Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Characterization of sulfur and chlorine behavior during pyrolysis of biomass and waste
Hala
Braidy
 ab,
Sylvie
Valin
*a and
Fabrice
Patisson
ab,
Sylvie
Valin
*a and
Fabrice
Patisson
 b
b
aUniv. Grenoble Alpes, CEA, LITEN, DTCH, 38000 Grenoble, France. E-mail: sylvie.valin@cea.fr
bUniversité de Lorraine, Institut Jean Lamour, Labex DAMAS, Nancy, 54011, France
First published on 5th June 2023
Abstract
The aim of this study is to characterize the behavior of sulfur and chlorine during the pyrolysis of biomasses (agricultural residues: corn residues and colza straw) and waste components (cardboard, wool, and PVC), selected for their different compositions and ash contents. Pyrolysis experiments were performed between 365 and 850 °C. The major parts of S for wool and cardboard, and Cl for PVC, initially present in organic forms, are easily released into the gas phase, even below 550 °C, with no interaction with ash-forming elements. For the agricultural residues, both the organically and inorganically associated S contribute to its release. Cl, initially mainly present in inorganic chloride salts (KCl, KClO4…), is only partly released. In the remaining char for colza straw, sulfur is associated with calcium as CaSO4, reduced to CaS above 800 °C; for corn residues, sulfur and chlorine are found as K2SO4, KCl and MgCl2.
1. Introduction
To reduce the dependence on fossil fuels and to push forward a circular economy, the gasification of renewable or non-valorized carbonaceous resources (biomass, waste) allows to produce a synthesis gas (mainly composed of H2, CO, CO2, H2O, CH4), which can then be used as a form of energy or transformed by catalytic synthesis into fuel or other chemical raw materials. However, volatile inorganic elements (S, Cl, N) found in carbonaceous resources are often released in the gas phase during gasification; these may be harmful to the post-gasification processes due to their corrosive nature (HCl, KCl, NaCl…) and their poisoning effect on catalysts (H2S, COS).In recent years, there has been an uptake in experimental and thermodynamic studies on the gasification of biomass and/or waste rich in inorganic elements, with a particular focus on characterizing gaseous inorganic pollutant emissions (H2S, COS, HCl, NaCl, NH3…). Various parameters related to the feedstock and the operating conditions (temperature, type of oxidant, equivalence ratio) during the gasification process influence the behavior of these emissions and their release mechanism.1–5 Moreover, different quantities and chemical forms of inorganic elements have been identified in carbonaceous resources correlating with the type of material of origin and making the link with their release under thermal treatment. Investigating the association of ash-forming elements in the fuel structure seemed an important aspect to understand their release behavior. Studies in the literature have investigated the relationship between the different chemical forms of sulfur in coal and their behavior during pyrolysis.6,7 Moreover, other studies focused on the release of sulfur during thermal conversion of biomass feedstock and showed that it is released through two mechanisms related to its chemical form: an initial release of organic S at low temperatures (<500 °C), followed by a decomposition of inorganic S at higher temperatures.2,3,5
Other studies1,3,4,8,9 were conducted to characterize the transformation of chlorine, alkali and alkaline earth metals in biomass feedstock, where they exist primarily in inorganic salt forms. These chloride salts may vaporize as alkali chlorides (KCl(g), NaCl(g)) at temperatures above 700 °C or may decompose and release Cl, which is rapidly converted into HCl(g) at lower temperatures. Below 700 °C, organic and inorganic chlorine partially transforms into HCl. In solid recovered fuels (SRF), where Cl exists in its two different forms, the distinction between inorganic and organic Cl did not correlate with the differentiation between volatile and non-volatile chlorine after pyrolysis.10
On the other hand, in our previous study,11 thermodynamic equilibrium calculations have shown that the major elements of ash (mainly Ca and Si) can influence the S distribution in gaseous and solid products. The reaction of calcium with other elements, particularly silicon, inhibits its ability to retain sulfur in the condensed solid phase and thus increases the release of sulfur in the gas phase. This influence of ash-forming elements was experimentally observed in12 for biochars generated from pyrolysis and gasification of oak and corn stover; silicon-poor oak biochar was found to retain a higher fraction of sulfur compared to the biochar of the silicon-rich corn stover. In bagasse gasification, the release of alkali metals present as water-soluble salts (KCl, K2SO4, K3PO4, K2CO3) and ion-exchangeable ions bound to the organic matrix (K+) was reduced by the presence of aluminosilicates in the feedstock.13 However, in another study on biomass gasification, the ash composition had a marginal effect on the release of S and Cl, but was relevant for the release of K.5
In biomass-derived feedstock such as paper and cardboard, or materials derived from fossil resources such as textiles, to our knowledge, there is less information available on the sulfur and chlorine nature in such types of resources and their resulting release mechanisms.
Therefore, the transformation of inorganic volatile elements (S, Cl, K…) during pyrolysis and gasification and their distribution between solid and volatile phases still need experimental investigation, considering their initial chemical forms, the characteristics of the resource, and its chemical composition, especially its ash content and composition. This study focuses on the sulfur and chlorine transformation during the pyrolysis of biomass and waste from different types and origins, pyrolysis being considered as the first step of gasification. It investigates a previously unexplored relationship between the type and composition of such feedstocks and the behavior of sulfur and chlorine. Two agricultural residues with different ash content and composition and three waste components were selected as feedstock. A leaching technique was applied to the selected resources to quantify the different initial chemical forms of sulfur and chlorine (organic/inorganic). Analytical experiments were conducted using a lab-scale induction heated reactor. Various analytical techniques were employed to quantitatively and qualitatively assess the behavior of sulfur and chlorine during pyrolysis. This innovative approach enables a more precise and comprehensive evaluation of the impact of the resource's chemical composition, especially the composition of ash, as well as the chemical forms of S and Cl associated with the type of raw material.
2. Experimental
2.1. Feedstock and its characterization
Five feedstocks were selected among agricultural residues and materials present in waste to investigate a large variety of composition, ash, sulfur and chlorine content:- Colza straw and corn residues (leaf, husk, stalk) are post-harvesting crops and residues deriving from agriculture.
- Cardboard, PVC, and wool (textile) are urban post-consumer residues deriving from industries or households.
To obtain the most homogeneous and representative samples possible, starting from agricultural residues batches of a few kg, quartering followed by grinding to mm size using a hammer mill was performed to obtain particles between 1 and 1.5 mm. The waste components were shredded up to particles between 3 and 10 mm (Fig. 1).
The elemental composition of the samples was determined according to the standards:
- ISO 18122 for the determination of the ash content.
- EN ISO 21663 for the determination of the total content of C, H, and N using a micro-analyzer.
- EN 15408 and EN ISO 10304-1 for the determination of the total content of S and Cl. This method is carried out in two steps; the sample is first oxidized by combustion with O2 in a bomb calorimeter (Parr 6200 Isoperibol Calorimeter) where sulfur and chlorine are transformed respectively into chlorides and sulfates, which are dissolved and/or absorbed in a 5 M NaOH solution. Then, the bomb is washed out and the recovered solution is analyzed by ion chromatography.
- ASTM D6349 for the determination of major inorganic element contents (Si, Ca, K, Na, Mg, Fe, Al, and P) by Inductively Coupled Plasma (ICP) Spectrometry.
A chemical fractionation method was applied to four of the selected feedstock: the two agricultural residues, cardboard, and wool, to investigate in which chemical form inorganic elements are present and how they are associated in the fuel structure. This method was originally developed for coal14 and then modified and applied for biomass fuels.15,16 It is based on selective leaching by increasingly aggressive solvents, i.e., water, 1 M ammonium acetate, and 1 M hydrochloric acid. The different types of ash-forming elements are distinguished according to their solubility in the different solvents.
In our study, the chemical fractionation method was limited to consecutive leaching in two solvents, i.e., pure water and 1 M ammonium acetate (NH4Ac) solution. First, an excess of Milli-Q water (15 mL g−1 of the solid sample) was used to leach out 10 g of solid fuel with agitation for 24 hours at ambient temperature. After this period, the solution was filtered and the water leachate was collected. The remaining solid was then leached out in a 1 M NH4Ac solution (pH = 7.45) (5 mL g−1 of solid) with agitation for 24 hours at ambient temperature. The suspension was filtered and the acetate leachate was collected. Water and acetate leachates were analyzed by ICP-OES for S quantification and by ionic chromatography for Cl− and cations (K+, Na+, and Ca2+) quantification. In addition, the content of S and Cl in the dried residual solid was determined with the same method as that used for the raw feedstock, in an attempt to complete the elemental balance of S and Cl in the fractionation method.
2.2. Pyrolysis experimental setup and procedure
Experiments were performed in a lab-scale setup that was described in detail in a previous study17 and was adapted for this study. It consists in an induction-heated set-up, in which the reaction takes place, followed by a condensable and gas products collection system (Fig. 2). The reactor is a quartz tube (365 mm in height, 22 mm in internal diameter, 3 mm in thickness) fixed to a porous quartz-sintered disc (25 mm in diameter, 4 mm in thickness, grade 0 in porosity with maximum pore size from 160 to 250 μm) that can withstand temperatures up to 900 °C. Quartz was selected as the reactor's material to avoid any adsorption or interaction with the released inorganic species.18 The reactor is placed inside a susceptor that consists in a 316L stainless-steel tube (560 mm in height, 30.15 mm in internal diameter). Inside the susceptor and under the quartz tube reactor, a metallic heat exchanger helps to preheat the entering gas (N2 continuously fed at a flow rate of 0.5 NL min−1 in order to flush the system and carry the volatile products). The susceptor is surrounded by a water-cooled copper coil inductor (420 mm long), which is connected to a 12 kW electrical generator that supplies energy to the induction circuit. An external quartz tube is placed between the coil and the susceptor to avoid any contact between them and to center the setup. Set point temperature is adjusted with a PID controller connected to a two-color optical pyrometer, which aims at the susceptor wall at the sample level, so that the susceptor reaches the set point value with a heating rate of approximately 85 °C s−1. However, the quartz reactor takes a longer time to reach the set point with a heating rate of 10 °C s−1 measured in its center. The temperature is homogeneous in the sample region, between the sintered disc and 32 mm above, with a maximum temperature difference of 10 °C.For each experiment, the reactor was filled up to 40 mm of its height with 2 to 4 g of dried feedstock. To determine the real temperature of the samples during each experiment, the temperature profile inside the sample was measured using three thermocouples at different axial positions (z = 2, 17, 32 mm above the quartz disc) inside the sample. The average of the measurements at the three axial positions was considered. Two types of experiments were performed.
- Experiments in which the reactor was heated up and kept at the desired temperature for 20 minutes. Tests were performed at two different set point temperature levels: 800 °C and 850 °C for all feedstocks.
- Experiments in which the heating was interrupted at different times and in which each sample therefore reached a different maximum temperature. These tests were intended to have a more comprehensive understanding of the transformation of S and Cl during heating.
Each experiment is shown in Fig. 3 as a function of its heating time, where it is referred to according to the maximum average temperature reached inside the sample.
The remaining solid product in the sample holder, referred to as the char, is collected for further analyses once the reactor has cooled down. The mass yield of the char (in %) is calculated as the ratio of the mass of the char to that of the dried feedstock.
2.3. Char characterization
The elemental composition of the char was analyzed according to the same methods as that used for the feedstocks. The retention of an inorganic element in the char is defined as the ratio of the mass of the element in the char to that of the element in the dried feedstock.The crystalline phase analysis of the char samples was conducted with a powder X-ray diffractometer. XRD patterns were evaluated using the DIFFRAC.EVA software with ICCD RDB databases (PDF-2 and PDF-4).
The chars' morphology and local surface elemental composition were studied using a scanning electron microscope (Philips XL30 with a 15 kV electron beam) coupled to an energy dispersive X-ray spectroscopy (SEM-EDX).
3. Results and discussion
3.1. Feedstock characteristics
| Unit | Colza straw | Corn residues | Cardboard | Wool | PVC | |
|---|---|---|---|---|---|---|
| a na: not analyzed. | ||||||
| Moisture | wt% | 11.3 | 9.7 | 7.5 | 11.7 | — |
| Ash at 550 °C | wt% | 10.1 | 3.5 | 12.8 | 1.2 | 3.5 |
| C | wt% | 43.9 | 45.8 | 42.3 | 49.1 | 39.5 |
| H | wt% | 5.47 | 6.02 | 5.36 | 6.54 | 5.23 |
| N | wt% | 0.96 | 1.24 | 0.28 | 14.47 | 0.09 |
| O by difference | wt% | 38.6 | 43.5 | 42.2 | 25.19 | 2.25 |
| S | wt% | 0.33 | 0.11 | 0.1 | 2.66 | 0.06 |
| Cl | wt% | 0.15 | 0.36 | 0.07 | 0.09 | 49.4 |
| K | wt% | 0.77 | 0.73 | 0.11 | 0.1 | <0.04 |
| Si | wt% | 0.54 | 0.27 | 2.0 | 0.6 | 0.39 |
| Ca | wt% | 3.74 | 0.45 | 4.17 | 0.78 | 0.29 |
| Na | wt% | 0.01 | 0.003 | 0.19 | 0.15 | <0.04 |
| Mg | wt% | 0.12 | 0.21 | 0.18 | 0.06 | <0.03 |
| Al | wt% | 0.04 | 0.01 | 0.77 | 0.11 | 0.12 |
| Fe | wt% | 0.03 | 0.01 | 0.74 | 0.11 | 0.09 |
| P | wt% | 0.13 | 0.15 | 0.03 | 0.04 | 0.008 |
| Pb | wt% | na | na | na | na | 1.1 |
Wool is a natural fiber of animal origin, and thus presents a high protein content (keratin), which has a polypeptide chain with amino acid side chains (cystine, cysteine, cysteic acid, and methionine).19 Among the selected feedstock, wool has the highest sulfur content (2.7 wt%) and the lowest ash content (1.2 wt%).
PVC is a fossil-based resource and one of the most widely-used types of plastics, it has a very high chlorine content of 49.4 wt%. It contains approximately 1 wt% of lead, which can be attributed to the usage of lead stabilizers during the manufacturing process. These stabilizers are commonly employed to enhance the stability and durability of PVC products.20
 | ||
| Fig. 4 Sulfur and chlorine distribution in recovered leachates and solid residue of the feedstocks (chemical fractionation results). | ||
According to,8 cations (K+, Na+, and Ca2+), anions (Cl−, HPO42−, H2PO4−, SO42−), and Si(OH)3O−, found in the water and NH4Ac leachates, come from inorganic forms of dissolved salts in plant fluids or precipitated soluble salts in dried biomass. S, Cl and P that are insoluble and thus found in the solid residue are included in organic covalent forms. In addition, metal cations bound to anionic organic groups in the organic molecules constituting the biomass (ex: COO−), typically, K+, Na+, Mn2+, Ca2+, Mg2+, Fe2+, and Al3+ are soluble in NH4Ac where they undergo ion exchange and are replaced with ammonium ions and thus released to the liquid phase. Other minerals such as calcium salts and silicon compounds that are precipitated naturally in the biomass and are closely embedded in carbonaceous material remain insoluble.
Significant differences can be noted in the distribution of sulfur and chlorine in the leachates and the solid residue between the agricultural residues, cardboard, and wool, which means, as mentioned before, differences in the relative quantities of their two chemical forms (organic and inorganic).
For the colza straw and corn residues, 67% and 51% of S, respectively, and more than 85% of Cl were found in the water (predominantly) and NH4Ac leachates. Thus, more than half of the sulfur and almost all the chlorine are present in the inorganic form of soluble salts in the agricultural residues. To get more information on the association of S and Cl with other key inorganic elements, Table 2 presents the fraction of major ash elements (K, Ca, and Si) measured in the water leachates where they could be associated to S or Cl. A high fraction (>75%) of the potassium is recovered in water where, similarly to S and Cl, it is present in an inorganic form of soluble salts. The molar ratios K/Cl and K/(S + Cl) in the initial feedstock and in the water leachates are higher than 1. This indicates that the number of moles of potassium is sufficiently high to be able to bond with chlorine and with sulfur. Possible forms of salts made of these elements are potassium chlorides KCl and KClO4.9 The molar ratio K/S is higher than 2 only for the corn residues for which S could thus be present in the form of potassium sulfates K2SO4.21 K can also be present in other soluble forms such as carbonates (K2CO3)5 and phosphates K2HPO4.22 For colza straw and corn residues, 20% and 50% of the calcium is recovered in the water, respectively. It can be present in the form of sulfates such as CaSO4,23 or even as calcium oxalates (CaC2O4).24 The insoluble part of Ca can be organically associated into the biomass (Ca–COO) or in carbonates (CaCO3).24 Silicon is only slightly leached in water, which shows that it mainly remains in the solid residue. It can be present as included minerals (silica SiO2) or excluded minerals coming from soil sand and clay (SiO2 and aluminosilicates often with some K, Na, or Ca).
| K | Ca | Si | |
|---|---|---|---|
| Colza straw | 77% | 20% | 3% |
| Corn residues | 77% | 50% | 0.25% |
For cardboard, less than 15% of sulfur is recovered in the leachates as inorganic sulfur, while 74% of sulfur is measured in the solid residue, indicating that S is mainly in an organic form in the cardboard. In the Kraft pulping process, sodium sulfide Na2S is used to dissolve the lignin that binds the cellulose fibers together in the lignocellulosic biomass. Therefore, at the end of the process, remainder of the sulfur additives could be found as minerals or be included in the lignin structure (organically associated).
For wool, all the sulfur is in the solid residue, which shows that S is mainly in an insoluble organic form. This is in agreement with what was expected, with sulfur mostly present in amino acids in this type of feedstock.
Lixiviation is not adapted to solid plastic materials. However, it is well known that the organic form of chlorine is dominant in PVC that contains organically chlorinated compounds.
3.2. Sulfur and chlorine behavior in pyrolysis
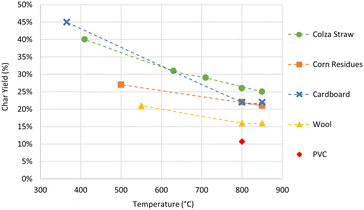
Fig. 6 illustrates the sulfur and chlorine retention in the chars as a function of maximum pyrolysis temperature. Error bars show the relative uncertainty calculated by the propagation method. For colza straw, 46% of the initial sulfur is retained in the char produced at the pyrolysis temperature of 410 °C. The sulfur retention decreases to about 35% in the chars produced at higher temperatures (630 °C, 710 °C, 800 °C and 850 °C). For corn residues, the sulfur retention in the char is between 24 and 30% in the pyrolysis experiments performed at 500 °C, 800 °C and 850 °C. For cardboard, 35% of the initial sulfur is found in the char produced at a pyrolysis temperature of 365 °C. However, at 800 °C and 850 °C, the sulfur retention decreases to 15%. On the other hand, only 5% to 7% of the initial sulfur is retained in the chars of wool produced at 550 °C, 800 °C and 850 °C.
These experimental results show a low influence of the final pyrolysis temperature between 500 and 850 °C for agricultural residues. This is in agreement with other studies that found a slight decrease of the total sulfur content of the char4 or a constant release of sulfur2 with increasing temperature from 550 °C to 900 °C during pyrolysis of wheat straw. Temperature increase from 550 to 850 °C has also a low influence on the retention of sulfur in the char for wool. Nonetheless, it is not the case for cardboard when comparing between pyrolysis temperatures of 365 °C and 800–850 °C.
To explain the different results seen among the four feedstocks, the sulfur retention in the char after pyrolysis can be related to the initial chemical form of sulfur in the feedstock. According to studies found in the literature,2,3 organic forms of sulfur are more volatile and easily released into the gas phase even at low temperatures below 500 °C, compared to inorganic forms. For wool, the fraction (5–7%) of the initial sulfur retained in the solid residue is close to the inorganic fraction (3%) of sulfur measured in the original resource. This shows that the dominating organic form of S in wool is probably completely released in the gas phase before reaching 550 °C. Moreover, for cardboard in which S is mainly in organic forms, S retention decreases as temperature increases, until it reaches 15% at 800 °C and 850 °C, which could correspond to its initial inorganic fraction. If S is considered to be included in the lignin structure in cardboard, the lignin decomposition on a wide temperature range of about 250 to 600 °C (ref. 26) could explain why S continues to be released above 365 °C.
For the agricultural residues, the fraction of S retained in the chars (24–35%) is lower than the fraction of inorganic S (51–67%) quantified by lixiviation. This shows that besides the organic S initially released at low temperatures below 500 °C, a part of inorganic S is also released. Indeed, pyrolysis studies1–3,5 showed that, during the devolatilization process, the organically associated S is released via the formation of highly reactive SH radicals that either remain in the char or extract H, C, or O from the char, to form later H2S, COS, or SO2:
| Fuel − S + Heat → H2S + COS + … + Char − S |
As for inorganic sulfur, at temperatures below 1000 °C, the evaporation rates of inorganic sulfates are low. However, previous authors2,27 showed that a part of these sulfates can be transformed to char-bound S and thus be released to the gas phase. This could explain why the fraction of S retained in the chars is lower than the fraction of inorganic S in our results and could confirm that both the organically and the inorganically associated S contribute to the amount of S released.
There is no significant evolution of the chlorine retention in the chars for the agricultural residues, considering the uncertainties. About 50% of the initial chlorine is retained in the char between 500 °C and 850 °C for the corn residues. In the colza straw, 67–75% of the chlorine remains in the char between 410 °C and 710 °C, while only 55% is retained in the char at 800 and 850 °C. In the case of the PVC pyrolysis experiment at 800 °C, two impingers filled with deionized water were placed downstream of the reactor, to collect the chlorinated species (HCl, KCl, NaCl) released in the gas phase. Around 90% of the initial chlorine was measured in the impingers, and therefore released in the gas phase. This allows us to consider that less than 10% of the initial chlorine is retained in the char in the case of PVC. The difference in the retention of chlorine between the agricultural residues and PVC chars can be related to its initial chemical form in the resource. PVC, having the totality of Cl in an organic form, undergoes three steps (dehydrochlorination, condensation and fragmentation) during the devolatilization stage, in the temperature range 150–500 °C, which results in the complete release of Cl as HCl.28,29 Furthermore, it has been reported that, at molar ratio Cl/Pb = 3,30 the hydrogen chloride (HCl) produced from the decomposition of PVC during pyrolysis can potentially reacts with lead oxide (PbO) to form lead(II) chloride (PbCl2) that subsequently melts and volatilizes at temperatures exceeding 501 °C.30,31 In our case, despite the presence of a notable quantity of lead in our PVC sample originating from lead stabilizers used in PVC manufacturing, the molar ratio of Cl to Pb is considerably higher, making it challenging to confirm any significant interaction between the two elements. On the other hand, more than 85% of chlorine is present in an inorganic form of soluble salts in the agricultural residues (Fig. 4). According to previous studies,1,3,4 under pyrolysis conditions, at temperatures under 700 °C, the release of a significant fraction of the Cl (40–60%) in the fuel as HCl is attributed to an ion-exchange reaction of KCl and oxygen-containing functional groups that are either present in the fuel or formed during the devolatilization step:
| R–COOH + KCl (s) → R–COOK + HCl (g) |
Above 700 °C, the evaporation of KCl is considered to be the main pathway for the release of Cl. According to our results, only a part of inorganic chlorine is released in the gas phase in pyrolysis experiments.
According to literature (Section 1), the behavior of inorganic forms of S and Cl can depend on the ash composition of the feedstock. To have a deeper understanding of the influence of the ash-forming elements on the sulfur and chlorine behavior during pyrolysis, the chars were analyzed for their inorganic composition. Fig. 7 presents the retention in the char of the major inorganic elements (K, Ca, Si) after pyrolysis for the two agricultural residues. Error bars show the relative uncertainty calculated by the propagation method.
 | ||
| Fig. 7 Major ash-forming elements retention in the char as a function of maximum pyrolysis temperature. | ||
K totally remains in the char below 700 °C for both agricultural residues. In the original feedstock, K is mainly present in soluble inorganic salts (KCl, KClO4, K2SO4…). Under 700 °C, the evaporation rates of these inorganic salts are low and as said in the preceding paragraph, K can combine with volatile organic compounds and transform into organic K (R–COOK). This could explain its total retention in the char at temperatures below 700 °C. At 800 °C and 850 °C, a low release is observed. By comparing with Fig. 6, the retentions of K and Cl follow a similar profile, which could suggest that the release of metal chlorides, particularly KCl, can start occurring at temperatures above 800 °C. This is in agreement with previous studies that have shown that only a small amount (5–10%) of K is released at temperatures below 700 °C,3–5 and that the release of alkali metals and chlorine is closely related to one another.3
All the silicon is retained in the chars at the different temperatures. As expected, Si is not volatile and remains in the silicate matrix.8
67 to 83% of the Ca is retained in the char between 365 and 850 °C for colza straw, while 71% to 84% of Ca remains in the corn residue char between 500 and 850 °C. Even at temperatures as low as 365 °C, there is a fraction of Ca released in the gas that is not negligible (15–35%). The temperature has no significant influence on the Ca retention considering the uncertainties. As mentioned before, 80% and 50% of the total Ca is organically bond or present as carbonates for colza straw and corn residues, respectively. Literature reports that significant fractions of Ca are volatilized during cane bagasse and cane trash pyrolysis at 500 °C,32 and cane bagasse gasification between 650 and 1170 °C.13 The reduction in Ca bound to the organic phase after gasification and the observation of acetate ions in the syngas indicated that during gasification, organically bound Ca (COO–Ca) was volatilized releasing Ca2+, and COO− ions. The thermal breakdown of carboxylates (COO) in the biomass from large molecular mass structures and the subsequent release of light carboxylates might be an important mechanism for the release of Ca that is organically bound (Ca–COO) from the pyrolysis of biomass at low temperatures. This provides an explanation for the release of Ca in our experiments.
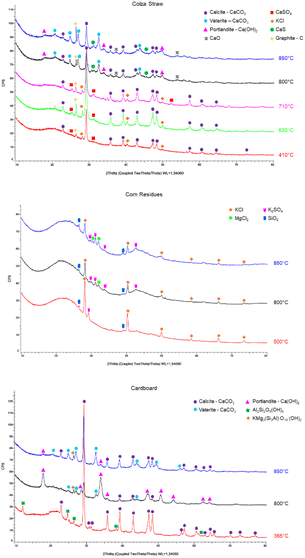 | ||
| Fig. 8 XRD diffractograms of colza straw, corn residues and cardboard chars at their different pyrolysis temperatures. | ||
 | ||
| Fig. 9 SEM observations of colza straw (a), corn residues (b), wool (c) and cardboard (d) chars at 800 °C. | ||
Since wool samples have a very low ash content, their chars showed no clear mineral phases and therefore their XRD results are not presented. The main part of wool chars consists in a carbonaceous matrix containing some inorganic elements, which may explain their amorphous nature seen in the XRD analysis. The SEM-EDX analysis showed the presence of sulfur along with calcium in the carbonaceous matrix.
For colza straw, in the samples pyrolyzed between 410 and 710 °C, peaks are observed at 2θ = 28.2°, 2θ = 40.3° and 2θ = 50° and are attributed to sylvite (KCl). In the SEM-EDX analysis, Cl is found in a phase at 710 °C containing high K content, which could correspond to KCl. However, for the chars produced at higher pyrolysis temperatures, 800 °C and 850 °C, no presence of sylvite is found in XRD. These observations could confirm that above 800 °C there is a release of Cl and K due to the metal chlorides volatilization. In the SEM-EDX analysis, at 800 °C and 850 °C, the carbonaceous matrix mainly contains Ca, with some K, Mg, S, P and Cl.
The SEM-EDX analysis shows the presence of sulfur with the carbonaceous matrix along with high amount of Ca and some K, P and Mg. In the XRD results, calcium sulfate CaSO4 (2θ = 25.3° and 2θ = 31.3°) is identified in the chars produced at 410 °C, 630 °C, and 710 °C. At 710 °C, the intensity of the two peaks is the highest, with a third additional peak present at 2θ = 52.3°; this can be due to the larger devolatilization of the carbon matrix at higher temperatures, inducing a higher concentration of CaSO4. No peaks corresponding to CaSO4 are found for the chars produced at 800 °C and 850 °C. On the other hand, the presence of calcium sulfide CaS is confirmed by the peaks at 2θ = 31.3° and 2θ = 45.5° in these chars. This could show that the inorganic sulfates, besides being partially transformed to organic S bound to the char and released in the gas as discussed previously, can also be reduced to inorganic sulfide at temperatures above 700 °C, as seen in previous studies.2 The main crystalline phase detected in the chars produced under 740 °C is calcite (CaCO3). However, for the chars produced at higher temperatures (800 °C and 850 °C), calcite and vaterite, two polymorphs of calcium carbonate, are the major predominant crystalline phases. Additional peaks are then observed at 2θ = 18°, 28.5°, and 34° and at 2θ = 37.5° which are attributed to the presence of portlandite (Ca(OH)2) and lime (CaO) respectively.
For the corn residues char at 500 °C, the SEM-EDX analysis show phases containing K and Cl appearing as fine particles attached to the char, which could correspond to KCl. As temperature rises to 800 °C and 850 °C, the concentration of these particles decrease. In addition, grains containing Si and O, which may correspond to silicon oxide SiO2, are found dispersed on the matrix in the different chars. These observations are consistent with the XRD results. Indeed, for all temperatures, silica (SiO2) is detected in the corn residue chars by XRD. Sylvite (KCl) is the major crystalline phase detected with several peaks. Their intensity decreases with the increase of the temperature above 500 °C, which can be explained by the partial release of KCl at these temperatures. Contrary to colza straw char, KCl is still detected in the chars produced at 800 and 850 °C. On the other hand, for the chars produced at 800 °C and 850 °C, magnesium chloride (MgCl2) is present with a peak at 2θ = 32° and two peaks (2θ = 30.3° and 2θ = 32°) at 850 °C. According to,32 while Cl may be bonded into the char organic matrix, it may also react with Mg and Ca to form refractory MgCl2 or CaCl2. Moreover, additional peaks appear at 2θ = 30°, 2θ = 31.2°, 2θ = 34° and 2θ = 43° corresponding to potassium sulfate K2SO4, the only sulfur-containing phase for this feedstock.
No crystalline structures containing S or Cl are identified in the diffractograms of the cardboard char samples pyrolyzed at the three different temperatures (365 °C, 800 °C and 850 °C). Moreover, in the SEM-EDX analysis, neither S nor Cl are observed in the chars at the different temperatures This could be due to the very low content of S and Cl remaining in the char samples (under 0.1 wt%). The main crystalline phase detected in the char produced at 365 °C is calcite CaCO3. In addition, three peaks are identified at 2θ = 12.2°, 2θ = 24.6° and 2θ = 26.5° attributed to kaolin (Al2Si2O5(OH)4). Both calcite and kaolin (clay) are inorganic minerals used in the paper industry as fillers and as surface coatings.33 For the chars produced at 800 °C and 850 °C, CaCO3 is present in two of its polymorphs: calcite and vaterite. In addition, a silicate of formula KMg3(Si3Al)O10(OH)2 is found. Portlandite Ca(OH)2 also appears to be present especially in the char at 800 °C.
4. Conclusions
The objective of this study was to characterize the sulfur and chlorine release during pyrolysis of biomass and waste and to evaluate the effect of the chemical forms of S and Cl, the chemical composition of the feedstock, and the temperature on this release. A leaching technique was applied to the selected resources to determine the repartition of initial sulfur and chlorine into organic and inorganic forms. In the two agricultural residues, S was found in its two chemical forms as organically bonded to the carbon matrix and as inorganic soluble sulfates (CaSO4 in the Ca-rich colza straw and K2SO4 in the K-rich corn residues). Cl is mainly in the form of inorganic salts (KCl, KClO4…). However, in the waste components wool and cardboard, sulfur was mainly found in an organic form, while in PVC Cl is known to be organically bonded in the polymer structure. Pyrolysis tests performed at variable temperatures between 420 and 850 °C show a resource-dependent retention of S and Cl in the char. For wool and cardboard, approximately 100% and 65% of S is released in the gas respectively, which corresponds to the fraction of S initially present in organic form. For PVC initially containing organically chlorinated compounds, more than 90% of the Cl is released into the gas as HCl. Thus, the organic forms of S and Cl are volatile and easily released into the gas phase even at low temperatures without interacting with other inorganic elements, even in high ash content resources. For agricultural residues, Cl is mainly in the form of inorganic salts (KCl, KClO4…), while about half of the S is in an organic form bound to the C matrix and the other half is in inorganic form (sulfates: K2SO4, CaSO4…). In pyrolysis, the release of inorganic forms in the gas is partial. Different crystallized S containing forms are identified in the chars (CaSO4 or CaS for colza straw, K2SO4 for corn residues…), which depend on the pyrolysis temperature and the ash composition. These results constitute a basis for further study on in situ cleaning methods based on interactions between inorganic elements to limit the release of inorganic volatile pollutants.Author contributions
Hala Braidy – investigation, visualization, writing – original draft; Sylvie Valin – writing – review & editing, supervision; Fabrice Patisson – writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Maguelone GRATEAU, Maria Antonia MAROTTA and Sebastien THIERY for their contribution during this paper preparation.Notes and references
- J. N. Knudsen, P. A. Jensen and K. Dam-Johansen, Energy Fuels, 2004, 18, 1385–1399 CrossRef CAS.
- J. N. Knudsen, P. A. Jensen, W. Lin, F. J. Frandsen and K. Dam-Johansen, Energy Fuels, 2004, 18, 810–819 CrossRef CAS.
- J. M. Johansen, J. G. Jakobsen, F. J. Frandsen and P. Glarborg, Energy Fuels, 2011, 25, 4961–4971 CrossRef CAS.
- P. A. Tchoffor, K. O. Davidsson and H. Thunman, Energy Fuels, 2013, 27, 7510–7520 CrossRef CAS.
- P. A. Tchoffor, F. Moradian, A. Pettersson, K. O. Davidsson and H. Thunman, Energy Fuels, 2016, 30, 10435–10442 CrossRef CAS.
- M. Wang, Y. Hu, J. Wang, L. Chang and H. Wang, J. Anal. Appl. Pyrolysis, 2013, 104, 585–592 CrossRef CAS.
- Y. Gu, J. Yperman, J. Vandewijngaarden, G. Reggers and R. Carleer, Fuel, 2017, 202, 494–502 CrossRef CAS.
- M. Zevenhoven, P. Yrjas, B.-J. Skrifvars and M. Hupa, Energy Fuels, 2012, 26, 6366–6386 CrossRef CAS.
- L. Deng, X. Huang, Y. Tie, J. Jiang, K. Zhang, S. Ma and D. Che, J. Energy Inst., 2022, 103, 117–127 CrossRef CAS.
- E. Daouk, R. Sani, D. Pham Minh and A. Nzihou, Fuel, 2018, 225, 54–61 CrossRef CAS.
- H. Braidy, S. Valin and F. Patisson, 30th European Biomass Conference and Exhibition Search PubMed.
- S. Cheah, S. C. Malone and C. J. Feik, Environ. Sci. Technol., 2014, 48, 8474–8480 CrossRef CAS PubMed.
- C. Andrea Jordan and G. Akay, Fuel, 2012, 91, 253–263 CrossRef CAS.
- S. A. Benson and P. L. Holm, Ind. Eng. Chem. Prod. Res. Dev., 1985, 24, 145–149 CrossRef CAS.
- L. L. Baxter, T. R. Miles, T. R. Miles, B. M. Jenkins, T. Milne, D. Dayton, R. W. Bryers and L. L. Oden, Fuel Process. Technol., 1998, 54, 47–78 CrossRef CAS.
- M. Zevenhoven-Onderwater, PhD thesis, Aabo Akademi, 2001.
- O. Sosa Sabogal, S. Valin, S. Thiery and S. Salvador, Chem. Eng. Res. Des., 2021, 173, 206–214 CrossRef CAS.
- V. F. de Almeida, A. Gómez-Barea, J. Arroyo-Caire and I. Pardo, Waste Biomass Valorization, 2020, 11, 6869–6884 CrossRef.
- A. C. Pina, N. Tancredi, C. O. Ania and A. Amaya, J. Mater. Sci. Eng. B, 2021, 268, 115115 CrossRef CAS.
- A. CHABROL and S. GIROIS, Tech. Ing., Plast. Compos., 2013, am3233 Search PubMed.
- S. V. Vassilev, D. Baxter, L. K. Andersen, C. G. Vassileva and T. J. Morgan, Fuel, 2012, 94, 1–33 CrossRef CAS.
- P. Piotrowska, M. Zevenhoven, K. Davidsson, M. Hupa, L.-E. Åmand, V. Barišić and E. Coda Zabetta, Energy Fuels, 2010, 24, 333–345 CrossRef CAS.
- F. Suárez-García, A. Martínez-Alonso, M. Fernández Llorente and J. M. D. Tascón, Fuel, 2002, 81, 1161–1169 CrossRef.
- A. Pettersson, M. Zevenhoven, B.-M. Steenari and L.-E. Åmand, Fuel, 2008, 87, 3183–3193 CrossRef CAS.
- M.-H. Cho, Y.-K. Choi and J.-S. Kim, Energy, 2015, 87, 586–593 CrossRef CAS.
- P. de Wild, PhD thesis, Rijksuniversiteit Groningen, 2011.
- P. A. Tchoffor, K. O. Davidsson and H. Thunman, Energy Fuels, 2014, 28, 6953–6965 CrossRef CAS.
- M. Bläsing, M. Weigand, J. Fasenacht and M. Müller, Fuel Process. Technol., 2015, 134, 85–91 CrossRef.
- A. Casazza, E. Spennati, A. Converti and G. Busca, Chem. Eng. Trans., 2019, 74, 1141–1146 Search PubMed.
- S.-J. Wang, H. Zhang, L.-M. Shao, S.-M. Liu and P.-J. He, Chemosphere, 2014, 117, 353–359 CrossRef CAS.
- W. Xu, G. Song, Q. Song and Q. Yao, Fuel Process. Technol., 2022, 227, 107089 CrossRef CAS.
- D. M. Keown, G. Favas, J. Hayashi and C.-Z. Li, Bioresour. Technol., 2005, 96, 1570–1577 CrossRef CAS PubMed.
- M. A. Hubbe and R. A. Gill, Bioresources, 2016, 11, 2886–2963 CAS.
| This journal is © The Royal Society of Chemistry 2023 |