Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Supramolecular multi-electron redox photosensitisers comprising a ring-shaped Re(I) tetranuclear complex and a polyoxometalate†
Maria
Takahashi
a,
Tsuyoshi
Asatani
a,
Tatsuki
Morimoto
b,
Yoshinobu
Kamakura
 a,
Kotaro
Fujii
a,
Kotaro
Fujii
 a,
Masatomo
Yashima
a,
Masatomo
Yashima
 a,
Naoki
Hosokawa
a,
Yusuke
Tamaki
a and
Osamu
Ishitani
a,
Naoki
Hosokawa
a,
Yusuke
Tamaki
a and
Osamu
Ishitani
 *ac
*ac
aDepartment of Chemistry, School of Science, Tokyo Institute of Technology, O-okayama 2-12-1-NE-1, Meguro-ku, Tokyo 152-8550, Japan. E-mail: ishitani@chem.titech.ac.jp
bSchool of Engineering, Tokyo University of Technology, 1404-1 Katakura, Hachioji, Tokyo 192-0982, Japan
cDepartment of Chemistry, Graduate School of Advanced Science and Engineering, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima, Hiroshima 739 8526, Japan
First published on 1st December 2022
Abstract
Redox photosensitisers (PSs) play essential roles in various photocatalytic reactions. Herein, we synthesised new redox PSs of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 supramolecules that comprise a ring-shaped Re(I) tetranuclear complex with 4+ charges and a Keggin-type heteropolyoxometalate with 4− charges. These PSs photochemically accumulate multi-electrons in one molecule (three or four electrons) in the presence of an electron donor and can supply electrons with different reduction potentials. PSs were successfully applied in the photocatalytic reduction of CO2 using catalysts (Ru(II) and Re(I) complexes) and triethanolamine as a reductant. In photocatalytic reactions, these supramolecular PSs supply a different number of electrons to the catalyst depending on the redox potential of the intermediate, which is made from the one-electron-reduced species of the catalyst and CO2. Based on these data, information on the reduction potentials of the intermediates was obtained.
1 supramolecules that comprise a ring-shaped Re(I) tetranuclear complex with 4+ charges and a Keggin-type heteropolyoxometalate with 4− charges. These PSs photochemically accumulate multi-electrons in one molecule (three or four electrons) in the presence of an electron donor and can supply electrons with different reduction potentials. PSs were successfully applied in the photocatalytic reduction of CO2 using catalysts (Ru(II) and Re(I) complexes) and triethanolamine as a reductant. In photocatalytic reactions, these supramolecular PSs supply a different number of electrons to the catalyst depending on the redox potential of the intermediate, which is made from the one-electron-reduced species of the catalyst and CO2. Based on these data, information on the reduction potentials of the intermediates was obtained.
Introduction
Redox reactions initiated by photochemical electron transfer have been widely used in various research fields, such as organic synthesis1,2 and artificial photosynthesis.3–7 In these reactions, redox photosensitisers (PSs), also referred to as photoredox catalysts,1,2 induce photochemical electron transfer from an electron donor to an acceptor as a starting process.7 For example, the triplet metal-to-ligand-charge-transfer (3MLCT) excited state of tris(2,2′-bipyridine) ruthenium(II) ([Ru(bpy)3]2+) is formed through photoirradiation; this excited state has superior reduction and oxidation capabilities, i.e., 2.12 eV greater (as excitation energy) compared to its ground state.8 Since both the oxidised and reduced states of the Ru complex are stable, they are used as effective PSs in numerous photochemical redox reactions. In recent years, Ir(III) complexes,1,7,9,10 Os(II) complexes,7,11 and Cu(I) heteroleptic complexes12–18 and some organic compounds2b,19–21 have also been used as PSs.Generally, the photoexcitation of a molecule can induce only one-electron transfer. Hence, typical PSs can initiate only a one-electron transfer from the electron donor to the acceptor. Since, on the other hand, one-electron reduction of CO2 requires very high amounts of energy (E0 = −1.9 V vs. NHE), the two-electron reduction of CO2 coupled with another chemical reaction, such as proton addition can be applied to lower the required energy to produce stable products such as CO (E0 = −0.53 V) and HCOOH (E0 = −0.61 V). Hence, catalysts that completely accept two electrons through redox-photosensitised reaction(s) and reduce CO2 should be used with PSs to achieve efficient photocatalytic CO2 reduction.7
In such two-component photocatalytic systems, an intermediate produced by the chemical reaction(s) of the one-electron-reduced species (OERS) of the catalyst must rapidly accept one more electron because the side reactions of the active intermediate induce decomposition of the catalyst and lower the durability of the photocatalytic system. For avoiding this decomposition process of the catalyst, a significantly higher number of PSs than catalysts has been used in many reported photocatalytic systems to suppress these side reactions because the usual PSs initiate only a one-electron transfer.7 In these systems, however, the decomposition of PSs cannot be avoided owing to the photochemical decomposition of the OERSs of the excess PSs, which accumulate in the reaction solution.
Therefore, developing PSs that can accumulate multi-electrons in one molecule and donate them to the catalytic reaction with suitable timing should inspire a new direction in photocatalytic redox reactions. However, only a limited number of PSs can accumulate multi-electrons. For example, Ru(II) complexes with quinone or pyridinium-cation moieties integrated into ligands22 or viologen moieties attached to ligands23 have been reported. However, such PSs have weak reduction power, and their application in photocatalytic reactions has been limited to low-energy reactions, such as H2 evolution.23–27 Another challenge associated with multi-electron-accumulating PSs is that the accumulated electrons in PSs with multiple numbers of the same or similar photosensitiser units in one molecule, such as ring-shaped Re(I) multinuclear complexes,28,29,30a,37 have the same or similar reduction powers. It cannot accumulate multi-electrons in the presence of a catalyst because its OERS rapidly passes an accepted electron to the catalyst, i.e., it works only as a one-electron transfer photosensitiser.28,29
In photocatalytic systems for CO2 reduction, the reduction potentials of the catalyst and the intermediate derived from the reaction of the reduced catalyst with a substrate such as CO2 should be different. Since the reduction potential of the intermediate, which is very important information for developing efficient photocatalytic systems, is often more positive than the first one owing to the following chemical reaction or reactions, the reduction potential cannot be determined using ordinary electrochemical methods. To the best of our knowledge, there have been no reports of a multi-electron-accumulating PS that can precisely supply two electrons to the catalyst and intermediate in photocatalytic CO2 reduction reactions.
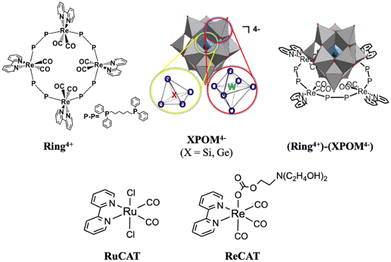
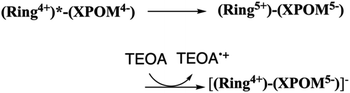
Herein, we report the first examples of supramolecular redox photosensitisers consisting of a ring-shaped Re(I) tetranuclear complex (Ring4+) and Keggin-type heteropolyoxometalate [XW12O40]4− (XPOM4−, X = Si, Ge) (Chart 1). Ring4+ has four positive charges owing to the one plus charge of each Re(I) unit and the space inside the ring structure.28–30a Its lowest 3MLCT excited state has a long lifetime even in solution at 25 °C (τ = 225 and 406 ns), of which the dual phosphorescence property is attributed to stable conformers,28 and high oxidation power. Its OERS is relatively stable, and its reduction power is high. In contrast, XPOM4−, in which twelve octahedral tungsten oxyanions surround a central silicate or germanate group, has four negative charges. They can electrochemically accumulate multi-electrons in a single molecule because their reduced states are stable.31–33
We found that strong electrostatic interactions between XPOM4− and Ring4+ formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ion pair (Ring4+)–(XPOM4−), as shown in Chart 1. In these supramolecules, the Ring4+ unit functions as an intramolecular PS that photochemically transfers multi-electrons to the XPOM4− unit in the presence of triethanolamine in the initial stage. It accumulates one more electron in one of the Re(I) complex moieties, resulting in three electrons with different reduction powers. Using this novel photochemical multi-electron accumulating system as a PS, we developed new photocatalytic CO2 reduction systems accompanied by Re(I)- or Ru(II)-complex catalysts (RuCAT and ReCAT in Chart 1). In addition, a comparison of the photosensitising abilities of the two types of (Ring4+)–(XPOM4−), where X = Si or Ge, provides information on redox potentials of the intermediates formed from the OERSs of the Re and Ru catalysts.
1 ion pair (Ring4+)–(XPOM4−), as shown in Chart 1. In these supramolecules, the Ring4+ unit functions as an intramolecular PS that photochemically transfers multi-electrons to the XPOM4− unit in the presence of triethanolamine in the initial stage. It accumulates one more electron in one of the Re(I) complex moieties, resulting in three electrons with different reduction powers. Using this novel photochemical multi-electron accumulating system as a PS, we developed new photocatalytic CO2 reduction systems accompanied by Re(I)- or Ru(II)-complex catalysts (RuCAT and ReCAT in Chart 1). In addition, a comparison of the photosensitising abilities of the two types of (Ring4+)–(XPOM4−), where X = Si or Ge, provides information on redox potentials of the intermediates formed from the OERSs of the Re and Ru catalysts.
Results and discussion
Synthesis of (Ring4+)–(XPOM4−) and their structures
(Ring4+)(PF6−)430 and (TBA+)4(XPOM4−) (X = Si or Ge; TBA+ = tetrabutylammonium cation)34,35 were synthesised according to previously reported procedures. An acetonitrile solution (6 mL) containing (Ring4+)(PF6−)4 (3.00 μmol) was added to another acetonitrile solution containing (TBA+)4(SiPOM4−) (3.00 μmol), giving (Ring4+)–(SiPOM4−) as yellow solids in a 96% yield (Fig. S1†). A similar method, except for the usage of (TBA+)4(GePOM4−) instead of (TBA+)4(SiPOM4−), gave (Ring4+)–(GePOM4−) also as yellow solids (yield = 97%). Their elemental analysis data clearly indicate that both of them were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ion pairs consisting of Ring4+ and XPOM4−.
1 ion pairs consisting of Ring4+ and XPOM4−.
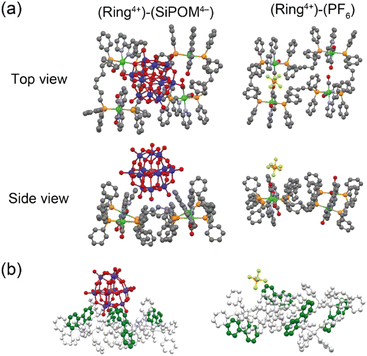
Single crystals of both (Ring4+)–(SiPOM4−), and (Ring4+)(PF6−)4 were obtained by recrystallisation using N,N-dimethylacetamide/methanol (crystallographic data of (Ring4+)–(SiPOM4−) and (Ring4+)(PF6−)4 are shown in Table S1†). Fig. 1a shows the crystal structures determined using single-crystal X-ray diffraction, which shows that Ring4+ and SiPOM4− form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ion pairs. There were observable differences between the Ring4+ moieties of (Ring4+)–(SiPOM4−) and (Ring4+)(PF6−)4.
1 ion pairs. There were observable differences between the Ring4+ moieties of (Ring4+)–(SiPOM4−) and (Ring4+)(PF6−)4.
For instance, the distances between two adjacent Re(I) ions in (Ring4+)–(SiPOM4−) (7.77, 11.41, 7.77, 11.41 Å) were smaller than those in (Ring4+)(PF6−)4 (7.99, 11.28, 8.12, 11.67 Å). All the bpy ligands in (Ring4+)–(SiPOM4−) were seated on the same side of the least-squares plane of the four Re(I) units in the same Ring4+. The SiPOM4− moiety was seated and closely surrounded by the four bpy units (Fig. 1b, left). In contrast, in (Ring4+)(PF6−)4, the two bpy units on the diagonal were seated on the same side, while the other two were on the opposite side (Fig. 1b, right). These results strongly indicate that all Re(I) ions with a positive charge and all bpy ligands that have a positive charge compared to the CO ligands owing to their different electron-withdrawing properties should compensate for the four negative charges of the SiPOM4− moiety. The closest distance between the Ring4+ and SiPOM4− in (Ring4+)–(SiPOM4−) was 3.26 Å for the C(bpy) and O(SiPOM4−), suggesting that interaction between Ring4+ and SiPOM4− exist because this distance is similar to the sum of the van der Waals radii of carbon (1.70 Å) and oxygen (1.52 Å).36
(Ring4+)–(XPOM4−) dissolved in DMSO solution
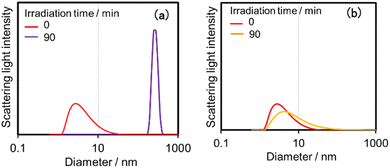
Both (Ring4+)–(SiPOM4−) and (Ring4+)–(GePOM4−) were soluble in dimethyl sulfoxide (DMSO); however, only in small amounts in acetonitrile and N,N-dimethylformamide. 1H NMR spectra of (Ring4+)–(SiPOM4−) and (Ring4+)(PF6−)4 in DMSO-d6 were different (Fig. S2, Table S2†). The peaks attributed to the protons at the 4,4′-positions (bpy-4 in Table S2†) of (Ring4+)–(SiPOM4−) broadened, and about 0.4 ppm downfield shifted compared to those of (Ring4+)(PF6−)4. The other peaks of the bpy ligand and phenyl groups of the phosphine ligands of (Ring4+)–(SiPOM4−) were slightly shifted (−0.09 to 0.09 ppm). The FT-IR spectra of (Ring4+)–(SiPOM4−) and (Ring4+)(PF6−)4 measured in DMSO were also different (Fig. S3a†): νCO = 1927 and 1846 cm−1 in the solution of (Ring4+)(PF6−)4 while νCO peaks at 1928 and 1852 cm−1 in the solution of (Ring4+)–(SiPOM4−), whose shapes were also different. These differences in the spectra indicate that Ring4+ and SiPOM4− dissolved in the DMSO solution interact closely, even in solution. 1H NMR and FT-IR spectra of (Ring4+)–(GePOM4−) in DMSO-d6 and DMSO, respectively, also showed similar differences from those of (Ring4+)(PF6−)4 (Table S2, Fig. S3b†).Since Ring4+ and XPOM4− interact with each other even in DMSO solutions, we measured particle sizes in DMSO solutions containing (Ring4+)(PF6−)4, (TBA+)4(SiPOM4−), (Ring4+)–(SiPOM4−), (TBA+)4(GePOM4−), or (Ring4+)–(GePOM4−) using dynamic light scattering (DLS). Fig. 2 shows the particle size distributions. In the solutions of (Ring4+)(PF6−)4, (TBA+)4(SiPOM4−), and (TBA+)4(GePOM4−), small particles with diameters (D) of approximately 1 nm were mainly observed, and these are attributable to solvated monomeric Ring4+, SiPOM4−, and GePOM4−, respectively, some of which may interact with the counter anions or cations because of the size similarity to the X-ray structure of each ion of (Ring4+)–(SiPOM4−) (Fig. 1). In contrast, in the solution containing (Ring4+)–(SiPOM4−), larger particles (2.9 ± 0.4 nm) were mainly observed compared to those of Ring4+ and SiPOM4− (Fig. 2a). Therefore, even in the DMSO solution, Ring4+ and SiPOM4− maintained the interaction and formed a supramolecule consisting of one molecule of Ring4+ and one molecule of SiPOM4−. In the DMSO solution of (Ring4+)–(GePOM4−), particles of similar size (3.2 ± 0.5 nm) were observed (Fig. 2b). Therefore, it can be deduced that strong electrostatic interactions maintain supramolecular interactions between Ring4+ and XPOM4− even in DMSO solutions and mainly form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 supramolecule consisting of one ion each.
1 supramolecule consisting of one ion each.
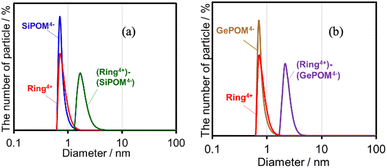 | ||
| Fig. 2 Particle size distributions of the DMSO solutions containing (a) (Ring4+)(PF6−)4, (TBA+)4(SiPOM4−), or (Ring4+)–(SiPOM4−), and (b) (Ring4+)(PF6−)4, (TBA+)4(GePOM4−), or (Ring4+)–(GePOM4−). | ||
Fig. 3 shows UV-vis absorption spectra of (Ring4+)–(XPOM4−) and their constituent ions in DMSO solutions. The similarity in the spectra of (Ring4+)–(XPOM4−) and the summation of those of (Ring4+)(PF6−)4 and (TBA+)4(XPOM4−) indicates that there was no strong electric interaction between the Ring4+ and XPOM4− units of (Ring4+)–(XPOM4−) in the ground states, although they exist closely in the solutions as described above. In both spectra of (Ring4+)–(XPOM4−), a broad peak was observed at λmax = 408 nm, which is attributed to singlet metal-to-ligand-charge-transfer (1MLCT) absorption of the Ring4+ unit.
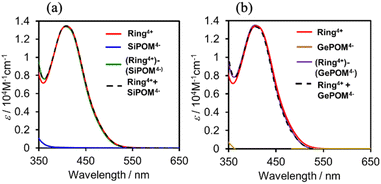 | ||
| Fig. 3 UV-vis absorption spectra of (a) (Ring4+)–(SiPOM4−) and (b) (Ring4+)–(GePOM4−) in the DMSO solutions: spectra of (Ring4+)(PF6−)4, (TBA+)4(XPOM4−), and summation of them were also shown. | ||
In the much shorter wavelength region, singlet ligand-to-metal-charge-transfer (1LMCT) absorption of the XPOM4− unit and singlet π–π* absorption of the Ring4+ unit were observed. Therefore, only the Ring4+ unit of the supramolecule can be selectively excited by light at λex ≥ 400 nm in photochemical reactions.
Fig. 4 shows emission spectra of (Ring4+)(PF6−)4 and (Ring4+)–(XPOM4−) dissolved in DMSO solutions using excitation light at λex = 400 nm. This outcome is due to the emission from Ring4+ or the Ring4+ unit of the supramolecules because the shapes of the emission spectra were very similar in all the solutions. However, the strengths of both (Ring4+)–(SiPOM4−) and (Ring4+)–(GePOM4−) were significantly weaker than that of (Ring4+)(PF6−)4. The photophysical properties of the samples are summarised in Table 1. The emission quantum yields (Φem) of the Ring4+ units in (Ring4+)–(SiPOM4−) and (Ring4+)–(GePOM4−) were only 15% and 13% compared to that of (Ring4+)(PF6−)4, respectively. Therefore, it can be inferred that most of the 3MLCT excited states of the Ring4+ units in the supramolecules were quenched by the XPOM4− unit.
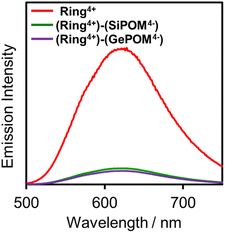 | ||
| Fig. 4 Emission spectra of (Ring4+)(PF6−)4 (red), (Ring4+)–(SiPOM4−) (green), and (Ring4+)–(GePOM4−) (purple): λex = 400 nm, concentration of the complex was 0.05 mM. | ||
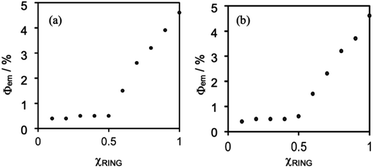
Fig. 5 shows Job plots of emission quantum yields (Φem) from the mixed solutions of the DMSO solutions containing (Ring4+)(PF6−)4 and (TBA+)4(XPOM4−). Their total amount was the same (0.10 mM) but their ratio (χRING = [Ring4+]/([Ring4+] + [XPOM4−])) was changed. At χRING > 0.5, smaller χRING made higher Φem, but Φem was almost constant at χRING ≤ 0.5. These results clearly indicate that almost all of the added XPOM4− were converted into the supramolecule with Ring4+ in the DMSO solution when [(TBA+)4(XPOM4−)] < [(Ring4+)(PF6−)4]. Even on the occasion of [(TBA+)4(XPOM4−)] = [(Ring4+)(PF6−)4], most of Ring4+ interacted with XPOM4− to form the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 supramolecule (Ring4+)–(XPOM4−) in both DMSO solutions containing either (TBA+)4(GePOM4−) or (TBA+)4(SiPOM4−).
1 supramolecule (Ring4+)–(XPOM4−) in both DMSO solutions containing either (TBA+)4(GePOM4−) or (TBA+)4(SiPOM4−).
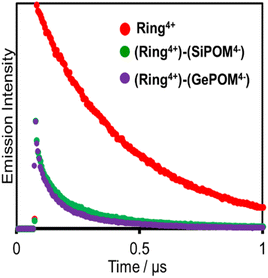
Fig. 6 shows emission decays of Ring4+ and (Ring4+)–(XPOM4−) dissolved in the DMSO solutions using the single-photon counting method, normalised by absorbed photon numbers (λex = 400 nm, λdet = 615 nm). Notably, the emission strength at time = 0 was very different between (Ring4+)–(SiPOM4−) and free Ring4+, i.e., the former was much weaker than the latter when the number of integrations was unified. This result clearly indicates that static quenching of the excited state of the Ring4+ unit by the SiPOM4− unit rapidly proceeded within the time resolution of the apparatus (200 ps). Based on careful investigation of these emission decay data as described in the ESI section,† we can conclude that the percentage of dissociation of (Ring4+)–(SiPOM4−) was only 3.9%, and more than 85% of the excited state of the Ring4+ unit was statically quenched by the SiPOM4− unit in the DMSO solution dissolving 0.05 mM of (Ring4+)–(SiPOM4−).
In the DMSO solution containing 0.05 mM of (Ring4+)–(GePOM4−), a similar emission quenching (Fig. S4†) were observed compared to the free Ring4+. These findings show that the supramolecular structure of (Ring4+)–(GePOM4−) was maintained when the Ring4+ unit was excited. Additionally, the 3MLCT excited state of the Ring4+ unit was efficiently quenched by the GePOM4− unit in the (Ring4+)–(GePOM4−) as well (more than 87%).
Electrochemical properties
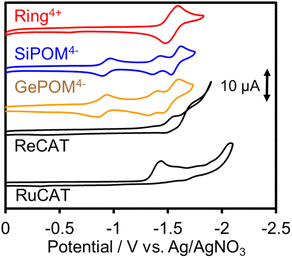
Since (Ring4+)–(XPOM4−)s were partially separated into Ring4+ and XPOM4− in solutions containing electrolyte, we separately measured cyclic voltammograms (CVs) of each unit. Since, as described above, the UV-vis absorption spectra does not change between (Ring4+)–(XPOM4−) and the summation of Ring4+ and XPOM4− (1:1), the redox potentials of each unit of (Ring4+)–(XPOM4−)s should scarcely change compared to those of separated Ring4+ and XPOM4−. Fig. 7 shows CVs of (Ring4+)(PF6−)4 and (TBA+)4(XPOM4−) in DMSO–TEOA (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solutions containing 0.1 M of (TBA+)(PF6−) as supporting electrolyte under a CO2 atmosphere. The voltammograms of catalysts used in photocatalytic reactions as described below are also added. In the DMSO solution of (Ring4+)(PF6−)4, a reversible redox wave was observed at E1/2 = −1.54 V vs. Ag/AgNO3 (ΔEp = 118 mV), as previously reported in a DMF solution.30a This redox is attributed to the one-electron reduction of each Re unit; that is, four electrons were introduced into Ring4+ at once (eqn (1)).
1 v/v) solutions containing 0.1 M of (TBA+)(PF6−) as supporting electrolyte under a CO2 atmosphere. The voltammograms of catalysts used in photocatalytic reactions as described below are also added. In the DMSO solution of (Ring4+)(PF6−)4, a reversible redox wave was observed at E1/2 = −1.54 V vs. Ag/AgNO3 (ΔEp = 118 mV), as previously reported in a DMF solution.30a This redox is attributed to the one-electron reduction of each Re unit; that is, four electrons were introduced into Ring4+ at once (eqn (1)).| [(–C6H12PPh2)ReI(bpy)(CO)2(PPh2–)]44+ + 4e− ⇆ [(–C6H12PPh2)ReI(bpy−˙)(CO)2(PPh2–)]4 | (1) |
In both solutions of (TBA+)4(XPOM4−), three reversible waves were observed at E1/2 = −0.93 V (ΔEp = 77 mV), E1/2 = −1.40 V (ΔEp = 78 mV), and E1/2 = −1.58 V (ΔEp = 90 mV) in the case of SiPOM4−, and E1/2 = −0.88 V (ΔEp = 68 mV), E1/2 = −1.33 V (ΔEp = 85 mV), and E1/2 = −1.50 V (ΔEp = 100 mV) in the case of GePOM4−, respectively. SiPOM4− can accept one, one, and two electrons in order, that is, four electrons in one molecule, according to the reactions shown in eqn (2)–(4), particularly under acidic conditions.38 In the DMSO–TEOA (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution under a CO2 atmosphere, TEOA and contaminated water probably worked as proton donors with the assistance of carbonate produced from the dissolved CO2, and carbonate esters produced from CO2 and TEOA.39 The similarity of the CVs of SiPOM4− and GePOM4− strongly suggests that similar three-step reduction processes (eqn (2)–(4)) also occurred in the CV of GePOM4−.
1 v/v) solution under a CO2 atmosphere, TEOA and contaminated water probably worked as proton donors with the assistance of carbonate produced from the dissolved CO2, and carbonate esters produced from CO2 and TEOA.39 The similarity of the CVs of SiPOM4− and GePOM4− strongly suggests that similar three-step reduction processes (eqn (2)–(4)) also occurred in the CV of GePOM4−.
| XW12O404− + e− ⇆ XW12O405− (X = Si, Ge) | (2) |
| XW12O405− + e− ⇆ XW12O406− | (3) |
| XW12O406− + 2e− + 2H+ ⇆ H2XW12O406− | (4) |
Although the exact reduction potential of the 3MLCT excited state of Ring4+ has not been determined because of a lack of information on the excitation energy of Re complexes, it is expected as approximately −1.0 to −1.1 V vs. Ag/AgNO3.30 This is more negative compared to the first reduction potential of SiPOM4− and GePOM4− (E1/2 = −0.93 V and −0.88 V, respectively). Considering that there is no strong electric interaction between the Ring4+ and XPOM4− units, we can conclude that the emission quenching observed in the supramolecule (Ring4+)–(XPOM4−) proceeds via electron transfer from the excited Ring4+ unit to XPOM4− (eqn (5)). The reduction potentials described above are listed in Table 2.
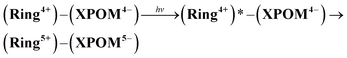 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solutions under a CO2 atmosphere
1 v/v) solutions under a CO2 atmosphere
| Complex | E red1/2/V vs. Ag/AgNO3 (ΔE/mV) | |||
|---|---|---|---|---|
| XPOM4−/XPOM5− | XPOM5−/XPOM6− | XPOM6−/H2XPOM6− | Ring4+/Ring0 | |
| (Ring4+)(PF6−)4 | — | — | — | −1.54 (118) |
| (TBA+)4(SiPOM4−) | −0.93 (77) | −1.40 (78) | −1.58 (90) | — |
| (TBA+)4(GePOM4−) | −0.88 (68) | −1.33 (85) | −1.50 (100) | — |
| E redp/V vs. Ag/AgNO3 | ||||
| RuCAT | −1.42 | |||
| ReCAT | −1.50 | |||
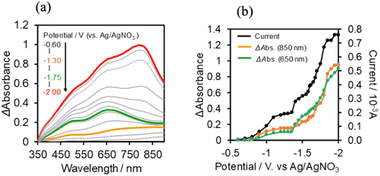
The UV-vis absorption spectra of the reduced XPOM4− were obtained using the flow electrolysis of (TBA+)4(XPOM4−). Fig. 8 shows the results for SiPOM4− measured in DMSO. It indicates a two-step reduction; the first one started at −0.8 V and finalised at −1.1 V and the second one started at −1.4 V and finalised at −1.7 V. They should be attributed to a first one-electron reduction (SiPOM4−/SiPOM5−) and the second one-electron reduction (SiPOM5−/SiPOM6−), of which redox potentials are E1/2 = −0.93 V and −1.40 V as described above (Table 2). The number of electrons injected into one molecule (n) at each step is calculated using eqn (6) with a flow rate (v = 0.15 mL min−1) and the currents to give n = 1.0 at E = −1.0 V and n = 1.1 at E = −2.3 V, respectively:
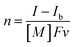 | (6) |
In the case of (TBA+)4(GePOM4−), 4-electrons reduction proceeds at a more positive potential than the reduction of Ring4+, and this reduction process couples with proton addition (PCET processes). Since the addition of TEOA to DMSO made the viscosity of the solution too high for use in flow electrolysis, ethanol (EtOH) was used as the proton source. A similar CV was obtained in the DMSO–EtOH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) mixed solution containing (TBA+)4(GePOM4−) to that in the DMSO–TEOA (5
1 v/v) mixed solution containing (TBA+)4(GePOM4−) to that in the DMSO–TEOA (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) mixed solution (Fig. S5†). Fig. 9b shows the I–V curve for the flow electrolysis of (TBA+)4(GePOM4−) in a DMSO–EtOH (5
1 v/v) mixed solution (Fig. S5†). Fig. 9b shows the I–V curve for the flow electrolysis of (TBA+)4(GePOM4−) in a DMSO–EtOH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution, where the current mainly changed in three steps: the current drastically increased at E = −0.8 V, −1.45 V, and −1.8 V. In each step, GePOM4− accepts 1.1, 1.0, and 1.8 electron(s), respectively. These results indicate that the three steps of total four-electron reduction proceeded as shown in eqn (2)–(4). Fig. 9a shows the UV-vis absorption spectral changes in the solution during electrolysis.
1 v/v) solution, where the current mainly changed in three steps: the current drastically increased at E = −0.8 V, −1.45 V, and −1.8 V. In each step, GePOM4− accepts 1.1, 1.0, and 1.8 electron(s), respectively. These results indicate that the three steps of total four-electron reduction proceeded as shown in eqn (2)–(4). Fig. 9a shows the UV-vis absorption spectral changes in the solution during electrolysis.
From these results and investigations, we determined the molar extinction coefficients of the reduced GePOM4− species, that is, ε850 (GePOM5−) = 1900 M−1 cm−1 (λ = 850 nm), ε650 (GePOM5−) = 1400 M−1 cm−1 (λ = 650 nm), ε850 (GePOM6−) = 2200 M−1 cm−1 (λ = 850 nm), ε650 (GePOM6−) = 4000 M−1 cm−1 (λ = 650 nm), ε850 (H2GePOM6−) = 9300 M−1 cm−1 (λ = 850 nm), and ε650 (H2GePOM6−) = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 (λ = 650 nm).
000 M−1 cm−1 (λ = 650 nm).
We can use the absorption spectrum of the OERS of the model mononuclear complex cis-(CO)-[Re(bpy)(CO)2(PEtPh2)2]+ (blue line in Fig. 10c: λmax = 362 nm, 490 nm, 515 nm) as that of the OERS of Ring4+ because the spectrum of the photochemically reduced Ring4+ was well fitted by using the spectra of cis-(CO)-[Re(bpy)(CO)2(PEtPh2)2]+ and its OERS.30a
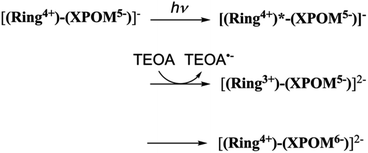
Photochemical reduction of (Ring4+)–(XPOM4−)
A DMSO–TEOA (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
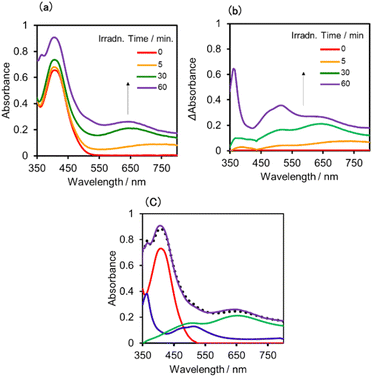
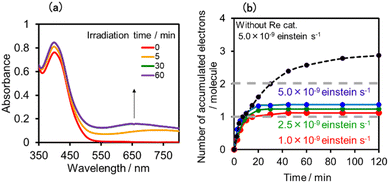
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution containing (Ring4+)–(SiPOM4−) was irradiated at λex = 436 nm with a light intensity of 5 × 10−9 einstein s−1. Fig. 10a and b show the spectral changes during irradiation (irradiation times of 5 min, 30 min, and 60 min) and differential spectra before and after the irradiation, respectively. Immediately after irradiation, a new broadband with absorption maxima was observed at λmax = 480 nm and 730 nm. This result was attributed to the formation of OERS of SiPOM4− as it had a spectrum similar to that of SiPOM5− obtained by flow electrolysis (orange line in Fig. 8a). Further irradiation causes a change in the absorption shape. For example, after irradiation for 30 min, the absorption maxima became λmax at 500 nm and 650 nm, which are attributed to the two-electron reduced species (TWERS) of the SiPOM4− unit because of the similarity of the spectrum to that of SiPOM6− (green line in Fig. 8a). Irradiation periods longer than 30 min caused another spectrum change with new absorption maxima at λmax = 360 nm, 490 nm (sh), and 515 nm (60 min irradiation: purple line in Fig. 10b).
1 v/v) solution containing (Ring4+)–(SiPOM4−) was irradiated at λex = 436 nm with a light intensity of 5 × 10−9 einstein s−1. Fig. 10a and b show the spectral changes during irradiation (irradiation times of 5 min, 30 min, and 60 min) and differential spectra before and after the irradiation, respectively. Immediately after irradiation, a new broadband with absorption maxima was observed at λmax = 480 nm and 730 nm. This result was attributed to the formation of OERS of SiPOM4− as it had a spectrum similar to that of SiPOM5− obtained by flow electrolysis (orange line in Fig. 8a). Further irradiation causes a change in the absorption shape. For example, after irradiation for 30 min, the absorption maxima became λmax at 500 nm and 650 nm, which are attributed to the two-electron reduced species (TWERS) of the SiPOM4− unit because of the similarity of the spectrum to that of SiPOM6− (green line in Fig. 8a). Irradiation periods longer than 30 min caused another spectrum change with new absorption maxima at λmax = 360 nm, 490 nm (sh), and 515 nm (60 min irradiation: purple line in Fig. 10b).
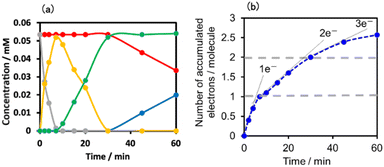
This outcome was due to the additional formation of OERS of the Ring4+ unit (blue line in Fig. 10c),30a that is, the three-electron reduced species of the supramolecule ([(Ring3+)–(SiPOM6−)]3−) was produced. All the observed spectra during irradiation can be well fitted by the spectra of Ring4+, SiPOM4−, SiPOM5−, SiPOM6−, and/or Ring3+. Fig. 10c shows a typical example of the fitting result of the spectrum after 60 min of irradiation, in which SiPOM6−, Ring4+, and Ring3+ were used.
The number of accumulated electrons in one molecule of (Ring4+)–(SiPOM4−) was obtained as ∼2.6 after irradiation for 60 min through the fitting result. This result indicates that the irradiation of (Ring4+)–(SiPOM4−) in the presence of TEOA as a reductant caused the stepwise injection of two electrons into the SiPOM4− unit (eqn (7) and (8)), and then one more electron was introduced into the Ring4+ unit, giving a three-electron reduced species, that is, [(Ring3+)–(SiPOM6−)]3− (eqn (9)).
| [(Ring4+)–(SiPOM4−)] + e− → [(Ring4+)–(SiPOM5−)]− | (7) |
| [(Ring4+)–(SiPOM5−)]− + e− → [(Ring4+)–(SiPOM6−)]2− | (8) |
| [(Ring4+)–(SiPOM6−)]2− + e− → [(Ring3+)–(SiPOM6−)]3− | (9) |
Fig. 11a shows the concentrations of the various redox states of each unit in this supramolecule at different irradiation times. Fig. 11b shows the accumulated electrons in one supramolecule. From the slope of each step, the formation quantum yields of [(Ring4+)–(SiPOM5−)]−, [(Ring4+)–( SiPOM6−)]2−, and [(Ring3+)–(SiPOM6−)]3− were obtained as 83%, 32%, and 10%, respectively.
Similar experiments were performed for (Ring4+)–(GePOM4−), and Fig. S6 and S7† shows the results. In the initial stage, similar spectral changes were observed, that is, a broad absorption appeared until 5 min of irradiation, and then the absorption maxima shifted to 650 nm after irradiation for 20 min. These two spectra can be attributed to the OERS species [(Ring4+)–(GePOM5−)]− and TWERS species [(Ring4+)–(GePOM6−)]2− (eqn (10) and (11)) because of their similarities to those of GePOM5− and GePOM6− obtained by flow electrolysis (Fig. 9). Fitting results indicated that the quantum yields of [(Ring4+)–(GePOM5−)]− and [(Ring4+)–(GePOM6−)]2− were 85% and 64%, respectively (Fig. S6b†). Further irradiation induced spectral changes from those of (Ring4+)–(SiPOM4−), that is, an increase in the absorption at λmax = ∼360 nm and λ > ∼750 nm, and the spectra after irradiation for longer than 30 min can be fitted using the spectra of Ring4+, Ring3+, GePOM6− and H2GePOM4− (Fig. S6c†). The photochemical reduction of [(Ring4+)–(GePOM6−)]2− should first give the three-electron reduced species [(Ring3+)–(GePOM6−)]3− (eqn (12)). Because the formation potential of H2GePOM6− (E = −1.50 V) is similar to that of Ring3+ (E = −1.54 V), intramolecular electron transfer, which should give [(Ring4+)–(GePOM7−)]3−, and then two molecules of the produced [(Ring4+)–(GePOM7−)]3− probably underwent disproportionation coupled with protonation, giving [(Ring4+)–(H2GePOM6−)]2−, which is a four-electron reduced species, and the TWRS species [(Ring4+)–(GePOM6−)]2− (eqn (13)).
| [(Ring4+)–(GePOM4−)] + e− → [(Ring4+)–(GePOM5−)]− | (10) |
| [(Ring4+)–(GePOM5−)]− + e− → [(Ring4+)–(GePOM6−)]2− | (11) |
| [(Ring4+)–(GePOM6−)]2− + e− → [(Ring3+)–(GePOM6−)]3− | (12) |
| [(Ring3+)–(GePOM6−)]3− ⇆ [(Ring4+)–(GePOM7−)]3− |
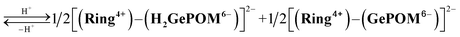 | (13) |
In the DMSO–TEOA (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) mixed solution, the emission from (Ring4+)–(XPOM4−) was almost completely quenched. This effect should be reductive quenching by TEOA: the Stern–Volmer plots in the case of (Ring4+)–(SiPOM4−) are shown in Fig. S8.† Therefore, there are two photochemical formation routes of OERS [(Ring4+)–(XPOM5−)]−: first, intermolecular reductive quenching of the excited Ring4+ unit by TEOA followed by intramolecular electron transfer to the XPOM5− unit (eqn (14)); second, intramolecular electron transfer from the excited Ring4+ unit to the XPOM5− unit, followed by reduction of the oxidised ring unit (Ring5+) by TEOA (eqn (15)). Although we could not determine the ratio between these two formation processes of [(Ring4+)–(XPOM5−)]− owing to the complexity of the emission behaviours of excited (Ring4+)–(XPOM4−), both excited (Ring4+)–(SiPOM4−) and (Ring4+)–(GePOM4−) were quantitatively quenched, and the corresponding OERS species formed in good quantum yields, as described above.
1 v/v) mixed solution, the emission from (Ring4+)–(XPOM4−) was almost completely quenched. This effect should be reductive quenching by TEOA: the Stern–Volmer plots in the case of (Ring4+)–(SiPOM4−) are shown in Fig. S8.† Therefore, there are two photochemical formation routes of OERS [(Ring4+)–(XPOM5−)]−: first, intermolecular reductive quenching of the excited Ring4+ unit by TEOA followed by intramolecular electron transfer to the XPOM5− unit (eqn (14)); second, intramolecular electron transfer from the excited Ring4+ unit to the XPOM5− unit, followed by reduction of the oxidised ring unit (Ring5+) by TEOA (eqn (15)). Although we could not determine the ratio between these two formation processes of [(Ring4+)–(XPOM5−)]− owing to the complexity of the emission behaviours of excited (Ring4+)–(XPOM4−), both excited (Ring4+)–(SiPOM4−) and (Ring4+)–(GePOM4−) were quantitatively quenched, and the corresponding OERS species formed in good quantum yields, as described above.
In contrast, the second reduction of TWERS, [(Ring4+)–(XPOM6−)]2−, should be produced via intermolecular reductive quenching of the excited Ring4+ unit in [(Ring4+)–(XPOM5−)]− by TEOA, followed by intramolecular electron transfer from the Ring3+ unit to the XPOM5− unit (eqn (16)), because the reduction potential of XPOM5− (Ered1/2 = −1.40 V) is much more negative than the oxidation potential of the 3MLCT excited state of Ring4+ (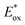 = −1.0 ∼ −1.1 V). Intramolecular oxidative quenching is a highly endothermic process.
= −1.0 ∼ −1.1 V). Intramolecular oxidative quenching is a highly endothermic process.
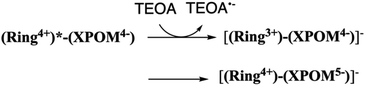 | (14) |
 | (15) |
 | (16) |
The third reduction process to form [(Ring5+)–(SiPOM6−)]3− and [(Ring4+)–(H2GePOM6−)]2− should proceed via intermolecular reductive quenching of the excited Ring4+ unit by TEOA for a similar reason. The quantum yields of the second and third reduction processes were lower than those of the first one. This result is reasonable because the intramolecular electron transfer from the reduced POM unit to the excited Ring4+ unit, which is an energy-consuming process owing to the subsequent back-electron transfer, should compete with the intermolecular electron transfer from TEOA.
Photocatalytic reduction of CO2 with RuCAT
Photocatalytic CO2 reduction was conducted using (Ring4+)–(XPOM4−) or Ring4+ as PS together with RuCAT, which has been frequently used as a catalyst for CO2 reduction in photocatalytic and electrocatalytic systems.7,42,43 A DMSO–TEOA (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
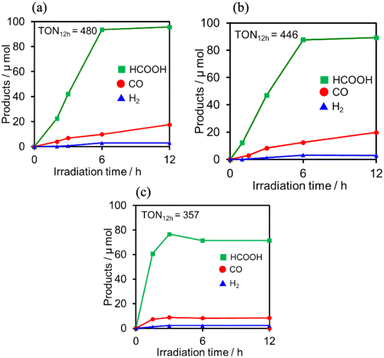
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution containing one of the PSs and RuCAT (0.05 mM each) was irradiated at λex = 436 nm, with a high light intensity of 2.5 × 10−7 einstein s−1 under a CO2 atmosphere, giving HCOOH as the main product with CO and H2 as minor products in all cases (Fig. 12). In the reaction using (Ring4+)–(SiPOM4−) as PS, HCOOH continuously formed for up to 6 h, and the turnover number and the selectivity of the HCOOH formation were TONHCOOH = 480 and SHCOOH = 86%, respectively (Fig. 12a). In the photocatalytic reaction using (Ring4+)–(GePOM4−) as PS, similar results were obtained; TONHCOOH = 446 (Fig. 12b). Notably, the use of Ring4+, a well-known PS as described in the Introduction section, instead of (Ring4+)–(XPOM4−) induced the lowest durability of photocatalysis (Fig. 12c, TONHCOOH = 357), although the formation speed of HCOOH was faster in the initial stage compared to that of (Ring4+)–(XPOM4−) as PS. The slower formation of HCOOH in the cases using (Ring4+)–(XPOM4−) could be caused by the slower reduction processes of their TWERS ([(Ring4+)–(XPOM6−)]2−), compared to that of free Ring4+.
1 v/v) solution containing one of the PSs and RuCAT (0.05 mM each) was irradiated at λex = 436 nm, with a high light intensity of 2.5 × 10−7 einstein s−1 under a CO2 atmosphere, giving HCOOH as the main product with CO and H2 as minor products in all cases (Fig. 12). In the reaction using (Ring4+)–(SiPOM4−) as PS, HCOOH continuously formed for up to 6 h, and the turnover number and the selectivity of the HCOOH formation were TONHCOOH = 480 and SHCOOH = 86%, respectively (Fig. 12a). In the photocatalytic reaction using (Ring4+)–(GePOM4−) as PS, similar results were obtained; TONHCOOH = 446 (Fig. 12b). Notably, the use of Ring4+, a well-known PS as described in the Introduction section, instead of (Ring4+)–(XPOM4−) induced the lowest durability of photocatalysis (Fig. 12c, TONHCOOH = 357), although the formation speed of HCOOH was faster in the initial stage compared to that of (Ring4+)–(XPOM4−) as PS. The slower formation of HCOOH in the cases using (Ring4+)–(XPOM4−) could be caused by the slower reduction processes of their TWERS ([(Ring4+)–(XPOM6−)]2−), compared to that of free Ring4+.
The CV of RuCAT measured in a DMSO–TEOA solution containing (TBA+)(PF6−) under a CO2 atmosphere showed a broad irreversible wave at Ep = −1.42 V vs. Ag/AgNO3 (Fig. 7). Therefore, although the OERS of the Ring4+ unit (Ring3+) and protonated four-electron reduced species of the GePOM4− (H2GePOM6−) unit can transfer an electron to RuCAT, the other reduced species of the SiPOM4− and GePOM4− units cannot perform electron transfer to RuCAT because of their more positive redox potentials (Table 2).40
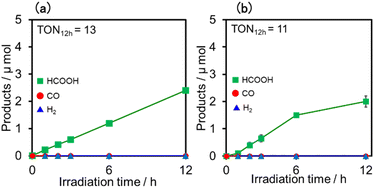
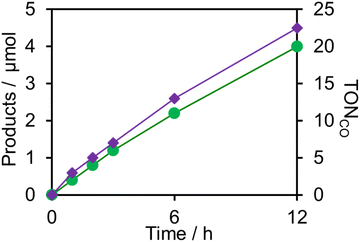
Upon irradiation with high light intensity, certain amounts of various species with different reducing powers are accumulated in the photocatalytic reactions, and we cannot separately investigate the roles of each reduced species of the supramolecular photocatalysts (ESI†41–43). Therefore, the photocatalytic reactions were conducted using a lower light intensity (5.0 × 10−9 einstein s−1) to investigate more details of the photocatalytic systems using either (Ring4+)–(SiPOM4−) or (Ring4+)–(GePOM4−) as PS by observing the exact amounts of the reduced species of the photosensitisers, as described below. HCOOH was photocatalytically produced as the main product in both cases (Fig. 13).
Similar photoreactions without PS or RuCAT produced only small amounts of HCOOH (Table 3). These results indicate that (Ring4+)–(XPOM4−) and RuCAT functioned as PS and catalyst, respectively. Using (Ring4+)–(SiPOM4−) as PS, HCOOH continuously formed even after irradiation for 12 h, and the turnover number and the selectivity of the HCOOH formation were TONHCOOH = 12 and SHCOOH = 97% after irradiation for 12 h (Fig. 13a). Although in the photocatalytic reaction using (Ring4+)–(GePOM4−) as PS, HCOOH was also produced as the main product, an induction period was observed in the initial stage, and the formation rate of HCOOH slowed down after irradiation for 6 h (Fig. 13b), in which TONHCOOH was 11 after 12 h irradiation (SHCOOH = 94%).44
| Photosensitiser | Catalyst | Time | Products/μmol | TONHCOOH | |
|---|---|---|---|---|---|
| h | HCOOH | CO | |||
a DMSO–TEOA (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution containing PS (0.05 mM) and/or RuCAT (0.05 mM) was irradiated at λex = 436 nm (5.0 × 10−9 einstein s−1) under a CO2 atmosphere.
b In the absence of RuCAT.
c In the absence of (Ring4+)–(XPOM4−). 1 v/v) solution containing PS (0.05 mM) and/or RuCAT (0.05 mM) was irradiated at λex = 436 nm (5.0 × 10−9 einstein s−1) under a CO2 atmosphere.
b In the absence of RuCAT.
c In the absence of (Ring4+)–(XPOM4−).
|
|||||
| (Ring4+)–(SiPOM4−) | RuCAT | 1 | 0.2 | Trace | 1 |
| 6 | 1.2 | Trace | 6 | ||
| 12 | 2.4 | Trace | 12 | ||
| (Ring4+)–(GePOM4−) | RuCAT | 1 | 0.1 | Trace | 0.5 |
| 6 | 1.5 | Trace | 7.5 | ||
| 12 | 2.0 | Trace | 10 | ||
| (Ring4+)–(SiPOM4−) | —b | 12 | Trace | Trace | |
| (Ring4+)–(GePOM4−) | —b | 12 | Trace | Trace | |
| —c | RuCAT | 12 | Trace | Trace | |
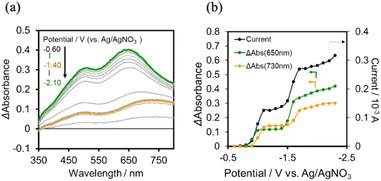
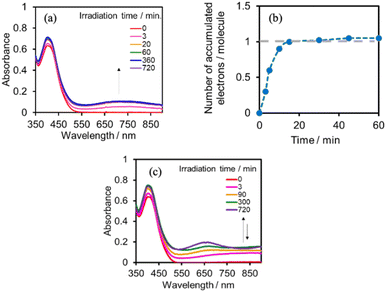
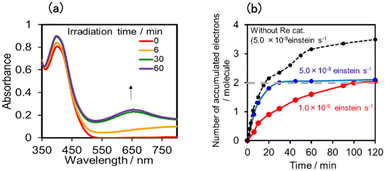
We investigated the electron-accumulation behaviour of (Ring4+)–(XPOM4−) during photocatalytic reactions with low light intensity (5.0 × 10−9 einstein s−1). Fig. 14a shows the UV-vis absorption spectra of the reaction solution with (Ring4+)–(SiPOM4−) as the PS during irradiation. In the initial stage, OERS ([(Ring4+)–(SiPOM5−)]−) was formed with a similar time scale to the photoreaction without RuCAT (Fig. 10a). The formation yield of the OERS was ∼100% after 18 min of irradiation (Fig. 14b). Although longer irradiation times slowly induced the formation of a small amount of TWERS ([(Ring4+)–(SiPOM6−)]2−), the yield did not change for up to 12 h. In other words, most of the produced TWERS were consumed during the photocatalytic reaction. Three-electron reduced species, that is, [(Ring3+)–(SiPOM6−)]3− was not observed at all. Based on these results and the similar formation potentials of the TWERS and the OERS of RuCAT (Table 2), we can conclude that the TWERS can slowly supply one electron to RuCAT (eqn (17)) and can efficiently supply another electron to intermediate(s) as well, which should be produced from the OERS of RuCAT and CO2 and/or H+, giving HCOOH (eqn (18) and (19)). This second supply of electrons from the TWERS should be sufficiently fast to suppress the decomposition of the Ru catalyst, which should be polymerization of reduced Ru complexes41–43 (eqn (20), ESI†), and recover the OERS. In other words, TWERS (E1/2red = −1.40 V) has sufficient reduction potential to reduce the intermediate(s) of the formation of HCOOH with high efficiency.
 | (17) |
 | (18) |
 | (19) |
 | (20) |
Notably, at higher light intensities (2.5 × 10−7 einstein s−1), a considerable amount of TWERS [(Ring4+)–(SiPOM6−)]2− accumulated in the reaction solution (Fig. 10b), owing to the much higher photochemical electron supply from the Ring4+ unit. Under such conditions, the three-electron reduced species [(Ring3+)–(SiPOM6−)]3− is photochemically produced during the photocatalytic reaction and supplies an electron to RuCAT (eqn (21)). This electron supply should be much faster than that from the TWERS because of the much higher reduction potential of [(Ring3+)–(SiPOM6−)]3− (Table 2).
 | (21) |
Fig. 14c shows the UV-vis absorption spectral changes of the solution in the case of (Ring4+)–(GePOM4−) during the photocatalytic reaction using a low light intensity (5.0 × 10−9 einstein s−1) for 60 min. Accumulation of OERS [(Ring4+)–(GePOM5−)]− was also observed in this photocatalytic reaction solution during irradiation. After irradiation for 10 min, one-electron reduction of nearly all of (Ring4+)–(GePOM4−) proceeded, in which the production speed of the OERS was slightly faster than that using (Ring4+)–(SiPOM4−) as PS. Further irradiation induced evident spectral changes; the accumulation of TWERS ([(Ring4+)–(GePOM6−)]2−) showed an absorption maximum at ∼650 nm. In addition, another species with an absorption band at longer wavelengths suggested the formation of a Ru polymer41–43 (eqn (20), ESI†).
DLS was applied to the photocatalytic reaction solutions (irradiated for 90 min) to detect the Ru polymer. In the case of (Ring4+)–(GePOM4−), not only particles with D = several nanometres, which are attributed to (Ring4+)–(GePOM4−) and partially to the accumulated oligomers, but also much larger particles with D = 140 nm, which are attributable to the Ru polymer (Fig. 15a). However, when using (Ring4+)–(SiPOM4−), such large particles were not observed after the photocatalytic reaction (Fig. 15b). Therefore, under these reaction conditions (low light intensity), Ru polymer formation was suppressed during the photocatalytic reaction using (Ring4+)–(SiPOM4−) as the PS but not entirely in the system using (Ring4+)–(GePOM4−).
These results indicate that in the photocatalytic system using (Ring4+)–(GePOM4−) as the PS, the TWERS [(Ring4+)–(GePOM6−)]2− supplies an electron to RuCAT at a slower rate than the system using (Ring4+)–(SiPOM4−) owing to its lower reduction ability (Table 2). The second electron donation from the TWERS ([(Ring4+)–(GePOM6−)]2−) to the intermediate produced from the OERS of RuCAT should also be slower than that from [(Ring4+)–(SiPOM6−)]2− because of the faster decline in photocatalysis and the formation of the Ru polymer, even in the presence of TWERS. This provides information on the redox properties of the reaction intermediate for photocatalytic CO2 reduction using RuCAT. Since the intermediate can be efficiently reduced by the TWERS of [(Ring4+)–(SiPOM4−)] ([(Ring4+)–(SiPOM6−)]2−) of which oxidation potential is E1/2 = −1.40 V, the reduction potential of the intermediate should be more positive than −1.40 V. In contrast, in the case using (Ring4+)–(GePOM4−) as PS, reduction of the intermediate by the TWERS ([(Ring4+)–(GePOM6−)]2−) was slow. In other words, the reduction potential of the intermediate should be similar to or slightly more negative than the redox potential of the [(Ring4+)–(GePOM6−)]2−/[(Ring4+)–(GePOM5−)]− couple, that is, E2/1 = −1.33 V. Notably, determining the reduction potential of the reaction intermediate is difficult in principle when the reduction potential of the starting catalyst is more negative than that of the short-lived intermediate, as described in the Introduction section.
Photocatalytic reduction of CO2 with ReCAT
fac-[ReI(N^N)(CO3)L]n+-type complexes are probably the most frequently used catalysts in both photocatalytic and electrocatalytic systems for CO2 reduction.7 In particular, fac-[Re(bpy)(CO3){OC(O)OC2H4N(C2H4OH)2}] (ReCAT) incorporates CO2 as a carbonate ester ligand with a deprotonated TEOA unit. This compound has been reported to function as an excellent catalyst for CO2 reduction in photocatalytic45 and electrocatalytic46 systems. The CV of ReCAT measured in a DMSO–TEOA solution containing (TBA+)(PF6−) under a CO2 atmosphere showed a broad irreversible wave at Epred = −1.50 V vs. Ag/AgNO3 (Fig. 7). Therefore, we can expect that the OERS of Ring4+ (Ring3+) and the (protonated) four-electron reduced species of GePOM4− (H2GePOM6−) should be able to transfer an electron to ReCAT while the OERS and TWERS of SiPOM4− and GePOM4− are unable to do so because of their more positive redox potentials (Fig. 7, Table 2).A DMSO–TEOA (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution containing (Ring4+)–(SiPOM4−) and ReCAT (0.05 mM each) was irradiated at λex = 436 nm with a low light intensity (5.0 × 10−9 einstein s−1) under a CO2 atmosphere, giving CO selectively and continuously for 12 h (TONCO = 20, SCO > 99%) (Fig. 16). A similar reaction, except when using (Ring4+)–(GePOM4−) as PS instead of (Ring4+)–(SiPOM4−), gave similar results (TONCO = 23, SCO > 99%). The quantum yields of CO formation were approximately 2% in each case using (Ring4+)–(SiPOM4−) or (Ring4+)–(SiPOM4−) as PS and ReCAT as the catalyst.47
1 v/v) solution containing (Ring4+)–(SiPOM4−) and ReCAT (0.05 mM each) was irradiated at λex = 436 nm with a low light intensity (5.0 × 10−9 einstein s−1) under a CO2 atmosphere, giving CO selectively and continuously for 12 h (TONCO = 20, SCO > 99%) (Fig. 16). A similar reaction, except when using (Ring4+)–(GePOM4−) as PS instead of (Ring4+)–(SiPOM4−), gave similar results (TONCO = 23, SCO > 99%). The quantum yields of CO formation were approximately 2% in each case using (Ring4+)–(SiPOM4−) or (Ring4+)–(SiPOM4−) as PS and ReCAT as the catalyst.47
Fig. 17a shows the changes in UV-vis absorption during the photocatalytic reaction using (Ring4+)–(SiPOM4−) as the PS. In the initial stage (irradiation time < 5 min), the OERS ([(Ring4+)–(SiPOM5−)]−) accumulated, similar to the photoreaction in the absence of ReCAT (Fig. 10a). The following processes were different in the presence and absence of ReCAT, that is, the accumulated amount of the TWERS ([(Ring4+)–(SiPOM5−)]2−) was lower, and the three-electron reduction species ([(Ring3+)–(SiPOM6−)]3−) was not observed in the presence of the catalyst. The yield of TWERS reached a maximum (40%) after 20 min irradiation, and 60% of the OERS remained in the solution. The yield of the accumulated TWERS was dependent on the irradiation light intensity; a lower light intensity induced a lower yield (Fig. 17b). The maximum yield of [(Ring4+)–(SiPOM5−)]2− was only 10% when the light intensity was 1.0 × 10−9 einstein s−1. Notably, the photocatalytic CO formation proceeded at this low light intensity (TONCO = 12 after 12 h of irradiation). Based on these results, we deduced that two electrons were donated from the three-electron reduced species [(Ring3+)–(SiPOM6−)]3− during photocatalytic CO2 reduction using (Ring4+)–(SiPOM4−) as the PS and ReCAT as the catalyst. The first electron transfer should proceed from [(Ring3+)–(SiPOM6−)]3− to ReCAT to give the TWERS and OERS of ReCAT (ReCAT−) because electron transfer from the TWERS to ReCAT was endergonic. The produced TWERS should provide one more electron to an intermediate produced from ReCAT− because TWERS is consumed during the photocatalytic reaction. Therefore, the intermediate can be reduced by the species with E1/2 = −1.40 V, but this second electron transfer was slower than that in the system using RuCAT and (Ring4+)–(SiPOM4−). Therefore, the reduction potential of the intermediate obtained from the OERS of ReCAT is close to −1.40 V.
In the photocatalytic reaction using (Ring4+)–(GePOM4−) as PS and ReCAT as a catalyst, all (Ring4+)–(GePOM4−) were converted into the TWERS ([(Ring4+)–(GePOM6−)]2−); that is, the TWERS were fully accumulated not only with an irradiation light intensity of 5.0 × 10−9 einstein s−1 (λex = 436 nm) but also with a lower intensity (1.0 × 10−9 einstein s−1) (Fig. 18a and b). This result indicates that the intermediate produced from the OERS of ReCAT cannot be reduced by TWERS [(Ring4+)–(GePOM6−)]2−, whose redox potential is E1/2 = −1.33 V. This result is consistent with the results obtained using (Ring4+)–(SiPOM4−) as the reduction of the intermediate proceeded by [(Ring4+)–(SiPOM6−)]2−, with a redox potential of E1/2 = −1.40 V, only at a slow rate. Therefore, in this photocatalytic reaction using (Ring4+)–(GePOM4−) as the PS, the intermediate must also be reduced only by [(Ring4+)–(H2GePOM6−)]2− (and partially by an α-amino radical produced by deprotonation of the oxidised TEOA40). Notably, the formation rate of [(Ring4+)–(H2GePOM6−)]2− was slightly higher than that of [(Ring3+)–(SiPOM6−)]3− under these reaction conditions (Fig. 11a and S7a). This effect is probably the reason for the similar CO formation rates in the two photocatalytic systems. Based on the results and the investigations, we can deduce that, in the photocatalytic reduction of CO2 using ReCAT, the reduction potential of the intermediate produced from ReCAT− is −1.33V < E ≤ −1.40 V. We recently clarified that the following process of ReCAT− to the intermediate is a unimolecular reaction with a rate constant of k = 1.8 s−1 at 298 K.48 Although several assumptions of the structure of the intermediate were reported, this is the first information about a redox potential of the intermediate in the photocatalytic reactions using Re(I)-complex catalysts to the best of our knowledge.
This method for determining the reduction potentials of the reaction intermediates was applied to another Re(I) complex, fac-[Re(Me2bpy)(CO3){OC(O)OC2H4N(C2H4OH)2}] (ReMeCAT, Me2bpy = 4,4′-dimethyl-2,2′-bipyridine, Fig. S10†), which has a more negative reduction potential (Epred = −1.64 V, Fig. S11†) than that of ReCAT (Epred = − 1.50 V). We can expect the intermediate produced from the OERS of ReMeCAT to have a more negative reduction potential than that of ReCAT if the intermediates have similar structures. In this case, the RingMe4+ unit with Me2bpy ligands instead of the bpy ligands must be used instead of the Ring4+ unit in the PS because the reduction power of Ring3+ (E1/2(Ring4+/3+) = −1.54 V) is not sufficient to reduce ReMeCAT. In contrast, RingMe3+ (E1/2(RingMe4+/3+) = − 1.78 V, Fig. S11†) can reduce ReMeCAT. A DMSO–TEOA (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) solution containing (RingMe4+)–(SiPOM4−) and ReMeCAT (0.05 mM each) was irradiated at λex = 436 nm with a low light intensity (5.0 × 10−9 einstein s−1) under a CO2 atmosphere, also giving CO selectively (TONCO = 43 after irradiation for 12 h, Table S4†). The UV-vis absorption changes of the photocatalytic reaction solution indicate that two electrons were accumulated in (RingMe4+)–(SiPOM4−) in the photostationary state of the photocatalytic reaction (Fig. S12;† the details of these experimental results are described in the ESI†). This clearly shows that TWERS [(RingMe4+)–(SiPOM6−)]2− cannot reduce the intermediate produced from the OERS of ReMeCAT. Therefore, we can conclude that the intermediate has a more negative reduction potential than −1.40 V. These results strongly support the reliability of this estimation method for the reduction potential of the reaction intermediate.
1 v/v) solution containing (RingMe4+)–(SiPOM4−) and ReMeCAT (0.05 mM each) was irradiated at λex = 436 nm with a low light intensity (5.0 × 10−9 einstein s−1) under a CO2 atmosphere, also giving CO selectively (TONCO = 43 after irradiation for 12 h, Table S4†). The UV-vis absorption changes of the photocatalytic reaction solution indicate that two electrons were accumulated in (RingMe4+)–(SiPOM4−) in the photostationary state of the photocatalytic reaction (Fig. S12;† the details of these experimental results are described in the ESI†). This clearly shows that TWERS [(RingMe4+)–(SiPOM6−)]2− cannot reduce the intermediate produced from the OERS of ReMeCAT. Therefore, we can conclude that the intermediate has a more negative reduction potential than −1.40 V. These results strongly support the reliability of this estimation method for the reduction potential of the reaction intermediate.
Conclusions
We synthesised supramolecular photosensitisers consisting of ring-shaped tetranuclear Re(I) complexes and Keggin-type heteropolyoxometalates in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. These supramolecular photosensitisers can photochemically accumulate multi-electrons in the presence of TEOA as the electron donor. In some combinations of photosensitisers and catalysts, supramolecular photosensitisers can donate two electrons to the catalyst and the intermediate to induce the photocatalytic reduction of CO2 to CO or HCOOH. In molecular photocatalytic systems, CO2 reduction generally proceeds via an intermediate produced by the OERS of the catalyst and CO2 and/or H+. In many cases, since the reduction potential of the intermediate is more positive than the first reduction potential of the catalyst, it is a challenge to obtain information on the redox properties of the intermediate. Moreover, electrons accumulated in supramolecular photosensitisers have different reduction powers; therefore, we can use these supramolecular photosensitisers to gain insight into the reduction potentials of the intermediates produced from the OERSs of the catalysts. The following conclusions were drawn from this study:
1 ratio. These supramolecular photosensitisers can photochemically accumulate multi-electrons in the presence of TEOA as the electron donor. In some combinations of photosensitisers and catalysts, supramolecular photosensitisers can donate two electrons to the catalyst and the intermediate to induce the photocatalytic reduction of CO2 to CO or HCOOH. In molecular photocatalytic systems, CO2 reduction generally proceeds via an intermediate produced by the OERS of the catalyst and CO2 and/or H+. In many cases, since the reduction potential of the intermediate is more positive than the first reduction potential of the catalyst, it is a challenge to obtain information on the redox properties of the intermediate. Moreover, electrons accumulated in supramolecular photosensitisers have different reduction powers; therefore, we can use these supramolecular photosensitisers to gain insight into the reduction potentials of the intermediates produced from the OERSs of the catalysts. The following conclusions were drawn from this study:
(i) The reduction of the intermediate from the OERS of ReCAT does not proceed using an electron donor (reduced PS) with Ered1/2 = −1.33 V but proceeds with Ered1/2 = −1.40 V.
(ii) The intermediate from the OERS of ReMeCAT cannot be reduced, even by the reduced PS with Ered1/2 = −1.40 V.
(iii) The reduction of the intermediate made from the OERS of RuCAT and CO2 barely proceeded with the reduced PS with Ered1/2 = −1.33 V.
These photochemical methods for supplying multi-electrons with different potentials from one molecule should also aid in clarifying the redox properties of the intermediates of other photocatalytic reactions, such as H2 evolution and photoredox catalytic reactions, providing multi-electron reduction products. In addition, the supramolecular photosensitizers developed in this study can suppress decomposition of intermediates produced from OERS of the catalyst owing to their rapid second electron donating abilities to increase durability of the photocatalysis.
Data availability
Data supporting the work within this manuscript is included within the full text of the manuscript and the provided ESI.†Author contributions
MT and TA did most of the experiments and equally contributed. TM and YT guided them. NH made crystals of the samples. YK measured the X-ray diffraction data and analyzed the crystal structures, and KF and MY supported YK. OI conceived and leaded this research, and leaded to write this paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by JSPS KAKENHI Grant Number JP20H00396 and JP17H06440 in Scientific Research on Innovative Areas “Innovations for Light-Energy Conversion (I4LEC)”, and partially supported by JSPS Core-to-Core Program.Notes and references
- (a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (b) M. H. Shaw, J. Twilton and D. W. C. MacMillan, J. Org. Chem., 2016, 81, 6898–6926 CrossRef CAS PubMed.
- (a) Y. Sakakibara and K. Murakami, ACS Catal., 2022, 12, 1857–1878 CrossRef CAS; (b) T. S. Teets, Y. Wu and D. Kim, Synlett, 2021, 33, 1154–1179 CrossRef.
- H. Inoue, T. Shimada, Y. Kou, Y. Nabetani, D. Masui, S. Takagi and H. Tachibana, ChemSusChem, 2011, 4, 173–179 CAS.
- L. Chen, G. Chen, C.-F. Leung, C. Cometto, M. Robert and T.-C. Lau, Chem. Soc. Rev., 2020, 49, 7271–7283 RSC.
- A. M. Appel, J. E. Bercaw, A. B. Bocarsly, H. Dobbek, D. L. DuBois, M. Dupuis, J. G. Ferry, E. Fujita, R. Hille, P. J. A. Kenis, C. A. Kerfeld, R. H. Morris, C. H. F. Peden, A. R. Portis, S. W. Ragsdale, T. B. Rauchfuss, J. N. H. Reek, L. C. Seefeldt, R. K. Thauer and G. L. Waldrop, Chem. Rev., 2013, 113, 6621–6658 CrossRef CAS PubMed.
- K. E. Dalle, J. Warnan, J. J. Leung, B. Reuillard, I. S. Karmel and E. Reisner, Chem. Rev., 2019, 119, 2752–2875 CrossRef CAS PubMed.
- Y. Yamazaki, H. Takeda and O. Ishitani, J. Photochem. Photobiol., C, 2015, 25, 106–137 CrossRef CAS.
- A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. von Zelewsky, Coord. Chem. Rev., 1988, 84, 85–277 CrossRef CAS.
- Y. Kuramochi and O. Ishitani, Inorg. Chem., 2016, 55, 5702–5709 CrossRef CAS PubMed.
- S. Ruccolo, Y. Qin, C. Schnedermann and D. G. Nocera, J. Am. Chem. Soc., 2018, 140, 14926–14937 CrossRef CAS PubMed.
- (a) Y. Tamaki, K. Koike, T. Morimoto, Y. Yamazaki and O. Ishitani, Inorg. Chem., 2013, 52, 11902–11909 CrossRef CAS PubMed; (b) M. Irikura, Y. Tamaki and O. Ishitani, Chem. Sci., 2021, 12, 13888–13896 RSC.
- S.-P. Luo, E. Mejía, A. Friedrich, A. Pazidis, H. Junge, A.-E. Surkus, R. Jackstell, S. Denurra, S. Gladiali, S. Lochbrunner and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 419–423 CrossRef CAS PubMed.
- (a) H. Takeda, K. Ohashi, A. Sekine and O. Ishitani, J. Am. Chem. Soc., 2016, 138, 4354–4357 CrossRef CAS PubMed; (b) H. Takeda, H. Kamiyama, K. Okamoto, M. Irimajiri, T. Mizutani, K. Koike, A. Sekine and O. Ishitani, J. Am. Chem. Soc., 2018, 140, 17241–17254 CrossRef CAS PubMed.
- M. Heberle, S. Tschierlei, N. Rockstroh, M. Ringenberg, W. Frey, H. Junge, M. Beller, S. Lochbrunner and M. Karnahl, Chem.–Eur. J., 2017, 23, 312–319 CrossRef CAS PubMed.
- M. Heberle, S. Tschierlei, N. Rockstroh, M. Ringenberg, W. Frey, H. Junge, M. Beller, S. Lochbrunner and M. Karnahl, Chem.–Eur. J., 2017, 23, 312–319 CrossRef CAS PubMed.
- A. Rosas-Hernández, C. Steinlechner, H. Junge and M. Beller, Green Chem., 2017, 19, 2356–2360 RSC.
- H. Takeda, Y. Monma, H. Sugiyama, H. Uekusa and O. Ishitani, Front. Chem., 2019, 7, 418 CrossRef CAS PubMed.
- M. S. Lazorski and F. N. Castellano, Polyhedron, 2014, 82, 57–70 CrossRef CAS.
- F. M. Wisser, M. Duguet, Q. Perrinet, A. C. Ghosh, M. Alves-Favaro, Y. Mohr, C. Lorentz, E. A. Quadrelli, R. Palkovits, D. Farrusseng, C. Mellot-Draznieks, V. de Waele and J. Canivet, Angew. Chem., Int. Ed. Engl., 2020, 59, 5116–5122 CrossRef CAS PubMed.
- L. Chen, Y. Qin, G. Chen, M. Li, L. Cai, Y. Qiu, H. Fan, M. Robert and T.-C. Lau, Dalton Trans., 2019, 48, 9596–9602 RSC.
- E. Boutin, L. Merakeb, B. Ma, B. Boudy, M. Wang, J. Bonin, E. Anxolabéhère-Mallart and M. Robert, Chem. Soc. Rev., 2020, 49, 5772–5809 RSC.
- T. Fukushima, T. Wada, H. Ohtsu and K. Tanaka, Dalton Trans., 2010, 39, 11526–11534 RSC.
- (a) K. Yamamoto, A. Call and K. Sakai, Chem.–Eur. J., 2018, 24, 16620–16629 CrossRef CAS PubMed; (b) K. Yamamoto, A. Call and K. Sakai, Chem.–Eur. J., 2018, 24, 16620–16629 CrossRef CAS PubMed.
- R. Konduri, H. Ye, F. M. MacDonnell, S. Serroni, S. Campagna and K. Rajeshwar, Angew. Chem., Int. Ed., 2002, 41, 3185–3187 CrossRef CAS PubMed.
- D. E. Polyansky, D. Cabelli, J. T. Muckerman, T. Fukushima, K. Tanaka and E. Fujita, Inorg. Chem., 2008, 47, 3958–3968 CrossRef CAS PubMed.
- J. Nomrowski and O. S. Wenger, J. Am. Chem. Soc., 2018, 140, 5343–5346 CrossRef CAS PubMed.
- J.-F. Lefebvre, J. Schindler, P. Traber, Y. Zhang, S. Kupfer, S. Gräfe, I. Baussanne, M. Demeunynck, J.-M. Mouesca, S. Gambarelli, V. Artero, B. Dietzek and M. Chavarot-Kerlidou, Chem. Sci., 2018, 9, 4152–4159 RSC.
- T. Morimoto, C. Nishiura, M. Tanaka, J. Rohacova, Y. Nakagawa, Y. Funada, K. Koike, Y. Yamamoto, S. Shishido, T. Kojima, T. Saeki, T. Ozeki and O. Ishitani, J. Am. Chem. Soc., 2013, 135, 13266–13269 CrossRef CAS PubMed.
- J. Rohacova and O. Ishitani, Chem. Sci., 2016, 7, 6728–6739 RSC.
- (a) T. Asatani, Y. Nakagawa, Y. Funada, S. Sawa, H. Takeda, T. Morimoto, K. Koike and O. Ishitani, Inorg. Chem., 2014, 53, 7170–7180 CrossRef CAS PubMed; (b) K. A. Walters and K. S. Schanze, Spectrum, 1998, 11, 1044–5536 Search PubMed; (c) K. Koike, J. Tanabe, S. Toyama, H. Tsubaki, K. Sakamoto, J. R. Westwell, F. P. A. Johnson, H. Hori, H. Saitoh and O. Ishitani, Inorg. Chem., 2000, 39, 2777–2783 CrossRef CAS PubMed.
- C. L. Hill, Chem. Rev., 1998, 98, 1–2 CrossRef CAS PubMed.
- I.-M. Mbomekallé, X. López, J. M. Poblet, F. Sécheresse, B. Keita and L. Nadjo, Inorg. Chem., 2010, 49, 7001–7006 CrossRef PubMed.
- X. López, J. M. Maestre, C. Bo and J.-M. Poblet, J. Am. Chem. Soc., 2001, 123, 9571–9576 CrossRef PubMed.
- A. Teze and G. Herve, Inorg. Synth., 1990, 27, 85–96 CAS.
- C. Rocchiccioli-Deltcheff, M. Fournier, R. Franck and R. Thouvenot, Inorg. Chem., 1983, 22, 207–216 CrossRef CAS.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- (a) S. Tanaka, Y. Matsubara, T. Asatani, T. Morimoto, O. Ishitani and K. Onda, Chem. Phys. Lett., 2016, 662, 120–126 CrossRef CAS; (b) Y. Shimoda, K. Miyata, M. Funaki, T. Ehara, T. Morimoto, S. Nozawa, S.-i. Adachi, O. Ishitani and K. Onda, Inorg. Chem., 2021, 60, 7773–7784 CrossRef CAS PubMed.
- M. Sadakane and E. Steckhan, Chem. Rev., 1998, 98, 219–238 CrossRef CAS PubMed.
- R. N. Sampaio, D. C. Grills, D. E. Polyansky, D. J. Szalda and E. Fujita, J. Am. Chem. Soc., 2020, 142, 2413–2428 CrossRef CAS PubMed.
- Deprotonation from the one-electron-oxidation species of TEOA gives another strong electron donor, an α-amino radical. This might reduce RuCAT owing to the strong reduction power. Oxidation of the α-amino radical should proceed by (Ring4+)–(XPOM4−) and their reduced forms (OERS, TWERS) as well. Therefore, the contribution of the α-amino radical to the reduction of the intermediates produced by OERS of the catalysts should be small, X. Ci and D. G. Whitten, in Photoinduced Electron Transfer Part C, ed. M. A. Fox and M. Chanon, Elsevier, Amsterdam, 1998, pp. 555–561 Search PubMed.
- J.-M. Lehn and R. Ziessel, J. Organomet. Chem., 1990, 382, 157–173 CrossRef CAS.
- Y. Kuramochi, J. Itabashi, K. Fukaya, A. Enomoto, M. Yoshida and H. Ishida, Chem. Sci., 2015, 6, 3063–3074 RSC.
- Y. Kuramochi, O. Ishitani and H. Ishida, Coord. Chem. Rev., 2018, 373, 333–356 CrossRef CAS.
- In the system using Ring4+ as PS instead of (Ring4+)–(XPOM4−), the initial formation rate of HCOOH was much higher (Fig. S7†) than those in the systems using (Ring4+)–(XPOM4−) (Fig. 13). This is reasonable because the formation quantum yield of OERS of Ring4+ is much higher than those of [(Ring3+)–(XPOM6−)]3− and [(Ring4+)–(H2XPOM6−)]2−, which initiate the photocatalytic reduction of CO2. After the 3 h irradiation, the formation of HCOOH was terminated in the system using Ring4+ (Fig. S7†). Its TON was 214, which is smaller than that observed under high light intensity (TON = 357, Fig. 12c). The decomposition of RuCAT, i.e., the formation of the Ru polymer, should be more efficient under irradiation with low light intensity.
- T. Nakajima, Y. Tamaki, K. Ueno, E. Kato, T. Nishikawa, K. Ohkubo, Y. Yamazaki, T. Morimoto and O. Ishitani, J. Am. Chem. Soc., 2016, 138, 13818–13821 CrossRef CAS PubMed.
- H. Kumagai, T. Nishikawa, H. Koizumi, T. Yatsu, G. Sahara, Y. Yamazaki, Y. Tamaki and O. Ishitani, Chem. Sci., 2019, 10, 1597–1606 RSC.
- In the absence of (Ring4+)–(XPOM4−), a DMSO–TEOA solution containing only ReCAT was irradiated for 12 h, giving CO with TONCO = 4. It has been reported that some Re(I) complexes work as “photocatalysts” in the absence of other metal complexes.7 In these cases, a part of the added Re complex changed its structure, giving a different Re(I) complex in the initial stage of the photocatalytic reaction. Each added and produced complex worked as a PS or catalyst in the reaction solution. In a system using only ReCAT, similar reactions should proceed to give CO. Notably, in the presence of (Ring4+)–(XPOM4−), the inner filter effect of (Ring4+)–(XPOM4−) strongly suppresses the excitation of ReCAT owing to the stronger absorption of (Ring4+)–(XPOM4−) (ε436nm = 10500 M−1 cm−1) as compared to that of ReCAT (ε436nm = 860 M−1 cm−1, Fig. S10†) at the excitation wavelength (λex = 436 nm). Therefore, the contribution of the photocatalysis of ReCAT itself should be very small in the photocatalytic reduction of CO2 when using both (Ring4+)–(XPOM4−) as the PS and ReCAT as the catalyst.
- K. Kamogawa, Y. Shimoda, K. Miyata, K. Onda, Y. Yamazaki, Y. Tamaki and O. Ishitani, Chem. Sci., 2021, 12, 9682–9693 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2153829 and 2153830. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc04252e |
| This journal is © The Royal Society of Chemistry 2023 |