Open Access Article
Open Access ArticleIn situ recrystallization of zero-dimensional hybrid metal halide glass-ceramics toward improved scintillation performance†
Bohan
Li
 a,
Jiance
Jin
a,
Jiance
Jin
 b,
Meijuan
Yin
a,
Kai
Han
b,
Yuchi
Zhang
a,
Xinlei
Zhang
a,
Anran
Zhang
b,
Zhiguo
Xia
b,
Meijuan
Yin
a,
Kai
Han
b,
Yuchi
Zhang
a,
Xinlei
Zhang
a,
Anran
Zhang
b,
Zhiguo
Xia
 *b and
Yan
Xu
*b and
Yan
Xu
 *a
*a
aDepartment of Chemistry, College of Sciences, Northeastern University, Shenyang, 110819, China. E-mail: xuyan@mail.neu.edu.cn
bState Key Laboratory of Luminescent Materials and Devices, Guangdong Provincial Key Laboratory of Fiber Laser Materials and Applied Techniques, School of Materials Science and Engineering, South China University of Technology, Guangzhou, 510641, China. E-mail: xiazg@scut.edu.cn
First published on 16th October 2023
Abstract
Zero-dimensional (0D) hybrid metal halide (HMH) glasses are emerging luminescent materials and have gained attention due to their transparent character and ease of processing. However, the weakening of photoluminescence quantum efficiency from crystal to glass phases poses limitations for photonics applications. Here we develop high-performance glass-ceramic (G-C) scintillators via in situ recrystallization from 0D HMH glass counterparts composed of distinct organic cations and inorganic anions. The G-C scintillators maintain excellent transparency and exhibit nearly 10-fold higher light yields and lower detection limits than those of glassy phases. The general in situ recrystallization within the glass component by a facile heat treatment is analyzed via combined experimental elaboration and structural/spectral characterization. Our results on the development of G-Cs can initiate more exploration on the phase transformation engineering in 0D HMHs, and therefore make them highly promising for large-area scintillation screen applications.
Introduction
Zero-dimensional (0D) hybrid metal halides (HMHs) are widely used as light-emitting diodes, sensors, scintillators, etc. because of their excellent optical properties and good structural tunability.1–9 Due to the unique molecular structure of 0D metal halide crystals, that is, consisting of mutually isolated inorganic anions and organic cations, the design synthesis of 0D HMH crystals with diverse structures and optical properties has been continuously pursued.10–18 Recently, our group initially reported on the glass formation process and scintillation applications of an (ETP)2MnBr4-based transparent medium, which has sparked broader interests in 0D HMH glasses.19 Generally, strictly manipulating the organic–inorganic components and avoiding irreversible decomposition before melting simultaneously is the key to obtaining luminescent 0D HMH glasses, which in turn leads to easy processing and high transparency.20–23Vitrification is a thermally dependent phenomenon that involves the conversion of a material from a solid to a stable liquid or glassy state, which is characterized by the absence of long-range molecular order.24–27 Only a few crystals feature a capacity to afford such stable transitions to either of these states following amorphization without compositional decomposition.28–31 Hybrid glasses representing a relatively novel class of materials, dominated by metal–organic framework glasses, coordination polymers glasses and HMH glasses discussed herein, are capable of undergoing glass transition by connecting both organic and inorganic parts in a cooperative manner.32–36 Unlike single-crystal materials that are challenging to grow to sufficient size, organic–inorganic hybrid glasses offer enhanced processability and moldability.37–40 These glasses can be used as a matrix to disperse pre-prepared MOF crystals or other nanoparticles to obtain functional hybrid crystal–glass composites with improved physicochemical properties and which have been extensively studied.41–43 However, there is still a lack of understanding on how to achieve in situ recrystallization from the melt within the hybrid glass matrix to obtain unique glass-ceramics (G-Cs) with outstanding and intriguing properties.44
Here, we have successfully designed and prepared a series of 0D HMH based glasses by modulating diverse organic cations and inorganic anions in their constituents. Flexible manufacturing of optical fibers and large-size glass wafers further demonstrates the excellent processability of these 0D HMH glasses. 31P solid-state NMR and Mn K-edge EXAFS for selected Mn-based 0D HMH glass show that the tetra-coordination structure of the phosphorus and Mn luminescent center was retained after vitrification, so that the characteristic emissions are preserved. But the simple melting-process can result in a remarkable decrease of quantum efficiency, which also inevitably occurred in previous research,22 restricting the applications of these 0D HMH glasses. In situ recrystallization in the 0D HMH glass phase has great potential to address the issue although it is challenging in experiments and mechanisms. Herein we integrated the melt-processing and a stepwise heating method to obtain corresponding G-Cs, exhibiting enhanced light yield, a lower detection limit in X-ray scintillation applications, and improved imaging resolution in X-ray imaging compared to conventional glass. Therefore, this work provides a simple and feasible pre-treatment strategy to efficiently modulate the nature of 0D HMH glasses with superior scintillation performances for optical radiation detection.
Results and discussion
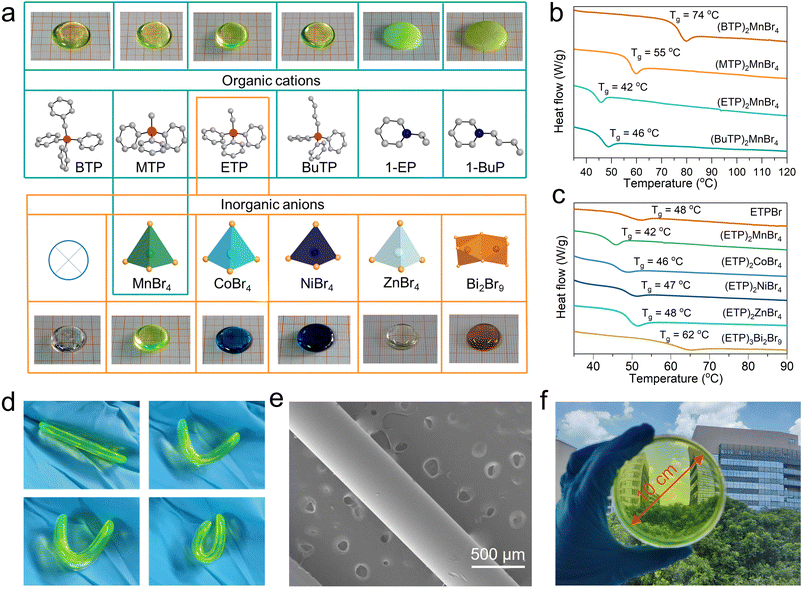
Six different ionic liquids were selected here as organic ligands to synthesize single crystals of (BTP)2MnBr4 (BTP = benzyltriphenylphosphonium), (MTP)2MnBr4 (MTP = methyltriphenylphosphonium), (ETP)2MnBr4 (ETP = ethyltriphenylphosphonium), (BuTP)2MnBr4 (BuTP = butyltriphenylphosphonium), (1-EP)2MnBr4 (1-EP = 1-ethylpyridine) and (1-BuP)2MnBr4 (1-BuP = 1-butylpyridine). Detailed crystallography data of these crystals were collected, and they are composed of different organic ligands with (MnBr4)2− inorganic tetrahedra (ESI Fig. 1 and 2 and ESI Table 1†), and the as-grown crystals of these 0D HMHs can be easily melted at different temperatures. Upon melting, (BTP)2MnBr4, (MTP)2MnBr4, (ETP)2MnBr4 and (BuTP)2MnBr4 give transparent blocks, whereas (1-EP)2MnBr4 and (1-BuP)2MnBr4 were obtained as opaque blocks (Fig. 1a). To clearly understand this phenomenon, we monitored the temperature rise and fall process of the HMHs using differential scanning calorimetry (DSC). As the temperature increases, all six 0D HMHs melt before the decomposition temperature, but they exhibit differences during the subsequent temperature decrease (ESI Fig. 3†). When the temperature decreases, the 0D HMHs with higher melting temperature (Tm), assembling from larger quaternary phosphonium organic ligands, changed from the molten state to the amorphous state, while the 0D HMHs with lower Tm and containing smaller pyridinium organic ligands recrystallized from the molten state to the crystalline state again (ESI Fig. 4†). The transparent blocks of (BTP)2MnBr4, (MTP)2MnBr4, (ETP)2MnBr4, and (BuTP)2MnBr4 with amorphous diffraction peaks display glass transition temperatures (Tg) instead of a fixed Tm in the monitored DSC curves. This Tg ranges from 42 °C to 74 °C, commonly observed in glass samples (Fig. 1b). The appearance of the Tg indicates that (BTP)2MnBr4, (MTP)2MnBr4, (ETP)2MnBr4, and (BuTP)2MnBr4 underwent a transformation from the crystalline to glassy state after the melting and cooling process. Conversely, (1-EP)2MnBr4 and (1-BuP)2MnBr4 show exothermic peaks due to recrystallization during cooling, which prevents the formation of the glassy state (ESI Fig. 5†).Besides organic cations, the physical and chemical properties of HMHs are dominantly determined by the inorganic anions in the crystal structure.45,46 We attempted to broaden the synthetic pathway for HMH glasses to encompass various metal types of 0D HMH compounds through alterations of the inorganic anionic components. As an example, we selected an ionic liquid of ETPBr as the organic ligand to synthesize various metal ions containing 0D HMH, including Co2+, Ni2+, Zn2+ and Bi3+ with their corresponding crystal structures shown in ESI Fig. 6 and 7,† and the corresponding crystal data shown in ESI Table 2.† Surprisingly, pure ETPBr without the introduction of any metal ions, as well as the HMHs of (ETP)2CoBr4, (ETP)2NiBr4, (ETP)2ZnBr4, and (ETP)3Bi2Br9 crystals can also form corresponding glass samples after melting, showing different body colors (ESI Fig. 8 and 9†). The ionic liquid of ETPBr without metal ions melts at approximately 207 °C, forming organic small molecule glass with a Tg of around 48 °C once cooled (Fig. 1c). When organic ligands are combined with different inorganic polyhedra, such as (MnBr4)2−, (CoBr4)2−, (NiBr4)2−, (ZnBr4)2− tetrahedra and (Bi2Br9)3− dimer to form 0D HMHs, the Tm decreases compared to ETPBr and can form related hybrid glasses possessing different UV absorption spectra (ESI Fig. 10†). Monitoring of the Tg for various 0D HMH glasses revealed a correlation between the Tg and the atomic number of the metal ion. One can find that Tg increases gradually with an increase in atomic number of metal ions.
Similarly, the alteration of the halogen ions can also preserve the glass transition ability of 0D HMHs. As an example, we synthesized (ETP)2MnCl4 and successfully obtained the corresponding glass sample with a specific Tg (ESI Fig. 11 and ESI Table 3†). The successful preparation of these diverse 0D HMH glasses indicates that screening of appropriate ionic liquids is critical to achieve the glass transition of 0D HMH crystals at a low Tg. The existence of low Tg for 0D HMH glass affords it a unique ability to bend and fold near the Tg, indicating an excellent workability under mild conditions (Fig. 1d). Using this property, we prepared optical fibers with a diameter of about 400 μm, and a uniform distribution of elements was observed in the fibers using EDS (Fig. 1e and ESI Fig. 12†). Likewise, we can create glass discs that are 10 cm in diameter and have excellent light transmission properties (Fig. 1f).
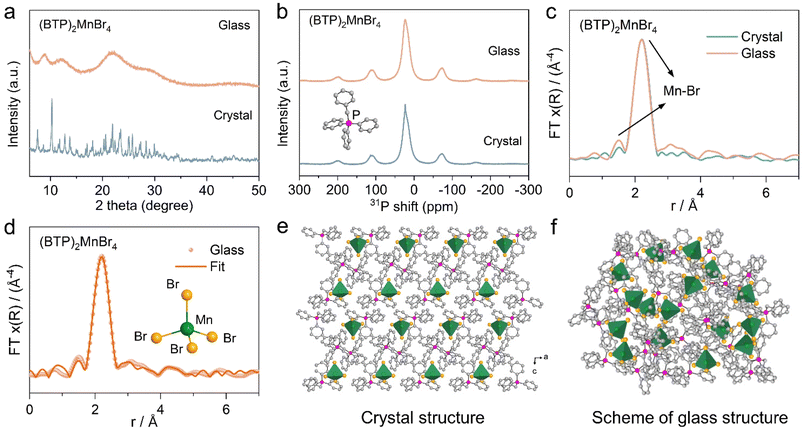
The conversion of 0D HMHs from crystals to glasses can be verified by XRD patterns (Fig. 2a). To further explore the structural transformations in detail, 31P solid-state nuclear magnetic resonance (NMR) and extended X-ray absorption fine structure (EXAFS) techniques were employed to investigate the coordination modes of P and Mn ions in the selected 0D HMH of (BTP)2MnBr4 as an example. Fig. 2b illustrates that the peak positions of 31P spectra for the (BTP)2MnBr4 glass sample align perfectly with those of the crystal, unequivocally confirming the retention of the four-coordinated P atom in (BTP)2MnBr4.47 To examine the phase transformation of (MnBr4)2− tetrahedra during crystalline–glass transition of (BTP)2MnBr4, we recorded the Mn K-edge EXAFS data of (BTP)2MnBr4 glass and crystal (ESI Fig. 13†), respectively. The Fourier transforms of the Mn K-edge EXAFS spectra of (BTP)2MnBr4 glass and crystal are similar, exhibiting two peaks at 1.5 Å and 2.2 Å, which can be attributed to the Mn–Br bond (Fig. 2c). The Mn–Br bond lengths in the (BTP)2MnBr4 glass were fitted to be approximately 2.53 Å and 2.63 Å, which are comparable to those of the (BTP)2MnBr4 crystal (Fig. 2d and ESI Table 4†).48 The near-identical Mn K-edge EXAFS curves of the (BTP)2MnBr4 glass and crystal confirm the complete preservation of the (MnBr4)2− tetrahedral component within the glass structure upon vitrification.49 Likewise, the similarity of glass and crystal phases in IR spectra and Raman spectra further demonstrate the retention of organic and inorganic components in the glass (ESI Fig. 14 and 15†).50,51 In considering the analysis results of XRD, 31P solid NMR, EXAFS, IR spectra, and Raman spectra of (BTP)2MnBr4 glass and crystal, we can conclusively describe the structure transition of (BTP)2MnBr4. When the (BTP)2MnBr4 crystals vitrify to glass, the regularly arranged crystal structure changed into a disordered glass structure, but the organic cations and the (MnBr4)2− tetrahedra in the crystal were preserved in the glass phase, supported by 31P solid NMR and Mn K-edge EXAFS (Fig. 2e and f) as discussed above.
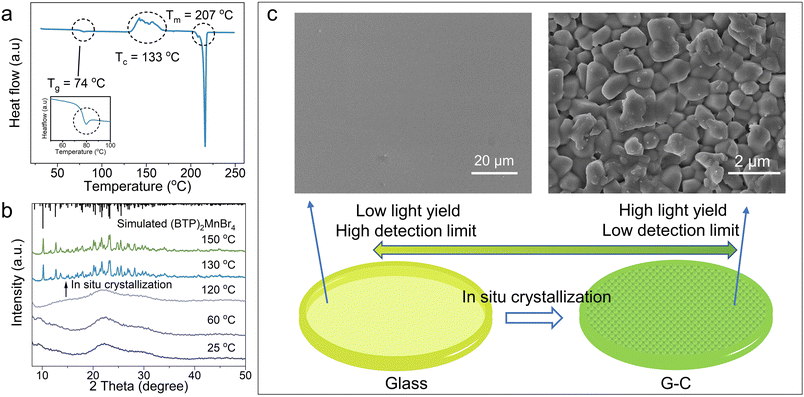
Due to the retention of the tetra-coordinated structure of Mn2+ in (BTP)2MnBr4 glass, its fluorescence emission and decay lifetime are similar to that of the crystal (ESI Fig. 16 and 17†). During the crystal–glass transition, the quantum efficiency and scintillation performance of the glass decreases, making the glass sample substantially less promising for optical functional applications (ESI Fig. 18 and ESI Table 5†). Intriguingly, the DSC curve of (BTP)2MnBr4 glass shows an exothermic peak at about 130 °C higher than Tg, indicating the recrystallization of the glass during a stepwise temperature heating (Fig. 3a). This result was further confirmed by temperature-dependent XRD characterization, proving the in situ transformation of the amorphous (BTP)2MnBr4 glass into its crystalline phase again upon heating (Fig. 3b). Although the exothermic peak of crystallization was not monitored on the DSC curves of the other 0D HMH glasses, all of them could afford the in situ crystallization of 0D HMH glass by heat treatment. The XRD of the 0D HMH glasses after heat treatment at different temperatures indicated that this phenomenon is general in 0D HMH glasses (ESI Fig. 19†).
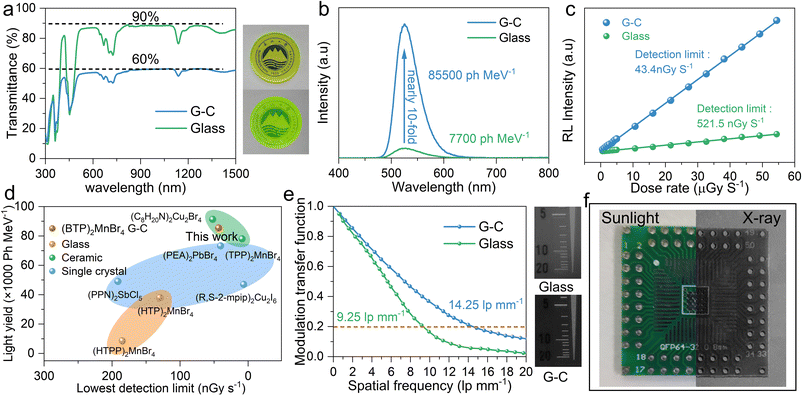
By holding the glass samples at 130–150 °C, we achieved in situ recrystallization inside the glass, resulting in (BTP)2MnBr4 G-C. Fig. 3c shows that the smooth surface of the glass changed into stacked microcrystals with a grain size of about 1 μm. After recrystallization at 130 °C, the transmittance of the glass is greatly preserved, and the as-formed G-C still has 60% transmittance between 500 and 1500 nm (Fig. 4a). As the recrystallization temperature increases, the grain size increases and the sample transmittance decreases (ESI Fig. 20 and 21†). By comparing the light yield with that of the standard scintillator LuAG:Ce, the G-C demonstrated a nearly 10-fold improvement over the glass sample, achieving an excellent light yield of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ph MeV−1 (Fig. 4b and ESI Fig. 22†). Furthermore, the (BTP)2MnBr4 G-C exhibited a lower detection limit than the glass sample, with a detection limit of 43.4 nGy s−1, well below the required metering rate for X-ray diagnostics (5.5 μGy s−1), significantly reducing the radiation dose required for use in medical examinations (Fig. 4c). When compared to other high-performance bulk hybrid scintillation materials, the large-area (BTP)2MnBr4 G-C via such a recrystallization processing technique demonstrates a higher yield and lower detection limit than those of scintillation glass, placing it on par with scintillation ceramics and single crystals (Fig. 4d).5,22,23,52–55
500 ph MeV−1 (Fig. 4b and ESI Fig. 22†). Furthermore, the (BTP)2MnBr4 G-C exhibited a lower detection limit than the glass sample, with a detection limit of 43.4 nGy s−1, well below the required metering rate for X-ray diagnostics (5.5 μGy s−1), significantly reducing the radiation dose required for use in medical examinations (Fig. 4c). When compared to other high-performance bulk hybrid scintillation materials, the large-area (BTP)2MnBr4 G-C via such a recrystallization processing technique demonstrates a higher yield and lower detection limit than those of scintillation glass, placing it on par with scintillation ceramics and single crystals (Fig. 4d).5,22,23,52–55
To demonstrate the practical application of the as-obtained HMH G-Cs as scintillators in X-ray imaging, we compared the spatial resolution of (BTP)2MnBr4 G-C to that of glass samples. The (BTP)2MnBr4 G-C exhibited excellent spatial resolution (14.25 lp mm−1), surpassing obviously that of the (BTP)2MnBr4 glass (9.25 lp mm−1) due to the outstanding transmittance and high light yield (Fig. 4e). Images of the X-ray standard resolution test template provide further evidence of the superior spatial resolution achieved by (BTP)2MnBr4 G-C (ESI Fig. 23†). Subsequent imaging experiments demonstrated the practicality of (BTP)2MnBr4 G-C in X-ray imaging applications. Clear circuit structures and the fine needles inserted into a chicken that were not visible under fluorescent light could be successfully captured under X-ray irradiation (Fig. 4f and ESI Fig. 24†).
Conclusions
In summary, we have developed a general in situ recrystallization process within the 0D HMH glass components to prepare high-performance glass-ceramics. Four quaternary phosphonium ionic liquids can be amorphized in combination with (MnBr4)2− to form 0D HMH glasses with very low Tg and good processing properties. By utilizing ethyltriphenylphosphonium (ETP) with vitrification capability as organic cations, the luminescent center in 0D HMH glasses can be expanded to diverse polyhedra composed of different metal and halogen ions. 31P solid-state NMR and EXAFS tests support that the 0D HMH glass retains the coordination environment of organic cations and inorganic polyhedra of HMH crystals after vitrification, but the photoluminescence quantum efficiency and scintillation performance are reduced. By applying an in situ recrystallization strategy through holding the glass phase at 130 °C, the G-Cs originating from 0D HMH glass were successfully generated, showing 10-fold light yield and reduced detection limit in comparison to the glass phase as scintillators. The excellent transmittance and greatly improved scintillation performance of large-area G-Cs make them great candidates for X-ray imaging applications. The concept of exploiting recrystallization of 0D HMH glass toward G-Cs is expected to open the gateway for developing high performance scintillators, and allow them to become an emerging optical functional material.Experimental section
Synthesis. Growth of (BTP)2MnBr4, (MTP)2MnBr4, (ETP)2MnBr4, (BuTP)2MnBr4, (1-EP)2MnBr4 and (1-BuP)2MnBr4 single crystals
Specifically, BTPBr (1 mmol), MTPBr (1 mmol), ETPBr (1 mmol), BuTPBr (1 mmol), 1-EPBr (1 mmol) or 1-BuP (1 mmol) and MnBr2 (0.5 mmol) were dissolved in ethanol under stirring conditions. Then, crystals of (BTP)2MnBr4, (MTP)2MnBr4, (ETP)2MnBr4, (BuTP)2MnBr4, (1-EP)2MnBr4 and (1-BuP)2MnBr4 were gradually precipitated with the volatilization of ethanol, and the crystals were collected.56Growth of (ETP)2CoBr4, (ETP)2NiBr4, (ETP)2ZnBr4 and (ETP)3Bi2Br9 single crystals
Specifically, ETPBr (1 mmol) and CoBr2 (0.5 mmol), NiBr2 (0.5 mmol), ZnBr2 (0.5 mmol) or BiBr3 (0.66 mmol) were dissolved in dichloromethane (99.9%, 2 mL) under stirring conditions. Then, the counter-solvent ether was added and left for one to two days, and the crystals of (ETP)2CoBr4, (ETP)2NiBr4, (ETP)2ZnBr4 and (ETP)3Bi2Br9 were gradually precipitated and collected.Fabrication of hybrid metal halide (HMH) glass
The HMH glass was obtained by heating HMH crystal at 130–250 °C under air conditions, and cooling the melt to room temperature.Fabrication of (BTP)2MnBr4 glass-ceramic (G-C)
The pre-prepared (BTP)2MnBr4 glass precursor was kept in an air atmosphere at 130–150 °C for 30 min, and the sample was removed after natural cooling, and the obtained sample was ground and polished to obtain (BTP)2MnBr4 G-C.Characterization
The single crystal data was collected using the XtaLAB AFC12 X-ray four-circle single crystal diffractometer (Rigaku) equipped with a CCD-detector, graphite monochromator and Cu Kα radiation source. Powder X-ray diffraction (PXRD) patterns and the variable temperature-dependent PXRD measurements were taken on an Aeris PXRD diffractometer (PANalytical Corporation, the Netherlands) operating at 40 kV and 15 mA with monochromatized Cu Kα radiation (λ = 1.5406 Å). Variable temperature-dependent PXRD was conducted from room temperature to 150 °C with a heating rate of 10 °C min−1 and data was collected after holding at 25 °C, 60 °C, 120 °C, 130 °C, and 150 °C for 10 minutes. The photoluminescence excitation (PLE), photoluminescence (PL) and PL decay spectra measurements were performed on an FLS1000 fluorescence spectrophotometer (Edinburgh Instruments Ltd., UK). The photoluminescence quantum yields were recorded using a Hamamatsu Quantaurus-QY spectrometer (model C11347-11). The PLQYs were calculated based on the equation: ηQE = IS/(ER − ES), in which IS denotes the luminescence emission spectral area of the sample, ER is the excitation light spectral area of the null integrating sphere, and ES is the excitation light spectral area of the excited sample. Differential scanning calorimetry (DSC) measurements were carried out on a TA DSC Q2000 mainly focusing on its thermotropic behavior. The sample was heated at a scan rate of 5 °C min−1. After cooling to room temperature, the second up-scan was performed using the same procedure as the first scan. Thermogravimetric analysis (TGA) was conducted on a TG 209F3 from 30 to 500 °C in an argon atmosphere with a ramping rate of 10 °C min−1. The light transmittance spectra were recorded using a Lambda 750 spectrophotometer (PerkinElmer, USA) in the range from 300 to 1500 nm. Raman data was collected on an XploRA PLUS (λ = 785 nm). Fourier-transform infrared (FTIR) was performed on a Bruker VERTEX 70. A solid state NMR experiment was carried out on a Bruker 600 MHz (14.1 T) magnet with AVANCE NEO consoles using a Bruker 3.2 mm HXY magic angle spinning (MAS) probe. The Larmor frequency for 31P was determined to be 242.99 MHz. 31P spectra were acquired with one pulse and referenced to 85% H3PO4 solution (0 ppm). The Mn K-edge extended X-ray absorption fine structure (EXAFS) spectra were collected at beamline BL14W1 of SSRF operated at 3.5 GeV in “top-up” mode with a constant current of 220 mA. The energy was calibrated according to the absorption edge of pure Mn foil. EXAFS data from the solid samples were recorded in transmission mode with high-flux ion chambers with a 32-element Ge solid-state detector, respectively. The duration of the measurement was about 0.5 h for each sample. The extended XAFS (EXAFS) data were analyzed using the DEMETER software package.40 Radioluminescence spectra at room temperature (RT) were recorded using an FLS1000 fluorescence spectrophotometer (Edinburgh Instruments Ltd., Edinburgh, U.K.). The (BTP)2MnBr4 glass, G-C and the commercial scintillator LuAG:Ce were tightly attached to the circular window of an integrating sphere with an X-ray source (Amptek Mini-X tube with a Mo target and a maximum power output of 6 W). The LY values can be calculated from the total ratio of the number of emitted photons for (BTP)2MnBr4 glass, G-C and the commercial scintillator LuAG:Ce at the equivalent absorbed X-ray energy. X-ray detection limits were calculated from the linear relationship between the RL intensity and the X-ray dose of the corresponding samples. Noise data were obtained in the absence of samples. The detection limit of the corresponding sample was obtained when the signal-to-noise ratio was 3. X-ray imaging was acquired by using a complementary metal oxide semiconductor camera. MTF measurements can be used to evaluate the fundamental spatial resolution performance of an imaging system. The spatial resolution can be determined by the spatial frequency value when MTF = 0.2. The MTF curve was calculated by the slanted-edge method.Data availability
All data needed to evaluate the conclusions are present in the paper and ESI.† The data that support the findings of this study are available from the corresponding author upon request.Author contributions
Y. X. and Z. G. X. initiated and guided the research. B. H. L. synthesized the samples and wrote the manuscript, and Y. X. and Z. G. X. revised it. B. H. L., J. C. J., M. J. Y., K. H. and X. L. Z. interpreted the theoretical and experimental results. Y. C. Z. and A. R. Z. performed the morphological and structural characterization. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22171040), the Fundamental Research Funds for the Central Universities, China (No. N2105006), and the Guangdong Provincial Science & Technology Project (2023A0505050084).References
- M. Li and Z. Xia, Chem. Soc. Rev., 2021, 50, 2626–2662 RSC.
- S. Sun, M. Lu, X. Gao, Z. Shi, X. Bai, W. W. Yu and Y. Zhang, Adv. Sci., 2021, 8, 2102689 CrossRef PubMed.
- P. Tao, S. J. Liu and W. Y. Wong, Adv. Opt. Mater., 2020, 8, 2000985 CrossRef CAS.
- L. J. Xu, X. Lin, Q. He, M. Worku and B. Ma, Nat. Commun., 2020, 11, 4329 CrossRef CAS PubMed.
- K. Han, K. Sakhatskyi, J. Jin, Q. Zhang, M. V. Kovalenko and Z. Xia, Adv. Mater., 2022, 34, 2110420 CrossRef CAS PubMed.
- T. M. Guo, Y. J. Gong, Z. G. Li, Y. M. Liu, W. Li, Z. Y. Li and X. H. Bu, Small, 2022, 18, 2103829 CrossRef CAS PubMed.
- P. K. Rajput, A. K. Poonia, S. Mukherjee, T. Sheikh, M. Shrivastava, K. V. Adarsh and A. Nag, J. Phys. Chem. C, 2022, 126, 9889–9897 CrossRef CAS.
- H. Wang, J. X. Wang, X. Song, T. He, Y. Zhou, O. Shekhah, L. Gutierrez-Arzaluz, M. Bayindir, M. Eddaoudi, O. M. Bakr and O. F. Mohammed, ACS Cent. Sci., 2023, 9, 668–674 CrossRef CAS PubMed.
- R. W. Huang, X. Song, S. Chen, J. Yin, P. Maity, J. Wang, B. Shao, H. Zhu, C. Dong, P. Yuan, T. Ahmad, O. F. Mohammed and O. M. Bakr, J. Am. Chem. Soc., 2023, 145, 13816–13827 CrossRef CAS PubMed.
- M. Li, J. Zhou, G. Zhou, M. S. Molokeev, J. Zhao, V. Morad, M. V. Kovalenko and Z. Xia, Angew. Chem., Int. Ed., 2019, 58, 18670–18675 CrossRef CAS PubMed.
- L. J. Xu, A. Plaviak, X. Lin, M. Worku, Q. He, M. Chaaban, B. J. Kim and B. Ma, Angew. Chem., Int. Ed., 2020, 59, 23067–23071 CrossRef CAS PubMed.
- Q. Li, B. Xu, Z. Chen, J. Han, L. Tan, Z. Luo, P. Shen and Z. Quan, Adv. Funct. Mater., 2021, 31, 2104923 CrossRef CAS.
- B. Su, S. Geng, Z. Xiao and Z. Xia, Angew. Chem., Int. Ed., 2022, 61, e202208881 CrossRef CAS PubMed.
- T. Huang, Q. Wei, W. Lin, H. Peng, S. Yao and B. Zou, Mater. Today Phys., 2022, 25, 100703 CrossRef CAS.
- J. Lu, J. Gao, S. Wang, M. J. Xie, B. Y. Li, W. F. Wang, J. R. Mi, F. K. Zheng and G. C. Guo, Nano Lett., 2023, 23, 4351–4358 CrossRef CAS PubMed.
- A. S. Kshirsagar, H. Arfin, S. Banerjee, B. Mondal and A. Nag, J. Phys. Chem. C, 2021, 125, 27671–27677 CrossRef CAS.
- H. Meng, B. Chen, W. Zhu, Z. Zhou, T. Jiang, X. Xu, S. Liu and Q. Zhao, Laser Photonics Rev., 2023, 17, 2201007 CrossRef CAS.
- D. Y. Li, J. H. Song, Z. Y. Xu, Y. J. Gao, X. Yin, Y. H. Hou, L. J. Feng, C. Y. Yue, H. Fei and X. W. Lei, Chem. Mater., 2022, 34, 6985–6995 CrossRef CAS.
- B. Li, Y. Xu, X. Zhang, K. Han, J. Jin and Z. Xia, Adv. Opt. Mater., 2022, 10, 2102793 CrossRef CAS.
- Y. Zhang, Y. Zhang, Y. Zhao, H. Jia, Z. Yang, B. Yin, Y. Wu, Y. Yi, C. Zhang and J. Yao, J. Am. Chem. Soc., 2023, 145, 12360–12369 CrossRef CAS PubMed.
- B. Li, J. Jin, M. Yin, X. Zhang, M. S. Molokeev, Z. Xia and Y. Xu, Angew. Chem., Int. Ed., 2022, 61, e202212741 CrossRef CAS PubMed.
- J. B. Luo, J. H. Wei, Z. Z. Zhang, Z. L. He and D. B. Kuang, Angew. Chem., Int. Ed., 2023, 62, e202216504 CrossRef CAS PubMed.
- Y. Xu, Z. Li, G. Peng, F. Qiu, Z. Li, Y. Lei, Y. Deng, H. Wang, Z. Liu and Z. Jin, Adv. Opt. Mater., 2023, 11, 2300216 CrossRef CAS.
- K. Ito, C. T. Moynihan and C. A. Angell, Nature, 1999, 398, 492–495 CrossRef CAS.
- N. Ma and S. Horike, Chem. Rev., 2022, 122, 4163–4203 CrossRef CAS PubMed.
- S. Horike, S. S. Nagarkar, T. Ogawa and S. Kitagawa, Angew. Chem., Int. Ed., 2020, 59, 6652–6664 CrossRef CAS PubMed.
- C. Ye, L. N. McHugh, C. Chen, S. E. Dutton and T. D. Bennett, Angew. Chem., Int. Ed., 2023, e202302406 CAS.
- T. D. Bennett and S. Horike, Nat. Rev. Mater., 2018, 3, 431–440 CrossRef.
- M. A. Ali, W. M. W. Winters, M. A. Mohamed, D. Tan, G. Zheng, R. S. K. Madsen, O. V. Magdysyuk, M. Diaz-Lopez, B. Cai, N. Gong, Y. Xu, I. Hung, Z. Gan, S. Sen, H. T. Sun, T. D. Bennett, X. Liu, Y. Yue and J. Qiu, Angew. Chem., Int. Ed., 2023, 62, e202218094 CrossRef CAS PubMed.
- G. Du, S. Wen, J. Zhao, P. Ran, D. Wang, L. Wei, X. Qiao, Y. Yang, J. Qiu and S. Zhou, Adv. Mater., 2023, 35, e2205578 CrossRef PubMed.
- T. Li, W. A. Dunlap-Shohl, E. W. Reinheimer, P. Le Magueres and D. B. Mitzi, Chem. Sci., 2019, 10, 1168–1175 RSC.
- J. Hou, M. L. Rios Gomez, A. Krajnc, A. McCaul, S. Li, A. M. Bumstead, A. F. Sapnik, Z. Deng, R. Lin, P. A. Chater, D. S. Keeble, D. A. Keen, D. Appadoo, B. Chan, V. Chen, G. Mali and T. D. Bennett, J. Am. Chem. Soc., 2020, 142, 3880–3890 CrossRef CAS PubMed.
- T. D. Bennett, Y. Yue, P. Li, A. Qiao, H. Tao, N. G. Greaves, T. Richards, G. I. Lampronti, S. A. Redfern, F. Blanc, O. K. Farha, J. T. Hupp, A. K. Cheetham and D. A. Keen, J. Am. Chem. Soc., 2016, 138, 3484–3492 CrossRef CAS PubMed.
- Z. Yin, Y. Zhao, S. Wan, J. Yang, Z. Shi, S. X. Peng, M. Z. Chen, T. Y. Xie, T. W. Zeng, O. Yamamuro, M. Nirei, H. Akiba, Y. B. Zhang, H. B. Yu and M. H. Zeng, J. Am. Chem. Soc., 2022, 144, 13021–13025 CrossRef CAS PubMed.
- R. S. K. Madsen, A. Qiao, J. Sen, I. Hung, K. Chen, Z. Gan, S. Sen and Y. Yue, Science, 2020, 367, 1473–1476 CrossRef CAS PubMed.
- Z. Fan, C. Das, A. Demessence, R. Zheng, S. Tanabe, Y. S. Wei and S. Horike, Chem. Sci., 2022, 13, 3281–3287 RSC.
- V. Nozari, O. Smirnova, J. M. Tuffnell, A. Knebel, T. D. Bennett and L. Wondraczek, Adv. Mater. Technol., 2022, 7, 2200343 CrossRef CAS.
- M. A. Ali, X. Liu, H.-T. Sun, J. Ren and J. Qiu, Chem. Mater., 2022, 34, 2476–2483 CrossRef CAS.
- R. Lin, X. Li, A. Krajnc, Z. Li, M. Li, W. Wang, L. Zhuang, S. Smart, Z. Zhu, D. Appadoo, J. Harmer, Z. Wang, A. G. Buzanich, S. Beyer, L. Wang, G. Mali, T. D. Bennett, V. Chen and J. Hou, Angew. Chem., Int. Ed., 2022, 61, e202112880 CrossRef CAS PubMed.
- S. Li, R. Limbach, L. Longley, A. A. Shirzadi, J. C. Walmsley, D. N. Johnstone, P. A. Midgley, L. Wondraczek and T. D. Bennett, J. Am. Chem. Soc., 2019, 141, 1027–1034 CrossRef CAS PubMed.
- R. Lin, M. Chai, Y. Zhou, V. Chen, T. D. Bennett and J. Hou, Chem. Soc. Rev., 2023, 52, 4149–4172 RSC.
- J. Hou, C. W. Ashling, S. M. Collins, A. Krajnc, C. Zhou, L. Longley, D. N. Johnstone, P. A. Chater, S. Li, M. V. Coulet, P. L. Llewellyn, F. X. Coudert, D. A. Keen, P. A. Midgley, G. Mali, V. Chen and T. D. Bennett, Nat. Commun., 2019, 10, 2580 CrossRef PubMed.
- C. W. Ashling, D. N. Johnstone, R. N. Widmer, J. Hou, S. M. Collins, A. F. Sapnik, A. M. Bumstead, P. A. Midgley, P. A. Chater, D. A. Keen and T. D. Bennett, J. Am. Chem. Soc., 2019, 141, 15641–15648 CrossRef CAS PubMed.
- D. X. Liu, H. L. Zhu, W. X. Zhang and X. M. Chen, Angew. Chem., Int. Ed., 2023, 62, e202218902 CrossRef CAS PubMed.
- M. Li, M. S. Molokeev, J. Zhao and Z. Xia, Adv. Opt. Mater., 2020, 8, 1902114 CrossRef CAS.
- A. N. Usoltsev, T. S. Sukhikh, A. S. Novikov, V. R. Shayapov, D. P. Pishchur, I. V. Korolkov, I. F. Sakhapov, V. P. Fedin, M. N. Sokolov and S. A. Adonin, Inorg. Chem., 2021, 60, 2797–2804 CrossRef CAS PubMed.
- J. D. Lewis, M. Ha, H. Luo, A. Faucher, V. K. Michaelis and Y. Román-Leshkov, ACS Catal., 2018, 8, 3076–3086 CrossRef CAS.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef CAS PubMed.
- S. Shimono, M. Sekine, Y. Niwa, H. Sagayama, K. Araki, Y. Hata and H. Kishimura, Mater. Res. Bull., 2023, 159, 112103 CrossRef CAS.
- A. Qiao, T. D. Bennett, H. Tao, A. Krajnc, G. Mali, C. M. Doherty, A. W. Thornton, J. C. Mauro, G. N. Greaves and Y. Yue, Sci. Adv., 2018, 4, eaao6827 CrossRef PubMed.
- T. D. Bennett, J. C. Tan, Y. Yue, E. Baxter, C. Ducati, N. J. Terrill, H. H. Yeung, Z. Zhou, W. Chen, S. Henke, A. K. Cheetham and G. N. Greaves, Nat. Commun., 2015, 6, 8079 CrossRef CAS PubMed.
- B. Jia, D. Chu, N. Li, Y. Zhang, Z. Yang, Y. Hu, Z. Zhao, J. Feng, X. Ren, H. Zhang, G. Zhao, H. Sun, N. Yuan, J. Ding, Y. Liu and S. F. Liu, ACS Energy Lett., 2022, 8, 590–599 CrossRef.
- Z. Song, Z. Jia, X. Guo, B. Yu, G. Liu, L. Meng, Y. Liu, Q. Lin and Y. Dang, Adv. Opt. Mater., 2023, 11, 2203014 CrossRef CAS.
- Q. He, C. Zhou, L. Xu, S. Lee, X. Lin, J. Neu, M. Worku, M. Chaaban and B. Ma, ACS Mater. Lett., 2020, 2, 633–638 CrossRef CAS.
- B. Su, J. Jin, K. Han and Z. Xia, Adv. Funct. Mater., 2023, 33, 2210735 CrossRef CAS.
- Y.-Y. Ma, Y.-R. Song, W.-J. Xu, Q.-Q. Zhong, H.-Q. Fu, X.-L. Liu, C.-Y. Yue and X.-W. Lei, J. Mater. Chem. C, 2021, 9, 9952–9961 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc04332k |
| This journal is © The Royal Society of Chemistry 2023 |