Evaluating electrophile and nucleophile understanding: a large-scale study of learners’ explanations of reaction mechanisms
Stephanie J. H.
Frost
 ,
Brandon J.
Yik
,
Brandon J.
Yik
 ,
Amber J.
Dood
,
Amber J.
Dood
 ,
Daniel Cruz-Ramírez
de Arellano
,
Daniel Cruz-Ramírez
de Arellano
 ,
Kimberly B.
Fields
and
Jeffrey R.
Raker
,
Kimberly B.
Fields
and
Jeffrey R.
Raker
 *
*
Department of Chemistry, University of South Florida, Tampa, Florida 33620, USA. E-mail: jraker@usf.edu
First published on 2nd February 2023
Abstract
A deep understanding of organic chemistry requires a learner to understand many concepts and have fluency with multiple skills. This understanding is particularly necessary for constructing and using mechanisms to explain chemical reactions. Electrophilicity and nucleophilicity are two fundamental concepts to learning and understanding reaction mechanisms. Prior research suggests that learners focus heavily on explicit structural features (e.g., formal charge) rather than implicit features (e.g., an open p-orbital) when identifying and describing the role of electrophiles and nucleophiles in reaction mechanisms; however, these findings come from small-scale, interview-based investigations with a limited number of reaction mechanisms. The work reported herein seeks to further explore the meaning learners ascribe to electrophiles and nucleophiles by evaluating 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 936 written explanations from constructed-response items asking what is happening in reaction mechanisms and why it happens for 85 unique reaction mechanisms across a yearlong postsecondary organic chemistry course. To analyze these data, we developed an electrophile rubric to capture learners’ level of explanation sophistication (Absent, Descriptive, Foundational, and Complex); this electrophile rubric is complementary to a nucleophile rubric previously reported in the literature. Our data show proportional levels of explanation sophistication for electrophiles and nucleophiles (τb = 0.402) across these written explanations of reaction mechanisms. We note that learners’ explanations of nucleophiles tend to be at a higher level than their explanations of electrophiles. While this finding does support prior literature reports, we also found that explanations of mechanisms involving reductions of pi-bonds (e.g., carbonyls) tended to be more sophisticated for electrophiles than for nucleophiles. Overall, our results support the claim that learners are able to discuss both electrophilicity and nucleophilicity; however, learners discuss electrophilicity and nucleophilicity at different levels of sophistication where nucleophilicity predominates for most reaction types.
936 written explanations from constructed-response items asking what is happening in reaction mechanisms and why it happens for 85 unique reaction mechanisms across a yearlong postsecondary organic chemistry course. To analyze these data, we developed an electrophile rubric to capture learners’ level of explanation sophistication (Absent, Descriptive, Foundational, and Complex); this electrophile rubric is complementary to a nucleophile rubric previously reported in the literature. Our data show proportional levels of explanation sophistication for electrophiles and nucleophiles (τb = 0.402) across these written explanations of reaction mechanisms. We note that learners’ explanations of nucleophiles tend to be at a higher level than their explanations of electrophiles. While this finding does support prior literature reports, we also found that explanations of mechanisms involving reductions of pi-bonds (e.g., carbonyls) tended to be more sophisticated for electrophiles than for nucleophiles. Overall, our results support the claim that learners are able to discuss both electrophilicity and nucleophilicity; however, learners discuss electrophilicity and nucleophilicity at different levels of sophistication where nucleophilicity predominates for most reaction types.
Introduction
A sufficient understanding of electrophiles and nucleophiles is vital for understanding organic chemistry reaction mechanisms (Nedungadi and Brown, 2021). An overwhelming conclusion in the organic chemistry education research literature is that learners have difficulty identifying electrophiles and nucleophiles as well as applying appropriate terminology to describe what is happening in reaction mechanisms (Bhattacharyya and Bodner, 2005; Kraft et al., 2010; Strickland et al., 2010; Grove et al., 2012a, 2012b; Defever et al., 2015; Crandell et al., 2020). At the heart of such difficulty is translating the representational system for chemical structure and reaction mechanisms (i.e., the electron-pushing formalism) into nuanced understandings (often implicit features) of electron deficient and electron sufficient atoms and moieties. While electrophiles and nucleophiles are paired, the research literature suggests that learners have a differential understanding of the two, with more proficiency associated with identifying and describing nucleophiles (Anzovino and Bretz, 2015, 2016; Galloway et al., 2017; Caspari, et al., 2018a; Graulich et al., 2019; Watts et al., 2020, 2022). At present, chemistry education researchers in this area have primarily used in-depth interviews to explore learners’ understanding of organic chemistry topics. While such research provides detail-rich descriptions about learners’ understanding, the findings of studies which explore a larger, broader sample of learners’ written work may better translate across multiple courses, reaction mechanisms, curricula, institutional contexts, etc.In this study, we report the evaluation of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 936 written explanations about what is happening and why in reaction mechanisms. Written explanations were collected for 85 reactions taught across two terms of a yearlong organic chemistry course sequence. The level of explanation sophistication for electrophiles and nucleophiles was determined for each reaction mechanism explanation response; further, the degree of association between levels of explanation sophistication of electrophiles and nucleophiles was determined. Associations were disaggregated for a series of reaction families (e.g., reactions involving a reduction of a pi-bond, reactions of aromatic species) with results suggesting that discrepancies between electrophiles and nucleophiles may be based on cued explicit features of the reaction mechanism such as formal charge and common moieties (e.g., carbonyl). The results of our study provide understanding for how to better structure assessments and how to better evaluate pedagogy in organic chemistry education. Additionally, our results provide a foundation for the development of automated technologies to assess learning in organic chemistry course contexts and how large-scale studies either within or across institutional contexts are necessary to better understand the broad claims of more information-rich studies with smaller numbers of study participants.
936 written explanations about what is happening and why in reaction mechanisms. Written explanations were collected for 85 reactions taught across two terms of a yearlong organic chemistry course sequence. The level of explanation sophistication for electrophiles and nucleophiles was determined for each reaction mechanism explanation response; further, the degree of association between levels of explanation sophistication of electrophiles and nucleophiles was determined. Associations were disaggregated for a series of reaction families (e.g., reactions involving a reduction of a pi-bond, reactions of aromatic species) with results suggesting that discrepancies between electrophiles and nucleophiles may be based on cued explicit features of the reaction mechanism such as formal charge and common moieties (e.g., carbonyl). The results of our study provide understanding for how to better structure assessments and how to better evaluate pedagogy in organic chemistry education. Additionally, our results provide a foundation for the development of automated technologies to assess learning in organic chemistry course contexts and how large-scale studies either within or across institutional contexts are necessary to better understand the broad claims of more information-rich studies with smaller numbers of study participants.
Electrophiles and nucleophiles
Electrophiles are defined as electron-seeking species and nucleophiles are defined as nucleus-seeking species; electrophiles are characterized as electron deficient, whereas nucleophiles are characterized as electron sufficient. These two sets of definitions differ in functional (the former) or structural (the latter) perspectives of conceptualizing electrophiles and nucleophiles (Anzovino and Bretz, 2015; Klein, 2021). It is important to note that there is an inherent overlap between electrophiles and nucleophiles, and Lewis acid–base characterizations: electrophiles are related to Lewis acids and nucleophiles are related to Lewis bases. Context guides and determines which terminology is used by chemists to characterize a species and its interactions with other species. A given species has varying functionality as an electrophile or Lewis acid and a nucleophile or Lewis base based on a broad set of features. Furthermore, identifying how a species is involved in a reaction mechanism is necessary to characterize the role of the species: for example, water can act as both a nucleophile (e.g., forming a bond with a carbocation) and a base (e.g., deprotonating an intermediate) in the mechanism of a unimolecular substitution reaction.Identification of electrophiles, nucleophiles, and the subsequent descriptions of their role in a reaction mechanism is both structurally and functionally oriented (Anzovino and Bretz, 2016; Graulich et al., 2019). Properties of molecules can be shown explicitly or implicitly (Graulich et al., 2019). Formal charges are an easily identifiable explicit feature for categorizing electrophiles and nucleophiles: a positive formal charge on an atom would indicate electrophilicity (i.e., a deficiency of electrons) and a negative formal charge on an atom would indicate nucleophilicity (i.e., an excess of electrons). In contrast, partial charges, are typically not drawn on species; therefore, implicit structural features may need to be interpreted to determine the presence of unequal distribution of charge, and thus electrophilicity or nucleophilicity. For example, understanding of bond polarity, resonance, and resonance hybrid structures are necessary for identifying partial charges within a given species. Further, some neutral species with lone pairs (i.e., electron sufficiency) show an explicit indication of nucleophilicity, whereas some neutral species with empty orbitals such as aluminum chloride (AlCl3) could be considered as an implicit indication of electrophilicity. Additionally, pi bonds and sigma bonds can have nucleophilic character, albeit with differing levels of explicitness. Large collections of reaction mechanisms are taught as “reactions of a pi bond” wherein, in the absence of particular chemoselectivity (e.g., alkenes versus alkynes, or alkenes versus aromatic species), pi bonds are explicitly electron sufficient, and thus nucleophilic. Sigma bonds are not all sufficiently electron-rich to act as nucleophiles, and thus the need to imply an implicit feature is needed. Lastly, polarizability (i.e., the property of an atom or collection of atoms to have an uneven distribution of electrons due to an external entity) characterizes more polarizable atoms or ions as being more nucleophilic (e.g., iodide, a large polarizable anion, is a stronger nucleophile than chloride if not considering solvent effects).
Prior work has shown a disparity between how electrophiles and nucleophiles are identified and described in various contexts with a particular reliance on explicit structural features (Bhattacharyya and Bodner, 2005; Strickland et al., 2010; Bhattacharyya, 2014; Anzovino and Bretz, 2015, 2016; Graulich, 2015; Akkuzu and Uyulgan, 2016; Putica and Trivic, 2016; Galloway et al., 2017; Caspari et al., 2018a; Dood et al., 2020a; Watts et al., 2020, 2022; Dood and Watts, 2022b). Anzovino and Bretz (2015, 2016) found that learners were more apt at identifying and providing examples of electrophiles and nucleophiles when charge was explicit: for example, the positive carbon in a carbocation species or the negatively charged oxygen of an alkoxide. Further, learners more easily identified and proposed the carbon of a carbonyl as an electrophile, while not explicitly explaining such electrophilicity using concepts such as polarity or resonance; Putica and Trivic (2016) had a similar finding. Additionally, when lone pairs were explicitly depicted in the Anzovino and Bretz (2015, 2016) studies, as with water, learners were more apt in identifying and proposing nucleophiles; Dood et al. (2020a) had a similar finding as well.
From a functional perspective, electrophiles are characterized as accepting electrons and nucleophiles are characterized as donating electrons. Thus, identification of a species depends on how it interacts with other species. Consequently, electrophiles and nucleophiles are not exclusively defined as species that can be isolated at the end of a reaction; electrophiles and nucleophiles are also intermediates that are formed and further react in the context of a reaction mechanism. As such, the curved arrows of the electron-pushing formalism provide context and cues to help learners identify electrophiles and nucleophiles: curved arrows point towards electrophiles, and curved arrows originate from nucleophiles (i.e., from electron sufficient to electron deficient) (Bhattacharyya and Bodner, 2005; Grove et al., 2012a, 2012b; Anzovino and Bretz, 2015).
The context of how two species interact may cue learners to the function of a species through the use of reaction mechanisms. Anzovino and Bretz (2015, 2016) found that when learners were asked to identify electrophiles and nucleophiles for a given set of reactants, those learners would propose a mechanism and predict the product(s) of the reaction before identifying which species were electrophiles or nucleophiles, if at all. Although study participants were not explicitly prompted by Watts et al. (2020) to identify any electrophiles or nucleophiles, Watts et al. (2020) reported that no participants invoked such terminology when given a writing-to-learn assignment focused on utilizing representations of a reaction mechanism diagram and a reaction coordinate diagram; the study participants did, however, discuss charge and the movement of electrons, and thus may not have invoked electrophilicity and nucleophilicity since it was not explicitly stated in the assessment prompt directions.
In reaction contexts, electrophiles and nucleophiles are paired. If there is an electrophile, there is a nucleophile that interacts with it in a reaction mechanism, and vice versa. Learners in multiple studies were more likely to identify and describe the role of nucleophiles than electrophiles for given reaction mechanisms, and explicitly identify a nucleophile (or electrophile) without identifying the other (Anzovino and Bretz, 2015, 2016; Bhattacharyya and Harris, 2018; Dood et al., 2020a). Anzovino and Bretz (2015, 2016) noted that of their eleven interviewees, only one interviewee identified any electrophile when describing what is happening in eight different mechanisms. In the context of reaction mechanisms, the findings of Anzovino and Bretz highlight a lack of identification and description of electrophiles in similar detail and depth to nucleophiles. Nedungadi and Brown (2021) noted that identification of electrophiles and nucleophiles is a critical learning outcome of undergraduate organic chemistry courses. Bhattacharyya and Harris (2018) suggested that a potential cause for the difference in identification of nucleophiles and electrophiles is due to the use of the active voice to describe bond formation between the nucleophile and electrophile (i.e., the nucleophile attacks the electrophile).
Reasoning in organic chemistry
While identification of electrophilic and nucleophilic species is a fundamental skill, the higher-order goal is for learners to identify and describe the action of such species in the context of reaction mechanisms. In other words, the goal is for chemists to explain how and why species interact in reaction mechanisms. A key objective in organic chemistry is for learners to be able to rationalize and argue for observed or predicted products for a given set of reacting species (NGSS Lead States, 2013; Nedungadi and Brown, 2021). The ability to construct explanations for reaction mechanisms is a key indicator of this objective. This requires learners to integrate and apply various organic chemistry concepts (such as electrophiles, nucleophiles, Lewis acids and bases, wedge-dash bond line formulas, nomenclature, curved arrows, etc.) in explanations of reaction mechanisms. Thus, requiring learners to construct explanations of reaction mechanisms can be used as both a learning activity and a measure of learning.Reasoning exhibited in explanations of organic chemistry reaction mechanisms has been characterized with an array of frameworks with varying operationalizations of reasoning sophistication (Dood and Watts, 2022a). The lower end of reasoning sophistication includes teleological and anthropomorphic reasoning. Teleological reasoning uses the consequence as the reason why an action occurs (i.e., the reaction occurs because products are more stable than reactants), and anthropomorphic reasoning provides human characteristics to inanimate entities (i.e., atoms want a filled octet) to rationalize reaction mechanisms (Talanquer, 2013; Dood et al., 2020a). Teleological reasoning has been reported on multiple occasions in the context of learners’ explanations of reaction mechanisms (Bhattacharyya and Bodner, 2005; Bhattacharyya, 2014; Galloway et al., 2017; DeCocq and Bhattacharyya, 2019; Dood et al., 2020a). For example, Caspari et al. (2018b) highlighted that many learners invoke teleological reasoning when explaining a mechanism by focusing on the backwards sequence of events. While teleological reasoning can be appropriately used, a more sound cause for certain phenomena (e.g., explanation of why a reaction mechanism occurs) is necessary (Caspari et al., 2018a). The higher end of sophistication of reasoning includes causal mechanistic reasoning where the causal aspect explains to why a reaction occurs and the mechanistic aspect explains to how a reaction occurs (Yan and Talanquer, 2015; Crandell et al., 2018, 2020). A general example of a causal mechanistic explanation for a bimolecular substitution reaction is: “the lone pair on the nucleophilic ethoxide is attracted to the partial positive carbon on the 1-bromoethane while the bromide leaves.” This example relates to the causal aspects with the attraction of ethoxide to 1-bromoethane and the mechanistic aspects with the electron movement. Much work has explored how learners engage with causal mechanistic reasoning in organic chemistry (Caspari et al., 2018a; Bodé et al., 2019; Crandell et al., 2020; Noyes et al., 2022); notably, learners attempt to engage in causal mechanistic reasoning regardless of correctness of their responses (Bodé et al., 2019; Deng and Flynn, 2021).
In developing a rubric to evaluate the level of explanation sophistication for nucleophiles (herein referred to as the nucleophile rubric), Yik et al. (2023) reported modifications of a reasoning framework preposed by Dood et al. (2020a). The nucleophile rubric includes an Absent level (i.e., a non-normative or no explanation level) not found in the other frameworks reviewed by Dood and Watts (2022a); a possible reasoning for why such a level has been excluded is that prior frameworks tended to be research-focused while the work by Yik et al. (2021) focused on developing an assessment tool for instructors and learners to use. An absence of understanding can be evaluated in responses to formative assessment constructed-response items using the framework from Yik et al. (2023). The nucleophile rubric also has Descriptive, Foundational, and Complex levels with increasing sophistication wherein the Descriptive level maps onto the teleological and anthropomorphic aspects of reasoning and the Complex level maps onto causal mechanistic reasoning. This is not to suggest that the levels of explanation sophistication operationalized in the nucleophile rubric are synonymous with the frameworks and associated levels of reasoning as reviewed by Dood and Watts (2022a); each of the frameworks serves a different purpose and has a different rationale for operationalizing reasoning. In other words, a sound Complex level of explanation sophistication could contain teleological reasoning or anthropomorphism as long as a causal mechanistic explanation was part of the explanation. Fundamentally, the choices made by Yik et al. (2023) communicate that the audience for their rubric (i.e., reasoning framework) are instructors and learners, and therefore demonstrate that their reasoning framework is intended for audiences beyond reasoning researchers. The work reported herein extends upon Yik et al. (2023) with analogous levels of explanation sophistication focused on electrophiles.
Assessing reaction mechanisms learning
Written explanations of reaction mechanisms are a primary means to evaluate the meaning ascribed by learners to reaction mechanism pictures. Unfortunately, many assessments in organic chemistry courses only ask learners to draw reaction mechanisms and do not ask for explanations of those reaction mechanisms. This has led to an array of studies that resoundingly conclude that learners are able to draw reaction mechanisms with little to no understanding of the lines, letters, symbols, arrows, etc. that comprise reaction mechanisms (Anderson and Bodner, 2008; Ferguson and Bodner, 2008; Grove et al., 2012a; Grove et al., 2012b; Bhattacharyya, 2014; Flynn and Ogilvie, 2015; Flynn and Featherstone, 2017; Galloway et al., 2017; Weinrich and Sevian, 2017; Nedungadi and Brown, 2021; Deglopper et al., 2022; Dood and Watts, 2022b). Written explanations provide both an opportunity to integrate and apply concepts and skills (i.e., a learning process) as well as to demonstrate learning (i.e., an assessment of learning) (Cooper, 2015).Several analogous variations of constructed-response items have been reported in the literature for prompting learners to describe what is happening and why for a range of reaction mechanisms (Kraft et al., 2010; Sevian and Talanquer, 2014; Cooper et al., 2016; Caspari et al., 2018a; Crandell et al., 2018, 2020; Dood et al., 2018; Bodé et al., 2019; Graulich et al., 2019; Deng and Flynn, 2021; Kranz et al., 2023; Lieber and Graulich, 2022; Lieber et al., 2022; Yik et al., 2023). While not exclusive to such purposes, these constructed-response items have been presented for use as formative assessments, for promoting learning, and for instructors to respond in real time to the measured learning (Dood et al., 2018, 2020a; Yik et al., 2021). Reported use of constructed-response items note how such explanations provide a more thorough, complex, and authentic assessment of the desired learning expected in organic chemistry courses (Dood et al., 2018; Yik et al., 2021, 2023). The knowledge generated from such constructed-response items has in turn informed the development of adaptive tutorials which rely on the constructed-response items for pre-post measures of learning (Dood et al., 2019, 2020b; Yik et al., 2021). For those reporting use of such constructed-response items in the literature, descriptions of the assessment loop being closed and re-cycled are typically reported; the “data” collected from the assessments are being used to create and revise learning experiences and environments for future organic chemistry learners (Dood et al., 2019, 2020b; Yik et al., 2021; Lieber et al., 2022). When constructed-response items and resulting data are intended for instructional and learning use, rubrics to evaluate responses are essential; such rubrics need to connect with the learning outcomes of the course and resolve to have a learner-centered focus (Andrade, 2000; Brookhart and Chen, 2015).
Research question
This study was framed by one research question:• How do learners’ level of explanation sophistication for electrophiles and nucleophiles relate when they are asked to explain what is happening and why for various organic chemistry reaction mechanisms?
To answer this research question, we report the development of a rubric to evaluate the level of explanation sophistication for electrophiles. This rubric mirrors the previously reported rubric for evaluating the level of explanation sophistication for nucleophiles (Yik et al., 2023).
Methods
Data for this study were collected across multiple organic chemistry courses and terms, and for a variety of organic chemistry reaction mechanisms. The electrophile rubric (reported herein) and the nucleophile rubric (Yik et al., 2023) were used to evaluate learners’ explanations of reaction mechanisms. Cross-tabulations, non-parametric evaluations of associations, and other descriptive techniques are used to answer our guiding research question. Exemplar explanations (i.e., responses to the constructed response item) are provided to give context to the descriptive and statistical analyses.This work was conducted under application Pro#00028802, “Comprehensive evaluation of the University of South Florida's undergraduate and graduate chemistry curricula,” as reviewed by the University of South Florida's Institutional Review Board on December 13, 2016; the activities were determined to not constitute research involving human subjects per Institutional Review Board criteria.
Data collection
Data were collected from the first and second terms of a yearlong post-secondary organic chemistry course sequence between Fall 2017 and Fall 2021. This includes seven semesters of the first-term course (Fall 2017, Spring 2018, Spring 2019, Fall 2019, Spring 2020, Fall 2020, and Fall 2021) and two semesters of the second-term course (Spring 2020 and Fall 2021). Data were collected at the University of South Florida, a large, research-intensive, public university in the southeastern United States. Data were collected in the courses of four instructors (authors DCR, KBF, and JRR, and F. Costanza – see Acknowledgements).The first-term course has between four and six sections each semester with 180 learners per section, and the second-term course has between three and five sections each semester with 150 learners per section. Each course holds lectures twice per week (75 minutes each) with one discussion section per week (50 minutes) led by a graduate teaching assistant. Between Fall 2017 and Spring 2019, all instructors used the Solomons et al. (2016)Organic Chemistry, 12th edition textbook, and between Fall 2019 and Fall 2021, all instructors used the Klein (2017, 2021)Organic Chemistry, 3rd edition or 4th edition textbook.
Learners responded to one of two comparable constructed-response item prompt variations (see Table 1) via Qualtrics, an online survey platform, to provide their responses. The prompts are modifications of the constructed-response item originally reported by Cooper et al. (2016), with variations used in multiple studies since (Crandell et al., 2018, 2020; Dood et al., 2019; Yik et al., 2021, 2023). A “more cued” prompt was given to help specify to learners to focus on each individual step in their explanation and minimize over-generalized statements (e.g., the molecules react to form the products). Learners received bonus points towards either their term examination or final examination scores for completing the assessment; learners received these bonus points based on completion and not correctness. Learners had the opportunity to complete up to four assessments (approximately one to three explanations of reaction mechanisms per assessment) each semester, and students may have completed assessments in both the first- and second-semester courses. All data are deidentified to protect confidentiality and for anonymity; thus, we are unable to connect learner responses within the dataset to conduct more nuanced or paired analyses of our data. A total of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 936 responses were collected. The same data were used in Yik et al. (2023); in addition, the same levels of explanation sophistication for nucleophiles for each of the responses in the data set reported in Yik et al. (2023) are also used in the study herein. Some of the explanations for unimolecular nucleophilic substitution reactions were also previously reported in studies by Dood et al. (2020a, 2020b).
936 responses were collected. The same data were used in Yik et al. (2023); in addition, the same levels of explanation sophistication for nucleophiles for each of the responses in the data set reported in Yik et al. (2023) are also used in the study herein. Some of the explanations for unimolecular nucleophilic substitution reactions were also previously reported in studies by Dood et al. (2020a, 2020b).
| Original | More cued |
|---|---|
| Part A: Describe in full what you think is happening on the molecular level for this reaction. Be sure to discuss the role of the reactant and the intermediate. | Part A: Describe in full detail the sequence of events that occur at the molecular level for this reaction. Be sure to discuss the role of each reactant and intermediate. |
| Part B: Using a molecular level explanation, explain why this reaction occurs. Be sure to discuss why the reactants form the products shown. | Part B: Using a molecular level explanation, explain why each of the reactants and intermediates interact. |
Written explanations were collected for 85 unique reaction mechanisms taught in the yearlong organic chemistry course sequence (see Table 2). Reaction mechanisms include: bimolecular substitution reactions (“SN2”), reactions involving a carbocation intermediate (“carbocation”; e.g., unimolecular nucleophilic substitution), reduction reactions of a pi-bond (“reduction”; e.g., reduction of a carbonyl with lithium aluminum hydride), aromatic reactions (“aromatic”; e.g., electrophilic aromatic substitution), and nucleophilic addition reactions (“nucleophilic addition”; e.g., conjugate addition). Reaction mechanisms are grouped by “family” based on similar mechanism components and features.
| Reaction family | Reaction | Variations | Number of explanations |
|---|---|---|---|
| SN2 | Alkylation | 1 | 411 |
| Conversion to good leaving group, then SN2 | 4 | 932 | |
| Epoxidation/ring-opening | 6 | 1284 | |
| Halohydrin formation | 3 | 717 | |
| Carbocation | Halogenation/hydrohalogenation | 5 | 1262 |
| Hydration/dehydration | 4 | 1276 | |
| Unimolecular nucleophilic substitution (SN1) | 8 | 5050 | |
| Reduction | Grignard | 6 | 961 |
| Reduction | 9 | 1825 | |
| Aromatic | Acylation | 1 | 197 |
| Addition–elimination | 1 | 136 | |
| Alkylation | 1 | 196 | |
| Azo coupling | 1 | 170 | |
| Electrophilic aromatic substitution | 6 | 657 | |
| Nucleophilic aromatic substitution | 2 | 225 | |
| Nucleophilic addition | Acyl substitution | 3 | 403 |
| Alpha-halogenation | 1 | 68 | |
| Condensation of an ester/diester | 2 | 402 | |
| Conjugate addition | 2 | 255 | |
| Electrophilic addition | 2 | 553 | |
| Enamine/imine synthesis | 2 | 367 | |
| Hemiacetal/acetal formation | 4 | 885 | |
| Hydration/dehydration | 2 | 137 | |
| Hydrolysis | 1 | 70 | |
| Saponification | 1 | 202 | |
Development and evaluation of a rubric for level of explanation sophistication for electrophiles
A rubric mirroring that for nucleophiles (Yik et al., 2023) was developed to evaluate the level of explanation sophistication for electrophiles. The four levels of sophistication (Absent, Descriptive, Foundational, and Complex) were operationalized for electrophiles based on the nucleophile rubric and the chemistry education research literature on electrophiles (see Table 3 for the electrophile rubric). We also note that a previous conceptualization of the rubric for electrophiles was reported in Raker et al. (2023); however, the electrophile rubric herein replaces that rubric, as the Raker et al. (2023) rubric was developed without application to data and without interrater reliability investigations.| Level | Description of level | Key features of level |
|---|---|---|
| Absent | • No response | • The electrophile is not identified |
| • Non-normative | • The electrophile–nucleophile step is missing from the explanation | |
| Descriptive | • Describes the electrophile engaging in bond forming processes | • The electrophilic molecule or atom is identified |
| • Simplistic description of bond forming processes | • Bond forming processes are described | |
| • Electrophilic behavior is described at a surface, atomic level | • Electrons (i.e., the absence of electrons) are not used to describe electrophilic behavior | |
| Foundational | • Electrophilic behavior is described at a surface, electronic level | • Electrons (i.e., the absence of electrons) are central to electrophilic behavior and arrows represent the movement of electrons |
| • Explicit features are mentioned | • Bond forming processes are described using electrons | |
| • Implicit features may be mentioned, but not fully explained | ||
| Complex | • Describes why the electrophile is involved in the bond forming processes | • Implicit electronic features (e.g., partial charges, empty p-orbitals, LUMO considerations) are used to describe electrophilic behavior |
| • Electrophilic behavior is described at a deeper, electronic level | • Bond forming processes are described using electrons, the absence of electrons, and electronic properties | |
| • Explicit features are used to infer implicit features and sufficiently explained | ||
As noted in the development in of the nucleophile rubric (Yik et al., 2023), the levels of explanation sophistication map well with other reasoning frameworks (Dood and Watts, 2022a) used in investigations of reaction mechanism understanding. In keeping with prior investigations of learners’ understanding of electrophiles and nucleophiles (Anzovino and Bretz, 2015, 2016), proton transfer reaction mechanism steps are also not evaluated using the electrophile rubric as with Yik et al. (2023).
An interrater reliability investigation was conducted with 200 randomly selected responses inclusive of all rubric levels of explanation sophistication (Absent through Complex) after SJHF independently coded all responses. SJHF and JRR independently coded the responses. Initial agreement was 41.5% (κ = 0.098). Initial agreement was low due to a need to clarify the Descriptive and Foundational levels. SJHF and JRR clarified the four rubric levels, interpretation of responses when explanations were unclear, and if the need for explicit identification (without descriptions of electrophilic behavior) of the electrophile would be coded at the Descriptive or Foundational level. SJHF and JRR agreed that if an explanation only identified the electrophile without further descriptions of electrophilic behavior, that would be categorized as Descriptive. SJHF then recoded all data at the Foundational level after these discussions since that was the primary change to the coding scheme. After a second discussion, final agreement was 86.5% (κ = 0.838). All data (N = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 936) are classified accordingly (see Table 4) and reported by each of the two course terms.
936) are classified accordingly (see Table 4) and reported by each of the two course terms.
| Level | First term (%) | Second term (%) | Overall (%) |
|---|---|---|---|
| n | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 559 559 |
6377 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 936 936 |
| Absent | 17.6 | 19.5 | 18.2 |
| Descriptive | 54.2 | 61.4 | 56.5 |
| Foundational | 18.5 | 9.6 | 15.7 |
| Complex | 9.7 | 9.5 | 9.6 |
The distribution of responses is given in Table 5 by course term and for the overall data set. For both terms, most explanations (>54%) are classified as Descriptive. There is no observed floor or ceiling effect, i.e., a majority of responses are not at the lowest nor highest level of the rubric, respectively (see Šimkovic and Träuble, 2019). However, a ceiling effect would inherently be valuable in the context of instruction, as it would imply that most learners were providing the highest level of explanation for the target course. The lack of a floor effect suggests that the curriculum in which the data were collected results in some learning; however, nearly 20% of responses are classified as Absent, and thus opportunities for improvement exist.
| Cell (%) | Nucleophile level | Total (%) | ||||
|---|---|---|---|---|---|---|
| Absent | Descriptive | Foundational | Complex | |||
| Electrophile level | Absent | 9.7 | 6.7 | 1.6 | 0.1 | 18.2 |
| Descriptive | 3.2 | 37.2 | 15.4 | 0.7 | 56.5 | |
| Foundational | 0.9 | 6.9 | 6.2 | 1.7 | 15.7 | |
| Complex | 0.3 | 3.8 | 4.1 | 1.4 | 9.6 | |
| Total (%) | 14.1 | 54.6 | 27.3 | 4.0 | ||
Given the wide array of reaction mechanisms (n = 85) for which data were collected, the interrater reliability as reported above, and the descriptive analyses in Table 5, the electrophile rubric and resulting analyses are suitable for the analyses presented in the Results & Discussion section. Parallel analyses were conducted with the nucleophile rubric, and mirrored results were obtained (Yik et al., 2023). Classifications for levels of explanation sophistication using both rubrics were combined for the analyses in this study.
Results & discussion
To address the overarching research question, cross-tabulations of the level of explanation sophistication for electrophiles and nucleophiles for the data set are reported overall, by course term, and by reaction family. Cross-tabulation analyses are interpreted to consider measures of association between the electrophile and nucleophile levels overall, by course term, and by reaction family. Finally, evaluation of the differences between level of explanation sophistication for electrophiles and nucleophiles was conducted. Representative explanations and reaction mechanisms are provided for context in interpreting study results; all explanations are presented as provided by the learners including any misspellings, colloquial language, punctuation, lack of punctuation, etc.Exemplar responses
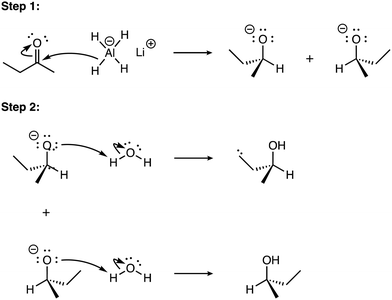
Three exemplar reaction mechanism explanations are provided, one for each of the non-Absent levels, to contextually situate the statistical analyses reported in upcoming sections. The three exemplar explanations are at differing levels of explanation sophistication for a common reaction mechanism, i.e., a unimolecular substitution reaction of tert-butyl bromide and ethanol to form 2-ethoxy-2-methylpropane (see Fig. 1).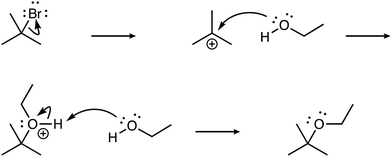 | ||
| Fig. 1 The reaction mechanism for the unimolecular substitution of tert-butyl bromide and ethanol to form 2-ethoxy-2-methylpropane. | ||
For the first example (Descriptive for both electrophile and nucleophile), the learner identifies both an electrophilic species and a nucleophilic species; however, there a discussion of electrons or electron deficiency/sufficiency is lacking.
Descriptive for both electrophile and nucleophile levels:
“The bond between carbon and bromine is breaking, as bromine is leaving the substrate. A carbocation is formed, and the oxygen from the ethanol is attacking the carbocation. This leaves a positive charge on the oxygen atom, so another ethanol will come in and take the proton from the oxygen, which will break the bond between itself and oxygen. The bromine on the substrate will leave as it is a good leaving group. Oxygen always wants to attack carbocations or accept protons, so it will always do that if at all possible.”
For the second example, i.e., Foundational for both electrophile and nucleophile levels, the learner invokes energetics, where there is recognition of a high-energy intermediate (i.e., the carbocation) that is lowered in energy when it forms a bond (i.e., neutralized). Regarding the nucleophile, the learner does not invoke implicit descriptions, but only descriptions of explicit electrons. The learner uses anthropomorphisms when describing the high-energy carbocation as “disliking” the positive charge.
Foundational for both electrophile and nucleophile levels:
“On the molecular level for this reaction, the bromine atom is taking the electrons from its bond to the alkane carbon, forming a negative charged bromine atom. Then, one of the lone electron pairs on the oxygen atom of ethanol attacks the positively charged carbon in order to neutralize it. Then, a different ethanol molecule attacks the hydrogen of the previous one that's not attached to the alkane, and the negative charge of the hydrogen–carbon bond is transferred to the oxygen molecule, giving it two lone electron pair sets, which neutralizes it. This reaction occurs because both ethanol molecules attack the main reactant in order to neutralize it. In the first instance, ethanol neutralizes the positive charge on the central carbon since carbon atoms greatly dislike any source of charge. In the second instance, oxygen atoms prefer to have two sets of lone electron pairs, and so another ethanol molecule removed a hydrogen from the oxygen in order to give it the desired two lone electron pairs.”
For the third example, i.e., Complex for both electrophile and nucleophile levels, the learner identifies a key implicit feature of carbocation stability: the methyl groups stabilize the carbocation through the inductive effect. Further, the learner implies that the lone pairs on the nucleophile (i.e., ethanol) are attracted to the electrophile (i.e., the carbocation).
Complex for both electrophile and nucleophile levels:
“In this reaction, the bromine atom leaves because a tertiary carbocation is the most stable, so it can afford to leave. This leaves behind a positive charge where the HO of the ethanol nucleophile attacks since it has extra lone pairs that it can use to form a bond. In the final step, in order to make the new substituted product more stable because it has a positive charge, extra ethanol solution deprotonates the hydrogen from the oxygen of the bonded ethanol to remove the positive charge it has (oxygen with three bonds has a positive charge) This reaction occurs because of the stability of the tertiary carbocation being balanced by nearby groups via induction. Also, bromine is a good leaving group because it's the conjugate base of a strong acid and it can deal with its negative charge better due to size and electronegativity. Additionally, the extra lone pairs on oxygen make it a base that is attracted to the positive charge of the carbocation. This causes a substitution which is then deprotonated because the positive charge of the substituted ethanol is attracted to the lone pairs of the extra ethanol in solution.”
Cross-tabulation analyses
Cross-tabulation of the levels of explanation sophistication for electrophiles and nucleophiles for the entire data set (i.e., overall) is reported in Table 5. The cross-tabulation analyses for the entire data set show proportional understanding between electrophiles and nucleophiles. Furthermore, it also suggests many learners are within one level of explanation sophistication of each other (e.g., Descriptive for electrophiles and Foundational for nucleophiles). Results of a chi-square test suggest a relationship between the two levels: χ2(9) = 7491, p < 0.001; thus, the level of explanation sophistication between electrophiles and nucleophiles is interdependent and proportional. In other words, Descriptive levels of explanation sophistication for both electrophiles and nucleophiles are observed (∼37.2% of all explanations), or Foundational levels for both (∼6.2% of all explanations), or Complex levels for both (∼1.4%).Kendall's tau-b provides a measure of the effect size of the relationship between electrophile and nucleophile explanation sophistication: τb = 0.402 (strong), p < 0.001. This finding suggests that while the levels of explanation of sophistication are related between electrophiles and nucleophiles, the relationship is not perfect (i.e., τb = 1).
Proportional and related understanding of electrophiles and nucleophiles mirrors the paired relationship between these two concepts. Nearly 55% of classification of levels of explanation sophistication for electrophiles and nucleophiles were the same while roughly 10% of all explanations are at the Absent level for both concepts. Although, most (∼37%) of the entire data set were at the Descriptive level of sophistication for both concepts. Measures of association between the electrophile and nucleophile levels for the complete data set are strong with the suggestion of interdependence; however, this association may be large due to the significant number of classifications at either both the Absent levels or the Descriptive levels.
Cross-tabulations for each of the terms (i.e., first and second) and by reaction family are reported in Appendix 1. The results of the chi-squared tests and Kendall's tau-b analyses for each term and reaction family are reported in Table 6. Similar trends for the complete data set are observed for each of the course terms and reaction families, that is, a proportional, strong association.
| n | χ 2 (df = 9)a | τ b (size)a | |
|---|---|---|---|
| a All statistical results are statistically significant (p < 0.001). | |||
| First term | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 559 559 |
4681 | 0.387 (strong) |
| Second term | 6377 | 2861 | 0.438 (strong) |
| SN2 | 4873 | 2348 | 0.459 (strong) |
| Carbocation | 7432 | 1774 | 0.306 (strong) |
| Reduction | 2786 | 1276 | 0.502 (strong) |
| Aromatic | 1581 | 485 | 0.384 (strong) |
| Nucleophilic addition | 3264 | 1988 | 0.504 (strong) |
Again, classification at proportional levels was observed for the two course terms and the five reaction families with between 8% and 15% at the Absent level for both electrophiles and nucleophiles, and between 29% and 47% at the Descriptive level for both. Thus, association measures may be conflated based on observation of the two cross-tabulations (i.e., Absent–Absent or Descriptive–Descriptive).
Differences in level of explanation sophistication
While the cross-tabulations and measures of association analyses suggest a proportional and strong relationship (albeit in part due to the large number of explanations classified as Absent–Absent or Descriptive–Descriptive), explanations that are not classified at proportional levels need to be further explored. Observations of the on-diagonal of Table 5 (see previous section) show that ∼54.5% of the evaluated explanations were at the same level of explanation sophistication for both electrophiles and nucleophiles. While that percentage is relatively high (a little more than half), this also means that nearly half of the explanations were different by one or more levels between the electrophile explanation sophistication classification and the nucleophile explanation sophistication classification.To further explore these “off-diagonal” classifications, explanations were further sorted as the “equal levels,” the “higher electrophile level,” or the “higher nucleophile level.” A summary of this analysis is provided in Table 7 for the overall data set, by course term, and by reaction family. Except for the reduction reaction family, there is a persistent pattern of responses where the electrophile level is lower than the nucleophile level. The difference between these percentages is the most with the aromatic reaction family (14.9% versus 36.2%); and the difference is the least with the reduction reaction family (19.2% versus 15.7%). Thus, a range of differences is observed between the electrophile or nucleophile level of explanation sophistication for a given response. This range of differences reinforces prior work in this area (Anzovino and Bretz, 2015, 2016; Watts et al., 2020). Our results support the prior work of others with a larger-scale measure; additionally, our work shows that findings of these prior works extend to a broader array of reaction mechanisms encompassing the entirety of a yearlong organic chemistry course.
| n | Equal levels (%) | Higher electrophile level (%) | Higher nucleophile level (%) | |
|---|---|---|---|---|
| Overall | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 936 936 |
54.5 | 19.2 | 26.4 |
| First term | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 599 599 |
53.3 | 20.8 | 26.0 |
| Second term | 6377 | 57.0 | 15.8 | 27.3 |
| SN2 | 4873 | 52.1 | 18.9 | 29.0 |
| Carbocation | 7432 | 49.6 | 23.3 | 27.1 |
| Reduction | 2786 | 65.1 | 19.2 | 15.7 |
| Aromatic | 1581 | 49.0 | 14.9 | 36.2 |
| Nucleophilic addition | 3264 | 62.6 | 12.1 | 25.3 |
To a degree, reaction family explains the observed disparity in levels between electrophile and nucleophile explanation sophistication. Across the complete data set, when the electrophile level was higher, there were often explicit formal charges on the electrophile in the mechanisms. Below is an example response for addition of bromine (Br2) across an alkene (i.e., cyclohexene) to form the two enantiomers of the trans-dibromo product (see Fig. 2).
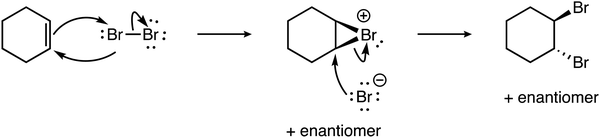 | ||
| Fig. 2 The reaction mechanism for the halogenation of cyclohexene and bromine to form (1R,2R)-1,2-dibromocyclohexane. | ||
The learner notes the three-membered ring (i.e., a bromonium ion) and its electron deficiency due to a positive formal charge, and thus indicates electrophilicity. In addition, the learner's explanation also demonstrates a complex understanding of electrophiles in describing how the polarizability of diatomic bromine results in enough electron deficiency that leads to the formation of the bromonium intermediate and the bromide ion. While the nucleophiles (i.e., the pi bond and the bromide anion) are identified in this reaction mechanism, little is stated about the electron sufficiency of these species, and thus only a Descriptive classification is made for the nucleophile component of the explanation.
“The double bond acts as a nucleophile and performs nucleophilic attack on one of the Bromine molecules of Br2 as the molecule becomes temporarily polar is it nears the double bond. The bromine molecule then forms a ringed intermediate with the two carbons of the previous double bond. This ring has an overall positive charge, which the bromine anion, formed by the splitting of the bromine molecule, performs a backside nucleophilic attack on. This then resulting in two bromine molecules added trans to each other across the double bond.” (electrophile: Complex; nucleophile: Descriptive).
From the opposite perspective, higher levels of explanation sophistication for nucleophiles relative to electrophiles were often observed when reactants or intermediates had formal negative charges on the nucleophile and when reactants or intermediates had no formal charges on the electrophile. For example, take this explanation for the epoxidation of (1R,2R)-2-chlorocyclohexanol with hydroxide followed by thiophenolate to form (1S,2S)-2-(phenylthiol)cyclohexanol (see Fig. 3).
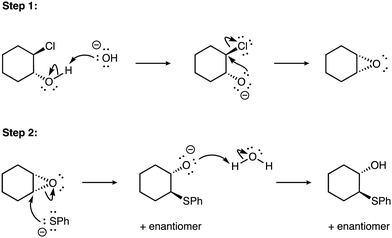 | ||
| Fig. 3 The reaction mechanism of (1R,2R)-2-chlorocyclohexanol with hydroxide followed by thiophenolate to form (1S,2S)-2-(phenylthiol)cyclohexanol. | ||
Here the hydroxide, alkoxide intermediate, and thiophenolate all have formal negative charges, likely cueing discussion of nucleophiles for the learner. These two exemplars reinforce prior work (Anzovino and Bretz, 2015, 2016) suggesting that learners rely on explicit, structural features (i.e., formal charge) to fully identify and describe the role of an electrophile or nucleophile.
“Part A: in step one, the oxygen of the OH− acts as a nucleophile and attacks the Hydrogen atom of the alcohol attached to the ring. This attack is followed by the shift of electron density to the oxygen, given it a negative charge because of excess electrons. Then since the oxygen has excess electrons, it is good for nucleophile role and it will attach the carbon attached to the Cl, as Cl is a good leaving group and would rather leave the molecule. The created an epoxide, which is a reactive electrophile. The next step 2 shows the nucleophile SPh that is negatively charged attacking the carbon attached to the epoxide. This electron density is then shifted to the oxygen, which allows the breaking of the three-membered ring. One oxygen obtains those electrons from the breaking of the epoxide and attacks a water molecule to obtain a hydrogen atom. This is true since the O− is more nucleophilic than the water. This created alcohol and keeping in mind that a backside occurred previously it is now showing inversion of configuration where the OH is in the same side (dash) as the epoxide was previously (also in-dash) and the SPh that attacked in the other side (solid wedge). Since we have stereochemistry, the enantiomer is also a product of this reaction.” (electrophile: Descriptive; nucleophile: Complex).
We note that there were more explanations for the reduction reaction family where the electrophile level of explanation sophistication was higher than the nucleophile level of explanation sophistication; this was the only reaction family in which this was observed. The reactions contained in this family were the reductions of carbonyls with a hydride species or a carbanion-type species (e.g., a Grignard reagent). Instruction on these reaction mechanisms places a large emphasis on the susceptibility of a carbonyl to form a bond with a nucleophile due to its minor resonance structure. For example, here is an explanation of the reaction mechanism for the reduction of 2-butanone with lithium aluminum hydride to form a racemic mixture of 2-butanol (Fig. 4).
When lithium aluminum hydride or sodium borohydride are the source of the nucleophile, learners may be operating from a definition that “hydrogen” (or as often stated in explanations “a proton”) is involved in Brønsted–Lowry acid–base reactions and is either neutral or electron deficient. Thus, lack of elaboration of the nucleophile in this reaction is the result of misapplication of understanding rather than an exclusive focus on the electrophile.
“The carbon attached to the ketone on the 2-butanone is electropositive, due to oxygen pulling the electrons away from the carbon. This is done through induction, oxygen is more electronegative and is able to hold the electrons. The other way is resonance, the electrons in the double bond are able to move freely from the double bond to the oxygen. The reactant LiAlH4, has an extremely negative aluminum element that naturally has a high positive charge. The aluminum will want to give away a hydrogen and therefore the hydrogen–aluminum bond attacks the electrophilic carbon as a nucleophilic attack on the compound. In order to add the hydrogen to the carbon, the electrons had to become localized on the oxygen to avoid breaking the octet rule for the carbon. This reaction is completed in water, which will then act as an acid, giving the oxygen a hydrogen. The oxygen wants the hydrogen to lose the negative charge, because it becomes more stable.” (electrophile: Complex; nucleophile: Descriptive).
Previous work has also shown a conflation of Brønsted–Lowry acidity and basicity with electrophilicity and nucleophilicity (Bhattacharyya and Bodner, 2005; Strickland et al., 2010; Cruz-Ramírez De Arellano and Towns, 2014; Anzovino and Bretz, 2015). While multiple instances are observed in our data, this finding echoes the one instance in which a learner in the Anzovino and Bretz (2015) study identified an electrophile without identifying a nucleophile was for a reduction of sodium borohydride with a carbonyl; this is possibly due to the way hydride reactions are introduced in the curriculum typically late in the first term when learners may still be learning to discern between proton transfers and reactions of nucleophilic hydride ions.
As the literature suggests (Anzovino and Bretz, 2015, 2016), charge and lone pairs (i.e., explicit structural features), and the carbon of a carbonyl (reinforced with its minor resonance structure) were key to the disparity of levels of explanation sophistication between electrophiles and nucleophiles. More explicit features were associated with higher level of explanation sophistication. Generally, we observed that the level of explanation sophistication was higher for nucleophiles than electrophiles, confirming prior research. However, electron-deficient species (i.e., electrophiles) were somewhat present in explanations both from a structural and functional role in many reaction mechanisms; this differs slightly from the finding that only one of eleven participants in the Anzovino and Bretz (2015, 2016) work who explicitly identified an electrophile across their interview study.
Implications for instructors
There are three key implications for instructors: (1) use of the electrophile rubric and nucleophile rubric as formative assessment tools, (2) assessment tool results to develop pedagogical content knowledge related to electrophiles and nucleophiles, and (3) organic chemistry course evaluation.Evidence for learning reaction mechanisms has primarily focused on recreating reaction mechanism drawings resulting in little meaning ascribed by learners (Bhattacharyya and Bodner, 2005; Grove et al., 2012a, 2012b; Bhattacharyya, 2014; Defever et al., 2015). Constructed-response items asking learners to explain what is happening and why for a given reaction mechanism puts such meaning at the forefront. The key to setting such meaning as a learning outcome ensures that adequate assessment tools are available to evaluate and promote such learning. The electrophile rubric reported herein and the nucleophile rubric previously reported (Yik et al., 2023) coupled with the constructed-response item prompt variations (Dood et al., 2020a, 2020b) provide a suitable foundation for adopting such outcomes and implementing such assessments. We reaffirm our prior work and the work of others in suggesting that writing-to-learn and demonstration of the complexity of reaction mechanism understanding is best done through formative assessment wherein feedback is quickly provided to a learner, and instruction is continuously augmented to better support learning (Dood et al., 2018, 2019, 2020a, 2020b; Yik et al., 2021; Dood and Watts, 2022a). Knowing what the symbolism of a reaction mechanism picture communicates and how to convey such meaning to others, not simply reproducing the picture, is key to the understanding of organic chemistry. The two rubrics provide a foundation for instructors to reaffirm their commitment to putting deeper learning, situated in the practice of chemistry, at the forefront of the desired learning in their courses.
Evidence of learning collected using the constructed-response items from this study are a source of information for an instructor to reflect upon their instruction and resulting learning by learners in their courses. We are frequently amazed by our learners who correctly invoke molecular orbitals, hyperconjugation, and nuanced aspects of steric effects, for example, when describing reaction mechanisms; however, as we observe and report in Table 5, very few explanations overall contain evidence for such understanding. We are equally amazed at how often learners try out, albeit incorrectly (in whole or in part), new concepts to make sense of reaction mechanisms. It is through these misapplications, mis-integrations, and yes, completely incorrect ideas that we reflect as educators on how to help learners navigate through the learning process to achieve the sophisticated understanding we strive to guide them to attain. Our work addresses the misunderstanding of organic chemists that if a learner can draw a reaction mechanism, then they ascribe the meaning we ascribe to that reaction mechanism drawing (Bhattacharyya and Bodner, 2005; Grove et al., 2012a, 2012b); when in reality, if we do not ask the learner explicitly what the reaction mechanism picture means, we have no understanding of what that learner knows about reaction mechanisms (Graulich, 2015; Caspari et al., 2018b). Until we ask learners the question, we have little means to further our pedagogical content knowledge, i.e., the ways instructors approach teaching their content knowledge (Belge Can and Boz, 2022; Jones et al., 2022).
Regular use of constructed-response items like those used in this study included across a course term, across a yearlong course sequence, or across multiple offerings of a course provide an information-rich set of learning evidence to evaluate the curriculum. Unlike course or program-level outcomes (e.g., core ideas, big ideas, anchoring concepts) wherein assessment items need to be written, evaluated, mapped onto such outcomes and then aggregated based on difficulty, complexity, etc., the electrophile rubric and nucleophile rubric provide a generalizable framework for evaluating a wide range of descriptions of what is happening and why in reaction mechanisms. In other words, the rubrics and associated constructed-response items provide a means to evaluate learner understanding when the exact reaction mechanisms (and corresponding reactant species) vary; notwithstanding, reaction mechanisms are confined to those that include at minimum an electrophile and a nucleophile. Thus, an educator could consider levels of explanation sophistication across a course to evaluate how learners progress in constructing more sophisticated explanations with different reaction mechanisms at each assessment interval. Or, an educator could compare levels of explanation sophistication from semester to semester for aromatic reactions even when varying the exact species in an electrophilic aromatic substitution each term. Either of these suggested uses of the rubrics to assess learning provides a means to evaluate and revise learning experiences based on resultant findings.
Implications for researchers
There are three key implications for researchers: (1) development and extension of assessment tools for measuring and promoting learning of reaction mechanisms, (2) investigation of how and why organic chemistry instructors implement constructed-response assessments, and (3) longitudinal and multi-institution evaluation of electrophile and nucleophile learning across organic chemistry course sequences. These implications for researchers purposefully mirror those implications proposed for instructors.The development and extension of assessment tools in chemistry education must be knowledge-based and knowledge-generating. A common critique of chemistry education research is that much is known about the failings of chemistry instructional experiences to result in desired learning outcomes. However, less is known about how to harness that knowledge to create new learning tools to promote and subsequently evaluate such learning. Concept inventories provide one avenue (e.g., McClary and Bretz, 2012; Graulich et al., 2019; Atkinson et al., 2020); however, widespread routine use of those inventories to evaluate learning in chemistry courses has not yet been reported. Additionally, many concepts and skills do not have a complementary inventory and multiple-choice items are typically central to concept inventories. Constructed-response items provide another avenue, often described as more authentic assessment experiences than say a learner choosing from a list of prescribed responses (Laverty et al., 2016; Stowe and Cooper, 2017; Underwood et al., 2018; Deglopper et al., 2022). However, there is a lack of suitable rubrics to evaluate learning with widespread adoption. Still, sufficient chemistry education research exists to inform such assessment tool development, yet much more basic research is still needed to understand learning in organic chemistry, such as learner understanding of radical or pericyclic reaction mechanisms. We believe it to be important to consider how suitable rubrics could be developed and extended from current rubrics to assess such learning.
We cannot expect to develop assessment tools and leave up to chance the adoption and lasting implementation of our assessments. There is an opportunity for assessment tool developers and the broader chemistry education community to investigate how such tools are adopted and implemented (Gibbons et al., 2022). Such knowledge is not limited to further dissemination of assessment tools and should inform the development of new assessment tools that address the needs of educators. The research-practice divide, routinely acknowledged in the context of professional conferences and workshops and in more concrete ways through editorials and commentaries (e.g., Johnson, 2022), suggests that researchers need to consider and better understand how the results of our work (if at all) impacts teaching and learning. Specifically for the work reported herein: What barriers prevent adoption of more constructed-response items for collecting evidence of reaction mechanism understanding? What knowledge are educators constructing based on asking their learners to describe what is happening and why for the reaction mechanisms taught in their courses? What are the next set of assessments tools that need to be created to assist educators in further measuring learning and evaluating their courses and curriculum? Assessment tool developers have a responsibility to ask these questions, and to conduct such studies.
The generalizability of constructed-response item variations and the associated electrophile rubric and nucleophile rubric provide an opportunity for longitudinal and multi-institution investigations. The implications of our work suggest that exact assessments do not need to be used at every assessment time point, nor across multiple institutions; thus, “common” assessments may no longer be necessary for large-scale evaluation work. Though a “common” assessment task or constructed-response prompt is necessary, a specific reaction mechanism and common reactant species are unnecessary for comparing explanation sophistication. This creates an opportunity to more easily evaluate the impact of multiple curricula enacted at multiple institutions. While there may be other methodological obstacles to overcome, customization of assessments to the reactions taught in a given course and curriculum are possible given the generalizability of the constructed response prompts and rubrics.
Limitations
There are three main limitations of this work: (1) homogeneity of the study site, curriculum, and sample; (2) incentive for providing reaction mechanism explanations; and, (3) time and effort to analyze explanations of reaction mechanisms.All data were collected at a single study site: the University of South Florida. At the same time, data were collected in multiple terms, with multiple instructors, and using two different textbooks. Nevertheless, a homogeneity limitation certainly exists. Therefore, the implications as presented should be considered in this light. For example, while the rubrics and resulting analyses are rooted in the chemistry education research literature, and thus based on a heterogenous set of learning contexts, it is possible that idiosyncrasies associated with a particular curricula may limit transferability of our results to those settings: for example, to a 3D-centered curriculum (e.g., Stowe et al., 2020), to a curriculum where explanation and science practices are emphasized, or to a curriculum wherein organic chemistry concepts are integrated into broadly defined courses on structure and reactivity, and thus do not fit the yearlong organic chemistry course sequence as it is at the study site (e.g., Flynn and Ogilvie, 2015). Adoption of constructed-response items and associated rubrics may need adaptation to meet curricular requirements and desired outcomes. However, this should be conceptualized as a strength and not exclusively a weakness, for the prompts and rubrics are malleable to allow for such modifications more easily than, for example, removing one or more items from a concept inventory.
It is important to reflect on the process in which the data were collected for this study. Learners were given bonus points towards either their term or final examination scores for responding to the assessment prompts. Correctness or achieving a certain level of explanation sophistication did not impact the number of bonus points a learner received; in fact, the electrophile rubric and nucleophile rubric were not fully realized until after data collection had concluded. Therefore, use of the rubrics as a true formative assessment tool for learners has not yet been implemented at the study site. We are aware that assessments drive learning, and thus the routine expectation of completing such assessments may have positively impacted learning; however, no feedback was provided to learners for them to evaluate their own learning via these assessments. Thus, we are left wondering as to whether more explanations would have included proportional understanding and at higher levels had the learners received feedback. We are also left to ponder as to whether fewer explanations would have disproportional understanding if learners knew to explicitly address both electrophiles and nucleophiles. Ambiguity with both of these conjectures is not a rationale for invalidating the results of this study. In a way, our results are suitably natural in that they reflect an insight into learning without an explicit socially desirable bias towards providing the answer the learners think the instructor (or researcher) wants to hear. Still, we acknowledge that we did observe learners stating “I hope this is what you want” and other similar phrases in the context of their explanations.
The time and effort necessary to evaluate written explanations in large-enrollment courses such as organic chemistry is sizable. As noted in the Methods section, for any given term, between 450 and 1080 learners are engaged in one of the two organic chemistry courses offered at the study site. Assuming only one reaction mechanism explanation is given on a summative assessment (e.g., term examination) in one course, the time and skills necessary to apply either or both rubrics is enormous. Nearly the entirety of the analyses reported herein, and in Yik et al. (2023), were done after the courses were completed and on a time scale longer than appropriate for returning feedback to learners in the context of a course. Although not listed in the Implications for Researchers, implementation of the constructed-response items and use of the rubrics in large-enrollment courses would benefit from automated, lexical analysis means for scoring explanations. There is precedent for using machine learning techniques to automate such analysis with examples for then incorporating resulting machine learning models into adaptive tutorial experiences for learners (Dood et al., 2018, 2019, 2020a, 2020b; Yik et al., 2021). However, a sufficient predictive model has not yet been identified (nor reported) that meets criteria for acceptable use for automating electrophile and nucleophile level of sophistication.
Conclusions
To address opportunities for improving instruction, a clear understanding of what our learners know and can do is necessary and potentially transformational. In previous work, Yik et al. (2023) noted the distribution of levels of explanation sophistication for nucleophiles across nearly 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 explanations of reaction mechanisms. This work extends the nucleophile work to include simultaneous evaluation of the level of explanation sophistication for electrophiles for those same explanations and considers the association between the two evaluations. This work provides a large-scale corroboration of previous literature reported findings regarding identification and description of electrophiles and nucleophiles in organic chemistry reaction mechanisms (Anzovino and Bretz, 2015, 2016; Putica and Trivic, 2016; Dood et al., 2020a; Watts et al., 2020, 2022). In particular, the findings of the study herein reaffirm how explicit structural features and the electron-pushing formalism in reaction mechanisms are necessary cues for learners to identify electrophiles and nucleophiles (Anzovino and Bretz, 2015, 2016). However, the findings of the study herein differ from previous reports in that learners do identify and describe the role of electrophiles in reaction mechanisms, and in some circumstances, at a higher level of sophistication than nucleophiles. The results of this work provide opportunities for instructors to implement such tools in their courses, reflect on the resulting evidence of learning when revising their courses, and consider measuring learning across their course and the broader organic chemistry curriculum. Lastly, the results of this work provide opportunities for researchers to further develop and extend assessment tools for measuring learning in organic chemistry instructional contexts, investigating how and why instructors adopt and implement assessment tools, and how to conceive and enact multi-course and multi-institutional studies of organic chemistry reaction mechanism learning.
000 explanations of reaction mechanisms. This work extends the nucleophile work to include simultaneous evaluation of the level of explanation sophistication for electrophiles for those same explanations and considers the association between the two evaluations. This work provides a large-scale corroboration of previous literature reported findings regarding identification and description of electrophiles and nucleophiles in organic chemistry reaction mechanisms (Anzovino and Bretz, 2015, 2016; Putica and Trivic, 2016; Dood et al., 2020a; Watts et al., 2020, 2022). In particular, the findings of the study herein reaffirm how explicit structural features and the electron-pushing formalism in reaction mechanisms are necessary cues for learners to identify electrophiles and nucleophiles (Anzovino and Bretz, 2015, 2016). However, the findings of the study herein differ from previous reports in that learners do identify and describe the role of electrophiles in reaction mechanisms, and in some circumstances, at a higher level of sophistication than nucleophiles. The results of this work provide opportunities for instructors to implement such tools in their courses, reflect on the resulting evidence of learning when revising their courses, and consider measuring learning across their course and the broader organic chemistry curriculum. Lastly, the results of this work provide opportunities for researchers to further develop and extend assessment tools for measuring learning in organic chemistry instructional contexts, investigating how and why instructors adopt and implement assessment tools, and how to conceive and enact multi-course and multi-institutional studies of organic chemistry reaction mechanism learning.
Author contributions
SJHF, BJY, and JRR conceptualized the study. SJHF, BJY, AJD, and JRR conceptualized the electrophile rubric. BJY, AJD, and JRR collected data with the assistance of DCR and KBF. BJY cleaned and prepared the data. SJHF classified responses using the electrophile rubric. BJY classified responses using the nucleophile rubric. SJHF analyzed the combined data set. SJHF and JRR conducted the interrater reliability study for the electrophile rubric. SJHF authored the manuscript. All authors read, edited, and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Appendix 1
Cross-tabulation analyses for course terms and reaction families (Tables 8–14).
Cell (%) (n = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 559) 559) |
Nucleophile level | Total (%) | ||||
|---|---|---|---|---|---|---|
| Absent | Descriptive | Foundational | Complex | |||
| Electrophile level | Absent | 8.8 | 6.8 | 1.8 | 0.1 | 17.6 |
| Descriptive | 3.0 | 35.9 | 14.7 | 0.6 | 54.2 | |
| Foundational | 1.1 | 8.2 | 7.4 | 1.9 | 18.6 | |
| Complex | 0.3 | 3.6 | 4.5 | 1.2 | 9.7 | |
| Total (%) | 13.2 | 54.6 | 28.3 | 3.9 | ||
| Cell (%) (n = 6377) | Nucleophile level | Total (%) | ||||
|---|---|---|---|---|---|---|
| Absent | Descriptive | Foundational | Complex | |||
| Electrophile level | Absent | 11.5 | 6.5 | 1.4 | 0.1 | 19.5 |
| Descriptive | 3.6 | 39.9 | 16.9 | 0.9 | 61.4 | |
| Foundational | 0.5 | 4.0 | 3.7 | 1.5 | 9.6 | |
| Complex | 0.3 | 4.1 | 3.2 | 1.9 | 9.5 | |
| Total (%) | 16.0 | 54.5 | 25.2 | 4.4 | ||
| Cell (%) (n = 4873) | Nucleophile level | Total (%) | ||||
|---|---|---|---|---|---|---|
| Absent | Descriptive | Foundational | Complex | |||
| Electrophile level | Absent | 8.3 | 7.4 | 1.3 | <0.1 | 17.1 |
| Descriptive | 1.3 | 38.4 | 19.4 | 0.4 | 59.4 | |
| Foundational | <0.1 | 3.6 | 3.6 | 0.5 | 7.7 | |
| Complex | <0.1 | 5.1 | 8.9 | 1.9 | 15.8 | |
| Total (%) | 9.6 | 54.5 | 33.2 | 2.7 | ||
| Cell (%) (n = 7432) | Nucleophile level | Total (%) | ||||
|---|---|---|---|---|---|---|
| Absent | Descriptive | Foundational | Complex | |||
| Electrophile level | Absent | 7.3 | 5.7 | 2.3 | 0.3 | 15.6 |
| Descriptive | 3.8 | 29.3 | 14.1 | 1.0 | 48.2 | |
| Foundational | 2.1 | 13.7 | 12.2 | 3.7 | 31.7 | |
| Complex | 0.3 | 1.9 | 1.5 | 0.8 | 4.6 | |
| Total (%) | 15.6 | 50.6 | 30.1 | 5.8 | ||
| Cell (%) (n = 2786) | Nucleophile level | Total (%) | ||||
|---|---|---|---|---|---|---|
| Absent | Descriptive | Foundational | Complex | |||
| Electrophile level | Absent | 14.9 | 8.2 | 0.6 | 0.0 | 23.7 |
| Descriptive | 4.9 | 47.4 | 6.3 | 0.3 | 58.8 | |
| Foundational | 0.3 | 2.8 | 1.6 | 0.4 | 5.0 | |
| Complex | 1.0 | 6.2 | 4.1 | 1.3 | 12.5 | |
| Total (%) | 21.0 | 64.5 | 12.5 | 2.0 | ||
| Cell (%) (n = 1581) | Nucleophile level | Total (%) | ||||
|---|---|---|---|---|---|---|
| Absent | Descriptive | Foundational | Complex | |||
| Electrophile level | Absent | 9.9 | 9.7 | 2.5 | 0.1 | 22.3 |
| Descriptive | 5.1 | 33.7 | 20.9 | 1.6 | 61.4 | |
| Foundational | 0.3 | 2.7 | 2.9 | 1.3 | 7.3 | |
| Complex | 0.6 | 3.2 | 3.0 | 2.4 | 9.1 | |
| Total (%) | 15.9 | 49.3 | 29.3 | 5.4 | ||
| Cell (%) (n = 2786) | Nucleophile level | Total (%) | ||||
|---|---|---|---|---|---|---|
| Absent | Descriptive | Foundational | Complex | |||
| Electrophile level | Absent | 12.7 | 5.4 | 1.0 | 0.0 | 19.1 |
| Descriptive | 2.2 | 46.4 | 17.6 | 0.6 | 66.8 | |
| Foundational | 0.1 | 1.7 | 1.8 | 0.7 | 4.4 | |
| Complex | 0.1 | 4.5 | 3.5 | 1.7 | 9.7 | |
| Total (%) | 15.1 | 57.9 | 23.9 | 3.1 | ||
Acknowledgements
We would like to thank the Organic Chemistry 1 (CHM 2210) and Organic Chemistry 2 (CHM 2211) learners at the University of South Florida who wrote the reaction mechanism explanations evaluated in this study. We would also like to thank Frankie Constanza (University of South Florida) for allowing access to learners in their course for our data collection.References
- Akkuzu N. and Uyulgan M. A., (2016), An epistemological inquiry into organic chemistry education: exploration of undergraduate students’ conceptual understanding of functional groups, Chem. Educ. Res. Pract., 17(1), 36–57 10.1039/c5rp00128e.
- Anderson T. L. and Bodner G. M., (2008), What can we do about “Parker”? A case study of a good student who didn’t “get” organic chemistry, Chem. Educ. Res. Pract., 9(2), 93–101 10.1039/b806223b.
- Andrade H. G., (2000), Using rubrics to promote thinking and learning, Educ. Leadersh., 57(5), 13–18.
- Anzovino M. E. and Bretz S., (2015), Organic chemistry students’ ideas about nucleophiles and electrophiles: the role of charges and mechanisms, Chem. Educ. Res. Pract., 16(4), 797–810 10.1039/c5rp00113g.
- Anzovino M. E. and Bretz S. L., (2016), Organic chemistry students’ fragmented ideas about the structure and function of nucleophiles and electrophiles: a concept map analysis, Chem. Educ. Res. Pract., 17(4), 1019–1029 10.1039/c6rp00111d.
- Atkinson M. B., Popova M., Croisant M., Reed D. J. and Bretz S. L., (2020), Development of the reaction coordinate diagram inventory: measuring student thinking and confidence, J. Chem. Educ., 97(7), 1841–1851 DOI:10.1021/acs.jchemed.9b01186.
- Belge Can H. and Boz Y., (2022), Development of pre-service teachers’ pedagogical content knowledge and the factors affecting that development: a longitudinal study, Chem. Educ. Res. Pract.23(4), 980–997 10.1039/D2RP00106C.
- Bhattacharyya G., (2014), Trials and tribulations: student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15(4), 594–609 10.1039/c3rp00127j.
- Bhattacharyya G. and Bodner G., (2005), “It gets me to the product”: How students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407 DOI:10.1021/ed082p1402.
- Bhattacharyya G. and Harris M. S., (2018), Compromised structures: verbal descriptions of mechanism diagrams, J. Chem. Educ., 95(3), 366–375 DOI:10.1021/acs.jchemed.7b00157.
- Bodé N. E., Deng J. M. and Flynn A. B., (2019), Getting past the rules and to the WHY: causal mechanistic arguments when judging the plausibility of organic reaction mechanisms, J. Chem. Educ., 96(6), 1068–1082 DOI:10.1021/acs.jchemed.8b00719.
- Brookhart S. M. and Chen F., (2015), The quality and effectiveness of descriptive rubrics, Educ. Rev., 67(3), 343–368 DOI:10.1080/00131911.2014.929565.
- Caspari I., Kranz D. and Graulich N., (2018a), Resolving the complexity of organic chemistry students’ reasoning through the lens of a mechanistic framework, Chem. Educ. Res. Pract., 19(4), 1117–1141 10.1039/c8rp00131f.
- Caspari I., Weinrich M. L., Sevian H. and Graulich N., (2018b), This mechanistic step is “productive”: organic chemistry students’ backward-oriented reasoning, Chem. Educ. Res. Pract., 19(1), 42–59 10.1039/C7RP00124J.
- Cooper M. M., (2015), Why Ask Why? J. Chem. Educ., 92(8), 1273–1279 DOI:10.1021/acs.jchemed.5b00203.
- Cooper M. M., Kouyoumdjian H. and Underwood S., (2016), Investigating Students’ Reasoning about Acid–Base Reactions, J. Chem. Educ., 93(10), 1703–1712 DOI:10.1021/acs.jchemed.6b00417.
- Crandell O. M., Kouyoumdjian H., M. Underwood S. and M. Cooper M., (2018), Reasoning about reactions in organic chemistry: starting it in general chemistry, J. Chem. Educ., 96(2), 213–226 DOI:10.1021/acs.jchemed.8b00784.
- Crandell O. M., Lockhart M. A. and Cooper M. M., (2020), Arrows on the page are not a good gauge: evidence for the importance of causal mechanistic explanations about nucleophilic substitution in organic chemistry, J. Chem. Educ., 97(2), 313–327 DOI:10.1021/acs.jchemed.9b00815.
- Cruz-Ramírez De Arellano D. and Towns M. H., (2014), Students’ understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15(4), 501–515 10.1039/c3rp00089c.
- DeCocq V. and Bhattacharyya G., (2019), TMI (Too much information)! Effects of given information on organic chemistry students’ approaches to solving mechanism tasks, Chem. Educ. Res. Pract., 20(1), 213–228 10.1039/C8RP00214B.
- Defever R. S., Bruce H. and Bhattacharyya G., (2015), Mental rolodexing: senior chemistry majors’ understanding of chemical and physical properties, J. Chem. Educ., 92(3), 415–426 DOI:10.1021/ed500360g.
- Deglopper K. S., Schwarz C. E., Ellias N. J. and Stowe R. L., (2022), Impact of assessment emphasis on organic chemistry students’ explanations for an alkene addition reaction, J. Chem. Educ., 99(3), 1368–1382 DOI:10.1021/acs.jchemed.1c01080.
- Deng J. M. and Flynn A. B., (2021), Reasoning, granularity, and comparisons in students’ arguments on two organic chemistry items, Chem. Educ. Res. Pract., 22(3), 749–771 10.1039/d0rp00320d.
- Dood A. J. and Watts F. M., (2022a), Mechanistic reasoning in organic chemistry: a scoping review of how students describe and explain mechanisms in the chemistry education research literature, J. Chem. Educ., 99(8), 2864–2876 DOI:10.1021/acs.jchemed.2c00313.
- Dood A. J. and Watts F. M., (2022b), Students’ strategies, struggles, and successes with mechanism problem solving in organic chemistry: a scoping review of the research literature, J. Chem. Educ., 100(1), 53–68.
- Dood A. J., Fields K. B. and Raker J. R., (2018), Using lexical analysis to predict Lewis acid-base model use in responses to an acid–base proton-transfer reaction, J. Chem. Educ., 95(8), 1267–1275 DOI:10.1021/acs.jchemed.8b00177.
- Dood A. J., Fields K. B., Cruz-Ramírez De Arellano D. and Raker J. R., (2019), Development and evaluation of a Lewis acid–base tutorial for use in postsecondary organic chemistry courses, Can. J. Chem., 97(10), 711–721 DOI:10.1139/cjc-2018-0479.
- Dood A. J., Dood J. C., Cruz-Ramírez De Arellano D., Fields K. B. and Raker J. R., (2020a), Analyzing explanations of substitution reactions using lexical analysis and logistic regression techniques, Chem. Educ. Res. Pract., 21(1), 267–286 10.1039/c9rp00148d.
- Dood A. J., Dood J. C., Cruz-Ramírez De Arellano D., Fields K. B. and Raker J. R., (2020b), Using the research literature to develop an adaptive intervention to improve student explanations of an SN1 reaction mechanism, J. Chem. Educ., 97(10), 3551–3562 DOI:10.1021/acs.jchemed.0c00569.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9(2), 102–113 10.1039/b806225k.
- Flynn A. B. and Featherstone R. B., (2017), Language of mechanisms: exam analysis reveals students’ strengths, strategies, and errors when using the electron-pushing formalism (curved arrows) in new reactions, Chem. Educ. Res. Pract., 18(1), 64–77 10.1039/C6RP00126B.
- Flynn A. B. and Ogilvie W. W., (2015), Mechanisms before reactions: a mechanistic approach to the organic chemistry curriculum based on patterns of electron flow, J. Chem. Educ., 92(5), 803–810 DOI:10.1021/ed500284d.
- Galloway K. R., Stoyanovich C. and Flynn A. B., (2017), Students’ interpretations of mechanistic language in organic chemistry before learning reactions, Chem. Educ. Res. Pract., 18(2), 353–374 10.1039/C6RP00231E.
- Gibbons R. E., Reed J. J., Srinivasan S., Murphy K. L. and Raker J. R., (2022), Assessment tools in context: results from a national survey of postsecondary chemistry faculty, J. Chem. Educ., 99(8), 2843–2852 DOI:10.1021/acs.jchemed.2c00269.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: How do students deal with the invisible? Chem. Educ. Res. Pract., 16(1), 9–21 10.1039/c4rp00165f.
- Graulich N., Hedtrich S. and Harzenetter R., (2019), Explicit versus implicit similarity – exploring relational conceptual understanding in organic chemistry, Chem. Educ. Res. Pract., 20(4), 924–936 10.1039/C9RP00054B.
- Grove N. P., Cooper M. M. and Cox E. L., (2012a), Does mechanistic thinking improve student success in organic chemistry? J. Chem. Educ., 89(7), 850–853 DOI:10.1021/ed200394d.
- Grove N. P., Cooper M. M. and Rush K. M., (2012b), Decorating with arrows: toward the development of representational competence in organic chemistry, J. Chem. Educ., 89(7), 844–849 DOI:10.1021/ed2003934.
- Johnson R., (2022), Bridging the divide between chemistry educators and chemistry education researchers, J. Chem. Educ., 99(11), 3631–3632 DOI:10.1021/acs.jchemed.2c01035.
- Jones T., Romanov A., Pratt J. M. and Popova M., (2022), Multi-framework case study characterizing organic chemistry instructors’ approaches toward teaching about representations, Chem. Educ. Res. Pract., 23(4), 930–947 10.1039/D2RP00173J.
- Klein D. R., (2017), Organic Chemistry, John Wiley & Sons, Inc.
- Klein D. R., (2021), Organic Chemistry, John Wiley & Sons, Inc.
- Kraft A., Strickland A. M., and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11(4), 281–292 10.1039/c0rp90003f.
- Kranz D., Schween M. and Graulich N., (2023), Patterns of reasoning – exploring the interplay of students’ work with a scaffold and their conceptual knowledge in organic chemistry, Chem. Educ. Res. Pract. 10.1039/D2RP00132B.
- Laverty J. T., Underwood S. M., Matz R. L., Posey L. A., Carmel J. H., Caballero M. D., et al., (2016), Characterizing College Science Assessments: The Three-Dimensional Learning Assessment Protocol, PLoS One, 11(9), e0162333 DOI:10.1371/JOURNAL.PONE.0162333.
- Lieber L. and Graulich N., (2022), Investigating students’ argumentation when judging the plausibility of alternative reaction pathways in organic chemistry, Chem. Educ. Res. Pract., 23(1), 38–54 10.1039/D1RP00145K.
- Lieber L. S., Ibraj K., Caspari-Gnann I. and Graulich N., (2022), Closing the gap of organic chemistry students’ performance with an adaptive scaffold for argumentation patterns, Chem. Educ. Res. Pract., 23(4), 811–828 10.1039/D2RP00016D.
- McClary L. M. and Bretz S. L., (2012), Development and assessment of a diagnostic tool to identify organic chemistry students’ alternative conceptions related to acid strength, Int. J. Sci. Educ., 34(15), 2317–2341 DOI:10.1080/09500693.2012.684433.
- Nedungadi S. and Brown C. E., (2021), Thinking like an electron: concepts pertinent to developing proficiency in organic reaction mechanisms, Chem. Teach. Int. Best Pract. Chem. Educ., 3(1), 9–17 DOI:10.1515/cti-2019-0020.
- NGSS Lead States, (2013), Next Generation Science Standards: For States, By States, National Academies Press.
- Noyes K., Carlson C. G., Stoltzfus J. R., Schwarz C. V., Long T. M. and Cooper M. M., (2022), A deep look into designing a task and coding scheme through the lens of causal mechanistic reasoning, J. Chem. Educ., 99(2), 874–885 DOI:10.1021/acs.jchemed.1c00959.
- Putica K. and Trivic D. D., (2016), Cognitive apprenticeship as a vehicle for enhancing the understanding and functionalization of organic chemistry knowledge, Chem. Educ. Res. Pract., 17(1), 172–196 10.1039/C5RP00179J.
- Raker J. R., Yik B. J. and Dood A. J., (2023), Development of a generalizable framework for machine-learning based evaluation of written explanations of reaction mechanisms from the postsecondary organic chemistry curriculum, in Student reasoning in organic chemistry: research advances and evidence-based instructional practices, Graulich N. and Shultz G. V. (ed.) The Royal Society of Chemistry.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: a learning progression on chemical thinking, Chem. Educ. Res. Pract., 15(1), 10–23 10.1039/c3rp00111c.
- Šimkovic M. and Träuble B., (2019), Robustness of statistical methods when measure is affected by ceiling and/or floor effect, PLoS One, 14(8), e0220889 DOI:10.1371/JOURNAL.PONE.0220889.
- Solomons T. W. G., Fryhle C. B. and Snyder S. A., (2016), Organic Chemistry, John Wiley & Sons, Inc.
- Stowe R. L. and Cooper M. M., (2017), Practicing what we preach: assessing “critical thinking” in organic chemistry, J. Chem. Educ., 94(12), 1852–1859 DOI:10.1021/acs.jchemed.7b00335.
- Stowe R. L., Esselman B. J., Ralph V. R., Ellison A. J., Martell J. D., Deglopper K. S. and Schwarz C. E., (2020), Impact of maintaining assessment emphasis on three-dimensional learning as organic chemistry moved online, J. Chem. Educ., 97(9), 2408–2420 DOI:10.1021/acs.jchemed.0C00757.
- Strickland A. M., Kraft A. and Bhattacharyya G., (2010), What happens when representations fail to represent? Graduate students’ mental models of organic chemistry diagrams, Chem. Educ. Res. Pract., 11(4), 293–301 10.1039/c0rp90009e.
- Talanquer V., (2013), When atoms want, J. Chem. Educ., 90(11), 1419–1424 DOI:10.1021/ed400311x.
- Underwood S. M., Posey L. A., Herrington D. G., Carmel J. H. and Cooper M. M., (2018), Adapting assessment tasks to support three-dimensional learning, J. Chem. Educ., 95(2), 207–217 DOI:10.1021/acs.jchemed.7B00645.
- Watts F. M., Schmidt-Mccormack J. A., Wilhelm C. A., Karlin A., Sattar A., Thompson B. C., et al., (2020), What students write about when students write about mechanisms: analysis of features present in students’ written descriptions of an organic reaction mechanism, Chem. Educ. Res. Pract., 21(4), 1148–1172 10.1039/c9rp00185a.
- Watts F. M., Park G. Y., Petterson M. N. and Shultz G. V., (2022), Considering alternative reaction mechanisms: students’ use of multiple representations to reason about mechanisms for a writing-to-learn assignment, Chem. Educ. Res. Pract., 23(2), 486–507 10.1039/d1rp00301a.
- Weinrich M. L. and Sevian H., (2017), Capturing students’ abstraction while solving organic reaction mechanism problems across a semester, Chem. Educ. Res. Pract., 18(1), 169–190 10.1039/C6RP00120C.
- Yan F. and Talanquer V., (2015), Students’ ideas about how and why chemical reactions happen: mapping the conceptual landscape, Int. J. Sci. Educ., 37(18), 3066–3092 DOI:10.1080/09500693.2015.1121414.
- Yik B. J., Dood A. J., Cruz-Ramírez De Arellano D., Fields K. B. and Raker J. R., (2021), Development of a machine learning-based tool to evaluate correct Lewis acid-base model use in written responses to open-ended formative assessment items, Chem. Educ. Res. Pract., 22(4), 866–885 10.1039/d1rp00111f.
- Yik B. J., Dood A. J., Frost S. J. H., Cruz-Ramírez De Arellano D., Fields K. B. and Raker J. R., (2023), Generalized rubric for level of explanation sophistication for nucleophiles in organic chemistry reaction mechanisms, Chem. Educ. Res. Pract., 24(1), 263–282 10.1039/D2RP00184E.
| This journal is © The Royal Society of Chemistry 2023 |