Analysis of students’ diagrams of water molecules in snowflakes to reveal their conceptual understanding of hydrogen bonds
Henry
Matovu
 *a,
Mihye
Won
*a,
Mihye
Won
 a,
David Franklin
Treagust
a,
David Franklin
Treagust
 a,
Mauro
Mocerino
a,
Mauro
Mocerino
 b,
Dewi Ayu Kencana
Ungu
b,
Dewi Ayu Kencana
Ungu
 a,
Chin-Chung
Tsai
a,
Chin-Chung
Tsai
 c and
Roy
Tasker
c and
Roy
Tasker
 d
d
aSchool of Education, Curtin University, Perth, Australia. E-mail: henry.matovu@postgrad.curtin.edu.au
bSchool of Molecular and Life Sciences, Curtin University, Perth, Australia
cProgram of Learning Sciences, and Institute for Research Excellence in Learning Sciences, National Taiwan Normal University, Taipei, Taiwan
dSchool of Science, Western Sydney University, Penrith, Australia
First published on 30th October 2022
Abstract
Recent studies have reported a growing trend of using student-generated diagrams for assessment in science teaching and research. However, many educators tend to use diagrams to explore students’ perceptions of scientists and their work rather than explore conceptual understanding of abstract concepts. In this study, we used diagrams to investigate students’ conceptual understanding of the nature of hydrogen bonds among water molecules in snowflakes. Participants were 70 first- and second-year university students. Following a sequence of interview prompts, the students drew diagrams to illustrate the interactions amongst water molecules in snowflakes. Sixty students’ diagrams were analyzed inductively using a constant comparison method. Most diagrams showed that the students did not have major challenges drawing the water molecule structure, recognizing polarity of a water molecule, or recognizing the intermolecular nature of hydrogen bonds. However, the diagrams revealed varied ways in which students conceptualized the formation of hydrogen bonds. A third of the diagrams revealed students’ alternative conceptions about the role of lone pairs of electrons in the formation of hydrogen bonds. Most diagrams which showed a good understanding of the nature of a hydrogen bond revealed students’ difficulties in recognizing molecular interactions in a 3D space. Our findings suggest that student-generated diagrams can provide a powerful way to understand students’ conceptions of abstract science concepts.
Introduction
The use of diagrams is a common practice in many science classrooms (Cheng and Gilbert, 2009; Tippett, 2016). In some science classes, educators provide preconstructed diagrams and ask students to interpret the diagrams without engaging students in creating their own diagrams. For example, in biology, educators may provide students with preconstructed and labelled diagrams of biological systems and processes as a means of mediating classroom discussions (Liu et al., 2014; Quillin and Thomas, 2015). In chemistry, educators commonly provide learners with diagrams of science equipment as pre-lab activities (e.g., Chittleborough and Treagust, 2008), or diagrams of molecular structures to help students link the visible (macroscopic) to the invisible (submicroscopic) entities. In other cases, educators ask learners to construct diagrams to develop observational skills (Quillin and Thomas, 2015; Ainsworth and Scheiter, 2021), increase learners’ engagement (e.g., Ainsworth et al., 2011), promote reasoning (Tippett, 2016), and for assessment (Chang et al., 2020). The present study builds on earlier literature on multiple representation research and the use student-generated diagrams for assessment to investigate students’ understanding of the nature of hydrogen bonds among water molecules in snowflakes; this literature provides the theoretical framework for this research.Theoretical framework
Diagrams as forms of multiple representations for learning and assessment
Multiple representations refer to using more than one way to represent the same or similar concept. Chemistry concepts are often explained in several ways such as by means of analogies, graphs, models, formulae, or diagrams (Treagust et al., 2017). For example, structures of molecules can be described using physical ball-and-stick models, space-filling models, formulae, or 2D structural drawings. Researchers concur that using multiple representations can support students’ learning more than using a single representation (Gilbert and Treagust, 2009). Because each form of representation has different characteristics and may convey different but complementary information about a concept, Ainsworth (1999; 2006) proposed several functions of multiple representations in learning – supporting students to use multiple problem-solving strategies, augmenting information in one form of representation by another, and helping students construct a deeper understanding of science concepts. However, because novice students have difficulty translating freely from one form of representation to another (Kozma and Russell, 1997; Kozma, 2003; Allred and Bretz, 2019), the effectiveness of using multiple representations for these students may be limited.Another line of multiple representation research argues that, rather than interpret externally provided science representations, students learn better when they are given an opportunity to construct and interpret their own representations, such as through drawings (Van Meter and Garner, 2005; Ainsworth et al., 2011). Indeed, a recent review of the use of diagrams in science learning settings has reported a shift from learning science from diagrams (interpreting preconstructed diagrams) to learning science with diagrams (reasoning while constructing diagrams) in recent years (Tippett, 2016). Constructing diagrams allows students to actively reason and develop mental models of science phenomena by selecting the most relevant spatial features and representing them visually (Wu and Rau, 2019). Moreover, through diagrams, students can flexibly construct, organise, represent, evaluate, and communicate their understanding of science concepts (Treagust et al., 2017). This action helps learners navigate the challenge of accumulating fragmented knowledge (Gilbert, 2006). Students can then build connections amongst concepts, and achieve meaningful learning by incorporating new learning content into already existing knowledge structures to build coherent scientific understanding (Wu and Rau, 2019). There is now a growing body of evidence that learning with drawings promotes deeper processing and integrated understanding of science concepts (e.g., Tytler et al., 2020; Andrade et al., 2021). In chemistry, for example, integrating the drawing of chemical species at the submicroscopic level in teaching and learning sessions improved students’ reasoning about chemical equations and stoichiometry (Davidowitz et al., 2010) and the particulate nature of matter (e.g., Derman and Ebenezer, 2020; Andrade et al., 2021).
In addition to promoting students’ reasoning and conceptual understanding, research also suggests that engaging students in drawing diagrams of science phenomena can act as a window into students’ mental models of spatial and dynamic aspects of the phenomena (Tippett, 2016). Different from other ways of assessing conceptual understanding which restrict students’ responses to the lists provided, the process of drawing diagrams allows students freedom to flexibly express their ideas and engage in ‘sense-making’ rather than ‘selecting options’ (Nyachwaya et al., 2011). As an exploratory research tool, students’ diagrams may reveal students’ alternative conceptions that existing diagnostic tests or other modes of assessment may miss (Ainsworth et al., 2011). Diagrams are especially useful when the students lack sufficient competency in the use of the relevant scientific terminologies (McLure et al., 2021a). For example, a multimodal study by Cooper et al. (2015) reported that students’ diagrams provided a better representation of students’ understanding of the nature of intermolecular forces compared to the students’ written text.
A recent literature review by Chang et al. (2020) found that, in science learning settings, there is a growing trend of using student-generated diagrams for assessment purposes. However, when educators use students’ diagrams for this purpose, many tend to evaluate students’ perceptions of scientists through a ‘Draw-A-Scientist-Test’ or its modified versions (Finson, 2002; Farland-Smith, 2012; Reinisch et al., 2017; Miller et al., 2018). Other studies evaluated students’ perceptions of science learning and teaching (e.g., Markic and Eilks, 2015) or students’ modelling abilities (Chang et al., 2020).
In chemistry education, few studies have used student-generated diagrams to investigate students’ understanding of key chemistry concepts; exceptions are the particulate nature of matter (e.g., Kelly et al., 2010; Kern et al., 2010; Nyachwaya et al., 2011; McLure et al., 2021b), atomic structure (e.g., Derman et al., 2019), and intermolecular forces (e.g., Williams et al., 2015; Noyes and Cooper, 2019). Using an open-ended drawing approach, Nyachwaya et al. (2011) tasked college students to balance chemical equations and illustrate the nature of particles involved in the reactions. Some students did not show the distinction between covalent and ionic bonds in chemical species, while other students exhibited difficulties interpreting symbolic language or depicting reasonable molecular geometry, relative atomic and ionic sizes, and oxidation states of the species involved in chemical reactions (Nyachwaya et al., 2011). Derman et al. (2019) reported that, when asked to illustrate their mental models of the structure of an atom, many pre-service teachers drew an atom as a central nucleus surrounded by shells (or orbits). In addition, most participants represented electrons as negatively charged particles on the shells/orbits, but did not represent the charges of protons, the space-filling character of atoms, or the quantum-mechanical theory of atomic structure (Derman et al., 2019). Related to the concept of intermolecular forces, learners used an online drawing tool to illustrate their understanding of the concepts of hydrogen bonds, dipole–dipole interactions, and London dispersion forces (Cooper et al., 2015; Williams et al., 2015; Becker et al., 2016; Noyes and Cooper, 2019). By analysing the students’ diagrams, the researchers uncovered several students’ alternative conceptions about intermolecular forces, such as hydrogen bonds in ethanol being intramolecular covalent bonds, or students’ difficulties in recognising the role of charges in London dispersion forces.
Taken together, previous studies have provided a useful starting point in terms of demonstrating the power of using diagrams to assess students’ conceptual understanding in chemistry. Previous studies mainly focused on students representing the nature and number of individual chemical species (e.g., atoms, ions, or molecules) participating in molecular reactions (e.g., Kelly et al., 2010; Kern et al., 2010). When researchers used diagrams to investigate students’ understanding of the nature of interactions among particles (e.g., Williams et al., 2015; Noyes and Cooper, 2019), the students’ diagrams were based on a small number of particles (two to three) and students were not tasked to reason about how the particles would interact in a context containing many particles. The position taken in our research is that by asking students to consider multiple molecules interacting with each other in a relevant context, they are engaged in thinking beyond individual particles. This action not only helps students appreciate the relevance of their chemistry knowledge but also overtly displays students’ thinking about the nature of the interactions.
Students’ difficulties in learning the concept of hydrogen bonds
The concept of hydrogen bonds is one of the many abstract, yet fundamental, chemistry concepts related to molecular interactions which many students find challenging to grasp. Achieving coherent understanding of the nature of hydrogen bonds is not a trivial task as students require a good understanding of shapes of molecules, electronegativity effects, and how these relate to the distribution of electrons in the molecules (Henderleiter et al., 2001). Without a coherent understanding of the concept of hydrogen bonds, students may rely on rote memorisation of facts to predict or explain properties of substances (Cooper et al., 2013).Studies have reported students’ difficulties in understanding the concept of hydrogen bonds and its importance in explaining many macroscopic properties of substances, such as boiling and melting points (Henderleiter et al., 2001; Schmidt et al., 2009). Using a two-tier diagnostic test, Schmidt et al. (2009) provided lists of compounds and asked students to identify with reasons which of the compounds would form hydrogen bonds. The authors reported that some senior high school students had difficulties identifying compounds that could form hydrogen bonds. Using interviews and lists of individual structural formulae already constructed by the researchers, Henderleiter et al. (2001) tasked undergraduate organic chemistry students to identify the situations in which hydrogen bonds can form among molecules, and to apply their understanding of the concept of hydrogen bonds to explain trends in boiling points of substances. Henderleiter et al. (2001) reported that some students had difficulties in predicting the formation of hydrogen bonds in molecules; for instance, some students explained that water molecules could form hydrogen bonds with methane molecules by polarizing the hydrogen atoms in the methane molecules. Students also tended to confuse the term hydrogen bond with covalent bonds that involve hydrogen atoms (Williams et al., 2015). For example, when tasked to illustrate their understanding of hydrogen bonds through diagrams, the majority (about 60%) of first-year university students enrolled in a general chemistry course illustrated hydrogen bonds in ethanol molecules as bonds occurring “within” the molecules rather than as intermolecular forces (Cooper et al., 2015).
Overall, previous studies employed a range of strategies and prompts to uncover students’ understanding of the concept of hydrogen bonds. Some of the studies provided contexts for students to demonstrate their understanding (Henderleiter et al., 2001; Schmidt et al., 2009). However, researchers argued that students could easily rely on their rote memorization of the concept of hydrogen bonds in these contexts by choosing from the lists of options provided (e.g., Schmidt et al., 2009), or invoke key words in their explanations of macroscopic properties without a clear understanding of how hydrogen bonds are formed (Cooper et al., 2013). For example, to predict the occurrence of hydrogen bonds, some students simply focused on identifying the presence of oxygen and hydrogen atoms in molecules or applying periodic trends in electronegativity without considering when such trends fail to apply (Schmidt et al., 2009). In addition, none of the previous studies on students’ conceptions of hydrogen bonds investigated in-depth how students conceptualised the formation of hydrogen bonds. Although recognising that hydrogen bonds are intermolecular in nature is important, this is only the first step to understand the nature of these intermolecular interactions. Yet, earlier studies did not go deeper to investigate students’ understanding of the reason why a hydrogen bond forms and the role of lone pairs of electrons. Moreover, by using key chemistry terms, such as hydrogen bonds or dipole-dipole interactions in their prompts, previous studies may have limited the students’ freedom to express their understanding of molecular interactions (e.g., Cooper et al., 2015). Also, previous studies did not provide prompts for students to coherently reason and demonstrate their understanding of the concept of hydrogen bonds through diagrams.
In this research, we employed student-generated diagrams to investigate students’ conceptual understanding of the nature of hydrogen bonds among many water molecules in snowflakes and to identify the difficulties that students may face as they link different chemistry concepts to illustrate the nature of these molecular interactions. Understanding students’ ideas and learning difficulties can be used to inform design interventions for addressing students’ learning difficulties and alternative conceptions in a more systematic way (Nyachwaya et al., 2011). To effectively engage with drawing activities, students require opportunities to discuss their ideas with peers and knowledgeable others (McLure et al., 2021a), and the drawing activities need to be scaffolded appropriately through training or prompts (Van Meter and Garner, 2005; Ainsworth and Scheiter, 2021). Therefore, in this research, the students were asked to explain their drawings to the researchers. The researchers also provided prompts for students to progressively build on their prior understanding of structure and polarity of water molecules while reasoning about the interactions among water molecules in the context of snowflakes. The present study was designed to answer the following research questions:
• How do students use diagrams to represent their understanding of the nature of hydrogen bonds among water molecules in snowflakes?
• What are the common conceptions of the nature of hydrogen bonds among water molecules in snowflakes inferred from these student-generated diagrams?
Methods
Research context
A total of 70 first and second-year undergraduate chemistry students enrolled in two chemistry units (Reactivity and Function in Chemistry for first-year students, and Chemistry of Biological Processes for second-year students) at a large public university in Australia participated in the study. The two chemistry units include a focus on the concepts of intermolecular forces and molecular shape and how these factors influence the chemistry of substances. Each unit is taught in the form of 1–2 hour lectures, 3–4 hour hands-on lab sessions, and 1–2 hour tutorial workshops led by tutors per week. During the tutorial workshops, students work in teams of 3–5 to complete learning tasks following a Process-Oriented Guided Inquiry Learning (POGIL) approach. In some activities, students manipulate ball-and-stick models to explore different forms of molecules such as conformations and enantiomers. Students also often draw diagrams of molecular structures while completing the tasks. However, the emphasis is placed on the symbolic representations but not on the submicroscopic interactions, and no specific emphasis is placed on representing molecules in 3D. In addition, reading materials such as Blackman et al. (2019) are recommended although they are not mandatory. This textbook is recommended for use in over 15 universities across Australia and New Zealand.Students enrolled in the two units were those taking chemistry-related degree programs such as Chemistry, Chemical Engineering, Food science, and Nutrition. The enrolled students were considered to have an adequate understanding of the basic chemistry concepts related to water molecules as all these students had completed and passed high school chemistry and at least one university level chemistry unit as a prerequisite to be in these two chemistry units. The participation of these students in the interviews and subsequent learning activities was seen as beneficial for the students in the two units, and the lecturers encouraged all students to participate in them.
The interviews and drawing activities were conducted outside the normal course timetables and students were free to choose the most convenient time for them to participate. Scheduling of the sessions was done using an online meeting scheduling tool (https://doodle.com). Students logged into the online system via a link that was provided by the researchers. The students were informed that they would complete the learning tasks in pairs, therefore, each slot could be occupied by two students. Since the students randomly selected slots, some students were paired with their friends while others with peers with whom they had not worked with prior to this activity.
Data collection
Before collecting data, ethics approval was obtained from the institutional Human Research Ethics Committee (HRE2020-0081). In addition, before participating in the study, students gave consent to use their diagrams and interview data for this study.In this study, the students were paired so that they could explain their ideas to each other and were given an opportunity to collaborate on the drawing task to produce a shared diagram if they wished. Each student was given a pen and paper and tasked to draw a diagram to illustrate their understanding of the nature of hydrogen bonds among water molecules in snowflakes. Because explaining the nature of hydrogen bonds requires students to integrate their knowledge of the bonding, structure, and polarity in water molecules, the researchers provided students with a series of verbal prompts so that the students could complete the drawing activity stepwise. The prompts were not intended to guide learners to the correct answers or lead them to a better understanding but to elicit students’ ideas about the concepts underlying the interactions amongst water molecules illustrated in their drawings. Also, because it is common for students to confuse the term ‘hydrogen bonds’ with intramolecular covalent bonds involving hydrogen atoms (e.g., Cooper et al., 2015), or to simply reproduce textbook definitions of the concept, in this study, we did not directly use the term hydrogen bonds in the interview prompts. Instead, we asked the students to illustrate how they imagined the water molecules interacting with one another in the context of snowflakes.
The verbal prompts used in this study were developed in meetings among three authors (HM, MW, and DU), and were checked by the other authors, two of whom had more than twenty-five years’ experience in teaching first- and second-year university chemistry units. The final verbal prompts employed by the researchers are provided in Table 1. Students’ diagrams in response to the verbal prompts were collected. In addition, all the students’ interactions were audio and video recorded. For each pair of students, the interview and drawing activity lasted 15–25 minutes.
| Target concept | Verbal prompt |
|---|---|
| Structure of a water molecule | Here we have some magnified images of snowflakes (the researcher shows some of the shapes of snowflakes). |
| (a) What do you notice about the shapes of snowflakes? | |
| (b) Snowflakes are made from water molecules. Imagine I am a year 11 (high school) student, what can you tell me about a water molecule? | |
| (c) Please draw the water molecule you have described at the centre of the piece of paper. | |
| (d) Why is the water molecule shaped like that? | |
| Polarity and nature of hydrogen bonds among water molecules | Let's imagine that this water molecule is at a very low temperature, say close to its freezing point, and we have another water molecule coming close enough to the first one to interact with it, |
| (e) How would you draw the interaction between those two water molecules? | |
| (f) Why would the molecules interact like that? | |
| (g) If we have another molecule coming close to the first one, is it still possible for it to interact with the first one? How would you draw the interaction between the third water molecule and the first one? Please explain why the molecules would interact like this. | |
| [This prompt was repeated until the student said that no more water molecules would interact with the first one, with reasons] |
As mentioned above, in the present study, students were given opportunities to discuss their ideas with one another and to collaborate while constructing the diagrams. However, most students preferred to construct individual diagrams and to provide individual explanations, irrespective of whether the students were friends prior to the activity or not, or whether the students’ ideas were similar or not. Therefore, after a few sessions, the authors dropped the prompts asking the students to discuss with each other every time. Instead, the authors asked each student to draw a diagram to represent their own understanding and to explain their diagram verbally (Table 1). After drawing, the individual students were asked to confirm if they were happy with their diagrams.
Students in four of the pairs collaborated during the drawing task, taking turns at drawing to create shared diagrams, and explaining to each other as they drew the diagrams. When students collaborate to construct a shared diagram, it is hard to discern which idea belongs to which student and, by working on the same diagram, students may be forced to change their ideas in the process. Therefore, the authors decided to exclude shared students’ diagrams from the analysis.
For a given student-generated diagram to be included in the analysis, the student needed to have been enrolled in the two target chemistry units and the student must have constructed the diagram individually to illustrate their understanding of water molecule interactions. A student who did not draw a diagram was not considered for analysis.
Analysis
Of the 70 student participants, data from 60 students was used for analysis. One student was not enrolled in the target chemistry units while one student did not draw a diagram and, therefore, had incomplete data. Diagrams created by eight of the students (4 pairs) were also excluded because the students collaborated to construct shared diagrams (one diagram from each pair). The remaining 60 students’ diagrams were analysed qualitatively. In our earlier work (e.g., McLure et al., 2021b; Tenzin et al., 2022), we identified a set of procedures to interpret students’ diagrams in relation to their conceptual understanding without extensively relying on students’ verbal explanations. We adopted similar procedures to analyse students’ diagrams in the present study. First, inductive analysis (Merriam and Tisdell, 2015) of the diagrams was conducted to identify categories related to the students’ conceptions of the nature of hydrogen bonds among water molecules in snowflakes. Two of the authors of this paper (HM & MW) carefully examined each student's diagram to identify the students’ conceptions of the structure and polarity of a water molecule and the nature of hydrogen bonds among water molecules in snowflakes. A constant comparison method (Merriam and Tisdell, 2015) was then adopted to identify categories related to students’ conceptions of the nature of hydrogen bonds among water molecules in snowflakes.Although the focus of this study was understanding students’ conceptions of the nature of hydrogen bonds as represented in their diagrams, the drawing activity was multimodal in nature (Jewitt, 2013) since students were tasked to draw diagrams as well as verbally explain their understanding. In such a case, students may choose to illustrate some ideas in their diagrams and represent other ideas using other modes (verbal explanations and gestures). Therefore, in analysing the students’ diagrams, the first two authors (HM & MW) constantly referred to the transcripts and videos of the interactions the students engaged in during the drawing activities to confirm the authors’ interpretations of the nature of students’ conceptions represented in the diagrams. For example, when students expressed difficulty in representing the 3D nature of molecular interactions on a piece of paper, the two authors supplemented the students’ diagrams with their verbal explanations and gestures to accurately represent the students’ understanding. The data and the coding scheme were constantly revisited in meetings between the two authors to refine the categories until consensus was reached that all the data was correctly represented and that no more categories were emerging out of the data. Once the two authors agreed upon the categorisation of students’ understanding, the coding scheme was further checked against the data and refined by a second pair of the authors (MM & RT). Amendments in the categorisation of the students’ diagrams were discussed with the first two authors until consensus was reached. The final categories of students’ diagrams were discussed and confirmed by four authors (HM, MW, DT, and MM).
Findings
Analysis of the diagrams in relation to the students’ conceptions of the nature of hydrogen bonds among water molecules in snowflakes generated four conceptual categories, A–D. Category A diagrams showed students’ difficulties in drawing the structure of a water molecule and/or reasoning about polarity in water molecules and the nature of intermolecular interactions. Category B diagrams revealed students’ difficulties in recognizing the role of lone pairs of electrons in the formation of hydrogen bonds. Both Categories C and D diagrams indicated hydrogen bonds as directional intermolecular forces between lone pairs of electrons on oxygen atoms and hydrogen atoms of neighbouring water molecules; Category C diagrams showed the molecular interactions in 2D space whilst Category D diagrams represented interactions in 3D space. The descriptors of the conceptual categories and the number of diagrams in each category are shown in Table 2.| Categories | Number of diagrams | Descriptors |
|---|---|---|
| (A) Uncertain of the structure of water molecules and/or the nature of intermolecular interactions | 10 | Diagrams show linear water molecules and multiple covalent bonds to hydrogen atoms; no indication of polarity in water molecules; intermolecular interactions are represented as covalent bonds between molecules rather than as electrostatic interactions; hydrogen bonds are formed between hydrogen and hydrogen atoms, or oxygen and oxygen atoms. |
| (B) Uncertain of the role of lone pairs of electrons in forming hydrogen bonds | 20 | Diagrams show bent structures and polarity in water molecules; hydrogen bonds are not covalent in nature – they are electrostatic interactions between molecules but the role of lone pairs of electrons in forming a hydrogen bond is unclear. The lone pairs form an electron dense region around the oxygen atom to form one or numerous hydrogen bonds, or individual electrons form hydrogen bonds. |
| (C) Molecules form hydrogen bonds in 2D space | 25 | Diagrams indicate bent structures and polarity in water molecules; hydrogen bonds are electrostatic interactions between oxygen and hydrogen atoms of different molecules; the role of lone pairs in forming hydrogen bonds is clear; water molecules form multiple hydrogen bonds but the interactions among water molecules are represented in 2D space. |
| (D) Molecules form hydrogen bonds in 3D space | 5 | Diagrams indicate hydrogen bonds as electrostatic interactions between lone pairs of electrons and hydrogen atoms in different molecules; each water molecule forms a tetrahedral structure (four hydrogen bonds) in 3D space. |
The conceptual categories A–D are further discussed below:
Category A: uncertain of the structure of water molecules and/or the nature of intermolecular interactions
The diagrams in this category (n = 10) showed that the students had difficulties recognising the difference between the nature of intermolecular and intramolecular interactions. Diagrams in category A did not indicate the existence of polarity in water molecules and its role in the formation of hydrogen bonds. These students’ diagrams showed hydrogen bonds as covalent interactions between molecules rather than as electrostatic interactions. Some diagrams in this category (n = 3) also showed that the students struggled with drawing the structure of a water molecule. These students had difficulties in reasoning about the number of bonds that individual atoms can form. For example, Craig's diagram (Fig. 1a) indicated that the student knew that each water molecule consisted of a central oxygen atom which was bonded to two hydrogen atoms. However, the oxygen atom formed double bonds with hydrogen atoms to complete an octet, just like a carbon atom in a carbon dioxide (CO2) molecule would. The diagram shows that Craig did not realise that the hydrogen atom could not form more than one covalent bond. Craig also had difficulties in recognising the non-covalent nature of intermolecular interactions. Although Craig had heard about hydrogen bonds, the student imagined that these were covalent bonds involving hydrogen atoms of different molecules. To represent a ‘hydrogen bond’, the student drew a solid line between hydrogen atoms in different water molecules. While drawing, Craig explained that “it must be hydrogen to hydrogen … because it is a hydrogen bond… it wouldn’t be oxygen to oxygen… because oxygen already has a full octet”.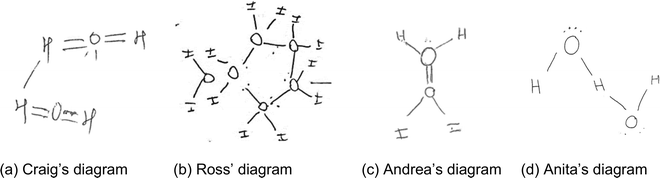 | ||
| Fig. 1 (a–d) Examples of students’ diagrams showing difficulties in reasoning about the structure of water molecules and/or the nature of intermolecular interactions. | ||
The rest of the diagrams in this category (n = 7), such as those of Ross (Fig. 1b), Andrea (Fig. 1c), and Anita (Fig. 1d), showed a reasonable understanding of the structure of a water molecule. The students drew a bent structure for a water molecule with two or four dots around the oxygen atom, suggesting lone pairs of electrons. However, the students had difficulty explaining why the molecule was bent in shape and did not recognize polarity in water molecules and its role in intermolecular interactions. When asked to illustrate how the water molecules in snowflakes interacted, the students made attempts to connect different water molecules, but they had difficulty in recognizing that a hydrogen bond was a non-covalent interaction between an oxygen atom in one molecule and a hydrogen atom in another molecule. For example, in his diagram, Ross indicated dots to represent the nonbonding electrons on oxygen atoms but was not entirely sure of why a water molecule was bent. When asked about the shape of the molecule, he explained that “isn’t it the orbital? … it is supposed to actually repel each other depending on its electronegativity…”. In terms of molecular interactions, Ross connected oxygen atoms in two neighbouring water molecules with a solid line coming off the nonbonding electrons. Ross’ diagram suggested that the oxygen atoms in water molecules would use the lone pairs of electrons to bond covalently with neighbouring water molecules. The student had difficulties distinguishing interactions between atoms to form molecules and interactions among molecules (such as hydrogen bonds). Ross verbally explained that “…the oxygen [atom] is just craving for more bonds because it has the two valence electrons.”
Andrea drew a bent structure of a water molecule (Fig. 1c) and explained that “the water molecule has a bond angle of 120 … I am not sure if it's because these two hydrogen bonds [the O–H bonds in a water molecule] repel each other”. Andrea also connected the oxygen atoms of neighbouring water molecules, but with a double bond. The oxygen atom in a water molecule looked like a carbon atom in an ethene molecule. While drawing her diagram, Andrea explained that “the oxygen molecules will attach to each other … because of the lone pairs.” Like Ross, Andrea had difficulty recognising the difference between intermolecular and intermolecular interactions. Both Ross and Andrea reasoned that the water molecules could interact covalently, and this was clearly represented in their diagrams.
Anita's diagram (Fig. 1d) showed that, the student knew about the composition and bent structure of a water molecule. Anita also knew that a hydrogen bond involved hydrogen and oxygen atoms. However, the student reasoned that a hydrogen bond resulted in a common hydrogen atom being covalently shared between two oxygen atoms. While drawing, Anita commented that, “I think it [the second molecule] will attach to the hydrogen, because it is hydrogen bonding … two water molecules share a hydrogen atom”.
Category B: uncertain of the role of lone pairs of electrons in forming hydrogen bonds
The diagrams in this category (n = 20) showed the bent shape of each water molecule. Most of the diagrams in this category indicated dots on the oxygen atoms to illustrate the presence of nonbonding electrons. Most diagrams in category B also showed that students recognised the presence of partial charges in water molecules. In addition, all the diagrams in category B showed that hydrogen bonds were intermolecular in nature and different from intramolecular bonds. For this purpose, the students connected molecules with dashed or zigzag lines, or simply showed water molecules close to one another to show that hydrogen bonds were not covalent bonds but simply electrostatic forces of attraction between oxygen and hydrogen atoms in different molecules. However, the diagrams in category B showed students’ difficulties in reasoning about the role of lone pairs of electrons in the formation of hydrogen bonds.Four of the diagrams in this category, such as those of Harrold (Fig. 2a) and Jasper (Fig. 2b), showed that a water molecule has two charged regions, a partially positive (δ+) and a partially negative (δ−) region. The hydrogen atoms of each water molecule collectively formed a partially positively charged region while the nonbonding electrons on the oxygen atom also collectively created a single partially negatively charged region. Water molecules in snowflakes interacted through electrostatic attraction between these oppositely charged regions without any directionality. Neighbouring water molecules were stacked around the central one. For example, Harrold's diagram showed that a water molecule was bent in shape and that the oxygen atom had two lone pairs of electrons. The student explained that these electron pairs repel each other and the position of the hydrogen atoms result in the bent shape of a water molecule, and that “the water molecule is polar, one side is slightly negative, the other side is positive (draws partial charges)”. To represent the interactions among molecules, Harrold illustrated that the hydrogen end of each incoming water molecule approached the region of lone pairs of electrons on the central molecule and vice versa. The student used zigzag lines to represent the interactions between the oppositely charged ends of different water molecules, suggesting that the hydrogen bonds were not covalent in nature. While constructing his diagram, Harrold explained that “opposites attract… so slightly negative will go to the slightly positive side of the other water molecule” without mentioning the role of lone pairs of electrons or individual hydrogen atoms. Harrold's explanation confirmed our interpretation of his diagram that the student recognized polarity in water molecules but had difficulties in recognizing the role of individual lone pairs of electrons and the individual hydrogen atoms in the formation of hydrogen bonds. A similar explanation of molecular interactions was provided by Jasper.
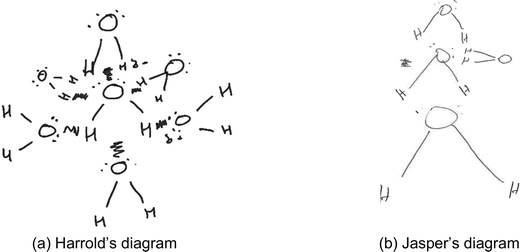 | ||
| Fig. 2 (a and b) Examples of students’ diagrams showing difficulties in reasoning about the directionality of hydrogen bonds. | ||
About half of the diagrams in category B (n = 8) also showed that the oxygen atom in a water molecule is partially negatively charged (δ−) and that each hydrogen atom was a partially positively charged (δ+) region. Like the diagrams in Fig. 2, these diagrams (Fig. 3) showed that the two lone pairs of electrons in a water molecule collectively formed a single partially negative (δ−) region. However, different from the diagrams in Fig. 2, each hydrogen atom was a separate partially positively charged region. Therefore, each water molecule had three partially charged regions. Oppositely charged regions in neighbouring water molecules would attract each other in a flat plane.
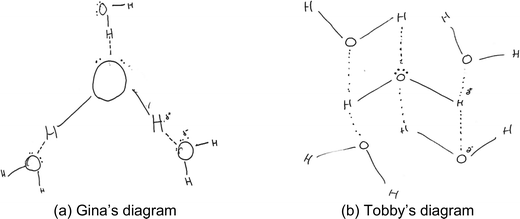 | ||
| Fig. 3 (a and b) Examples of students' diagrams showing three partially charged regions in a water molecule forming hydrogen bonds. | ||
For example, Gina's diagram showed that, to form hydrogen bonds among water molecules, the partially negative region around the oxygen atom of the central water molecule attracted a single hydrogen atom of a neighbouring water molecule (Fig. 3a), and each hydrogen atom of the central molecule attracted an oxygen atom of a neighbouring molecule. To support her diagram, Gina first explained that a water molecule was bent because “based on the electron repulsion theory, the nonbonding electrons force the hydrogen atoms closer together (gestures the bent shape with both hands)”. While drawing the interactions between molecules, Gina first connected water molecules to the hydrogen atoms of the central molecule reasoning that “there is a hydrogen bond… an intermolecular force between the positive end of this [central] water molecule and the negative end of this [attaching] water molecule, they attract each other”. According to Gina, the oxygen and hydrogen ends of a water molecule created a dipole. However, the two lone pairs of electrons collectively formed a single hydrogen bond. Gina's verbal explanations were aligned with her diagram representing bent shapes of water molecules, partial charges on the atoms, and a single dotted line from each oxygen atom to represent intermolecular hydrogen bonds.
On the other hand, Tobby's diagram (Fig. 3b) also clearly showed the bent shape and polarity in water molecules, but the oxygen atom of the central molecule had a negative charge all around it that would attract hydrogen atoms of neighbouring molecules from different directions. According to Tobby, “…there's more electrons present on the central oxygen compared to the hydrogen, which means that this [hydrogen atom] becomes… slightly delta positive and this [oxygen atom]… slightly negative, and… opposites attract.” Just like his diagram, Tobby's verbal explanation did not emphasise the role of lone pairs of electrons. The diagram also indicated that the student had difficulties in recognising how many hydrogen bonds each hydrogen atom could form. From his diagram, each hydrogen atom of a central molecule appeared to have enough positive charge to attract more than one oxygen atom from neighbouring water molecules (Fig. 3b).
The rest of the diagrams in category B (n = 8) also showed that each hydrogen atom in a water molecule is a separate partially positive (δ+) region, and that hydrogen bonds were electrostatic interactions amongst molecules. However, different from the diagrams in Fig. 2 and 3, these eight diagrams (such as those in Fig. 4) showed that each of the four nonbonding electrons on the oxygen atom in a water molecule can individually form a hydrogen bond with a hydrogen atom in a neighbouring water molecule. For example, diagrams by Morris (Fig. 4a) and Aaron (Fig. 4b) showed that, being negatively charged, each individual nonbonding electron on the oxygen atom can attract a partially positively charged hydrogen atom from a neighbouring molecule.
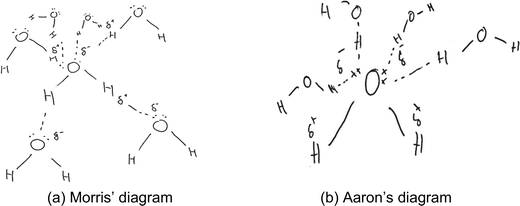 | ||
| Fig. 4 (a and b) Examples of students’ diagrams showing hydrogen bond formation with individual nonbonding electrons. | ||
When asked to provide his reasoning, Morris explained that “I have attached one of the lone electrons to the positive hydrogen, that's how they bond… the hydrogen only takes one electron … two hydrogens for each pair”. Although the student talked about the hydrogen atoms ‘bonding’ to the individual electrons, he indicated these intermolecular hydrogen bonds as dotted lines to distinguish them from the intramolecular covalent bonds. A similar line of reasoning was evident in Aaron's diagram and verbal explanation, except that Aaron did not draw hydrogen bonds from the hydrogen atoms of the central water molecule.
Category C: molecules form hydrogen bonds in 2D space
The diagrams in this category (n = 25) showed the bent shape of a water molecule. The diagrams also showed that the students recognised that each water molecule is polar in nature and has two pairs of nonbonding electrons on the oxygen atom. Similar to category B, the diagrams in category C also showed a hydrogen bond as an electrostatic force of attraction between different water molecules. Unlike diagrams in category B, the role of lone pairs of electrons in forming hydrogen bonds was clearly shown in the diagrams in category C. Diagrams in category C showed that a hydrogen bond formed between a lone pair of electrons on an oxygen atom in one molecule and a partially positive hydrogen atom of a neighbouring water molecule. Since a water molecule has multiple sites (individual hydrogen atoms and lone pairs of electrons) for forming hydrogen bonds, each water molecule can form multiple hydrogen bonds with other molecules.Most diagrams in this category (n = 20), such as those of Monica (Fig. 5a) and Carlos (Fig. 5b), showed that each water molecule in a snowflake formed a maximum of four hydrogen bonds with neighbouring water molecules using two lone pairs of electrons on the oxygen atom and two hydrogen atoms. In her diagram, Monica illustrated partial charges on the different atoms and labelled the hydrogen bond as the force of attraction between a lone pair and a partially positive hydrogen atom. Monica explained that “oxygen and hydrogen have different electronegativities which allow molecules to interact via hydrogen bonds… the lone pairs of electrons help the hydrogen atoms to be attracted to the oxygen”. Similarly, after drawing the first four water molecules attached to a central molecule, Carlos explained that any more water molecules would be attached “not to the first one [central molecule] but on those outside” suggesting that the student recognized that each molecule had only four opportunities for forming hydrogen bonds. However, these students’ diagrams, did not indicate the positioning of water molecules in 3D space, implying that the water molecules were interacting in a flat plane. For example, the drawings by Monica and Carlos showed a square planar arrangement created by water molecules interacting with the central molecule in 2D. Also, the students did not mention molecules interacting in 3D.
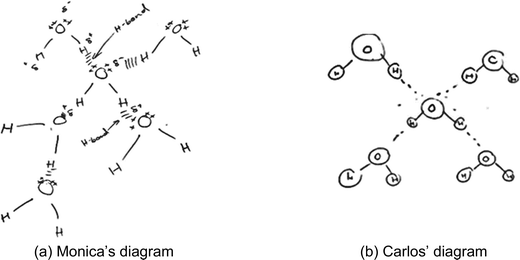 | ||
| Fig. 5 (a and b) Examples of students’ diagrams showing a water molecule forming hydrogen bonds with four other molecules in 2D. | ||
The remaining five diagrams in this category not only showed that molecules interacted in 2D but also that the students did not recognize that, in snowflakes, each water molecule interacted with four other water molecules through hydrogen bonds. For example, Calvin's diagram (Fig. 6a) indicated that each water molecule had two lone pairs of electrons and two hydrogen atoms, and that each hydrogen atom was attracted to a lone pair of electrons. Both hydrogen atoms and lone pairs of electrons participated in the formation of hydrogen bonds and each water molecule formed four hydrogen bonds. In his explanation, Calvin elaborated that “the hydrogen and oxygen ends of water molecules differ in polarity… they attract … hydrogen to lone pair”. However, Calvin's diagram showed that he was uncertain of the orientation of the water molecules around the central one – water molecules arranged themselves in a 2D plane.
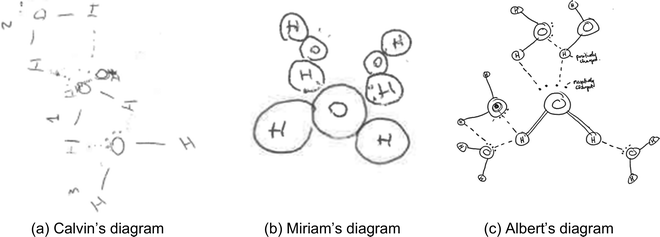 | ||
| Fig. 6 (a–c) Examples of students' diagrams showing a water molecule forming hydrogen bonds with multiple (but not four) other molecules in 2D. | ||
Miriam's diagram (Fig. 6b) showed that the student recognised a hydrogen bond as an intermolecular force rather than a covalent bonding interaction; by drawing the attached water molecules in those positions on the central one, the diagram showed that the student recognised the role of lone pairs. After connecting a hydrogen atom to the central water molecule, the student also verbally explained that “there will be a lone pair here [on the oxygen], and another lone pair here”. However, Miriam's diagram also showed that only the lone pairs of electrons on the central molecule would participate in forming hydrogen bonds. As a result, one water molecule in a snowflake could form a maximum of two hydrogen bonds with neighbouring molecules in 2D. This interpretation was further confirmed when the student explained that it was not possible for more water molecules to interact with the first molecule because “there were no more lone pairs”.
Albert's diagram (Fig. 6c) showed that both hydrogen atoms and lone pairs of electrons in a water molecule could participate in forming hydrogen bonds. Albert's representation of the first water molecule was different from the rest. It appeared that the student was trying to produce a realistic diagram of the molecular models he was familiar with. When asked about his representation, the student explained that “[the double lines] are meant to be like a ball-and-stick model.” Albert completed the diagram by adopting a simpler convention of representing water molecules using a single line to show the covalent bond between oxygen and hydrogen atoms. The student verbally explained that “[a hydrogen atom is] slightly more positively charged, which is attracting the negative charge of the oxygen free electrons, lone pairs”. Despite this understanding, Albert visualised the molecular interactions in 2D. As a result, in his diagram, the student misjudged the distances between atoms in different molecules – water molecules could approach each other so closely that a hydrogen atom in one water molecule could form more than one hydrogen bond and a water molecule could form more than four hydrogen bonds with neighbouring molecules.
Category D: molecules form hydrogen bonds in 3D space
Like most diagrams in category C, the diagrams in category D (n = 5) showed a reasonable understanding of the nature of hydrogen bonds among water molecules in snowflakes in that hydrogen bonds were electrostatic in nature and were formed between hydrogen atoms and lone pairs of electrons in different molecules. Using both hydrogen atoms and both lone pairs of electrons on the oxygen atom, each water molecule in a snowflake formed a maximum of four hydrogen bonds with other water molecules. Unlike category C, the diagrams in category D also emphasized that the water molecules interacted in a three-dimensional space rather than in a two-dimensional plane. For example, the diagram by Matt (Fig. 7a) showed that the nonbonding electron pairs on the centre oxygen atom in a water molecule were in different planes. Therefore, through hydrogen bonds, water molecules formed a 3D tetrahedral structure. When drawing, Matt explained that “it [the arrangement of molecules] will be a bit more 3D than that but, yeah, that would be a tetrahedral form”. Similarly, although the first diagram by Timothy (Fig. 7b) appeared like those in category C, Timothy drew a second diagram to emphasize that the four electron domains in a water molecule were in 3D space. Therefore, water molecules would form 3D structures by interacting with other water molecules through hydrogen bonds. In fact, after drawing the four water molecules around one, Timothy explained that “…a better way to draw it would be in 3D like (draws the water molecule with electron domains on different sides), you know how we draw 3D?… is that 3D?”.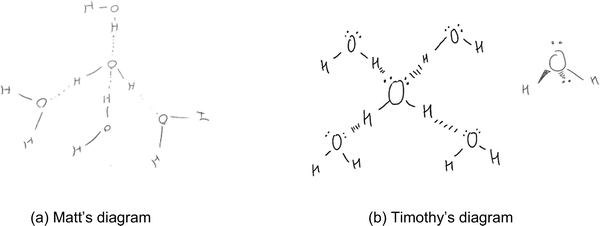 | ||
| Fig. 7 (a and b) Examples of students' diagrams showing water molecules forming hydrogen bonds in 3D. | ||
Some students (n = 3) acknowledged that water molecules would interact in 3D, but they could not effectively represent such interactions with their diagrams on a piece of paper. Instead, they used gestures and verbal explanations to express their ideas. For example, after drawing four water molecules connected to a central one with hydrogen bonds, one of the students explained that “…these four water molecules could in turn bond onto other three individual molecules (uses gestures to show interactions from different angles)… it would create a shape that is 3D lattice.”
Summary of analyses
Overall, the analysis of the student-generated diagrams in this study showed that most students (50 out of 60) had a good understanding of the structure and polarity of a water molecule. However, the nature of hydrogen bonds among water molecules in snowflakes was conceptualised in a wide range of ways. While half (n = 30) of the student-generated diagrams showed a hydrogen bond as being intermolecular, electrostatic, and directional in nature (categories C and D), the other half of diagrams revealed students’ difficulties or alternative conceptions about the structure of a water molecule and/or the nature of intermolecular interactions (category A), or the role of lone pairs of electrons in the formation of hydrogen bonds (category B). Also, the number of students who visualised hydrogen bonds among water molecules in 3D space (5 out of 60) was very small considering that the participants in this study were those majoring in chemistry and chemistry-related degree programs and had experienced teaching with molecular models.Discussion
Using student-generated diagrams, this study investigated university chemistry students’ conceptual understanding of the nature of hydrogen bonds among water molecules in snowflakes. Irrespective of the students’ conceptions of the nature of hydrogen bonds, most of the diagrams showed the bent structure of water molecules and showed the nature of intramolecular bonds in water molecules to be covalent. While constructing the diagrams, many students explained that drawing was not an easy task for them because they had never considered representing molecular interactions when many molecules are involved. The relatively novel approach of visually illustrating their understanding through diagrams challenged students to integrate their prior understanding in a way that they may not have been used to before. Studies also have reported that even though students may be able to express their understanding in symbolic forms, they may have difficulties to visually represent chemical processes at the submicroscopic level (Nyachwaya et al., 2011; Dickson et al., 2016).Despite the difficulties these students may have faced in representing the molecular interactions among water molecules in snowflakes, with appropriate prompts, these first and second-year undergraduate students made efforts to illustrate how they visualised the interactions. Many students represented hydrogen bonds as dashed (or dotted) lines. Other students simply positioned hydrogen atoms close to or touching the lone pairs of electrons on oxygen atoms. These representations were efforts by the students to illustrate that a hydrogen bond was a force of attraction between molecules, different from intramolecular bonds. Many students also recognised that water molecules were polar in nature, and some included partial charges on the oxygen and hydrogen atoms in their diagrams. Our findings suggest that, when prompted appropriately, students can engage in drawing diagrams that illustrate their conceptual understanding.
This study found that the students had varied ways of conceptualizing the exact nature of hydrogen bonds among water molecules, even though most recognised the hydrogen bonds as intermolecular forces. Half of the diagrams (30 out of 60) showed a reasonable scientific understanding of the nature of hydrogen bonds among water molecules in snowflakes. The diagrams showed a hydrogen bond as an electrostatic attraction between a hydrogen atom of one water molecule and a lone pair of electrons on the oxygen atom in a neighbouring water molecule. Some diagrams revealed students’ difficulties in conceptualising the nature of hydrogen bonds among water molecules in snowflakes, including difficulties in distinguishing hydrogen bonds from covalent bonds (n = 10), recognising the role of lone pairs of electrons in the formation of hydrogen bonds (n = 20), and recognising molecular interactions in 3D space (n = 25).
Previous studies (e.g., Henderleiter et al., 2001; Schmidt et al., 2009; Cooper et al., 2015) have reported a range of students’ alternative conceptions and learning difficulties related to the concept of a hydrogen bond including: students recognising hydrogen bonds as bonds “within” rather than as forces “between” ethanol molecules (Cooper et al., 2015; Williams et al., 2015), difficulties in identifying molecules in which hydrogen bonds form (Schmidt et al., 2009), or difficulties in using the concept of hydrogen bonds to explain macroscopic properties of substances (Henderleiter et al., 2001). In the present study, challenging students to construct their own representations of molecular interactions uncovered more students’ alternative conceptions of the structure and polarity of a water molecule and the nature of hydrogen bonds. The alternative conceptions about the structure of water molecules included: water molecules forming linear structures like CO2, hydrogen atoms in water molecules forming multiple covalent bonds with one oxygen atom, and two oxygen atoms in different water molecules covalently sharing a common hydrogen atom between them. Alternative conceptions related to the nature of hydrogen bonds in water molecules included: hydrogen bonds as covalent bonds formed when oxygen atoms in water molecules use their nonbonding electrons to bond to other oxygen atoms in other molecules, individual nonbonding electrons on oxygen atoms forming hydrogen bonds, hydrogen atoms on a water molecule forming a single partially positively charged region which can form hydrogen bonds without directionality, lone pairs of electrons collectively forming a single partially negative region that can form one or multiple hydrogen bonds without directionality, and hydrogen bonds as molecular interactions in 2D space. These alternative conceptions have not been reported in previous studies.
The differences in research designs can be used to explain the differences in students’ alternative conceptions of the nature of hydrogen bonds revealed in this study and those reported in earlier studies. Unlike most studies that explicitly ask students to discuss their understanding of key terminologies such as intermolecular forces or hydrogen bonds (e.g., Henderleiter et al., 2001; Schmidt et al., 2009; Cooper et al., 2015), in the present study, we did not directly use the term ‘hydrogen bond’ in our interview prompts. Instead, we asked students to illustrate how a central water molecule in a snowflake would interact with other molecules in its vicinity. By avoiding key chemistry terms such as ‘hydrogen bonds’, the students were free to illustrate their true understanding, imaginations, or predictions of the nature of molecular interactions in snowflakes rather than reproduce textbook definitions of the concept. Researchers (e.g., Cooper et al., 2015) have suggested that the term ‘hydrogen bond’ may be confused by students as any bond that involves a hydrogen atom, such as an intramolecular oxygen–hydrogen bonds in water or in alcohol molecules. By asking students to draw diagrams of molecular interactions, they made efforts to imagine and represent their conceptions.
It is often difficult to pin-point the sources of students’ learning difficulties and alternative conceptions (Taber, 2019). However, in this study, some of the alternative conceptions exhibited in students’ diagrams (for example, a hydrogen atom with more than one covalent bond) may stem from incomplete understanding of basic concepts such as atomic structure and bonding, concepts which are taught at lower levels of formal education. Studies have also reported that students’ alternative concepts can be retained over long periods of time (Dickson et al., 2016) and can be retained even after explicit instruction (e.g., Rushton et al., 2008). Drawing flat diagrams without any indication of the 3D nature of molecular interactions may be a result of students’ familiarity with 2D drawings without explicit emphasis on the limitations of these models or connections to what the drawings actually represent in 3D (e.g., Nicoll, 2003). Students’ difficulties in visualising 3D interactions based on 2D diagrams have also been widely recognised in the literature (Wu and Shah, 2004). In the present study, difficulties with conceptualizing basic concepts such as bonding in water molecules were evident in category A diagrams. In addition, although students used ball-and-stick molecular models in the chemistry units from which we recruited the participants, drawing diagrams to represent molecules and their interactions in 3D was hardly emphasized by the instructors and tutors. When discussing the concept of hydrogen bonds, the recommended textbook materials (e.g., Blackman et al., 2019) did not emphasise the 3D nature of interactions between water molecules either. By providing opportunities for students to interact with 3D molecular model kits, the instructors and tutors may have assumed that the students would intuitively recognise that molecules interact in 3D. However, this was not case as most students drew flat diagrams and did not mention molecules interacting in 3D.
Other learning difficulties may result from the way in which the concept of the nature of a hydrogen bond is taught and assessed. For example, when educators teach, they often emphasise that a hydrogen bond is an electrostatic attraction between a hydrogen atom in one molecule and a highly electronegative atom bearing lone pairs of electrons from a different molecule (Henderleiter et al., 2001). However, such a definition does not emphasise the location of the hydrogen bond. In addition, when discussing molecules that can form hydrogen bonds such as alcohols and carboxylic acids, some chemistry textbooks illustrate a dotted line connecting one oxygen atom in one molecule and a hydrogen atom in another molecule without showing the role of lone pairs of electrons (e.g., Brady and Senese, 2004; Brown et al., 2013; Blackman et al., 2019). Although not a compulsory resource, Blackman et al. (2019) was one of the recommended texts in the teaching of the chemistry units we recruited the participants from. Therefore, some of these textbook illustrations may have been partly responsible for students considering both lone pairs of electrons on an oxygen atom as a single negatively charged region. Moreover, the nature of assessment also normally does not go beyond one molecule connecting to one other molecule through hydrogen bonds. By asking students to connect only two molecules, it is easy for the students to attain a full score even though they may have alternative conceptions if tasked to connect multiple molecules. Therefore, in this study, when the students were tasked to connect multiple water molecules, they felt challenged and had to think about what happens amongst many water molecules.
Overall, the wide range of students’ conceptions of the nature of hydrogen bonds among water molecules identified in the present study highlights the benefits of using student-generated diagrams to investigate the level of students’ conceptual understanding of science concepts. The drawing tasks challenged students to link together their prior knowledge of the nature of bonding, structure, and polarity in a water molecule, and hydrogen bonds amongst water molecules in the context of snowflakes. The students made genuine efforts to express their understanding. The study, therefore, also lends support to previous studies that advocate for the inclusion of drawing tasks in science learning sessions to improve student engagement (Ainsworth et al., 2011) and reasoning (Tytler et al., 2020; Andrade et al., 2021; McLure et al., 2021a).
Implications
Understanding students’ conceptions of a science concept is a key step in identifying students’ difficulties and designing teaching interventions to address them. In this study, we used the context of snowflakes and student-generated diagrams to investigate students’ conceptual understanding of the nature of hydrogen bonds among many water molecules. The drawing activity provided students with an opportunity to retrieve, organize, and link their knowledge of chemistry concepts to communicate their understanding of hydrogen bonds among water molecules in snowflakes. By analysing the students’ diagrams, we were able to identify a range of students’ difficulties and alternative conceptions of the nature of hydrogen bonds rather than their recalled knowledge of key chemistry terms.In terms of assessment, educators may want to employ drawing activities to investigate students’ understanding of abstract science concepts. It is worth noting that the use of diagrams as an assessment tool of students’ conceptions has garnered some debate over the past 20 years. Some researchers (e.g., Ehrlén, 2009) suggest that drawings may not be a good way to accurately understand students’ conceptions because students may inconsistently rely on social-cultural resources to represent their ideas in drawings. Consequently, researchers such as Becker et al. (2016) argue that students’ diagrams can only be understood when analysed in combination with other modes such as interviews (verbal) or written explanations. Indeed, making sense of students’ diagrams on their own is a challenging task because it requires content knowledge, representational skills, and experience in interpreting students’ diagrams.
However, previous research has developed a set of procedures that researchers can use to make sense of student-generated diagrams without extensively referring to their verbal explanations (McLure et al., 2021b; Tenzin et al., 2022). In addition, although drawing 2D diagrams may not afford students an opportunity to conveniently represent molecular interactions in 3D space, spatial aspects are even harder to discern in many of the alternative representation modes such as verbal and written explanations. Compared to other conventional representation methods, we believe drawing diagrams is at least as effective in evaluating how students visualise spatial relations among molecules. Moreover, triangulation of the data indicated that students’ diagrams were well aligned with their verbal explanations, except for three students who relied on words and gestures to communicate their understanding of 3D interactions of molecules. In future, in addition to diagrams, alternative ways of representing spatial relations (e.g., gestures) could be explored further.
Different from other studies investigating students’ conceptions with diagrams (e.g., Nyachwaya et al., 2011; Cooper et al., 2015), this study offered staged prompts to accompany drawing tasks and it might have contributed to increasing the value of the drawings as a conceptual assessment tool. In the present study, the staged prompts engaged the students in imagining and reasoning about molecular interactions and, even though they were not used to the task, the students drew diagrams that reflected their conceptions of science concepts. Research reported that providing students with scaffolding prompts while drawing increases the degree of correlation between the student-generated diagrams and their written explanations (Noyes and Cooper, 2019). When designing diagram-based conceptual assessments, future researchers may wish to provide staged prompts to accompany drawing tasks. The prompts should be designed carefully to make visible the nature of students’ conceptual understanding rather than recall of science terminologies. In addition, future researchers may also wish to experiment with different ways of administering the staged prompts to large groups of students at once (e.g., in written form). This is because conducting interviews (as we did) in large classes is very time-consuming and impractical on a regular basis.
For students to represent their ideas in diagrams, the present study used the easy-to-use pen and paper. We made this choice to avoid the additional difficulty that some students may encounter in using technology to represent their ideas. However, digital drawing is becoming more popular in teaching and learning settings (Ainsworth and Scheiter, 2021). In future studies, educators may want to consider using different tools for drawing or to investigate the role of technology in using student-generated diagrams for assessment purposes.
The findings of the present study also have implications on the level of knowledge targeted by drawing assessments, as well as the teaching of chemistry. Assessing students’ conceptual understanding at a deeper level, for example by asking students to connect multiple water molecules rather than just two water molecules, can be beneficial in understanding students’ learning difficulties and alternative conceptions. In terms of teaching practice, since many students visualized the molecular interactions in 2D, the present study highlights a need for educators to emphasise the 3D nature of molecular interactions in their teaching and the limitations of the different modal representations (such as 2D drawings). It is also important for educators to explicitly guide students in ways of representing molecular interactions at the submicroscopic level. In addition, educators need to design learning interventions to support students’ 3D visualisation of molecular concepts. Educators may want to investigate the potential of 3D visualisation technologies such as immersive virtual reality in supporting students’ visualisation of molecular concepts.
Limitations
The limitations of the study were two-fold. First, the study was conducted at a single university in Australia. Therefore, it is not clear whether the findings of this study in relation to the students’ conceptions of the nature of hydrogen bonds between water molecules would be evident in other universities. Future studies may benefit from involving participants from multiple institutions to derive more generalisable findings. Secondly, participants in this study were those majoring in chemistry and related courses. Therefore, the findings may not be generalisable to all student populations. Applying a similar assessment protocol to students in a different setting, for example, in high school, would help researchers explore how students develop these ideas across populations. Nevertheless, the present study has identified several alternative conceptions of the nature of hydrogen bonds that have not been reported earlier. These alternative conceptions include; a hydrogen bond resulting in two oxygen atoms of different molecules sharing a common hydrogen atom between them, hydrogen bonds being covalent bonds formed when oxygen atoms in water molecules use their nonbonding electrons to bond to other oxygen atoms, individual nonbonding electrons on the oxygen atoms forming hydrogen bonds, hydrogen atoms on a water molecule forming a single partially positive region which can form hydrogen bonds without directionality, lone pairs of electrons collectively forming a single partially negative region that can form one or multiple hydrogen bonds without directionality, and hydrogen bonds as molecular interactions in a 2D space.Conclusion
This study revealed a wide range of alternative conceptions of the nature of hydrogen bonds amongst many water molecules in snowflakes, including the shape of water molecule, the role of lone pairs in forming hydrogen bonds, and the molecular interactions in a 2D plane. These alternative conceptions were uncovered largely due to the unique question prompt (interactions amongst many water molecules) and the distinctive mode of representation (diagrams). Different from the routine written test items, the drawing task demanded that students go beyond repeating scientific vocabulary or applying test-taking skills. The students made genuine efforts to imagine, visualise, and illustrate the interactions amongst many water molecules in snowflakes to demonstrate their understanding of the nature of hydrogen bonds. Considering the powerful insights we gained from the analysis of student-drawn diagrams, chemistry educators may wish to adopt similar strategies to examine and support students’ conceptual understanding.Conflicts of interest
The authors have no conflict of interest to declare.Acknowledgements
This study was supported by Australian Research Council Discovery Projects, Drawing science diagrams to enhance students’ scientific creativity (DP 180100143) and Using immersive virtual reality to enhance students’ scientific visualisation (DP 190100160).References
- Ainsworth S., (1999), The functions of multiple representations, Comput. Educ., 33(2), 131–152.
- Ainsworth S., (2006), DeFT: A conceptual framework for considering learning with multiple representations, Learn. Instr., 16(3), 183–198.
- Ainsworth S. E. and Scheiter K., (2021), Learning by drawing visual representations: Potential, purposes, and practical implications, Curr. Dir. Psychol. Sci., 30(1), 61–67.
- Ainsworth S., Prain V. and Tytler R., (2011), Drawing to learn in science, Science, 333(6046), 1096–1097.
- Allred Z. D. R. and Bretz S. L., (2019), University chemistry students’ interpretations of multiple representations of the helium atom, Chem. Educ. Res. Pract., 20(2), 358–368.
- Andrade D. V. F., Freire S. and Baptista M., (2021), Constructing scientific explanations for chemical phenomena through drawings among 8th-grade students, Eurasia J. Math. Sci. Technol., 17(1), em1937.
- Becker N., Noyes K. and Cooper M., (2016), Characterizing students’ mechanistic reasoning about London dispersion forces, J. Chem. Educ., 93(10), 1713–1724.
- Blackman A., Bottle S. E., Schmid S., Mocerino M. and Wille U., (2019), Chemistry, Wiley.
- Brady J. and Senese F., (2004), Chemistry: Matter and its changes, Wiley.
- Brown T. L., LeMay H. E., Bursten E. B., Murphy C. and Woodward P., (2013), Chemistry: The central science, Pearson.
- Chang H.-Y., Lin T.-J., Lee M.-H., Lee S. W.-Y., Lin T.-C., Tan A.-L. and Tsai C.-C., (2020), A systematic review of trends and findings in research employing drawing assessment in science education, Stud. Sci. Educ., 56(1), 77–110.
- Cheng M. and Gilbert J. K., (2009), in Gilbert J. K. and Treagust D. (ed.), Multiple representations in chemical education, Dordrecht: Springer Netherlands, pp. 55–73.
- Chittleborough G. and Treagust D., (2008), Correct interpretation of chemical diagrams requires transforming from one level of representation to another, Res. Sci. Educ., 38(4), 463–482.
- Cooper M. M., Corley L. M. and Underwood S. M., (2013), An investigation of college chemistry students' understanding of structure–property relationships, J. Res. Sci. Teach., 50(6), 699–721.
- Cooper M. M., Williams L. C. and Underwood S. M., (2015), Student understanding of intermolecular forces: A multimodal study, J. Chem. Educ., 92(8), 1288–1298.
- Davidowitz B., Chittleborough G. and Murray E., (2010), Student-generated submicro diagrams: a useful tool for teaching and learning chemical equations and stoichiometry, Chem. Educ. Res. Pract., 11(3), 154–164.
- Derman A. and Ebenezer J., (2020), The effect of multiple representations of physical and chemical changes on the development of primary pre-service teachers cognitive structures, Res. Sci. Educ., 50(4), 1575–1601.
- Derman A., Koçak N. and Eilks I., (2019), Insights into components of prospective science teachers’ mental models and their preferred visual representations of atoms, Educ. Sci., 9(2), 154.
- Dickson H., Thompson C. D. and O'Toole P., (2016), A picture is worth a thousand words: Investigating first year chemistry students’ ability to visually express their understanding of chemistry concepts, Int. J. Innov. Sci. Math. Educ., 24(1), 12–23.
- Ehrlén K., (2009), Drawings as representations of children's conceptions, Int. J. Sci. Educ., 31(1), 41–57.
- Farland-Smith D., (2012), Development and field test of the modified Draw-a-Scientist Test and the Draw-a-Scientist Rubric, Sch. Sci. Math., 112(2), 109–116.
- Finson K. D., (2002), Drawing a scientist: What we do and do not know after fifty years of drawings, Sch. Sci. Math., 102(7), 335–345.
- Gilbert J. K., (2006), On the nature of “context” in chemical education, Int. J. Sci. Educ., 28(9), 957–976.
- Gilbert J. K. and Treagust D. F., (2009), Multiple representations in chemical education, Springer.
- Henderleiter J., Smart R., Anderson J. and Elian O., (2001), How do organic chemistry students understand and apply hydrogen bonding? J. Chem. Educ., 78(8), 1126.
- Jewitt C., (2013), in Price S., Jewitt C. and Brown B. (ed.), The SAGE handbook of digital technology research, London: SAGE, pp. 250–265.
- Kelly R. M., Barrera J. H. and Mohamed S. C., (2010), An analysis of undergraduate general chemistry students’ misconceptions of the submicroscopic level of precipitation reactions, J. Chem. Educ., 87(1), 113–118.
- Kern A. L., Wood N. B., Roehrig G. H. and Nyachwaya J., (2010), A qualitative report of the ways high school chemistry students attempt to represent a chemical reaction at the atomic/molecular level, Chem. Educ. Res. Pract., 11(3), 165–172.
- Kozma R., (2003), The material features of multiple representations and their cognitive and social affordances for science understanding, Learn. Instr., 13(2), 205–226.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34(9), 949–968.
- Liu Y., Won M. and Treagust D. F., (2014), in Eilam B. and Gilbert J. K. (ed.), Science teachers’ use of visual representations, Switzerland Springer International Publishing, pp. 103–121.
- Markic S. and Eilks I., (2015), in Kahveci M. and Orgill M. (ed.), Affective dimensions in chemistry education, Berlin, Heidelberg: Springer, pp. 259–278.
- McLure F., Won M. and Treagust D. F., (2021a), Analysis of students’ diagrams explaining scientific phenomena, Res. Sci. Educ., 52(4), 1225–1241.
- McLure F., Won M. and Treagust D. F., (2021b), What students’ diagrams reveal about their sense-making of plate tectonics in lower secondary science, Int. J. Sci. Educ., 43(16), 2684–2705.
- Merriam S. B. and Tisdell E. J., (2015), Qualitative research: A guide to design and implementation, John Wiley & Sons.
- Miller D. I., Nolla K. M., Eagly A. H. and Uttal D. H., (2018), The development of children's gender-science stereotypes: A Meta-analysis of 5 decades of U.S. Draw-A-Scientist studies, Child Dev., 89(6), 1943–1955.
- Nicoll G., (2003), A qualitative investigation of undergraduate chemistry students' macroscopic interpretations of the submicroscopic structures of molecules, J. Chem. Educ., 80(2), 205.
- Noyes K. and Cooper M. M., (2019), Investigating student understanding of London dispersion forces: A longitudinal study, J. Chem. Educ., 96(9), 1821–1832.
- Nyachwaya J. M., Mohamed A.-R., Roehrig G. H., Wood N. B., Kern A. L. and Schneider J. L., (2011), The development of an open-ended drawing tool: an alternative diagnostic tool for assessing students' understanding of the particulate nature of matter, Chem. Educ. Res. Pract., 12(2), 121–132.
- Quillin K. and Thomas S., (2015), Drawing-to-learn: A framework for using drawings to promote model-based reasoning in biology, CBE Life Sci. Educ., 14(1), es2.
- Reinisch B., Krell M., Hergert S., Gogolin S. and Krüger D., (2017), Methodical challenges concerning the Draw-A-Scientist Test: A critical view about the assessment and evaluation of learners’ conceptions of scientists, Int. J. Sci. Educ., 39(14), 1952–1975.
- Rushton G. T., Hardy R. C., Gwaltney K. P. and Lewis S. E., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students, Chem. Educ. Res. Pract., 9(2), 122–130.
- Schmidt H.-J., Kaufmann B. and Treagust D. F., (2009), Students’ understanding of boiling points and intermolecular forces, Chem. Educ. Res. Pract., 10(4), 265–272.
- Taber K. S., (2019), Alternative conceptions and the learning of chemistry, Isr. J. Chem., 59(6–7), 450–469.
- Tenzin S., Won M. and Treagust D., (2022), Hair-raising fun: Making sense of student-generated diagrams, Sci. Teach., 90(1), 48–54.
- Tippett C. D., (2016), What recent research on diagrams suggests about learning with rather than learning from visual representations in science, Int. J. Sci. Educ., 38(5), 725–746.
- Treagust D. F., Won M. and McLure F., (2017), in Amin G. T. and Levrini O. (ed.), Converging perspectives on conceptual change: Mapping an emerging paradigm in the learning sciences, London: Routledge, pp. 121–128.
- Tytler R., Prain V., Aranda G., Ferguson J. and Gorur R., (2020), Drawing to reason and learn in science, J. Res. Sci. Teach., 57(2), 209–231.
- Van Meter P. and Garner J., (2005), The promise and practice of learner-generated drawing: Literature review and synthesis, Educ. Psychol. Rev., 17(4), 285–325.
- Williams L. C., Underwood S. M., Klymkowsky M. W. and Cooper M. M., (2015), Are noncovalent interactions an Achilles heel in chemistry education? A comparison of instructional approaches, J. Chem. Educ., 92(12), 1979–1987.
- Wu S. P. W. and Rau M. A., (2019), How students learn content in science, technology, engineering, and mathematics (STEM) through drawing activities, Educ. Psychol. Rev., 31(1), 87–120.
- Wu H.-K. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88(3), 465–492.
| This journal is © The Royal Society of Chemistry 2023 |
