DOI:
10.1039/D3SD00110E
(Critical Review)
Sens. Diagn., 2023,
2, 988-1076
An overview of Schiff base-based fluorescent turn-on probes: a potential candidate for tracking live cell imaging of biologically active metal ions
Received
9th May 2023
, Accepted 10th June 2023
First published on 12th June 2023
Abstract
The development of new fluorescent turn-on probes has attracted much attention due to the selective and sensitive detection of biologically important metal ions and their applicability in live cell imaging. Since it has been established that fluorescent probes work well in cellular media, many intriguing investigations are under progress. It is desirable to use quick and easy methods for detecting metal ions in biological and environmental systems, and the probes should provide enhanced photophysical properties with sensitivity, selectivity, a low detection threshold, a quick response time, operational simplicity, and real-time analysis, which are non-lethal to living cells, demanding extensive research. There are several novel fluorescent turn-on probes reported thus far; however, Schiff base-based probes have gained particular interest because of their intriguing optical characteristics, great thermal stability, and ease of synthesis. Moreover, they stand out in the bioactive domain as promising fluorescent chemosensors for biologically prominent metal cations and are well known for their low cytotoxicity. Schiff bases exhibit strong luminescence properties in synergistic systems with many ideal fluorophores such as quinoline, anthracene, coumarin, fluorescein, rhodamine, boron-dipyrromethene (BODIPY), dansyl, and naphthalimide. As a result, these synthetically flexible compounds with coordination sites such as N and O can rigidly bind to a wide range of transition metal ions with turn-on fluorescence properties. This review compiles the detailed sensing properties of numerous Schiff base-based probes for biologically important metal cations such as Fe(II), Zn(II), Cu(II), Ni(II), Co(II), Ag(I), Au(III), Hg(II), Cd(II) and Pd(II) via a fluorescence turn-on mechanism. Further, it covers the detection approaches elucidating the optoelectronic characteristics as well as their application in cellular imaging, which will provide a better understanding of the accumulation of metal ions in organelles.
 A. Afrin | Afrin A. currently works as a Junior Research Fellow (JRF) at the Department of Chemistry, National Institute of Technology Calicut. She had completed master's degree in chemistry from Cochin University of Science and Technology in the year 2020. Her current research areas include modulation of solid-state emissive properties of donor–acceptor conjugates. |
 Anjitha Jayaraj | Anjitha Jayaraj obtained her MSc degree from National Institute of Technology Calicut in 2020. She is currently pursuing her research carrier from the same institute, under the guidance of Dr Chinna Ayya Swamy P. Her present study focuses on designing N-heterocyclic carbene metal-based catalysts for hydrofunctionalisation reactions. |
 M. S. Gayathri | Gayathri M. S. completed her post-graduation in chemistry from National Institute of Technology Calicut. She was the project student under the guidance of Dr Chinna Ayya Swamy P. Her area of focus of study was synthesis of novel Schiff base complexes for the detection of metal ions. |
 Chinna Ayya Swamy P. | Dr Swamy obtained MSc in General chemistry from the University of Hyderabad in 2009 and PhD from the IISc, India, in the field of main group organometallics in 2014. After completion of the PhD, he has spent five years abroad as a post-doctoral fellow in the area of supramolecular chemistry and organometallics chemistry. Later, he joined as Assistant Professor in the Department of Chemistry, National Institute of Technology Calicut in 2020. Currently, he works on the design and synthesis of supramolecular architecture for stabilizing low-valent main group compounds, metal-based asymmetric catalysis and chemosensors for biologically important ions. |
1. Introduction
Bio-imaging probes have become an integral part in the determination of the activities of biomolecules in biomedical research, and therefore, the design of such non-invasive probes is a current requirement in the research field.1 The metal ions present in biological systems regulate a variety of functions important including neurophysiology, protein cofactors, dioxygen binding, and transport for maintaining human health.2 Excessiveness or deficiency, however, can be harmful and even fatal. Moreover, the urbanism unscrupulously contaminates the natural environment with metal ions, affecting people's health by denaturing biological processes. As a matter of fact, metal ion detection has always been a hot topic in the research community.3 There are several spectrophotometric methods and electroanalytical techniques such as atomic absorption/emission spectroscopy (AAS/AES),4 inductively coupled plasma mass spectroscopy (ICP-MS),5 inductively coupled plasma atomic emission spectroscopy (ICP-AES),6 and electrochemical7 and colorimetric methods8 available to detect various metal ions. However, the application of these techniques is limited because they require expensive instrumentation and complicated pre-treatments take time, resulting in low detection speed and the inability to detect and inspect cells and living tissues on site.9 Fluorescent chemosensors have made notable progress to the meteoric rise of analytical chemistry notably in the cellular media due to their non-lethal nature in living cells with enhanced photophysical properties of sensitivity and selectivity, low detection limits, rapid response, operational simplicity, real-time analysis.10–15 Furthermore, the visual validation aided by this technique, as well as the cost-effectiveness, made them serve for the past few decades.
Fluorescent chemosensors are probes that have a binding site and a fluorophore, as well as a possible communication mechanism between the two sites.16 Noncovalent interactions including hydrogen bonding, electrostatic attractions, and coordination events are used by most fluorescent probes to bind analytes. Active fluorescent probes are being developed for a wide range of applications with a focus on the detection of metal ions rather than anions or neutral molecules.17 Furthermore, these novel fluorescent sensors based on simple organic compounds have the potential to be useful in monitoring biological events under both in vitro and in vivo conditions.18 Subsequently, they became capable of monitoring the subcellular localization and dynamics of biological targets, possessing good biocompatibility, high spatiotemporal resolution, and ease of chemical modification. The common detection mechanisms altering the photophysical processes include aggregation-induced emission (AIE), excited-state intramolecular proton transfer (ESIPT), photoinduced electron transfer (PET), intramolecular charge transfer (ICT), chelation-induced enhanced fluorescence (CHEF), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, fluorescence resonance energy transfer (FRET) and spin–orbit coupling.19,20
N isomerization, fluorescence resonance energy transfer (FRET) and spin–orbit coupling.19,20
In general, chemosensors are classified into two types based on optical signals: fluorescent off–on and on–off systems. The non-fluorescent free compound will recognize fluorescent analytes in off–on probes and vice versa in on–off probes.21–23 Another method is the use of ratiometric sensors, which analyse the shift in emission and are useful for the quantification of ions.24–26 Further, many novel designs with sensor systems such as chemodosimeters are being developed with the goal of improving the selectivity and sensitivity. Here, the probe on interaction with a specific analyte undergoes an irreversible chemical reaction and produces different optical properties, and the chemodosimeter function depends on these properties.27–29 All of these have proven to be vital tools in subcellular habitats for clinical diagnostic, physiological, and pathological functions involving metal ions.30
Reportedly, major research endeavors in the detection of ions with varying fluorophores are being made in order to study their intriguing luminescence properties. Quinoline,31 anthracene,32 coumarin,33 fluorescein,34 rhodamine35 and bipyridine are among the commonly investigated probes. However, many of them have low solubility, poor recognition sensitivity, and complex synthesis under extreme conditions.36 As a result, there is always room for improvement in designing probes to overcome these constraints. Schiff bases or imines are one type of compound that has been highlighted in many fields of chemistry due to its ease of synthesis and structural modification.37–39 Furthermore, Schiff bases with coordination sites such as N and O enable them to rigidly bind to many transition metal ions with fluorescence turn-on response.40–43 Hence, Schiff bases in synergistic systems with other ideal fluorophores act as fascinating turn-on probes exhibiting selective affinity for metal cations, and are employed in cellular imaging.44–46
Recently, we have reported on a simple acyclic salen Schiff base showing selective fluorescence turn-on response for the detection of Zn(II) ions in the nanomolar range, which is employed for confocal fluorescence microscopic imaging in HeLa cells.47 Recognizing the importance of this hot research topic, many notable reviews have been published,48–50 focusing on classifying fluorophore combinations rather than differentiating metal ion analytes. This review examines the recent advances in the development of Schiff base-based fluorescent turn-on chemosensors, including synthesis, design principle with photophysical properties, sensing mechanism, and analyte recognition studies with many parameters, primarily emphasizing on biological applications.
2. Schiff base-based fluorescent turn-on probes for Zn(II) ions
The second most prevalent transition-metal ion, zinc, plays a very important role in the biological system, including the transcription of genes, activity of metalloenzymes, transmission of brain signals, and DNA-binding proteins.51–54 Moreover, the irregularity of Zn(II) ions causes numerous diseases such as neurodegenerative illness, including epilepsy, stroke, Alzheimer's, and Parkinson's.55 In addition to its numerous other uses, the excess amount of Zn(II) ions are highly hazardous, and cause diabetes, skin conditions, and prostatic adenocarcinoma.56 It also decreases soil microbial activity, which results in phytotoxic consequences.57 Due to their high sensitivity, precise measurement, and spatiotemporal resolution compared to other detection methods, fluorescence techniques are employed for Zn(II) sensing.58,59 Therefore, the creation of simple, widely available, and selective fluorescent Zn(II) probes is essential for both environmental and biological studies.60
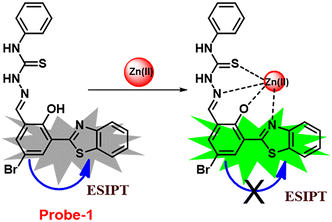
In this regard, a ratiometric probe based on benzothiazole was synthesized by Shuang et al. in 2021,61 which shows very selective recognition of Zn(II) ions via the fluorescence turn-on mechanism. The probe-1 was synthesized by reacting aminothiophenol with 4-bromosalicylaldehyde under acidic conditions, leading to the formation of substituted benzothiazole. Further, the Duff reaction of this benzothiazole was conducted followed by reacting with substituted urea, yielding the final probe (probe-1). The designed principle of probe-1 is that 2-(2′-hydroxyphenyl)benzothiazole fluorophore acts as a signaling moiety and the Schiff base unit acts as a receptor site. When Zn(II) ions are added to probe-1, the maximum emission wavelength shifts by 82 nm from 570 to 488 nm, and the color changes from orange to green, with no change detected for the other competitive metal ions. This color change incorporated emission of probe-1 upon binding to Zn(II) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which can be explained on the basis of inhibition of ESIPT process and can result in a CHEF due to the engagement of the phenolic O atom in the coordination of probe-1 with Zn(II) ions (Fig. 1). The detection limit of probe-1 with Zn(II) ions determined via fluorescence spectra is 37.7 nM. Furthermore, probe-1 has a low cytotoxicity, which qualifies its efficacy for tracing and visualizing Zn(II) ions in vitro in HeLa cells and live SH-SY5Y neuroblastoma cells.
1, which can be explained on the basis of inhibition of ESIPT process and can result in a CHEF due to the engagement of the phenolic O atom in the coordination of probe-1 with Zn(II) ions (Fig. 1). The detection limit of probe-1 with Zn(II) ions determined via fluorescence spectra is 37.7 nM. Furthermore, probe-1 has a low cytotoxicity, which qualifies its efficacy for tracing and visualizing Zn(II) ions in vitro in HeLa cells and live SH-SY5Y neuroblastoma cells.
 |
| | Fig. 1 Chemical structure and proposed sensing mechanism of probe-1 with Zn(II) (redrawn the ChemDraw structure from ref. 61). | |
Fluorescent probe-2 was developed by combining ortho-vanillin with 1-(3-aminopropyl)imidazole via a simple condensation reaction62 by Mobin and co-workers in 2019. Probe-2 was structurally characterized by various spectroscopic techniques such as nuclear magnetic resonance (NMR), high-resolution mass spectrometry (HRMS), and infra-red (IR), and single-crystal X-ray diffraction (XRD) analysis to confirm its molecular entity. Synthesized probe-2 is selective, sensitive and reversible towards the Zn(II) ions via the fluorescence turn-on mechanism and shows no or minimal response upon the addition of various metal ions. The restriction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N imine bond isomerization on effective coordination by Zn(II) ions could be the cause of the fluorescence turn-on, and a new bathochromic band arises in the absorption spectrum. The detection limit was determined to be 31.044 nM using the equation LOD = 3σ/slope. Moreover, the single-crystal XRD identified formation of dinuclear Zn(II) complexes indicates that probe-2 strongly binds to the Zn(II) ions. The detection of Zn(II) ions by probe-2 in biological media was investigated on HeLa and DU-145 cancer cell lines, since probe-2 was proven to be non-toxic and highly biocompatible for cellular proliferation by the MTT cell viability assay, which is helpful for the detection or monitoring of Zn(II) ions in biological samples (Fig. 2).
N imine bond isomerization on effective coordination by Zn(II) ions could be the cause of the fluorescence turn-on, and a new bathochromic band arises in the absorption spectrum. The detection limit was determined to be 31.044 nM using the equation LOD = 3σ/slope. Moreover, the single-crystal XRD identified formation of dinuclear Zn(II) complexes indicates that probe-2 strongly binds to the Zn(II) ions. The detection of Zn(II) ions by probe-2 in biological media was investigated on HeLa and DU-145 cancer cell lines, since probe-2 was proven to be non-toxic and highly biocompatible for cellular proliferation by the MTT cell viability assay, which is helpful for the detection or monitoring of Zn(II) ions in biological samples (Fig. 2).
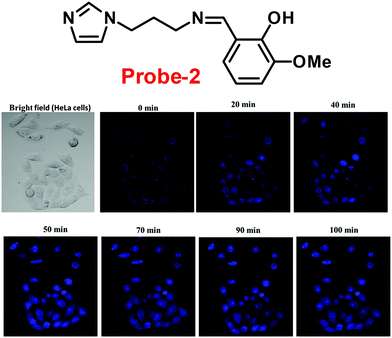 |
| | Fig. 2 Chemical structure of probe-2 (top) and confocal fluorescence images of probe-2 (25 μM, 2 h) with Zn(II) ions on HeLa cells with different time intervals (bottom) (redrawn and reprinted with permission from ref. 62, Copyright 2019 Royal Society of Chemistry). | |
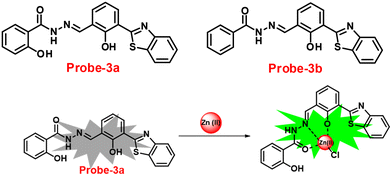
A novel fluorescent chemosensor, probe-3, was synthesized via a simple condensation reaction of the aldehyde and amine, which leads to the formation of probe-3a and probe-3b by Mondal et al.63 in 2019. The probes selectively exhibit strong ratiometric changes in the emission spectra, upon addition of Zn(II) ions, resulting in a blue shift from 550 nm to 478 nm, which occurs as a consequence of the ESIPT process in a successful (Fig. 3) stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a low detection limit value of 0.16 μM for probe-3a. The binding constant of probe-3a with Zn(II) ions was estimated to be 1.6 × 105 M−1via fluorescence titration according to the Benesi–Hildebrand (B–H) plot, indicating that probe-3a–Zn(II) is an adequately stable complex. The binding mechanism of probes with Zn(II) was strongly supported by 1H NMR, density functional theory (DFT), time-dependent density-functional theory (TD-DFT), and electrospray ionisation mass spectrometry (ESI-MS). Consequently, dip-stick experimentation, which enabled real-time monitoring without the use of an instrument, resulted in an instant qualitative detection of Zn(II) with the probes, and was confirmed by the color change in the absorption and fluorescence. Further, the probes were subjected to the sensing of Zn(II) ions in biological media, and an experiment was carried out on human breast cancer cell lines (MCF-7) and tissues. These cells exhibited distinct fluorescence colors upon introducing Zn(II), which supports the recognition of Zn(II) ions in biological samples (Fig. 4).
1 with a low detection limit value of 0.16 μM for probe-3a. The binding constant of probe-3a with Zn(II) ions was estimated to be 1.6 × 105 M−1via fluorescence titration according to the Benesi–Hildebrand (B–H) plot, indicating that probe-3a–Zn(II) is an adequately stable complex. The binding mechanism of probes with Zn(II) was strongly supported by 1H NMR, density functional theory (DFT), time-dependent density-functional theory (TD-DFT), and electrospray ionisation mass spectrometry (ESI-MS). Consequently, dip-stick experimentation, which enabled real-time monitoring without the use of an instrument, resulted in an instant qualitative detection of Zn(II) with the probes, and was confirmed by the color change in the absorption and fluorescence. Further, the probes were subjected to the sensing of Zn(II) ions in biological media, and an experiment was carried out on human breast cancer cell lines (MCF-7) and tissues. These cells exhibited distinct fluorescence colors upon introducing Zn(II), which supports the recognition of Zn(II) ions in biological samples (Fig. 4).
 |
| | Fig. 3 Chemical structures of probe-(3a–3b) and proposed sensing mechanism of probe-3a with Zn(II) (redrawn the ChemDraw structure from ref. 63). | |
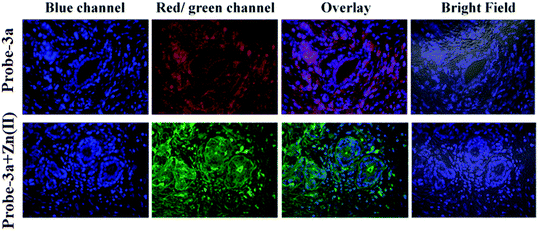 |
| | Fig. 4 Confocal bio-imaging of human breast tissues on treatment with 15 μM probe-3a with Zn(II) (reprinted with permission from ref. 63, Copyright 2019 Royal Society of Chemistry). | |
The one-step condensation reaction of 5-phenylsalicylaldehyde and 2-aminobenzohydrazide yielded a Schiff base derivative probe-4 by Lu et al. in 2018 (ref. 64) with remarkable selectivity and sensitivity towards Zn(II) ions. The addition of Zn(II) ions to the aqueous solution of probe-4 caused an intense green fluorescence emission with a substantial red shift, even in the presence of other competing metal ions (Fig. 5). The three-atom cage containing Zn(II) narrowed probe-4's free rotation and twisting, resulting in strong stiffness and high co-planarity of the molecular assembly, which led to the induction of ICT and CHEF upon complexation with Zn(II) ions. The detection limit value of probe-4 with Zn(II) ions was determined to be 72 nM, which is much lower than that of WHO-recommended level (76 μM Zn(II) ions) in drinking water. The stoichiometry of probe-4 with Zn(II) ions was determined via Job's plot and disclosed as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio. The reversibility of probe-4 towards Zn(II) was confirmed via addition of EDTA to probe-4–Zn(II) in CH3CN–H2O, which leads to the regeneration of probe-4 alone, these results strongly supported the reversibility of probe-4. Finally, probe-4 was demonstrated as a feasible fluorescent sensor for real-time monitoring of Zn(II) in biological systems via bio-imaging in living HeLa cells and zebrafish models, with minimal cellular damage.
1 binding ratio. The reversibility of probe-4 towards Zn(II) was confirmed via addition of EDTA to probe-4–Zn(II) in CH3CN–H2O, which leads to the regeneration of probe-4 alone, these results strongly supported the reversibility of probe-4. Finally, probe-4 was demonstrated as a feasible fluorescent sensor for real-time monitoring of Zn(II) in biological systems via bio-imaging in living HeLa cells and zebrafish models, with minimal cellular damage.
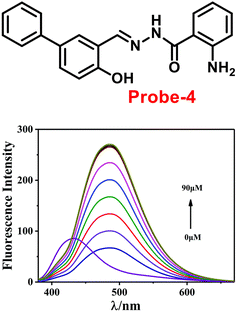 |
| | Fig. 5 Chemical structure of probe-4 (top) and fluorescence spectra of probe-4 (10 μM) in the presence of increasing concentrations of Zn(II) (bottom) (redrawn and reprinted with permission from ref. 64, Copyright 2018 Royal Society of Chemistry). | |
In 2017, Annaraj and colleagues65 developed quinoline-based probe-5 by easily combining 2-hydroxyquinoline-3-carbaldehyde and 2-amino-4-methyl phenol, which exhibits very high sensitivity and selectivity towards Zn(II) ions via fluorescence turn-on behavior and no response to other competitive ions such as Cd(II), Pb(II), Hg(II), Co(II), Cu(II), Co(II), Pb(II), and Ni(II) ions. The effective anchoring of Zn(II) to probe-5via two –OH and imine nitrogen resulted in limited free rotation and enhanced the CHEF action by inhibiting PET, which were responsible for the fluorescence turn-on (Fig. 6). The fluorescence response of probe-5 with Zn(II) ions was found to be linearly rising upon the incremental addition of Zn(II), and the LOD was determined to be 72 nM. The significant Stokes shift value and red emission favored this probe and indicated that it is a biocompatible probe for the bio-imaging of Zn(II) ions in A549 cells and zebrafish embryos.
 |
| | Fig. 6 Chemical structure and proposed sensing mechanism of probe-5 with Zn(II) (redrawn the ChemDraw structure from ref. 65). | |
Chattopadhyay and co-workers synthesized pyrazole-based Schiff base chemosensor probe-6 in a good yield, via a simple condensation reaction between 3-amino-5-phenylpyrazole and 2,6-diformyl-4-methyl-phenol66 in 2017. The pure nature of probe-6 displayed very poor emission, which exhibits green luminescence turn-on towards Zn(II) ions via the generation of dinuclear Zn(II) complexes (Fig. 7). In the presence of other competing ions, there was no change in the fluorescence spectra, caused by the addition of Zn(II) ions, which was explained by the CHEF mechanism. According to Job's plot calculation, Zn(II) ions form a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio, and probe-6 was found to detect Zn(II) ions at extremely low levels up to 27.80 nM.
1 binding ratio, and probe-6 was found to detect Zn(II) ions at extremely low levels up to 27.80 nM.
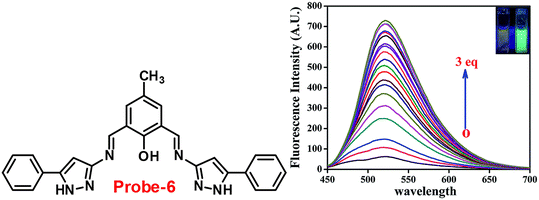 |
| | Fig. 7 Chemical structure of probe-6 (left) and fluorescence spectra of probe-6 with addition of Zn(II) ions (right) (redrawn and reprinted with permission from ref. 66, Copyright 2017 Royal Society of Chemistry). | |
It is essential to develop and synthesize a probe that can detect Zn(II) ions even in the presence of other competing metal ions. In this regard, Misra and co-workers focused on the synthesis of probe-7 from 3-hydoxy-2-naphthoic hydrazide and ortho-vanillin under reflux conditions67 in 2016, which was structurally confirmed by multinuclear NMR and HRMS. Probe-7 can be utilised as an active fluorescent probe for Zn(II) ions with aggregation-induced emission enhancement (AIEE). Probe-7 could not only detect Zn(II) ions by acute chromogenic and selective fluorescence turn-on responses, but also differentiate among its substantial AIEE activity in a high water ratio, and Zn(II) induced AIEE activity through separate luminescence signals without interfering the competitive metal ions, which was explained by the CHECF and PET off mechanisms (Fig. 8). The limit of detection (LOD) of probe-7 for Zn(II) ions was estimated from fluorescence spectra, which was found to be 0.11 μM. The results strongly suggested that probe-7 is highly biocompatible in biological media as well as detecting Zn(II) ions in physiological media.
 |
| | Fig. 8 Chemical structure and proposed sensing mechanism of probe-7 with Zn(II) (redrawn the ChemDraw structure from ref. 67). | |
Benzothiazole-based fluorescent probes8a–c were designed and synthesized by Chen and co-workers in 2017 (ref. 68) for the detection of Zn(II) ions via the fluorescence turn-on mechanism. The probes were structurally characterized via various analytical techniques such as multinuclear NMR and HRMS. There was a considerable change in the emission of these probes8a–c upon binding with Zn(II) ions from a very poor yellow color emission to a strong blue color emission, whereas no change was seen in fluorescence spectra upon addition of other competitive metal ions (Fig. 9). However, probe-8a displayed a very high sensitivity towards Zn(II) ions as compared with probe-8b and 8c. The LOD value of probe-8a with Zn(II) ions was calculated from the equation 3σ/slope and found to be as low as 7 nM with high sensitivity with respect to probe-8b and 8c. Furthermore, confocal laser scanning micrographs of HeLa cells demonstrate that probe-8a has high cell permeability and can specifically recognize Zn(II) ions in living cells.
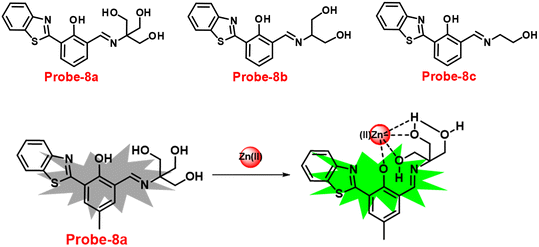 |
| | Fig. 9 Chemical structures of probe-8(a–c) and sensing mechanism of probe-8a with Zn(II) (redrawn the ChemDraw structure from ref. 68). | |
Wang and colleagues developed quinoline-conjugated fluorescent turn-on probe-9 that is simple, quick to respond, and highly selective for Zn(II) ions69 in 2021. Probe-9 was synthesized via condensation of 2-quinolinecarboxaldehyde and 2-picolinyl hydrazide. The native state of probe-9 showed very low emission, whereas the rapid amplification of luminescence intensity was observed on complexation with Zn(II) ions, which could be explained by the effective suppression of PET. Thus, the CHEF effect improves the molecule's planarity and stiffness by limiting free rotation (Fig. 10). Further, probe-9 was also utilized as a fluorescent probe for detecting Zn(II) ions across a wide pH range. The binding ability and mechanism was determined by Job's plot analysis, FT-IR spectrum, and 1H NMR titration. Furthermore, probe-9 is being developed into test strips for quick, convenient, quantitative, and qualitative Zn(II) ion determination. On the basis of the IUPAC guideline for Zn(II) ions, the detection limit of the probe is particularly essential in the field of supramolecular chemistry, and the authors determined the limit of detection using the equation of 3σ/slope to be 72 nM, which is much lesser than that of IUPAC recommendation. Further, the binding constant of probe-9 with Zn(II) ions was calculated to be 6.65 × 103 M−1 using the B–H equation.
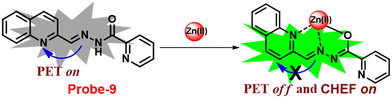 |
| | Fig. 10 Chemical structure and proposed sensing mechanism of probe-9 with Zn(II) (redrawn the ChemDraw structure from ref. 69). | |
Gorden and co-workers successfully synthesized and characterized a pentadentate Schiff base ligand (probe-10)70 in 2019, which has the ability to act as a fluorescent turn-on probe for Zn(II) ions. Probe-10 was synthesized via a simple condensation reaction between 3,5-di-tert-butyl-2-hydroxybenzaldehyde and pyridine-2,6-diyldimethanamine in EtOH under reflux conditions. The synthesized probe-10 was very poorly emissive in nature, and upon adding Zn(II) ions, there was an enhancement in the emission and the quantum yields increased by 1.6% (Fig. 11). However, under similar conditions, no change or very minimal changes were perceived for other competitive metal ions. The titration of probe-10 with competitive metal ions produced an unusual result in which the accumulation of heterometals such as Ca(II), Mg(II), K(I), and Cd(II) causes an increase in emission as compared with other metal ions. The binding constant of probe-10 with Zn(II) ions was estimated as 1.8 × 10−1 M−1. The LOD of probe-10 in the presence of Zn(II) was determined to be 7.2 μM.
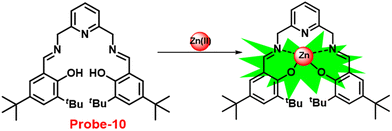 |
| | Fig. 11 Chemical structure and proposed sensing mechanism of probe-10 with Zn(II) (redrawn the ChemDraw structure from ref. 70). | |
Sreekanth et al. developed a chemosensor with thiophene-appended carbohydrazide, which exhibits luminescence turn-on recognition for Zn(II) ions via the CHEF process71 in 2021. Probe-11 (Fig. 12) was produced via a simple condensation reaction of thiophene-2,5-dicarbohydrazide and 2-hydroxynaphthaldehyde in EtOH under reflux conditions, yielding a yellow colour solid. Fluorescence titration data revealed that in the presence of other metal ions, no changes in fluorescence spectra were observed even at very high concentrations, whereas upon addition of Zn(II) ions, the emission intensity was gradually increased. The LOD of probe-11 with Zn(II) ions was estimated to be 0.15 μM using the 3σ/slope method from fluorescence spectra, and the association constant was calculated using the B–H equation to be 1.15 × 104 M−1. The stoichiometry of probe-11 with Zn(II) was estimated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using Job's plot. Further, the binding interaction and fluorescence turn-on response of probe-11 with Zn(II) ions were strongly held by the DFT calculations. The reversibility of probe-11 + Zn(II) was studied using EDTA, producing a better output, which was then converted into a logic circuit. The remarkable properties of probe-11 can be used to detect Zn(II) ions in both biological and environmental samples.
1 using Job's plot. Further, the binding interaction and fluorescence turn-on response of probe-11 with Zn(II) ions were strongly held by the DFT calculations. The reversibility of probe-11 + Zn(II) was studied using EDTA, producing a better output, which was then converted into a logic circuit. The remarkable properties of probe-11 can be used to detect Zn(II) ions in both biological and environmental samples.
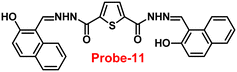 |
| | Fig. 12 Chemical structure of probe-11 (redrawn the ChemDraw structure from ref. 71). | |
Roy et al. synthesized two Schiff base compounds named Probe-12a and Probe-12bvia the condensation reaction of 2-hydrazinylquinoline with 2-hydroxy-5-methylbenzaldehyde and 2-hydroxy-benzaldehyde respectively72 in 2019. Probe-12a and probe-12b are as very sensitive and selective towards Zn(II) ions; however, their sensing abilities vary from each other, for example, upon addition of one equivalent of Zn(II) ions, the emission intensity of probe-12a at 490 nm was enriched by 4.5-fold upon excitation at 410 nm, whereas probe-12b exhibited the increment of fluorescence intensity by 30 folds upon excitation at 515 nm due to the suppression of PET process (Fig. 13). The more sensing ability of probe-12b might be attributed to the existence of the methyl group, and thus, probe-12b acts as better luminescent probe for Zn(II) ions. Further, the authors demonstrated the interaction of several competitive metal ions and the results suggest that there was no change observed in the fluorescence spectra. The binding constant and limit of detection of probe-12b with Zn(II) ions were estimated from the emission titration spectra via the B–H and 3σ/slope equation respectively and found to be 5.90 × 104 M−1 and 220.6 nM correspondingly. Furthermore, the authors tested probe-12b in a practical application, detecting Zn(II) in water using the dip strike method, and the results indicated that probe-12b could be a potential candidate for detecting Zn(II) ions in solid state as well. Finally, Zn(II) ions were detected in biological samples using probe-12b, which was incubated with C6 cells, and upon treatment with Zn(II) ions, the probe showed strong green colour fluorescence enhancement inside the cells. As a result, probe-12b was identified as a possible candidate for detecting Zn(II) ions in both environmental and biological samples.
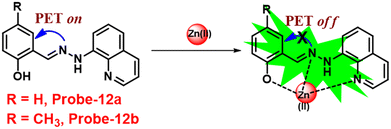 |
| | Fig. 13 Chemical structure and proposed sensing mechanism of probe-12a–b with Zn(II) (redrawn the ChemDraw structure from ref. 72). | |
It is critical to investigate the variations in Zn(II) ion levels in clinical, medical, and environmental circles. Taking this into account, Shen and his colleagues synthesized biocompatible and tissue permeable dual-channel fluorescent off–on Schiff base probe-13 for Zn(II) ions73 in 2021. When exposed to Zn(II), probe-13 produced a blue luminescence signal (455 nm) due to the suppression of PET process, and upon addition of various competitive metal ions, no change in fluorescence spectra was observed, which strongly demonstrated that probe-13 is ideal for the detection of Zn(II) ions (Fig. 14). Further, authors evaluated the applications of probe-13 for the detection of Zn(II) ions in biological samples via bio-imaging on live cells, larval zebrafish, and plants. Probe-13 has a LOD of 56 nM for Zn(II), which was calculated from the fluorescence spectra. Further, authors performed the sensitive response characterization in the pH range of 7.0 to 9.4 (pKa = 8.40). Furthermore, probe-13 is the best because of its great stability and reversibility, which may be used to detect Zn(II) ions in environmental and biological materials.
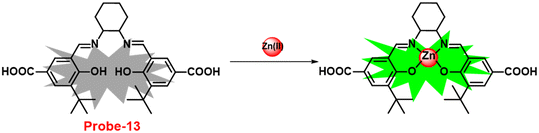 |
| | Fig. 14 Chemical structure and proposed sensing mechanism of probe-13 with Zn(II) (redrawn the ChemDraw structure from ref. 73). | |
Brooker et al. reported the first excursion into the new generations of innovative macrocyclic-based Schiff bases in 2018 via a simple condensation reaction between carbazole-dialdehyde and ethylene diamine and created [2 + 2] Schiff base macrocycles.74 Authors mainly focused on the synthesis of two probes (14a and 14b) (Fig. 15), which show selectivity as well as sensitivity, and can also be utilized as powerful turn-on blue luminescent sensors for Zn(II) ions in DMF at 335 nm, which might be attributed to the enhancement of CHEF and suppression of PET. In the presence of other competitive metal ions, probe-14a as well as 14b shows no change or very minimal change in the fluorescence spectra, which strongly suggested that the probes are potential for the recognition of Zn(II) ions, on addition of other metal ions. The detection limits of probes with Zn(II) ions were calculated to be in the nanomolar range via fluorescence titration spectra. Further, the binding ability of probes towards Zn(II) ions were validated via1H NMR, ESI and DFT calculations.
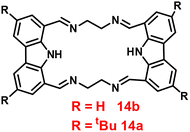 |
| | Fig. 15 Chemical structure of probe-14a and probe-14b. | |
An ESIPT-based 2-(20-aminophenyl)benzothiazole derivative, probe-15, as the fluorescent turn-on probe, was synthesized by Thennaraasu and coworkers in 2016.75 The effect of Zn(II) ions on ESIPT inhibition was examined by evaluating the absorbance and fluorescence spectra of probe-15 (Fig. 16), and the results strongly suggested that the fluorescence band of probe-15 changed from 556 nm to 459 nm with enhanced fluorescence signals. However, in the presence of competitive metal ions (10 eq.), probe-15 displayed no or very little fluorescence changes. From the above-mentioned results, one can conclude that only Zn(II) ions caused a noteworthy change in the fluorescence profiles of probe-15, while no other metal ions caused significant variations and could be applied as potential candidates for Zn(II) ions with other competitive metal ions. Furthermore, in order to know the binding mode and fluorescence enhancement of probe-15 with Zn(II) ions, authors conducted FT-IR, DFT, and 1H-NMR studies, which strongly supported the contribution of –NH, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, and –OH groups in ESIPT and –C
N–, and –OH groups in ESIPT and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– isomerization processes, which were suppressed upon binding with Zn(II) ions, resulting in fluorescence improvement. In addition, the stoichiometric ratio of probe-15 with Zn(II) ions was calculated via Job's plot to be 1
N– isomerization processes, which were suppressed upon binding with Zn(II) ions, resulting in fluorescence improvement. In addition, the stoichiometric ratio of probe-15 with Zn(II) ions was calculated via Job's plot to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was further confirmed by ESI-mass data. The LOD of probe-15 with Zn(II) ions was estimated to be 4.5 nM by the 3σ/slope method. Further, the binding constant was estimated by the B–H method to be 2.4 × 104 M−1. Probe-15 demonstrated very good thermal stability and the decomposition of the probe-15–Zn(II) complex was found to be very minimal (1.15%) at a temperature of 259.38 °C, which endows probe-15 with the potential for the detection of environmental samples.
1, which was further confirmed by ESI-mass data. The LOD of probe-15 with Zn(II) ions was estimated to be 4.5 nM by the 3σ/slope method. Further, the binding constant was estimated by the B–H method to be 2.4 × 104 M−1. Probe-15 demonstrated very good thermal stability and the decomposition of the probe-15–Zn(II) complex was found to be very minimal (1.15%) at a temperature of 259.38 °C, which endows probe-15 with the potential for the detection of environmental samples.
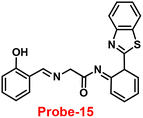 |
| | Fig. 16 Chemical structure of probe-15. | |
Coumarin is a phytochemical, and a majority of its conjugates have a diverse range of pharmacological effects, which make it useful in biology due to its less cytotoxicity. A Schiff base-based probe (probe-16) was obtained by condensation of 1,2-bis(2-aminophenylthio) ethane with 8-formyl-7-hydroxy-4-methylcoumarin by Sinha et al. in 2016.76 In the presence of Zn(II) ions with probe-16, the emission intensity was increased at 514 nm, while it is unresponsive to other cations. The binding mechanism of probe-16 with Zn(II) ions was explained by the restriction of ESIPT and CHEF (Fig. 17). The association constant of probe-16 with Zn(II) is 6.49 × 104 M−1 by the B–H method and the 3σ/slope method was used to estimate the LOD of Zn(II) ions, which was found to be as minimal as 0.068 μM. The probe-16 showed lower cytotoxicity, which suggests that probe-16 has a potential application for the in vitro and in vivo recognition of Zn(II) ions. The authors performed the bio-imaging on SCC084 (human oral carcinoma) cells for the sensing of Zn(II) ions and the whole cell line emission enhancement was obtained, which demonstrated the potential of probe-16 for the recognition of Zn(II) ions in a cellular medium (Fig. 18).
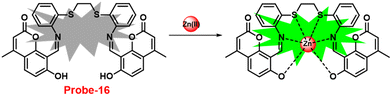 |
| | Fig. 17 Chemical structure and proposed sensing mechanism of probe-16 with Zn(II) (redrawn the ChemDraw structure from ref. 76). | |
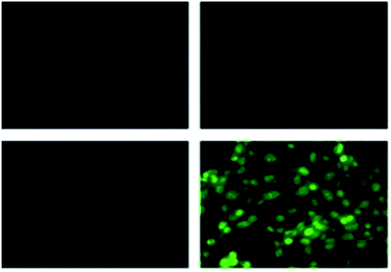 |
| | Fig. 18 Fluorescence microscopic images of SCC084 (human oral carcinoma) with probe-16 and Zn(II) ions + probe-16 (reprinted with permission from ref. 76, Copyright 2016 Royal Society of Chemistry). | |
A flexible ligand, N1,N3-bis(3-methoxysalicylidene)diethylenetriamine (probe-17),77 was successfully synthesized by Goswami and coworkers in 2017, which gave various coordination modes to give trinuclear Zn complexes with Zn(NO3)2·6H2O, ZnBr2, and ZnI2. All the synthesized compounds were structurally characterized by multinuclear NMR, FT-IR spectroscopy, elemental analysis (EA) and single-crystal XRD analysis. The authors performed the fluorescence spectroscopic techniques to understand the sensing potential of probe-17 (Fig. 19), and the results established great sensitivity and selectivity towards Zn(II) ions in aqueous solutions at pH 7.4. The emission intensity was increased by 19-fold with maxima at 467 nm when different amounts of Zn(II) were added, whereas no or minimal difference was found for the other metal ions. The binding ability and fluorescence enhancement ability could be attributed to the CHEF, which occurs when probe-17 binds to Zn(II) ions and forms intramolecular hydrogen bonds among phenolic OH and amine nitrogen, allowing for ESIPT. Further, probe-17 serves as a dependable sensor with a LOD in the nanomolar range (6.72 nM), and it is utilized for imaging in HCT 116 cells for the real-time monitoring. The binding constant of probe-17 with Zn(II) was estimated using the B–H equation and displayed as 31.647 × 104 M−1. The binding ratio of probe-17 with Zn(II) ions was estimated with Job's plot and found out to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was strongly validated by ESI-MS and DFT calculations. In addition, the complex-Zn(II) turned out to detect pyrophosphate (PPi) with among all other phosphates in an aqueous medium with both chromogenic and fluorogenic response with a LOD of 5.12 nM.
1, which was strongly validated by ESI-MS and DFT calculations. In addition, the complex-Zn(II) turned out to detect pyrophosphate (PPi) with among all other phosphates in an aqueous medium with both chromogenic and fluorogenic response with a LOD of 5.12 nM.
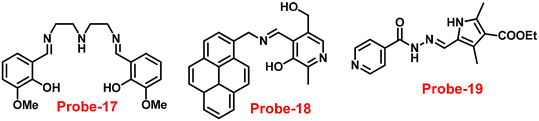 |
| | Fig. 19 Chemical structures of probe-(17–19) (redrawn the ChemDraw structure from ref. 77–79). | |
Sahoo et al. designed Schiff base-based probe-18 (Fig. 19) by the condensation of vitamin B6 cofactor pyridoxal with the 1-pyrenemethylamine, which functions as a three-in-one sensor for three bioactive analytes with the recognition unit being pyrene fluorophore and the vitamin B6 cofactor pyridoxal78 in 2018. The structural characterization of target compound (probe-18) was established by 1H-NMR, attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR), HRMS, and EA. When the fluorescence spectra were acquired, probe-18 showed significant fluorescence amplification at 485 nm when Zn(II) ions were added. Furthermore, fluorescence titration experiments of probe-18 with gradual incremental addition of Zn(II) caused in the probe-18's luminescence was increased vertically and a new band arising at 485 nm. However, in the presence of other metal ions, probe-18 resulted in very minimal or no response in fluorescence spectra. The suppression of the PET mechanism, in which the transfer of a lone pair of electrons from the nitrogen atom (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) to the pyrene fluorophore in the excited state is hampered by binding with Zn(II), could explain this vertical amplification of bands. Interestingly, probe-18 detects three essential bioactive analytes Zn(II), H2PO4−, and cysteine, with LOD values of 2.3 μM, 0.21 μM, and 0.16 μM, respectively. In vitro cellular imaging was also used to detect intracellular Zn(II) ions using probe-18. Further, probe-18 was utilized in recognition of Zn(II) ions in HeLa cells. Hence, based on the efficacy of probe-18, it can be applied for the recognition of Zn(II) ions in biological and environmental samples.
N–) to the pyrene fluorophore in the excited state is hampered by binding with Zn(II), could explain this vertical amplification of bands. Interestingly, probe-18 detects three essential bioactive analytes Zn(II), H2PO4−, and cysteine, with LOD values of 2.3 μM, 0.21 μM, and 0.16 μM, respectively. In vitro cellular imaging was also used to detect intracellular Zn(II) ions using probe-18. Further, probe-18 was utilized in recognition of Zn(II) ions in HeLa cells. Hence, based on the efficacy of probe-18, it can be applied for the recognition of Zn(II) ions in biological and environmental samples.
Schiff base with pyrrole units is a versatile chelating combination that has received a lot of attention, in this regard, Wang and colleagues designed and synthesized a hydrazone with a pyrrole unit, probe-19, (Fig. 19) for the selective and sensitive detection with the luminescence turn-on mechanism of Zn(II) ions in 2018.79 The target compound was obtained upon condensation reaction with ethyl 5-formyl-2,4-dimethyl-pyrrole-3-carboxylate and isonicotinohydrazide in ethanol under reflux conditions. Probe-19 was structurally characterised via multinuclear NMR, ESI-MS, and EA. Probe-19 displayed poor emission in the native state and in the presence of Zn(II) ions, the emission intensity was enhanced at 550 nm with moderately high quantum yields due to the presence of CHEF process. Probe-19 exhibited very low LOD for Zn(II) ions, for example, 0.18 μM, which was calculated by the 3σ/slope method. Job's plot was established using fluorescence spectra that showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for probe-19 with Zn(II) ions and a binding constant (Ka) of 1.25 × 104 M−1. Due to the presence of very low detection limit, probe-19 was applied for the real-time monitoring of Zn(II) ions in imaging intracellular cells. Further, probe-19 shows potential for application in the detection of biological samples, which was performed on U251 cell lines. The cells were treated with probe-19 and showed no fluorescence, whereas upon incorporation with the Zn(II) ions, the color changed into strong green color fluorescence inside the cell line.
1 stoichiometry for probe-19 with Zn(II) ions and a binding constant (Ka) of 1.25 × 104 M−1. Due to the presence of very low detection limit, probe-19 was applied for the real-time monitoring of Zn(II) ions in imaging intracellular cells. Further, probe-19 shows potential for application in the detection of biological samples, which was performed on U251 cell lines. The cells were treated with probe-19 and showed no fluorescence, whereas upon incorporation with the Zn(II) ions, the color changed into strong green color fluorescence inside the cell line.
Kim et al. synthesized highly potential probe-20 based on the imidazole derivative in 2019,80 which shows luminescence turn on–off detection of Zn(II) and S2− ions with very low LOD values of 1.59 μM and 8.03 μM, correspondingly. The synthesis of the target compound was followed by a very simple condensation reaction between (4)-amino-4(5)-(aminocarbonyl)imidazole hydrochloride and 4-diethylaminosalicylaldehyde in MeOH under reflux conditions. The native state of probe-20 exhibited very poor emission as a result of PET and upon addition of Zn(II) ion fluorescence enrichment was observed, which was attributed to the suppression of PET and CHEF (Fig. 20). To find out the stoichiometry of probe-20 with Zn(II) ions, authors performed Job's plot and results discovered the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio. The fluorescence changes of probe-20 for Zn(II) were investigated further to rule out the interference of other cations, whereas the fluorescence quenching was caused by Fe(III), Cu(II), Cr(III), Fe(II), and Co(II) (78–100%), and competitive metal ion shows no change in the fluorescence spectra. The binding constant of probe-20 with Zn(II) ions was calculated from the fluorescence spectra using B–H equation as 2.0 × 103 M−1. Probe-20 displayed good reversibility with EDTA, which extends its real-time applicability and also in live cell imaging (Fig. 21).
1 binding ratio. The fluorescence changes of probe-20 for Zn(II) were investigated further to rule out the interference of other cations, whereas the fluorescence quenching was caused by Fe(III), Cu(II), Cr(III), Fe(II), and Co(II) (78–100%), and competitive metal ion shows no change in the fluorescence spectra. The binding constant of probe-20 with Zn(II) ions was calculated from the fluorescence spectra using B–H equation as 2.0 × 103 M−1. Probe-20 displayed good reversibility with EDTA, which extends its real-time applicability and also in live cell imaging (Fig. 21).
 |
| | Fig. 20 Chemical structure and proposed sensing mechanism of probe-20 with Zn(II) (redrawn the ChemDraw structure from ref. 80). | |
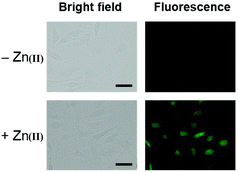 |
| | Fig. 21 Fluorescence imaging of HeLa cells incubated with probe-20 followed by the addition of Zn(II) (reprinted with permission from ref. 80, Copyright 2019 Royal Society of Chemistry). | |
Ghosh and his colleagues developed an iminophenol probe (probe-21)81 with 1,2,3-traizole in the proximal position that exhibits a significant increase in emission upon interaction with Zn(II) ions as compared with other metal ions in 2021. The authors proposed the sensing mechanism responsible for fluorescence turn-on, which is the chelation of Zn(II) ions in the Schiff base core. From Job's plots, the binding ratio of probe-21 (Fig. 22) and Zn(II) ions was estimated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was established via ESI-MS and DFT studies. The binding constant was calculated from the equation of B–H to be 9.06 × 104 M−1. The LOD was determined using fluorescence titration as 1.8 μM. Moreover, picric acid (PA) was successfully detected by a zinc-ensemble over a series of other nitroaromatics and was confirmed by DFT studies. Yang and co-workers synthesized a simple probe (probe-22)82 from 4-diethylaminosalicylaldehyde and 7-amino-4-methyl coumarin, which acts as a selective and sensitive probe for Zn(II) ions in 2016. Probe-22 (Fig. 22) was found to be very poorly emissive in nature and an increase in emission was observed at 500 nm with Zn(II) ions, increasing the quantum yields (ϕ = 0.1651). Though, upon addition of other metal ions, there was no change in the fluorescence spectra specifying probe-22 was very selective to Zn(II) ions. Further, the fluorescence enhancement might be attributed to the suppression of C
1, which was established via ESI-MS and DFT studies. The binding constant was calculated from the equation of B–H to be 9.06 × 104 M−1. The LOD was determined using fluorescence titration as 1.8 μM. Moreover, picric acid (PA) was successfully detected by a zinc-ensemble over a series of other nitroaromatics and was confirmed by DFT studies. Yang and co-workers synthesized a simple probe (probe-22)82 from 4-diethylaminosalicylaldehyde and 7-amino-4-methyl coumarin, which acts as a selective and sensitive probe for Zn(II) ions in 2016. Probe-22 (Fig. 22) was found to be very poorly emissive in nature and an increase in emission was observed at 500 nm with Zn(II) ions, increasing the quantum yields (ϕ = 0.1651). Though, upon addition of other metal ions, there was no change in the fluorescence spectra specifying probe-22 was very selective to Zn(II) ions. Further, the fluorescence enhancement might be attributed to the suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and the inhibition of PET. The binding ratio among probe-22 and Zn(II) ions was calculated using Job's plot as 2
N isomerization and the inhibition of PET. The binding ratio among probe-22 and Zn(II) ions was calculated using Job's plot as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and was strongly held by ESI-MS, 1H NMR, and DFT calculations. Furthermore, the LOD and the binding constant of probe-22 with Zn(II) ions were found as log
1 and was strongly held by ESI-MS, 1H NMR, and DFT calculations. Furthermore, the LOD and the binding constant of probe-22 with Zn(II) ions were found as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 6.04 and 2.59 μM, respectively. Since, probe-22 has a moderately high association constant with Zn(II) ions and a low detection limit, it could be applicable to biological and environmental samples.
K = 6.04 and 2.59 μM, respectively. Since, probe-22 has a moderately high association constant with Zn(II) ions and a low detection limit, it could be applicable to biological and environmental samples.
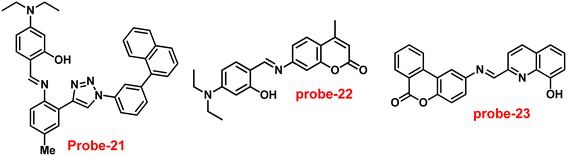 |
| | Fig. 22 Chemical structures of probe-(21–23) (redrawn the ChemDraw structure from ref. 81–83). | |
Singh et al. focused on dual coumarin-based Schiff base probe-23 (ref. 83) for the selective and sensitive detection of Zn(II) ions in 2016. Probe-23 (Fig. 22) was synthesized via a simple condensation reaction between 6-amino-3,4-benzocoumarin and 8-hydroxy quinolone-2-carboxaldehyde in ethanol under reflux conditions. The titration of probe-23 was conducted using a UV-visible and fluorescence spectrophotometer, and the results indicated a colorimetric response towards Fe(III) and a fluorescence turn-on behavior for Zn(II) ions. The emission turn-on mechanism might be due to the presence of strong complexation via O and N heteroatoms, which resulted in an increase in the radiative decay as well as suppression of PET and cis–trans isomerism. The B–H relation was used to calculate the binding constant as 3.8 × 103 M−1. The LOD of probe-23 with Zn(II) ions was calculated from the fluorescence titration spectra via the 3σ/slope method and showed to be 10 μM. The binding ratio of probe-23 and Zn(II) was calculated from Job's plot as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The reversible studies of probe-23 + Zn(II) were conducted with EDTA and the restored probe-23, which is again utilized for the detection of Zn(II) ions. Furthermore, probe-23's fluorescence emission response can be examined as a binary logic function.
1. The reversible studies of probe-23 + Zn(II) were conducted with EDTA and the restored probe-23, which is again utilized for the detection of Zn(II) ions. Furthermore, probe-23's fluorescence emission response can be examined as a binary logic function.
Kuwar and colleagues designed and synthesized nicotin-based Schiff base probe-24, which showed very selective and sensitive nature towards Zn(II) ions in 2018.84 The pure form of probe-24 (Fig. 23) displayed very poor emission due to the presence of cis–trans isomerism and PET, which was suppressed by CHEF effects upon addition of Zn(II) ions; as a result, a substantial enrichment of emission at 517 nm in a binary mixture of acetonitrile/water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) was detected. However, in the presence of other metal ions, there was no or minimal fluorescence changes observed. Further, authors determined the detection limit via fluorescence titration spectra to be 4.35 nM. The binding constant of probe-24 and Zn(II) was estimated by non-linear curve fitting of the fluorescence titration spectra and found to be log
50, v/v) was detected. However, in the presence of other metal ions, there was no or minimal fluorescence changes observed. Further, authors determined the detection limit via fluorescence titration spectra to be 4.35 nM. The binding constant of probe-24 and Zn(II) was estimated by non-linear curve fitting of the fluorescence titration spectra and found to be log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 5.63 (0.15). Further, stoichiometry was determined from Job's plot, and the results were exhibited to be 1
K = 5.63 (0.15). Further, stoichiometry was determined from Job's plot, and the results were exhibited to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Finally, authors conducted the cytotoxicity of probe-24 on A549 cells, and the results indicated very low toxicity, which strongly supported the use of probe-24 to monitor Zn(II) ions in biological samples (Fig. 24).
1. Finally, authors conducted the cytotoxicity of probe-24 on A549 cells, and the results indicated very low toxicity, which strongly supported the use of probe-24 to monitor Zn(II) ions in biological samples (Fig. 24).
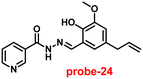 |
| | Fig. 23 Chemical structure of probe-24 (redrawn the ChemDraw structure from ref. 84). | |
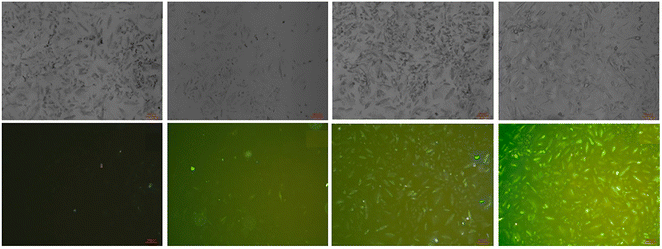 |
| | Fig. 24 Bioimaging study images of probe-24 on A549 cells with Zn(II) ions (reprinted with permission from ref. 84, Copyright 2018 Elsevier). | |
A single molecular structure of imine was designed by Kim et al. from 5-methylisoxazole-3-amine and 2-hydroxy-1-naphthaldehyde, which contains an imine as a chromophore and a naphthol group as a fluorophore in 2019.85 The native state of probe-25 was poorly emissive in nature; on titrating with Zn(II) ions, the emission intensity was gradually increased, which attributed to the CHEF effect (Fig. 25). However, upon introduction of other competitive metal ions to probe-25, there was no change in fluorescence spectra, which strongly suggested the very selectivity towards Zn(II) ions. Job's plot supported the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding of probe-25 with Zn(II), which was strongly supported by the ESI-MS, DFT and 1H NMR data. The LOD and association constant of probe-25 with Zn(II) were evaluated from the fluorescence titration spectra as 1.29 μM and 7.9 × 104 M−1 respectively. Further, authors performed the recognition of Zn(II) ions in biological applications; for this, Zebrafish was incubated with probe-25 and data supported that strong luminescence was generated inside the Zebrafish (Fig. 26).
1 binding of probe-25 with Zn(II), which was strongly supported by the ESI-MS, DFT and 1H NMR data. The LOD and association constant of probe-25 with Zn(II) were evaluated from the fluorescence titration spectra as 1.29 μM and 7.9 × 104 M−1 respectively. Further, authors performed the recognition of Zn(II) ions in biological applications; for this, Zebrafish was incubated with probe-25 and data supported that strong luminescence was generated inside the Zebrafish (Fig. 26).
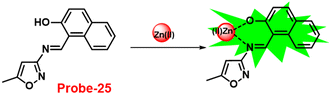 |
| | Fig. 25 Chemical structure and proposed sensing mechanism of probe-25 with Zn(II) (redrawn the ChemDraw structure from ref. 85). | |
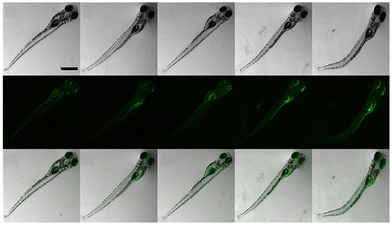 |
| | Fig. 26 Bioimaging study images of probe-25 on zebrafish with Zn(II) ions (reprinted with permission from ref. 85, Copyright 2019 Elsevier). | |
In 2018, Yang and colleagues developed new fluorescent probe-26 with rhodamine and chromone moieties as a fluorescent “turn-on” probe for Zn(II) ions that works via inhibited PET86 (Fig. 27). When Zn(II) is added to probe-26, the emission intensity at 490 nm increases in an ethanol/HEPES solution (7/3, pH = 7.2). Further, the authors determined the stoichiometry between probe-26 and Zn(II) ions to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 via Job's plot, which was supported by ESI-MS, 1H NMR and DFT data. The LOD and association constants were calculated using fluorescence titration data and reported as 0.33 μM and 9.98 × 104 M−1 correspondingly. Finally, the authors used probe-26 in a practical application, such as test strips, and developed a practical, efficient, and low-cost Zn(II) ion testing tool.
1 via Job's plot, which was supported by ESI-MS, 1H NMR and DFT data. The LOD and association constants were calculated using fluorescence titration data and reported as 0.33 μM and 9.98 × 104 M−1 correspondingly. Finally, the authors used probe-26 in a practical application, such as test strips, and developed a practical, efficient, and low-cost Zn(II) ion testing tool.
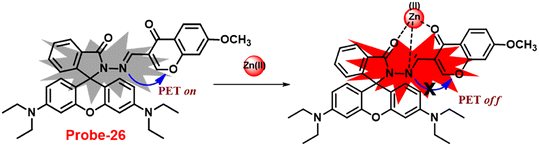 |
| | Fig. 27 Chemical structure and proposed sensing mechanism of probe-26 with Zn(II) (redrawn the ChemDraw structure from ref. 86). | |
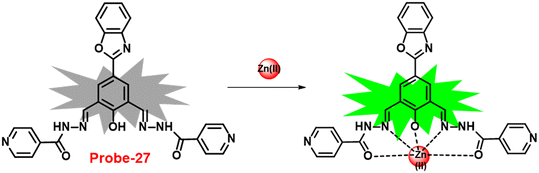
In 2018, Viswanthamurthi et al. established a powerful tracker for Zn(II) and PPi ions in biological systems by appending benzoxazole to dipodal Schiff base (probe-27).87Probe-27 was synthesized from benzoxazole, which underwent a double Duff reaction followed by the reaction with isonicotinic hydrazide in a methanol solution at room temperature and gave the final product as a yellow coloured solid. When probe-27 was titrated with Zn(II) ions, the emission intensity progressively increased and attained maxima upon addition of one eq. via the ICT mechanism (Fig. 28). However, upon addition of competitive metal ions, there was no difference in the fluorescence profile, which strongly supports the selectivity of probe-27 towards Zn(II) ions. Fluorescence titrations were used to determine the LOD and binding value (Ka) of the probe-27–Zn(II) complex, which were 0.52 μM and 4.53 × 104 M−1 respectively. Further, authors determined the complexation ratio between probe-27 and Zn(II) ions to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 via Job's plot, which was further supported by ESI-MS, 1H NMR and DFT results. Furthermore, the emission intensity at 525 nm of the probe-27–Zn(II) ensemble was reduced dramatically upon addition of a 100 μM solution of PPi anions, but remained stable for other anions, which showed the potential of probe-27 to detect both the biologically important metal ions (Zn(II)) and anion (PPi) (Fig. 29).
1 via Job's plot, which was further supported by ESI-MS, 1H NMR and DFT results. Furthermore, the emission intensity at 525 nm of the probe-27–Zn(II) ensemble was reduced dramatically upon addition of a 100 μM solution of PPi anions, but remained stable for other anions, which showed the potential of probe-27 to detect both the biologically important metal ions (Zn(II)) and anion (PPi) (Fig. 29).
 |
| | Fig. 28 Chemical structure and proposed sensing mechanism of probe-27 with Zn(II) (redrawn the ChemDraw structure from ref. 87). | |
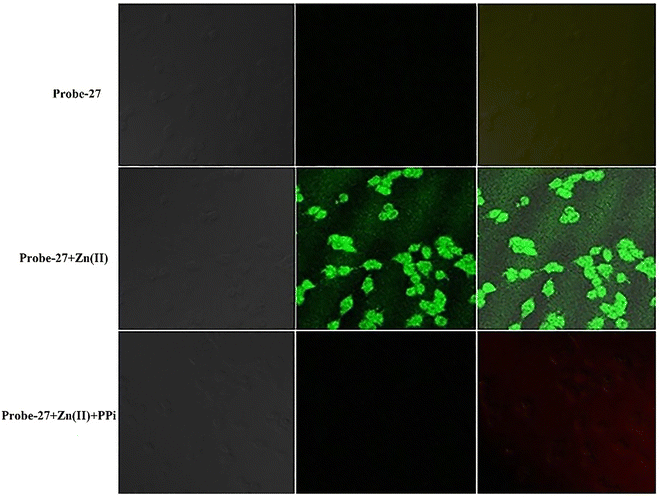 |
| | Fig. 29 Fluorescence images of cells (HeLa) treated with probe-27, probe-27 + Zn(II) and probe-27 + Zn(II) + PPi (reprinted with permission from ref. 87, Copyright 2019 Elsevier). | |
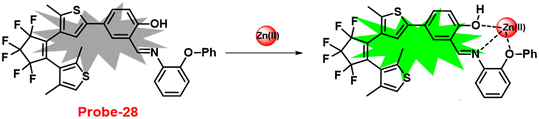
Pu and co-workers reported a fast response diarylethene derivative containing phenoxyaniline and Schiff base (probe-28),88 which exhibited the fluorescence “turn-on” mechanism for the recognition of Zn(II) ions in 2019. Probe-28 was titrated with several metal ions including Zn(II) ions and upon addition of Zn(II) ions, the emission intensity was gradually increased to 105 times at 582 nm and the fluorescence color has changed from non-fluorescence to bright yellow due to the suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and CHEF (Fig. 30). However, no difference was detected in fluorescence spectra of probe-28 in the presence of other metal ions. Job's plot analysis and HRMS revealed a 1
N isomerization and CHEF (Fig. 30). However, no difference was detected in fluorescence spectra of probe-28 in the presence of other metal ions. Job's plot analysis and HRMS revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry for probe-28 and Zn(II) ions. Furthermore, probe-28 was successfully demonstrated for the analysis of real-time applications due to the presence of very low detection limits such as 13.4 nM. The fluorescence titration data yields a binding constant of 3.49 × 104 M−1 from the B–H equation and gratifyingly, it could be applied to design a logic gate.
1 binding stoichiometry for probe-28 and Zn(II) ions. Furthermore, probe-28 was successfully demonstrated for the analysis of real-time applications due to the presence of very low detection limits such as 13.4 nM. The fluorescence titration data yields a binding constant of 3.49 × 104 M−1 from the B–H equation and gratifyingly, it could be applied to design a logic gate.
 |
| | Fig. 30 Chemical structure and proposed sensing mechanism of probe-28 with Zn(II) (redrawn the ChemDraw structure from ref. 88). | |
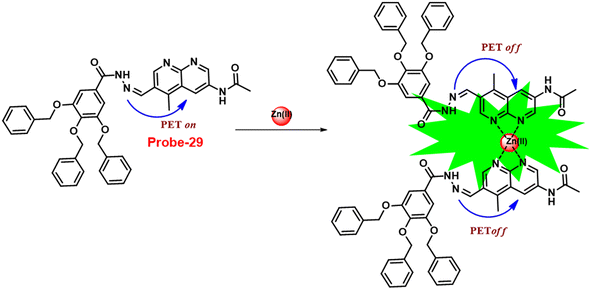
In 2019, Yang and colleagues designed an imine probe (probe-29)89 from a 4-methyl-7-acetamide-1,8-naphthyridyl moiety and a trihydroxybenzoyl hydrazine unit that acts as a sensitive probe for Zn(II) ions via the fluorescence turn-on mechanism. Probe-29 alone demonstrated weak emission, but the addition of Zn(II) resulted in the formation of a new band centred at 504 nm with increased intensity, which attributed to the suppression of PET and CHEF process (Fig. 31). On the basis of nonlinear B–H equation from emission titration values, Job's plot method yielded a coordinative stoichiometry ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a binding constant (Ka) of 1.14 × 105 M−1. The LOD of probe-29 with Zn(II) ions was determined to be 7.52 nM. The perfect reversibility and renewability of probe-29 for the recognition of Zn(II) was confirmed using Na2EDTA, a chelating agent, in which the fluorescence emission of complex probe-29–Zn(II) was significantly quenched with the addition of Na2EDTA and can be utilized for various practical applications.
1 with a binding constant (Ka) of 1.14 × 105 M−1. The LOD of probe-29 with Zn(II) ions was determined to be 7.52 nM. The perfect reversibility and renewability of probe-29 for the recognition of Zn(II) was confirmed using Na2EDTA, a chelating agent, in which the fluorescence emission of complex probe-29–Zn(II) was significantly quenched with the addition of Na2EDTA and can be utilized for various practical applications.
 |
| | Fig. 31 Chemical structure and proposed sensing mechanism of probe-29 with Zn(II) (redrawn the ChemDraw structure from ref. 89). | |
Lin and colleagues developed a Schiff base, probe-30, from imidazo[2,1-b]thiazole-6-carboxylic acid and 3-ethoxy-2-hydroxybenzaldehyde for the selective and sensitive sensing of Zn(II) via the fluorescence turn-on mechanism90 in 2019. Probe-30 showed very poor emission with low emission intensity (ϕ = 0.016). However, the addition of Zn(II) ions to probe-30 could cause a significant change in the emission intensity (ϕ = 0.3) at 511 nm, resulting in a 42-fold increase in intensity, due to the presence of CHEF process and the absence of PET (Fig. 32 and 33). Probe-30 binds to Zn(II) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as confirmed by 1H NMR, DFT/TD-DFT, mass spectral analysis and Job's plot. The association constant of probe-30 with Zn(II) ions was estimated with a nonlinear B–H as 2.2 × 105 M−1. The LOD was estimated using emission titration data to be 1.2 nM by the 3σ/slope method. In contrast, this recognition process was reversible, as the fluorescence titration experiment of probe-30–[Zn(II)] to PPi results in lower emission, which results strongly supported by regeneration of probe-30. Finally, authors claims that the fluorescence signals of probe-30 were used to build a molecular INHIBIT logic gate.
1, as confirmed by 1H NMR, DFT/TD-DFT, mass spectral analysis and Job's plot. The association constant of probe-30 with Zn(II) ions was estimated with a nonlinear B–H as 2.2 × 105 M−1. The LOD was estimated using emission titration data to be 1.2 nM by the 3σ/slope method. In contrast, this recognition process was reversible, as the fluorescence titration experiment of probe-30–[Zn(II)] to PPi results in lower emission, which results strongly supported by regeneration of probe-30. Finally, authors claims that the fluorescence signals of probe-30 were used to build a molecular INHIBIT logic gate.
 |
| | Fig. 32 Chemical structure and proposed sensing mechanism of probe-30 with Zn(II) (redrawn the ChemDraw structure from ref. 90). | |
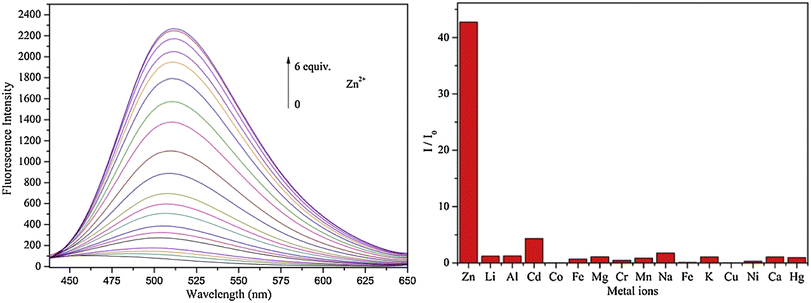 |
| | Fig. 33 Fluorescence spectra of probe-30 with Zn(II) ions (left) and competitive binding study of probe-30 with different metal ions (right) (reprinted with permission from ref. 90, Copyright 2019 Elsevier). | |
Yang and his coworkers demonstrated benzocoumarin-based single probe-31, which was synthesized form the 3-amino-5,6-benzocoumarin and 2-hydroxy-1-naphthaldehyde in ethanol under reflux conditions91 in 2019. The native state of probe-31 was poorly emissive in nature and showed enhancement in fluorescence emission upon addition of Zn(II) ions (Fig. 35). The LOD of probe-31 towards Zn(II) was established as 3.6 μM via fluorescence titration data, and the binding constant as 3.21 × 104 M−1 using B–H equation. According to Job's plot, the stoichiometric ratio between probe-31 and Zn(II) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was strongly supported by ESI-MS and 1H NMR. Finally, authors proposed the mechanism of fluorescence turn-on due to the complexation and simultaneous blocking of two processes, i.e., PET and C
1, which was strongly supported by ESI-MS and 1H NMR. Finally, authors proposed the mechanism of fluorescence turn-on due to the complexation and simultaneous blocking of two processes, i.e., PET and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 34).
N isomerization (Fig. 34).
 |
| | Fig. 34 Chemical structure and proposed sensing mechanism of probe-31 with Zn(II) (redrawn the ChemDraw structure from ref. 91). | |
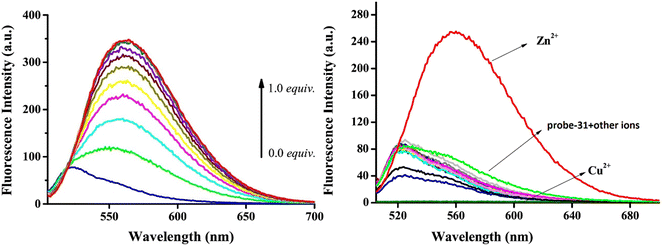 |
| | Fig. 35 Fluorescence spectra of probe-31 with incremental addition of Zn(II) ions (left) and fluorescence spectra of probe-31 in the presence of different metal ions (right) (reprinted with permission from ref. 91, Copyright 2019 Elsevier). | |
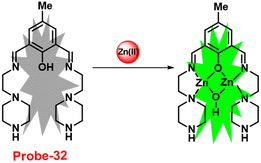
A Schiff-base ligand (probe-32) based on 2-hydroxy-5-methylisophthalaldehyde was synthesized by Saha et al. for the sensing of Zn(II) ions via the fluorescence turn-on mechanism in 2019.92 The probe-32 displayed very poor emission, upon addition of Zn(II) ions, the gradual increase in emission intensity and reached 16-fold after saturation (Fig. 36). The probe-32 showed colorimetric response towards Zn(II) ions, in which color changed from light yellow to green, whereas in the presence of Cu(II) ions, it turned from light yellow to colorless. Further, authors calculated detection limits via emission titration data and found as 1.059 nM for Zn(II) ions. Further, fluorescence titration experiments, ESI-MS analysis, and DFT studies demonstrated probe-32–Zn(II) complex have 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding. Furthermore, the use of Na2EDTA solution allows the probe-32 to be fully regenerate from probe-32 + Zn(II) complex. Finally, the authors utilized the probe-32 for the real-time quantitative detection of Zn(II) in water samples and biological systems by a quick change in emission intensity of probe-32 in the pH range 6–8 against Zn(II) ions. Further, the probe-32 was subjected for the recognition of Zn(II) ions in biological samples, the authors incubated MDA-MB-468 cells which exhibited no fluorescence and upon addition of Zn(II) to cells, which generated strong green color fluorescence around cells and results strongly suggested that the probe-32 was potential for the sensing of Zn(II) ions in biological samples (Fig. 37).
2 binding. Furthermore, the use of Na2EDTA solution allows the probe-32 to be fully regenerate from probe-32 + Zn(II) complex. Finally, the authors utilized the probe-32 for the real-time quantitative detection of Zn(II) in water samples and biological systems by a quick change in emission intensity of probe-32 in the pH range 6–8 against Zn(II) ions. Further, the probe-32 was subjected for the recognition of Zn(II) ions in biological samples, the authors incubated MDA-MB-468 cells which exhibited no fluorescence and upon addition of Zn(II) to cells, which generated strong green color fluorescence around cells and results strongly suggested that the probe-32 was potential for the sensing of Zn(II) ions in biological samples (Fig. 37).
 |
| | Fig. 36 Chemical structure and proposed sensing mechanism of probe-32 with Zn(II) (redrawn the ChemDraw structure from ref. 92). | |
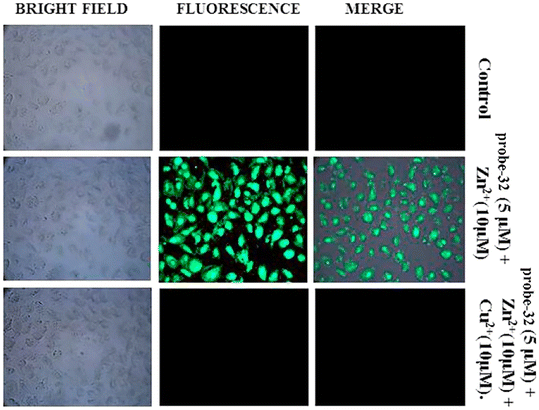 |
| | Fig. 37 Bio-imaging images of untreated MDA-MB-468 (control), cells treated with probe-32 (5 μM) + Zn(II) (10 μM) and with probe-32 (5 μM) + Zn(II) (10 μM) + Cu(II) (10 μM) (reprinted with permission from ref. 92, Copyright 2019 Elsevier). | |
The Schiff base (probe-33) was synthesized by Mondal et al. via the condensation reaction of 3,5-dichlorosalicylaldehyde and 2,2/-(butane-1,4-diylbis(sulfanediyl))dianiline in methanol under reflux conditions in 2021.93 The native state of probe-33 (Fig. 38) exhibited very poor emission and, upon addition of Zn(II) ions, displayed fluorescence turn-on response and no difference was detected with other competitive metal ions. Fluorescence spectral titration results indicated that the LOD for Zn(II) is 1.73 nM, which is much lesser as compared with the WHO recommended level. Further, the authors, calculated the association constant of probe-33 with Zn(II) ions from the titration data and found as 6.91 × 104 M−1. The reversibility of probe-33 was performed using the EDTA as chemical inputs, an INHIBIT logic gate was built and regeneration of probe-33 was observed. Job's plot suggested 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation of probe-33 with Zn(II) ions, which was further confirmed by single crystal XRD analysis. Finally, the authors utilized probe-33 for the detection of Zn(II) in the intracellular region of human breast cancer cells (MCF-7) (Fig. 39).
1 complexation of probe-33 with Zn(II) ions, which was further confirmed by single crystal XRD analysis. Finally, the authors utilized probe-33 for the detection of Zn(II) in the intracellular region of human breast cancer cells (MCF-7) (Fig. 39).
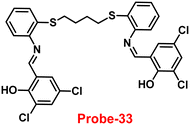 |
| | Fig. 38 Chemical structure of probe-33 (redrawn the ChemDraw structure from ref. 93). | |
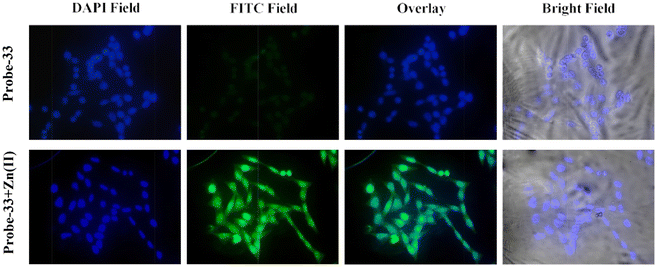 |
| | Fig. 39 Fluorescence images of MCF-7 cells after incubation with probe-33 (top) and the probe-33–Zn(II) complex (bottom) (reprinted with permission from ref. 93, Copyright 2021 Elsevier). | |
A series of ‘naked-eye’ bis-Schiff base fluorescent chemosensors (probe-34a, probe-34b and probe-34c)94 were synthesized based on ESIPT by Wang et al. in 2020. The probes were synthesized via a simple condensation reaction between aldehyde and amines and were structurally characterized by multinuclear NMR, ESI-MS and other analytical techniques. The native state of probes showed very poor emission; upon addition of Zn(II) ions, emission intensity was increased and color changes from colorless to bluish green, which may be the result of Zn(II) chelating with the target probes and limiting C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 40 and 41). However, there were no such observations noticed upon addition of other competitive metal ions. For the detection of probe-34a, probe-34b and probe-34c, with Zn(II) ions showed very less LOD (67.2 nM) with good anti-interference capabilities and quick response. A binding ratio of 1
N isomerization (Fig. 40 and 41). However, there were no such observations noticed upon addition of other competitive metal ions. For the detection of probe-34a, probe-34b and probe-34c, with Zn(II) ions showed very less LOD (67.2 nM) with good anti-interference capabilities and quick response. A binding ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was determined for the three probes with Zn(II) ions based on Job's plot analysis of the fluorescence data, which was further supported by the 1H NMR data. An inverted fluorescence microscopy imaging experiment was conducted and the findings showed that the probes exhibit good cell membrane permeability and hypotoxicity. Probe-34a was used for the detection of Zn(II) ions in SW620 cancer cells, and the results showed that the probe-34a alone exhibited no fluorescence; upon addition of Zn(II) ions, strong luminescence was observed inside the cells. From the above-mentioned results, authors claimed that probe-34a is potential for the recognition of Zn(II) ions in both biological and environmental samples.
1 was determined for the three probes with Zn(II) ions based on Job's plot analysis of the fluorescence data, which was further supported by the 1H NMR data. An inverted fluorescence microscopy imaging experiment was conducted and the findings showed that the probes exhibit good cell membrane permeability and hypotoxicity. Probe-34a was used for the detection of Zn(II) ions in SW620 cancer cells, and the results showed that the probe-34a alone exhibited no fluorescence; upon addition of Zn(II) ions, strong luminescence was observed inside the cells. From the above-mentioned results, authors claimed that probe-34a is potential for the recognition of Zn(II) ions in both biological and environmental samples.
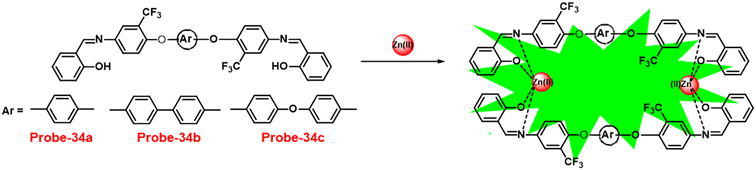 |
| | Fig. 40 Chemical structures of probe-(34a–c) and proposed sensing mechanism of probe-34a with Zn(II) (redrawn the ChemDraw structure from ref. 94). | |
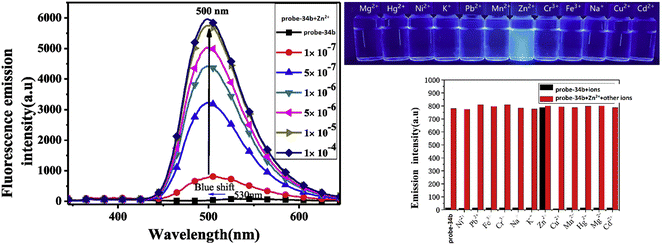 |
| | Fig. 41 Fluorescence spectra changes of probe-34b on adding Zn(II) ions (left), fluorescence colour change of probe-34b with different metal ions under a UV lamp (right top) and metal ion competitive studies of probe-34b (right bottom) (reprinted with permission from ref. 94, Copyright 2020 Elsevier). | |
Gudasi and colleagues developed a simple and inexpensive optical sensor (probe-35)95via a condensation reaction between 2-hydroxy-1-naphthaldehyde and 5-di-tert-butyl-2-hydroxybenzohydrazide in MeOH in 2020. The pure form of probe-35 exhibited very poor emission, and with the increase in the concentration of Zn(II) ions, the fluorescence intensity was increased by 17-fold and a 60 nm blue shift in the emission maxima was observed, which turned the colorless solution into a vivid yellow solution in 20% aqueous acetonitrile. However, under similar conditions, upon addition of various competitive metal ions to probe-35, there were no observable changes present in the fluorescence spectra, which indicate that probe-35 was very selective towards Zn(II) ions. The authors proposed the mechanism for fluorescence turn-on response, which might be attributed to the ESIPT and ICT processes (Fig. 42). The stoichiometry ratio between probe-35 and Zn(II) ions was determined to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 via Job's plot, which was strongly supported by B–H plots, 1H NMR and ESI-MS analyses. The binding constant and LOD value of probe-35 with Zn(II) ions were estimated from titration data and displayed as 7.79 × 106 M−1 and 0.31 μM respectively. Finally, authors decided to apply probe-35 to sense Zn(II) ions from the biological samples, in which cytotoxicity experiments revealed that probe-35 showed very less cytotoxicity. Probe-35 was incubated with HeLa cells, which showed no emission, and upon treatment with Zn(II) ions, the fluorescence intensity was seen inside the cells. From the above-mentioned results, authors concluded that probe-35 shows potential to sense Zn(II) ions in environmental and biological samples (Fig. 43).
1 via Job's plot, which was strongly supported by B–H plots, 1H NMR and ESI-MS analyses. The binding constant and LOD value of probe-35 with Zn(II) ions were estimated from titration data and displayed as 7.79 × 106 M−1 and 0.31 μM respectively. Finally, authors decided to apply probe-35 to sense Zn(II) ions from the biological samples, in which cytotoxicity experiments revealed that probe-35 showed very less cytotoxicity. Probe-35 was incubated with HeLa cells, which showed no emission, and upon treatment with Zn(II) ions, the fluorescence intensity was seen inside the cells. From the above-mentioned results, authors concluded that probe-35 shows potential to sense Zn(II) ions in environmental and biological samples (Fig. 43).
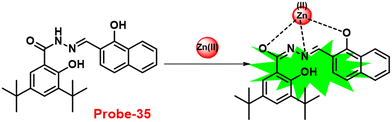 |
| | Fig. 42 Chemical structure and proposed sensing mechanism of probe-35 with Zn(II) (redrawn the ChemDraw structure from ref. 95). | |
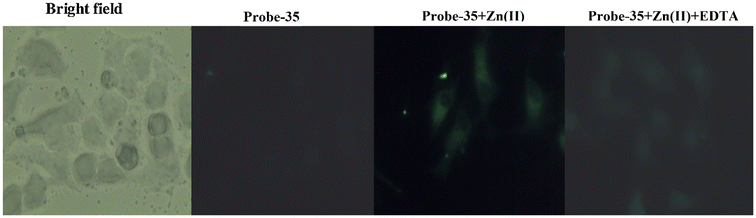 |
| | Fig. 43 Bio-imaging images of HeLa cells with probe-35 with the addition of Zn(II) (reprinted with permission from ref. 95, Copyright 2020 Elsevier). | |
In 2021, Rani et al. developed a pyrene-based Schiff base probe-36 (ref. 96) for sensitive and selective detection of Zn(II) ions. The synthesis of probe-36 (Fig. 44) involves two steps: first, the synthesis of malonohydrazide, which was treated with 2 eq. of pyrene carboxyaldehyde, as a result of which the product has two pyrene units on either side of the molecule. The native state of probe-36 was poorly emissive in nature due to the presence of PET and was suppressed with the Zn(II) ions and caused fluorescence “turn-on”, whereas probe-36 did not respond significantly to other competitive ions. Further, probe-36 and Zn(II) ion interaction produced an effective fluorescence enhancement that was observable with the naked eye as a blue color emission under a UV lamp (Fig. 45). The LOD of probe-36 with Zn(II) was estimated to be 5.1 nM via fluorescence titration spectra (3σ/slope), this is significantly lower than the WHO-recommended standard of 76 μM for safe drinking water. The association constant of probe-36 with Zn(II) ions was estimated from the fluorescence titration spectra and displayed as 3 × 105 M−1. Job's plot suggested a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio for the probe-36–Zn(II) complex. Finally, the authors concentrated on the identification of Zn(II) ions in biological samples, and a cytotoxicity experiment using probe-36 was carried out and the results strongly indicated the poor cytotoxicity of probe-36 under biological conditions. The results encouraged to further investigate in this regard. Therefore, probe-36 was incubated in HeLa cells and no emission was observed, and the incorporation of Zn(II) ions to the cells produced a strong blue color emission inside the cells (Fig. 46), which strongly supported the potential of probe-36 for the recognition of Zn(II) ions in biological samples.
1 stoichiometric ratio for the probe-36–Zn(II) complex. Finally, the authors concentrated on the identification of Zn(II) ions in biological samples, and a cytotoxicity experiment using probe-36 was carried out and the results strongly indicated the poor cytotoxicity of probe-36 under biological conditions. The results encouraged to further investigate in this regard. Therefore, probe-36 was incubated in HeLa cells and no emission was observed, and the incorporation of Zn(II) ions to the cells produced a strong blue color emission inside the cells (Fig. 46), which strongly supported the potential of probe-36 for the recognition of Zn(II) ions in biological samples.
 |
| | Fig. 44 Chemical structure of probe-36 (redrawn the ChemDraw structure from ref. 96). | |
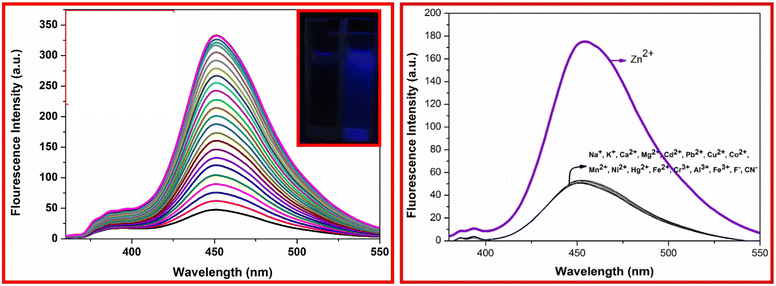 |
| | Fig. 45 Emission spectra of probe-36 upon addition of Zn(II) ions (left) and competitive binding study of probe-36 with different metal ions (reprinted with permission from ref. 96, Copyright 2021 Elsevier). | |
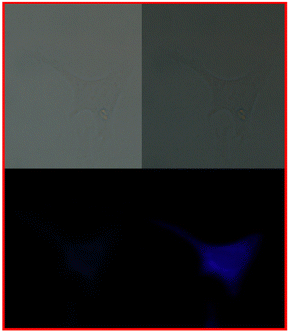 |
| | Fig. 46 Fluorescence images of live HeLa cells with probe-36 with Zn(II) ions (reprinted with permission from ref. 96, Copyright 2021 Elsevier). | |
Xue and co-workers obtained quinolone-based Schiff base (probe-37)97 for the recognition of Zn(II) ions via the fluorescence turn-on mechanism in 2018. The pure form of probe-37 displayed poor emission, and upon addition of Zn(II) ions, the increase in emission intensity was noticed with good quantum yields and exhibited a significant large Stokes shift >200 nm due to the absence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 47). The LOD of probe-37 with Zn(II) ions calculated from fluorescence spectra and was found to be 89.3 nM. A 2
N isomerization (Fig. 47). The LOD of probe-37 with Zn(II) ions calculated from fluorescence spectra and was found to be 89.3 nM. A 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was found for probe-37 with Zn(II) ions, which was validated by fluorescence measurements, UV-vis, ESI-MS, absorption analysis, 1H NMR spectra and DFT studies. The reversibility of probe-37 was tested using Na2EDTA. Finally, the authors tested the sensing of Zn(II) ions in cellular media, in which a cytotoxicity experiment was conducted with probe-37 that was found to exhibit very poor cytotoxicity on HeLa cells. The results encouraged the authors to recognize Zn(II) ions in biological samples. Probe-37 incubated with Zn(II) ions exhibited very bright luminescence inside the cells, which strongly suggested the potential of probe-37 for the sensing of biological Zn(II) samples (Fig. 48).
1 stoichiometry was found for probe-37 with Zn(II) ions, which was validated by fluorescence measurements, UV-vis, ESI-MS, absorption analysis, 1H NMR spectra and DFT studies. The reversibility of probe-37 was tested using Na2EDTA. Finally, the authors tested the sensing of Zn(II) ions in cellular media, in which a cytotoxicity experiment was conducted with probe-37 that was found to exhibit very poor cytotoxicity on HeLa cells. The results encouraged the authors to recognize Zn(II) ions in biological samples. Probe-37 incubated with Zn(II) ions exhibited very bright luminescence inside the cells, which strongly suggested the potential of probe-37 for the sensing of biological Zn(II) samples (Fig. 48).
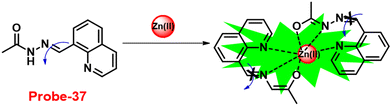 |
| | Fig. 47 Chemical structure and proposed sensing mechanism of probe-37 with Zn(II) (redrawn the ChemDraw structure from ref. 97). | |
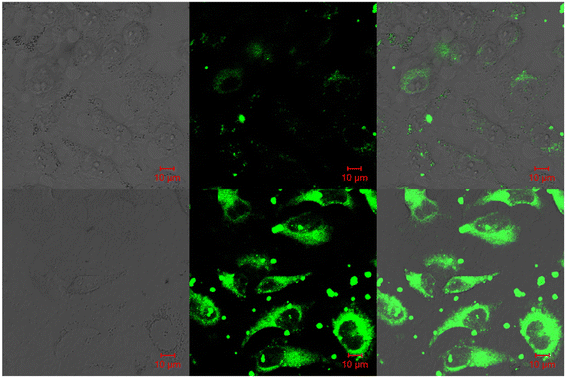 |
| | Fig. 48 Confocal fluorescence images of HeLa cells incubated with probe-37 in the presence of Zn(II) ions (reprinted with permission from ref. 97, Copyright 2018 Elsevier). | |
Following the above-mentioned report, Xu and co-workers focused on the synthesis of bis-quinoline based probe-38,98 which showed selective fluorescence turn-on for Zn(II) ions and chromogenic response for Co(II) ions in 2020. The synthesized probe-38 showed very poor emission, and upon incorporation of Zn(II) ions, the fluorescence intensity increased, whereas other competitive metal ions showed no change in fluorescence spectra and the results strongly supported that probe-38 is very selective towards Zn(II) ions via fluorescence turn-on, which was caused by metal chelation effects (CHEF) (Fig. 49). Further, probe-38 can also function as a chromogenic probe for Zn(II) ion recognition with least interference from other competitive metal ions, which demonstrates a long wavelength emission (570 nm) with a substantial large Stokes shift over 170 nm. The limit of detection of probe-38 was estimated to be 0.66 μM from the fluorescence spectra for Zn(II). Further, the association constant calculated for probe-38 with Zn(II) from the absorption titration data was 1.03 × 104 M−1. The stoichiometric ratio of probe-38 with Zn(II) ions was calculated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by Job's plot, which was confirmed via1H NMR and ESI-MS data. Finally, the authors established the potential of probe-38 for the sensing of Zn(II) ions in biological samples and outcomes strongly supported that probe-38 was capable for the recognition of Zn(II) ions in biological samples and was confirmed by intracellular imaging of Zn(II) ions (Fig. 50).
1 by Job's plot, which was confirmed via1H NMR and ESI-MS data. Finally, the authors established the potential of probe-38 for the sensing of Zn(II) ions in biological samples and outcomes strongly supported that probe-38 was capable for the recognition of Zn(II) ions in biological samples and was confirmed by intracellular imaging of Zn(II) ions (Fig. 50).
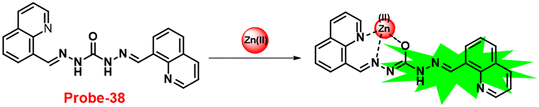 |
| | Fig. 49 Chemical structure and proposed sensing mechanism of probe-38 with Zn(II) (redrawn the ChemDraw structure from ref. 98). | |
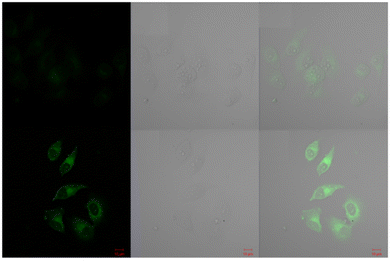 |
| | Fig. 50 Confocal fluorescence images of HeLa cells of probe-38 with Zn(II) ions (reprinted with permission from ref. 98, Copyright 2020 Elsevier). | |
In 2021, Wang et al. designed a luminous probe with great selectivity, sensitivity and significant Stokes shift for the sensing of Zn(II) ions using a triphenylamine Schiff base (probe-39).99 The target compound was synthesized via a Schiff base condensation reaction between 2-hydroxy-1-naphthaldehyde and an amine derivative of triphenylamine in ethanol under reflux conditions. The native state of probe-39 was poorly emissive in nature; upon addition of Zn(II) ions, the turn-on response was observed with greater fluorescence intensity in DMF-H2O. Probe-39 has shown strong anti-interference performance even when several other metal ions or anions were present, which strongly supported the selectivity of probe-39. The LOD value of probe-39 with Zn(II) ions was determined from the fluorescence titration values as 19.134 nM in DMF–H2O, and probe-39 accomplished the detection of Zn(II) ions with remarkable linear correlation. Additionally, probe-39–Zn(II) complex has a stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with an association constant of 3.24 × 104 M−1. Further, the fluorescence studies of probe-39 were carried out at various pH values and found to show better results in the range of 5–8. Furthermore, the binding mechanism (Fig. 51) and fluorescence turn-on mechanism were confirmed via DFT calculations, 1H NMR titration, FT-IR, and ESI-MS and were used to demonstrate the interaction properties. The authors utilized probe-39 for the recognition of Zn(II) ions in living cells along with environmental areas (Fig. 52). Consequently, probe-39 has potential applications for sensing Zn(II) ions in the environment and biological systems.
1 with an association constant of 3.24 × 104 M−1. Further, the fluorescence studies of probe-39 were carried out at various pH values and found to show better results in the range of 5–8. Furthermore, the binding mechanism (Fig. 51) and fluorescence turn-on mechanism were confirmed via DFT calculations, 1H NMR titration, FT-IR, and ESI-MS and were used to demonstrate the interaction properties. The authors utilized probe-39 for the recognition of Zn(II) ions in living cells along with environmental areas (Fig. 52). Consequently, probe-39 has potential applications for sensing Zn(II) ions in the environment and biological systems.
 |
| | Fig. 51 Chemical structure and proposed sensing mechanism of probe-39 with Zn(II) (redrawn the ChemDraw structure from ref. 99). | |
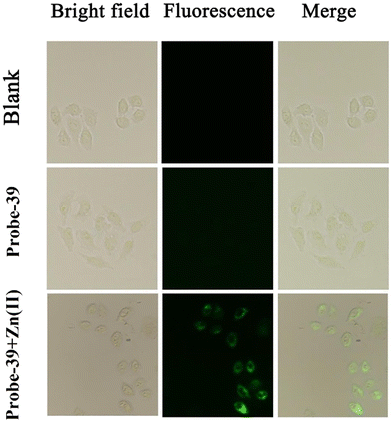 |
| | Fig. 52 Fluorescence microscopic images of HeLa cells treated with probe-39 and probe-39–Zn(II) ions (reprinted with permission from ref. 99, Copyright 2021 Elsevier). | |
Venkatesan et al. developed a novel fluorescent turn-on probe for the detection of Zn(II) ions using both cyclic (probe-40a) and noncyclic (probe-40b) Schiff's bases in 2019. Under two distinct catalytic conditions, the condensation of 4-(diethylamino)salicylaldehyde and 2 aminobenzenethiol in sulphuric acid produces the product probe-40a, while with acetic acid yields probe-40b.100 The native state of probes displayed very poor emissive properties due to the presence of ESIPT in CH3OH/H2O, while upon addition of Zn(II) ions, there is increase in the intensity of emission due to the CHEF process (Fig. 53). However, on titrating with other metal ions, no change was observed in the fluorescence spectra. The results of Job's plot and B–H plot analysis showed the development of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, with association constants (Ka) of 4.9 × 104 M−1 and 2.1 × 104 M−1 with probe-40a and probe-40b, respectively. The reversibility of probes was tested using EDTA and data showed very good results. Probe-40a and probe-40b were found to have detection limits of 67 nM and 0.36 μM, respectively, which were calculated from the fluorescence titration data. The detection of Zn(II) ions in various water samples and pharmaceutical multivitamin tablets was accomplished with probe-40a and probe-40b.
1 stoichiometry, with association constants (Ka) of 4.9 × 104 M−1 and 2.1 × 104 M−1 with probe-40a and probe-40b, respectively. The reversibility of probes was tested using EDTA and data showed very good results. Probe-40a and probe-40b were found to have detection limits of 67 nM and 0.36 μM, respectively, which were calculated from the fluorescence titration data. The detection of Zn(II) ions in various water samples and pharmaceutical multivitamin tablets was accomplished with probe-40a and probe-40b.
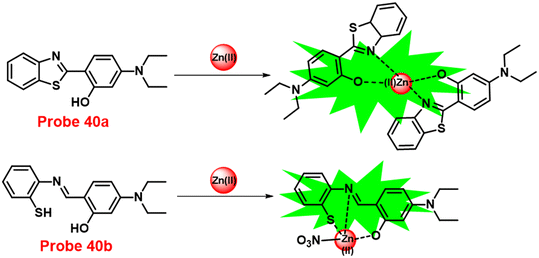 |
| | Fig. 53 Chemical structure and proposed sensing mechanism of probe-40a and probe-40b with Zn(II) (redrawn the ChemDraw structure from ref. 100). | |
Jia and co-workers synthesized a multi-responsive tetraphenylethylene-functionalised salicylaldehyde-based Schiff base (probe-41),101 which displayed AIE-activity and could be utilized for the detection of Zn(II) ions via the fluorescence turn-on mechanism. Probe-41 displayed reversible mechanofluorochromism (MFC), as shown by the color change of emission from yellowish green to orange-yellow following grinding. The solution sate of probe-41 showed very poor emission, and upon addition of Zn(II) ions, the fluorescence intensity drastically increased as a result of suppression of ESIPT (Fig. 54), and other metal ions do not affect the fluorescence spectra, which strongly suggested the selectivity and sensitivity towards Zn(II). The complexation ratio between probe-41 and Zn(II) were determined to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was validated by Job's plot, ESI-MS and 1H NMR. Further, authors observed a large Stokes shift of emission spectra during the titration process, which showed clearly visible changes in the fluorescence (from blue to bright orange). The sensing mechanism was proposed, based on the inhibition of PET and ESIPT. Further, the LOD value of probe-41 and Zn(II) was estimated to be as low as 80.5 nM.
1, which was validated by Job's plot, ESI-MS and 1H NMR. Further, authors observed a large Stokes shift of emission spectra during the titration process, which showed clearly visible changes in the fluorescence (from blue to bright orange). The sensing mechanism was proposed, based on the inhibition of PET and ESIPT. Further, the LOD value of probe-41 and Zn(II) was estimated to be as low as 80.5 nM.
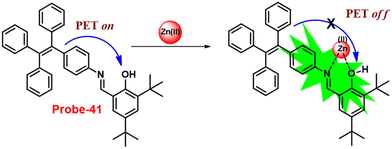 |
| | Fig. 54 Chemical structure and proposed sensing mechanism of probe-41 with Zn(II) (redrawn the ChemDraw structure from ref. 101). | |
In 2021, Sun et al. reported the synthesis of a novel pyrene-incorporated Schiff base (probe-42) with an AIEE property that was used for the OTFT and solution-state detection of Zn(II) ions.102Probe-42 was synthesized using an one-pot reflux reaction of pyrene-1-carboxaldehyde and 4-amino-5-phenyl-4H-1,2,4-triazole-3-thiol. The presence of J-aggregation, crystalline changes, and nanofiber formation in the AIEE studies of probe-42 (Fig. 55) (in CH3CN) at various water fractions (fw: 0–97.5%) was validated by UV-vis absorption/photoluminescence (UV/PL), powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM), and dynamic light scattering (DLS). The pure form of probe-42 displayed poor emission and upon addition of Zn(II) ions, a gradual increment in the fluorescence spectra with enhanced quantum yields was observed, which attributed to the suppression of PET. However, in the presence of other competitive metal ions, no change in fluorescence spectra was found. The LOD value of Zn(II) with probe-42 was calculated to be 0.79 nM, from the standard deviation and linear fittings of fluorescence titration spectra. The stoichiometry of probe-42 with Zn(II) ions was estimated with Job's plot and found to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was confirmed by HRMS and 1H NMR data. The MTT assay and IC50 studies, as well as in vitro/in vivo imaging for probe-42 with Zn(II) ions, were conducted on B16F10 cell lines and in zebrafish, to determine the probe's biocompatibility and it demonstrated applications of probe-42 in biological samples (Fig. 56). The OTFT devices was created upon incorporation of probe-42 with pentacene, which validated the reversibility discovered to be more than 90% change in drain-source current in response to Zn(II) with a LOD of 5.46 μM. The OTFT devices additionally exhibited Zn(II) ion detection in samples of tap water and lake water.
1, which was confirmed by HRMS and 1H NMR data. The MTT assay and IC50 studies, as well as in vitro/in vivo imaging for probe-42 with Zn(II) ions, were conducted on B16F10 cell lines and in zebrafish, to determine the probe's biocompatibility and it demonstrated applications of probe-42 in biological samples (Fig. 56). The OTFT devices was created upon incorporation of probe-42 with pentacene, which validated the reversibility discovered to be more than 90% change in drain-source current in response to Zn(II) with a LOD of 5.46 μM. The OTFT devices additionally exhibited Zn(II) ion detection in samples of tap water and lake water.
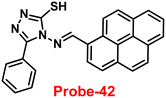 |
| | Fig. 55 Chemical structure of probe-42 (redrawn the ChemDraw structure from ref. 102). | |
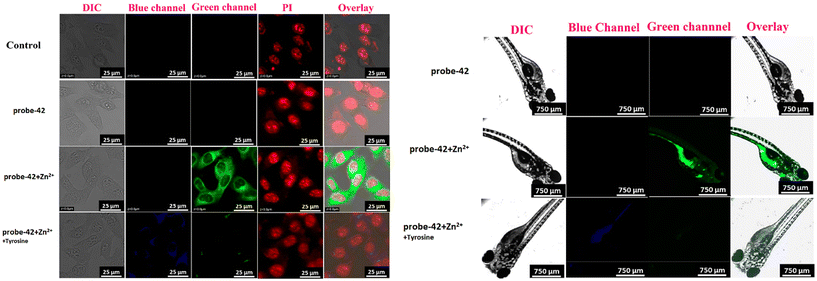 |
| | Fig. 56 Cellular images of probe-4(II) Zn(II) and probe-4(II) Zn(II) + tyrosine and zebra fish imaging of probe-4(II) Zn(II) and probe-4(II) Zn(II) + tyrosine (reprinted with permission from ref. 102, Copyright 2021 American Chemical Society). | |
In 2019, Upadhyay and colleagues synthesized a novel coumarin-based Schiff-base sensor, probe-43, for the selective and sensitive fluorescence turn-on recognition of Zn(II) ions.103 A “turn-on” green emissive response was produced by probe-43 (Fig. 57) after selective Zn(II)-triggered hydrolysis in ethanol, which was then reconstructed into fragments. This response was verified by a number of physicochemical tests, and single-crystal XRD studies. The idea of hydrolysis and restructuring was supported by spectroscopic data combined with ESI-MS, which suggests that the probe-43–Zn(II) ensemble assumed the form of dimmer in solutions. XRD examinations revealed more evidence for the probe-43–Zn(II) ensemble, demonstrating that this Schiff base represents another unique Schiff base created in situ during the growth of single crystals. However, upon addition of other competitive metal ions to probe-43, there was no change in the fluorescence spectra, which strongly supports the selectivity of probe-43 towards Zn(II) ions. The authors proposed that upon binding, probe-43 and Zn(II) ions cause the reduction of intramolecular interactions (π–π interactions), which results in a blue-shift emission band, and the suppression of PET is responsible for the increase in quantum yields. The detection limits of probe-43 with Zn(II) ions were calculated to be in the sub-nanomolar range from the fluorescence titration spectra. Further, probe-43 was effectively utilized for the detection of Zn(II) optically and also via live cell imaging by selective hydrolytic fluorogenic events in SiHa cells (Fig. 58).
 |
| | Fig. 57 Chemical structure of probe-43 (redrawn the ChemDraw structure from ref. 103). | |
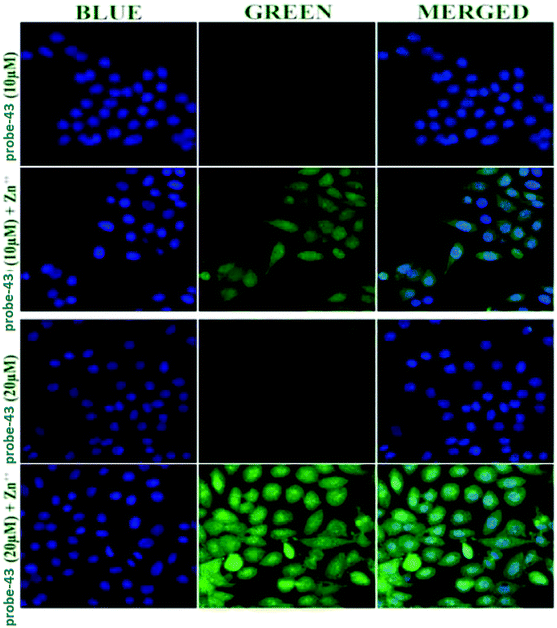 |
| | Fig. 58 Fluorescence microscopic images of SiHa cells treated with probe-43 in the presence of Zn(II) ions (reprinted with permission from ref. 103, Copyright 2019 Royal Society of Chemistry). | |
Das and co-workers obtained a macrocyclic Schiff base (probe-44)104 for the selective and sensitive sensing of Zn(II) ions via the fluorescence turn-on mechanism in 2016. The macrocyclic ligand probe-44 (Fig. 59) was synthesized by treating Zn(NO3)2 with an acyclic side-off compartmental ligand, which forms a dinuclear macrocyclic zinc(II) complex, which further undergoes hydrolysis of the starting ligand via Zn(II)-catalyzed processes. The authors used various analytical and spectroscopic techniques to confirm the formation of macrocyclic ligand probe-44 in the solution. The pure form of probe-44 displayed a strong green color emission, and addition of Zn(II) ions causes the appearance of a new fluorescence emission band at 481 nm, whose strength gradually increases. As a result, ligand probe-44 functions as a ratiometric fluorescence chemodosimeter for the targeted recognition of Zn(II) ions, even in the presence of other competitive metal ions.
 |
| | Fig. 59 Chemical structure of probe-44 (redrawn the ChemDraw structure from ref. 104). | |
In 2019, Kim et al. synthesized a new Schiff base chemosensor probe-45 using 1-aminohydantoin hydrochloride and 3-methoxysalicylaldehyde in ethanol with stirring under room temperature conditions.105 The native form of probe-45 was weak emissive in nature; upon incorporation of Zn(II) ions, the emission intensity was gradually improved in aqueous solutions due to the CHEF effect (Fig. 60). However, in the presence of other competitive metal ions, there was no or minimal change in the fluorescence spectra, which demonstrated the selectivity towards Zn(II) ions. The LOD value of probe-45 with Zn(II) ions was calculated to be 11.9 μM from the fluorescence titration spectra using the 3σ/slope, which was much less than WHO-recommended levels (76.0 μM). The reversibility of probe-45 was demonstrated using EDTA, and it was found that Zn(II) binding could be reversible. Finally, authors applied probe-45 for the sensing of Zn(II) ions in biological media and showed a very significant role in the detection of Zn(II) in live cells (Fig. 61). Additionally, probe-45 could be used for the detection of Cu(II) colorimetrically, which changes the color from colorless to pink.
 |
| | Fig. 60 Chemical structure and proposed sensing mechanism of probe-45 with Zn(II) (redrawn the ChemDraw structure from ref. 63). | |
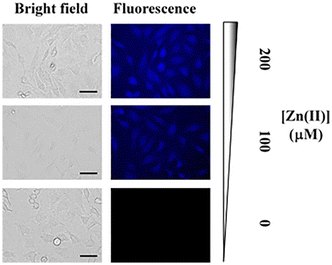 |
| | Fig. 61 Fluorescence microscopic images of HeLa cells in probe-45 in the presence of Zn(II) ions (reprinted with permission from ref. 105, Copyright 2019 Elsevier). | |
A novel bis(salamo)-type fluorescent probe (probe-46)106 based on naphthalenediol was synthesized by Dong and colleagues for the selective recognition of Zn(II) via the fluorescence turn-on mechanism in 2019. The native form of probe-46 displayed poor emissive properties, and upon incorporation of Zn(II) ions, the emission intensity was enhanced gradually, because of the inhibition of PET (Fig. 62), whereas under similar conditions in the presence of other competitive metal ions, no change was observed in the emission spectra. The LOD and association constant of probe-46 with Zn(II) ions were estimated to be 0.52 μM and 2.71 × 104 M−1 respectively from the fluorescence titration spectra. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 stoichiometry was estimated by Job's plot for the complex between probe-46 and Zn(II) ions and was supported by fluorescence and UV-vis titrations; ESI-MS, single crystal as well as theoretical calculations were used to understand their detection mechanisms. Further, the binding mode of probe-46 with Zn(II) ions was confirmed via Hirshfeld surface analysis, EA, IR, UV-Vis spectroscopy, and fluorescence spectroscopy.
3 stoichiometry was estimated by Job's plot for the complex between probe-46 and Zn(II) ions and was supported by fluorescence and UV-vis titrations; ESI-MS, single crystal as well as theoretical calculations were used to understand their detection mechanisms. Further, the binding mode of probe-46 with Zn(II) ions was confirmed via Hirshfeld surface analysis, EA, IR, UV-Vis spectroscopy, and fluorescence spectroscopy.
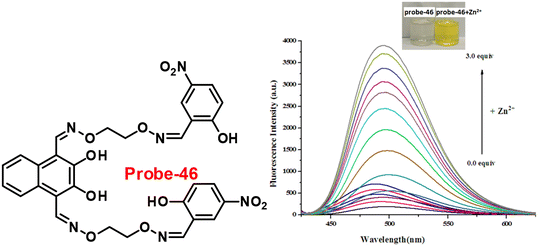 |
| | Fig. 62 Chemical structure of probe-46 (left) and changes in the fluorescence spectra of probe-46 upon increasing the Zn(II) ion concentration (right) (reprinted with permission from ref. 106, Copyright 2019 Elsevier). | |
In 2018, Pu et al. developed a potential chromogenic and fluorogenic chemosensor of Cu(II) and Zn(II) ions established on diarylethene conjugated with 2-(methylthio)benzenamine Schiff's moiety (probe-47).107Probe-47 (Fig. 63) showed discrimination of two metal ions such as Zn(II) and Cu(II) via fluorescence turn-on and colorimetric studies respectively. Probe-47 displayed poor emission; upon incorporation of Zn(II) ions, the fluorescence was gradually increased up to 53.8-fold as well as a shift in luminous color from light red to bright yellow. However, in the presence of other competitive metal ions, no change was observed in the fluorescence spectra of probe-47 and Cu(II) ions were found to be fluorescence quenched up to 98%, resulting in a color change from light red to non-fluorescent. The coordination stoichiometry between probe-47 and Cu(II)/Zn(II) was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in both cases. With the help of these discoveries, a different input switch and an embedded logic circuit were effectively built.
1 in both cases. With the help of these discoveries, a different input switch and an embedded logic circuit were effectively built.
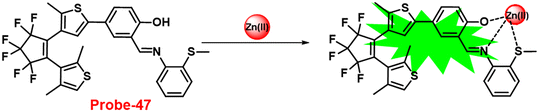 |
| | Fig. 63 Chemical structure and proposed sensing mechanism of probe-47 with Zn(II) (redrawn the ChemDraw structure from ref. 107). | |
In 2018, Wang and co-workers obtained novel coumarin-based fluorescent probe-48 (Fig. 64),108 which could be useful for the sensing of Zn(II) ions via the fluorescence turn-on mechanism. The target compound was synthesized via a simple condensation reaction between thiosemicarbazide and 8-formyl-7-hydroxy-4-methylcoumarin. Probe-48 showed a colorimetric response towards Fe(III) ions and color variations from colorless to brown but fluorescence turn-on behavior towards the Zn(II) ions (Fig. 65). In the presence of other metal ions, probe-48 only showed a high ability to coordinate toward Zn(II) and Fe(III) ions, with the LOD values as low as 6 nM and 5.38 μM, correspondingly. The stoichiometry between probe-48 and Zn(II)/Fe(III) was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was confirmed by Job's plot. Moreover, probe-48 could quickly, easily, and practically monitor Zn(II) and Fe(III) ions using conventional test strips. Fluorescence cell images also identified the potential of probe-48 to be used as a bio-imaging fluorescent sensor to identify Zn(II) ions in human cancer cells (Fig. 66).
1, which was confirmed by Job's plot. Moreover, probe-48 could quickly, easily, and practically monitor Zn(II) and Fe(III) ions using conventional test strips. Fluorescence cell images also identified the potential of probe-48 to be used as a bio-imaging fluorescent sensor to identify Zn(II) ions in human cancer cells (Fig. 66).
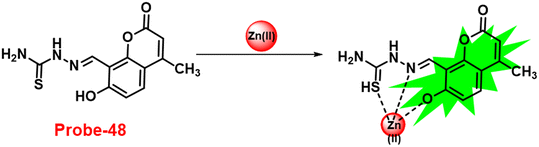 |
| | Fig. 64 Chemical structure and proposed sensing mechanism of probe-48 with Zn(II) (redrawn the ChemDraw structure from ref. 108). | |
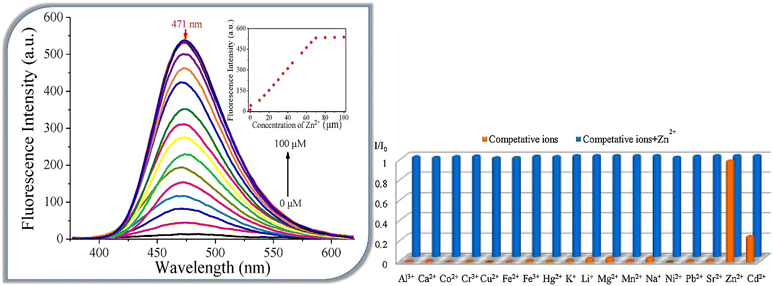 |
| | Fig. 65 Changes in the fluorescence spectra of probe-48 upon incremental addition of Zn(II) ions (left) and competitive binding study of probe-48 with different metal ions (right) (reprinted with permission from ref. 108, Copyright 2018 Elsevier). | |
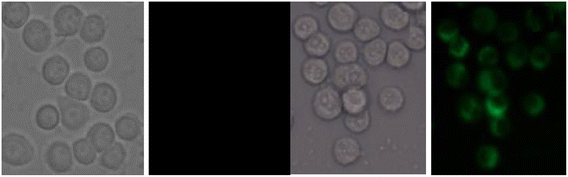 |
| | Fig. 66 Bioimaging images of cancer cells treated with probe-48 followed by the addition of Zn(II) ions (reprinted with permission from ref. 108, Copyright 2018 Elsevier). | |
Swamy et al. synthesized two acyclic Schiff base probes,47probe-49a and probe-49b, that could be used for the detection of Zn(II) via fluorescence turn-on and as colorimetric chemosensors for Zn(II), Cu(II), and Co(II) ions by altering from colorless to yellow, and no change was observed in the emission spectra in the presence of other competitive metal ions in 2022. The fluorescence turn-on mechanism proposed for these probes was the conquest of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– isomerization, PET blocking, and the ESIPT process (Fig. 67). The recognition mechanism of Zn(II) ions with probe-49a/probe-49b was further supported by 1H NMR titrations. Furthermore, Job's plot analysis displayed 1
N– isomerization, PET blocking, and the ESIPT process (Fig. 67). The recognition mechanism of Zn(II) ions with probe-49a/probe-49b was further supported by 1H NMR titrations. Furthermore, Job's plot analysis displayed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding for probes with Zn(II) ions, which was established by DFT, 1H NMR, UV-vis, and fluorescence spectroscopy studies. In addition, the LOD values of probe-49a and probe-49b for Zn(II) ions were found to be 7.69 and 5.35 nM, correspondingly, which were much lesser than permitted by the U.S. Environmental Protection Agency (US-EPA). The probes could be used as an efficient luminescent probe for the recognition of Zn(II) ions in living cells, based on the fluorescence microscopic imaging in HeLa cells, which strongly demonstrates the immense potential of this simple chemosensor in cellular imaging (Fig. 68).
1 binding for probes with Zn(II) ions, which was established by DFT, 1H NMR, UV-vis, and fluorescence spectroscopy studies. In addition, the LOD values of probe-49a and probe-49b for Zn(II) ions were found to be 7.69 and 5.35 nM, correspondingly, which were much lesser than permitted by the U.S. Environmental Protection Agency (US-EPA). The probes could be used as an efficient luminescent probe for the recognition of Zn(II) ions in living cells, based on the fluorescence microscopic imaging in HeLa cells, which strongly demonstrates the immense potential of this simple chemosensor in cellular imaging (Fig. 68).
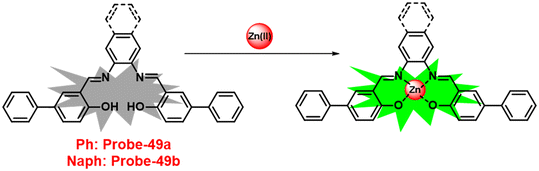 |
| | Fig. 67 Chemical structure and proposed sensing mechanism of probe-49a and probe-49b with Zn(II) (redrawn the ChemDraw structure from ref. 47). | |
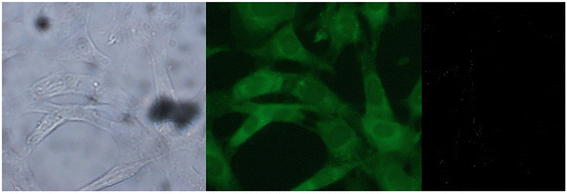 |
| | Fig. 68 Bio-imaging images of HeLa cells treated with probe-49a followed by addition of Zn(II) ions (reprinted with permission from ref. 47, Copyright 2022 Elsevier). | |
3. Schiff base-based fluorescent turn-on probes for Hg(II) ions
As we know, heavy metals have lethal effects on human life, in this regard mercury, which has attracted special attention due to its extreme toxicity.109,110 Even at extremely low concentrations, it can have significant negative impacts on human body, such as impairing the ability of proteins and enzymes to function, movement disorders, cognitive dysfunction, brain damage, and kidney failure.111–113 There are several conventional methods such as ICP-MS and AES/AAS for the recognition of Hg(II) ions in waste water.114 Recently, researchers focused on selective and sensitive probes for the recognition of Hg(II) ions, which can be utilized for both in environmental waste samples and in living cells.115–119
In 2021, Jiao and co-workers constructed a rhodamine-based Schiff base (probe-50)120 fluorescent turn-on probe for Hg(II) ions, which was obtained from rhodamine B and acenaphtho[1,2-b]quinoxaline-9-amine. The target compound was structurally characterized by several analytical techniques such as 1H NMR, 13C NMR, HRMS, and single-crystal XRD. The native state of probe-50 displayed poor emission, whereas upon incorporation of Hg(II) ions, the spirolactam ring undergoes a ring-opening process (Fig. 69), and the fluorescence intensity at 586 nm is considerably enhanced 70-fold in C2H5OH/HEPES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Fig. 70). However, on titrating with other competitive metal ions, there was no change in the emission spectra, these results strongly suggested that probe-50 is very selective towards Hg(II) ions (Fig. 70). The LOD of probe-50 with Hg(II) ions was estimated as 10 nM from the emission titration spectra. Furthermore, probe-50 finds a method for monitoring variations in Hg(II) ion content in biological systems by confocal laser microscopy, such as fluorescence imaging of HeLa live cells and zebra fish (Fig. 71).
1, v/v) (Fig. 70). However, on titrating with other competitive metal ions, there was no change in the emission spectra, these results strongly suggested that probe-50 is very selective towards Hg(II) ions (Fig. 70). The LOD of probe-50 with Hg(II) ions was estimated as 10 nM from the emission titration spectra. Furthermore, probe-50 finds a method for monitoring variations in Hg(II) ion content in biological systems by confocal laser microscopy, such as fluorescence imaging of HeLa live cells and zebra fish (Fig. 71).
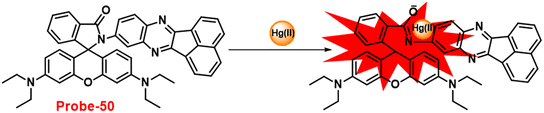 |
| | Fig. 69 Chemical structure and proposed sensing mechanism of probe-50 with Hg(II) (redrawn the ChemDraw structure from ref. 120). | |
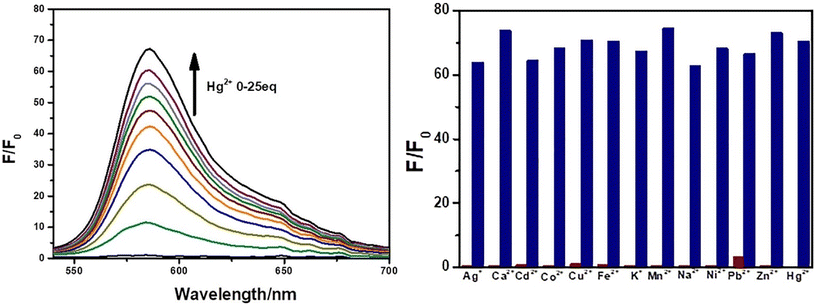 |
| | Fig. 70 Emission spectra of probe-50 upon incremental addition of Hg(II) ions (left) and changes in the emission spectra of probe-50 with different metal ions (right) (reprinted with permission from ref. 120, Copyright 2021 Elsevier). | |
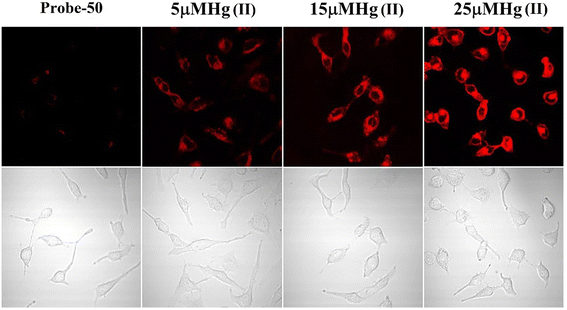 |
| | Fig. 71 Confocal fluorescence images of HeLa cells treated with probe-50 and incubated with different concentrations of Hg(II) ions (reprinted with permission from ref. 120, Copyright 2021 Elsevier). | |
Niu et al. developed an effective Schiff-base fluorescent chemosensor, probe-51,121 made from low-cost starting materials such as benzophenone and 2-aminobenzenethiol, which was characterised by IR, 1H NMR, and 13C NMR, revealing highly selective and sensitive sensing of Hg(II) ions. Due to the precise binding of Hg(II) with imine N and thiol S atoms, probe-51 shows a distinct turn-on emission behavior for Hg(II) ions throughout a wide range of pH values, whereas in the presence of other metal ions, there was no change in the fluorescence spectra. The suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and ESIPT could be attributed to the fluorescence turn-on response of probe-51 (Fig. 72). Further, probe-51–Hg(II) was also regenerated back to probe-51 when EDTA was added, which provided the potentiality of probe-51. The 2
N isomerization and ESIPT could be attributed to the fluorescence turn-on response of probe-51 (Fig. 72). Further, probe-51–Hg(II) was also regenerated back to probe-51 when EDTA was added, which provided the potentiality of probe-51. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding for probe-51–Hg(II) was determined by utilizing the method of continuous variations and Job's plot, which was further validated by the B–H method. The binding constant was found to be 4.484 × 105 M−1, and the fluorescence titration profile shows that probe-51 has a detection limit of 22 nM for Hg(II), which is as low as to detect submillimolar concentrations of Hg(II) ions seen in practice.
1 binding for probe-51–Hg(II) was determined by utilizing the method of continuous variations and Job's plot, which was further validated by the B–H method. The binding constant was found to be 4.484 × 105 M−1, and the fluorescence titration profile shows that probe-51 has a detection limit of 22 nM for Hg(II), which is as low as to detect submillimolar concentrations of Hg(II) ions seen in practice.
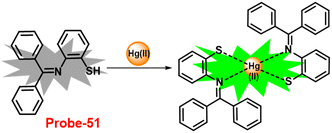 |
| | Fig. 72 Chemical structure and proposed sensing mechanism of probe-51 with Hg(II) (redrawn the ChemDraw structure from ref. 63). | |
In 2016, Yang et al. conducted a study aimed to synthesise a rhodamine based chemosensor (probe-52) for the selective and sensitive recognition of Hg(II) ions in ethanol/HEPES (pH 7.3, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v, ex = 480 nm) via the fluorescence turn-on mechanism.122Probe-52 (Fig. 73) was synthesized from rhodamine hydrazide and furfural in ethanol. Probe-52 was structurally characterized by ESI-MS, 1H, 13C NMR, FT-IR (KBr) and EA. The native state of probe-52 exhibited very weak emission, on titrating with Hg(II) ions, and the fluorescence intensity gradually increased as “green” luminescence enhanced by 320-fold. However, the authors tested other metal ions, which showed no change in the fluorescence spectra. Further, the association constant of probe-52 with Hg(II) was computed to be 1.01 × 104.94 M−1 and the solution color changed from colorless to green fluorescence when exposed to a UV lamp. Meanwhile, Cys can significantly reduce the green fluorescence of probe-52–Hg(II); however, no change in fluorescence spectra was observed for GSH, SO42−, SO32−, CN−, CO32−, or HCY. The LOD values for Hg(II) and Cys were calculated to be 0.09 μM and 0.08 μM, respectively, and they could be applied as dual fluorescent probes that can distinguish Hg(II) ions from other metal ions as well as Cys from other biothiols, and thus, are highly utilizable in aqueous media and bio-imaging studies (Fig. 74).
7, v/v, ex = 480 nm) via the fluorescence turn-on mechanism.122Probe-52 (Fig. 73) was synthesized from rhodamine hydrazide and furfural in ethanol. Probe-52 was structurally characterized by ESI-MS, 1H, 13C NMR, FT-IR (KBr) and EA. The native state of probe-52 exhibited very weak emission, on titrating with Hg(II) ions, and the fluorescence intensity gradually increased as “green” luminescence enhanced by 320-fold. However, the authors tested other metal ions, which showed no change in the fluorescence spectra. Further, the association constant of probe-52 with Hg(II) was computed to be 1.01 × 104.94 M−1 and the solution color changed from colorless to green fluorescence when exposed to a UV lamp. Meanwhile, Cys can significantly reduce the green fluorescence of probe-52–Hg(II); however, no change in fluorescence spectra was observed for GSH, SO42−, SO32−, CN−, CO32−, or HCY. The LOD values for Hg(II) and Cys were calculated to be 0.09 μM and 0.08 μM, respectively, and they could be applied as dual fluorescent probes that can distinguish Hg(II) ions from other metal ions as well as Cys from other biothiols, and thus, are highly utilizable in aqueous media and bio-imaging studies (Fig. 74).
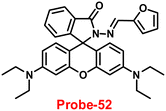 |
| | Fig. 73 Chemical structure of probe-52 (redrawn the ChemDraw structure from ref. 122). | |
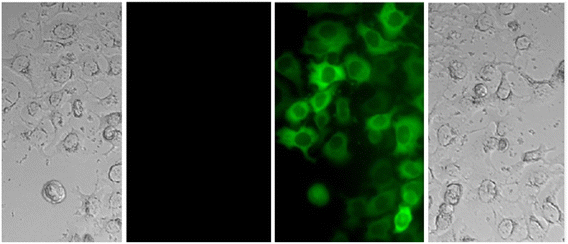 |
| | Fig. 74 Fluorescence microscopic images of SW480 cells with probe-52 in the presence of Hg(II) ions and Cys (reprinted with permission from ref. 122, Copyright 2016 Elsevier). | |
In 2016, Yang reported a facile synthesis method of a novel π-conjugated linear bis-Schiff base (probe-53) containing an α-cyanostilbene unit.123 The structural characterization of probe-53 was carried out by several analytical techniques. Probe-53 (Fig. 75) possesses very interesting AIE effects and is utilized for the sensing of Hg(II) ions with strong affinities, as evidenced by a visible color change or fluorescence emission of the sample in THF and THF/water solutions. The authors proposed that the sensing mechanism of fluorescence turn-on could be explained by the suppression of the restricted intramolecular rotations (RIR) of aromatic rotors and inhibition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, which block the non-fluorescence pathway of probe-53. The competitive studies of probe-53 revealed high selectivity towards Hg(II) ions even with other metal ions. Further, to find out the recognition of Hg(II) ions in biological media, authors performed the sensing studies on HeLa cells, which strongly suggested that probe-53 could be able to sense Hg(II) ions in biological samples (Fig. 76). Due to the very high sensitivity and selectivity of probe-53 towards Hg(II) ions via the fluorescence turn-on behavior with a low LOD (3.4 nM in THF, 0.24 μM in THF/water), it became a compatible sensor in both environmental and biological samples.
N isomerization, which block the non-fluorescence pathway of probe-53. The competitive studies of probe-53 revealed high selectivity towards Hg(II) ions even with other metal ions. Further, to find out the recognition of Hg(II) ions in biological media, authors performed the sensing studies on HeLa cells, which strongly suggested that probe-53 could be able to sense Hg(II) ions in biological samples (Fig. 76). Due to the very high sensitivity and selectivity of probe-53 towards Hg(II) ions via the fluorescence turn-on behavior with a low LOD (3.4 nM in THF, 0.24 μM in THF/water), it became a compatible sensor in both environmental and biological samples.
 |
| | Fig. 75 Chemical structure of probe-53 (redrawn the ChemDraw structure from ref. 123). | |
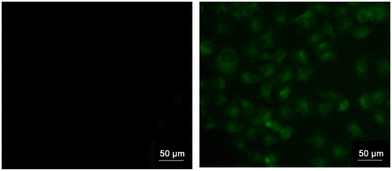 |
| | Fig. 76 Fluorescence microscopic images of HeLa cells incubated with probe-53 (left) and probe-53 + Hg(II) ions (right) (reprinted with permission from ref. 123, Copyright 2016 Elsevier). | |
Luxami and co-workers obtained a novel napthalimide-based Schiff base, probe-54,124 which exhibited high selectivity and sensitivity towards Hg(II) ions via the fluorescence turn-on mechanism in 2017. The target compound was synthesized via a reaction between aminonaphthamide and salicylaldehyde in ethanol under reflux conditions, which was confirmed by multinuclear NMR and HRMS spectrometry. Probe-54 (Fig. 77) demonstrated a spectacular ratiometric absorbance response as well as a turn-on emission behavior towards nanomolar concentrations of Hg(II) ions without interference from other metal ions. A B–H equation was utilized to calculate the binding constant for probe-54 with Hg(II) in CH3OH to be 6.89 × 106 M−1. It was found that Probe-54 had the lowest LOD value of 0.5 nM for Hg(II) based on the titration results. Further, probe-54 showed good DNA intercalator abilities by fluorescence enhancement when bound to Ct-DNA and had a higher binding constant than amonafide, indicating that it might be used as a therapeutic candidate.
 |
| | Fig. 77 Chemical structure of probe-54 (redrawn the ChemDraw structure from ref. 124). | |
In 2017, Yao et al. synthesized two sulfur-rich Schiff base-based chemosensors, namely, probe-55a and probe-55b, with thiooxo-rhodamine and bithiophene units under sulfur-rich conditions, which provided better selectivity and highly sensitive detection of Hg(II) via colorimetric and fluorometric responses.125 The two probes were synthesized via a simple condensation reaction between thiooxo-rhodamine B hydrazone and [2,2-bithiophene]-5-carboxaldehyde in an ethanol solution under reflux conditions and characterized by several analytical techniques. The pure state of probes (Fig. 78) was found to be poorly emissive in nature, but upon addition of Hg(II) ions, the emission intensity gradually increased, whereas in the presence of other metal ions, no change in the emission spectra strongly supported the selectivity of probes. The determined LOD values for probe-55a and probe-55b were 0.62 ppb and 0.59 ppb, respectively with a high binding constant attributed to the sulphur environment. The fluorescence signal changing behavior of probe-55a was found to follow the FRET and probe-55b only demonstrated the traditional ‘Off–On’ response to Hg(II) addition. By comparing the bithiophene and rhodamine B-normalised spectra, probe-55a's FRET process was established. The overlap region between the bithiophene and rhodamine B absorption and emission spectra suggested that FRET between the donor bithiophene and the recipient rhodamine (acceptor) could occur in this region. Both chemosensors demonstrated rapid response, high selectivity, and excellent sensitivity to Hg(II), with visible color changes recognizable with the naked eye. In addition, the detection of trace Hg(II) ions in tap water and actual lake water with good recovery efficiency is a practical application of probe-55a and probe-55b. Additionally, the probes could be employed with low cytotoxicity by confocal fluorescence microscopy to see Hg(II) in live HeLa cells (Fig. 79).
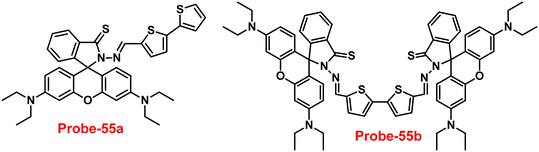 |
| | Fig. 78 Chemical structures of probe-55a and probe-55b (redrawn the ChemDraw structure from ref. 125). | |
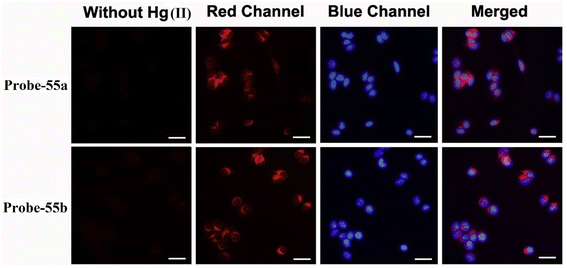 |
| | Fig. 79 Confocal fluorescence images of probe-55a and probe-55b in HeLa cells in the presence of Hg(II) ions (reprinted with permission from ref. 125, Copyright 2018 Elsevier). | |
In 2018, Singh and colleagues demonstrated a fluorescence turn-on signal for Hg(II) facilitated by a sensibly developed new Schiff base probe (probe-56).126 The pure state of probe-56 exhibited poor emission and dramatic rise in fluorescence intensity on titrating with Hg(II) ions, which could be explained by the conventional CHEF mechanism (Fig. 80), whereas in the presence of other competitive metal ions, no change in the emission intensity was observed. The binding constant and LOD of probe-56 with Hg(II) ions were estimated as 2.5 × 104 M−1 and 2 μM respectively from the fluorescence titration spectra. Furthermore, probe-56 displayed very intriguing features that discriminate two heavy metal ions such as Hg(II) ions with fluorescence turn-on and Ag(I) ions via the fluorescence turn-off mechanism. Due to the potentiality of probe-56, it could be applied for the recognition of both heavy metal ions simultaneously.
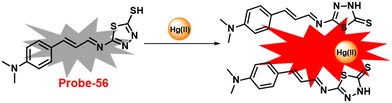 |
| | Fig. 80 Chemical structure and proposed sensing mechanism of probe-59 with Hg(II) (redrawn the ChemDraw structure from ref. 126). | |
A facile fluorescent “turn-on” probe for Hg(II) ions was prepared via single-step condensation reactions between 2,3-diamino naphthalene and o-salicylaldehyde, and m, or p-hydroxybenzaldehyde, as demonstrated by Shen and co-workers in 2019.127 The emission response of the probes towards Hg(II) ions can be explained by the geometry of probes that match the size of Hg(II) ions, and these results indicate that the probe containing a salicylaldehyde group is having more affinity towards Hg(II) with a binding ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Increases in Hg(II) ion concentration were found to significantly increase the fluorescence intensity at the maximum emission peak of probes in an aqueous solution of ACN and water. This finding was interpreted as the result of an ESIPT-coupled AIEE process that was made possible by the coordination of the probes with Hg(II) ions (Fig. 81). The probes exhibit high sensitivity and selectivity over other interfering metal ions with a LOD value over 12.5 ppb.
1. Increases in Hg(II) ion concentration were found to significantly increase the fluorescence intensity at the maximum emission peak of probes in an aqueous solution of ACN and water. This finding was interpreted as the result of an ESIPT-coupled AIEE process that was made possible by the coordination of the probes with Hg(II) ions (Fig. 81). The probes exhibit high sensitivity and selectivity over other interfering metal ions with a LOD value over 12.5 ppb.
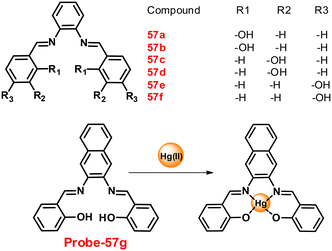 |
| | Fig. 81 Chemical structures of probe-57(a–f) and proposed sensing mechanism of probe-57g with Hg(II) (redrawn the ChemDraw structure from ref. 127). | |
In 2020, Lee et al. designed a rhodamine-based Schiff base (probe-58),128 which was utilized for the synthesis of fluorescent organic nanoparticles by a simple re-precipitation method. The optical properties of nanoparticles including UV-vis, emission and TCS-PC measurements are remarkably different from that of the parent organic moiety. According to the sensing studies, titrating with Hg(II) ions significantly enhance the emission of nanoparticles by nearly 7-fold more than its initial value among the series of heavy metal ions. The increased emission can be due to the CHEF, on better interactions of probe-58 and Hg(II) ions with more reactive imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) groups and oxygen (O) of carbonyl groups when the spirolactum ring of the rhodamine core is opened (Fig. 82). The LOD value of Hg(II) ions was estimated to be 1.729 ng mL−1 and the binding ratio of probe-58 with Hg(II) ions to be 1
N) groups and oxygen (O) of carbonyl groups when the spirolactum ring of the rhodamine core is opened (Fig. 82). The LOD value of Hg(II) ions was estimated to be 1.729 ng mL−1 and the binding ratio of probe-58 with Hg(II) ions to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Moreover, the addition of a 0–2 g mL−1 Hg(II) ion solution increases the lifetime value from 5.63 ns to 6.12 ns, indicating the formation of complexes in the excited state. The intracellular recognition of Hg(II) ions using probe-58 is another advantage of prepared nanoparticles that will be probed for potential applications in the biomedical field (Fig. 83).
1. Moreover, the addition of a 0–2 g mL−1 Hg(II) ion solution increases the lifetime value from 5.63 ns to 6.12 ns, indicating the formation of complexes in the excited state. The intracellular recognition of Hg(II) ions using probe-58 is another advantage of prepared nanoparticles that will be probed for potential applications in the biomedical field (Fig. 83).
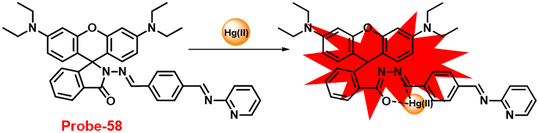 |
| | Fig. 82 Chemical structure and proposed sensing mechanism of probe-58 with Hg(II) (redrawn the ChemDraw structure from ref. 128). | |
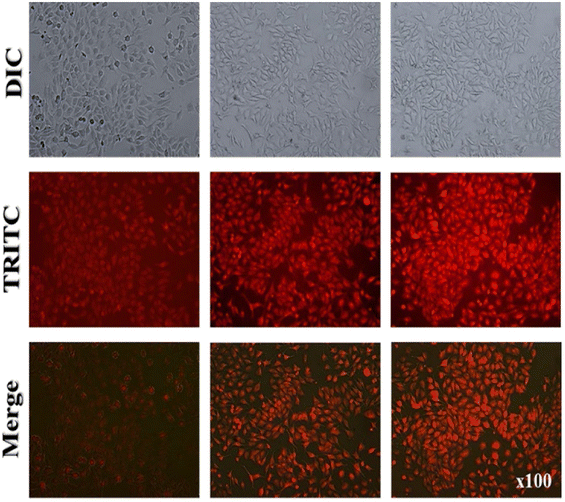 |
| | Fig. 83 Fluorescence microscopic images of probe-58 in living A375 cells with Hg(II) ions. (reprinted with permission from ref. 128, Copyright 2020 Elsevier). | |
In 2021, Sekar and colleagues created a novel carbazole–hydrazinobenzothiazole sensor (probe-59)129 that detects Hg(II) ions by emitting green fluorescence emission from a pool of other metal ions with the lowest LOD value of about 0.14 μM. Probe-59 was synthesized from 9-ethyl-9H-carbazolyl-3-carbaldehyde and hydrazine benzothiazole in methanol under reflux conditions and was structurally characterized via various analytical techniques. The synthesized pure probe-59 displayed poor emission upon coordination with Hg(II) ions via nitrogen atoms of the hydrazine unit with the sulphur atom of the benzothiazole unit resulting in the fluorescence enhancement and was determined by using 1H NMR, ESI-MS, and IR titration studies. Upon complexation with Hg(II) ions, the switch on the ICT between the benzothiazole moiety and the carbazole unit could be related to its sensing behavior (Fig. 84). Using one- and three-dimensional fluorescence spectral analysis, it was also determined whether probe-59 could be used to target metal ions in proteins and subcellular organelles in cells and other organisms. The potential ability in electrochemical sensing of Hg(II) ions was investigated by CV analysis of probe-59 with different metal ions. The results indicated that probe-59 exhibits a shift to a higher oxidation potential in the presence of Hg(II), demonstrating the possibility of an electrochemical process caused by the CHEF effect and showing no effect for the other metal ions.
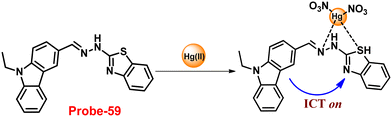 |
| | Fig. 84 Chemical structure and proposed sensing mechanism of probe-59 with Hg(II) (redrawn the ChemDraw structure from ref. 129). | |
In 2019, Trivedi and colleagues prepared simple pyrene-based probes 60a–c from pyrene-1-carboxaldehyde and different types of amines, and characterized by FT-IR, ESI-Mass, UV-vis, 1H NMR, and 13C NMR.130 The synthesized compounds are very weak emissive in nature; upon addition of Hg(II) ions, the emission was gradually increased in which probe-60a showed better efficiency. Conversely, with other metal ions, there was no change in fluorescence spectra. The authors proposed the binding ability of probes with Hg(II) ions; first, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-(imine) link of the thiazole moiety is hydrolyzed by Hg(II), whereas pyrene-carboxaldehyde molecules operate as ‘turn-on’ chemodosimeters for Hg(II) ions (Fig. 85). The detection limit of probe-60a with Hg(II) ions was found to be 0.270 μM. Additionally, the probe-60a's chemodosimetric irreversible hydrolysis was investigated by FT-IR spectroscopy, UV/Vis, liquid chromatography with tandem mass spectrometry (LC-MS), fluorescence, 1H NMR, and DFT analysis.
N-(imine) link of the thiazole moiety is hydrolyzed by Hg(II), whereas pyrene-carboxaldehyde molecules operate as ‘turn-on’ chemodosimeters for Hg(II) ions (Fig. 85). The detection limit of probe-60a with Hg(II) ions was found to be 0.270 μM. Additionally, the probe-60a's chemodosimetric irreversible hydrolysis was investigated by FT-IR spectroscopy, UV/Vis, liquid chromatography with tandem mass spectrometry (LC-MS), fluorescence, 1H NMR, and DFT analysis.
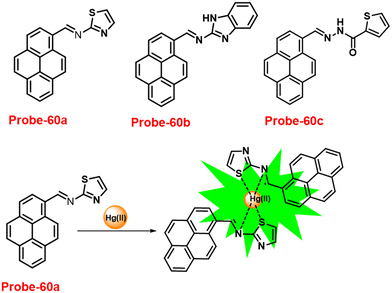 |
| | Fig. 85 Chemical structures of probe-60a–c (top) and sensing mechanism of probe-60a with Hg(II) (bottom) (redrawn the ChemDraw structure from ref. 130). | |
Duan et al. fabricated novel Schiff-based luminescent probes (probe-61a and probe-61b) (Fig. 86), with triazolyl and rhodamine hydrazide conjugates131 that selectively recognize Hg(II) ions, exhibiting major color change from colorless to pink and emission turn-on mechanism, while upon titration with other competitive metal ions, there was no change in the fluorescence spectra in 2020. The addition of 1.0 eq. of Hg(II) ions caused 600-fold and 350-fold enhancement in the emission intensity of probe-61a and probe-61b, respectively, which could be attributed to a reduction in the PET process and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization on Hg(II) ion coordination. The LOD value was measured to be 13.4 nM and 15.6 nM respectively for both probe-61a and probe-61b toward Hg(II) ions using the equation DL = 3σ/slope. Due to their potentiality, these probes can be utilized in future applications for the recognition of Hg(II) in various media. The DFT studies predicted the equivalent molecular geometric arrangement, orbital electron distribution, and orbital energy of both probes with their metal complexes. Job's plot analysis gave a binding ratio of 1
N isomerization on Hg(II) ion coordination. The LOD value was measured to be 13.4 nM and 15.6 nM respectively for both probe-61a and probe-61b toward Hg(II) ions using the equation DL = 3σ/slope. Due to their potentiality, these probes can be utilized in future applications for the recognition of Hg(II) in various media. The DFT studies predicted the equivalent molecular geometric arrangement, orbital electron distribution, and orbital energy of both probes with their metal complexes. Job's plot analysis gave a binding ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and further, the probes were fruitfully used for imaging Hg(II) ions in live breast cancer cells and MCF-7 cells, illustrating the probe's applications for biological-Hg(II) ion detection (Fig. 87).
1, and further, the probes were fruitfully used for imaging Hg(II) ions in live breast cancer cells and MCF-7 cells, illustrating the probe's applications for biological-Hg(II) ion detection (Fig. 87).
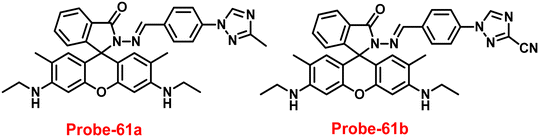 |
| | Fig. 86 Chemical structures of probe-61a and probe-61b (redrawn the ChemDraw structure from ref. 131). | |
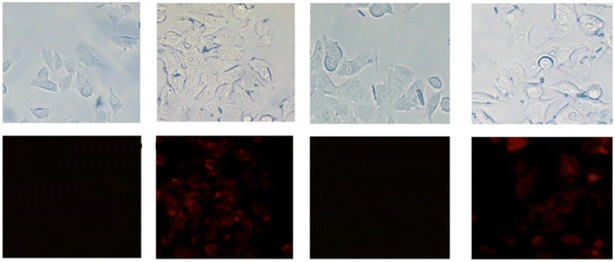 |
| | Fig. 87 Fluorescence images of MCF-7 cells treated with probe-61a (left) and probe-61b (right) with Hg(II) ions. (reprinted with permission from ref. 131, Copyright 2020 American Chemical Society). | |
In 2016, Lee et al. developed probe-62 (ref. 132) by combining quinolone-hydrazino-1,8-naphthalimide conjugates for sensitive and selective sensing of Hg(II) ions. The target compound was synthesized with 2-(2-(dimethylamino)ethyl)-6-hydrazinyl-1H-benzo[d,e]isoquinoline-1,3(2H)-dione and 8-hydroxy-2-quinolinecarboxaldehyde in ethanol under reflux conditions and was structurally characterized by various analytical techniques. The native state of probe-62 displayed poor emission, upon incorporation of Hg(II) ions due to the absence of PET; the emission intensity progressively enhanced 6-fold at 535 nm (Fig. 88) and no change was observed with other metal ions. The LOD value of probe-62 for Hg(II) ions was observed to be 0.24 μM, and Job's plot analysis discovered a complex ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a binding constant of 4.12 × 105 M−1. In addition, probe-62 changes color in response to the anions CN− and F−, agreeing for dual mode detection of several analytes.
1 with a binding constant of 4.12 × 105 M−1. In addition, probe-62 changes color in response to the anions CN− and F−, agreeing for dual mode detection of several analytes.
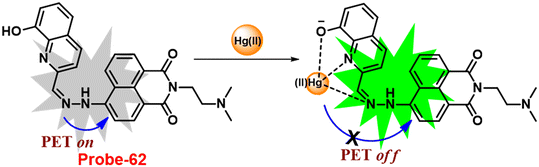 |
| | Fig. 88 Chemical structure and proposed sensing mechanism of probe-62 with Hg(II) (redrawn the ChemDraw structure from ref. 132). | |
In 2017, Wu et al. synthesized a Schiff base sensor (probe-63)133 by the combination of rhodamine B hydrazide and 2-(phenylselanyl)benzaldehyde, and it was structurally characterized by 1H NMR, 13C NMR, and HRMS data. In CH3OH/H2O (v/v 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions, probe-63 demonstrates high selectivity and sensitivity towards Hg(II) ions and the color changed to pink with 48-fold fluorescence amplification, which is caused by the ring opening of the rhodamine unit (Fig. 89), whereas no change was observed in the absorption and emission spectra with other competitive metal ions. In addition, the molecule binds to Hg(II) in a 1
1) solutions, probe-63 demonstrates high selectivity and sensitivity towards Hg(II) ions and the color changed to pink with 48-fold fluorescence amplification, which is caused by the ring opening of the rhodamine unit (Fig. 89), whereas no change was observed in the absorption and emission spectra with other competitive metal ions. In addition, the molecule binds to Hg(II) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was validated by Job's plot and was supported by the ESI-MS and DFT calculations. The LOD value of probe-63 from the fluorescence titration spectra was calculated to be 12 nM and could be utilized effectively for Hg(II) sensing over a wide pH range of 4.0–10.0. Finally, the authors applied probe-63 for sensing Hg(II) ions in in vitro and in vivo studies, including test strips, bio imaging in HeLa cells and zebrafish with the help of confocal fluorescence microscopy (Fig. 90).
1, which was validated by Job's plot and was supported by the ESI-MS and DFT calculations. The LOD value of probe-63 from the fluorescence titration spectra was calculated to be 12 nM and could be utilized effectively for Hg(II) sensing over a wide pH range of 4.0–10.0. Finally, the authors applied probe-63 for sensing Hg(II) ions in in vitro and in vivo studies, including test strips, bio imaging in HeLa cells and zebrafish with the help of confocal fluorescence microscopy (Fig. 90).
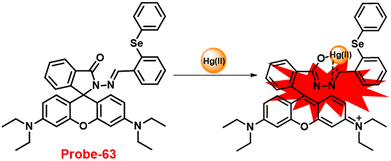 |
| | Fig. 89 Chemical structure and proposed sensing mechanism of probe-63 with Hg(II) (redrawn the ChemDraw structure from ref. 133). | |
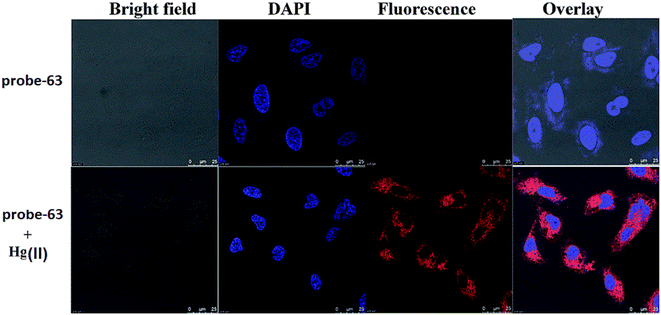 |
| | Fig. 90 Confocal microscopic images of probe-63 treated with Hg(II) ions in HeLa cells. (reprinted with permission from ref. 133, Copyright 2017 Royal Society of Chemistry). | |
In 2019, Wang et al. developed a novel chemosensor, probe-64,134 using a commercially accessible pyrazole derivative and rhodamine 6G as the precursors and structurally described it via various analytical and spectroscopic analysis. Probe-64 showed high fluorescence “turn-on” behavior towards Hg(II) ions in an aqueous DMSO solution via a complexation reaction, and the color changed from colorless to pink even in the presence of other interfering metal ions, which strongly suggested that probe-64 was very selective towards Hg(II) ions. Probe-64 exhibits good fluorescence amplification on introducing Hg(II) ions, because of the extended conjugated structure, which may be related to the hindered PET process and CHEF behavior (Fig. 91). The LOD value was as low as 0.2 nM, making them appropriate for the quantitative determination of Hg(II) ions in aqueous media. Job's plot revealed a binding ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the probe-64–Hg(II) complex. It was further demonstrated that it was a straightforward and efficient solid-state probe because the color of the sensor substantially changed when it was impregnated on filter paper and submerged in a Hg(II) solution.
1 for the probe-64–Hg(II) complex. It was further demonstrated that it was a straightforward and efficient solid-state probe because the color of the sensor substantially changed when it was impregnated on filter paper and submerged in a Hg(II) solution.
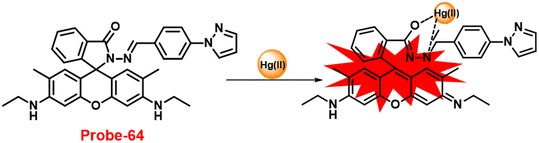 |
| | Fig. 91 Chemical structure and proposed sensing mechanism of probe-64 with Hg(II) (redrawn the ChemDraw structure from ref. 134). | |
In 2017, Chen et al. focused on the synthesis of a unique Schiff base probe (probe-65)135via a simple condensation reaction of 7-hydroxy-4-methyl-2-oxo-2H-chromene-8-carbaldehyde and 2-(2-aminophenyl)benzimidazole in ethanol under reflux conditions, which was characterized by multinuclear ESI-MS, IR, and NMR. The pure form of probe-65 displayed poor emission, upon addition of Hg(II) ions the sudden increment in fluorescence, implies that Hg(II) ions stimulated hydrolysis of a Hg(II)–probe-65 complex transforms the imine group to the aldehyde group (Fig. 92). A Job's plot reveals 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding, and the probe-65–Hg(II) binding constant was calculated as 8.75 × 104 M−1. Furthermore, the probe-65 was successfully used in cell imaging, indicating that it has a potential application for sensing Hg(II) ions in living cells (Fig. 93).
1 binding, and the probe-65–Hg(II) binding constant was calculated as 8.75 × 104 M−1. Furthermore, the probe-65 was successfully used in cell imaging, indicating that it has a potential application for sensing Hg(II) ions in living cells (Fig. 93).
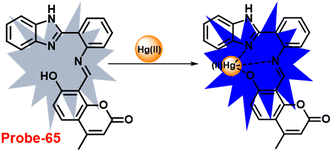 |
| | Fig. 92 Chemical structure and proposed sensing mechanism of probe-65 with Hg(II) (redrawn the ChemDraw structure from ref. 135). | |
 |
| | Fig. 93 Bio-imaging of probe-65 treated with Hg(II) ions in HeLa cells. (reprinted with permission from ref. 135, Copyright 2017 Elsevier). | |
Chatterjee et al. synthesized a 1,1′-unsymmetrical substituted ferrocene-based chemosensor (66a–c),136 which shows a sensitive and selective fluorescence turn-on behavior towards Hg(II) ions via selective functionalization of ferrocenyl species with a fluorescent active rhodamine moiety in 2021. The probes (Fig. 94) possess active organometallic complexes with rhodamine moieties, demonstrating the exceptional recognition ability of metal ions in solutions and at the subcellular level. The native state of the probes was found to be very poorly emissive in nature, and upon incorporation of Hg(II) ions, the emission properties drastically change with quantum yields, while with other competitive metal ions, there was no change in the emission spectra. The DFT studies and spectroscopic analysis have been used to establish the binding nature of the unsymmetrical ferrocene-based rhodamine organometallic probes with Hg(II) ions. The study also showed the significant intracellular metal identification and imaging properties, which can be used in applications related to the bio-imaging of heavy metal ions. The bio-imaging investigation of the THP-1 cancer cell line revealed significant chemical bioaccumulation and interactions with heavy metal ions (Fig. 95). The unsymmetrical ferrocenyl-rhodamine derivatives showing turn-on response were also used to monitor various molecular logic operations.
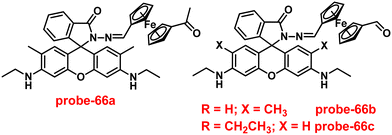 |
| | Fig. 94 Chemical structures of probe-66a–c (redrawn the ChemDraw structure from ref. 136). | |
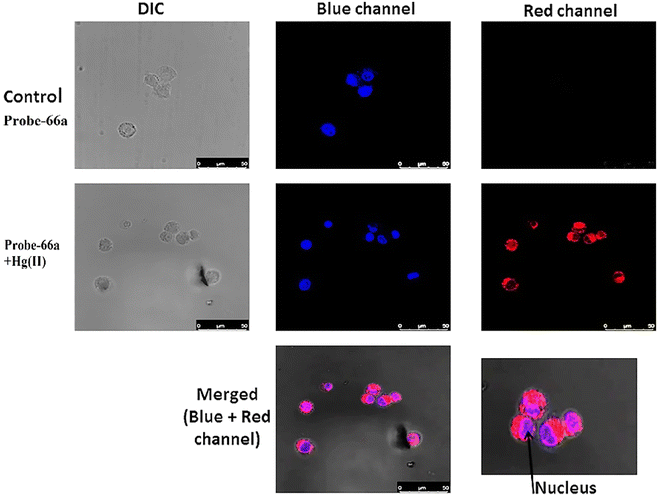 |
| | Fig. 95 Confocal fluorescence images of THP-1 cells of probe-66a with Hg(II) ions. (reprinted with permission from ref. 136, Copyright 2021 Elsevier). | |
In 2016, Zang and colleagues developed a rhodamine B conjugate with a NS2-containing sensor (probe-67),137 which could be used as a dual probe for Cu(II) colorimetrically and Hg(II) ions fluorogenically. The target compound was structurally characterized via various analytical and spectroscopic techniques. The native mode of probe-67 displayed poor fluorescence intensity; upon titration with Hg(II) ions, the emission intensity drastically enhanced under aqueous conditions, while Cu(II) ions showed an exceptional colorimetric response. The interference of other examined environmentally relevant species did not affect probe-67, which strongly suggested that probe-67 has a potential ability to detect Hg(II) ions via the fluorescence turn-on mechanism (Fig. 96) and Cu(II) via colorimetric response. Probe-67 showed very good selectivity towards Cu(II) and Hg(II) in aqueous solutions at a physiological pH, with very low LOD values of 1.63 μM and 2.36 μM, correspondingly. The authors proposed the sensing mechanism of probe-67; when spirolactam was opening upon metal binding, the coordination mechanism results in ring opening, which causes a high UV-vis absorption and fluorescence amplification in both recognition processes. A proof-of-concept test illustrates the possible use of probe-67 for the recognition of trace Hg(II) and Cu(II) in challenging environment water samples. Furthermore, probe-67, which has good membrane permeability, is successfully used in microscopic imaging to detect Hg(II) ions in PC3 cells (Fig. 97).
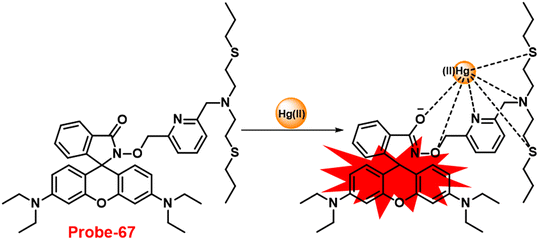 |
| | Fig. 96 Chemical structure and proposed sensing mechanism of probe-67 with Hg(II) (redrawn the ChemDraw structure from ref. 137). | |
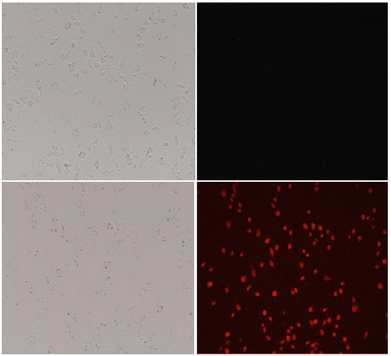 |
| | Fig. 97 Confocal fluorescence images of PC3 cells of probe-67 with Hg(II) ions. (reprinted with permission from ref. 137, Copyright 2016 Elsevier). | |
In 2021, Lee et al. synthesized a new fluorescent chemosensor, probe-68, based on rhodamine 6G for the selective sensing of Hg(II) ions.138 The sensing studies were carried out with Hg(II) ions and other metal ions in the combined organic aqueous phase, and the fluorescent chemosensor (probe-68) (Fig. 98) exhibits exceptional sensitivity and selectivity for Hg(II) ions. The sensing mechanism of probe-68 demonstrated enhanced fluorescence with the Hg(II) ions, which caused the spirocyclic form (spirolactum) to change into an open-ring form. According to Job's plot and 1H NMR experiment, the stoichiometric ratio of the probe-68–Hg(II) complex was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For probe-68–Hg(II) complexation, the calculated association constant and LOD were 6 × 104 M−1 and 30.37 nM, respectively. The Hg(II) metal ion was successfully detected by a spike and recovery method from actual water samples in practical use. Probe-68 was also utilized for the sensing of Hg(II) ions in biological media, which was carried out by the MTT assay for the cytotoxicity and the data showed very low cytotoxicity, providing valuable information for the identification of intracellular Hg(II) ions in MDA-MB-231 and A375 breast cancer cells (Fig. 99).
1. For probe-68–Hg(II) complexation, the calculated association constant and LOD were 6 × 104 M−1 and 30.37 nM, respectively. The Hg(II) metal ion was successfully detected by a spike and recovery method from actual water samples in practical use. Probe-68 was also utilized for the sensing of Hg(II) ions in biological media, which was carried out by the MTT assay for the cytotoxicity and the data showed very low cytotoxicity, providing valuable information for the identification of intracellular Hg(II) ions in MDA-MB-231 and A375 breast cancer cells (Fig. 99).
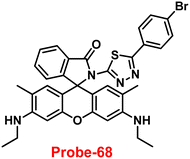 |
| | Fig. 98 Chemical structure of probe-68 (redrawn the ChemDraw structure from ref. 138). | |
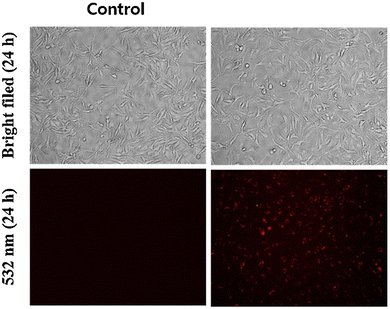 |
| | Fig. 99 Bio-imaging images of living MDA-MB-231 cells with probe-68 and probe-68 + 5 μM Hg(II) ions (reprinted with permission from ref. 138, Copyright 2021 Elsevier). | |
In 2017, Li et al. developed a rhodamine-based sensor, probe-69, for detecting Hg(II) ions139 by the reflux reaction of rhodamine 6G hydrazide and 7-diethylamino-3-(1-hydroxy-3-oxobut-1-enyl)-2H-chromen-2-one and structurally characterized it by various analytical and spectroscopic techniques. Probe-69 (Fig. 100) demonstrated remarkable selectivity towards Hg(II) under neutral aqueous conditions as a colorimetric and ratiometric fluorescent sensor. More remarkably, probe-69 could sense Hg(II) with emission amplification at moderately high pH values. Under such conditions, probe-69 showed colorimetric response towards Cu(II) ions, whereas upon titrating with other metal ions, there was no change in emission spectra as well as absorption spectra. Job's plot analysis revealed that the binding of probe-69 to Hg(II) is of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry from the absorption spectra. Probe-69 could find use as a Hg(II) detector in living cells (Fig. 101) or water samples.
1 stoichiometry from the absorption spectra. Probe-69 could find use as a Hg(II) detector in living cells (Fig. 101) or water samples.
 |
| | Fig. 100 Chemical structure of probe-69 (redrawn the ChemDraw structure from ref. 139). | |
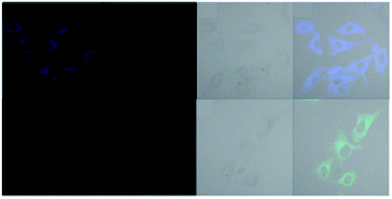 |
| | Fig. 101 Bio-imaging images of HeLa cells with probe-69 (reprinted with permission from ref. 139, Copyright 2017 Royal Society of Chemistry). | |
4. Schiff base-based fluorescent turn-on probes for Cd(II) ions
Despite their toxicity, heavy metals have potential uses. For instance, cadmium is used in pigments, electroplating, metallurgy, batteries, and agriculture.140–143 In contrast, it has been discovered that cadmium metal is extremely hazardous and dangerous to people health even at very low levels.144–146 As a result, it is imperative to design and synthesize probes that respond to Cd(II) ions for quantitative and qualitative examination in both biological and environmental samples due to the presence of its benefits and drawbacks.147–149
In 2019, the widespread health hazardous effects of Cd(II) influenced Wan et al. to develop a quinoline-containing Schiff base (probe-70) (Fig. 102),150 which showed very high selectivity and sensitivity for the on-site recognition of Cd(II) ions. The target compound was obtained via a simple condensation reaction between ethylenediamine and quinoline aldehyde in an ethanol solution under reflux conditions, which was structurally characterized by various analytical and spectroscopic techniques. The native state of probe-70 showed poor emission, and on titrating with Cd(II) ions, the emission intensity gradually increased and reached maxima after addition of 1 eq., whereas in the presence of other competitive metal ions, evident changes cannot be observed in the fluorescence spectra, which strongly suggested the potential of probe-70 for the selective detection of Cd(II) ions (Fig. 103). Probe-70 was sparklingly soluble in water and titration was conducted in methanol water combination at pH 7. Job's plot measurement indicated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation among probe-70 and Cd(II) ions, which was strongly supported by 1H NMR and ESI-MS analysis. The LOD value of probe-70 was calculated from emission titration spectra to be 2.4 nM.
1 stoichiometric complexation among probe-70 and Cd(II) ions, which was strongly supported by 1H NMR and ESI-MS analysis. The LOD value of probe-70 was calculated from emission titration spectra to be 2.4 nM.
 |
| | Fig. 102 Chemical structure of probe-70 (redrawn the ChemDraw structure from ref. 150). | |
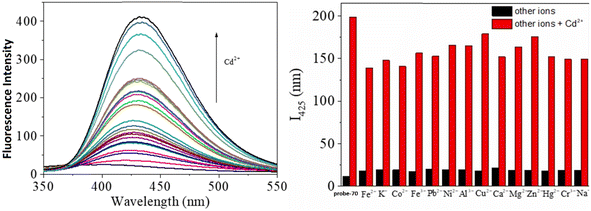 |
| | Fig. 103 Change in the emission spectra of probe-70 upon increasing the concentration of Cd(II) ions (left) and with different metal ions (right) (reprinted with permission from ref. 150, Copyright 2019 Elsevier). | |
In order to overcome the limitation of previously synthesized Cd(II) probes for the recognition of Cd(II) over Zn(II) in aqueous solutions, Rajesh et al. developed a quinoline-based Schiff base (probe-71)151 in 2021, which detected Cd(II) in a semi aqueous medium and was synthesized by the reaction between 2-hydroxy-1-naphthalene carboxaldehyde and 2-hydrazino quinolone. The absorption data of probe-71 with Cd(II) showed colorimetric response and changes from colorless to yellow-orange in ACN–H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). However, the fluorescence intensity of probe-71 displayed poor emission, and upon addition of Cd(II) ions, the emission intensity was gradually increased 37-fold its original intensity at 510 nm. Under similar conditions, with other competitive metal ions, there was no change in the fluorescence spectra. The sensing mechanism of probe-71 with Cd(II) was explained by the restriction of C
2, v/v). However, the fluorescence intensity of probe-71 displayed poor emission, and upon addition of Cd(II) ions, the emission intensity was gradually increased 37-fold its original intensity at 510 nm. Under similar conditions, with other competitive metal ions, there was no change in the fluorescence spectra. The sensing mechanism of probe-71 with Cd(II) was explained by the restriction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and CHEF effects (Fig. 104). The LOD value of probe-71 with Cd(II) ions was estimated to be 49.5 nM from the titration spectra using the 3σ/slope equation. The binding constant was estimated to be 1.77 × 105 M−1. The stoichiometry of probe-71 with Cd(II) ions was estimated to be 2
N isomerization and CHEF effects (Fig. 104). The LOD value of probe-71 with Cd(II) ions was estimated to be 49.5 nM from the titration spectra using the 3σ/slope equation. The binding constant was estimated to be 1.77 × 105 M−1. The stoichiometry of probe-71 with Cd(II) ions was estimated to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 via Job's plot, which was confirmed by DFT, 1H NMR and ESI-MS data.
1 via Job's plot, which was confirmed by DFT, 1H NMR and ESI-MS data.
 |
| | Fig. 104 Chemical structure and proposed sensing mechanism of probe-71 with Cd(II) (redrawn the ChemDraw structure from ref. 151). | |
In 2021, Lu et al. concentrated on the synthesis of a purine-constructed Schiff base-based fluorescent turn-on probe (probe-72)152 for the recognition of Cd(II) ions in an EtOH–H2O solution. Probe-72 was synthesized via a simple condensation reaction between 6-hydrazinyl-8-methyl-9-(naphthalen-1-yl)-9H-purine and quinoline-2-carbaldehyde in an ethanol solution under reflux conditions and structurally characterized by various analytical techniques. The pure form of probe-72 showed very poor emission due to the presence of PET and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerism; upon incorporation of Cd(II) ions, the luminescence intensity was drastically increased and on titrating with other competitive metal ions, no change was observed in the emission spectra (Fig. 105). From the emission spectra, it was observed that the intensity of the peak at 530 nm increased with the gradual addition of Cd(II) and the detection limit was found to be 41 nM. Job's plot revealed the 1
N isomerism; upon incorporation of Cd(II) ions, the luminescence intensity was drastically increased and on titrating with other competitive metal ions, no change was observed in the emission spectra (Fig. 105). From the emission spectra, it was observed that the intensity of the peak at 530 nm increased with the gradual addition of Cd(II) and the detection limit was found to be 41 nM. Job's plot revealed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric complexations between probe-72 and Cd(II) ions, which was strongly supported by 1H NMR, IR and ESI-MS data. Further, the authors focused on the effect of pH on probe-72 and showed that they did not produce any fluorescence change within the pH range of 6–11, whereas upon adding Cd(II), the fluorescence intensity exclusively amplified at pH 2–11, indicating that the detection of Cd(II) ions with probe-72 was suitable for physiological pH. Apart from acting as a fluorescent probe, inoculating probe-72 in HeLa cells in in vivo cell imaging was achieved by sensing Cd(II) ions by fluorescence microscopy without causing any harm to the cell line (Fig. 106).
2 stoichiometric complexations between probe-72 and Cd(II) ions, which was strongly supported by 1H NMR, IR and ESI-MS data. Further, the authors focused on the effect of pH on probe-72 and showed that they did not produce any fluorescence change within the pH range of 6–11, whereas upon adding Cd(II), the fluorescence intensity exclusively amplified at pH 2–11, indicating that the detection of Cd(II) ions with probe-72 was suitable for physiological pH. Apart from acting as a fluorescent probe, inoculating probe-72 in HeLa cells in in vivo cell imaging was achieved by sensing Cd(II) ions by fluorescence microscopy without causing any harm to the cell line (Fig. 106).
 |
| | Fig. 105 Chemical structure and proposed sensing mechanism of probe-72 with Cd(II) (redrawn the ChemDraw structure from ref. 152). | |
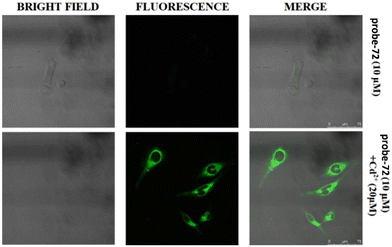 |
| | Fig. 106 Fluorescence microscopic imaging of probe-72 in HeLa cells (top) in the presence of Cd(II) (bottom) (reprinted with permission from ref. 152, Copyright 2021 Elsevier). | |
In 2021, Khan and his colleagues created a novel Schiff base using a benzothiazole unit, which was obtained from the condensation reaction between 2-hydrazinobenzothiazole and 2-hydroxy-1-naphthaldehyde in ethanol under reflux conditions and was described by various spectroscopic and analytical techniques.153Probe-73 exhibited poor emission; however, on adding Cd(II) ions, the emission intensity drastically increased and attained maxima after addition of one eq., which is due to the combined effect of ICT and CHEF (Fig. 107). However, with other metal ions, there was no change in fluorescence spectra, which strongly suggested probe-73 selective towards Cd(II) ions. B–H and the Job's plot approach both confirm that the binding ratio between probe-73 and Cd(II) is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A binding constant of 1.17 × 104 M−1 and detection limits in the micromolar range were calculated for probe-73 with Cd(II) ions from the fluorescence titration spectra. Furthermore, the authors utilized probe-73 for the sensing of Cd(II) ions in biological media; the HeLa cells were incubated with probe-73 and showed no fluorescence, and in the presence of Cd(II) ions, the emission was generated inside the cells (Fig. 108), which strongly suggested that probe-73 shows potential for the recognition of Cd(II) ions in biological media.
1. A binding constant of 1.17 × 104 M−1 and detection limits in the micromolar range were calculated for probe-73 with Cd(II) ions from the fluorescence titration spectra. Furthermore, the authors utilized probe-73 for the sensing of Cd(II) ions in biological media; the HeLa cells were incubated with probe-73 and showed no fluorescence, and in the presence of Cd(II) ions, the emission was generated inside the cells (Fig. 108), which strongly suggested that probe-73 shows potential for the recognition of Cd(II) ions in biological media.
 |
| | Fig. 107 Chemical structure and proposed sensing mechanism of probe-73 with Cd(II) (redrawn the ChemDraw structure from ref. 63). | |
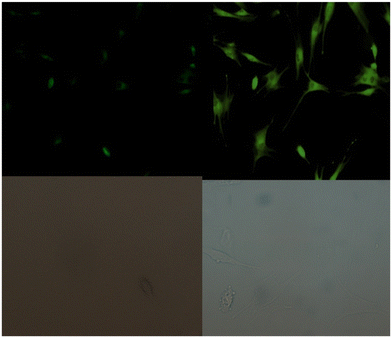 |
| | Fig. 108 Fluorescence microscopic imaging of probe-73 in HeLa cells (reprinted with permission from ref. 153, Copyright 2021 Elsevier). | |
In 2020, a Schiff base fluorescent chemosensor (probe-74)154 for the sensing of Cd(II) ions was prepared by Shuang et al. via a one-pot and one-step condensation reaction between 2,3-naphthalene diamine and imidazole-2-carboxaldehyde in MeOH under reflux conditions. The native state of probe-74 showed poor emission and showed response towards Cd(II) ratiometrically over Zn(II) ions, whereas no response was observed for the other metals. The emission maxima were shifted towards the blue region for 74 nm with the consequently enhanced emission by the complexation of Cd(II) ions with probe-74, because of the suppression of the ICT process (Fig. 109). Some changes were observed in the ratio of fluorescence intensities (F398/F472), on adding 1 eq. of metal ions and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding of probe-74 with Cd(II), enabling the ratiometric sensing of Cd(II) ions with a LOD value of 0.38 μM. Additionally, confocal fluorescence microscopy was used to examine the use of probe-74 to sense Cd(II) ions in human liver cancer cells (SMMC-7721), zebrafish, and live tissues of Arabidopsis thaliana, revealing that it can be used to monitor Cd(II) ions in various biological contexts with low toxicity (Fig. 110).
1 binding of probe-74 with Cd(II), enabling the ratiometric sensing of Cd(II) ions with a LOD value of 0.38 μM. Additionally, confocal fluorescence microscopy was used to examine the use of probe-74 to sense Cd(II) ions in human liver cancer cells (SMMC-7721), zebrafish, and live tissues of Arabidopsis thaliana, revealing that it can be used to monitor Cd(II) ions in various biological contexts with low toxicity (Fig. 110).
 |
| | Fig. 109 Chemical structure and proposed sensing mechanism of probe-74 with Cd(II) (redrawn the ChemDraw structure from ref. 154). | |
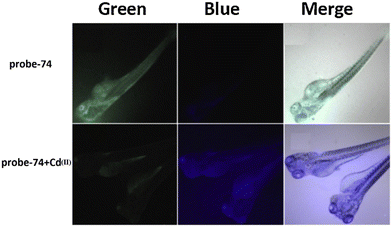 |
| | Fig. 110 Fluorescence microscopic imaging of probe-74 in zebra fish (reprinted with permission from ref. 154, Copyright 2020 Elsevier). | |
5. Schiff base-based fluorescent turn-on probes for Au(III) ions
Due to the relevance of gold in the fields of chemistry, biology, environmental protection, and medicine, it has gained a great deal of research interest.155–158 Gold complexes are also used in biological systems and for the treatment of diseases such as tuberculosis, AIDS, cancer, asthma, brain lesions, rheumatoid arthritis, malaria, and asthma because of their catalytic activity, which is especially useful in chemical synthesis.159–164 Therefore, it is crucial to recognize gold metal ions, and researchers concentrated on developing probes to find Au(I) and Au(III) ions.
The widespread use of Au(III) as a catalyst in numerous organic reactions, as well as the health risks associated with Au(III), prompted the researchers to develop a selective sensitive approach for detecting Au(III) ions. In this regard, Mondal et al. synthesized a rhodamine-appended naphthalene-based Schiff base (probe-75) in 2020 (Fig. 111),165 which acts as a sensitive, selective fluorescence turn-on sensor for the detection of Au(III) ions. Probe-75 was prepared by the condensation reaction of rhodamine B hydrazide and 4-hydroxy-1-napthaldehyde followed by treatment with 1-fluoro-2,4-dinitrobenzene in acetonitrile under reflux conditions, which was confirmed by several spectroscopic and analytical techniques. The native state of probe-75 was poorly emissive in nature; on titrating with Au(III) ions, the emission intensity was drastically enhanced and attained saturation after addition of one eq. with a shift in the color of the solution from colorless to orange, while with other competitive metal ions, there was no influence on the fluorescence spectra. Furthermore, probe-75 showed a colorimetric response towards Au(III) ions and the color shifts from colorless to pink color in CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), which could be utilized for real-time monitoring. A further binding mechanism of probe-75 was proposed by the authors and ring opening of spirolactum and suppression of PET process, which was supported by the ESI-MS, FT-IR and DFT calculations. The LOD value of probe-75 with Au(III) was calculated to be 1.51 μM from the fluorescence titration data using the equation 3σ/slope. Considering the ability of probe-75 to act as a selective and sensitive fluorescent turn-on probe, in vivo cell imaging was achieved by inoculating probe-75 in MC3T3 cells with very low cytotoxicity (determined by the MTT assay) (Fig. 112).
1, v/v), which could be utilized for real-time monitoring. A further binding mechanism of probe-75 was proposed by the authors and ring opening of spirolactum and suppression of PET process, which was supported by the ESI-MS, FT-IR and DFT calculations. The LOD value of probe-75 with Au(III) was calculated to be 1.51 μM from the fluorescence titration data using the equation 3σ/slope. Considering the ability of probe-75 to act as a selective and sensitive fluorescent turn-on probe, in vivo cell imaging was achieved by inoculating probe-75 in MC3T3 cells with very low cytotoxicity (determined by the MTT assay) (Fig. 112).
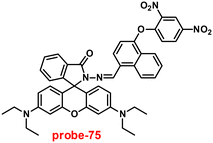 |
| | Fig. 111 Chemical structure of probe-75 (redrawn the ChemDraw structure from ref. 165). | |
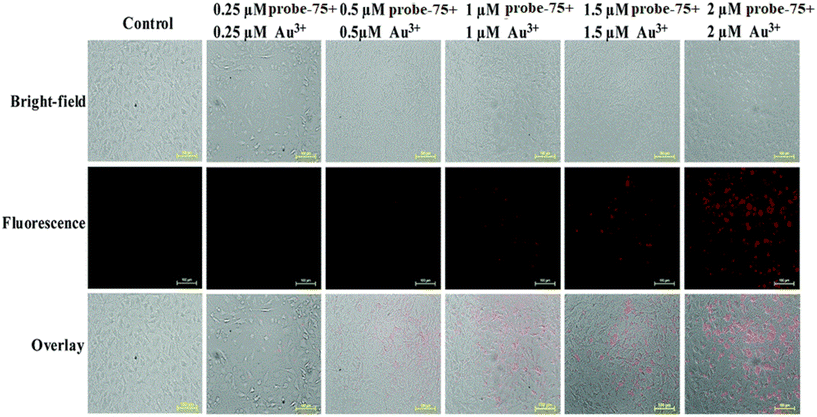 |
| | Fig. 112 Fluorescence microscopic imaging of MC3T3 cells with probe-75 and Au(III) ions. (reprinted with permission from ref. 165, Copyright 2020 Royal Society of Chemistry). | |
In order to develop a selective fluorescent probe for the detection of Au(III) ions, BODIPY dye was chosen as a fluorophore core considering its high photo stability and quantum yield, which results in intense absorption and emission bands. In 2016, the authors focused on the synthesis of BODIPY-appended 2-hydrazinopyrazine (probe-76a) and 2-acetylpyrazine group (probe-76b) for the selective recognition of Au(III) ions.166 First, probe-76 was used to test the sensing studies with Au(III) at different pH values and the results indicated distinguishable photophysical properties at pH 7. The probes showed poor emission, and upon addition of Au(III) ions, it showed enhanced fluorescence intensity, whereas other competitive metal ions do not show any noticeable changes in the fluorescence spectra. In the case of absorption spectra, color changes were observed from purple to light orange on adding Au(III) ions to probe-76. Furthermore, probe-76b showed potentiality towards Au(III) ions under strong acidic conditions and slow response time in comparison to probe-76a, which could be explained by the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond's sensitivity to acidic pH, while probe-76b with a greater number of C
N bond's sensitivity to acidic pH, while probe-76b with a greater number of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds exhibited lower extent of hydrolysis by Au(III) than that of probe-76a (Fig. 113). The 1H-NMR data also suggested the formation of aldehyde groups by hydrolysis of the C
N bonds exhibited lower extent of hydrolysis by Au(III) than that of probe-76a (Fig. 113). The 1H-NMR data also suggested the formation of aldehyde groups by hydrolysis of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group in the presence of Au(III) ions. Furthermore, the authors demonstrated the use of probe-76a in PC12 cells and zebrafish (with very low cytotoxicity determined by the MTT assay); in vivo cell imaging was obtained via Au(III) monitoring using laser confocal fluorescence microscopy (Fig. 114).
N group in the presence of Au(III) ions. Furthermore, the authors demonstrated the use of probe-76a in PC12 cells and zebrafish (with very low cytotoxicity determined by the MTT assay); in vivo cell imaging was obtained via Au(III) monitoring using laser confocal fluorescence microscopy (Fig. 114).
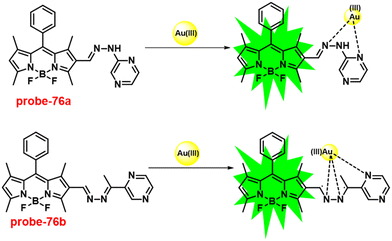 |
| | Fig. 113 Chemical structures and proposed sensing mechanism of probe-76a and probe-76b with Au(III) (redrawn the ChemDraw structure from ref. 166). | |
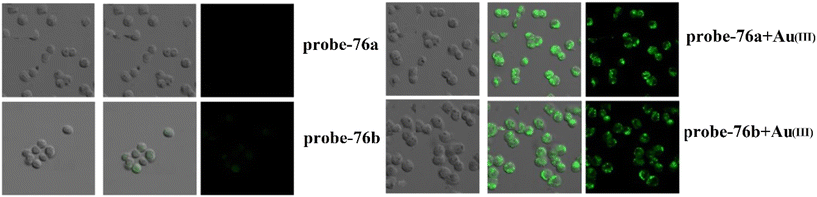 |
| | Fig. 114 Fluorescence microscopic imaging of PC12 cells with probe-76a and probe-76b treated with Au(III) ions. (reprinted with permission from ref. 166, Copyright 2016 Elsevier). | |
Although there are lot of gold ion probes developed so far, most of them were unable to distinguish between Au(III) and Au(I) ions. Initially, Feng et al. designed a two-photon fluorescent probe for the detection of gold ions, which was used for targeting mitochondria, but this probe was found to have similar issues due to coordination sites (N^N). In order to overcome the inability of previously reported probes to distinguish between Au(III) and Au(I), Feng et al. synthesized probe-77 in 2017 (Fig. 115),167 which replaced the coordination site N^N with N^O sites. The target compound was prepared by the reaction of a carbazole-containing aldehyde derivative with benzenesulfonohydrazide in methanol and the structure was supported by 1H and 13C-NMR. Probe-77 showed very weak fluorescence, and upon incorporation of Au(III) ions, a peak was acquired at 547 nm along with 16-fold enhancement of intensity, whereas other competitive metal ions including Au(I) were unable to impart any changes in the fluorescence intensity, which indicate that probe-77 was highly selective towards Au(III) ions. From the absorption spectra, it was noticed that an intense peak at 436 nm was obtained and titration of Au(III) ions with probe-77 resulted in a blue-shift. Additionally, the binding nature of probe-77 with Au(III) ions was supported by MALDI-TOF mass, 1H-NMR and DFT calculations. Further, inoculating probe-77 into zebrafish, in vivo live cell imaging of Au(III) ions was achieved by two-photon microscopy, and very low cytotoxicity of probe-77 was found against MCF-7 and HeLa cells (Fig. 116).
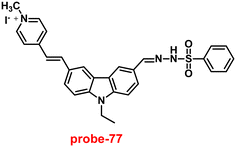 |
| | Fig. 115 Chemical structure of probe-77 (redrawn the ChemDraw structure from ref. 167). | |
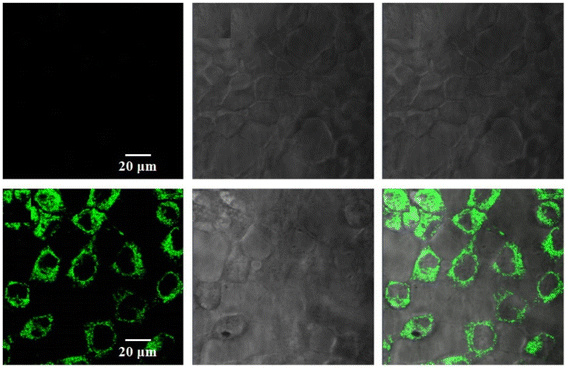 |
| | Fig. 116 Fluorescence microscopic imaging of MCF-7 cells with probe-77 treated with Au(III) ions (reprinted with permission from ref. 167, Copyright 2017 Elsevier). | |
6. Schiff base-based fluorescent turn-on probes for Ag(I) ions
One of the precious metals, silver, is used in a variety of industries, including chemistry, electrical engineering, photography, and pharmaceuticals.168,169 Due to the potentiality of silver, researchers devoted much effort to the synthesis of various probes for selective detection.170 There are several analytical methods such as AAS,171 surface-enhanced Raman scattering,172 ion-selective electrodes,173 voltammetry,174–176 ICP-AES,177 ICP-MS,178 and potentiometry179 available for the identification of silver ions.
In 2018, Kim and co-workers designed and synthesized an octopamine-appended cinnamaldehyde derivative (probe-78)180via a reaction between octopamine and 4-dimethylaminocinnamaldehyde in MeOH under reflux conditions and structurally characterized it by ESI-MS, 1H and 13C-NMR. Fluorescence emission spectra of probe-78 displayed poor emission, and in the presence of Ag(I) ions, the emission intensity was gradually increased, whereas the other competitive metal ions Ga(III), Cd(II), Fe(II), Al(III), In(III), Cu(II), Mg(II), Pd(II), Au(III), Hg(II), Ni(II), Co(II) Fe(II) and Al(III) do not change the fluorescence intensity, which indicate the selective nature of probe-78. Job's plot revealed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation between probe-78 and Ag(I) ions, which was further validated by ESI-MS, 1H NMR and DFT data. The DFT calculation indicated the lowering of band gap of probe-78 upon binding with Ag(I), which ascertained that probe-78 + Ag(I) complex formation causes a bathochromic shift. The sensing mechanism (Fig. 117) of probe-78 with Ag(I) was confirmed by 1H NMR and the binding ratio was estimated to be 1
1 stoichiometric complexation between probe-78 and Ag(I) ions, which was further validated by ESI-MS, 1H NMR and DFT data. The DFT calculation indicated the lowering of band gap of probe-78 upon binding with Ag(I), which ascertained that probe-78 + Ag(I) complex formation causes a bathochromic shift. The sensing mechanism (Fig. 117) of probe-78 with Ag(I) was confirmed by 1H NMR and the binding ratio was estimated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by Job's plot. Further, the authors demonstrated the influence of the pH detecting ability of probe-78 with Ag(I), and at pH 7, it showed higher fluorescence intensity than that at other pH values, which strongly suggested that probe-78 was potential for the detection in physiological media. The LOD value of probe-78 with Ag(I) ions from the fluorescence titration spectra using the equation 3σ/slope was found to be 1.49 μM.
1 by Job's plot. Further, the authors demonstrated the influence of the pH detecting ability of probe-78 with Ag(I), and at pH 7, it showed higher fluorescence intensity than that at other pH values, which strongly suggested that probe-78 was potential for the detection in physiological media. The LOD value of probe-78 with Ag(I) ions from the fluorescence titration spectra using the equation 3σ/slope was found to be 1.49 μM.
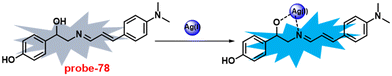 |
| | Fig. 117 Chemical structure and proposed sensing mechanism of probe-78 with Ag(I) (redrawn the ChemDraw structure from ref. 180). | |
In order to develop a fluorescent probe for the selective and sensitive recognition of Ag(I) in an aqueous medium, Nandhakumar et al. synthesized an oxygen-, sulphur- and nitrogen-containing heterocyclic chemosensor, probe-79,181 in 2018 via a condensation reaction between methyl carbazate and 5-nitro-2-thiophenecarboxaldehyde in ethanol–acetic acid, and the structure was established by 1H and 13C-NMR. To establish the sensing ability of probe-79, the emission spectra displayed poor emission, the fluorescence intensity was gradually increased and the emission band shifted to 405 nm on titration with Ag(I) ions. However, probe-79 does not alter the fluorescence spectra when other metal ions are present, which strongly confirms the selectivity. On varying the pH of the solution, it was found that probe-79 effectively detects Ag(I) via showing spectral changes within the pH range of 4–9. Therefore, probe-79 could be applied for the sensing of Ag(I) ions at physiological pH (7.4). Fluorescence titration of probe-79 with Ag(I) in MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), revealed 1
1, v/v), revealed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation, which was confirmed by Job's plot analysis, IR studies and ESI-MS. The proposed mechanism of probe-79 with Ag(I) might be attributed to ICT (Fig. 118). Finally, the authors aimed to detect Ag(I) ions in biological samples, by inoculation of probe-79 in E. coli cells followed by insertion of Ag(I); live cell imaging was obtained by laser confocal scanning microscopy (Fig. 119).
1 stoichiometric complexation, which was confirmed by Job's plot analysis, IR studies and ESI-MS. The proposed mechanism of probe-79 with Ag(I) might be attributed to ICT (Fig. 118). Finally, the authors aimed to detect Ag(I) ions in biological samples, by inoculation of probe-79 in E. coli cells followed by insertion of Ag(I); live cell imaging was obtained by laser confocal scanning microscopy (Fig. 119).
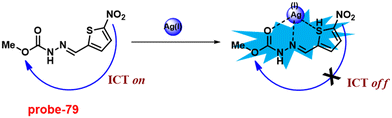 |
| | Fig. 118 Chemical structure and proposed sensing mechanism of probe-79 with Ag(I) (redrawn the ChemDraw structure from ref. 181). | |
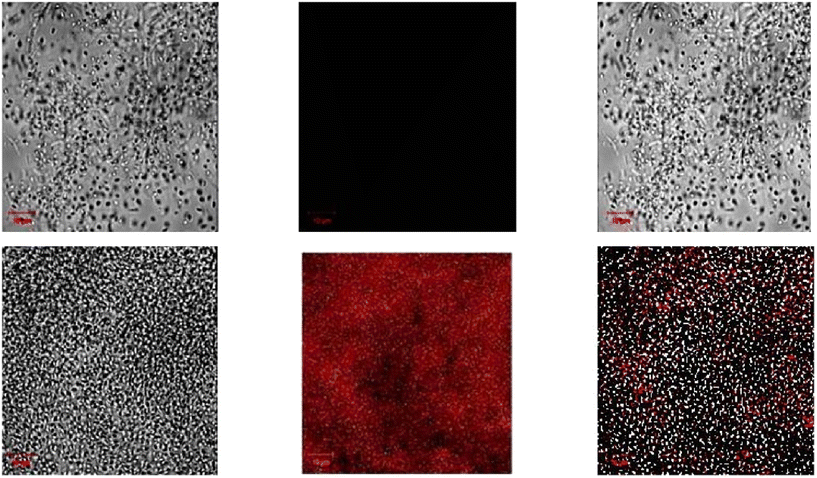 |
| | Fig. 119 Cellular images of probe-79 in E. coli cells by laser confocal scanning microscopy (reprinted with permission from ref. 181, Copyright 2018 Elsevier). | |
Considering the benefits of using the fluorescent probe rather than intensity-based probes to detect heavy metal ions, Nandhakumar et al. developed a quinolone-based Schiff base (probe-80)182 in 2018 for ratiometric fluorescence turn-on for the Ag(I) ions, which can be utilized for the quantification of metal ions. Probe-80 was prepared by the reaction between 2-hydroxyquinoline-3-carbaldehyde and 2-aminophenol in a methanol-triethylamine medium, and the structure was supported by several analytical and spectroscopic techniques. From the fluorescence emission spectra of probe-80 in a MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution, it was found that dual emission bands appeared at 410 and 500 nm with very low intensity, while on titration with Ag(I) ions, the emission was progressively increased and saturated after addition of 1 eq., whereas no considerable change was observed in the fluorescence emission of probe-80 in the presence of other competitive ions, which strongly supported the selectivity of probe. The fluorescence titration data showed the ratiometric response, in which the intensity of the peak at 410 nm increased and the peak at 500 nm decreased simultaneously. Moreover, authors focused on the titration experiments on varying pH, and it was noticed that probe-80 and probe-80 + Ag(I) remained stable in the pH range of 4 to 9, which indicated that probe-80 could be utilised for the detection of Ag(I) under physiological pH (7.4). Fluorescence titration data revealed the 1
1, v/v) solution, it was found that dual emission bands appeared at 410 and 500 nm with very low intensity, while on titration with Ag(I) ions, the emission was progressively increased and saturated after addition of 1 eq., whereas no considerable change was observed in the fluorescence emission of probe-80 in the presence of other competitive ions, which strongly supported the selectivity of probe. The fluorescence titration data showed the ratiometric response, in which the intensity of the peak at 410 nm increased and the peak at 500 nm decreased simultaneously. Moreover, authors focused on the titration experiments on varying pH, and it was noticed that probe-80 and probe-80 + Ag(I) remained stable in the pH range of 4 to 9, which indicated that probe-80 could be utilised for the detection of Ag(I) under physiological pH (7.4). Fluorescence titration data revealed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric complexation between probe-80 and Ag(I), which was also supported by Job's plot and ESI-MS. The authors also proposed the sensing mechanism of probe-80, where the Ag(I) ions coordinated through the lone pair of N-atoms and O-atoms, which leads to enhanced fluorescence as a result of inhibition of PET (Fig. 120). The association and LOD values of probe-80 were found from the fluorescence titration spectra as 2.41 × 104 M−2 and 14 μM respectively. Further, for the practical application, fluorescence cell imaging of probe-80 incubated with E. coli cells was obtained in the presence of Ag(I) ions using a laser scanning microscope as red fluorescence (Fig. 121).
2 stoichiometric complexation between probe-80 and Ag(I), which was also supported by Job's plot and ESI-MS. The authors also proposed the sensing mechanism of probe-80, where the Ag(I) ions coordinated through the lone pair of N-atoms and O-atoms, which leads to enhanced fluorescence as a result of inhibition of PET (Fig. 120). The association and LOD values of probe-80 were found from the fluorescence titration spectra as 2.41 × 104 M−2 and 14 μM respectively. Further, for the practical application, fluorescence cell imaging of probe-80 incubated with E. coli cells was obtained in the presence of Ag(I) ions using a laser scanning microscope as red fluorescence (Fig. 121).
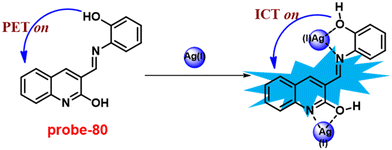 |
| | Fig. 120 Chemical structure and proposed sensing mechanism of probe-80 with Ag(I) ions (redrawn the ChemDraw structure from ref. 182). | |
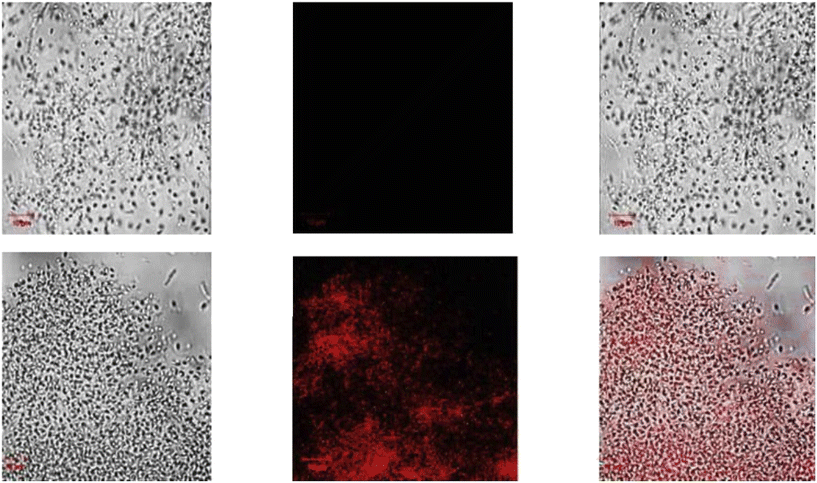 |
| | Fig. 121 Laser confocal scanning microscopic images of probe-80 in E. coli cells in the presence of Ag+ ions. (reprinted with permission from ref. 182, Copyright 2018 Elsevier). | |
In 2020, a fluorometric turn-on probe for the selective recognition of Ag(I) ions was synthesized by Patra and colleagues and characterized by XRD analysis, multinuclear NMR, ESI-MS, UV-vis spectra, and EA.183 The native state of probe-81 exhibited poor emission; on adding Ag(I) ions, the emission intensity was drastically enhanced, whereas with other competitive metal ions, there is no influence on the fluorescence spectra, which strongly suggested that probe-81 showed very selective fluorescence turn-on towards Ag(I) ions (Fig. 122). Further, probe-81 displayed a chromogenic response towards Cu(II) ions. The absorption titration data, Job's plot analysis, and ESI-MS analysis supported the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between probe-81 and Ag(I) ions. The colorimetric detection of probe-81 with Cu(II) and Ag(I) ions was estimated to be 1.7 and 2.2 μM, respectively, and the Ag(I) fluorometric detection limit was up to 1.6 μM. Probe-81, which works over a wide pH range, can be used to measure and identify the ions Cu(II) and Ag(I) in environmental samples as well as water samples.
1 binding between probe-81 and Ag(I) ions. The colorimetric detection of probe-81 with Cu(II) and Ag(I) ions was estimated to be 1.7 and 2.2 μM, respectively, and the Ag(I) fluorometric detection limit was up to 1.6 μM. Probe-81, which works over a wide pH range, can be used to measure and identify the ions Cu(II) and Ag(I) in environmental samples as well as water samples.
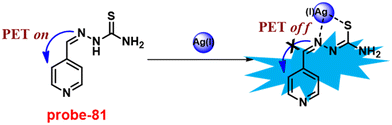 |
| | Fig. 122 Chemical structure and proposed sensing mechanism of probe-81 with Ag(I) (redrawn the ChemDraw structure from ref. 183). | |
7. Schiff base-based fluorescent turn-on probes for Cu(II) ions
Transition metal ions play crucial roles in biological systems, in which Cu(II) ions receive particular attention because they are used in numerous essential physiological functions in living things.184–190 However, excessive copper levels that accumulate in organelles can cause Menkes disease,191 Wilson's disease,192 familial amyotrophic lateral sclerosis, Alzheimer's disease, Parkinson's disease, and prion disorders.193,194 For the real-time response tool to analyze the level of Cu(II) ions for in vitro and in vivo research, a vital probe is therefore needed.
In 2018, Xu et al. designed and synthesized coumarin-based monocarbazone Schiff bases for selective fluorescent turn-on probes for Cu(II) ions.195 The target probe-82 was synthesized by mixing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 proportion of 3H-benzo[h] chromene-3-carbaldehyde and carbazide in ethyl alcohol, and the reaction mixture was stirred under reflux conditions to give a final product in good yields and was confirmed by various analytical techniques. Probe-82 displayed very good aggregate induce ratiometric emission in methanol and water mixtures. The sensing studies were carried out in MeOH and water mixtures, where probe-82 showed very poor emission (ϕ = 0.061) with a peak at 450 nm, and upon addition of Cu(II) ion, a very bright emission was generated at 511 nm with enhanced quantum yields (ϕ = 0.25), simultaneously decreasing the emission intensity at 450 nm. To find out better selectivity and sensitivity of probe-82, it was tested with several interfering metal ions and no difference in fluorescence spectra was observed. The binding mechanism of Cu(II) ions and probe-82 was examined and found to be ICT combined with metal-induced association (Fig. 123). The detection limit of probe-82 with Cu(II) was determined to be 10.4 nM using the formula 3σ/slope. Moreover, for the practical application, bio-imaging of probe-82 was carried out and the results suggested that probe-82 successfully detects Cu(II) ions in biological media, which was confirmed by fluorescence microscopy (Fig. 124).
1 proportion of 3H-benzo[h] chromene-3-carbaldehyde and carbazide in ethyl alcohol, and the reaction mixture was stirred under reflux conditions to give a final product in good yields and was confirmed by various analytical techniques. Probe-82 displayed very good aggregate induce ratiometric emission in methanol and water mixtures. The sensing studies were carried out in MeOH and water mixtures, where probe-82 showed very poor emission (ϕ = 0.061) with a peak at 450 nm, and upon addition of Cu(II) ion, a very bright emission was generated at 511 nm with enhanced quantum yields (ϕ = 0.25), simultaneously decreasing the emission intensity at 450 nm. To find out better selectivity and sensitivity of probe-82, it was tested with several interfering metal ions and no difference in fluorescence spectra was observed. The binding mechanism of Cu(II) ions and probe-82 was examined and found to be ICT combined with metal-induced association (Fig. 123). The detection limit of probe-82 with Cu(II) was determined to be 10.4 nM using the formula 3σ/slope. Moreover, for the practical application, bio-imaging of probe-82 was carried out and the results suggested that probe-82 successfully detects Cu(II) ions in biological media, which was confirmed by fluorescence microscopy (Fig. 124).
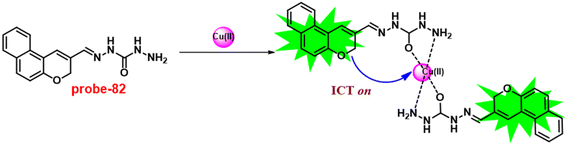 |
| | Fig. 123 Chemical structure and proposed sensing mechanism of probe-82 with Cu(II) (redrawn the ChemDraw structure from ref. 195). | |
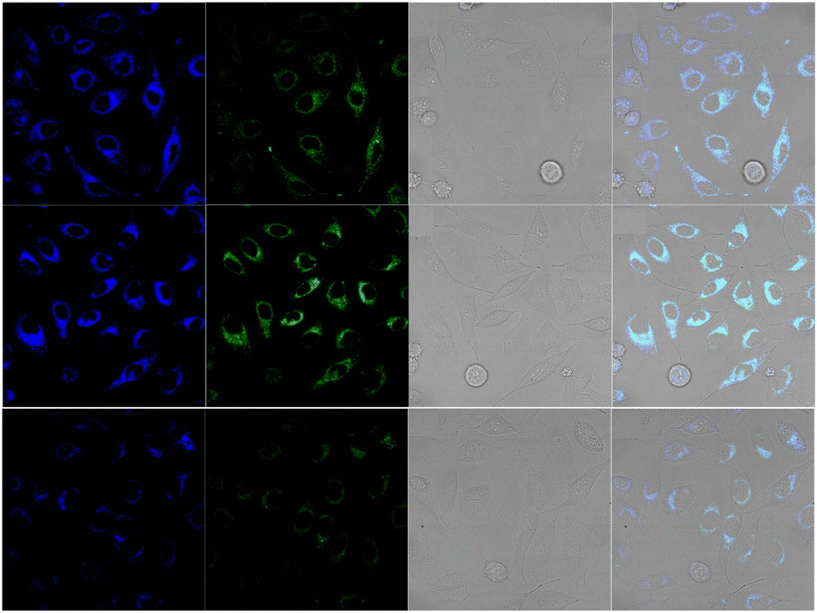 |
| | Fig. 124 Bio-imaging of probe-82 on HeLa cells by laser confocal scanning microscopy (reprinted with permission from ref. 195, Copyright 2018 Elsevier). | |
In 2019, Wang et al. focused on the synthesis of a Congo red-conjugated Schiff base derivative and characterized it using several analytical techniques.196Probe-83 was exhibited to be very selective and sensitive towards Cu(II) ions via colorimetric and fluorescence turn-on responses, which was based on the CHEF mechanisms in water and ethanol. Probe-83 (Fig. 125) displayed a change in color from brown to colorless upon addition of Cu(II) ions, which was confirmed by changes in UV-vis absorption spectra. However, in the presence of other competitive metal ions, no response was observed in either fluorescence turn-on or colorimetry, which indicates that probe-83 was very selective towards Cu(II) ions. The binding stoichiometry was obtained as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and confirmed by Job's plot and ESI-MS. Probe-83 exhibited very good detection limit as well as association constants of 0.1 nM and 1.03 × 106 M−1 correspondingly. As noted, detection limit values are much lower than that of WHO approved. The reversibility of probe-83 was observed upon addition of a sodium sulfide solution to probe-83 + Cu(II). Further, cellular imaging of probe-83 was performed on HepG2 cells, and it was found to have negligible cytotoxicity with very high cell permeability. The HepG2 cells were incubated with probe-83 for 30 min, which did not show visible fluorescence, whereas upon treatment with Cu(II) ions, strong fluorescence images were observed using a fluorescence microscope (Fig. 126). Thus, probe-83 found to be a potential candidate for the recognition of Cu(II) ions in biological media.
1 and confirmed by Job's plot and ESI-MS. Probe-83 exhibited very good detection limit as well as association constants of 0.1 nM and 1.03 × 106 M−1 correspondingly. As noted, detection limit values are much lower than that of WHO approved. The reversibility of probe-83 was observed upon addition of a sodium sulfide solution to probe-83 + Cu(II). Further, cellular imaging of probe-83 was performed on HepG2 cells, and it was found to have negligible cytotoxicity with very high cell permeability. The HepG2 cells were incubated with probe-83 for 30 min, which did not show visible fluorescence, whereas upon treatment with Cu(II) ions, strong fluorescence images were observed using a fluorescence microscope (Fig. 126). Thus, probe-83 found to be a potential candidate for the recognition of Cu(II) ions in biological media.
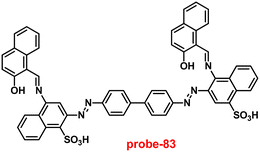 |
| | Fig. 125 Chemical structure of probe-83 (redrawn the ChemDraw structure from ref. 196). | |
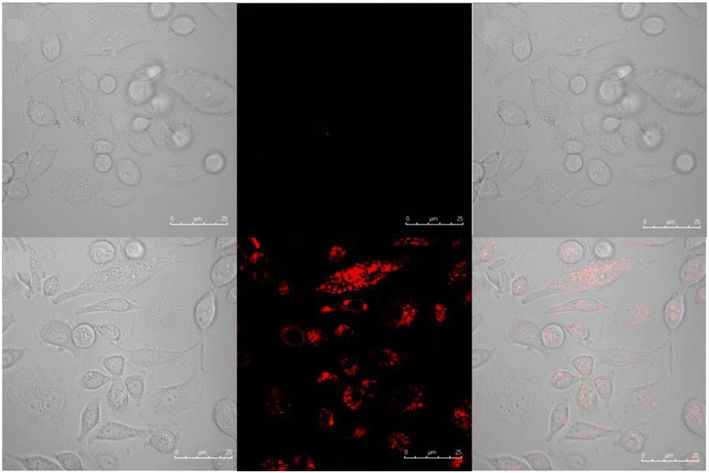 |
| | Fig. 126 Fluorescence microscopic images of HepG2 cells treated with probe-83 and in the presence of cu(II) ions (reprinted with permission from ref. 196, Copyright 2019 Elsevier). | |
A novel bisthiophene-conjugated Schiff base was designed and synthesised via a simple condensation reaction of diamino malononitrile and 2-formyl thiophene in ethanol, and probe-84 was structurally characterized via analytical techniques by Kumar and co-workers in 2019.197 The sensing studies of probe-84 were conducted in MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (8
H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) media, which exhibited a chromogenic behavior towards Cu(II) ions, and the color changed from yellow to colorless, even though in the presence of other competitive metal ions, it does not show any changes in absorption spectra. The fluorescence studies of probe-84 displayed fluorescence enhancement upon incremental addition of Cu(II) ions, which was explained by the CHEF mechanism (Fig. 127) and no change in emission intensity with other metal ions. The LOD value was calculated via fluorescence spectra as 14.5 nM, which was much lower than that of WHO guidelines (0.02 μM) in drinking water. The stoichiometry of probe-84 with Cu(II) ions was determined by Job's plot as 1
2, v/v) media, which exhibited a chromogenic behavior towards Cu(II) ions, and the color changed from yellow to colorless, even though in the presence of other competitive metal ions, it does not show any changes in absorption spectra. The fluorescence studies of probe-84 displayed fluorescence enhancement upon incremental addition of Cu(II) ions, which was explained by the CHEF mechanism (Fig. 127) and no change in emission intensity with other metal ions. The LOD value was calculated via fluorescence spectra as 14.5 nM, which was much lower than that of WHO guidelines (0.02 μM) in drinking water. The stoichiometry of probe-84 with Cu(II) ions was determined by Job's plot as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The association constant was assessed by the B–H method as 7.31 × 104 M−1. The nature of binding mode of probe-84 with Cu(II) ions was strongly supported by FTIR, NMR, ESI-mass, as well as DFT data.
1. The association constant was assessed by the B–H method as 7.31 × 104 M−1. The nature of binding mode of probe-84 with Cu(II) ions was strongly supported by FTIR, NMR, ESI-mass, as well as DFT data.
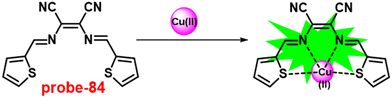 |
| | Fig. 127 Chemical structure and proposed sensing mechanism of probe-84 with Cu(II) (redrawn the ChemDraw structure from ref. 63). | |
Mohan and co-workers designed and synthesized a very simple and efficient fluorescent chemosensor for Cu(II) ions via pyrene-conjugated Schiff base derivatives in 2018.198 The target probe-85 was synthesized upon a reaction between 1-pyrenecarboxaldehyde and 3-hydroxy-2-napthoic hydrazide. The sensing studies of probe-85 (Fig. 128) were conducted in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, towards several metal ions by fluorescence spectroscopic methods. There was a gradual increase in fluorescence intensity at 450 nm on addition of Cu(II) ions to probe-85 due to the absence of PET process, whereas in the presence of above-mentioned cations, no changes were observed in the emission spectra. The results strongly suggested that probe-85 showed selective response to Cu(II) ions with other interfering metal ions. The sensing studies were conducted in a wide range of pH (5–9), which does not affect the sensing ability of probe-85 with Cu(II) ions. The binding constant and LOD values were found to be 1.16 × 104 M−1 and 0.26 μM respectively. The reversibility studies of probe-85 were performed using EDTA, which showed the emission quenching on titration with EDTA to probe-85 + Cu(II). The cellular imaging experiments of probe-85 were conducted on living RAW 264.7 cells in the presence of Cu(II) ions by fluorescence microscopy. Probe-85 was incubated with cells, which showed insignificant fluorescence emission, whereas upon treatment with Cu(II) ions, it produced a very strong fluorescence emission under GFP (Fig. 129). Thus, probe-85 was demonstrated as a potential candidate for the recognition of Cu(II) ions in biological media.
H2O, towards several metal ions by fluorescence spectroscopic methods. There was a gradual increase in fluorescence intensity at 450 nm on addition of Cu(II) ions to probe-85 due to the absence of PET process, whereas in the presence of above-mentioned cations, no changes were observed in the emission spectra. The results strongly suggested that probe-85 showed selective response to Cu(II) ions with other interfering metal ions. The sensing studies were conducted in a wide range of pH (5–9), which does not affect the sensing ability of probe-85 with Cu(II) ions. The binding constant and LOD values were found to be 1.16 × 104 M−1 and 0.26 μM respectively. The reversibility studies of probe-85 were performed using EDTA, which showed the emission quenching on titration with EDTA to probe-85 + Cu(II). The cellular imaging experiments of probe-85 were conducted on living RAW 264.7 cells in the presence of Cu(II) ions by fluorescence microscopy. Probe-85 was incubated with cells, which showed insignificant fluorescence emission, whereas upon treatment with Cu(II) ions, it produced a very strong fluorescence emission under GFP (Fig. 129). Thus, probe-85 was demonstrated as a potential candidate for the recognition of Cu(II) ions in biological media.
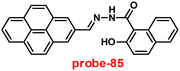 |
| | Fig. 128 Chemical structure of probe-85 (redrawn the ChemDraw structure from ref. 198). | |
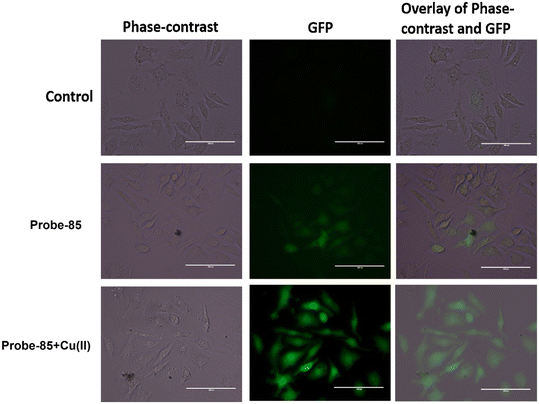 |
| | Fig. 129 Fluorescence microscopic images of RAW 264.7 cells treated with probe-85 and in the presence of Cu(II) ions (reprinted with permission from ref. 198, Copyright 2018 Elsevier). | |
In 2017, a 1,8-naphthalimide hydrazine-conjugated pyrrole-based Schiff base, probe-86, was synthesised by Wang et al. via a simple condensation reaction, which exhibited very selective ratiometric fluorescence response towards Cu(II) ions in acetonitrile and water mixtures.199 The absorption studies of probe-86 (Fig. 130) changed color from colorless to orange color on adding Cu(II) ions and resulted in drastic changes in absorption bands and no response for other metal ions. The binding mode of probe-86 was determined by ESI-MS, IR, single-crystal XRD and DFT studies. The proposed sensing mechanism of probe-86 was identified via a hydrolysis manner, which is a vigorous approach to design potential candidates for efficient ratiometric fluorescent sensors for Cu(II) ions. The LOD value of probe-86 was estimated to be 1.50 μM from the linear response range covering 3–10 μM. Finally, the authors established the efficacy of probe-86 for the recognition of Cu(II) ions in biological media. The outcomes disclosed a strong emission in the presence of Cu(II) ions, which was observed by fluorescence microscopy (Fig. 131).
 |
| | Fig. 130 Chemical structure of probe-86 (redrawn the ChemDraw structure from ref. 199). | |
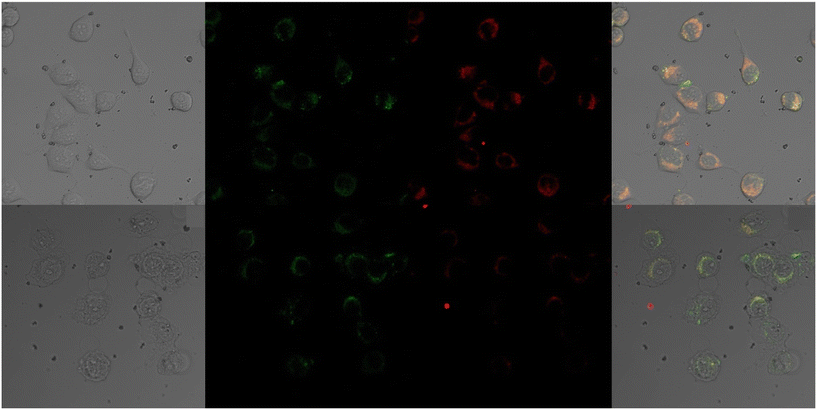 |
| | Fig. 131 Fluorescence microscopic images of HeLa cells treated with probe-86 and in the presence of Cu(II) ions (reprinted with permission from ref. 199, Copyright 2017 Elsevier). | |
Kong and co-workers synthesized a coumarin-naphthol-based Schiff base unit for selective fluorescence turn-on for sensing Cu(II) ions in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 proportion of DMSO and HEPES buffer. The target compound probe-87 (ref. 200) was synthesized upon treatment of 7-diethylaminocoumarin-3-aldehyde with 3-amino-2-naphthol in EtOH, and the reaction was refluxed for 6 h. The sensing studies of probe-87 obtained better sensitivity and selectivity towards particular metal ions. Upon incremental addition of Cu(II) ions to probe-87, the absorption band at 345 nm progressively falls, whereas the adsorption band at 452 nm increased regularly. However, fluorescence titration data revealed that in the presence of Cu(II) ions, the emission peak at 520 nm gradually increased and reached maximum at 0.5 eq. with 60-fold enhancement, which detected that Cu(II) ions encouraged the decomposition of imines to discharge the fluorescence compound 7-diethylaminocoumarin-3-aldehyde (Fig. 132). The LOD value of probe-87 was estimated to be 0.82 μg L−1 (12.7 nM) using the equation 3σ/slope, which is much lesser as recommended by WHO (2.0 mg L−1) in drinking water. Job's plot revealed that the stoichiometry of probe-87 with Cu(II) was 1
99 proportion of DMSO and HEPES buffer. The target compound probe-87 (ref. 200) was synthesized upon treatment of 7-diethylaminocoumarin-3-aldehyde with 3-amino-2-naphthol in EtOH, and the reaction was refluxed for 6 h. The sensing studies of probe-87 obtained better sensitivity and selectivity towards particular metal ions. Upon incremental addition of Cu(II) ions to probe-87, the absorption band at 345 nm progressively falls, whereas the adsorption band at 452 nm increased regularly. However, fluorescence titration data revealed that in the presence of Cu(II) ions, the emission peak at 520 nm gradually increased and reached maximum at 0.5 eq. with 60-fold enhancement, which detected that Cu(II) ions encouraged the decomposition of imines to discharge the fluorescence compound 7-diethylaminocoumarin-3-aldehyde (Fig. 132). The LOD value of probe-87 was estimated to be 0.82 μg L−1 (12.7 nM) using the equation 3σ/slope, which is much lesser as recommended by WHO (2.0 mg L−1) in drinking water. Job's plot revealed that the stoichiometry of probe-87 with Cu(II) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and the binding constant was estimated to be 1.74 × 105 M−1. The binding mechanism was analysed from the 1H NMR studies. The low cytotoxicity of probe-87 was confirmed by the MTT assay and could be applied as the potential candidate for the recognition of Cu(II) ions in biological media, which was confirmed by fluorescence microscopy (Fig. 133). Thus, probe-87 will work as an excellent material in the field of biomedical research and bioimaging of Cu(II)-related syndromes.
2 and the binding constant was estimated to be 1.74 × 105 M−1. The binding mechanism was analysed from the 1H NMR studies. The low cytotoxicity of probe-87 was confirmed by the MTT assay and could be applied as the potential candidate for the recognition of Cu(II) ions in biological media, which was confirmed by fluorescence microscopy (Fig. 133). Thus, probe-87 will work as an excellent material in the field of biomedical research and bioimaging of Cu(II)-related syndromes.
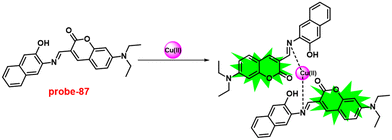 |
| | Fig. 132 Chemical structure and proposed sensing mechanism of probe-87 with Cu(II) (redrawn the ChemDraw structure from ref. 200). | |
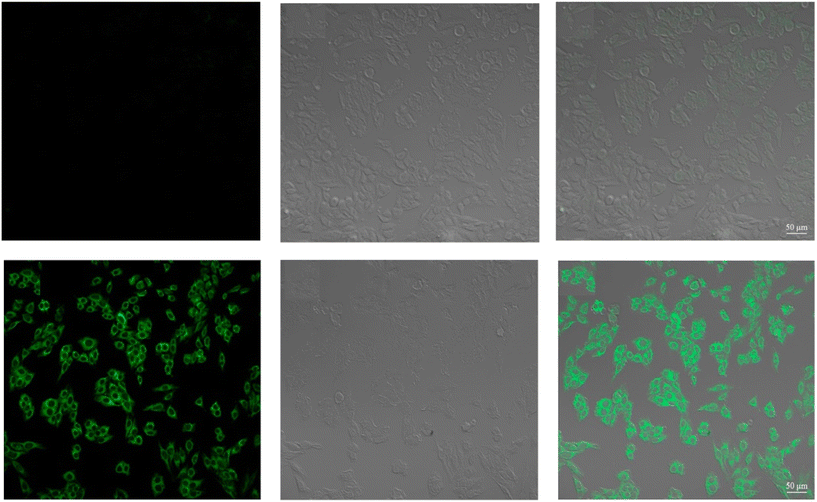 |
| | Fig. 133 Fluorescence microscopic images of HepG2 cells treated with probe-87 and in the presence of Cu(II) ions (reprinted with permission from ref. 200, Copyright 2017 Elsevier). | |
A photochromic diarylethene-conjugated Schiff base entity was synthesised by Pu et al. in 2018.201Probe-88 was extensively studied for the photochromic properties and fluorescence sensing of various metal ions, acids and bases in ACN. Upon addition of Cu(II) ions to probe-88, the fluorescence band gradually enhanced with 90-fold intensity and shifted to a blue region for 56 nm, and emission color alterations from dark red to brick red, due to the restriction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and enhancement of the CHEF process (Fig. 134). However, no change was perceived with excess amounts of other interfering metal ions. As a consequence, this probe-88 was a very highly selective fluorescent chemosensor for the recognition of Cu(II) ions. Job's plot of probe-88 exhibited maximum absorption at 0.3 of molar fraction, and the ratio of probe-88-Cu(II) was found to be 2
N isomerisation and enhancement of the CHEF process (Fig. 134). However, no change was perceived with excess amounts of other interfering metal ions. As a consequence, this probe-88 was a very highly selective fluorescent chemosensor for the recognition of Cu(II) ions. Job's plot of probe-88 exhibited maximum absorption at 0.3 of molar fraction, and the ratio of probe-88-Cu(II) was found to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The binding constant was calculated as 4 × 104 M−1 from the B–H equation. The LOD value of probe-88 was measured as 1.49 μM using the equation 3σ/slope. Further, for practical applications, probe-88 was utilised for the sensing of Cu(II) in drinking water with high precision.
1. The binding constant was calculated as 4 × 104 M−1 from the B–H equation. The LOD value of probe-88 was measured as 1.49 μM using the equation 3σ/slope. Further, for practical applications, probe-88 was utilised for the sensing of Cu(II) in drinking water with high precision.
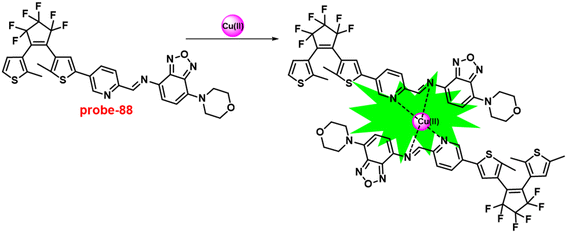 |
| | Fig. 134 Chemical structure and proposed sensing mechanism of probe-88 with Cu(II) (redrawn the ChemDraw structure from ref. 201). | |
In 2018, Wang et al. designed and synthesized a new carbazole-based Schiff base moiety for the ultrasensitive recognition of Cu(II) ions in CH3CN.202 The sensing studies of probe-89 were performed in acetonitrile as a solvent using the UV-vis and fluorescence spectroscopic studies. In the UV-vis spectra, upon addition of Cu(II) to probe-89, the absorption band at 400 nm completely diminished and the color changed from yellow to colorless. The emission studies revealed that the fluorescence enhanced up to 160-fold with 10 eq. Cu(II) ions added to probe-89 over other competitive metal ions due to the ICT process (Fig. 135). The LOD value was obtained as 27.4 nM from the equation LOD = 3σ/slope, which is a much lower value as recommended by the US-EPA. The association constant was determined to be 1.26 × 10−6 M−1 for probe-89 and Cu(II) ions through the B–H plot. Job's plot determined a stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for probe-89 and Cu(II) ions. Due to the very fast detection ability, probe-89 has potential applications in biomedical and environmental monitoring applications.
1 for probe-89 and Cu(II) ions. Due to the very fast detection ability, probe-89 has potential applications in biomedical and environmental monitoring applications.
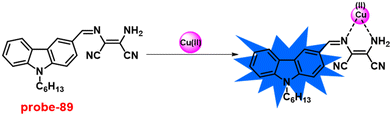 |
| | Fig. 135 Chemical structure and proposed sensing mechanism of probe-89 with Cu(II) (redrawn the ChemDraw structure from ref. 202). | |
In 2018, Wang and co-workers focused on the synthesis of a benzoimidazole-based Schiff base for selective and sensitive fluorescent turn-on probes for Cu(II) ions in CH3CN-Tris solutions.203 The UV-vis and fluorescence analysis confirmed that with Cu(II) ions, probe-90 showed fluorescence enrichment by about 50-fold, due to the suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation (Fig. 136) and ratiometric absorption in aqueous solutions over a varied pH array in the presence of other interfering metal ions with a very low detection of limit of 0.49 μM. Job's plot established the binding ratios of probe-90 with Cu(II) as 1
N isomerisation (Fig. 136) and ratiometric absorption in aqueous solutions over a varied pH array in the presence of other interfering metal ions with a very low detection of limit of 0.49 μM. Job's plot established the binding ratios of probe-90 with Cu(II) as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, as supported by ESI-MS and DFT calculations. Further, the binding value was calculated from the B–H plot to be 2.5 × 108 M−2, which revealed that probe-90 had great attraction for Cu(II) ions. Further, for the practical applications of probe-90, cellular imaging investigations were carried out for the detection of Cu(II) ions in biological media. Thus, authors choose HepG2 cell lines, in which they found no fluorescence in the absence of Cu(II) ions, but a bright green colour emission in the presence of Cu(II). This results suggested that probe-90 have good permeability into cells for the detection of Cu(II) ions (Fig. 137).
2, as supported by ESI-MS and DFT calculations. Further, the binding value was calculated from the B–H plot to be 2.5 × 108 M−2, which revealed that probe-90 had great attraction for Cu(II) ions. Further, for the practical applications of probe-90, cellular imaging investigations were carried out for the detection of Cu(II) ions in biological media. Thus, authors choose HepG2 cell lines, in which they found no fluorescence in the absence of Cu(II) ions, but a bright green colour emission in the presence of Cu(II). This results suggested that probe-90 have good permeability into cells for the detection of Cu(II) ions (Fig. 137).
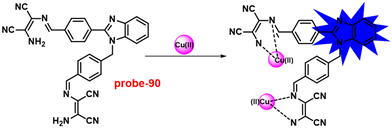 |
| | Fig. 136 Chemical structure and proposed sensing mechanism of probe-90 with Cu(II) (redrawn the ChemDraw structure from ref. 203). | |
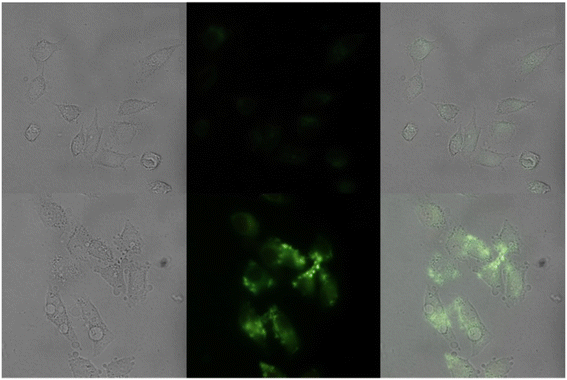 |
| | Fig. 137 Fluorescence microscopic images of HepG2 cells treated with probe-90 and in the presence of Cu(II) ions (reprinted with permission from ref. 203, Copyright 2018 Elsevier). | |
In 2018, Wu and coworkers aimed to synthesise a dimethylamino-imidazole conjugate-based Schiff base for the selective fluorescence turn-on detection of Cu(II) ions in acetonitrile/water (v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in a Tris-HCl solution at pH 7.4.204 The sensing studies of probe-91 demonstrated colorimetric response, color changes from colorless to yellow colour and fluorescence turn-on response towards Cu(II) ions over other competitive metal ions, due to the suppression of PET (Fig. 138). The LOD values of probe-91 were calculated from the absorption and fluorescence spectra to be 0.46 μM and 15 nM, which were much lesser as approved by WHO for drinking water (31.5 μM). Job's plot data provided strong information regarding the binding stoichiometry such as 1
2) in a Tris-HCl solution at pH 7.4.204 The sensing studies of probe-91 demonstrated colorimetric response, color changes from colorless to yellow colour and fluorescence turn-on response towards Cu(II) ions over other competitive metal ions, due to the suppression of PET (Fig. 138). The LOD values of probe-91 were calculated from the absorption and fluorescence spectra to be 0.46 μM and 15 nM, which were much lesser as approved by WHO for drinking water (31.5 μM). Job's plot data provided strong information regarding the binding stoichiometry such as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the association constant was found to be 4.3 × 107 M−1. The reversibility experiment was performed using S2− as the external source for the regeneration of probe-91, and the results indicated that probe-91 has good reversible properties. Further, the mode of binding was supported by 1H NMR, ESI-MS and DFT calculations.
1 and the association constant was found to be 4.3 × 107 M−1. The reversibility experiment was performed using S2− as the external source for the regeneration of probe-91, and the results indicated that probe-91 has good reversible properties. Further, the mode of binding was supported by 1H NMR, ESI-MS and DFT calculations.
 |
| | Fig. 138 Chemical structure and proposed sensing mechanism of probe-91 with Cu(II) (redrawn the ChemDraw structure from ref. 204). | |
In 2019, a novel fluorene-conjugated Schiff base moiety was designed, synthesized and structurally characterised using several analytical techniques.205Probe-92 was found to have very high selectivity towards Cu(II) ions with a very low LOD of 1.54 nM over other interfering metal ions. The absorption titration of probe-92 towards Cu(II) ions displayed colorimetric response and color shifts from pale yellow to colorless, which gave visual detection. Upon addition of Cu(II) ions, probe-92 showed fluorescence turn-on ascribed to the suppression of the isomerisation of imines and blocking of the ICT quenching process (Fig. 139) and no change was observed towards other metal ions. The stoichiometry ratio was determined via Job's plot as well as ESI-MS and displayed to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Authors successfully demonstrated the practical applicability of probe-92 by the recognition of Cu(II) ions in real samples of copper in electroplating wastewater. Additionally, probe-92 was displayed as a very good chromogenic and fluorescent turn-on probe for CN− with a very low LOD of 0.18 μM.
1. Authors successfully demonstrated the practical applicability of probe-92 by the recognition of Cu(II) ions in real samples of copper in electroplating wastewater. Additionally, probe-92 was displayed as a very good chromogenic and fluorescent turn-on probe for CN− with a very low LOD of 0.18 μM.
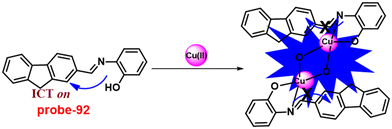 |
| | Fig. 139 Chemical structure and proposed sensing mechanism of probe-92 with Cu(II) (redrawn the ChemDraw structure from ref. 205). | |
In 2019, Amine et al. aimed at the synthesis of an azo group-conjugated Schiff base scaffold for sensitive and selective fluorescence turn-on for Cu(II) ions in methanol and H2O mixtures (7/3, v/v). Probe-93 was obtained via a simple condensation reaction between 2-hydroxy-5-(p-tolyldiazenyl)benzaldehyde and N-(3-aminopropyl)imidazole in ethanol under reflux conditions.206 The sensing studies of probe-93 were conducted and enhanced emission was observed upon addition of Cu(II) ions (Fig. 141) without any interference with other metal ions, which is due to the collective consequence of the CHEF and suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 140). Thus, the results indicate very high selectivity towards Cu(II) ions. Job's plot gave strong information regarding the stoichiometric ratio of probe-93 with Cu(II) ions, which was found to be 2
N isomerization (Fig. 140). Thus, the results indicate very high selectivity towards Cu(II) ions. Job's plot gave strong information regarding the stoichiometric ratio of probe-93 with Cu(II) ions, which was found to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as confirmed by ESI-MS and 1H NMR. Finally, the LOD value was estimated to be 1.8 μM that is much lesser than the WHO endorsement level (20 μM) for drinking water.
1, as confirmed by ESI-MS and 1H NMR. Finally, the LOD value was estimated to be 1.8 μM that is much lesser than the WHO endorsement level (20 μM) for drinking water.
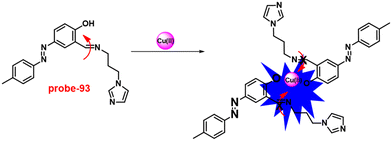 |
| | Fig. 140 Chemical structure and proposed sensing mechanism of probe-93 with Cu(II) (redrawn the ChemDraw structure from ref. 206). | |
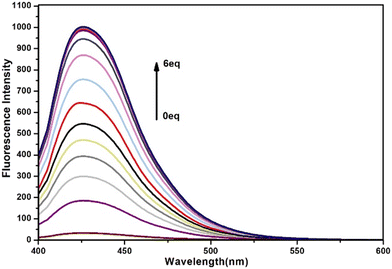 |
| | Fig. 141 Change in the emission spectra of probe-93 on increasing the concentration of Cu(II) ions (left) and with different metal ions (right) (reprinted with permission from ref. 206, Copyright 2019 Elsevier). | |
Xie et al. designed and synthesized a triphenylamine-based Schiff base conjugate for the selective fluorescence turn-on detection of Cu(II) ions in 2017.207Probe-94 was synthesised via a simple condensation reaction between triphenylamine aldehyde and 2-aminophenol in EtOH under reflux conditions. The emission of probe-94 was very poor, whereas upon addition of Cu(II) ions to it a very bright emission at 450 nm was observed in acetonitrile/water, which results in the suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation (Fig. 142). Further, the presence of excess amounts of different metal ions does not show any variation in the fluorescence spectra, which indicate that probe-94 was very selective towards Cu(II) ions. The association constant of the complex in CH3CN and CH3CN/H2O (v/v = 4/6, pH = 7.4) HEPES buffer was calculated from the B–H plot as 1.96 × 107 M−1 and 5.43 × 107 M−1 respectively. Job's plot revealed that the binding ratio of probe-94 and Cu(II) ions was found to be 1
N isomerisation (Fig. 142). Further, the presence of excess amounts of different metal ions does not show any variation in the fluorescence spectra, which indicate that probe-94 was very selective towards Cu(II) ions. The association constant of the complex in CH3CN and CH3CN/H2O (v/v = 4/6, pH = 7.4) HEPES buffer was calculated from the B–H plot as 1.96 × 107 M−1 and 5.43 × 107 M−1 respectively. Job's plot revealed that the binding ratio of probe-94 and Cu(II) ions was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and further confirmed by 1H NMR and ESI-mass. The LOD value was estimated from the fluorescence spectra and displayed as 0.18 μM and 0.85 μM in CH3CN and CH3CN/H2O (v/v = 4/6, pH = 7.4) HEPES buffer correspondingly. Finally, authors demonstrated the practical applicability of probe-94, which could be employed for the sensing of Cu(II) ions in diverse water samples.
1 and further confirmed by 1H NMR and ESI-mass. The LOD value was estimated from the fluorescence spectra and displayed as 0.18 μM and 0.85 μM in CH3CN and CH3CN/H2O (v/v = 4/6, pH = 7.4) HEPES buffer correspondingly. Finally, authors demonstrated the practical applicability of probe-94, which could be employed for the sensing of Cu(II) ions in diverse water samples.
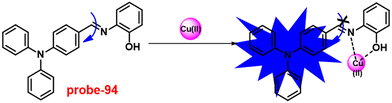 |
| | Fig. 142 Chemical structure and proposed sensing mechanism of probe-94 with Cu(II) (redrawn the ChemDraw structure from ref. 207). | |
In 2019, Xiao and co-workers aimed to synthesise a naphthalene and pyridine-comprehending Schiff base conjugate that works as a very selective probe for Cu(II) ions in aqueous media.208 The sensing studies of probe-95 demonstrated selective fluorescence turn-on towards Cu(II) ions, which is ascribed to the PET process (Fig. 143), and there was no alteration in fluorescence emission with other competitive metal ions. The nature of binding mode of probe-95 was estimated via Job's plot to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of probe-95 and Cu(II) ions, which was further supported by ESI-MS studies. The LOD and association constant of probe-95 were found to be 3.90 nM and 1.38 × 105 M−2 towards Cu(II) ions. The reversibility of probe-95 was performed using the S2− anion and the results strongly supported that upon addition of S2− anion, probe-95 + Cu(II) reproduced the probe and the results indicate that probe-95 has very good reversible properties.
1 of probe-95 and Cu(II) ions, which was further supported by ESI-MS studies. The LOD and association constant of probe-95 were found to be 3.90 nM and 1.38 × 105 M−2 towards Cu(II) ions. The reversibility of probe-95 was performed using the S2− anion and the results strongly supported that upon addition of S2− anion, probe-95 + Cu(II) reproduced the probe and the results indicate that probe-95 has very good reversible properties.
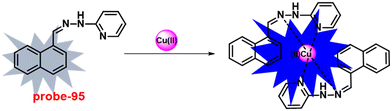 |
| | Fig. 143 Chemical structure and proposed sensing mechanism of probe-95 with Cu(II) (redrawn the ChemDraw structure from ref. 208). | |
In 2016, Ko et al. designed an anthracene-pyridine-conjugated Schiff base moiety via a facile process for the fluorogenic turn-on behavior of Cu(II) ions.209 The sensing data demonstrated that upon addition of Cu(II) ions to probe-96, gradual fluorescence enhancement was observed 23-fold times as a result of suppression in PET process (Fig. 144). There was no change in fluorescence spectra with excess amounts of other competitive metal ions that indicate very high selectivity towards Cu(II) ions. Further, the absorption spectra also show changes in the presence of Cu(II) ions, i.e. a band at 405 nm was progressively decreased, whereas a band at 325 nm was increased simultaneously. The binding stoichiometry was calculated from Job's plot to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was supported by 1H NMR, and a binding constant was displayed as 2.12 × 106 M−2. The LOD value was calculated from the fluorescence spectra as 0.1 nM. Further, the formation of a probe-96 complex was well characterized by quantum yield enhancement, SEM, and theoretical calculations. For the practical relevance of probe-96, the detection of Cu(II) ions in biological media (Raw 264.7 cells) was performed by fluorescence microscopy. The results strongly indicated that there was no fluorescence emission when probe-96 was used alone, but upon its treatment with Cu(II) ions bright fluorescence emission was observed inside the cell (Fig. 145). Moreover, probe-96 was found to have very low cytotoxicity as well as great permeability, which opened up the possibility for its use in drug delivery into cells.
1, which was supported by 1H NMR, and a binding constant was displayed as 2.12 × 106 M−2. The LOD value was calculated from the fluorescence spectra as 0.1 nM. Further, the formation of a probe-96 complex was well characterized by quantum yield enhancement, SEM, and theoretical calculations. For the practical relevance of probe-96, the detection of Cu(II) ions in biological media (Raw 264.7 cells) was performed by fluorescence microscopy. The results strongly indicated that there was no fluorescence emission when probe-96 was used alone, but upon its treatment with Cu(II) ions bright fluorescence emission was observed inside the cell (Fig. 145). Moreover, probe-96 was found to have very low cytotoxicity as well as great permeability, which opened up the possibility for its use in drug delivery into cells.
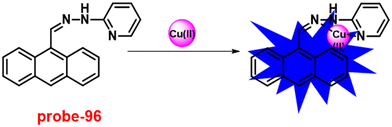 |
| | Fig. 144 Chemical structure and proposed sensing mechanism of probe-96 with Cu(II) (redrawn the ChemDraw structure from ref. 209). | |
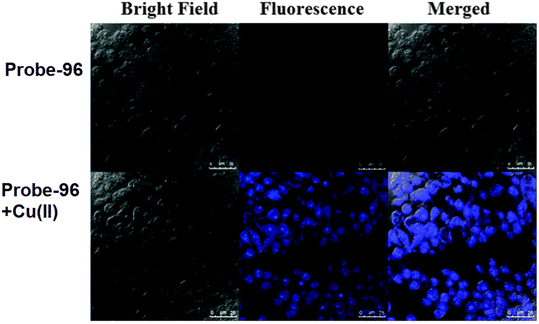 |
| | Fig. 145 Fluorescence microscopic images of Raw 264.7 cells treated with probe-96 (top) and in the presence of Cu(II) ions (bottom) (reprinted with permission from ref. 209, Copyright 2016 Royal Society of Chemistry). | |
Guchhait et al. obtained a pyrene-based Schiff base conjugate for the selective chromogenic and fluorometric sensing of Cu(II) ions. In 2016, probe-97 (Fig. 146) was synthesized upon reaction between pyrene-1-carboxaldehyde hydrazone and 2-hydroxy-1-naphthaldehyde in MeOH at room temperature.210 Target probe-97 was structurally characterised by several NMR and analytical techniques. Probe-97 was found to have very interesting solid structures such as a 3D polymeric network with several different type of interactions such as CH⋯π, π⋯π (pyrene and pyrene) and intermolecular H-bonding, which influence the photophysical properties and sensing mechanism. The UV-vis spectra of probe-97 showed drastic differences, and the color changes form colorless to yellow color, and hence, this probe-97 could be applied for potential colorimetric detection of Cu(II) ions. However, upon addition of Cu(II) ions to probe-97, the broad emission band from 520 nm to 560 nm was significantly decreased to a assured point and became saturated. On titrating with Cu(II) ions, the fluorescence band at 466 nm progressively enhanced (Fig. 146). The observed findings are elucidated as follows: in the presence of Cu(II) ions, the excimer break down and once complexation between Cu(II) ions with probe-97 gave rise to an enhancement emission band at 466 nm. Further, probe-97 demonstrated the efficient sensing of Cu(II) ions in biological media, which was confirmed by confocal fluorescence microscopy.
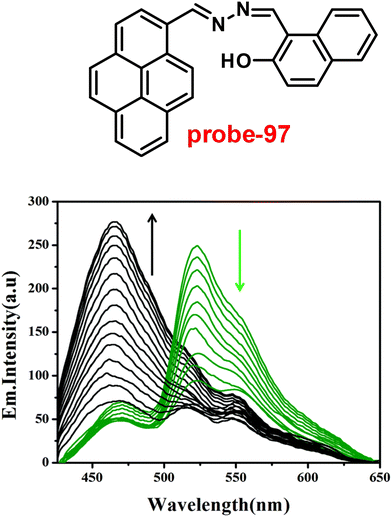 |
| | Fig. 146 Chemical structure of probe-97 (top) and change in the emission spectra of probe-97 on increasing the concentration of Cu(II) ions (bottom) (redrawn and reprinted with permission from ref. 210, Copyright 2016 Royal Society of Chemistry). | |
In 2017, Kuwar synthesized a new terephthaldehyde-based Schiff base, probe-98, and characterized it using several analytical techniques.211Probe-98 was found to show very sensitive and selective fluorescence turn-on response to Cu(II) ions, even with the interfering metal ions. The absorption titration exhibited a colorimetric response to probe-98, and the color changes from colorless to red color with Cu(II) ions. The binding stoichiometry was determined by Job's plot as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which was confirmed via fluorescence enhancement at 386 nm. The LOD and association value (Ka) of probe-98 with Cu(II) ions were estimated to be 0.62 μM and 6.67 × 104 M−1, correspondingly. The fluorescence turn-on mechanism was proposed, which might be due to the suppression of C
2, which was confirmed via fluorescence enhancement at 386 nm. The LOD and association value (Ka) of probe-98 with Cu(II) ions were estimated to be 0.62 μM and 6.67 × 104 M−1, correspondingly. The fluorescence turn-on mechanism was proposed, which might be due to the suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and PET process along with the CHEF (Fig. 147). Further, probe-98 was found to show excellent performance towards the recognition of Cu(II) ions in biological media in live L929 cells (Fig. 148).
N isomerisation and PET process along with the CHEF (Fig. 147). Further, probe-98 was found to show excellent performance towards the recognition of Cu(II) ions in biological media in live L929 cells (Fig. 148).
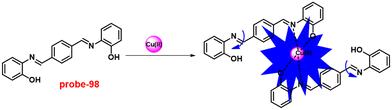 |
| | Fig. 147 Chemical structure and proposed sensing mechanism of probe-98 with Cu(II) (redrawn the ChemDraw structure from ref. 211). | |
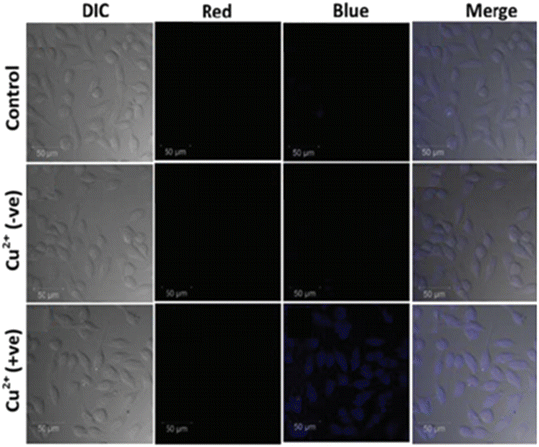 |
| | Fig. 148 Fluorescence microscopic images of L929 cells treated with probe-98 and in the presence of Cu(II) ions (reprinted with permission from ref. 211, Copyright 2017 Royal Society of Chemistry). | |
In 2019, Kang and co-workers obtained rhodamine and Schiff base conjugates for sensitive and selective fluorescence turn-on towards Cu(II) ions.212 The sensing studies of probe-99 demonstrated that the absorption band at 552 nm was gradually increased with the Cu(II) ions, whereas emission spectra fluorescence enhancement was observed with the increase in quantum yields, due to the ring opening of rhodamine (Fig. 149). However, the competitive binding studies of probe-99 with metal ions showed no changes in the absorption and emission spectra, which indicates the selective and sensitive response towards Cu(II) ions. Finally, probe-99 was tested for the recognition of Cu(II) ions in biological media, and the outcomes revealed that probe-99 was found to have very high potential for cellular imaging in living cells as well as in live mice.
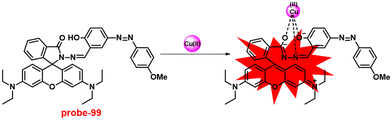 |
| | Fig. 149 Chemical structure and proposed sensing mechanism of probe-99 with Cu(II) (redrawn the ChemDraw structure from ref. 212). | |
A new luminous probe-100(a–c) (Fig. 150) was created by Yamato and his coworkers in 2016. It is a pyrene with a long-chain Schiff base derivative at position 1.213Probe-100b and probe-100c were also developed to compare their binding efficiency for the sensing of Cu(II) ions. Probe-100a contained a methoxy group instead of the diethylaminocarbonylmethoxy group, and probe-100c contained a simply phenylimino moiety instead of the hydrazido carbonyl group. By forming a coordination connection with the hydrazidocarbonyl group, the ligands probe-100a and probe-100b can bind and detect Cu(II) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand-to-metal binding mode. In the CH3CN/CH2Cl2 solvent system, on adding 10 eq. of Cu(II) the emission intensity of ligands probe-100a and probe-100b increases by 65-fold and 25-fold, respectively. By inhibiting the PET while bound to Cu(II), probe-100a causes a blue emission. Additionally, due to the significant suppression of PET and the distinct binding method of the ligand/metal complex formation, probe-100a is highly sensitive for the detection of Cu(II) compared to probe-100b and probe-100c.
1 ligand-to-metal binding mode. In the CH3CN/CH2Cl2 solvent system, on adding 10 eq. of Cu(II) the emission intensity of ligands probe-100a and probe-100b increases by 65-fold and 25-fold, respectively. By inhibiting the PET while bound to Cu(II), probe-100a causes a blue emission. Additionally, due to the significant suppression of PET and the distinct binding method of the ligand/metal complex formation, probe-100a is highly sensitive for the detection of Cu(II) compared to probe-100b and probe-100c.
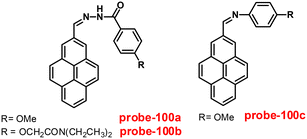 |
| | Fig. 150 Chemical structures of probe-100a–c (redrawn the ChemDraw structure from ref. 213). | |
In 2016, Jiang et al. synthesized novel probe-101, which could be applied for the selective recognition of Cu(II) ions and cysteine.214 On titrating with Cu(II) ions, the Schiff base unit of probe-101 (Fig. 151) can coordinate with Cu(II) and cause a highly selective fluorescence “turn-on” process. The terminal olefin of probe-101 could interact with Cys and cause fluorescence quenching. The reversible nature of the produced probe-101-Cys exhibited improved Cu(II) binding properties and regained probe-101 fluorescence. The detection limits of probe-101 with Cu(II) and Cys were 25 nM and 11 nM respectively. Fluorescence imaging tests were performed in HeLa cells to further highlight probe-101's usefulness in biological systems.
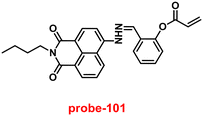 |
| | Fig. 151 Chemical structure of probe-101 (redrawn the ChemDraw structure from ref. 214). | |
8. Schiff base-based fluorescent turn-on probes for Ni(II) ions
One of the transition metals, Ni(II) ions, is essential for many metallo-enzymes including carbon monoxide dehydrogenases, acireductone dioxygenases and hydrogenases. It also plays a role in respiration, biosynthesis, and metabolism in living things.215–219 Apart from the biological applications of Ni(II) ions, it has potential utility in the field of battery industry, such as electroplating, Ni–Cd batteries, and electroforming.220,221 However, the excess amount of Ni(II) accumulation in organelles causes several health issues such as respiratory problems, allergies, pneumonitis, lung cancer, and central nervous disorders in humans.222–224 Consequently, there is a necessity of selective and sensitive probe for the recognition and monitoring of Ni(II) ions in environmental and biological samples.
In 2019, Patra and co-workers aimed to synthesize novel probe-102 for the sensitive and selective fluorescence turn-on behavior towards Ni(II) ions.225Probe-102 in its original form was observed to be poorly emissive in nature, while upon addition of Ni(II) ions, the luminescence was gradually increased by 3-fold and was saturated after addition of one eq., due to suppression of PET, ESIPT and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 152), while the emission spectra showed fluorescence quenching in the presence of Cu(II) ions. UV-vis studies show that in the presence of Ni(II) and Cu(II), a color change was observed from colorless to yellow, which is evidently observed from the rise of a new band towards the long wavelength region. Job's plot gave strong information regarding the complexation ratio of probe-102 with Ni(II) ions, which was found to be 1
N isomerization (Fig. 152), while the emission spectra showed fluorescence quenching in the presence of Cu(II) ions. UV-vis studies show that in the presence of Ni(II) and Cu(II), a color change was observed from colorless to yellow, which is evidently observed from the rise of a new band towards the long wavelength region. Job's plot gave strong information regarding the complexation ratio of probe-102 with Ni(II) ions, which was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as supported by ESI-MS analysis and DFT studies. The detection limit of probe-102 was analyzed from the fluorescence spectrum and found to be 1.71 μM for Ni(II) ions, and much lesser than that approved by the WHO rules for drinking water. The reversibility of probe-102 with Ni(II) and Cu(II) ions was obtained from the addition of Na2EDTA experiments. Further, the authors demonstrated the applicability of probe-102 for the recognition of Ni(II) ions in biological samples and its excellent detection ability towards Ni(II) in cellular media, which was supported by fluorescence microscopy (Fig. 153).
1, as supported by ESI-MS analysis and DFT studies. The detection limit of probe-102 was analyzed from the fluorescence spectrum and found to be 1.71 μM for Ni(II) ions, and much lesser than that approved by the WHO rules for drinking water. The reversibility of probe-102 with Ni(II) and Cu(II) ions was obtained from the addition of Na2EDTA experiments. Further, the authors demonstrated the applicability of probe-102 for the recognition of Ni(II) ions in biological samples and its excellent detection ability towards Ni(II) in cellular media, which was supported by fluorescence microscopy (Fig. 153).
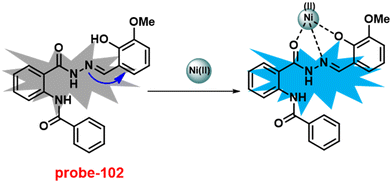 |
| | Fig. 152 Chemical structure and proposed sensing mechanism of probe-102 with Ni(II) (redrawn the ChemDraw structure from ref. 225). | |
 |
| | Fig. 153 Fluorescence microscopic images of HeLa cells treated with probe-102 and in the presence of Ni(II) ions (reprinted with permission from ref. 225, Copyright 2019 Royal Society of Chemistry). | |
In 2018, Sivaraman and his colleagues developed a new probe (probe-103)226 with readily accessible “off–on–off” colorimetric and fluorescence responses via a condensation reaction between 2-aminophenylsulphide and 5-chlorosalicylaldehyde and structurally characterized it via various analytical and spectroscopic techniques. The pure form of probe-103 was very weak emissive in nature; on titrating with Ni(II) ions, the emission intensity was gradually enhanced because of CHEF (Fig. 154) and revealed no influence on emission spectra with other competitive metal ions. However, the UV-vis titration data of probe-103 showed dramatic color change from colorless to dark yellow upon addition of Ni(II) ions. By using Job's plot analysis, the binding ratio was determined to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between probe-103 and Ni(II) ions. In addition, the LOD value for probe-103 was calculated to be as low as 8.67 nM by the fluorescence approach and 0.361 μM by the UV-vis method, which suggests a potential use for probe-103 in detecting Ni(II) in the environment. The collected spectroscopic data of probe-103 with Ni(II) were also used to demonstrate the molecular logic gates. Probe-103 was also effectively used for Ni(II) fluorescence imaging in HeLa cells (Fig. 155). In order to show the practical applications of probe-103 for the detection of Ni(II) ions, test kits were also created by dip-coating the probe-103 solution onto filter paper and allowing it to dry in the air.
1 between probe-103 and Ni(II) ions. In addition, the LOD value for probe-103 was calculated to be as low as 8.67 nM by the fluorescence approach and 0.361 μM by the UV-vis method, which suggests a potential use for probe-103 in detecting Ni(II) in the environment. The collected spectroscopic data of probe-103 with Ni(II) were also used to demonstrate the molecular logic gates. Probe-103 was also effectively used for Ni(II) fluorescence imaging in HeLa cells (Fig. 155). In order to show the practical applications of probe-103 for the detection of Ni(II) ions, test kits were also created by dip-coating the probe-103 solution onto filter paper and allowing it to dry in the air.
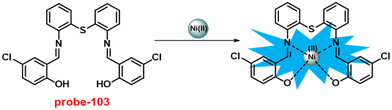 |
| | Fig. 154 Chemical structure and proposed sensing mechanism of probe-103 with Ni(II) (redrawn the ChemDraw structure from ref. 226). | |
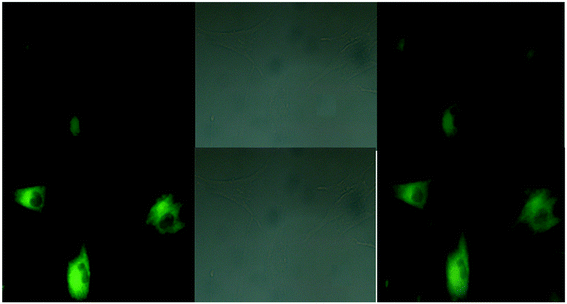 |
| | Fig. 155 Fluorescence microscopic images of HeLa cells treated with probe-103 and in the presence of Ni(II) ions (reprinted with permission from ref. 226, Copyright 2018 Royal Society of Chemistry). | |
Patra and co-workers synthesized selective and sensitive fluorescent turn-on probe-104, which gave response towards Ni(II) ions, from 3-amino-2-phenyl-4(3H)-quinazolinone and 2-pyridene carboxaldehyde in methanol under reflux conditions and structurally characterized it by various analytical and spectroscopic techniques.227 The purest form of probe-104 showed very poor emission, and upon introduction of Ni(II) ions, the fluorescence intensity was gradually increased, whereas other competitive metal ions showed no effect on the fluorescence spectra. Further, probe-104 displayed a colorimetric response towards Ni(II) ions. The DFT analyses and isolation of a single crystal of the metal complex gave additional confirmation of the recognition mechanism (Fig. 156). The binding stoichiometry of probe-104 and Ni(II) was calculated to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 based on Job's plot calculation. The detection limit was substantially lower than that indicated by the WHO rules for drinking water, reaching up to 1.8 μM and 1.18 μM for colorimetric and fluorometric analyses respectively. The chemosensor has also been successfully used to make molecular logic gates, image living cells, and analyze real samples using a smartphone. HeLa cells were used in cell imaging studies to confirm probe-104's affinity for Ni(II) ions present in an intracellular medium.
1 based on Job's plot calculation. The detection limit was substantially lower than that indicated by the WHO rules for drinking water, reaching up to 1.8 μM and 1.18 μM for colorimetric and fluorometric analyses respectively. The chemosensor has also been successfully used to make molecular logic gates, image living cells, and analyze real samples using a smartphone. HeLa cells were used in cell imaging studies to confirm probe-104's affinity for Ni(II) ions present in an intracellular medium.
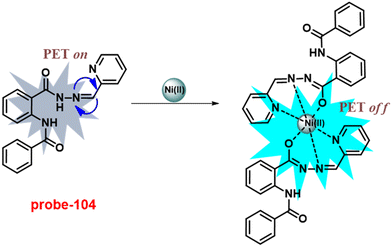 |
| | Fig. 156 Chemical structure and proposed sensing mechanism of probe-104 with Ni(II) (redrawn the ChemDraw structure from ref. 227). | |
9. Schiff base-based fluorescent turn-on probes for Pd(II) ions
Palladium can bind to proteins (including casein, silk fibroin, and many enzymes), amino acids that contain thiols, vitamin B6, DNA, and other macromolecules due to its thiophilic nature.228–232 This can disrupt a number of cellular processes including DNA degradation, harm to cell mitochondria, and inhibition of enzyme activity.233,234 The Pd(II) complexes are enormously used as catalysts in various cross-coupling reactions, and the byproducts containing trace amounts of Pd(II) are extremely toxic as well as carcinogenic,235,236 which is very mandatory to sense the trace amount of Pd(II) in environmental and biological samples.
In 2017, Sinha et al. designed and synthesized rhodamine-appended allylic iminephenol (probe-105) for the sensitive and selective detection of Pd(II) at a very low LOD value via the fluorescence turn-on mechanism.237 The native form of probe-105 displayed poor emission, and on adding Pd(II) ions, the emission intensity increased by 22 times and reached a maximum after addition of one eq. (Fig. 157), and with other metal ions, no changes were observed in the fluorescence spectra, which strongly suggested that probe-105 was very selective towards Pd(II) ions. The absorption spectral data were found to show colorimetric response, and the color changes from colorless to maroon due to the presence of LMCT in CH3CN–H2O (v/v = 1/4). The LOD value of probe-105 with Pd(II) ions was estimated from the fluorescence titration spectra to be 50 nM, which was much lesser than the WHO recommended level. Job's plot suggested 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between probe-105 and Pd(II), which was supported by 1H NMR, ESI-MS and DFT calculations. Furthermore, DFT calculations also supported experimental data including the lengthening of the C
1 stoichiometry between probe-105 and Pd(II), which was supported by 1H NMR, ESI-MS and DFT calculations. Furthermore, DFT calculations also supported experimental data including the lengthening of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond causing a red shift of the absorption peak. In addition, by incubating probe-105 with a Pd(II) ion solution in HCT116 cells, in vivo fluorescence cell imaging could be obtained (Fig. 158).
O bond causing a red shift of the absorption peak. In addition, by incubating probe-105 with a Pd(II) ion solution in HCT116 cells, in vivo fluorescence cell imaging could be obtained (Fig. 158).
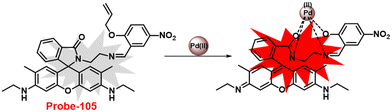 |
| | Fig. 157 Chemical structure and proposed sensing mechanism of probe-105 with Pd(II) (redrawn the ChemDraw structure from ref. 237). | |
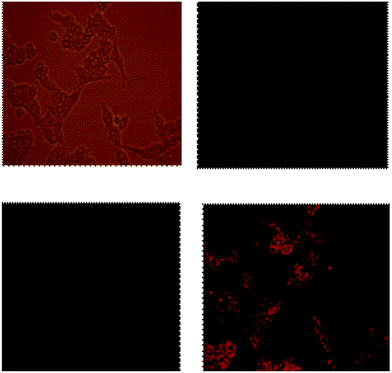 |
| | Fig. 158 Fluorescence microscopic images of HCT 116 cells treated with probe-105 and in the presence of Pd(II) ions (reprinted with permission from ref. 237, Copyright 2017 Royal Society of Chemistry). | |
In view of the previous success of rhodamine derivatives as a fluorescent sensing probe of Pd(II) ions, Sinha et al. were influenced to synthesize a coumarinyl-rhodamine Schiff base (probe-106),238 which was obtained from the condensation reaction of 4-methyl-7-hydroxy-8-formyl-coumarin and N-(rhodamine-B)lactam-1,2-ethylenediamine in ethanol under reflux conditions, and it was structurally characterized via several analytical and spectroscopic techniques in 2019. The native state of probe-106 (Fig. 159) displayed poor emission; on adding Pd(II) ions, the luminescence intensity gradually enhanced, whereas in the presence of competitive ions, no considerable change was observed in the fluorescence spectra. Under similar conditions, the absorption spectra of probe-106 with Pd(II) ions displayed colorimetric responses and the color changes from pink to pale yellow. The LOD value of probe-106 with Pd(II) ions was estimated from the fluorescence titration data as 18.8 nM. Job's plot ascertained the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between probe-106 and Pd(II) ions, which was validated by FTIR, 1H NMR, DFT and ESI-MS data. The authors proposed the sensing mechanism of probe-106, which involved the formation of [CR–Pd(II)] + complex using hydroxy-O and imine-N of a coumarinyl–rhodamine moiety involving the opening of a spirolactam ring. The DFT calculations suggested the reduction in band gap of probe-106 upon binding with Pd(II) ions, which was indicative of the increase in conjugation on ring opening and subsequent enhancement of fluorescence intensity as well as color change. Furthermore, after incubating probe-106 in MCF7 cells, the live imaging of Pd(II) ions was performed using a fluorescence microscopic technique. Moreover, probe-106 provided in vivo cell imaging (Fig. 159) along with selective and sensitive sensing of Pd(II) ions at such low detection limits.
1 stoichiometry between probe-106 and Pd(II) ions, which was validated by FTIR, 1H NMR, DFT and ESI-MS data. The authors proposed the sensing mechanism of probe-106, which involved the formation of [CR–Pd(II)] + complex using hydroxy-O and imine-N of a coumarinyl–rhodamine moiety involving the opening of a spirolactam ring. The DFT calculations suggested the reduction in band gap of probe-106 upon binding with Pd(II) ions, which was indicative of the increase in conjugation on ring opening and subsequent enhancement of fluorescence intensity as well as color change. Furthermore, after incubating probe-106 in MCF7 cells, the live imaging of Pd(II) ions was performed using a fluorescence microscopic technique. Moreover, probe-106 provided in vivo cell imaging (Fig. 159) along with selective and sensitive sensing of Pd(II) ions at such low detection limits.
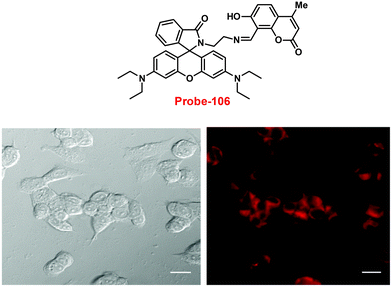 |
| | Fig. 159 Chemical structure of probe-106 (top) and fluorescence microscopic images of MCF7 cells treated with probe-106 and in the presence of Pd(II) ions (bottom) (redrawn and reprinted with permission from ref. 238, Copyright 2019 Royal Society of Chemistry). | |
Pathak et al. developed probe-107, a Schiff base based on rhodamine–aminopyrone, via the reaction of 4-amino antipyrine and glyoxal, which was then followed by condensation with hydrazinated rhodamine B in ethanol under reflux conditions, and the structure was verified using a variety of analytical and spectroscopic techniques in 2019.239 The pure state of probe-107 (Fig. 160) found to be very poorly emissive in nature, on titrating with Pd(II) ions, the luminescence intensity gradually enhanced, whereas with other competitive metal ions, it failed to impart any considerable effect on fluorescence spectra (Fig. 161). Conversely, in the presence of Pd(II) ions, probe-107 exhibited a colorimetric response, in which the color changes from colorless to pink. The LOD value was found to be 11.9 μM which was determined by a fluorescence titration experiment. Job's plot revealed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation between probe-107 and Pd(II), which was further supported by 1H NMR, DFT and ESI-MS data. The DFT calculation also strongly supported the experimental data such as the reduction in the band gap of probe-107 with Pd(II) ions, which was due to the formation of complex via hydroxy-O and enamine-N of a rhodamine moiety and carbonyl-O and imine-N of aminopyrone. The fluorescence-enhanced mechanism was proposed by authors, which was spirolactum ring opening upon metal binding. Furthermore, apart from acting as a sensitive and selective fluorescent turn-on sensor, incubating probe-107 in MDA-MB-468 cells, in vivo cell imaging of Pd(II) was obtained without causing any harm (determined by the MTT assay) (Fig. 162).
1 stoichiometric complexation between probe-107 and Pd(II), which was further supported by 1H NMR, DFT and ESI-MS data. The DFT calculation also strongly supported the experimental data such as the reduction in the band gap of probe-107 with Pd(II) ions, which was due to the formation of complex via hydroxy-O and enamine-N of a rhodamine moiety and carbonyl-O and imine-N of aminopyrone. The fluorescence-enhanced mechanism was proposed by authors, which was spirolactum ring opening upon metal binding. Furthermore, apart from acting as a sensitive and selective fluorescent turn-on sensor, incubating probe-107 in MDA-MB-468 cells, in vivo cell imaging of Pd(II) was obtained without causing any harm (determined by the MTT assay) (Fig. 162).
 |
| | Fig. 160 Chemical structures of probe-107–109 (redrawn the ChemDraw structure from ref. 239–241). | |
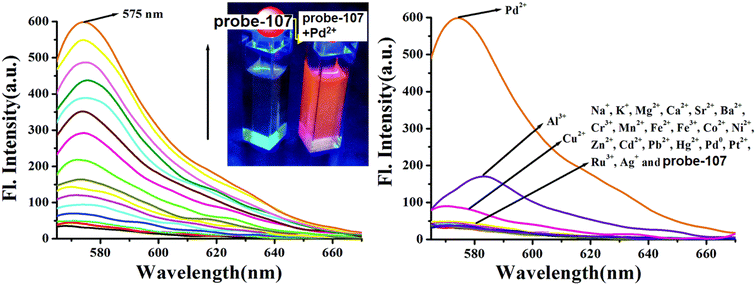 |
| | Fig. 161 Change in the emission spectra of probe-107 on increasing the concentration of Pd(II) ions (left) and with different metal ions (right) (reprinted with permission from ref. 239, Copyright 2019 Royal Society of Chemistry). | |
 |
| | Fig. 162 Fluorescence microscopic images of MDA-MB-468 cells treated with probe-107 and in the presence of Pd(II) ions (reprinted with permission from ref. 239, Copyright 2019 Royal Society of Chemistry). | |
Sinha et al. designed and synthesized ethylenediamine-bridged 2-hydroxy benzophenone–rhodamine-B (probe-108)240 by the reaction between ethylenediamine derivatives of rhodamine B and 2-hydroxy benzophenone and structurally characterised it by several analytical and spectroscopic techniques in 2019. The native state of probe-108 (Fig. 160) was poorly emissive in nature and showed strong luminescence intensity in the presence of Pd(II) ions, whereas upon addition of other competitive metal ions, no intervening effect was seen in the fluorescence spectra. However, probe-108 with Pd(II) ions showed colorimetric responses, showing changes from colorless to pink color. The ESI-MS and 1H NMR data revealed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation between probe-108 and Pd(II) ions, which was further supported by Job's plot analysis. Further, the effect of the pH on the detection capability of probe-108 with Pd(II) ions was evaluated, and the results indicated no effect on the fluorescence spectra in the pH range of 8–12, which shows the potential of probe-108 for the detection of Pd(II) ions in physiological media. The detection limit of probe-108 with Pd(II) ions was calculated from the fluorescence titration experiment to be as low as 34 nM. Considering the selective and sensitive fluorescence turn-on response of probe-108 towards Pd(II) ions, the authors applied for the detection of Pd(II) ions in biological media; firstly, probe-108 was incubated in MDA-MB-231 cell lines and red fluorescence image was obtained upon addition of Pd(II) ions using a microscope providing intracellular imaging (Fig. 163). The DFT calculation suggested a reduction in the band gap of probe-108 upon binding with Pd(II), which signified the formation of [BR–Pd(II)] + complex via four coordination sites (hydroxy-O, imine-N of rhodamine moiety and hydroxy-O of benzophenone and imine-N which bridged these two moieties).
1 stoichiometric complexation between probe-108 and Pd(II) ions, which was further supported by Job's plot analysis. Further, the effect of the pH on the detection capability of probe-108 with Pd(II) ions was evaluated, and the results indicated no effect on the fluorescence spectra in the pH range of 8–12, which shows the potential of probe-108 for the detection of Pd(II) ions in physiological media. The detection limit of probe-108 with Pd(II) ions was calculated from the fluorescence titration experiment to be as low as 34 nM. Considering the selective and sensitive fluorescence turn-on response of probe-108 towards Pd(II) ions, the authors applied for the detection of Pd(II) ions in biological media; firstly, probe-108 was incubated in MDA-MB-231 cell lines and red fluorescence image was obtained upon addition of Pd(II) ions using a microscope providing intracellular imaging (Fig. 163). The DFT calculation suggested a reduction in the band gap of probe-108 upon binding with Pd(II), which signified the formation of [BR–Pd(II)] + complex via four coordination sites (hydroxy-O, imine-N of rhodamine moiety and hydroxy-O of benzophenone and imine-N which bridged these two moieties).
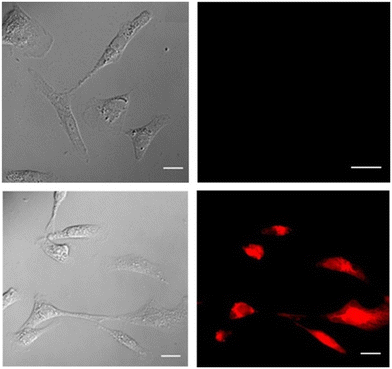 |
| | Fig. 163 Bio-imaging of MDA-MB-231 cells incubated with probe-108 and in the presence of Pd(II) ions (reprinted with permission from ref. 240, Copyright 2019 American Chemical Society). | |
With the aim to overcome the oxidation state specificity of chemosensors in the detection of Pd(II) ions, in 2018, Sinha and coworkers developed an allyl-functionalized rhodamine Schiff base (probe-109) (Fig. 160)241via the reaction of 2-allyloxy5-nitrobenzaldehyde and N-(rhodamine-6G) lactam hydrazine, which could show selective and sensitive detection for the Pd(II) ions via colorimetric as well as fluorescence turn-on response under physiological conditions (stable at pH 5–10), which was confirmed by analytical and spectroscopic methods. The fluorescence data of probe-109 demonstrated that the emission intensity increases with high quantum yields on gradual addition of Pd(II) and the saturation point was reached after adding 1.1 eq., whereas upon addition of different competitive metal ions, it failed to impart any considerable changes in fluorescence spectra. The detection of Pd in all oxidation states (0, II, and IV) by probe-109 was possible, as it was able to bind Pd(0) and Pd(II) through an allyl group and could bind Pd(IV) through O, N, and O donor sites of lactones, imines and ethers respectively. The LOD value of Pd(II) was established to be 95 nM, which was calculated from the fluorescence titration spectra. Finally, the live cell imaging of detection of Pd(II) by probe-109 was carried out in RAW 264.7 cells. The incubation of cells with probe-109 followed by incubation with Pd(II) resulted in an enhancement in the fluorescence intensity, as observed by confocal microscopy, without causing any harm. The potency of probe-109 in both in vitro and in vivo recognition of Pd(II) ions and fluorescence cell imaging was well established (Fig. 164).
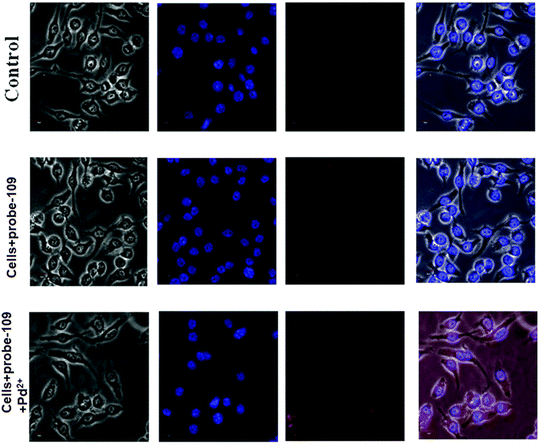 |
| | Fig. 164 Fluorescence microscopic images of RAW 264.7 cells treated with probe-109 and in the presence of Pd(II) ions (reprinted with permission from ref. 241, Copyright 2018 Royal Society of Chemistry). | |
10. Schiff base-based fluorescent turn-on probes for Fe(II)/Fe(III) ions
Since it actively participates in numerous metabolic processes including oxygen transport in haemoglobin and myoglobin, electron transfer from nutrients to oxygen, and DNA repair and synthesis in the living system, iron is a key metal ion in all organisms.242–245 In addition, iron plays a critical part in the manufacture of haemoglobin, wound healing, and healthy muscle function. However, the body's higher iron levels harm tissues and encourage the production of dangerous hydroxyl radicals, which can result in a variety of degenerative illnesses such as atherosclerosis, cancer, and neurological disorders.246–250 Additionally, anemia can result from an iron shortage. An adult human must ingest 1.5 g of iron through their meals each day if they are menstruating due to the possibility of loss of iron, and in accordance with WHO guidelines, 1–3 mg L−1 of iron is acceptable in drinking water.251
Nadakumar et al. synthesized a naphthalene-based Schiff base derivative for specific fluorescence turn-on recognition of Fe(II) ions in a mixture of acetonitrile and water in 2016.252Probe-110 was obtained from the reaction of 1-naphthaldehyde and 2-aminopyridine in ethanol at room temperature for 5 h. The target compound was structurally characterized via various NMR and other analytical techniques. The fluorescence sensing studies established that with Fe(II) ions, the emission intensity of probe-110 increased with a 16 nm red shift in the emission band, and the LOD value as low as 0.15 μM, which was calculated from the 3σ/slope equation. However, no change was seen upon addition of several metal ions to probe-110, which strongly validate the selectivity towards Fe(II) ions. The proposed mechanism for turn-on response was due to the diminution of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and the increase in the ICT process (Fig. 165). The binding constant of probe-110 with Fe(II) ions was estimated from the absorption spectra to be 5.02 × 104 M−2. Further, stoichiometry determined via Job's plot and the ratio of probe-110 to Fe(II) ions was displayed as 2
N isomerisation and the increase in the ICT process (Fig. 165). The binding constant of probe-110 with Fe(II) ions was estimated from the absorption spectra to be 5.02 × 104 M−2. Further, stoichiometry determined via Job's plot and the ratio of probe-110 to Fe(II) ions was displayed as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Finally the practical ability of probe-110 was tested for the recognition of Fe(II) ions in tomato juice, tablets, dark chocolate and tap water and the results strongly suggested that probe-110 is efficient for detecting and monitoring Fe(II) ions.
1. Finally the practical ability of probe-110 was tested for the recognition of Fe(II) ions in tomato juice, tablets, dark chocolate and tap water and the results strongly suggested that probe-110 is efficient for detecting and monitoring Fe(II) ions.
 |
| | Fig. 165 Chemical structure and proposed sensing mechanism of probe-110 with Fe(II) (redrawn the ChemDraw structure from ref. 252). | |
Simple probe-111 for the sensing of Fe(II) based on pyrene conjugated with a Schiff base was synthesised by Wu et al. in 2016.253Probe-111 (Fig. 166) was synthesized via a reaction between 2-aminobenzenethiol and pyrene-1-carbaldehyde in MeOH under reflux conditions for 12 h, which was confirmed via NMR and HRMS. The sensing studies of probe-111 were conducted in acetonitrile by several heavy metal ions and biologically important metals. The fluorescence emission of probe-111 was found to be very weak at 448 nm and upon addition of Fe(II) and Fe(III) ions, the emission intensity was spontaneously increased by 16 times. However, in the case of Fe(III) ions, the luminescence band was moved towards red region with 115 nm; as a result, it shows bright green emission, whereas Fe(II) ions show only blue color emission under UV conditions. Thus, the emission enhancement was attributed to the establishment of rigid system and the CHEF effect was controlled. Further, the UV-vis titration data of probe-111 displayed a colorimetric response towards Fe(III) ions and the color changes from colorless to yellow color, which could be used as a simple way for recognition through the naked eye. Thus, probe-111 exhibited discrimination of Fe(II) and Fe(III) ions. The binding constant of probe-111 for Fe(II), Fe(III) was calculated from the Hill equation and was estimated to be 3.04 × 109 M−1 and 3.97 × 106 M−1 correspondingly. Job's plots revealed binding ratios of probe-111 and Fe(II) and Fe(III) as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was validated by ESI-MS and 1H NMR. The LOD value of probe-111 with Fe(II) and F− was determined from the fluorescence spectra to be 0.3 ppm and 25.7 ppb, respectively, which is much lesser as recommended by WHO. Further, the reversibility of probe-111 + Fe(II) was monitored upon addition of TMEDA, whereas in the case of a fluoride complex, reversibility was established using calcium nitrate. Finally, probe-111 was subjected to real-time monitoring Fe(II) ions in lake and drinking water and the results indicated that probe-111 was able to show response towards Fe(II) ions. Thus, probe-111 was found to be an efficient and potential candidate for the detection of Fe(II) ions in biological and environmental samples.
1, which was validated by ESI-MS and 1H NMR. The LOD value of probe-111 with Fe(II) and F− was determined from the fluorescence spectra to be 0.3 ppm and 25.7 ppb, respectively, which is much lesser as recommended by WHO. Further, the reversibility of probe-111 + Fe(II) was monitored upon addition of TMEDA, whereas in the case of a fluoride complex, reversibility was established using calcium nitrate. Finally, probe-111 was subjected to real-time monitoring Fe(II) ions in lake and drinking water and the results indicated that probe-111 was able to show response towards Fe(II) ions. Thus, probe-111 was found to be an efficient and potential candidate for the detection of Fe(II) ions in biological and environmental samples.
 |
| | Fig. 166 Chemical structures of probe-111 and probe-112 (redrawn the ChemDraw structure from ref. 253 and 254). | |
A novel rhodamine-based Schiff base (probe-112) was synthesized, characterized and investigated for its sensing capabilities by Gupta et al. in 2016.254Probe-112 (Fig. 166) was found to exhibit a colorimetric response upon exposure to Cu(II) and Al(III) ions and on–off emission behavior upon addition of Fe(III) ions. Probe-112 was characterized by FT-IR, NMR and ESI mass spectra. The absorption spectra demonstrated significant spectral changes with Al(III) and Cu(II) and resulted in the corresponding color changes, which make probe-112 as a colorimetric sensor. The selectivity studies were done by visual inspection, absorption and emission spectral analysis and ESI-MS and DFT experiments. Emission spectrum of probe-112 was analyzed, which exhibited very weak luminescence upon excitation at 520 nm. Upon adding Fe(III) ions, a substantial change in emission profile was noted with the appearance of a strong emission band at 587 nm with 43-fold increment, which could be due to the formation of a ring-opened probe-112–Fe(III) complex. However, no spectral changes were observed in the presence of other metal ions. The binding stoichiometry was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by Job's plot analysis with an association constant of 5 × 104 M−1 from the absorption spectra. For investigating the practical utility of probe-112, paper strips carrying the alcoholic solutions of probe-112 were constructed and it was found that the paper strips were also giving positive responses in sensing. The red-shift in the fluorescence spectra of the probe-112–Fe(III) complex was verified by the DFT calculations, which revealed that the binding of Fe(III) to probe-112 lowered the band gap.
1 by Job's plot analysis with an association constant of 5 × 104 M−1 from the absorption spectra. For investigating the practical utility of probe-112, paper strips carrying the alcoholic solutions of probe-112 were constructed and it was found that the paper strips were also giving positive responses in sensing. The red-shift in the fluorescence spectra of the probe-112–Fe(III) complex was verified by the DFT calculations, which revealed that the binding of Fe(III) to probe-112 lowered the band gap.
A simple chemo sensor (probe-113)255 based on a benzothiazole and Schiff base derivative was synthesised by Pitchumani and co-workers in 2016. Probe-113 was synthesized from 2-amino-4-methylbenzothiazole and salicylaldehyde in ethanol at room temperature and a target compound was characterised by NMR and several analytical techniques. The sensing studies of probe-113 showed that titration with Fe(III) ions resulted in fluorescence intensity enhancement, whereas other metal ions imparted no significant response, which shows the selectivity of probe-113 with a very low LOD value of 0.89 nM towards Fe(III) ions. The proposed mechanism for fluorescence enhancement was recognized for the generation of a new ICT process upon metal binding (Fig. 167) to probe-113, which was supported by TD-DFT calculations. The complexation ratio of probe-113 with Fe(III) was determined via absorption spectra to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was proved by ESI-MS and TD-DFT studies. The association constant was calculated from the absorption spectra as 3.6 × 106 M−1, the value established the strong binding nature among probe-113 and Fe(III) ions. Further, probe-113 was subjected for the sensing of Fe(III) ions in biological samples in cellular media, which was confirmed by fluorescence microscopy. The results demonstrated that the presence of Fe(III) ions in cells, which was already incubated with probe-113, gave a bright emission inside the cells that indicate that probe-113 was a potential candidate for the detection of Fe(III) ions in biological samples (Fig. 168).
1, which was proved by ESI-MS and TD-DFT studies. The association constant was calculated from the absorption spectra as 3.6 × 106 M−1, the value established the strong binding nature among probe-113 and Fe(III) ions. Further, probe-113 was subjected for the sensing of Fe(III) ions in biological samples in cellular media, which was confirmed by fluorescence microscopy. The results demonstrated that the presence of Fe(III) ions in cells, which was already incubated with probe-113, gave a bright emission inside the cells that indicate that probe-113 was a potential candidate for the detection of Fe(III) ions in biological samples (Fig. 168).
 |
| | Fig. 167 Chemical structure and proposed sensing mechanism of probe-113 with Fe(III) (redrawn the ChemDraw structure from ref. 255). | |
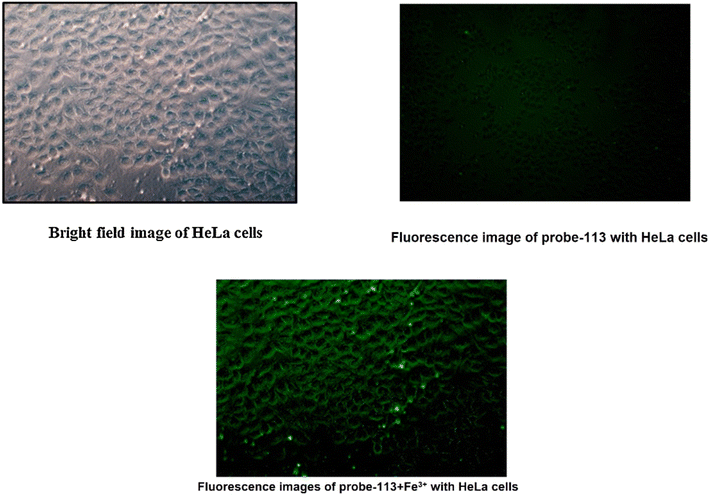 |
| | Fig. 168 Fluorescence microscopic images of HeLa cells treated with probe-113 and in the presence of Fe(III) ions (reprinted with permission from ref. 255, Copyright 2016 Elsevier). | |
Rhodamine conjugated with a Schiff base was developed by Nayab et al. in 2017,256 which displayed very sensitive and selective fluorescence turn-on behavior towards Fe(III) ions. Probe-114 was synthesized from 3,5-difluorobenzaldehyde and rhodamine-B in methanol and refluxed for 12 h to give the target compound as a white color solid and analyzed it by various NMR and ESI-MS. The sensing studies of probe-114 were conducted by UV-vis, fluorescence, 1H NMR, and ESI-MS spectral studies. On titration with Fe(III) ions, probe-114 exhibited color alterations from colorless to red color, which was confirmed by spectral changes observed in the UV-vis spectra, whereas other metal ions found no change. These results were strongly supported by the DFT data and reduction in the band gap after addition of Fe(III) ions. In the fluorescence spectra, on adding Fe(III) ions, the intensity was enhanced by 53-fold, which could be attributed to the transformation of a spirolactam ring to an acyclic form (Fig. 169). However, there was no change in emission spectra upon addition of competitive metal ions, which gave extraordinary selectivity towards Fe(III) ions. The detection limits and binding constant of probe-114 were determined from the absorption spectra to be 6.94 μM and 1.13 × 105 M−1. Job's plot demonstrated the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of probe-114 and Fe(III) ions and was confirmed by UV-vis, FTIR and ESI-MS studies. The reversibility of probe-114 was established upon addition of EDTA to the Fe-complex of probe-114 that was found to exhibit a change from red to colorless as well as weak fluorescence emission. Though, re-addition of Fe(III) ions subsequently resulted in the retrieval of absorbance band and very bright luminescence. Thus, probe-114 was found to have very promising photophysical properties and applied for the recognition of Fe(III) ions in biological and environmental samples.
1 ratio of probe-114 and Fe(III) ions and was confirmed by UV-vis, FTIR and ESI-MS studies. The reversibility of probe-114 was established upon addition of EDTA to the Fe-complex of probe-114 that was found to exhibit a change from red to colorless as well as weak fluorescence emission. Though, re-addition of Fe(III) ions subsequently resulted in the retrieval of absorbance band and very bright luminescence. Thus, probe-114 was found to have very promising photophysical properties and applied for the recognition of Fe(III) ions in biological and environmental samples.
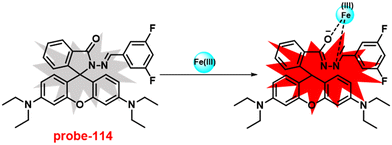 |
| | Fig. 169 Chemical structure and proposed sensing mechanism of probe-114 with Fe(III) (redrawn the ChemDraw structure from ref. 256). | |
Following the above-mentioned report, Adhikari et al. aimed to synthesise rhodamine B containing a Schiff base and conducted sensing studies by UV-vis and fluorescence spectroscopic methods, based on the suppression of PET and increase in the process of CHEF and FRET in 2017. Target probe-115 (Fig. 170) was obtained from the amino group of rhodamine and aldehyde derivatives in ethanol under reflux conditions.257 In the absorption titration, on adding Fe(III) ions there was an intensification in the absorbance with a simultaneous color change of the solution from colorless to pink color. The color of emission changes from blue to orange color with the 95-fold fluorescence intensity enhancement on titrating with Fe(III) ions and very low detection limit as low as 20 nM. However, in the presence of other competitive metal ions, there was no change in emission spectra and the results strongly suggested that probe-115 showed very sensitive and selective fluorescence turn-on response towards Fe(III) ions. Probe-115 was found to show a ratiometric response and resulted in two iso-emissive points at 481 and 542 nm, which was designated as the molecular transfer from non-bound to metal bounded complexes. The association constant was estimated for probe-115 with Fe(III) ions to be 3.7025 × 106 M−1 from emission titration data using the Hill equation. The binding stoichiometry between probe-115 with Fe(III) was estimated from Job's plot as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and was supported by the ESI-MS spectrum. Finally, authors demonstrated that probe-115 show ability to detect Fe(III) ions in biological media and was found to be very efficient for intracellular Fe(III) ions in HeLa cells and established by fluorescence microscopic images. Further, the above finding was strongly verified by the DFT studies (Fig. 171).
1 and was supported by the ESI-MS spectrum. Finally, authors demonstrated that probe-115 show ability to detect Fe(III) ions in biological media and was found to be very efficient for intracellular Fe(III) ions in HeLa cells and established by fluorescence microscopic images. Further, the above finding was strongly verified by the DFT studies (Fig. 171).
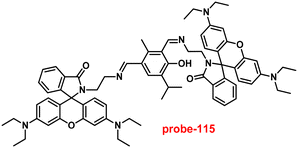 |
| | Fig. 170 Chemical structure of probe-115 (redrawn the ChemDraw structure from ref. 257). | |
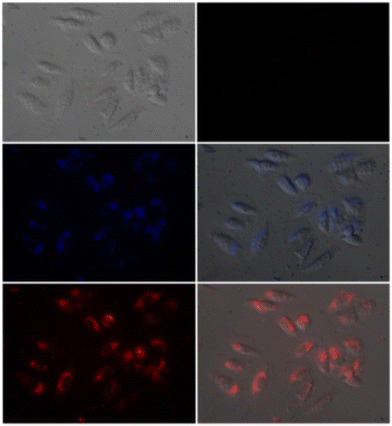 |
| | Fig. 171 Fluorescence microscopic images of HeLa cells treated with probe-115 and in the presence of Fe(III) ions (reprinted with permission from ref. 257, Copyright 2017 Elsevier). | |
Niu et al. obtained a diketopyrrolopyrrole-conjugated Schiff base derivative for the dual response such as colorimetric and fluorescence turn-on towards Fe(III) ions in 2018.258Probe-116 was obtained from the reaction between the aldehyde of diketopyrrolopyrrole and o-aminophenol in anhydrous ethanol under reflux conditions for 12 h. While other interfering metal ions do not cause any color changes, the absorption titration data of probe-116 showed significant selectivity towards Fe(III) ions and color shifted from purple to red, which could be seen with the naked eye. In the case of emission spectra, upon addition of Fe(III) ions, remarkable fluorescence emission intensity enhancement by ∼9.5 fold at 645 nm was observed (Fig. 173) and the quantum yield was increased from 0.012 to 0.54, although there was no change or very slight alteration in the emission with other metal ions. The fluorescence enhancement could be attributed to the suppression of PET and CHEF effects (Fig. 172). The detection limits and association constants were calculated for probe-116 with Fe(III) to be 2.4 × 104 M−1 and 14.3 nM respectively. Further, the binding mode of probe-116 and Fe(III) ions was validated by Job's plot analysis, 1H NMR and FTIR spectra. The reversibility of probe-116 was obtained upon treatment with S2− or CN−. Probe-116 possesses great stability, detection limit, quick response in a range of pH values, as well as good reversibility and it became a potential candidate for the recognition of Fe(III) ions in environmental and biological samples.
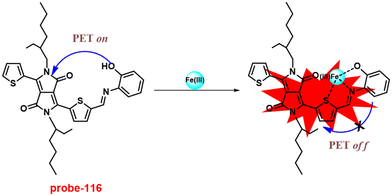 |
| | Fig. 172 Chemical structure and proposed sensing mechanism of probe-116 with Fe(III) (redrawn the ChemDraw structure from ref. 258). | |
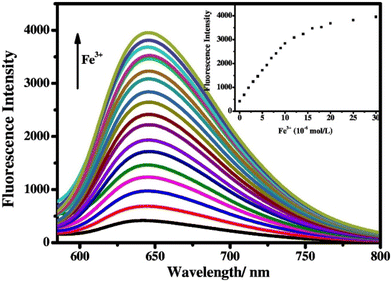 |
| | Fig. 173 Change in emission spectra of probe-116 on increasing the concentration of Fe(III) ions (left) and with other ions (right) (reprinted with permission from ref. 258, Copyright 2018 Elsevier). | |
Following the above-mentioned report, Niu et al. aimed to synthesise a biphenyl-based Schiff base conjugate exhibiting chromogenic and fluorescence turn-on dual responses towards Fe(III) ions in 2019. The synthesis of a target compound (probe-117)259 was obtained from 3,3′-dihydroxybenzidine and 1-naphthaldehyde in ethanol under reflux conditions for 6 h and a yellow solid was obtained as a product, which was verified by NMR and various spectroscopic analysis. The absorption titration data of probe-117 showed selective response towards Fe(III) ions and color variations from yellow to colorless in DMSO/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solutions, whereas no change in the absorption spectra with other metal ions. On titrating with Fe(III) ions to probe-117, the emission intensity was enhanced with a gradual increment in quantum yields, due to the suppression of C
1, v/v) solutions, whereas no change in the absorption spectra with other metal ions. On titrating with Fe(III) ions to probe-117, the emission intensity was enhanced with a gradual increment in quantum yields, due to the suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and PET (Fig. 174). However, on adding other competitive metal ions, there was no alteration in the emission spectra, which established the very high selectivity of probe-117 towards Fe(III) ions. The complexation ratio of probe-117 with Fe(III) ions was found to be 1
N isomerisation and PET (Fig. 174). However, on adding other competitive metal ions, there was no alteration in the emission spectra, which established the very high selectivity of probe-117 towards Fe(III) ions. The complexation ratio of probe-117 with Fe(III) ions was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and confirmed by Job's plot, 1H NMR, HRMS analysis and theoretical studies. The LOD value of the complex with Fe(III) ions was estimated from the fluorescence titration spectra as low as 0.178 μM. For the practical purpose, probe-117 was utilized for the detection of Fe(III) ions in drinking water and food samples with good precision.
2 and confirmed by Job's plot, 1H NMR, HRMS analysis and theoretical studies. The LOD value of the complex with Fe(III) ions was estimated from the fluorescence titration spectra as low as 0.178 μM. For the practical purpose, probe-117 was utilized for the detection of Fe(III) ions in drinking water and food samples with good precision.
 |
| | Fig. 174 Chemical structure and proposed sensing mechanism of probe-117 with Fe(III) (redrawn the ChemDraw structure from ref. 259). | |
A dual functional sensor based on oligothiophene–phenylamine for Al(III) and Fe(III) ions was obtained by Niu et al. in 2018.260Probe-118 gave a very quick and selective fluorescence turn-on response towards Al(III) and Fe(III) ions without causing any intervening effect from competitive metal ions; however, Fe(III) ions were found to show better response than Al(III) ions. UV-vis studies were carried out in the presence of various metal ions with Fe(III) ions with probe-118. Upon addition of 2.0 eq. of Al(III) and Fe(III) ions, probe-118 showed outstanding enhancement in the absorbance, which could be due to the construction of the metal complex of probe-118. Nonetheless, with other competitive metal ions, there was no change in the absorption and emission spectra (Fig. 176). The fluorescence emission studies demonstrated that the addition of Al(III) and Fe(III) ions induced a fluorescence turn-on (Fig. 175) behavior accompanied by a color transformation from colorless to green and emission bands at 536 and 572 nm. The LOD value of probe-118 with Fe(III) ions was determined to be 0.172 μM with a binding constant of 1.04 × 104 M−1. Job's plot revealed the stoichiometry of probe-118 with Fe(III) ions as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was further validated by ESI-MS, 1H NMR and FT-IR. The practical application of probe-118 was established by the recognition of Fe(III) ions via a paper strip as well as in environmental and food samples.
1, which was further validated by ESI-MS, 1H NMR and FT-IR. The practical application of probe-118 was established by the recognition of Fe(III) ions via a paper strip as well as in environmental and food samples.
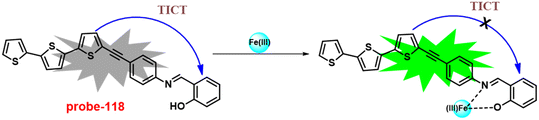 |
| | Fig. 175 Chemical structure and proposed sensing mechanism of probe-118 with Fe(III) ions (redrawn the ChemDraw structure from ref. 260). | |
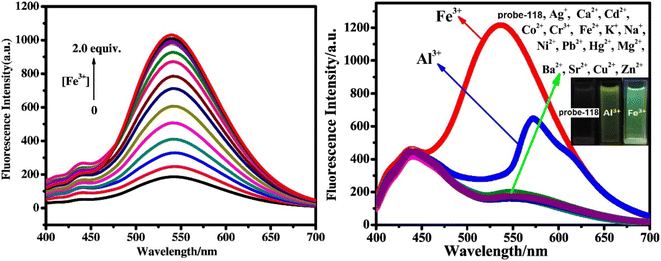 |
| | Fig. 176 Change in the emission spectra of probe-118 on increasing the concentration of Fe(III) ions (left) and with other ions (right) (reprinted with permission from ref. 260, Copyright 2018 Elsevier). | |
In 2021, Tabassum et al. obtained a diaminobenzophenone-based Schiff base derivative (probe-119), which was structurally confirmed by 1H & 13C NMR, UV-vis, FT-IR and ESI-MS analysis.261 The titration studies were conducted by UV-vis and fluorescence spectroscopic analyses. In the absorption titration of probe-119, titration with Fe(II) ions caused colorimetric responses, showing color alteration from light yellow to brown, which can be identified through naked eyes. The fluorescence titration data demonstrated a significant fluorescence turn-on response towards Fe(II) ions, whereas with other competitive metal ions there was no change observed in the emission spectra. The emission turn-on behavior could be attributed to the suppression of PET, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and CHEF, which rose after the formation of metal coordination (Fig. 177). The binding constant of probe-119 was estimated from the change in absorption spectra upon addition of Fe(II) ions as 6.173 × 107 M−2. The association stoichiometry of probe-119 with Fe(II) ions was estimated from Job's plot to be 2
N isomerization and CHEF, which rose after the formation of metal coordination (Fig. 177). The binding constant of probe-119 was estimated from the change in absorption spectra upon addition of Fe(II) ions as 6.173 × 107 M−2. The association stoichiometry of probe-119 with Fe(II) ions was estimated from Job's plot to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was supported by 1H NMR and theoretical calculations. The LOD value was appraised from the fluorescence titration data and exhibited to be 0.0363 μM, which is much lesser than the WHO suggested value (5 μM) for Fe(II) in the drinking water. As probe-119 possesses a good binding constant and low detection limits, it was further applied for monitoring Fe(II) ions in diverse samples such as lake, well and river water.
1, which was supported by 1H NMR and theoretical calculations. The LOD value was appraised from the fluorescence titration data and exhibited to be 0.0363 μM, which is much lesser than the WHO suggested value (5 μM) for Fe(II) in the drinking water. As probe-119 possesses a good binding constant and low detection limits, it was further applied for monitoring Fe(II) ions in diverse samples such as lake, well and river water.
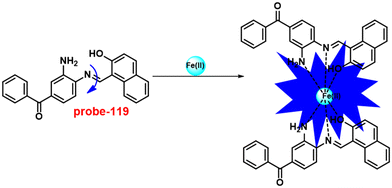 |
| | Fig. 177 Chemical structure and proposed sensing mechanism of probe-119 with Fe(II) (redrawn the ChemDraw structure from ref. 261). | |
Recently, Annaraj et al. have designed a rhodamine-containing Schiff base derivative for the selective recognition of Fe(III) ions and the tracking of Fe(III) ions in cellular media in 2020.262 The absorption titration data showed that adding Fe(III) and Cu(II) ions to probe-120 (Fig. 178) resulted in spectral changes accompanied by a color variation of solution from colorless to red color, which could originate from the cleavage of spirolactam, whereas there was no change in the presence of other competitive metal ions. The fluorescence titration data exhibited bright emission upon addition of Fe(III) ions to probe-120 at 554 nm, which could be due to the presence of a new ICT process, whereas very minor enrichment was noticed in the presence of Fe(II) ions. However, with other competitive metal ions, no significant change was observed in the emission spectra that strongly supported the selectivity towards Fe(III) ions. The detection limits of probe-120 towards Cu(II) and Fe(III) ions were calculated to be 263 pM and 3.1 nM respectively. The association constant of probe-120 for Cu(II) and Fe(III) was determined by the B–H equation as 5.2 × 10−2 M−1 and 1.1 × 10−2 M−1 correspondingly and the binding ratio was calculated from Job's plot to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was strongly validated by 1H NMR, ESI-MS and DFT calculations. Further, with the ability of probe-120 towards the detection of Fe(III) ions in cellular media, the fluorescence bio-imaging demonstrated its insignificant toxicity for the sensing of Fe(III) ions in zebrafish embryos. Thus, the results strongly indicate that probe-120 is a potential candidate for the recognition of Fe(III) ions in zebrafish embryos.
1, which was strongly validated by 1H NMR, ESI-MS and DFT calculations. Further, with the ability of probe-120 towards the detection of Fe(III) ions in cellular media, the fluorescence bio-imaging demonstrated its insignificant toxicity for the sensing of Fe(III) ions in zebrafish embryos. Thus, the results strongly indicate that probe-120 is a potential candidate for the recognition of Fe(III) ions in zebrafish embryos.
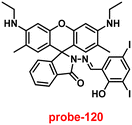 |
| | Fig. 178 Chemical structure of probe-120 (redrawn the ChemDraw structure from ref. 262). | |
In 2018, Selvakumar and co-workers263 designed and synthesized a naphthalene diamine-based β-diketone conjugate, which could exhibit a selective fluorescence turn-on response towards Fe(II) and a colorimetric response towards Fe(II) and Cu(II) ions. Probe-121 was obtained by the Schiff base condensation reaction of naphthalene-1,5-diamine and acetylacetone in ethanol, and the target compound synthesis was confirmed via various spectroscopic analysis. The sensing studies of probe-121 were conducted via UV-vis and fluorescence spectroscopic techniques. The absorption titration data demonstrated that upon addition of Fe(II) and Cu(II) ions to probe-121, absorbance was increased and competitive metal ions did not prompt any response. The fluorescence titration results of probe-121 with Fe(II) ions show that the emission intensity was gradually increased due to the suppression of the PET process (Fig. 179), whereas other competitive metal ions do not show any response with probe-121, which suggests a greater selectivity towards Fe(II). The complexometric ratio of probe-121 with Cu(II) and Fe(II) ions was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 via Job's plot and supported by DFT studies, and the association constants were estimated as 1.66 × 1010 and 9.22 × 108 M−1, correspondingly. The LOD values were estimated from the emission titration data and was estimated as 0.5 μM for Fe(II) ions. Further, probe-121 was found to show a binding interface with bovine serum albumin, which was detected by fluorescence quenching and FRET. Finally, the authors claimed that probe-121 possesses potential ability for the instantaneous recognition of Fe(II) and Cu(II) metal ions.
2 via Job's plot and supported by DFT studies, and the association constants were estimated as 1.66 × 1010 and 9.22 × 108 M−1, correspondingly. The LOD values were estimated from the emission titration data and was estimated as 0.5 μM for Fe(II) ions. Further, probe-121 was found to show a binding interface with bovine serum albumin, which was detected by fluorescence quenching and FRET. Finally, the authors claimed that probe-121 possesses potential ability for the instantaneous recognition of Fe(II) and Cu(II) metal ions.
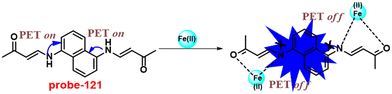 |
| | Fig. 179 Chemical structure and proposed sensing mechanism of probe-121 with Fe(II) (redrawn the ChemDraw structure from ref. 263). | |
Bhosale and co-worker aimed to synthesise benzothiazole and pyrene Schiff base derivatives for selective fluorescence turn-on behavior towards Fe(III) ions in 2017. The target molecule (probe-122)264 was synthesised by a condensation reaction between 2-amino-6-methoxy benzothiazole and pyrene aldehyde in ethanol under reflux conditions and the product was established by NMR and other analytical analyses. The sensing studies were conducted by using absorption and fluorescence spectroscopic techniques in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solvent combinations. From the UV-vis absorption data, on adding Fe(II) and Fe(III) ions, probe-122 exhibited a drastic change in absorption bands with colorimetric responses and the color variations from yellow to colorless and, no interference was observed with other metal ions. Probe-122 exhibits very weak emission due to the PET process (Fig. 180) and on titrating with Fe(II) and Fe(III) ions, the bright emission was observed with 189- and 141-fold enhancement respectively at 456 nm, which could be contributed to the suppression of the PET process. Upon addition of other competitive metal ions, there was no alteration in the emission spectra, which strongly suggested the selectivity of probe-122 towards Fe(II) and Fe(III) ions. The LOD values of probe-122 with Fe(II) and Fe(III) ions was calculated from the fluorescence titration data to be 2.61 μM and 2.06 μM, correspondingly. The association constant was calculated from the B–H equation and displayed for Fe(III) as 2.59 × 104 M−1 and the binding stoichiometry was obtained from Job's plot as 1
1, v/v) solvent combinations. From the UV-vis absorption data, on adding Fe(II) and Fe(III) ions, probe-122 exhibited a drastic change in absorption bands with colorimetric responses and the color variations from yellow to colorless and, no interference was observed with other metal ions. Probe-122 exhibits very weak emission due to the PET process (Fig. 180) and on titrating with Fe(II) and Fe(III) ions, the bright emission was observed with 189- and 141-fold enhancement respectively at 456 nm, which could be contributed to the suppression of the PET process. Upon addition of other competitive metal ions, there was no alteration in the emission spectra, which strongly suggested the selectivity of probe-122 towards Fe(II) and Fe(III) ions. The LOD values of probe-122 with Fe(II) and Fe(III) ions was calculated from the fluorescence titration data to be 2.61 μM and 2.06 μM, correspondingly. The association constant was calculated from the B–H equation and displayed for Fe(III) as 2.59 × 104 M−1 and the binding stoichiometry was obtained from Job's plot as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for probe-122 and Fe(III) ions. The reversible nature of probe-122 with Fe(III) ions was obtained upon addition of an EDTA solution. Thus, the authors claimed that probe-122 possesses a good association constant, and the detection limits make it a promising candidate for monitoring Fe(III) ions.
1 for probe-122 and Fe(III) ions. The reversible nature of probe-122 with Fe(III) ions was obtained upon addition of an EDTA solution. Thus, the authors claimed that probe-122 possesses a good association constant, and the detection limits make it a promising candidate for monitoring Fe(III) ions.
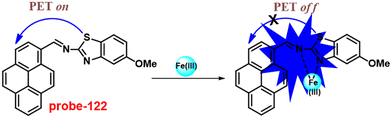 |
| | Fig. 180 Chemical structure and proposed sensing mechanism of probe-122 with Fe(III) ions (redrawn the ChemDraw structure from ref. 264). | |
A novel rhodamine-based Schiff base derivative for selective fluorescence turn-on for Fe(III) ions was obtained by Xu et al. in 2017.265Probe-123 was synthesized by a simple condensation reaction of rhodamine amine with aldehyde of a photochromic unit in ethanol under reflux conditions. The incorporation of photochromic units was found to have different color changes from blue to colorless under UV conditions and vice versa under visible light. The sensing studies of probe-123 (Fig. 181) demonstrated that upon titration of probe-123 with Fe(III) ions, the absorption bands at 360 and 530 nm were gradually increased and the color of solution was transformed from colorless to pink and very slight spectral changes were observed with the addition of Al(III) and Cr(III), whereas in the case of other competitive metal ions, no changes in the absorption spectra were observed. The fluorescence spectra of probe-123 were found to exhibit very weak emission and after addition of Fe(III) ions, the bright emission was generated at 585 nm with 168-fold. Conversely, no alteration in emission spectra was noticed for other competitive metal ions but a very slight enhancement for Al(III) and Cr(III) ions. These results strongly suggested that probe-123 shows very high selectivity towards Fe(III) ions. The binding stoichiometry of probe-123 with Fe(III) ions was calculated from Job's plot as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the LOD value of probe-123 with Fe(III) ions was estimated from the emission titration spectra to be 65 nM. The binding constant was estimated from the B–H equation as 5.97 × 104 M−1. The reversibility of probe-123 + Fe(III) ions was established upon addition of an EDTA solution.
1 and the LOD value of probe-123 with Fe(III) ions was estimated from the emission titration spectra to be 65 nM. The binding constant was estimated from the B–H equation as 5.97 × 104 M−1. The reversibility of probe-123 + Fe(III) ions was established upon addition of an EDTA solution.
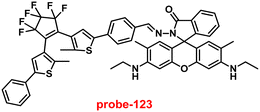 |
| | Fig. 181 Chemical structure of probe-123 (redrawn the ChemDraw structure from ref. 265). | |
Liang and team developed highly selective probe-124 (ref. 266) for monitoring Fe(III) ions in living cells as well as aqueous solutions in 2020. Target fluorescent probe-124 was synthesised via a condensation reaction between naphthalimide–monoaldehyde and 2-hydrazine pyridine. The emission profile of probe-124 exhibited very weak fluorescence, while upon complexation with Fe(III) ions, the turn-on fluorescence response was observed due to the rigidity and planarity with inhibition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 182). The selectivity of probe-124 was determined with other metal ions, nonetheless no remarkable emission changes were observed for other metal ions apart from Fe(III) ions, which reveals the selectivity and recognition of probe-124 even in the presence of other competitive metal ions (Fig. 183). The pH studies were carried out to identify the range over which the sensing was efficient and the results revealed that probe-124 exhibited extreme high emission intensity in the array of 3.2 to 6.4. Absorbance studies demonstrated that titration with Fe(III) ions induced significant changes in the spectra with the generation of a new band at 426 nm, which indicates the successful complexation of the metal ion with the ligand and color alteration from orange to light yellow, which was detectable by the naked eye. The LOD value was estimated based on the 3σ/slope method to be 38.3 nM with a binding constant of 1.77 × 106 M−1. Probe-124 offers good water solubility with its non-toxic nature and better cell membrane penetrability and was applied for the sensing of Fe(III) ions in biological media. In short, probe-124 could be considered as a promising candidate for the recognition of exogenous and endogenous detection of Fe(III) ions in biological samples and makes its practical applications very significant (Fig. 184).
N isomerization (Fig. 182). The selectivity of probe-124 was determined with other metal ions, nonetheless no remarkable emission changes were observed for other metal ions apart from Fe(III) ions, which reveals the selectivity and recognition of probe-124 even in the presence of other competitive metal ions (Fig. 183). The pH studies were carried out to identify the range over which the sensing was efficient and the results revealed that probe-124 exhibited extreme high emission intensity in the array of 3.2 to 6.4. Absorbance studies demonstrated that titration with Fe(III) ions induced significant changes in the spectra with the generation of a new band at 426 nm, which indicates the successful complexation of the metal ion with the ligand and color alteration from orange to light yellow, which was detectable by the naked eye. The LOD value was estimated based on the 3σ/slope method to be 38.3 nM with a binding constant of 1.77 × 106 M−1. Probe-124 offers good water solubility with its non-toxic nature and better cell membrane penetrability and was applied for the sensing of Fe(III) ions in biological media. In short, probe-124 could be considered as a promising candidate for the recognition of exogenous and endogenous detection of Fe(III) ions in biological samples and makes its practical applications very significant (Fig. 184).
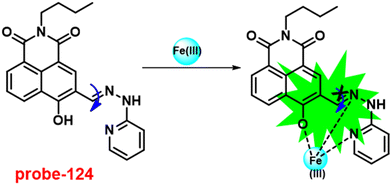 |
| | Fig. 182 Chemical structure and proposed sensing mechanism of probe-124 with Fe(III) (redrawn the ChemDraw structure from ref. 266). | |
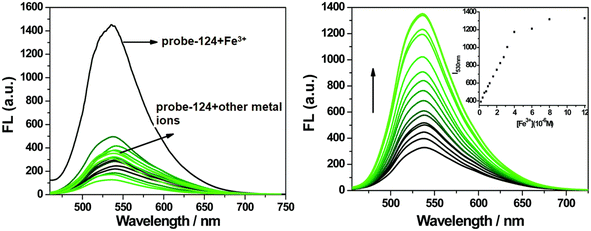 |
| | Fig. 183 Change in the emission spectra of probe-124 on increasing the concentration of Fe(III) ions (left) and with other ions (right) (reprinted with permission from ref. 266, Copyright 2020 Royal Society of Chemistry). | |
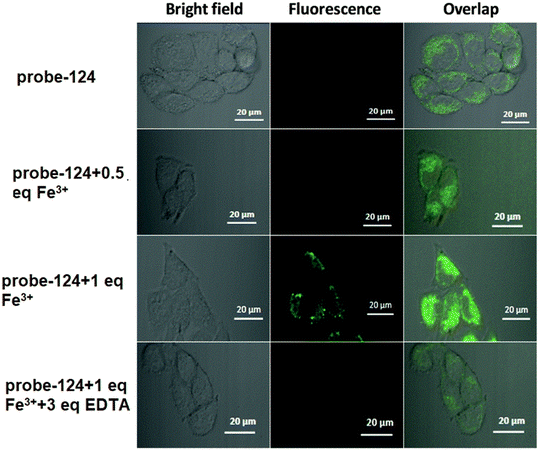 |
| | Fig. 184 Fluorescence microscopic images of bel-7420 cells treated with probe-124 and in the presence of Fe(III) ions (reprinted with permission from ref. 266, Copyright 2020 Royal Society of Chemistry). | |
A quinoline-based Schiff base conjugate showing selective fluorescence turn-on and colorimetric responses towards Fe(III) ions was designed by Tian and co-workers in 2017.267 Target probe-125 was synthesized by a reaction between quinone aldehyde and 4-methoxyaniline. The UV-vis titration data of probe-125 demonstrated that on titrating with Fe(III) ions, probe-125 resulted in an increase in absorbance with a color alteration from colorless to red color, while after addition of other competitive metal ions, there was no such significant change. The fluorescence spectra of probe-125 showed very weak emission, while in the presence of Fe(III) ions, the emission intensity progressively increased by 44-fold due to the suppression of PET as well as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 185). However, no other metal ions were found to have similar observations, which indicate that probe-125 was very selective towards Fe(III) ions. The association constant of probe-125 towards Fe(III) ions was found to be 7.22 × 104 M−1 using the B–H equation. Further, the complexation ratio was estimated from Job's plot as 1
N isomerization (Fig. 185). However, no other metal ions were found to have similar observations, which indicate that probe-125 was very selective towards Fe(III) ions. The association constant of probe-125 towards Fe(III) ions was found to be 7.22 × 104 M−1 using the B–H equation. Further, the complexation ratio was estimated from Job's plot as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was strongly supported by ESI-MS and 1H NMR. The LOD value was calculated from the fluorescence titration for probe-125 with Fe(III) ions to be 0.048 μM. Thus, due to the abnormal photophysical properties of probe-125, it was applied for the recognition of Fe(III) ions in environmental as well as biological samples.
1, which was strongly supported by ESI-MS and 1H NMR. The LOD value was calculated from the fluorescence titration for probe-125 with Fe(III) ions to be 0.048 μM. Thus, due to the abnormal photophysical properties of probe-125, it was applied for the recognition of Fe(III) ions in environmental as well as biological samples.
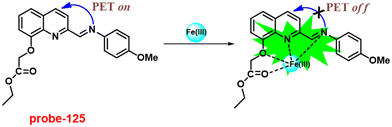 |
| | Fig. 185 Chemical structure and proposed sensing mechanism of probe-125 with Fe(III) (redrawn the ChemDraw structure from ref. 267). | |
11. Conclusion and future perspectives
The primary focus of this article is to review the recent design and synthesis of several novel Schiff base conjugates with fluorescent moieties for the sensitive and selective recognition of biological metal ions, especially transition metal ions. Transition metal ions play an important role in biological systems; hence recognition and monitoring are very mandatory. In this concern, bio-imaging can give more information regarding the accumulation of metal ions in particular organelles. There are several probes reported previously for the detection of transition metals, among which Schiff base-based chemosensors have attracted special attention due to their easy way of synthesis, stability towards heat and light and interesting optical properties such as poor emission in the absence of metal, whereas in the presence of specific metal ions (cavity size of Schiff bases), they show very bright luminescence. Here the sensing mechanism of Schiff bases with various metal ions is mainly based on the PET, RET, FRET, CHEF and ICT processes. Interestingly, after the incorporation of fluorescent moieties (signaling unit) in conjugation with Schiff bases, very high fluorescence was observed upon their binding to specific metal ions, which is utilized for the sensing of metal ions in cellular media via confocal fluorescence microscopic images. According to the recent literature reports, Schiff base-based fluorescent probes have very low cytotoxicity, and hence, they could be used to detect metal ions in biological media via cellular imaging with very good reversibility. Furthermore, their derivatives demonstrated a very efficient luminous response in biological media, allowing them to easily pass through the cells without interfering with or compromising cellular functions. Aside from the fluorescence turn-on response to metal ions, there are numerous Schiff base-based probes that can perform detection via colorimetric ability, in which the response can be seen with the naked eye and was used to detect metal ions in polluted ecological systems. Moreover, synthesizing materials by inclusion of Schiff bases in nanoparticles via self-assembly is also considered promising in the realm of supramolecular chemistry, and these materials are used as potential candidates for use in the fields of medicine, biotechnology, and biochemistry. However, a close examination of the recent literature reveals that the researchers are focusing mainly on the numerous design strategies based on Schiff base units for the selective and sensitive sensing of transition metal ions; however, the probes have innumerable restrictions in order to respond to metal ions in individual cell lines as well as specific organelles. There are few known Schiff base conjugates showing very low selectivity as well as poor permeability, and only few Schiff base-based probes for selective and sensitive detection of paramagnetic metal ions such as Fe(II), Cr(III), Fe(III), Cu(II), Ni(II), Co(II), and Mn(II) ions in biological media with moderately excellent quantum yields are known in the literature. In addition, many synthetic probes do not have the potential to remove hazardous metal ions from ecological systems due to the lack of selectivity, which is a key issue in water research. As a result, this gap in work continues to motivate researchers to strive on enhanced probes.
In sensing chemistry, designing probes by focusing on the incorporation of several artificial fluorescent dyes such as quinoline, anthracene, coumarin, fluorescein, rhodamine, and bipyridine on Schiff bases via synthetic and structural modifications shows the potential detection of various metal ions via the fluorescence turn-on mechanism with interesting applications. For instance, such probes work with fast response to specific metal ions, with exceptionally low detection limits showing strong luminescence characteristics in the biological media. Moreover, these molecular systems can absorb and emit in the red or near-infrared range and be utilized for biological applications because of their ease of penetration via cellular media and the blood–brain barrier, which will provide a better picture about the accumulation of metal ions. Here, we have discussed some of the intriguing Schiff base-based probes for the detection of metal ions focusing on biological media as well as their imaging applications (Table 1). For example, the incorporation of a rhodamine moiety on the Schiff base unit, which exhibits red or NIR emission, can be effectively implemented for the tracking of metal ions in human organelles and will give the dye the exceptional feature to make it ideal for sensing and identifying early-stage diseases such as Alzheimer's and Parkinson's. Moreover, the monitoring of other biologically important paramagnetic metal ions is also possible with the help of other Schiff base–incorporated fluorescent dyes, which showed highly fluorescence turn-on.
Table 1 Chart comparing the key characteristics of the reported Schiff base-based fluorescent turn-on probes for the above-mentioned biologically important metal ions
| Sl. no. |
Probe |
Target metal ions |
Association constant |
Mechanism |
Detection limit |
Live cell imaging |
Ref. |
| 1 |
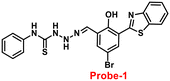
|
Zn(II) |
4.155 × 104 M−1 |
CHEF on |
37.7 nM |
HeLa cells and live SH-SY5Y neuroblastoma cells |
61
|
| 2 |
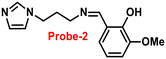
|
Zn(II) |
NA |
Restriction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization N isomerization |
31.044 nM |
HeLa and DU-145 cancer cell lines |
62
|
| 3 |
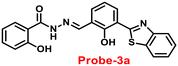
|
Zn(II) |
1.6 × 105 M−1 |
Suppression of ESIPT |
0.16 μM |
Human breast cancer cell lines (MCF-7) |
63
|
| 4 |
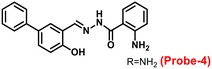
|
Zn(II) |
NA |
ICT and CHEF |
72 nM |
HeLa cells and zebrafish models |
64
|
| 5 |
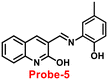
|
Zn(II) |
3.7 × 102 M−1 |
PET off and CHEF |
72 nM |
A549 cells and zebrafish embryos |
65
|
| 6 |
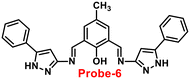
|
Zn(II) |
NA |
CHEF |
27.80 nM |
NA |
66
|
| 7 |
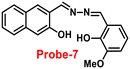
|
Zn(II) |
NA |
CHEF |
0.11 μM |
NA |
67
|
| 8 |
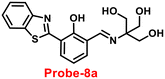
|
Zn(II) |
4.53 × 104 M−1 |
CHEF |
7 nM |
HeLa cells |
68
|
| 9 |
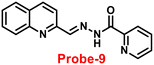
|
Zn(II) |
6.65 × 103 M−1 |
CHEF |
72 nM |
NA |
69
|
| 10 |
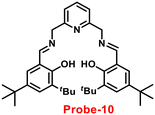
|
Zn(II) |
1.8 × 101 M−1 |
NA |
7.2 μM |
NA |
70
|
| 11 |
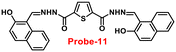
|
Zn(II) |
1.15 × 104 M−1 |
CHEF |
0.15 μM |
NA |
71
|
| 12 |
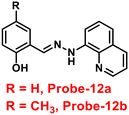
|
Zn(II) |
5.90 × 104 M−1 |
PET |
220.6 nM |
C6 cells |
72
|
| 13 |
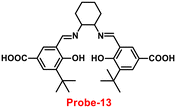
|
Zn(II) |
6.05 μM |
ICT-off |
56 nM |
Live cells, larval zebrafish, and plants |
73
|
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
| ESIPT |
| 14 |
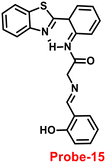
|
Zn(II) |
2.4 × 104 M−1 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
4.5 nM |
NA |
75
|
| ESIPT |
| 15 |
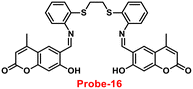
|
Zn(II) |
6.49 × 104 M−1 |
ESIPT and CHEF |
0.068 μM |
SCC084 (human oral carcinoma) cells |
76
|
| 16 |
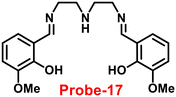
|
Zn(II) |
31.647 × 104 M−1 |
CHEF and ESIPT |
6.72 nM |
HCT 116 cells |
77
|
| 17 |
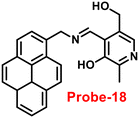
|
Zn(II) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β = 10.03(1) β = 10.03(1) |
PET |
2.34 μM |
HeLa cells |
78
|
| 18 |
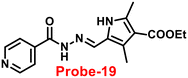
|
Zn(II) |
1.25 × 104 M−1 |
CHEF |
0.18 μM |
U251 cells |
79
|
| 19 |
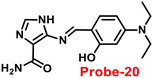
|
Zn(II) |
2.0 × 103 M−1 |
PET off and CHEF |
1.59 μM |
HeLa cells |
80
|
| 20 |
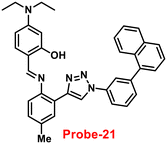
|
Zn(II) |
9.06 × 104 M−1 |
CHEF |
1.8 μM |
NA |
81
|
| 21 |
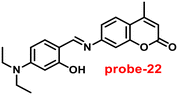
|
Zn(II) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 6.04 K = 6.04 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and PET N isomerisation and PET |
2.59 μM |
NA |
82
|
| 22 |
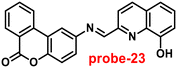
|
Zn(II) |
3.8 × 103 M−1 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and PET N isomerisation and PET |
10 μM |
NA |
83
|
| 23 |
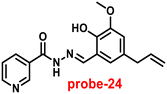
|
Zn(II) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 5.63(0.15) K = 5.63(0.15) |
CHEF and PET off |
4.35 nM |
A549 cells |
84
|
| 24 |
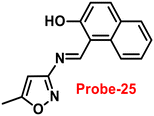
|
Zn(II) |
7.9 × 104 M−1 |
CHEF |
1.29 μM |
Zebrafish |
85
|
| 25 |
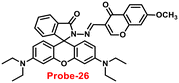
|
Zn(II) |
9.98 × 104 M−1 |
PET |
0.33 μM |
NA |
86
|
| 26 |
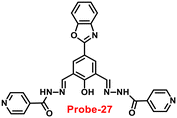
|
Zn(II) |
4.53 × 104 M−1 |
ICT |
0.52 μM |
HeLa cells |
87
|
| 27 |
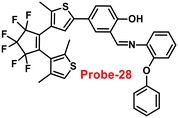
|
Zn(II) |
3.49 × 104 M−1 |
Suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and CHEF on N isomerisation and CHEF on |
13.4 nM |
NA |
88
|
| 28 |
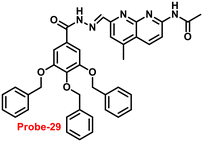
|
Zn(II) |
1.14 × 105 M−1 |
Suppression of PET and CHEF on |
7.52 nM |
NA |
89
|
| 29 |
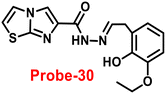
|
Zn(II) |
2.2 × 105 M−1 |
Suppression of PET and CHEF on |
1.2 nM |
NA |
90
|
| 30 |
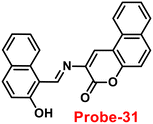
|
Zn(II) |
3.21 × 104 M−1 |
Suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and PET N isomerisation and PET |
3.6 μM |
NA |
91
|
| 31 |
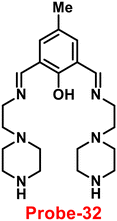
|
Zn(II) |
7.14 × 104 M−1 |
CHEF |
1.059 nM |
MDA-MB-468 cells |
92
|
| 32 |
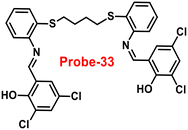
|
Zn(II) |
6.91 × 104 M−1 |
CHEF and suppression of ESIPT |
1.73 nM |
MCF-7 cells |
93
|
| 33 |
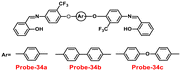
|
Zn(II) |
1.42 × 106 M−1 |
CHEF and restriction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
67.2 nM |
SW620 cancer cells |
94
|
| 34 |
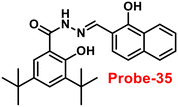
|
Zn(II) |
7.79 × 106 M−1 |
ESIPT and ICT |
0.31 μM |
HeLa cells |
95
|
| 35 |
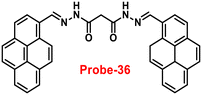
|
Zn(II) |
3 × 105 M−1 |
PET |
5.1 nM |
HeLa cells |
96
|
| 36 |
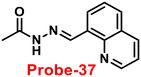
|
Zn(II) |
NA |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
89.3 nM |
HeLa cells |
97
|
| 37 |
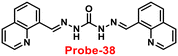
|
Zn(II) |
1.03 × 104 M−1 |
CHEF |
0.66 μM |
HeLa cells |
98
|
| 38 |
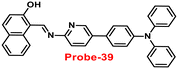
|
Zn(II) |
3.24 × 104 M−1 |
PET and CHEF |
19.134 nM |
HeLa cells |
99
|
| 39 |
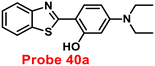
|
Zn(II) |
4.9 × 104 M−1 |
ESIPT and CHEF |
67 nM |
NA |
100
|
| 40 |
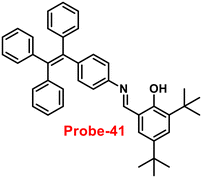
|
Zn(II) |
NA |
PET |
80.5 nM |
NA |
101
|
| 41 |
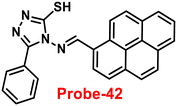
|
Zn(II) |
4.205 × 10−7 M−2 |
PET |
0.79 nM |
B16F10 cell lines and in zebrafish |
102
|
| 42 |
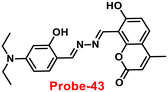
|
Zn(II) |
NA |
Reduction of π–π interactions and PET |
30.3 nM |
SiHa cells |
103
|
| 43 |
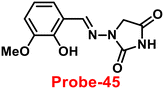
|
Zn(II) |
4.5 × 103 M−1 |
CHEF |
11.9 μM |
HeLa cells |
105
|
| 44 |
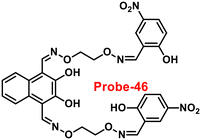
|
Zn(II) |
2.71 × 104 M−1 |
PET off |
0.52 μM |
NA |
106
|
| 45 |
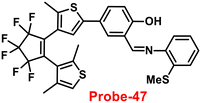
|
Zn(II) |
4.692 × 105 M−1 |
NA |
38.6 nM |
NA |
107
|
| 46 |
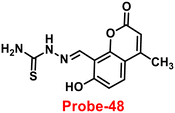
|
Zn(II) |
2.847 × 103 M−1 |
ESIPT |
6 nM |
Cancer cells |
108
|
| 47 |
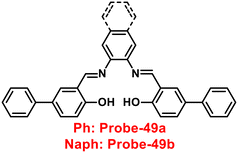
|
Zn(II) |
NA |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation, PET and ESIPT N isomerisation, PET and ESIPT |
7.69 and 5.35 nM |
HeLa cells |
109
|
| 48 |
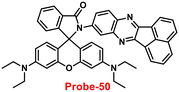
|
Hg(II) |
NA |
Ring opening of spirolactum |
10 nM |
HeLa cells and zebra fish |
120
|
| 49 |
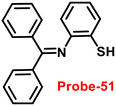
|
Hg(II) |
4.484 × 105 M−1 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and ESIPT N isomerisation and ESIPT |
22 nM |
NA |
121
|
| 50 |
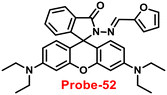
|
Hg(II) |
1.01 × 104.94 M−1 |
Ring opening of spirolactum |
0.09 μM |
SW480 cells |
122
|
| 51 |

|
Hg(II) |
NA |
Suppression of RIR and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
3.4 nM |
HeLa cells |
123
|
| 52 |
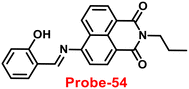
|
Hg(II) |
6.89 × 106 M−1 |
Hydrolysis |
0.5 nM |
NA |
124
|
| 53 |
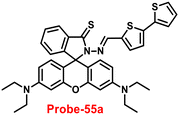
|
Hg(II) |
3.94 × 105 M−1 |
Ring opening |
0.62 ppb |
HeLa cells |
125
|
| 54 |
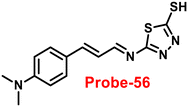
|
Hg(II) |
2.5 × 104 M−1 |
CHEF |
2 μM |
NA |
126
|
| 55 |
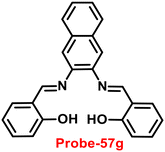
|
Hg(II) |
NA |
ESIPT |
12.5 ppb |
NA |
127
|
| 56 |
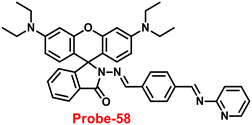
|
Hg(II) |
4.149 × 103 M−1 |
CHEF caused by ring opening |
1.729 ng mL−1 |
A375 cells |
128
|
| 57 |
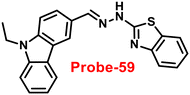
|
Hg(II) |
1.88 × 105 M−1 |
ICT on |
0.14 μM |
NA |
129
|
| 58 |
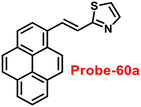
|
Hg(II) |
5.8 × 105 M−2 |
Schiff base hydrolysis |
0.270 μM |
NA |
130
|
| 59 |
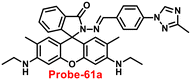
|
Hg(II) |
NA |
PET and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
13.4 nM |
Live breast cancer cells and MCF-7 cells |
131
|
| 60 |
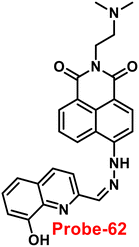
|
Hg(II) |
4.12 × 105 M−1 |
PET |
0.24 μM |
NA |
132
|
| 61 |
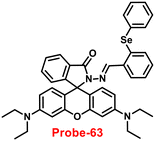
|
Hg(II) |
7.44 × 104 M−1 |
Ring opening |
12 nM |
HeLa cells and zebrafish |
133
|
| 62 |
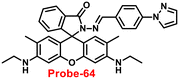
|
Hg(II) |
NA |
PET and CHEF |
0.2 nM |
NA |
134
|
| 63 |
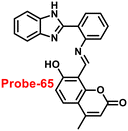
|
Hg(II) |
8.75 × 104 M−1 |
Hydrolysis |
70 nM |
HeLa cells |
135
|
| 64 |
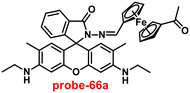
|
Hg(II) |
NA |
Ring opening |
NA |
THP-1 cells |
136
|
| 65 |
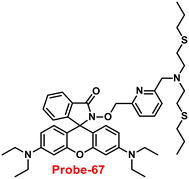
|
Hg(II) |
3.28 × 105 M−1 |
Ring opening |
2.36 μM |
PC3 cells |
137
|
| 66 |
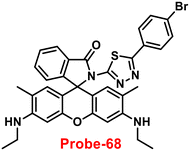
|
Hg(II) |
6 × 104 M−1 |
Ring opening |
30.37 nM |
MDA-MB-231 and A375 breast cancer cells |
138
|
| 67 |
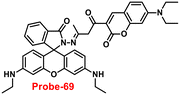
|
Hg(II) |
3.34 × 104 M−1 |
Ring opening |
2.96 μM |
HeLa cells |
139
|
| 68 |
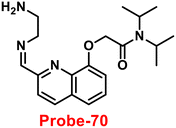
|
Cd(II) |
NA |
NA |
2.4 nM |
NA |
150
|
| 69 |
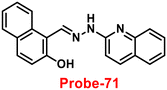
|
Cd(II) |
1.77 × 105 M−1 |
CHEF and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
0.14 nM |
NA |
151
|
| 70 |
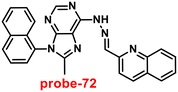
|
Cd(II) |
0.862 × 105 M−1 |
PET and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
41 nM |
HeLa cells |
152
|
| 71 |
|
Cd(II) |
1.17 × 104 M−1 |
ICT on and CHEF |
μM level |
HeLa cells |
153
|
| 72 |
|
Cd(II) |
1.628 × 104 M−1 |
ICT off |
0.38 μM |
Human liver cancer cells (SMMC-7721), zebrafish, and live tissues of Arabidopsis thaliana |
154
|
| 73 |
|
Au(III) |
NA |
Ring opening and suppression of PET |
1.51 μM |
MC3T3 cells |
165
|
| 74 |
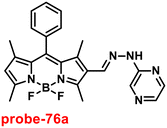
|
Au(III) |
NA |
Hydrolysis |
60 nM |
PC12 cells and zebrafish |
166
|
| 75 |
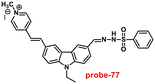
|
Au(III) |
NA |
Schiff base hydrolysis |
22 nM |
MCF-7 and HeLa cells |
167
|
| 76 |
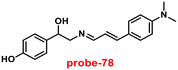
|
Ag(I) |
7.00 × 104 M−1 |
NA |
1.49 μM |
NA |
180
|
| 77 |
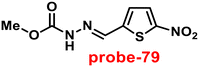
|
Ag(I) |
1.58 × 106 M−1 |
ICT off |
0.12 μM |
E. coli cells |
181
|
| 78 |
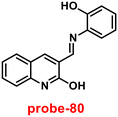
|
Ag(I) |
2.41 × 104 M−2 |
PET off and ICT on |
14 μM |
E. coli cells |
182
|
| 79 |
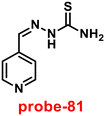
|
Ag(I) |
2.8 × 109 M−2 |
PET off |
1.6 μM |
NA |
183
|
| 80 |
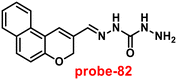
|
Cu(II) |
NA |
ICT on |
10.4 nM |
HeLa cells |
195
|
| 81 |
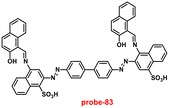
|
Cu(II) |
1.03 × 106 M−1 |
CHEF |
0.1 nM |
HepG2 cells |
196
|
| 82 |
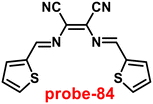
|
Cu(II) |
7.31 × 104 M−1 |
CHEF |
14.5 nM |
NA |
197
|
| 83 |
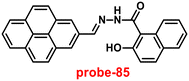
|
Cu(II) |
1.16 × 104 M−1 |
PET off |
0.26 μM |
RAW 264.7 cells |
198
|
| 84 |
|
Cu(II) |
NA |
Hydrolysis |
1.50 μM |
HeLa cells |
199
|
| 85 |
|
Cu(II) |
1.74 × 105 M−1 |
Decomposition of imine bond |
12.7 nM |
HepG2 cells |
200
|
| 86 |
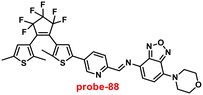
|
Cu(II) |
4 × 104 M−1 |
CHEF and restriction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
1.49 μM |
NA |
201
|
| 87 |
|
Cu(II) |
1.26 × 10−6 M−1 |
ICT |
27.4 nM |
NA |
202
|
| 88 |
|
Cu(II) |
2.5 × 108 M−2 |
Suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
0.49 μM |
HepG2 cells |
203
|
| 89 |
|
Cu(II) |
4.3 × 107 M−1 |
Suppression of PET |
15 nM |
NA |
204
|
| 90 |
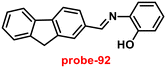
|
Cu(II) |
2.84 × 105 M−1 |
ICT quenching |
1.54 nM |
NA |
205
|
| 91 |
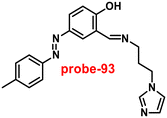
|
Cu(II) |
NA |
CHEF and restriction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
1.8 μM |
NA |
206
|
| 92 |
|
Cu(II) |
1.96 × 107 M−1 |
Restriction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
0.18 μM |
NA |
207
|
| 93 |
|
Cu(II) |
1.38 × 105 M−2 |
Suppression of PET |
3.90 nM |
NA |
208
|
| 94 |
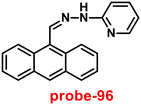
|
Cu(II) |
2.12 × 106 M−1 |
Suppression of PET |
0.1 nM |
Raw 264.7 cells |
209
|
| 95 |
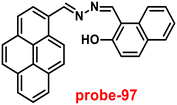
|
Cu(II) |
4.8 × 106 M−1 |
Break down the excimer formation |
0.1 μM |
HEK 293 cells |
210
|
| 96 |
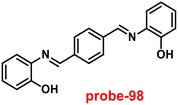
|
Cu(II) |
6.67 × 104 M−1 |
Suppression of PET and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
0.62 μM |
L929 cells |
211
|
| 97 |
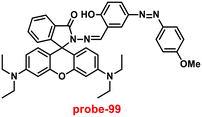
|
Cu(II) |
NA |
Spirolactum ring opening |
NA |
HepG2 cells |
212
|
| 98 |
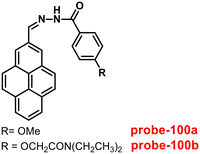
|
Cu(II) |
1.29 × 105 M−1 for 100a; 1.55 × 104 M−1 for 100b |
PET |
88 nM for 100a; 0.49 μM for 100b |
NA |
213
|
| 99 |
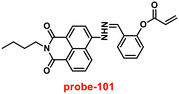
|
Cu(II) |
NA |
NA |
25 nM |
HeLa cells |
214
|
| 100 |
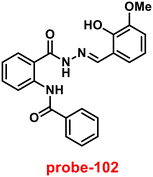
|
Ni(II) |
1.1 × 104 M−1 |
PET, ESIPT and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
1.71 μM |
HeLa cells |
225
|
| 101 |
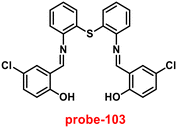
|
Ni(II) |
4.18 × 105 M−1 |
CHEF |
8.67 nM |
HeLa cells |
226
|
| 102 |
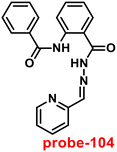
|
Ni(II) |
1.45 × 103 M−1/2 |
PET off |
1.18 μM |
HeLa cells |
227
|
| 103 |
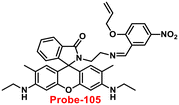
|
Pd(II) |
NA |
Ring opening of spirolactum |
50 nM |
HCT 116 cells |
237
|
| 104 |
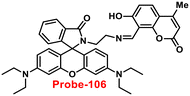
|
Pd(II) |
9.1 × 104 M−1 |
Ring opening of spirolactum |
18.8 nM |
MCF7 cells |
238
|
| 105 |
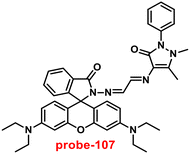
|
Pd(II) |
8.36 × 103 M−1 |
Ring opening of spirolactum |
11.9 μM |
MDA-MB-468 cells |
239
|
| 106 |
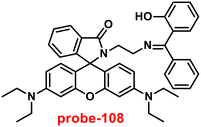
|
Pd(II) |
1.99 × 104 M−1 |
Ring opening of spirolactum |
34 nM |
MDA-MB-231 cells |
240
|
| 107 |
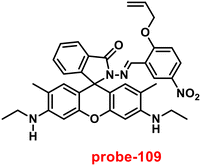
|
Pd(II) |
NA |
Ring opening of spirolactum |
95 nM |
RAW 264.7 cells |
241
|
| 108 |
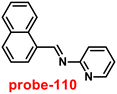
|
Fe(II) |
5.02 × 104 M−2 |
ICT |
0.15 μM |
NA |
252
|
| 109 |
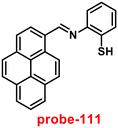
|
Fe(II) |
3.04 × 109 M−1 |
CHEF |
0.3 ppm |
NA |
253
|
| 110 |
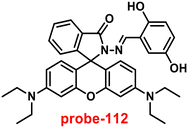
|
Fe(III) |
5 × 104 M−1 |
Ring opening of spirolactum |
19 nM |
NA |
254
|
| 111 |
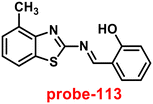
|
Fe(III) |
3.6 × 106 M−1 |
ICT |
0.89 nM |
HeLa cells |
255
|
| 112 |
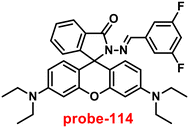
|
Fe(III) |
1.13 × 105 M−1 |
Ring opening of spirolactum |
6.94 μM |
NA |
256
|
| 113 |
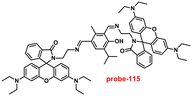
|
Fe(III) |
3.7025 × 106 M−1 |
PET suppression and CHEF, FRET on |
20 nM |
HeLa cells |
257
|
| 114 |
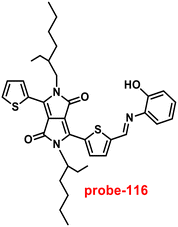
|
Fe(III) |
2.4 × 104 M−1 |
Suppression of PET and CHEF |
14.3 nM |
NA |
258
|
| 115 |
|
Fe(III) |
3.099 × 104 M−2 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and PET N isomerisation and PET |
0.178 μM |
NA |
259
|
| 116 |
|
Fe(III) |
1.04 × 104 M−1 |
TICT |
0.172 μM |
NA |
260
|
| 117 |
|
Fe(II) |
6.173 × 107 M−2 |
PET, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation, CHEF N isomerisation, CHEF |
0.0363 μM |
NA |
261
|
| 118 |
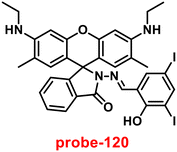
|
Fe(III) |
1.1 × 10−2 M−1 |
ICT |
3.1 nM |
Zebrafish |
262
|
| 119 |
|
Fe(II) |
9.22 × 108 M−1 |
PET off |
0.5 μM |
NA |
263
|
| 120 |
|
Fe(III) |
2.59 × 104 M−1 |
PET off |
2.06 μM |
NA |
264
|
| 121 |
|
Fe(III) |
5.97 × 104 M−1 |
Ring opening |
65 nM |
NA |
265
|
| 122 |
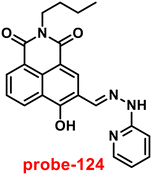
|
Fe(III) |
1.77 × 106 M−1 |
Inhibition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
38 nM |
bel-7420 cells |
266
|
| 123 |
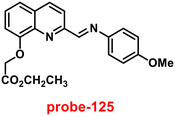
|
Fe(III) |
7.22 × 104 M−1 |
PET and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation N isomerisation |
0.048 μM |
NA |
267
|
The designing of receptor conjugates for the detection and monitoring of metal ions in biological systems needs to consider several parameters, and it should be stable in physiological media and meet the requirements of clinical diagnostics for the development of customized medicines in the future. Further, the design and synthesis of effective probes for the detection of analytes (especially biologically important metal ions) in complex biological fluids such as blood, serum, and urine is another hurdle in this field. Moreover, effective disease indicator recognition frequently necessitates multiplex exploration, which loads a significant number of relevant individual element detections. Regarding these, the design of particular fluorophores on Schiff bases as well as structural modification of Schiff bases needs to be reconsidered to show better photophysical properties and selective detection. Further, the incorporation of ammonium, phosphonium, and morpholine on Schiff bases can be utilized for the detection of metal ions in particular organelles.
Overall, in recent years, the development of Schiff base-based probes in the field of metal sensing chemistry has substantially favored the accurate monitoring of hazardous metal ions and health-related biomolecules, which is critical for safeguarding human health and the environment. Because of the functionalization of the Schiff base with fluorescent molecules, which works as a signaling agent and generates an exponential increase in its usage in sensing, the domains of advanced functional materials, environmental protection, and healthcare can continue to improve for years to come.
Abbreviations
| ICP-MS | Inductively coupled plasma mass spectroscopy |
| ICP-AES | Inductively coupled plasma atomic emission spectroscopy |
| AAS/AES | Atomic absorption/emission spectroscopy |
| AIE | Aggregation-induced emission |
| ESIPT | Excited-state intramolecular proton transfer |
| PET | Photoinduced electron transfer |
| ICT | Intramolecular charge transfer |
| CHEF | Chelation induced enhanced fluorescence |
| FRET | Fluorescence resonance energy transfer |
| nm | Nanometer |
| nM | Nanomolar |
| μM | Micromolar |
| NMR | Nuclear magnetic resonance |
| HRMS | High-resolution mass spectrometry |
| FT-IR | Fourier transform infrared |
| ATR-FTIR | Attenuated total reflectance-Fourier transform infrared spectroscopy |
| XRD | X-ray diffraction |
| LOD | Limit of detection |
| B–H | Benesi–Hildebrand |
| DFT | Density functional theory |
| TD-DFT | Time-dependent density-functional theory |
| ESI-MS | Electrospray ionisation mass spectrometry |
| WHO | World Health Organisation |
| EDTA | Ethylene diamine tetraacetate |
| AIEE | Aggregation-induced emission enhancement |
| DMF | Dimethylformamide |
| PPi | Pyrophosphate |
| PA | Picric acid |
| UV/PL | UV-vis absorption/photoluminescence |
| PXRD | Powder X-ray diffraction |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscopy |
| AFM | Atomic force microscopy |
| DLS | Dynamic light scattering |
| US-EPA | U.S. Environmental Protection Agency |
| EA | Elemental analysis |
| Cys | Cysteine |
| THF | Tetrahydrofuran |
| ppb | Parts per billion |
| ppm | Parts per million |
| ns | Nanoseconds |
| CV | Cyclic voltammogram |
| LC-MS | Liquid chromatography with tandem mass spectrometry |
| DMSO | Dimethylsulfoxide |
| BODIPY | Boron-dipyrromethene |
| MALDI-TOF | Matrix-assisted laser desorption ionization-time of flight mass spectrometry |
| ACN | Acetonitrile |
| LMCT | Ligand to metal charge transfer |
| MFC | Mechanofluorochromism |
| RIR | Restricted the intramolecular rotations |
| TEA | Triethylamine |
Author contributions
A. A. contributed to the conceptualization, planning, execution and drafting of the article. A. J. contributed to investigating standard detection techniques and future perspectives. C. A. S. P. contributed with proposed the conception, supervision, resources, review and editing of the article. All the authors thoroughly read the final manuscript draft and gave permission for its submission.
Conflicts of interest
The authors declare no competing financial interest.
Acknowledgements
C. A. S. P. thanks, SERB/SRG/2021/002112 for funding and National Institute of Technology for support. Ms. A. J. thanks National Institute of Technology, Calicut for institute fellowship and Ms. A. A. thanks for SERB/EEQ/2021/000180.
References
- Y. Hang, J. Boryczka and N. Wu, Chem. Soc. Rev., 2022, 51, 329 RSC.
- X. Zheng, W. Cheng, C. Ji, J. Zhang and M. Yin, Rev. Anal. Chem., 2020, 39, 231 CrossRef CAS.
- S. A. A. Razavi and A. Morsali, Coord. Chem. Rev., 2020, 415, 213299 CrossRef CAS.
- R. A. Bisergaevam and Y. N. Sirieva, J. Phys.: Conf. Ser., 2020, 1691, 012055 CrossRef.
- T. Xu, J. Hu and H. Chen, Microchem. J., 2019, 149, 103972 CrossRef CAS.
- Y. Qing, Y. Hang, R. Wanjaul, Z. Jiang and B. Hu, Anal. Sci., 2003, 19, 1417 CrossRef CAS PubMed.
- L. Cui, J. Wu and H. Ju, Biosens. Bioelectron., 2015, 63, 276 CrossRef CAS PubMed.
- J. F. Zhang, Y. Zhou, J. Yoon and J. S. Kim, Chem. Soc. Rev., 2011, 40, 3416 RSC.
- L. Prodi, F. Bolletta, M. Montalti and N. Zaccheroni, Coord. Chem. Rev., 2000, 205, 59 CrossRef CAS.
- J. S. Wu, W. M. Liu, J. C. Ge, H. Y. Zhang and P. F. Wang, Chem. Soc. Rev., 2011, 40, 3483 RSC.
- D. Udhayakumari and V. Inbaraj, J. Fluoresc., 2020, 30, 1203 CrossRef CAS PubMed.
- K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2013, 114, 4564 CrossRef PubMed.
- M. Formica, V. Fusi, L. Giorgi and M. Micheloni, Coord. Chem. Rev., 2012, 256, 170 CrossRef CAS.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105 RSC.
- S. Chowdhury, B. Rooj, A. Dutta and U. Mandal, J. Fluoresc., 2018, 28, 999 CrossRef CAS PubMed.
- P. Bhalla, K. Malhotra, N. Tomer and R. Malhotra, Inorg. Chem. Commun., 2022, 146, 110026 CrossRef CAS.
- P. A. Gale and C. Caltagirone, Chem. Soc. Rev., 2015, 44, 4212 RSC.
- P. Jiang and Z. Guo, Coord. Chem. Rev., 2004, 248, 205 CrossRef CAS.
- G. Sivaraman, M. Iniya, T. Anand, N. G. Kotla, O. Sunnapu, S. Singaravadivel, A. Gulyani and D. Chellappa, Coord. Chem. Rev., 2018, 357, 50 CrossRef CAS.
- J. Wu, W. Liu, J. Ge, H. Zhang and P. Wang, Chem. Soc. Rev., 2011, 40, 3483 RSC.
- J. Liu, Y. Q. Xie, Q. Lin, B. B. Shi, P. Zhang, Y. M. Zhang and T. B. Wei, Sens. Actuators, B, 2013, 186, 657 CrossRef CAS.
- S. A. Ingale and F. Seela, J. Org. Chem., 2012, 77, 9352 CrossRef CAS PubMed.
- I. Ravikumar and P. Ghosh, Inorg. Chem., 2011, 50, 4229 CrossRef CAS PubMed.
- M. Xiong, Z. Yang, R. J. Lake, J. Li, S. Hong, H. Fan, X.-B. Zhang and Y. Lu, Angew. Chem., Int. Ed., 2022, 59, 1891 CrossRef PubMed.
- J. H. Wang, Y. M. Liu, J. B. Chao, H. Wang, Y. Wang and S. Shuang, Sens. Actuators, B, 2020, 303, 127216 CrossRef CAS.
- L. Chen, D. Liu, J. Peng, Q. Du and H. He, Coord. Chem. Rev., 2020, 404, 213113 CrossRef CAS.
- P. Chen, W. Bai and Y. Bao, J. Mater. Chem., 2019, 7, 11731 CAS.
- S. Xu, K. Chen and H. Tian, J. Mater. Chem., 2005, 15, 2676 RSC.
- S. Subedi, L. N. Neupane, H. Yu and K. H. Lee, Sens. Actuators, B, 2021, 338, 129814 CrossRef CAS.
- L. Fu, F. L. Jiang, D. Fortin, P. D. Harvey and Y. Liu, Chem. Commun., 2011, 47, 5503 RSC.
- D. Maity, A. Mukherjee, S. K. Mandal and P. Roy, J. Lumin., 2019, 210, 508 CrossRef CAS.
- J. H. Kim, J. Y. Noh, I. H. Hwang, J. Kang, J. Kim and C. Kim, Tetrahedron Lett., 2013, 54, 2415 CrossRef CAS.
- H. Li, L. Cai and Z. Chen, Adv. Chem. Sens., 2021, 1, 121 Search PubMed.
- C. Liu, S. Huang, H. Yao, S. He, Y. Lu, L. Zhao and X. Zeng, RSC Adv., 2014, 4, 16109 RSC.
- H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465 RSC.
- K. T. A. Priyangga, Y. S. Kurniawan, K. Ohto and J. Jumina, J. Multidiscip. Appl. Nat. Sci., 2022, 2, 23 CrossRef.
- J. L. Segura, M. J. Mancheno and F. Zamora, Chem. Soc. Rev., 2016, 45, 5635 RSC.
- J. Wang, Q. Meng, Y. Yang, S. Zhong, R. Zhang, Y. Fang, Y. Gao and X. Cui, ACS Sens., 2022, 7, 2521 CrossRef CAS PubMed.
- C. M. Da Silva, L. V. Modolo, R. B. Alves, M. A. de Resende, C. V. Martins and A. de Fátima, J. Adv. Res., 2011, 2, 1 CrossRef.
- J. Cheng, K. Wei, X. Ma, X. Zhou and H. Xiang, J. Phys. Chem. C, 2013, 117, 16552 CrossRef CAS.
- P. G. Cozzi, Chem. Soc. Rev., 2004, 33, 410 RSC.
- S. Khan, X. Chen, A. Almahri, E. S. Allehyani, F. A. Alhumaydhi, M. M. Ibrahim and S. Ali, J. Environ. Chem. Eng., 2021, 9, 106381 CrossRef CAS.
- V. K. Gupta, A. K. Singh and L. K. Kumawat, Sens. Actuators, B, 2014, 195, 98 CrossRef CAS.
- J. Berrones-Reyes, B. M. Munoz-Flores, A. Gomez-Trevino, M. A. Treto-Suarez, D. Paez-Hernandez, E. Schott, X. Zarate and V. M. Jimenez-Perez, Mater. Chem. Phys., 2019, 233, 89 CrossRef CAS.
- Y. Wang, Z. Y. Ma, D. L. Zhang, J. L. Deng, X. Chen, C. Z. Xie, X. Qiao, Q. Z. Li and J. Y. Xu, Spectrochim. Acta, Part A, 2018, 195, 57 CrossRef PubMed.
- H. Tian, X. Qiao, Z. L. Zhang, C. Z. Xie, Q. Z. Li and J. Y. Xu, Spectrochim. Acta, Part A, 2019, 207, 31 CrossRef CAS PubMed.
- A. Jayaraj, M. S. Gayathri, G. Sivaraman and P. C. A. Swamy, J. Photochem. Photobiol., B, 2022, 226, 112371 CrossRef CAS PubMed.
- P. Middya, A. Saha and S. Chattopadhyay, Inorg. Chim. Acta, 2022, 545, 121246 CrossRef.
- A. Kumar, M. Saini, B. Mohan and M. Kamboj, Microchem. J., 2022, 181, 107798 CrossRef CAS.
- M. Kaur, S. Kumar, M. Yusuf, J. Lee, R. J. Brown, K. H. Kim and A. K. Malik, Coord. Chem. Rev., 2021, 449, 214235 CrossRef.
- J. M. Berg and Y. Shi, Science, 1996, 271, 1081 CrossRef CAS PubMed.
- X. Xie and T. G. Smart, Nature, 1991, 349, 521 CrossRef CAS PubMed.
- Q. Ai, BioMetals, 2001, 14, 315 CrossRef PubMed.
- B. L. Vallee and K. H. Falchuk, Physiol. Rev., 1993, 73, 79 CrossRef CAS PubMed.
-
(a) A. Helal and H. S. Kim, Tetrahedron Lett., 2009, 50, 5510 CrossRef CAS;
(b) J. H. Weiss, S. L. Sensi and J. Y. Koh, Trends Pharmacol. Sci., 2000, 21, 395 CrossRef CAS PubMed.
-
(a) A. Azam, H. M. Chawla and S. Pandey, Tetrahedron Lett., 2010, 51, 4710 CrossRef CAS;
(b) S. H. Mashraqui, T. Khan, S. Sundaram, R. Betkar and M. Chandiramani, Tetrahedron Lett., 2007, 48, 8487 CrossRef CAS.
-
(a) A. Voegelin, S. Pfister, A. C. Scheinost, M. A. Marcus and R. Kretzschmar, Environ. Sci. Technol., 2005, 39, 6616 CrossRef CAS PubMed;
(b) E. Callender and K. C. Rice, Environ. Sci. Technol., 2000, 34, 232 CrossRef CAS.
- Z. Xu, J. Yoon and D. R. Spring, Chem. Soc. Rev., 2010, 39, 1996 RSC.
- E. Tomat and S. J. Lippard, Curr. Opin. Chem. Biol., 2010, 14, 225 CrossRef CAS PubMed.
-
(a) D. Buccella, J. A. Horowitz and S. J. Lippard, J. Am. Chem. Soc., 2011, 133, 4101 CrossRef CAS PubMed;
(b) S. K. Rastogi, P. Pal, D. E. Aston, T. E. Bitterwolf and A. L. Branen, ACS Appl. Mater. Interfaces, 2011, 3, 1731 CrossRef CAS PubMed;
(c) A. Krezel, J. Wojcik, M. Maciejczyk and W. Bal, Inorg. Chem., 2011, 50, 72 CrossRef CAS PubMed;
(d) E. Tomat and S. J. Lippard, Inorg. Chem., 2010, 49, 9113 CrossRef CAS PubMed;
(e) S. Y. Kim and J. I. Hong, Tetrahedron Lett., 2009, 50, 2822 CrossRef CAS;
(f) L. Xue, H. H. Wang and H. Jiang, Inorg. Chem., 2008, 47, 4310 CrossRef CAS PubMed;
(g) B. Mohan, P. Balakrishnan, D. Umadevi and S. Shanmugaraju, Inorg. Chim. Acta, 2022, 533, 120798 CrossRef CAS.
- Q. Wu, L. Feng, J. B. Chao, Y. Wang and S. Shuang, Analyst, 2021, 146, 4348 RSC.
- S. N. Ansari, A. K. Saini, B. P. Kumari and S. M. Mobin, Inorg. Chem. Front., 2019, 6, 736 RSC.
- S. Gharami, K. Aich, D. Sarkar, P. Ghosh, N. Murmu and T. K. Mondal, New J. Chem., 2019, 43, 1857 RSC.
- Z. Lu, W. Fan, Y. Lu, C. Fan, H. Zhao, K. Guo, W. Chua and Y. Lu, New J. Chem., 2018, 42, 12198 RSC.
- A. S. Murugan, N. Vidhyalakshmi, U. Ramesh and J. Annaraj, J. Mater. Chem. B, 2017, 5, 3195 RSC.
- S. Lohar, S. Pal, M. Mukherjee, A. Maji, N. Demitri and P. Chattopadhyay, RSC Adv., 2017, 7, 25528 RSC.
- M. Shyamal, P. Mazumdar, S. Maity, S. Samanta, G. P. Sahoo and A. Misra, ACS Sens., 2016, 1, 739 CrossRef CAS.
- C. Chang, F. Wang, T. Wei and X. Chen, Ind. Eng. Chem. Res., 2017, 56, 8797 CrossRef CAS.
- J.-T. Wang, Y.-Y. Pei, M.-Y. Yan, Y. G. Li, G.-G. Yang, C.-H. Qu, W. Luo, J. Wang and Q.-F. Li, Microchem. J., 2021, 160, 105776 CrossRef CAS.
- K. M. Wyss, E. E. Hardy and A. E. V. Gorden, Inorg. Chim. Acta, 2019, 492, 156 CrossRef CAS.
- M. M. Mathew and A. Sreekanth, Inorg. Chim. Acta, 2021, 516, 120149 CrossRef.
- D. Maity, A. Mukherjee, S. K. Mandal and P. Roy, J. Lumin., 2019, 210, 508 CrossRef CAS.
- X. He, F. Ding, X. Sun, Y. Zheng, W. Xu, L. Ye, H. Chen and J. Shen, Inorg. Chem., 2021, 60, 5563 CrossRef CAS PubMed.
- S. J. Malthus, S. A. Cameron and S. Brooker, Inorg. Chem., 2018, 57, 2480 CrossRef CAS PubMed.
- S. Janakipriya, S. Tamilmani and S. Thennarasu, RSC Adv., 2016, 6, 71496 RSC.
- C. Patra, A. K. Bhanja, A. Mahapatra, S. Mishra, K. D. Sahab and C. Sinha, RSC Adv., 2016, 6, 76505 RSC.
- B. Naskar, R. Modak, D. K. Maiti, M. G. B. Drew, A. Bauza, A. Frontera, C. D. Mukhopadhyay, S. Mishra, K. D. Sahae and S. Goswami, Dalton Trans., 2017, 46, 9498 RSC.
- Y. Upadhyay, T. Anand, L. T. Babu, P. Paira, G. Crisponi, S. K. A. Kumar, R. Kumar and S. K. Sahoo, Dalton Trans., 2018, 47, 742 RSC.
- Y. Wang, X. Hou, Z. Li, Q. Zhou, M. Lei, S. Hu, X. Wu, C. Li, Z. Xu and Y. Wang, Anal. Methods, 2018, 10, 5790 RSC.
- J. Y. Yun, J. B. Chae, M. Kim, M. H. Lim and C. Kim, Photochem. Photobiol. Sci., 2019, 18, 166 CrossRef CAS PubMed.
- S. Ghosh, N. Baildya and K. Ghosh, New J. Chem., 2021, 45, 10923 RSC.
- J.-C. Qin, L. Fan and Z.-Y. Yang, Sens. Actuators, B, 2016, 228, 156 CrossRef CAS.
- N. Roy, A. Dutta, P. Mondal, P. C. Paul and T. S. Singh, Sens. Actuators, B, 2016, 236, 719 CrossRef CAS.
- M. Patil, S. Bothra, S. K. Sahoo, H. A. Rather, R. Vasita, R. Bendre and A. Kuwar, Sens. Actuators, B, 2018, 270, 200 CrossRef CAS.
- J. M. Jung, D. Yun, H. Lee, K.-T. Kim and C. Kim, Sens. Actuators, B, 2019, 297, 126814 CrossRef CAS.
- L.-M. Liu and Z.-Y. Yang, J. Photochem. Photobiol., A, 2018, 364, 558 CrossRef CAS.
- A. Gomathi, P. Viswanathamurthi and K. Natarajan, J. Photochem. Photobiol., A, 2019, 370, 75 CrossRef.
- F. Liu, C. Fan and S. Pu, J. Photochem. Photobiol., A, 2019, 371, 248 CrossRef CAS.
- C.-R. Li, G.-Q. Wang, L. Fan, S. L. Li, J.-C. Qin and Z.-Y. Yang, J. Photochem. Photobiol., A, 2019, 375, 231 CrossRef CAS.
- Y. Xu, H. Wang, J. Zhao, X. Yang, M. Pei, G. Zhang, Y. Zhang and L. Lin, J. Photochem. Photobiol., A, 2019, 383, 112026 CrossRef CAS.
- C. Liu, J.-C. Qin, J. Xue, L.-M. Tian, T.-R. Li and Z.-Y. Yang, J. Photochem. Photobiol., A, 2019, 385, 112091 CrossRef CAS.
- J. Mandal, P. Ghorai, K. Pal, P. Karmakar and A. Saha, J. Lumin., 2019, 205, 14 CrossRef CAS.
- S. Acharyya, S. Gharami, D. Sarkar, P. Ghosh, N. Murmu and T. K. Mondal, J. Mol. Struct., 2021, 1224, 129179 CrossRef CAS.
- X. Wang, G. Ding, Y. Duan, M. Wang, G. Zhu, X. Li, Y. Zhang and X. Qin, Tetrahedron, 2020, 76, 131108 CrossRef CAS.
- M. Budri, G. Naik, S. Patil, P. Kadolkar, K. Gudasi and S. Inamdar, J. Photochem. Photobiol., A, 2020, 390, 112298 CrossRef CAS.
- B. K. Rani and S. A. John, J. Photochem. Photobiol., A, 2021, 418, 113372 CrossRef CAS.
- W.-N. Wu, P.-D. Mao, Y. Wang, X.-L. Zhao, Z.-Q. Xu, Z.-H. Xu and Y. Xue, Spectrochim. Acta, Part A, 2018, 188, 324 CrossRef CAS PubMed.
- L.-L. Gao, S.-P. Li, Y. Wang, W.-N. Wu, X.-L. Zhao, H.-J. Li and Z.-H. Xu, Spectrochim. Acta, Part A, 2020, 230, 118025 CrossRef CAS PubMed.
- D. Wang, Q. Yin, M. Zheng, Y. Xie, W. He, Z. Li, S. Hou and H. Wang, Spectrochim. Acta, Part A, 2021, 251, 119480 CrossRef CAS PubMed.
- V. Venkatesan, R. S. Kumar, S. K. A. Kumar and S. K. Sahoo, Inorg. Chem. Commun., 2019, 102, 171 CrossRef CAS.
- J. Jia and H. Zhao, Org. Electron., 2019, 73, 55 CrossRef CAS.
- M. Shellaiah, Y.-T. Chen, N. Thirumalaivasan, B. Aazaad, K. Awasthi, K. W. Sun, S.-P. Wu, M.-C. Lin and N. Ohta, ACS Appl. Mater. Interfaces, 2021, 13, 28610 CrossRef CAS PubMed.
- A. Pandey, S. K. Asthana, A. Prakash, J. K. Roy, I. Tiwaria and K. K. Upadhyay, Dalton Trans., 2019, 48, 2068 RSC.
- S. Das, J. Adhikary, P. Chakraborty, T. Chakraborty and D. Das, RSC Adv., 2016, 6, 98620 RSC.
- M. S. Kim, T. G. Jo, M. Yang, J. Han, M. H. Lim and C. Kim, Spectrochim. Acta, Part A, 2019, 211, 34 CrossRef CAS PubMed.
- L.-Z. Liu, L. Wang, M. Yu, Q. Zhao, Y. Zhang, Y.-X. Sun and W.-K. Dong, Spectrochim. Acta, Part A, 2019, 222, 117209 CrossRef CAS PubMed.
- S. Guo, G. Liu, C. Fan and S. Pu, Sens. Actuators, B, 2018, 266, 603 CrossRef CAS.
- L. Wang, W. Li, W. Zhi, Y. Huang, J. Han, Y. Wang, Y. Ren and L. Ni, Sens. Actuators, B, 2018, 260, 243 CrossRef CAS.
-
M. Berlin, R. K. Zalups and B. A. Fowler, “Mercury”, in Handbook on the Toxicology of Metals, ed. G. F. Nordberg, B. A. Fowler, M. Nordberg and L. T. Friberg, Elsevier, New York, NY, USA, 3rd edn, 2007, ch. 33 Search PubMed.
- S. Bose-O'Reilly, L. Beate, R. M. Gothe, C. Beinhoff, U. Siebert and G. Drasch, Environ. Res., 2008, 107, 89 CrossRef.
- T. W. Clarkson, L. Magos and G. J. Myers, N. Engl. J. Med., 2003, 349, 1731 CrossRef CAS PubMed.
- T. W. Clarkson and L. Magos, Crit. Rev. Toxicol., 2006, 36, 609 CrossRef CAS PubMed.
- M. R. Knecht and M. Sethi, Anal. Bioanal. Chem., 2009, 394, 33 CrossRef CAS PubMed.
- Y. M. Yang, Q. Zhao, W. Feng and F. Y. Li, Chem. Rev., 2013, 113, 192 CrossRef CAS PubMed.
-
(a) N. Kumari, N. Dey and S. Bhattacharya, Analyst, 2014, 139, 2370 RSC;
(b) N. Kumari, N. Dey and S. Bhattacharya, RSC Adv., 2014, 4, 4230 RSC.
-
(a) S. Saha, H. Agarwalla, H. Gupta, M. Baidya, E. Suresh, S. K. Ghosh and A. Das, Dalton Trans., 2013, 42, 15097 RSC;
(b) P. Mahato, S. Saha, P. Das, H. Agarwalla and A. Das, RSC Adv., 2014, 4, 36140 RSC.
- U. G. Reddy, V. Ramu, S. Roy, N. Taye, S. Chattopadhyay and A. Das, Chem. Commun., 2014, 50, 14421 RSC.
- N. Kumari, N. Dey, S. Jha and S. Bhattacharya, ACS Appl. Mater. Interfaces, 2013, 5, 2438 CrossRef CAS PubMed.
- S. Saha, P. Mahato, U. G. Reddy, E. Suresh, A. Chakrabarty, M. Baidya, S. K. Ghosh and A. Das, Inorg. Chem., 2012, 51, 336 CrossRef CAS PubMed.
- C. Cui, X. Gao, X. Jia, Y. Jiao and C. Duan, Inorg. Chim. Acta, 2021, 520, 120285 CrossRef CAS.
- Q. Su, Q. Niu, T. Sun and T. Li, Tetrahedron Lett., 2016, 57, 4297 CrossRef CAS.
- B. Liu, X. Hu, J. Chai and B. Yang, Sens. Actuators, B, 2016, 228, 94 CrossRef CAS.
- W. Fang, G. Zhang, J. Chen, L. Kong, L. Yang, H. Bi and J. Yang, Sens. Actuators, B, 2016, 229, 338 CrossRef CAS.
- R. Goel, S. Sharma, K. Paul and V. Luxami, Sens. Actuators, B, 2017, 246, 776 CrossRef CAS.
- Y. Fang, X. Li, J.-Y. Li, G.-Y. Wang, Y. Zhou, N.-Z. Xu, Y. Hu and C. Yao, Sens. Actuators, B, 2018, 255, 1182 CrossRef CAS.
- R. Singh and G. Das, Sens. Actuators, B, 2018, 258, 478 CrossRef CAS.
- Z.-L. Wu, D. Shi, L. Huang, W.-Y. He, X.-Y. Sun, B. Liu and J.-S. Shen, Sens. Actuators, B, 2019, 281, 311 CrossRef CAS.
- P. G. Mahajan, J. S. Shin, N. C. Dige, B. D. Vanjare, Y. Han, N. G. Choi, S. J. Kim, S. Y. Seo and K. H. Lee, J. Photochem. Photobiol., A, 2020, 397, 112579 CrossRef CAS.
- D. B. C. Leslee, K. Sekar and M. M. Kothottil, J. Photochem. Photobiol., A, 2021, 415, 113303 CrossRef.
- V. Tekuri, S. K. Sahoo and D. R. Trivedi, Spectrochim. Acta, Part A, 2019, 218, 19 CrossRef CAS PubMed.
- W. Zhong, L. Wang, D. Qin, J. Zhou and H. Duan, ACS Omega, 2020, 5, 24285 CrossRef CAS PubMed.
- Y.-K. La, J.-A. Hong, Y.-J. Jeong and J. Lee, RSC Adv., 2016, 6, 84098 RSC.
- P. Venkatesan, N. Thirumalivasan and S.-P. Wu, RSC Adv., 2017, 7, 21733 RSC.
- G. Yang, X. Meng, S. Fang, H. Duan, L. Wang and Z. Wang, RSC Adv., 2019, 9, 8529 RSC.
- Y. Gao, C. Zhang, S. Peng and H. Chen, Sens. Actuators, B, 2017, 238, 455 CrossRef CAS.
- S. Dewangan, T. Barik, B. Halder, A. Mishra, R. Dhiman, T. Sasamori and S. Chatterjee, J. Organomet. Chem., 2021, 948, 121922 CrossRef CAS.
- M. Li, Y. Sun, L. Dong, Q.-C. Feng, H. Xu, S.-Q. Zang and T. C. W. Mak, Sens. Actuators, B, 2016, 226, 332 CrossRef CAS.
- B. D. Vanjare, P. G. Mahajan, H.-I. Ryoo, N. C. Dige, N. G. Choi, Y. Han, S. J. Kim, C.-H. Kim and K. H. Lee, Sens. Actuators, B, 2021, 330, 129308 CrossRef CAS.
- Z.-Q. Xu, X.-J. Mao, Y. Wang, W.-N. Wu, P.-D. Mao, X.-L. Zhao, Y.-C. Fan and H.-J. Li, RSC Adv., 2017, 7, 42312 RSC.
- P. Wang, D. Zhou and B. Chen, Spectrochim. Acta, Part A, 2019, 207, 276 CrossRef CAS PubMed.
- N. Shaily, A. Kumar and N. Ahmed, New J. Chem., 2017, 41, 14746 RSC.
- M. Huang, C. Lv, Q. Huang, J. Lai and H. Sun, RSC Adv., 2019, 9, 36011 RSC.
- B. Makwanal, D. Vyas, K. Bhatt, S. Darji and V. Jain, Appl. Nanosci., 2016, 6, 555 CrossRef.
- Z. Liu, C. Zhang, W. He, Z. Yang, X. Gao and Z. Guo, Chem. Commun., 2010, 46, 6138 RSC.
- A. Maity, U. Ghosh, D. Giri, D. Mukherjee, T. Maiti and S. Patra, Dalton Trans., 2019, 48, 2108 RSC.
-
B. R. Singh, Cadmium in Soils and Plants, ed. M. J. McLaughlin and B. R. Singh, 1999, vol. 257 Search PubMed.
- L. Xue, G. Li, Q. Liu, H. Wang, C. Liu, X. Ding, S. He and H. Jiang, Inorg. Chem., 2011, 50, 3680 CrossRef CAS PubMed.
- C. Lu, Z. Xu, J. Cui, R. Zhang and X. Qian, J. Organomet. Chem., 2007, 72, 3554 CAS.
- L. Xu, M.-L. He, H.-B. Yang and X. Qian, Dalton Trans., 2013, 42, 8218 RSC.
- X. Wan, H. Ke, J. Tang and G. Yang, Talanta, 2019, 199, 8 CrossRef CAS PubMed.
- D. Mohanasundaram, R. Bhaskar, G. G. V. Kumar, J. Rajesh and G. Rajagopal, Microchem. J., 2021, 164, 106030 CrossRef CAS.
- W. Chen, H. Xu, L. Ju and H. Lu, Tetrahedron, 2021, 88, 132123 CrossRef CAS.
- S. A. Khan, Q. Ullah, A. S. A. Almalki, S. Kumar, R. J. Obaid, M. A. Alsharif, S. Y. Alfaifi and A. A. Hashmi, J. Mol. Liq., 2021, 328, 115407 CrossRef CAS.
- J. H. Wang, Y. M. Liu, J. B. Chao, H. Wang, Y. Wang and S. M. Shuang, Sens. Actuators, B, 2020, 303, 127216 CrossRef CAS.
-
Gold: Progress in Chemistry, Biochemistry and Technology, ed. H. Schmidbaur, Wiley, Chichester, 1999 Search PubMed.
-
Gold Chemistry Applications and Future Directions in the Life Sciences, ed. F. Mohr, Wiley-VCH, Weinheim, 2009 Search PubMed.
- S. Singha, D. Kim, H. Seo, S. W. Cho and K. H. Ahn, Chem. Soc. Rev., 2015, 44, 4367 RSC.
- J. F. Zhang, Y. Zhou, J. Yoon and J. S. Kim, Chem. Soc. Rev., 2011, 40, 3416–3429 RSC.
- N. Duman, S. E. Evans, O. Karadag, S. As-çıoglu, B. S. Ener, S. Kiraz and S. S. Ahin, Int. J. Dermatol., 2014, 53, 1286 CrossRef PubMed.
- I. Ott, Coord. Chem. Rev., 2009, 253, 1670 CrossRef CAS.
- M. Navarro, Coord. Chem. Rev., 2009, 253, 1619 CrossRef CAS.
-
L. Messori and G. Marcon, Gold Complexes in the Treatment of Rheumatoid Arthritis, Metal Ions and their Complexes in Medication, CRC Press, 2004 Search PubMed.
- C. F. Shaw, Chem. Rev., 1999, 99, 2589 CrossRef CAS PubMed.
- A. Locke and E. R. Main, JAMA, J. Am. Med. Assoc., 1928, 90, 259 CrossRef.
- S. Mondal, S. K. Manna, S. Pathak, A. Ghosh, P. Datta, D. Mandal and S. Mukhopadhyay, New J. Chem., 2020, 44, 7954 RSC.
- E. Wang, L. Pang, Y. Zhou, J. Zhang, F. Yu, H. Qiao and X. Pang, Biosens. Bioelectron., 2016, 77, 812 CrossRef CAS PubMed.
- W. Wang, Y. Huang, S. Wang, Y. Zhou, W. Huang, Y. Feng, W. Zhang, W. Yu, Q. Zhou, M. Chen and M. Fang, Spectrochim. Acta, Part A, 2017, 187, 110 CrossRef CAS PubMed.
- T. W. Purcell and J. J. Peters, Environ. Toxicol. Chem., 1998, 17, 539 CrossRef CAS.
- M. Á. Corral, M. M. Dorado and I. R. Garcia, Chem. Rev., 2008, 108, 3174 CrossRef PubMed.
- J. F. Zhang, Y. Zhou, J. Yoon and J. S. Kim, Chem. Soc. Rev., 2011, 40, 3416 RSC.
-
(a) G. Chakrapani, P. L. Mahanta, D. S. R. Murty and B. Gomathy, Talanta, 2001, 53, 1139 CrossRef CAS PubMed;
(b) S. Dadfarnia, A. M. Haji Shabani and M. Gohari, Talanta, 2004, 64, 682 CrossRef CAS PubMed;
(c) G. S. Pomales, T. K. Mudalige, J. H. Lim and S. W. Linder, J. Agric. Food Chem., 2013, 61, 7250 CrossRef PubMed.
- E. Tan, P. Yin, X. Lang, X. Wang, T. You and L. Guo, Analyst, 2012, 137, 3925 RSC.
- K. Wygladacz, A. Radu, C. Xu, Y. Qin and E. Bakker, Anal. Chem., 2005, 77, 4706 CrossRef CAS PubMed.
- R. K. Shervedani and M. K. Babadi, Talanta, 2006, 69, 741 CrossRef CAS PubMed.
- Y. H. Li, H. Q. Xie and F. Q. Zhou, Talanta, 2005, 67, 28 CrossRef CAS PubMed.
- A. Mohadesi and M. A. Taher, Talanta, 2007, 71, 615 CrossRef CAS PubMed.
- R. P. Singh and E. R. Pambid, Analyst, 1990, 115, 301 RSC.
-
(a) K. Ndung, M. A. Ranville, R. P. Franks and A. R. Flegal, Mar. Chem., 2006, 98, 109 CrossRef;
(b) L. Yang and R. E. R. Sturgeon, J. Anal. At. Spectrom., 2002, 7, 88 RSC;
(c) R. K. Katarina, T. Takayanagi, M. Oshima and S. Motomizu, Anal. Chim. Acta, 2006, 558, 246 CrossRef CAS.
- M. Shamsipur, M. Javanbakht, V. Lippolis, A. Garau, G. D. Filippo, M. R. Ganjali and A. Yari, Anal. Chim. Acta, 2002, 462, 225 CrossRef CAS.
- J. H. Kang, J. B. Chae and C. Kim, R. Soc. Open Sci., 2018, 5, 180293 CrossRef PubMed.
- N. Bhuvanesh, S. Suresh, P. R. Kumar, E. M. Mothi, K. Kannan, V. R. Kannan and R. Nandhakumar, J. Photochem. Photobiol., A, 2018, 360, 6 CrossRef CAS.
- N. Bhuvanesh, S. Suresh, J. Prabhu, K. Kannan, V. R. Kannan and R. Nandhakumar, Opt. Mater., 2018, 82, 123 CrossRef CAS.
- M. Sahu, A. K. Manna, K. Rout, J. Mondal and G. K. Patra, Inorg. Chim. Acta, 2020, 508, 119633 CrossRef CAS.
- Y. K. Jang, U. C. Nam, H. L. Kwon, I. H. Hwang and C. Kim, Dyes Pigm., 2013, 99, 6 CrossRef CAS.
- X. Chen, M. J. Jou, H. Lee, S. Kou, J. Lim, S.-W. Nam, S. Park, K.-M. Kim and J. Yoon, Sens. Actuators, B, 2009, 137, 597 CrossRef CAS.
- J. A. Cotruvo, A. T. Aron, K. M. Ramos-Torres and C. J. Chang, Chem. Soc. Rev., 2015, 44, 4400 RSC.
- C. J. Chang, Nat. Chem. Biol., 2015, 11, 744 CrossRef CAS PubMed.
- A. T. Aron, K. M. Ramos-Torres, J. A. Cotruvo and C. J. Chang, Acc. Chem. Res., 2015, 48, 2434 CrossRef CAS PubMed.
- L. Krishnamoorthy, J. A. Cotruvo, J. Chan, H. Kaluarachchi, A. Muchenditsi, V. S. Pendyala, S. Jia, A. T. Aron, C. M. Ackerman, M. N. V. Wal, T. Guan, L. P. Smaga, S. L. Farhi, E. J. New, S. Lutsenko and C. J. Chang, Nat. Chem. Biol., 2016, 12, 586 CrossRef CAS PubMed.
- Y. J. Na, Y. W. Choi, J. Y. Yun, K.-M. Park, P.-S. Chang and C. Kim, Spectrochim. Acta, Part A, 2015, 136, 1649 CrossRef CAS PubMed.
- E. Madsen and J. D. Gitlin, Annu. Rev. Neurosci., 2007, 30, 317 CrossRef CAS PubMed.
- K. J. Barnham, C. L. Masters and A. I. Bush, Nat. Rev. Drug Discovery, 2004, 3, 205 CrossRef CAS PubMed.
- P. G. Georgopoulos, A. Roy, M. J. Yonone-Lioy, R. E. Opiekun and P. J. Lioy, J. Toxicol. Environ. Health, Part B, 2001, 4, 341 CAS.
-
(a) Y.-F. Tan, N. O'Toole, N. L. Taylor and A. H. Millar, Plant Physiol., 2010, 152, 747 CrossRef CAS PubMed;
(b) R. Uauy, M. Araya and B. Lonnerdal, Preface, Am. J. Clin. Nutr., 2008, 88, 819S CrossRef CAS PubMed;
(c) A. Malik, Environ. Int., 2004, 30, 261 CrossRef CAS PubMed.
- Y. Wang, H. Wu, W.-N. Wu, Y.-P. Yu, X.-L. Zhao, Z.-H. Xu, Z.-Q. Xu and Y.-C. Fan, J. Lumin., 2018, 204, 289 CrossRef CAS.
- H. Ni, Q. Wang, L. Jin, W. Wang, L. Dai and C. Zhao, J. Lumin., 2019, 206, 125–131 CrossRef CAS.
- V. Venkatesan, R. S. Kumar, S. K. A. Kumar and S. K. Sahoo, J. Mol. Struct., 2019, 1198, 126906 CrossRef CAS.
- A. Saravanan, G. Subashini, S. Shyamsivappan, T. Suresh, K. Kadirvelu, N. Bhuvanesh, R. Nandhakumar and P. S. Mohan, J. Photochem. Photobiol., A, 2018, 364, 424 CrossRef.
- Y. Wang, P.-D. Mao, W.-N. Wu, X.-J. Mao, X.-L. Zhao, Z.-Q. Xu, Y.-C. Fan and Z.-H. Xu, Sens. Actuators, B, 2017, 251, 813 CrossRef CAS.
- S. Wang, Z. Wang, Y. Yin, J. Luo and L. Kong, J. Photochem. Photobiol., A, 2017, 333, 213 CrossRef CAS.
- W. Gao, H. Li and S. Pu, J. Photochem. Photobiol., A, 2018, 364, 208 CrossRef CAS.
- J. Yin, Q. Bing, L. Wang and G. Wang, Spectrochim. Acta, Part A, 2018, 189, 495 CrossRef CAS PubMed.
- Q. Bing, L. Wang, D. Li and G. Wang, Spectrochim. Acta, Part A, 2018, 202, 305 CrossRef CAS PubMed.
- H. Fang, P.-C. Huang and F.-Y. Wu, Spectrochim. Acta, Part A, 2018, 204, 568 CrossRef CAS PubMed.
- F. N. Moghadam, M. Amirnasr, S. Meghdadi, K. Eskandari, A. Buchholz and W. Plass, Spectrochim. Acta,
Part A, 2019, 207, 6 CrossRef PubMed.
- S. Slassi, M. Aarjane, A. El-Ghayoury and A. Amine, Spectrochim. Acta, Part A, 2019, 215, 348 CrossRef CAS PubMed.
- X. Wang, W. Shi, L. Feng, J. Ma, Y. Li, X. Kong, Y. Chen, Y. Hui and Z. Xie, Inorg. Chem. Commun., 2017, 79, 50 CrossRef CAS.
- N. Xiao and C. Zhang, Inorg. Chem. Commun., 2019, 107, 107467 CrossRef CAS.
- T. Simon, M. Shellaiah, V. Srinivasadesikan, C.-C. Lin, F.-H. Ko, K. W. Sun and M.-C. Lin, New J. Chem., 2016, 40, 6101 RSC.
- S. Ghosh, A. Ganguly, Md. R. Uddin, S. Mandal, Md. A. Alam and N. Guchhait, Dalton Trans., 2016, 45, 11042 RSC.
- P. Torawane, K. Keshav, M. K. Kumawat, R. Srivastava, T. Anand, S. Sahoo, A. Borse and A. Kuwar, Photochem. Photobiol. Sci., 2017, 16, 1464 CrossRef CAS PubMed.
- M. Yang, L. Ma, J. Li and L. Kang, RSC Adv., 2019, 9, 16812 RSC.
- Z. Kowser, C.-C. Jin, X. Jiang, S. Rahman, P. E. Georghiou, X.-L. Ni, X. Zeng, C. Redshaw and T. Yamato, Tetrahedron, 2016, 72, 4575 CrossRef CAS.
- R. Shen, J. J. Yang, H. Luo, B. Wang and Y. Jiang, Tetrahedron, 2017, 73, 373 CrossRef CAS.
- B. Zambelli, F. Musiani, S. Benini and S. Ciurli, Acc. Chem. Res., 2011, 44, 520 CrossRef CAS PubMed.
- S. W. Ragsdale, J. Biol. Chem., 2009, 284, 18571 CrossRef CAS PubMed.
- R. J. Maier, Biochem. Soc. Trans., 2005, 33, 83 CrossRef CAS PubMed.
- Y. Lee, H. Liu, J. Lee, S. Kim, J. Sessler and Y. Kim, Chem. – Eur. J., 2010, 16, 5895 CrossRef CAS PubMed.
- P. Bandyopadhyay and A. K. Ghosh, J. Phys. Chem. B, 2010, 114, 11462 CrossRef CAS PubMed.
- K. S. Kasprzak, F. W. Sunderman and K. Salnikowa, Mutat. Res., 2003, 533, 67 CrossRef CAS PubMed.
-
P. H. Kuck, Mineral Commodity Summaries: Nickel, United States Geological Survey, 2006 Search PubMed.
-
(a) E. Denkhaus and K. Salnikow, Crit. Rev. Oncol. Hematol., 2002, 42, 35 CrossRef CAS PubMed;
(b)
Nickel and Its Surprising Impact in Nature, ed. H. Sigal, A. Sigal and R. O. K. Sigal, John Wiley and Sons Ltd, UK, 2007 Search PubMed.
- W. Lee, K. A. Davis, R. L. Rettmer and R. F. Labbe, Am. J. Clin. Nutr., 1988, 48, 286 CrossRef CAS PubMed.
-
(a) X. Q. Liu, X. Zhou, X. Shu and J. Zhu, Macromolecules, 2009, 42, 7634 CrossRef CAS;
(b) S. C. Dodani, Q. He and C. J. Chang, J. Am. Chem. Soc., 2009, 131, 18020 CrossRef CAS PubMed.
- A. K. Manna, K. Rout, S. Chowdhury and G. K. Patra, Photochem. Photobiol. Sci., 2019, 18, 1512 CrossRef CAS PubMed.
- G. G. V. Kumar, M. P. Kesavan, M. Sankarganesh, K. Sakthipandi, J. Rajesh and G. Sivaraman, New J. Chem., 2018, 42, 2865 RSC.
- A. K. Manna, S. Chowdhury and G. K. Patra, Dalton Trans., 2019, 48, 12336 RSC.
-
S. J. Lippard and J. M. Berg, Principles of Bioinorganic Chemistry, University Science Books, 1994 Search PubMed.
-
C. Melber, D. Keller and I. Mangelsdorf, Environmental Health Criteria 226, Palladium, World Health Organization, Geneva, 2002, p. 1 Search PubMed.
-
Z. Berner, C. Menzel, A. Zeller, J. D. Eckhardt, D. Stüben and A. Hartwig, Analytical Procedure for the Quantification of in vitro Induced Pt- and Pd-DNA Adducts in Human Lung Cells, in Palladium Emissions in the Environment, ed. F. Zereini and F. Alt, Springer, Berlin, Heidelberg, 2006, DOI:10.1007/3-540-29220-9_16.
-
International Programme on Chemical Safety, Palladium; Environmental Health Criteria Series 226, World Health Organization, Geneva, 2002 Search PubMed.
- T. Gebel, H. Lantzsch, K. Plebow and H. Dunkelberg, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 1997, 389, 183 CrossRef CAS PubMed.
-
(a) J. Kielhorn, C. Melber, D. Keller and I. Mangelsdorf, Int. J. Hyg. Environ. Health, 2002, 205, 417 CrossRef CAS PubMed;
(b) J. C. Wataha and C. T. Hanks, J. Oral Rehabil., 1996, 23, 309 CrossRef CAS PubMed.
-
(a) C. L. S. Wiseman and F. Zereini, Sci. Total Environ., 2009, 407, 2493 CrossRef CAS PubMed;
(b) T. Z. Liu, S. D. Lee and R. S. Bhatnagar, Toxicol. Lett., 1979, 60, 469 CrossRef.
-
C. Melber, D. Keller and I. Mangelsdorf, Environmental Health Criteria for Palladium, World Health Organization, Geneva, 2002 Search PubMed.
-
Palladium Emissions in the Environment Analytical Methods, Environmental Assessment and Health Effects, ed. F. Alt and F. Zereini, Springer-Verlag, Berlin, 2006 Search PubMed.
- A. K. Bhanja, S. Mishra, K. D. Sahab and C. Sinha, Dalton Trans., 2017, 46, 9245 RSC.
- A. K. Adak, R. Purkait, S. K. Manna, B. C. Ghosh, S. Pathak and C. Sinha, New J. Chem., 2019, 43, 3899 RSC.
- S. Mondal, S. K. Manna, S. Pathak, A. A. Masum and S. Mukhopadhyay, New J. Chem., 2019, 49, 3513 RSC.
- A. K. Adak, B. Dutta, S. K. Manna and C. Sinha, ACS Omega, 2019, 4, 18987 CrossRef CAS PubMed.
- A. K. Bhanja, S. Mishra, K. Kar, K. Naskar, S. Maity, K. D. Sahab and C. Sinha, New J. Chem., 2018, 42, 17351 RSC.
- J.-W. Lee and J. D. Helmann, Nature, 2006, 440, 363 CrossRef CAS PubMed.
- B. He, A. B. Pun, D. Zherebetskyy, Y. Liu, F. Liu, L. M. Klivansky, A. M. McGough, B. Zhang, K. Lo, T. P. Russell, L. N. Wang and Y. Liu, J. Am. Chem. Soc., 2014, 136, 15093 CrossRef CAS PubMed.
- J. P. Sumner and R. Kopelman, Analyst, 2005, 130, 528 RSC.
-
(a) R. S. Eisenstein, Annu. Rev. Nutr., 2000, 20, 627 CrossRef CAS PubMed;
(b) T. A. Rouault, Nat. Chem. Biol., 2006, 2, 406 CrossRef CAS PubMed.
-
(a) C. Brugnara, Clin. Chem., 2003, 49, 1573 CrossRef CAS PubMed;
(b) N. R. Chereddy, K. Suman, P. S. Korrapati, S. A. Thennarasu and B. Manda, Dyes Pigm., 2012, 95, 606 CrossRef CAS.
- D. A. Weinstein, C. N. Roy, M. D. Fleming, M. F. Loda, J. I. Wolfsdorf and N. C. Andrews, Blood, 2002, 100, 3776 CrossRef CAS PubMed.
- X. Huang, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2003, 533, 153 CrossRef CAS PubMed.
- R. Agarwal, N. Vasavada, N. G. Sachs and S. Chase, Kidney Int., 2004, 65, 82279 Search PubMed.
- W. H. Hoerl, J. Am. Soc. Nephrol., 2007, 18, 382 CrossRef CAS PubMed.
- S. Epsztejn, H. Glickstein, V. Picard, I. N. Slotki, W. Breuer, C. Beaumont and Z. I. Cabantchik, Blood, 1999, 94, 3593 CrossRef CAS PubMed.
- S. Santhoshkumar, K. Velmurugan, J. Prabhu, G. Radhakrishnan and R. Nandhakumar, Inorg. Chim. Acta, 2016, 439, 1 CrossRef CAS.
- C.-F. Wan, Y.-J. Chang, C.-Y. Chien, Y.-W. Sie, C.-H. Hu and A.-T. Wu, J. Lumin., 2016, 178, 115 CrossRef CAS.
- V. K. Gupta, N. Mergu and L. K. Kumawat, Sens. Actuators, B, 2016, 223, 101 CrossRef CAS.
- T. Nandhini, P. Kaleeswaran and K. Pitchumani, Sens. Actuators, B, 2016, 230, 199 CrossRef CAS.
- P. S. Nayab and M. Shkir, Sens. Actuators, B, 2017, 245, 395 CrossRef CAS.
- S. Adhikari, A. Ghosh, M. Ghosh, S. Guria and D. Das, Sens. Actuators, B, 2017, 251, 942 CrossRef CAS.
- S. Zhang, T. Sun, D. Xiao, F. Yuan, T. Li, E. Wang, H. Liu and Q. Niu, Spectrochim. Acta, Part A, 2018, 189, 594 CrossRef CAS PubMed.
- Z. Zuo, X. Song, D. Guo, Z. Guo and Q. Niu, J. Photochem. Photobiol., A, 2019, 382, 111876 CrossRef CAS.
- Z. Guo, Q. Niu and T. Li, Spectrochim. Acta, Part A, 2018, 200, 76 CrossRef CAS PubMed.
- R. Kouser, S. Zehra, R. A. Khan, A. Alsalme, F. Arjmand and S. Tabassum, Spectrochim. Acta, Part A, 2021, 247, 119156 CrossRef CAS PubMed.
- A. S. Murugan, M. Kiruthika, E. R. A. Noelson, P. Yogapandi, G. G. Kumar and J. Annaraj, Arabian J. Chem., 2021, 14, 102910 CrossRef.
- G. T. Selvan, C. Varadaraju, R. T. Selvan, Israel V. M. V. Enoch and P. M. Selvakumar, ACS Omega, 2018, 3, 7985 CrossRef PubMed.
- S. D. Padghan, A. L. Puyad, R. S. Bhosale, S. V. Bhosale and S. V. Bhosale, Photochem. Photobiol. Sci., 2017, 16, 1591 CrossRef CAS PubMed.
- H. Xu, H. Ding, G. Li, C. Fan, G. Liu and S. Pu, RSC Adv., 2017, 7, 29827 RSC.
- L. Hou, T. Liu, Y. Gong, J. Li, C. Deng, C. Zhang, Y. Wang, S. Shuang and W. Liang, New J. Chem., 2020, 44, 19642 RSC.
- B. Li, J. Tian, D. Zhang and F. Tian, Luminescence, 2017, 8, 1 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, fluorescence resonance energy transfer (FRET) and spin–orbit coupling.19,20
N isomerization, fluorescence resonance energy transfer (FRET) and spin–orbit coupling.19,20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which can be explained on the basis of inhibition of ESIPT process and can result in a CHEF due to the engagement of the phenolic O atom in the coordination of probe-1 with Zn(II) ions (Fig. 1). The detection limit of probe-1 with Zn(II) ions determined via fluorescence spectra is 37.7 nM. Furthermore, probe-1 has a low cytotoxicity, which qualifies its efficacy for tracing and visualizing Zn(II) ions in vitro in HeLa cells and live SH-SY5Y neuroblastoma cells.
1, which can be explained on the basis of inhibition of ESIPT process and can result in a CHEF due to the engagement of the phenolic O atom in the coordination of probe-1 with Zn(II) ions (Fig. 1). The detection limit of probe-1 with Zn(II) ions determined via fluorescence spectra is 37.7 nM. Furthermore, probe-1 has a low cytotoxicity, which qualifies its efficacy for tracing and visualizing Zn(II) ions in vitro in HeLa cells and live SH-SY5Y neuroblastoma cells.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N imine bond isomerization on effective coordination by Zn(II) ions could be the cause of the fluorescence turn-on, and a new bathochromic band arises in the absorption spectrum. The detection limit was determined to be 31.044 nM using the equation LOD = 3σ/slope. Moreover, the single-crystal XRD identified formation of dinuclear Zn(II) complexes indicates that probe-2 strongly binds to the Zn(II) ions. The detection of Zn(II) ions by probe-2 in biological media was investigated on HeLa and DU-145 cancer cell lines, since probe-2 was proven to be non-toxic and highly biocompatible for cellular proliferation by the MTT cell viability assay, which is helpful for the detection or monitoring of Zn(II) ions in biological samples (Fig. 2).
N imine bond isomerization on effective coordination by Zn(II) ions could be the cause of the fluorescence turn-on, and a new bathochromic band arises in the absorption spectrum. The detection limit was determined to be 31.044 nM using the equation LOD = 3σ/slope. Moreover, the single-crystal XRD identified formation of dinuclear Zn(II) complexes indicates that probe-2 strongly binds to the Zn(II) ions. The detection of Zn(II) ions by probe-2 in biological media was investigated on HeLa and DU-145 cancer cell lines, since probe-2 was proven to be non-toxic and highly biocompatible for cellular proliferation by the MTT cell viability assay, which is helpful for the detection or monitoring of Zn(II) ions in biological samples (Fig. 2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a low detection limit value of 0.16 μM for probe-3a. The binding constant of probe-3a with Zn(II) ions was estimated to be 1.6 × 105 M−1via fluorescence titration according to the Benesi–Hildebrand (B–H) plot, indicating that probe-3a–Zn(II) is an adequately stable complex. The binding mechanism of probes with Zn(II) was strongly supported by 1H NMR, density functional theory (DFT), time-dependent density-functional theory (TD-DFT), and electrospray ionisation mass spectrometry (ESI-MS). Consequently, dip-stick experimentation, which enabled real-time monitoring without the use of an instrument, resulted in an instant qualitative detection of Zn(II) with the probes, and was confirmed by the color change in the absorption and fluorescence. Further, the probes were subjected to the sensing of Zn(II) ions in biological media, and an experiment was carried out on human breast cancer cell lines (MCF-7) and tissues. These cells exhibited distinct fluorescence colors upon introducing Zn(II), which supports the recognition of Zn(II) ions in biological samples (Fig. 4).
1 with a low detection limit value of 0.16 μM for probe-3a. The binding constant of probe-3a with Zn(II) ions was estimated to be 1.6 × 105 M−1via fluorescence titration according to the Benesi–Hildebrand (B–H) plot, indicating that probe-3a–Zn(II) is an adequately stable complex. The binding mechanism of probes with Zn(II) was strongly supported by 1H NMR, density functional theory (DFT), time-dependent density-functional theory (TD-DFT), and electrospray ionisation mass spectrometry (ESI-MS). Consequently, dip-stick experimentation, which enabled real-time monitoring without the use of an instrument, resulted in an instant qualitative detection of Zn(II) with the probes, and was confirmed by the color change in the absorption and fluorescence. Further, the probes were subjected to the sensing of Zn(II) ions in biological media, and an experiment was carried out on human breast cancer cell lines (MCF-7) and tissues. These cells exhibited distinct fluorescence colors upon introducing Zn(II), which supports the recognition of Zn(II) ions in biological samples (Fig. 4).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio. The reversibility of probe-4 towards Zn(II) was confirmed via addition of EDTA to probe-4–Zn(II) in CH3CN–H2O, which leads to the regeneration of probe-4 alone, these results strongly supported the reversibility of probe-4. Finally, probe-4 was demonstrated as a feasible fluorescent sensor for real-time monitoring of Zn(II) in biological systems via bio-imaging in living HeLa cells and zebrafish models, with minimal cellular damage.
1 binding ratio. The reversibility of probe-4 towards Zn(II) was confirmed via addition of EDTA to probe-4–Zn(II) in CH3CN–H2O, which leads to the regeneration of probe-4 alone, these results strongly supported the reversibility of probe-4. Finally, probe-4 was demonstrated as a feasible fluorescent sensor for real-time monitoring of Zn(II) in biological systems via bio-imaging in living HeLa cells and zebrafish models, with minimal cellular damage.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio, and probe-6 was found to detect Zn(II) ions at extremely low levels up to 27.80 nM.
1 binding ratio, and probe-6 was found to detect Zn(II) ions at extremely low levels up to 27.80 nM.




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using Job's plot. Further, the binding interaction and fluorescence turn-on response of probe-11 with Zn(II) ions were strongly held by the DFT calculations. The reversibility of probe-11 + Zn(II) was studied using EDTA, producing a better output, which was then converted into a logic circuit. The remarkable properties of probe-11 can be used to detect Zn(II) ions in both biological and environmental samples.
1 using Job's plot. Further, the binding interaction and fluorescence turn-on response of probe-11 with Zn(II) ions were strongly held by the DFT calculations. The reversibility of probe-11 + Zn(II) was studied using EDTA, producing a better output, which was then converted into a logic circuit. The remarkable properties of probe-11 can be used to detect Zn(II) ions in both biological and environmental samples.


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, and –OH groups in ESIPT and –C
N–, and –OH groups in ESIPT and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– isomerization processes, which were suppressed upon binding with Zn(II) ions, resulting in fluorescence improvement. In addition, the stoichiometric ratio of probe-15 with Zn(II) ions was calculated via Job's plot to be 1
N– isomerization processes, which were suppressed upon binding with Zn(II) ions, resulting in fluorescence improvement. In addition, the stoichiometric ratio of probe-15 with Zn(II) ions was calculated via Job's plot to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was further confirmed by ESI-mass data. The LOD of probe-15 with Zn(II) ions was estimated to be 4.5 nM by the 3σ/slope method. Further, the binding constant was estimated by the B–H method to be 2.4 × 104 M−1. Probe-15 demonstrated very good thermal stability and the decomposition of the probe-15–Zn(II) complex was found to be very minimal (1.15%) at a temperature of 259.38 °C, which endows probe-15 with the potential for the detection of environmental samples.
1, which was further confirmed by ESI-mass data. The LOD of probe-15 with Zn(II) ions was estimated to be 4.5 nM by the 3σ/slope method. Further, the binding constant was estimated by the B–H method to be 2.4 × 104 M−1. Probe-15 demonstrated very good thermal stability and the decomposition of the probe-15–Zn(II) complex was found to be very minimal (1.15%) at a temperature of 259.38 °C, which endows probe-15 with the potential for the detection of environmental samples.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was strongly validated by ESI-MS and DFT calculations. In addition, the complex-Zn(II) turned out to detect pyrophosphate (PPi) with among all other phosphates in an aqueous medium with both chromogenic and fluorogenic response with a LOD of 5.12 nM.
1, which was strongly validated by ESI-MS and DFT calculations. In addition, the complex-Zn(II) turned out to detect pyrophosphate (PPi) with among all other phosphates in an aqueous medium with both chromogenic and fluorogenic response with a LOD of 5.12 nM.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) to the pyrene fluorophore in the excited state is hampered by binding with Zn(II), could explain this vertical amplification of bands. Interestingly, probe-18 detects three essential bioactive analytes Zn(II), H2PO4−, and cysteine, with LOD values of 2.3 μM, 0.21 μM, and 0.16 μM, respectively. In vitro cellular imaging was also used to detect intracellular Zn(II) ions using probe-18. Further, probe-18 was utilized in recognition of Zn(II) ions in HeLa cells. Hence, based on the efficacy of probe-18, it can be applied for the recognition of Zn(II) ions in biological and environmental samples.
N–) to the pyrene fluorophore in the excited state is hampered by binding with Zn(II), could explain this vertical amplification of bands. Interestingly, probe-18 detects three essential bioactive analytes Zn(II), H2PO4−, and cysteine, with LOD values of 2.3 μM, 0.21 μM, and 0.16 μM, respectively. In vitro cellular imaging was also used to detect intracellular Zn(II) ions using probe-18. Further, probe-18 was utilized in recognition of Zn(II) ions in HeLa cells. Hence, based on the efficacy of probe-18, it can be applied for the recognition of Zn(II) ions in biological and environmental samples.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for probe-19 with Zn(II) ions and a binding constant (Ka) of 1.25 × 104 M−1. Due to the presence of very low detection limit, probe-19 was applied for the real-time monitoring of Zn(II) ions in imaging intracellular cells. Further, probe-19 shows potential for application in the detection of biological samples, which was performed on U251 cell lines. The cells were treated with probe-19 and showed no fluorescence, whereas upon incorporation with the Zn(II) ions, the color changed into strong green color fluorescence inside the cell line.
1 stoichiometry for probe-19 with Zn(II) ions and a binding constant (Ka) of 1.25 × 104 M−1. Due to the presence of very low detection limit, probe-19 was applied for the real-time monitoring of Zn(II) ions in imaging intracellular cells. Further, probe-19 shows potential for application in the detection of biological samples, which was performed on U251 cell lines. The cells were treated with probe-19 and showed no fluorescence, whereas upon incorporation with the Zn(II) ions, the color changed into strong green color fluorescence inside the cell line.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio. The fluorescence changes of probe-20 for Zn(II) were investigated further to rule out the interference of other cations, whereas the fluorescence quenching was caused by Fe(III), Cu(II), Cr(III), Fe(II), and Co(II) (78–100%), and competitive metal ion shows no change in the fluorescence spectra. The binding constant of probe-20 with Zn(II) ions was calculated from the fluorescence spectra using B–H equation as 2.0 × 103 M−1. Probe-20 displayed good reversibility with EDTA, which extends its real-time applicability and also in live cell imaging (Fig. 21).
1 binding ratio. The fluorescence changes of probe-20 for Zn(II) were investigated further to rule out the interference of other cations, whereas the fluorescence quenching was caused by Fe(III), Cu(II), Cr(III), Fe(II), and Co(II) (78–100%), and competitive metal ion shows no change in the fluorescence spectra. The binding constant of probe-20 with Zn(II) ions was calculated from the fluorescence spectra using B–H equation as 2.0 × 103 M−1. Probe-20 displayed good reversibility with EDTA, which extends its real-time applicability and also in live cell imaging (Fig. 21).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was established via ESI-MS and DFT studies. The binding constant was calculated from the equation of B–H to be 9.06 × 104 M−1. The LOD was determined using fluorescence titration as 1.8 μM. Moreover, picric acid (PA) was successfully detected by a zinc-ensemble over a series of other nitroaromatics and was confirmed by DFT studies. Yang and co-workers synthesized a simple probe (probe-22)82 from 4-diethylaminosalicylaldehyde and 7-amino-4-methyl coumarin, which acts as a selective and sensitive probe for Zn(II) ions in 2016. Probe-22 (Fig. 22) was found to be very poorly emissive in nature and an increase in emission was observed at 500 nm with Zn(II) ions, increasing the quantum yields (ϕ = 0.1651). Though, upon addition of other metal ions, there was no change in the fluorescence spectra specifying probe-22 was very selective to Zn(II) ions. Further, the fluorescence enhancement might be attributed to the suppression of C
1, which was established via ESI-MS and DFT studies. The binding constant was calculated from the equation of B–H to be 9.06 × 104 M−1. The LOD was determined using fluorescence titration as 1.8 μM. Moreover, picric acid (PA) was successfully detected by a zinc-ensemble over a series of other nitroaromatics and was confirmed by DFT studies. Yang and co-workers synthesized a simple probe (probe-22)82 from 4-diethylaminosalicylaldehyde and 7-amino-4-methyl coumarin, which acts as a selective and sensitive probe for Zn(II) ions in 2016. Probe-22 (Fig. 22) was found to be very poorly emissive in nature and an increase in emission was observed at 500 nm with Zn(II) ions, increasing the quantum yields (ϕ = 0.1651). Though, upon addition of other metal ions, there was no change in the fluorescence spectra specifying probe-22 was very selective to Zn(II) ions. Further, the fluorescence enhancement might be attributed to the suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and the inhibition of PET. The binding ratio among probe-22 and Zn(II) ions was calculated using Job's plot as 2
N isomerization and the inhibition of PET. The binding ratio among probe-22 and Zn(II) ions was calculated using Job's plot as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and was strongly held by ESI-MS, 1H NMR, and DFT calculations. Furthermore, the LOD and the binding constant of probe-22 with Zn(II) ions were found as log
1 and was strongly held by ESI-MS, 1H NMR, and DFT calculations. Furthermore, the LOD and the binding constant of probe-22 with Zn(II) ions were found as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 6.04 and 2.59 μM, respectively. Since, probe-22 has a moderately high association constant with Zn(II) ions and a low detection limit, it could be applicable to biological and environmental samples.
K = 6.04 and 2.59 μM, respectively. Since, probe-22 has a moderately high association constant with Zn(II) ions and a low detection limit, it could be applicable to biological and environmental samples.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The reversible studies of probe-23 + Zn(II) were conducted with EDTA and the restored probe-23, which is again utilized for the detection of Zn(II) ions. Furthermore, probe-23's fluorescence emission response can be examined as a binary logic function.
1. The reversible studies of probe-23 + Zn(II) were conducted with EDTA and the restored probe-23, which is again utilized for the detection of Zn(II) ions. Furthermore, probe-23's fluorescence emission response can be examined as a binary logic function.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) was detected. However, in the presence of other metal ions, there was no or minimal fluorescence changes observed. Further, authors determined the detection limit via fluorescence titration spectra to be 4.35 nM. The binding constant of probe-24 and Zn(II) was estimated by non-linear curve fitting of the fluorescence titration spectra and found to be log
50, v/v) was detected. However, in the presence of other metal ions, there was no or minimal fluorescence changes observed. Further, authors determined the detection limit via fluorescence titration spectra to be 4.35 nM. The binding constant of probe-24 and Zn(II) was estimated by non-linear curve fitting of the fluorescence titration spectra and found to be log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 5.63 (0.15). Further, stoichiometry was determined from Job's plot, and the results were exhibited to be 1
K = 5.63 (0.15). Further, stoichiometry was determined from Job's plot, and the results were exhibited to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Finally, authors conducted the cytotoxicity of probe-24 on A549 cells, and the results indicated very low toxicity, which strongly supported the use of probe-24 to monitor Zn(II) ions in biological samples (Fig. 24).
1. Finally, authors conducted the cytotoxicity of probe-24 on A549 cells, and the results indicated very low toxicity, which strongly supported the use of probe-24 to monitor Zn(II) ions in biological samples (Fig. 24).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding of probe-25 with Zn(II), which was strongly supported by the ESI-MS, DFT and 1H NMR data. The LOD and association constant of probe-25 with Zn(II) were evaluated from the fluorescence titration spectra as 1.29 μM and 7.9 × 104 M−1 respectively. Further, authors performed the recognition of Zn(II) ions in biological applications; for this, Zebrafish was incubated with probe-25 and data supported that strong luminescence was generated inside the Zebrafish (Fig. 26).
1 binding of probe-25 with Zn(II), which was strongly supported by the ESI-MS, DFT and 1H NMR data. The LOD and association constant of probe-25 with Zn(II) were evaluated from the fluorescence titration spectra as 1.29 μM and 7.9 × 104 M−1 respectively. Further, authors performed the recognition of Zn(II) ions in biological applications; for this, Zebrafish was incubated with probe-25 and data supported that strong luminescence was generated inside the Zebrafish (Fig. 26).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 via Job's plot, which was supported by ESI-MS, 1H NMR and DFT data. The LOD and association constants were calculated using fluorescence titration data and reported as 0.33 μM and 9.98 × 104 M−1 correspondingly. Finally, the authors used probe-26 in a practical application, such as test strips, and developed a practical, efficient, and low-cost Zn(II) ion testing tool.
1 via Job's plot, which was supported by ESI-MS, 1H NMR and DFT data. The LOD and association constants were calculated using fluorescence titration data and reported as 0.33 μM and 9.98 × 104 M−1 correspondingly. Finally, the authors used probe-26 in a practical application, such as test strips, and developed a practical, efficient, and low-cost Zn(II) ion testing tool.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 via Job's plot, which was further supported by ESI-MS, 1H NMR and DFT results. Furthermore, the emission intensity at 525 nm of the probe-27–Zn(II) ensemble was reduced dramatically upon addition of a 100 μM solution of PPi anions, but remained stable for other anions, which showed the potential of probe-27 to detect both the biologically important metal ions (Zn(II)) and anion (PPi) (Fig. 29).
1 via Job's plot, which was further supported by ESI-MS, 1H NMR and DFT results. Furthermore, the emission intensity at 525 nm of the probe-27–Zn(II) ensemble was reduced dramatically upon addition of a 100 μM solution of PPi anions, but remained stable for other anions, which showed the potential of probe-27 to detect both the biologically important metal ions (Zn(II)) and anion (PPi) (Fig. 29).

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and CHEF (Fig. 30). However, no difference was detected in fluorescence spectra of probe-28 in the presence of other metal ions. Job's plot analysis and HRMS revealed a 1
N isomerization and CHEF (Fig. 30). However, no difference was detected in fluorescence spectra of probe-28 in the presence of other metal ions. Job's plot analysis and HRMS revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry for probe-28 and Zn(II) ions. Furthermore, probe-28 was successfully demonstrated for the analysis of real-time applications due to the presence of very low detection limits such as 13.4 nM. The fluorescence titration data yields a binding constant of 3.49 × 104 M−1 from the B–H equation and gratifyingly, it could be applied to design a logic gate.
1 binding stoichiometry for probe-28 and Zn(II) ions. Furthermore, probe-28 was successfully demonstrated for the analysis of real-time applications due to the presence of very low detection limits such as 13.4 nM. The fluorescence titration data yields a binding constant of 3.49 × 104 M−1 from the B–H equation and gratifyingly, it could be applied to design a logic gate.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a binding constant (Ka) of 1.14 × 105 M−1. The LOD of probe-29 with Zn(II) ions was determined to be 7.52 nM. The perfect reversibility and renewability of probe-29 for the recognition of Zn(II) was confirmed using Na2EDTA, a chelating agent, in which the fluorescence emission of complex probe-29–Zn(II) was significantly quenched with the addition of Na2EDTA and can be utilized for various practical applications.
1 with a binding constant (Ka) of 1.14 × 105 M−1. The LOD of probe-29 with Zn(II) ions was determined to be 7.52 nM. The perfect reversibility and renewability of probe-29 for the recognition of Zn(II) was confirmed using Na2EDTA, a chelating agent, in which the fluorescence emission of complex probe-29–Zn(II) was significantly quenched with the addition of Na2EDTA and can be utilized for various practical applications.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as confirmed by 1H NMR, DFT/TD-DFT, mass spectral analysis and Job's plot. The association constant of probe-30 with Zn(II) ions was estimated with a nonlinear B–H as 2.2 × 105 M−1. The LOD was estimated using emission titration data to be 1.2 nM by the 3σ/slope method. In contrast, this recognition process was reversible, as the fluorescence titration experiment of probe-30–[Zn(II)] to PPi results in lower emission, which results strongly supported by regeneration of probe-30. Finally, authors claims that the fluorescence signals of probe-30 were used to build a molecular INHIBIT logic gate.
1, as confirmed by 1H NMR, DFT/TD-DFT, mass spectral analysis and Job's plot. The association constant of probe-30 with Zn(II) ions was estimated with a nonlinear B–H as 2.2 × 105 M−1. The LOD was estimated using emission titration data to be 1.2 nM by the 3σ/slope method. In contrast, this recognition process was reversible, as the fluorescence titration experiment of probe-30–[Zn(II)] to PPi results in lower emission, which results strongly supported by regeneration of probe-30. Finally, authors claims that the fluorescence signals of probe-30 were used to build a molecular INHIBIT logic gate.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was strongly supported by ESI-MS and 1H NMR. Finally, authors proposed the mechanism of fluorescence turn-on due to the complexation and simultaneous blocking of two processes, i.e., PET and C
1, which was strongly supported by ESI-MS and 1H NMR. Finally, authors proposed the mechanism of fluorescence turn-on due to the complexation and simultaneous blocking of two processes, i.e., PET and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 34).
N isomerization (Fig. 34).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding. Furthermore, the use of Na2EDTA solution allows the probe-32 to be fully regenerate from probe-32 + Zn(II) complex. Finally, the authors utilized the probe-32 for the real-time quantitative detection of Zn(II) in water samples and biological systems by a quick change in emission intensity of probe-32 in the pH range 6–8 against Zn(II) ions. Further, the probe-32 was subjected for the recognition of Zn(II) ions in biological samples, the authors incubated MDA-MB-468 cells which exhibited no fluorescence and upon addition of Zn(II) to cells, which generated strong green color fluorescence around cells and results strongly suggested that the probe-32 was potential for the sensing of Zn(II) ions in biological samples (Fig. 37).
2 binding. Furthermore, the use of Na2EDTA solution allows the probe-32 to be fully regenerate from probe-32 + Zn(II) complex. Finally, the authors utilized the probe-32 for the real-time quantitative detection of Zn(II) in water samples and biological systems by a quick change in emission intensity of probe-32 in the pH range 6–8 against Zn(II) ions. Further, the probe-32 was subjected for the recognition of Zn(II) ions in biological samples, the authors incubated MDA-MB-468 cells which exhibited no fluorescence and upon addition of Zn(II) to cells, which generated strong green color fluorescence around cells and results strongly suggested that the probe-32 was potential for the sensing of Zn(II) ions in biological samples (Fig. 37).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation of probe-33 with Zn(II) ions, which was further confirmed by single crystal XRD analysis. Finally, the authors utilized probe-33 for the detection of Zn(II) in the intracellular region of human breast cancer cells (MCF-7) (Fig. 39).
1 complexation of probe-33 with Zn(II) ions, which was further confirmed by single crystal XRD analysis. Finally, the authors utilized probe-33 for the detection of Zn(II) in the intracellular region of human breast cancer cells (MCF-7) (Fig. 39).

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 40 and 41). However, there were no such observations noticed upon addition of other competitive metal ions. For the detection of probe-34a, probe-34b and probe-34c, with Zn(II) ions showed very less LOD (67.2 nM) with good anti-interference capabilities and quick response. A binding ratio of 1
N isomerization (Fig. 40 and 41). However, there were no such observations noticed upon addition of other competitive metal ions. For the detection of probe-34a, probe-34b and probe-34c, with Zn(II) ions showed very less LOD (67.2 nM) with good anti-interference capabilities and quick response. A binding ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was determined for the three probes with Zn(II) ions based on Job's plot analysis of the fluorescence data, which was further supported by the 1H NMR data. An inverted fluorescence microscopy imaging experiment was conducted and the findings showed that the probes exhibit good cell membrane permeability and hypotoxicity. Probe-34a was used for the detection of Zn(II) ions in SW620 cancer cells, and the results showed that the probe-34a alone exhibited no fluorescence; upon addition of Zn(II) ions, strong luminescence was observed inside the cells. From the above-mentioned results, authors claimed that probe-34a is potential for the recognition of Zn(II) ions in both biological and environmental samples.
1 was determined for the three probes with Zn(II) ions based on Job's plot analysis of the fluorescence data, which was further supported by the 1H NMR data. An inverted fluorescence microscopy imaging experiment was conducted and the findings showed that the probes exhibit good cell membrane permeability and hypotoxicity. Probe-34a was used for the detection of Zn(II) ions in SW620 cancer cells, and the results showed that the probe-34a alone exhibited no fluorescence; upon addition of Zn(II) ions, strong luminescence was observed inside the cells. From the above-mentioned results, authors claimed that probe-34a is potential for the recognition of Zn(II) ions in both biological and environmental samples.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 via Job's plot, which was strongly supported by B–H plots, 1H NMR and ESI-MS analyses. The binding constant and LOD value of probe-35 with Zn(II) ions were estimated from titration data and displayed as 7.79 × 106 M−1 and 0.31 μM respectively. Finally, authors decided to apply probe-35 to sense Zn(II) ions from the biological samples, in which cytotoxicity experiments revealed that probe-35 showed very less cytotoxicity. Probe-35 was incubated with HeLa cells, which showed no emission, and upon treatment with Zn(II) ions, the fluorescence intensity was seen inside the cells. From the above-mentioned results, authors concluded that probe-35 shows potential to sense Zn(II) ions in environmental and biological samples (Fig. 43).
1 via Job's plot, which was strongly supported by B–H plots, 1H NMR and ESI-MS analyses. The binding constant and LOD value of probe-35 with Zn(II) ions were estimated from titration data and displayed as 7.79 × 106 M−1 and 0.31 μM respectively. Finally, authors decided to apply probe-35 to sense Zn(II) ions from the biological samples, in which cytotoxicity experiments revealed that probe-35 showed very less cytotoxicity. Probe-35 was incubated with HeLa cells, which showed no emission, and upon treatment with Zn(II) ions, the fluorescence intensity was seen inside the cells. From the above-mentioned results, authors concluded that probe-35 shows potential to sense Zn(II) ions in environmental and biological samples (Fig. 43).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio for the probe-36–Zn(II) complex. Finally, the authors concentrated on the identification of Zn(II) ions in biological samples, and a cytotoxicity experiment using probe-36 was carried out and the results strongly indicated the poor cytotoxicity of probe-36 under biological conditions. The results encouraged to further investigate in this regard. Therefore, probe-36 was incubated in HeLa cells and no emission was observed, and the incorporation of Zn(II) ions to the cells produced a strong blue color emission inside the cells (Fig. 46), which strongly supported the potential of probe-36 for the recognition of Zn(II) ions in biological samples.
1 stoichiometric ratio for the probe-36–Zn(II) complex. Finally, the authors concentrated on the identification of Zn(II) ions in biological samples, and a cytotoxicity experiment using probe-36 was carried out and the results strongly indicated the poor cytotoxicity of probe-36 under biological conditions. The results encouraged to further investigate in this regard. Therefore, probe-36 was incubated in HeLa cells and no emission was observed, and the incorporation of Zn(II) ions to the cells produced a strong blue color emission inside the cells (Fig. 46), which strongly supported the potential of probe-36 for the recognition of Zn(II) ions in biological samples.


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 47). The LOD of probe-37 with Zn(II) ions calculated from fluorescence spectra and was found to be 89.3 nM. A 2
N isomerization (Fig. 47). The LOD of probe-37 with Zn(II) ions calculated from fluorescence spectra and was found to be 89.3 nM. A 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was found for probe-37 with Zn(II) ions, which was validated by fluorescence measurements, UV-vis, ESI-MS, absorption analysis, 1H NMR spectra and DFT studies. The reversibility of probe-37 was tested using Na2EDTA. Finally, the authors tested the sensing of Zn(II) ions in cellular media, in which a cytotoxicity experiment was conducted with probe-37 that was found to exhibit very poor cytotoxicity on HeLa cells. The results encouraged the authors to recognize Zn(II) ions in biological samples. Probe-37 incubated with Zn(II) ions exhibited very bright luminescence inside the cells, which strongly suggested the potential of probe-37 for the sensing of biological Zn(II) samples (Fig. 48).
1 stoichiometry was found for probe-37 with Zn(II) ions, which was validated by fluorescence measurements, UV-vis, ESI-MS, absorption analysis, 1H NMR spectra and DFT studies. The reversibility of probe-37 was tested using Na2EDTA. Finally, the authors tested the sensing of Zn(II) ions in cellular media, in which a cytotoxicity experiment was conducted with probe-37 that was found to exhibit very poor cytotoxicity on HeLa cells. The results encouraged the authors to recognize Zn(II) ions in biological samples. Probe-37 incubated with Zn(II) ions exhibited very bright luminescence inside the cells, which strongly suggested the potential of probe-37 for the sensing of biological Zn(II) samples (Fig. 48).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by Job's plot, which was confirmed via1H NMR and ESI-MS data. Finally, the authors established the potential of probe-38 for the sensing of Zn(II) ions in biological samples and outcomes strongly supported that probe-38 was capable for the recognition of Zn(II) ions in biological samples and was confirmed by intracellular imaging of Zn(II) ions (Fig. 50).
1 by Job's plot, which was confirmed via1H NMR and ESI-MS data. Finally, the authors established the potential of probe-38 for the sensing of Zn(II) ions in biological samples and outcomes strongly supported that probe-38 was capable for the recognition of Zn(II) ions in biological samples and was confirmed by intracellular imaging of Zn(II) ions (Fig. 50).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with an association constant of 3.24 × 104 M−1. Further, the fluorescence studies of probe-39 were carried out at various pH values and found to show better results in the range of 5–8. Furthermore, the binding mechanism (Fig. 51) and fluorescence turn-on mechanism were confirmed via DFT calculations, 1H NMR titration, FT-IR, and ESI-MS and were used to demonstrate the interaction properties. The authors utilized probe-39 for the recognition of Zn(II) ions in living cells along with environmental areas (Fig. 52). Consequently, probe-39 has potential applications for sensing Zn(II) ions in the environment and biological systems.
1 with an association constant of 3.24 × 104 M−1. Further, the fluorescence studies of probe-39 were carried out at various pH values and found to show better results in the range of 5–8. Furthermore, the binding mechanism (Fig. 51) and fluorescence turn-on mechanism were confirmed via DFT calculations, 1H NMR titration, FT-IR, and ESI-MS and were used to demonstrate the interaction properties. The authors utilized probe-39 for the recognition of Zn(II) ions in living cells along with environmental areas (Fig. 52). Consequently, probe-39 has potential applications for sensing Zn(II) ions in the environment and biological systems.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, with association constants (Ka) of 4.9 × 104 M−1 and 2.1 × 104 M−1 with probe-40a and probe-40b, respectively. The reversibility of probes was tested using EDTA and data showed very good results. Probe-40a and probe-40b were found to have detection limits of 67 nM and 0.36 μM, respectively, which were calculated from the fluorescence titration data. The detection of Zn(II) ions in various water samples and pharmaceutical multivitamin tablets was accomplished with probe-40a and probe-40b.
1 stoichiometry, with association constants (Ka) of 4.9 × 104 M−1 and 2.1 × 104 M−1 with probe-40a and probe-40b, respectively. The reversibility of probes was tested using EDTA and data showed very good results. Probe-40a and probe-40b were found to have detection limits of 67 nM and 0.36 μM, respectively, which were calculated from the fluorescence titration data. The detection of Zn(II) ions in various water samples and pharmaceutical multivitamin tablets was accomplished with probe-40a and probe-40b.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was validated by Job's plot, ESI-MS and 1H NMR. Further, authors observed a large Stokes shift of emission spectra during the titration process, which showed clearly visible changes in the fluorescence (from blue to bright orange). The sensing mechanism was proposed, based on the inhibition of PET and ESIPT. Further, the LOD value of probe-41 and Zn(II) was estimated to be as low as 80.5 nM.
1, which was validated by Job's plot, ESI-MS and 1H NMR. Further, authors observed a large Stokes shift of emission spectra during the titration process, which showed clearly visible changes in the fluorescence (from blue to bright orange). The sensing mechanism was proposed, based on the inhibition of PET and ESIPT. Further, the LOD value of probe-41 and Zn(II) was estimated to be as low as 80.5 nM.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was confirmed by HRMS and 1H NMR data. The MTT assay and IC50 studies, as well as in vitro/in vivo imaging for probe-42 with Zn(II) ions, were conducted on B16F10 cell lines and in zebrafish, to determine the probe's biocompatibility and it demonstrated applications of probe-42 in biological samples (Fig. 56). The OTFT devices was created upon incorporation of probe-42 with pentacene, which validated the reversibility discovered to be more than 90% change in drain-source current in response to Zn(II) with a LOD of 5.46 μM. The OTFT devices additionally exhibited Zn(II) ion detection in samples of tap water and lake water.
1, which was confirmed by HRMS and 1H NMR data. The MTT assay and IC50 studies, as well as in vitro/in vivo imaging for probe-42 with Zn(II) ions, were conducted on B16F10 cell lines and in zebrafish, to determine the probe's biocompatibility and it demonstrated applications of probe-42 in biological samples (Fig. 56). The OTFT devices was created upon incorporation of probe-42 with pentacene, which validated the reversibility discovered to be more than 90% change in drain-source current in response to Zn(II) with a LOD of 5.46 μM. The OTFT devices additionally exhibited Zn(II) ion detection in samples of tap water and lake water.






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 stoichiometry was estimated by Job's plot for the complex between probe-46 and Zn(II) ions and was supported by fluorescence and UV-vis titrations; ESI-MS, single crystal as well as theoretical calculations were used to understand their detection mechanisms. Further, the binding mode of probe-46 with Zn(II) ions was confirmed via Hirshfeld surface analysis, EA, IR, UV-Vis spectroscopy, and fluorescence spectroscopy.
3 stoichiometry was estimated by Job's plot for the complex between probe-46 and Zn(II) ions and was supported by fluorescence and UV-vis titrations; ESI-MS, single crystal as well as theoretical calculations were used to understand their detection mechanisms. Further, the binding mode of probe-46 with Zn(II) ions was confirmed via Hirshfeld surface analysis, EA, IR, UV-Vis spectroscopy, and fluorescence spectroscopy.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in both cases. With the help of these discoveries, a different input switch and an embedded logic circuit were effectively built.
1 in both cases. With the help of these discoveries, a different input switch and an embedded logic circuit were effectively built.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was confirmed by Job's plot. Moreover, probe-48 could quickly, easily, and practically monitor Zn(II) and Fe(III) ions using conventional test strips. Fluorescence cell images also identified the potential of probe-48 to be used as a bio-imaging fluorescent sensor to identify Zn(II) ions in human cancer cells (Fig. 66).
1, which was confirmed by Job's plot. Moreover, probe-48 could quickly, easily, and practically monitor Zn(II) and Fe(III) ions using conventional test strips. Fluorescence cell images also identified the potential of probe-48 to be used as a bio-imaging fluorescent sensor to identify Zn(II) ions in human cancer cells (Fig. 66).


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– isomerization, PET blocking, and the ESIPT process (Fig. 67). The recognition mechanism of Zn(II) ions with probe-49a/probe-49b was further supported by 1H NMR titrations. Furthermore, Job's plot analysis displayed 1
N– isomerization, PET blocking, and the ESIPT process (Fig. 67). The recognition mechanism of Zn(II) ions with probe-49a/probe-49b was further supported by 1H NMR titrations. Furthermore, Job's plot analysis displayed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding for probes with Zn(II) ions, which was established by DFT, 1H NMR, UV-vis, and fluorescence spectroscopy studies. In addition, the LOD values of probe-49a and probe-49b for Zn(II) ions were found to be 7.69 and 5.35 nM, correspondingly, which were much lesser than permitted by the U.S. Environmental Protection Agency (US-EPA). The probes could be used as an efficient luminescent probe for the recognition of Zn(II) ions in living cells, based on the fluorescence microscopic imaging in HeLa cells, which strongly demonstrates the immense potential of this simple chemosensor in cellular imaging (Fig. 68).
1 binding for probes with Zn(II) ions, which was established by DFT, 1H NMR, UV-vis, and fluorescence spectroscopy studies. In addition, the LOD values of probe-49a and probe-49b for Zn(II) ions were found to be 7.69 and 5.35 nM, correspondingly, which were much lesser than permitted by the U.S. Environmental Protection Agency (US-EPA). The probes could be used as an efficient luminescent probe for the recognition of Zn(II) ions in living cells, based on the fluorescence microscopic imaging in HeLa cells, which strongly demonstrates the immense potential of this simple chemosensor in cellular imaging (Fig. 68).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Fig. 70). However, on titrating with other competitive metal ions, there was no change in the emission spectra, these results strongly suggested that probe-50 is very selective towards Hg(II) ions (Fig. 70). The LOD of probe-50 with Hg(II) ions was estimated as 10 nM from the emission titration spectra. Furthermore, probe-50 finds a method for monitoring variations in Hg(II) ion content in biological systems by confocal laser microscopy, such as fluorescence imaging of HeLa live cells and zebra fish (Fig. 71).
1, v/v) (Fig. 70). However, on titrating with other competitive metal ions, there was no change in the emission spectra, these results strongly suggested that probe-50 is very selective towards Hg(II) ions (Fig. 70). The LOD of probe-50 with Hg(II) ions was estimated as 10 nM from the emission titration spectra. Furthermore, probe-50 finds a method for monitoring variations in Hg(II) ion content in biological systems by confocal laser microscopy, such as fluorescence imaging of HeLa live cells and zebra fish (Fig. 71).


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and ESIPT could be attributed to the fluorescence turn-on response of probe-51 (Fig. 72). Further, probe-51–Hg(II) was also regenerated back to probe-51 when EDTA was added, which provided the potentiality of probe-51. The 2
N isomerization and ESIPT could be attributed to the fluorescence turn-on response of probe-51 (Fig. 72). Further, probe-51–Hg(II) was also regenerated back to probe-51 when EDTA was added, which provided the potentiality of probe-51. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding for probe-51–Hg(II) was determined by utilizing the method of continuous variations and Job's plot, which was further validated by the B–H method. The binding constant was found to be 4.484 × 105 M−1, and the fluorescence titration profile shows that probe-51 has a detection limit of 22 nM for Hg(II), which is as low as to detect submillimolar concentrations of Hg(II) ions seen in practice.
1 binding for probe-51–Hg(II) was determined by utilizing the method of continuous variations and Job's plot, which was further validated by the B–H method. The binding constant was found to be 4.484 × 105 M−1, and the fluorescence titration profile shows that probe-51 has a detection limit of 22 nM for Hg(II), which is as low as to detect submillimolar concentrations of Hg(II) ions seen in practice.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v, ex = 480 nm) via the fluorescence turn-on mechanism.122Probe-52 (Fig. 73) was synthesized from rhodamine hydrazide and furfural in ethanol. Probe-52 was structurally characterized by ESI-MS, 1H, 13C NMR, FT-IR (KBr) and EA. The native state of probe-52 exhibited very weak emission, on titrating with Hg(II) ions, and the fluorescence intensity gradually increased as “green” luminescence enhanced by 320-fold. However, the authors tested other metal ions, which showed no change in the fluorescence spectra. Further, the association constant of probe-52 with Hg(II) was computed to be 1.01 × 104.94 M−1 and the solution color changed from colorless to green fluorescence when exposed to a UV lamp. Meanwhile, Cys can significantly reduce the green fluorescence of probe-52–Hg(II); however, no change in fluorescence spectra was observed for GSH, SO42−, SO32−, CN−, CO32−, or HCY. The LOD values for Hg(II) and Cys were calculated to be 0.09 μM and 0.08 μM, respectively, and they could be applied as dual fluorescent probes that can distinguish Hg(II) ions from other metal ions as well as Cys from other biothiols, and thus, are highly utilizable in aqueous media and bio-imaging studies (Fig. 74).
7, v/v, ex = 480 nm) via the fluorescence turn-on mechanism.122Probe-52 (Fig. 73) was synthesized from rhodamine hydrazide and furfural in ethanol. Probe-52 was structurally characterized by ESI-MS, 1H, 13C NMR, FT-IR (KBr) and EA. The native state of probe-52 exhibited very weak emission, on titrating with Hg(II) ions, and the fluorescence intensity gradually increased as “green” luminescence enhanced by 320-fold. However, the authors tested other metal ions, which showed no change in the fluorescence spectra. Further, the association constant of probe-52 with Hg(II) was computed to be 1.01 × 104.94 M−1 and the solution color changed from colorless to green fluorescence when exposed to a UV lamp. Meanwhile, Cys can significantly reduce the green fluorescence of probe-52–Hg(II); however, no change in fluorescence spectra was observed for GSH, SO42−, SO32−, CN−, CO32−, or HCY. The LOD values for Hg(II) and Cys were calculated to be 0.09 μM and 0.08 μM, respectively, and they could be applied as dual fluorescent probes that can distinguish Hg(II) ions from other metal ions as well as Cys from other biothiols, and thus, are highly utilizable in aqueous media and bio-imaging studies (Fig. 74).

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, which block the non-fluorescence pathway of probe-53. The competitive studies of probe-53 revealed high selectivity towards Hg(II) ions even with other metal ions. Further, to find out the recognition of Hg(II) ions in biological media, authors performed the sensing studies on HeLa cells, which strongly suggested that probe-53 could be able to sense Hg(II) ions in biological samples (Fig. 76). Due to the very high sensitivity and selectivity of probe-53 towards Hg(II) ions via the fluorescence turn-on behavior with a low LOD (3.4 nM in THF, 0.24 μM in THF/water), it became a compatible sensor in both environmental and biological samples.
N isomerization, which block the non-fluorescence pathway of probe-53. The competitive studies of probe-53 revealed high selectivity towards Hg(II) ions even with other metal ions. Further, to find out the recognition of Hg(II) ions in biological media, authors performed the sensing studies on HeLa cells, which strongly suggested that probe-53 could be able to sense Hg(II) ions in biological samples (Fig. 76). Due to the very high sensitivity and selectivity of probe-53 towards Hg(II) ions via the fluorescence turn-on behavior with a low LOD (3.4 nM in THF, 0.24 μM in THF/water), it became a compatible sensor in both environmental and biological samples.





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Increases in Hg(II) ion concentration were found to significantly increase the fluorescence intensity at the maximum emission peak of probes in an aqueous solution of ACN and water. This finding was interpreted as the result of an ESIPT-coupled AIEE process that was made possible by the coordination of the probes with Hg(II) ions (Fig. 81). The probes exhibit high sensitivity and selectivity over other interfering metal ions with a LOD value over 12.5 ppb.
1. Increases in Hg(II) ion concentration were found to significantly increase the fluorescence intensity at the maximum emission peak of probes in an aqueous solution of ACN and water. This finding was interpreted as the result of an ESIPT-coupled AIEE process that was made possible by the coordination of the probes with Hg(II) ions (Fig. 81). The probes exhibit high sensitivity and selectivity over other interfering metal ions with a LOD value over 12.5 ppb.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) groups and oxygen (O) of carbonyl groups when the spirolactum ring of the rhodamine core is opened (Fig. 82). The LOD value of Hg(II) ions was estimated to be 1.729 ng mL−1 and the binding ratio of probe-58 with Hg(II) ions to be 1
N) groups and oxygen (O) of carbonyl groups when the spirolactum ring of the rhodamine core is opened (Fig. 82). The LOD value of Hg(II) ions was estimated to be 1.729 ng mL−1 and the binding ratio of probe-58 with Hg(II) ions to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Moreover, the addition of a 0–2 g mL−1 Hg(II) ion solution increases the lifetime value from 5.63 ns to 6.12 ns, indicating the formation of complexes in the excited state. The intracellular recognition of Hg(II) ions using probe-58 is another advantage of prepared nanoparticles that will be probed for potential applications in the biomedical field (Fig. 83).
1. Moreover, the addition of a 0–2 g mL−1 Hg(II) ion solution increases the lifetime value from 5.63 ns to 6.12 ns, indicating the formation of complexes in the excited state. The intracellular recognition of Hg(II) ions using probe-58 is another advantage of prepared nanoparticles that will be probed for potential applications in the biomedical field (Fig. 83).


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-(imine) link of the thiazole moiety is hydrolyzed by Hg(II), whereas pyrene-carboxaldehyde molecules operate as ‘turn-on’ chemodosimeters for Hg(II) ions (Fig. 85). The detection limit of probe-60a with Hg(II) ions was found to be 0.270 μM. Additionally, the probe-60a's chemodosimetric irreversible hydrolysis was investigated by FT-IR spectroscopy, UV/Vis, liquid chromatography with tandem mass spectrometry (LC-MS), fluorescence, 1H NMR, and DFT analysis.
N-(imine) link of the thiazole moiety is hydrolyzed by Hg(II), whereas pyrene-carboxaldehyde molecules operate as ‘turn-on’ chemodosimeters for Hg(II) ions (Fig. 85). The detection limit of probe-60a with Hg(II) ions was found to be 0.270 μM. Additionally, the probe-60a's chemodosimetric irreversible hydrolysis was investigated by FT-IR spectroscopy, UV/Vis, liquid chromatography with tandem mass spectrometry (LC-MS), fluorescence, 1H NMR, and DFT analysis.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization on Hg(II) ion coordination. The LOD value was measured to be 13.4 nM and 15.6 nM respectively for both probe-61a and probe-61b toward Hg(II) ions using the equation DL = 3σ/slope. Due to their potentiality, these probes can be utilized in future applications for the recognition of Hg(II) in various media. The DFT studies predicted the equivalent molecular geometric arrangement, orbital electron distribution, and orbital energy of both probes with their metal complexes. Job's plot analysis gave a binding ratio of 1
N isomerization on Hg(II) ion coordination. The LOD value was measured to be 13.4 nM and 15.6 nM respectively for both probe-61a and probe-61b toward Hg(II) ions using the equation DL = 3σ/slope. Due to their potentiality, these probes can be utilized in future applications for the recognition of Hg(II) in various media. The DFT studies predicted the equivalent molecular geometric arrangement, orbital electron distribution, and orbital energy of both probes with their metal complexes. Job's plot analysis gave a binding ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and further, the probes were fruitfully used for imaging Hg(II) ions in live breast cancer cells and MCF-7 cells, illustrating the probe's applications for biological-Hg(II) ion detection (Fig. 87).
1, and further, the probes were fruitfully used for imaging Hg(II) ions in live breast cancer cells and MCF-7 cells, illustrating the probe's applications for biological-Hg(II) ion detection (Fig. 87).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a binding constant of 4.12 × 105 M−1. In addition, probe-62 changes color in response to the anions CN− and F−, agreeing for dual mode detection of several analytes.
1 with a binding constant of 4.12 × 105 M−1. In addition, probe-62 changes color in response to the anions CN− and F−, agreeing for dual mode detection of several analytes.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions, probe-63 demonstrates high selectivity and sensitivity towards Hg(II) ions and the color changed to pink with 48-fold fluorescence amplification, which is caused by the ring opening of the rhodamine unit (Fig. 89), whereas no change was observed in the absorption and emission spectra with other competitive metal ions. In addition, the molecule binds to Hg(II) in a 1
1) solutions, probe-63 demonstrates high selectivity and sensitivity towards Hg(II) ions and the color changed to pink with 48-fold fluorescence amplification, which is caused by the ring opening of the rhodamine unit (Fig. 89), whereas no change was observed in the absorption and emission spectra with other competitive metal ions. In addition, the molecule binds to Hg(II) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was validated by Job's plot and was supported by the ESI-MS and DFT calculations. The LOD value of probe-63 from the fluorescence titration spectra was calculated to be 12 nM and could be utilized effectively for Hg(II) sensing over a wide pH range of 4.0–10.0. Finally, the authors applied probe-63 for sensing Hg(II) ions in in vitro and in vivo studies, including test strips, bio imaging in HeLa cells and zebrafish with the help of confocal fluorescence microscopy (Fig. 90).
1, which was validated by Job's plot and was supported by the ESI-MS and DFT calculations. The LOD value of probe-63 from the fluorescence titration spectra was calculated to be 12 nM and could be utilized effectively for Hg(II) sensing over a wide pH range of 4.0–10.0. Finally, the authors applied probe-63 for sensing Hg(II) ions in in vitro and in vivo studies, including test strips, bio imaging in HeLa cells and zebrafish with the help of confocal fluorescence microscopy (Fig. 90).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the probe-64–Hg(II) complex. It was further demonstrated that it was a straightforward and efficient solid-state probe because the color of the sensor substantially changed when it was impregnated on filter paper and submerged in a Hg(II) solution.
1 for the probe-64–Hg(II) complex. It was further demonstrated that it was a straightforward and efficient solid-state probe because the color of the sensor substantially changed when it was impregnated on filter paper and submerged in a Hg(II) solution.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding, and the probe-65–Hg(II) binding constant was calculated as 8.75 × 104 M−1. Furthermore, the probe-65 was successfully used in cell imaging, indicating that it has a potential application for sensing Hg(II) ions in living cells (Fig. 93).
1 binding, and the probe-65–Hg(II) binding constant was calculated as 8.75 × 104 M−1. Furthermore, the probe-65 was successfully used in cell imaging, indicating that it has a potential application for sensing Hg(II) ions in living cells (Fig. 93).





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For probe-68–Hg(II) complexation, the calculated association constant and LOD were 6 × 104 M−1 and 30.37 nM, respectively. The Hg(II) metal ion was successfully detected by a spike and recovery method from actual water samples in practical use. Probe-68 was also utilized for the sensing of Hg(II) ions in biological media, which was carried out by the MTT assay for the cytotoxicity and the data showed very low cytotoxicity, providing valuable information for the identification of intracellular Hg(II) ions in MDA-MB-231 and A375 breast cancer cells (Fig. 99).
1. For probe-68–Hg(II) complexation, the calculated association constant and LOD were 6 × 104 M−1 and 30.37 nM, respectively. The Hg(II) metal ion was successfully detected by a spike and recovery method from actual water samples in practical use. Probe-68 was also utilized for the sensing of Hg(II) ions in biological media, which was carried out by the MTT assay for the cytotoxicity and the data showed very low cytotoxicity, providing valuable information for the identification of intracellular Hg(II) ions in MDA-MB-231 and A375 breast cancer cells (Fig. 99).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry from the absorption spectra. Probe-69 could find use as a Hg(II) detector in living cells (Fig. 101) or water samples.
1 stoichiometry from the absorption spectra. Probe-69 could find use as a Hg(II) detector in living cells (Fig. 101) or water samples.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation among probe-70 and Cd(II) ions, which was strongly supported by 1H NMR and ESI-MS analysis. The LOD value of probe-70 was calculated from emission titration spectra to be 2.4 nM.
1 stoichiometric complexation among probe-70 and Cd(II) ions, which was strongly supported by 1H NMR and ESI-MS analysis. The LOD value of probe-70 was calculated from emission titration spectra to be 2.4 nM.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). However, the fluorescence intensity of probe-71 displayed poor emission, and upon addition of Cd(II) ions, the emission intensity was gradually increased 37-fold its original intensity at 510 nm. Under similar conditions, with other competitive metal ions, there was no change in the fluorescence spectra. The sensing mechanism of probe-71 with Cd(II) was explained by the restriction of C
2, v/v). However, the fluorescence intensity of probe-71 displayed poor emission, and upon addition of Cd(II) ions, the emission intensity was gradually increased 37-fold its original intensity at 510 nm. Under similar conditions, with other competitive metal ions, there was no change in the fluorescence spectra. The sensing mechanism of probe-71 with Cd(II) was explained by the restriction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and CHEF effects (Fig. 104). The LOD value of probe-71 with Cd(II) ions was estimated to be 49.5 nM from the titration spectra using the 3σ/slope equation. The binding constant was estimated to be 1.77 × 105 M−1. The stoichiometry of probe-71 with Cd(II) ions was estimated to be 2
N isomerization and CHEF effects (Fig. 104). The LOD value of probe-71 with Cd(II) ions was estimated to be 49.5 nM from the titration spectra using the 3σ/slope equation. The binding constant was estimated to be 1.77 × 105 M−1. The stoichiometry of probe-71 with Cd(II) ions was estimated to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 via Job's plot, which was confirmed by DFT, 1H NMR and ESI-MS data.
1 via Job's plot, which was confirmed by DFT, 1H NMR and ESI-MS data.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerism; upon incorporation of Cd(II) ions, the luminescence intensity was drastically increased and on titrating with other competitive metal ions, no change was observed in the emission spectra (Fig. 105). From the emission spectra, it was observed that the intensity of the peak at 530 nm increased with the gradual addition of Cd(II) and the detection limit was found to be 41 nM. Job's plot revealed the 1
N isomerism; upon incorporation of Cd(II) ions, the luminescence intensity was drastically increased and on titrating with other competitive metal ions, no change was observed in the emission spectra (Fig. 105). From the emission spectra, it was observed that the intensity of the peak at 530 nm increased with the gradual addition of Cd(II) and the detection limit was found to be 41 nM. Job's plot revealed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric complexations between probe-72 and Cd(II) ions, which was strongly supported by 1H NMR, IR and ESI-MS data. Further, the authors focused on the effect of pH on probe-72 and showed that they did not produce any fluorescence change within the pH range of 6–11, whereas upon adding Cd(II), the fluorescence intensity exclusively amplified at pH 2–11, indicating that the detection of Cd(II) ions with probe-72 was suitable for physiological pH. Apart from acting as a fluorescent probe, inoculating probe-72 in HeLa cells in in vivo cell imaging was achieved by sensing Cd(II) ions by fluorescence microscopy without causing any harm to the cell line (Fig. 106).
2 stoichiometric complexations between probe-72 and Cd(II) ions, which was strongly supported by 1H NMR, IR and ESI-MS data. Further, the authors focused on the effect of pH on probe-72 and showed that they did not produce any fluorescence change within the pH range of 6–11, whereas upon adding Cd(II), the fluorescence intensity exclusively amplified at pH 2–11, indicating that the detection of Cd(II) ions with probe-72 was suitable for physiological pH. Apart from acting as a fluorescent probe, inoculating probe-72 in HeLa cells in in vivo cell imaging was achieved by sensing Cd(II) ions by fluorescence microscopy without causing any harm to the cell line (Fig. 106).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A binding constant of 1.17 × 104 M−1 and detection limits in the micromolar range were calculated for probe-73 with Cd(II) ions from the fluorescence titration spectra. Furthermore, the authors utilized probe-73 for the sensing of Cd(II) ions in biological media; the HeLa cells were incubated with probe-73 and showed no fluorescence, and in the presence of Cd(II) ions, the emission was generated inside the cells (Fig. 108), which strongly suggested that probe-73 shows potential for the recognition of Cd(II) ions in biological media.
1. A binding constant of 1.17 × 104 M−1 and detection limits in the micromolar range were calculated for probe-73 with Cd(II) ions from the fluorescence titration spectra. Furthermore, the authors utilized probe-73 for the sensing of Cd(II) ions in biological media; the HeLa cells were incubated with probe-73 and showed no fluorescence, and in the presence of Cd(II) ions, the emission was generated inside the cells (Fig. 108), which strongly suggested that probe-73 shows potential for the recognition of Cd(II) ions in biological media.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding of probe-74 with Cd(II), enabling the ratiometric sensing of Cd(II) ions with a LOD value of 0.38 μM. Additionally, confocal fluorescence microscopy was used to examine the use of probe-74 to sense Cd(II) ions in human liver cancer cells (SMMC-7721), zebrafish, and live tissues of Arabidopsis thaliana, revealing that it can be used to monitor Cd(II) ions in various biological contexts with low toxicity (Fig. 110).
1 binding of probe-74 with Cd(II), enabling the ratiometric sensing of Cd(II) ions with a LOD value of 0.38 μM. Additionally, confocal fluorescence microscopy was used to examine the use of probe-74 to sense Cd(II) ions in human liver cancer cells (SMMC-7721), zebrafish, and live tissues of Arabidopsis thaliana, revealing that it can be used to monitor Cd(II) ions in various biological contexts with low toxicity (Fig. 110).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), which could be utilized for real-time monitoring. A further binding mechanism of probe-75 was proposed by the authors and ring opening of spirolactum and suppression of PET process, which was supported by the ESI-MS, FT-IR and DFT calculations. The LOD value of probe-75 with Au(III) was calculated to be 1.51 μM from the fluorescence titration data using the equation 3σ/slope. Considering the ability of probe-75 to act as a selective and sensitive fluorescent turn-on probe, in vivo cell imaging was achieved by inoculating probe-75 in MC3T3 cells with very low cytotoxicity (determined by the MTT assay) (Fig. 112).
1, v/v), which could be utilized for real-time monitoring. A further binding mechanism of probe-75 was proposed by the authors and ring opening of spirolactum and suppression of PET process, which was supported by the ESI-MS, FT-IR and DFT calculations. The LOD value of probe-75 with Au(III) was calculated to be 1.51 μM from the fluorescence titration data using the equation 3σ/slope. Considering the ability of probe-75 to act as a selective and sensitive fluorescent turn-on probe, in vivo cell imaging was achieved by inoculating probe-75 in MC3T3 cells with very low cytotoxicity (determined by the MTT assay) (Fig. 112).

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond's sensitivity to acidic pH, while probe-76b with a greater number of C
N bond's sensitivity to acidic pH, while probe-76b with a greater number of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds exhibited lower extent of hydrolysis by Au(III) than that of probe-76a (Fig. 113). The 1H-NMR data also suggested the formation of aldehyde groups by hydrolysis of the C
N bonds exhibited lower extent of hydrolysis by Au(III) than that of probe-76a (Fig. 113). The 1H-NMR data also suggested the formation of aldehyde groups by hydrolysis of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group in the presence of Au(III) ions. Furthermore, the authors demonstrated the use of probe-76a in PC12 cells and zebrafish (with very low cytotoxicity determined by the MTT assay); in vivo cell imaging was obtained via Au(III) monitoring using laser confocal fluorescence microscopy (Fig. 114).
N group in the presence of Au(III) ions. Furthermore, the authors demonstrated the use of probe-76a in PC12 cells and zebrafish (with very low cytotoxicity determined by the MTT assay); in vivo cell imaging was obtained via Au(III) monitoring using laser confocal fluorescence microscopy (Fig. 114).



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation between probe-78 and Ag(I) ions, which was further validated by ESI-MS, 1H NMR and DFT data. The DFT calculation indicated the lowering of band gap of probe-78 upon binding with Ag(I), which ascertained that probe-78 + Ag(I) complex formation causes a bathochromic shift. The sensing mechanism (Fig. 117) of probe-78 with Ag(I) was confirmed by 1H NMR and the binding ratio was estimated to be 1
1 stoichiometric complexation between probe-78 and Ag(I) ions, which was further validated by ESI-MS, 1H NMR and DFT data. The DFT calculation indicated the lowering of band gap of probe-78 upon binding with Ag(I), which ascertained that probe-78 + Ag(I) complex formation causes a bathochromic shift. The sensing mechanism (Fig. 117) of probe-78 with Ag(I) was confirmed by 1H NMR and the binding ratio was estimated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by Job's plot. Further, the authors demonstrated the influence of the pH detecting ability of probe-78 with Ag(I), and at pH 7, it showed higher fluorescence intensity than that at other pH values, which strongly suggested that probe-78 was potential for the detection in physiological media. The LOD value of probe-78 with Ag(I) ions from the fluorescence titration spectra using the equation 3σ/slope was found to be 1.49 μM.
1 by Job's plot. Further, the authors demonstrated the influence of the pH detecting ability of probe-78 with Ag(I), and at pH 7, it showed higher fluorescence intensity than that at other pH values, which strongly suggested that probe-78 was potential for the detection in physiological media. The LOD value of probe-78 with Ag(I) ions from the fluorescence titration spectra using the equation 3σ/slope was found to be 1.49 μM.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), revealed 1
1, v/v), revealed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation, which was confirmed by Job's plot analysis, IR studies and ESI-MS. The proposed mechanism of probe-79 with Ag(I) might be attributed to ICT (Fig. 118). Finally, the authors aimed to detect Ag(I) ions in biological samples, by inoculation of probe-79 in E. coli cells followed by insertion of Ag(I); live cell imaging was obtained by laser confocal scanning microscopy (Fig. 119).
1 stoichiometric complexation, which was confirmed by Job's plot analysis, IR studies and ESI-MS. The proposed mechanism of probe-79 with Ag(I) might be attributed to ICT (Fig. 118). Finally, the authors aimed to detect Ag(I) ions in biological samples, by inoculation of probe-79 in E. coli cells followed by insertion of Ag(I); live cell imaging was obtained by laser confocal scanning microscopy (Fig. 119).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution, it was found that dual emission bands appeared at 410 and 500 nm with very low intensity, while on titration with Ag(I) ions, the emission was progressively increased and saturated after addition of 1 eq., whereas no considerable change was observed in the fluorescence emission of probe-80 in the presence of other competitive ions, which strongly supported the selectivity of probe. The fluorescence titration data showed the ratiometric response, in which the intensity of the peak at 410 nm increased and the peak at 500 nm decreased simultaneously. Moreover, authors focused on the titration experiments on varying pH, and it was noticed that probe-80 and probe-80 + Ag(I) remained stable in the pH range of 4 to 9, which indicated that probe-80 could be utilised for the detection of Ag(I) under physiological pH (7.4). Fluorescence titration data revealed the 1
1, v/v) solution, it was found that dual emission bands appeared at 410 and 500 nm with very low intensity, while on titration with Ag(I) ions, the emission was progressively increased and saturated after addition of 1 eq., whereas no considerable change was observed in the fluorescence emission of probe-80 in the presence of other competitive ions, which strongly supported the selectivity of probe. The fluorescence titration data showed the ratiometric response, in which the intensity of the peak at 410 nm increased and the peak at 500 nm decreased simultaneously. Moreover, authors focused on the titration experiments on varying pH, and it was noticed that probe-80 and probe-80 + Ag(I) remained stable in the pH range of 4 to 9, which indicated that probe-80 could be utilised for the detection of Ag(I) under physiological pH (7.4). Fluorescence titration data revealed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric complexation between probe-80 and Ag(I), which was also supported by Job's plot and ESI-MS. The authors also proposed the sensing mechanism of probe-80, where the Ag(I) ions coordinated through the lone pair of N-atoms and O-atoms, which leads to enhanced fluorescence as a result of inhibition of PET (Fig. 120). The association and LOD values of probe-80 were found from the fluorescence titration spectra as 2.41 × 104 M−2 and 14 μM respectively. Further, for the practical application, fluorescence cell imaging of probe-80 incubated with E. coli cells was obtained in the presence of Ag(I) ions using a laser scanning microscope as red fluorescence (Fig. 121).
2 stoichiometric complexation between probe-80 and Ag(I), which was also supported by Job's plot and ESI-MS. The authors also proposed the sensing mechanism of probe-80, where the Ag(I) ions coordinated through the lone pair of N-atoms and O-atoms, which leads to enhanced fluorescence as a result of inhibition of PET (Fig. 120). The association and LOD values of probe-80 were found from the fluorescence titration spectra as 2.41 × 104 M−2 and 14 μM respectively. Further, for the practical application, fluorescence cell imaging of probe-80 incubated with E. coli cells was obtained in the presence of Ag(I) ions using a laser scanning microscope as red fluorescence (Fig. 121).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between probe-81 and Ag(I) ions. The colorimetric detection of probe-81 with Cu(II) and Ag(I) ions was estimated to be 1.7 and 2.2 μM, respectively, and the Ag(I) fluorometric detection limit was up to 1.6 μM. Probe-81, which works over a wide pH range, can be used to measure and identify the ions Cu(II) and Ag(I) in environmental samples as well as water samples.
1 binding between probe-81 and Ag(I) ions. The colorimetric detection of probe-81 with Cu(II) and Ag(I) ions was estimated to be 1.7 and 2.2 μM, respectively, and the Ag(I) fluorometric detection limit was up to 1.6 μM. Probe-81, which works over a wide pH range, can be used to measure and identify the ions Cu(II) and Ag(I) in environmental samples as well as water samples.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 proportion of 3H-benzo[h] chromene-3-carbaldehyde and carbazide in ethyl alcohol, and the reaction mixture was stirred under reflux conditions to give a final product in good yields and was confirmed by various analytical techniques. Probe-82 displayed very good aggregate induce ratiometric emission in methanol and water mixtures. The sensing studies were carried out in MeOH and water mixtures, where probe-82 showed very poor emission (ϕ = 0.061) with a peak at 450 nm, and upon addition of Cu(II) ion, a very bright emission was generated at 511 nm with enhanced quantum yields (ϕ = 0.25), simultaneously decreasing the emission intensity at 450 nm. To find out better selectivity and sensitivity of probe-82, it was tested with several interfering metal ions and no difference in fluorescence spectra was observed. The binding mechanism of Cu(II) ions and probe-82 was examined and found to be ICT combined with metal-induced association (Fig. 123). The detection limit of probe-82 with Cu(II) was determined to be 10.4 nM using the formula 3σ/slope. Moreover, for the practical application, bio-imaging of probe-82 was carried out and the results suggested that probe-82 successfully detects Cu(II) ions in biological media, which was confirmed by fluorescence microscopy (Fig. 124).
1 proportion of 3H-benzo[h] chromene-3-carbaldehyde and carbazide in ethyl alcohol, and the reaction mixture was stirred under reflux conditions to give a final product in good yields and was confirmed by various analytical techniques. Probe-82 displayed very good aggregate induce ratiometric emission in methanol and water mixtures. The sensing studies were carried out in MeOH and water mixtures, where probe-82 showed very poor emission (ϕ = 0.061) with a peak at 450 nm, and upon addition of Cu(II) ion, a very bright emission was generated at 511 nm with enhanced quantum yields (ϕ = 0.25), simultaneously decreasing the emission intensity at 450 nm. To find out better selectivity and sensitivity of probe-82, it was tested with several interfering metal ions and no difference in fluorescence spectra was observed. The binding mechanism of Cu(II) ions and probe-82 was examined and found to be ICT combined with metal-induced association (Fig. 123). The detection limit of probe-82 with Cu(II) was determined to be 10.4 nM using the formula 3σ/slope. Moreover, for the practical application, bio-imaging of probe-82 was carried out and the results suggested that probe-82 successfully detects Cu(II) ions in biological media, which was confirmed by fluorescence microscopy (Fig. 124).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and confirmed by Job's plot and ESI-MS. Probe-83 exhibited very good detection limit as well as association constants of 0.1 nM and 1.03 × 106 M−1 correspondingly. As noted, detection limit values are much lower than that of WHO approved. The reversibility of probe-83 was observed upon addition of a sodium sulfide solution to probe-83 + Cu(II). Further, cellular imaging of probe-83 was performed on HepG2 cells, and it was found to have negligible cytotoxicity with very high cell permeability. The HepG2 cells were incubated with probe-83 for 30 min, which did not show visible fluorescence, whereas upon treatment with Cu(II) ions, strong fluorescence images were observed using a fluorescence microscope (Fig. 126). Thus, probe-83 found to be a potential candidate for the recognition of Cu(II) ions in biological media.
1 and confirmed by Job's plot and ESI-MS. Probe-83 exhibited very good detection limit as well as association constants of 0.1 nM and 1.03 × 106 M−1 correspondingly. As noted, detection limit values are much lower than that of WHO approved. The reversibility of probe-83 was observed upon addition of a sodium sulfide solution to probe-83 + Cu(II). Further, cellular imaging of probe-83 was performed on HepG2 cells, and it was found to have negligible cytotoxicity with very high cell permeability. The HepG2 cells were incubated with probe-83 for 30 min, which did not show visible fluorescence, whereas upon treatment with Cu(II) ions, strong fluorescence images were observed using a fluorescence microscope (Fig. 126). Thus, probe-83 found to be a potential candidate for the recognition of Cu(II) ions in biological media.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (8
H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) media, which exhibited a chromogenic behavior towards Cu(II) ions, and the color changed from yellow to colorless, even though in the presence of other competitive metal ions, it does not show any changes in absorption spectra. The fluorescence studies of probe-84 displayed fluorescence enhancement upon incremental addition of Cu(II) ions, which was explained by the CHEF mechanism (Fig. 127) and no change in emission intensity with other metal ions. The LOD value was calculated via fluorescence spectra as 14.5 nM, which was much lower than that of WHO guidelines (0.02 μM) in drinking water. The stoichiometry of probe-84 with Cu(II) ions was determined by Job's plot as 1
2, v/v) media, which exhibited a chromogenic behavior towards Cu(II) ions, and the color changed from yellow to colorless, even though in the presence of other competitive metal ions, it does not show any changes in absorption spectra. The fluorescence studies of probe-84 displayed fluorescence enhancement upon incremental addition of Cu(II) ions, which was explained by the CHEF mechanism (Fig. 127) and no change in emission intensity with other metal ions. The LOD value was calculated via fluorescence spectra as 14.5 nM, which was much lower than that of WHO guidelines (0.02 μM) in drinking water. The stoichiometry of probe-84 with Cu(II) ions was determined by Job's plot as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The association constant was assessed by the B–H method as 7.31 × 104 M−1. The nature of binding mode of probe-84 with Cu(II) ions was strongly supported by FTIR, NMR, ESI-mass, as well as DFT data.
1. The association constant was assessed by the B–H method as 7.31 × 104 M−1. The nature of binding mode of probe-84 with Cu(II) ions was strongly supported by FTIR, NMR, ESI-mass, as well as DFT data.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, towards several metal ions by fluorescence spectroscopic methods. There was a gradual increase in fluorescence intensity at 450 nm on addition of Cu(II) ions to probe-85 due to the absence of PET process, whereas in the presence of above-mentioned cations, no changes were observed in the emission spectra. The results strongly suggested that probe-85 showed selective response to Cu(II) ions with other interfering metal ions. The sensing studies were conducted in a wide range of pH (5–9), which does not affect the sensing ability of probe-85 with Cu(II) ions. The binding constant and LOD values were found to be 1.16 × 104 M−1 and 0.26 μM respectively. The reversibility studies of probe-85 were performed using EDTA, which showed the emission quenching on titration with EDTA to probe-85 + Cu(II). The cellular imaging experiments of probe-85 were conducted on living RAW 264.7 cells in the presence of Cu(II) ions by fluorescence microscopy. Probe-85 was incubated with cells, which showed insignificant fluorescence emission, whereas upon treatment with Cu(II) ions, it produced a very strong fluorescence emission under GFP (Fig. 129). Thus, probe-85 was demonstrated as a potential candidate for the recognition of Cu(II) ions in biological media.
H2O, towards several metal ions by fluorescence spectroscopic methods. There was a gradual increase in fluorescence intensity at 450 nm on addition of Cu(II) ions to probe-85 due to the absence of PET process, whereas in the presence of above-mentioned cations, no changes were observed in the emission spectra. The results strongly suggested that probe-85 showed selective response to Cu(II) ions with other interfering metal ions. The sensing studies were conducted in a wide range of pH (5–9), which does not affect the sensing ability of probe-85 with Cu(II) ions. The binding constant and LOD values were found to be 1.16 × 104 M−1 and 0.26 μM respectively. The reversibility studies of probe-85 were performed using EDTA, which showed the emission quenching on titration with EDTA to probe-85 + Cu(II). The cellular imaging experiments of probe-85 were conducted on living RAW 264.7 cells in the presence of Cu(II) ions by fluorescence microscopy. Probe-85 was incubated with cells, which showed insignificant fluorescence emission, whereas upon treatment with Cu(II) ions, it produced a very strong fluorescence emission under GFP (Fig. 129). Thus, probe-85 was demonstrated as a potential candidate for the recognition of Cu(II) ions in biological media.



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 proportion of DMSO and HEPES buffer. The target compound probe-87 (ref. 200) was synthesized upon treatment of 7-diethylaminocoumarin-3-aldehyde with 3-amino-2-naphthol in EtOH, and the reaction was refluxed for 6 h. The sensing studies of probe-87 obtained better sensitivity and selectivity towards particular metal ions. Upon incremental addition of Cu(II) ions to probe-87, the absorption band at 345 nm progressively falls, whereas the adsorption band at 452 nm increased regularly. However, fluorescence titration data revealed that in the presence of Cu(II) ions, the emission peak at 520 nm gradually increased and reached maximum at 0.5 eq. with 60-fold enhancement, which detected that Cu(II) ions encouraged the decomposition of imines to discharge the fluorescence compound 7-diethylaminocoumarin-3-aldehyde (Fig. 132). The LOD value of probe-87 was estimated to be 0.82 μg L−1 (12.7 nM) using the equation 3σ/slope, which is much lesser as recommended by WHO (2.0 mg L−1) in drinking water. Job's plot revealed that the stoichiometry of probe-87 with Cu(II) was 1
99 proportion of DMSO and HEPES buffer. The target compound probe-87 (ref. 200) was synthesized upon treatment of 7-diethylaminocoumarin-3-aldehyde with 3-amino-2-naphthol in EtOH, and the reaction was refluxed for 6 h. The sensing studies of probe-87 obtained better sensitivity and selectivity towards particular metal ions. Upon incremental addition of Cu(II) ions to probe-87, the absorption band at 345 nm progressively falls, whereas the adsorption band at 452 nm increased regularly. However, fluorescence titration data revealed that in the presence of Cu(II) ions, the emission peak at 520 nm gradually increased and reached maximum at 0.5 eq. with 60-fold enhancement, which detected that Cu(II) ions encouraged the decomposition of imines to discharge the fluorescence compound 7-diethylaminocoumarin-3-aldehyde (Fig. 132). The LOD value of probe-87 was estimated to be 0.82 μg L−1 (12.7 nM) using the equation 3σ/slope, which is much lesser as recommended by WHO (2.0 mg L−1) in drinking water. Job's plot revealed that the stoichiometry of probe-87 with Cu(II) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and the binding constant was estimated to be 1.74 × 105 M−1. The binding mechanism was analysed from the 1H NMR studies. The low cytotoxicity of probe-87 was confirmed by the MTT assay and could be applied as the potential candidate for the recognition of Cu(II) ions in biological media, which was confirmed by fluorescence microscopy (Fig. 133). Thus, probe-87 will work as an excellent material in the field of biomedical research and bioimaging of Cu(II)-related syndromes.
2 and the binding constant was estimated to be 1.74 × 105 M−1. The binding mechanism was analysed from the 1H NMR studies. The low cytotoxicity of probe-87 was confirmed by the MTT assay and could be applied as the potential candidate for the recognition of Cu(II) ions in biological media, which was confirmed by fluorescence microscopy (Fig. 133). Thus, probe-87 will work as an excellent material in the field of biomedical research and bioimaging of Cu(II)-related syndromes.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and enhancement of the CHEF process (Fig. 134). However, no change was perceived with excess amounts of other interfering metal ions. As a consequence, this probe-88 was a very highly selective fluorescent chemosensor for the recognition of Cu(II) ions. Job's plot of probe-88 exhibited maximum absorption at 0.3 of molar fraction, and the ratio of probe-88-Cu(II) was found to be 2
N isomerisation and enhancement of the CHEF process (Fig. 134). However, no change was perceived with excess amounts of other interfering metal ions. As a consequence, this probe-88 was a very highly selective fluorescent chemosensor for the recognition of Cu(II) ions. Job's plot of probe-88 exhibited maximum absorption at 0.3 of molar fraction, and the ratio of probe-88-Cu(II) was found to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The binding constant was calculated as 4 × 104 M−1 from the B–H equation. The LOD value of probe-88 was measured as 1.49 μM using the equation 3σ/slope. Further, for practical applications, probe-88 was utilised for the sensing of Cu(II) in drinking water with high precision.
1. The binding constant was calculated as 4 × 104 M−1 from the B–H equation. The LOD value of probe-88 was measured as 1.49 μM using the equation 3σ/slope. Further, for practical applications, probe-88 was utilised for the sensing of Cu(II) in drinking water with high precision.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for probe-89 and Cu(II) ions. Due to the very fast detection ability, probe-89 has potential applications in biomedical and environmental monitoring applications.
1 for probe-89 and Cu(II) ions. Due to the very fast detection ability, probe-89 has potential applications in biomedical and environmental monitoring applications.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation (Fig. 136) and ratiometric absorption in aqueous solutions over a varied pH array in the presence of other interfering metal ions with a very low detection of limit of 0.49 μM. Job's plot established the binding ratios of probe-90 with Cu(II) as 1
N isomerisation (Fig. 136) and ratiometric absorption in aqueous solutions over a varied pH array in the presence of other interfering metal ions with a very low detection of limit of 0.49 μM. Job's plot established the binding ratios of probe-90 with Cu(II) as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, as supported by ESI-MS and DFT calculations. Further, the binding value was calculated from the B–H plot to be 2.5 × 108 M−2, which revealed that probe-90 had great attraction for Cu(II) ions. Further, for the practical applications of probe-90, cellular imaging investigations were carried out for the detection of Cu(II) ions in biological media. Thus, authors choose HepG2 cell lines, in which they found no fluorescence in the absence of Cu(II) ions, but a bright green colour emission in the presence of Cu(II). This results suggested that probe-90 have good permeability into cells for the detection of Cu(II) ions (Fig. 137).
2, as supported by ESI-MS and DFT calculations. Further, the binding value was calculated from the B–H plot to be 2.5 × 108 M−2, which revealed that probe-90 had great attraction for Cu(II) ions. Further, for the practical applications of probe-90, cellular imaging investigations were carried out for the detection of Cu(II) ions in biological media. Thus, authors choose HepG2 cell lines, in which they found no fluorescence in the absence of Cu(II) ions, but a bright green colour emission in the presence of Cu(II). This results suggested that probe-90 have good permeability into cells for the detection of Cu(II) ions (Fig. 137).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in a Tris-HCl solution at pH 7.4.204 The sensing studies of probe-91 demonstrated colorimetric response, color changes from colorless to yellow colour and fluorescence turn-on response towards Cu(II) ions over other competitive metal ions, due to the suppression of PET (Fig. 138). The LOD values of probe-91 were calculated from the absorption and fluorescence spectra to be 0.46 μM and 15 nM, which were much lesser as approved by WHO for drinking water (31.5 μM). Job's plot data provided strong information regarding the binding stoichiometry such as 1
2) in a Tris-HCl solution at pH 7.4.204 The sensing studies of probe-91 demonstrated colorimetric response, color changes from colorless to yellow colour and fluorescence turn-on response towards Cu(II) ions over other competitive metal ions, due to the suppression of PET (Fig. 138). The LOD values of probe-91 were calculated from the absorption and fluorescence spectra to be 0.46 μM and 15 nM, which were much lesser as approved by WHO for drinking water (31.5 μM). Job's plot data provided strong information regarding the binding stoichiometry such as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the association constant was found to be 4.3 × 107 M−1. The reversibility experiment was performed using S2− as the external source for the regeneration of probe-91, and the results indicated that probe-91 has good reversible properties. Further, the mode of binding was supported by 1H NMR, ESI-MS and DFT calculations.
1 and the association constant was found to be 4.3 × 107 M−1. The reversibility experiment was performed using S2− as the external source for the regeneration of probe-91, and the results indicated that probe-91 has good reversible properties. Further, the mode of binding was supported by 1H NMR, ESI-MS and DFT calculations.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Authors successfully demonstrated the practical applicability of probe-92 by the recognition of Cu(II) ions in real samples of copper in electroplating wastewater. Additionally, probe-92 was displayed as a very good chromogenic and fluorescent turn-on probe for CN− with a very low LOD of 0.18 μM.
1. Authors successfully demonstrated the practical applicability of probe-92 by the recognition of Cu(II) ions in real samples of copper in electroplating wastewater. Additionally, probe-92 was displayed as a very good chromogenic and fluorescent turn-on probe for CN− with a very low LOD of 0.18 μM.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 140). Thus, the results indicate very high selectivity towards Cu(II) ions. Job's plot gave strong information regarding the stoichiometric ratio of probe-93 with Cu(II) ions, which was found to be 2
N isomerization (Fig. 140). Thus, the results indicate very high selectivity towards Cu(II) ions. Job's plot gave strong information regarding the stoichiometric ratio of probe-93 with Cu(II) ions, which was found to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as confirmed by ESI-MS and 1H NMR. Finally, the LOD value was estimated to be 1.8 μM that is much lesser than the WHO endorsement level (20 μM) for drinking water.
1, as confirmed by ESI-MS and 1H NMR. Finally, the LOD value was estimated to be 1.8 μM that is much lesser than the WHO endorsement level (20 μM) for drinking water.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation (Fig. 142). Further, the presence of excess amounts of different metal ions does not show any variation in the fluorescence spectra, which indicate that probe-94 was very selective towards Cu(II) ions. The association constant of the complex in CH3CN and CH3CN/H2O (v/v = 4/6, pH = 7.4) HEPES buffer was calculated from the B–H plot as 1.96 × 107 M−1 and 5.43 × 107 M−1 respectively. Job's plot revealed that the binding ratio of probe-94 and Cu(II) ions was found to be 1
N isomerisation (Fig. 142). Further, the presence of excess amounts of different metal ions does not show any variation in the fluorescence spectra, which indicate that probe-94 was very selective towards Cu(II) ions. The association constant of the complex in CH3CN and CH3CN/H2O (v/v = 4/6, pH = 7.4) HEPES buffer was calculated from the B–H plot as 1.96 × 107 M−1 and 5.43 × 107 M−1 respectively. Job's plot revealed that the binding ratio of probe-94 and Cu(II) ions was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and further confirmed by 1H NMR and ESI-mass. The LOD value was estimated from the fluorescence spectra and displayed as 0.18 μM and 0.85 μM in CH3CN and CH3CN/H2O (v/v = 4/6, pH = 7.4) HEPES buffer correspondingly. Finally, authors demonstrated the practical applicability of probe-94, which could be employed for the sensing of Cu(II) ions in diverse water samples.
1 and further confirmed by 1H NMR and ESI-mass. The LOD value was estimated from the fluorescence spectra and displayed as 0.18 μM and 0.85 μM in CH3CN and CH3CN/H2O (v/v = 4/6, pH = 7.4) HEPES buffer correspondingly. Finally, authors demonstrated the practical applicability of probe-94, which could be employed for the sensing of Cu(II) ions in diverse water samples.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of probe-95 and Cu(II) ions, which was further supported by ESI-MS studies. The LOD and association constant of probe-95 were found to be 3.90 nM and 1.38 × 105 M−2 towards Cu(II) ions. The reversibility of probe-95 was performed using the S2− anion and the results strongly supported that upon addition of S2− anion, probe-95 + Cu(II) reproduced the probe and the results indicate that probe-95 has very good reversible properties.
1 of probe-95 and Cu(II) ions, which was further supported by ESI-MS studies. The LOD and association constant of probe-95 were found to be 3.90 nM and 1.38 × 105 M−2 towards Cu(II) ions. The reversibility of probe-95 was performed using the S2− anion and the results strongly supported that upon addition of S2− anion, probe-95 + Cu(II) reproduced the probe and the results indicate that probe-95 has very good reversible properties.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was supported by 1H NMR, and a binding constant was displayed as 2.12 × 106 M−2. The LOD value was calculated from the fluorescence spectra as 0.1 nM. Further, the formation of a probe-96 complex was well characterized by quantum yield enhancement, SEM, and theoretical calculations. For the practical relevance of probe-96, the detection of Cu(II) ions in biological media (Raw 264.7 cells) was performed by fluorescence microscopy. The results strongly indicated that there was no fluorescence emission when probe-96 was used alone, but upon its treatment with Cu(II) ions bright fluorescence emission was observed inside the cell (Fig. 145). Moreover, probe-96 was found to have very low cytotoxicity as well as great permeability, which opened up the possibility for its use in drug delivery into cells.
1, which was supported by 1H NMR, and a binding constant was displayed as 2.12 × 106 M−2. The LOD value was calculated from the fluorescence spectra as 0.1 nM. Further, the formation of a probe-96 complex was well characterized by quantum yield enhancement, SEM, and theoretical calculations. For the practical relevance of probe-96, the detection of Cu(II) ions in biological media (Raw 264.7 cells) was performed by fluorescence microscopy. The results strongly indicated that there was no fluorescence emission when probe-96 was used alone, but upon its treatment with Cu(II) ions bright fluorescence emission was observed inside the cell (Fig. 145). Moreover, probe-96 was found to have very low cytotoxicity as well as great permeability, which opened up the possibility for its use in drug delivery into cells.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which was confirmed via fluorescence enhancement at 386 nm. The LOD and association value (Ka) of probe-98 with Cu(II) ions were estimated to be 0.62 μM and 6.67 × 104 M−1, correspondingly. The fluorescence turn-on mechanism was proposed, which might be due to the suppression of C
2, which was confirmed via fluorescence enhancement at 386 nm. The LOD and association value (Ka) of probe-98 with Cu(II) ions were estimated to be 0.62 μM and 6.67 × 104 M−1, correspondingly. The fluorescence turn-on mechanism was proposed, which might be due to the suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and PET process along with the CHEF (Fig. 147). Further, probe-98 was found to show excellent performance towards the recognition of Cu(II) ions in biological media in live L929 cells (Fig. 148).
N isomerisation and PET process along with the CHEF (Fig. 147). Further, probe-98 was found to show excellent performance towards the recognition of Cu(II) ions in biological media in live L929 cells (Fig. 148).


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand-to-metal binding mode. In the CH3CN/CH2Cl2 solvent system, on adding 10 eq. of Cu(II) the emission intensity of ligands probe-100a and probe-100b increases by 65-fold and 25-fold, respectively. By inhibiting the PET while bound to Cu(II), probe-100a causes a blue emission. Additionally, due to the significant suppression of PET and the distinct binding method of the ligand/metal complex formation, probe-100a is highly sensitive for the detection of Cu(II) compared to probe-100b and probe-100c.
1 ligand-to-metal binding mode. In the CH3CN/CH2Cl2 solvent system, on adding 10 eq. of Cu(II) the emission intensity of ligands probe-100a and probe-100b increases by 65-fold and 25-fold, respectively. By inhibiting the PET while bound to Cu(II), probe-100a causes a blue emission. Additionally, due to the significant suppression of PET and the distinct binding method of the ligand/metal complex formation, probe-100a is highly sensitive for the detection of Cu(II) compared to probe-100b and probe-100c.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 152), while the emission spectra showed fluorescence quenching in the presence of Cu(II) ions. UV-vis studies show that in the presence of Ni(II) and Cu(II), a color change was observed from colorless to yellow, which is evidently observed from the rise of a new band towards the long wavelength region. Job's plot gave strong information regarding the complexation ratio of probe-102 with Ni(II) ions, which was found to be 1
N isomerization (Fig. 152), while the emission spectra showed fluorescence quenching in the presence of Cu(II) ions. UV-vis studies show that in the presence of Ni(II) and Cu(II), a color change was observed from colorless to yellow, which is evidently observed from the rise of a new band towards the long wavelength region. Job's plot gave strong information regarding the complexation ratio of probe-102 with Ni(II) ions, which was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as supported by ESI-MS analysis and DFT studies. The detection limit of probe-102 was analyzed from the fluorescence spectrum and found to be 1.71 μM for Ni(II) ions, and much lesser than that approved by the WHO rules for drinking water. The reversibility of probe-102 with Ni(II) and Cu(II) ions was obtained from the addition of Na2EDTA experiments. Further, the authors demonstrated the applicability of probe-102 for the recognition of Ni(II) ions in biological samples and its excellent detection ability towards Ni(II) in cellular media, which was supported by fluorescence microscopy (Fig. 153).
1, as supported by ESI-MS analysis and DFT studies. The detection limit of probe-102 was analyzed from the fluorescence spectrum and found to be 1.71 μM for Ni(II) ions, and much lesser than that approved by the WHO rules for drinking water. The reversibility of probe-102 with Ni(II) and Cu(II) ions was obtained from the addition of Na2EDTA experiments. Further, the authors demonstrated the applicability of probe-102 for the recognition of Ni(II) ions in biological samples and its excellent detection ability towards Ni(II) in cellular media, which was supported by fluorescence microscopy (Fig. 153).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between probe-103 and Ni(II) ions. In addition, the LOD value for probe-103 was calculated to be as low as 8.67 nM by the fluorescence approach and 0.361 μM by the UV-vis method, which suggests a potential use for probe-103 in detecting Ni(II) in the environment. The collected spectroscopic data of probe-103 with Ni(II) were also used to demonstrate the molecular logic gates. Probe-103 was also effectively used for Ni(II) fluorescence imaging in HeLa cells (Fig. 155). In order to show the practical applications of probe-103 for the detection of Ni(II) ions, test kits were also created by dip-coating the probe-103 solution onto filter paper and allowing it to dry in the air.
1 between probe-103 and Ni(II) ions. In addition, the LOD value for probe-103 was calculated to be as low as 8.67 nM by the fluorescence approach and 0.361 μM by the UV-vis method, which suggests a potential use for probe-103 in detecting Ni(II) in the environment. The collected spectroscopic data of probe-103 with Ni(II) were also used to demonstrate the molecular logic gates. Probe-103 was also effectively used for Ni(II) fluorescence imaging in HeLa cells (Fig. 155). In order to show the practical applications of probe-103 for the detection of Ni(II) ions, test kits were also created by dip-coating the probe-103 solution onto filter paper and allowing it to dry in the air.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 based on Job's plot calculation. The detection limit was substantially lower than that indicated by the WHO rules for drinking water, reaching up to 1.8 μM and 1.18 μM for colorimetric and fluorometric analyses respectively. The chemosensor has also been successfully used to make molecular logic gates, image living cells, and analyze real samples using a smartphone. HeLa cells were used in cell imaging studies to confirm probe-104's affinity for Ni(II) ions present in an intracellular medium.
1 based on Job's plot calculation. The detection limit was substantially lower than that indicated by the WHO rules for drinking water, reaching up to 1.8 μM and 1.18 μM for colorimetric and fluorometric analyses respectively. The chemosensor has also been successfully used to make molecular logic gates, image living cells, and analyze real samples using a smartphone. HeLa cells were used in cell imaging studies to confirm probe-104's affinity for Ni(II) ions present in an intracellular medium.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between probe-105 and Pd(II), which was supported by 1H NMR, ESI-MS and DFT calculations. Furthermore, DFT calculations also supported experimental data including the lengthening of the C
1 stoichiometry between probe-105 and Pd(II), which was supported by 1H NMR, ESI-MS and DFT calculations. Furthermore, DFT calculations also supported experimental data including the lengthening of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond causing a red shift of the absorption peak. In addition, by incubating probe-105 with a Pd(II) ion solution in HCT116 cells, in vivo fluorescence cell imaging could be obtained (Fig. 158).
O bond causing a red shift of the absorption peak. In addition, by incubating probe-105 with a Pd(II) ion solution in HCT116 cells, in vivo fluorescence cell imaging could be obtained (Fig. 158).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between probe-106 and Pd(II) ions, which was validated by FTIR, 1H NMR, DFT and ESI-MS data. The authors proposed the sensing mechanism of probe-106, which involved the formation of [CR–Pd(II)] + complex using hydroxy-O and imine-N of a coumarinyl–rhodamine moiety involving the opening of a spirolactam ring. The DFT calculations suggested the reduction in band gap of probe-106 upon binding with Pd(II) ions, which was indicative of the increase in conjugation on ring opening and subsequent enhancement of fluorescence intensity as well as color change. Furthermore, after incubating probe-106 in MCF7 cells, the live imaging of Pd(II) ions was performed using a fluorescence microscopic technique. Moreover, probe-106 provided in vivo cell imaging (Fig. 159) along with selective and sensitive sensing of Pd(II) ions at such low detection limits.
1 stoichiometry between probe-106 and Pd(II) ions, which was validated by FTIR, 1H NMR, DFT and ESI-MS data. The authors proposed the sensing mechanism of probe-106, which involved the formation of [CR–Pd(II)] + complex using hydroxy-O and imine-N of a coumarinyl–rhodamine moiety involving the opening of a spirolactam ring. The DFT calculations suggested the reduction in band gap of probe-106 upon binding with Pd(II) ions, which was indicative of the increase in conjugation on ring opening and subsequent enhancement of fluorescence intensity as well as color change. Furthermore, after incubating probe-106 in MCF7 cells, the live imaging of Pd(II) ions was performed using a fluorescence microscopic technique. Moreover, probe-106 provided in vivo cell imaging (Fig. 159) along with selective and sensitive sensing of Pd(II) ions at such low detection limits.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation between probe-107 and Pd(II), which was further supported by 1H NMR, DFT and ESI-MS data. The DFT calculation also strongly supported the experimental data such as the reduction in the band gap of probe-107 with Pd(II) ions, which was due to the formation of complex via hydroxy-O and enamine-N of a rhodamine moiety and carbonyl-O and imine-N of aminopyrone. The fluorescence-enhanced mechanism was proposed by authors, which was spirolactum ring opening upon metal binding. Furthermore, apart from acting as a sensitive and selective fluorescent turn-on sensor, incubating probe-107 in MDA-MB-468 cells, in vivo cell imaging of Pd(II) was obtained without causing any harm (determined by the MTT assay) (Fig. 162).
1 stoichiometric complexation between probe-107 and Pd(II), which was further supported by 1H NMR, DFT and ESI-MS data. The DFT calculation also strongly supported the experimental data such as the reduction in the band gap of probe-107 with Pd(II) ions, which was due to the formation of complex via hydroxy-O and enamine-N of a rhodamine moiety and carbonyl-O and imine-N of aminopyrone. The fluorescence-enhanced mechanism was proposed by authors, which was spirolactum ring opening upon metal binding. Furthermore, apart from acting as a sensitive and selective fluorescent turn-on sensor, incubating probe-107 in MDA-MB-468 cells, in vivo cell imaging of Pd(II) was obtained without causing any harm (determined by the MTT assay) (Fig. 162).


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation between probe-108 and Pd(II) ions, which was further supported by Job's plot analysis. Further, the effect of the pH on the detection capability of probe-108 with Pd(II) ions was evaluated, and the results indicated no effect on the fluorescence spectra in the pH range of 8–12, which shows the potential of probe-108 for the detection of Pd(II) ions in physiological media. The detection limit of probe-108 with Pd(II) ions was calculated from the fluorescence titration experiment to be as low as 34 nM. Considering the selective and sensitive fluorescence turn-on response of probe-108 towards Pd(II) ions, the authors applied for the detection of Pd(II) ions in biological media; firstly, probe-108 was incubated in MDA-MB-231 cell lines and red fluorescence image was obtained upon addition of Pd(II) ions using a microscope providing intracellular imaging (Fig. 163). The DFT calculation suggested a reduction in the band gap of probe-108 upon binding with Pd(II), which signified the formation of [BR–Pd(II)] + complex via four coordination sites (hydroxy-O, imine-N of rhodamine moiety and hydroxy-O of benzophenone and imine-N which bridged these two moieties).
1 stoichiometric complexation between probe-108 and Pd(II) ions, which was further supported by Job's plot analysis. Further, the effect of the pH on the detection capability of probe-108 with Pd(II) ions was evaluated, and the results indicated no effect on the fluorescence spectra in the pH range of 8–12, which shows the potential of probe-108 for the detection of Pd(II) ions in physiological media. The detection limit of probe-108 with Pd(II) ions was calculated from the fluorescence titration experiment to be as low as 34 nM. Considering the selective and sensitive fluorescence turn-on response of probe-108 towards Pd(II) ions, the authors applied for the detection of Pd(II) ions in biological media; firstly, probe-108 was incubated in MDA-MB-231 cell lines and red fluorescence image was obtained upon addition of Pd(II) ions using a microscope providing intracellular imaging (Fig. 163). The DFT calculation suggested a reduction in the band gap of probe-108 upon binding with Pd(II), which signified the formation of [BR–Pd(II)] + complex via four coordination sites (hydroxy-O, imine-N of rhodamine moiety and hydroxy-O of benzophenone and imine-N which bridged these two moieties).

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and the increase in the ICT process (Fig. 165). The binding constant of probe-110 with Fe(II) ions was estimated from the absorption spectra to be 5.02 × 104 M−2. Further, stoichiometry determined via Job's plot and the ratio of probe-110 to Fe(II) ions was displayed as 2
N isomerisation and the increase in the ICT process (Fig. 165). The binding constant of probe-110 with Fe(II) ions was estimated from the absorption spectra to be 5.02 × 104 M−2. Further, stoichiometry determined via Job's plot and the ratio of probe-110 to Fe(II) ions was displayed as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Finally the practical ability of probe-110 was tested for the recognition of Fe(II) ions in tomato juice, tablets, dark chocolate and tap water and the results strongly suggested that probe-110 is efficient for detecting and monitoring Fe(II) ions.
1. Finally the practical ability of probe-110 was tested for the recognition of Fe(II) ions in tomato juice, tablets, dark chocolate and tap water and the results strongly suggested that probe-110 is efficient for detecting and monitoring Fe(II) ions.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was validated by ESI-MS and 1H NMR. The LOD value of probe-111 with Fe(II) and F− was determined from the fluorescence spectra to be 0.3 ppm and 25.7 ppb, respectively, which is much lesser as recommended by WHO. Further, the reversibility of probe-111 + Fe(II) was monitored upon addition of TMEDA, whereas in the case of a fluoride complex, reversibility was established using calcium nitrate. Finally, probe-111 was subjected to real-time monitoring Fe(II) ions in lake and drinking water and the results indicated that probe-111 was able to show response towards Fe(II) ions. Thus, probe-111 was found to be an efficient and potential candidate for the detection of Fe(II) ions in biological and environmental samples.
1, which was validated by ESI-MS and 1H NMR. The LOD value of probe-111 with Fe(II) and F− was determined from the fluorescence spectra to be 0.3 ppm and 25.7 ppb, respectively, which is much lesser as recommended by WHO. Further, the reversibility of probe-111 + Fe(II) was monitored upon addition of TMEDA, whereas in the case of a fluoride complex, reversibility was established using calcium nitrate. Finally, probe-111 was subjected to real-time monitoring Fe(II) ions in lake and drinking water and the results indicated that probe-111 was able to show response towards Fe(II) ions. Thus, probe-111 was found to be an efficient and potential candidate for the detection of Fe(II) ions in biological and environmental samples.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by Job's plot analysis with an association constant of 5 × 104 M−1 from the absorption spectra. For investigating the practical utility of probe-112, paper strips carrying the alcoholic solutions of probe-112 were constructed and it was found that the paper strips were also giving positive responses in sensing. The red-shift in the fluorescence spectra of the probe-112–Fe(III) complex was verified by the DFT calculations, which revealed that the binding of Fe(III) to probe-112 lowered the band gap.
1 by Job's plot analysis with an association constant of 5 × 104 M−1 from the absorption spectra. For investigating the practical utility of probe-112, paper strips carrying the alcoholic solutions of probe-112 were constructed and it was found that the paper strips were also giving positive responses in sensing. The red-shift in the fluorescence spectra of the probe-112–Fe(III) complex was verified by the DFT calculations, which revealed that the binding of Fe(III) to probe-112 lowered the band gap.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was proved by ESI-MS and TD-DFT studies. The association constant was calculated from the absorption spectra as 3.6 × 106 M−1, the value established the strong binding nature among probe-113 and Fe(III) ions. Further, probe-113 was subjected for the sensing of Fe(III) ions in biological samples in cellular media, which was confirmed by fluorescence microscopy. The results demonstrated that the presence of Fe(III) ions in cells, which was already incubated with probe-113, gave a bright emission inside the cells that indicate that probe-113 was a potential candidate for the detection of Fe(III) ions in biological samples (Fig. 168).
1, which was proved by ESI-MS and TD-DFT studies. The association constant was calculated from the absorption spectra as 3.6 × 106 M−1, the value established the strong binding nature among probe-113 and Fe(III) ions. Further, probe-113 was subjected for the sensing of Fe(III) ions in biological samples in cellular media, which was confirmed by fluorescence microscopy. The results demonstrated that the presence of Fe(III) ions in cells, which was already incubated with probe-113, gave a bright emission inside the cells that indicate that probe-113 was a potential candidate for the detection of Fe(III) ions in biological samples (Fig. 168).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of probe-114 and Fe(III) ions and was confirmed by UV-vis, FTIR and ESI-MS studies. The reversibility of probe-114 was established upon addition of EDTA to the Fe-complex of probe-114 that was found to exhibit a change from red to colorless as well as weak fluorescence emission. Though, re-addition of Fe(III) ions subsequently resulted in the retrieval of absorbance band and very bright luminescence. Thus, probe-114 was found to have very promising photophysical properties and applied for the recognition of Fe(III) ions in biological and environmental samples.
1 ratio of probe-114 and Fe(III) ions and was confirmed by UV-vis, FTIR and ESI-MS studies. The reversibility of probe-114 was established upon addition of EDTA to the Fe-complex of probe-114 that was found to exhibit a change from red to colorless as well as weak fluorescence emission. Though, re-addition of Fe(III) ions subsequently resulted in the retrieval of absorbance band and very bright luminescence. Thus, probe-114 was found to have very promising photophysical properties and applied for the recognition of Fe(III) ions in biological and environmental samples.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and was supported by the ESI-MS spectrum. Finally, authors demonstrated that probe-115 show ability to detect Fe(III) ions in biological media and was found to be very efficient for intracellular Fe(III) ions in HeLa cells and established by fluorescence microscopic images. Further, the above finding was strongly verified by the DFT studies (Fig. 171).
1 and was supported by the ESI-MS spectrum. Finally, authors demonstrated that probe-115 show ability to detect Fe(III) ions in biological media and was found to be very efficient for intracellular Fe(III) ions in HeLa cells and established by fluorescence microscopic images. Further, the above finding was strongly verified by the DFT studies (Fig. 171).



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solutions, whereas no change in the absorption spectra with other metal ions. On titrating with Fe(III) ions to probe-117, the emission intensity was enhanced with a gradual increment in quantum yields, due to the suppression of C
1, v/v) solutions, whereas no change in the absorption spectra with other metal ions. On titrating with Fe(III) ions to probe-117, the emission intensity was enhanced with a gradual increment in quantum yields, due to the suppression of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and PET (Fig. 174). However, on adding other competitive metal ions, there was no alteration in the emission spectra, which established the very high selectivity of probe-117 towards Fe(III) ions. The complexation ratio of probe-117 with Fe(III) ions was found to be 1
N isomerisation and PET (Fig. 174). However, on adding other competitive metal ions, there was no alteration in the emission spectra, which established the very high selectivity of probe-117 towards Fe(III) ions. The complexation ratio of probe-117 with Fe(III) ions was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and confirmed by Job's plot, 1H NMR, HRMS analysis and theoretical studies. The LOD value of the complex with Fe(III) ions was estimated from the fluorescence titration spectra as low as 0.178 μM. For the practical purpose, probe-117 was utilized for the detection of Fe(III) ions in drinking water and food samples with good precision.
2 and confirmed by Job's plot, 1H NMR, HRMS analysis and theoretical studies. The LOD value of the complex with Fe(III) ions was estimated from the fluorescence titration spectra as low as 0.178 μM. For the practical purpose, probe-117 was utilized for the detection of Fe(III) ions in drinking water and food samples with good precision.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was further validated by ESI-MS, 1H NMR and FT-IR. The practical application of probe-118 was established by the recognition of Fe(III) ions via a paper strip as well as in environmental and food samples.
1, which was further validated by ESI-MS, 1H NMR and FT-IR. The practical application of probe-118 was established by the recognition of Fe(III) ions via a paper strip as well as in environmental and food samples.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and CHEF, which rose after the formation of metal coordination (Fig. 177). The binding constant of probe-119 was estimated from the change in absorption spectra upon addition of Fe(II) ions as 6.173 × 107 M−2. The association stoichiometry of probe-119 with Fe(II) ions was estimated from Job's plot to be 2
N isomerization and CHEF, which rose after the formation of metal coordination (Fig. 177). The binding constant of probe-119 was estimated from the change in absorption spectra upon addition of Fe(II) ions as 6.173 × 107 M−2. The association stoichiometry of probe-119 with Fe(II) ions was estimated from Job's plot to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was supported by 1H NMR and theoretical calculations. The LOD value was appraised from the fluorescence titration data and exhibited to be 0.0363 μM, which is much lesser than the WHO suggested value (5 μM) for Fe(II) in the drinking water. As probe-119 possesses a good binding constant and low detection limits, it was further applied for monitoring Fe(II) ions in diverse samples such as lake, well and river water.
1, which was supported by 1H NMR and theoretical calculations. The LOD value was appraised from the fluorescence titration data and exhibited to be 0.0363 μM, which is much lesser than the WHO suggested value (5 μM) for Fe(II) in the drinking water. As probe-119 possesses a good binding constant and low detection limits, it was further applied for monitoring Fe(II) ions in diverse samples such as lake, well and river water.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was strongly validated by 1H NMR, ESI-MS and DFT calculations. Further, with the ability of probe-120 towards the detection of Fe(III) ions in cellular media, the fluorescence bio-imaging demonstrated its insignificant toxicity for the sensing of Fe(III) ions in zebrafish embryos. Thus, the results strongly indicate that probe-120 is a potential candidate for the recognition of Fe(III) ions in zebrafish embryos.
1, which was strongly validated by 1H NMR, ESI-MS and DFT calculations. Further, with the ability of probe-120 towards the detection of Fe(III) ions in cellular media, the fluorescence bio-imaging demonstrated its insignificant toxicity for the sensing of Fe(III) ions in zebrafish embryos. Thus, the results strongly indicate that probe-120 is a potential candidate for the recognition of Fe(III) ions in zebrafish embryos.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 via Job's plot and supported by DFT studies, and the association constants were estimated as 1.66 × 1010 and 9.22 × 108 M−1, correspondingly. The LOD values were estimated from the emission titration data and was estimated as 0.5 μM for Fe(II) ions. Further, probe-121 was found to show a binding interface with bovine serum albumin, which was detected by fluorescence quenching and FRET. Finally, the authors claimed that probe-121 possesses potential ability for the instantaneous recognition of Fe(II) and Cu(II) metal ions.
2 via Job's plot and supported by DFT studies, and the association constants were estimated as 1.66 × 1010 and 9.22 × 108 M−1, correspondingly. The LOD values were estimated from the emission titration data and was estimated as 0.5 μM for Fe(II) ions. Further, probe-121 was found to show a binding interface with bovine serum albumin, which was detected by fluorescence quenching and FRET. Finally, the authors claimed that probe-121 possesses potential ability for the instantaneous recognition of Fe(II) and Cu(II) metal ions.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solvent combinations. From the UV-vis absorption data, on adding Fe(II) and Fe(III) ions, probe-122 exhibited a drastic change in absorption bands with colorimetric responses and the color variations from yellow to colorless and, no interference was observed with other metal ions. Probe-122 exhibits very weak emission due to the PET process (Fig. 180) and on titrating with Fe(II) and Fe(III) ions, the bright emission was observed with 189- and 141-fold enhancement respectively at 456 nm, which could be contributed to the suppression of the PET process. Upon addition of other competitive metal ions, there was no alteration in the emission spectra, which strongly suggested the selectivity of probe-122 towards Fe(II) and Fe(III) ions. The LOD values of probe-122 with Fe(II) and Fe(III) ions was calculated from the fluorescence titration data to be 2.61 μM and 2.06 μM, correspondingly. The association constant was calculated from the B–H equation and displayed for Fe(III) as 2.59 × 104 M−1 and the binding stoichiometry was obtained from Job's plot as 1
1, v/v) solvent combinations. From the UV-vis absorption data, on adding Fe(II) and Fe(III) ions, probe-122 exhibited a drastic change in absorption bands with colorimetric responses and the color variations from yellow to colorless and, no interference was observed with other metal ions. Probe-122 exhibits very weak emission due to the PET process (Fig. 180) and on titrating with Fe(II) and Fe(III) ions, the bright emission was observed with 189- and 141-fold enhancement respectively at 456 nm, which could be contributed to the suppression of the PET process. Upon addition of other competitive metal ions, there was no alteration in the emission spectra, which strongly suggested the selectivity of probe-122 towards Fe(II) and Fe(III) ions. The LOD values of probe-122 with Fe(II) and Fe(III) ions was calculated from the fluorescence titration data to be 2.61 μM and 2.06 μM, correspondingly. The association constant was calculated from the B–H equation and displayed for Fe(III) as 2.59 × 104 M−1 and the binding stoichiometry was obtained from Job's plot as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for probe-122 and Fe(III) ions. The reversible nature of probe-122 with Fe(III) ions was obtained upon addition of an EDTA solution. Thus, the authors claimed that probe-122 possesses a good association constant, and the detection limits make it a promising candidate for monitoring Fe(III) ions.
1 for probe-122 and Fe(III) ions. The reversible nature of probe-122 with Fe(III) ions was obtained upon addition of an EDTA solution. Thus, the authors claimed that probe-122 possesses a good association constant, and the detection limits make it a promising candidate for monitoring Fe(III) ions.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the LOD value of probe-123 with Fe(III) ions was estimated from the emission titration spectra to be 65 nM. The binding constant was estimated from the B–H equation as 5.97 × 104 M−1. The reversibility of probe-123 + Fe(III) ions was established upon addition of an EDTA solution.
1 and the LOD value of probe-123 with Fe(III) ions was estimated from the emission titration spectra to be 65 nM. The binding constant was estimated from the B–H equation as 5.97 × 104 M−1. The reversibility of probe-123 + Fe(III) ions was established upon addition of an EDTA solution.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 182). The selectivity of probe-124 was determined with other metal ions, nonetheless no remarkable emission changes were observed for other metal ions apart from Fe(III) ions, which reveals the selectivity and recognition of probe-124 even in the presence of other competitive metal ions (Fig. 183). The pH studies were carried out to identify the range over which the sensing was efficient and the results revealed that probe-124 exhibited extreme high emission intensity in the array of 3.2 to 6.4. Absorbance studies demonstrated that titration with Fe(III) ions induced significant changes in the spectra with the generation of a new band at 426 nm, which indicates the successful complexation of the metal ion with the ligand and color alteration from orange to light yellow, which was detectable by the naked eye. The LOD value was estimated based on the 3σ/slope method to be 38.3 nM with a binding constant of 1.77 × 106 M−1. Probe-124 offers good water solubility with its non-toxic nature and better cell membrane penetrability and was applied for the sensing of Fe(III) ions in biological media. In short, probe-124 could be considered as a promising candidate for the recognition of exogenous and endogenous detection of Fe(III) ions in biological samples and makes its practical applications very significant (Fig. 184).
N isomerization (Fig. 182). The selectivity of probe-124 was determined with other metal ions, nonetheless no remarkable emission changes were observed for other metal ions apart from Fe(III) ions, which reveals the selectivity and recognition of probe-124 even in the presence of other competitive metal ions (Fig. 183). The pH studies were carried out to identify the range over which the sensing was efficient and the results revealed that probe-124 exhibited extreme high emission intensity in the array of 3.2 to 6.4. Absorbance studies demonstrated that titration with Fe(III) ions induced significant changes in the spectra with the generation of a new band at 426 nm, which indicates the successful complexation of the metal ion with the ligand and color alteration from orange to light yellow, which was detectable by the naked eye. The LOD value was estimated based on the 3σ/slope method to be 38.3 nM with a binding constant of 1.77 × 106 M−1. Probe-124 offers good water solubility with its non-toxic nature and better cell membrane penetrability and was applied for the sensing of Fe(III) ions in biological media. In short, probe-124 could be considered as a promising candidate for the recognition of exogenous and endogenous detection of Fe(III) ions in biological samples and makes its practical applications very significant (Fig. 184).


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization (Fig. 185). However, no other metal ions were found to have similar observations, which indicate that probe-125 was very selective towards Fe(III) ions. The association constant of probe-125 towards Fe(III) ions was found to be 7.22 × 104 M−1 using the B–H equation. Further, the complexation ratio was estimated from Job's plot as 1
N isomerization (Fig. 185). However, no other metal ions were found to have similar observations, which indicate that probe-125 was very selective towards Fe(III) ions. The association constant of probe-125 towards Fe(III) ions was found to be 7.22 × 104 M−1 using the B–H equation. Further, the complexation ratio was estimated from Job's plot as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was strongly supported by ESI-MS and 1H NMR. The LOD value was calculated from the fluorescence titration for probe-125 with Fe(III) ions to be 0.048 μM. Thus, due to the abnormal photophysical properties of probe-125, it was applied for the recognition of Fe(III) ions in environmental as well as biological samples.
1, which was strongly supported by ESI-MS and 1H NMR. The LOD value was calculated from the fluorescence titration for probe-125 with Fe(III) ions to be 0.048 μM. Thus, due to the abnormal photophysical properties of probe-125, it was applied for the recognition of Fe(III) ions in environmental as well as biological samples.


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization
N isomerization










![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β = 10.03(1)
β = 10.03(1)



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 6.04
K = 6.04![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and PET
N isomerisation and PET
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and PET
N isomerisation and PET
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 5.63(0.15)
K = 5.63(0.15)



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and CHEF on
N isomerisation and CHEF on


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and PET
N isomerisation and PET


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation










![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation, PET and ESIPT
N isomerisation, PET and ESIPT

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and ESIPT
N isomerisation and ESIPT

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation







![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation









![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation















![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation














![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation and PET
N isomerisation and PET

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation, CHEF
N isomerisation, CHEF




![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation
N isomerisation





