 Open Access Article
Open Access ArticleFacile access to chiral γ-butyrolactones via rhodium-catalysed asymmetric hydrogenation of γ-butenolides and γ-hydroxybutenolides†
Yuxuan
Zhou‡
a,
Siyuan
Guo‡
a,
Qiyuan
Huang
a,
Qiwei
Lang
*a,
Gen-Qiang
Chen
 *ab and
Xumu
Zhang
*ab and
Xumu
Zhang
 *a
*a
aShenzhen Grubbs Institute, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen 518000, People's Republic of China. E-mail: langqw@sustech.edu.cn; chengq@sustech.edu.cn; zhangxm@sustech.edu.cn
bAcademy for Advanced Interdisciplinary Studies, Southern University of Science and Technology, Shenzhen 518000, People's Republic of China
First published on 15th April 2023
Abstract
The highly efficient Rh/ZhaoPhos-catalysed asymmetric hydrogenation of γ-butenolides and γ-hydroxybutenolides was successfully developed. This protocol provides an efficient and practical approach to the synthesis of various chiral γ-butyrolactones, which are synthetically valuable building blocks of diverse natural products and therapeutic substances, with excellent results (up to >99% conversion and 99% ee). Further follow-up transformations have been revealed to accomplish creative and efficient synthetic routes for several enantiomerically enriched drugs via this catalytic methodology.
γ-Butyrolactone is an essential framework existing in large numbers of natural products1 and pharmaceutical compounds2 and shows unique and excellent biological activities (Fig. 1). Furthermore, enantiomerically pure γ-butyrolactones could serve as building blocks for the construction of a wide range of complex molecules,3 and diverse transformations have been developed,4 especially in the highly versatile furanone structure, to synthesize physiologically and therapeutically important reagents, for example brivaracetam,5 arctigenin,6 pilocarpine,7etc.
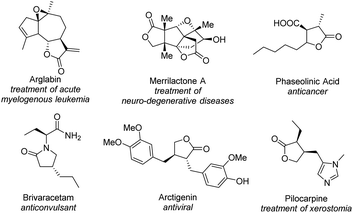 | ||
| Fig. 1 Natural products containing the γ-butyrolactone core, and pharmaceutical compounds derived from γ-butyrolactones. | ||
As commercially available or easily accessible compounds, γ-butenolides8 are important synthetic precursors towards γ-butyrolactones, and an impressive range of transformations have been developed for the enantioselective construction of chiral γ-butyrolactones.9 With the advancement in the field of asymmetric metal catalysis, enantioselective 1,4-addition and reduction of γ-butenolides have been considered representative methodologies of preparing optically active γ-butyrolactones. In contrast to the well-developed asymmetric 1,4-additions10 (Scheme 1a), with transition metal-catalysed 1,4-reduction of γ-butenolides as an alternative it would be difficult to achieve satisfactory catalytic conversion and stereoselectivity as well as broad substrate scope because of the lack of efficient metal catalysts.11 The Buchwald group reported a CuH-catalysed enantioselective conjugate reduction of γ-butenolides;12 however, the requirement of using high catalyst loading and a strong base additive could limit the application of this method in organic synthesis and the pharmaceutical industry. Recently, our group developed a bisphosphine-thiourea chiral ligand, ZhaoPhos,13 which exhibited extraordinary potential in rhodium14 and iridium-catalyzed15 asymmetric hydrogenation due to its powerful hydrogen-bonding and anion-binding ability resulting from the thiourea moiety. Inspired by this novel strategy, we herein demonstrated a Rh/ZhaoPhos catalysed asymmetric hydrogenation of easily accessible γ-butenolides and γ-hydroxybutenolides, which could be a straightforward method of approaching chiral γ-butyrolactones with excellent catalytic results (Scheme 1b).
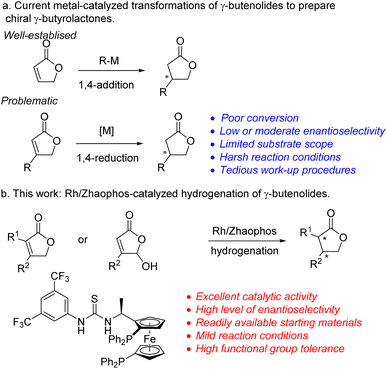 | ||
| Scheme 1 Current methods of preparing chiral γ-butyrolactones from γ-butenolides, and proposed Rh/ZhaoPhos-catalyzed hydrogenation. | ||
We initiated our investigation with evaluation of various combinations of the rhodium precatalyst and different chiral phosphine ligands for the asymmetric hydrogenation of 4-phenylfuran-2(5H)-one 1a. After examining a series of commonly used chiral phosphine ligands and using THF as the solvent, very poor enantioselectivity was achieved with ligands such as DuanPhos, SegPhos, BINAP and so on (Table 1, entries 1–7). Employing ZhaoPhos as a ligand could facilitate the hydrogenation with a significantly improved enantioselective manner, and the desired product 2a was obtained in 79% conversion and 97% ee under 50 atm of H2 at room temperature within 24 hours (entry 8). Furthermore, the solvent was crucial to the control of the reaction rate, and by screening several protic and aprotic solvents (Table 2, entries 1–9), we found that by employing DCM as solvent complete hydrogenation could be achieved under standard conditions, which afforded 2a with >99% conversion and in 98% ee (entry 8).
| Entrya | Ligand | Conv.b (%) | eec (%) |
|---|---|---|---|
a Unless otherwise noted, all hydrogenations were carried out with a [Rh(NBD)2BF4]/ligand/1a (0.1 mmol) ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in 1.0 mL of THF under H2 (50 atm) at room temperature for 24 h.
b Determined by 1H NMR.
c Determined by HPLC analysis, and the absolute configuration of 2a was determined as S by comparing the optical rotation data with the literature. 100 in 1.0 mL of THF under H2 (50 atm) at room temperature for 24 h.
b Determined by 1H NMR.
c Determined by HPLC analysis, and the absolute configuration of 2a was determined as S by comparing the optical rotation data with the literature.
|
|||
| 1 | (RC,SP)-DuanPhos | 14 | 1 |
| 2 | (S)-SegPhos | 100 | 2 |
| 3 | (S)-BINAP | 78 | 0 |
| 4 | (R,S)-JosiPhos | 100 | 2 |
| 5 | (R)-MeO-BIPHEP | 13 | 10 |
| 6 | WalPhos | 31 | 16 |
| 7 | (R)-C3*-TunePhos | 8 | 13 |
| 8 | ZhaoPhos | 79 | 97 |
| Entrya | Solvent | Conv.b (%) | eec (%) |
|---|---|---|---|
a Unless otherwise noted, all hydrogenations were carried out with a [Rh(NBD)2BF4]/ligand/1a (0.1 mmol) ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in 1.0 mL of solvent under H2 (50 atm) at room temperature for 24 h.
b Determined by 1H NMR.
c Determined by HPLC analysis, and the absolute configuration of 2a was determined as S by comparing the optical rotation data with the literature. 100 in 1.0 mL of solvent under H2 (50 atm) at room temperature for 24 h.
b Determined by 1H NMR.
c Determined by HPLC analysis, and the absolute configuration of 2a was determined as S by comparing the optical rotation data with the literature.
|
|||
| 1 | MeOH | 29 | 96 |
| 2 | EtOH | 19 | 97 |
| 3 | CF3CH2OH | 59 | 98 |
| 4 | EtOAc | >99 | 97 |
| 5 | Toluene | 91 | 95 |
| 6 | THF | 79 | 97 |
| 7 | 1,4-Dioxane | 30 | 16 |
| 8 | DCM | >99 | 98 |
| 9 | DCE | 25 | 96 |
With optimized reaction conditions in hand, we then continued to investigate the substrate generality (Table 3). A considerable number of β-aryl substituents (2a–l) on γ-butenolides were investigated, and the hydrogenation proceeded smoothly to yield the desired products with excellent yields (95 ∼ 99%) and in high enantioselectivities (97 ∼ 98% ee), in which both electron-withdrawing (2b–2d) and electron-donating (2e–2j) substituents on the aryl ring at different positions were well tolerated. The hydrogenation of substrates with the bulky naphthyl moiety (1k) and heterocyclic unit (thienyl, 1l) was feasible, giving the corresponding product with excellent results (95 ∼ 99% yields and 98% ee). In addition, the alkyl substituents (2m–n) were explored as well, and it is noteworthy that α-methyl substituted lactone 2n could be produced with 84% or 82% ee respectively from the substrates featuring either endo- (1n) or exocyclic (1n′) unsaturation, in which this transformation was rarely achieved with good yield and in acceptable enantioselectivity according to previous studies.16 The reactions of α,β-disubstituted γ-butenolides 1o and 1p were also conducted; however, no desired products were obtained.
a Unless otherwise noted, all hydrogenations were carried out with a [Rh(NBD)2BF4]/ligand/1a (0.1 mmol) ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in 1.0 mL of DCM under H2 (50 atm) at room temperature for 24 h.
b Isolated yield.
c Determined by HPLC analysis, and the absolute configuration was determined by comparing the optical rotation data with the literature. 100 in 1.0 mL of DCM under H2 (50 atm) at room temperature for 24 h.
b Isolated yield.
c Determined by HPLC analysis, and the absolute configuration was determined by comparing the optical rotation data with the literature.
|
|---|

|
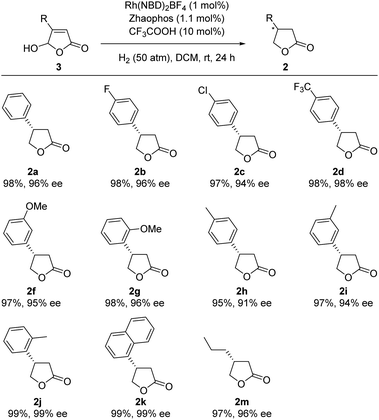
Apart from the hydrogenation of γ-butenolides, γ-hydroxybutenolides 3 were also successfully reduced under the same reaction conditions with CF3COOH as an additive (see the ESI† for details), and consequently afforded γ-butyrolactones 2 directly in high yields (95 ∼ 99%) and with excellent enantioselectivities (up to 99% ee) (Table 4). We suggested that CF3COOH could activate the hydroxy group of lactol, which could be protonated to form an oxonium salt, and then hydrogenated by the catalyst.15e,f γ-Hydroxybutenolides could be naturally occurring as well as easily prepared, and on account of realizing these transformations, the scope of the starting material for preparing chiral γ-butyrolactones was extremely expanded, which further improved the synthetic value of the Rh/ZhaoPhos catalyst in the field of asymmetric hydrogenation.
a Unless otherwise noted, all hydrogenations were carried out with a [Rh(NBD)2BF4]/ligand/CF3COOH/1a (0.1 mmol) ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in 1.0 mL of DCM under H2 (50 atm) at room temperature for 24 h.
b Isolated yield.
c Determined by HPLC analysis, and the absolute configuration was determined comparing the optical rotation data with the literature. 100 in 1.0 mL of DCM under H2 (50 atm) at room temperature for 24 h.
b Isolated yield.
c Determined by HPLC analysis, and the absolute configuration was determined comparing the optical rotation data with the literature.
|
|---|
|
To demonstrate the synthetic utilities of this method, a gram-scale hydrogenation of substrate 1m (Scheme 2a) was conducted with a low catalyst loading of 0.02 mol%. To our delight, the hydrogenation underwent smoothly under 70 atm of H2 at 50 °C using CF3COOH (10 mol%) as the additive (adding CF3COOH could not only stabilize the cationic rhodium catalyst to increase the TON of the reaction, but also activate the carbonyl of α,β-unsaturated lactone to facilitate the hydrogenation), and the chiral γ-butyrolactone 2m was afforded in 98% yield and with 95% ee. We further transformed this chiral building block via a ring-opening/recyclization strategy to achieve the total synthesis of brivaracetam in high optical purity (96.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.1 dr and >99% ee) within only 3 steps (67% overall yield). Moreover, the 2-step synthesis of arctigenin analogue 7 was realized using γ-butenolide 1e as the starting material (79% overall yield, Scheme 2b), which presented a shorter synthetic route compared to previous studies.6c
3.1 dr and >99% ee) within only 3 steps (67% overall yield). Moreover, the 2-step synthesis of arctigenin analogue 7 was realized using γ-butenolide 1e as the starting material (79% overall yield, Scheme 2b), which presented a shorter synthetic route compared to previous studies.6c
In summary, we have developed a highly enantioselective Rh/ZhaoPhos-catalyzed hydrogenation, providing a series of synthetically useful chiral γ-butyrolactones with readily available γ-butenolides and γ-hydroxybutenolides as starting materials. In addition, this strategy was applicable to the construction of various natural products and therapeutic substances, and scalable and concise syntheses of the pharmaceutical drugs brivaracetam and arctigenin were accomplished through this methodology, which demonstrated its practical utilities.
Data availability
All experimental procedures, characterization, and computational data for this study, can be found in the ESI.†Author contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
X. Zhang is indebted to the Southern University of Science and Technology (start-up fund), Shenzhen Science and Technology Innovation Committee (No. KQTD20150717103157174), Stable Support Plan Program of the Shenzhen Natural Science Fund (Program Contract No. 20200925161222002), Key-Area Research and Development Program of Guangdong Province (No. 2020B010188001), Innovative Team of Universities in Guangdong Province (No. 2020KCXTD016), and National Natural Science Foundation of China (No. 21991113). G.-Q. Chen gratefully acknowledges the National Natural Science Foundation of China (No. 22171129), the Guangdong Basic and Applied Basic Research Foundation (2022B1515020055) and Shenzhen Science and Technology Innovation Committee (JCYJ20210324104202007) for financial support. The authors acknowlege the assistance of SUSTech Core Research Facilities.References
- (a) R. Haritakun, P. Rachtawee, R. Chanthaket, N. Boonyuen and M. Isaka, Butyrolactones from the fungus Aspergillus terreus BCC 4651, Chem. Pharm. Bull., 2010, 58, 1545–1548 CrossRef CAS PubMed; (b) D. J. Faulkner, Marine natural products, Nat. Prod. Rep., 2001, 18, 1–49 RSC.
- (a) M. Yamawaki, K. Nishi, S. Nishimoto, S. Yamauchi, K. Akiyama, T. Kishida, M. Maruyama, H. Nishiwaki and T. Sugahara, Immunomodulatory effect of (−)-matairesinol in vivo and ex vivo, Biosci., Biotechnol., Biochem., 2011, 75, 859–863 CrossRef CAS PubMed; (b) S. Ōmura, H. Tanaka, Y. Okada and H. Marumo, Isolation and structure of nanaomycin D, an enantiomer of the antibiotic kalafungin, J. Chem. Soc., Chem. Commun., 1976, 320–321 RSC; (c) O. C. Snead III and K. M. Gibson, Gamma-hydroxybutyric acid, N. Engl. J. Med., 2005, 352, 2721–2732 CrossRef PubMed.
- J. Hur, J. Jang and J. Sim, A review of the pharmacological activities and recent synthetic advances of γ-butyrolactones, Int. J. Mol. Sci., 2021, 22, 2769 CrossRef CAS PubMed.
- B. Mao, M. Fananas-Mastral and B. L. Feringa, Catalytic Asymmetric Synthesis of Butenolides and Butyrolactones, Chem. Rev., 2017, 117, 10502–10566 CrossRef CAS PubMed.
- (a) M. Gayke, H. Narode, G. Eppa, R. S. Bhosale and J. S. Yadav, Synthetic Approaches toward the Synthesis of Brivaracetam: An Antiepileptic Drug, ACS Omega, 2022, 7, 2486–2503 CrossRef CAS PubMed; (b) S. Liao, H. Chen, G. Wang, S. Wu, Z. Yang, W. Luo, Z. Liu, X. Gao, J. Qin, C.-h. Li and Z. Wang, Identification, characterization, synthesis and strategy for minimization of potential impurities observed in the synthesis of brivaracetam, Tetrahedron, 2020, 76, 131273 CrossRef CAS; (c) S. P. Chavan, S. A. Kawale and P. N. Chavan, Formal synthesis of brivaracetam: a key to construct the pyrrolidone scaffold using Pd-catalyzed oxidative cyclization and ring-closing metathesis reaction, Tetrahedron Lett., 2019, 60, 151249 CrossRef CAS.
- (a) S. Duan, S. Huang, J. Gong, Y. Shen, L. Zeng, Y. Feng, W. Ren, Y. Leng and Y. Hu, Design and synthesis of novel arctigenin analogues for the amelioration of metabolic disorders, ACS Med. Chem. Lett., 2015, 6, 386–391 CrossRef CAS PubMed; (b) D. Wu, L. Jin, X. Huang, H. Deng, Q. K. Shen, Z. S. Quan, C. Zhang and H. Y. Guo, Arctigenin: pharmacology, total synthesis, and progress in structure modification, J. Enzyme Inhib. Med. Chem., 2022, 37, 2452–2477 CrossRef CAS PubMed; (c) L. M. Recnik, R. J. Thatcher, S. Mallah, C. P. Butts, G. L. Collingridge, E. Molnar, D. E. Jane and C. L. Willis, Synthesis and pharmacological characterisation of arctigenin analogues as antagonists of AMPA and kainate receptors, Org. Biomol. Chem., 2021, 19, 9154–9162 RSC.
- (a) D. A. Horne, B. Fugmann, K. Yakushijin and G. Buchi, A synthesis of pilocarpine, J. Org. Chem., 2002, 58, 62–64 CrossRef; (b) T. Schmidt, N. Heise, K. Merzweiler, H. P. Deigner, A. Al-Harrasi and R. Csuk, Concise Synthesis of Both Enantiomers of Pilocarpine, Molecules, 2021, 26, 3676 CrossRef CAS PubMed; (c) S. G. Davies, P. M. Roberts, P. T. Stephenson, H. R. Storr and J. E. Thomson, A practical and scaleable total synthesis of the jaborandi alkaloid (+)-pilocarpine, Tetrahedron, 2009, 65, 8283–8296 CrossRef CAS.
- S. Chatterjee, R. Sahoo and S. Nanda, Recent reports on the synthesis of gamma-butenolide, gamma-alkylidenebutenolide frameworks, and related natural products, Org. Biomol. Chem., 2021, 19, 7298–7332 RSC.
- J. Y. Kato, N. Funa, H. Watanabe, Y. Ohnishi and S. Horinouchi, Biosynthesis of gamma-butyrolactone autoregulators that switch on secondary metabolism and morphological development in Streptomyces, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 2378–2383 CrossRef CAS PubMed.
- (a) M. K. Brown, S. J. Degrado and A. H. Hoveyda, Highly enantioselective Cu-catalyzed conjugate additions of dialkylzinc reagents to unsaturated furanones and pyranones: preparation of air-stable and catalytically active Cu-peptide complexes, Angew. Chem., Int. Ed., 2005, 44, 5306–5310 CrossRef CAS PubMed; (b) T. Gendrineau, O. Chuzel, H. Eijsberg, J. P. Genet and S. Darses, C1-symmetric monosubstituted chiral diene ligands in asymmetric rhodium-catalyzed 1,4-addition reactions, Angew. Chem., Int. Ed., 2008, 47, 7669–7672 CrossRef CAS PubMed; (c) Y. Luo and A. J. Carnell, Chemoenzymatic synthesis and application of bicyclo[2.2.2]octadiene ligands: increased efficiency in rhodium-catalyzed asymmetric conjugate additions by electronic tuning, Angew. Chem., Int. Ed., 2010, 49, 2750–2754 CrossRef CAS PubMed; (d) T. Hayashi and K. Yamasaki, Rhodium-catalyzed asymmetric 1,4-addition and its related asymmetric reactions, Chem. Rev., 2003, 103, 2829–2844 CrossRef CAS PubMed; (e) H. J. Edwards, J. D. Hargrave, S. D. Penrose and C. G. Frost, Synthetic applications of rhodium catalysed conjugate addition, Chem. Soc. Rev., 2010, 39, 2093–2105 RSC; (f) G. Chen, N. Tokunaga and T. Hayashi, Rhodium-catalyzed asymmetric 1,4-addition of arylboronic acids to coumarins: asymmetric synthesis of (R)-tolterodine, Org. Lett., 2005, 7, 2285–2288 CrossRef CAS PubMed.
- (a) J. J. Verendel, J. Q. Li, X. Quan, B. Peters, T. Zhou, O. R. Gautun, T. Govender and P. G. Andersson, Chiral hetero- and carbocyclic compounds from the asymmetric hydrogenation of cyclic alkenes, Chem.–Eur. J., 2012, 18, 6507–6513 CrossRef CAS PubMed; (b) T. Ohta, T. Miyake, N. Seido, H. Kumobayashi and H. Takaya, Asymmetric Hydrogenation of Olefins with Aprotic Oxygen Functionalities Catalyzed by BINAP-Ru(II) Complexes, J. Org. Chem., 2002, 60, 357–363 CrossRef; (c) P. M. Donate, D. Frederico, R. da Silva, M. G. Constantino, G. Del Ponte and P. S. Bonatto, Asymmetric synthesis of γ-butyrolactones by enantioselective hydrogenation of butenolides, Tetrahedron: Asymmetry, 2003, 14, 3253–3256 CrossRef CAS.
- G. Hughes, M. Kimura and S. L. Buchwald, Catalytic enantioselective conjugate reduction of lactones and lactams, J. Am. Chem. Soc., 2003, 125, 11253–11258 CrossRef CAS PubMed.
- Q. Zhao, S. Li, K. Huang, R. Wang and X. Zhang, A novel chiral bisphosphine-thiourea ligand for asymmetric hydrogenation of beta,beta-disubstituted nitroalkenes, Org. Lett., 2013, 15, 4014–4017 CrossRef CAS PubMed.
- (a) Z. Zhang, Z. Han, G. Gu, X.-Q. Dong and X. Zhang, Enantioselective Synthesis of Chiral 3-Substituted-3-silylpropionic Esters via Rhodium/Bisphosphine-Thiourea-Catalyzed Asymmetric Hydrogenation, Adv. Synth. Catal., 2017, 359, 2585–2589 CrossRef CAS; (b) G. Liu, Z. Han, X. Q. Dong and X. Zhang, Rh-Catalyzed Asymmetric Hydrogenation of beta-Substituted-beta-thio-alpha,beta-unsaturated Esters: Expeditious Access to Chiral Organic Sulfides, Org. Lett., 2018, 20, 5636–5639 CrossRef CAS PubMed; (c) X. Yin, Y. Huang, Z. Chen, Y. Hu, L. Tao, Q. Zhao, X. Q. Dong and X. Zhang, Enantioselective Access to Chiral 2-Substituted 2,3-Dihydrobenzo[1,4]dioxane Derivatives through Rh-Catalyzed Asymmetric Hydrogenation, Org. Lett., 2018, 20, 4173–4177 CrossRef CAS PubMed; (d) Z. Han, G. Liu, R. Wang, X. Q. Dong and X. Zhang, Highly efficient Ir-catalyzed asymmetric hydrogenation of benzoxazinones and derivatives with a Bronsted acid cocatalyst, Chem. Sci., 2019, 10, 4328–4333 RSC; (e) C. Yin, T. Yang, Y. Pan, J. Wen and X. Zhang, Rh-Catalyzed Asymmetric Hydrogenation of Unsaturated Medium-Ring NH Lactams: Highly Enantioselective Synthesis of N-Unprotected 2,3-Dihydro-1,5-benzothiazepinones, Org. Lett., 2020, 22, 920–923 CrossRef CAS PubMed; (f) Q. Zhao, C. Chen, J. Wen, X. Q. Dong and X. Zhang, Noncovalent Interaction-Assisted Ferrocenyl Phosphine Ligands in Asymmetric Catalysis, Acc. Chem. Res., 2020, 53, 1905–1921 CrossRef CAS PubMed; (g) H. Yang, Y. Zhou, Z. Zhang, J. Wen and X. Zhang, Iridium-Catalyzed Hydroiodination and Formal Hydroamination of Olefins with N-Iodo Reagents and Molecular Hydrogen: An Umpolung Strategy, Org. Lett., 2022, 24, 1842–1847 CrossRef CAS PubMed; (h) L. S. Zheng, C. Yin, F. Wang, G. Q. Chen and X. Zhang, Enantioselective synthesis of cis-hexahydro-gamma-carboline derivatives via Ir-catalyzed asymmetric hydrogenation, Chem. Commun., 2022, 58, 3286–3289 RSC.
- (a) L.-S. Zheng, C. Yin, F. Wang, G.-Q. Chen and X. Zhang, Enantioselective synthesis of cis-hexahydro-γ-carboline derivatives via Ir-catalysed asymmetric hydrogenation, Chem. Commun., 2022, 58, 3286–3289 RSC; (b) F. Wang, Y. Chen, P. Yu, G.-Q. Chen and X. Zhang, Asymmetric Hydrogenation of Oximes Synergistically Assisted by Lewis and Bronsted Acids, J. Am. Chem. Soc., 2022, 144, 17763–17768 CrossRef CAS PubMed; (c) G. Liu, L. Zheng, K. Tian, H. Wang, L. Wa Chung, X. Zhang and X.-Q. Dong, Ir-Catalyzed Asymmetric Hydrogenation of Unprotected Indoles: Scope Investigations and Mechanistic Studies, CCS Chem., 2022, 1–13, DOI:10.31635/ccschem.022.202101643; (d) Z. Han, G. Liu, X. Yang, X.-Q. Dong and X. Zhang, Enantiodivergent Synthesis of Chiral Tetrahydroquinoline Derivatives via Ir-Catalyzed Asymmetric Hydrogenation: Solvent-Dependent Enantioselective Control and Mechanistic Investigations, ACS Catal., 2021, 11, 7281–7291 CrossRef CAS; (e) T. Yang, Y. Sun, H. Wang, Z. Lin, J. Wen and X. Zhang, Iridium-Catalyzed Enantioselective Hydrogenation of Oxocarbenium Ions: A Case of Ionic Hydrogenation, Angew. Chem., Int. Ed., 2020, 59, 6108–6114 CrossRef CAS PubMed; (f) Y. Sun, Q. Zhao, H. Wang, T. Yang, J. Wen and X. Zhang, Asymmetric Hydrogenation of Cationic Intermediates for the Synthesis of Chiral N,O-Acetals, Chem.–Eur. J., 2020, 26, 11470–11477 CrossRef CAS PubMed; (g) T. Yang, X. Guo, Q. Yin and X. Zhang, Intramolecular asymmetric reductive amination: synthesis of enantioenriched dibenz[c,e]azepines, Chem. Sci., 2019, 10, 2473–2477 RSC; (h) Z. Han, G. Liu, R. Wang, X.-Q. Dong and X. Zhang, Highly efficient Ir-catalyzed asymmetric hydrogenation of benzoxazinones and derivatives with a Bronsted acid cocatalyst, Chem. Sci., 2019, 10, 4328–4333 RSC; (i) Z. Han, Y.-Q. Guan, G. Liu, R. Wang, X. Yin, Q. Zhao, H. Cong, X.-Q. Dong and X. Zhang, Iridium-Catalyzed Asymmetric Hydrogenation of Tetrasubstituted α-Fluoro-β-enamino Esters: Efficient Access to Chiral α-Fluoro-β-amino Esters with Two Adjacent Tertiary Stereocenters, Org. Lett., 2018, 20, 6349–6353 CrossRef CAS PubMed.
- Q. Lang, G. Gu, Y. Cheng, Q. Yin and X. Zhang, Highly Enantioselective Synthesis of Chiral γ-Lactams by Rh-Catalyzed Asymmetric Hydrogenation, ACS Catal., 2018, 8, 4824–4828 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc00491k |
| ‡ Yuxuan Zhou and Siyuan Guo contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |



