scission occurs. Fig. 9(b) shows micelles with 200 ≤ Nag ≤ 600 for Wi = 48, and Fig. 9(d) shows some polymers which are randomly chosen for Wi = 44. Note that, for clarity, we use the data for ϕ = 0.1 in Fig. 9(b) because the number of micelles with 200 ≤ Nag ≤ 600 significantly decreases for large Wi due to flow-induced scission. We have confirmed that wormlike micelles exhibit similar alignment properties for ϕ = 0.1 as in the case of ϕ = 0.05. We observe in Fig. 9(d) that polymers considerably align in the flow direction, whereas wormlike micelles slightly tilt from the flow direction in Fig. 9(b). These observations are consistent with the quantitative results shown in Fig. 8. We then conclude that visualization also demonstrates the suppression of micellar alignment for Wi ≳ Wic. In the next subsection, we consider the mechanism of the suppression of micellar alignment focusing on the effective longest relaxation time under shear flow.
4.2 Effective longest relaxation time
We have shown that, for high shear rates, flow-induced scission occurs (Fig. 5) and suppresses micellar alignment compared with polymers (Fig. 8 and 9). This subsection demonstrates that flow-induced scission suppresses micellar alignment for fixed Nag by reducing the effective longest relaxation time. As mentioned above, we focus on micelles with Nag ≳ NΛ. Such micelles have a common longest relaxation time τΛ and exhibit the most significant alignment of all the micelles in the system.Let us now demonstrate that the decrease in the effective longest relaxation time τΛ causes the suppression of micellar alignment for high shear rates. Here, we propose a scenario that flow-induced scission reduces τb for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ≳
≳ ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c and then shifts τΛ and NΛ shown in Fig. 7 towards lower values. To verify this scenario, we focus on the fact that micelles with Nag ≳ NΛ are characterized by a common longest relaxation time τΛ. On the basis of this fact, we investigate whether NΛ changes for
c and then shifts τΛ and NΛ shown in Fig. 7 towards lower values. To verify this scenario, we focus on the fact that micelles with Nag ≳ NΛ are characterized by a common longest relaxation time τΛ. On the basis of this fact, we investigate whether NΛ changes for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ≳
≳ ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c to confirm the reduction of τΛ. Fig. 10 shows
c to confirm the reduction of τΛ. Fig. 10 shows ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) as a function of Nag for various
as a function of Nag for various ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . Note that, for clarity, we subtract the average value
. Note that, for clarity, we subtract the average value  over micelles with Nag ≥ NΛ from
over micelles with Nag ≥ NΛ from ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) for each
for each ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) . In Fig. 10, we show NΛ at equilibrium by a vertical dotted line for comparison. For
. In Fig. 10, we show NΛ at equilibrium by a vertical dotted line for comparison. For ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ≤
≤ ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c, the behavior of
c, the behavior of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) changes around NΛ. For Nag ≲ NΛ,
changes around NΛ. For Nag ≲ NΛ, ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) depends on Nag because the micellar alignment is characterized by τr(Nag). In contrast, since τΛ is a common longest relaxation time of micelles with Nag ≳ NΛ,
depends on Nag because the micellar alignment is characterized by τr(Nag). In contrast, since τΛ is a common longest relaxation time of micelles with Nag ≳ NΛ, ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) is independent of Nag for Nag ≳ NΛ. Most notably, for
is independent of Nag for Nag ≳ NΛ. Most notably, for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) >
> ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c, the plateau regime of
c, the plateau regime of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) extends to a lower Nag as
extends to a lower Nag as ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) increases. This indicates that NΛ decreases as
increases. This indicates that NΛ decreases as ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) increases. In other words, the effective longest relaxation time also decreases for
increases. In other words, the effective longest relaxation time also decreases for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) >
> ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c. We, therefore, conclude that flow-induced scission promotes micellar scission and reduces the effective longest relaxation time τΛ, leading to the suppression of micellar alignment even for fixed Nag.
c. We, therefore, conclude that flow-induced scission promotes micellar scission and reduces the effective longest relaxation time τΛ, leading to the suppression of micellar alignment even for fixed Nag.
5. Conclusions
We have conducted DPD simulations of nonionic surfactant solutions under shear flow to investigate the effect of scission on the alignment of wormlike micelles. The most important conclusion is that flow-induced scission suppresses micellar alignment even for fixed aggregation numbers Nag. One may think that the suppression is obvious because flow-induced scission shortens micelles, i.e., decreases Nag, thus promoting their rotational relaxation. However, we emphasize that the suppression of micellar alignment can occur even for fixed Nag. We have verified the conclusion by evaluating the shear-rate![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) dependence of the average lifetime τb and the mean orientation angle
dependence of the average lifetime τb and the mean orientation angle ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) for fixed Nag (Fig. 5). Specifically, when
for fixed Nag (Fig. 5). Specifically, when ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) exceeds a threshold shear rate
exceeds a threshold shear rate ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c, τb becomes smaller than the value τ0b at equilibrium and
c, τb becomes smaller than the value τ0b at equilibrium and ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) does not show such a decrease as when
does not show such a decrease as when ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ≲
≲ ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c. Comparing the alignment of micelles with that of polymers (i.e., chains without scission kinetics) has provided further evidence that flow-induced scission suppresses micellar alignment for fixed Nag (Fig. 8). To introduce the Weissenberg number Wi = τ
c. Comparing the alignment of micelles with that of polymers (i.e., chains without scission kinetics) has provided further evidence that flow-induced scission suppresses micellar alignment for fixed Nag (Fig. 8). To introduce the Weissenberg number Wi = τ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) defined as the product of the longest relaxation time τ and
defined as the product of the longest relaxation time τ and ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) , we have evaluated τ of micelles using the method proposed in our previous study.32 This method, which considers the effect of scission kinetics on micellar relaxation, has allowed us to compare the alignment of micelles and polymers appropriately. When Wi is smaller than the threshold Weissenberg number Wic = τ
, we have evaluated τ of micelles using the method proposed in our previous study.32 This method, which considers the effect of scission kinetics on micellar relaxation, has allowed us to compare the alignment of micelles and polymers appropriately. When Wi is smaller than the threshold Weissenberg number Wic = τ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c for flow-induced scission,
c for flow-induced scission, ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) of both micelles and polymers follows a single function of Wi. This collapse indicates the similar alignment behavior of micelles and polymers when flow-induced scission does not occur. In contrast,
of both micelles and polymers follows a single function of Wi. This collapse indicates the similar alignment behavior of micelles and polymers when flow-induced scission does not occur. In contrast, ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) of micelles is larger than that of polymers for Wi ≳ Wic. Such deviation of
of micelles is larger than that of polymers for Wi ≳ Wic. Such deviation of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) above Wic confirms that flow-induced scission suppresses micellar alignment for fixed Nag. Visualization also has shown that wormlike micelles tilt more from the flow direction than polymers for given Wi (≳Wic) (Fig. 9).
above Wic confirms that flow-induced scission suppresses micellar alignment for fixed Nag. Visualization also has shown that wormlike micelles tilt more from the flow direction than polymers for given Wi (≳Wic) (Fig. 9).
To explain the mechanism of the alignment suppression due to flow-induced scission, we have considered the effective longest relaxation time of micelles. Then we have demonstrated that the decrease in the effective longest relaxation time due to flow-induced scission plays a crucial role in suppressing micellar alignment. The concept of the micellar longest relaxation time (Fig. 7) predicts that the reduction of τb due to flow-induced scission decreases the effective longest relaxation time τΛ under shear flow. We have verified this prediction by evaluating Nag dependence of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) (Fig. 10). According to the concept, when Nag is larger than a certain aggregation number NΛ, micelles are characterized by a common longest relaxation time τΛ. Indeed, we have found that when
(Fig. 10). According to the concept, when Nag is larger than a certain aggregation number NΛ, micelles are characterized by a common longest relaxation time τΛ. Indeed, we have found that when ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) is smaller than
is smaller than ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c,
c, ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) is independent of Nag for Nag ≳ NΛ. In contrast, for
is independent of Nag for Nag ≳ NΛ. In contrast, for ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ≳
≳ ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) c, the plateau regime of
c, the plateau regime of ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) extends to a lower Nag as
extends to a lower Nag as ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) increases. This observation shows that flow-induced scission reduces NΛ and consequently reduces the effective Weissenberg number Wi = τΛ
increases. This observation shows that flow-induced scission reduces NΛ and consequently reduces the effective Weissenberg number Wi = τΛ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) of wormlike micelles with fixed Nag (≳NΛ) compared with the case without flow-induced scission, which is a major origin of the alignment suppression.
of wormlike micelles with fixed Nag (≳NΛ) compared with the case without flow-induced scission, which is a major origin of the alignment suppression.
In the present study, we have quantitatively shown that flow-induced scission suppresses micellar alignment even for fixed Nag and qualitatively explained the mechanism of this phenomenon through the decrease in the effective longest relaxation time under shear flow. However, we have yet to quantitatively explain the alignment behavior of micelles for Wi ≳ Wic, where flow-induced scission occurs. For example, it is not completely clear why ![[small phi variant, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d7.gif) exhibits the nonmonotonic dependence on Wi (Fig. 8). To explain the degree of alignment suppression, we first need to quantify the effective longest relaxation time. In addition, it is likely that we need to consider the direct coupling between the orientational dynamics and scission kinetics of individual micelles under shear flow. These are left for future studies.
exhibits the nonmonotonic dependence on Wi (Fig. 8). To explain the degree of alignment suppression, we first need to quantify the effective longest relaxation time. In addition, it is likely that we need to consider the direct coupling between the orientational dynamics and scission kinetics of individual micelles under shear flow. These are left for future studies.
Author contributions
Yusuke Koide: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing – original draft, writing – review & editing. Susumu Goto: conceptualization, funding acquisition, project administration, resources, supervision, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present study was supported in part by JSPS Grants-in-Aid for Scientific Research (20H02068 and 21J21061). The DPD simulations were mainly conducted under the auspices of the NIFS Collaboration Research Programs (NIFS20KNSS145). A part of the simulations was conducted using the JAXA Supercomputer System Generation 3 (JSS3).Notes and references
- C. M. White and M. G. Mungal, Annu. Rev. Fluid Mech., 2008, 40, 235–256 CrossRef.
- J. L. Zakin, B. Lu and H.-W. Bewersdorff, Rev. Chem. Eng., 1998, 14, 253–320 CAS.
- L. Warwaruk and S. Ghaemi, J. Fluid Mech., 2021, 917, A7 CrossRef CAS.
- S. A. Vanapalli, S. L. Ceccio and M. J. Solomon, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 16660–16665 CrossRef CAS PubMed.
- J. M. J. Den Toonder, A. A. Draad, G. D. C. Kuiken and F. T. M. Nieuwstadt, Appl. Sci. Res., 1995, 55, 63–82 CrossRef.
- A. S. Pereira, R. M. Andrade and E. J. Soares, J. Non-Newtonian Fluid Mech., 2013, 202, 72–87 CrossRef CAS.
- E. J. Soares, J. Non-Newtonian Fluid Mech., 2020, 276, 104225 CrossRef CAS.
- Z. Chu, C. A. Dreiss and Y. Feng, Chem. Soc. Rev., 2013, 42, 7174 RSC.
- S. Tamano, H. Ikarashi, Y. Morinishi and K. Taga, J. Non-Newtonian Fluid Mech., 2015, 215, 1–7 CrossRef CAS.
- B. Yu, F. Li and Y. Kawaguchi, Int. J. Heat Fluid Flow, 2004, 25, 961–974 CrossRef CAS.
- A. Krope and L. C. Lipus, Appl. Therm. Eng., 2010, 30, 833–838 CrossRef CAS.
- C. A. Dreiss, Soft Matter, 2007, 3, 956–970 RSC.
- L. Zhong, M. Oostrom, M. J. Truex, V. R. Vermeul and J. E. Szecsody, J. Hazard. Mater., 2013, 244–245, 160–170 CrossRef CAS PubMed.
- P. A. Cornwell, Int. J. Cosmet. Sci., 2018, 40, 16–30 CrossRef CAS PubMed.
- C. M. Schroeder, R. E. Teixeira, E. S. Shaqfeh and S. Chu, Phys. Rev. Lett., 2005, 95, 018301 CrossRef PubMed.
- C. M. Schroeder, R. E. Teixeira, E. S. G. Shaqfeh and S. Chu, Macromolecules, 2005, 38, 1967–1978 CrossRef CAS.
- C.-C. Huang, R. G. Winkler, G. Sutmann and G. Gompper, Macromolecules, 2010, 43, 10107–10116 CrossRef CAS.
- R. G. Winkler, J. Chem. Phys., 2010, 133, 164905 CrossRef PubMed.
- J. B. Hayter and J. Penfold, J. Phys. Chem., 1984, 88, 4589–4593 CrossRef CAS.
- V. Croce, T. Cosgrove, C. A. Dreiss, S. King, G. Maitland and T. Hughes, Langmuir, 2005, 21, 6762–6768 CrossRef CAS PubMed.
- S. Förster, M. Konrad and P. Lindner, Phys. Rev. Lett., 2005, 94, 017803 CrossRef PubMed.
- M. Takeda, T. Kusano, T. Matsunaga, H. Endo, M. Shibayama and T. Shikata, Langmuir, 2011, 27, 1731–1738 CrossRef CAS PubMed.
- C. R. López-Barrón, A. K. Gurnon, A. P. R. Eberle, L. Porcar and N. J. Wagner, Phys. Rev. E, 2014, 89, 042301 CrossRef PubMed.
- A. K. Gurnon, C. López-Barrón, M. J. Wasbrough, L. Porcar and N. J. Wagner, ACS Macro Lett., 2014, 3, 276–280 CrossRef CAS PubMed.
- B. Arenas-Gómez, C. Garza, Y. Liu and R. Castillo, J. Colloid Interface Sci., 2020, 560, 618–625 CrossRef PubMed.
- J. P. King, C. S. G. Butler, S. W. Prescott, A. V. Sokolova, L. de Campo, A. P. Williams and R. F. Tabor, Phys. Fluids, 2022, 34, 083104 CrossRef CAS.
- C.-C. Huang, H. Xu and J.-P. Ryckaert, Europhys. Lett., 2008, 81, 58002 CrossRef.
- C.-C. Huang, J.-P. Ryckaert and H. Xu, Phys. Rev. E, 2009, 79, 041501 CrossRef PubMed.
- J. Wei, Y. Kawaguchi, B. Yu, F. Li and C. Zhang, Nonlinear Dyn., 2010, 61, 503–515 CrossRef.
- A. Sambasivam, A. V. Sangwai and R. Sureshkumar, Phys. Rev. Lett., 2015, 114, 158302 CrossRef PubMed.
- W. Carl, R. Makhloufi and M. Kröger, J. Phys. II, 1997, 7, 931–946 CrossRef CAS.
- Y. Koide and S. Goto, J. Chem. Phys., 2022, 157, 084903 CrossRef CAS PubMed.
- M. Kröger and R. Makhloufi, Phys. Rev. E, 1996, 53, 2531 CrossRef PubMed.
- C.-C. Huang, H. Xu and J.-P. Ryckaert, J. Chem. Phys., 2006, 125, 094901 CrossRef PubMed.
- J. T. Padding, W. J. Briels, M. R. Stukan and E. S. Boek, Soft Matter, 2009, 5, 4367–4375 RSC.
- J. T. Padding, E. S. Boek and W. J. Briels, J. Chem. Phys., 2008, 129, 074903 CrossRef CAS PubMed.
- P. Español and P. Warren, Europhys. Lett., 1995, 30, 191 CrossRef.
- R. D. Groot and P. B. Warren, J. Chem. Phys., 1997, 107, 4423–4435 CrossRef CAS.
- K. Kremer and G. S. Grest, J. Chem. Phys., 1990, 92, 5057–5086 CrossRef CAS.
- W. Jiang, J. Huang, Y. Wang and M. Laradji, J. Chem. Phys., 2007, 126, 044901 CrossRef PubMed.
- A. W. Lees and S. F. Edwards, J. Phys. C: Solid State Phys., 1972, 5, 1921 CrossRef.
- D. J. Evans and G. Morriss, Statistical Mechanics of Nonequilibrium Liquids, Cambridge University Press, 2008 Search PubMed.
- A. Vishnyakov, M.-T. Lee and A. V. Neimark, J. Phys. Chem. Lett., 2013, 4, 797–802 CrossRef CAS PubMed.
- M.-T. Lee, R. Mao, A. Vishnyakov and A. V. Neimark, J. Phys. Chem. B, 2016, 120, 4980–4991 CrossRef CAS PubMed.
- N. I. Fisher, Statistical Analysis of Circular Data, Cambridge University Press, 1995 Search PubMed.
- C. Aust, M. Kröger and S. Hess, Macromolecules, 1999, 32, 5660–5672 CrossRef CAS.
- Y. Kobayashi, N. Arai and A. Nikoubashman, Soft Matter, 2020, 16, 476–486 RSC.
- E. L. Kaplan and P. Meier, J. Am. Stat. Assoc., 1958, 53, 457–481 CrossRef.
- R. J. Hommel and M. D. Graham, J. Non-Newtonian Fluid Mech., 2021, 295, 104606 CrossRef CAS.
- B. O'Shaughnessy and J. Yu, Phys. Rev. Lett., 1995, 74, 4329–4332 CrossRef PubMed.
- J. T. Padding and E. S. Boek, Europhys. Lett., 2004, 66, 756 CrossRef CAS.
- R. E. Teixeira, H. P. Babcock, E. S. G. Shaqfeh and S. Chu, Macromolecules, 2005, 38, 581–592 CrossRef CAS.
- M. Q. Tu, M. Lee, R. M. Robertson-Anderson and C. M. Schroeder, Macromolecules, 2020, 53, 9406–9419 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |