 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cellulose as sacrificial agents for enhanced photoactivated hydrogen production†
María Isabel
Alvarado-Ávila
a,
Stefano
De Luca
a,
Ulrica
Edlund
b,
Fei
Ye
 a and
Joydeep
Dutta
a and
Joydeep
Dutta
 *a
*a
aFunctional NanoMaterials Group, Department of Applied Physics, School of Engineering Sciences, KTH Royal Institute of Technology, Hannes Alfvéns väg 12, 114 19 Stockholm, Sweden. E-mail: joydeep@kth.se
bFibre and Polymer Technology, KTH Royal Institute of Technology, SE 100 44 Stockholm, Sweden
First published on 21st March 2023
Abstract
The search for new energy sources together with the need to control greenhouse gas emissions has led to continued interest in low-emitting renewable energy technologies. In this context, water splitting for hydrogen production is a reasonable alternative to replace fossil fuels due to its high energy density producing only water during combustion. Cellulose is abundant in nature and as residuals from human activity, and therefore a natural, ecological, and carbon-neutral source for hydrogen production. In the present work, we propose a sustainable method for hydrogen production using sunlight and cellulose as sacrificial agents during the photocatalytic water splitting process. Platinum (Pt) catalyst activates hydrogen production, and parameters such as pH of the system, cellulose concentration, and Pt loading were studied. Using different biomasses, we found that the presence of hemicellulose and xyloglucan as part of the molecular composition considerably increased the H2 production rate from 36 μmol L−1 in one hour for rapeseed cellulose to 167.44 μmol L−1 for acid-treated cellulose isolated from Ulva fenestrata algae. Carboxymethylation and TEMPO-oxidation of cellulosic biomass both led to more stable suspensions with higher rates of H2 production close to 225 μmol L−1, which was associated with their water solubility properties. The results suggest that cellulosic biomass can be an attractive alternative as a sacrificial agent for the photocatalytic splitting of water for H2 production.
1. Introduction
According to the International Energy Agency (IEA), global energy demands increased by 4.6% during 2021, overcoming the 4% decline in 2020 due to the economic slowdown attributed to the Covid-19 pandemic.1 This has led to an increase in the consumption of non-renewable energy sources like coal and fossil fuels, increasing the concentration of carbon dioxide in the atmosphere to over the threshold of 400 ppm. The share of fossil fuels in the global energy mix has been around 80% for decades.2 Efforts are being made across the globe to replace and reduce the use of fossil fuels by increasing the use of renewable energies from solar photovoltaics to windmills and green H2 usage, amongst others. Hydrogen is one of the best candidates to replace the use of carbon-based fuels and achieve total decarbonization in maritime transportation,3 aircraft,4 road transportation, and in heating of building and manufacturing activities.5 Not only due to its superior energy density (120 MJ kg−1 compared to 44 MJ kg−1 of gasoline) but also because the only byproduct of combustion is water which would assist in the reduction of greenhouse gas emission, water splitting for hydrogen production is being extensively studied by researchers.Several methods to produce hydrogen have been developed over the last decades. The most used commercial process is steam methane reforming, which contributes to 80 to 85% of global hydrogen production, followed by coal gasification and electrolysis.6–8 Economical and efficient methods to produce hydrogen where renewable energies like solar, wind, and biomass are involved are attracting continued attention. The use of solar energy for hydrogen production is considered the most promising because of being an unlimited resource with irradiation reaching 6 kW h m−2.9 Water splitting via photocatalysis and photo/electro-catalysis began to attract attention since Fujishima and Honda found that titanium dioxide (TiO2), working as a photoanode, split water in a photo/electro-chemical cell even when no current was applied.10 It is well known that semiconductors like titanium dioxide (TiO2), cadmium sulfide (CdS), zinc oxide (ZnO), tungsten oxide (WO3), etc., with band gaps around or lower than 3 eV are suitable for water oxidation allowing oxygen and hydroxyl radical production.11–13 Photocatalyst stability in water solution is another requirement for its implementations, and hence TiO2 has been extensively investigated as a photocatalyst due to its ability to absorb light mainly in the UV range with exceptional stability in a wide pH range.14–16
Nevertheless, to solve the electron–hole recombination problems, incorporating a co-catalyst like metal nanoparticles17 or another semiconductor18,19 acting as an electron scavenger to lower the activation barrier for the reaction has been studied.20–24 On the other hand, the addition of sacrificial agents like sodium sulfide (Na2S), ethanol (C2H5OH), methanol (CH3OH), and different type of alcohols acting as hole scavengers25,26 improves the separation of charges.27 Even though methanol or isopropanol has been extensively used as electron acceptors during the photocatalytic water splitting process,28,29 it is also a valuable resource and expensive fuel in the industry by itself, limiting its economic viability. Cellulosic biomass is a more reasonable alternative as a sacrificial agent, especially since it is generally considered to be a carbon-neutral emission source where the carbon dioxide absorbed during the plant growth is then released during hydrogen production.30
Cellulose is plentiful in human-created residuals like straw, tree branches, wastepaper, cotton waste, fruit peels, etc., making it a sustainable source to produce hydrogen. Lignocellulose represents a major fraction of above 90% of all terrestrial plant biomass (170 billion × 109 tons per annum). Lignocellulosic biomass contains cellulose (38–50%), which is composed of linear chains of β-1,4 linked D-glucose units and is insoluble in water, along with hemicelluloses (23–38%) and lignin (15–25%). The current use of cellulose as a fuel precursor for alcohol, sugar, and hydrogen production is based on complex thermochemical processes like steam gasification, fast pyrolysis, hydrolysis, and fermentation.31–33 Lignocellulose biomasses can serve as sacrificial agents (electron donors) to reduce e−–h+ recombination in a photocatalytic reaction leading to a more efficient process since many organic compounds like alcohols, polyols, sugar as well as organic acids act as electron donors.34 Electrons in a semiconductor can be excited from the valence band (VB) to the conduction band (CB) upon absorption of photons with energy higher than the band gap. The photo-generated electrons and holes have certain electrical potential and if the potential of the holes is positive enough, they can oxidize water molecules according to two types of reactions shown hereunder:35
| 2H2O → O2 + 4H+ + 4e−, E0 = 1.23 V | (1) |
| H2O → ˙OH + H+ + e−, E0 = 2.73 V | (2) |
The reactions are reported together with the respective potential vs. Standard Hydrogen Electrode (SHE) at which they occur in acidic pH, labeled as E0. Reaction (1) requires a considerably lower potential than reaction (2). Moreover, while reaction (1) leads to the formation of O2, reaction (2) leads to the formation of hydroxyl radicals ˙OH. The electrons on the right side of both reactions are captured by holes generated in the VB of the photocatalyst, leaving protons in the medium, while photoexcited electrons are used to reduce such protons according to the following reaction, resulting in the evolution of H2:
| 2H+ + 2e− → H2, E0 = 0 V | (3) |
The potentials needed for H2O oxidation and H2 evolution reactions are affected by the pH of the system.
Photocatalytic degradation of cellulose-containing biomass can be a simple and promising path for hydrogen production by utilizing cellulose and the created intermediates as sacrificial agents, but only a few studies with this approach have been reported in the literature.36–40 A comprehensive study of the degradation of different types of biomasses with different cellulose, hemicellulose, and lignin concentrations is limited. In this work, we report photocatalytic hydrogen production by using different sources of cellulose and cellulose derivatives as sacrificial agents. TiO2 and platinum (Pt) were used as a photocatalyst and co-catalyst, respectively. Parameters like pH, Pt loading, and biomass concentration were studied to better understand the hydrogen production mechanism and limitations of using cellulosic biomass as a sacrificial agent.
2. Experimental
2.1. Materials
Titanium dioxide (TiO2) nanoparticles (commercial P25), sodium dodecylbenzene sulfonate (SDBS), sodium borohydride (NaBH4), sodium hydroxide (NaOH), sodium sulfide nonahydrate (Na2S·9H2O), D-glucose (dextrose), and hexachloroplatinic acid (H2PtCl6) were purchased from Sigma Aldrich Chemie GmbH (Taufkirchen, Munich, Germany). Hydrochloric acid (HCl) and nitric acid (HNO3) were from Merck (Darmstadt, Germany) and terephthalic acid disodium salt (TPA) and 2-hydroxyterephthalic acid (hTPA) from Tokyo Chemical Industry (Zwijndrecht, Belgium). All the chemicals were used as received and aqueous solutions were prepared with deionized water (DI water).Table 1 shows different types of cellulosic biomasses studied in this work, including bleached and un-bleached lignocellulose extracted from rapeseed straw, α-cellulose from spruce, cellulose extracted from U. fenestrata algae, acid-treated cellulose extracted from U. fenestrata algae and TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl radical)-oxidized CNF (cellulose nanofibers). These celluloses were prepared according to the processes described in the references shown in Table 1 except for sodium carboxymethyl cellulose (CMC-Na) that was acquired from Carl Roth, and had an average degree of substitution of 0.8.
| Biomass | Carbohydrate and lignin composition | Crystallinity index | Reference |
|---|---|---|---|
| Lignocellulose extracted from rapeseed straw | Cellulose 80% | Not available | 41 |
| Hemicelluloses 11% | |||
| Lignin 8% | |||
| Lignocellulose extracted from rapeseed straw – H2O2 bleached | Cellulose 85% | 83% | 41 |
| Hemicelluloses 8% | |||
| Lignin 6% | |||
| α-Cellulose fibers from spruce pulp | Cellulose 99% | 71% | Supplied by Södra Cell AB |
| Cellulose extracted from U. fenestrata | Cellulose 85% | 48% | 42 |
| Xyloglucan 15% | |||
| Acid-treated cellulose extracted from U. fenestrata | Cellulose 91% | 63% | 42 |
| Xyloglucan 9% | |||
| TEMPO-oxidized CNF prepared from never-dried spruce pulp | Cellulose 99% | Not available | 43 |
| CMC-Na | The sodium salt of cellulose with carboxymethyl pendant groups, very pure. Degree of substitution = 0.8 | Not available | Supplied by Carl Roth |
2.2. Photocatalyst preparation
As received, P25 TiO2 was covered with platinum nanoparticles (Pt NPs) following a modified version of the procedure reported by Yao et al.44–46 In brief, 120 mg of sodium dodecylbenzene sulfonate (SDBS) surfactant was dissolved in 30 mL of deionized (DI) water. When the SDBS was completely dissolved, 150 mg of P25 was added under continuous stirring. Finally, a solution of 3 mM hexachloroplatinic acid was prepared, and pre-determined volumes were added to obtain different Pt mass loadings on TiO2. The solution was sonicated for 30 min and stirred for 1 h at room temperature. Following this, the recipient containing the solution was transferred to an oil bath preheated to 70 °C, and 15.8 mg of NaBH4 was added to reduce the Pt in the solution and thereafter aged for 2 h. Following the aging process, the solution was cooled down to room temperature, centrifuged to recover the photocatalyst, and then rinsed several times with DI water and ethanol prior to further use. Dry powder was obtained by heating overnight at 60 °C in an atmospheric oven and stored for further use. Three photocatalysts were prepared with a Pt loading of 0.2, 0.5, and 1 wt% labeled as 0.2Pt–TiO2, 0.5Pt–TiO2 and 1Pt–TiO2, respectively.2.3. Characterization
Microstructure and elemental analysis of the photocatalysts were carried out by using a ZEISS Ultra-55 scanning electron microscope (SEM) equipped with energy-dispersive X-ray spectroscopy (SEM-EDX) working at 10 kV. The titanium dioxide and Pt-coated Titanium dioxide nanoparticles were also studied and crystal structure was determined using high resolution transmission electron microscopy (HR-TEM, JEOL JEM-2100F, Akishima, Tokyo). Optical absorption was measured by PerkinElmer Lambda 750 UV/Vis spectrophotometer. Photoluminescence (PL) measurements were used to determine the hydroxyl radicals (˙OH) production using a PerkinElmer LS 55 Fluorescence spectrometer. Hydroxyl radicals were determined using terephthalic acid disodium (TPA) salt as a selective scavenger of ˙OH. TPA reacts with ˙OH producing highly fluorescent 2-hydroxyterephthalic acid (hTPA) that can be determined by PL spectroscopy. In brief, 7.5 mg of the photocatalyst was added to 25 mL of 200 μM TPA solution and magnetically stirred in darkness for 30 min to reach adsorption equilibrium. Nitrogen gas was bubbled for 10 min to remove dissolved oxygen. Then the solution was irradiated with a commercial UVA light source (OSRAM Ultra-Vitalux 300 W lamp) for 30 min, and samples were extracted every 10 minutes. PL measurements were used to determine hTPA concentration using an excitation wavelength of 325 nm (calibration curve for hTPA concentration is provided as Fig. S1 in the ESI†). DelsaNano C was used to determine the zeta potentials and particle size using dynamic light scattering technique. Total Pt loading was determined by digesting the particles in aqua regia and following this, after 50 times dilution, inductively coupled plasma-optical emission spectroscopy (ICP-OES) was used to determine the absolute quantities (Thermo Scientific iCAP 6500).2.4. Photocatalytic hydrogen production
The experiments were run in a 25 mL three-necked round-bottomed Pyrex glass flask filled with 25 mL of the studied suspension in water with 0.3 mg mL−1 of photocatalyst and 0.25 mg mL−1 of respective cellulose samples (Table 1). The composition of sacrificial agents varied from 99% cellulose to 85%, with or without the presence of hemicelluloses and lignin. The crystallinity index (CI) measures the relative amount of crystalline cellulose in a two-phase model to describe cellulose chains with crystalline and amorphous regions.47The initial suspension pH was adjusted to 5 using HCl (0.1 M) and NaOH (0.1 M). Before the start of each experiment, the system was sonicated for 30 min and then purged using nitrogen gas for another 30 min to reduce dissolved oxygen. The light source simulating natural sunlight was placed at a distance such that the irradiation flux on the sample was around 1000 W m−2. Hydrogen gas concentration was continuously measured using a H2 microsensor from Unisense, Denmark. The calibration curve can be found in ESI (Fig. S2†). An external air blower was used to keep the temperature stable. The reactor was sealed with rubber caps, and the sensors were connected directly into the system through syringe probes.
3. Results and discussions
Microstructure of TiO2 (P25) photocatalyst and TiO2 with 0.2, 0.5, and 1% Pt loading are shown in Fig. 1. From the SEM micrographs, no clear evidence of the Pt NPs deposition could be observed possibly due to the small sizes. Energy dispersive X-ray spectroscopy (EDX) profiles below every micrograph in Fig. 1 however conclusively show Ti and Pt distributions confirming the presence of Pt on titania NPs. In addition, ICP-OES was used to determine the total amount of platinum loading on the TiO2 during the synthesis process, showing that 0.095, 0.36, and 0.86 wt% of Pt were deposited on 0.2Pt–TiO2, 0.5Pt–TiO2 and 1Pt–TiO2, respectively. All the photocatalysts tested showed a homogeneous platinum distribution on the titanium dioxide substrate attributed to the role of SDBS as an anionic surfactant stabilizing the metal clusters.44,48 The primary particle size of TiO2 was noted as 21 nm by the supplier. Hydrodynamic diameter and distribution at different pH of the titania particles measured by DLS are shown in Fig. S3a.† TiO2 tends to agglomerate at neutral pH forming clusters with sizes around 522 nm and 218 nm. On the other hand, after loading Pt, the diameter of the clusters decreased to 108, 132, and 114 nm when the amount of Pt was 0.2, 0.5, and 1%, respectively. It is well known that the point of zero-charge of TiO2 is around pH 6.7 to 7.49,50 This is reflected in Fig. S3b and c,† where the hydrodynamic size decreased drastically in acidic pH, demonstrating that the charge of the particle surface increases preventing the aggregation of the nanoparticles. From Fig. S3a,† it is possible to observe that the incorporation of Pt on the TiO2 surface prevents aggregation and decreases the hydrodynamic diameter. It has been reported that platinum nanoparticles present a negative surface charge over almost all pH ranges,51–53 modifying the zero-charge point of the photocatalyst. Fig. S3d† shows the role of SDBS during the platinum deposition on TiO2, stabilizing the nanoparticles and narrowing the hydrodynamic particle size distribution.The morphology of TiO2 and Pt nanoparticle-coated TiO2 at different magnifications is shown in Fig. 2. Both the truncated tetrahedral anatase and short-rod-shaped rutile phase of TiO2 are observed, suggesting that the particles are of mixed anatase and rutile phases (Fig. 2b). Selected-area electron diffraction (SAED) pattern of TiO2 nanoparticles (Fig. 2c) was used to calculate the interplanar spacings of TiO2 crystals. The obtained result of d-spacing, 0.348 nm, 0.227 nm, 0.185 nm, 0.166 nm, 0.147 nm, and 0.128 nm, can be indexed to anatase (101), rutile (200), anatase (200), (211), (204) and (215) planes,54 respectively, which proves the major phase of TiO2 used in this work is anatase with the tetragonal crystal structure. In Fig. 2d, Pt nanoparticles with a size of about 3–5 nm can be clearly observed to be attached on the edge of TiO2 nanoparticle. A high-resolution image of Fig. 2e further exhibits that the Pt particles grown on TiO2 have a lattice spacing of ∼0.23 nm, which is from the (111) planes of Pt. In the diffraction pattern of 0.5Pt–TiO2 (Fig. 2f), several diffracted spots were selected to calculate the lattice spacing in real space. The spacings of 0.23 nm and 0.197 nm can be indexed to (111) and (200) planes of face-centered-cubic structured Pt, labeled in Fig. 2f.
The XRD patterns of the prepared samples were obtained to learn about the crystalline phase proportion and the crystallite size of the photocatalysts (see Fig. S4a†). XRD curves of all samples were dominated with peaks belonging to TiO2 anatase and rutile phases (ICDD file no. 21-1272 and ICDD file no. 21-1276, respectively shown in Fig. S4b†). The phase composition was calculated using the relative intensity method (eqn (4)),55 where A% corresponds to the weight fraction of the anatase phase, IR represents the intensity of the strongest peak of the rutile phase (110), and IA the intensity of the strongest peak if anatase phase corresponding to (101). From this, it was found that anatase represented 85% of all phases and rutile 15% of TiO2. No evident platinum was registered on loaded TiO2 samples. The absence of Pt can be ascribed to the small size of Pt NPs and its high dispersion on the TiO2 particles.56 Crystallite size was calculated using the Scherrer equation (eqn (5)) where L is the crystallite size, K corresponds to a constant with a value of 0.9, λ is the wavelength of the X-ray used (1.54 Å), θ is the Bragg angle, and FWHM is the full width at half-maximum of the peak, in this case, peaks with the highest intensity were considered for both phases. The crystallite size of the commercial TiO2 estimated from the rutile and anatase phases was 18.48 nm and 18.40 nm respectively. No changes were found when Pt was loaded on the photocatalyst, indicating that Pt NPs did not affect the crystal structure of TiO2 (no electronic doping leading to crystal structure changes), and it was only deposited on the surface.
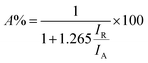 | (4) |
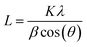 | (5) |
The optical properties of the photocatalyst in terms of adsorption are fundamental in the evaluation of its performance.57 A strong adsorption in the UV range around 320 nm, characteristic of anatase and rutile phases of TiO2, as can be seen in Fig. 3. Platinum nanoparticles induce broad adsorption over the entire visible region. These results agree with previous studies58,59 and show the plasmon-induced enhancement of absorption from the Pt cluster on the TiO2 surface.
 | ||
| Fig. 3 UV-vis spectra for different photocatalysts, commercial TiO2, TiO2 loaded with 0.2 wt% Pt (0.2Pt–TiO2), 0.5 wt% Pt (0.5Pt–TiO2), and 1 wt% Pt (0.2Pt–TiO2). | ||
The influence of Pt loading on TiO2 for hydrogen production was studied using CMC-Na as a sacrificial agent. Fig. 4a shows the performance of TiO2 as a function of Pt deposited. As expected H2 production was negligible when no Pt was involved, and no signal could be detected, showing that Pt has a catalytic role for hydrogen evolution during the photocatalytic process. When the amount of Pt loading was 0.2% and 0.5%, outstanding improvement can be noticed. However, when 1% Pt was dispersed on the photocatalyst surface, only a marginal improvement was observed when the production was compared with H2 obtained by using 0.5% Pt nanocomposites. Higher Pt contents could lead to the agglomeration of Pt NPs, and the incorporation of excessive metal sites may increase the electron–hole recombination due to an increase in the free electron density.60 It is also possible to form aggregates over time, generating a shading effect from the agglomerate suspended particles diminishing light penetration. To verify the role of Pt on TiO2, the obtained photogenerated ˙OH was studied by PL spectroscopy using terephthalic acid (TPA) as a scavenger. It is well known that TPA reacts with ˙OH radicals producing 2-hydroxy terephthalic acid (hTPA),61,62 which has an emission peak around 432 nm when is excited with a 323 nm light source. Fig. 4b shows the hTPA concentration calculated from PL intensity at 432 nm (see Table S1†) and the calibration curve is presented in Fig. S1.† hTPA concentration of TPA solution gradually increased over irradiation time with the lowest concentration corresponding to bare TiO2, increasing proportionately with Pt loading. This implies that the amount of ˙OH generated is more significant when Pt is present in the photocatalyst following the order 1Pt–TiO2 > 0.5Pt–TiO2 > 0.2Pt–TiO2 > TiO2. This result confirms that Pt acts by suppressing the recombination of the hole–electron pair and thus improves the ˙OH generation at the same time. Even though 1% Pt loading presents higher ˙OH production, the agglomeration of Pt NPs may increase the electron–hole pair recombination making the charge separation more difficult, during the photocatalytic process. From these results, we decided to carry out all the photocatalytic hydrogen production tests using 0.5Pt–TiO2 as the photocatalyst.
Photocatalytic hydrogen production using celluloses listed in Table 1 and the photocatalyst prepared with 0.5% Pt loading were compared. The production rates are shown in Fig. 5a and b. When no sacrificial agent was added, only water splitting contributing to the hydrogen production process is observed. This modest H2 production improves when a sacrificial agent is added during the photocatalytic reaction. Cellulose extracted from rapeseed straw, either H2O2-bleached or un-bleached, showed lower performance for H2 production. On the other hand, CMC-Na and TEMPO-oxidized CNF exhibited the best hydrogen production rate. Comparing the crystallinity index (CI) between the different types of celluloses, it could be observed that lower CI led to increased H2 production. Lower CI is associated with weaker chemical bonds,63 and it has been found that during enzymatic hydrolysis of cellulose to glucose, for biofuel conversion, a completely amorphous sample is hydrolyzed faster than a partially crystalline cellulose.64 A similar trend was reported by Caravaca et al. during cellulose reforming,40 establishing that a CI of 80% or lower is the optimum value for fescue grass hydrolysis. Higher CI values lead to a difficult interaction between the photocatalyst particles and the interior sites of cellulose material. The smallest H2 production was recorded with H2O2-bleached rapeseed straw cellulose. Before bleaching the rapeseed straw cellulose, the process includes an alkaline treatment where hemicelluloses, mainly xylan is mostly removed.41 Removing these amorphous regions could lead to an increase in the crystallinity index and, subsequently, lead to a reduction in the hydrogen production rate.
From Fig. 5b, it is also possible to observe that lignin-free celluloses from green macroalgae, isolated from U. fenestrata, contributed to improved H2 production. Lignin provides elasticity and mechanical strength to the cellulose microfibrils protecting them from chemical and enzymatic degradation.65 Hence, lignin is likely to affect the interaction between the photocatalyst and cellulose, protecting the cellulose microfibrils and making it tougher to break and disperse, leading to an unstable suspension. Xyloglucan is partially soluble in water and interrupts the formation of cellulose crystals leading to a low CI.66 This could explain the improved hydrogen production rate using U. fenestrata celluloses. However, a considerable difference in hydrogen production rate was found when the cellulose was acid-treated, increasing from 2.43 μmol h−1 for U. fenestrata to 3.78 μmol h−1 for acid-treated U. fenestrata cellulose. CI is higher for acid-treated U. fenestrata cellulose, however, so is the glucose content, which could improve the hydrogen production rate.42 On the other hand, α-cellulose fibers from spruce pulp showed a lower performance with a production rate of 1.98 μmol h−1 despite containing 99% cellulose with no lignin, suggesting that the higher CI led to lower availability of cellulose as a sacrificial agent.
The total dispersion of completely water-insoluble cellulose is difficult to obtain because of the long rigid chains and the inter-molecular and intra-molecular forces that lead to an unstable system, as was the case of H2O2-bleached and un-bleached cellulose extracted from rapeseed straw discussed above. Considering this, modification of cellulose structure to improve its water-solubility properties (widely discussed in the literature, see for instance67–69), where different groups substitute hydroxyl groups, could be beneficial in photocatalytic hydrogen production. Cellulose hydroxyl groups are substituted with carboxymethyl (–CH2–COO−) groups in CMC-Na and carboxylate (RCOO−) groups in TEMPO-oxidized cellulose. The addition of these polar groups increases the solubility of cellulose, resulting in better suspension in aqueous media, and avoiding agglomeration and thus aggregation of the particles reducing its availability as an active component of the system. The degradation kinetics of cellulose is thus affected depending on the degree of substitution, the substitution pattern, and the degree of polymerization. Erdal et al. studied the degradation of CMC, establishing that the hydrolysis dramatically decreased when the degree of substitution was higher than 1.70
From the above, it is expected that using these cellulose derivatives as sacrificial agents will ameliorate hydrogen production. This is also clearly observed in Fig. 5b where CMC-Na and TEMPO-oxidized cellulose show the best performance for photocatalytic H2 production with rates of 7.01 and 4.27 μmol h−1, respectively. Meanwhile, Fig. 5a shows a pronounced hydrogen production slope when CMC-Na and TEMPO-oxidized cellulose were used. A similar trend was found during the first minutes with acid-treated U. fenestrata cellulose and U. fenestrata cellulose. Zhao et al. reported that the photo-reforming of lignocellulose showed a similar pathway compared to the enzymatic oxidation processes,71 where due to a kinetic factor of accessibility cellulose first is hydrolyzed into soluble glucose, formic acid, hydroxymethyl furfural (HMF), etc. These soluble products have faster diffusion rate consuming hydroxyl radicals that provide protons to the system,72 which then get reduced to hydrogen gas. Fig. 6 corresponds to a schematic diagram showing different species involved in the degradation of the biomass samples. According to Machado et al., hydroxyl radicals (˙OH) cause a structural fragmentation of lignin structure attacking the double bond of the aromatic rings.73 On the other hand, hemicelluloses (like xyloglucan) and cellulose follow similar mineralization paths where ˙OH breaks the glycosidic linkages producing xylose and glucose, respectively. Xylose and glucose continue their degradation forming intermediates like arabinose, erythrose, galactose, glyceraldehyde, hydroxymethylfurfural (HMF), etc. until mineralization is complete and products like formic acid, carbon dioxide, carbon monoxide, and hydrogen are obtained.74,75 This emphasizes the importance of ˙OH produced during the photocatalytic process as shown in Fig. 4b.
 | ||
| Fig. 6 Schematic representation of cellulose and hemicellulose degradation and molecules participating during the photocatalysis process. | ||
Based on the above discussions, the high activity observed during the first minutes when U. fenestrata and acid-treated U. fenestrata celluloses were used can be attributed to the consumption of the soluble xyloglucan present in their microstructure. Meanwhile, an induction period was observed before the H2 production started when H2O2-bleached and un-bleached celluloses extracted from rapeseed straw were used. Given the above observations, the performance of CMC-Na, Tempo-oxidized cellulose, U. fenestrata, and acid-treated U. fenestrata were compared with the performance of classical sacrificial agents keeping the concentration of photocatalyst constant with 0.3 mg mL−1 and 0.25 mg mL−1 sacrificial agent used, respectively. Sodium sulfide (Na2S) and commercial glucose were considered for this comparison. From Fig. 7, it is possible to observe that glucose improves hydrogen yield when U. fenestrata and acid-treated U. fenestrata samples were considered, which is attributed to the glucose content in the acid-treated U. fenestrata mentioned above. On the other hand, Na2S was used as an inorganic sacrificial agent presenting a different reaction mechanism than glucose, where Na2S is more easily oxidizable as direct interaction with the holes generated on the photocatalyst76,77 can occur, producing S22− ions thus reducing the need of the presence of ˙OH radicals. Despite this, the performance of Na2S is similar to CMC-Na which can be attributed to its high substitution degree of around 0.8 making it highly soluble showing its potential to be used as a biomass sacrificial agent.
The influence of the sacrificial agent concentration during photocatalysis was tested for CMC-Na by keeping the photocatalyst concentration constant at 0.3 mg mL−1. Several suspensions were prepared between 0.25 and 5 mg mL−1. From Fig. 8a, we can observe that the optimum cellulose concentration corresponds to 2.5 mg mL−1. Higher cellulose concentrations showed a rapid decrease in H2 production attributed to an increase in the shadowing effect of photocatalysis particles by the cellulose particles reducing the interaction of photons used for the generation of photoexcited electrons and holes on the semiconductor surface.
To study the influence of pH on the hydrogen production rates, different CMC-Na suspensions were prepared with pH from 2 to 5 and tested using 0.3 mg mL−1 of 0.5Pt–TiO2 and 0.25 mg mL−1 of CMC-Na. From Fig. 8b, it is possible to notice an increase in H2 production moving from pH 5 to pH 4, and the maximum output was recorded at pH 2. These results suggest that acidic pH is beneficial for the system's performance. This can be attributed to the presence of excess protons in the system lowering the overpotential needed for hydrogen evolution. However, at pH 2–3, the suspension was unstable, and agglomeration of particles occur. Even though the hydrogen production at pH 2 was almost double what was obtained at pH 5, the agglomeration became more severe with time, and the hydrogen production practically stopped due to the precipitation of the sacrificial agent together with the corrosion of the photocatalysts.78 To explain the instability of the solution, the zeta potential values of CMC-Na were measured and are shown in Fig. S5.† From these results obtained at pH 2 and 3, particles have a net charge close to 0, making the suspension unstable thus enabling agglomeration. On the other hand, when the pH is higher than 4, the zeta potential values are more negative, decreasing the tendency of the agglomeration of the particles.
Long-term performance and recovery of the photocatalyst were studied considering 4 running cycles of 8 hours each, 2.5 mg mL−1 of CMC-Na was renewed in each cycle run, and 0.3 mg L−1 of 0.5Pt–TiO2 was continuously recovered. Fig. 9 shows a linear tendency in the hydrogen production rate during the 8 h of light exposure. When subsequent other runs were performed the H2 production remained stable during the first two cycles where only 10% of the capacity was reduced after 16 hours. Nevertheless, after the second run photocatalyst presented a significant decrease in the H2 production rate of approximately of 46% which kept constant in run 4. The instability associated with H2 production it was related to incomplete biomass degradation and difficulties in recovering the photocatalyst at the end of every cycle run.
 | ||
| Fig. 9 Long-term stability and recyclability of the 0.5Pt–TiO2 photocatalyst using CMC-Na as sacrificial agent. | ||
4. Conclusions
This work investigated photocatalytic hydrogen production by using different cellulose types as sacrificial agents using platinum loaded on TiO2 as a photocatalyst. It was demonstrated that hydrogen could successfully be produced using all the cellulosic biomass and cellulose derivatives tested, and its performance is strongly related to the composition of the biomass used and the amount of platinum loading. Optimal performance towards H2 production was obtained when 0.5 wt% of platinum was loaded. Hydrogen production was strongly affected by the crystallinity index and the presence of soluble structures, like xyloglucan in the cellulosic material which is consumed during the first minutes of the photocatalytic process. Lignin-free celluloses from green macroalgae, isolated from U. fenestrata algae was the best sacrificial agent showing a hydrogen production rate of 140.85 μmol L−1 that could be further improved in the cellulose obtained from U. fenestrata was acid-treated yielding 167.44 μmol L−1 hydrogen in 1 hour of exposure. Cellulose derivatives with incorporated polar substituents showed almost two times higher hydrogen production rates than the un-modified ones. The enhancement in the performance was associated with the higher solubility of the derivative products due to the carboxymethyl (–CH2–COO−) and carboxylate (RCOO−) groups, improving the dispersion and the stability of the system when the pH was 5. A successful deployment of photocatalytic generation of H2 from cellulose would have several positive effects associated with some relevant Sustainable Development Goals. Possible issues may arise when it comes to the photocatalyst used, which should allow good efficiency while being affordable enough, without posing any significant environmental threat throughout all its life cycle.Conflicts of interest
There are no conflicts to declare.Acknowledgements
MI Alvarado-Ávila would like to thank the National Commission for Scientific and Technological Research (ANID) for the Doctoral scholarship “Beca Chile” 2018-72190683. The authors also want to acknowledge partial financial support from Swedish Energy Agency (Energimyndigheten) through a project entitled HESAC (Project No. 45504-1).References
- IEA, Global Energy Review 2021, IEA, Paris, 2021 Search PubMed.
- bp, Statistical Review of World Energy, 2022, https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2022-full-report.pdf Search PubMed.
- S. Atilhan, S. Park, M. M. El-Halwagi, M. Atilhan, M. Moore and R. B. Nielsen, Curr. Opin. Chem. Eng., 2021, 31, 100668 CrossRef.
- B. Khandelwal, A. Karakurt, P. R. Sekaran, V. Sethi and R. Singh, Prog. Aeronaut. Sci., 2013, 60, 45–59 CrossRef.
- A. M. Oliveira, R. R. Beswick and Y. Yan, Curr. Opin. Chem. Eng., 2021, 33, 100701 CrossRef.
- A. P. Simpson and A. E. Lutz, Int. J. Hydrogen Energy, 2007, 32, 4811–4820 CrossRef CAS.
- S. Danwittayakul and J. Dutta, Int. J. Hydrogen Energy, 2012, 37, 5518–5526 CrossRef CAS.
- S. Danwittayakul, K. Lakshman, S. Al-Harthi and J. Dutta, Appl. Catal., A, 2014, 471, 63–69 CrossRef CAS.
- E. Kabir, P. Kumar, S. Kumar, A. A. Adelodun and K.-H. Kim, Renewable Sustainable Energy Rev., 2018, 82, 894–900 CrossRef.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- X. Ning and G. Lu, Nanoscale, 2020, 12, 1213–1223 RSC.
- S. U. Khan, M. Al-Shahry and W. B. Ingler Jr, Science, 2002, 297, 2243–2245 CrossRef CAS PubMed.
- F. Wang, C. Di Valentin and G. Pacchioni, J. Phys. Chem. C, 2012, 116, 8901–8909 CrossRef CAS.
- K.-S. Mun, S. D. Alvarez, W.-Y. Choi and M. J. Sailor, ACS Nano, 2010, 4, 2070–2076 CrossRef CAS.
- F. Loosli, P. Le Coustumer and S. Stoll, Sci. Total Environ., 2015, 535, 28–34 CrossRef CAS PubMed.
- B. S. Vadlamani, T. Uppal, S. C. Verma and M. Misra, Sensors, 2020, 20, 5871 CrossRef CAS.
- S. Sarkar, A. Makhal, T. Bora, S. Baruah, J. Dutta and S. K. Pal, Phys. Chem. Chem. Phys., 2011, 13, 12488–12496 RSC.
- S. Akel, R. Dillert, N. O. Balayeva, R. Boughaled, J. Koch, M. El Azzouzi and D. W. Bahnemann, Catalysts, 2018, 8, 647 CrossRef.
- S. Sarkar, A. Makhal, K. Lakshman, T. Bora, J. Dutta and S. Kumar Pal, J. Phys. Chem. C, 2012, 116, 14248–14256 CrossRef CAS.
- J. Ran, J. Zhang, J. Yu, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2014, 43, 7787–7812 RSC.
- T. Bora, H. H. Kyaw and J. Dutta, Electrochim. Acta, 2012, 68, 141–145 CrossRef CAS.
- T. Bora, H. H. Kyaw, S. Sarkar, S. K. Pal and J. Dutta, Beilstein J. Nanotechnol., 2011, 2, 681–690 CrossRef CAS PubMed.
- S. Kumar, F. Ye, B. Mazinani, S. Dobretsov and J. Dutta, Int. J. Mol. Sci., 2021, 22, 4513 CrossRef CAS PubMed.
- M. Batvandi, A. Haghighatzadeh, B. Mazinani and J. Dutta, Appl. Phys. A: Mater. Sci. Process., 2022, 128, 853 CrossRef CAS.
- X. Cai, Z. Zeng, Y. Liu, Z. Li, X. Gu, Y. Zhao, L. Mao and J. Zhang, Appl. Catal., B, 2021, 297, 120391 CrossRef CAS.
- J. Velázquez, R. Fernández-González, L. Díaz, E. P. Melián, V. Rodríguez and P. Núñez, J. Alloys Compd., 2017, 721, 405–410 CrossRef.
- A. Makhal, S. Sarkar, T. Bora, S. Baruah, J. Dutta, A. Raychaudhuri and S. K. Pal, Nanotechnology, 2010, 21, 265703 CrossRef PubMed.
- H. Tian, S.-Z. Kang, X. Li, L. Qin, M. Ji and J. Mu, Sol. Energy Mater. Sol. Cells, 2015, 134, 309–317 CrossRef CAS.
- G. L. Chiarello, M. H. Aguirre and E. Selli, J. Catal., 2010, 273, 182–190 CrossRef CAS.
- S. E. Hosseini, M. Abdul Wahid, M. Jamil, A. A. Azli and M. F. Misbah, Int. J. Energy Res., 2015, 39, 1597–1615 CrossRef CAS.
- S. Su, W. Li, Z. Bai, H. Xiang and J. Bai, Int. J. Hydrogen Energy, 2010, 35, 4459–4465 CrossRef CAS.
- Y.-C. Lo, M.-D. Bai, W.-M. Chen and J.-S. Chang, Bioresour. Technol., 2008, 99, 8299–8303 CrossRef CAS PubMed.
- J. Zou, H. Yang, Z. Zeng, C. Wu, P. T. Williams and H. Chen, Int. J. Hydrogen Energy, 2016, 41, 10598–10607 CrossRef CAS.
- A. M. Balu and R. Luque, in Producing Fuels and Fine Chemicals from Biomass Using Nanomaterials, 2013, pp. 1–4 Search PubMed.
- S. Siahrostami, G.-L. Li, V. Viswanathan and J. K. Nørskov, J. Phys. Chem. Lett., 2017, 8, 1157–1160 CrossRef CAS.
- G. Zhang, C. Ni, X. Huang, A. Welgamage, L. A. Lawton, P. K. Robertson and J. T. Irvine, Chem. Commun., 2016, 52, 1673–1676 RSC.
- D. W. Wakerley, M. F. Kuehnel, K. L. Orchard, K. H. Ly, T. E. Rosser and E. Reisner, Nat. Energy, 2017, 2, 1–9 Search PubMed.
- C. Chang, N. Skillen, S. Nagarajan, K. Ralphs, J. T. Irvine, L. Lawton and P. K. Robertson, Sustainable Energy Fuels, 2019, 3, 1971–1975 RSC.
- A. Speltini, M. Sturini, D. Dondi, E. Annovazzi, F. Maraschi, V. Caratto, A. Profumo and A. Buttafava, Photochem. Photobiol. Sci., 2014, 13, 1410–1419 CrossRef CAS.
- A. Caravaca, W. Jones, C. Hardacre and M. Bowker, Proc. R. Soc. A, 2016, 472, 20160054 CrossRef CAS PubMed.
- A. Svärd, R. Moriana, E. Brännvall and U. Edlund, ACS Sustainable Chem. Eng., 2018, 7, 790–801 CrossRef.
- N. Wahlström, U. Edlund, H. Pavia, G. Toth, A. Jaworski, A. J. Pell, F. X. Choong, H. Shirani, K. P. R. Nilsson and A. Richter-Dahlfors, Cellulose, 2020, 27, 3707–3725 CrossRef.
- A. Isogai, T. Saito and H. Fukuzumi, Nanoscale, 2011, 3, 71–85 RSC.
- W. Yao, J. Yang, J. Wang and Y. Nuli, Electrochem. Commun., 2007, 9, 1029–1034 CrossRef CAS.
- R. A. Al-Alawi, K. Laxman, S. Dastgir and J. Dutta, Appl. Surf. Sci., 2016, 377, 200–206 CrossRef CAS.
- M. I. A. Ávila, E. Toledo-Carrillo and J. Dutta, Clean. Eng. Technol., 2020, 1, 100016 CrossRef.
- K. Nisizawa, J. Ferment. Technol., 1973, 51, 267–304 CAS.
- H. Ma, B. Yin, S. Wang, Y. Jiao, W. Pan, S. Huang, S. Chen and F. Meng, ChemPhysChem, 2004, 5, 68–75 CrossRef CAS PubMed.
- Z. Cao, T. Zhang, P. Ren, D. Cao, Y. Lin, L. Wang, B. Zhang and X. Xiang, Catalysts, 2020, 10, 69 CrossRef CAS.
- G. Konecoglu, T. Safak, Y. Kalpakli and M. Akgun, Adv. Environ. Res., 2015, 4, 25–38 CrossRef.
- E. Drzymała, G. Gruzeł, A. Pajor-Świerzy, J. Depciuch, R. Socha, A. Kowal, P. Warszyński and M. Parlinska-Wojtan, J. Nanopart. Res., 2018, 20, 1–13 CrossRef PubMed.
- G. Marzun, C. Streich, S. Jendrzej, S. Barcikowski and P. Wagener, Langmuir, 2014, 30, 11928–11936 CrossRef CAS PubMed.
- H.-S. Oh, J.-G. Oh, Y.-G. Hong and H. Kim, Electrochim. Acta, 2007, 52, 7278–7285 CrossRef CAS.
- A. Srivastava, M. Deepa, S. Bhandari and H. Fuess, Nanoscale Res. Lett., 2009, 4, 54–62 CrossRef CAS PubMed.
- R. A. Spurr and H. Myers, Anal. Chem., 1957, 29, 760–762 CrossRef CAS.
- S. Oh, H. Ha, H. Choi, C. Jo, J. Cho, H. Choi, R. Ryoo, H. Y. Kim and J. Y. Park, J. Chem. Phys., 2019, 151, 234716 CrossRef PubMed.
- T. Bora, K. K. Lakshman, S. Sarkar, A. Makhal, S. Sardar, S. K. Pal and J. Dutta, Beilstein J. Nanotechnol., 2013, 4, 714–725 CrossRef CAS PubMed.
- L. M. Ahmed, I. Ivanova, F. H. Hussein and D. W. Bahnemann, Int. J. Photoenergy, 2014, 2014, 503516 CrossRef.
- L. Hou, M. Zhang, Z. Guan, Q. Li and J. Yang, J. Nanopart. Res., 2018, 20, 1–8 CrossRef CAS.
- P. Pichat, J. M. Herrmann, J. Disdier, M. N. Mozzanega and H. Courbon, in Studies in Surface Science and Catalysis, ed. S. Kaliaguine and A. Mahay, Elsevier, 1984, vol. 19, pp. 319–326 Search PubMed.
- W. Dong, D. Wang, L. Jiang, H. Zhu, H. Huang, J. Li, H. Zhao, C. Li, B. Chen and G. Deng, Mater. Lett., 2013, 98, 265–268 CrossRef CAS.
- S. E. Page, W. A. Arnold and K. McNeill, J. Environ. Monit., 2010, 12, 1658–1665 RSC.
- S. Park, J. O. Baker, M. E. Himmel, P. A. Parilla and D. K. Johnson, Biotechnol. Biofuels, 2010, 3, 10 CrossRef PubMed.
- M. Hall, P. Bansal, J. H. Lee, M. J. Realff and A. S. Bommarius, FEBS J., 2010, 277, 1571–1582 CrossRef CAS PubMed.
- S. I. Mussatto and J. A. Teixeira, Current Research. Technology and Education Topics in Applied Microbiology and Microbial Biotechnology, ed. A. Méndez-Vilas, 2010, vol. 2, pp. 897–907, https://hdl.handle.net/1822/16762 Search PubMed.
- Q. Zhou, M. W. Rutland, T. T. Teeri and H. Brumer, Cellulose, 2007, 14, 625–641 CrossRef CAS.
- S. Schmidt, T. Liebert and T. Heinze, Green Chem., 2014, 16, 1941–1946 RSC.
- C. T. O'Brien, T. Virtanen, S. Donets, J. Jennings, O. Guskova, A. H. Morrell, M. Rymaruk, L. Ruusuvirta, J. Salmela and H. Setala, Polymer, 2021, 223, 123681 CrossRef.
- S. Thiangtham, J. Runt and H. Manuspiya, Carbohydr. Polym., 2019, 208, 314–322 CrossRef CAS PubMed.
- N. B. Erdal and M. Hakkarainen, Biomacromolecules, 2022, 23, 2713–2729 CrossRef CAS PubMed.
- H. Zhao, C.-F. Li, X. Yu, N. Zhong, Z.-Y. Hu, Y. Li, S. Larter, M. G. Kibria and J. Hu, Appl. Catal., B, 2022, 302, 120872 CrossRef CAS.
- H. Hao, L. Zhang, W. Wang and S. Zeng, ChemSusChem, 2018, 11, 2810–2817 CrossRef CAS PubMed.
- A. E. H. Machado, A. M. Furuyama, S. Z. Falone, R. Ruggiero, D. d. S. Perez and A. Castellan, Chemosphere, 2000, 40, 115–124 CrossRef CAS PubMed.
- R. Chong, J. Li, Y. Ma, B. Zhang, H. Han and C. Li, J. Catal., 2014, 314, 101–108 CrossRef CAS.
- H. Zhao, X. Yu, G. Hu, N. Zhong, Z.-Y. Hu, S. Larter, Y. Li, M. G. Kibria and J. Hu, Green Chem., 2021, 23, 8124–8130 RSC.
- C. Li, H. Wang, J. Ming, M. Liu and P. Fang, Int. J. Hydrogen Energy, 2017, 42, 16968–16978 CrossRef CAS.
- X. Li, J. Xu, L. Li, S. Zhao, M. Mao, Z. Liu and Y. Li, Catal. Lett., 2021, 151, 2408–2419 CrossRef CAS.
- M. Wang, S. Shen, L. Li, Z. Tang and J. Yang, J. Mater. Sci., 2017, 52, 5155–5164 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3se00109a |
| This journal is © The Royal Society of Chemistry 2023 |






