Open Access Article
Open Access ArticleRegulating the orientation of a single coordinate bond by the synergistic action of mechanical forces and electric field†
Wei
Zhang
a,
Zhibin
Zhao
a,
Min
Tan
a,
Adila
Adijiang
a,
Shurong
Zhong
a,
Xiaona
Xu
a,
Tianran
Zhao
a,
Emusani
Ramya
a,
Lu
Sun
a,
Xueyan
Zhao
a,
Zhiqiang
Fan
 *b and
Dong
Xiang
*b and
Dong
Xiang
 *ac
*ac
aInstitute of Modern Optics, Center of Single Molecule Sciences, Key Laboratory of Micro-scale Optical Information Science and Technology, Nankai University, Tianjin 300350, China
bSchool of Physics and Electronic Science, Changsha University of Science and Technology, Changsha 410114, China
cSchool of Materials Science and Engineering, Smart Sensing Interdisciplinary Science Center, Nankai University, Tianjin 300350, China. E-mail: zqfan@csust.edu.cn; xiangdongde@nankai.edu.cn
First published on 2nd October 2023
Abstract
The molecular binding orientation with respect to the electrode plays a pivotal role in determining the performance of molecular devices. However, accomplishing in situ modulation of single-molecule binding orientation remains a great challenge due to the lack of suitable testing systems and characterization approaches. To this end, by employing a developed STM-BJ technique, we demonstrate that the conductance of pyridine-anchored molecular junctions decreases as the applied voltage increases, which is determined by the repeated formation of thousands of gold–molecule–gold dynamic break junctions. In contrast, the static fixed molecular junctions (the distance between two electrodes is fixed) with identical molecules exhibit a reverse tendency as the bias voltage increases. Supported by flicker noise measurements and theoretical calculations, we provide compelling evidence that the orientation of nitrogen–gold bonds (a universal coordinate bond) in the pyridine-anchored molecular junctions can be manipulated to align with the electric field by the synergistic action of the mechanical stretching force and the electric fields, whereas either stimulus alone cannot achieve the same effect. Our study provides a framework for characterizing and regulating the orientation of a single coordinate bond, offering an approach to control electron transport through single molecular junctions.
Introduction
Modulation of the electron transport properties through molecular junctions at the single-molecule level is crucial to the development of single-molecule devices.1–4 Previous reports have proved that molecule conductance can be effectively regulated by external stimuli, such as gating voltage,5,6 light,7–9 mechanical force,10,11 and temperature.12,13 One impressive feature of the molecular junctions is that the distance between the source and drain electrodes is quite small down to sub-nanometer and thus the electric field can be exceptionally large even when only a small voltage is applied.14,15 By utilizing the intense electric field, molecules can undergo hydrogen tautomerization,16 redox reactions,17 chemical catalysis,18,19 bond rupture,20–23 and conductance switching.24–26 It will be wonderful if we can continuously control in situ the single chemical bond orientation by directly controlling the electric field (bias voltage). However, it has barely been reported primarily due to the absence of suitable testing systems and approaches.Meanwhile, for certain molecules with π-system coupling to electrodes, even a small change in the binding orientation can lead to a substantial alteration in the molecular junction conductance,27 making them an ideal system for investigating molecular binding orientation. Utilizing these specific molecules, we demonstrated that the orientation of a coordinate bond can be regulated by an external electrical field in a dynamic break junction, but cannot be regulated in a fixed junction. Employing the scanning tunneling microscopy break junction (STM-BJ) technique with a developed electrode suspension function, two types of contrasting molecular break junctions were constructed and the conductance evolution upon increased bias was monitored to quantify the change of bond orientation.28,29 The first type consists of pyridine-anchored molecular break junctions, where the π-binding can occur between the aromatic ring and electrode, and thus the conductance of the molecular junction is highly sensitive to the bonding orientation of the anchoring group. As the bias voltage increases, a decrease in the conductance of pyridine-anchored molecular junctions was observed by the repeated formation and breakage of thousands of single-molecule junctions. The second kind consists of amino-anchored molecular break junctions that are devoid of the π-binding between an aromatic ring and electrode. In line with this, we found that at larger bias voltages, the conductance of amino-anchored molecular break junctions essentially remained unaltered. Assisted by flicker noise measurement and density functional theory (DFT) calculation, it is revealed that the conductance change in the pyridine-anchored molecular junction originates from the alteration of coordinate bond orientation regulated by the synergistic action of electric field and mechanical stretching.
To reveal the role of mechanical stretching in regulating the bonding orientation, we established static fixed junctions by halting the movement of the top electrode once a molecule-bridged junction was formed. In contrast to the behavior demonstrated in the dynamic break junctions, the conductance derived from the I–V curves of both the pyridine-anchored and amino-anchored molecular junctions increases as the applied bias voltage increases. These differences indicate that the electric field applied in the experiment alone is insufficient to regulate the orientation of the nitrogen–gold bond. Instead, a synergistic action of mechanical forces and the electric field enables the regulation of coordinate bond orientation.
Results and discussion
Conductance of dynamic molecular break junctions
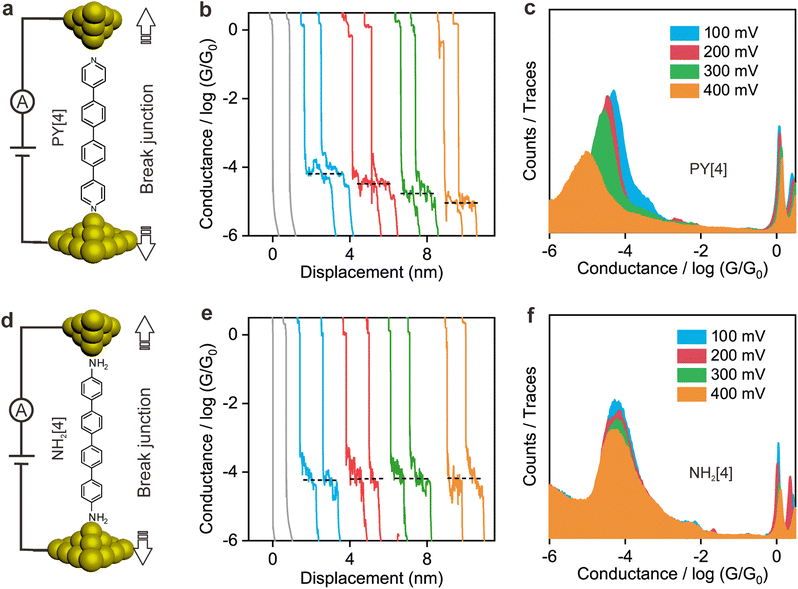
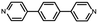
Fig. 1a illustrates the strategy for the conductance measurement of the 4,4′-di(4-pyridyl)biphenyl (labeled as PY[4]) molecular junction using the STM-BJ technique. All the molecules utilized in the experiments were dissolved in 1,2,4-trichlorobenzene (Aladdin, >99%) with a concentration of 0.5 mmol L−1 for electrical characterization. The STM tip for current measurement was prepared by burning one end of a gold wire (250 μm in diameter) with a butane-mixed flame, see ESI Fig. S1 and S2† for detailed information regarding sample preparation. Two types of electrical measurements were performed in the solution environment after the molecules' self-assembling process. One is the conductance measurement based on thousands of dynamic molecule break junctions employing the STM-BJ technique. By controlling the displacement of the STM tip, a molecule-bridged junction can be repeatedly formed via the nitrogen–gold link and broken subsequently. Thousands of conductance–displacement traces were recorded during the stretching process, and were used to construct conductance histograms, which determined the probable conductance of the junctions.Fig. 1b shows typical conductance–displacement traces under different bias voltages. The gray traces show the conductance of a bare gold contact measured in the pure 1,2,4-trichlorobenzene (TCB) solvent. Integer multiples of G0 (G0 = 2e2/h ≈ 77.5 μS) can be observed with no additional conductance plateau below 1 G0. In contrast, the conductance plateaus below 1 G0 were clearly observed when the conductance measurement was performed in PY[4] containing solution. A close examination of the traces reveals that the conductance plateaus shift down as the bias voltage increases. Fig. 1c presents the one-dimensional (1D) conductance histograms of PY[4] molecular junctions at the bias voltages of 100 mV, 200 mV, 300 mV, and 400 mV, respectively. Each of them was constructed from approximately 3000 conductance–displacement traces without data selection. The peak position indicates the probable conductance of the PY[4] molecular junction. As the bias voltage increases from 100 mV to 400 mV, the conductance continuously shifts from 4.5 × 10−5G0 (≈3.51 nS) to 8.6 × 10−6G0 (≈0.66 nS). The corresponding two-dimensional (2D) conductance histogram is shown in ESI Fig. S3,† which confirms that the conductance of molecular junction decreases as the bias voltage increases. The conductance evolution under negative bias exhibits a similar trend, i.e., the conductance of the molecule junction decreases as the absolute value of the bias increases.
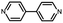
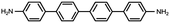
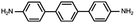
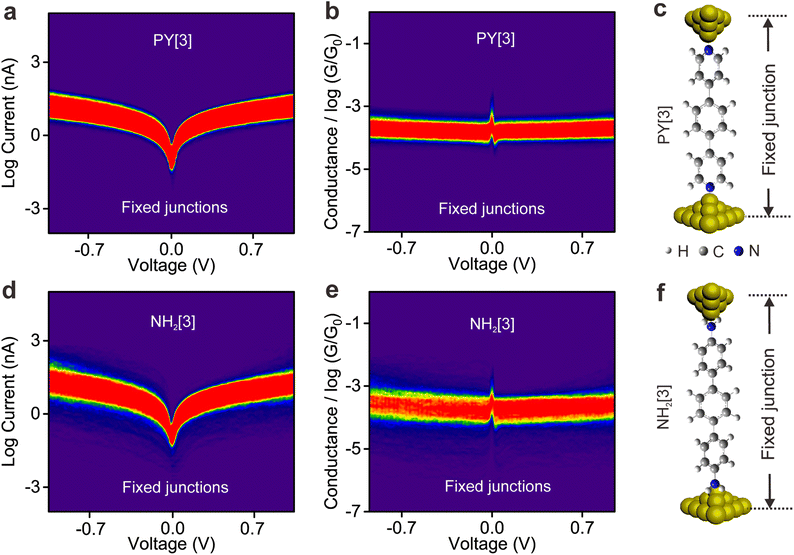
In contrast, for p,p′-diaminoquaterphenyl (labeled as NH2[4]) molecules which possess similar backbone structures to PY[4] but different anchoring groups (i.e., amino group) at both ends, the conductance remains almost unchanged when the applied bias voltage changed from 100 mV to 400 mV (Fig. 1d–f), and the corresponding 2D conductance histograms are shown in ESI Fig. S4.† Similarly, the conductance of the NH2[4] molecule remains constant when the applied negative bias changed from −100 mV to −400 mV. To verify the reliability of the observation that pyridyl-terminated molecules and amino-terminated molecules show discrepant behavior, we investigated a series of molecules with two different anchoring groups, i.e., 1,4-bis(pyrid-4-yl)benzene (labeled PY[3]), 4,4′-bipyridine (labeled as PY[2]), 4,4′′-diamino-p-terphenyl (labeled as NH2[3]), and p,p′-diaminoquaterphenyl (labeled as NH2[2]), respectively.
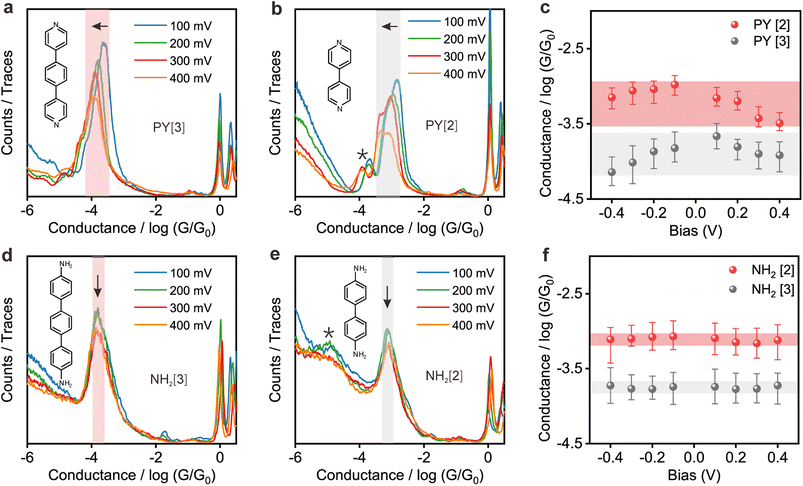
Fig. 2a and b show the 1D conductance histograms of PY[3] and PY[2] molecular junctions, respectively. Similar to PY[4], the conductance of PY[3] and PY[2] molecular junctions decreases when the applied bias voltage increases, as demonstrated by the conductance histograms. On close examination of Fig. 2b, additional minor peaks can be seen that are left-shifted as the bias voltage increases and situated at low conductance locations, as indicated by the star symbol. We assign the small peaks to the new type of molecular junctions (i.e., PY[2] dimer formed via π–π stacking)30–32 rather than the varied configuration of single PY[2] junctions upon mechanical stretching, since the low conductance plateau is parallel to the high conductance plateau (i.e., two conductance plateaus appeared simultaneously rather than in sequence), as shown in ESI Fig. S7.† This claim is further supported by the flicker noise measurement which will be explained in more detail later.
In contrast, as illustrated in Fig. 2d and e, the applied bias voltages do not affect the conductance of NH2[3] and NH2[2] molecular junctions. Further testing revealed that the conductance of the molecular junctions using thiol as an anchoring group did not exhibit bias dependence, see ESI Fig. S9.† These results confirm that the bias-dependent molecular conductance is strongly affected by the terminal anchoring groups of the molecules. Notably, minor peaks, as marked by the star symbol, can also be observed in the conductance histogram for NH2[2] molecular junctions, see Fig. 2e. We attribute the small peak to the NH2[2] dimer formation via an intermolecular hydrogen bond.9,33 The individual chemical structures of the molecules and the corresponding conductance under positive bias are summarized in Table 1. Accordingly, the probable conductances of different types of molecular junctions under negative bias are summarized in ESI Table S1.†
More intuitive analysis was carried out to investigate the bias voltage influence on the conductance of the molecules with different anchoring groups. Fig. 2c demonstrates a negative correlation between the conductance of pyridine-anchored molecules and the applied bias voltage, that is, the conductance of molecular junction drops as the absolute magnitude of the bias voltage increases. In contrast, the conductance of the amino-anchored molecules remains relatively constant, and is almost independent of the bias voltage, as shown in Fig. 2f. As an initial conjecture, the distinct behavior of these two types of molecules originates from either the energy structural changes as the molecule is exposed to an external electric field34,35 or the distinguished conformational changes (e.g., rotation of aromatic rings) regulated by the bias voltage.13 However, this speculation was ruled out by I–V measurements of the fixed molecular junctions, which demonstrates that the two types of molecular junctions exhibit the same behavior upon voltage sweeping, as shown below.
Conductance of static fixed molecular junctions
To elucidate the underlying mechanism for the distinct behavior of two types of molecules, we performed I–V measurement based on the fixed molecular junctions. To perform the I–V measurement, the movement of the top electrode was paused automatically via a feedback system and the molecular junction was allowed to remain fixed for a while (referred to as the electrode-suspension time) once a molecule-bridged junction was formed, which can be judged by the detected conductance. Subsequently, the voltage sweeping mode was triggered, and the corresponding I–V curves were recorded and used to plot the I–V density image, see ESI Fig. S10† for detailed information.Fig. 3a and b illustrate the 2D logarithmic I–V and conductance–voltage (G–V) density plotting, which was constructed from approximately 3000 individual I–V curves of PY[3] molecular junctions. In contrast to earlier findings shown in dynamic break junctions (Fig. 2c), the G–V plot shows that the conductance increases with an increasing bias voltage (Fig. 3b), and Fig. 3c shows the schematic of the fixed PY[3] molecular junctions. For comparison, we also plotted the I–V and G–V density images of NH2[3] molecular junctions, as shown in Fig. 3d–f. It is evident that NH2[3] molecular junctions displayed an increased conductance with increasing bias voltage as in the case of PY[3]. Additional I–V measurement shows that the conductance of molecular fixed junctions for NH2[2] and NH2[4] also increases upon an increased bias voltage. This behavior is anticipated because the electrode's Fermi level typically approaches the molecular orbital (HOMO or LUMO) when a higher bias voltage is applied, leading to a lower energy barrier and higher conductance,34,36 see ESI Fig. S11.†
The interesting observation is that the conductance of the pyridine-terminated molecular break junction decreased with increasing applied bias voltage, but the conductance of static fixed junction shows the opposite trend. Notably, the molecule will be exposed to a similar electric field in both break junctions and fixed junctions as the same bias is applied, indicating that the distinct behaviors observed in the two types of junctions do not solely originate from the applied bias voltage. Also, the opposite behavior displayed by two types of junctions effectively eliminates the possibility that the rotation of aromatic ring modulated by the electric field results in conductance changes. Instead, we hypothesize that the distinct behavior of break junctions and fixed junctions originates from the properties of the anchoring group that are strongly influenced by the synergistic action of the electric field and mechanical forces.
Bias dependence of flicker noise spectroscopy
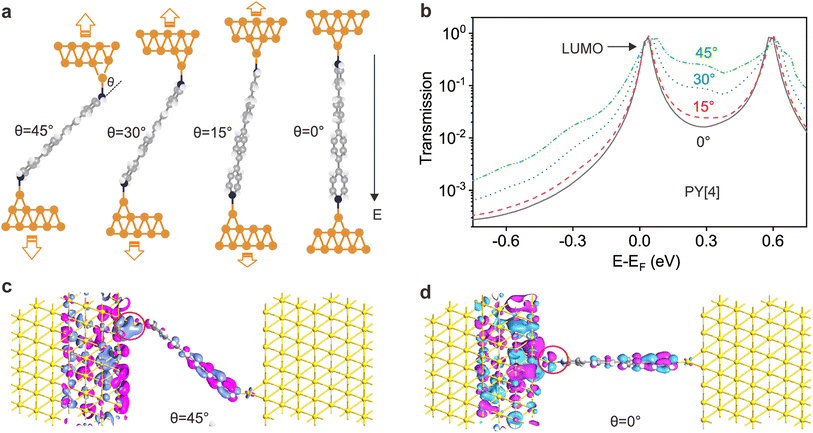
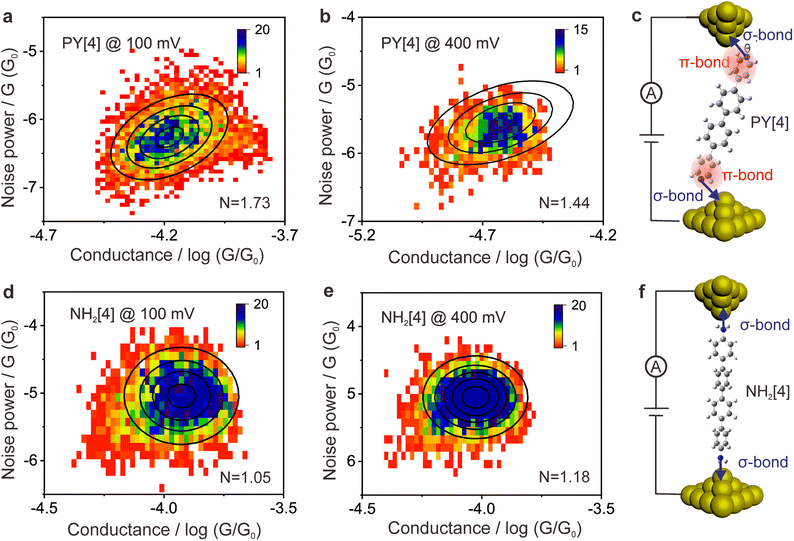
The ability to differentiate between through-space or through-bond electron transport pathways using the flicker noise spectrum has been established.37,38 In particular, noise power scaling as GN (N = 2.0) refers to electron transport through space while noise power scaling as G1.0 corresponds to the through-bond. Here, G represents the average conductance of each junction. Meanwhile, the electron transport channel significantly correlated with the contact geometry,37,38 and thus the flicker noise spectrum can be used to judge the molecule bonding geometry. The flicker noise measurements were then performed on two different kinds of molecular junctions, see ESI Fig. S12† for further information. Fig. 4a and b show 2D histograms of the normalized noise power plotted against the average conductance of PY[4] molecular junctions. The noise power scales as G1.73 at a bias voltage of 100 mV (Fig. 4a) and scales as G1.44 at 400 mV (Fig. 4b), which indicates that electron transport through a bond predominates over that through space in PY[4] molecular junctions under a higher bias voltage. In contrast, the noise power scales as G1.05 at a bias voltage of 100 mV and almost retains the same at a bias voltage of 400 mV for NH2[4] molecular junctions (Fig. 4d and e). The exponent remains unchanged (N ≈ 1) at both low and high voltages, indicating that the electron transport channel is almost unchanged, i.e., the electrons mainly transport through a bond for NH2[4] molecular break junctions at both low and high bias voltages.For pyridine-anchored molecules, the pyridyl can couple with a gold electrode through both the σ bond of its N atom (nitrogen–gold, σ-binding) and the π-bond of its pyridine ring (π-binding),27,39–41 as schematically shown in Fig. 4c. For the π-binding configuration, the electron is mainly transported through space between the pyridine ring and electrode, which can be seen as a weak coupling compared to the one of σ bond.26,27 The electronic coupling between the molecular π*-electron and the gold d-electrons decreases as θ (the angle between the nitrogen–gold bond and the plane of the pyridine ring) decreases.42 The π-coupling is particularly strongest when the nitrogen–gold bond is perpendicular to the pyridine ring (i.e., the angle θ = 90°), and π-coupling is weakest when it is parallel to the pyridine plane (i.e., θ = 0°). The decrease of noise power (N) upon a higher bias voltage (Fig. 4a and b) indicates that the electron transport channel through space (π-coupling) is suppressed. In other words, the decrease of noise power indicates that the nitrogen–gold bond gradually aligns parallel to the direction of the pyridine plane (the same as that of the electric field) upon a higher bias in the dynamical break junctions.
In contrast, for the NH2[4] molecular junction, the donor–acceptor bond (σ-binding) is primarily formed between the electrode and the anchoring group,42,43 as schematically presented in Fig. 4f. Due to the lack of π-binding, the electron will primarily be transported through a bond, which results in N ≈ 1 and it remains unaltered upon a higher bias, as presented in Fig. 4d and e. The absence of π-coupling in NH2[4] molecular junctions can be attributed to two possible factors: (1) compared to PY[4], the aromatic ring in NH2[4] is far away from the electrodes by a C–N distance and thus the π-binding between the aromatic ring and the electrode is relatively weak;27 (2) the amine–gold link is naturally parallel to the plane of the pyridine ring,44 and this configuration makes the aromatic ring distant from the electrode and causes a weak π-coupling for the NH2[4] molecular junction.
Flicker noise spectrum of the PY[2] molecular junction at a low conductance plateau was also carried out, as we mentioned previously. The 2D histogram of the normalized noise power versus the average conductance of the molecular junction is displayed in ESI Fig. S13.† It can be found that N = 1.95, which indicates that the electron is transported across space rather than through a bond at this state. Combining the observation that the probable low conductance is one order of magnitude smaller than the probable high conductance (Fig. 2b) and the feature of the 2D conductance histogram (Fig. S7†), we believe that the minor peak with low conductance results from the PY [2] dimer formation via π–π stacking rather than a single PY[2] molecule with a perpendicular bonding configuration at the interface.
Theoretical calculation
To confirm the proposed mechanism, the transmission of the molecular junctions with different tilt angles was computed based on density functional theory combined with non-equilibrium Green's function. Fig. 5a shows the schematic of molecular junctions with varied tilt angles between the nitrogen–gold bond and pyridine plane, and the correspondingly computed transmission spectra are shown in Fig. 5b. It reveals that the lowest unoccupied molecular π*-orbital (LUMO) is close to the Fermi energy level (EF), indicating that the LUMO predominantly contributes to the conductance of the PY[4] molecular junction. Fig. 5b demonstrates that the transmission peak broadens as the tilt angle (θ) increases. This feature signifies that the strength of the electronic coupling between the gold electrode and the molecular π*-orbital is enhanced when the nitrogen–gold bond tilts out of the pyridine plane, which supports our conjecture, i.e., change in the orientation of the nitrogen–gold bond will result in the alternation of molecular junction conductance.Moreover, we can find that the whole transmission coefficient decreases as the tilt angle decreases from 45° to 0°, which indicates that the conductance of the junction will decrease as the orientation of the nitrogen–gold bond changes from being out of the pyridine plane (θ = 45°) to being parallel to the pyridine plane (θ = 0°). This finding is in line with the calculated results of the electron transport pathway, which demonstrates that the volumes of electron transport through the nitrogen–gold bonds decrease when the tilt angle decreases, see ESI Fig. S14.† Transmission eigenstates at 0.3 eV for the PY[4] molecular junction at the tilt angle of 45° and 0° are shown in Fig. 5c and d. It is evident that the spatial distributions of transmission eigenstates at the interface become localized when the tilt angle changes from 45° to 0°, which will result in a decreased conductance. In contrast, for the NH2[4] molecular junction, both HOMO and LUMO are far from the Fermi level of the electrode and the wave function at the Fermi level is insensitive to the tilt angle of the molecule compared to PY[4], see ESI Fig. S15.†
We further calculated the electrostatic potential distribution of the molecules with different anchoring groups, see ESI Fig. S16.† It shows that the electrostatic potential around the amino group is positively charged, while the nitrogen atom of the pyridyl group is negatively charged. When the molecule comes in contact with the electrode, a coordination bond will be formed between the anchoring group and the electrode. Under a fixed electric field, the direction of the force undertaken by the amino group and the pyridyl group should be opposite. In that case the external field exerts a pushing force on the pyridyl-based bond (N–Au) and makes the bond tilt angle θ decrease (N–Au gradually parallel to the pyridyl plane) with the assistance of mechanical stretching; the amino-based bond will not experience such a pushing force and thus will not promote the decrease of θ. The change of bond tilt angle will lead to the pronounced variation of the molecular junction conductance, as demonstrated in Fig. 5 in the main text. Thus, the different behaviors of amino-terminated and pyridyl-terminated molecule junctions under the joint action of the electric field and mechanical stretching can also be explained by the distribution of electrostatic potential.
Therefore, the theoretical model calculations, electrostatic potential calculations as well as the measured noise spectra strongly support our hypothesis that the pronounced conductance change of the pyridine-anchored molecular junction highly likely results from the orientation change of the nitrogen–gold bond. With the assistance of mechanical stretching, the orientation of the nitrogen–gold bond gradually aligns with the direction of the increasing electric field. This is anticipated because the nitrogen–gold bond is a coordinate bond, where the electrons for the bond formation are solely supplied by the N atom (so-called semi-polar bond) and is highly sensitive to the direction of the external electric field.21 This bond orientation change reduces the coupling strength by suppressing the π coupling channel between the pyridine ring and electrode. The orientation change of the nitrogen–gold bonds with increasing bias not only makes the molecular coupling strength weaker in line with the decreased conductance, but also gives rise to the following features: (1) the length of the conductance plateau decreases, since a weak coupling facilitates the breakage of the junction and thus makes the lifetime of the junction short, which agrees well with the experimental observation as presented in ESI Fig. S2–S7;† and (2) the intensity of the peak in the conductance histogram decreases due to the decreased lifetime of the junction and decreased length of the conductance plateau, which is consistent with the feature seen in Fig. 1c and 2a, b.
We would like to emphasize that (1) the electric field activation alone does not result in a change in the orientation of the nitrogen–gold bond. This claim is supported by the I–V test, which shows that in a static fixed junction, the conductance decrease is not observed when the electric field is increased during the voltage sweeping process (Fig. 3); (2) the sole activation of mechanical stretching cannot trigger the change of nitrogen–gold orientation in a pyridyl-anchored molecular junction either. If mechanical stretching alone has the capability to alter the nitrogen–gold orientation, the conductance of the junction would no longer depend on the bias voltage; (3) only the synergistic action of dynamical mechanical stretching and electric field activation can regulate the orientation of the pyridine–gold link in the coordinate bond terminated molecular break junction.
As a discussion, although the conductance of the molecular junctions using thiol and amino as anchoring groups does not exhibit bias dependence, we cannot draw the conclusion that the orientation of the S–Au or amino–Au bond can't be modulated by the synergistic action of mechanical forces and electric field, because the π-coupling was absent in these molecular junctions and the orientational change of the S–Au/N–Au bond might not result in a notable conductance variation. Our additional calculations show that the binding energies of PY[4] and NH2[4] on the electrode surface are approximately 1.604 eV and 0.509 eV, respectively. The diverse binding energy may result in different behaviors of amino and pyridyl-anchored molecular junctions as well. For the amino-anchored molecular junction with weak bonding strength, the orientation of the N–Au bond may be modulated by the sole mechanical stretching already, resulting in the observation that the conductance does not depend on the bias voltage any more. To address whether it is feasible to regulate the orientation of amino–Au and S–Au bond by the same strategy, additional experiments are highly desired.
Conclusion
In summary, we investigated the influence of electric field strength and mechanical stretching on the bonding orientation of two types of molecular junctions: dynamic break junctions and static fixed junctions. Employing the single-molecule break junction technique, we observed a decrease in the conductance of pyridine-anchored molecular junctions as the bias voltage increases. In contrast, the conductance of the static fixed molecular junctions with identical molecules increases as the bias voltage increases by utilizing the electrode suspension function of the device. This opposite behavior in the two types of junctions indicates that the conductance of the junction is modulated by the synergistic interplay of mechanical stretching and electric field. With the assistance of flicker noise spectra measurements and DFT-based calculations, we revealed that the electric field, coupled with the mechanical stretching, can regulate the orientation of the pyridine–gold bond to align with the direction of the increased electric field which reduces the coupling strength between the pyridine π*-system and electrode, ultimately resulting in a pronounced decrease in conductance. In contrast, this phenomenon was not observed in amino-anchored and thiol-anchored molecular junctions due to the absence of π-coupling. Our study provides a framework for characterizing and regulating the orientation of a single chemical bond in single molecular junctions. This approach shows promise for achieving precise control over the behavior of single molecules, facilitating the modulation of electron transport across single-molecule junctions and opening up a path to the realization of molecular functional devices.Data availability
Further details of the experimental procedure, fabrication of the STM tip and substrate, conductance of single-molecule break junctions under different biases, I–V measurement of fixed molecular junctions, flicker noise power spectroscopy, DFT calculation of electron transport through molecular junctions and molecular electrostatic potential distribution are available in the ESI.†Author contributions
D. X., initiated the project. W. Z., performed electrical measurement and carried out the electrostatic potential calculation. Z. Z., performed AFM measurement. Z. F., performed DFT calculations. W. Z. and D. X., wrote the manuscript with contributions from all authors.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
We acknowledge the financial support from the National Key R&D Program of China (2021YFA1200103), the National Natural Science Foundation of China (22273041, 91950116, 11804170), and the Natural Science Foundation of Tianjin (19JCZDJC31000, 19JCJQJC60900, 22JCYBJC01310).Notes and references
- S. V. Aradhya and L. Venkataraman, Single-molecule junctions beyond electronic transport, Nat. Nanotechnol., 2013, 8, 399–410 CrossRef CAS PubMed
.
- C. Jia, A. Migliore, N. Xin, S. Huang, J. Wang, Q. Yang, S. Wang, H. Chen, D. Wang, B. Feng, Z. Liu, G. Zhang, D. H. Qu, H. Tian, M. A. Ratner, H. Q. Xu, A. Nitzan and X. Guo, Covalently bonded single-molecule junctions with stable and reversible photoswitched conductivity, Science, 2016, 352, 1443–1445 CrossRef CAS PubMed
.
- D. Xiang, X. Wang, C. Jia, T. Lee and X. Guo, Molecular-Scale Electronics: From Concept to Function, Chem. Rev., 2016, 116, 4318–4440 CrossRef CAS PubMed
.
- M. Wang, T. Wang, O. S. Ojambati, T. J. Duffin, K. Kang, T. Lee, E. Scheer, D. Xiang and C. A. Nijhuis, Plasmonic phenomena in molecular junctions: principles and applications, Nat. Rev. Chem., 2022, 6, 681–704 CrossRef PubMed
.
- M. L. Perrin, E. Burzurí and H. S. van der Zant, Single-molecule transistors, Chem. Soc. Rev., 2015, 44, 902–919 RSC
.
- D. Xiang, H. Jeong, D. Kim, T. Lee, Y. Cheng, Q. Wang and D. Mayer, Three-Terminal Single-Molecule Junctions Formed by Mechanically Controllable Break Junctions with Side Gating, Nano Lett., 2013, 13, 2809–2813 CrossRef CAS PubMed
.
- L. Chen, A. Feng, M. Wang, J. Liu, W. Hong, X. Guo and D. Xiang, Towards single-molecule optoelectronic devices, Sci. China: Chem., 2018, 61, 1368–1384 CrossRef CAS
.
- A. Giguère, M. Ernzerhof and D. Mayou, Surface Plasmon Polariton-Controlled Molecular Switch, J. Phys. Chem. C, 2018, 122, 20083–20089 CrossRef
.
- Z. Zhao, C. Guo, L. Ni, X. Zhao, S. Zhang and D. Xiang, In situ photoconductivity measurements of imidazole in optical fiber break-junctions, Nanoscale Horiz., 2021, 6, 386–392 RSC
.
- C. Bruot, J. Hihath and N. Tao, Mechanically controlled molecular orbital alignment in single molecule junctions, Nat. Nanotechnol., 2012, 7, 35–40 CrossRef CAS PubMed
.
- I. Franco, C. B. George, G. C. Solomon, G. C. Schatz and M. A. Ratner, Mechanically Activated Molecular Switch through Single-Molecule Pulling, J. Am. Chem. Soc., 2011, 133, 2242–2249 CrossRef CAS PubMed
.
- M. Kamenetska, J. R. Widawsky, M. Dell'Angela, M. Frei and L. Venkataraman, Temperature dependent tunneling conductance of single molecule junctions, J. Chem. Phys., 2017, 146, 092311 CrossRef
.
- W. Lee, S. Louie, A. M. Evans, N. M. Orchanian, I. B. Stone, B. Zhang, Y. Wei, X. Roy, C. Nuckolls and L. Venkataraman, Increased Molecular Conductance in Oligo[n]phenylene Wires by Thermally Enhanced Dihedral Planarization, Nano Lett., 2022, 22, 4919–4924 CrossRef CAS PubMed
.
- D. Miura, K. Iwata, S. Kurokawa and A. Sakai, Break Voltage of the 1G0 Contact of Noble Metals and Alloys, e-J. Surf. Sci. Nanotechnol., 2009, 7, 891–897 CrossRef CAS
.
- H. Song, M. A. Reed and T. Lee, Single Molecule Electronic Devices, Adv. Mater., 2011, 23, 1583–1608 CrossRef CAS PubMed
.
- P. Liljeroth, J. Repp and G. A. Meyer, Current-Induced Hydrogen Tautomerization and Conductance Switching of Naphthalocyanine Molecules, Science, 2007, 317, 1203–1206 CrossRef CAS PubMed
.
- S. Shaik, D. Mandal and R. Ramanan, Oriented electric fields as future smart reagents in chemistry, Nat. Chem., 2016, 8, 1091–1098 CrossRef CAS PubMed
.
- A. Zhang, X. Zhuang, J. Liu, J. Huang, L. Lin, Y. Tang, S. Zhao, R. Li, B. Wang, B. Fang and W. Hong, Catalytic cycle of formate dehydrogenase captured by single-molecule conductance, Nat. Catal., 2023, 6, 266–275 CrossRef CAS
.
- S. Shaik, D. Danovich, J. Joy, Z. Wang and T. Stuyver, Electric-Field Mediated Chemistry: Uncovering and Exploiting the Potential of (Oriented) Electric Fields to Exert Chemical Catalysis and Reaction Control, J. Am. Chem. Soc., 2020, 142, 12551–12562 CrossRef CAS PubMed
.
- H. Li, T. A. Su, V. Zhang, M. L. Steigerwald, C. Nuckolls and L. Venkataraman, Electric field breakdown in single molecule junctions, J. Am. Chem. Soc., 2015, 137, 5028–5033 CrossRef CAS PubMed
.
- M. Omidian, S. Leitherer, N. Néel, M. Brandbyge and J. Kröger, Electric-Field Control of a Single-Atom Polar Bond, Phys. Rev. Lett., 2021, 126, 216801 CrossRef CAS PubMed
.
- J. Lin, Y. Lv, K. Song, X. Song, H. Zang, P. Du, Y. Zang and D. Zhu, Cleavage of non-polar C(sp2)–C(sp2) bonds in cycloparaphenylenes via electric field-catalyzed electrophilic aromatic substitution, Nat. Commun., 2023, 14, 293 CrossRef CAS
.
- B. Zhang, C. Schaack, C. R. Prindle, E. A. Vo, M. Aziz, M. L. Steigerwald, T. C. Berkelbach, C. Nuckolls and L. Venkataraman, Electric fields drive bond homolysis, Chem. Sci., 2023, 14, 1769–1774 RSC
.
- G. Mezei, Z. Balogh, A. Magyarkuti and A. Halbritter, Voltage-Controlled Binary Conductance Switching in Gold-4,4′-Bipyridine-Gold Single-Molecule Nanowires, J. Phys. Chem. Lett., 2020, 11, 8053–8059 CrossRef CAS
.
- L. Tučková, A. Jaroš, C. Foroutan-Nejad and M. Straka, A quest for ideal electric field-driven MX@C70 endohedral fullerene memristors: which MX fits the best?, Phys. Chem. Chem. Phys., 2023, 25, 14245–14256 RSC
.
- Y. Wei, L. Li, J. E. Greenwald and L. Venkataraman, Voltage-Modulated van der Waals Interaction in Single-Molecule Junctions, Nano Lett., 2023, 23, 567–572 CrossRef CAS PubMed
.
- Z. Yu, Y.-X. Xu, J.-Q. Su, P. M. Radjenovic, Y.-H. Wang, J.-F. Zheng, B. Teng, Y. Shao, X.-S. Zhou and J.-F. Li, Probing Interfacial Electronic Effects on Single-Molecule Adsorption Geometry and Electron Transport at Atomically Flat Surfaces, Angew. Chem., Int. Ed., 2021, 60, 15452–15458 CrossRef CAS
.
- H. Ohnishi, Y. Kondo and K. Takayanagi, Quantized conductance through individual rows of suspended gold atoms, Nature, 1998, 395, 780–783 CrossRef CAS
.
- B. Xu and N. J. Tao, Measurement of single-molecule resistance by repeated formation of molecular junctions, Science, 2003, 301, 1221–1223 CrossRef CAS
.
- Y. Tang, Y. Zhou, D. Zhou, Y. Chen, Z. Xiao, J. Shi, J. Liu and W. Hong, Electric Field-Induced Assembly in Single-Stacking Terphenyl Junctions, J. Am. Chem. Soc., 2020, 142, 19101–19109 CrossRef CAS PubMed
.
- R. Frisenda, V. A. E. C. Janssen, F. C. Grozema, H. S. J. van der Zant and N. Renaud, Mechanically controlled quantum interference in individual π-stacked dimers, Nat. Chem., 2016, 8, 1099–1104 CrossRef CAS PubMed
.
- S. Wu, M. T. González, R. Huber, S. Grunder, M. Mayor, C. Schönenberger and M. Calame, Molecular junctions based on aromatic coupling, Nat. Nanotechnol., 2008, 3, 569–574 CrossRef CAS PubMed
.
- X. Zhao, X. Zhang, K. Yin, S. Zhang, Z. Zhao, M. Tan, X. Xu, Z. Zhao, M. Wang, B. Xu, T. Lee, E. Scheer and D. Xiang, In Situ Adjustable Nanogaps and In-Plane Break Junctions, Small Methods, 2023, 7, 2201427 CrossRef CAS PubMed
.
- C. Guo, X. Chen, S.-Y. Ding, D. Mayer, Q. Wang, Z. Zhao, L. Ni, H. Liu, T. Lee and B. Xu, Molecular orbital gating surface-enhanced Raman scattering, ACS Nano, 2018, 12, 11229–11235 CrossRef CAS PubMed
.
- S.-X. Li, D.-L. Chen, Z.-P. Zhang, Z.-W. Long and S.-J. Qin, Study on the ground state properties and excitation properties of C18 under different external electric fields, Acta Phys. Sin., 2020, 69, 103101 CrossRef
.
- Z. Zhao, W. Wang, X. Zhou, L. Ni, K. Kang, T. Lee, H. Han, H. Yuan, C. Guo and M. Wang, Crystal Size Effect on Carrier Transport of Microscale Perovskite Junctions via Soft Contact, Nano Lett., 2020, 20, 8640–8646 CrossRef CAS PubMed
.
- O. Adak, E. Rosenthal, J. Meisner, E. F. Andrade, A. N. Pasupathy, C. Nuckolls, M. S. Hybertsen and L. Venkataraman, Flicker Noise as a Probe of Electronic Interaction at Metal–Single Molecule Interfaces, Nano Lett., 2015, 15, 4143–4149 CrossRef CAS PubMed
.
- J. Li, Z. Zhuang, P. Shen, S. Song, B. Z. Tang and Z. Zhao, Achieving Multiple Quantum-Interfered States via Through-Space and Through-Bond Synergistic Effect in Foldamer-Based Single-Molecule Junctions, J. Am. Chem. Soc., 2022, 144, 8073–8083 CrossRef CAS PubMed
.
- T. Dadosh, Y. Gordin, R. Krahne, I. Khivrich, D. Mahalu, V. Frydman, J. Sperling, A. Yacoby and I. Bar-Joseph, Measurement of the conductance of single conjugated molecules, Nature, 2005, 436, 677–680 CrossRef CAS PubMed
.
- C. A. Martin, D. Ding, H. S. J. van der Zant and J. M. v. Ruitenbeek, Lithographic mechanical break junctions for single-molecule measurements in vacuum: possibilities and limitations, New J. Phys., 2008, 10, 065008 CrossRef
.
- S. T. Schneebeli, M. Kamenetska, Z. Cheng, R. Skouta, R. A. Friesner, L. Venkataraman and R. Breslow, Single-molecule conductance through multiple π-π-stacked benzene rings determined with direct electrode-to-benzene ring connections, J. Am. Chem. Soc., 2011, 133, 2136–2139 CrossRef CAS PubMed
.
- S. Quek, M. Kamenetska, M. Steigerwald, H. Choi, S. Louie, M. Hybertsen, J. Neaton and L. Venkataraman, Mechanically controlled binary conductance switching of a single-molecule junction, Nat. Nanotechnol., 2009, 4, 230–234 CrossRef CAS PubMed
.
- M. S. Hybertsen, L. Venkataraman, J. E. Klare, A. C. Whalley, M. L. Steigerwald and C. Nuckolls, Amine-linked single-molecule circuits: systematic trends across molecular families, J. Phys.: Condens. Matter, 2008, 20, 374115 CrossRef PubMed
.
- S. Y. Quek, L. Venkataraman, H. J. Choi, S. G. Louie, M. S. Hybertsen and J. B. Neaton, Amine-gold linked single-molecule circuits: experiment and theory, Nano Lett., 2007, 7, 3477–3482 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc03892k |
| This journal is © The Royal Society of Chemistry 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 conductance–time traces and correspondingly a discrete Fourier transform.
000 conductance–time traces and correspondingly a discrete Fourier transform.