Pre-service chemistry teachers’ knowledge of the coordination number and the oxidation number in coordination compounds
N.
Turan-Oluk

Department of Mathematics and Science Education – Chemistry Education, Gazi University, Ankara, Turkey. E-mail: nurcanturan@gazi.edu.tr
First published on 10th October 2022
Abstract
The purpose of this study is to determine pre-service chemistry teachers’ knowledge of the oxidation number and coordination number in coordination compounds. Data were collected from 31 pre-service chemistry teachers through a 4-question scale, and the second question of the scale consisted of 8 sub-questions. The results showed that the participants had difficulties in defining both the coordination number and the oxidation number of the central atoms/ions in coordination compounds, and they had misconceptions about these concepts. Also, it was revealed that the participants had difficulties in determining both the oxidation number of the central atom and the coordination number in coordination compounds. Participants stated that they had problems in determining the oxidation number because they did not remember the ligand charges, and the coordination number because they did not remember the number of ligand teeth.
1. Introduction
When students encounter a concept, they make sense of it with their current knowledge and experience. For meaningful learning, students need to make a correct and valid association between their prior knowledge and newly encountered information. If this is not achieved, students may construct concepts that are not compatible with scientific reality. Such concepts of students are expressed as misconceptions (Nakhleh, 1992). Misconceptions are barriers to developing a proper understanding of science and they inhibit students’ future learning of science (Davis, 1997). Since these misconceptions hinder the understanding of scientific knowledge, it is very important to identify and eliminate them (Barke et al., 2008).When the literature on misconceptions is examined, it is seen that there are many studies on various topics of chemistry, especially on big ideas of chemistry at different academic levels (Sreenivasulu and Subramaniam, 2019). While the majority of these studies focused on school-level students (Mayer, 2011, Allen and Worokwu, 2020), relatively few were conducted with university students (Kelly et al., 2010; Brandriet and Bretz, 2014; Uce, 2015; Sreenivasulu and Subramaniam, 2019).
Studies on chemistry education have generally focused on big ideas of chemistry – for example, chemical bonding (Peterson and Treagust, 1989; Luxford and Bretz, 2014); atomic structure (Griffiths and Preston, 1992); electrochemistry (Sanger and Greenbowe, 1997; Chia et al., 2022); chemical equilibria (Hackling and Garnett, 1985); intermolecular forces (Widarti et al., 2019); and reaction kinetics (Yan and Subramaniam, 2018). There are relatively fewer studies that have focused on chemical concepts that are important but solely encountered in the university chemistry curriculum (Granville, 1985; Milenković et al., 2016; Sreenivasulu and Subramaniam, 2019; Sindhu, 2021). The literature also calls for research on such chemical concepts that are not included in the ‘big ideas’ topics (Sreenivasulu and Subramaniam, 2019).
Although coordination compounds have been known since ancient times, the study of coordination chemistry as a discipline is relatively new (Constable, 2019). In fact, coordination compounds are important in everyday life. For example, an iron oxalate complex is used to clean the rust out of water-cooled automobile engines. For instance, commercial salad dressing frequently contains EDTA, which is a chelating ligand, as a food additive to prevent rancidity by forming a compound with the product's metal ions (Kotz and Purcell, 1991). Also, there are several applications for coordination compounds in both industrial chemistry and medicine. The majority of catalysts are complexes of transition metals; for instance, compounds of platinum and gold are utilized in medicine (Canbaz, 2016); prussian blue, aureolin, and alizarin red are just a few examples of the various coordination compounds that have been used as pigments (Miessler and Tarr, 1999).
When the chemistry education literature is examined, it is seen that the studies on coordination compounds are very limited (Barke et al., 2008; Sam et al., 2015; Sreenivasulu and Subramaniam, 2019). In one study, M. E. Cass, an advanced inorganic chemistry course instructor, introduced an activity he developed to teach ligands (Cass, 2004). In another study, flowcharts for determining the type of isomer and naming in coordination compounds were developed and findings on their use were presented (Yesiloglu et al., 2021). Another study related to this area is on organometallics (Gorunova et al., 2018).
When the studies on the oxidation number are examined, although there are studies on misconceptions about this concept, it is seen that the focus is on the determination of the oxidation number in simple salts or the oxidation number method in redox reactions (Garnett and Treagust, 1992), or in more general studies on transition metals it is only considered as a misconception. For example, Sreenivasulu and Subramaniam (2014) conducted a study on transition metal chemistry with university students (Sreenivasulu and Subramaniam, 2014). They determined that the participants described this subject as difficult, and they identified 24 common misconceptions about the formation of complex ions, oxidation numbers of metals, ionization energy, and formation of colored compounds (Sreenivasulu and Subramaniam, 2014).
Similarly, Balasundram and Karpudewan (2021), in their study on university students’ understanding of transition metals including the formation of complex ions, the ionization energy of transition metals, the formation of colors in transition metal ions, and the reactivity of transition metals, examined the effect of Popplet on reducing alternative conceptions (Balasundram and Karpudewan, 2021). It was identified that the students had the misconception that metals do not form complex ions when in a zero oxidation number (Karpudewan and Balasundram, 2019; Balasundram and Karpudewan, 2021).
The concept of the oxidation number was invented by inorganic chemists. The oxidation number is useful for balancing redox reactions and is needed for systematizing transition metal chemistry (Calzaferri, 1999). It is one of the most important heuristic concepts in chemistry and plays a significant role in teaching chemistry (Steinborn, 2004). It was determined that the oxidation number concept was usually examined in the context of simple salts (Ukah et al., 2021) or redox reactions (Brandriet and Bretz, 2014), not within the scope of coordination compounds. In the context of redox reactions, it was determined that students had difficulties in determining the oxidation number of the species in the reaction (Garnett et al., 1990; Shehu, 2015; Laliyo et al., 2019).
The structural chemistry of a coordination compound/ion is based on two key properties of the central metal atom/ion: its oxidation number and coordination number (Baxter, 2020). These concepts are highly interrelated and are usually seen as similar concepts (Lemma, 2012). The coordination number is a term encountered in transition metal chemistry. Although this concept is a very important subject in the university chemistry curriculum, there are very few studies on misconceptions about this concept (Sreenivasulu and Subramaniam, 2019). In addition, it is seen that the oxidation number and coordination number concepts are mixed (Parkin, 2006; Sreenivasulu and Subramaniam, 2019; Yesiloglu et al., 2021) or used interchangeably even in academic studies (Smith, 2005).
2. Theoretical background
2.1. Concepts of the coordination number and oxidation number in coordination compounds
In coordination compounds, the central atom/ion is surrounded by two or more ligands (molecules or ions) via coordinate covalent bonds. The coordinate covalent bonds between the central metal atom/ion and the ligands can be considered as Sigma bonds. According to Lewis acid–base theory, ligands act as electron-pair donors and thus are considered as Lewis bases, while the central metal ion acts as an electron pair acceptor, that is, a Lewis acid. An atom in the ligand that can donate an electron pair and directly bond to the metal center is called the donor atom.For example, the compound CrCl3·6H2O is a coordination compound, in which the metal is bonded to the ligands through coordinate covalent bonds, consisting of the complex ion [Cr(H2O)6]3+ and the counter ion Cl−. This unit within the square bracket is the coordination entity or ion formed by the coordination of H2O ligands to the central Cr3+ ion (Fig. 1). There are three Cl− counter ions (as they balance the charge on the complex ion) that are located outside the square bracket.
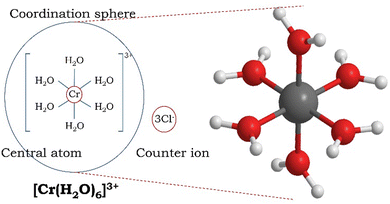 | ||
| Fig. 1 The structural formula of a complex ion, [Cr(H2O)6]3+, shows the central metal ion and the ligands (left: octahedral geometry around the chromium(III) ion; right: ball and stick model). | ||
The term “denticity” refers to the quantity of donor atoms from a specific ligand bonded to the same central atom (Connelly et al., 2005). The term “bidentate” is used to describe ligands that have two donor atoms.
In 1893, Alfred Werner proposed a model for coordination compounds. Werner hypothesized two types of valence: the first as a primary or ionizable valence (Hauptvalenz) and the second as a secondary or non-ionizable valence (Nebenvalenz) (Mickey, 1981; Constable, 2019). Although primary valences can be satisfied only by anions, secondary valences can be satisfied by anions and neutral molecules (Bailar, 1987). As an example, in the [CuCl2]− anion, the primary valence (oxidation number) is 1+ and the secondary valence (coordination number) is 2. Similarly, in the ions [CuCl4]2− and [CuCl5]3− the secondary valence values are four and five, respectively, but their primary valence value is the same, 2+.
The primary valence in Werner's theory is known as the oxidation number. The oxidation number of a central atom in a coordination compound is defined as the charge that it will bear when all ligands are removed together with the pairs of electrons shared with the central atom (Connelly et al., 2005). It can be calculated as the “charge of complex minus the sum of the charges of all ligands” (Steinborn, 2004). Although there is debate on the usage of the terms “oxidation state” and “oxidation number” (Loock, 2011), these terms are now considered to be synonymous (Jensen, 2011). In this study, the term oxidation number was preferred.
According to Werner's theory, one characteristic of a coordination compound is the secondary valency of the central atom, but this usage has not survived and it is now called the coordination number (Kettle, 2013). The coordination number of a metal ion is the number of σ-bonds between the ligand donor atoms and the central metal ion (IUPAC). Because coordinate bond formation results from a Lewis acid–base reaction, the coordination number can also be viewed as equal to the number of electron pairs donated by ligand donor atoms or accepted by the metal in forming a coordination compound. The coordination number can also be described as the number of atoms directly bonded to the central atom (Smith, 2005).
2.2. The importance of the oxidation number in the nomenclature of coordination compounds
The primary valence (oxidation number) refers to the charge over the metal ion (Bhatt, 2015). According to principles outlined in the book Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005 (Red Book), the oxidation number of the central atom may be indicated by appending a Roman numeral to the central atom name (Connelly et al., 2005). The oxidation number of the central atom in a coordination compound may be positive, zero, or negative, and by convention, it is indicated as (III), (0), and (–II), respectively (Sloan, 1987). But the oxidation number zero is not usually shown (Connelly et al., 2005).In a coordination compound, the oxidation number of the central atom is found by subtracting the sum of the charges of any ligands from the charge of the complex (Steinborn, 2004; Atkins and Overton, 2010). For example, in the [Co(en)2NH3Cl]2+ complex ion, the oxidation number for the central metal ion should be +3 because the charge of the complex ion is +2 minus the sum charges of all the ligands which is −1 (+2 – (−1) = +3). As can be understood from the definition and the example, knowing the ligand charges correctly is a prerequisite for determining the oxidation number correctly. In addition, failure to correctly determine the oxidation number will lead to incorrect naming of the compound. Yesiloglu et al. (2021) developed a flow chart for naming coordination compounds and examined the naming errors. They determined that the second most common error was due to the incorrect determination of the oxidation number (Yesiloglu et al., 2021). In addition, Sreenivasulu and Subramaniam (2019) determined that the concepts of the coordination number and oxidation number were confused with each other (Sreenivasulu and Subramaniam, 2019). To talk about coordination chemistry, we need to know coordination nomenclature correctly, and to do the naming accordingly, and we need to know and apply the concepts in the naming rules correctly. Additionally, the terms call for the conceptual synthesis of concepts from several chemistry disciplines, such as chemical bonding and stoichiometry (Sreenivasulu and Subramaniam, 2019). For these reasons, it is important to determine university students’ knowledge of the oxidation number and coordination number concepts and whether they can use these concepts correctly in naming coordination compounds.
This study aims to determine pre-service chemistry teachers’ knowledge of the oxidation number and coordination number concepts.
For this purpose, this study aims to address the following questions:
(1) How do pre-service chemistry teachers define the oxidation number and coordination number concepts? (Here, an explanation that demonstrates knowledge is needed rather than a textbook definition.)
(2) Can pre-service chemistry teachers correctly determine the central atom's oxidation number and coordination number in the given coordination compounds?
(3) Where do pre-service chemistry teachers have difficulty in determining the coordination number and the oxidation number?
3. Method
3.1. Participants
Data were collected from 31 pre-service chemistry teachers. 18 of them were in their third year of graduate education and 13 were in their second year. They attend a public university's four-year, eight-semester chemistry teacher training program as part of their teacher preparation. The curriculum includes chemistry courses (such as general chemistry, inorganic chemistry, and organic chemistry laboratory), general education courses (such as classroom management, measurement, and assessment), and chemistry education courses (such as special teaching techniques and students’ alternative conceptions in chemistry). They took an Inorganic Chemistry II course covering the structure and chemical bonding theories of coordination compounds in the 4th semester of their education. The present study was carried out in the 5th semester of the chemistry education program. In this study, a multi-purpose sampling method was used, which is quite consistent with the principles of qualitative research. According to Patton (1987), the random sampling method allows making valid generalizations about the universe, while the purposive sampling method is more suitable for investigating a particular situation or obtaining very rich data. Before starting the study, the participants were informed about the content and duration of the study; their participation was voluntary, they could withdraw from the study at any time and their identities would be kept confidential. The participants also gave their written consent. The scientific ethics, principles, and rules were followed at all stages of this research.3.2. Development of the data collection tool and data collection procedure
The purpose of the study was to determine the pre-service chemistry teachers’ knowledge of the oxidation number and coordination number concepts. For this purpose, a scale that consisted of four questions was developed (see Appendix I). The first question in the scale asked was to define the “oxidation number” and the “coordination number”. The second question in the scale consisted of eight sub-questions, each of which asked was to determine the “oxidation number” and “coordination number” in the given coordination compound and to name it. In addition, while answering this question, the participants were verbally asked to indicate the reasons for difficulties in determining the oxidation number and coordination number. The 3rd and 4th questions in the scale included the opinions of the participants about whether or not they had difficulty in determining the oxidation number and coordination number. The scale was prepared in this format to measure the ability of the participants to define the given concepts and to determine whether they could use these concepts in practice.To ensure the content validity of this scale, the compounds in the 2nd question were selected to correspond to each type of coordination compound, e.g., the coordination sphere is anionic (3rd and 4th sub-questions of the 2nd question), cationic (2nd, 6th, 7th and 8th sub-questions of the 2nd question), or neutral (1st sub-question of the 2nd question). In order to observe the impact on determining the coordination number, samples with bidentate ligands were added when choosing compounds (2nd, 4th, 6th and 8th sub-questions of the 2nd question). To demonstrate how the participants determined the oxidation number in compounds in which anions and cations were coordination spheres, a related example was added (5th sub-question of the 2nd question).
Afterward, the scale was reviewed by two chemistry education experts who had done studies on coordination compounds. In the trial version of the scale, it was desired to determine the oxidation number and coordination number for the given compounds, but the names of these compounds were not requested. During the expert examination, it was revealed that with this form of the scale, “the effect of correctly identifying these concepts on naming” could not be determined. Therefore, the naming of the given compounds was added to the scale in line with the expert opinion.
The scale was administered to the participants after explaining the basic concepts of coordination compounds, naming and isomerism in their normal processes. During the application, reminder documents were not given to the participants. The participants were asked to indicate the points they had difficulty based on the sub-question, by specifying the sub-question number and justification on the blank part of the paper.
3.3. Data analysis
Qualitative analysis includes working with data, organizing and breaking them into useful units, examining them to find patterns, discovering what matters and deciding what to say to others (Bogdan and Biklen, 1992). The open-ended questions in the scale (1st, 3rd, and 4th questions) were analyzed and evaluated using the content analysis method. With content analysis, any raw data similar to each other are compiled under certain codes, which are then presented as main categories (Strauss and Corbin, 1990a, 1990b). Categories were derived from the data set rather than directly from a preconceived perspective in the present study. In the present study, the participants’ statements were carefully read, and raw categories were developed by the researcher. In later stages of the analysis, new categories were added, with old categories being occasionally changed or combined into a new one(s). At the end of the analysis, the final categories were formed.In question 1, participants’ responses were compared with the standard IUPAC definitions of the term “coordination number” (—Sigma bonds between ligands and the central atom) and the “oxidation number” (the oxidation number of a central atom in a coordination compound is defined as the charge that it will bear when all ligands are removed together with the pairs of electrons shared with the central atom). The definition from the book was not expected from the participants in this situation, but their own explanations that matched the description were also considered as valid.
Students’ responses were grouped into the following categories suggested by Abraham et al. (Abraham et al., 1994):
• Sound Understanding: responses that contained all the components of the verified response. A student's response about the “coordination number” was classified as Sound Understanding if it met the standard IUPAC definition. In addition, any response that referred to the number of secondary valences, the number of electron pairs donated by ligands or accepted by the central metal ion, was also considered as Sound Understanding. Similarly, students’ responses about the “oxidation number” were classified as Sound Understanding if it met the standard IUPAC definition. Additionally, any response that referred to the number of primary valences was considered as Sound Understanding. In other words, the students were not required to provide explicit definitions of the books; rather, definitions with this meaning were recognized as evidence of sound understanding. Examples of responses provided by the participants that were considered to demonstrate “sound understanding” for the coordination number were “the number of coordinated covalent bonds made,” “the number of donor atoms to which the central atom is bonded,” and “the number of Sigma bonds made by the central atom.” The participants' definition of “the result should be zero when the charges of the ligands and the central atom's oxidation number are added together, if it has a charge the outcome should match the charge of the compound,” as well as their mathematical representation of the concept, were considered as sound understanding.
• Certain Misconceptions: responses that contained unreasonable or incorrect information.
• No Comprehension: repeated the question; contained irrelevant information, unclear answers, or not answered.
The second question in the scale consisted of eight sub-questions, each of which asked was to determine the “oxidation number” and “coordination number” and to name the given coordination compound. The participants’ responses to the second question of the scale were assessed as true/false according to the answer key. A matrix table with 8 × 3 cells was prepared for each participant. One point was given for each correct answer and 0 point was given for the wrong or unanswered ones. Both the total scores of the participants and the frequency of sub-questions that were answered correctly were recorded. After the scoring analysis, the responses were examined in detail to determine what types of errors were made by the participants. The errors were coded and categorized.
4. Results
The findings of the study are presented in three sections. In Section 4.1, how the participants defined the coordination number and oxidation number concepts and misconceptions about these concepts are presented. Section 4.2 gives the results regarding whether participants could correctly identify the coordination number and the oxidation number of the central atom in the eight coordination compounds/ions and participants’ performance in the nomenclature of the coordination compounds/ions in terms of the correct use of the oxidation number in naming. It also outlines the types of errors that participants made in determining the oxidation number and coordination number.Finally, in Section 4.3 the participants’ views on the difficulties they encountered while determining the coordination number and oxidation number are presented.
4.1. Participants’ definitions of the coordination number and oxidation number, and their misconceptions
Analysis of the participants’ responses showed that only 10% of them correctly defined the “oxidation number” and 81% had misconceptions about this concept. Five different misconceptions were identified among the participants regarding this concept, and the most frequent ones were “exchange of electrons during reactions in the coordination compound” and “the number of electrons an atom gains or loses”, respectively (see Table 1). In the correct answers regarding this concept, it was determined that one participant made a correct definition as “when added with the oxidation numbers of other atoms, the result should be zero. If the compound has a charge, the total result must equal the charge” and two participants explained it with mathematical equations instead of definitions such as “oxidation number = (charge of coordination sphere) − (sum of charges of ligands)”.| Concept | Misconceptions | Percentagea |
|---|---|---|
| a N = 31. | ||
| Oxidation number | The exchange of electrons in the coordination compound during reactions | 32 |
| The number of electrons an atom gains or loses | 29 | |
| The number of valence electrons that the central atom has | 13 | |
| The ion charge | 3 | |
| The number of electrons that an atom uses to form a bond | 3 | |
| Total misconception | 80 | |
| Coordination number | The number of ligands attached to the central atom | 71 |
When the definitions of the participants regarding the concept of the coordination number were examined, it was seen that the findings were quite interesting. It was determined that most of the participants (71%) had misconceptions about this concept and all of them had the same misconception, that is, the number of ligands attached to the central atom. Participants ignored the bidentate or polydentate ligands when making this definition.
4.2. Identification of the coordination number and the oxidation number of the central atom/ion in coordination compounds/ions
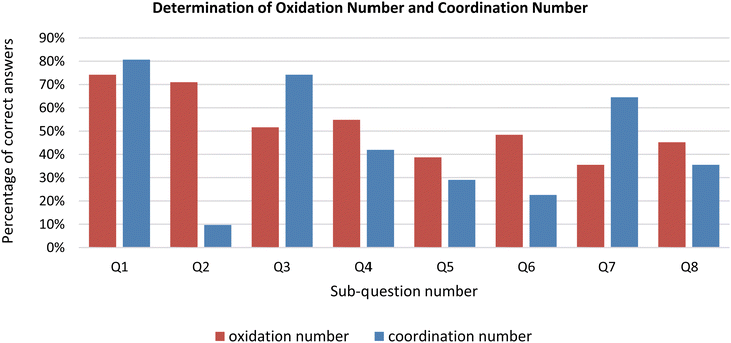
The percentage of correct determination of the oxidation number of the central atom/ion in the given coordination compounds was 52% and this percentage was 45% for the coordination number in the same compounds. To examine the effect of the oxidation number concept on naming, the participants were also asked to name the given compound in addition to determining the oxidation number of the central atom/ion. The percentage of correct expression of the oxidation number in naming was 41%. It was found that every participant who failed to correctly identify the oxidation level also misidentified the compound's name. In some sub-questions (11%), although the participants determined the central atom/ion oxidation number correctly, they could not express it correctly in naming.As stated above, it was determined that the participants had difficulties in determining the coordination number as well (the correct determination rate was only 45%). Additionally, it was observed that the correct determination of the coordination number varied considerably based on sub-questions. The percentages of correct determination were 81% for the 1st sub-question, 10% for the 2nd, and 74%, 42%, 29%, 23%, 65%, and 35% for the others, respectively. When the reason for this high variation was examined, it was seen that the type of ligand in the sub-questions was the major factor. In sub-questions 1, 3 and 7, it was seen that the percentages of determining the coordination number were quite high (see Fig. 2). The reason for the high success rate in these questions may be that these questions contain only monodentate and generally familiar ligands (such as Cl−, NH3). Consistent with this result, it was determined that the participants were more unsuccessful in determining the coordination number in questions 4, 6, and 8, which included oxalato (ox) or ethylenediamine (en) bidentate ligands. The question with the lowest percentage of correct determination of the coordination number is the second sub-question. Because of the sulfato ligand in this question, the participants had a lot of difficulty with this question.
It was also seen that some participants included the counterion when determining the coordination number. For example, it was seen that they calculated the coordination number in the 6th sub-question ([Co(en)2Cl2]Cl) as “2 en + 3 Cl−, coordination number = 5” and in the 8th sub-question ([Co(en)(NH3)3Cl]SO4) as “1 en + 3 NH3 + 1 Cl− + 1 SO42−, coordination number = 6”. In this calculation, it was also seen that the participants neglected that the ethylenediamine (en) ligand was bidentate.
When the determination of the oxidation number of the central atom/ion was examined separately for each sub-question, the percentages of correct determination were 74% for the 1st sub-question, 71% for the 2nd, and 52%, 55%, 39%, 48%, 35%, and 45% for the others, respectively. In the 1st and 2nd questions, which included the ligands they frequently encountered, it was noted that the success rate of the participants in determining the oxidation step was over 70%. In the 7th sub-question with the lowest percentage, the participants stated that they had difficulty in determining the oxidation number of the central atom because they did not know the charge of the NO2− ligand. This result showed us that the participants were able to determine the oxidation number provided they knew the charges of the ligands. In the 5th sub-question with the second-lowest percentage, both the cation and the anion of the coordination compound were coordination spheres. When the participants’ errors in determining the oxidation number were examined, it was determined that the participants mostly thought of the charge of the coordination sphere as the oxidation number of the central atom/ion.
4.3. Participants’ views on the difficulties they encountered while determining the coordination number and oxidation number
In the scale, the 3rd and 4th questions asked the opinions of the participants about whether they had difficulty in determining the oxidation number and coordination number. Their responses indicated that they had challenges in determining both the oxidation number (77%) and coordination number (87%). The most frequently expressed reasons for difficulty in determining the oxidation number were (a) not knowing the ligand charges and (b) not knowing how to calculate the oxidation number in the coordination compound. For example, a participant determined the oxidation number correctly in all other sub-questions, except in the 3rd and 4th sub-questions. This participant stated that she had difficulty with these sub-questions because she did not remember the charges of the CO and oxalato (ox) ligands. The views of some participants regarding their struggles in determining the oxidation number are given below.Since I don’t remember the ligand charges, I have difficulty in determining the oxidation number [Mary]
I don’t know the ligand charges so I have difficulty [Susan]
Because I don’t remember how to find the oxidation number in the coordination compound I had difficulty [Anna]
Actually, I know how to calculate the oxidation number, but I can’t calculate it correctly when there are ligands in the compound that I can’t remember the charge on. For example, in sub-question 7, I could not remember the charge of the NO2 ligand and therefore could not calculate the oxidation number [April]
Most of the participants stated that they had difficulty in determining the coordination number because they did not know/remember the ligand types (monodentate, bidentate ligand). Indeed, there were bidentate ligands in the 2nd, 6th, and 8th sub-questions and it was seen that the percentages of correct determination of the coordination number in these sub-questions were quite low. The following views of some participants reflect this difficulty.
I had difficulty in determining the coordination number, as I could not remember the type of ligands (monodentate or bidentate) [Angel]
I had difficulty in determining the coordination number. Because I got confused about whether some ligands (ox, en, etc.) are bidentate or not [Rose]
I had difficulty in determining the coordination number when there are polydentate ligands [Nicole]
Since some participants define the coordination number as the number of ligands attached to the central atom, they determine the coordination number incorrectly, especially in questions involving bidentate ligands, but they do not think that they have difficulties. Below are examples of participant statements for this situation.
Since the coordination number is equal to the ligand number, I count the ligands in the compound. So there is nothing difficult [Wendy]
I have no difficulties, because the coordination number is equal to the ligand number [Nick]
5. Discussion
This study examined the pre-service chemistry teachers’ knowledge of the oxidation number and coordination number concepts in coordination compounds. It was determined that the participants had difficulties in defining and determining both the coordination number and the oxidation number of the central atom/ion, and they had misconceptions about these concepts.It was found that the majority of participants (71%) had misconceptions about the coordination number, and that they all thought it was related to the number of ligands that were connected to the central atom. Furthermore, it was noticed that the majority of the participants who held this misconception reported that they had no trouble finding the coordination number. Participants who stated that not knowing the number of teeth was a challenge did not have this misconception, because those who have this misconception think that it is very easy to determine the coordination number and it is sufficient to count the number of ligands.
The majority of the students attempted to explain the coordination number of a central metal atom/ion in terms of the number of ligands. For example, in the 6th sub-question in the second question, ([Co(en)2Cl2]+) four ligands are coordinated to the central Co3+ and the majority of the participants determined the coordination number as 4, whereas the actual coordination number (the number of Sigma bonds between the ligand donor atoms and the central metal ion) is 6 because of the bidentate ethylenediamine (en) ligand. In an inorganic chemistry book, the coordination number was defined as “the number of ligands directly bonded to the central atom in a coordination entity” (Madan, 1987). This definition is valid only if all ligands are “monodentate ligands” and such unelaborated definitions may well be a source of misconceptions among participants. Unfortunately, in this study too, it was found that the vast majority of the participants had this misconception. This misconception was also identified as the most common misconception (73.54%, 103 out of 140 students) among university students in another study on the concept of the coordination number in coordination compounds (Sreenivasulu and Subramaniam, 2019).
Analysis of the participants’ responses to the sub-questions revealed that the participants were more successful in determining the coordination number than in determining the oxidation number in the 1st, 3rd, and 7th sub-questions (see Fig. 2). The reason for the high success rate in these questions may be that these questions contain only monodentate and generally familiar ligands (such as Cl− and NH3). When the success of the participants in determining the coordination number was examined on the basis of sub-questions and the opinions of the participants on the points they had difficulty in determining the coordination number were evaluated together, it was seen that they gave wrong answers to these questions, because they did not know the number of teeth when there were polydentate ligands in the question or they had the misconception of “coordination number = number of ligands”.
For example, in the 6th sub-question dealing with the compound [Co(en)2Cl2]Cl, it was seen that all the participants who got this question wrong determined the coordination number as four because they ignored that the ethylenediamine (en) ligand was bidentate. For the same reason, it was determined that they found the coordination number as five in the eighth sub-question, which dealt with the [Co(en)(NH3)3Cl]SO4 compound.
The question that the participants had the most difficulty in determining the coordination number was the second sub-question which included the sulfato ligand. It was seen that all participants who made a mistake in this question containing the [Co(SO4)(NH3)4]+ ion determined the coordination number as 5. Some ligands like the SO42− ion can act as both a monodentate ligand and a bidentate ligand (Housecroft and Sharpe, 2012). Such ligands may change their mode of coordination to maintain the coordination number (Bailar, 1987). For example, in the compound [Co(en)2(H2O)SO4]NO3, sulfato acts as a monodentate ligand, while in the [Co(en)2SO4]NO3 compound it is bidentate.
According to the present study's findings, just 10% of the participants understood what an “oxidation number” was, and 81% had a misconception about it. Regarding this idea, there were five different misconceptions found among the participants. Providing an incomplete explanation for the oxidation number may lead to incorrect interpretations or misconceptions (Widarti et al., 2016). In simple inorganic salts, the oxidation number is defined as “the atom's charge after ionic approximation of its bonds” (Karen et al., 2016). But in coordination compounds, it is described as “the charge remaining on a metal atom when all ligands are removed heterolytically” (Parkin, 2006). In the present study, it was determined that most of the participants defined the oxidation number by considering simple inorganic salts – although it was stated in the question that they should answer by considering coordination compounds. They may have adopted this expression for the definition without adequately understanding the underlying nuances (Clough and Driver, 1985).
Another identified misconception about the oxidation number was that “the oxidation number is the ion charge”. This is also a misconception found by Garnett et al. in a study of simple salts: the oxidation number of a polyatomic species is equal to the charges on the species (Garnett et al., 1990). Similarly, the common misunderstanding in simple salts among students is that the polyatomic ion's charge corresponds to an oxidation number (Brandriet and Bretz, 2014). Additionally, according to De Jong and Treagust (2002), students have difficulty with the explanation and assigning of oxidation numbers. This expression reminds us of the well-known rules of determining the oxidation number for simple inorganic compounds, which include “the sum of the oxidation numbers of all the atoms in a molecule or ion must equal the total charge” (Holleran and Jespersen, 1980) and “for simple ion, the oxidation number is equal to the net charge on the ion” (IUPAC-Gold Book). Students may have responded in this way because they could not distinguish between charge and the oxidation number (Brandriet and Bretz, 2014). Thus, it can be reasonably deduced that this misconception may also stem from the participants’ adoption of oxidation number determination rules for simple inorganic compounds to the coordination compounds without differentiating. This shows us that participants fail to adapt a concept they learned in the context of one topic to another topic, and they have not learned the concept fully, because if a student is able to apply the concepts he has learned to different circumstances and then adapts them to the new conditions, he is assumed to be a learned person (Sözbilir and Neacşu, 2014). It is not surprising that students give wrong answers on a subject they have learned before. This situation may be explained in a number of ways. Students may not have paid attention during the lesson, may not have understood what the teacher said, or may have just forgotten. Forgetting is a more difficult problem for teachers to overcome. But even in this case, teachers should use every opportunity to bring in previous work and relate it to the new subject (Taber, 2002).
Consistent with the above findings, the participants had difficulties in determining the oxidation number of the central atom in coordination compounds. For example, in the 5th sub-question of the scale, only 22% of the participants could correctly determine the oxidation number. In this sub-question, both the cation and the anion of the coordination compound were coordination spheres. Similar findings were obtained in a previous study (Yesiloglu et al., 2021), which showed participants’ difficulties in determining the oxidation number in coordination compounds in which the anion and cation were coordination spheres. As expected, determining the oxidation number of the central atoms is more difficult in coordination compounds containing both complex cations and anions. In fact, it was found that even in simple salts, if the given compounds are complicated, they have trouble determining the oxidation state and make a lot of errors (Basuki et al., 2018).
When the participants’ errors in determining the oxidation number were examined, it was seen that the participants mostly wrote the charge of the coordination sphere as the oxidation number of the central atom. In the second question, it was determined that one of the participants who had this misconception found the oxidation number of the central atom in the [Co(SO4)(NH3)4]+ compound to be (1). Similarly, she found the oxidation number of the iron in the [Fe(CN)5(CO)]3− compound as (−3) and the oxidation number of the cobalt in the [Co(ox)3]3− compound as (−3). This mistake was also observed in previous studies on the misconceptions of students about coordination chemistry (Sreenivasulu and Subramaniam, 2019; Yesiloglu et al., 2021). Determination of the “oxidation numbers” in simple inorganic salts is explained in detail and the related rules are given in the books. In many inorganic chemistry books, it is emphasized that the “oxidation number” of the central atom should be specified in naming coordination compounds (Madan, 1987; Kettle, 2013), but in very few books (Atkins and Overton, 2010; Baxter, 2020) determination of the oxidation number in coordination compounds is explained and exemplified. Since this concept is also used in simple compounds, in most cases it is assumed that students are familiar with it and know how to determine it. However, students might not be successful as we think in conceptualizing the oxidation number concept that differs in different contexts. Atkins proposed two different methods, the “neutral-ligand method” and the “donor-pair method”, to determine the “oxidation number” of coordination compounds (Atkins and Overton, 2010). In the “neutral-ligand method”, all ligands are considered to be neutral and classified as X-type (one-electron radical donors, like halogens) and L-type (two-electron donors, like CO) according to the number of electrons they provide to the central atom. In the “donor-pair method”, ligands are considered to be electron-pair donors and can be neutral or anionic. In this method, the oxidation number is calculated according to the following rule.
• oxidation number of the metal atom = total charge of the complex − charges of ligands
Since the ligands are introduced as anionic and neutral ligands in naming conventions (see Red Book), using this method to determine the “oxidation number” may be helpful in avoiding misconceptions in learners.
In this study, it was revealed that the participants had difficulties in both determining the oxidation number of the central atom and determining the coordination number in coordination compounds. The participants stated that they had problems in determining the oxidation number because they did not remember the ligand charges and the coordination number because they did not remember the number of ligand teeth. Based on the statements of the participants, we can say that they did not learn both concepts meaningfully, because the main achievements of meaningful learning are the ability to keep new information in long-term memory, to be able to recall it when desired, to facilitate subsequent learning, and to enable the information to be used in logical judgment processes while solving unorthodox problems (Şahin, 2002).
Implications and limitations
Students need to be able to determine the oxidation number of the central atom to name coordination compounds correctly. In teaching, it is generally assumed that students are familiar with this concept from simple compounds and know how to determine it. However, in teaching the nomenclature of coordination compounds, it is emphasized only that the oxidation number of the central atom should be specified without an adequate explanation for the oxidation number in the context of coordination compounds. The results of this study showed that the students had problems in transferring the oxidation number concept that they learned in simple compounds to coordination compounds. For this reason, it is suggested that the determination of the oxidation number in coordination compounds before naming them should be discussed separately and explained with examples from coordination compounds.The coordination number concept is also very important, especially in determining geometric isomerism. Therefore, students’ inability to determine the coordination number correctly in a coordination compound may cause them to incorrectly determine the type of isomerism. For this concept, it will be useful for the students to be equipped especially on ligand types (monodentate, bidentate, etc.), to determine the coordination number correctly. Additionally, the participants’ tendency to define the coordination number as “the number of ligands attached to the central atom” may be due to the use of examples that only include monodentate ligands during the introduction of the coordination number concept. In this context, it is recommended that the number of Sigma bonds be emphasized instead of the number of ligands during the concept introduction phase and to emphasize the need for considering the number of ligand teeth in determining the coordination number.
In the present study, the knowledge of the chemistry students about the coordination number and oxidation number was investigated. It is recommended that future studies might examine the relationship of these concepts with the shapes of coordination compounds or the formation of coordination compounds.
All data collection tools being in written format and the lack of individual participant interviews are the biggest limitations of the study. Also, the study being limited to only 31 participants is another important limitation that decreases the external validity of the study. The decreased quota for chemistry teachers made finding an adequate number of samples quite difficult in our country. The participants’ definitions of the coordination number and oxidation number concepts may have been affected by how they were taught. This could not be controlled, so it is a limitation of the study.
Conflicts of interest
There are no conflicts to declare.Appendix I the scale of the knowledge of the oxidation number and coordination number in coordination compounds
(1) Explain the following concepts taking into account the coordination compounds.• Oxidation Number
• Coordination Number
(2) Determine the oxidation number and coordination number of the central atoms in the following coordination compounds.
| Coordination compound/ion | Name | Oxidation number | Coordination number | |
|---|---|---|---|---|
| 1 | [PtCl2(NH3)2] | |||
| 2 | [Co(SO4)(NH3)4]+ | |||
| 3 | [Fe(CN)5(CO)]3− | |||
| 4 | [Co(ox)3]3− | |||
| 5 | [Pt(NH3)4][PtCl4] | |||
| 6 | [Co(en)2Cl2]Cl | |||
| 7 | [CoCl(NO2)(NH3)4]+ | |||
| 8 | [Co(en)(NH3)3Cl]SO4 |
(3) I have difficulty in determining the oxidation number of the central atom in coordination compounds.
Yes □No □
Cause of Difficulty (If your answer is yes)
(4) I have difficulty in determining the coordination number of the central atom in coordination compounds.
Yes □No □
Cause of Difficulty (If your answer is yes)
References
- Abraham M. R., Williamson V. M. and Westbrook S. L., (1994), A cross-age study of the understanding of five chemistry concepts, J. Res. Sci. Teach., 31(2), 147–165.
- Allen O. G. and Worokwu C., (2020), Effect of simulation and guided-inquiry strategies in elimination of students’ misconception in chemistry teaching, J. Glob. Res. Educ. Soc. Sci., 14(3), 16–21.
- Atkins P. and Overton T., (2010), Shriver and Atkins’ inorganic chemistry, USA: Oxford University Press.
- Bailar Jr J. C., (1987), Comprehensive coordination chemistry: the synthesis, reactions, properties & applications of coordination compounds, in Wilkinson G. (ed.), Development of coordination chemistry since 1930, Brighton: Pergamon Press, vol. 1, pp. 21–30.
- Balasundram N. and Karpudewan M., (2021), Exploring the use of a writing-to-learn activity embedded with multiple modes using ‘Popplet’ on pre-university students’ alternative conceptions on transition metals, Chem. Educ. Res. Pract., 22(2), 263–281.
- Barke H. D., Hazari A. and Yitbarek S., (2008), Misconceptions in chemistry: Addressing perceptions in chemical education, Springer Science & Business Media.
- Basuki R., Amanda H., Bemis R., Lisma A., & Yusnaidar Y., (2018), Incomplete explanation in determining oxidation number: A case study on chemistry program students. J. Pendidik. IPA Indones., 7(3), 333–340.
- Baxter S., (2020), Coordination chemistry and its applications, Los Angeles: Tritech Digital Media.
- Bhatt V., (2015), Essentials of coordination chemistry: A simplified approach with 3D visuals, Academic Press.
- Bogdan R. C. and Biklen S. K., (1992), Qualitative research for education, 2nd edn, Boston: Allyn & Bacon.
- Brandriet A. R. and Bretz S. L., (2014), Measuring meta-ignorance through the lens of confidence: examining students’ redox misconceptions about oxidation numbers, charge, and electron transfer, Chem. Educ. Res. Pract., 15(4), 729–746.
- Calzaferri G., (1999), Oxidation numbers, J. Chem. Educ., 76(3), 362.
- Canbaz H., (2016), Contemporary teaching methods in mongolian secondary school chemistry, Int. J. Eng. Sci. (IJES), 5(4), 81–84.
- Cass M. E., (2004), Student-directed explorations to learn about ligands in an inorganic chemistry course, J. Chem. Educ., 81(8), 1145.
- Chia V. Y., Hölttä-Otto K. and Anariba F., (2022), Using the electrochemistry designette to visualize students’ competence and misconceptions on electrochemical principles, J. Chem. Educ., 99(3), 1533–1538.
- Clough E. E. and Driver R., (1985), Secondary students’ conceptions of the conduction of heat: bringing together scientific and personal views, Phys. Educ., 20(4), 176–182.
- Connelly N. G.; Damhus T.; Hartshorn R. M. and Hutton A. T., (2005), Nomenclature of inorganic chemistry: IUPAC Recommendations, UK: RSC Publishing, pp. 142–198.
- Constable E. C., (2019), What's in a Name?—A Short History of Coordination Chemistry from Then to Now, Chem, 1(1), 126–163.
- Davis B. G., (1997), Misconceptions as barriers to understanding science. Science teaching reconsidered: A hand book, Washington, DC: National Academy, pp. 27–32.
- De Jong O. and Treagust D., (2002), The teaching and learning of electrochemistry, in Gilbert J. K., De Jong O., Justi R., Treagust D. F. and van Driel J. H. (ed.), Chemical education: towards research-based practice, Dordrecht: Kluwer Academic Publishers, pp. 317–337.
- Garnett P. J. and Treagust D. F., (1992), Conceptual difficulties experienced by senior high school students of electrochemistry: Electric circuits and oxidation-reduction equations, J. Res. Sci. Teach., 29(2), 121–142.
- Garnett P. J., Garnett P. J. and Treagust D. F., (1990), Common misconceptions in electrochemistry: Can we improve students’ understanding of this topic, Chemeda: Aust. J. Chem. Educ., 27(3), Y11.
- Gorunova O. N., Novitskiy I. M., Grishin Y. K., Gloriozov I. P., Roznyatovsky V. A., Khrustalev V. N. et al., (2018), When applying the mercury poisoning test to palladacycle-catalyzed reactions, one should not consider the common misconception of mercury (0) selectivity, Organometallics, 37(17), 2842–2858.
- Granville M. F., (1985), Student misconceptions in thermodynamics, J. Chem. Educ., 62(10), 847.
- Griffiths A. K. and Preston K. R., (1992), Grade-12 students’ misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29(6), 611–628.
- Hackling M. W. and Garnett P. J., (1985), Misconceptions of chemical equilibrium, Eur. J. Sci. Educ., 7(2), 205–214.
- Holleran E. M. and Jespersen N. D., (1980), Elementary oxidation-number rules, J. Chem. Educ., 57(9), 670.
- Housecroft C. E. and Sharpe A. G., (2012), Inorganic chemistry, Edinburg: Pearson Education, vol. 1.
- IUPAC-Gold book, (1997), Online version (2019-) Compendium of Chemical Terminology, 2nd edn, Compiled by A. D. McNaught and A. Wilkinson, Oxford: Blackwell Scientific Publications, created by S. J. Chalk, ISBN 0-9678550-9-8 DOI:10.1351/goldbook. (accessed March 2022).
- Jensen W. B., (2011), Oxidation states versus oxidation numbers, J. Chem. Educ., 88(12), 1599–1600.
- Karen P., McArdle P. and Takats J., (2016), A comprehensive definition of oxidation state (IUPAC Recommendations 2016), Pure Appl. Chem., 88(8), 831–839.
- Karpudewan M. and Balasundram N., (2019), Addressing alternative conceptions about transition metals among form six students using information and communication technology based instruction, Eurasia J. Math. Sci. Technol. Educ., 15(7), em1731.
- Kelly R. M., Barrera J. H. and Mohamed S. C., (2010), An analysis of undergraduate general chemistry students’ misconceptions of the submicroscopic level of precipitation reactions, J. Chem. Educ., 87(1), 113–118.
- Kettle S. F. A., (2013), Physical inorganic chemistry: A coordination chemistry approach, Springer.
- Kotz J. and Purcell K. F., (1991), Chemistry & Chemical Reactivity, 2nd edn, Orlando, FL: Saunders College Publishing.
- Laliyo L. A. R., Botutihe D. N. and Panigoro C., (2019), The development of two-tier instrument based on distractor to assess conceptual understanding level and student misconceptions in explaining redox reactions, Int. J. Learn. Teach. Educ. Res., 18(9), 216–237.
- Lemma A., (2012), Diagnosing the diagnostics: Misconceptions of twelfth grade students on selected chemistry concepts in two preparatory Schools in eastern Ethiopia, Afr. J. Chem. Educ., 2(2), 16–31.
- Loock H. P., (2011), Expanded definition of the oxidation state, J. Chem. Educ., 88(3), 282–283.
- Luxford C. J. and Bretz S. L., (2014), Development of the bonding representations inventory to identify student misconceptions about covalent and ionic bonding representations, J. Chem. Educ., 91(3), 312–320.
- Madan R. D., (1987), Modern inorganic chemistry, S. Chand Publishing.
- Mayer K., (2011), Addressing students’ misconceptions about gases, mass, and composition, J. Chem. Educ., 88(1), 111–115.
- Mickey C. D., (1981), Some aspects of coordination chemistry, J. Chem. Educ., 58(3), 257–262.
- Miessler G. L., & Tarr D. A., (1999), Coordination compounds, Inorganic chemistry, 2nd edn, United States: Prentice Hall.
- Milenković D. D., Hrin T. N., Segedinac M. D. and Horvat S., (2016), Development of a three-tier test as a valid diagnostic tool for identification of misconceptions related to carbohydrates, J. Chem. Educ., 93(9), 1514–1520.
- Nakhleh M., (1992), Why some students don’t learn chemistry: Chemical misconceptions, J. Chem. Educ., 69(3), 191–196.
- Parkin G., (2006), Valence, oxidation number, and formal charge: three related but fundamentally different concepts, J. Chem. Educ., 83(5), 791.
- Patton M. Q., (1987), How to use qualitative methods in evaluation (No. 4), Sage.
- Peterson R. F. and Treagust D. F., (1989), Grade-12 students’ misconceptions of covalent bonding and structure, J. Chem. Educ., 66(6), 459.
- Şahin F., (2002), Kavram haritalarının değerlendirme aracı olarak kullanılması ile ilgili bir araştırma, Pamukkale Univ. J. Educ., 11(11), 17–32.
- Sam A.; Niebert K.; Hanson R. and Twumasi A. K., (2015), The model of educational reconstruction: scientists’ and students’ conceptual balances to improve teaching of coordination chemistry in higher education, Int. J. Acad. Res. Reflect, 7(5), 67–77.
- Sanger M. J. and Greenbowe T. J., (1997), Common student misconceptions in electrochemistry: Galvanic, electrolytic, and concentration cells, J. Res. Sci. Teach., 34(4), 377–398.
- Shehu G., (2015), Two ideas of redox reaction: Misconceptions and their challenges in chemistry education, IOSR J. Res. Method Educ., 5(1), 15–20.
- Sindhu R. S., (2021), Teachers’ misconception concerning valence and valency in chemistry, Sci. Educ. Int., 32(4), 308–310.
- Sloan T. E., (1987), in Wilkinson G. (ed.), Nomenclature of coordination compounds. Comprehensive coordination chemistry: The synthesis, reactions, properties & applications of coordination compounds, Brighton: Pergamon Press, vol. 1, pp. 109–134.
- Smith D. W., (2005), Valence, covalence, hypervalence, oxidation state, and coordination number, J. Chem. Educ., 82(8), 1202.
- Sözbilir M. & Neacşu I., (2014), Alternative assessment and evaluation methods: A practical guide book for teachers, Erzurum/Bucharest: Erzurum Provincial Directorate of National Education.
- Sreenivasulu B.; Subramaniam R., (2014), Exploring undergraduates’ understanding of transition metals chemistry with the use of cognitive and confidence measures, Res. Sci. Educ., 44(6), 801–828.
- Sreenivasulu B. and Subramaniam R., (2019), Mapping the conceptual space formed by students’ understanding of coordination number of a transition metal complex: an exploratory study, Chem. Educ. Res. Pract., 20(3), 468–483.
- Steinborn D., (2004), The concept of oxidation states in metal complexes, J. Chem. Educ., 81(8), 1148.
- Strauss A. L. and Corbin J., (1990a), Basics of qualitative research: Grounded theory procedures and techniques, Newbury Park, CA: Sage.
- Strauss A. L. and Corbin J., (1990b), Basics of qualitative research: grounded theory procedures and techniques, Newbury Park, CA: Sage.
- Taber K., (2002), Chemical misconceptions: prevention, diagnosis and cure, Royal Society of Chemistry, vol. 1.
- Uce M., (2015), Constructing models in teaching of chemical bonds: Ionic bond, covalent bond, double and triple bonds, hydrogen bond and molecular geometry, Educ. Res. Rev., 10(4), 491–500.
- Ukah P. N., Uchegbu R. I. and Ehujuo C. A., (2021), Development of a diagnostic test in iupac nomenclature of chemical substances for senior secondary school chemistry students, IOSR J. Res. Method Educ., 11(3), 46–51.
- Widarti H. R., Marfuaf S. and Retnosari R., (2019), Identıfyıng students’ misconception about intermolecular forces topic in organıc chemıstry I course, Unnes Sci. Educ. J., 8(1), 46–56.
- Widarti H. R., Permanasari A. and Mulyani S., (2016), Student misconception on redox titration (a challenge on the course implementation through cognitive dissonance based on the multiple representations). J. Pendidik. IPA Indones., 5(1), 56–62.
- Yan Y. K. and Subramaniam R., (2018), Using a multi-tier diagnostic test to explore the nature of students’ alternative conceptions on reaction kinetics, Chem. Educ. Res. Pract., 19(1), 213–226.
- Yesiloglu S. N., Turan-Oluk N. and Tufan Y., (2021), Development and use of flowcharts for identifying types of isomers and naming coordination compounds, J. Chem. Educ., 98(6), 1988–1996.
| This journal is © The Royal Society of Chemistry 2023 |