Exploring senior high-school students’ understanding of electrochemical concepts: patterns of thinking across Turkish and Indonesian contexts
Canan
Nakiboglu
 *a,
Sri
Rahayu
*a,
Sri
Rahayu
 b,
Nuri
Nakiboğlu
b,
Nuri
Nakiboğlu
 c and
David F.
Treagust
c and
David F.
Treagust
 d
d
aChemistry Education Division, Necatibey Education Faculty, Balikesr University, Balıkesir, Turkey. E-mail: nakiboglu2002@yahoo.com
bChemistry Department, Faculty of Mathematics & Science, Universitas Negeri Malang, Malang, Indonesia
cChemistry Department Science and Art Faculty, Balikesr University, Balıkesir, Turkey
dSchool of Education, Curtin University, GPO Box U1987, Perth, WA 6845, Australia
First published on 13th September 2023
Abstract
This study focuses on examining senior high-school students’ conceptual understanding and difficulties concerning electrochemistry and comparing patterns of thinking across Turkish and Indonesian contexts. The Electrochemistry Concept Questionnaire (ECQ) was applied to 516 Indonesian and 516 Turkish high school students right after the teaching of the electrochemistry topics. The ECQ contains 18 multiple-choice questions and these questions belong to five different categories: reactions occurring during electrolysis, differences between electrolytic and voltaic cells, movement of ions in voltaic cells, poles in voltaic cells, and voltaic cell reactions. At the end of the study, it was determined that both Indonesian and Turkish senior high-school students’ understanding of electrochemistry concepts was relatively weak and they shared common difficulties concerning electrochemical concepts. While there was no significant difference between the average scores of the students from both countries on the test, it was determined that there were some significant differences on the basis of questions. It has been concluded that students from both countries have alternative conceptions similar to those determined in previous studies such as “during electrolysis, the electric current produces ions” and “electrons migrate through the solution from one electrode to the other”. At the end of the study, the reasons for the similar results and the significantly different results for the students of the two countries to comprehend electro-concepts were discussed.
Introduction
The goal of science education reforms in the USA and other Western countries like the UK since the 1960s has been to improve the quality of science education in schools. These reforms have the key aim of teaching science in a way that all children might become scientifically literate persons (DeBoer, 2000; Herscovitz et al., 2012) as well as preparing students who are interested in becoming scientists (Ayas et al., 2010). Such reforms have spread out around the world including in Asian countries. Scientifically literate people can be described as those with sufficient scientific knowledge (e.g. concept and principle) and the ability when encountering changes to have the competence to solve real-world problems (Bond, 1989). Teaching any science subject, such as chemistry, should contribute to the goal of achieving scientific literacy and chemical literacy in particular (Tsaparlis, 2000; Shwartz et al., 2006). So, attaining a sufficient conceptual understanding of chemistry is crucial for students. Concepts and principles are the basic building blocks of scientific knowledge and understanding a concept is a prerequisite to making complex inferences or accomplishing any scientific work using it (Mi et al., 2020).Chemistry is one of the most important branches of science. It lies at the heart of many matters of public concern, such as the protection of the environment, and the supply of energy needed to keep society running (Brown et al., 2015). Chemistry topics generally involve studying the matter and understanding the properties of matter that enable learners to understand what happens around them (Sirhan, 2007). Learning chemistry is not just about learning the content available only in textbooks or the requirements of the curriculum. For the learning to be effective, those taught must be able to put that knowledge into practice in everyday life, get involved in activities concerning chemical issues, and make rational and informed decisions regarding their own experiences (Gilbert and Treagust, 2009). However, chemistry is considered a difficult subject for many students because of its complex nature (Johnstone, 2000; Sirhan, 2007). The basis of the abstract nature of chemistry is the explanations based on the particulate structure of matter (Rahayu and Kita, 2010; Taber and García-Franco, 2010). This is directly related to the sub-microscopic dimension of the triple representation of chemistry proposed by Johnstone. On the other hand, both algorithmic problem-solving and symbolic representations of chemical concepts correspond to the symbolic dimension of this triple representation. Teaching without associating these two different dimensions with the teachers in the lessons or the symbolic dimension being in the foreground can cause important problems ranging from the students not understanding the basic concepts to misunderstanding. As a result of the difficult and complex nature of chemistry and also the fact that it is one of the most conceptually difficult subjects in the school curriculum, it is of major importance that anyone teaching chemistry should be aware of the areas of difficulty in the subject.
Electrochemistry is ranked by teachers and students as one of the most difficult curriculum domains taught and learned in secondary school chemistry (e.g.De Jong and Treagust, 2003). In many chemistry curricula and textbooks, it is common to divide electrochemistry into two topics: redox reactions (oxidation and reduction) and electrochemical cells (galvanic and electrolytic). Several researchers have identified students’ misconceptions and difficulties related to galvanic and electrolytic cells (Garnett and Treagust, 1992b; Sanger and Greenbowe, 1997a; Schmidt et al., 2007; Supasorn, 2015; Loh and Subramaniam, 2018; Tsaparlis, 2018). However, there have been a limited number of studies conducted as comparative research in electrochemistry. Comparative research studies are more of a perspective or orientation than a separate research technique. It has been stated that comparative studies provide a basis for making statements about empirical regularities and for evaluating and interpreting cases according to material and theoretical criteria. It is also meant to describe and explain the similarities and differences in situations or outcomes among large-scale social units such as regions, nations, societies, and cultures that can be used to gain insights into different teaching or schooling approaches (Miri and Shahrokh, 2019). Therefore, this is the departure point of this study, as explained in detail in the subsection of the rationale of this study.
Theoretical framework and students’ conceptions about electrochemistry
Conceptual learning is a very complex process and many factors affect this process. Especially, due to the abstract nature of chemistry, the factors affecting learning are even more complex. Failure to understand can occur for a variety of reasons. These may be due to a lack of prior knowledge of the topic, not knowing what is relevant, or failure to pay attention to the relevant relationship within new information and the relationship between it and prior knowledge. A simple factual error or overgeneralization of concepts and events can also lead to a failure to understand. Although their emergence is based on different sources, it can be said that misconception is a kind of failure to understand (Nakiboğlu, 2006). During the teaching of chemistry, different types of misconceptions are encountered. Knowing the difference between these types of misconceptions can be helpful in recognizing students’ learning difficulties during lessons and in instructional planning. We see that different classifications are made for different types of misconceptions. The fact that most of these classifications are somehow related to the origin of the misconception can be considered as the common point of the classifications. In one of these classifications, Skelly and Hall (1993) grouped misconceptions into two main groups as experiential and instructional. Experiential misconceptions are misconceptions based on people's daily experiences. They called misconceptions that occur as a result of some instructional activities inside or outside the classroom instructional misconceptions.On the other hand, it is seen that the terms misconception and alternative conceptions are used interchangeably from time to time (Nakiboğlu, 2006). Taber (2000) argued that the term misconception can be used for students’ misunderstanding during teaching, and when students have communication problems for some reason, for example, the teacher cannot explain well, mutters, or the student cannot concentrate. He stated that it may also occur due to reasons such as poor hearing or not being able to read the board. Sometimes, this communication emphasizes that all of the problems can occur and that the student may misunderstand and such errors can be easily corrected with a little remedial explanation. The term alternative conception using the constructivist approach was defined as follows: “It is a concept that does not match the accepted scientific version.” Taber also stated that alternative conceptions and alternative frameworks cannot be misconceptions because they are not simple communication errors and cannot be easily corrected.
When the studies on students' understandings of electrochemistry are examined, it is seen that students' conceptions on this subject cannot be called simple communication errors, nor can they be easily corrected. The reason for the difficulties in learning the subjects and concepts of electrochemistry is that the students have difficulty in visualizing chemical events and chemical processes at the submicroscopic level, and they cannot establish a relationship between the three levels of chemistry (macro-submicro and symbolic) (Nakiboglu and Nakiboglu, 2018b). Materials used in daily life and the many observable electrochemical events in the laboratory have sub-microscopic representations because they are explained by particle-size relationships (electric current, electrons, and ions). In addition, while explaining these events, representations in the symbolic dimension such as symbols, equations, and battery diagrams are used. Failure to present all these in an integrated manner can cause serious problems in learning.
Another reason for the difficulty in learning electrochemistry has to do with the nature of electrochemistry. Prior knowledge plays a key role in teaching electrochemistry. According to the constructivist learning theory, learning is a cognitive process and is built on prior knowledge (Bodner, 1986). Deficiencies or/and alternative conceptions in students' prior knowledge can cause serious problems in the subjects to be taught later. At this point, it can be said that electrochemistry also requires a lot of prerequisite knowledge, and the convenient construction of electrochemistry subjects and concepts in the student's knowledge structure can only be achieved if this prerequisite knowledge is learned in a meaningful way by the students. This prerequisite knowledge for electrochemistry teaching is the particulate structure of matter and bond concepts, ion formation, oxidation–reduction reactions, oxidation states, electric current and electric circuits, potential, energy and energy units, solution chemistry, concentration, activity, conductivity, electroneutrality, thermodynamics (especially work and free energy exchange) and chemical equilibrium.
Electrochemistry is a part of chemistry courses in the upper secondary school curricula of different counties (Rahayu et al., 2011; Sia et al., 2012; Nakiboğlu and Nakiboğlu, 2018a, 2018b; Lu et al., 2020). Besides, the concepts and topics of electrochemistry provide information to interpret some daily life events such as corrosion, the batteries used in different devices, and electro-plating. For this reason, it is also related to chemical literacy. On the other hand, electrochemical concepts and subjects have always been shown to be the most difficult by both high school students and chemistry teachers (Bojczuk, 1982; Finley et al., 1982; Butts and Smith, 1987; de Jong and Treagust, 2003; Lu et al., 2020).
When the studies on teaching of electrochemistry in the literature are examined, topics and concepts that students have conceptual difficulties with and alternative conceptions about in electrochemistry can be grouped as follows: electrical circuits (charge law, electric current, and potential difference), oxidation–reduction reactions (oxidation number, oxidation state, and charge), electrochemical cells (current transition in electrolyte solution and salt bridge, electroneutrality, electrolysis, galvanic cells, and concentration cells), chemical and electrochemical equilibrium. Studies were carried out concerning alternative concepts and learning difficulties in each of these groups, and their reasons are briefly explained correspondingly below.
Regarding electric circuits, Garnett and Treagust (1992a) have stated that the understanding of electrical circuits is a prerequisite for understanding the operation of galvanic and electrolytic cells and therefore it is crucial. Alternative conceptions about electric circuits are examined in three groups. These are charge law, electric current, and potential difference. Garnett and Treagust (1992a) determined that students' conceptions of electrical circuits were related to the nature of the electric current in metallic conductors and in electrolytes and they also found that 12th-grade students from Australia did not fully understand metallic conductivity. One of the difficulties experienced by students involves the concept of different potentials (Özkaya 2002). Özkaya (2002) found that Turkish prospective chemistry teachers have difficulty understanding that the half-cell potential referred to in electrochemistry is the potential difference between the solution and the electrode immersed in it, and this potential difference cannot be measured, although the potential difference between two half cells can be measured. Concerning this, Allsop and George (1982) also reported that students in the UK had difficulty using standard reduction potentials to predict chemical reactions. In their study, Bradley and Moodie (2023) discussed the meaning and language associated with the use of the signs + and - with particular reference to electrochemical cells and circuits. They suggested that the undifferentiated use of plus and minus signs in that context creates possibilities for students to form misconceptions and in this way, the plus and minus signs are also “hidden persuaders”.
Reduction–oxidation reaction is also another essential prerequisite for the understanding of the galvanic and electrolytic cells (Garnett and Treagust, 1992a). One of the important problems with reduction–oxidation reactions is that students misunderstand the difference between oxidation state and charge, and consequently mistakes are made in balancing the reduction–oxidation reaction equations (Adu-Gyamfi et al., 2015; Anselme, 1997). De Jong et al. (1995) who studied with two Dutch chemistry teachers concerned with the topic of redox reactions determined several specific teaching problems arising in the lessons of the chemistry teachers. This study was the first exploration into the unknown field of problems in teaching redox reactions. They noted that the students thought that the ion charge and the oxidation number were similar, or that the oxidation number would be fixed, such as the ion charge value. Brandriet and Bretz (2014) studied with the US college students and they similarly revealed that students do not know the difference between oxidation state and ion charge and they can use these two terms interchangeably. Basuki (2020) investigated first-year undergraduate chemistry students’ understanding of assigning oxidation numbers and found that the students were confused about the nature of oxidation numbers and had several misconceptions relating to the inappropriate assumptions in assigning oxidation numbers. Besides, Garnett and Treagust (1992a) determined that students used different definitions to identify reduction–oxidation reactions and that they thought that reduction and oxidation processes could occur independently. Many of these various misconceptions identified by the reduction–oxidation reaction in different countries were also revealed in a study of secondary school Indonesian students. In this study conducted by Apriadi, Redhana and Suardana (2018), researchers reached 10 different misconceptions. These are (1) determining oxidation numbers based on the number of atoms present in molecules or ions; (2) the electrons released during oxidation are electrons that are around or near the atomic nucleus; (3) the reaction at the cathode is an oxidation reaction; (4) the reaction at the battery anode is a redox reaction; (5) the formation of negative ions during reduction occurs because the electrons in the atom are reduced; (6) determine the name of the compound that does not match the oxidation number; (7) reducing agent is a substance that undergoes oxidation; (8) the oxidizing agent is a substance that undergoes reduction; (9) ionic charge equal to zero; and (10) redox reactions are the same as auto redox reactions.
Understanding the galvanic cell requires both a systematic understanding of the formation of current in the galvanic cell from the microscopic perspective of the directional movement of electrons (external circuit) and the directional movement of ions (internal circuit), as well as a conceptual knowledge of electrolytes, electrodes, electrode reactions, and potential differences (De Jong and Treagust, 2003; Lu et al., 2020). When we look at students' alternative conceptions about electrochemical cells in general, it is seen that they are mostly related to the electrolytic solution, salt bridge, the determination of the charge of the anode and cathode, and naming the components of an electrochemical cell. In fact, it is seen that some of the misconceptions here are related to the problems concerning the electric currents explained above. According to Garnett and Treagust (1992a, 1992b) in general, students are aware that current cannot flow without a closed circuit, but they think that electrons play a role in current flow in the electrolytic solution, due to the metallic conductivity effect they have learned before. Accordingly, problems arise for students to explain the salt bridge. Therefore, students think that the moving or transferring electrons through cations and anions move across the electrodes and salt bridge. In studies, it has been determined that the salt bridge is thought to play the role of an electron donor in the completion of the circuit (Garnett and Treagust, 1992b). Similar misconceptions concerning salt bridges have also been revealed in the study of Sanger and Greenbowe with US students (1997b) and in the study of Lin et al. (2002) with Taiwanese students. These authors detected several misconceptions concerning the function of a salt bridge. Lin et al. (2002) found that students thought that the principle and function of the salt bridge and the copper wire were similar; and the students believed that there must be a salt bridge to complete the circuit. One of the students' problems with the anode and cathode is the idea, also identified by Sanger and Greenbowe (1997a), that the anode is positively charged because it loses electrons, and the cathode is negatively charged because it gains electrons.
Another problem is related to the differentiation of electrolytic and galvanic cells (Nakiboğlu and Nakiboğlu, 2022a). In the study in which Turkish prospective chemistry teachers’ understanding and misconceptions about electrolytic cells in electrochemistry were investigated by Ekiz et al. (2011), they determined that prospective chemistry teachers could not distinguish electrolytic cells from galvanic cells. Allsop and George (1982) also indicated that the students were unable to produce an acceptable diagram of an electrochemical cell and to predict the cell reactions. In addition to the alternative conceptions specific to galvanic cells described above, it was determined that the students also had difficulties with the electrolytic cell and electrolysis. Although students' alternative concepts of electrolysis are mostly discussed together with other electrochemistry subjects (De Jong and Treagust, 2003; Özkaya et al., 2003; Schmidt et al., 2007), it is also seen that some studies, albeit a small number, focus on determining the students' understanding of the electrolysis phenomenon (Ahtee et al., 2002; Sia et al., 2012; Nakiboğlu and Nakiboğlu, 2022b). Ahtee et al. (2002) cited that when the word electrolysis is mentioned, most of the pupils think that it has something to do with physics and students do not readily integrate their knowledge across physics and chemistry. It can be stated that a significant part of the learning difficulties or alternative conceptions related to electrolysis is based on the fact that students do not fully comprehend the difference between electrolytic and galvanic cells. As a result, students cannot correctly interpret the events that occur during electrolysis. In addition, it is based on the students' over-generalization of the expressions used during the lecture. When explaining electrolysis to students, expressions such as the reverse or reverse of the event in galvanic cells are used. In this case, students misinterpret the expression to reverse and think that the events at the anode and cathode are reversed. Thus, alternative conceptions about electrolysis determined by different researchers can be summarized as follows. Garnett and Treagust (1992a, 1992b) have determined that students have thought that the anode is negatively charged and so attracts cations, while the cathode is positively charged and thus attracts anions. Sanger and Greenbowe (1997b) have found that the students have suggested that when identical electrodes are used in electrolysis, the same reactions would occur at both electrodes. Another study by Acar and Tarhan (2007) revealed that Turkish high school students have thought that “the polarity of the terminals of the applied voltage does not affect the site of the anode and cathode; water does not affect the products of the electrolysis of aqueous solutions; the products of electrolysis of a molten salt and its aqueous solution are the same; and when identical electrodes are used in electrolysis, the products are the same at both electrodes” (p. 363).
Sanger and Greenbowe (1997b) investigated alternative concepts of concentration cells by introductory chemistry students attending an American midwestern university. They considered the students' difficulties concerning concentration cells, taking into account (a) identification of the anode and cathode in concentration cells, and (b) prediction of the products and the electromotive force of concentration cells. One of the misconceptions determined by them was that “the direction of electron flow in concentration cells is not dependent on the relative concentration of the ions”. The reason for this thought is that the students did not expect a potential difference due to the presence of the same ions in both cells. That is, the students think that since the same type of electrolyte is used in concentration cells, no reaction will occur and the concentration difference will not cause an electron transfer. Another misconception identified was that “because there is no net reaction in concentration cells, the reaction quotient cannot be calculated”. The source of this problem can be explained as follows. While the students add up the half-cell reactions, they try to remove the same species from both sides of the equation without considering the concentration difference, because the species in the reactants and products are the same. Thus, they think that a clear reaction equation cannot be written.
Birss and Truax (1990) suggested that one of the possible problems in electrochemistry is related to the concept of equilibrium. Claiming that the difficulty with the concept of equilibrium potential is a language problem, they focused on what the term “equilibrium” corresponds to in the electrochemical process and stated that there are two different terms of “equilibrium” to explain the events in cells. They named one of them as “electrochemical equilibrium” and the other as “chemical equilibrium”. Özkaya (2002) in his study with Turkish prospective chemistry teachers investigated to what extent they know the difference between chemical and electrochemical equilibrium in galvanic cells and whether they have alternative concepts in this regard. He found that the prospective chemistry teachers thought that when a metal is immersed into an electrolyte involving its ions, the electrical potentials of the metal and electrolyte become equal because an electrochemical equilibrium is established between the metal and its ions in the electrolyte.
Rationale and research questions
It is seen that the students' alternative conceptions concerning electrochemistry presented above are investigated in different countries. These research investigations have revealed that students from different countries and at different levels have common alternative conceptions of electrochemistry (Barral et al., 1992; Garnett and Treagust, 1992a, 1992b; Ogude and Bradley, 1994; Sanger and Greenbowe, 1997a,1997b; Özkaya, 2002; Rahayu et al., 2011; Nakiboğlu and Nakiboğlu, 2018a, 2018b). For this reason, it can be said that students’ difficulties and problems in learning about electrochemistry concepts and topics are universal. To show that the problems in electrochemistry education are universal, it is important to evaluate students from different contexts using the same instruments. There are not many studies that have compared students from different countries on electrochemistry using the same instrument. Rahayu et al. (2011) have compared the understanding of basic electrochemical concepts (in both electrolytic and voltaic cells) among senior high-school students from Indonesia and Japan which are two Asian countries. They aimed to assess the level of understanding of electrochemical concepts among students from the two groups as well as to identify any differences in the consistency of their understanding. They found that the students had many similar problems. Another comparison to be made between a Western country (Türkiye) and an Asian country can provide important information. Although Türkiye is located in both Asia and Europe as a country, the education programs it follows are mostly close to Europe.Comparative studies can be helpful in the broader and better understanding of the reasons for the differences in outcomes. Thus, the results of this study can provide an opportunity to compare responses and knowledge in the two educational contexts and to explore the extent of similarities and differences. Thus, we can understand how electrochemistry in both countries is treated in schools and determine whether students have common thinking patterns. By comparison, one may learn from others where there are possibilities for improvement and how these may be brought about and one can get ideas to consider implementing in a national context, taking the local cultural, historical, and pedagogical traditions into account (Lie, 2005). From this point of view, in general, this study aims at comparing Indonesian and Turkish senior high-school students’ (SHS) conceptual understanding and difficulties concerning electrochemistry using the same instrument and also to examine patterns of thinking across Turkish and Indonesian contexts. For this purpose, the research questions of the study were formed as follows.
1. What is the level of overall understanding of electrochemical concepts of Indonesian and Turkish SHS students? Are there significant differences in the overall understanding of the concepts in electrochemistry between Indonesian and Turkish SHS students?
2. What are the Indonesian and Turkish SHS students' alternative conceptions in electrochemistry?
3. How consistent are the Indonesian and Turkish SHS students’ understanding of the electrochemistry concepts related to the five conceptual categories? Are there significant differences between them?
Methods
This study employs a retrospective causal–comparative ex-post facto design (Gay and Airasian, 2000). In retrospective causal–comparative research design, it tried to determine which are the factor or factors (independent variables) that cause an observed event or situation (dependent variable) (Sözbilir, 2019). The dependent variable in this study is the level of understanding of the electrochemical concepts of two different student groups, and an attempt to describe the factors (school-related variables and/or cultural factors) that may affect them will be made, depending on whether there is a significant difference between the levels or the levels are not different.The context of the study
The education system of both countries and the situation of electrochemistry subjects in the chemistry curriculum are explained separately below.The Turkish context
Education in Türkiye is governed by a national system and compulsory education lasts 12 years. Primary and secondary education is financed by the state and free of charge in public schools, between the ages of 6 and 18. Primary school, lower and upper secondary schools are four years. Teaching science begins in the 3rd grade as an integrated course that includes chemistry–physics–biology and astronomy. Teaching chemistry as a separate course begins in the 9th grade of upper-secondary school.The upper-secondary school (lycee or high school) includes grades 9–12, and ages 15–18. It encloses different categories of educational institutions such as Anatolian high school, Science high school, and Vocational high school. These educational institutions are under the Turkish Ministry of National Education and can be in the form of public or private schools. Although in the 9th, 10th, 11th, and 12th grades of Anatolian and Science high schools, chemistry lessons are taught as compulsory courses, the chemistry lessons are taught only in 9th and 10th grades of Vocational high schools as compulsory. While there was one chemistry curriculum for all high school types until 2018, a different chemistry curriculum has been prepared and implemented in science high schools since 2018. Among the establishment purposes of science high schools should be a resource for the training of students as scientists. Thus, although both programs contain the same units, topics, and the order of units and topics, the content is given in a little more detail in science high schools, and science high school programs include more experimental work for students (Nakiboğlu, 2021).
Electrochemistry, an essential component of the Turkish upper secondary school chemistry curriculum, is taught in the unit “Oxidation States’ in the 11th grade and “Chemistry and Electricity” in the 12th-grade. Our attention in this paper is the 42 hours of instructional time for teaching the “Chemistry and Electricity” unit in the 12th-grade chemistry curriculum which corresponds to 29% of the entire 12th-grade curriculum. Contents of the Turkish 12th-grade chemistry curriculum concerning electrochemistry are presented in Table 1.
| Basic competency | Objectives |
|---|---|
| a The objective is only included in 2018 science high school chemistry curriculum. | |
| 11.1.5. Oxidation states | 11.1.5.1. Explains the relationship between oxidation states and electron configurations. |
| 12.1.1. Electric current in reduction–oxidation reactions | 12.1.1.1. To recognize the redox reactions. |
| 12.1.1.2. To explain the relationship between redox reactions and electrical energy. | |
| 12.1.2. Electrodes and electrochemical cells | 12.1.2.1. To explain the concepts of electrode and electrochemical cell. |
| 12.1.3. Electrode potentials | 12.1.3.1. To explain the spontaneity of redox reactions using standard electrode potentials. |
| 12.1.4. Electricity generation from chemicals | 12.1.4.1. To explain the voltage and service life of galvanic cells under standard conditions by giving examples. |
| 12.1.4.2. To explain the importance of solar cells, fuel cells and lithium-ion batteries by relating them to their usage areas. | |
| 12.1.5. Electrolysis | 12.1.5.1. To explain electrolysis in terms of electric current, time and mass of matter undergoing change. |
| 12.1.5.2. To explain the process of obtaining chemical substances by electrolysis method. | |
| 12.1.6. Corrosion | 12.1.6.1. To explain the electrochemical basis of corrosion prevention methods. |
The Indonesian context
The education system in Indonesia is also centralized, and includes 12 years of compulsory education between 6 and 18 years old — 6 years for Primary school level, 3 years for lower secondary, and 3 years for upper secondary levels. Science lessons begin in the 4th grade as an integrated course that includes chemistry–physics–biology and astronomy. Teaching chemistry as a separate course begins in the 10th grade of upper-secondary school.The upper-secondary school includes grades 10–12 and ages 16–18 years. These educational institutions are under the Indonesian Ministry of National Education and can be in the form of public or private schools. Indonesian schools adopted Curriculum 2013. The Curriculum 2013 suggests that all school levels implement a student-centered approach to teaching–learning.
Electrochemistry is an important component of the Indonesian high school chemistry curriculum and is taught in the “Redox Reactions and Electrochemistry” unit for 30 × 45 minutes (22 hours and 30 minutes) in the 12th grade. Contents of the Indonesian 12th-grade chemistry curriculum concerning electrochemistry are presented in Table 2.
| Basic competency | Objectives |
|---|---|
| 3.1. Balance the redox reactions equations. | 3.1.1 To balance redox reactions using half reaction method (electron ions). |
| 3.2. Analyze the processes that occur in the voltaic and electrolysis cells and explain its uses. | 3.1.2 To balance redox reactions using the oxidation number change method. |
| 3.3. Analyze the factors that influence the occurrence of corrosion and how to overcome them. | 3.2.1 To explain the arrangement of voltaic/galvani cells and the functions of each part. |
| 3.4. Apply the stoichiometry of redox reactions and Faraday's law to calculate the quantities associated with an electrolytic cell. | 3.2.2 To explain the characteristics of a redox reaction that takes place spontaneously through experimental data. |
| 3.2.3 To explain how the redox reactions in a voltaic cell can generate electrical energy. | |
| 3.2.4 To write down the symbol/notation of the cell and the reactions that occur in the voltaic cell. | |
| 3.2.5 To calculate cell potential based on potential standard data. | |
| 3.2.6 To describe the metal activities series (voltaic series). | |
| 3.2.7 To explain the working principle of voltaic cells which are widely used in daily live (batteries, accumulators, etc.). | |
| 3.2.8 To describe and explain the composition of an electrolytic cell and the function of each component. | |
| 3.2.9 To write down the reaction that occur at the anode and cathode in a solution or liquid with an active electrode or an inert electrode. | |
| 3.3.1 To explain the meaning of corrosion and factors that influence the occurrence of corrosion. | |
| 3.4.1. To apply Faraday's law concepts in electrolytic cell calculations. | |
| 4.3 Determine the order of oxidizing or reducing power based on experimental data. | 4.3.1 To apply the concept of balancing redox reactions experiments. |
| 4.4 Design a voltaic cell using nearby materials. | 4.4.1 To design simple experiment about electrochemistry cells. |
| 4.5 Propose ideas to prevent and overcome the occurrence of corrosion. | 4.5.1 To explain ways to prevent corrosion occur. |
| 4.6 Present a procedure for gilding metal objects | 4.6.1 To apply the concept of electrolytic cell calculations in solving problems. |
The electrochemistry concept questionnaire
In causal–comparative design, the performance of the groups should be compared using a valid dependent variable measure (Gay and Airasian, 2000, p. 353). For this reason, the same valid and reliable instrument based on the chemistry curricula of both countries was used in data collection. Our previous experience with this instrument in the comparative Indonesian and Japanese analyses of students’ learning indicated that it would be a very useful instrument for the comparative study reported in this paper because we had gained important insights into students’ learning of these concepts of electrochemistry when presented in different languages and in different school systems.The Electrochemistry Concept Questionnaire (ECQ) was developed by Rahayu et al. (2011) according to the Indonesian high school chemistry curriculum, and its validity and reliability were ensured (Cronbach's alpha reliability for the original instrument was 0.63). We accepted that position and provided additional measures of reliability. The ECQ contains 18 multiple-choice questions and these questions are in five different categories. These categories and their corresponding question numbers are shown in Table 3.
| Conceptual categories | Question no. |
|---|---|
| Reactions occurring during electrolysis | 1, 2, 17 |
| Differences between electrolytic and voltaic cells | 3, 4, 9, 18 |
| Movement of ions in voltaic cells | 5, 7, 8, 10 |
| Poles in voltaic cells | 11, 12, 13, 14 |
| Voltaic cell reactions | 6, 15, 16 |
It was seen that the measurements were carefully developed based on relevant existing knowledge and the questionnaire included only relevant questions that measure electrochemistry knowledge. It was confirmed that the construction validity of the ECQ was achieved. The Turkish authors of this study examined the scale items and they saw that the measurements were carefully developed based on the relevant existing knowledge and the questionnaire included only relevant questions that measure electrochemistry knowledge. So it was confirmed that the construct validity of ECQ was achieved.
The original version of ECQ was prepared in English and later translated into Indonesian. The explanations for the development of the test and its adaptation to Indonesian language are explained in the article by Rahayu et al. (2011). They followed five steps while developing the ECQ and tried to maximize validity and reliability with these steps. In the first step, the previous research studies focusing on the electrochemistry concepts of the students were reviewed, and then the content validity was carried out by examining the programs of the countries. Besides chemistry teachers and lecturers were involved to confirm the content coverage in the ECQ. Since the original test was used in this study, all steps except the first step were applied exactly. Apart from these, a pilot study was carried out in the study. The procedures for this purpose are given below.
Since the ECQ was in English, firstly, the test was adapted to Turkish in the present study. The following procedures were carried out for the adaptation study. First of all, it was examined which grade level students the test is suitable for and how much it overlaps with the achievements of the Turkish high school chemistry curriculum. At the end of the examination, it was determined that all the questions fully coincided with an outcome in Electrodes and Electrochemical Cells, which is the second topic of the “Chemistry and Electricity” unit of the 12th-grade chemistry curriculum, and the two outcomes in the fifth topic, Electrolysis as seen from Table 1. These achievements are 12.1.2.1, 12.1.5.1, and 12.1.5.2.
After determining that the test fully corresponds to the achievements of the chemistry curriculum, its Turkish translation was made by a chemistry doctoral student who had an advanced degree in English and by one of the authors of the study (CN). After the translations were compared to each other and finalized, they were checked in terms of both content and language by one of the study authors (NN) who was the electro-analytical chemistry expert, and the necessary corrections were made. The test, whose Turkish translation was completed, was examined by two high school chemistry teachers and it was confirmed that the test overlapped with the topics related to the chemistry and electricity unit taught in the 12th-grade chemistry lessons. So, the content validity of the ECQ was ensured. In addition, face validity was ensured by the examination of the test by both Turkish authors and Turkish chemistry teachers.
Finally, the comprehensibility of the questions was tested by applying the test to 56 12th grade students studying at different high schools and it was determined that there was no problem in the intelligibility of the questions.
Participants of the study and data collection
The basic causal–comparative design involves selecting two groups differing in some independent variable and these two groups of participants are usually referred to as a comparison group. The definition and selection of the comparison group is a very important part of the causal–comparative procedure. The important consideration is to select samples that are representative of their respective populations (Gay and Airasian, 2000, p. 354). Thus, it can be said that the selected student group represents its own population since teaching is carried out according to the national chemistry program in both educational contexts. In addition, due to the fact that electrochemistry subjects are seen in the 12th grade for both educational contexts, the comparison groups are close to each other in terms of age and learning experience. After teaching the electrochemistry unit in both groups, the instrument was administered to the students.The sample consists of a total of 1032 senior high school students, 516 of whom are Indonesian and 516 are Turkish. Students in the Turkish sample come from four different public schools and 221 were female and 231 were male, and 24 students did not specify their gender. Of the 516 Indonesian students in total, 359 were female and 157 were male. 516 Indonesian students consisted of 237 students who entered the chemistry education study program as freshmen students and 279 12th grade high school students. During this research, the university freshmen, who had completed high school the previous year, were in the first semester and had not yet studied the electrochemistry topic at the university which was in the second semester of their first year. The rest of the sample, the 12th grade high school students, had just studied electrochemistry and completed the electrochemistry test. Hence, based on the level of prior experiences learning electrochemistry, the knowledge of the answers to the ECQ questions for freshmen and high school students is based only on the electrochemical knowledge they learned in the 12th-grade high school chemistry course.
All ethical principles were complied with in the study. For the Turkish sample, necessary permission was obtained from the Balikesir University Science and Engineering Ethics Committee. Before the application, necessary explanations were made to the students and the students signed a voluntary participation form.
For the Indonesian sample, necessary permission to conduct the study was sought, and permission was received from the Head of the Chemistry Department and the lecturers who provided the schedule for students to take the electrochemistry test and the students. The students were also informed about the purpose of the questionnaire and that they had a choice of whether or not to participate. For the 12th grade high school sample, permission was obtained from the Education Office in the local city where this study was conducted. Then, the permission letter was submitted to the high schools. After the schools approved our research plan, we contacted the chemistry teachers. The students were informed about the objectives of the study, procedures, and volunteers of the participants. The researchers also made it understood that the student's identity would be kept confidential. Furthermore, principals and teachers decided that there was no need to take permission from parents regarding student volunteerism in the research.
Data analysis
The SPSS 18.0 package program was used in the analysis of all data. All of the data were entered by a researcher and then checked by two graduate students one by one, and incorrectly entered data were identified and corrected. Thus, it was ensured that all data were entered into SPSS completely correctly.One of the authors of the study, CN, and a graduate student analyzed the SPSS data separately. When the results were compared, it was determined that they were exactly the same. Thus, the intercoder reliability was obtained (Gay and Airasian, 2000, p. 175). Before comparing the data of the two groups, it was checked whether the data showed normal distribution and the tests were selected according to these data. Thus, descriptive and inferential statistics are used together. The names of the tests used and why they were chosen are explained in detail in the findings section.
Findings
In this section, the findings are presented in order to answer the research questions.The level of SHS students’ overall understanding of electrochemical concepts
In the first research question, it was investigated firstly the level of overall understanding of electrochemical concepts of Indonesian and Turkish SHS students and secondly whether there was a significant difference in the overall understanding of the concepts in electrochemistry between Indonesian and Turkish SHS students. With regard to research question 1, the descriptive statistics of students’ overall understanding of the concepts in electrochemistry for two samples are presented in Table 4.| Turkish sample | Indonesian sample | |
|---|---|---|
| Number of cases | 516 | 516 |
| Mean | 7.02 | 7.21 |
| Std. deviation | 2.97 | 2.72 |
| Minimum | 0 | 1 |
| Maximum | 18 | 18 |
| Cronbach α | 0.59 | 0.54 |
| Ferguson-δ | 0.954 | 0.942 |
Although the ECQ reliability has been proven for the original ECQ before Rahayu et al. (2011), Cronbach's alpha reliability values were recalculated separately for both groups after the application in this study. This value was found to be 0.59 and 0.54 in Turkish and Indonesian samples, respectively, and these values are over the threshold value of 0.50 recommended by Nunally and Bernstein (1994 cited in Sia et al., 2012). Besides, the generally accepted standard for high reliability of a measure is α ≥ 0.70, it was cited that α is not necessarily the most appropriate measure of reliability for all assessments (Brandriet and Bretz, 2014). Adams and Wieman (2011) have also asserted that low reliability can be reasonable for Formative Assessment of Instruction tools. Therefore, additional measures of reliability were examined. Ferguson-δ values for ECQ were also calculated. Ferguson-δ is a measure of the discrimination of the overall test scores and reflects the extent to which students earn scores compared to the total possible scores. If the Ferguson-δ meets to be δ ≥ 0.90, the test is considered to have sufficient discrimination (Brandriet and Bretz, 2014). As seen from Table 4, the Ferguson-δ values were found to be 0.954 and 0.942 in the Turkish and Indonesian samples, respectively.
When Table 4 is examined, the average score obtained by the students from both countries in the test was determined to be 7.02 and 7.21 for Türkiye and Indonesia, respectively. Since the maximum score to be taken from the test is 18, these results show that students' understandings of the concepts within both samples are about 40% on average (39% for Turkish and 40% for Indonesian students).
It has been examined as to whether there is a difference between these values, which are quite close to each other. In order to decide whether parametric or non-parametric tests will be used for comparison, it was first examined whether the data of both countries showed a normal distribution. For this purpose, the Kolmogorov–Smirnov test was performed and skewness values were examined. Test of normality test results are shown in Table 5.
| Samples | Kolmogorov–Smirnov | ||
|---|---|---|---|
| Statistic | df | Sig. | |
| Turkish sample | 0.107 | 516 | 0.000 |
| Indonesian sample | 0.093 | 516 | 0.000 |
As seen in Table 5, since p = 0.000, and the p is less than 0.05, there is no normal distribution for both samples. Additionally, according to skewness, it was found that 0.382/0.108 = 3.537 for the Turkish sample and 0.341/0.108 = 3.157 for the Indonesian sample. As both values are greater than 1.96, there is no normal distribution. So, it was decided that the data of both countries did not show a normal distribution, and the Mann–Whitney Test, one of the non-parametric tests was conducted to compare the average score on the ECQ (comprising 18 items) for the Indonesian and Turkish students and finding is presented in Table 6.
| Ranks | U | p | |||
|---|---|---|---|---|---|
| Country | N | Mean rank | Sum of ranks | ||
| Türkiye | 516 | 502.37 | 259![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224.50 224.50 |
125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 838.500 838.500 |
0.126 |
| Indonesia | 516 | 530.63 | 273![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 803.50 803.50 |
||
| Total | 1032 | ||||
It can be seen from Table 6 that there is not a significant difference in the average scores achieved by the Indonesian students (M = 7.21, SD = 2.72) and the Turkish students (M = 7.02 SD = 2.92) since p > 0.05 (p = 0.126). Since there was no difference between the average scores of the students from both countries regarding their achievement in the test, it was investigated what the students' achievement of each question on the test was and whether there was a difference between the scores of two countries. Percentage, frequencies and mean of Turkish and Indonesian students’ correct answers are shown in Table 7 and Fig. 1 compares the distribution of students’ answer to the 18 questions in the ECQ.
| Question number | Turkish sample (n = 516) | Indonesian sample (N = 516) | ||
|---|---|---|---|---|
| Frequency | Percentage | Frequency | Percentage | |
| 1 | 70 | 13.6 | 43 | 8.3 |
| 2 | 116 | 22.5 | 76 | 14.7 |
| 3 | 164 | 31.8 | 161 | 31.2 |
| 4 | 335 | 64.9 | 229 | 44.4 |
| 5 | 298 | 57.8 | 162 | 31.4 |
| 6 | 69 | 13.4 | 94 | 18.2 |
| 7 | 130 | 25.2 | 180 | 34.9 |
| 8 | 308 | 59.7 | 111 | 21.5 |
| 9 | 322 | 62.4 | 358 | 69.4 |
| 10 | 144 | 27.9 | 193 | 37.4 |
| 11 | 173 | 33.5 | 325 | 63.0 |
| 12 | 149 | 28.9 | 275 | 53.3 |
| 13 | 231 | 44.8 | 372 | 72.1 |
| 14 | 243 | 47.1 | 325 | 63.0 |
| 15 | 193 | 37.4 | 103 | 20.0 |
| 16 | 218 | 42.2 | 251 | 48.6 |
| 17 | 287 | 55.6 | 303 | 58.7 |
| 18 | 170 | 32.9 | 160 | 31.0 |
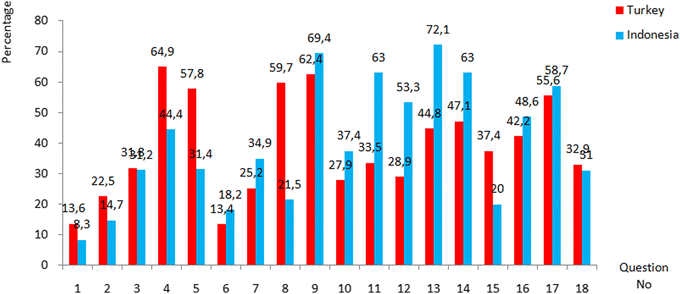 | ||
| Fig. 1 The percentage of Indonesian and Turkish students who correctly answered the 18 questions in the ECQ. | ||
As seen in Table 7, the percentage of Indonesian students’ correct answers ranged between 8.3% (Q1) and 72.1% (Q13) and the Turkish students’ correct answers ranged between 13.4% (Q6) and 64.91% (Q4). The percentage of correct answers in only 6 questions (Q9, Q11, Q 12, Q13, Q14 and Q17) for the Indonesian sample and in 5 questions (Q4, Q5, Q8, Q9 and Q17) for the Turkish sample is over 50%. The fact that the average scores of the students from both countries for each question are below 40% indicates that the students have alternative conceptions about electrochemistry that the test is trying to measure. Therefore, in the second research question, alternative conceptions of the students were examined.
When Fig. 1 is examined, it is seen that while Indonesian students' achievement in questions 1, 2, 4, 5, 8, 15, and 18 is higher than Turkish students, they are equal in Q3, and Turkish students' achievement in other questions is higher. In order to determine whether these differences are significant, the Mann–Whitney Test was conducted to compare the percentage of Indonesian and Turkish students who correctly answered the 18 questions, and the findings are presented in Table 8.
| Question no. | Country | Mean rank | Sum of ranks | U | p |
|---|---|---|---|---|---|
| Q1 | Türkiye | 530.00 | 273![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480.00 480.00 |
1.262 × 105 | 0.007 |
| Indonesia | 503.00 | 259![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 548.00 548.00 |
|||
| Q2 | Türkiye | 536.50 | 276![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 834.00 834.00 |
1.228 × 105 | 0.001 |
| Indonesia | 496.50 | 256![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 194.00 194.00 |
|||
| Q3 | Türkiye | 518.00 | 267![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 288.00 288.00 |
1.324 × 105 | 0.841 |
| Indonesia | 515.00 | 265![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740.00 740.00 |
|||
| Q4 | Türkiye | 569.50 | 293![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 862.00 862.00 |
1.058 × 105 | 0.000 |
| Indonesia | 463.50 | 239![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 166.00 166.00 |
|||
| Q5 | Türkiye | 584.50 | 301![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 602.00 602.00 |
9.804 × 104 | 0.000 |
| Indonesia | 448.50 | 231![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426.00 426.00 |
|||
| Q6 | Türkiye | 504.00 | 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 064.00 064.00 |
1.267 × 105 | 0.033 |
| Indonesia | 529.00 | 272![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 964.00 964.00 |
|||
| Q7 | Türkiye | 491.50 | 253![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 614.00 614.00 |
1.202 × 105 | 0.001 |
| Indonesia | 541.50 | 279![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 414.00 414.00 |
|||
| Q8 | Türkiye | 615.00 | 317![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340.00 340.00 |
8.230 × 104 | 0.000 |
| Indonesia | 418.00 | 215![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 688.00 688.00 |
|||
| Q9 | Türkiye | 498.50 | 257![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 226.00 226.00 |
1.238 × 105 | 0.018 |
| Indonesia | 534.50 | 275![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 802.00 802.00 |
|||
| Q10 | Türkiye | 492.00 | 253![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 872.00 872.00 |
1.205 × 105 | 0.001 |
| Indonesia | 541.00 | 279![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 156.00 156.00 |
|||
| Q11 | Türkiye | 440.50 | 227![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 298.00 298.00 |
9.391 × 104 | 0.000 |
| Indonesia | 592.50 | 305![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730.00 730.00 |
|||
| Q12 | Türkiye | 453.50 | 234![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 006.00 006.00 |
1.006 × 105 | 0.000 |
| Indonesia | 579.50 | 299![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 022.00 022.00 |
|||
| Q13 | Türkiye | 446.00 | 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 136.00 136.00 |
9.675 × 104 | 0.000 |
| Indonesia | 587.00 | 302![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 892.00 892.00 |
|||
| Q14 | Türkiye | 475.50 | 245![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 358.00 358.00 |
1.120 × 105 | 0.000 |
| Indonesia | 557.50 | 287![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670.00 670.00 |
|||
| Q15 | Türkiye | 561.50 | 289![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 734.00 734.00 |
1.099 × 105 | 0.000 |
| Indonesia | 471.50 | 243![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 294.00 294.00 |
|||
| Q16 | Türkiye | 500.00 | 258![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.00 000.00 |
1.246 × 105 | 0.039 |
| Indonesia | 533.00 | 275![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 028.00 028.00 |
|||
| Q17 | Türkiye | 508.50 | 262![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 386.00 386.00 |
1.290 × 105 | 0.314 |
| Indonesia | 524.50 | 270![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 642.00 642.00 |
|||
| Q18 | Türkiye | 521.50 | 269![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 094.00 094.00 |
1.305 × 105 | 0.505 |
| Indonesia | 511.50 | 263![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 934.00 934.00 |
It can be seen from Table 8, there is no significant difference in the percentage of Indonesian and Turkish students who correctly answered Q3, Q17, and Q18 since p > 0.05 (p = 0.841, p = 0.374, and p = 0.505). It is seen that there is a significant difference between the percentages of correct answers by the students of the two countries for all questions except these three questions.
The Indonesian and Turkish SHS students' alternative conceptions in electrochemistry
In this sub-section, to find the answer to the second research question it was examined whether the students of both countries have alternative conceptions by considering the percentages of correct answers given to the questions. For this purpose, findings on alternative concepts are presented under the sub-titles of the conceptual categories.Reactions occurring during electrolysis
In the Q1 and Q2 of the test, which are the three questions (Q1, Q2, and Q17) in the category of reactions occurring during electrolysis, it was examined whether the students knew exactly the reactions that occurred during electrolysis. Although it is determined that there is a significant difference in favour of Türkiye between the students of both countries in Q1, it is seen that the number of correct answers for the students of both countries is extremely low (8.3% for Indonesian and 13.6% for Turkish students). In Q1, the choice of the option “HCl is decomposed by electrolysis into H+ and Cl− ions” shows that the students both do not know that hydrochloric acid is a strong electrolyte and that they have the alternative conception of “hydrochloric acid decomposed to produce its constituent ions during electrolysis”. A similar alternative conception emerged in the Q2. The percentages of correct answers to Q2 are 14.7% and 22.5% for Indonesian and Turkish students, respectively. A significant difference was found between these values in favour of Türkiye. In Q17, the reactions occurring in the cathode and anode during electrolysis were given and the students were asked to determine the anode and cathode in the electrolytic cell based on these reactions. It was determined that 58.7% of Indonesian and 55.6% of Turkish students answered Q17 correctly and there was no significant difference between the students of the two countries. Although approximately 60% of the students answer this question correctly, around 40% could not identify the cathode based on the reactions occurring in the cathode and anode during electrolysis.Differences between electrolytic and voltaic cells
There are four questions (Q3, Q4, Q9, and Q18) in this category. In Q3, students' understanding of electric current in aqueous solution in electrolytic cell was examined and the percentages of correct answers were 31.2% and 31.8% for Indonesian and Turkish students, respectively. It was determined that there was no significant difference between the findings of the students from the two countries. The correct answer expected from the students in this question is “Positively and negatively charged ions flow through the solution in opposite directions.” On the other hand, it is seen that a significant part of the students have alternative conceptions of “Electrons flow through the solution from one electrode to the other.” and “Ions accept electrons at one electrode and carry them through the solution to the other electrode”. The Q4 is related to electron flow in a voltaic cell and 44.4% of Indonesian and 64.9% of Turkish students answered this question correctly. In this question, which was determined to be a significant difference in favour of Turkish students, about 56% of Indonesian students and about 35% of Turkish students had alternative conception and they thought that electrons moved from one electrode to another through the salt bridge in voltaic cell.Q9 investigated students' alternative conceptions concerning distinguishing the galvanic and electrolytic cells according to whether being the cell reactions in the voltaic and electrolytic cells spontaneously. 69.4% of Indonesian students and 62.4% of Turkish students answered correctly, and it was found that these students thought that in the electrolysis cell, electrical energy changes into chemical energy whereas in the voltaic cell, chemical energy changes into electrical energy. In this question, which was determined to be a significant difference in favour of Indonesian students, it is seen that approximately 30% of Indonesian students and approximately 38% of Turkish students have alternative conceptions on this subject. In these alternative conceptions, it was determined that the students thought “The cell reaction can occur spontaneously in both a voltaic and electrolysis cells.” or “In the voltaic cell, electrical energy changes into chemical energy whereas in the electrolysis cell, chemical energy changes into electrical energy.”
In Q18, the difference between galvanic and electrolytic cells was examined according to the ion movement caused by the presence of an electric field between the electrodes, and students' alternative conceptions were determined. The percentages of correct answers for Q18 are 31.0% and 32.9% for Indonesian and Turkish students, respectively and there is no significant difference between the findings of the students from the two countries. The other options other than the correct option were chosen by approximately 70% of the students. Based on the expressions in these options, a significant portion of the students thought that electrons flow through the solution from one electrode to the other electrode and that the ions carry the electrons through the solution.
Movement of ions in voltaic cells
The students' conceptions of how they interpret the ion movement in maintaining charge balance in voltaic cells were examined in Q5, Q7, Q8, and Q10. In Q5, based on the role of the salt bridge, students' alternative conceptions of the transport of electric current in the cell by means of ions when a voltaic cell is working were investigated. The percentages of correct answers to Q5 are 31.4% and 57.8% for Indonesian and Turkish students, respectively, and a significant difference was found between these values in favour of Türkiye. Students have alternative conceptions about the type of ions in the salt bridge that moved to the cathode and the anode in a voltaic cell. The students think that anions in the salt bridge moved to the cathode while cations moved to the anode or they can move in both directions or not move in any direction. Q7 investigated whether the students know whether the electrolyte in the salt bridge is taking part in the cell reaction. Although it is determined that there is a significant difference in favour of Indonesia, between the students of both countries in Q7, as it is seen from Table 7 the number of correct answers for the students of both countries is extremely low (34.9% for Indonesian and 25.2% for Turkish students). Students from both countries have alternative conceptions of both understanding that the electrolyte in the salt bridge is a strong or weak electrolyte and that this electrolyte takes part in the cell reaction.In Q8, students' conceptions concerning their knowledge about maintaining electro-neutrality in each of the half-cells via movements of ions were investigated. It is seen that 21.5% of the Indonesian students and 59.7% of the Turkish students think that the total electric charges of the cations and anions in the two half-cells are always the same. There is a significant difference between the students of both countries in favour of Türkiye, approximately 40% of Turkish students and approximately 78% of Indonesian students have confusion about this issue. It is seen that some of the students confuse the equality of the number of cations and anions with the charge balance.
With Q10, the students’ conceptions of the ion movement in the voltaic cell were examined again in order to provide the charge balance. In this question, it was also investigated whether the students had alternative conceptions about the movement of electrons in solution and about charge balance. Although there is a significant difference between the students of both countries in favour of Indonesia, the percentage of correct answers for the students of both countries is extremely low (37.4% for Indonesian and 27.9% for Turkish students). In this question, it was revealed that most of the students had the alternative conception, in which electrons move in the solution from one half-cell to the other through the porous disc.
Poles in voltaic cells
Four questions (Q11, Q12, Q13, and Q14) in the conceptual category measure poles in the voltaic cells. This category investigated whether the students determined the cathode and anode in galvanic cells according to the oxidation and reduction reaction occurring in the cells, the charge of the cell pole, and by looking at the electrode contains which type of ions flow. Looking at the percentages of correct answers in Table 7 for Q11, it is seen that 63.0% of Indonesian students and 33.5% of Turkish students think that the cathode is the electrode where positive ions accept electrons, that is, the electrode where reduction occurs, and the positive pole in the cell. There is a significant difference between the students of both countries in favour of Indonesia and most of the Turkish students have confusion about distinguishing the poles in voltaic cells. In Q12, there is a significant difference between the students of both countries in favour of Indonesia and the percentage of correct answers was found that 53.3% for Indonesian students and 28.9% for Turkish students. It is seen that most Turkish students did not understand that the cathode is the electrode to which positively charged ions flow. For Q13, it was found that 72.1% of Indonesian students and 44.8% of Turkish students think that the anode is the negative pole of the cell and the electrode at which the oxidation reaction occurs. There is a significant difference between the students of both countries in favour of Indonesian students and most of the Turkish students have problems distinguishing the types of reactions occurring at the cathode and anode in voltaic cells. In Q 14, it was asked which electrode is the negative pole. 63.0% of Indonesian students and 47.1% of Turkish students answered this question correctly. There is a significant difference between the students of both countries in favour of Indonesia.Voltaic cell reactions
Three questions (Q6, Q15, and Q16) were asked in order to determine the level of comprehension of the students regarding the formation of voltaic cell reactions. Although it is determined that there is a significant difference in favour of Indonesia between the students of both countries in Q6, it is seen that the number of correct answers for the students of both countries is extremely low (18.2% for Indonesian and 13.4% for Turkish students). The Q6 investigates whether the students understand the oxidation and reduction reactions occur at the same time in the half-cells of a voltaic cell. It was found that most of the students from both countries thought that oxidation and reduction reactions occur separately at different times in the half-cells of a voltaic cell. Q15 investigated the alternative conceptions of the students about understanding whether the electrode reaction is an electrode surface reaction or not. In this question, it was found that 20.0% of Indonesian students and 37.4% of Turkish students think that a chemical reaction occurs on the surface of the electrode when a voltaic cell is working. Most of the Indonesian and Turkish students have alternative conceptions that a chemical reaction occurs in the electrolyte solution and/or inside the electrode when a voltaic cell is working. There is a significant difference between the students of both countries in favour of the Turkish students in Q15. In Q16, it was examined whether they comprehended that the inert electrode would not react when the voltaic cell was working. It was found that the percentages of correct answers were 48.6% and 42.2% for Indonesian and Turkish students, respectively and there was a significant difference between the students of both countries in favour of Indonesia. Students have alternative conceptions concerning inert electrodes and over 50% of students from both countries think that when a voltaic cell is working, the inert electrode will be involved in the cell reaction.Consistency in Indonesian and Turkish SHS students’ understanding of electrochemical concepts
The third research question investigated whether there was consistency in Indonesian and Turkish SHS students in their understanding of the electrochemical concepts concerning the five conceptual categories. The percentage and frequencies of the students who correctly answered all items in each of the five conceptual categories were calculated and are shown in Table 9.| Conceptual Categories | Question no | Frequency (%) | Mean scores | Standard deviation | |||
|---|---|---|---|---|---|---|---|
| Indonesian | Turkish | Indonesian | Turkish | Indonesian | Turkish | ||
| Reactions occurring during electrolysis | 1, 2, 17 | 11 (2.1) | 49 (9.5) | 0.82 | 0.92 | 0.70 | 0.89 |
| Differences between electrolytic and voltaic cells | 3, 4, 9, 18 | 16 (3.1) | 54 (5.2) | 1.76 | 1.92 | 0.95 | 1.11 |
| Movement of ions in voltaic cells | 5, 7, 8, 10 | 8 (1.6) | 14 (2.7) | 1.25 | 1.71 | 0.95 | 0.96 |
| Poles in voltaic cells | 11, 12, 13, 14 | 148 (28.7) | 70 (13.6) | 2.51 | 1.54 | 1.28 | 1.41 |
| Voltaic cell reactions | 6, 15, 16 | 15 (2.9) | 9 (1.7) | 0.87 | 0.93 | 0.82 | 0.79 |
When Table 9 examined, it is seen that the level of consistency for the Indonesian students ranged from 1.6% to 28.7%, with the highest consistency in understanding displayed about the poles in voltaic cells. For Turkish students, the percentage of students who displayed consistent understanding in all five categories ranging from 1.7% to 13.6%, with the highest consistency in understanding displayed about the poles in voltaic cells. In order to identify any differences in consistency between the two samples of students, the Mann–Whitney test was conducted to compare the percentage of Indonesian and Turkish students who correctly answered all items in each of the five conceptual categories of ECQ. The findings are presented in Table 10.
| Conceptual categories | Question No. | Ranks | U | p | |||
|---|---|---|---|---|---|---|---|
| Country | N | Mean rank | Sum of ranks | ||||
| Reactions occurring during electrolysis | Q1, Q2, Q17 | Türkiye | 516 | 522.49 | 269![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 603.50 603.50 |
1.300 × 105 | 0.478 |
| Indonesia | 516 | 510.51 | 263![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 424.50 424.50 |
||||
| Differences between electrolytic and voltaic cells | Q3, Q4, Q9, Q18 | Türkiye | 516 | 535.64 | 276![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 388.50 388.50 |
1.233 × 105 | 0.031 |
| Indonesia | 516 | 497.36 | 256![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 639.50 639.50 |
||||
| Movement of ions in voltaic cells | Q5, Q7, Q8, Q10 | Türkiye | 516 | 585.40 | 302![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 065.00 065.00 |
9.758 × 104 | 0.000 |
| Indonesia | 516 | 447.60 | 230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963.00 963.00 |
||||
| Poles in voltaic cells | Q11, Q12, Q13, Q14 | Türkiye | 516 | 417.72 | 215![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 543.00 543.00 |
8.2157 × 104 | 0.000 |
| Indonesia | 516 | 615.28 | 317![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485.00 485.00 |
||||
| Voltaic cell reactions | Q6, Q15, Q16 | Türkiye | 516 | 529.69 | 273![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320.00 320.00 |
1.263 × 105 | 0.129 |
| Indonesia | 516 | 503.31 | 259![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 708.00 708.00 |
||||
An examination of Table 10 shows that there were no significant differences in the consistency between the Indonesian and Turkish students in two (reactions occurring during electrolysis and voltaic cell reactions) of the five conceptual categories. On the other hand, both Indonesian and Turkish students' mean scores are quite low for both categories. While the mean scores concerning category of reactions occurring during electrolysis are 0.82 for Indonesian students and 0.92 for Turkish students, the mean scores concerning category of voltaic cell reactions are 0.87 for Indonesian students and 0.93 for Turkish students. Table 10 shows that the mean scores of the Turkish students were significantly higher than those of the Indonesian students for two conceptual categories (“Differences between electrolytic and voltaic cells” and “Movement of ions in voltaic cells”). The mean score of the Indonesian students was significantly higher than those of the Indonesian students for the category of “Poles in voltaic cells”.
Conclusions and implications
At the end of this study, in which the conceptual understanding and difficulties of Indonesian and Turkish SHS students about electrochemistry were examined comparatively, the following conclusions were reached and are summarised, taking into account the research questions below.Response to the first research question: the level of overall understanding of electrochemical concepts of Indonesian and Turkish SHS students
Both the Indonesian and Turkish SHS students' overall understanding of the concepts in electrochemistry was weak and they had major difficulties concerning electrochemical concepts and topics. This result is in line with the results of the studies conducted by students from many countries, that electrochemistry subjects and concepts are difficult for students (Allsop and George, 1982; Barral et al., 1992; Garnett and Treagust, 1992a, 1992b; Ogude and Bradley, 1994; Sanger and Greenbowe, 1997a; Ahtee et al., 2002; Lin et al., 2002; Özkaya 2002; Schmidt et al., 2007; Rahayu et al., 2011; Akram et al., 2014; Supasorn, 2015; Loh and Subramaniam, 2018; Nakiboğlu and Nakiboğlu, 2018a, 2018b; Rahayu et al., 2022). Although the countries compared here have two different geographical regions and cultures, such as Indonesia, an Asian country, and Türkiye, which is located in both Asia and Europe, there was no significant difference between the average scores achieved. Although there was no significant difference between the mean scores related to the whole test of both countries' students, it has been determined that there is a significant difference between them in the comparisons made at the questions and conceptual categories, except for three questions (Q3, Q17, and Q18). When the questions determined to have a significant difference between the two countries in terms of student achievement were examined, it was concluded that this difference was not entirely in favour of one country. While Indonesian students are more successful than Turkish students in nine questions (Q6, Q7, Q9, Q10, Q11, Q12, Q13, Q14, and Q16) of the ECQ, Turkish students are more successful in six questions (Q1, Q2, Q4, Q5, Q8, and Q15). Explanations of what these differences between countries are and their reasons have been presented below while discussing the conclusions for the alternative conceptions detected from the test.Response to the second research question: whether the students from both countries have alternative conceptions.
Both countries' students shared the same alternative conceptions dealing with electrolysis, electricity flow, voltaic cells, and the pole of electrodes. In the category of reactions occurring during electrolysis, it was determined that the students of both countries had the alternative conception of “electrolyte decomposes to produce its constituent ions during electrolysis”. This result is in line with the result of the study in which the same test was applied to Indonesian and Japanese students previously Rahayu et al. (2011) and also results of other studies (Ogude and Bradley, 1994; Schmidt et al., 2007; Sia et al., 2012). They found that the students had the same alternative conceptions and even the percentage of the correct answer of the Indonesian students in this study was very close to those of the Indonesian students placed in the present study. Besides, similar findings were obtained in the study of Ogude and Bradley (1994) and they found that the electric current broke the electrolyte into positive and negative ions. In their studies concerning basic electrolysis concepts, Sia et al. (2012) concluded that high school students displayed an extremely limited understanding of electrolytic processes involving molten compounds and aqueous solutions of compounds. Schmidt et al. (2007) also ask a similar question to Q1 of the present study and they revealed that students thought that compounds are decomposed into ions in electrolysis. They cited that students did not consider electron transfer reactions at the electrodes, since they saw the ions as the end product of the electrolysis. Besides, Schmidt et al. (2007) indicated that the idea that during the electrolysis the electric current produces ions could be linked with the history of chemistry. They stated that when Faraday coined the term, he thought of it as a process in which electric current is used to decompose substances, and therefore it would have been thought that other chemistry terms ending in “-lysis” could be explained in the same way. A similar relationship can be established in the Indonesian and Turkish contexts because, the term “elektrolisis” is in the Indonesian case and the term “elektroliz” is in the Turkish case end with “-lisis” and “-liz” which were adapted from the western term “electrolysis”. The reason for this alternative conception of Turkish students may be related to the definitions of electrolysis in textbooks. The following quotation is included in a 12th-grade chemistry textbook that is most used in Turkish schools.“As with the decomposition of water into its elements, electrolysis is the decomposition of a compound or mixture into its elements with the help of electrical energy, the cell in which electrolysis takes place is called an electrolytic cell. (Çiçek et al., 2021, p. 25)”
An excerpt from the Indonesian textbook on this situation is given below.
“Electrolysis is a process of utilizing electrical energy to carry out non-spontaneous redox reactions. An electrolytic cell is a device used in the electrolysis process, which consists of a direct current source and positive and negative electrodes. Substances that are electrolyzed are electrolytes in the form of solutions or liquids (molten) of pure substances. If a liquid or electrolyte solution is electrified in a direct current source through the electrode rod, the ions in the liquid or solution will move towards the electrode with the opposite charge” (Sudarmo, 2015)
It seems there is a similarity between Turkish and Indonesian textbooks in which both textbooks emphasize on the movement of ions in the electrolyte solution. However, most Indonesian textbooks also make a point that electrolysis uses a direct current source and happens in a non-spontaneous redox reaction. In this case, students should have prior knowledge of what is the spontaneous reaction in electrochemistry means before they continue to think about the opposite direction of electron movement between electrodes.
This alternative conception concerning “HCl is decomposed by electrolysis into H+ and Cl− ions” is also based on students' knowing the concept of electrolyte, and from this point of view, it can be said that knowledge is a prerequisite for students. Therefore, one of the reasons for the alternative conception may be due to the lack of prior knowledge on the HCl is a strong electrolyte. Another piece of evidence to suggest that students' lack of knowledge about electrolytes can be associated with the very low percentage of correct answers by students from both countries in the Q7 which is related to the type of electrolyte in the salt bridge. The concept of electrolyte, which has an important role in the student's understanding of the behaviour of solutions, is an important concept in the chemistry program of many countries. Some researchers examined student alternative conceptions and levels of understanding of electrolytes, and also indicated that the concept of electrolyte was prerequisite for understanding of the further topics (Lu and Bi, 2016; Siswaningsih and Muchtar, 2017; Lu et al., 2019).
Another alternative conception concerning electrolysis is related to the students’ problems recognizing the anode and cathode in electrolytic cells. Although more than half of the students from both countries answered the question 17 correctly, it was concluded that approximately 40% of the students could not distinguish the minus and plus poles of an electrolytic cell by looking at the electrochemical reactions. This conclusion is also in line with the result of the other studies concerning electrolysis and electrolytic cells (Garnet and Treagust, 1992b; Sia et al., 2012; Rahayu et al., 2022). In this case, the problem seems to be with students did not concentrate on the electron transfer at the electrodes while determining the charge of the cell pole. A similar situation was seen in the question (Q14) about voltaic cells when students had problems choosing the negative and positive poles of the cell based on similar thinking in the voltaic cell. It is seen that Indonesian students are more successful than Turkish students in determining the cell poles and their signs.
The possible reasons for the problems experienced by the students in the Turkish sample in distinguishing the minus and plus poles of the cells can be explained as follows. First of all, although the anode and cathode are explained in Turkish textbooks and it is stated on which electrodes the reduction and oxidation take place, it is not explained what the poles’ sign will be by associating it with electron transfer. The representations of cell do not include markings for the anode and cathode. Besides, in the analysis of the 12th-grade chemistry textbook by Nakiboğlu and Nakiboğlu (2018b) regarding the representations in the “chemistry and electricity” unit, it was determined that the reactions occurring in the electrochemical and electrolytic cells were not explained by associating the triple representation of chemistry too much. Therefore, students cannot relate electrochemical reactions to the microscopic level and conceptually interpret them. In Indonesian textbooks, signs and cell poles mostly appear in the pictures for illustrating how the voltaic cell proceeds. Besides textbooks, an Indonesian teacher is urged to provide worksheets and ask students through the worksheet to memorize cell poles, sign, and redox processes. For example, when teaching a voltaic cell, a teacher provides donkey bridges like “KaReAnOks” (abbreviation from Katode-Reduksi, Anode-Oksidasi) for the process in the cathode is reduction occurs, the anode is oxidation occurring, and “KaPAN” (abbreviation from Kathode-Positif, Anode-Negatif) for the positive and negative poles. A similar way is also applied to electrolysis, for example, students memorize “KNAP” (abbreviation from Katode-Negatif, Anode-Positif) for charge poles in which the cathode is the negative pole and the anode is the positive pole.
One of the important alternative concepts identified at the end of the study is related to “the transport of the electric current in the electrolyte solutions”. In Q3, when this situation was examined in electrolytic cells, it was determined that approximately 70% of the students from both countries could not answer correctly. A similar situation arose in Q10 regarding voltaic cells, with around 70% of Turkish students and slightly more than 60% of Indonesian students giving wrong answers. These results can be interpreted as the fact that students from both countries cannot distinguish between metallic and electrolytic conductivity. Alternative conceptions such as “Electrons flow through the solution from one electrode to the other.”, “Ions accept electrons at one electrode and carry them through the solution to the other electrode” and “In the solution, electrons are transferred from one ion to the next.” expressing this confusion overlaps with the misconceptions identified in previous studies (Garnett and Treagust 1992a, 1992b; Sanger and Greenbowe, 1997a; Özkaya et al., 2003; Schmidt et al., 2007; Rahayu et al., 2011; Sia et al., 2012). When the Turkish secondary school chemistry curriculum (MNE, 2018) is examined, it is seen that metallic conductivity is not explained in detail based on electron movements. Also, in the 9th and 10th-grade chemistry textbooks, there is only one sentence explanation that the electrical conductivity of metals is provided by the electron movement. Information on the explanation of both metallic and electrolytic conductivity by comparison with each other is included in the 10th-grade physics textbooks (Kaderoğlu et al., 2021, p. 16). On the other hand, as also indicated by Garnett et al. (1990), chemistry and physics are presented as unrelated disciplines and therefore students cannot use the electrical conductivity information they learned in physics courses in explaining the electrical current flow through the aqueous solution. Besides, it should be cited that circuits and the nature of electric current are other prerequisite knowledge to understand the operation of cells (Garnett et al., 1990).
Other important alternative conceptions determined at the end of the study are related to the salt bridge. The salt bridge and its associated alternative concepts and sources of this thought can be classified as follows. First, the motion directions of the ions in the salt bridge and the flow of electrons through the salt bridge can be considered. Regarding alternative conceptions about the direction of ions transport in the salt bridge, which is also determined by some studies (Garnett and Treagust 1992a, 1992b; Garnett et al. 1990; Sanger and Greenbowe, 1997a; Schmidt et al., 2007; Rahayu et al., 2011; Sia et al., 2012), students think that anions in the salt bridge moved to the cathode while cations moved to the anode or they can move in both directions or not move in any direction (Q5). It can be said that this situation is due to the inability to understand what the assignment of the salt bridge in the electrochemical cells is and the inability to establish a relationship with this position of salt bridge with the principle of electroneutrality and charge balance in the solution.
Alternative conceptions such as “When the voltaic cell is working, the direction of flow of electrons is from the anode to the cathode through the salt bridge or from the cathode to the anode through the salt bridge (Q4).” coincides with the alternative conceptions identified in previous studies (Garnett and Treagust 1992a, 1992b; Garnett et al., 1990; Sanger and Greenbowe, 1997a; Schmidt et al., 2007; Rahayu et al., 2011; Sia et al., 2012). It can be stated that the basis of such alternative conceptions of salt bridge is primarily the confusion of the metallic conductivity with electrolytic conductivity and/or the alternative conceptions that students have about current and conductivity discussed above.
Turkish students were more successful in both Q5 about the motion directions of the ions in the salt bridge and Q4 about the flow of electrons through the salt bridge and the correct answer percentages were approximately 65% for Q4 and approximately 60% for Q5. This shows that the role of salt bridge in electrochemical cells is better understood by Turkish students. The reason can be that when Turkish 12th-grade chemistry textbooks are examined, the task of the salt bridge is explained in detail by establishing a relationship between electroneutrality and charge balance (Çiçek et al., 2021, p. 27). While the success of Indonesian students for Q4 is about 45%, the number of students who answer Q5 correctly is about 32%. This result can be supported by the result of Q8 that approximately 60% of the Turkish students think that the total electric charges of the cations and anions in the two half-cells are always the same.
In Indonesian textbooks, the existence of salt bridge is explained in various ways. While some textbooks explain what a salt bridge is and the task of a salt bridge in detail, other textbooks are less detailed.
At the end of the study, another problem determined for students' understanding of the salt bridge is that the students have incorrect knowledge or alternative conception about the electrolyte in the salt bridge, as can be seen from the low correct answer rates for Q7. Approximately 75% of Turkish students and approximately 65% of Indonesian students think that the electrolyte in the salt bridge is a strong or weak electrolyte and that this electrolyte takes part in the cell reaction. This situation can be attributed to the fact that the concept of electrolyte is not fully understood by both student groups. Because, as we discussed in Q1 and Q2, the alternative conception that “electrolytes decompose during electrolysis”, electrolyte and solution chemistry is a prerequisite for understanding cell operation.
One of the conclusions of the present study is related to the students being able to distinguish between electrochemical and electrolytic cells. It was determined that a significant part of the students did not have problems differentiating galvanic and electrolytic cells according to whether the cell reactions were spontaneous or not. Approximately 70% of Indonesian students and approximately 63% of Turkish students thought that in the electrolysis cell, electrical energy changes into chemical energy whereas in the voltaic cell, chemical energy changes into electrical energy. According to Rahayu et al. (2011), the findings of the previous study on this situation were quite similar to the findings of this study. It can be said that one of the reasons why the Turkish sample is successful in distinguishing cells is that 12th-grade chemistry textbooks specifically address this situation and include a T-Chart, which compares two cell types and is prepared for understanding cell differences (Nakiboğlu, 2022). Another reason is that as can be seen from the Turkish chemistry curriculum in Table 1, the subject of “spontaneity” is specifically addressed and examined.
In Indonesian textbooks, the subject of spontaneity is addressed and explained in voltaic cells and electrolysis. Spontaneous reactions in voltaic cells will produce electricity, while electrolysis occurs in the non-spontaneous reaction that needs electricity.
Other important alternative conceptions identified in the present study are related to where electrochemical reactions occur in the galvanic cell and inert electrodes. While the galvanic cell is operating, electrochemical reactions take place on the electrode surface, whether the electrode is reactive or inert. The difference may be a reaction on the inert electrode surface, but the inert electrode is not involved in the cell reaction. At the end of the present study, it was concluded that a very important part of the students of both countries did not know that the electrochemical reaction takes place on the electrode surface in galvanic cells and had the alternative conception that the inert electrode takes part in the cell reaction. Regarding this situation, Ali et al. (2022) stated that another interesting common belief of students on the function of inert electrodes is that no reaction will occur at the surface of inert electrodes. As the reason for this, they indicated that misinterpretation of the word “inert” in their everyday language prevented students from thinking that there was a reaction in the inert electrodes even though they did not participate in the reaction. Besides, the understanding of the oxidation and reduction reactions that occur at the same time in the half-cells of a voltaic cell was found to be quite low for students of both countries. More than 80% of the students from both countries thought that oxidation and reduction reactions occur separately at different times in the half-cells of a voltaic cell.
Finally, two important general reasons for the problems experienced in electrochemistry in the Turkish sample can be added and these problems are associated with the teaching methods and problem-solving styles used by the teachers in the classroom. In the Turkish education system, a two-stage selection exam is applied for students to enter higher education. In the studies conducted on the types of questions in these exams, the exam questions are mostly mathematical questions and are more in the form of exercises rather than problem-solving (Sarıkaya Gacanoğlu and Nakiboğlu, 2022). The students in the study group are the students who prepare for these exams because they are 12th-grade students. It is also known that they prefer presentation rather than student-centered teaching in lessons and they include mathematical questions in lessons and exams. Since students focus on solving questions rather than understanding the subjects in depth, it can be said that it is effective in their low conceptual understanding of electrochemistry. The fact that students successfully solve such algorithmic questions does not mean that they have learned the subject meaningfully (Nakhleh, 1993). Regarding this, Ogude and Bradley (1994) indicated that although many students were able to successfully solve quantitative electrochemical problems in most chemistry exams, few of them were able to answer qualitative questions that required a deeper conceptual knowledge of electrochemistry. In terms of problem-solving with algorithms, Indonesian students are also easier to do it compared to conceptual understanding.
Response to the third research question: consistency of understanding by students from both countries
The questions in the ECQ test are grouped into five conceptual categories. These categories are reactions occurring during electrolysis, differences between electrolytic and voltaic cells, movement of ions in voltaic cells, poles in voltaic cells and voltaic cell reactions. As noted, the percentages of Indonesian and Turkish students who correctly answered all items in each of the five conceptual categories of ECQ were quite low for all categories. While the level of consistency for the Indonesian students ranged from 1.6% to 28.7%, the percentage of Turkish students who displayed consistent understanding in all five categories ranged from 1.7% to 13.6%. This analysis supports the conclusion reached in the results of the first research question, and that both the Indonesian and Turkish SHS students' concepts of electrochemistry were weak and they have major difficulties concerning electrochemistry concepts and topics. Similarly, this situation supports the conclusions reached about alternative concepts related to electrochemistry, which were put forward by the second research question.The interesting conclusion regarding the findings in this section is the difference in the higher scores of the two groups in some categories. For example, Indonesian students scored higher in “Poles in voltaic cells” than Turkish students, while they scored lower in “movement of ions in voltaic cells”. Although there is a difference between the two countries in terms of the duration of teaching electrochemistry subjects, in this study, we did not have details of the teaching so are unable to explain differences in correct responses between students in the two countries but are a targeted source for further research.
Recommendations
Based on the present research's conclusions, the following important recommendations can be made in terms of students’ prerequisite knowledge, teaching strategies, and laboratory work and media.Integrating students’ prerequisite knowledge
An important point determined both in the present research and in previous studies is that teaching electrochemistry subjects and concepts includes much prerequisite knowledge including several fundamental concepts in disciplines such as physics. The prerequisite concepts and topics are electrolyte and solution chemistry, metallic and electrolytic conductivity and their explanation of microscopic level, electroactivity and reactivity (to understand electrolyte selection in the salt bridge), free energy exchange, and chemical equilibrium. In addition, at the beginning of teaching the electrochemistry unit, the similar and different aspects of electrochemical reactions and redox reactions should be explained to the students.Teaching strategies that connect the three levels of representation of chemistry concepts
As well as reviewing the prerequisite knowledge at the beginning of the teaching, is that different teaching methods and approaches should be used in relation to electrochemistry teaching. Because electrochemistry topics and concepts include both macroscopic phenomena and explanations based on sub-microscopic levels should be made during the explanation of these phenomena. The symbolic level of chemistry is very important in establishing the relationship between these two levels, and the symbolic dimension is also extremely important for solving questions about electrochemistry. Gilbert and Treagust (2009) argued that greater attention needs to be paid to the links between macroscopic and sub-microscopic understandings of electrochemistry. Thus, in order to eliminate alternative conceptions of students about electrochemistry or to provide meaningful learning, it is seen that electrochemistry teaching is done by using different teaching methods and approaches. In his review study, Tsaparlis (2018) examined the effective teaching strategies at the secondary and tertiary levels used for enhancing conceptual understanding and increasing positive attitudes toward electrochemistry. Although it has been determined that approaches such as cooperative learning instruction (Acar and Tarhan, 2007), case-based instruction (Tarkın and Uzuntiryaki-Kondakcı, 2017), and problem-based learning (Günter and Alpat, 2017) contribute to students' learning and increase their attitudes towards chemistry, it is seen that the studies mostly used in electrochemistry instruction are laboratory-based approaches (May and Gupta, 1997; Tsaparlis and Gorezi, 2005, 2007; Hawkins and Phelps, 2013; Supasorn, 2015; Hunter et al., 2019) and visual approaches such as graphical materials and computer animations (Sanger and Greenbowe, 2000; Brandt et al., 2001; Yang et al., 2003; Osman and Lee, 2014; Su, 2018; Hunter et al., 2019).Students’ use of the laboratory and media
Both hands-on activities in the laboratory and computer animations, which are also seen from the results of these studies, have important effects on students' understanding of electrochemistry concepts. Computer animations could help students to understand chemical procedures and concepts in electrochemistry by increasing their ability to visualize the operations that occur at the submicroscopic level (Nakhleh 1992; Chiu and Wu 2009; Cole et al., 2019). The use of graphic organizers in the teaching of electrochemistry topics also could make important contributions. Nakiboğlu and Nakiboğlu (2021) investigated the views of prospective chemistry teachers (PCTs) on the use of graphic organizers supported with interactive PowerPoint presentation technology in teaching electrochemical concepts. They found that PCTs thought that this kind of visual teaching could enhance the comprehension and motivation of students.Author contributions
S. R. and D. F. T. developed the electrochemistry concept questionnaire (ECQ). C. N. and N. N. translated into Turkish and carried out a pilot study with a Turkish sample. C. N. conceptualised and designed the methodology. All authors formally validated their own data. C. N. analysed and visualised all data. All the authors contributed to the original draft preparation and reviewing and editing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The data regarding the Turkish students of the study were collected within the scope of Balıkesir University Scientific Research Project (2017/165). Authors C. N. and N. N. would like to thank Balıkesir University Scientific Research Project Unit for their support.References
- Acar B. and Tarhan L., (2007), Effect of cooperative learning strategies on students' understanding of concepts in electrochemistry, Int. J. Sci. Math. Educ., 5, 349–373.
- Adams W. K. and Wieman C. E. Development and validation of instruments to measure learning of expert-like thinking, Int. J. Sci. Educ., 2011, 33, 1289–1312.
- Adu-Gyamfi K., Ampiah J. G. and Agyei D. D., (2015), High school chemistry students’ alternative conceptions of H2O, OH−, and H+ in balancing redox reactions, Int. J. Dev. Sustain., 4(6), 744–758.
- Ahtee M., Asunta T. and Palm H., (2002), Student teachers’ problems in teaching ‘electrolysis’ with a key demonstration, Chem. Educ. Res. Pract., 3(3), 317–326.
- Akram M., Surif J. B. and Ali M., (2014), Conceptual difficulties of secondary school students in electrochemistry, Asian Soc. Sci., 10(19), 276.
- Ali M. T., Woldu A. R. and Yohannes A. G., (2022), High school students’ learning difficulties in electrochemistry: a mini review, AJCE, 12(2), 202–237.
- Allsop R. T. and George N. H., (1982), Redox in Nuffield advanced chemistry, Educ. Chem., 19, 57–59.
- Anselme J.-P., (1997), Understanding oxidation-reduction in organic chemistry, J. Chem. Educ., 74(1), 69–72.
- Apriadi N. N. S., Redhana I. W. and Suardana I. N. (2018), Identifikasi miskonsepsi siswa kelas x pada topik reaksi redoks [Identify the misconceptions of grade X students on the topic of redox reactions], J. Pendidik. Kim. Indones, 2(2), 70–77.
- Ayas A., Özmen H. and Çalık M., (2010), Students’ conceptions of the particulate nature of matter at secondary and tertiary level, Int. J. Sci. Math. Educ., 8, 165–184 DOI:10.1007/s10763-009-9167-x.
- Barral F. L., Fernández E. G.-R. and Otero J. R. G., (1992), Secondary students' interpretations of the process occurring in an electrochemical cell, J. Chem. Educ., 69(8), 655–657.
- Basuki R., (2020), Conceptual difficulties experienced by first-year undergraduate chemistry students in assigning oxidation number: a case study of high school chemistry textbooks, Indones. J. Chem., 20(1), 223–236.
- Birss V. I. and Truax D. R., (1990), An effective approach to teaching electrochemistry, J. Chem. Educ., 67(5), 403–409 DOI:10.1021/ed067p403.
- Bodner G. M., (1986), Constructivism: A theory of knowledge, J. Chem. Educ., 63(10), 873.
- Bond D., (1989), In pursuit of chemical literacy: a place for chemical re-actions, J. Chem. Educ., 66(2), 157–160 DOI:10.1021/ed066p157.
- Bradley J. and Moodie P., (2023), Hidden persuaders in a systemic approach to learning about electrochemical cells and circuits, Afr. J. Chem. Educ., 13(1), 15–28.
- Brandriet A. R. and Bretz S. L., (2014), The development of the Redox Concept Inventory as a measure of students’ symbolic and particulate redox understandings and confidence, J. Chem. Educ., 91(8), 1132–1144.
- Brandt L., Elen J., Hellemans J., Heerman L., Couwenberg I., Volckaert L. and Morisse H., (2001), The impact of concept mapping and visualization on the learning of secondary school chemistry students, Int. J. Sci. Educ., 23,(12) 1303–1313.
- Brown T. L., LeMay H. E., Bursten B. E., Murphy C. J., Woodward P. M. and Stoltzfus M. W., (2015), Chemistry: The central science, 13th edn, England: Pearson.
- Bojczuk M., (1982), Topic difficulties in O- and A-Level chemistry, School Sci. Rev., 63(224), 545–551.
- Butts B. and Smith R., (1987), What do students perceive as difficult in HSC chemistry? Aust. Sci. Teachers J., 32(4), 45–51.
- Chiu M. H. and Wu H. K., (2009), The roles of multimedia in the teaching and learning of the triplet relationship in chemistry, in Gilbert J. K. and Treagust D. F. (ed.), Multiple representations in chemical education, Milton Keynes, UK: Springer, pp. 251–283.
- Çiçek K., Demirol M., Bozyil O., Danişman O. and Yildiz S., (2021), Secondary school chemistry 12th grade textbook, Ankara (in Turkish): Ministry of National Education Publications.
- Cole M. H., Rosenthal D. P. and Sanger M. J., (2019), Two studies comparing students’ explanations of an oxidation—reduction reaction after viewing a single computer animation: the effect of varying the complexity of visual images and depicting water molecules, Chem. Educ. Res. Pract., 20(4), 738–759.
- DeBoer G. E., (2000), Scientific literacy: Another look at its historical and contemporary meanings and its relationship to science education reform, J. Res. Sci. Teach., 37(6), 582–601 DOI:10.1002/1098-2736(200008)37:63.0.CO;2-L.
- De Jong O. and Treagust D., (2003), The teaching and learning of electrochemistry, in Gilbert J. K., de Jong O., Justi R., Treagust D. F. and van Driel J. H. (ed.), Chemical Education: Towards research based practice, Dordrecht: Kluwer Academic Publishers, pp. 317–337.
- De Jong O., Acampo J. and Verdonk A., (1995), Problems in teaching the topic of redox reactions: actions and conceptions of chemistry teachers, J. Res. Sci. Teach., 32(10) 1097–1110.
- Ekiz B., Kutucu E. S., Akkus H. and Boz Y., (2011), Pre-service chemistry teachers’ understanding of electrolytic cells, in Psillos D. and Sperandeo R. M. (ed.), Proceedings of the European Science Education Research Association (ESERA 2011): Science Learning and Citizenship (Part 12: Pre-service science teacher education), ESERA, pp. 51–54.
- Finley F. N., Stewart J. and Yarroch W. L., (1982), Teachers' perceptions of important and difficult science content, Sci. Educ., 66(4), 531–538.
- Garnett P. J. and Treagust D. F., (1992a), Conceptual difficulties experienced by senior high school students of electrochemistry: lectric circuits and oxidation-reduction equations, J. Res. Sci. Teach., 29(2), 121–142.
- Garnet P. J. and Treagust D. F., (1992b), Conceptual difficulties experienced by senior high school students of electrochemistry: electrochemical (Galvanic) and Electrolytic cells, J. Res. Sci. Teach., 29(10), 1079–1099.
- Garnett P. J., Garnett P. J. and Treagust D. F., (1990), Implications of research on students’ understanding of electrochemistry for improving science curricula and classroom practice, Int. J. Sci. Educ., 12(2), 147–156.
- Gay L. R. and Airasion P., (2000), Educational research: Competencies for analysis and application, New Jersey: Prentice-Hall.
- Gilbert J. K. and Treagust D. F., (2009), Multiple representations in chemical education. Milton Keynes, UK: Springer.
- Günter, T. and Alpat, S. K., (2017), The effects of problem-based learning (PBL) on the academic achievement of students studying ‘Electrochemistry’, Chem. Educ. Res. Pract., 18(1), 78–98.
- Hawkins I. and Phelps A. J., (2013), Virtual laboratory vs. traditional laboratory: which is more effective for teaching electrochemistry? Chem. Educ. Res. Pract., 14(4), 516–523.
- Herscovitz O., Kaberman Z., Saar L. and Dori Y. J., (2012), The relationship between metacognition and the ability to pose questions in chemical education, Metacognition Sci. Educ., 165–195 DOI:10.1007/978-94-007-2132-6_8.
- Hunter V., Hawkins I. and Phelps A. J., (2019), Comparing the influence of visualization type in an electrochemistry laboratory on the student discourse: who do they talk to and what do they say?, Chem. Educ. Res. Pract., 20(4), 851–861.
- Johnstone A., (2000), Teaching of chemistry-logical or psychological, Chem. Educ.: Res. Practice Europe, 1(1), 9–15 10.1039/A9RP90001B.
- Kaderoğlu A., Kaya N., Karaaslan V. E. and Koç Y. S., (2021), Secondary school physics 10th grade textbook, Ankara. (in Turkish): Ministry of National Education Publications.
- Lie S., (2005), How Can Large International Comparative Studies Contribute to the Quality of Science Education?, Research and the Quality of Science Education, Dordrecht: Springer Netherlands, pp. 27–40.
- Lin H. S., Yang T. C., Chiu H. L. and Chou C. Y., (2002), Students' difficulties in learning electrochemistry, Proc. Natl. Sci. Counc. Repub. China, 12(3), 100–105.
- Loh A. S. L. and Subramaniam R., (2018), Mapping the knowledge structure exhibited by a cohort of students based on their understanding of how a galvanic cell produces energy, J. Res. Sci. Teach., 55(6), 777–809.
- Lu S. and Bi H., (2016), Development of a measurement instrument to assess students' electrolyte conceptual understanding, Chem. Educ. Res. Pract., 17(4), 1030–1040.
- Lu S., Bi H. and Liu X., (2019), A phenomenographic study of 10th grade students’ understanding of electrolytes, Chem. Educ. Res. Pract., 20(1), 204–212.
- Lu H., Jiang Y. and Bi H., (2020), Development of a measurement instrument to assess students' proficiency levels regarding galvanic cells, Chem. Educ. Res. Pract., 21(2), 655–667.
- May M. A. and Gupta V. K., (1997), Electrochemistry "discovery" course for undergraduates, J. Chem. Educ., 74(7), 824–828.
- Mi S., Lu S. and Bi H., (2020), Trends and foundations in research on students’ conceptual understanding in science education: a method based on the structural topic model, J. Balt. Sci. Educ., 19(4), 551–568 DOI:10.33225/jbse/20.19.551.
- Ministry of National Education [MNE], (2018), Secondary education chemistry curriculum (9–12th grades), Ankara: Board of Education.
- Miri S. M. and Shahrokh Z. D., (2019), A Short Introduction to Comparative Research. Available online: https://www.researchgate.net/publication/336278925_A_Short_Introduction_to_Comparative_Research (accessed on 23 July 2023).
- Nakhleh M. B., (1992), Why some students don’t learn chemistry: Chemical misconceptions, J. Chem. Educ., 69(3), 191–196.
- Nakhleh M. B., (1993), Are our students conceptual thinkers or algorithmic problem solvers? Identifying conceptual students in general chemistry, J. Chem. Educ., 70(1), 52.
- Nakiboğlu C., (2006), Alternative conceptions in science and technology teaching. (in Turkish), in Bahar M. (ed.), in Fen ve Teknoloji Öğretimi, Ankara: Pegem A Yayıncılık, pp. 190–217.
- Nakiboğlu C., (2021), Analysis of the experiments in the Science high school chemistry textbooks in terms of the purpose of the 2018 Science High School Chemistry Curriculum for experimental study, J. Turk. Chem. Soc.:Chem. Educ., 6(2), 209–239 DOI:10.37995/jotcsc.991061.
- Nakiboğlu C., (2022), Usage of graphic organizers in upper-secondary school chemistry textbooks, MM Int. J. Educ. Sci., 6(2), 1–31 DOI:10.46762/mamulebd.1196017.
- Nakiboğlu N. and Nakiboğlu C., (2018a), Examination of 12th grade students’ cognitive structures about electrochemical concepts through Word Association Test, in Arslan, H., Dorczak, R. and Alına-Andreea, D. U. (ed.), Educational Policy and Research, Kraków: the Institute of Public Affairs Jagiellonian University, pp. 293–300.
- Nakiboglu C. and Nakiboglu N., (2018b), Evaluation of “Chemistry and Electricity” unit of 12th grade chemistry textbook in terms of the chemistry triplet, in Arslan, H., Dorczak, R. and Alına-Andreea, D. U. (ed.), Educational Policy and Research, Educational Policy and Research, pp. 301–306.
- Nakiboğlu C. and Nakiboğlu N., (2021), Views of prospective chemistry teachers on the use of graphic organizers supported with interactive PowerPoint presentation technology in teaching electrochemistry concepts. Int. J. Physc. Chem. Ed., 13(3), 47–63.
- Nakiboğlu C. and Nakiboğlu N., (2022a), Investigation of prospective chemistry teachers' evaluations of galvanic and electrolytic cell difference, Ankara: Ankara International Congress on Scientific Research-VII, pp. 1784–1792.
- Nakiboğlu C. and Nakiboğlu N., (2022b), The use of V-diagram in teaching the topic of electrolysis and investigation of student opinions on the topic, Ankara: Ankara International Congress on Scientific Research-VII, pp. 1793–1800.
- Ogude A. N. and Bradley J. D., (1994), Ionic conduction and electrical neutrality in operating electrochemical cells, J. Chem. Educ., 71, 29–34.
- Osman K. and Lee T. T., (2014), Impact of interactive multimedia module with pedagogical agents on students’ understanding and motivation in the learning of electrochemistry, Int. J. Sci. Math. Educ., 12, 395–421.
- Özkaya A. R., (2002), Conceptual difficulties experienced by prospective teachers in electrochemistry: half-cell potential, cell potential, and chemical and electrochemical equilibrium in galvanic cells, J. Chem. Educ., 79(6), 735.
- Özkaya A. R., Üce M. and Şahin M., (2003), Prospective teachers’ conceptual understanding of electrochemistry: Galvanic and electrolytic cells, Univ. Chem. Educ., 7, 1–12.
- Rahayu S. and Kita M., (2010), An analysis of Indonesian and Japanese students’ understandings of macroscopic and sub microscopic levels of representing matter and its changes, Int. J. Sci. Math. Educ., 8, 667–688.
- Rahayu S., Treagust D. F., Chandrasegaran A. L., Kita M. and Ibnu S., (2011), Assessment of electrochemical concepts: a comparative study involving senior high-school students in Indonesia and Japan, Res. Sci. Technol. Educ., 29(2), 169–188.
- Rahayu S., Treagust D. F. and Chandrasegaran A. L., (2022), High school and preservice chemistry teacher education students’ understanding of voltaic and electrolytic cell concepts: evidence of consistent learning difficulties across years, Int. J. Sci. Math. Educ., 20(8), 1859–1882.
- Sanger M. J. and Greenbowe T. J., (1997a), Common student misconception in electrochemistry: current flow in electrolyte solutions and the salt bridge, J. Chem. Educ., 74(7), 819–823.
- Sanger M. J. and Greenbowe T. J., (1997b), Common student misconceptions in electrochemistry: Galvanic, electrolytic, and concentration cells, J. Res. Sci. Teach., 34(4), 377–398.
- Sanger M. J. and Greenbowe T. J., (2000), Addressing student misconceptions concerning electron flow in aqueous solutions with instruction including computer animations and conceptual change strategies, Int. J. Sci. Educ., 22(5), 521–547.
- Sarıkaya Gacanoğlu Ş. and Nakiboğlu C., (2022), Analysis of the chemistry questions in the higher education institutions examination according to the 2018 Chemistry Curriculum acquisitions (in Turkish), J. Turk. Chem. Soc.:Chem. Educ., 7(2), 217–242 DOI:10.37995/jotcsc.1165863.
- Schmidt H.-J., Marohn A. and Harrison A. G., (2007), Factors that prevent learning in electrochemistry, J. Res. Sci. Teach., 44(2), 258–283.
- Shwartz Y., Ben-Zvi R. and Hofstein A., (2006), The use of scientific literacy taxonomy for assessing the development of chemical literacy among high-school students, Chem. Educ. Res. Pract., 7(4), 203–225 10.1039/b6rp90011a.
- Sia D. T., Treagust D. F. and Chandrasegaran A. L., (2012), High school students’ proficiency and confidence levels in displaying their understanding of basic electrolysis concepts, Int. J. Sci. Math. Educ., 10, 1325–1345.
- Sirhan G., (2007), Learning difficulties in chemistry: An overview, J. Turkish Sci. Educ., 4(2), 2–20.
- Siswaningsih W. and Muchtar H. K., (2017), An investigation into students’ misconception on electrolyte and non-electrolytes solutions with two tier diagnostic test-based pictorial, Adv. Sci. Lett., 23(11), 10555–10558.
- Skelly K. M. and Hall D., (1993), The development and validation of a categorization of sources of misconceptions in chemistry, in Novak, J. (ed.), Proceedings, Third International Seminar on Misconceptions and Educational Strategies in Science and Mathematics, August 1–4, 199, Ithaca, NY: Cornell University, pp. 1496–1535.
- Sözbilir M., (2019), Causal comparative research method, in Özmen, H. and Karamustafaoğlu, O. (ed.), Eğitimde araştırma yöntemleri, Ankara: Pegem Akademi, pp. 179–195 (in Turkish).
- Su K. D., (2018), Enhancing students’ corresponding reasoning of cognitive performances by animated concept mapping in electrochemistry, J. Baltic Sci. Educ., 17(4), 662–673.
- Sudarmo U., (2015), Kimia 3 untuk SMA/MA Kelas XII [Chemistry 3 for SMA/MA Grade XII], Jakarta: Erlangga Publisher.
- Supasorn S., (2015), Grade 12 students’ conceptual understanding and mental models of galvanic cells before and after learning by using small-scale experiments in conjunction with a model kit, Chem. Educ. Res. Pract., 16, 393–407.
- Taber S. K., (2000), Molar and Molecular conceptions of research into learning chemistry: towards a synthesis, Paper presented at Variety in Chemistry Teaching Meeting, University of Lancaster.
- Taber K. S. and García-Franco A., (2010), Learning processes in chemistry: Drawing upon cognitive resources to learn about the particulate structure of matter, J. Learn. Sci., 19(1), 99–142.
- Tarkın A. and Uzuntiryaki-Kondakcı E., (2017), Implementation of case-based instruction on electrochemistry at the 11th grade level, Chem. Educ. Res. Pract.18(4), 659–681.
- Tsaparlis G., (2000), The states-of-matter approach (SOMA) to introductory chemistry, Chem. Educ. Res. Pract., 1(1), 161–168 10.1039/a9rp90017a.
- Tsaparlis G., (2018), Teaching and Learning Electrochemistry, Israel J. Chem., 58, 1–16 DOI:10.1002/ijch.201800071.
- Tsaparlis G. and Gorezi M., (2005), A modification of a conventional expository physical chemistry laboratory to accommodate an inquiry/project-based component: method and students’ evaluation, Can. J. Sci. Math. Tech. Educ., 5, 111–131.
- Tsaparlis G. and Gorezi M., (2007), Addition of a project-based component to a conventional expository physical chemistry laboratory, J. Chem. Educ., 84(4), 668–670.
- Yang E. M., Andre T., Greenbowe T. J. and Tibell L., (2003), Spatial ability and the impact of visualization/animation on learning electrochemistry, Int. J. Sci. Educ., 25(3), 329–349.
| This journal is © The Royal Society of Chemistry 2024 |
