Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Programmable synthesis of difluorinated hydrocarbons from alkenes through a photocatalytic linchpin strategy†
Zhi-Qi
Zhang
abc,
Cheng-Qiang
Wang
c,
Long-Ji
Li
ac,
Jared L.
Piper
d,
Zhi-Hui
Peng
*d,
Jun-An
Ma
 *ab,
Fa-Guang
Zhang
*ab,
Fa-Guang
Zhang
 *b and
Jie
Wu
*b and
Jie
Wu
 *c
*c
aJoint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Binhai New City, Fuzhou 350207, P. R. China. E-mail: majun_an68@tju.edu.cn
bDepartment of Chemistry, Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Frontiers Science Center for Synthetic Biology (Ministry of Education), Tianjin University, Tianjin 300072, P. R. China. E-mail: zhangfg1987@tju.edu.cn
cDepartment of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543, Republic of Singapore. E-mail: chmjie@nus.edu.sg
dPfizer Worldwide Research and Development Medicine, Eastern Point Rd, Groton, CT 06340, USA. E-mail: Zhihui.Peng@pfizer.com
First published on 11th October 2023
Abstract
The introduction of difluoromethylene moieties into organic molecules has garnered significant attention due to their profound influence on the physicochemical and biological properties of compounds. Nonetheless, the existing approaches for accessing difluoroalkanes from readily available feedstock chemicals remain limited. In this study, we present an efficient and modular protocol for the synthesis of difluorinated compounds from alkenes, employing the readily accessible reagent, ClCF2SO2Na, as a versatile “difluoromethylene” linchpin. By means of an organophotoredox-catalysed hydrochlorodifluoromethylation of alkenes, followed by a ligated boryl radical-facilitated halogen atom transfer (XAT) process, we have successfully obtained various difluorinated compounds, including gem-difluoroalkanes, gem-difluoroalkenes, difluoromethyl alkanes, and difluoromethyl alkenes, with satisfactory yields. The practical utility of this linchpin strategy has been demonstrated through the successful preparation of CF2-linked derivatives of complex drugs and natural products. This method opens up new avenues for the synthesis of structurally diverse difluorinated hydrocarbons and highlights the utility of ligated boryl radicals in organofluorine chemistry.
Introduction
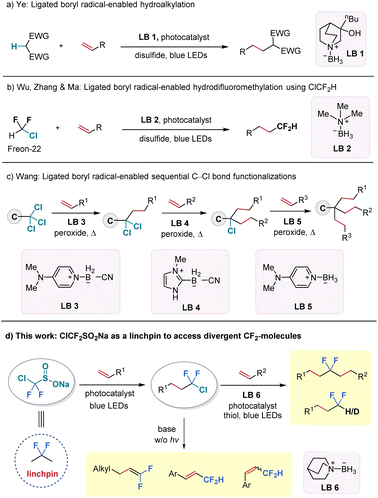
The incorporation of difluoroalkyl groups into organic molecules has garnered significant and sustained attention due to their impact on the physicochemical and biological properties of these molecules.1–6 To date, most efforts to construct difluorinated scaffolds rely on deoxyfluorination of carbonyl compounds7–9 or transition metal or Lewis acid-catalysed/mediated difluoroalkylation and difluorination reactions,10–13 with a particular focus on aryl-CF2 systems. However, synthetic approaches to access gem-difluoroalkanes {C(sp3)–CF2–C(sp3)} from abundant feedstock chemicals remain scarce.14–20 Although a few examples have been reported to form aliphatic difluorinated moieties from unactivated alkenes, these approaches have been limited to using alkyl CF2–Br/I as the starting CF2 precursors.21,22 Thus, there is a pressing need for novel, efficient, and robust methods to expand the diversity of gem-difluorinated compounds, particularly those derived from unactivated aliphatic systems.Ligated boryl radicals, denoted by the general formula L+-R2B˙−, are stabilized by coordination to amines, phosphines, sulphides, or N-heterocyclic carbenes (NHCs) and share seven electrons in their valence shell.23–25 Notably, ligated boryl radicals exhibit a strong nucleophilic character and offer considerable potential for halogen atom transfer (XAT) and hydrogen atom transfer (HAT) processes by tuning the Lewis base motifs.26–30 For instance, the Ye group successfully activated electron-deficient C–H bonds by employing a quinuclidine-borane (LB 1) as the HAT reagent to realize a radical hydroalkylation reaction of unactivated alkenes in 2021 (Fig. 1a).31 However, the XAT process triggered by ligated-boryl radicals was only known to activate alkyl iodides and bromides for decades,32–36 until recently, when our group successfully activated the C–Cl bond (BDEcal. = 87 kcal mol−1) from chlorodifluoromethane (ClCF2H, Freon-22) under blue light irradiation, using commercially available and inexpensive trimethylamine-borane (LB 2) as the XAT reagent (Fig. 1b).37 At the same time, the Wang group reported a three-step process for sequential C–Cl bond functionalization of activated trichloromethyl groups with the choice of an appropriate ligated borane reagent in each step (LB 3–5) (Fig. 1c).38
Encouraged by these recent scientific advancements, we aimed to establish a protocol for ligated boryl radical-promoted C–Cl bond functionalization of C(sp3)–CF2–Cl substrates to provide a novel and streamlined method for accessing gem-difluorinated aliphatic backbones, which were previously challenging to obtain. Along this endeavour, we turned our attention to an easily accessible reagent—sodium chlorodifluoromethylsulfinate (ClCF2SO2Na) as the CF2-precursor.39–41 The proposed sequence involves two radical carbon–carbon bond formation transformations and has the potential to connect two alkene fragments through the valuable CF2-unit (Fig. 1d). If successful, this protocol would open an interesting avenue for synthesizing structurally divergent difluorinated hydrocarbons, with ClCF2SO2Na serving as a practical “linchpin”.42–48 While the “linchpin” strategy has been explored in recent years to link two molecular skeletons through linkers such as alkyne, alkene, and sulfone groups, its application in organofluorine chemistry remains underdeveloped.49–51 Herein, we collaborated with colleagues from Pfizer to prepare ClCF2SO2Na in kilogram-scale. By merging radical chlorodifluoromethylation of alkenes with a subsequent ligated boryl radical-facilitated hydrodifluoroalkylation of different alkenes, we were able to easily access a wide variety of internal gem-difluoro alkanes under mild visible-light irradiation conditions. Furthermore, photo-induced hydrodechlorination and base-promoted elimination reactions enabled the synthesis of a broad range of terminal gem-difluorinated alkanes and alkenes (Fig. 1d).
Results and discussion
Development of hydrochlorodifluoromethylation of alkenes
Our investigation began with the development of a radical chlorodifluoromethylation protocol for alkenes with ClCF2SO2Na. A screening of photocatalysts, thiols, and solvents established that the desired product 1j was obtained in up to 86% isolated yield by conducting the reaction with Mes-Acr-Me+ClO4− as the photocatalyst and methyl thiosalicylate as the HAT mediator in a mixed solvent of CHCl3/CF3CH2OH (9/1, 0.2 M, see the ESI Table 1† for optimization details). As shown in Fig. 2A, this protocol accommodated a wide range of unactivated mono-, di-, and trisubstituted alkenes (1a–1s) with good to high yields. Notably, the reaction conditions tolerated various functional groups, including those bearing silicon (1b), phosphine (1c), amine (1d), silyl ether (1k and 1m), free hydroxyl group (1l), aldehyde (1i), tosylate (1n), hetero/aliphatic-cyclic rings (1f, 1g, and 1r), alkyne (1k), and bromide (1p). Additionally, a substrate bearing two olefin motifs was fully chlorodifluoromethylated at both sites in a 71% yield (1t). Notably, selected complex alkenes derived from natural products or drug molecules were found to be well-tolerated under standard conditions, affording the corresponding difluorinated derivatives (1u–1ae) in good yields.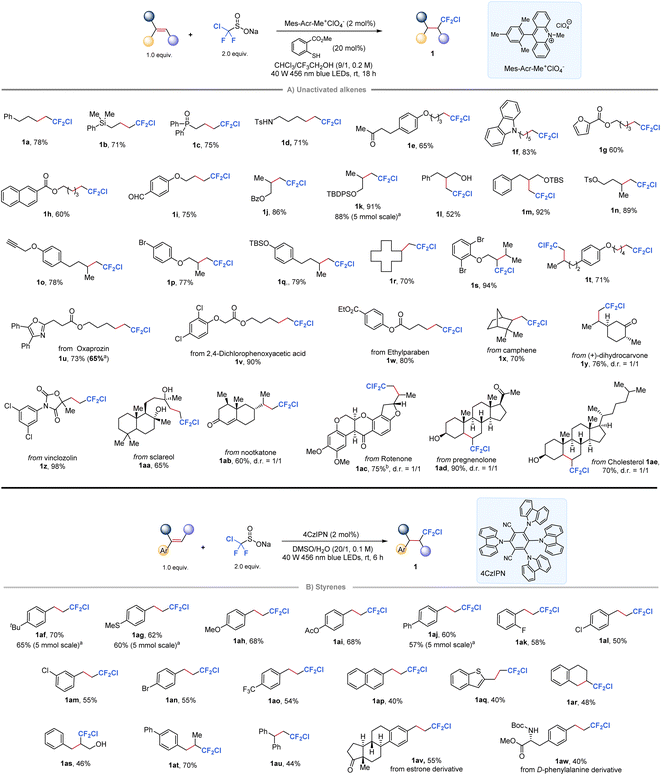 | ||
| Fig. 2 Hydrochlorodifluoromethylation of alkenes with ClCF2SO2Na. a5.0 mmol of alkenes, 3.0 equiv. of ClCF2SO2Na; b3.0 equiv. of ClCF2SO2Na. | ||
When the scope of this protocol was extended to styrenes, however, low yields were observed for the target chlorodifluoromethylation products due to undesired polymerization side reactions in the presence of the acridinium photocatalyst. Notably, cyanoarenes have been previously shown to serve as effective organophotocatalysts for the hydrofluoroalkylation of styrenes, with the solvent choice playing a critical role.52 Taking this into account, we investigated various organic photocatalysts and solvents, ultimately finding that the desired reaction could be achieved using 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) in DMSO (Fig. 2B, see the ESI Table 2† for screening details). The protocol was shown to be effective for a range of styrenes substituted at ortho-, meta-, or para-positions of the phenyl ring, yielding the desired products in moderate to good yields (1af–1ao). Furthermore, the protocol successfully delivered chlorodifluoromethylation products from substrates including 2-naphthyl (1ap), heteroaromatic (1aq), cyclic internal alkene (1ar), 1,2-disubstituted (1ar–1at) and 1,1-disubstituted (1au) styrene derivatives. Importantly, the reaction could be easily scaled up to gram-scale with minimal loss (1k, 1af, 1ag, and 1aj), highlighting the practical synthetic utility of this protocol. In addition, relatively complex styrenes derived from estrone or phenylalanine are also accommodated in this reaction to give 1av and 1aw in moderate yields.
Synthesis of gem-difluorinated alkanes from alkenes
With a series of chlorodifluoromethyl alkanes in hand, our study then focused on the selective activation of the C–Cl bond by choosing compound 1k as the model substrate with unactivated alkenes. A systematic optimization of the reaction parameters, including photocatalysts, ligated boranes (XAT reagents), disulfides (HAT reagents), solvents, and light source, revealed that the desired product 2a could be obtained in 90% yield by utilizing quinuclidine-ligated borane LB 6 in combination with a simple phenyl thiol under blue light irradiation conditions (see the ESI Table 3† for optimization details). Fig. 3 illustrates the assembly of CF2-linked aliphatic hydrocarbons through an organophotoredox-catalysed radical chlorodifluoromethylation coupled with ligated boryl radical-enabled hydrodifluoroalkylation, starting from ClCF2SO2Na. As a proof of concept study, we briefly explored the scope of ClCF2–alkanes (1k, 1af, 1ag, and 1aj) to connect with unactivated alkenes. The study found that a wide range of mono-substituted alkenes with various functional groups, such as tosylate (2b), free hydroxyl group (2c), ester (2e and 2k), amide (2f), carbonyl group (2j and 2x), chloride (2t), and free carboxylic acid (2p), were all compatible to deliver the corresponding difluorinated products in moderate to high yields. Phosphorus (2d), nitrogen (2i), oxygen (2u), and silicon (2o and 2v) atom-tethered alkenes were also competent reaction partners. Furthermore, cyclic structures, including carbazole (2g and 2l), furan (2m), oxetane (2n), cyclododecane (2r) and adamantane (2y), underwent the reaction smoothly. It should be noted that the reaction between ethylene, the largest-volume and cost-effective industrial chemical that has an annual production of over 150 million tons, and 1y led to the formation of gem-difluoroalkane (2q) with a yield of 46%. Additionally, the linchpin strategy demonstrated effective utilization in the late-stage functionalization of complex alkenes derived from pharmaceutical molecules and natural products. Unactivated alkenes containing the core structures of norborene (2z), camphene (2aa), carprofen (2ab), or sclareol (2ac) smoothly reacted with 1y with good yields. Importantly, it was feasible to use the linchpin strategy to connect two bioactive olefins derived from oxaprozin and L-menthol (2ad), or L-(−)-borneol (2ae), or ibuprofen (2af) or 2,4-dichlorophenoxyacetic acid (2ag), which clearly indicates the broad applicability of this strategy. Among the tested alkenes, relatively lower yields were observed for substrates containing allylic alcohols or silanes (i.e., 2u, 2v and 2x) due to the simultaneous formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond-reduced by-products. Lower conversions accounted for the moderate yields of some substrates (i.e., 2b, 2i and 2y), and dechlorination of intermediate 1 would be the major process after prolonging the reaction time. Alternatively, low solubility of some alkene starting materials or alkyl-CF2Cl intermediates in tBuCN might result in moderate yields of some products (i.e., 2ab, 2ad–2ag).
C bond-reduced by-products. Lower conversions accounted for the moderate yields of some substrates (i.e., 2b, 2i and 2y), and dechlorination of intermediate 1 would be the major process after prolonging the reaction time. Alternatively, low solubility of some alkene starting materials or alkyl-CF2Cl intermediates in tBuCN might result in moderate yields of some products (i.e., 2ab, 2ad–2ag).
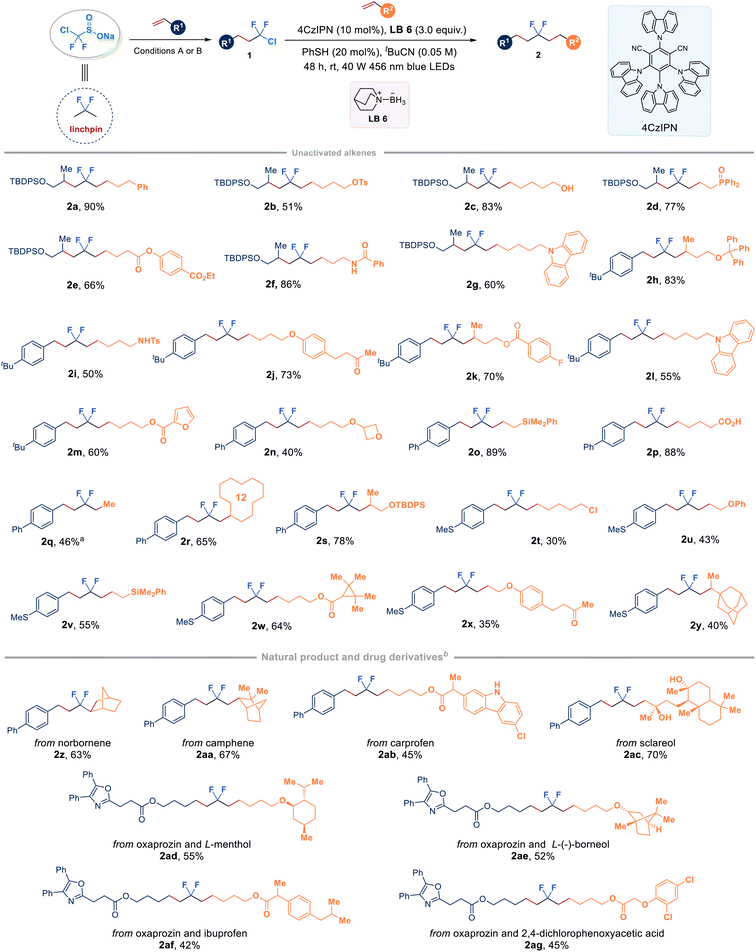 | ||
| Fig. 3 Synthesis of internal gem-difluoro alkanes from two alkenes and ClCF2SO2Na. a1aj (0.2 mmol, 1.0 equiv.), 1 atm balloon of ethylene. b50 equiv. of H2O was added. | ||
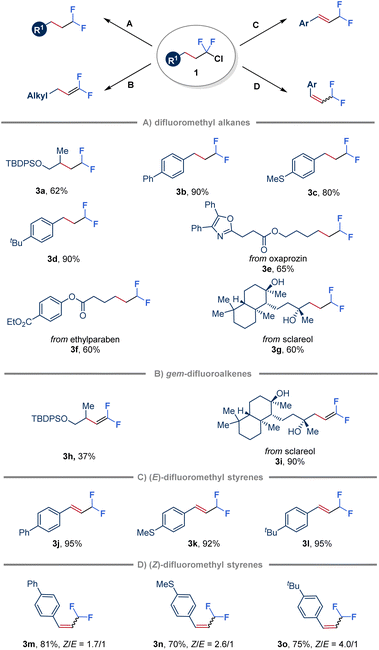
To further demonstrate the synthetic application of the ClCF2–alkane intermediates, various transformations have been explored (Fig. 4). Initially, in the absence of an alkene, hydrodechlorination triggered by quinuclidine-ligated borane (LB 6) was achieved by employing a more sterically hindered hydrogen atom donor, bis(2,4,6-triisopropylphenyl) disulfide (TRIPS)2 (Fig. 4A). Substrates derived from both unactivated alkenes and styrenes were found to be suitable for this transformation (3a–3d). Moreover, ClCF2–alkanes obtained from natural products, such as oxaprozin, ethylparaben and sclareol, were also efficiently converted to the corresponding products through hydrodechlorination with moderate yields (3e–3g). Furthermore, gem-difluoroalkenes (3h and 3i) were obtained in variable yields by simply treating ClCF2–alkane 1 with tBuOK (Fig. 4B).53,54 Notably, ClCF2–alkanes derived from styrenes reacted with tBuOK to give conjugated (E)-β-difluoromethyl styrenes (3j–3l) in high yields attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond migration to more conjugated systems (Fig. 4C). In addition, inspired by recent advances in E to Z photoisomerization of alkenes through an energy transfer process,55 adding Ir(ppy)3 as a triplet photosensitizer in a one-pot two-step manner led to the formation of (Z)-β-difluoromethyl styrenes as the major products (3m–3o) with a Z/E ratio of up to 4
C bond migration to more conjugated systems (Fig. 4C). In addition, inspired by recent advances in E to Z photoisomerization of alkenes through an energy transfer process,55 adding Ir(ppy)3 as a triplet photosensitizer in a one-pot two-step manner led to the formation of (Z)-β-difluoromethyl styrenes as the major products (3m–3o) with a Z/E ratio of up to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 4D).
1 (Fig. 4D).
Mechanistic considerations
In order to gain a better understanding of the C–Cl bond activation mechanism, a series of experiments were conducted (Fig. 5). First, the isolation of byproduct quinuclidine-BH2Cl 4 (confirmed by the X-ray crystallographic analysis) provides support for the proposed key XAT process (Fig. 5a). A radical clock experiment using β-pinene resulted in the formation of the ring-opened product 5 in 57% yield (Fig. 5b). In addition, a radical trapping experiment was also performed using 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), which completely inhibited the formation of the difluoroalkylation product 2a. Instead, the TEMPO-intercepted species 6 was detected by high resolution mass spectrometry (HRMS) (Fig. 5c). These results strongly suggest the involvement of alkyl-CF2 radicals in the reaction process. Furthermore, two parallel reactions were conducted using quinuclidine-BH2Cl and quinuclidine-BD2Cl (about 60% deuterated) with excess deuterium oxide as an additive (Fig. 5d). The results showed that product 3b, with much higher deuterium incorporation, was observed in the latter case (36% vs. 81% deuterium incorporation, respectively). This finding suggests that the hydrogen atom of the CF2H-product was most likely derived from quinuclidine-BH3 through a HAT process. In addition, the very low value of quantum yield (Φ = 0.0013) supports the possibility of an in-cage mechanism (See the ESI† for details). Stern–Volmer quenching experiments indicated that the excited photocatalyst could be quenched more effectively by PhSSPh than by ligated borane or the alkyl-CF2Cl intermediate (See the ESI† for details).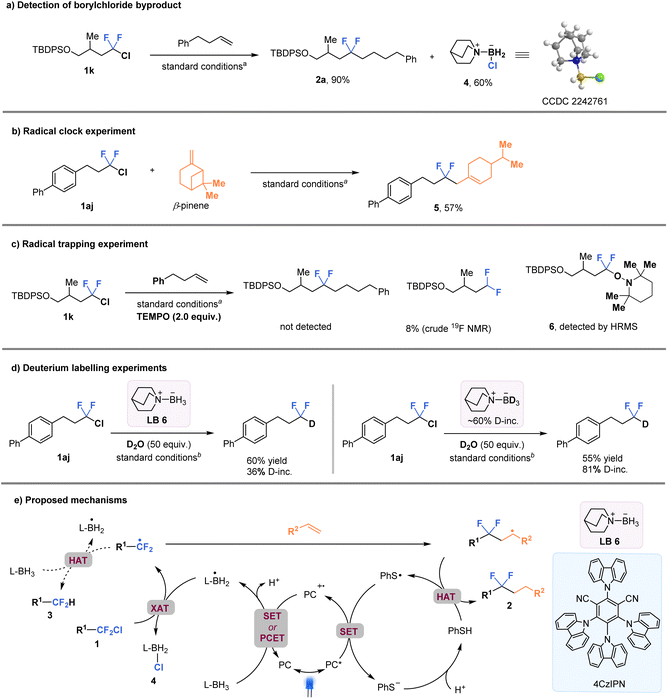 | ||
| Fig. 5 Control experiments for mechanism studies and proposed mechanisms. aStandard conditions in Fig. 3. bStandard conditions in Fig. 4A. | ||
A tentative mechanism is proposed based on all the experimental data and previous literature reports31,37,38 (Fig. 5e). The excited-state photocatalyst [4CzIPN]* [E1/2 (PC˙+/PC*) = −1.18 V vs. SCE in MeCN] is presumed to reduce the aryl thiol radical to thiol anion species [E(PhS˙/PhS−) = 0.16 V vs. SCE]. Then PC˙+ would trigger the formation of the key ligated boryl radical intermediate (L-BH2˙) through either a single electron transfer (SET)/deprotonation sequence or a concerted proton-coupled electron transfer (PCET) process. Subsequently, the nucleophilic boryl radical activates the C–Cl bond of substrate 1 through a XAT process, resulting in the formation of the corresponding CF2-alkyl radical (R1-CF2˙). The R1-CF2˙ undergoes intermolecular radical addition with the alkene substrate, followed by a HAT process with thiophenol, to generate the desired gem-difluoroalkane 2. Alternatively, the CF2-alkyl radical may undergo HAT with amine-borane, leading to the formation of difluoromethyl alkane 3 in the absence of alkenes.
Conclusions
In summary, we have successfully developed a general and widely applicable method for synthesizing diverse difluorinated alkanes and alkenes by leveraging easily accessible ClCF2SO2Na as a practical difluoromethylene linchpin. This strategy involves an organophotoredox-catalysed hydrochlorodifluoromethylation reaction, followed by a tertiary amine-borane-triggered XAT process under blue light irradiation conditions. Our approach offers several advantages, including broad substrate scope, excellent functional group tolerance, metal-free character, mild reaction conditions, and CF2-link-derivatization of complex bioactive alkenes, which demonstrate the potential utilities of this linchpin protocol. Our ongoing research involves merging ligated boryl radicals with transition metal catalysis and exploring its potential applications.Data availability
The ESI† contains method description, product characterization data, NMR spectra, and mechanism study details.Author contributions
Z. Q. Z., J. A. M., F. G. Z. and J. W. conceived and designed the investigations. Z. Q. Z., C. Q. W. and L. J. L. performed the experiments. J. L. P. and Z. H. P. gave the guidance. Z. Q. Z., J. A. M., F. G. Z. and J. W. wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support provided by the National Natural Science Foundation of China (22071170 to J. W., 92156025 to J. A. M., and 22271212 to F. G. Z.) the National Key Research and Development Program of China (2019YFA0905100 to J. A. M.), and Pfizer (A-0004153-00-00 to J. W.).References
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC.
- J.-A. Ma and D. Cahard, Chem. Rev., 2008, 108, PR1–PR43 CrossRef CAS PubMed.
- E. P. Gillis, K. J. Eastman, M. D. Hill, Y. Wang, R. Callejo, A. M. Z. Slawin and D. O'Hagan, Beilstein J. Org. Chem., 2014, 10, 18–25 CrossRef PubMed.
- Y. Zafrani, G. Sod-Moriah, D. Yeffet, A. Berliner, D. Amir, D. Marciano, S. Elias, S. Katalan, N. Ashkenazi, M. Madmon, E. Gershonov and S. Saphier, J. Med. Chem., 2019, 62, 5628–5637 CrossRef CAS PubMed.
- R. Britton, V. Gouverneur, J.-H. Lin, M. Meanwell, C. Ni, G. Pupo, J.-C. Xiao and J. Hu, Contemporary synthetic strategies in organofluorine chemistry, Nat. Rev. Methods Primers, 2021, 1, 47–68 CrossRef CAS.
- F.-L. Qing, X.-Y. Liu, J.-A. Ma, Q. Shen, Q. Song and P. Tang, CCS Chem., 2022, 4, 2518–2549 CrossRef CAS.
- G. S. Lal, G. P. Pez, R. J. Pesaresi, F. M. Prozonic and H. Cheng, J. Org. Chem., 1999, 64, 7048–7054 CrossRef CAS.
- A. L'Heureux, F. Beaulieu, C. Bennett, D. R. Bill, S. Clayton, F. LaFlamme, M. Mirmehrabi, S. Tadayon, D. Tovell and M. Couturier, J. Org. Chem., 2010, 75, 3401–3411 CrossRef PubMed.
- S. C. Sondej and J. A. Katzenellenbogen, J. Org. Chem., 2002, 51, 3508–3513 CrossRef.
- B. Chen and D. A. Vicic, Top. Organomet. Chem., 2014, 52, 113 CrossRef.
- Z. Feng, Y.-L. Xiao and X. Zhang, Acc. Chem. Res., 2018, 51, 2264–2278 CrossRef CAS PubMed.
- D. R. Carvalho and A. H. Christian, Org. Biomol. Chem., 2021, 19, 947–964 RSC.
- J. B. I. Sap, C. F. Meyer, N. J. W. Straathof, N. Iwumene, C. W. am Ende, A. A. Trabanco and V. Gouverneur, Chem. Soc. Rev., 2021, 50, 8214–8247 RSC.
- Q.-Q. Min, Z. Yin, Z. Feng, W.-H. Guo and X. Zhang, J. Am. Chem. Soc., 2014, 136, 1230–1233 CrossRef CAS PubMed.
- S. Ge, S. I. Arlow, M. G. Mormino and J. F. Hartwig, J. Am. Chem. Soc., 2014, 136, 14401–14404 CrossRef CAS PubMed.
- W.-X. Lv, Q. Li, J. Li, Z. Li, E. Lin, D. Tan, Y. Cai, W. Fan and H. Wang, Angew. Chem., Int. Ed., 2018, 57, 16544–16548 CrossRef CAS PubMed.
- H. Wang and N. T. Jui, J. Am. Chem. Soc., 2018, 140, 163–166 CrossRef CAS PubMed.
- S. Kawamura, C. J. Henderson, Y. Aoki, D. Sekine, S. Kobayashi and M. Sodeoka, Chem. Commun., 2018, 54, 11276–11279 RSC.
- X.-S. Hu, J.-X. He, S.-Z. Dong, Q.-H. Zhao, J.-S. Yu and J. Zhou, Nat. Commun., 2020, 11, 5500 CrossRef CAS PubMed.
- S. Akiyama, N. Oyama, T. Endo, K. Kubota and H. Ito, J. Am. Chem. Soc., 2021, 143, 5260–5268 CrossRef CAS PubMed.
- W.-J. Yue, C. S. Day, A. J. B. Rucinski and R. Martin, Org. Lett., 2022, 24, 5109–5114 CrossRef CAS PubMed.
- X. Ren, X. Gao, Q. Min, S. Zhang and X. Zhang, Chem. Sci., 2022, 13, 3454–3460 RSC.
- J. A. Baban, V. P. J. Marti and B. P. Roberts, J. Chem. Soc., Perkin Trans. 2, 1985, 1723–1733 RSC.
- J. A. Baban and B. P. Roberts, J. Chem. Soc., Perkin Trans. 2, 1988, 1195–1200 RSC.
- H.-S. Dang and B. P. Roberts, J. Chem. Soc., Perkin Trans. 1, 1993, 891–898 RSC.
- T. Taniguchi, Eur. J. Org Chem., 2019, 2019, 6308–6319 CrossRef CAS.
- T. Taniguchi, Chem. Soc. Rev., 2021, 50, 8995–9021 RSC.
- L. Capaldo, T. Noël and D. Ravelli, Chem Catal., 2022, 2, 957–966 CrossRef CAS.
- F. Juliá, T. Constantin and D. Leonori, Chem. Rev., 2022, 122, 2292–2352 CrossRef PubMed.
- T.-Y. Peng, F.-L. Zhang and Y.-F. Wang, Acc. Chem. Res., 2023, 56, 169–186 CrossRef CAS PubMed.
- G. Lei, M. Xu, R. Chang, I. Funes-Ardoiz and J. Ye, J. Am. Chem. Soc., 2021, 143, 11251–11261 CrossRef CAS PubMed.
- X. Pan, E. Lacote, J. Lalevée and D. P. Curran, J. Am. Chem. Soc., 2012, 134, 5669–5674 CrossRef CAS PubMed.
- T. Kawamoto, T. Okada, D. P. Curran and I. Ryu, Org. Lett., 2013, 15, 2144–2147 CrossRef CAS PubMed.
- V. I. Supranovich, V. V. Levin, M. I. Struchkova, A. A. Korlyukov and A. D. Dilman, Org. Lett., 2017, 19, 3215–3218 CrossRef CAS PubMed.
- T. Wan, L. Capaldo, D. Ravelli, W. Vitullo, F. J. de Zwart, B. de Bruin and T. Noël, J. Am. Chem. Soc., 2023, 145, 991–999 CrossRef CAS PubMed.
- C.-L. Ji, X. Zhai, Q.-Y. Fang, C. Zhu, J. Han and J. Xie, Chem. Soc. Rev., 2023, 52, 6120–6138 RSC.
- Z.-Q. Zhang, Y.-Q. Sang, C.-Q. Wang, P. Dai, X.-S. Xue, J. L. Piper, Z.-H. Peng, J.-A. Ma, F.-G. Zhang and J. Wu, J. Am. Chem. Soc., 2022, 144, 14288–14296 CrossRef CAS PubMed.
- Q. Zhao, B. Li, X. Zhou, Z. Wang, F.-L. Zhang, Y. Li, X. Zhou, Y. Fu and Y.-F. Wang, J. Am. Chem. Soc., 2022, 144, 15275–15285 CrossRef CAS PubMed.
- J.-L. ClaveL, B. Langlois, R. Nantermet, M. Tordeux and C. Wakselman, J. Chem. Soc., Perkin Trans. 1, 1992, 3371–3375 RSC.
- Y. F. Zhang, R. L. Kirchmeier and J. M. Shreeve, Inorg. Chem., 1992, 31, 492–494 CrossRef CAS.
- D. Meng, L. Li, A. Brown, J.-N. Desrosiers, S. Duan, C. M. Hayward, Z. He, J. Hu, T. Makowski, M. Maloney, S. Monfette, H. Perfect, J. L. Piper, M. Zhou and D. W. Widlicka, Cell Rep. Phys. Sci., 2021, 2, 10349 Search PubMed.
- J. Dong, L. Krasnova, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2014, 53, 9430–9448 CrossRef CAS PubMed.
- X. Wang, M. Yang, S. Ye, Y. Kuang and J. Wu, Chem. Sci., 2021, 12, 6437–6441 RSC.
- D. Filippini and M. Silvi, Nat. Chem., 2022, 14, 66–70 CrossRef CAS PubMed.
- B. Yang, S. Lu, Y. Wang and S. Zhu, Nat. Commun., 2022, 13, 1858 CrossRef CAS PubMed.
- J. Yu, X. Zhang, X. Wu, T. Liu, Z. Zhang, J. Wu and C. Zhu, Chem, 2023, 9, 472–482 CAS.
- Z. Wu, M. Wu, K. Zhu, J. Wu and Y. Lu, Chem, 2023, 9, 978–988 CAS.
- A. Matsumoto, N. I. Maeda and K. Maruoka, J. Am. Chem. Soc., 2023, 145, 20344–20354 CrossRef CAS PubMed.
- M. D. Kosobokov, V. V. Levin, A. A. Zemtsov, M. I. Struchkova, A. A. Korlyukov, D. E. Arkhipov and A. D. Dilman, Org. Lett., 2014, 16, 1438–1441 CrossRef CAS PubMed.
- K. T. O'Brien, J. W. Nadraws and A. B. Smith III, Org. Lett., 2021, 23, 1521–1524 CrossRef PubMed.
- F. Berger, M. B. Plutschack, J. Riegger, W. Yu, S. Speicher, M. Ho, N. Frank and T. Ritter, Nature, 2019, 567, 223–228 CrossRef CAS PubMed.
- D. Louvel, A. Souibgui, A. Taponard, J. Rouillon, M. Mosbah, Y. Moussaoui, G. Pilet, L. Khrouz, C. Monnereau, J. C. Vantourout and A. Tlilia, Adv. Synth. Catal., 2022, 364, 139–148 CrossRef CAS.
- G. Chelucci, Chem. Rev., 2012, 112, 1344–1462 CrossRef CAS PubMed.
- X. Zhang and S. Cao, Tetrahedron Lett., 2017, 58, 375–392 CrossRef CAS.
- T. Neveselý, M. Wienhold, J. J. Molloy and R. Gilmour, Chem. Rev., 2022, 122, 2650–2694 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and NMR spectra of all new compounds. CCDC 2242761. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc03951j |
| This journal is © The Royal Society of Chemistry 2023 |