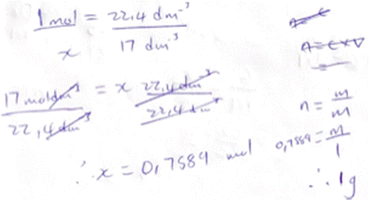Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The efficacy of instruction in application of mole ratios and submicro- and macro-scopic equivalent forms of the mole within the unit factor method
Angela Elisabeth Stott
Faculty of Education, University of the Free State, 205 Nelson Mandela Drive, Park West, Bloemfontein, Free State, South Africa. E-mail: StottAE@ufs.ac.za
First published on 4th January 2023
Abstract
The unit factor method, a generic strategy for solving any proportion-related problem, is known to be effective at reducing cognitive load through unit-cancellation providing step-by-step guidance. However, concerns have been raised that it can be applied mindlessly. This primarily quantitative prepost study investigates the efficacy of instruction aimed at addressing such concerns. This was done by making submicro- and macro-scopic equivalent forms of the mole concept, and the meanings of mole ratios, explicit, and emphasising the application of these within the unit factor method to solve stoichiometry calculations. Data were collected from 161 South African Physical Sciences teachers’ answers to four calculation, and 14 conceptual, questions in each of a pre- and a post-test written at the start and end, respectively, of a two-day workshop at which such instruction was implemented. These data were analysed for changes in strategy type and calculation and conceptual knowledge, i.e., heuristic power. A small (n = 7) group retained deficient calculation strategies in which they failed to recognise the need to apply proportion to the mole ratio. For the remainder, a weak but significant correlation was found between their conceptual and calculation improvements. There was high uptake of the unit factor method in the posttest, although a group (n = 33) which began with relatively good calculation knowledge largely rejected this method. Statistically significant improvements in both conceptual and calculation knowledge were found regardless of the extent of uptake of the unit factor method, however the calculation improvement measured was significantly lower for the group which showed moderate uptake of the unit factor method, suggesting they may have needed a longer intervention. Based on the findings, speculations are made regarding the nature of knowledge and the mechanism of development of heuristic power. Long-term effects of such an intervention would, however, still need to be determined.
Introduction
Algorithms may be seen as necessary evils in that they are required to reduce cognitive load (Hartman and Nelson, 2021), yet they may be applied incorrectly due to rote, rather than meaningful, usage (Nyachwaya et al., 2014), and may detract from conceptual development (Skemp, 1987). Ideally, an algorithm should provide metacognitive support (Vo et al., 2022), e.g. by directing the user from one step to the next (Gulacar et al., 2021), while also directing attention to the integration of relevant concepts at the macroscopic, submicroscopic, and symbolic levels of representation (Johnstone, 2000). This is an investigation into the efficacy of an instructional sequence designed with the intention of guiding deployment of an algorithm, the unit factor method, in this desired manner through explicit application of the meaning of the mole ratio and the mole concept's role in connecting these levels of representation, and so propelling participants along Niaz's (1995) algorithmic-conceptual continuum of developing heuristic power. Each of these concepts is explained below. Since this study focuses on the efficacy of this instructional sequence within a series of 2 day in-service workshops for South African physical sciences teachers, the difficulties they are known to experience with stoichiometry calculations are discussed first.Literature review and conceptual framework
South African physical sciences teachers’ difficulties with stoichiometry calculations
Mathematics competence, particularly regarding use of proportion, largely determines the extent of challenge experienced in performing stoichiometry calculations (Ralph and Lewis, 2018). It is therefore not surprising that South African physical sciences (SA PS) teachers, who are known to have difficulty with mathematics in general (Taylor, 2019), and proportion in particular (Selvaratnam, 2011), largely fail to obtain the correct answer to basic stoichiometry calculation exercises (Stott, 2021). These teachers’ failure to recognise the need to use proportion was found to be a considerable contributor to their limited success with stoichiometry calculations (Stott, 2021). This failure is consistent with Gulacar et al.'s (2021) finding that not knowing what to do in subproblems within the calculation process is a critical contributor to difficulties experienced during stoichiometry calculations.Cognitive load theory and the necessity of algorithms
These difficulties are unsurprising, given the mathematical and conceptual complexity of stoichiometry (Ramful and Narod, 2014) and the limitations to working memory experienced by novices, described by cognitive load theory (Sweller, 2011). Central to cognitive load theory in the context of chemistry education (see, for example, Hartman and Nelson, 2021), is the view that algorithms are crucial for novices to engage fluently in well-defined calculations. The terms relevant to this premise, as well as the way each is operationalised in this study, are explained in Table 1. The term algorithm is used, here, to refer to a wide range of procedures which include both narrowly applicable formulae and more generic methods, such as the unit factor method.| Term | Explanation | Ref. | Operationalisation in this study |
|---|---|---|---|
| Algorithm | “Procedures with sequential steps to achieve a goal” | (Hartman and Nelson, 2015, p. 8) | E.g., the unit factor method |
| Fluency | The ability to attain the correct answer to well-defined calculations, such as asked in chemistry examinations | (Hartman and Nelson, 2015) | Calculation success, indicated by score/16 |
| Novices within a particular domain | People who do not possesses an extensive and highly structured knowledge system about that domain in their long-term memory | (Hartman and Nelson, 2015) | Most of the teachers included in this study, as suggested by their low pretest scores |
| Well-defined calculations | Calculation questions for which experts in the field agree that specific rules and procedures could be used to obtain correct answers | (Spiro and Deschryver, 2009) | Typical grade 10 and 11 end-of-chapter stoichiometry calculation exercises, used in the pre- and post-tests |
The reason why algorithms are necessary for novices to attain fluency is that their knowledge, stored in long term memory, is of limited extent and degree of structure (Hartman and Nelson, 2015). This reduces their ability to chunk information, resulting in high levels of cognitive load which prohibits effective engagement in all types of problems (Sweller, 2011; Tsaparlis, 2021). For well-defined calculations this prohibition can be overcome by repeated use of an algorithm until automaticity has been achieved (Hartman and Nelson, 2015). Algorithm automaticity can be attained by scaffolding practice such that initially a high degree of structure is provided, and this is faded as automaticity is reached (Graulich et al., 2021). This necessary use of algorithms comes, however, at a potential cost: an overreliance on memorised procedures at the expense of development of conceptual understanding, with associated inappropriate application of these algorithms, particularly to novel contexts (Skemp, 1987).
Conceptual understanding and levels of representation in chemistry
In the context of chemistry education, development of conceptual understanding is believed to be closely related to development of an understanding of interrelations between the various levels of representation: submicroscopic, macroscopic and symbolic (Johnstone, 2000). Indeed, the mole concept, central to stoichiometry, serves as a connection between these levels of representation and can therefore only be understood if attention is paid to interrelations between these levels (Fang et al., 2014). This belief in the conceptual value of such interrelations has, for example, led to recent calls for greater use and interconnection of these forms of representation in Chemistry textbooks (Ramnarain and Chanetsa, 2016) and their modelling and explicit instruction in teacher education programmes (Mweshi et al., 2020). From the perspective of cognitive load theory, it is, however, unsurprising that teachers find it difficult to pay attention to each of these forms of representation, as well as their interconnection, in the classroom (Koopman, 2017), and therefore unsurprising that the mole concept specifically (Malcolm et al., 2019), and stoichiometry more broadly (Stott, 2020), tend to be poorly understood.The unit factor method
The instructional sequence investigated was designed to introduce participants to use of the unit factor method as both an algorithm and a way to direct attention towards the meaning of the mole reacting ratio given in a balanced equation and the interconnections between the submicroscopic, macroscopic and symbolic forms of representation. To explain the rationale behind this sequence, the unit factor method is explained, and exemplified, below, and potential strengths are discussed, as well as threats to its effective use as an agent for reducing cognitive load while also directing attention towards conceptual understanding.The unit factor method (Herron and Wheatley, 1978), also called the factor label method (Poole, 1989), is an application of dimensional analysis (DeMeo, 2008), i.e., the conversion of units of measurement through use of unit equivalence. As illustrated in Fig. 1, in the unit factor method, the value given in a proportion-related question is multiplied by a conversion factor which is, in effect, unity, due to its numerator and denominator being equivalent to one another. The conversion factor's denominator has the unit of the given variable, and its numerator the unit of the required variable. In this way the given unit cancels out and the required unit remains in the answer. These units can therefore be used to guide the student towards a productive sequence of steps. In this way the common difficulty identified by Gulacar et al. (2021), of students not knowing what to do next when solving stoichiometry calculations, may be mitigated. It is therefore unsurprising that the unit factor method is generally considered effective at helping students of a wide range of mathematical competencies to obtain the correct answers to well-defined stoichiometry problems (Herron and Wheatley, 1978; Gabel and Sherwood, 1983), even with limited conceptual knowledge (Robinson, 2003; Cook and Cook, 2005). It is also unsurprising that DeMeo (2008) found this to be the most popular method for teaching stoichiometry calculations in his sample, drawn largely from chemistry teachers from the United States of America. An additional potential benefit of the unit factor method in contexts, such as South Africa, where failure to recognise the need to apply proportion to the mole ratio is a common error (Stott, 2021), is that it is a generic proportion method, and so is well suited to drawing attention to the need to use proportion.
 | ||
| Fig. 1 An example of a well-defined stoichiometry calculation problem and its solution using the unit factor method. | ||
Viewed from another angle, the unit factor method may be seen as potentially providing metacognitive support, at least within the analysis and solution phases of problem solving (Vo et al., 2022). This is because identification of the given and required information and their units, i.e., analysis of the problem, is central to this method, as is the relationship between the given and required quantities, required for the solution of the problem. It is hoped that the solver's attention will be directed towards the relationship between given and required quantities by the need to cancel the given, and introduce the required, unit. However, this very feature, powerful for reducing cognitive load as it provides metacognitive support, has invited criticism for this method through: (1) its ability to allow users to calculate the correct answer despite possessing little conceptual knowledge of stoichiometry (Robinson, 2003); (2) the finding that users may focus so much on the units that they fail to think about the associated concepts (Tang et al., 2014); (3) the speculation that the method might stunt conceptual development through reliance on unit cancelling at the expense of attention to the relevant concepts (DeToma, 1994; Robinson, 2003; Cook and Cook, 2005; DeMeo, 2008; Page et al., 2018).
In potential rebuttal of these criticisms, it is argued that these problems are not inherent to the unit factor method. Instead, the conversion factor(s) can be used as vehicles for drawing attention to the mole ratio and connections within and between submicro- and macro-scopic quantities of chemicals, expressed symbolically. This is because these conversion factors express mole reaction ratios or equivalent forms of the mole concept, as discussed below.
The mole concept's role in connecting the macro- and submicro-scopic levels
The purpose of the mole concept is to bridge the submicro–macro-scopic gap in chemistry. This gap exists because people conceptualise chemical reactions on the submicroscopic level, e.g., regarding molecules reacting with one another, for which particle counts – how many – is the best measure of quantity. However, people work, practically, with chemicals on the macroscopic level where macroscopic quantities, such as mass – how heavy – and volume – how large – are needed. The mole concept bridges this submicro–macro-scopic gap in two ways: (1) it allows elements’ mass numbers to be reusable on both the submicroscopic level (as the mass, in amu, of a single atom or molecule) and the macroscopic level (as the mass, in grams, of a mole of atoms or molecules) and (2) it enables use of the particle count quantity – how many – at the macroscopic level too. In order to help learners to understand this, it is necessary to help them to appreciate: (1) the enormity of Avagadro's number (6.02 × 1023); (2) the concept of using group words, such as pair, dozen, or Avagadro's number, interchangeably with certain numbers; and (3) why 6.02 × 1023 was deemed sufficiently valuable to be assigned group-word status, namely that 1 amu, the fundamental unit of mass since it is the mass of a nucleon, the category of submicroscopic particles which have significant mass, equals .
.
This discussion has stated what the mole concept's purpose is and how it achieves this purpose, but not what the mole is. A common explanation given to learners is to equate the mole to Avagadro's number, i.e., that the mole is a group word meaning 6.02 × 1023 (Fang et al., 2014). However, this is ontologically incorrect, since the mole is the amount of substance which has Avagadro's number of particles in it, rather than being Avagadro's number itself, hence the phrase amount of substance in both the latest IUPAC definition, and the older definition still in use in the South African Curriculum. According to the latest IUPAC definition, “The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly 6.02214076 × 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol−1 and is called the Avogadro number. The amount of substance, symbol n, of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles” (BIMP, 2019, p. 134). The older definition, even more opaque to a learner is: “a mole is the amount of substance having the same number of particles as there are atoms in 12 g carbon-12” (DBE, 2011, p. 24).
Heuristic power
Niaz (1995) described development of competence in stoichiometry as progressing from algorithmic dependence to conceptual competence through stages of increasing heuristic power, with this progression being led by evolving conceptual knowledge. Based on this framework, it seems unreasonable to expect a 2 day workshop to propel all its participants to conceptual competence, particularly those who started the workshop at a stage of heavy algorithmic dependence. However, it does seem reasonable to expect an intervention to propel a large proportion of participants along the conceptual – algorithmic continuum, for it to be considered at all efficacious and to view the extent of this propulsion as an indication of the extent of the instructional sequence's efficacy. Increase in heuristic power, operationalised as an increase in both the ability to solve calculation and to answer conceptual, questions, correctly, can therefore be viewed as an indicator of intervention efficacy.Problem statement
The issue at the heart of this study is whether explicit instruction into a focus on the meaning of the mole reaction ratio and the submicro–macro-scopic connections which the mole concept provides, and an application of these within the unit factor method, can result in uptake of this method in a manner which increases heuristic power. This would suggest that the benefits of an algorithm (calculation success through reduced cognitive load) had been attained without the curses of an algorithm (inflexible application without conceptual understanding).Research questions
Considering the discussion above, this paper reports on an investigation into the efficacy of instruction aimed at developing an understanding of the mole reaction ratio and submicro- and macro-scopic equivalent forms of the mole concept and applying this to compile appropriate conversion factors within the unit factor method, and so to improve Stoichiometry heuristic power. This was operationalised within a series of two-day professional development workshops for South African physical sciences teachers. In reference to these, this investigation was guided by the research questions: (1) To what extent did the teachers’ stoichiometry strategy use, and heuristic power, change during this intervention? (2) How was uptake of the unit factor method related to changes in heuristic power?Method
This is a pragmatically conducted, primarily quantitative, survey study, informed by the framework for integrated methodologies (FraIM) (Plowright, 2011). According to FraIM, warrantability, characterised by logic and coherence, serves as a proxy for validity. Besides this constraint, research choices are made pragmatically in manners best suited to answering the research questions. Consistent with this framework, attention is paid to transparent reporting of the methods used to answer the research questions, so that the reader can judge the warrantability of the claims made, and although a quantitative focus is used, a qualitative illustrative example is also drawn on.Instructional sequence
The instructional sequence under investigation had three foci: (1) the mole concept's central role in connecting the various levels of representation in stoichiometry; (2) the meaning of the mole reacting ratio given in balanced equations; (3) application of these connections and meanings within the unit factor method. This was done by incrementally answering, and guiding learners to understand and apply the answers to the following questions:• What are submicro- and macro-scopic levels of representation of chemicals, and how does use of these necessitate use of the mole concept?
• What is the mole and how does it connect the submicro- and macro-scopic levels of representation? What is Avagadro's number, how large is it, and why was it chosen to represent the number of particles in a mole?
• What are equivalent ways of describing a mole of various types of particles?
• What is the unit factor method? Why is it valid to multiply a value by a fraction in which the numerator and denominator are equivalent? How can such fractions be arranged to perform a unit conversion, in general, and use equivalent ways of describing a mole, in particular?
• What is proportion? How do ratios and actual amounts differ from one another?
• What is the meaning of a balanced chemical equation? How can the mole reacting ratio given by the balanced equation be expressed in equivalent ways using a variety of units?
• How can proportion, e.g., by means of the unit factor method, be applied to the mole reacting ratio and its equivalent ratios to convert between ratios and actual amounts, and so solve a reaction-based stoichiometry question?
The way this instructional sequence was operationalised in the intervention under investigation is described in the section about the intervention, and an example of partial uptake of the methods promoted is given in the findings section. This instructional sequence is also exemplified in an online program which can be found at https://www.learnscience.co.za/challenge-page/mole-concept.
Intervention
This study refers to a series of eight 2 day (16 hour) in-service stoichiometry professional development interventions conducted across the Free State (FS) province of South Africa (SA) from November 2017 to March 2018 and to which FS physical sciences teachers across the province were invited, by their subject advisors. The intervention was prefaced by a pretest and followed by a posttest and biographical survey. The intervention was guided by the instructional sequence referred to above. This was done through a series of four sessions, each of which consisted of roughly one-hour-long periods of interactive teaching followed by roughly two-hour-long periods in which the participants applied what they had learnt to answer corresponding questions in a fill-in workbook. The participants were also expected to work through sections of the workbook as homework on the evening of the first day. This workbook had been designed with the same foci on concepts, proportion, and the unit factor method, as adopted in the intervention. See a two-page extract of the 42-page workbook in Appendix A. This workbook provides multiple opportunities to practice each type of stoichiometry calculation question relevant to the South African grades 10 and 11 physical sciences curriculum. Each section in the book begins with relevant conceptual questions, followed by a calculation example, using the unit factor method. A consistent colour-coding system is used throughout the book to indicate given and required information and units. These colours are used in the examples provided at the start of a section as well as for fill-in lines given for the first few questions of that section. Annotations are also given regarding the equivalent values required in conversion factors. This colour coding and the provided annotations may be classified as instructional prompts since they are embedded in the task design and are content-dependent (Graulich et al., 2021). These supports are reduced, and eventually removed, in later questions in each section, and at the end of each related group of sections unguided questions are provided about all these sections. This approach matches Graulich et al.'s (2021) description of use of scaffolding prompts.Sample
The sample used in this research is a subgroup of the 220 teachers who attended these interventions. It consists of the same teachers as included in Stott (2021) on the basis of their completion of the pretest, biographical survey and provision of written informed consent to the anonymous use of their data, minus the 10 of that sample who did not also answer the posttest. Some characteristics of the sample are given in Table 2 to enable judgement of the likelihood that findings from this study may be generalisable to other contexts. There are two routes to qualify to teach physical sciences in South Africa: the BSc (38.5% of this sample), or BEd routes. In either of these, it is not necessary to major in chemistry. Almost half (46%) of this sample did major in chemistry, while most of the remainder (39%) studied only one year of chemistry at the tertiary level. Only 7 (4%) had not studied any chemistry at tertiary level and only 14 (8%) had not taught stoichiometry at school level for at least one year. Before data collection commenced, the ethics committee for educational research at the University of the Free State evaluated the research proposal for compliance with relevant laws and institutional guidelines for ethical research and awarded ethical clearance for the study (UFS-HSD2017/1520).| Socioeconomic status of the learners taught | Possess a BSc degree | Stoichiometry teaching experience category | N |
|---|---|---|---|
| a 3 years or less. b 3–10 years. c 10 years or more. | |||
| High (teach at a quintile 5 school) | No (n = 9) | Inexperienceda | 6 |
| n = 19 (12%) | Moderately experiencedb | 3 | |
| Experiencedc | 0 | ||
| Yes (n = 10) | Inexperienced | 4 | |
| Moderately experienced | 1 | ||
| Experienced | 5 | ||
| Low (teach at quintile 1–4 schools) | No (n = 90) | Inexperienced | 46 |
| n = 142 (88%) | Moderately experienced | 20 | |
| Experienced | 24 | ||
| Yes (n = 52) | Inexperienced | 26 | |
| Moderately experienced | 16 | ||
| Experienced | 10 | ||
Data collection
The pre- and post-tests each contained sixteen multiple choice questions which were identical between the tests, and four calculation questions which differed slightly between the tests, although the difficulty (Horvat et al., 2016) and complexity (Knaus et al., 2011) levels of corresponding questions are considered comparable, as indicated in Appendix B. The multiple choice questions have been given, justified and analysed, in Stott (2020). Two of these questions measured algorithmic manipulation rather than conceptual understanding, and so have been excluded from this study. The remaining 14 questions fall into the following categories: (1) minimal conceptual interpretation (2 questions); (2) knowledge of foundational facts (4 questions); common conceptual errors (2 questions); understanding of equivalent submicroscopic and macroscopic values and ratio-amount distinctions (6 questions). The scores extracted from these questions in the manners described below comprise the study's quantitative data, and descriptions of selected calculations comprise the study's qualitative data, as explained in the section on qualitative analysis. All test questions and answers were collected at the end of the test sessions and after this the teachers were not given access to either these questions or their answers. The presenter also took care to avoid any discussion of these questions during or after the intervention, to decrease the limitation arising from prior exposure to the questions from pre- to post-tests, or by teachers from later implementations of the intervention receiving questions from their peers.Analysis
 | ||
| Fig. 2 An overview of the constructs determined per participant in preparation for the statistical analysis performed. | ||
| Strategy | Recognition of the need to use of proportion | Explicit statement of, and substitution into, formulae | Example of algorithm which would result in this classification if used to a high degree | Remarks |
|---|---|---|---|---|
| a Recognised the need to apply proportion to the mole ratio for more than half of the questions. b 7/10 or higher for statement of, and substitution into, formulae. | ||||
| Proportion reliant | Higha | Low | Unit factor method | Non-deficient |
| Formula and proportion | High | Highb | Use of formulae and proportion | |
| Formula reliant | Low | High | Use of formulae | Deficient since they do not account for the mole ratio |
| Minimal | Low | Low | None | |
Findings
Changes in strategy use and heuristic power
| Posttest unit factor method usage category | Number of posttest questions for which the unit factor method was used (/4) | Number of teachers (/161) | Fraction of sample (%) |
|---|---|---|---|
| Little to none | 0 & 1 | 33 | 20.5 |
| Moderate | 2 & 3 | 58 | 36 |
| High | 4 | 70 | 43.5 |
| Ending strategy use category | N (/161) | % | Mean (SD) pre-calculation score (/16) | Mean (SD) post-calculation score (/16) | Mean (SD) pre–post calculation improvement (/16) | Mean (SD) pre- conceptual score (/14) | Mean (SD) post- conceptual score (/14) | Mean (SD) pre–post conceptual improvement (/14) |
|---|---|---|---|---|---|---|---|---|
| Deficient | 7 | 4 | 3.6 (1.9) | 4.7 (2.2) | 1.1 (3.8) | 3.1 (0.8) | 5.7 (2.0) | 2.1 (2.2) |
| Non-deficient | 154 | 96 | 7.1 (4.4) | 11.5 (3.3) | 4.4 (4.0) | 5.1 (2.6) | 8.44 (2.7) | 3.0 (2.4) |
| T-test | t(9) = −4.1, p < 0.05 | t(7) = −7.3, p < 0.05 | t(159) = −2.1, p < 0.05 | t(12) = −4.95, p < 0.05 | t(7) = −3.25, p < 0.05 | t(159) = −0.89, p = 0.37 | ||
| Ending strategy use category | n | Correlation between conceptual and calculation improvement (r) | p |
|---|---|---|---|
| Deficient | 7 | −0.5 | 0.26 |
| Nondeficient | 154 | 0.21 (weak) | <0.05 |
| Ending strategy use category | N (/161) | % | Mean (SD) pre-calculation score (/16) | Mean (SD) post-calculation score (/16) | T-test for pre- and post-calculation scores | Mean (SD) pre-conceptual score (/14) | Mean (SD) post-conceptual score (/14) | T-test for pre- and post-conceptual scores |
|---|---|---|---|---|---|---|---|---|
| Deficient | 7 | 4 | 3.6 (1.9) | 4.7 (2.2) | t(6) = −0.74, p = 0.48 | 3.1 (0.8) | 5.7 (2.0) | t(6) = 2.58, p < 0.05 |
| Non-deficient | 154 | 96 | 7.1 (4.4) | 11.5 (3.3) | t(153) = −13.56, p < 0.05 | 5.1 (2.6) | 8.44 (2.7) | t(153) = −13.75, p < 0.05 |
Unit factor method uptake and changes in heuristic power
| Posttest unit factor method usage category | N (/161) | % | Mean (SD) pretest calculation score (/16) | Anova | Tukey–Kramer: a | Mean (SD) pretest conceptual score (/14) | Anova | |
|---|---|---|---|---|---|---|---|---|
| a Critical q value: 3.31 for alpha = 0.05. | ||||||||
| Little to none | 33 | 20.5 | 9.8 (4.2) | F(2, 151) = 8.56, p < 0.05 |  |
5.6 (2.4) | F(2, 151) = 2.75, p = 0.07 | |
| Moderate | 58 | 36 | 5.8 (4.3) |  |
4.5 (2.9) | |||
| High | 70 | 43.5 | 7.1 (4.1) | 5.4 (2.4) | ||||
| Posttest unit factor method usage category | N (/161) | % | Mean (SD) posttest calculation score (/16) | Anova | Tukey–Kramer: a | Mean (SD) posttest conceptual score (/14) | Anova | |
|---|---|---|---|---|---|---|---|---|
| a Critical q value: 3.31 for alpha = 0.05. | ||||||||
| Little to none | 29 | 18.8 | 13.0 (2.5) | F(2, 151) = 12.88, p < 0.05 |  |
9.2 (2.8) | F(2, 151) = 1.79, p = 0.17 | |
| Moderate | 55 | 35.7 | 9.9 (3.7) |  |
7.9 (2.9) | |||
| High | 70 | 43.5 | 12.2 (2.7) | 8.6 (2.5) | ||||
| Posttest unit factor method usage category | N (/161) | % | Mean (SD) pre–post calculation improvement (/16) | Anova | Mean (SD) pre–post conceptual improvement (/14) | Anova |
|---|---|---|---|---|---|---|
| Little to none | 29 | 18.8 | 3.2 (3.5) | F(2, 151) = 2.59, p = 0.08 | 3.6 (2.4) | F(2, 151) = 2.47, p = 0.09 |
| Moderate | 55 | 35.7 | 4.1 (4.0) | 2.4 (2.3) | ||
| High | 70 | 43.5 | 5.2 (4.0) | 3.1 (2.3) |
In contrast to this aimless approach in the pretest, the unit factor method was clearly used to focus the teacher's thinking productively in the posttest. They began by tabulating equivalent reacting values relevant to the question (molar, molecular and volume) for each substance, after which they used these values to compile conversion factors within the unit factor method. This suggests development of understanding of the relationship between measurements on the macro- and submicro-scopic levels, although this view is somewhat undermined by their incorrect reference to H2 atoms, as well as the error present in the tabulated row referring to molecules, which likely arose pragmatically as speculated in the table.
Consistent with the remarks made above, this teacher showed considerable pre- to post-test conceptual (4 to 8/14) and calculation (2 to 14/16) improvements. However, they showed little conceptual improvement regarding equivalent values (0 to 1/6) despite their ability to use these correctly in the calculations in the posttest, as shown in this example.
Discussion
Changes in strategy use and heuristic power
Unit factor method uptake and changes in heuristic power
Regarding uptake of the unit factor method and enhanced heuristic power in Stoichiometry, the findings suggests that: (1) increase in heuristic power across the duration of the intervention was dependent on participants developing awareness of the need to use proportion in Stoichiometry, but this awareness did not necessarily have to be developed through uptake of the unit factor method; (2) participants who started with good calculation knowledge were particularly likely to reject uptake of the unit factor method; (3) moderate uptake of the unit factor method, possibly resulting from the intervention period being too short for development of confidence in the method, was characterised by more modest gains in calculation skill during the intervention.These assertions are unsurprising, given that (1) multiple valid methods exist in Stoichiometry, including the collection of methods referred to here as formula and proportion strategies, (2) which most of the teachers who rejected the unit factor method were competent in at the start of the intervention (see Fig. 3), and which is favoured in South African chemistry marking guidelines at the school level (Stott, 2021); (3) teacher development tends to be a slow process (Luft and Hewson, 2014), and learning a new algorithm can be time-consuming, as discussed in the limitations section below.
Limitations
The findings of this study are limited to the duration of these 2 day workshops. The requirement for continued use of an algorithm until automaticity has been obtained, for a person to attain fluency (Hartman and Nelson, 2015), may undermine the long-term efficacy of such an intervention. This would certainly be the case for teachers whose use of the method would end at the end of the workshop. Providing the teachers with enough write-and-wipe scaffolded fill-in workbooks for use in their classes likely reduced the extent to which the uptake ended at the end of the investigated intervention. However, the current failure to recognise the unit factor method within the South African physical sciences examination marking guidelines (Stott, 2021), increases the likelihood that uptake ended at the end of the workshop. Additional limitations have been discussed within the text above.The argument could be made that the measured learning gains resulted from time on task, rather than from the efficacy of the instructional sequence used. Improvement related to time on task is, however, not by any means assured (Rudduck, 1986; Luft and Hewson, 2014), particularly in the developing world (Rogan, 2004; Stott, 2020). This view is supported by the low initial mean scores these teachers attained for these basic school-level calculations in the pretest, despite all but 7 having studied chemistry at tertiary level, and all but 14 having taught at least one year of stoichiometry at the high school level.
Conclusion
The objective of this study was to contribute to understanding the efficacy of providing explicit instruction into the meaning of the mole reaction ratio and the mole concept's connection between the macro- and submicro-scopic levels of representation in Chemistry and application of this knowledge to compile conversion factors within the unit factor method when solving Stoichiometry calculations. Such instruction is aimed at enhancing the participants’ heuristic power and so enhancing their ability to apply both algorithms and conceptual knowledge correctly (Niaz, 1995), for example to attain the correct answer to calculation and conceptual questions. The findings suggest that such instruction can effectively enhance heuristic power, although it appears that: (1) a two-day workshop is too short for optimal efficacy for approximately a third of teachers in this context; (2) the efficacy of the instructional sequence is dependent on development of an understanding of the importance of proportion in Stoichiometry, although this does not need to be coupled with uptake of the unit factor method; (3) the implicit understanding of how to apply mole-related equivalent values within the unit factor method in calculations may develop without development of explicit understanding of this equivalence.Conflicts of interest
There are no conflicts to declare.Appendix A: a part of the workbook used in the intervention
Appendix B: the questions used
| Question | Topic | Pretest | Posttest | Difficulty level (according to Horvat et al., 2016) | Concepts | Interactivity value (according to Horvat et al., 2016) | Complexity rating (applying Knaus et al.'s (2011) rubric) | Marks and mark allocation | Formulae relevant to formula-accessible calculations |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Yield determined by limiting reagent | A reaction mixture contains 17 g N2 and 0.5 mol H2. One of these is limiting. They react according to the given equation. What is the maximum number of moles of NH3 produced? | A reaction mixture contains 3.5 mol N2 and 18 g H2. One of these is limiting. They react according to the given equation. What is the maximum number of moles of NH3 that can be produced? | Medium, since given and required units differ, with one being moles | Limiting reagent, stoichiometric calculation | 0, since only 2 concepts are applied | 3 | (1) Application of mole ratio |  (not included in the checklist since the pretest could be answered without its use) (not included in the checklist since the pretest could be answered without its use) |
| (2) To the correctly chosen limiting reagent | |||||||||
| (3) Correct answer | |||||||||
| (3 Marks) | |||||||||
| 2 | Number of H atoms needed to react with a given volume of N2 | How many atoms of hydrogen are needed to fully react with 17 dm3 of nitrogen gas at STP? | How many atoms of hydrogen are needed to fully react with nitrogen to form 13 dm3 NH3 at STP? | Difficult, since given and required units differ, with neither being moles | Avogadro's law, stoichiometric calculation, submicroscopic particles | 1, since 3 concepts are applied | 6 + 1 = 7 | (1) Volume to molar conversion |  |
| (2) Application of mole ratio | N = nNA | ||||||||
| (3) Conversion to molecules | |||||||||
| (4) Conversion to atoms | |||||||||
| (5) Correct answer | |||||||||
| (5 Marks) | |||||||||
| 3 | Percentage yield | 7 g H2 reacts completely with N2 to form 28 g NH3. What is the percentage yield? | 7 g N2 react completely with H2 to form 8 g NH3. What is the percentage yield? | Difficult, since given and required units differ, with neither being moles | Stoichiometric calculation, percent yield | 0, since only 2 concepts are applied | 5 | (1) Application of mole ratio |  |
| (2) To find theoretical yield |  |
||||||||
| (3) % yield formula | |||||||||
| (4) Correct answer | |||||||||
| (4 Marks) | |||||||||
| 4 | Yield without limiting reagent | Calculate the maximum mass of SO3 that could be produced from 1.9 mol of oxygen and excess sulphur. | Calculate the maximum mass of SO3 that could be produced from 6 mol of oxygen and excess sulphur. | Medium, since given and required units differ, with one being moles | Chemical equation, stoichiometric calculation | 0, since only 2 concepts are applied | 3 | (1) Balanced equation |  |
| (2) MSO3 = 80 g mol−1 | |||||||||
| (3) Application of mole ratio | |||||||||
| (4) Correct answer | |||||||||
| (4 Marks) |
References
- BIMP, (2019), in Inglis B., Ullrich J. and Milton M. J. T. (ed.), The international system of units, SI Brochure, 9th edn, International Bureau of Weights and Measures, https://www.bipm.org/documents/20126/41483022/SI-Brochure-9-EN.pdf.
- Cook E. and Cook R. L., (2005), Cross-proportions: A conceptual method for developing quantitative problem-solving skills, J. Chem. Educ., 82(8), 1187 DOI:10.1021/ed082p1187.
- DBE, (2011), Physical sciences curriculum and assessment policy statement, Pretoria: Department of Basic Education.
- DeMeo S., (2008), Multiple solution methods for teaching science in the classroom: Improving quantitative problem solving using dimensional analysis and proportional reasoning, Universal-Publishers.
- DeToma R. P., (1994), Symbolic algebra and stoichiometry, J. Chem. Educ., 71(7), 568 DOI:10.1021/ed071p568.
- diSessa A. A., (2018), A friendly introduction to “knowledge in pieces”: Modeling types of knowledge and their roles in learning, Invited Lectures from the 13th International Congress on Mathematical Education, pp. 65–84 DOI:10.1007/978-3-319-72170-5_5.
- Fang S.-C., Hart C. and Clarke D., (2014), Unpacking the meaning of the mole concept for secondary school teachers and students, J. Chem. Educ., 91(3), 351–356.
- Gabel D. L. and Sherwood R. D., (1983), Facilitating problem solving in high school chemistry, J. Res. Sci. Teach., 20(2), 163–177 DOI:10.1002/tea.3660200207.
- Graulich N., Langner A., Vo K. and Yuriev E., (2021), Scaffolding Metacognition and Resource Activation During Problem Solving: A Continuum Perspective, in Tsaparlis G. (ed.), Problems and Problem Solving in Chemistry Education: Analysing Data, Looking for Patterns and Making Deductions, The Royal Society of Chemistry, pp. 38–67 10.1039/9781839163586-00038.
- Gulacar O., Cox C. and Fynewever H., (2021), Deconstructing the Problem-solving Process: Beneath Assigned Points and Beyond Traditional Assessment, in Tsaparlis G. (ed.), Problems and Problem Solving in Chemistry Education: Analysing Data, Looking for Patterns and Making Deductions, The Royal Society of Chemistry, pp. 68–92 10.1039/9781839163586-00068.
- Hartman J. R. and Nelson E. A., (2015), “Do we need to memorize that?” or cognitive science for chemists, Found. Chem., 17(3), 263–274 DOI:10.1007/s10698-015-9226-z.
- Hartman J. R. and Nelson E. A., (2021), A paradigm shift: The implications of working memory limits for physics and chemistry instruction, arXiv, preprint, arXiv:2102.00454 DOI:10.48550/arXiv.2102.00454.
- Herron J. D. and Wheatley G. H., (1978), A unit factor method for solving proportion problems, Math. Teach., 71(1), 18–21 DOI:10.5951/MT.71.1.0018.
- Horvat S., Segedinac M. D., Milenković D. D. and Hrin T. N., (2016), Development of procedure for the assessment of cognitive complexity of stoichiometric tasks, Macedonian J. Chem. Chem. Eng., 35(2), 275–284.
- Johnstone A. H., (2000), Teaching of chemistry-logical or psychological? Chem. Educ. Res. Pract., 1(1), 9–15 10.1039/A9RP90001B.
- Knaus K., Murphy K., Blecking A. and Holme T., (2011), A Valid and Reliable Instrument for Cognitive Complexity Rating Assignment of Chemistry Exam Items, J. Chem. Educ., 88(5), 554–560 DOI:10.1021/ed900070y.
- Koopman O., (2017), Investigating how science teachers in South Africa engage with all three levels of representation in selected chemistry topics, Afr. J. Res. Math., Sci. Technol. Educ., 21(1), 15–25.
- Luft J. A. and Hewson P. W., (2014), Research on teacher professional development programs in science, Handbook of Research on Science Education, Volume II, Routledge, pp. 903–924.
- Malcolm S. A., Mavhunga E. and Rollnick M., (2019), The Validity and Reliability of an Instrument to Measure Physical Science Teachers’ Topic Specific Pedagogical Content Knowledge in Stoichiometry, Afr. J. Res. Math., Sci. Technol. Educ., 22(2), 181–194.
- Mweshi E., Munyati O. and Nachiyunde K., (2020), Teachers’ Understanding of the Link between the Atomic Theory and the Mole Concept, Afr. J. Res. Math., Sci. Technol. Educ., 24(3), 411–422.
- Niaz M., (1995), Progressive transitions from algorithmic to conceptual understanding in student ability to solve chemistry problems: A Lakatosian interpretation, Sci. Educ., 79(1), 19–36.
- Niaz M. and Robinson W. R., (1993), Teaching algorithmic problem solving or conceptual understanding: Role of developmental level, mental capacity, and cognitive style, J. Sci. Educ. Technol., 2(2), 407–416.
- Nyachwaya J. M., Warfa A.-R. M., Roehrig G. H. and Schneider J. L., (2014), College chemistry students’ use of memorized algorithms in chemical reactions, Chem. Educ. Res. Pract., 15(1), 81–93.
- Page M. F. Z., Escott P., Silva M. and Barding G. A., (2018), The effect of teaching the entire academic year of high school chemistry utilizing abstract reasoning, Chem. Educ. Res. Pract., 19(2), 500–507 10.1039/c7rp00252a.
- Plowright D., (2011), Using mixed methods: Frameworks for an integrated methodology, SAGE Publications.
- Poole R. L., (1989), Teaching stoichiometry: A two cycle approach, J. Chem. Educ., 66(1), 57 DOI:10.1021/ed066p57.
- Ralph V. R. and Lewis S. E., (2018), Chemistry topics posing incommensurate difficulty to students with low math aptitude scores, Chem. Educ. Res. Pract., 19(3), 867–884.
- Ramful A. and Narod F. B., (2014), Proportional reasoning in the learning of chemistry: levels of complexity, Math. Educ. Res. J., 26(1), 25–46.
- Ramnarain U. and Chanetsa T., (2016), An analysis of South African Grade 9 natural sciences textbooks for their representation of nature of science, Int. J. Sci. Educ., 38(6), 922–933.
- Robinson W. R., (2003), Chemistry problem-solving: Symbol, macro, micro, and process aspects, ACS Publications DOI:10.1021/ed080p978.
- Rogan J., (2004), Professional Development: Implications for developing countries, in O-Saki K., Hosea K. and Ottevanger W. (ed.), Reforming Science and Mathematics Education in Sub-Saharan Africa: Obstacles and Opportunities, University of Dar es Salaam, pp. 155–170.
- Rudduck J., (1986), Making the most of the short inservice course, in Hopkins D. (ed.), Inservice Training and Educational Development: An International Survey, Croom Helm, pp. 302–311.
- Selvaratnam M., (2011), High school Physical Sciences teachers’ competence in some basic cognitive skills, S. Afr. J. Chem., 64, 231–236.
- Skemp R. R., (1987), Relational understanding and instrumental understanding, in Skemp R. R. (ed.), The Psychology of Learning Mathematics, Routledge, pp. 232–244 DOI:10.4324/9780203396391.
- Spiro R. J. and Deschryver M., (2009), Constructivism: When it's the wrong idea and when it's the only idea, in Tobias S. and Duffy T. M. (ed.), Constructivist Instruction: Success or Failure? Routledge, pp. 106–124.
- Stott A. E., (2020), Influence of Context on Stoichiometry Conceptual and Algorithmic Subject Matter Knowledge among South African Physical Sciences Teachers, J. Chem. Educ., 97(5), 1239–1246 DOI:10.1021/acs.jchemed.9b01056.
- Stott A. E., (2021), South African physical sciences teachers’ use of formulae and proportion when answering reaction-based stoichiometry calculation questions, Chem. Educ. Res. Pract., 22(2), 443–456 10.1039/D0RP00291G.
- Sweller J., (2011), Cognitive load theory, Psychology of learning and motivation, Elsevier, vol. 55, pp. 37–76.
- Talanquer V., (2006), Commonsense chemistry: A model for understanding students’ alternative conceptions, J. Chem. Educ., 83(5), 811.
- Tang H., Kirk J. and Pienta N. J., (2014), Investigating the effect of complexity factors in stoichiometry problems using logistic regression and eye tracking, J. Chem. Educ., 91(7), 969–975 DOI:10.1021/ed4004113.
- Taylor N., (2019), Inequalities in teacher knowledge in South Africa, in Spaull N. and Jansen J. (ed.), South African schooling: The enigma of inequality, Springer, pp. 263–282 DOI:10.1007/978-3-030-18811-5.
- Tsaparlis G., (2021), It Depends on the Problem and on the Solver: An Overview of the Working Memory Overload Hypothesis, Its Applicability and Its Limitations, in Tsaparlis G. (ed.), Problems and Problem Solving in Chemistry Education, The Royal Society of Chemistry, pp. 93–126 10.1039/9781839163586-00093.
- Vo K., Sarkar M., White P. J. and Yuriev E., (2022), Problem solving in chemistry supported by metacognitive scaffolding: teaching associates’ perspectives and practices, Chem. Educ. Res. Pract., 23(2), 436–451 10.1039/D1RP00242B.
| This journal is © The Royal Society of Chemistry 2023 |