Open Access Article
Open Access ArticleThermoresponsive behavior of cyclodextrin inclusion complexes with weakly anionic alkyl ethoxy carboxylates†
Larissa
dos Santos Silva Araújo
 ab,
Giuseppe
Lazzara
ab,
Giuseppe
Lazzara
 a and
Leonardo
Chiappisi
a and
Leonardo
Chiappisi
 *b
*b
aDipartimento di Fisica e Chimica, Università degli Studi di Palermo, Viale delle Scienze pad 17, 90128, Palermo, Italy
bInstitut Max von Laue-Paul Langevin, 71 avenue des Martyrs, 38042, Grenoble, France. E-mail: chiappisil@ill.eu
First published on 27th January 2023
Abstract
This study investigates the temperature responsive behavior of inclusion complexes formed by weakly anionic alkyl ethoxy carboxylates and α (αCD) and β-cyclodextrins (βCD). Small-angle neutron scattering (SANS) was performed to probe the structural behaviour at the 1–100 nanometer scale of the hierarchical assemblies at different temperatures. The phase transitions and thermodynamics were systematically monitored as a function of the degree of ionization of the surfactant by differential scanning calorimetry (DSC). Herein, we investigate the effect of the surfactant degree of ionization on the thermoresponsive properties of the inclusion complex supramolecular assemblies. Inclusion complexes formed with the ionized surfactant spontaneously assemble into multilayered structures, which soften with increasing temperature. We also found that the presence of charges is not only required to impart order to the supramolecular assemblies, but also induced in-plane crystallization of the inclusion complexes. Finally, the use of a weakly anionic surfactant allows us to probe the interplay between the charge density and temperature on the assembly of surfactant-cyclodextrin inclusion complexes. This study helps to improve the design of multi-responsive supramolecular systems based on cyclodextrins.
1 Introduction
Cyclodextrins (CD) are cyclic oligosaccharides able to form inclusion complexes with various molecules. α-cyclodextrin (αCD), β-cyclodextrin (βCD) and γ-cyclodextrin (γCD) are the most commonly used types, containing six, seven and eight α-D-glucopyranose units, linked by α-1,4 glycoside bonds forming a truncated cone-shaped ring. The hydrophilic rims decorated with the primary hydroxyl groups and a hydrophobic cavity containing high-energy water provide these molecules with unique features which are at the origin of their very rich supramolecular assembly behavior.1 Because of their versatility, cyclodextrins are widely used as hosts to build functionalized supramolecular materials, with polymers,2–4 and surfactants.1,5,6Cyclodextrin–surfactant inclusion complexes systems exhibit particular physicochemical properties, remarkable versatility, and enriched structural behaviour arising from a delicate balance of repulsive interactions of electrostatic or steric origin between surfactants and hydrogen-bond mediated interactions between cyclodextrins.5,7–10 Non-covalent interactions are at the basis of the reversible and stimuli-responsive behaviour of cyclodextrin–surfactant systems. Moreover, chemical modifications of CDs and the large variety of surfactants available allow the experimentalist to integrate the system with the desired functionalities. In a reversible process, the assembly of inclusion complexes into highly-ordered structures relies on directional hydrogen bonding between adjacent CDs and CD–water interactions. Hydrogen bonding also plays a critical role in the balance of the electrostatic interactions and macro phase stability, therefore, also influencing the assemblies' morphologies.9,11
The knowledge of a system's thermodynamic and structural behaviour and its particular characteristics provide essential tools to regulate and modulate the interactions involved in the assembly.12 It can also provide essential information on the role of the guests and hosts and their characteristics' effect on the structure and responsiveness.
The complex interplay of interactions governing the supramolecular assembly of surfactant–CD inclusion complexes is a widely exploited strategy to design responsive materials relying on environmental conditions. For instance, inclusion complexes which respond to light,13–15 pH,16 redox potential,17–19 solvent exchanges20,21 or temperature22,23 have been prepared for the design of stimuli-responsive assembled materials.12,24
Because of the large effect on the solubility, molecular vibrational states and strength of interactions, particularly hydrogen bonds, temperature is one of the most common and efficient stimuli employed in controlling molecular conformation and self-assembly processes.24 Temperature-controlled phase transitions of highly ordered assemblies of mixtures of CD with dimethyl tetrammonium bromide,9 tetradecyl dimethylammonium propane sulfonate,25 sodium 6-(4-((4-(dimethylamino)phenyl)diazenyl)phenoxy)hexanoate,26 1-decyl-3-methylimidazolium bromide27 and sodium dodecyl sulphate (SDS)28,29 have been investigated before. For these mixtures, thermo-switchable phases of microtubes, lamellas, hydrogels and vesicles have been reported, and the micrococonfinement potential via a co-assembly process by SDS@2βCD has also been reported.30,31
In a recent study, we described the properties of inclusion complex formed by cyclodextrins with weakly anionic alkyl ether carboxylic acids (AEC).16 In particular, the possibility offered by the weakly anionic head group allowed us to probe the effect of surfactant charge density on the formation of inclusion complexes through the pH of the solution without any change in the chemistry of the system. Very interestingly, multilayered aggregates are formed irrespective of the surfactant charge. However, electrostatic repulsion is essential for the formation of well-defined, ordered structures.
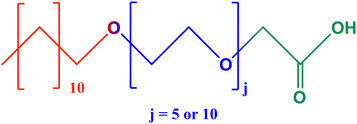
The aim of the present work is to investigate the effect of surfactant ionization on the thermoresponsiveness of the surfactant/CD supramolecular assemblies. We investigated the structural behaviour of the inclusion complexes assemblies between two alkyl ether carboxylic acids, the pentaoxyethylene dodecyl ether carboxylic acid (C12E5Ac) and the decaoxyethylene dodecyl ether carboxylic acid (C12E10Ac) (chemical structure in Fig. 1), with αCD and βCD by small-angle neutron scattering (SANS) over a wide temperature range of 15–70 °C. The temperature-induced phase transitions and thermodynamics were monitored by differential scanning calorimetry (DSC). We report the differences in the thermal responses of the structures resultant of the chemical architecture of surfactants, cyclodextrins, and the surfactant's degree of ionization, given as the sodium hydroxide and surfactant molar ratio (α = [NaOH]/[Stot]).
This study delivers a micro and nanometer perspective on the temperature responsiveness of the microstructure and CD–surfactant inclusion complexes' lattice assembly, opening the prospects on the guest role and evaluation of the host exchange in cyclodextrin complexes and also in the development of stimuli-responsive materials in the cosmetic, food, and pharmaceutical fields.
2 Experimental section
2.1 Materials
Pentaoxyethylene dodecyl ether carboxylic acid (C12E5Ac) and decaoxyethylene dodecyl ether carboxylic acid (C12E10Ac) are technical surfactants provided by KAO chemicals under the commercial names AKYPO RLM45CA (444 g mol−1, 92% purity) and AKYPO RLM100 (686 g mol−1, 90% purity), with 7.3 and 9.8 wt% water content, respectively.16 The hydrophobic part of the surfactants is a mixture of C12, C14 and C16 in approx. Ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25. The ethylene oxide units (EO) are Gaussian distributed over a mean of 4.6 and 9.4 for AKYPO RLM45CA and AKYPO RLM100, respectively, and a degree of carboxymethylation of 0.95 for both surfactants determined by NMR.32,33 αCD and βCD were acquired from TCI Europe. The water content of the cyclodextrins was determined from thermo-gravimetry and it is 10.1 for α and 11.5 wt% for βCD. Sodium hydroxide (Fluka, puriss) was used to adjust the pH of the solutions. The water content in the reagents was considered for sample preparation. All mentioned chemicals were used as received. All the solutions were prepared with D2O (D content > 99.8%) from Eurisotop (Gif-sur-Yvette, France).
0.25. The ethylene oxide units (EO) are Gaussian distributed over a mean of 4.6 and 9.4 for AKYPO RLM45CA and AKYPO RLM100, respectively, and a degree of carboxymethylation of 0.95 for both surfactants determined by NMR.32,33 αCD and βCD were acquired from TCI Europe. The water content of the cyclodextrins was determined from thermo-gravimetry and it is 10.1 for α and 11.5 wt% for βCD. Sodium hydroxide (Fluka, puriss) was used to adjust the pH of the solutions. The water content in the reagents was considered for sample preparation. All mentioned chemicals were used as received. All the solutions were prepared with D2O (D content > 99.8%) from Eurisotop (Gif-sur-Yvette, France).
2.2 Sample preparation
The samples were prepared by mixing aqueous solutions of the respective components, adding the cyclodextrin to the surfactant solution to obtain the desired concentrations in the samples at ambient conditions. Samples were heated for 30 minutes at 60 °C and left to stabilize for 24 hours.2.3 SANS
SANS patterns were recorded using 1 mm path quartz cells on D2234 at the Institut Laue-Langevin (Grenoble, France), with a two 3He detector setup, one fixed at 1.4 m sample-to-detector distance and, the second, used in two sample-to-detectors centre distance configurations of 5.6 and 17.6 m distance with corresponding collimation of 5.6 and 17.6 m, covering a total q-range of 0.03 to 6.5 nm−1, where q = 4π![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(θ/2)/λ. The samples were measured at temperatures 15, 25, 45 and 70 °C.
sin(θ/2)/λ. The samples were measured at temperatures 15, 25, 45 and 70 °C.
2.4 DSC
DSC measurements were performed with a sensitive multicell DSC (micro-DSC, TA instruments) under nitrogen flow in the range from 10 to 95 °C with scan rate of 1 °C min−1. The hastelloy sample cells of 1 cm3 contained approximately 500 mg of sample solution. The ΔH is expressed in J g−1 of surfactant. It is noteworthy that no signal was detected for the pure CD solutions, i.e., in the absence of surfactant. The analysis consisted in two heating and cooling cycles. The data were analysed using the freely available tool pyDSC.353 Results and discussion
3.1 SANS
In order to elucidate the microstructural aspects of the aggregates' responsiveness to changes in temperature, small-angle neutron scattering (SANS) measurements were performed. SANS curves of nonionized (α = 0) and ionized (α = 1) surfactant–CD systems at different temperatures are depicted in Fig. 2. We opted for showing in the main text both the intensity-scaled and non-scaled scattering curves: the former allows to highlight of structural features such as correlation peaks and slope changes, and the latter provides a comparison of the evolution of the scattering intensity, proportional to the mass of the aggregates. A further I(q)q2vs. q representation of the data is given in the ESI† (Fig. S3).Most scattering curves exhibit a q−2 power law, the characteristic signature of locally flat structures (Fig. 2. III and IV). The increase of the degree of ionization (α), e.g. increase in charge density, provides order to the multilayered structure but without affecting the overall shape and mass of the supramolecular aggregates, as evidenced by the presence of the peaks at mid-q in the SANS curves with a peak-to-peak ratio q1/q2 = 2 and by the fact that the data exhibit the same scattering intensity at low-q values. Depending on the molecular architecture of the host and guest, the typical spacing of the CD–surfactant layers is 10–15 nm. A table summarizing the different characteristic distances observed in the systems is given in the ESI.† It is important to note that the pure CD solutions are featureless at the given experimental conditions and that the surfactant assembles into simple vesicles (C12E5Ac) and globular micelles (C12E10Ac) (see ref. 32 and Fig. S2 in the ESI†).
At α = 0, no particular effect of the temperature was observed, except for C12E5Ac-αCD. For this system, the SANS patterns at 15, 25, and 45 °C are equivalent, exhibiting an extended q−2 power law at mid q, a decay of the scattering intensity at ∼0.6 nm−1. Such scattering patterns, which closely resemble that of the aqueous solutions of the surfactant,32 indicate the presence of CD-decorated vesicles in the solution. At 70 °C, a q−4 power-law at low-q values is observed, and a pronounced correlation peak appears at q = 0.98 nm−1 corresponding to a characteristic size of 6.4 nm. This value corresponds to the length of the surfactant chain, and a correlation peak at q ∼ 1 nm−1 is found in several dense-packed C12E5Ac systems.36–38 Moreover, C12E5Ac was shown to undergo liquid-liquid phase separation around 55 °C.32 Accordingly, the presence of αCD does not prevent phase separation of the surfactant from the solution. Interestingly, upon the addition of βCD, no temperature-induced structural changes are observed by raising the sample temperature up to 70 °C.
Temperature effects are more marked at α = 1, where increasing the temperature from 15 to 45 °C causes the correlation peaks – characteristic of the multilayer structure – to broaden. The broadening of the correlation peak can be ascribed either to a reduction of number of layers in the supramolecular aggregate, or by reducing the long-range order, i.e., by increasing the flexibility of the layer. Both interprepations are consistent with the recorded SANS data. However, a weakening of the later, CD–CD hydrogen bonds which are at the origin of the high rigidity of the inclusion complex layer, is better supported by the endothermic peak observed by differential scanning calorimetry (see next section). Further heating the C12E10Ac-αCD and C12E5Ac-βCD complexes at 70 °C, causes the peaks characteristic of the multilayered structure to fully disappear, and a strong single peak appears at q ≈ 0.36 and 0.44 nm−1, respectively. Such changes in the scattering pattern are consistent with a transition from multilayered aggregates to unilamellar ones, such as disks or vesicles, as found in similar systems.28,29 In detail, while the two structure peaks at temperatures ≤45 °C arise from intra-aggregate correlations, the peak found at 70 °C arises from a typical inter-particle distance of 18 and 14 nm. Similarly, inclusion complexes between sodium dodecyl sulfate and βCD show a structural phase transition from tubular structures to vesicles between 40 and 43 °C.28,29 The values of the repeating distances found in the investigated complexes are summarized in Table S1 in the ESI.†
Generally, no changes are observed in the CD packing upon the temperature increase for βCD. However, the temperature effect on the CD–CD and CD–water interactions in the lattice assembly can be observed in the high q region for C12E10Ac-αCD systems, at α = 0 and α = 1, and C12E5Ac-αCD ionized systems. For C12E5Ac-αCD, the singular peak observed at q = 4.43 nm−1 at low temperatures diverges in two peaks at 3.6 and 5.3 nm−1 as the temperature increase indicates changes in the packing lattice. Interestingly, the positions of the peaks at 70 °C are equivalent to the scattering profile observed for the respective nonionic system. Similar transitions are observed for C12E10Ac systems with the same CD at α = 0 and α = 1.
The structural effect of the different ethylene oxide units and the different behaviour upon thermal stimulus is evidenced at the high q region of α = 0 with α-CD of both surfactants in Fig. 2I-a and II-a. Whereas in Fig. 2I-a the scattering curves point to no temperature responsive of the surfactant with smaller number of EO and similar profile for all the temperatures, in Fig. 2II-a, it is possible to observe the lattice phase transition evidenced by the changes in the CD–CD organization.
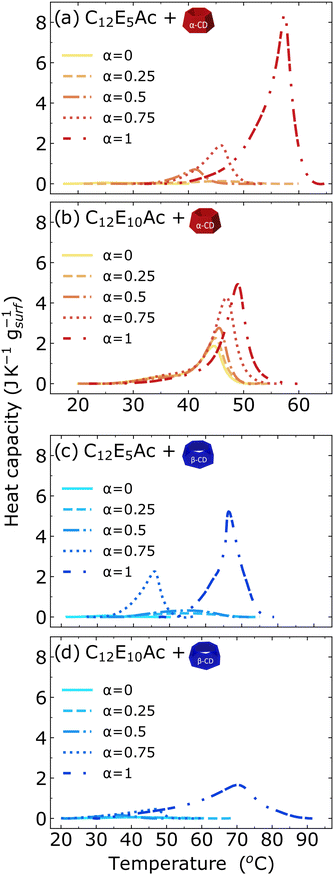
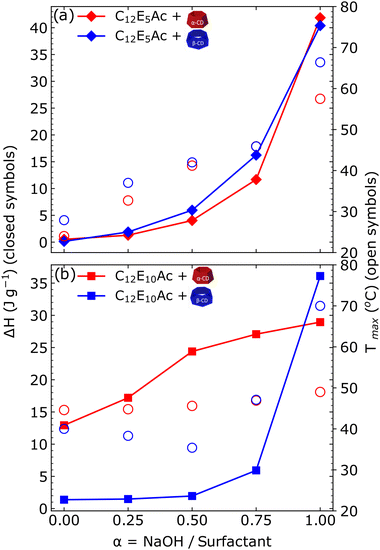
3.2 Differential scanning calorimetry
Calorimetric methods are well-established techniques to characterize thermodynamically supramolecular structures of surfactants, polymers, inclusion complexes and self-assembled structures. DSC allows to characterize the enthalpic change associated with the structural reorganization observed by small-angle scattering experiments. The DSC thermograms of the different mixtures are shown in Fig. 3, and the obtained enthalpic changes (ΔH) and temperature of the melting peak are given in Fig. 4.The general trend in the systems is the increase of ΔH with the increase of the surfactants' degree of ionization α. This observation well correlates with the increased crystallinity previously found in self-assembled supramolecular complexes of CD and alkyl ether carboxylic acids with increasing degree of ionization.16 The values of ΔH are very similar for all mixtures, varying from a basically undetectable transition at low degree of ionization to a value of ΔH of 40 J mol−1 of surfactant. An exception is made by the complex formed between C12E10Ac and αCD, which shows a significant endothermic peak also for the surfactant in the nonionic state. The temperature of the transition increases for mixtures of C12E5Ac with increasing degree of ionization, while an almost constant value of ≈40 °C is found for C12E10Ac. The increasing melting temperature observed indicates a higher crystal stability with increasing degree of ionization. The fact that this phenomenon is observed for the surfactant with the shorter ethylene oxide spacer only, points towards the importance of the location of the charge in the crystallization of surfactant/CD inclusion complexes.
A further interesting point is given by the fact that the thermograms of αCD complexes show the same onset, except for the mixture at a full degree of ionization. This indicates that for a given surfactant/CD inclusion complex, increasing the degree of ionization causes the amount of structure which are able to melt to increase, but not the energy of the crystal.
Taking into account the results from the SANS analysis, it can be stated that the endothermic peak probed by DSC is correlated with structural changes on the 10–20 nm scale. For all βCD inclusions complexes and the complex of αCD with the non-ionized C12E5Ac, no changes in the CD–CD packing, probed in the high-q region of the scattering pattern could be observed.
Our results agree well with similar studies performed on SDS@2βCD mixtures,28 where similar values for the melting enthalpy and a melting temperature of approx. 40 °C were found.
4 Conclusions
In summary, we have studied the thermoresponsive behaviour of the hierarchical assemblies of α and β-cyclodextrins and weakly anionic alkyl oligoethylene oxide carboxylic acids. The presence of a weakly acidic surfactant head group allowed us to probe the effect of the surfactant degree of ionization on the thermoresponsive properties of the inclusion complex supramolecular assemblies. This represents an important advantage with respect to previous studies performed either with strongly ionic or nonionic surfactants.9,25,27–29Small-angle neutron scattering experiments indicate that the presence of electrostatic repulsion is required for the formation of well-defined, ordered supramolecular structures. Increasing the temperature up to 70 °C softens the structures, providing a loss of internal order. The structural data were complemented with differential scanning calorimetry, which allowed us to probe the effect of the surfactant's degree of ionization on the inclusion complex crystal stability. In particular, the experiments evidenced the importance of the presence of charges to induce the crystallization of the layered inclusion complexes. In our previous investigation on the effect of surfactant charge density on the structure of the supramolecular assemblies,16 we pointed out that a certain degree of ionization of the surfactant is required for the formation of ordered aggregates. We rationalized the finding in terms of electrostatic repulsion between the layers, which promotes the transition from a disordered multilayered structure to a more ordered one. The novel results presented herein point towards a more complex situation. Not only the presence of charges provides electrostatic repulsion to the bilayers, but it induces crystallization of the same, thus, very likely, increasing the membrane rigidity.
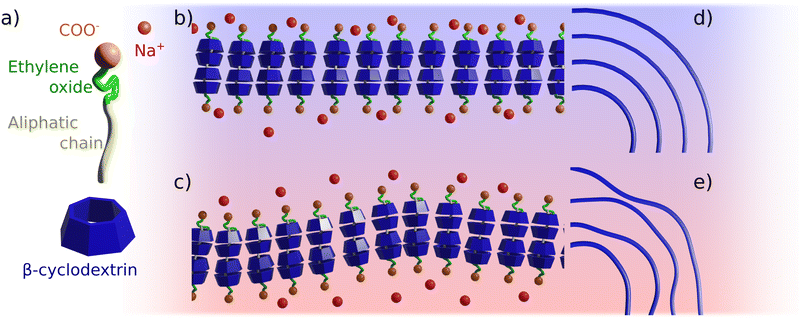
Temperature might affect the system in many different ways, e.g., by changing the solubility of the cyclodextrins and of the inclusion complexes, it causes a partial dehydration of the surfactant head group, it may cause unthreading events, and, finally, it affects the CD/CD in-plane interactions. It is useful to make some consideration to contextualize the relative importance of the different phenomena. No changes in solubility was detected by visual inspection of the samples, and the fact that the scattering intensity at low-q remains unchanged, allow us to rule out that the observed effects arise from solubility changes in the system and from unthreading events. This observation is in agreement with thermodynamic studies, which show that the equilibrium constant for the formation of CD–surfactant inclusion complexes – albeit decreasing with increasing temperature – remains significant with Keq ≫ 104 M−1.39,40,41 At the given experimental conditions, assuming a binding constant of 104 M−1, only 0.2 mol% of the surfactant would be unthreaded. Furthermore, increasing the temperature causes the oligo-ethylene oxide units of the surfactant to dehydrate, at least at a low degree of ionization. However, the head group area at low temperature is 58 and 86 Å2 at low pH and 123 and 130 Å2 at high pH, for C12E5Ac and C12E10Ac, respectively.32 The area occupied by α and βCD is 181 and 243 Å2, respectively. Accordingly, we do not expect any effect of temperature on the geometry of the inclusion complexes, since the packing is determined by the size of the cyclodextrins. Finally, the most likely event to occur is the weakening of the hydrogen bonds between adjacent cyclodextrin molecules, with their partial replacement with CD solvent ones. These bonds represents the weakest interactions in the system, thus the first ones who can be disrupted by increasing the systems' temperature. The weakening of these later, CD–CD bonds is also in agreement with the observed DSC and SANS results. A schematic representation of this phenomenon is given in Fig. 5.
To conclude, cyclodextrin/surfactant complexes have been shown to be extremely versatile systems, owing to the broad variety of stimuli they can respond to. Accordingly, they are excellent candidates for the design of multi-responsive systems. Recent studies have been focused on the characterization of CD inclusion complexes which respond to more than one trigger.25,26,42 Hence, alkyl oligoethylene oxide carboxylic acids-cyclodextrin complexes and their multiple responsiveness demonstrate the importance of understanding the interplay of interactions and the thermal and ionic effects on the formation and morphology of supramolecular assemblies of cyclodextrin/surfactant complexes. It also reinforces the importance of unveiling fundamental properties and dynamics aiming for the smart use of guests in developing building blocks that can further assemble to finally be able to direct them to the suitable application.
Author contributions
LSSA prepared samples, performed experiments, and wrote the manuscript. GL and LC reviewed the data critically and edited the manuscript. LSSA and LC conceived the experiments. GL and LC contributed to funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. S. S. A. is grateful to the ILL and the University of Palermo for a doctoral fellowship through the ILL PhD program. The ILL is acknowledged for providing beamtime on the D22 SANS instruments (data available at https://doi.ill.fr/10.5291/ILL-DATA.INTER-532), and the support of Lionel Porcar during the SANS experiments is heartily acknowledged. The partnership for soft condensed matter (PSCM) is acknowledged for providing the calorimeter and the laboratory infrastructure for sample preparation and pre-characterization.Notes and references
- A. J. Valente and O. Söderman, The formation of host-guest complexes between surfactants and cyclodextrins, Adv. Colloid Interface Sci., 2014, 205, 156–176 CrossRef CAS PubMed.
- R. De Lisi, G. Lazzara and S. Milioto, Temperature-controlled poly(propylene) glycol hydrophobicity on the formation of inclusion complexes with modified cyclodextrins. A DSC and ITC study, Phys. Chem. Chem. Phys., 2011, 13, 12571–12577 RSC.
- A. Harada, Y. Takashima and M. Nakahata, Supramolecular Polymeric Materials via Cyclodextrin-Guest Interactions, Acc. Chem. Res., 2014, 47, 2128–2140 CrossRef CAS PubMed.
- J. Wankar, N. G. Kotla, S. Gera, S. Rasala, A. Pandit and Y. A. Rochev, Recent Advances in Host-Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering, Adv. Funct. Mater., 2020, 30, 1909049 CrossRef CAS.
- L. dos Santos Silva Araújo, G. Lazzara and L. Chiappisi, Cyclodextrin/surfactant inclusion complexes: An integrated view of their thermodynamic and structural properties, Adv. Colloid Interface Sci., 2021, 289, 1–12 CrossRef PubMed.
- S. Yang, Y. Yan, J. Huang, A. V. Petukhov, L. M. J. Kroon-Batenburg, M. Drechsler, C. Zhou, M. Tu, S. Granick and L. Jiang, Giant capsids from lattice self-assembly of cyclodextrin complexes, Nat. Commun., 2017, 8, 15856 CrossRef CAS PubMed.
- W. Saenger and T. Steiner, Cyclodextrin Inclusion Complexes: Host-Guest Interactions and Hydrogen-Bonding Networks, Acta Crystallogr., Sect. A: Found. Crystallogr., 1998, A54, 798–805 CrossRef CAS.
- L. Liu and Q. X. Guo, The driving forces in the inclusion complexation of cyclodextrins, J. Inclusion Phenom., 2002, 42, 1–14 CrossRef CAS.
- J. Carlstedt, A. Bilalov, E. Krivtsova, U. Olsson and B. Lindman, Cyclodextrin–surfactant coassembly depends on the cyclodextrin ability to crystallize, Langmuir, 2012, 28, 2387–2394 CrossRef CAS PubMed.
- J. Landman, S. Ouhajji, S. Prévost, T. Narayanan, J. Groenewold, A. P. Philipse, W. K. Kegel and A. V. Petukhov, Inward growth by nucleation: Multiscale self-assembly of ordered membranes, Sci. Adv., 2018, 4, 1–8 Search PubMed.
- H. Wang, J. C. Wagner, W. Chen, C. Wang and W. Xiong, Spatially dependent H-bond dynamics at interfaces of water/biomimetic self-assembled lattice materials, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 23385–23392 CrossRef CAS PubMed.
- K. Liu, C. Ma, T. Wu, W. Qi, Y. Yan and J. Huang, Recent advances in assemblies of cyclodextrins and amphiphiles: construction and regulation, Curr. Opin. Colloid Interface Sci., 2020, 45, 44–56 CrossRef CAS.
- Y. Wang, N. Ma, Z. Wang and X. Zhang, Photocontrolled reversible supramolecular assemblies of an azobenzene-containing surfactant with α-cyclodextrin, Angew. Chem., Int. Ed., 2007, 46, 2823–2826 CrossRef CAS PubMed.
- P. Xing, H. Chen, M. Ma, X. Xu, A. Hao and Y. Zhao, Light and cucurbit[7]uril complexation dual-responsiveness of a cyanostilbene-based self-assembled system, Nanoscale, 2016, 8, 1892–1896 RSC.
- X. Zhang, X. Ma, K. Wang, S. Lin, S. Zhu, Y. Dai and F. Xia, Recent Advances in Cyclodextrin-Based Light-Responsive Supramolecular Systems, Macromol. Rapid Commun., 2018, 39, 1–12 Search PubMed.
- L. dos Santos Silva Araújo, L. Watson, D. A. Traore, G. Lazzara and L. Chiappisi, Hierarchical assembly of pH-responsive surfactant-cyclodextrin complexes, Soft Matter, 2022, 18, 6529–6537 RSC.
- B. Jiang, H. Guo, L. Zhao, B. Xu, C. Wang, C. Liu and H. Fan, Fabrication of a β-cyclodextrin-based self-assembly containing a redox-responsive ferrocene, Soft Matter, 2019, 16, 125–131 RSC.
- C. Zuo, X. Dai, S. Zhao, X. Liu, S. Ding, L. Ma, M. Liu and H. Wei, Fabrication of dual-redox responsive supramolecular copolymers using a reducible β -cyclodextran-ferrocene double-head unit, ACS Macro Lett., 2016, 5, 873–878 CrossRef CAS PubMed.
- H. Zhang, W. An, Z. Liu, A. Hao, J. Hao, J. Shen, X. Zhao, H. Sun and L. Sun, Redox-responsive vesicles prepared from supramolecular cyclodextrin amphiphiles, Carbohydr. Res., 2010, 345, 87–96 CrossRef CAS PubMed.
- C. Zhou, X. Cheng, Q. Zhao, Y. Yan, J. Wang and J. Huang, Self-assembly of nonionic surfactant tween 20@2β-CD inclusion complexes in dilute solution, Langmuir, 2013, 29, 13175–13182 CrossRef CAS PubMed.
- A. A. Rafati and F. Safatian, Thermodynamic studies of inclusion complex between cetyltrimethylammonium bromide (CTAB) and β-cyclodextrin (β -CD) in water/n-butanol mixture, using potentiometric technique, Phys. Chem. Liq., 2008, 46, 587–598 CrossRef CAS.
- C. Perry, P. Hébraud, V. Gernigon, C. Brochon, A. Lapp, P. Lindner and G. Schlatter, Pluronic and β-cyclodextrin in water: From swollen micelles to self-assembled crystalline platelets, Soft Matter, 2011, 7, 3502–3512 RSC.
- G. Lazzara, G. Olofsson, V. Alfredsson, K. Zhu, B. Nyström and K. Schillén, Temperature-responsive inclusion complex of cationic PNIPAAM diblock copolymer and γ -cyclodextrin, Soft Matter, 2012, 8, 5043–5054 RSC.
- A. Wang, W. Shi, J. Huang and Y. Yan, Adaptive soft molecular self-assemblies, Soft Matter, 2016, 12, 337–357 RSC.
- L. Jiang, Y. Yan and J. Huang, Zwitterionic surfactant/cyclodextrin hydrogel: microtubes and multiple responses, Soft Matter, 2011, 7, 10417 RSC.
- J. Wang, Q. Li, S. Yi and X. Chen, Visible-light/temperature dual-responsive hydrogel constructed by α-cyclodextrin and an azobenzene linked surfactant, Soft Matter, 2017, 13, 6490–6498 RSC.
- J. Zhang and X. Shen, Temperature-induced reversible transition between vesicle and supramolecular hydrogel in the aqueous ionic liquid-β-cyclodextrin system, J. Phys. Chem. B, 2013, 117, 1451–1457 CrossRef CAS PubMed.
- C. Zhou, X. Cheng, Y. Yan, J. Wang and J. Huang, Reversible transition between SDS@2β -CD microtubes and vesicles triggered by temperature, Langmuir, 2014, 30, 3381–3386 CrossRef CAS PubMed.
- S. Ouhajji, J. Landman, S. Prévost, L. Jiang, A. P. Philipse and A. V. Petukhov, In situ observation of self-assembly of sugars and surfactants from nanometres to microns, Soft Matter, 2017, 13, 2421–2425 RSC.
- J. W. De Folter, P. Liu, L. Jiang, A. Kuijk, H. E. Bakker, A. Imhof, A. Van Blaaderen, J. Huang, W. K. Kegel, A. P. Philipse and A. V. Petukhov, Self-organization of anisotropic and binary colloids in thermo-switchable 1D microconfinement, Part. Part. Syst. Charact., 2015, 32, 313–320 CrossRef CAS.
- L. Jiang, J. W. De Folter, J. Huang, A. P. Philipse, W. K. Kegel and A. V. Petukhov, Helical colloidal sphere structures through thermo-reversible co-assembly with molecular microtubes, Angew. Chem., Int. Ed., 2013, 52, 3364–3368 CrossRef CAS PubMed.
- L. Chiappisi, Polyoxyethylene alkyl ether carboxylic acids: An overview of a neglected class of surfactants with multiresponsive properties, Adv. Colloid Interface Sci., 2017, 250, 79–94 CrossRef CAS PubMed.
- D. W. Hayward, L. Chiappisi, J. H. Teo, S. Prévost, R. Schweins and M. Gradzielski, Neutralisation rate controls the self-assembly of pH-sensitive surfactants, Soft Matter, 2019, 15, 8611–8620 RSC.
- I. Laue-Langevin, D22-Large dynamic range small-angle diffractometer (Institut Laue-Langevin), 2003, https://www.ill.eu/users/instruments/instruments-list/d22/description/instrument-layout.
- A. Cisse, J. Peters, G. Lazzara and L. Chiappisi, PyDSC: a simple tool to treat differential scanning calorimetry data, J. Therm. Anal. Calorim., 2021, 145, 403–409 CrossRef CAS.
- L. Chiappisi, I. Hoffmann and M. Gradzielski, Membrane stiffening in Chitosan mediated multilamellar vesicles of alkyl ether carboxylates, J. Colloid Interface Sci., 2022, 627, 160–167 CrossRef CAS PubMed.
- L. Chiappisi, S. Prévost, I. Grillo and M. Gradzielski, From crab shells to smart systems: Chitosan-alkylethoxy carboxylate complexes, Langmuir, 2014, 30, 10615–10616 Search PubMed.
- S. Micciulla, D. W. Hayward, Y. Gerelli, A. Panzarella, R. von Klitzing, M. Gradzielski and L. Chiappisi, One-step procedure for the preparation of functional polysaccharide/fatty acid multilayered coatings, Commun. Chem., 2019, 2, 1–12 CrossRef CAS.
- M. Benko and Z. Király, Thermodynamics of inclusion complex formation of β-cyclodextrin with a variety of surfactants differing in the nature of headgroup, J. Chem. Thermodyn., 2012, 54, 211–216 CrossRef CAS.
- R. Lu, J. Hao, H. Wang and L. Tong, Determination of association constants for cyclodextrin–surfactant inclusion complexes: A numerical method based on surface tension measurements, J. Colloid Interface Sci., 1997, 192, 37–42 CrossRef CAS PubMed.
- P. Brocos, X. Banquy, N. Díaz-Vergara, S. Pérez-Casas, Á. Piñeiro and M. Costas, A critical approach to the thermodynamic characterization of inclusion complexes: Multiple-temperature isothermal titration calorimetric studies of native cyclodextrins with sodium dodecyl sulfate, J. Phys. Chem. B, 2011, 115, 14381–14396 CrossRef CAS PubMed.
- J. Shen, J. Pang, T. Kalwarczyk, R. Hołyst, X. Xin, G. Xu, X. Luan and Y. Yang, Manipulation of multiple-responsive fluorescent supramolecular materials based on the inclusion complexation of cyclodextrins with Tyloxapol, J. Mater. Chem. C, 2015, 3, 8104–8113 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sm01621d |
| This journal is © The Royal Society of Chemistry 2023 |