Open Access Article
Open Access ArticleEnantioselective [3+2]-cycloaddition of 2,3-disubstituted cyclobutenones: vicinal quaternary stereocenters construction and skeletal functionalization†
Licheng
Lu
and
Ping
Lu
 *
*
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai 200433, P. R. China. E-mail: plu@fudan.edu.cn
First published on 15th July 2023
Abstract
Cycloaddition is a fundamental transformation, featuring the assembly of complex cyclic molecules with multiple stereocenters. We report here a silver-catalyzed [3+2]-cycloaddition of 2,3-disubstituted cyclobutenones with an array of azomethine ylide precursors iminoesters, furnishing azabicycles in good yields and enantioselectivities. Up to three contiguous all-carbon quaternary centers, including two angular stereocenters, could be constructed efficiently, due to high reactivity of strained cyclobutenones. Subsequent skeletal remodeling provided versatile molecules with distinct structural characters.
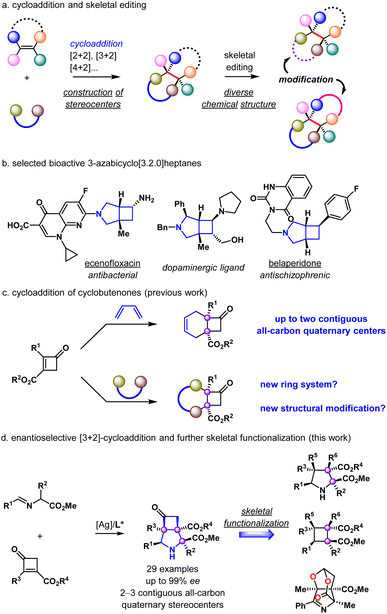
Introduction
Stereoselective organic synthesis is a continuously evolving field in the organic community, and substantial progress has been achieved with the development of creative and elegant methodologies. As a fundamental organic reaction, cycloaddition features a powerful construction of complex cyclic molecules with multiple stereocenters (Scheme 1a).1,2 However, the efficient enantioselective construction of the all-carbon quaternary stereocenters is still synthetically challenging due to inherent steric and conformational demands encountered in the preparation.3–5 On the other hand, skeletal functionalization, especially skeletal editing has emerged as an appealing approach to accessing new chemical space.6–12 In this context, 3-azabicyclo[3.2.0]heptane derivatives have garnered our interest. These derivatives have been used as bioisosteres of piperazine in the design of biologically active compounds (Scheme 1b).13,14 We envisioned that an enantioselective construction of such a bicyclic ring system would create vicinal stereocenters and provide a potentially valuable platform for skeletal modification.Cyclobutenones have been used as highly reactive dienophiles due to their inherent ring strain.15 Enantioselective functionalization of performed four-membered ring substrates has become an efficient strategy to synthesize enantioenriched cyclobutane derivatives.16–24 Cyclobutenones have been utilized as dienophiles in the enantioselective Diels–Alder reaction and natural product synthesis (Scheme 1c).25,26 We postulated that new ring systems could be prepared with versatile cycloaddition partners and the corresponding adducts would offer a new entry for structural modifications. 1,3-Dipolar cycloaddition has demonstrated its powerful utility in organic chemistry.27,28 We envisioned that a [3+2]-cycloaddition of 2,3-disubstituted cyclobutenones with azomethine ylide precursors iminoesters would afford the 3-azabicyclo[3.2.0]heptane derivatives containing two angular quaternary centers in a stereocontrolled manner (Scheme 1d). The regioselective ring-opening reaction of cyclobutanones could provide densely substituted pyrrolidines,29,30 and cyclobutanes could be subsequently obtained employing the nitrogen deletion method.31 Similarly, the ring-opening reaction of 3-azabicyclo[3.2.0]heptanes would generate acyclic dienes via simultaneous openings of both pyrrolidine and cyclobutane rings. Herein, we report our work on these speculations, benefiting from the enhanced reactivity of strained cyclobutenones.
Results and discussion
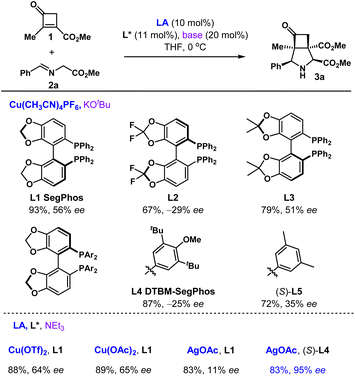
We started our studies with the reaction of 2-methyl-3-methoxycabonylcyclobutenone 1 and methyl (E)-2-(benzylideneamino)acetate 2a (Table 1). Inspired by the work of Carretero,32 the copper catalytic system was investigated. After an initial evaluation of chiral ligands (see Table S1† for full details), the copper-catalysed cycloaddition afforded the desired 3-azabicyclo[3.2.0]heptane 3a in 93% yield and 56% ee using (S)-Segphos (L1) as a ligand and t-BuOK as a base. Further optimization of Segphos-type ligands (L2–L5) gave no improvement. The enantioselectivity was slightly enhanced using mild base NEt3, and cyclobutanone 3a could be obtained in 89% yield and 65% ee. When switching from copper to silver catalytic system, satisfactory results could be achieved. In the presence of AgOAc/L4, the [3+2]-cycloaddition provided cyclobutanone 3a in 83% yield and 95% ee. The absolute configuration of 3a was unambiguously determined by single crystal X-ray diffraction analysis.| a Conditions: 1 (0.2 mmol), 2a (0.4 mmol), LA (10 mol%), L* (11 mol%), base (20 mol%), THF, 0 °C, 9–12 h. |
|---|
|
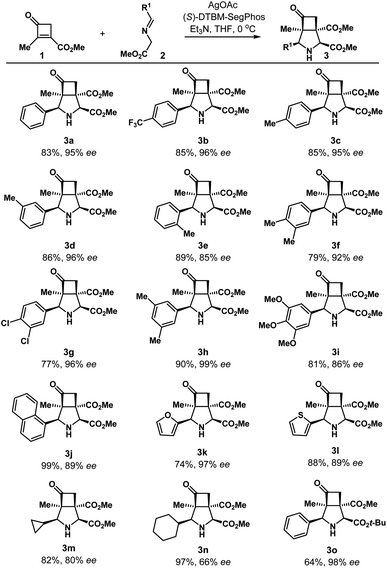
The substrate scope of iminoesters 2 investigated is summarized in Table 2. A variety of iminoesters derived from aryl aldehydes were applicable, affording the corresponding cycloadducts smoothly in good enantioselectivities. Both electron-rich and deficient substituents at the para-, meta-, ortho-, and multi-position on the aromatic ring provided azabicycles 3b–3i as single diastereomers in 77–90% yields and 85–99% ee. In addition, 1-naphthyl, 2-furyl, and 2-thienyl derived iminoesters furnished the cycloadducts 3j–3l in good yields and enantioselectivities (74–99% yields, 89–97% ee). Alkyl iminoesters with cyclopropyl (2m) and cyclohexyl (2n) substituents were also examined, and the enantioselectivity was in the range of 66–80% ee. Besides, the cycloaddition of 1a with t-butyl iminoester 2o provided the product 3o in 64% yield and 98% ee.
| a Conditions: 1 (0.2 mmol), 2 (0.4 mmol), AgOAc (10 mol%), L-4 (11 mol%), NEt3 (20 mol%), THF, 0 °C, 9–12 h. |
|---|
|
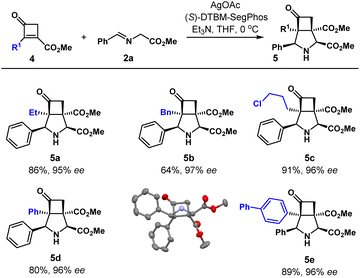
The substrate scope of cyclobutenones 4, which could be easily prepared from [2+2]-cycloaddition of enol ethers and keteniminiums, was then investigated (Table 3). A group of 2-alkyl and aryl substituted cyclobutenones 4 was tolerated well under the current conditions. Ethyl (5a), benzyl (5b), 3-chloropropyl (5c), phenyl (5d), and (1,1′-biphenyl)-4-yl (5e) substituents were effectively installed in the angular position.
| a Conditions: 4 (0.2 mmol), 2a (0.4 mmol), AgOAc (10 mol%), L-4 (11 mol%), NEt3 (20 mol%), THF, 0 °C, 9–11 h. |
|---|
|
The enantioselectivity was in the range of 95–97%. The absolute configuration of 5d was unambiguously determined by single crystal X-ray diffraction analysis.
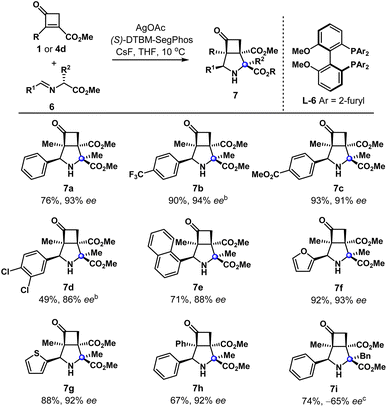
The reaction of (R)-iminoesters 6 and cyclobutenones 1 was then explored to synthesize bicyclic products 7 containing three contiguous quaternary stereocenters (Table 4). It has to mention that CsF was utilized as a base instead of NEt3 to afford the reproducible results (Table S6†), and the reaction of 1 with 6a provided the cycloadduct 7a in 76% yield and 93% ee. The absolute configuration of 7a was unambiguously determined by single crystal X-ray diffraction analysis. A range of iminoesters 6, derived from (hetero)aryl aldehydes worked well under optimal conditions, providing the corresponding cycloadducts 7b–7g in 49–93% yields and 86–94% ee. Of mention, in the case of electron-deficient phenyl-substituted iminoesters 6, NEt3 was used to provide slightly better results (7b and 7d). In addition, the reaction of 4d with 6a provided the product 7h in 67% yield and 92% ee. The reaction of 1a with 2-benzyl iminoester 6h worked as well, affording product 7i in 74% yield and −65% ee.
Control experiments were conducted to investigate the effect of chirality of iminoesters 6 (Scheme 2). To our surprise, an obvious difference of enantioselectivity was observed when in the reaction of 1 with iminoester 6a. The product 7a was isolated in 50% yield and only 73% ee when iminoester (S)-6a was used. Instead, both racemic or (R)-6a gave the product 7a in 65–76% yield and 90–93% ee. A subtle difference of enantioselectivity was also observed in the case of iminoester 6g. We assumed these results may be attributed to memory of chirality (MOC) effect.33,34
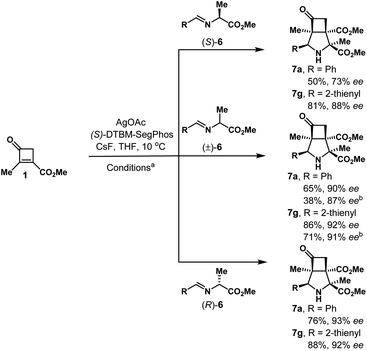 | ||
| Scheme 2 The reaction of 1 with enantioenriched or racemic iminoesters 6. aConditions: 1 (0.2 mmol), 6 (0.4 mmol), AgOAc (10 mol%), L-4 (11 mol%), CsF (40 mol%), THF, 10 °C, 21–22 h. b6 (0.2 mmol). | ||
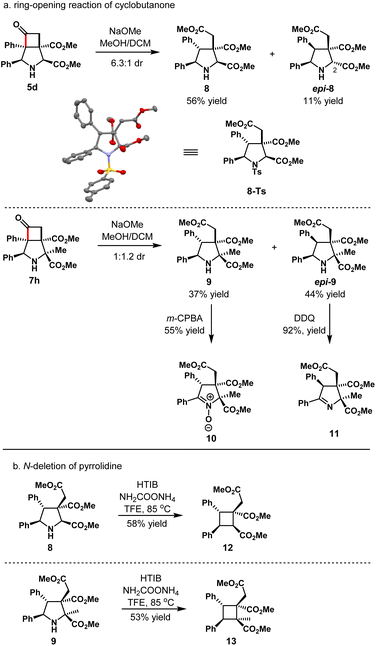
Skeletal functionalization has presented as an appealing strategy to build new chemical space. Selective ring-opening reactions of either cyclobutanone or pyrrolidine ring moiety could offer various densely functionalized molecules with structural diversities. Along with these lines, treatment of 3-azabicyclo[3.2.0]heptane 5d with NaOMe led to the products 8 and epi-8 in 67% yield and 6.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Scheme 3). The absolute configuration of 8 was unambiguously determined by single crystal X-ray diffraction analysis of its tosyl derivative. For minor diastereomers epi-8, epimerization at position 2 took place as well, and its structure was determined by single crystal X-ray diffraction analysis as well. Similarly, the ring-opening reaction of 7h provided diastereomers 9 in 81% yield and 1
1 dr (Scheme 3). The absolute configuration of 8 was unambiguously determined by single crystal X-ray diffraction analysis of its tosyl derivative. For minor diastereomers epi-8, epimerization at position 2 took place as well, and its structure was determined by single crystal X-ray diffraction analysis as well. Similarly, the ring-opening reaction of 7h provided diastereomers 9 in 81% yield and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 dr. Oxidation of 9 with m-CPBA gave the product 10 in 55% yield. In the same way, the product epi-10 could be obtained in 89% yield by oxidation of epi-9 with m-CPBA (not shown). Meanwhile, oxidation of epi-9 with DDQ gave the product 11 in 92% yield.
1.2 dr. Oxidation of 9 with m-CPBA gave the product 10 in 55% yield. In the same way, the product epi-10 could be obtained in 89% yield by oxidation of epi-9 with m-CPBA (not shown). Meanwhile, oxidation of epi-9 with DDQ gave the product 11 in 92% yield.
Densely substituted cyclobutane 12 could be obtained in 58% yield as a single diastereomer via nitrogen deletion of pyrrolidine 8 with the use of hydroxy(tosyloxy)iodobenzene (HTIB) and ammonium carbamate under Antonchick's conditions.31 Similarly, the cyclobutane 13 with two vicinal quaternary stereocenters could be furnished in 53% yield smoothly.
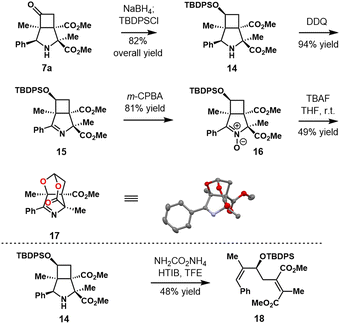
In addition, the reduction of 7a and sequential silylation gave the product 14 in 82% yield (Scheme 4). DDQ oxidation of 14 led to imine 15 smoothly, and further oxidation using m-CPBA provided product 16 in 81% yield. Interestingly, removal of silyl group using TBAF furnished an unexpected skeletal rearrangement product 17 in 49% yield.35 The structure of 17 was unambiguously determined by single crystal X-ray diffraction analysis. Meanwhile, treatment of 14 with HTIB and ammonium carbamate gave diene 18 in 48% yield via a simultaneous cleavage of both the pyrrolidine and cyclobutane rings process.
Conclusions
We developed here a silver-catalysed enantioselective [3+2]-cycloaddition of cyclobutenones and azomethine ylide precursors. Up to three contiguous all-carbon quaternary stereocenters could be efficiently constructed, owing to highly reactive cyclobutenones. Further transformations were investigated to access new chemical space.Data availability
General information, detailed experimental procedures, characterization data for all new compounds, and NMR spectra are in the ESI.†Author contributions
P. Lu conceptualized the project. L. Lu performed all the experimental work; both authors interpreted the data and co-wrote the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22071028, 21921003 to P.L.) and Fudan University (FDUROP to L.L.)Notes and references
- N. Nishiwaki, Methods and applications of cycloaddition reactions in organic syntheses, John Wiley & Sons, 2014 Search PubMed.
- S. Kobayashi and K. A. Jorgensen, Cycloaddition reactions in organic synthesis, Wiley-VCH, 2001 Search PubMed.
- K. W. Quasdorf and L. E. Overman, Nature, 2014, 516, 181–191 CrossRef CAS PubMed.
- F. Zhou, L. Zhu, B.-W. Pan, Y. Shi, Y.-L. Liu and J. Zhou, Chem. Sci., 2020, 11, 9341–9365 RSC.
- R. Long, J. Huang, J. Gong and Z. Yang, Nat. Prod. Rep., 2015, 32, 1584–1601 RSC.
- J. Jurczyk, J. Woo, S. F. Kim, B. D. Dherange, R. Sarpong and M. D. Levin, Nat. Synth., 2022, 1, 352–364 CrossRef PubMed.
- C. Zippel, J. Seibert and S. Brase, Angew. Chem., Int. Ed., 2021, 60, 19522–19524 CrossRef CAS.
- S. H. Kennedy, B. D. Dherange, K. J. Berger and M. D. Levin, Nature, 2021, 593, 223–227 CrossRef CAS.
- J. Jurczyk, M. C. Lux, D. Adpressa, S. F. Kim, Y. H. Lam, C. S. Yeung and R. Sarpong, Science, 2021, 373, 1004–1012 CrossRef CAS PubMed.
- J. Woo, A. H. Christian, S. A. Burgess, Y. Jiang, U. F. Mansoor and M. D. Levin, Science, 2022, 376, 527–532 CrossRef CAS PubMed.
- S. C. Patel and N. Z. Burns, J. Am. Chem. Soc., 2022, 144, 17797–17802 CrossRef CAS PubMed.
- H. Qin, W. Cai, S. Wang, T. Guo, G. Li and H. Lu, Angew. Chem., Int. Ed., 2021, 60, 20678–20683 CrossRef CAS.
- N. A. Meanwell and O. Loiseleur, J. Agric. Food Chem., 2022, 70, 10942–10971 CrossRef CAS PubMed.
- N. A. Meanwell and O. Loiseleur, J. Agric. Food Chem., 2022, 70, 10972–11004 CrossRef CAS PubMed.
- P. H. Chen and G. Dong, Chem.–Eur. J., 2016, 22, 18290–18315 CrossRef CAS PubMed.
- J. Chen, Q. Zhou, H. Fang and P. Lu, Chin. J. Chem., 2022, 40, 1346–1358 CrossRef CAS.
- M. Guisan-Ceinos, A. Parra, V. Martin-Heras and M. Tortosa, Angew. Chem., Int. Ed., 2016, 55, 6969–6972 CrossRef CAS PubMed.
- J. He, Q. Shao, Q. Wu and J. Q. Yu, J. Am. Chem. Soc., 2017, 139, 3344–3347 CrossRef CAS PubMed.
- C. Zhong, Y. Huang, H. Zhang, Q. Zhou, Y. Liu and P. Lu, Angew. Chem., Int. Ed., 2020, 59, 2750–2754 CrossRef CAS.
- F. W. Goetzke, A. M. L. Hell, L. van Dijk and S. P. Fletcher, Nat. Chem., 2021, 13, 880–886 CrossRef CAS PubMed.
- M. Yan, Q. Zhou and P. Lu, Angew. Chem., Int. Ed., 2023, 62, e202218008 CrossRef CAS PubMed.
- H. A. Clement, M. Boghi, R. M. McDonald, L. Bernier, J. W. Coe, W. Farrell, C. J. Helal, M. R. Reese, N. W. Sach, J. C. Lee and D. G. Hall, Angew. Chem., Int. Ed., 2019, 58, 18405–18409 CrossRef CAS.
- Z. Liang, L. Wang, Y. Wang, L. Wang, Q. Chong and F. Meng, J. Am. Chem. Soc., 2023, 145, 3588–3598 CrossRef CAS PubMed.
- Q. Gao and S. Xu, Angew. Chem., Int. Ed., 2023, 62, e202218025 CrossRef CAS PubMed.
- P. Yan, C. Zhong, J. Zhang, Y. Liu, H. Fang and P. Lu, Angew. Chem., Int. Ed., 2021, 60, 4609–4613 CrossRef CAS PubMed.
- P. Yan, J. Zhang, L. Lu, H. Fang and P. Lu, ACS Catal., 2022, 12, 15416–15423 CrossRef CAS.
- N. De and E. J. Yoo, ACS Catal., 2017, 8, 48–58 CrossRef.
- T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366–5412 CrossRef CAS PubMed.
- R. C. Gadwood, I. M. Mallick and A. J. DeWinter, J. Org. Chem., 2002, 52, 774–782 CrossRef.
- P. Yan, Q. Zhou, J. Chen and P. Lu, Chin. J. Chem., 2020, 38, 1103–1106 CrossRef CAS.
- C. Hui, L. Brieger, C. Strohmann and A. P. Antonchick, J. Am. Chem. Soc., 2021, 143, 18864–18870 CrossRef CAS PubMed.
- J. Corpas, A. Ponce, J. Adrio and J. C. Carretero, Org. Lett., 2018, 20, 3179–3182 CrossRef CAS PubMed.
- V. Alezra and T. Kawabata, Synthesis, 2016, 48, 2997–3016 CrossRef CAS.
- One referee suggested that the chiral iminoester might act as a ligand, thus leading to these observations. For more details, see Table S7.†.
- For a proposed mechanism, see Scheme S1.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2263230–2263236. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc02485g |
| This journal is © The Royal Society of Chemistry 2023 |