Open Access Article
Open Access ArticleAryl-to-alkyl radical relay Heck reaction of amides with vinyl arenes†
Yu-jia
Du‡
,
Xia-xin
Sheng‡
,
Jun-hua
Li
 ,
Jia-ming
Chen
,
Sen
Yang
* and
Ming
Chen
,
Jia-ming
Chen
,
Sen
Yang
* and
Ming
Chen
 *
*
Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology, School of Petrochemical Engineering, Changzhou University, 21 Gehu Road, Changzhou, 213164, China. E-mail: chenming0228@cczu.edu.cn
First published on 27th February 2023
Abstract
A palladium-catalyzed aryl-to-alkyl radical relay Heck reaction of amides at α-C(sp3)-H sites with vinyl arenes is described. This process displays a broad substrate scope with respect to both amide and alkene components and provides access to a diverse class of more complex molecules. The reaction is proposed to proceed via a hybrid palladium-radical mechanism. The core of the strategy is that the fast oxidative addition of aryl iodide and fast 1,5-HAT overcome the slow oxidative addition of alkyl halides, and the photoexcitation effect suppresses the undesired β-H elimination. It is anticipated that this approach would inspire the discovery of new palladium-catalyzed alkyl-Heck methods.
Introduction
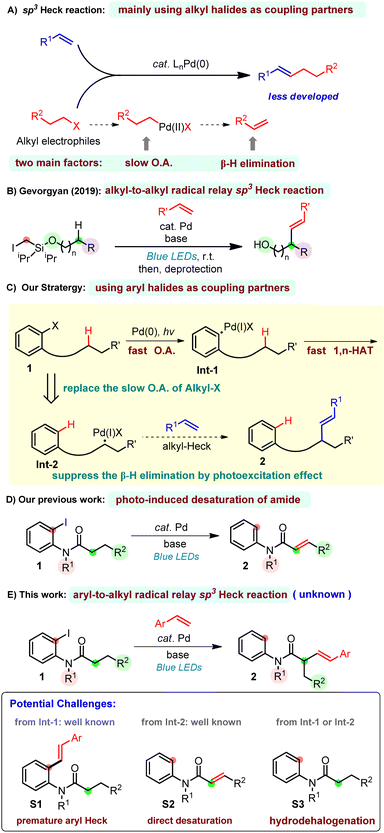
The Nobel-Prize-winning Mizoroki–Heck reaction1 is a fundamental synthetic transformation in chemical synthesis, enabling the direct cross coupling of aryl and vinyl halides (triflates or sulfonates) with simple alkenes.2 The utility of the palladium-catalyzed Heck reaction with aryl/vinyl halide sites has been well demonstrated in synthesis.2,3 However, the alkyl Heck reaction is less developed because of two main factors: the oxidative addition is slow with low-valent transition metals4 and the resulting alkylmetal species can undergo premature β-hydride elimination which leads to side products5 (Scheme 1A). To overcome these significant challenges, elegant contributions have been presented showing that β-hydride elimination can be suppressed through the use of first-row-transition metals, such as nickel, which is less likely to induce β-hydride elimination.6 The application of new ligand designs7 was also reported to be capable of constraining the β-hydride elimination. This, however, will lead to other adverse consequences as β-hydride elimination is eventually required as an elementary step in Heck reactions. To date, the Heck reaction of activated8 and perfluorinated9 alkyl halides has been established by Ni or Pd catalysis, and Pd-catalyzed Heck reactions of unactivated primary and secondary alkyl halides have been achieved by Fu,10 Alexanian11 and Zhou.12 Recently, Gevorgyan13 and Fu14 separately developed a hybrid palladium radical species,15 which has enabled a tertiary alkyl-Heck reaction to occur with high efficiency at room temperature.Despite these developments, new strategies for alkyl-Heck reactions are still important. Inspired by Gevorgyan's seminal work on alkyl-to-alkyl radical relay Heck reactions using an easily installed/removed Si-based auxiliary through the selective I-atom/radical translocation pathway (Scheme 1B),13b we thus conceived the approach of merging a fast oxidative addition rate of aryl halides with a rapid 1,n-HAT (hydrogen atom transfer) to overcome the slow oxidative addition of alkyl halides.16 Notably, the aryl-to-alkyl radical relay step was known in direct desaturation reactions, however, whether this step can happen in the presence of olefin is unpredictable, since the aryl Heck reaction is more favored. If feasible, the alkyl palladium species would be formed smoothly which subsequently underwent a photo-induced Heck reaction. The β-hydride elimination, on the other hand, can be suppressed by the photoexcitation effect (Scheme 1C).14a In 2022, our group developed a direct desaturation of amide by the cooperation of visible light and palladium catalysis (Scheme 1D).16c Herein, we disclose the first example of a palladium-catalyzed aryl-to-alkyl radical relay heck reaction of amide at α-C(sp3)-H sites with vinyl arenes using aryl iodide as the alkyl-Heck coupling partner to construct α-alkenyl amides (Scheme 1E). To the best of our knowledge, alkyl-Heck reactions through such a process have not been realized, which became the motivation of this study. The success of this reaction faces several potential challenges, the first and greatest one is the premature Heck reaction on the aryl site (S1), which was well developed.2,3 To compete with this, the 1,5-HAT rate has to be faster than the aryl-Heck reaction. Other possible pathways are the direct desaturation via β-hydride elimination16c,17 (S2) and hydrodehalogenation (S3).
Results and discussion
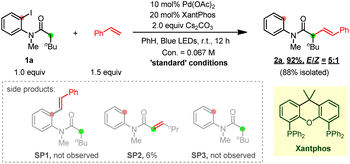
To test this hypothesis, our investigation began by using N-(2-iodophenyl)-N-methylhexanamide 1a and styrene as the model substrates (Table 1). After extensive optimization, the desired Heck product 2a was obtained in 88% isolated yield with a trans/cis (E/Z) selectivity of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
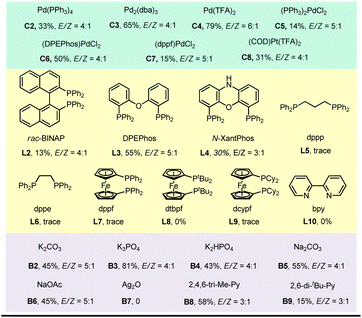
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at room temperature through an aryl-to-alkyl radical relay process when Pd(OAc)2/4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (xantphos) was used as the metal/ligand combination and Cs2CO3 as the base. The direct desaturated SP2 was isolated in 6% yield. Fortunately, both the premature ipso aryl-Heck product SP1 and the hydrodehalogenated SP3 were not obtained suggesting an excellent chemical selectivity of the current reaction. The necessity of each reactant was then explored through control experiments. Clearly, the palladium catalyst, the phosphine ligand, the base and blue light are all essential to this transformation (entries 1–4). Besides Pd(OAc)2, a variety of other Pd(0) and Pd(II) complexes were also tested (entry 5). While Pd2(dba)3, Pd(TFA)2 and (DPEphos)PdCl2 also gave moderate to good yields, Pd(PPh3)4, (PPh3)2PdCl2 and (dppf)PdCl2 were much less efficient. Notably, the same group Pt-catalyst (COD)Pt(TFA)2 could also deliver the desired product in 31% yield. Next, a series of ligands have been examined (entry 6). Use of rac-BINAP, DPEPhos and N-XantPhos (L2–L4) proved less efficient, and other bidentate P/N ligands (L5–L10) gave none or trace desired product. Both inorganic (B2–B6) and organic bases (B8–B9) dramatically promoted the reaction, among which K3PO4 gave a comparative efficiency with Cs2CO3, while K2CO3, K2HPO4, Na2CO3, NaOAc and 2,4,6-tri-Me-pyridine produced moderate yields. Ag2O totally shut down the reaction.
1 at room temperature through an aryl-to-alkyl radical relay process when Pd(OAc)2/4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (xantphos) was used as the metal/ligand combination and Cs2CO3 as the base. The direct desaturated SP2 was isolated in 6% yield. Fortunately, both the premature ipso aryl-Heck product SP1 and the hydrodehalogenated SP3 were not obtained suggesting an excellent chemical selectivity of the current reaction. The necessity of each reactant was then explored through control experiments. Clearly, the palladium catalyst, the phosphine ligand, the base and blue light are all essential to this transformation (entries 1–4). Besides Pd(OAc)2, a variety of other Pd(0) and Pd(II) complexes were also tested (entry 5). While Pd2(dba)3, Pd(TFA)2 and (DPEphos)PdCl2 also gave moderate to good yields, Pd(PPh3)4, (PPh3)2PdCl2 and (dppf)PdCl2 were much less efficient. Notably, the same group Pt-catalyst (COD)Pt(TFA)2 could also deliver the desired product in 31% yield. Next, a series of ligands have been examined (entry 6). Use of rac-BINAP, DPEPhos and N-XantPhos (L2–L4) proved less efficient, and other bidentate P/N ligands (L5–L10) gave none or trace desired product. Both inorganic (B2–B6) and organic bases (B8–B9) dramatically promoted the reaction, among which K3PO4 gave a comparative efficiency with Cs2CO3, while K2CO3, K2HPO4, Na2CO3, NaOAc and 2,4,6-tri-Me-pyridine produced moderate yields. Ag2O totally shut down the reaction.
| Entry | Variation from ‘standard’ conditions | Yield a(%) of 2a |
|---|---|---|
| a Each reaction was run on a 0.1 mmol scale in a sealed 4 mL vial for 12 h. b Yields of 2a and trans/cis ratios were determined by 1H NMR using CH2Br2 as the internal standard. TFA = trifluoroacetate, COD = 1,5-cyclooctadiene, dba = dibenzylideneacetone, Py = pyridine. | ||
| 1 | Without Pd(OAc)2 (C1) | 0 |
| 2 | Without XantPhos (L1) | 0 |
| 3 | Without Cs2CO3 (B1) | 16, E/Z = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | Without blue LEDs | 0 |
| 5 | C2–8 instead of Pd(OAc)2 | Listed below |
| 6 | L2–10 instead of XantPhos | Listed below |
| 7 | B2–9 instead of Cs2CO3 | Listed below |
| 8 | Carried out in air | Trace |
| 9 | 5 mol% Pd(OAc)2/10 mol% L1, 24 h | 33, E/Z = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | Solvent = THF | 73, E/Z = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | Solvent = dioxane | 90, E/Z = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | Solvent = DCM | 60, E/Z = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | Solvent = PhCF3 | 41, E/Z = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | 1.0 equiv. Cs2CO3 | 88, E/Z = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | 3.0 equiv. Cs2CO3 | 90, E/Z = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16 | 1.0 equiv. styrene | 80, E/Z = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 17 | 2.0 equiv. styrene | 84, E/Z = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
||
When the reaction was carried out in ambient air, only a trace amount of desired product was furnished indicating that oxygen may inhibit the reaction (entry 8). Attempts on reducing the catalyst/ligand loading remained unfruitful (entry 9). A survey of different solvents suggested benzene and dioxane to be optimal, though other solvents, such as dichloromethane, benzotrifluoride and more polar THF, also delivered the product in medium to good yields (entries 10–13). Varying the amount of Cs2CO3 had a negligible effect on this reaction (entries 14–15). Investigating the loading of styrene suggested that 1.5 equivalent was optimal (entries 16–17).
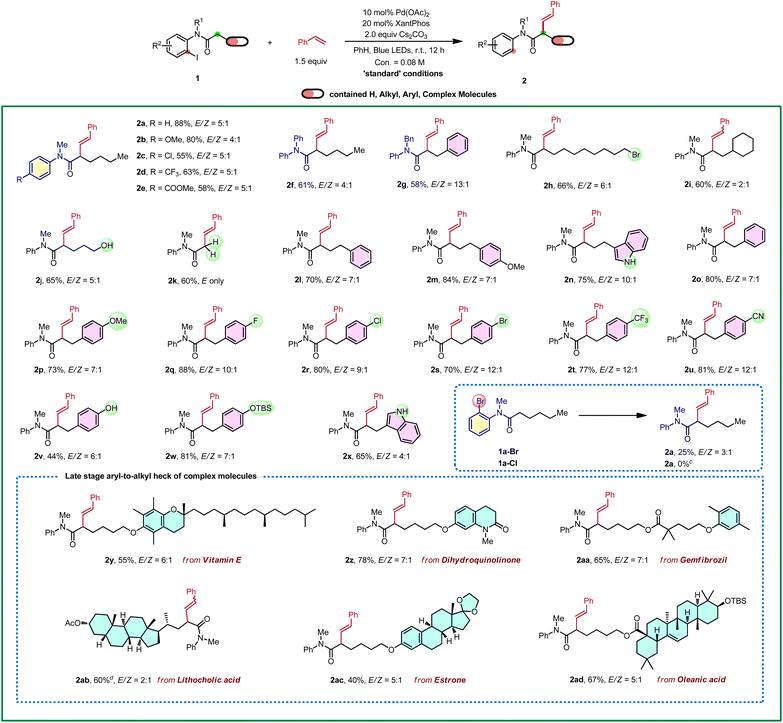
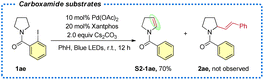
With the optimized conditions in hand, the substrate scope of amides was explored first (Table 2). Substitutions with both electron-withdrawing and electron-donating groups on the aniline moiety can all be tolerated with electron-rich arenes being more efficient (2a–2e). Then, substitutions at the nitrogen were explored, and the corresponding desired products were delivered in good yields (2f and 2g). Gratifyingly, a wide range of functional groups were tolerated at the carbonyl fragment including acid-sensitive moieties, such as TBS ether (2w and 2ad), ketal (2ac), and nucleophile/base sensitive moieties, such as alkyl halide (2h) and ester (2aa-2ab). In addition, free alcohol (2j), phenol (2v) and indol (2n and 2x) were compatible. Remarkably, the substrate with the acetyl group gave a single trans isomer product 2k. Moreover, common functional groups, such as methoxyl (2m, 2p), fluoride (2q), chloride (2r), bromide (2s), trifluoromethyl (2t), nitrile (2u) and lactam (2z) remained intact. Bromo-analogue (1a-Br) was a potentially viable substrate which gave 25% yield of the product with a large amount of 1a-Br remained. Chloro-analogue (1a-Cl) gave no desired product. Notably, late-stage modifications of biologically active molecules are often a key to identify medicinal agents. Amides derived from or tethered with bioactive natural products, such as vitamin E (2y), dihydroquinolinone (2z), gemfibrozil (2aa), lithocholic acid (2ab), estrone (2ac) and oleanic acid (2ad) all afforded the desired products in moderate to good yields. Next, 2-iodobenzamides 1ae was employed as the substrate, on the basis of the above optimized conditions, and it only produced the corresponding enamide side product S2-1ae in 70% yield and no Heck-type desired product was observed.18
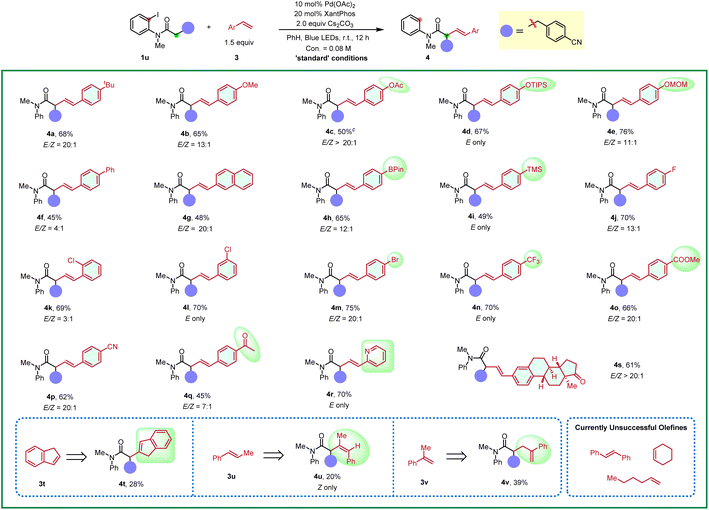
Furthermore, the substrate scope of styrene-type alkenes was examined in Table 3. Here, amide 1u was employed as the coupling partner due to its good yields and high trans/cis selectivity. On the whole, styrenes with both electron-withdrawing and electron-donating substituents were well tolerated under the standard conditions, affording the alkyl Heck products in good yields with good to excellent trans/cis selectivity. A wide range of functional groups proved to be compatible, including methoxy (4b), acetoxyl (4c), silyl ether (4d), acetal (4e), pinacol boronate (4h), trimethylsilyl (4i), fluoro (4j), chloro (4k and 4l), bromo (4m), trifluoromethyl (4n), ester (4o), cyano (4p), and ketone (4q and 4s) groups. Note that substitutions at the para, meta or ortho position of styrenes all reacted smoothly (4k, 4l). Extended aromatic rings, such as 4-vinyl-1,1′-biphenyl (3f), 2-vinylnaphthalene (3g), were also viable substrates. Notably, the reaction is compatible with 2-vinyl pyridine (3r) which has a good coordination ability, and the corresponding product was afforded in a good yield and excellent trans/cis selectivity (4r). An investigation of the substituents on the alkene moiety was also conducted. Both 1,2-disubstituted alkenes, such as 1H-indene (3t), (E)-prop-1-en-1-ylbenzene (3u) and 1,1-disubstituted, prop-1-en-2-ylbenzene (3v) were feasible substrates, albeit giving the corresponding products 4t–4v in 20–40% yields but as the single isomer. Aliphatic alkenes, such as hex-1-ene and cyclohexene were unfruitful, which might undergo direct desaturation. (E)-1,2-diphenylethene also gave the direct desaturation side product.
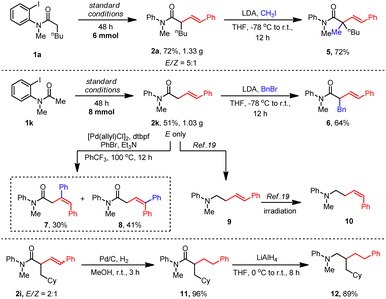
To test the practicality of this method, gram–scale reactions of 1a and 1k were carried out. More than one gram of the desired alkyl Heck products 2a and 2k were obtained with satisfactory yields (Scheme 2). Then, a variety of transformations were employed to extend the utility of this Heck reaction. First, α-alkylation occurred smoothly with methylation to give 5 and benzylation to form 6. Interestingly, a further aryl-Heck reaction of 2k with PhBr occurred at both olefine sides to produce 7 and 8. The carbonyl group of amide 2k could be reduced easily by LiAlH4 to form an amine product 9 of which the E olefine could be transformed to a Z isomer 10 under irradiation.19 Reduction of the olefine in 2i gave 11 in 96% yield which was further reduced by LiAlH4 to obtain an amine product 12.
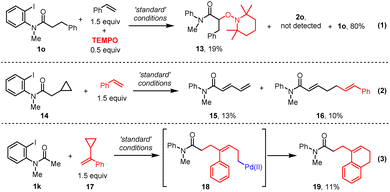
Based on the precedent literature for photo-induced palladium catalyzed alkyl-Heck reactions,13a–c,14a the mechanism is expected to be a hybrid Pd/radical pathway. A series of mechanistic studies were thus carried out. First, a radical-trapping experiment was conducted. In the presence of 50 mol% TEMPO, the α-radical was trapped by TEMPO to give 13 and no desired product was obtained (Scheme 3, eqn (1)). Next, the radical nature of this transformation was further confirmed by a radical-clock experiment with amide 14 and styrene, which produced both a ring-opening dienamide 15 and further a relay alkyl Heck product 1620(Scheme 3, eqn (2)). Finally, when cyclo-propane substituted styrene was subjected to this Heck reaction, benzocyclohexene product 1920was obtained through a ring-opening process followed by radical cyclization or electrophilic palladation of intermediate 18 (Scheme 3, eqn (3)). Altogether, these results provided evidence for the aryl-to-alkyl radical translocation process and radical nature of this relay Heck reaction.
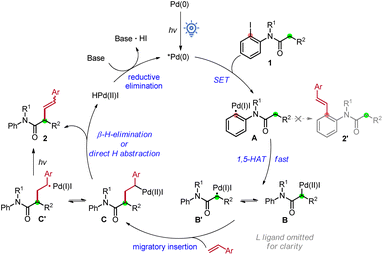
The reaction mechanism is proposed in Scheme 4. First, the Pd(0) complex is excited using visible light to form its excited state species which abstract the iodide on 1 in a SET process to form the aryl hybrid Pd-radical intermediate A. Subsequently, the latter undergoes a fast 1,5-HAT event to generate translocated alkyl hybrid Pd-radical species B′. Premature aryl Heck product 2′ was not observed here. Note that C–I bond reductive elimination or the I-atom transfer product was not observed in our reaction system. The species B/B′ then undergoes migratory insertion into the styrene to form the alkyl hybrid Pd-intermediate C/C′. The smooth occurrence of the reaction indicates that this step is much faster than β-H elimination of B. β-Hydride elimination of C or direct abstraction of a β-hydrogen atom by the Pd(I) species occurs of C′ afterwards to produce the product 2 and regenerate the Pd catalyst via reductive elimination. The formation of Z-(2) may be from the direct H abstraction of intermediate C′, and another possibility is that E-(2) could be transformed to a Z isomer under irradiation.19
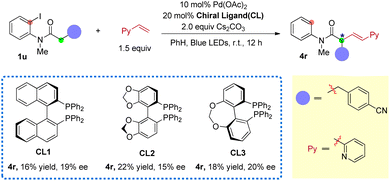
Based on the reaction mechanism, an enantioselective aryl-to-alkyl radical relay Heck reaction was attempted using chiral ligands (Scheme 5). The reaction of amide 1u with 2-vinyl pyridine was employed as the model reaction due to its good yields and high trans/cis selectivity. After screening of some chiral ligands, when BINAP-type chiral ligands (CL1-3) were employed as the ligands (instead of xantphos), 16–22% yield of the desired product 4r was obtained with some extent of enantio-control (from 15% to 20% ee) (for screening of more chiral ligands, see ESI S54†). This indicates the feasibility of developing an enantioselective photo-induced aryl-to-alkyl radical relay sp3 Heck reaction using chiral ligands. Efforts on improving both the enantioselectivity and conversion are ongoing.
Conclusions
In summary, a photo-induced palladium catalyzed aryl-to-alkyl radical relay Heck reaction at the α-C(sp3)-H site of amide has been developed. This reaction escaped the shackles of traditional alkyl halides as alkyl Heck reagents, but adopted inexpensive and commercially more available aryl halides, which are also considered to be more active in Heck reactions. The tolerance of a large variety of functional groups makes this method attractive for use in the synthesis of complex bioactive molecules. This method is scalable and operationally simple under mild conditions at room temperature. Additionally, the promising preliminary results obtained in this study will motivate the development of an enantioselective radical relay sp3 Heck reaction of amide. Mechanistic studies reveal a radical nature of this transformation. Development of intermolecular aryl-to-alkyl Heck reactions will be the focus of future work.Data availability
The experiments data were provide in ESI,† and we did not have the computational data.Author contributions
Y. D., X. S. and M. C. conceived the project and wrote the manuscript with feedback from the other authors. Y. D. and X. S. performed the experiments. M. C. and S. Y. supervised the project. All authors discussed the results and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Prof. M. Chen thanks Changzhou University for a startup fund and the financial support by NSFC/China (22101034), the Changzhou Leading Innovative Talent Project (CQ20210112), the Jiangsu specially appointed professors program and the start-up funding to M. Chen by Changzhou University (ZMF21020031). Dr S. Yang thanks the support by the Scientific Research Foundation of Jiangsu Provincial Education Department (21KJD150002) and Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology (BM2012110). Dr J-H. Li thanks the support by NSFC/China (21901012). We also thank the Analysis and Testing Center, NERC Biomass of Changzhou University for the assistance in NMR analysis. Jiaming Chen is acknowledged for checking the experiments. This work is dedicated to the 45th anniversary of the founding of Changzhou University.Notes and references
- (a) R. F. Heck and J. P. Nolley, J. Org. Chem., 1972, 37, 2320–2322 CrossRef CAS; (b) T. Mizoroki and K. Mori, Bull. Chem. Soc. Jpn., 1971, 44, 581 CrossRef CAS.
- (a) C. C. C. Johansson Seechurn, M. O. Kitching and T. J. Colacot, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef CAS; (b) M. Oestreich, The Mizoroki-Heck Reaction, John Wiley & Sons, West Sussex, U.K, 2009 CrossRef.
- W. Ma, P. Gandeepan, J. Li and L. Ackermann, Org. Chem. Front., 2017, 4, 1435–1467 RSC.
- (a) S. Patai, The Chemistry of the Metal-Carbon Bond, ed. J. K. Stille, Wiley, New York, 1985, vol. 2 Search PubMed; (b) R. G. Pearson and P. E. Figdore, J. Am. Chem. Soc., 1980, 102, 1541–1547 CrossRef CAS; (c) J. P. Collman, Acc. Chem. Res., 1975, 8, 342–347 CrossRef CAS; (d) A. C. Frisch and M. Beller, Angew. Chem., Int. Ed., 2005, 44, 674–688 CrossRef CAS; (e) T.-Y. Luh, M.-k. Leung and K.-T. Wong, Chem. Rev., 2000, 100, 3187–3204 CrossRef CAS; (f) A. Ariafard and Z. Lin, Organometallics, 2006, 25, 4030–4033 CrossRef CAS.
- (a) S. Brase and A. deMeijere, in Metal-Catalyzed Cross-Coupling Reactions, ed. F. Diederich and P. J. Stang, Wiley-VCH, Weinheim, 1998, ch. 3 Search PubMed; (b) F. Ozawa, T. Ito and A. Yamamoto, J. Am. Chem. Soc., 1980, 102, 6457–6463 CrossRef CAS; (c) J. Hartwig, Organotransition Metal Chemistry: From Bonding to Catalysis, University Science Books, Sausalito, CA, 2009, ch. 10, p. 398402 Search PubMed; (d) A. C. Bissember, A. Levina and G. C. Fu, J. Am. Chem. Soc., 2012, 134, 14232–14237 CrossRef CAS PubMed.
- N. Koga, S. Obara, K. Kitaura and K. Morokuma, J. Am. Chem. Soc., 1985, 107, 7109–7116 CrossRef CAS.
- (a) K. S. Bloome, R. L. McMahen and E. J. Alexanian, J. Am. Chem. Soc., 2011, 133, 20146–20148 CrossRef CAS; (b) N. Kambe, T. Iwasaki and J. Terao, Chem. Soc. Rev., 2011, 40, 4937–4947 RSC; (c) A. Rudolph and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 2656–2670 CrossRef CAS PubMed.
- For nickel-catalyzed Heck-type reactions of alkyl halides, see: (a) S. A. Lebedev, V. S. Lopatina, E. S. Petrov and I. P. Beletskaya, J. Organomet. Chem., 1988, 344, 253–259 CrossRef CAS for a recent report on nickel-catalyzed Heck-type reactions of primary benzyl chlorides, see:; (b) R. Matsubara, A. C. Gutierrez and T. F. Jamison, J. Am. Chem. Soc., 2011, 133, 19020–19023 CrossRef CAS PubMed; (c) C. Liu, S. Tang, D. Liu, J. Yuan, L. Zheng, L. Meng and A. Lei, Angew. Chem., Int. Ed., 2012, 51, 3638 CrossRef CAS PubMed For palladium-catalyzed Heck-type reactions of alkyl halides with no β C–H bond, see:; (d) F. Glorius, Tetrahedron Lett., 2003, 44, 5751–5754 CrossRef CAS; (e) M. Mori, I. Oda and Y. Ban, Tetrahedron Lett., 1982, 23, 5315–5318 CrossRef CAS.
- (a) Z. Feng, Q.-Q. Min, H.-Y. Zhao, J.-W. Gu and X. Zhang, Angew. Chem., Int. Ed., 2015, 54, 1270–1274 CrossRef CAS PubMed; (b) F. Zhang, Q.-Q. Min and X. Zhang, Synthesis, 2015, 47, 2912–2923 CrossRef CAS; (c) N. Surapanich, C. Kuhakarn, M. Pohmakotr and V. Reutrakul, Eur. J. Org. Chem., 2012, 2012, 5943–5952 CrossRef CAS; (d) Z. Li, E. Merino and C. Nevado, Top. Catal., 2017, 60, 545–553 CrossRef CAS; (e) Z. Li, A. García-Domínguez and C. Nevado, J. Am. Chem. Soc., 2015, 137, 11610–11613 CrossRef CAS PubMed.
- L. Firmansjah and G. C. Fu, J. Am. Chem. Soc., 2007, 129, 11340–11341 CrossRef CAS.
- (a) K. S. Bloome, R. L. McMahen and E. J. Alexanian, J. Am. Chem. Soc., 2011, 133, 20146–20148 CrossRef CAS PubMed; (b) C. M. McMahon and E. J. Alexa-nian, Angew. Chem., Int. Ed., 2014, 53, 5974–5977 CrossRef CAS PubMed; (c) A. R. O. Venning, M. R. Kwiatkowski, J. E. Roque Pena, B. C. Lainhart, A. A. Guruparan and E. J. Alexanian, J. Am. Chem. Soc., 2017, 139, 11595–11600 CrossRef CAS PubMed.
- Y. Zou and J. Zhou, Chem. Commun., 2014, 50, 3725–3728 RSC.
- (a) D. Kurandina, M. Parasram and V. Gevorgyan, Angew. Chem., Int. Ed., 2017, 56, 14212–14216 CrossRef CAS PubMed; (b) P. Chuentragool, D. Yadagiri, T. Morita, S. Sarkar, M. Parasram, Y. Wang and V. Gevorgyan, Angew. Chem., Int. Ed., 2019, 58, 1794–1798 CrossRef CAS; (c) D. Kurandina, M. Rivas, M. Radzhabov and V. Gevorgyan, Org. Lett., 2018, 20, 357–360 CrossRef CAS; (d) P. Chuentragool, D. Kurandina and V. Gevorgyan, Angew. Chem., Int. Ed., 2019, 58, 11586–11598 CrossRef CAS.
- (a) G.-Z. Wang, R. Shang, W.-M. Cheng and Y. Fu, J. Am. Chem. Soc., 2017, 139, 18307–18312 CrossRef CAS; (b) G.-Z. Wang, R. Shang and Y. Fu, Org. Lett., 2018, 20, 888–891 CrossRef CAS PubMed; (c) B. Zhao, R. Shang, G.-Z. Wang, S. Wang, H. Chen and Y. Fu, ACS Catal., 2020, 10, 1334–1343 CrossRef CAS.
- (a) L. Chen, Y. Hisaeda and H. Shimakoshi, Adv. Synth. Catal., 2019, 361, 2877–2884 CrossRef CAS; (b) G. A. M. Jardim, J. A. Damtas, A. A. Barboza, M. W. Paixao and M. A. B. Ferreira, Synthesis, 2022, 54, 4629–4645 CrossRef CAS; (c) M. Li, Y.-F. Qiu, C.-T. Wang, X.-S. Li, W.-X. Wei, Y.-Z. Wang, Q.-F. Bao, Y.-N. Ding, W.-Y. Shi and Y.-M. Liang, Org. Lett., 2020, 22, 6288–6293 CrossRef CAS PubMed.
- (a) M. Parasram, P. Chuentragool, D. Sarkar and V. Gevorgyan, J. Am. Chem. Soc., 2016, 138, 6340–6343 CrossRef CAS PubMed; (b) P. Chuentragool, M. Parasram, Y. Shi and V. Gevorgyan, J. Am. Chem. Soc., 2018, 140, 2465–2468 CrossRef CAS PubMed; (c) S. Yang, H. Fan, L. Xie, G. Dong and M. Chen, Org. Lett., 2022, 24, 6460–6465 CrossRef CAS PubMed; (d) R. Guo, H. Xiao, S. Li, Y. Luo, J. Bai, M. Zhang, Y. Guo, X. Qi and G. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202208232 CAS; (e) S. Sarkar, W. Sidhant, X. Jia and V. Gevorgyan, Chem, 2022, 8, 3096–3108 CrossRef CAS PubMed.
- (a) A.-F. Voica, A. Mendoza, W. R. Gutekunst, J. O. Fraga and P. S. Baran, Nat. Chem., 2012, 4, 629–635 CrossRef CAS; (b) W. Jin and S. Yu, Org. Lett., 2021, 23, 6931–6935 CrossRef CAS PubMed; (c) Y. Xia, K. Jana and A. Studer, Chem – Eur. J., 2021, 27, 16621–16625 CrossRef CAS PubMed; (d) L. M. Stateman, R. M. Dare, A. N. Paneque and D. A. Nagib, Chem, 2022, 8, 210–224 CrossRef CAS PubMed; (e) W.-L. Yu, Z.-G. Ren, K.-X. Ma, H.-Q. Yang, J.-J. Yang, H. Zheng, W. Wu and P.-F. Xu, Chem. Sci., 2022, 13, 7947–7954 RSC; (f) C. Wang, L. M. Azofra, P. Dam, M. Sebek, N. Steinfeldt, J. Rabeah and O. El-Sepelgy, ACS Catal., 2022, 12, 8868–8876 CrossRef CAS; (g) M. Ratushnyy, N. Kvasovs, S. Sarkar and V. Gevorgyan, Angew. Chem., Int. Ed., 2020, 59, 10316 CrossRef CAS PubMed.
- The carboxamide substrate 1ae only gave the direct desaturation product S2-1ae in 70% yield, and the radical relay Heck product 2ae was not observed
. - F. D. Lewis, J. M. Wagner-Brennan and A. M. Miller, Can. J. Chem., 1999, 77, 595–604 CrossRef CAS.
- The low yield of the reaction came from the decomposition of starting materials and the formation of other side-products with messy NMR.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sc06852d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |