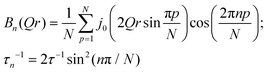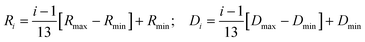Lateral diffusion of lipids in the DMPG membrane across the anomalous melting regime: effects of NaCl†
V. K.
Sharma
 *ab,
J.
Gupta
ab and
E.
Mamontov
*ab,
J.
Gupta
ab and
E.
Mamontov
 c
c
aSolid State Physics Division, Bhabha Atomic Research Centre, Mumbai 400085, India. E-mail: sharmavk@barc.gov.in; vksphy@gmail.com; Tel: +91-22-25594604
bHomi Bhabha National Institute, Anushaktinagar, Mumbai 400094, India
cNeutron Scattering Division, Oak Ridge National Laboratory, Oak Ridge, Tennessee 37830, USA
First published on 23rd November 2022
Abstract
The anionic dimyristoyl phosphatidylglycerol (DMPG) membrane in solvents with a low ionic strength is known to exhibit an unusually wide melting regime between the gel and fluid phase characterized by various anomalous macroscopic characteristics, such as low turbidity and high electrical conductivity and viscosity. A recent neutron spin echo study [Kelley, E. G. et al., Struct. Dyn., 7 (2020) 054704] revealed that during the extended melting phase transition the DMPG membrane becomes softer and exhibits faster collective bending fluctuation compared to the higher temperature fluid phase. In contrast, in the present work, using incoherent quasielastic neutron scattering through the anomalous phase transition regime we find that single-particle lateral and internal lipid motions in the DMPG membrane show regular temperature dependence, with no enhanced dynamics evident in the anomalous melting regime. Further, we find that incorporation of NaCl in DMPG suppresses the anomalous extended melting regime, concurrently enhancing the single-particle lipid dynamics, both the lateral diffusivity and (to a lesser extent) the internal lipid motion. This seems rather counterintuitive and in variance with the dynamic suppression effect exerted by a salt on a zwitterionic membrane. However, since incorporation of a salt in anionic DMPG leads to enhanced cooperativity, the disrupted cooperativity in the salt-free DMPG is associated with the baseline lipid dynamics that is suppressed to begin with, whereas addition of salt partially restores the cooperativity, thus enhancing lipid dynamics compared to the salt-free baseline DMPG membrane state. These results provide new insights into the ion-membrane interaction and divulge a correlation between microscopic dynamics and the structure of the lipid bilayer.
Introduction
The basic matrix of the cell membrane is a few nanometre thick lipid bilayer that is highly dynamic and exhibits a rich phase behaviour. The physics associated with the phase transitions of lipid membranes is incredibly interesting and is of biological relevance.1,2 Generally, these membranes undergo a main phase transition from a low temperature ordered phase to a high temperature fluid phase. This main phase transition strongly depends on the molecular structure of the phospholipid, viz., acyl chain length, degree of unsaturation, and structure and polarity of the head group.1,2 Usually, zwitterionic phospholipids, e.g., phosphatidylcholine (PC), show the main phase transition that is highly cooperative and occurs at a well-defined temperature, Tm. However, for the charged lipids, e.g., phosphatidylglycerol (PG) and phosphatidylserine (PS), the transition is found to be rather complex and dependent strongly on the pH of the medium, lipid concentration, and salt concentration.3–6 PG is the most abundant anionic phospholipid present in prokaryotic cell membranes and has been extensively studied as a model for negatively charged membranes. The phase behaviour of PG lipids is particularly unusual in buffers with low ionic strength. The melting transition of PG with saturated hydrocarbon chains up to 15 carbon atoms exhibits broadening with decreasing tail length but is highly cooperative for tail lengths of 16 carbon atoms (dipalmitoylphosphatidylglycerol, DPPG) at low ionic strength of the surrounding medium.7 In particular, dimyristoylphosphatidylglycerol (DMPG) has been widely investigated7–14 due to its anomalous broad transition regime between the gel and fluid phase called an intermediate phase, which is, unusually, also associated with the change in the macroscopic properties of the lipid dispersion. This intermediate phase showed various interesting properties such as low turbidity, high viscosity, high conductivity, and so on.8–10 This is in contrast with zwitterionic phospholipids, where no such significant change in the macroscopic properties is observed during the phase transition. The non-intuitive behaviour of DMPG dispersion has sparked decades of research into the nanoscale alterations in the membrane that may affect the macroscopic properties of the solution.7–9,11,13,15–18 Various biophysical methods have been used to decipher the correlation between the changes in the macroscopic properties and the change in the ordering of the lipids at the nanoscale. Initial studies suggested that DMPG formed an extended three-dimensional bilayer network or a continuous sponge-like structure, referred to as the aggregation model, in the intermediate phase.7,8 However, subsequent studies did not support these structures and models and revealed that, in the melting regime, DMPG forms highly perforated porous vesicles showing a pore size of ∼15 nm, which is responsible for the decrease in the optical contrast.4,9,16,18,19 A correlation peak is observed in small angle X-ray scattering (SAXS) data16 at low Q, which is related with the in-plane correlations between the pores. These pores are speculated to be stabilized due to a change in the spontaneous curvature of the membrane.12 It has also been hypothesized that increased area fluctuations associated with lipid melting also contribute to stabilization of the pores. High viscosity in the intermediate region has been explained based on the increase in the size and ionization of the vesicles.12,18,19 Recent neutron spin echo measurements20 have shown that during the phase transition membranes are softer and more dynamic compared to the higher temperature fluid phase. This is a very interesting direct observation on the enhanced bending fluctuation of the membrane during the melting regime. However, there are scarcely any reports on the lateral and internal motions of the DMPG during this melting regime. It is of interest to observe the effects of the perforated structure of the membrane on its dynamics. This type of system (triggering the formation of pores at a selected temperature) could be very useful for biotechnological applications such as drug delivery.As discussed above, at low ionic strength and high pH (>6), DMPG shows a very broad main phase transition associated with various anomalous macroscopic properties, which are mainly due to the electrostatic repulsion between charged PG groups, and it should be highly sensitive to changes in pH and salt concentration. It has been shown5,6 that for the concentrated DMPG vesicle solution (≥1 mM) there is no significant effect of changes in pH above 6. When the pH is decreased below 6, the intermediate regime starts to vanish, and the transition temperature increases as expected due to association of protons with the phosphate head group.5,6 Even at high pH (>6), in the presence of 100 mM NaCl, the main phase transition of DMPG becomes strongly cooperative, and a sharp peak is observed at 297 K, which is similar in appearance to that of the zwitterionic dimyristoyl phosphatidylcholine (DMPC) with the same alkyl tails as DMPG.4,18 The disappearance of the broad melting regime at high ionic strength or low pH indicates that the intermediate phase is related to the presence of charged head groups or high surface potential, which was independently confirmed by the conductivity measurements.18 The presence of 100 mM NaCl also eliminates the anomalous behaviour of the macroscopic properties (e.g., turbidity, viscosity, etc.). It is of interest to investigate the effects of NaCl on the dynamics of the DMPG membrane. Besides the peculiar phase behaviour, there is an upsurge of interest in the effects of salt on lipid bilayer systems, as cell membranes are surrounded by an aqueous buffer containing various ions including Na+, K+, and Cl−. Interactions between these ions and the lipid membrane play an important role in various physiological processes, including membrane fusion and transport across the membrane. Hence, it is not surprising to find many studies focusing on the effects of salt on the biophysical properties of the lipid membrane using various experimental as well as computational techniques.21–27 It is, however, interesting to note that, despite such extensive investigations, the effects of salts on the dynamics of the lipid membrane remain controversial even at present. For example, quasielastic neutron scattering (QENS),28 as well as fluorescence correlation spectroscopy (FCS) experiment with molecular dynamics (MD) simulation,22 has shown that addition of salt restricts the dynamics of the zwitterionic PC lipid bilayer. However, in contrast, nuclear magnetic resonance (NMR) experiments29 revealed no effects on the dynamics of the zwitterionic membrane in the presence of salt. They have also shown that in comparison to the zwitterionic membrane, addition of salt has strong effects on the dynamics of the anionic lipid membrane.29 For the anionic DOPG membrane, a decrease in the lateral diffusion coefficient was observed due to the addition of salt, which was explained based on the decrease in the free area per lipid molecule.29
In this work, we have used incoherent elastic and quasielastic neutron scattering to investigate the dynamics of the DMPG membrane across the broad peculiar melting regime. Our measurements showed no anomalous temperature dependence on the lateral and internal motion of the individual lipid. This is in contrast with the recent observation on the collective bending fluctuation of the lipid membrane, which showed that membranes are softer and more dynamic during the phase transition than in the fluid phase. We have also investigated the effects of incorporation of 100 mM NaCl on the membrane dynamics. Our measurements suggest that addition of NaCl enhances both lateral and internal motions. This is in contrast with the effects of salt on the dynamics of the zwitterionic membrane, which is generally found to restrict the lateral and internal motions of the membranes.22,28 This is also in contrast with the NMR investigation of the effects of salts on the lateral diffusion of the anionic DOPG membrane.29
Materials and methods
Materials
DMPG lipid (powder) and DMPC lipid (powder) were procured from Avanti Polar Lipids (Alabaster, AL). Sodium chloride and D2O (99.9%) were purchased from Sigma Aldrich (St. Louis, MO) and Cambridge Isotope Laboratories (Andover, MA), respectively.Preparation of unilamellar vesicles
Using the small angle scattering technique, Riske et al. have shown that an aqueous dispersion of anionic DMPG lipid generally forms unilamellar vesicles (ULVs).9 The extrusion method is a well-established and most efficient method to prepare ULVs with a narrow size distribution.30,31 We have prepared ULVs of DMPG using the extrusion method as discussed elsewhere,32–34 and the unilamellar structure of the prepared vesicles has been confirmed using small angle neutron scattering (Fig. S2, ESI†). In the extrusion method, the DMPG powder was taken in a glass vial and dissolved in chloroform. The chloroform was first evaporated using a dry gentle nitrogen stream, and then the sample was kept under vacuum for about 24 h to remove all traces of the organic solvent. This results in dry lipid films on the glass vial. We have prepared two sets of dry DMPG films on the glass vials. One of them was suspended in pure D2O, whereas the other was hydrated by 100 mM NaCl D2O at 320 K. Both lipid suspensions were vortexed for about 2 minutes and then subjected to 3 freeze–thaw cycles. 7 (w/w) % samples of DMPG in D2O and DMPG in 100 mM NaCl D2O ULVs were prepared by an extrusion method, in which the lipid suspension went through a mini-extruder (from Avanti Polar Lipids) with a porous polycarbonate membrane (pore diameter ∼100 nm) more than 21 times. During the extrusion, the lipid suspension was kept at 330 K. The same protocol was used to prepare DMPC ULVs in D2O.Differential scanning calorimetry
Differential scanning calorimetry (DSC) experiments were carried out on DMPG vesicles with and without NaCl using a NANO DSC Series III System with a Platinum Capillary Cell (TA Instruments). DSC data were collected for both the samples in the temperature range from 280 to 315 K. The scan rate for the cooling cycle was 0.5 K min−1. For reference, DSC measurements were also carried out on DMPC ULVs. To check the reversibility of the phase transition, DSC measurements were carried out in both heating and cooling cycles.Dynamic light scattering (DLS) measurements
DLS measurements were carried out on DMPG ULVs in the absence and presence of 100 mM NaCl using a Zetasizer Nano ZS system (Malvern Instruments, UK) equipped with a 633 nm He–Ne laser. Samples for DLS were diluted to 0.2 mg mL−1 and were passed through a 0.45 μm MILLEX-HV syringe filter to avoid any dust particles. Samples were filled in disposable sizing cuvettes and measurements were carried out on a scattering angle of 173°. DLS measurements were carried out at 283 K, 293 K, 300 K and 310 K. For direct comparisons, DLS measurements were also carried out on zwitterionic DMPC ULVs under identical experimental conditions. Samples were thermally equilibrated for 5 minutes before each measurement. Three sets of measurements were carried out at each temperature.Neutron scattering experiments
Neutron scattering measurements were carried out using the high-energy-resolution time of flight backscattering spectrometer BASIS35 at the Spallation Neutron Source (SNS) of ORNL. In the used configuration of the spectrometer, Si (111) reflections were utilized, which provide an energy resolution of 3.4 μeV (full width at half-maximum) and a range of accessible energy transfers of ±100 μeV and a Q range of 0.3–1.9 Å−1. Annular aluminium sample holders (height = 55 mm; diameter = 29 mm) with an internal spacing of 0.5 mm, which require about 2.5 mL of the sample, were used for the neutron scattering experiments. These sample holders were selected to give no more than 10% scattering from the samples, thereby minimizing multiple scattering effects. Two kinds of measurements, energy-resolved (within the resolution of the spectrometer) elastic scattering intensity scan, also known as the elastic fixed window scan (EFWS), and QENS measurements, were carried out on DMPG ULVs in the absence and presence of 100 mM NaCl. EFWS scan data were collected as a function of temperature from 313 K to 280 K with a step of 1 K. QENS measurements were carried out on both vesicle solutions at 310 K, 300 K, 293 K, and 280 K. These temperatures were selected based on the phase behaviour of DMPG ULVs, where the lipid membranes are in the gel phase (at 280 K), intermediate regime (at 293 K and 300 K), and fluid phase (at 310 K). QENS data were also recorded on the solvent at the same temperatures for reference. To determine the instrument resolution, QENS measurements were also performed on a vanadium standard. MANTID36 was used to carry out standard data reduction, which included background subtraction and detector efficiency corrections.QENS data analysis
For a hydrogenous system, scattering intensity measured in a QENS experiment is dominated by the incoherent scattering from the hydrogen atoms due to the exceptionally high incoherent neutron scattering cross section of hydrogen compared to both coherent and incoherent neutron scattering cross section of any other element. As our interest is the dynamics of lipids, it is imperative to preclude any additional hydrogen atoms apart from the lipid molecules. Hence, we have avoided any buffer (which generally contain hydrogen atoms) and used only deuterated water (D2O) to prepare the vesicle sample. QENS data were also collected from the pure D2O and were subtracted from the spectra collected for the vesicle solutions. It may be noted that the dynamics of hydrated water in direct contact with the lipid head group is not necessarily the same as the dynamics of bulk D2O. However, the fraction of such hydrated water in the present case is very small compared to the total amount of water in the sample. Furthermore, the use of deuterated water makes its contribution negligible, and hence it is not considered explicitly here. Hence, the scattering intensity originating from the lipid membrane can be obtained as follows:| Imem(Q,ω) ≈ Isolution(Q,ω) − ϕIsolvent(Q,ω) | (1) |
Lipid membranes are very dynamic in character and exhibit hierarchical dynamics extending over a wide span of spatial and temporal regimes.37 A time-of-flight backscattering spectrometer such as BASIS is suitable for probing membrane dynamics on the time scale from nanoseconds to a few picoseconds and on the length scale of a few Angstroms to nanometres.34,38 On this time and length scales, two distinct stochastic motions of the lipids, namely (i) lateral motion within the leaflets and (ii) localised internal motion, are expected to contribute to the measured QENS spectra.39 These motions are associated with single-particle dynamics, giving rise to the incoherent neutron scattering signal. Assuming both motions are independent of one another, the incoherent dynamic structure factor for the lipid membrane is a convolution of the incoherent scattering expression for individual lateral and internal motions, and can be written as34
| Smem(Q, ω) = Slat(Q, ω) ⊗ Sint(Q,ω) | (2) |
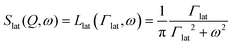 | (3) |
The general scattering expression corresponding to localised internal motion of lipids can be written as34
| Sint(Q, ω) = A(Q)δ(ω) + (1 − A(Q))Lint(Γint,ω) | (4) |
Combining eqn (2) through (4), the resultant dynamic structure factor suitable for the analysis of the lipid motion in the membrane can be given by
| Smem(Q, ω) = [A(Q)Llat(Γlat, ω) + (1 − A(Q))Ltot(Γlat + Γint, ω)] | (5) |
Model for internal motion
In contrast with the lateral lipid motion, the internal motion of lipids is spatially localized and highly dependent on the local configurations of the lipid in the membrane. Lipid molecules are well arranged and tightly packed when the membrane is measured in the ordered phase. In this phase, a majority of the lipids' acyl chains are arranged in the trans conformation, which is compatible with uniaxial rotational diffusion (URD) of the alkyl chain around the main axis. It is likely that not all of the chains are involved in the dynamics measurable in a QENS experiment at a certain temperature. Consequently, a generalised dynamic structure factor for reorientational motion in the ordered phase in this instance can be expressed as43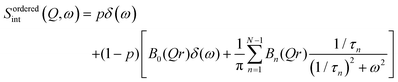 | (6) |
Here p is the fraction of hydrogen atoms immobile on the QENS measurement time scale, r is the radius of the circle, j0 is spherical Bessel function of the zeroth order, N is the number of jump sites, and τ is the average time spent between the successive jumps. The scattering function given by eqn (6) can be employed for URD for N ≥ 6 and Qa ≤ π.39 In this case, the rotational diffusion constant, Dr, would be
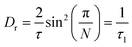 . A sufficiently large number, N = 12, was used, which is adequate for adopting the URD model. The resultant EISF becomes
. A sufficiently large number, N = 12, was used, which is adequate for adopting the URD model. The resultant EISF becomes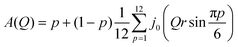 | (7) |
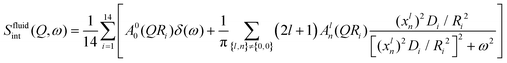 | (8) |
The resultant EISF for the fluid phase can be written as
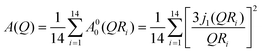 | (9) |
Results and discussion
Phase behaviour, size and stability of DMPG vesicles: effects of NaCl
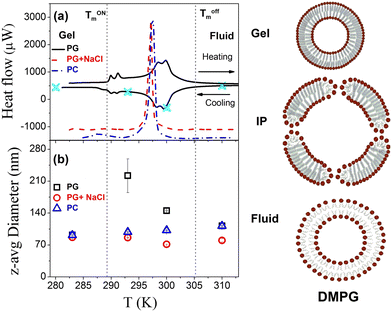
DSC thermograms as observed for DMPG vesicles in both heating and cooling cycles are shown in Fig. 1(a). It is evident that the DMPG membrane shows several broad features, which start from 289 K and end at 306 K, associated with the peculiar broad main phase transition in this membrane. We shall refer to these temperatures as Tonm and Toffm, respectively, since they separate the gel and fluid phases from the intermediate phase between these temperatures. Both the temperature points are shown as the dashed lines in Fig. 1(a). These temperatures have been shown to coincide with the changes in the macroscopic properties (e.g. viscosity, conductivity, turbidity) of the dispersion.7,18 The enthalpy associated with this broad transition is found to be ∼20 kJ mol−1, which is consistent with the literature.7 It has been shown that vesicle preparation and storage time do have a subtle effect on the shape of the DSC curve.19 The absolute values of Tonm and Toffm do not depend on whether DMPG vesicles have been extruded or unextruded. However, the shape of DSC curves is found to be different in these respective cases. In the case of unextruded DMPG vesicles, a sharp peak is observed just around Tonm, which is absent in the present case of extruded DMPG vesicles.19,20 Our DSC data profile is very similar to the reported DSC profile for the extruded vesicles, though at a different concentration.20 As shown in Fig. 1(a), it is evident that the DSC thermogram for the DMPG membrane in the presence of 100 mM NaCl is starkly different compared to that of the pure DMPG membrane, indicating that the presence of NaCl modulates the phase behaviour of the DMPG membrane. In the presence of 100 mM NaCl, a very sharp peak is observed at ∼297 K, which is similar to that of the zwitterionic DMPC membrane. For reference, the DSC thermogram measured on the zwitterionic DMPC membrane is also shown in Fig. 1(a).To investigate the effect of temperature on the size and stability of DMPG vesicles, DLS measurements were carried out on DMPG ULVs with and without 0.1 M NaCl at different temperatures. The observed intensity autocorrelation functions, g2(τ) (Fig. S1, ESI†), suggest that the effects of temperature on the size of DMPG vesicles are very distinct for DMPG with and without 0.1 M NaCl. In DMPG vesicles with 0.1 M NaCl, g2(τ) is found to decay faster as the temperature is increased, suggesting faster diffusion of the whole vesicles. This is in contrast with pure DMPG vesicles, where no such monotonous temperature dependence is observed. In the intermediate phase (293 K, 300 K), despite the higher temperature compared to the temperature point in the gel phase (280 K), the intensity autocorrelation function decays overall slower (more prominent at 293 K), qualitatively suggesting an increase in the size of the vesicles. Moreover, for the intermediate phase (293 K and 300 K), the intensity autocorrelation function shows a shoulder at long times suggesting the presence of different populations of multiple sizes of vesicles. This shoulder disappears with change in temperature as lipids in DMPG vesicles enter either the gel (at 280 K) or fluid (at 310 K) phase. To begin with, a cumulant method46 is used to describe the data for both the ULV systems. Cumulant analysis could describe all the observed data except for the DMPG vesicles in the intermediate phase, where a shoulder at long time could not be explained. Translational diffusion coefficients of the whole vesicles are obtained from the averaged relaxation rates using 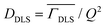 . The averaged hydrodynamic diameters of the ULVs are calculated from the respective values of DDLS using the Stokes–Einstein relation. The temperature dependent variation of the z-average diameter for DMPG vesicles in the absence and presence of 0.1 M NaCl is shown in Fig. 1(b). It is evident that the z-average diameter of DMPG vesicles in the presence of 100 mM NaCl is similar at all studied temperatures, i.e., not much affected by the gel–fluid phase transition. However, in the case of pure DMPG vesicles, a sudden increase in the z-average hydrodynamic diameter in the intermediate region was observed. For reference, DLS experiments have also been carried out on DMPC vesicles, and the obtained temperature dependence of the z-average size of zwitterionic DMPC vesicles is also shown in Fig. 1(b). It is found to be very similar to the temperature dependence of the z-average size of anionic DMPG vesicles in the presence of 100 mM NaCl.
. The averaged hydrodynamic diameters of the ULVs are calculated from the respective values of DDLS using the Stokes–Einstein relation. The temperature dependent variation of the z-average diameter for DMPG vesicles in the absence and presence of 0.1 M NaCl is shown in Fig. 1(b). It is evident that the z-average diameter of DMPG vesicles in the presence of 100 mM NaCl is similar at all studied temperatures, i.e., not much affected by the gel–fluid phase transition. However, in the case of pure DMPG vesicles, a sudden increase in the z-average hydrodynamic diameter in the intermediate region was observed. For reference, DLS experiments have also been carried out on DMPC vesicles, and the obtained temperature dependence of the z-average size of zwitterionic DMPC vesicles is also shown in Fig. 1(b). It is found to be very similar to the temperature dependence of the z-average size of anionic DMPG vesicles in the presence of 100 mM NaCl.
CONTIN47 analysis was also carried out for DMPG vesicles in the absence and presence of 100 mM NaCl at all the measured temperatures. It is found to describe the data well for both the vesicles and provides intensity weightage of size distribution of the DMPG vesicles with and without 100 mM NaCl at all the measured temperatures as shown in Fig. S1 (ESI†). It is evident that DMPG vesicles in the gel phase are monodisperse at around 100 nm. However, in the intermediate phase (293 K, 300 K) a bi-model distribution was observed, with one mode centred at around 100 nm and another one centred at around 600 nm for 293 K and at 4000 nm for 300 K with relatively low population. In the fluid phase (310 K), the size distribution again becomes monodisperse. However, in the case of DMPG vesicles with 100 mM NaCl, the size distribution was monodisperse at all the measured temperatures. Macroscopically, the DMPG vesicle solution becomes transparent with large polydispersity in the intermediate phase, suggesting that there is an abrupt change in the size of the vesicles in this region. Earlier studies4,9,12,16,17 suggested that in the intermediate region DMPG vesicles form perforated vesicles with large holes, which leads to a decrease in the refractive index contrast between the bilayer and the solvent that explains the low turbidity of the vesicle solution. In the intermediate region, the vesicle solution becomes relatively more viscous which was explained based on the increase in the size and ionization of vesicles.12,18,19 Simple aggregation and fusion of the vesicles were ruled out in the intermediate phase.4,11,16 In the fluid phase, the size of the vesicles decreases from the intermediate region and is found to be slightly larger than in the gel phase. Schematics of the change in the size and structure of DMPG vesicles with temperature are also shown in Fig. 1.
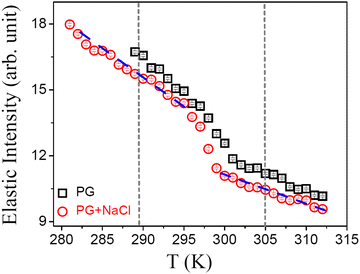
It is evident from DSC and DLS results that DMPG vesicles show an anomalous melting regime, in contrast with the results for DMPG vesicles in the presence of NaCl or pure DMPC. It is of interest to investigate whether these observations are associated with the change in the microscopic dynamics of the lipid membrane. The neutron scattering elastic scan, also known as the elastic fixed window scan (EFWS), is a suitable method to investigate phase transition associated with the microscopic dynamics of the system and is useful to compare with the DSC data. EFWS data provide additional information over the DSC data on whether the observed phase transitions are associated with a change in the microscopic dynamics of the system or not. Any sudden change in the elastic intensity is a signature of a phase transition associated with an alteration in the microscopic dynamics at that temperature. EFWS measurements were carried out on DMPG ULVs with and without 100 mM NaCl. Q-Averaged elastic intensities for DMPG ULVs with and without NaCl are shown in Fig. 2. For the DMPG membrane, it is evident that as the temperature is decreased the elastic intensity increased steadily. However, at ∼302 K, there is a sudden change in the slope of the elastic intensity. This is consistent with the DSC data which show a broad phase transition around this temperature. However, in the presence of NaCl, the EFWS data are found to be modulated. The increase in the elastic intensity around the main phase transition is bit sharper and larger compared to that in the pure DMPG membrane. Moreover, it occurs at a slightly lower temperature (∼298 K). These observations are independently corroborated by the EFWS measured with a higher energy resolution HFBS spectrometer (ΔE = 0.8 μeV), with better data statistics as shown in Fig. S3 (ESI†). It is evident that there is no abrupt change in the elastic intensity at Tonm and Toffm, but the intensity decreases much more rapidly in the temperature range between Tonm and Toffm compared to the gel and fluid phase. EFWS results together with the DSC data (Fig. 1a) indicate that a change in the phase behaviour of the DMPG membrane due to the presence of NaCl is also associated with the change in the microscopic dynamics of the DMPG membrane. Hence, in order to get more insight into the dynamical processes, QENS measurements were carried out on the DMPG membrane with and without 100 mM NaCl. QENS experiments were carried out at 280 K, 293 K, 300 K, and 310 K, thus assessing the gel, intermediate, and fluid phase of the DMPG membrane. These temperatures were chosen based on the observed phase behaviour of the DMPG membrane and are marked with a cross sign in Fig. 1(a).
Microscopic dynamics of DMPG vesicles: effect of NaCl
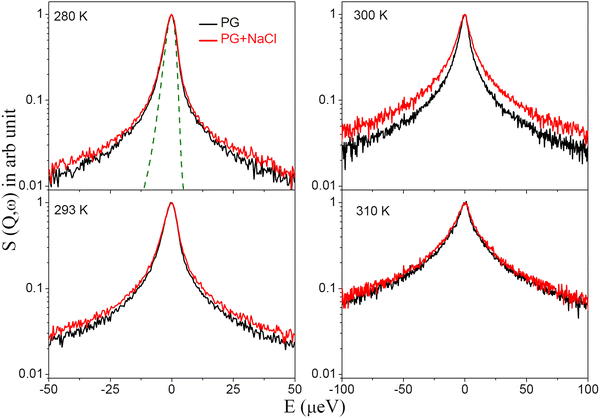
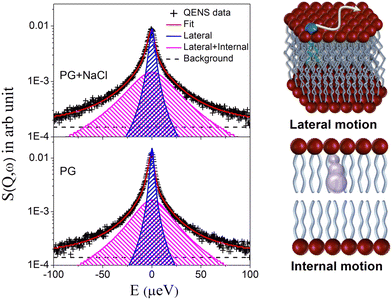
QENS spectra for the DMPG membrane in different phases, namely the gel, intermediate, and fluid phase, are obtained after subtracting the solvent contribution using eqn (1) and are shown in Fig. 3 for a representative Q value of 1.3 Å−1. The spectra are normalized to the peak amplitude. The instrument resolution as measured using a vanadium standard is also shown by a dashed line. Significant quasielastic broadening is observed for the DMPG membrane in all the phases, indicating stochastic motions of the lipids in the membrane. It is evident that quasielastic broadening increases monotonously with an increase in temperature, suggesting faster dynamics as expected for any thermally activated stochastic motion. In the case of a lipid membrane, besides the universal thermal activation of the dynamics, an increase in temperature augments conformational disorder in the membrane, resulting in an increase in the area per lipid, which enhances the membrane dynamics, ensuing an increase in quasielastic broadening. This is consistent with the EFWS results (Fig. 2 and Fig. S3, ESI†), which showed a monotonous decrease in the elastic intensity with temperature. No anomalous behaviour is observed in the intermediate phase. This is in sharp contrast with the recent observation of anomalous temperature dependence, where the membrane becomes softer in the intermediate phase compared to the fluid phase.20 It may be noted that those measurements are concerned with the collective fluctuations of the membranes, which are in a longer spatial and temporal regime compared to the dynamics analysed in the present study. QENS measurements were also carried out on the DMPG membrane in the presence of 100 mM NaCl to investigate the effects of incorporation of salt. For direct comparison, the QENS spectra for the DMPG membrane in the presence of 100 mM NaCl at the same Q value are shown in Fig. 3. It is evident from Fig. 3 that incorporation of 100 mM NaCl enhances quasielastic broadening at all the measured temperatures, with the most significant change evident at 300 K, where the neat DMPG membrane is in the intermediate phase, indicating that the presence of NaCl enhances the dynamics of the anionic DMPG membrane.To extract quantitative information on the microscopic dynamics of the membrane, the QENS data for the DMPG membrane with and without 100 mM NaCl were analysed. The model scattering expression as given in eqn (5) was convoluted with the instrument resolution function, and least squares fits of the model to the QENS data were carried out for both the membrane systems, with A(Q), Γlat, and Γint as the parameters. DAVE software48 developed at the NIST Center for Neutron Research was used for the analysis of the QENS data. It is found that the scattering expression as given in eqn (5) describes the QENS data well for both the membrane systems in the entire Q range at all the measured temperatures suggesting that lateral and internal motions of the DMPG contribute to the observed QENS spectra. Typical fitted QENS spectra for the DMPG membrane in the absence and presence of NaCl at 300 K at Q = 1.1 Å−1 along with schematics of both lateral and internal motions are depicted in Fig. 4. Individual components corresponding to the lateral and a combination of lateral and internal motions are also shown. Apparently, both the components look broader in the case of the DMPG membrane with NaCl in comparison to the DMPG membrane, indicating that addition of NaCl enhances both the motions at 300 K. To gain more insight into both the dynamical processes, the obtained fitting parameters Γlat, A(Q),and Γint were further analysed.
Lateral motion
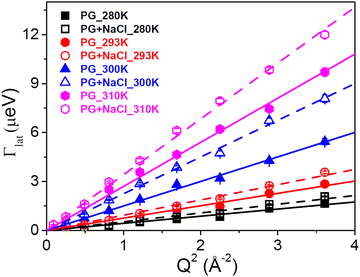
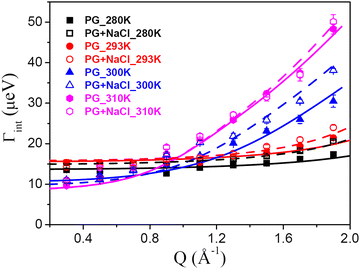
The translational motion of the whole lipid within the leaflet is referred to as the lateral motion of the lipids. This motion is of paramount interest as it plays a vital role in various physiologically relevant membrane processes including cell signalling, membrane trafficking, and cell recognition. Variation of HWHM of the Lorentzian corresponding to the lateral motion of the lipid, Γlat, with Q2 for the DMPG membrane in the absence and presence of NaCl at different temperatures is shown in Fig. 5. It is evident that for both the membrane systems at all the measured temperatures, Γlat follows a linear dependence on Q2, indicating that the lateral motion obeys the Fickian type diffusion law. Linear fits based on the Fickian diffusion model (Γlat = DlatQ2) are shown as lines in Fig. 5. The obtained lateral diffusion coefficients for DMPG membranes with and without NaCl are shown in Fig. 6. For the pure DMPG membrane, it is found that with the increase in temperature, the lateral diffusion coefficient of DMPG lipid monotonously increases. No unusual (e.g., faster than in the fluid phase) lateral diffusion is observed in the intermediate phase or during the phase transition, in contrast to the reported enhanced collective motion of the DMPG membrane during the phase transition (or in the intermediate phase) compared to the high temperature fluid phase.20 Viscosity measurements for the DMPG membrane in the low ionic strength solvents show an anomalous peak-like behaviour.18 In the transition regime, the viscosity of DMPG vesicles is about 10 times higher than that observed in the ordered and fluid phase.18 However, our QENS results do not show any such abrupt change in the membrane dynamics. This reflects the fact that the macroscopically measured viscosity of the vesicle solution is not directly correlated with the membrane dynamics at the nanometre length scales. Our previous QENS measurements on the zwitterionic membrane28 have suggested that solvent viscosity may play an important role in the tuning of the membrane dynamics. It may be noted that in the present measurement, although the viscosities of the solvents are very similar, the macroscopic viscosity of the whole vesicle solution dramatically increases only due to the large size of perforated vesicles in the intermediate phase.16,18 Our measurements suggested that microscopic lateral motion of lipids on the nanometre length scale is uncoupled to the change in the macroscopic viscosity of the vesicle solution.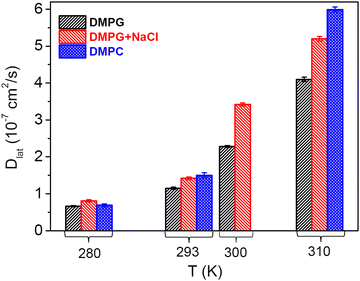 | ||
| Fig. 6 Lateral diffusion coefficients for the DMPG membrane in the absence and presence of NaCl at different temperatures. It is evident that the presence of 100 mM NaCl enhances the lateral diffusion coefficient at all the measured temperatures. For direct comparison, data obtained from the lateral diffusion coefficient for the DMPC membrane49 are also shown. | ||
As mentioned earlier, DMPG lipid has the same alkyl chains as DMPC and differs only in the head group. In the presence of 100 mM NaCl, the phase behaviour of DMPG is very similar to that of DMPC.4,18 Hence, lateral diffusion coefficients as obtained from the recent QENS study49 on the 7 (w/w) % DMPC membrane are also compared in Fig. 6. It is evident that the lateral diffusion coefficient for the DMPC membrane is much higher than that for the pure DMPG membrane, especially above the main phase transition temperature. Addition of NaCl is found to enhance the lateral diffusion of the DMPG lipid across all phases. This suggests that enhancement of the lateral diffusion of DMPG in the presence of NaCl is mainly associated with the decreased surface charge density of the membrane or screened Coulombic interactions between the head groups. This enhancement is most prominent at 300 K (intermediate phase of the DMPG membrane). Conductivity measurements18 revealed that the degree of ionization, and, therefore, the charge on the head group, increases significantly in the intermediate phase. This results in an increase in Coulombic interactions between the head groups, which may contribute to the suppression of the dynamics of lipid. Addition of NaCl screens coulombic interactions, which may enhance the lateral motion of the lipid. In the intermediate phase, an additional factor would be a change in the vesicular structure of the DMPG membrane. It has been shown12,16,17 that in the intermediate phase, DMPG vesicles have stable and large pores, which are stabilised due to a change in the membrane curvature. The distances between these pores are typically 30–40 nm.16 Although QENS measurements probe diffusion on the length scale of up to a few nanometres, the lipids residing in the vicinity of the pores are prone to be affected due to the perforated structure and have hindered lateral diffusivity. This may restrict the overall non-localised lateral diffusion of lipids in the DMPG membrane. However, in the presence of 100 mM NaCl, these large and stable pores disappear, which results in the additional enhancement in the lateral motion of the lipid. The difference in the phase behaviour between DMPG and DMPG with 100 mM NaCl could be yet another contributing factor. At 300 K, pure DMPG is in the transitional region, whereas in the presence of 100 mM NaCl, it is already in the fluid phase. These factors explain why the dynamics of DMPG is significantly different at 300 K for the DMPG membrane with and without 100 mM NaCl. The results are compared with the recent QENS study28 on the effects of salt on the dynamics of the zwitterionic DMPC membrane, where a sharp reduction in the lateral diffusion is observed due to addition of salt. Our measurements suggest that the effects of a salt on the membrane dynamics strongly depend on the polarity of the lipid head group. NMR studies on another anionic lipid membrane, i.e., DOPG membrane,29 showed that addition of a salt leads to a decrease in the lateral diffusion coefficient, which is just opposite to the effect observed herein in the case of DMPG. This might be due to a difference in the saturation and chain length of the lipid alkyl tails, which results in a different phase behaviour, and the experimental methods employed, as NMR is more sensitive to the macroscopic diffusion and QENS is more sensitive to the microscopic diffusion on the length scale of up to a few nanometres. The DOPG membrane does not show the anomalous broad melting regime like DMPG. As discussed above, unlike in the case of DOPG, the charge on the DMPG head group will be significantly higher in the intermediate phase, which also affects the membrane dynamics.
Internal motion
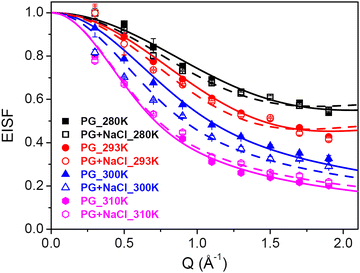
The other type of motion observed within the timescale of the QENS measurements is the internal dynamics of the lipid molecules. In contrast to lateral motion, the internal motion of lipids is localised in character. The localised dynamics are sensitive to lipid packing and molecular geometry and therefore reflect the changes in the molecular ordering. Internal motion can be characterised by the EISF and HWHM (Γint), which are obtained from the QENS data analysis. The Q-dependence of EISF and HWHM associated with the internal motions of the DMPG membrane with and without 100 mM NaCl at all the measured temperatures is depicted in Fig. 7 and 8, respectively. It is evident from the Q-dependent behaviour of EISF and Γint that the dynamics at 300 K and 310 K differ from the dynamics at 280 and 293 K. In the low temperature phase (280 and 293 K), the HWHM is approximately constant, which is a typical signature of reorientational motion.50 This is also supported by the fact that in the ordered phase, lipid molecules are well ordered and predominantly in all-trans conformation. In this arrangement, alkyl chains undergo URD on a circle with a radius of gyration r. The fractional URD model (eqn (7)) has been used to describe the dependence of EISF on Q for the DMPG membrane with and without 100 mM NaCl, and the fits are shown by the black and red lines. Strictly speaking, the DMPG membrane at 293 K is in the intermediate phase. It has been shown9 that at Tonm, only the beginning of the melting process is initiated, and the fluid phase exists only above Toffm. The temperature of 293 K is near Tonm, where the majority of DMPG lipids have a conformation similar to that in the gel phase. We have employed the URD model for DMPG vesicles at 293 K to enable a direct comparison between DMPG vesicles with no salt and DMPG vesicles in the presence of 100 mM NaCl, which are in the gel phase at 293 K. It is found that the fractional URD model describes the data well for both the membrane systems at 280 and 293 K. The obtained fit parameters, namely, the radius of rotation and the fraction of mobile hydrogen atoms, for the DMPG membrane with and without 100 mM NaCl are given in Table 1. For the DMPG membrane, at 280 K, the radius of rotation (or gyration) and the fraction of mobile hydrogen atoms are found to be 1.4 (±0.1) Å and 51 (±2) %, respectively. This suggests that about 51% of hydrogen atoms of the lipid molecules are participating in the fractional uniaxial rotational motion on a circle of radius ∼1.4 Å. It is found that addition of NaCl does not significantly affect the radius of gyration and the fraction of mobile hydrogen atoms. For consistency, the fractional URD model should also describe the behaviour of HWHM (Γint). The fractional URD model (Eqn (6)) has been employed to describe the behaviour of Γint with Dr as a parameter, and the fits are shown as lines. It is evident from Fig. 8 that the fractional URD model describes data well, and the obtained values of the rotational diffusion coefficients are given in Table 1. At 280 K, the Dr value is found to be 13.7 (±0.2) μeV, which is slightly increased to 14.9 (±0.3) μeV due to the addition of NaCl. At 293 K, no significant change in the rotational diffusion coefficient is observed. This could be due to the perforated vesicle,16 as lipids on the edge of the pore are more flexible than lipids in the membrane core. Hence, on average, the internal motion is faster in pure DMPG. In the presence of NaCl, though we expect an increase in the internal dynamics of the lipid, this effect was compensated by a different vesicular structure (no pores in the membrane). Hence, no significant change in the internal dynamics is observed at 293 K.| T (K) | Lipid system | Fraction of mobile (%) (1-px) | Radius of gyration (R) | D int (μeV) |
|---|---|---|---|---|
| 280 | DMPG | 51 ± 2 | 1.4 ± 0.1 | 13.7 ± 0.2 |
| DMPG + NaCl | 49 ± 2 | 1.6 ± 0.1 | 14.9 ± 0.3 | |
| 293 | DMPG | 62 ± 3 | 1.5 ± 0.1 | 15.6 ± 0.3 |
| DMPG + NaCl | 61 ± 3 | 1.7± 0.1 | 15.8 ± 0.4 |
The Q-dependence of HWHM corresponding to the internal motion, Γint, for both the membrane systems at 300 and 310 K is very different from that observed 280 and 293 K, particularly at higher Q values. At higher temperatures (300 and 310 K), Γint monotonously increases at higher Q values, while in the zero-Q limit, there is a flattening towards a finite nonzero value. This is a typical signature of LTD.45 We have used the LTD model to describe EISF and Γint for both the membrane systems, and it is found to describe the data well, as evident from Fig. 7 and 8. The values of Rmin and Dmin are found to be insignificant, which represents the minimal movement of hydrogen atoms at the first carbon position held by the head group. The obtained fitting parameters for the maximum size of confining volume (Rmax) and associated diffusivity (Dmax) are given in Table 2. It is evident that as the temperature is increased from 300 K to 310 K, Rmax and Dmax increase for both the systems, suggesting faster movement in a bigger space. At 300 K, it is found that in the presence of NaCl, Rmax and Dmax are larger than those for the pure DMPG membrane, suggesting that the presence of salt enhances the internal dynamics of the membrane. This could be explained based on the change in the physical state of the membrane, as evident from Fig. 1. At 300 K, the membrane is in the intermediate region in the case of pure DMPG vesicles. However, in the presence of salt, the membrane is in the fluid phase. This explanation is further supported by the fact that at 310 K, where the membranes in both the systems are in the fluid phase, no significant effects of addition of NaCl on the internal motion of lipid molecules are observed.
| T (K) | Lipid system | R max (Å) | D max (×10−7 cm2 s−1) |
|---|---|---|---|
| 300 | DMPG | 3.4 ± 0.2 | 35.1 ± 0.7 |
| DMPG + NaCl | 4.1 ± 0.2 | 46.7 ± 0.8 | |
| 310 | DMPG | 5.1 ± 0.3 | 64.4 ± 0.9 |
| DMPG + NaCl | 5.1 ± 0.3 | 64.0 ± 0.9 |
In contrast to other widely used macroscopic methods, information regarding diffusivities related to both lateral and internal motions of the lipids is obtained using QENS techniques. Unlike the reported enhanced collective bending fluctuation in the intermediate phase of the DMPG membrane,20 our measurements show no anomalous temperature dependence for lateral and internal motions of individual lipids across the broad melting regime of the DMPG membrane. Incorporation of NaCl significantly modulates the phase behaviour and at the same time accelerates the microscopic dynamics of the DMPG membrane. The enhancement of the single particle dynamics of the anionic DMPG membrane is in contrast with the recently reported QENS results on the zwitterionic DMPC membrane28 suggesting the significance of lipid polarity for membrane dynamics and salt–membrane interaction.
Conclusions
Using incoherent quasielastic neutron scattering, we investigated the dynamics of lipids in an anionic dimyristoyl phosphatidylglycerol (DMPG) membrane on the pico-to-nanosecond time scale and Angstrom-to-nanometer length scale through the anomalous extended melting transition regime. Unlike zwitterionic phospholipid membranes, such as dimyristoyl phosphatidylcholine (DMPC), which exhibit a relatively sharp main phase transition between the ordered and disordered phases, the charged DMPG membrane is characterized by an extended melting regime between the gel and fluid phases. This transition regime exhibits various anomalous macroscopically measurable characteristics, such as low turbidity and high electrical conductivity and viscosity. The anomalous properties of the DMPG membrane in the transition regime were corroborated by a recent neutron spin echo study,20 which revealed that in the extended melting regime the membrane becomes softer and exhibits faster collective bending fluctuations compared to the higher temperature fluid phase. Contrary to the expectations, in the current study we found that the lateral diffusivity and internal motions of lipids in the DMPG membrane exhibited a monotonic increase with temperature from the gel phase to the transition state to the fluid phase, showing no anomaly (e.g., no enhanced dynamics) in the transition regime. This observation was corroborated by the temperature-dependent scans of the energy-resolved elastic neutron scattering intensity exhibiting monotonic temperature dependence with no anomaly in the transition regime, despite clear evidence of the anomalous extended melting regime presented by differential scanning calorimetry. Likewise, dynamic light scattering indicated a large increase in the DMPG vesicle size in the transition melting regime, compared to both the gel and fluid phases. We conclude that, while changes in the membrane structure and morphology in the extended melting regime are associated with softening and the enhanced collective dynamics in the membrane, the single-particle lipid dynamics associated with the lateral diffusivity and internal lipid motion is not affected. Incorporation of NaCl in DMPG achieved by using 100 mM NaCl aqueous solvent suppresses the anomalous extended melting regime, as evidenced by the differential scanning calorimetry measurements showing a sharp meting transition and dynamic light scattering measurements showing the vesicle size essentially unchanged between 280 and 310 K. Concurrently, incorporation of NaCl enhances the single-particle lipid dynamics, both the lateral diffusivity and (to a lesser extent) the internal lipid motion. At a first glance, this is rather counterintuitive and in variance with the dynamic suppression effect exerted by a salt on a zwitterionic membrane.28 However, one needs to realize that, unlike in the zwitterionic membrane, incorporation of salt in anionic DMPG leads to enhanced cooperativity, as evidenced by the main phase transition reminiscent of the sharp melting transition in zwitterionic membranes. That is, the disrupted cooperativity in the salt-free DMPG is associated with the baseline lipid dynamics that is suppressed to begin with. Addition of salt partially restores the cooperativity, thus enhancing lipid dynamics compared to the salt-free baseline membrane state, despite the increased viscosity of the aqueous solvent.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The neutron scattering experiments at Oak Ridge National Laboratory's (ORNL's) Spallation Neutron Source (SNS) were supported by the Scientific User Facilities Division, Office of Science (Basic Energy Sciences), U.S. Department of Energy (DOE).References
- E. J. H. Sackmann, Handbook of Biological Physics, 1995, 1, 213–304 Search PubMed.
- T. Heimburg, Thermal Biophysics of Membranes, John Wiley and Sons, 2007, pp. 75–97 Search PubMed.
- R. M. Epand and S. W. Hui, FEBS Lett., 1986, 209, 257–260 CrossRef CAS.
- M. T. Lamy-Freund and K. A. Riske, Chem. Phys. Lipids, 2003, 122, 19–32 CrossRef CAS.
- K. A. Riske, H. G. Dobereiner and M. Teresa Lamy-Freund, J. Phys. Chem. B, 2002, 106, 239–246 CrossRef CAS.
- A. Watts, K. Harlos, W. Maschke and D. Marsh, Biochem. Biophys. Acta, 1978, 510, 63–74 CAS.
- M. F. Schneider, D. Marsh, W. Jahn, B. Kloesgen and T. Heimburg, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 14312–14317 CrossRef CAS.
- T. Heimburg and R. L. J. B. Biltonen, Biochemistry, 1994, 33, 9477–9488 CrossRef CAS.
- K. A. Riske, L. Q. Amaral and M. T. Lamy-Freund, Biochim. Biophys. Acta, Biomembr., 2001, 1511, 297–308 CrossRef CAS.
- K. A. Riske, M. J. Politi, W. F. Reed and M. T. Lamy-Freund, Chem. Phys. Lipids, 1997, 89, 31–44 CrossRef CAS.
- J.-M. I. Alakoskela and P. K. J. Kinnunen, Langmuir, 2007, 23, 4203–4213 CrossRef CAS.
- J.-M. Alakoskela, M. J. Parry and P. K. J. Kinnunen, Langmuir, 2010, 26, 4892–4900 CrossRef CAS.
- F. Spinozzi and L. Q. Amaral, Langmuir, 2016, 32, 13556–13565 CrossRef CAS PubMed.
- C. Loew, K. A. Riske, M. T. Lamy and J. Seelig, Langmuir, 2011, 27, 10041–10049 CrossRef CAS.
- K. Tajima, Y. Imai, A. Nakamura and M. Koshinuma, Adv. Colloid Interface Sci., 2000, 88, 79–97 CrossRef CAS.
- K. A. Riske, L. Q. Amaral, H. G. Döbereiner and M. T. Lamy, Biophys. J., 2004, 86, 3722–3733 CrossRef CAS.
- K. A. Riske, L. Q. Amaral and M. T. Lamy, Langmuir, 2009, 25, 10083–10091 CrossRef CAS PubMed.
- R. P. Barroso, K. A. Riske, V. B. Henriques and M. T. Lamy, Langmuir, 2010, 26, 13805–13814 CrossRef CAS.
- T. A. Enoki, V. B. Henriques and M. T. Lamy, Chem. Phys. Lipids, 2012, 165, 826–837 CrossRef CAS PubMed.
- E. G. Kelley, M. Nagao, P. D. Butler, L. Porcar and B. Farago, Struct. Dyn., 2020, 7, 054704 CrossRef CAS PubMed.
- G. Pabst, A. Hodzic, J. Štrancar, S. Danner, M. Rappolt and P. Laggner, Biophys. J., 2007, 93, 2688–2696 CrossRef CAS PubMed.
- R. A. Böckmann, A. Hac, T. Heimburg and H. Grubmüller, Biophys. J., 2003, 85, 1647–1655 CrossRef.
- H. I. Petrache, S. Tristram-Nagle, D. Harries, N. Kučerka, J. F. Nagle and V. A. Parsegian, J. Lipid Res., 2006, 47, 302–309 CrossRef CAS.
- S. A. Pandit, D. Bostick and M. L. Berkowitz, Biophys. J., 2003, 84, 3743–3750 CrossRef CAS.
- A. A. Gurtovenko and I. Vattulainen, J. Phys. Chem. B, 2008, 112, 1953–1962 CrossRef CAS.
- M. Rappolt, G. Pabst, H. Amenitsch and P. Laggner, Colloids Surf., A, 2001, 183–185, 171–181 CrossRef CAS.
- S. Jaksch, O. Holderer, H. Frielinghaus, A. Koutsioubas, P. Zolnierczuk, D. W. Hayward, S. Förster and P. Müller-Buschbaum, Frontiers in Physics, 2021, 9 Search PubMed.
- V. K. Sharma and E. Mamontov, J. Appl. Phys., 2022, 132, 074702 CrossRef CAS.
- A. Filippov, G. Orädd and G. Lindblom, Chem. Phys. Lipids, 2009, 159, 81–87 CrossRef CAS PubMed.
- H. Jousma, H. Talsma, F. Spies, J. G. H. Joosten, H. E. Junginger and D. J. A. Crommelin, Int. J. Pharm., 1987, 35, 263–274 CrossRef CAS.
- S. G. M. Ong, M. Chitneni, K. S. Lee, L. C. Ming and K. H. Yuen, Pharmaceutics, 2016, 8, 36 CrossRef.
- V. K. Sharma, E. Mamontov, M. Tyagi, S. Qian, D. K. Rai and V. S. Urban, J. Phys. Chem. Lett., 2016, 7, 2394–2401 CrossRef CAS.
- V. K. Sharma, S. Ghosh, P. Mandal, T. Yamada, K. Shibata, S. Mitra and R. Mukhopadhyay, Soft Matter, 2017, 13, 8969–8979 RSC.
- V. K. Sharma, E. Mamontov, D. B. Anunciado, H. O’Neill and V. S. Urban, J. Phys. Chem. B, 2015, 119, 4460–4470 CrossRef CAS.
- E. Mamontov and K. W. Herwig, Rev. Sci. Instrum., 2011, 82, 085109 CrossRef CAS PubMed.
- O. Arnold, J. C. Bilheux, J. M. Borreguero, A. Buts, S. I. Campbell, L. Chapon, M. Doucet, N. Draper, R. Ferraz Leal, M. A. Gigg and V. E. Lynch, et al. , Nucl. Instrum. Methods Phys. Res., Sect. A, 2014, 764, 156–166 CrossRef CAS.
- V. K. Sharma and E. Mamontov, Prog. Lipid Res., 2022, 87, 101179 CrossRef CAS.
- V. K. Sharma, E. Mamontov, M. Ohl and M. Tyagi, Phys. Chem. Chem. Phys, 2017, 19, 2514–2524 RSC.
- V. K. Sharma, E. Mamontov, D. B. Anunciado, H. O'Neill and V. S. Urban, Soft Matter, 2015, 11, 6755–6767 RSC.
- S. Busch, C. Smuda, L. C. Pardo and T. Unruh, J. Am. Chem. Soc., 2010, 132, 3232–3233 CrossRef CAS PubMed.
- H. Srinivasan, V. K. Sharma, S. Mitra and R. Mukhopadhyay, J. Phys. Chem. C, 2018, 122, 20419–20430 CrossRef CAS.
- C. Armstrong, M. Trapp, J. Peters, T. Seydel and M. Rheinstädter, Soft Matter, 2011, 7, 8358–8362 RSC.
- A. J. Dianoux, F. Volino and H. Hervet, Mol. Phys., 1975, 30, 1181–1194 CrossRef CAS.
- M. Doxastakis, V. G. Sakai, S. Ohtake, J. K. Maranas and J. J. de Pablo, Biophys. J., 2007, 92, 147–161 CrossRef CAS PubMed.
- F. Volino and A. J. Dianoux, Mol. Phys., 1980, 41, 271–279 CrossRef CAS.
- D. E. Koppel, J. Chem. Phys., 1972, 57, 4814–4820 CrossRef CAS.
- S. W. Provencher, Comput. Phys. Commun., 1982, 27, 229 CrossRef.
- R. T. Azuah, L. R. Kneller, Y. Qiu, P. L. Tregenna-Piggott, C. M. Brown, J. R. Copley and R. M. Dimeo, J. Res. Natl. Inst. Stand. Technol., 2009, 114, 341 CrossRef CAS.
- V. K. Sharma, E. Mamontov and M. Tyagi, Biochim. Biophys. Acta, Biomembr., 2020, 1862, 183100 CrossRef CAS.
- M. Bee, Quasielastic Neutron Scattering: Principles and Applications in Solid State Chemistry, Biology, and Materials Science, Adam Hilger, Bristol, England, Philadelphia, 1988 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sm01425d |
| This journal is © The Royal Society of Chemistry 2023 |